2
Comparative Evaluation of Procedures and Regulations for Biocontainment Facilities
This chapter reviews the guidelines, procedures, and regulations that govern the operations of biocontainment facilities at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) and other facilities. It is intended to provide context for the history of operations at USAMRIID and for the operations of the planned facility. The review included information presented in the Environmental Impact Statement (EIS), materials provide by USAMRIID (Army regulations, polices, standard operating procedures [SOPs], and suite-specific safety manuals), and discussions with USAMRIID personnel.
In this chapter and elsewhere in this report, the term “biosurety” refers collectively to systems and procedures used to safeguard biological select agents and toxins (BSAT) against theft, loss, diversion, or unauthorized access or use and to ensure that operations are conducted in a safe, secure, and reliable manner. There are several aspects of biosurety discussed in this chapter—biocontainment, biosafety, and biosecurity. Figure 2-1 provides USAMRIID’s illustration of how these aspects provide the foundation for its biosurety program.
BIOCONTAINMENT
Biocontainment laboratories are designed to prevent the accidental release of pathogenic organisms during scientific research. There are four biosafety levels (BSLs) of containment: BSL-1, BSL-2, BSL-3, and BSL-4. These levels indicate increasing levels of containment and are inclusive, so all safety features found at BSL-1 are found at BSL-2. BSL-3 builds on BSL-2. BSL-4—the highest level of biosafety—includes all of the features required at lower levels, plus additional engineering controls such as filtration of all exhaust air through two
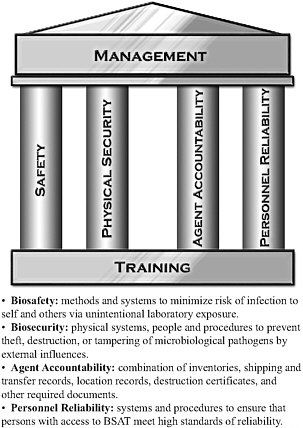
FIGURE 2-1 USAMRIID’s Biosurety Program. The program includes systems and procedures to properly safeguard BSAT against theft, loss, diversion, or unauthorized access or use, and to ensure that operations are conducted in a safe, secure, and reliable manner.
Source: Skvorak 2009.
HEPA (high-efficiency particulate air) filters in series, personal protective equipment (PPE; such as the one-piece encapsulated suit), SOPs (such as special decontamination processes), and administrative controls (for example, training requirements and medical surveillance enhancements). The proposed new USAMRIID facility will include BSL-3 and BSL-4 laboratories.
The Army has been a leader in developing cutting-edge requirements for high- and maximum-containment laboratories. As new containment laboratories have proliferated across the country in recent years, there has been a steady development of better facilities, increasingly robust biosafety practices and procedures, and a continuing cross-fertilization of new technologies. With this development has come a significant reduction in laboratory-acquired infections at USAMRIID and at other biocontainment facilities (Rusnak et al. 2004a).
Efforts to codify guidelines for improved biosafety began in the 1970s (Centers for Disease Control and Prevention [CDC] 1974; London School of Tropical Medicine 1974; 41 Fed. Reg. 27902 [July 7, 1974]; National Research Council [NRC] 1974). These guidelines primarily addressed the interrelationships among engineering aspects, secondary containment systems, and biosafety principles (practices and procedures). The guidelines detailed the four levels of biosafety for work with microbial agents in the laboratory and in parallel animal facilities. The focus was to protect laboratory workers and the surrounding community by creating a safe work environment that kept the microbes inside the clinical or research facility. By the late 1990s there was recognition that more attention was needed for securing particularly hazardous pathogens (BSAT) from potential acquisition by persons who did not have legitimate need to possess them (CDC/National Institutes of Health [NIH] 1999). Following the anthrax mailing events in fall 2001, significant attention was focused on who should have access to these agents (42 CFR 73 Select Agent Rule 2003, 2005).
As more laboratory facilities aiming to conduct research with these agents were constructed, more attention was paid to the process of commissioning the buildings before they were put into operation. Commissioning is the process whereby detailed scrutiny is given to individual and component operational systems (e.g., air supply, exhaust fans, dampers, and ducts) to assure the owner that the facility has been built as specified by the approved architectural plans. Lessons learned from the commissioning of one facility become the new baseline for the next. After the commissioning step comes the approval that the new facility in fact meets all of the requirements as specified for the intended biocontainment level.
In the United States, the “gold standard” for biosafety is the CDC/NIH (2007) guideline Biosafety in Microbiological and Biomedical Laboratories (BMBL). This guideline, currently in its fifth edition, is the basis for assessing all biosafety programs and is used by the CDC Division of Select Agents and Toxins to implement oversight and grant permits for all work with select agents and toxins. In addition to BMBL guidelines, USAMRIID is subject to Army regulations (AR 385-69), Department of the Army guidelines (DA PAM 385-69), NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH 2009), and CDC oversight for Select Agents and Toxins (42 CFR 73).
The EIS for the new USAMRIID facility was published in December 2006. At that time, the BMBL was in its fourth edition (CDC/NIH 1999), so the EIS does not specifically address any changes that might be reflected in the 2007 edition. For example, there has been one very significant change in engineering controls specified for BSL-3 labs (CDC/NIH 2007, p. 55):
A ducted air ventilation system is required. This system must provide sustained directional airflow by drawing air into the laboratory from “clean” areas toward “potentially contaminated” areas. The laboratory shall be designed such that under failure conditions the airflow will not be reversed.
This is one example of the continuous improvements that have been made in the design, construction, and operation of containment facilities. Although this requirement was not in place in 2006, the new USAMRIID facility will be designed to adhere to these and other guidelines set forth in the most current BMBL. The BMBL also provides guidance on the performance of risk assessments and the selection of appropriate safeguards with experience, changes in research protocols, or the use of new agents.
The new USAMRIID building(s) will be designed, constructed, verified, and operated according to all the design and engineering standards specified by CDC/NIH (2007) and the applicable requirements of the Biological Defense Safety Program set forth in AR 385-69 and DA PAM 385-69. The facility will be credentialed according to the specifications of the CDC Division of Select Agents and Toxins and/or the counterpart regulations of the U.S. Department of Agriculture (USDA) and Animal and Plant Health Inspection Service (APHIS) that apply to BSAT. USAMRIID reports that none of the research to be performed at the new facility will be classified, nor will there be projects using dry aerosols, such as powdered Bacillus anthracis (U.S. Army Medical Research and Materiel Command/U.S. Army Garrison [USAMRMC/USAG] 2006).
Another aspect of containment is the handling of wastes resulting from biological research. The committee reviewed current practices at USAMRIID, as well as the plans for the new facility as presented in the EIS. The wastewater collection, conveyance, and treatment systems at Fort Detrick are segregated into two separate systems: one that serves to contain and treat what is for the most part domestic wastewater generated on the base, and the other, which collects and treats all wastewaters associated with BSAT research, including its animal research operations. While not explicitly stated in the EIS, wastewater associated with BSAT research is autoclaved and/or infused with potent oxidizing chemicals (e.g., hypochlorite) and surfactants (e.g., quaternary amines) according to BMBL practices, prior to underground transmission through a recently rehabilitated, corrosion-resistant collection system. This process serves to disinfect the water by killing the organisms and deactivating the toxins. Chemically treated BSAT wastewaters are then stored above ground in large holding tanks prior to high-energy thermal treatment using batch steam injection. The thermally treated BSAT wastewater is then decanted into the existing Fort Detrick sanitary sewer, prior to permitted discharge under the National Pollutant Discharge Elimination System into the Monocacy River.
The wastewater volumes generated by the existing and proposed facilities are formidable (between 107 and 108 gallons per year). The EIS states that future USAMRIID facilities will use a limited amount of existing infrastructure (about 20 percent of subsurface collection system), but that on-site thermal treatment processes will be redesigned and decentralized. Stage 1 and 2 construction call for satellite steam-injection facilities in or immediately adjacent to buildings housing BSL-3 and BSL-4 laboratories. This is a significant departure from past USAMRIID operations, and will substantially reduce the hazard potential associated with the handling of relatively large wastewater volumes. Independent of
satellite thermal treatment, current BMBL physical/chemical laboratory disinfection practices will continue to be applied to all liquid wastes and wastewater generated by the proposed USAMRIID facilities.
Like its wastewater counterpart, there are distinctly separate systems to contain, convey, and treat solid wastes generated at USAMRIID. Solid wastes generated by BSAT research are categorized and specially sequestered at the laboratory of origin; they are contained, tracked, and destroyed on site, so they remain separate from the otherwise conventional solid waste generated on the base. BMBL practices require the careful segregation of all solid materials containing or contacting microbes, their culture media, and any vessel involved in their assay (no matter how dilute). Well-marked secondary and tertiary containments (such as barrels, drums, and mylar biohazard bags [“red bags”]) are used to identify and hold solid materials, the potentially infectious fraction of which are autoclaved in bulk prior to transport from the laboratory of origin. Each solid waste load containing potentially infectious material is traced by a chemical marker that verifies its exposure to accepted inactivation conditions during an autoclave cycle. Non-infectious solid materials are then subject to oxidative incineration at a central facility, and the residuals disposed at landfills according to licensed civil engineering practices (such as those of the American Society of Civil Engineers [ASCE 1996]). According to the USAMRIID EIS, there are no planned significant departures from past solid waste management practices, which the committee finds safe and appropriate when executed in accordance with existing SOPs and BMBL practices.
BIOSAFETY AT USAMRIID RELATIVE TO NIH AND CDC RULES AND GUIDELINES
Biosafety is defined by the World Health Organization as “… containment principles, technologies and practices which are implemented to prevent unintentional exposure to pathogens and toxins, or their accidental release” (WHO 2004, p. 47). Biosafety utilizes a number of control methodologies to include engineering controls, PPE, work practices (such as SOPs), and administrative controls (such as immunizations, medical surveillance, and training) to address the variety of hazards anticipated in the laboratory. These methodologies work synergistically to protect laboratory workers (and people they may come in contact with, such as co-workers and family members) as well as the environment. These control methodologies begin with basic principles and increase in scope and intensity as the nature of the hazards increase. The BMBL describes the requirements for the four levels of increasing biosafety (BSL-1 through BSL-4). As noted in the previous section, USAMRIID is subject to the BMBL guidelines (CDC/NIH 2007), as well as Army standards AR 385-69 and DA PAM 385-69, NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH 2009), and CDC oversight for Select Agents and Toxins (42 CFR 73).
DA PAM 385-69: Safety Standards for Microbiological and Biomedical Laboratories (2009) prescribes the technical safety requirements for the use, handling, transportation, transfer, storage, and disposal of infectious agents and toxins rated at BSL-2, -3, or -4. The standards mandate the use of the most recent edition of the BMBL.
USAMRIID provided the committee with 15 SOPs detailing various aspects of handling and inventorying BSAT materials, processing laboratory waste, decontaminating workspaces, and certifying waste-treatment equipment (autoclaves). These SOPs meet or exceed all requirements of the BMBL as well as the requirements of the Select Agent Program (42 CFR 73) promulgated by the U.S. Department of Health and Human Services (HHS) and USDA. For example, relative to certifying containment equipment, the USAMRIID SOP entitled Biological Safety Cabinet, Chemical Fume Hood, Class I Safety Enclosures, and HEPA Filtered Clean Benches Certification Program requires that any of these devices used in BSL-3 or BSL-4 laboratories be certified semi-annually. This schedule exceeds the National Sanitation Foundation Standard 49 for Class II BSC Certification as well as the BMBL requirement that these devices should be certified on an annual basis.
USAMRIID also provided examples of current safety program regulations and examples of suite-specific safety manuals. These materials meet or exceed guidelines and recommendations from CDC/NIH (2007), the Occupational Safety and Health Administration (CFR 1910.1030), and the Select Agent Program (42 CFR 73), and the Army, in fact, turned these recommendations into USAMRIID regulations. Notable in one suite-specific manual (Safety Manual for the BSL-2 Lab) is a section devoted to safety communications. The following statement indicates a commitment to ongoing attention to safety with emphasis on continuous improvement by incorporating “lessons learned” into current practice: “Accounts of laboratory mishaps, along with the lessons to be learned from them are discussed during the quarterly Safety Committee meeting and are available at the Safety Office” (p. 10). However, human error will continue to result in occasional exposures to infectious agents (see discussion of the glanders and tularemia cases later in this chapter).
The documents provided by USAMRIID are dated recently, most within the past 12 months. However, the revision history noted on the documents indicates these SOPs/regulations have been in place for long periods and are subject to regular review and revision as dictated by changes in external requirements or internal changes in risk assessment or from lessons learned. Also notable is Policy Letter 08-18, Commander’s Safety Policy, which clearly states the Commander’s commitment to safety and reminds all staff member of their rights and responsibilities for maintaining a safe environment for employees of the organization as well as the community at large.
There are two related “markers” that are indicative of the effectiveness of biological safety programs associated with laboratories that work with infectious pathogens: 1) Have laboratorians become infected with the agents in use? or 2)
Have there been any illnesses in the surrounding community attributable to the release of infectious materials from the laboratory? In the first instance, activities in the laboratory are considered to be the cause of the infection (for example, inappropriate protective equipment, accidents, animal bites). An infected laboratory worker might serve as the means for spreading the pathogen to family members or other community members. Alternatively, failures of the engineering controls that maintain containment in the facility have the potential for releasing infectious materials into the community.
Table 2-1 contains a list of laboratory-acquired infections that have occurred at USAMRIID since the 1940s; notable are the diminishing numbers over the decades since research first began. These reduced numbers clearly reflect the positive changes in biosafety practices, procedures, use of equipment, and better engineering controls. None of the reported laboratory-acquired infections were related to engineering systems failures. Rather, the reported infections resulted from human activities. Perhaps more important, no community infections related to work done at USAMRIID have been reported.
The pathogens recently studied at USAMRIID and anticipated to be studied in the near future are listed in Box 2-1. These agents are variously transmitted through physical contact, mucous membrane exposure, ingestion, bite from an arthropod vector, or inoculation. Others are spread by the aerosol route. The majority of these agents are not spread from human to human. These agents are carefully contained inside biological safety cabinets using BSL-3 practices and procedures. Risk assessments on specific agents, or any new agents, determine whether work is done in BSL-2, BSL-3, or BSL-4 laboratories.
USAMRIID currently has a robust biosafety program that consistently updates training, SOPs, and other written policies and manuals. In addition, USAMRIID has constituted and registered an Institutional Biosafety Committee (IBC) in compliance with the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH 2009; p. 2-32, part 2.3.4).
There are several select toxins that may be studied at the new USAMRIID. They are non-reproducing biochemicals that pose some risk to the user, and are studied in high-containment laboratories because of the added security associated with such facilities.
The nature of recombinant DNA research at USAMRIID is similar to that conducted in many federal, private, and university laboratories across the country. The protocols are closely monitored by the IBC and by NIH, which oversees such work through the Recombinant DNA Advisory Committee and its guidelines. The IBC membership includes knowledgeable USAMRIID scientists, a representative from the Barquist Army Health Clinic, and a virologist not affiliated with USAMRIID.
TABLE 2-1 Timeline of Significant Advances in Biocontainment, Biosafety, Biosurety, and Biosecurity and Listing (by Decade) of Laboratory-Acquired Infections at USAMRIID
|
Decade |
Laboratory-Acquired Infections at USAMRIID (route of exposure if known)a |
Significant Biosafety-Related Army Eventsb |
Other Significant Biosafety-Related Events |
|
1940s |
27 anthrax (cutaneous) 43 tularemia 51 brucellosis (inhalation) 7 glanders (1 cutaneous, 6 inhalation) |
|
|
|
1950s |
4 anthrax (1 inhalation, 3 cutaneous) 107 tularemia (primarily inhalation) 43 brucellosis (inhalation) 32 Q fever (primarily inhalation) 18 Venezuelan equine encephalitis (VEE; primarily inhalation) 1 plague |
|
|
|
Decade |
Laboratory-Acquired Infections at USAMRIID (route of exposure if known)a |
Significant Biosafety-Related Army Eventsb |
Other Significant Biosafety-Related Events |
|
1960s |
11 tularemia (inhalation, cutaneous) 1 brucellosis (inhalation) 23 Q fever (primarily inhalation) 7 VEE (primarily inhalation) |
|
|
|
1970s |
9 Rocky Mountain Spotted Fever (inhalation) 1 tularemia 1 VEE |
|
|
|
|
|
|
|
|
1980s |
1 tularemia 1 dengue fever 1 Q fever |
|
|
|
Decade |
Laboratory-Acquired Infections at USAMRIID (route of exposure if known)a |
Significant Biosafety-Related Army Eventsb |
Other Significant Biosafety-Related Events |
|
1990s |
1 chikungunya (needle stick) 1 vaccinia (cutaneous) 1 plague 3 staphylococcal entertoxin B |
|
|
|
BOX 2-1 Pathogenic Agents Recently Studied or Potentially Studied in the Near Future at USAMRIIDa Bacillus anthracis Brucella species Burkholderia mallei Burkholderia pseudomallei Clostridium botulinum Coxiella burnetii Crimean-Congo hemorrhagic fever virus Dengue virus Ebola virus Eastern equine encephalitis Francisella tularensis Guanarito virus Hantaviruses Influenza viruses Japanese encephalitis virus Junin virus Lassa fever virus Machupo virus Marburg virus Non-Variola pox viruses Rift Valley fever virus Staphylococcus enterotoxin B Venezuelan equine encephalitis Vibrio species West Nile virus Western equine encephalitis Yellow fever virus Yersinia enterocolitica Yersinia pestis |
The Department of Defense’s (DOD’s) biological safety and security program was reviewed by a Defense Science Board (DSB) task force in 2009. The review involved a comparison of DOD biological laboratories with similar facilities in academia, industry, and the Federal Government. Twenty-two laboratories were considered, including USAMRIID. The task force found that the safety and security of the DOD facilities was as good or better than that found in comparably sized facilities. It also made the observation that several BSL laboratories are more modern than the DOD laboratories. It further stated that “if USAMRIID is to stay in the forefront and address evolving threats, investment in new infrastructure must be sufficient” (DSB 2009, p. 39).
BIOSURETY
Biosurety programs involve systems and procedures to properly safeguard BSAT against theft, loss, diversion, or unauthorized access or use. The EIS states USAMRIID will undergo CDC inspection and approval prior to beginning work. As laws, regulatory requirements, and guidelines change and are adopted by CDC, USAMRIID would have to be compliant to pass inspection and be operational. This would include new requirements such as The Possession, Use, and Transfer of Select Agents and Toxins (42 CFR Part 73 [2005]), which was promulgated by HHS and USDA and forms the basis of current programmatic and operational biosecurity requirements in the United States (the BMBL)
(CDC/NIH 2007). Registered select agent facilities also are subject to announced and unannounced inspections by CDC, USDA, or both, depending on the agents studied at the facility. These inspections include detailed scrutiny of the receipt, storage, use, and transfer of BSAT.
CFR 42 Parts 72 and 73 establish specific requirements for institutes that possess, use, store, and transfer BSAT. In accordance with AR 385-69, USAMRIID must develop a program compliant with the regulations that in summary require:
-
registration of facilities/entities (application and inspection) with CDC/ USDA APHIS,
-
assignment of a responsible official to manage the biosecurity program,
-
investigation and adjudication by the Department of Justice (DOJ) of personnel with access to BSAT,
-
restricted access to BSAT,
-
development and implementation of a security plan (physical and IT security, inventory control), biosafety plan, and incident response plan,
-
provision of documented biosafety and biosecurity/security training to personnel,
-
maintenance of records,
-
inspections, and
-
notification of CDC or APHIS of theft, loss, or release of materials.
The biosurety program at USAMRIID focuses on accountability of both personnel and materials. Taken together these criteria complement each other and are more stringent than the existing requirements set forth by DOJ and HHS/USDA.
Personnel reliability is another aspect of biosurety, which involves systems and procedures to ensure that individuals with access to BSAT meet high standards of reliability. The issue of personnel reliability goes beyond the investigation and adjudication by the DOJ that is required of all personnel having access to BSAT. The Army has taken the lead in establishing a robust biosurety program, which has been fully adopted by USAMRIID (see Table 2-1). The Biological Personnel Reliability Program was initiated in 2003, and every new employee undergoes a 10-week process of personal interviews with questions on drug and alcohol use, mental health, financial issues, and any interactions with law enforcement. Personnel records are reviewed. A medical evaluation is performed that includes physical, emotional, and psychological assessments. After enrollment in the program, personnel are monitored carefully for disqualifying attributes including inappropriate attitude, conduct, or behavior. This Biological Personnel Reliability Program is considered to be a model by other institutions.
While this program is robust at screening and potentially deterring and detecting insider threats, it is the consensus of the committee that no program can stop all threats of theft (or misuse) of BSAT posed by those who have been granted access to BSAT and who are determined to take action. Risk in any ac-
tivity cannot be reduced to zero. The committee also recognizes that there are very few reports in the literature regarding employee theft or misuse of biological agents at USAMRIID or in the United States despite the large number of personnel working with these agents and the number of years research has been conducted. Preventing further incidents is an ongoing challenge that will need to balance strengthened biosecurity measures against the placement of undue additional stress on laboratory personnel. The current program will be strengthened by further training laboratory personnel on their individual and collective biosecurity responsibilities, reinforcing ethical norms of safe and responsible scientific conduct, and paying increased attention to behavioral signals that may identify personnel as “at risk” of becoming potential threats to their coworkers and the program. A recent NRC report, Responsible Research with Biological Select Agents and Toxins (NRC 2009), recommends training in scientific ethics and dual-use research, and that the training be designed to foster community responsibility. Research enterprises in academic, corporate, and military settings have been slow to respond to increasing calls for training of life scientists in ethical aspects of their work (Dando 2009). It would appear that USAMRIID has some difficulty assigning priority to training in ethics and responsibility in research, but this is not unique to military installations.
Before 2001 there was no requirement for a centralized BSAT database for inventory accountability. Since then there has been a dynamic inventory of agents in frequent usage, which includes review of research notebooks and physical inventory audits. BSAT transfers between laboratories require oversight by two persons. BSAT transfers outside of USAMRIID follow CDC/APHIS tracking requirements, packaging specifications of the Department of Transportation, and are moved through commercial “white glove” service.
In November 2008, USAMRIID undertook a 100-percent BSAT inventory verification to double-check the accuracy of its automated inventory management system. A significant overage of vials (9,079, including 141 BSAT vials) was discovered and reported in January 2009, and a “stand-down” was ordered until the individual laboratories were recertified as being compliant. Additional inventory controls have since been established and implemented (Skvorak 2009).
BIOSECURITY
Physical security at Fort Detrick is commensurate with the maximum level of fortification typical of high-value domestic military bases. The entire facility is surrounded by fencing, and there are security check points at all entrances. Vehicle and personnel checks are performed for all who enter the fort. There are additional security check points at the entrance to the laboratory buildings, as will be the case for the new USAMRIID facility. Incoming packages are subject to X-ray and/or physical inspection. Access to the individual laboratory suites requires use of swipe cards, key pads, and/or biometric readers, depending on
the nature of the laboratory. There are exterior and interior closed-circuit television systems. Access to BSAT requires further security access. As indicated above, all persons having access to BSAT must undergo FBI verification and adjudication, then extensive further scrutiny through the Biological Personnel Reliability Program process.
CASE STUDIES OF RECENT EVENTS AT USAMRIID
There are four fairly recent events at USAMRIID that can be used as case studies for identifying weaknesses in the institute’s programs and procedures. They include two cases of laboratory-acquired infections (tularemia and glanders), the discovery that the material in the “anthrax letters” mailed in 2001 originated from a source in a USAMRIID laboratory, and the discovery that the institute’s BSAT inventory was inaccurate.
Recent Laboratory-Acquired Infections
In late November 2009, a laboratory worker at USAMRIID contracted tularemia pneumonia as a result of her research with the causative agent Francisella tularensis. She began to exhibit symptoms of illness (fever, chills, myalgia, and headache) several days after having worked with the agent. Vaccination for tularemia is available to all BSAT workers. This researcher had not been vaccinated against F. tularensis because she had a non-laboratory related clinical case of tularemia in 1992, and had positive hemagglutinin titers suggesting that she retained immunity to the bacteria. Because her children were ill, she attributed her symptoms to catching their illness. Thus, she sought medical attention from the usual military channels for non-occupational illnesses about a week after symptoms began.
However, after her fever and symptoms persisted she sought care from USAMRIID’s special immunization program (SIP) clinic, the occupational health provider for USAMRIID. The SIP clinic confirmed a diagnosis of tularemia. The Frederick County Public Health Officer was informed by the on-base Barquist Army Health Clinic once a presumptive diagnosis was made, and was periodically updated. No other cases of tularemia occurred.
Based on these observations and comments on the case from USAMRIID, the Frederick County Health Officer, and the public, a number of lessons may be learned. First, even though there were circumstantial reasons for the worker to suspect her illness was unrelated to work in the laboratory, this case suggests that workers with fevers should be required to report to the SIP clinic before seeking care elsewhere because it has the specialized clinical staff and resources to identify diseases related to USAMRIID’s laboratory research. Second, it is important to maintain communications among USAMRIID, the Barquist Army Health Clinic, and the Frederick County Health Department to ensure timely reporting of cases to the State. In particular, because there is routine and frequent turnover of medical
staff at the military facilities, it is important that procedures are in place to ensure that communications are maintained. This incident should be carefully reviewed by USAMRIID for other lessons to be learned. Questions to consider include: 1) Were work practices appropriate? 2) Was respiratory protection appropriate, what level of protection would be appropriate, and was it employed? 3) Were practices in the laboratory regularly audited? 4) Are vaccination practices appropriate? 5) Was the worker aware of the procedures for reporting incidents and was she comfortable that incidents can be reported without fear of reprisal? 6) Are changes in current practices, PPE, engineering controls, or administrative controls for work with F. tularensis necessary?
In 2000, another incident also highlights that human actions are probably the weakest link in biosafety. The case involved occupational exposure to Burkholderia mallei, the causative agent of glanders. This was the first known case of human glanders in the United States since 1945. B. mallei typically infects equids (horses, mules, and donkeys). The infected USAMRIID worker sought medical attention outside the military system, but did not disclose the type of work with which he was involved, which contributed to the delayed diagnosis of his illness. No other cases occurred. The scientist admitted to not wearing gloves while working with the agent, so the route of exposure was assumed to be percutaneous. This case illustrates a breach of biosafety procedures for working safely with BSL-3 agents, as well as a violation of the laboratory’s requirement that illnesses be reported promptly. As noted earlier in this chapter, special training and SOPs are in place to prevent such events, but it remains the responsibility of an individual to adhere to ethical norms for safe and responsible scientific conduct.
In response to the circumstances of this case, USAMRIID conducted safety “stand-down” training with all employees and conducted laboratory environmental sampling, which found no evidence of surface or air contamination. In addition, the employee involved was provided additional training and was required to demonstrate competency before being allowed to work independently (Wadding 2009).
2001 Anthrax Attacks
In 2001, letters tainted with B. anthracis spores were mailed to lawmakers on Capitol Hill and members of the news media. The mailings were linked to 22 cases of anthrax, five of which were fatal. The spores are believed to have originated from the laboratories at USAMRIID because the strain of B. anthracis used in the attacks appeared to be the same as one used at the institute for research. The laboratory worker was suspected by the FBI because he had ready access to the specific B. anthracis strain and because aspects of his personality and work habits were considered suspicious. (The FBI’s public findings came after the publication of the EIS.) The incident reinforced existing serious concerns about biosurety. There has been rigorous federal attention paid to the vet-
ting of individuals who have access to these agents (42 CFR 73 Select Agent Rule 2003, 2005), inventory and accountability, and the development of a Biological Personnel Reliability Program (see earlier discussion in the section on Biosurety).
Inaccurate Inventory of BSAT
In early 2009, a USAMRIID researcher found four vials of BSAT material that were not included in the institute’s inventory database. USAMRIID undertook a complete BSAT inventory to double-check the accuracy of the automated inventory management system in accordance with new Army and DOD requirements that the inventory system account for 100 percent of BSAT material in its possession. A “stand-down” was ordered until every freezer and refrigerator was inventoried and all BSAT materials were identified and accounted for. A significant overage of vials (over 9,000 vials) was discovered and reported. Many of these “newly found” vials contained small volumes of working stocks left behind in freezers by departing scientists. Since this incident, additional inventory controls have been established and implemented. For example, SOPs for BSAT inventory shipping, receiving, and transfers were revised; BSAT inventory audits will now be performed annually; and consideration is being given to creating a centralized BSAT storage facility within USAMRIID (Skvorak 2009).
FINDINGS
-
USAMRIID’s current procedures and regulations for its biocontainment facilities meet or exceed the standards of NIH and CDC for such facilities and other accepted rules and guidance for handling and containing pathogens during use, inventorying, and storage; treating and safely disposing of laboratory solid waste; and handling and decontaminating wastewater.
-
Measures have been taken to improve safety at USAMRIID when problems have been identified. The new facilities will be operated under even more stringent guidelines than were in place previously regarding physical security, engineering infrastructure and redundancies, biosafety, and biosecurity. Thus, the committee has a high degree of confidence that the new USAMRIID facility will have the appropriate and effective physical security, biosurety program, and biosafety operating practices and procedures in place to protect its workers and the public from exposures to pathogens and any new pathogens studied in its laboratories. However, the recent tularemia case shows that risk cannot be reduced to zero.
-
USAMRIID has strived to improve safety procedures. Lessons learned from exposure and/or disease incidents have directed some of the improvements, as indicated by the decrease in laboratory-acquired infections from the 1940s to the present, so that laboratory-acquired infections are now infrequent.
RECOMMENDATIONS
-
USAMRIID should continue to set high standards for advancing security, operational, and biosurety measures.
-
Although USAMRIID has sought to set high standards for biosurety and biosafety, recent examples of laboratory-acquired infections (glanders, tularemia) and breaches in containment (B. anthracis spores) point to human error or deliberate misuse. The committee recommends further formalized training in responsibility and accountability at USAMRIID, similar to that required for NIH-sponsored training programs. The widely used text for this training (On Being a Scientist: Responsible Conduct in Research [National Academy of Sciences 1995]) includes modules for aspects such as error and negligence in science and conflicts of interest, but can be supplemented with case studies and discussions of relevant issues, such as whistle-blowing, whistle-blower protection, and dual use awareness. The circumstances surrounding the laboratory-acquired infections also should be carefully evaluated to determine what lessons can be learned for preventing future cases.



















