10
Reference Values for Tetrachloroethylene
The Environmental Protection Agency (EPA) draft Integrated Risk Information System (IRIS) assessment of tetrachloroethylene provides the agency’s assessment of the potential human health effects of exposure to the chemical. For noncancer effects, EPA proposes to establish an oral reference dose (RfD) and an inhalation reference concentration (RfC), which the agency defines as estimates (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure and a continuous inhalation exposure, respectively, of the human population (including sensitive subgroups) that are likely not to pose an appreciable risk of deleterious effects during a lifetime. The proposed RfC for tetrachloroethylene is 0.016 mg/m3, and the proposed RfD is 0.004 mg/kg per day. This chapter discusses how those reference values were determined.
SELECTION OF CRITICAL END POINT AND STUDIES
EPA selected neurotoxicity—specifically, outcomes of visual and cognitive dysfunction—as the critical noncancer health effect of tetrachloroethylene. As described in Chapter 3, epidemiologic and human studies involving controlled exposures have provided evidence of those effects. The experimental-animal literature available when the draft IRIS document was written also provided strong evidence that tetrachloroethylene is neurotoxic. One study (Mattsson et al. 1998) offered support of effects on visual function. New studies have provided further support of effects on the visual system and signal-detection tasks (Oshiro et al. 2008; Boyes et al. 2009) in animals. Although the committee supports EPA’s decision to use neurotoxicity as a critical end point, it recommends more focused assessments of specific criteria related to study design and methods as part of the process of selecting critical studies for deriving reference values.
The committee found that EPA reviewed all the relevant studies available at the time that the draft was written and agrees with many of the limitations that are noted, beginning on page 4-101. The committee also found, however, that the draft sometimes failed to consider weaknesses in study methods or inconsis-
tencies in results, two factors that should carry great weight in selecting key studies for calculating an RfC. For example, test outcomes (neurologic signs, emotional lability, choice reaction time, cancellation d2, and digit symbol) in a study by Seeber (1989) were worse in the low-exposure group compared with the high-exposure group. EPA’s discussion of the study (Section 4.6.1.2.2) did not mention that discrepancy. In another example, the committee judged the study by Echeverria et al. (1995) to be stronger than is characterized in the draft assessment (see detailed discussion in Chapter 3 of the present report). EPA discounted the study because (p. 4-77 to 4-78) “the lack of an unexposed control group limits the ability of the study to fully characterize the magnitude of the effects on visuospatial ability and to detect exposure-related symptoms or effects on tests of non-visuospatial cognitive ability. It also limits the extrapolation of the results to other populations exposed to tetrachloroethylene.” The committee judged that although there was no unexposed comparison group, the use of an internal comparison group (the group with the lowest exposure) has the advantage that any selection and confounding factors related to working in drycleaning facilities are present in both groups and reduces potential confounding by unmeasured factors.
The committee applied several criteria in selecting the epidemiologic studies that it considered most useful in establishing reference values for tetrachloroethylene. Three general criteria were addressed: the validity of individual studies, the internal consistency of results with the hypothesis of a causal role for tetrachloroethylene (for example, is there an association in a low-exposure group but not in the high-exposure group?), and the consistency of the findings with what is known from other sources (how the study fits into the overall picture of what is known). Those criteria are discussed in detail in Chapter 3.
EPA selected the study by Altmann et al. (1995), conducted in Mülheim, Germany, for calculating the RfC because it involved environmental exposures that are more relevant than occupational exposures for determining values designed to protect public health and it used a standardized computer-assisted testing battery. Those study factors are reasonable considerations, but they are not the most relevant for selecting a critical study. The committee concluded that the validity of the 1995 Altmann et al. study was seriously compromised by methodologic deficiencies, which are discussed in detail in Chapter 3 and summarized briefly below.
-
The most important concern is that the referent group was inappropriate in that it did not represent the counterfactual example. It was selected from among employees of the Public Health Office or the Medical Institution of Environmental Hygiene in Mülheim and matched to exposed subjects by age and sex. This selection bias resulted in a reference group clearly was more educated than the exposed group, and because the authors used only three categories of education, it is unlikely that differences in education were adequately controlled for. Because several of the primary outcomes are influenced by education, it is likely that substantial confounding remained. For example, there was no association between
-
tetrachloroethylene and visual evoked potentials (VEPs). That is important because visual deficits have been the most consistently reported effects of tetrachloroethylene, and they are outcomes that are essentially unrelated to education. Measures of vigilance, attention, and visual memory are strongly associated with education, and the exposed group had poorer performance in them, whereas measures of eye-hand coordination and finger tapping, which are weakly related to education, were similar in the two groups.
-
The rationale for the selection of study participants was poorly described, and several of the exposure measurements in those supposedly exposed were not reported. Without that information, it is impossible to determine whether this was a biased sample (that is, whether others were excluded for reasons other than study design).
-
Tetrachloroethylene was measured in air samples from homes for 7 days. Figure 1B of the paper is supposed to show indoor air concentrations for exposed participants and referents, but no concentrations are shown for the referent group. The amount of time that residents spent in their apartments is unknown. Time out of the apartments before neurobehavioral testing was unknown but was believed to account for lower blood concentrations of tetrachloroethylene before testing.
-
In the analyses, exposure was defined by group membership (yes-no variable) rather than by markers of exposure. Therefore, no dose-effect relationship was established in the exposed group. As stated above, group differences in neurobehavioral performance were more likely to be related to residual confounding by education or pre-exposure intellectual capacity.
-
The Neurobehavioral Evaluation System battery used to assess brain dysfunction related to exposure appropriately included four subtests that have been shown to be associated with solvent exposure in other research. However, the battery has no norms in this population, and some of the tests have not been well validated with regard to what they reveal about brain damage from any cause. The absence of norms makes it especially important to have basic, standardized measures of intellectual function that can be used to characterize the longstanding cognitive abilities (native intellectual capacity) of the two groups so that differences between the groups can be correctly attributed to exposure.
On the basis of the study selection criteria noted earlier—which emphasized validity, methodology, and consistency with the literature—the human studies that the committee judged most appropriate to use as points of departure for derivation of the RfC are Altmann et al. (1990), Cavalleri et al. (1994), Gobba et al. (1998), and Echeverria et al. (1995). The details of those studies and the reasons for their selection are discussed fully in Chapter 3 and summarized briefly here. The study by Altmann et al. (1990), who used controlled exposures in an experimental chamber, was chosen because it used random assignment to exposure groups, which reduced the potential for confounding of any associations between exposure and outcomes, and the exposure dosage was known. The study by Cavalleri et al.
(1994) was useful because it examined an occupational cohort of 33 dry-cleaners and it included followup assessments 2 years later, as reported by Gobba et al. (1998). Some members of the cohort continued to be exposed to the same workplace concentrations of tetrachloroethylene, and others worked in facilities where exposures had been reduced. The 1998 study by Gobba et al. was useful in that it allowed assessment of color vision before and after alterations in workplace exposure to tetrachloroethylene and because exposure concentrations could be estimated. The primary advantages of the study by Echeverria et al. (1995) were the reduction in potential confounding and confounding due to the use of an internal referent group and the ability to examine exposed workers for a dose-response effect with respect to measures of visuospatial performance on the basis of estimated cumulative lifetime exposure to tetrachloroethylene.
Among the animal studies considered by the committee, the one by Boyes et al. (2009) was judged to be appropriate to use as a point of departure for derivation of the RfC. The most sensitive end point in the study was the F2 (frequency-doubling) component of the evoked potential spectrum, a measure thought to reflect the activity of cortical neurons that respond to both stimulus offset and onset. The investigators also conducted a toxicokinetic analysis relating exposure concentration and duration to brain concentration. From that analysis, brain concentrations of tetrachloroethylene were linked to visual function.
DOSE METRICS
With respect to neurotoxicity, EPA’s use of the blood area under the curve (AUC) for tetrachloroethylene with various routes of exposure appears to be justified. The physiologically based pharmacokinetic (PBPK) model simulations presented in Figures 3-4, 3-5, 3-6a, and 3-6b of the draft IRIS assessment (EPA 2008) do suggest that the three PBPK models collectively describe the variation in blood and exhaled-breath concentrations of tetrachloroethylene observed in controlled human exposures. That provides confidence that later calculations of the tetrachloroethylene AUC during various exposure scenarios are accurately captured. A better dose metric for use in the neurotoxicity assessment might be the AUC for tetrachloroethylene in the brain. However, given the rapid partitioning of tetrachloroethylene between blood and well-perfused tissues and the lack of experimental data on brain tetrachloroethylene concentrations, the use of the blood AUC as a surrogate was appropriate. (As noted in Chapter 3, there are now some data that might be used in developing PBPK models of brain concentration.)
ROUTE-TO-ROUTE EXTRAPOLATION
EPA has chosen to use the venous-blood AUC as the route-to-route dose metric for extrapolating an inhalation exposure of tetrachloroethylene to a corresponding oral equivalent dose. The rationale behind that approach is sound and
adequately explained in the draft. However, the implementation of the approach raises serious methodologic concerns related to inappropriate use of the selected PBPK models and uncertainties in the fraction of an oral tetrachloroethylene dose that is metabolized. All three of the selected PBPK models were formulated and validated specifically against inhalation exposures. There was no attempt to validate model predictions against blood tetrachloroethylene concentrations after oral dosing. To use the PBPK models, EPA has empirically assumed a value for the rate of oral absorption of tetrachloroethylene, which is entered as a constant in the models. That approach is inferior to direct estimation as was used in other published PBPK models, such as the Gearhart et al. (1993) or Dallas et al. (1995) models (the latter only for rats and dogs). The latter PBPK models might have been better choices to begin this exercise. Better still, a harmonized PBPK modeling approach to synthesize important aspects of the various models into a single model would have provided the greatest confidence in the route-to-route extrapolation. See Chapter 11 for further discussion of the limitations of the PBPK modeling and the proposal to develop a harmonized model.
CHARACTERIZATION OF UNCERTAINTIES
The committee reviewed EPA’s application of uncertainty factors in deriving sample reference values on the basis of different studies. It found that the narrative made it clear what uncertainty factors were used but that there were some instances in which a supporting rationale was not provided for departure from the default option and other instances in which departures from the default option should have been considered.
Extrapolation from Lowest Observed-Adverse-Effect Level to No-Observed-Adverse-Effect Level
A factor of 10 was used consistently by EPA when a lowest observedadverse-effect level (LOAEL) from a study was used instead of a no-observedadverse-effect level (NOAEL). That is consistent with EPA policy. A benchmark dose (BMD) can be treated as a NOAEL, but no studies of neurotoxicity that could support a BMD calculation had been published when the draft was written. More recent studies of neurotoxicity would support such a calculation (Oshiro et al. 2008; Benignus et al. 2009; Boyes et al. 2009).
Extrapolation from Animals to Humans
The uncertainty factor for extrapolating animal data to humans is considered to have toxicokinetic and toxicodynamic aspects. EPA judged that an uncertainty factor of 3 was adequate to address these uncertainties. EPA applied that approach consistently, but the rationale for doing so was not adequately
described. Specifically, the draft cites an EPA (1994) document, but it would have enhanced transparency if it summarized briefly why an uncertainty factor of 3, rather than the default factor of 10, was used.
Human Variation
The application of a default factor of 10 to account for interindividual variation is justified because of the paucity of data on sensitive populations, including developing and aging organisms. Its use is appropriate and in accordance with EPA guidance.
Extrapolation from Subchronic Exposure to Chronic Exposure
The criteria for selecting the value of the uncertainty factor for extrapolating from subchronic exposure to chronic exposure were not clear, and this uncertainty was handled inconsistently in the draft IRIS assessment. It was noted (p. 5-13) that “a factor to address the potential for more severe toxicity from chronic or lifetime exposure to tetrachloroethylene is not used in this assessment. The epidemiologic studies, except for Schreiber et al. (2002), are all of median duration of exposures of more than 15% of a 70-year lifespan. There are no data to suggest that continuing exposure to tetrachloroethylene can increase the severity of effects; duration-response trends are not generally evident in the human studies.” On the basis of that rationale, no uncertainty factor for extrapolating to lifetime exposure was applied to the Altmann et al. (1995) study. However, in the discussion of the studies that support the RfC, a factor of 10 was applied to the Schreiber et al. study of day-care workers even though the mean exposure period was said to be 4 years, during which 23% of the time would involve exposure. It is not clear how that pattern would differ from residential exposure of people who work outside the home during the day. More directly, however, if EPA believes that longer exposures do not increase neurotoxicity or, by implication, that shorter exposures do not diminish it, one may question why a factor of 10 is applied to the results of the Schreiber et al. (2002) occupational study but not to the results of other occupational studies. Overall, the committee found that the literature provides little information about the possibility of cumulative toxicity from chronic exposure to tetrachloroethylene. No animal studies of chronic, life-long exposure were located, and except for Gobba et al. (1998) the epidemiologic studies did not involve long-term followup.
There is inconsistent use of the uncertainty factor when the sample reference value is based on the results of animal studies—Mattsson et al. (1998) and Rosengren et al. (1986). A factor of 10 was applied to the Mattsson et al. results and a factor of 3 to the Rosengren et al. results even though the two studies were of similar duration. EPA’s rationale (p. 5-15) was that “a subchronic to chronic factor of 3, rather than 10, was applied for Rosengren et al. (1986) in light of the large overall uncertainty for this study associated with extrapolating from a
LOAEL to NOAEL, from animal to humans, for human variation, and for database deficiencies; the total uncertainty factor was 3,000.” That justification is not clear. The reason for modifying the uncertainty factor may be that it is EPA’s policy to limit the overall uncertainty to 3,000 in deriving RfCs (EPA 2002). If that is the reason, it should be stated explicitly. If not, better justification should be provided.
The committee believes that an uncertainty factor of 3 should have been considered for animal studies like that of Mattsson et al. (1998) in which exposure occurred for 6 hours/day 4 days/week for 13 weeks. If that exposure regimen is treated in the same manner as acute exposure by applying a higher factor, doing so should be justified. Some discussion of the issue would improve the draft IRIS assessment.
Database Deficiencies
In the derivation of RfCs on the basis of neurotoxicity, EPA used a factor of 3 for database deficiencies because of the inadequacy of the experimental literature designed to characterize hazard and dose-response. Key deficiencies identified were inadequate data to address childhood or other life-stage susceptibility, a paucity of animal studies (especially studies of developing animals and of chronic, low-level exposures) designed to investigate neurotoxicity or to define and characterize dose-response relationships, and inadequate database on cognitive testing. It was unclear whether a factor of 3 was adequate to address these uncertainties because there was some overlap with the factor of 10 applied for human variation, which also addressed developmental concerns.
The committee recommends that EPA revisit and defend more clearly its decision to apply a factor of 3 for database deficiencies in light of new data and the committee's findings in Chapter 3. New studies include, for example, recent papers from researchers in EPA's National Health and Environmental Effects Research Laboratory provide excellent data from well-designed studies using controlled, acute exposures that link deficits in visual function and signal detection with atmospheric tetrachlorethylene concentrations and instantaneous concentrations in the brain. This includes papers by Oshiro et al. (2008) and Boyes et al. (2009) investigating function and by Shafer et al. (2005) on mechanisms, which is described in the IRIS document but not fully integrated. These studies link neural or behavioral effects to actual brain concentrations of tetrachloroethylene or to their estimated concentration using PBPK modeling. Thus, the animal literature on controlled acute exposure is now stronger. Notable gaps in the animal literature still include the paucity of studies of developmental or chronic exposures. Another consideration is that the committee found the human study of exposed children (Schreiber et al. 2002) to be methodologically flawed. The committee judges these to be serious gaps in the database, which suggests that a factor of 3 may be inadequate to account for database deficiencies.
Human Equivalences
Human equivalences are said to reflect adjustments from a less than continuous exposure to continuous exposure, such as might occur in a residence. That assumes continuous exposure although few people are in their homes 24 hours/day. The human equivalence factor is supposed to involve an adjustment from exposures 5 days/week to exposures 7 days/week by multiplying by 5/7 or from 8 hours/day to 24 hours/day when experimental exposures (as in animal studies) are less than continuous. For human occupational exposures, a 10/20 factor is applied to accommodate an increased respiration rate during work; however, when this factor is applied, the adjustment to a 24-hour day is not applied, but the adjustment to a 7-day week is. For oral exposures but apparently not for inhalation exposures, there is an allometric adjustment for body-weight differences. Those considerations are in accord with EPA policy but are far from intuitive and should be summarized in the document where they are applied in the tables. The draft’s Figure 5-7 clearly presents that approach in estimating cancer risk, but the figure does not apply to risk posed by inhalation. It is sometimes difficult to see how the “human equivalence” factor is determined for a particular study, and some rationale for its calculation would increase understanding of EPA’s approach. For example, the adjustment for the Mattsson et al. study is not described, but it appears to be an adjustment from exposures 6 hours/day 5 days/week to 24 hours/day 7 days/week.
GRAPHICAL PRESENTATION OF REFERENCE VALUES
The draft IRIS assessment provides graphical presentations of noncancer reference values for tetrachloroethylene (Figures 5-1, 5-2, and 5-4). One figure (Figure 5-1) illustrates reference values based on different neurotoxicity studies and two figures (Figures 5-2 and 5-5) compares EPA’s selected reference value based on neurotoxicity with other reference values based on other noncancer effects. The committee strongly supports the use of such graphical aids. In general, the approach is intended to make it clear which uncertainty factors were applied, to which studies they were applied, and the effects of particular assumptions. However, the figures in the draft document fail to accomplish that. The shading used in the legend for the figures does not match the shading in the figures, so it is impossible to determine which uncertainty factors were used. By including a small number of studies, the figure on neurotoxicity sample reference values (Figure 5-1) also misses an opportunity to show the degree to which the literature converges on a limited range of sample values. Convergence of estimated values from studies that are methodologically sound, even if they are not listed as key, would support the RfC proposed by EPA.
To synthesize the literature, the committee considered a graphical approach that shows how sample reference values that might be derived from the
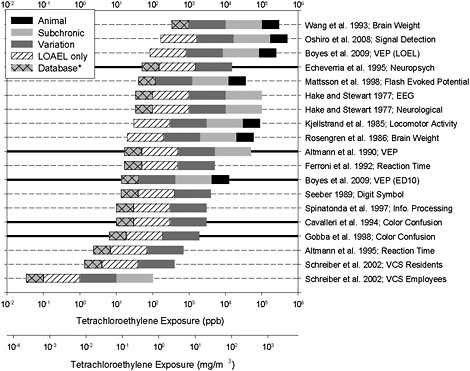
FIGURE 10-1 Distribution of sample reference values. Each horizontal bar represents a single study. Thick, horizontal lines represent studies identified by the committee as most applicable to the development of an RfC. The right end of a bar is at the “point of departure” and is based on concentrations used in the referenced study after conversion to “human equivalencies” or, in the case of animal studies, after adjustment for continuous exposure. Uncertainty factors are illustrated in different shadings: a factor of 3 if it is necessary to extrapolate from animals to humans (black); a factor of 10 if it is necessary to extrapolate from acute or subchronic exposure to chronic exposure (light gray); a factor of 10 for individual variation to account for sensitive individuals (dark gray); a factor of 10 if the study did not contain a NOAEL (diagonal lines) and a factor of 3 for uncertainty in the data base as applied by EPA (light gray, cross-hatched). *A maximum total uncertainty factor of 3,000 was applied for the purpose of this exercise. Where this might be exceeded, the maximum was achieved by omitting the “database” uncertainty so that other uncertainties could be visualized. The committee has recommended that EPA review the uncertainty factors to ensure that they are appropriately explained and used consistently, so some of the individual values used here could be subject to change. In some cases, EPA might judge that the total uncertainty exceeds 3,000 and would, therefore, not use that study to derive a sample reference value. Source: Graphic developed by M. Christopher Newland.
different studies of neurotoxicity compare with one another (see Figure 10-1). That was done by using the studies described in the draft and two studies (Oshiro et al. 2008; Boyes et al. 2009) published since the draft was written. The
approach enables the visualization of the range of concentrations studied, the identification of clusters of studies, and the isolation of especially low or high reference value estimates that might be derived from a particular study. The figure includes seven data points derived from animal studies (identified by a black bar on the right), three from controlled human exposures (identified by a light gray bar and the absence of a black bar), and studies of environmental or occupational exposures. The convergence of sample reference values into clusters would confer confidence on the use of a critical study if other studies led to similar conclusions.
The points of departure for the pre-2004 studies came from Tables 4-4 and 5-2 of the draft document, so they were human adjusted equivalent concentrations or, in the case of animal studies, adjustments for continuous exposures as appropriate. Uncertainty factors were based on how they are typically applied (see pp. 5-11 onward in the draft) even when the committee disagreed with their application. For example, the committee recommends an uncertainty factor different from that applied by EPA for the Schreiber et al. (2002) study. EPA applied an uncertainty factor of 10 to the Schreiber et al. results to extrapolate from “subchronic to chronic exposure,” but the study involved long-term environmental exposure of day-care workers, so the committee believed that this uncertainty factor not necessary. EPA’s factor of 10 was retained in the graphical display, and the RfD calculated for occupational exposure by using this factor appears unusually low.
Studies published since the EPA draft was written are also included in Figure 10-1. One study (Oshiro et al. 2008) identified a LOAEL of 500 ppm (acute). The dependent measure was a signal-detection task. Uncertainty factors for the study would include a factor for extrapolation from animals to humans (3), one for extrapolation from acute to chronic (10), one for sensitive populations (10), one for absence of a NOAEL (10), and the routine one for database uncertainties (3)—for a total uncertainty factor of 9,000. For the purposes of this exercise to show the full database, a maximum total uncertainty factor of 3,000 was applied. The committee has recommended that EPA review the uncertainty factors to ensure that they are appropriately explained and used consistently. In some cases, EPA might judge that the total uncertainty exceeds 3,000 and would, therefore, not use that study to derive a sample reference value. In the graph, the total uncertainty factor was reduced to 3,000 by not showing the uncertainty factor of 3 for database uncertainties. The second study (Boyes et al. 2009) reported a LOAEL of 250 ppm for VEPs evoked by a grid of vertical bars; this is similar to the contrast-sensitivity task. The same factors used in the Oshiro et al. study would be applied here, so a total uncertainty factor of 3,000 (similarly reduced from 9,000) was applied to calculate a sample reference value of 83.3 ppb. The Boyes et al. study also used PBPK modeling to estimate the shape of the relationship between brain concentration and VEP. An ED10, the brain concentration that produced a 10% change in VEP (the last figure in the Boyes et al. paper), was estimated. To estimate a point of departure from the ED10, the exposure concentrations, in parts per billion, that would produce this
brain concentration were estimated by back-calculating from relationships between brain and atmospheric concentrations in the authors’ Figures 1 and 2. An ED10 of 0.687 mg/mL comes from their Figure 7. From Figure 1, it can be estimated that the brain:blood ratio is 33:12 (at peak), so 0.687 mg/L in brain corresponds to 0.25 mg/L in blood. From Figure 2, it can be estimated that 50 ppm in air corresponds to a peak (and near asymptote) of 1 mg/L in blood. Therefore, the blood tetrachloroethylene concentration of 0.25 mg/L should result from 12.5 ppm in air. The committee recognized that those are rough estimates that assume linearity and that a more precise estimate could be obtained with modeling. The estimate is included here only as an illustration. The 12.5-ppm point of departure yields a sample reference value of 14 ppb after application of uncertainty factors for extrapolation from animals to humans (3), acute exposure (10), variation in sensitivity (10), and database uncertainty (3). It is unclear whether an uncertainty factor should be applied for the absence of a NOAEL in the study.
Some observations can be made from the figure. The majority of sample reference values are centrally clustered, but there is a wide spread to both the lower and higher ends. Although the overall range of the 19 sample values is 0.03-333 ppb (0.0002 - 2.6 mg/m3), it is reduced to about 6 to about 50 ppb (0.04 - 0.34 mg/m3) when restricted to the five strongest studies. EPA’s RfC of 2 ppb (0.016 mg/m3) calculated on the basis of the Altmann et al. (1995) study falls below the range and is higher than only the two other human studies, which were conducted by Schreiber et al. The Altmann et al. (1995) and Schreiber et al. (2002) studies are discussed and critiqued elsewhere, where it is noted that the makeup of the critical comparison groups is confounded and that this makes it difficult to attribute differences seen in dependent variables to tetrachloroethylene. The figure enhances transparency by showing how studies converge on a range of reference value estimates and how the study or studies selected as the one(s) to be used for establishing the final RfC compares with other studies.
The three studies that yield sample reference values above 50 ppb are the ones that identified effects at relatively high exposure concentrations because the end points were relatively insensitive or, like in the Oshiro et al. (2008) study, are of very high quality but used high exposure concentrations, so that a low end of the dose-effect curve was not readily identifiable. While the Boyes et al. (2009) study is considered a critical one by the committee, the sample reference value based on the LOAEL from the study (as opposed to the ED10) was considered to have too much uncertainty associated with it to be used as a point of departure. The consistency in the middle ranges among epidemiologic studies and controlled-exposure human studies, as well as in animal studies, provides support for points of departure in these ranges. Despite the use of different exposure regimens and concentrations among animal studies, human chamber studies, and occupational and environmental studies, there is a reasonable coherence in the sample reference values. Finally, to keep the maximum uncertainty factor to 3,000, the “database” factor of 3 was omitted from four estimates for the pur-
poses of the exercise in Figure 10-1. Reinstating this factor would not substantively change the conclusion about the consistency in reference concentrations.
The graphical display in Figure 10-1 shows a distribution of sample reference values based on neurotoxic effects observed in epidemiologic studies, controlled human experiments involving healthy volunteers, and animal experiments involving different species. Exposure ranged from chronic to acute. The studies involved different neurotoxic end points that are differentially sensitive to tetrachloroethylene exposure. Whereas uncertainty factors applied to a point of departure adjust uncertainties specific to their corresponding studies, the collective distribution of reference values provides an overarching measure of uncertainties, weight of evidence, sensitivities, and other sources of variation among different studies.
This approach could also be applied to EPA’s other graphical presentations of reference values based on other noncancer end points. Such an approach would allow organ-specific reference values to be put in context with one another. For example, the degree to which sample reference values for an organ system cluster, or fail to do so, might be viewed as evidence of the degree to which different studies tap similar toxic mechanisms, kinetics, end points, or other important characteristics of a chemical.












