4
Physician and Patient Participation in Cancer Clinical Trials
The ability to translate scientific discoveries into clinical advances relies on a robust clinical trials infrastructure, which is largely dependent on a critical mass of patients and physicians willing to participate in clinical trials. However, current indications suggest that participation in clinical trials is the exception rather than the rule both for patients and for physicians. It is estimated that only 3 percent of adults with cancer participate in clinical trials, and people who are members of racial and ethnic minorities, elderly and low-income individuals, and people who live in rural areas remain underrepresented (EDICT, 2008). Without adequate rates of participation by patients and physicians, it is unlikely that important research questions with the potential to improve patient outcomes will be answered efficiently and effectively. Furthermore, the trend toward targeted therapy and personalized medicine necessitates larger numbers of patients willing to participate in clinical trials, since these trials are increasingly reliant on stratified populations. According to the National Cancer Institute (NCI), the true effectiveness of cancer therapies will not be known unless more people are involved in clinical trials (NCI, 2001).
The committee concluded that the value of clinical trials in advancing patient care necessitates a paradigm change in the current approach to clinical trials. Building on discussions at a prior Institute of Medicine (IOM) workshop (IOM, 2009b), the committee emphasized that the therapies offered through clinical trials should ideally be considered the preferred treatment choice for physicians and patients, if they are available. Broad participation in a publicly sponsored clinical trials system—by investigators, community physicians, cancer centers, and patients—will enhance the system’s impact by efficiently providing practice-changing evidence.
Although many important clinical trials are undertaken by the pharmaceutical industry, relying solely on pharmaceutical companies and contract research organizations to maintain a clinical trials infrastructure would be detrimental for a variety of reasons. First, companies are primarily responsible to their shareholders and have less incentive to conduct certain types of clinical trials that are in the best interests of society. An industry-only clinical trials infrastructure may neglect important areas of research, including research on the comparative effectiveness of different therapeutics, research on novel indications for older drugs, determination of dose intensity, the development of combination products from multiple companies, research on the development of drugs for the treatment of rare diseases, evaluation of different surgical and radiation treatment methods, research on screening and prevention strategies, and research on rehabilitation and quality of life following therapy. In addition, industry trials are increasingly moving away from the United States (Agres, 2005; Glickman et al., 2009; IOM, 2009b; Normile, 2008), which could lower U.S. patient access to clinical trials and, in some instances, lower the applicability of the findings of clinical trials to the U.S. population. This movement of clinical trials overseas threatens the capacity of the United States to maintain a clinical trials infrastructure and workforce. Academic centers and community practices play a crucial role in training and mentoring the next generation of clinical investigators, but recent evidence suggests that the number of U.S.-based principal investigators is declining (Getz, 2007), potentially shrinking the training pipeline for new clinical investigators and negatively affecting the U.S. economy.
Rather than rely on a pharmaceutical industry-centered clinical trials infrastructure, the committee concluded that incentives must be realigned so that clinical investigators and patients will choose to participate in a publicly sponsored clinical trials system. The committee took a broad view of the disincentives preventing high rates of participation. For clinical investigators, the committee emphasized issues related to reimbursement, extensive regulatory burdens, and academic procedures related to tenure, promotion, and career development. For patients, the committee discussed third-party coverage for participation in clinical trials and patient and physician attitudes about participation in clinical trials, including knowledge of the availability of clinical trials. Many of these issues have been addressed in prior evaluations of the Clinical Trials Cooperative Group Program (NCI, 1997, 2005b), but low rates of participation in cancer clinical trials remains a significant barrier to the efficient translation of scientific discoveries into advances in patient care.
CLINICAL INVESTIGATOR PARTICIPATION
Retaining a workforce competent in conducting clinical trials is essential to maintaining a strong publicly funded clinical trials infrastructure.
However, the current system does not foster clinical investigator participation in publicly sponsored clinical trials. Misaligned incentives, both in academia and in physician practices, inhibit robust participation. Realigning incentives for clinical investigators so that they may participate in clinical trials is essential to increasing patient accrual.
Physicians who participate in Cooperative Group trials do so despite significant barriers and disincentives and have been referred to as an “all-volunteer army” because the costs of conducting trials outstrip the reimbursements provided by the NCI (IOM, 2009b; see also Chapter 3). Many of the reasons that investigators continue to participate in trials include the desire to advance cancer research and improve future patient care, to be involved in the design and conduct of clinical trials, and to offer patients access to state-of-the-art care, including access to investigational compounds.
Despite the importance of cancer clinical trials, the disincentives limiting provider participation are numerous. The American Cancer Society Cancer Action Network (ACS CAN) breaks down the disincentives into financial barriers, regulatory burdens, awareness of clinical trial options, and physician perceptions of clinical trials (ACS CAN, 2009). In addition to the barriers that are common to all investigators, academic physicians and community physicians confront somewhat unique barriers. Clinical trial involvement is not well rewarded in tenure and promotion processes in academia and is not aligned with the time investment required for conducting large, multi-institutional trials. Community practitioners lack the needed infrastructure and support to actively participate in clinical trials.
Financial and Regulatory Barriers
Participating Sites
In 2009, the American Society of Clinical Oncology (ASCO) conducted an Internet poll of Cooperative Group sites seeking to understand whether financial or other barriers are preventing participation in Cooperative Group clinical trials. The survey found that 32 percent of respondents (155 of 478 sites)1 indicated that they plan on limiting participation2 in the Cooperative Group Program (Blayney, 2009). Seventy-five percent of survey respondents who indicated that they were limiting participation cited
inadequate per case reimbursement as an important factor in this decision. An additional 38 sites (or 8 percent or those surveyed) were considering these limitations. Although many respondents indicated a preference for participation in Cooperative Group trials, 49 respondents indicated that their sites were increasing their rate of participation in industry trials. Jeffrey Abrams, associate director of the Cancer Therapy Evaluation Program, has also noted that cancer centers are curtailing their participation in Cooperative Group trials. In a recent IOM workshop, Abrams said some cancer centers have capped the number of accruals that can go to Cooperative Group trials, because they believe that it is too much of an economic burden (IOM, 2009b).
As the findings of the survey suggest, cancer centers and other sites enrolling patients in Cooperative Group clinical trials may be limiting participation because of inadequate reimbursement. Although Cooperative Group trials are recognized for their fundamental importance in setting the standard of care, institutions are faced with increasing cost restraints, making it difficult to participate in inadequately reimbursed activities, such as Cooperative Group trials. Despite the resource-intensive nature of clinical trials, the per patient reimbursement of $2,000 has remained the same since 1999 (IOM, 2009b). In June 2008, the NCI began using a complexity rating scheme to increase the rate of reimbursement for complex clinical trials, with the maximum reimbursement of $3,000 (Mooney, 2008). However, the extra $1,000 of reimbursement for complex clinical trials is still far below estimated costs required to conduct clinical trials, which in 2004 were estimated to be a median cost of $3,500 and $6,000 per patient for Phase III and Phase II trials, respectively (C-Change, 2005). For many cancer clinical trials, this amount appears to be inadequate to cover most labor costs, per subject enrollment costs, and additional research-related paperwork and reporting requirements (ACS CAN, 2009). If an academic medical center or a physician practice stands to lose thousands of dollars per patient by participating in the current publicly sponsored clinical trials system, it is not surprising that physician preferences are to treat patients with the standard of care or with a therapeutic agent off protocol, rather than being involved in a significantly more costly and more burdensome clinical trial.
Individual Physicians
According to one survey, only 13 percent of physicians are clinical investigators (Taylor, 2004). Aside from the lack of opportunity to participate as a clinical investigator, that survey found that the primary reasons for not acting as a clinical investigator include the time commitment involved (32 percent), a lack of personal support (30 percent), not having
the resources to run a successful trial (26 percent), and the paperwork burden (24 percent). However, only 17 percent of physicians surveyed said that they had no interest in becoming a clinical investigator (Taylor, 2004).
Physicians who enroll patients in Cooperative Group clinical trials face increased time and effort not reflected in current reimbursement policies. To participate in clinical trials, physicians must find applicable trials for their patients, explain these trials to their patients, and obtain informed consent, which can add significant time and effort to a physician’s workload (Comis et al., 2003). On average, 4 hours of a physician’s time is required before a patient can be enrolled in a trial, and some of that time is devoted to patients who ultimately choose not to participate in the clinical trial (Mansour, 1994). If a patient enrolls in a trial, the data collection and documentation requirements are substantially more onerous for patients in a trial than for patients receiving standard therapy outside of a trial (Comis et al., 2003). The complexity of the protocol, the recruitment and selection of study participants, high-intensity visit schedules, protocols that deviate from the standard of care, and the complexity and acuity of the patient population all add to the costs of treating patients within a clinical trial setting (ACS CAN, 2009). At an IOM workshop, one community physician noted that he gives double bookings for patients participating in complex clinical trials (for example, one with two targeted molecules) to have time to sort through all of the toxicities and to adjust the doses for each drug (IOM, 2009b). In addition, radiologists and pathologists must spend additional time conducting tests and analyses for clinical trials and resist doing so because they are not compensated for the extra work and time required.
As discussed in Chapter 3, the regulatory requirements for clinical trials are highly complex. These requirements can also prevent robust provider participation. Clinical investigators must contend with ambiguous and complex regulations in clinical trials, including the reporting requirements of the Food and Drug Administration (FDA), in addition to those of the National Institutes of Health (NIH), such as requirements for proof of adherence to good clinical practice guidelines and human subject protections; reporting of adverse events; and adherence to data monitoring, audits, and quality control requirements (ACS CAN, 2009). Oncologists are less likely to refer patients for participation in a clinical trial if they perceive the paperwork to be onerous and trial entry requirements to be too stringent (Siminoff et al., 2000). Given the low reimbursement levels and the voluntary nature of the Cooperative Group Program, the added burden of the regulatory requirements on clinical investigators is a major disincentive to participation.
In light of the additional time and resources required for physician participation in a clinical trial, the committee recognized the importance
of establishing a mechanism to reimburse physicians for their time commensurate with the level of work involved with participation in a trial. Specifically, the committee recommends that NCI increase the per case reimbursement rate. The committee also recommends that the American Medical Association establish new Current Procedural Terminology codes (CPT codes), reimbursed by the Centers for Medicare & Medicaid Services (CMS), private insurers, and other third-party payors, to pay an enhanced reimbursement for offering, enrolling, managing, and following a patient in a clinical trial. New CPT codes, with a higher reimbursement rate, that acknowledge the additional time and resources needed to counsel and care for a patient in a clinical trial would address an important deterrent to physician participation in clinical trials. With a proper definition of eligible trials, use of such a code could be easily audited.
The committee also discussed the importance of funding principal investigators who participate in Cooperative Group research. The committee distinguished two types of principal investigators: first, principal investigators who develop and oversee a Cooperative Group clinical trial, and second, principal investigators who oversee all Cooperative Group trials at a participating institution. Both of these types of principal investigators are important to the design, implementation, and monitoring of Cooperative Group trials. Therefore, the committee recommends that NCI provide funding to site and trial principal investigators to cover the time they need to develop and oversee approved clinical trials. In a similar step, the Operational Efficiency Working Group recommended that NCI officially recognize investigators for leadership in the design and conduct of Cooperative Group trials (Doroshow and Hortobagyi, 2009).
Participation by Community Physicians
The majority of cancer patients are treated in community settings, whereas the majority of cancer patients who enroll in clinical trials are treated within academic settings (Cox and McGarry, 2003; Somkin et al., 2005). However, community physicians also play a vital role in recruiting patients into clinical trials, especially large-scale trials of methods for cancer screening, adjuvant therapies, and first-line therapies for metastatic disease. One of the strengths of the Cooperative Group Program is the extensive involvement of physicians and patients in community practices. Participation by physicians and patients within community settings helps to ensure that the results of clinical trials are meaningful to a broad segment of the U.S. population and provides the patients with access to promising, innovative therapies as they are developed. NCI’s Cooperative Group Program is responsible for enrolling 85 percent of patients who enter NCI-sponsored trials, and about 65 percent of these patients enter from community-based
practices that include Community Clinical Oncology Program and academic medical center affiliates (C-Change and Coalition of Cancer Cooperative Groups, 2006). Despite the importance of enrolling community-based patients into clinical trials, a number of barriers prevent more community physicians from participating in clinical trials. During the IOM workshop on multicenter clinical trials, one speaker noted that doctors have virtually no incentives to enroll patients in a clinical trial but have many disincentives (IOM, 2009b). As with all physicians, financial burdens, regulatory complexity, awareness of trial availability, and attitudes about participation are barriers to clinical trial participation for community physicians. However, physicians in community practices may have fewer resources to support participation in a clinical trial, including a lack of logistical support and a lack of clinical research nurses. Some modest resources are available to support community practitioners, such as the Community Oncology Research grant, which gives up to $30,000 to support three community-based practices that enhance their clinical trials programs (ASCO, 2008). Despite the availability of these support mechanisms, a large discrepancy between the per case reimbursement and the actual cost of participation remains, and this is a major disincentive to participation. As mentioned above, a primary mechanism for improving community physician involvement in clinical trials includes better reimbursement for physicians enrolling patients in clinical trials.
In addition, the committee emphasized the importance of recognizing the research staff who participate in cancer clinical trials, including physicians, nurses, clinical research associates, pharmacists, and others who conduct clinical trials. The committee recommends that NCI work with a nonprofit foundation to develop a certification program, as recommended by the Clinical Trials Working Group (CTWG). Such a program could be one component of site credentialing for participation in Cooperative Group trials (see also the section on participation patterns). A certification program could distinguish investigators who actively participate in clinical trials and have met other metrics of high-quality care. Patients may seek out certified physicians, encouraging physicians to become certified and become more involved in clinical trials.
Singling out investigators who participate in clinical trials is consistent with the perspective that well-designed, properly implemented clinical trials are the optimal treatment option. In well-designed trials, patients randomized to the control group typically receive the current standard of care, whereas those allocated to the new treatment receive a treatment hypothesized to be similar to or better than the standard of care (Ellis, 2000). In one survey of oncology leaders at community integrated health centers, eight leaders agreed that trial participation is imperative to high-quality care, whereas only one leader did not support that assessment (Somkin et al., 2005).
Several analyses have attempted to assess whether clinical trial participants have better outcomes than nonparticipants, with mixed results (Braunholtz et al., 2001; Davis et al., 1985; Peppercorn et al., 2004; Robinson et al., 2009; Roy et al., 2000; Stiller, 1994). Although some studies suggest that individuals who participate in clinical trials have better outcomes than non-participants in specific medical areas, systematic reviews looking at clinical trials overall have not found such an effect. But these reviews did show that patients participating in a randomized controlled trial did not have worse outcomes than those receiving a similar treatment outside the trial (Vist et al., 2005, 2008). The National Comprehensive Cancer Network (NCCN) guidelines also state that “NCCN believes that the best management of any cancer patient is in a clinical trial” (NCCN, 2009).
Quality improvement programs, such as the ASCO Quality Oncology Practice Initiative (QOPI), may also single out high-performing practicing oncologists to patients and other stakeholders. QOPI is a voluntary self-assessment program that certifies oncology practices for high-quality care. ASCO analyzes practice data for evidence-based quality measures and provides feedback to practices to identify areas of improvement (ASCO certification program emphasizes quality of care, 2009). For example, QOPI could use a metric to assess how many of a physician’s patients are enrolled in clinical trials. Currently, Kaiser Permanente uses trial enrollment as a quality metric as part of its practice-based accountabilities (Wallace, 2009).
Participation by Academic Clinicians
Tenure and promotion policies and declining numbers of clinical investigators may prevent higher levels of involvement by academic investigators in clinical research. Tenure and promotion policies tend to value individual investigator-initiated, basic research more than multi-institutional, team-oriented clinical research. In addition, the shrinking physician scientist pipeline suggests that additional efforts may be needed to encourage, train, and retain clinical investigators.
Recognizing and Rewarding Clinical and Team Research
Clinical investigators require a specialized skills set, training, and orientation to be successful. They must be able to navigate the complex regulatory environment, work in teams, share the rewards of their work, and defer financial compensation while spending years in training (Andrews et al., 2009). Despite these unique skills and orientation, physician scientists focused on clinical research may not receive academic recognition and advancement commensurate with the value of their work. This may be due
to a number of factors, including a lack of awareness by promotions committees of what such research entails; the collaborative nature of research, which makes it difficult to mark individual accomplishments; the time span needed to obtain results from clinical research; and the underfunding of the Cooperative Groups (IOM, 2009b). Because Cooperative Group research is primarily accomplished in multi-institutional settings, promotion committees may be unaware of the intellectual rigor and complexity involved in trial design and protocol implementation. Likewise, promotion committees may not have a sense of the time commitment required for clinical trial research or the importance of Cooperative Group research in advancing the field of oncology research and patient care. In addition, physician scientists are less likely than basic scientists to have protected paid time to perform their research (NCI, 2005a).
In particular, clinical research—such as involvement in the Cooperative Group Program—requires a team orientation. According to the President’s Cancer Panel, team approaches are the paradigm for achieving progress in translating basic science discoveries into applications that improve clinical practice (NCI, 2005a). However, traditional academic metrics and incentives structures tend to reward individuals rather than teams (Altshuler and Altshuler, 2004). For example, current scientific journal authorship guidelines allow for singling out only first and last authors as leaders in publication. Altshuler and Altshuler called for a deconstruction of the author list so that the particular contributions of each author may be indicated. It is also possible to list all investigators instead of just the principal investigator in the Computer Retrieval of Information on Scientific Projects database3 (IOM, 2009b).
Because traditional academic metrics focus on individual accomplishments, investigators may participate minimally in team-oriented research activities. For example, investigators may limit participation in multi-institutional, late-phase clinical trials, such as those conducted through the Cooperative Group Program, so that they can dedicate more time to activities that are viewed as having higher value within their institution in terms of both funding and recognition. Academic clinicians are incentivized to conduct smaller, individual investigator-initiated studies that lead to R01 grants, the primary mechanism of support for NIH-funded cancer research (NCI, 2005a). The inadequate value given to team-based, clinical research in academic tenure and promotion decisions prevents more robust participation in Cooperative Group trials. Therefore, the committee recommends that academic medical centers develop policies and evaluation
metrics that recognize and reward clinical/team research in promotion and tenure decisions. Similar to the committee’s recommendation, the Operational Efficiency Working Group of the Clinical Trials Advisory Committee recommended that NCI create incentives for institutions to include accrual in Cooperative Group clinical trials as a service criterion for tenure and promotion.
Although there are mechanisms to support team-oriented research (such as NIH P01 Program Project, P30 Center, P50 SPORE grants, and U54 Cooperative Agreements4), they are a small fraction of the funding for individual project grants (NCI, 2005a). Without incentives to support team-oriented clinical research, the translation of discoveries in basic science into clinical knowledge and care will be slowed (Schrier, 1997). Recognizing the impact of this dilemma on the Cooperative Group Program, the CTWG recommended that NCI and academic incentives be realigned so that they promote collaborative team science (NCI, 2005b).
The Cooperative Group Program relies on clinical, team-oriented research. However, the committee found that cancer center Support Grants (CCSGs), which support the research capacities of cancer centers, do not adequately incentivize participation in multi-institutional Cooperative Group trials. Rather, CCSG review criteria favor investigator-initiated trials emerging from basic discoveries within a cancer center’s own institution. To fulfill current CCSG review guidelines, cancer centers that have a clinical component are expected to provide leadership for and participate in Cooperative Group trials.5 Part of the CCSG review is the assessment of a cancer center’s funding base. The U10 award that supports a Cooperative Group’s operations and statistical offices is considered equivalently with other peer-reviewed funding. However, the per case reimbursements that the Cooperative Groups provide to cancer centers are not counted in the CCSG review’s benchmark ratio,6 part of the funding base assessment that helps to determine the CCSG award amount. Because per case reimbursements are not adequately rewarded in CCSG reviews and do not fully cover the costs
of participation in a clinical trial, cancer center directors may discourage their investigators from actively participating in Cooperative Group trials. To improve participation in Cooperative Group trials, the committee recommends that NCI recognize and reward Cooperative Group efforts in Cancer Center Support Grant (CCSG; P30) site visits, and allow the CCSG research base to include the federal per case funding received by cancer centers that participate in Cooperative Group trials.
Ensuring the Clinical Investigator Pipeline
The pipeline of physician scientists is decreasing. In the United States, the physician scientist population is smaller now than it was 25 years ago (Ley and Rosenberg, 2005). In 1983, there were 18,535 physician scientists in the United States; by 1998, that number had fallen to 14,479, a 22 percent decline (Varki and Rosenberg, 2003). Reasons for the shrinking pipeline include the changing health care environment, the complexity of rapid advances in biomedical science and the consequent retooling necessary after clinical training, the length and rigor of research training required, the scarcity of funding for subspecialty training positions, competition for research funding, the perception that successful clinician scientists are those who focus on basic research and not clinical research, and senior faculty pessimism over the survival of physician scientists (Schrier, 1997).
Some progress toward reversing the trend has occurred. In 1998, NIH established career development rewards (the K23 and K24 grant programs) for young and established physicians undertaking clinical research. In 2002, NIH offered competitive loan repayment programs that offered at least 2 years of tax-free debt relief for young scientists with commitments to clinically oriented research training (Ley and Rosenberg, 2005). Several awards specifically focus on strengthening the physician scientist pipeline for oncology. For example, through the Damon Runyon Clinical Investigator Award, early career physician scientists receive $450,000 to support the development of their cancer research program to conduct patient-oriented cancer research under the mentorship of leading scientists (Damon Runyon Cancer Research Foundation, 2009). In addition, The ASCO Cancer Foundation (TACF) and NCI partnered to provide funding and recognition of clinical investigators leading cancer research programs at academic cancer centers through the NCI-TACF clinical investigator award. The award provides 2 years of salary support (10 to 15 percent) for up to 10 clinical investigators who play leadership roles in clinical trials at NCI-designated cancer centers. The intention of the award is to recognize clinical investigators who are not principal investigators on an NIH grant but who are actively involved in NCI-funded collaborative clinical trials, promoting collaborative team science and the retention of clinical investigators (NCI-TACF
clinical investigator award, 2009). In terms of mentorship, the American Association for the Advancement of Science and the journal Science recently launched CTScieNet, the Clinical and Translational Science Network. This site is both a career development portal and an evolving communications infrastructure whose goal is to educate trainees and new investigators in translational research skills and to link scientists by connecting communities of scientists through professional networks (Andrews et al., 2009).
The Cooperative Group infrastructure is recognized for its importance in mentoring and training young investigators because it brings together senior clinical investigators, experienced biostatisticians, data management experts, clinicians, and laboratory and population scientists (Mauer et al., 2007). According to Gregory Reaman, past chair of the Children’s Oncology Group, young investigators are taking the lead in some pediatric trials and more experienced investigators are stepping back and acting as mentors (Reaman, 2009).
Although career development awards and mentorship activities are encouraging, the committee found that these actions do not appear to be resulting in robust improvements in ensuring a pipeline of well-trained, motivated investigators willing to make career commitments to clinical research. The committee recommends that all stakeholders, including academic medical centers, community practices, professional societies, and NCI, work to ensure that clinical investigators have adequate training and mentoring, paid protected research time, the necessary resources, and recognition.
Physician Awareness of Clinical Trials
A lack of physician awareness of clinical trials also limits trial participation. According to one survey, the most common reason cited for physician nonparticipation in clinical trials was a lack of knowledge about clinical trials (Taylor, 2004). Primary care and specialty physicians who are not affiliated with research institutions may be even less aware of patient eligibility for clinical trials (EDICT, 2008). Because physicians are the primary conduit to patient entry into clinical trials, physician knowledge and endorsement of clinical trials are essential to enrolling patients in clinical trials (Schain, 1994). Comis and colleagues (2009) found that patient participation in a clinical trial was directly related to the level of physician involvement reported by the patient. Although there are clinical trial registries, the committee found that these registries were inadequate for broadly informing physicians of clinical trial availability at the point of care. The committee concluded that user-friendly electronic tools could be valuable for better informing physicians of relevant clinical trials at the point of care, potentially leading to increased physician and patient participation in
clinical trials. A further discussion of current clinical trial registries and the potential impact of electronic medical record (EMR) systems that quickly alert physicians about relevant clinical trials can be found in the section on ensuring adequate patient accrual at participating sites.
Physician Perspectives on Clinical Trials
Although physicians’ lack of knowledge about clinical trials is a documented barrier to participation in clinical trials, changing physicians’ perspectives may be equally important in increasing rates of participation in the publicly sponsored clinical trials system. Some physicians may be reluctant to refer patients to clinical trials because they believe that their involvement in a clinical trial will be an excessive administrative or financial burden to their practice (EDICT, 2008). In addition to perspectives about uncompensated time and effort associated with participation in a clinical trial, physicians may limit patient participation because of their own beliefs and assumptions about patient eligibility related to factors of age, comorbidities, cost, and adherence (EDICT, 2008). Physicians may also feel more comfortable presenting a single therapeutic approach to a patient rather than discussing different treatment options—including clinical trials—for fear that they may lose contact with and control over a patient’s follow-up if the patient participates in a clinical trial (Mansour, 1994). The committee noted the importance of changing physicians’ perspectives so that they will be more likely to encourage their patients to participate in clinical trials. The committee recommends that physicians strive to make participation in clinical trials a key component of clinical practice. Emphasizing that evidence-based care requires participation in clinical research, the committee calls on physicians to take part in the accumulation of evidence by enrolling patients on clinical trials (see also the section on participation patterns).
ENSURING ADEQUATE PATIENT ACCRUAL AT PARTICIPATING SITES
Ensuring the rapid accrual of patients into available clinical trials is essential for the efficient translation of research advances into clinical practice. Without a high level of accrual of patients into trials, it is unlikely that important research questions with the potential to improve patient outcomes will be answered efficiently and effectively. However, many trials never reach their accrual goals and thus generate no meaningful results to be published or disseminated. To ensure the rapid conduct and completion of clinical trials, the enrollment of patients on to clinical trials must be improved. At the same time, it is essential that clinical trials conducted by the Cooperative Groups maintain high-quality standards. Therefore, the
committee recommends that NCI, Cooperative Groups, and physicians take steps to increase the speed, volume, and diversity of patient accrual and ensure high-quality performance at all sites participating in Cooperative Group trials. In addition, NCI, Cooperative Groups, and physicians should encourage greater enrollment in high-priority trials, regardless of where the trial originates.
Several opportunities to facilitate patient accrual exist. As noted earlier, patients and physicians often lack awareness of clinical trial availability. Encouraging the development of a user-friendly, transparent, up-to-date, and easily accessible centralized registry could improve both physician and patient awareness of the available trials. In combination with electronic tools, such as clinical decision support software, a centralized registry could cue physicians to important, applicable clinical trials at the point of care.
In addition to facilitating access to quality information about available clinical trials, it is also possible to limit overly stringent eligibility requirements for clinical trial participation. Participants in previous IOM workshop discussions suggested that overly stringent eligibility criteria unnecessarily inhibit patient accrual and may limit the applicability of the findings of clinical trials to the general population (IOM, 2009b). Programmatic changes to the Cooperative Group Program could also facilitate patient accrual. Sites participating in Cooperative Group trials overseen by multiple groups must currently be separately credentialed and audited by each group. The establishment of a centralized credentialing body could ease administrative burdens and encourage more sites to actively accrue patients to high-priority, applicable trials.
Information on Clinical Trial Availability
Registries of clinical trials are primary resources that patients and their providers use to locate information about clinical trials (IOM, 2006). A number of registries exist, and the goals of these databases vary by user. The most comprehensive registry to date is ClinicalTrials.gov. Since February 2000, all entities conducting clinical trials of experimental treatments for serious or life-threatening diseases and conditions have been required to submit specific information to this public clinical trial registry, which was established by the National Library of Medicine of the U.S. Department of Health and Human Services as a result of Section 113 of the FDA Modernization Act of 1997. The FDA Amendments Act of 2007 (FDAAA) expanded the scope of clinical trials required to be registered with ClinicalTrials.gov to include all controlled clinical investigations (except Phase I trials) of drugs, biologics, and devices subject to FDA regulation. The law applies to research for all conditions and to research conducted by all sponsor types (e.g., industry, government, and academia) (reviewed by Zarin and Tse, 2008). About
80,551 trials sponsored by NIH, other federal agencies, and private industry are registered with ClinicalTrials.gov (NIH, 2008). However, FDAAA does not mandate the public reporting of trials with investigational interventions not regulated by FDA, such as surgical therapies (Zarin and Tse, 2008), which may be relevant to cancer patients.
In 2004, the International Committee of Medical Journal Editors announced that beginning on July 1, 2005, member journals would require as a precondition for publication registration of the clinical trials described in the journal articles (De Angelis et al., 2004). This policy led to a 73 percent worldwide increase in the number of trial registrations of all intervention types (Zarin et al., 2005).
Other registries with information on clinical trials are also available. TrialCheck.org is a cancer clinical trials registry supported by the Coalition of Cancer Cooperative Groups. TrialCheck is updated daily and is the only clinical trials database integrated into an EMR system (Comis, 2007). In addition, companies such as EmergingMed provide web-based tools to help match patients’ personal profiles to the enrollment criteria of available clinical trials, including both private and public trials (EmergingMed, 2009). NCI also has a clinical trials registry that contains information on more than 8,000 active clinical trials and 19,000 closed trials (NCI, 2009b). Georgia and North Carolina are trying to regionalize their clinical trials search engines and make the information more accurate and up-to-date to facilitate patient and physician access (IOM, 2009b).
Despite these encouraging steps, patients and physicians have difficulty navigating the available clinical trials registries. No centralized system currently exists to disseminate clinical trial information to patients and providers, making it difficult for patients with cancer and their providers to find appropriate trials of treatments for their particular disease and in their geographic location (IOM, 2009a). In addition, it has been reported that information on multiple trial search sites is often inaccurate, outdated, incomplete, or not regionalized (IOM, 2009b; Mathieu et al., 2009). Although the development of ClinicalTrials.gov, TrialCheck, EmergingMed and other registries are important first steps to providing the public with information on ongoing trials, they are not sufficient. A more comprehensive and transparent registry of clinical trials for drugs, biologics, and other therapeutic modalities is needed to enable patients and their providers to locate applicable, reliable clinical trial information. Better, user-friendly electronic tools that include information on high-priority trials, that are up-to-date, and that are easily, widely accessible by both patients and physicians could increase the level of awareness of trials and make it easier for physicians and patients to enroll in the most appropriate studies. Therefore, the committee recommends that NCI and Cooperative Groups develop electronic tools that cue physicians practic-
ing oncology via EMR systems about trials for which a particular patient is eligible.
For electronic tools to be highly successful, clinical research studies need automated connections to and interoperability with EMR systems, in addition to the seamless import and integration of protocol-directed assessments and interventions into existing clinical decision support systems (Masys, 2009). Such electronic tools with the right features for physician work flow could increase physician awareness about applicable clinical trials in real time. As mentioned above, TrialCheck is the only clinical trials database integrated into an EMR system (Comis, 2007). However, some impediments prevent the adoption and dissemination of user-friendly tools to notify physicians and their patients about applicable clinical trials. Current infrastructure limitations include the absence of interoperable EMRs and very low rates of adoption of clinical decision support tools (less than 10 percent of U.S. health care institutions have adopted these tools) (reviewed by Jha et al., 2006). However, the health information provisions of the American Recovery and Reinvestment Act of 2009 (ARRA) provided $19 billion to stimulate the meaningful use of EMR systems. The Office of the National Coordinator for Health Information Technology notes that the “focus on meaningful use is a recognition that better health care does not come solely from the adoption of technology itself, but through the exchange and use of health information to best inform clinical decisions at the point of care” (HHS, 2009), suggesting that meaningful use will include provisions for interoperability and the inclusion of tools such as decision support. In addition to the funding provided through ARRA, rapidly advancing information technologies7 (Masys, 2009) could facilitate the development of tools to inform physicians and patients of clinical trial availability. Patients could also benefit from education about participating in a clinical trial (see the section on expanding patient access to clinical trials).
Eligibility Requirements
Patients must meet certain eligibility criteria for entry into clinical trials. Historically, stringent eligibility criteria have excluded many patients, including, for example, those with prior cancers or certain prior treatments. However, there are some indications that the current eligibility criteria are unnecessarily stringent; from 1999 to 2005, the median number of eligibility criteria increased from 31 to 49 (Malakoff, 2008). It is estimated
that in current cancer clinical trials, only 20 to 40 percent of patients presenting at community or academic centers are eligible for participation in clinical trials. Those who are excluded include patients who have previously received multiple treatments, as well as those who have no sites of measurable disease, have poor performance status, or have advanced age (Melisko et al., 2005). During the IOM workshop on multicenter trials, there was general agreement that overly stringent eligibility criteria, such as previous treatment or previous cancer, unnecessarily prevented high rates of participation by patients (IOM, 2009b). The argument against relaxing the eligibility criteria is the potential to complicate trial data. Using less restrictive eligibility criteria may make it more difficult to interpret clinical trial findings, attribute adverse events, and may require the collection of additional safety data. However, the adoption of less restrictive eligibility criteria for most studies would permit more rapid accrual and also allow broader generalizations to be made, could better mimic the conditions encountered in medical practice, and could reduce the complexity and costs of clinical trials without compromising patient safety or requiring major increases in sample size (George, 1996). The committee recommends that NCI, Cooperative Groups, and physicians encourage the development of patient eligibility criteria that allow the broadest participation possible. Eliminating needless patient eligibility criteria would allow more flexibility and increase the rates of accrual. More patients could potentially benefit from enrollment in clinical trials, which could increase accrual and facilitate the timely completion of clinical trials.
Patient Advocate Involvement
Cancer patient advocates have been working with the Cooperative Groups since the early 1990s, and approximately 120 patient advocates currently serve as members of the 10 Cooperative Groups (Collyar, 2008). Examples of patient advocacy activities within Cooperative Group operations include incorporation of the patient or family perspective in the design and implementation of trials, patient education and communication, and patient recruitment, among other activities (Table 4-1). Patient advocates can provide feedback in areas such as eligibility criteria, study design and procedures, safety and confidentiality issues, feasibility, informed-consent processes, and other factors important to potential research participants that can help facilitate the development, implementation, and recruitment processes (Demmy et al., 2004). The involvement of patient advocates in the design and conduct of clinical trials has the potential to hasten accrual because trials that appeal to a broader population of patients may be designed (ENACCT-CCPH, 2008). Therefore, the committee recommends that NCI, Cooperative Groups, and physicians
TABLE 4-1 Cooperative Group Patient Advocate Teams
encourage greater participation by patient advocates in the design of clinical trials and in patient recruitment for trials.
One potential way to achieve greater participation by patients in the design of clinical trials is via community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained.8 Using their knowledge of the community to understand health problems and design trials that the community is likely
to value and participate in, these groups help recruit research participants. Additionally, community-based participatory research activities help to inform community members about how research is done and what comes out of it, with the goal of providing immediate benefits to the community from the results, when possible (ENACCT-CCPH, 2008).
Participation Patterns
A small percentage of physicians and sites enroll the majority of patients who participate in clinical trials. Many sites enroll only a few patients in trials to maintain their status in the program. Each Cooperative Group has specific criteria for accruing a certain number of patients to Cooperative Group trials. For example, Cancer and Leukemia Group B (CALGB) has three types of memberships with different thresholds for accrual: main member institutions are expected to have at least 50 registrations annually to CALGB trials, whereas at-large members are expected to have 30 registrations, and affiliates are expected to accrue at least 6 patients into CALGB trials (CALGB, 2009). However, discussions at an IOM workshop noted that it is difficult to encourage physicians and sites to participate more actively because there are few incentives to encourage greater involvement. Likewise, keeping sites open with very few accruals to Cooperative Group trials is usually not financially feasible. According to Laurence Baker, chair of the Southwest Oncology Group, the group “spends a lot of time talking about reducing the number of institutions and how we should police ourselves and reduce institutions that insufficiently participate” (IOM, 2009b).
Exacerbating the low rates of participation by sites and investigators is the amount of resources dedicated to credentialing and auditing the individual sites that participate in Cooperative Group research. Each Cooperative Group has its own rules and procedures for credentialing participating sites as part of its fulfillment of Cooperative Group Program guidelines. Both the operations centers and the statistics and data management centers of the Cooperative Groups have on-site auditing responsibilities (CTEP, 2006). In addition, the NCI Clinical Trials Monitoring Branch provides direct oversight of each Group’s monitoring program, which requires on-site auditing as well. Cooperative Group audits document the accuracy of the data submitted to the groups, verify investigator compliance with protocol and regulatory requirements, and provide an opportunity for the auditing team to discuss concerns about data quality and data management with sites. Cooperative Group guidelines require all institutions to be audited once every 36 months. To be in compliance with this requirement, each Group must conduct a comprehensive review of its membership and provide an annual accounting to NCI’s Cancer Therapy Evaluation Program of audit activities (CTEP, 2006).
With institutions participating in multiple Cooperative Group trials overseen by different groups, the various credentialing processes pose significant burdens to the participating site, the Cooperative Groups, and NCI. Having a single process for credentialing sites and investigators, with a corresponding registry, could ease administrative burdens. The committee recommends that NCI establish a centralized credentialing system for sites that participate in Cooperative Group trials. Additionally, the committee recommends that the Cooperative Groups eliminate investigators and sites with low rates of accrual or inadequate data management skills or quality. Centralized credentialing, in concert with the elimination of investigators or sites with low rates of accrual and inadequate data management capacity, could improve the efficiency of accrual while maintaining high standards for the participating sites. In addition to easing administrative burdens, centralized credentialing could also facilitate enrollment of patients in high-priority trials, as recommended earlier.
ASCO’s recent policy statement on clinical trial sites is useful for establishing centralized credentialing criteria. The statement outlined ASCO’s perspective on minimum site requirements and attributes that single out high-performing clinical trial sites (Zon et al., 2008) (Box 4-1). Among the attributes of exemplary sites are high rates of accrual, quality assurance, and promotion of clinical trials awareness programs. The Joint Commission, which accredits and certifies health care organizations and programs in the United States, may be another resource. As outlined earlier, the committee emphasizes the important role of physicians in the accumulation of data to support evidence-based care by offering high-quality clinical trials to their patients. Therefore, the committee recommends that physicians strive to achieve the ASCO exemplary attributes for academic and community clinical trial sites, including high accrual rates of 10 percent or more.
EXPANDING PATIENT ACCESS TO CLINICAL TRIALS
Ensuring patient access to clinical trials is essential to improving and advancing high-quality, evidence-based care. Without clinical trials that accrue patients in a timely manner, the rapid diffusion of clinical advances into practice is hampered and interventions of questionable benefit may remain part of clinical practice without adequate evidence supporting their use. For example, in 1999, evidence for the lack of benefit of bone marrow transplantation for breast cancer was found, after several years of delay because of poor trial accrual (Bennett et al., 2001). While the trial was ongoing, many women received this treatment outside of the clinical trial, enduring the severe adverse effects of this therapy, including treatment-related deaths, without evidence to guide the treatment decision.
|
BOX 4-1 American Society of Clinical Oncology Exemplary Attributes for Clinical Trial Sites Exemplary attributes:
NOTE: Accrual rate is defined as number of patients enrolled annually/number of new patients seen annually. SOURCE: Zon et al., 2008. |
Only 2 to 3 percent of adults with cancer participate in oncology clinical trials. Furthermore, elderly individuals, people who are members of racial and ethnic minority groups, low-income individuals, and people who reside in rural areas remain underrepresented in clinical trials (EDICT, 2008). This minimal participation has been attributed to a number of factors, including stringent eligibility criteria and physicians’ perspectives and awareness of clinical trials (as described in the preceding sections), as well as inadequate and uncertain insurance coverage and patient attitudes about and knowledge of clinical trials (as further delineated below) and complex social and institutional barriers delaying the implementation of clinical trials (see Chapter 3).
Patient Participation in Clinical Trials
A variety of factors prevent robust patient participation in clinical trials (see Table 4-2). One survey demonstrated that a majority of cancer patients either are unaware of the possibility of participation in clinical trials or are unsure that participation in clinical trials is an option for them (HarrisInteractive, 2001). However, surveys indicate that once they are informed about clinical trials and their eligibility for participation, people are interested in participating in clinical trials. Of the 85 percent of cancer patients who were unaware or unsure that participation in clinical trials was an option, about 75 percent said that they would have been willing
TABLE 4-2 Why Cancer Patients Who Are Aware of Clinical Trials Do Not Participate
|
Percent Who Responded “Major Reason” |
“Aware” But Did Not Participate Percent |
|
Belief that they would be better off taking “the standard treatment” |
37 |
|
Fear that they might get a placebo rather than actual treatment |
31 |
|
Belief that “the standard treatment” would be more effective |
30 |
|
Fear of being treated “like a guinea pig” |
22 |
|
Distance they would have to travel to obtain treatment |
21 |
|
Belief that the cost of treatment would not be covered by insurance |
20 |
|
Amount they would have to pay out-of-pocket |
18 |
|
Fear that their doctor would not be able to choose best treatment |
18 |
|
The effort involved in the informed consent process |
6 |
|
SOURCE: HarrisInteractive, 2001. Reprinted, with permission, from HarrisInteractive, 2001. Copyright 2001 by Harris Interactive Inc. |
|
to enroll (HarrisInteractive, 2001). A patient advocate has noted that the general population is not usually aware of and does not pay attention to clinical trial awareness campaigns until they are afflicted with a condition with inadequate treatment options (IOM, 2009b). Other surveys also suggest that if they are asked to participate, adults are willing to participate in clinical trials. In a survey of American adults, 32 percent indicated that they would be very willing to participate in cancer clinical trials if they were asked to do so, and 38 percent of adults said that they are inclined to participate if they were asked but have some questions or reservations about participating (Comis, 2003). One study determined that a lack of awareness and low prioritization of clinical trials by physicians, policy makers, and patients remain significant challenges to advancing effective clinical trials (C-Change and Coalition of Cancer Cooperative Groups, 2006).
People who do enroll in clinical trials do so for different reasons. According to a review of the literature by Comis and colleagues (Comis et al., 2003), a combination of altruism and hope for better treatment motivates patients to enroll in clinical trials. These decisions are complex and multifaceted and involve a weighing of beliefs for and against the trial by using a “personal balance account” (Verheggen et al., 1998). Factors that influence participation include patient and physician attitudes about clinical trials, the informed-consent process, an unwillingness to receive a placebo treatment, and a perception of personal benefit (reviewed by Comis et al. [2003] and Cox and McGarry [2003]). The geographic distance from a site offering a clinical trial, concerns over toxicity, time constraints, eligibility requirements, and inconvenience to the patient may also contribute to decisions over clinical trial participation (Melisko et al., 2005). The majority of
TABLE 4-3 Positive Experiences with Clinical Trials
|
Percent Who |
Clinical Trials Participants Percentage |
|
Say they were treated with dignity and respect |
97 |
|
Rate the quality of care received “excellent” or “good” |
97 |
|
Describe their overall experience as positive |
93 |
|
Do not feel that they were treated like a “guinea pig” |
82 |
|
Believe they were not subjected to more tests and procedures than they thought necessary |
81 |
|
Would recommend participation to someone else with cancer |
76 |
|
SOURCE: HarrisInteractive, 2001. Reprinted, with permission, from HarrisInteractive, 2001. Copyright 2001 by Harris Interactive Inc. |
|
clinical trial participants indicate that they viewed their experience in the trial positively (HarrisInteractive, 2001) (Table 4-3).
In addition to a low general rate of participation in clinical trials, individuals who are members of racial and ethnic minority groups are underrepresented in clinical trials (Figure 4-1), which may be related to historical, educational, cultural, linguistic, economic, geographic, social, and health system barriers (Colon-Otero et al., 2007; IOM, 1999, 2009a; Underwood, 2000). This low rate of participation may prevent segments of the population from benefiting from advances in cancer research and creating uncertainties over the applicability of research findings to diverse populations (Colon-Otero et al., 2007). According to an IOM report:
The inclusion of ethnic minority and medically underserved individuals in clinical trials and the dissemination of information to their community and health care providers are critical links connecting scientific innovation with improvements in health and health care delivery. Enhancement of these links is clearly within the purview of NCI and NIH. Although many factors pose challenges to such improvements (e.g., mistrust of the scientific establishment among many members of ethnic minority communities), without a concerted effort to enhance this process, ethnic minority and medically underserved communities will continue to lag behind the American majority in benefiting from the tremendous recent scientific achievements and medical breakthroughs in cancer prevention, treatment, and control. (IOM, 1999)
Physician-patient communication is critical to improving clinical trial participation. To communicate effectively with their patients, physicians must meet ethical mandates, convey medical knowledge, and demonstrate credibility, without creating misunderstandings or overwhelming their
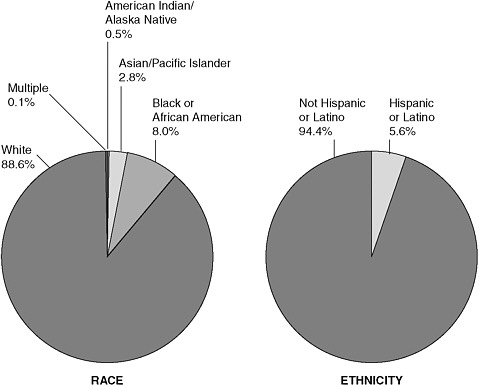
FIGURE 4-1 Enrollment by race and ethnicity for publicly funded NCI clinical trials.
SOURCE: Coalition of Cancer Cooperative Groups, 2006.
patients (Albrecht et al., 2008). A physician’s decision to not offer a patient the possibility of participation in a clinical trial is a significant reason for low rates of patient accrual (reviewed by Ellis, 2000). One study found that in two urban NCI-designated comprehensive cancer centers, patients were offered clinical trials in only 20 percent of the interactions, but when the patients perceived that they were offered a trial, 75 percent of patients assented to trial participation (Albrecht et al., 2008).
Researchers, physicians, patient advocates, and policy makers have emphasized the importance of patient participation in clinical trials. However, among the general public, few people are aware of clinical trials. It is thus important that patients have access to information about clinical trials and the importance of participation. Educational initiatives about clinical trials may facilitate understanding about clinical research and dispel misconceptions. For example, patients may incorrectly assume that cancer clinical trials often use placebos as a comparator, but the comparator is usu-
ally the current standard of care. With education initiatives that promote clinical trials as a treatment option, it is important that clinical trials actually be available and accessible. For instance, it may be an insurmountable burden, in terms of both time and cost, for a patient to travel to a cancer center for participation in a clinical trial. Trial sites in community settings, such as through the Community Clinical Oncology Program, could ensure higher rates of access. As indicated earlier in this chapter, a centralized, accessible, up-to-date registry could improve patient access to information about clinical trials and help patients locate the trials being conducted in their area. In addition, the committee recommends that CMS, federal and state health benefits plans, and private health insurers work with health care providers to educate patients more effectively about the availability, payment coverage, and value of clinical trials.
Insurance Coverage
A lack of insurance coverage for participation in clinical trials is also a barrier preventing robust provider and patient participation. Compared with the rate of insurance among the general U.S. population, patients enrolled in Cooperative Group clinical trials are significantly less likely to be uninsured (Table 4-4) (Sateren et al., 2002). Those who are insured may also face barriers because coverage of care in clinical trials is variable and may be uncertain. Patients who are interested and willing to enroll in a trial may decline because of an inability to pay for care that is not or that may not be covered. Others may still enroll, but they then might experience significant financial hardship as a result.
A large proportion of the care provided to cancer patients enrolled in clinical trials is considered routine and would be eligible for reimbursement outside of a trial (IOM, 2000). However, it may be problematic to deter-
TABLE 4-4 Participation in Cooperative Group Clinical Trials by Type of Insurance Coverage (Percent)
|
Insurance Type |
U.S. Population |
Clinical Trial Population |
|
Private |
70.2 |
71.6 |
|
All government |
24.3 |
32.5 |
|
Medicare |
13.2 |
20.8 |
|
Medicaid |
10.3 |
9.5 |
|
Military |
3.2 |
3.2 |
|
Total covered |
83.7 |
94.6 |
|
Not covered |
16.3 |
5.4 |
|
SOURCE: Sateren et al., 2002. Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Sateren, W. et al: Journal of Clinical Oncology Vol. 20(8), 2002:2109–2017. |
||
mine the costs of routine care associated with clinical trials because there may be uncertainty about what items and services will be covered. According to ACS CAN, “one of the fundamental challenges arises from the fact that routine care may be difficult to define precisely and may vary substantially by geographic region and type of provider” (ACS CAN, 2009).
Many health insurance policies generally exclude coverage for participation in clinical trials. Additionally, the Federal Employees Health Benefit Program does not require participating insurers to cover the costs of routine care incurred during participation in a clinical trial (ACS CAN, 2009). Insurers may deny coverage associated with clinical trial participation because they consider clinical trials to be experimental and want to limit coverage of therapies with little experimental evidence of effectiveness (GAO, 1999; IOM, 2009b). Insurers may also be reluctant to cover the costs of routine care related to clinical trial participation because they believe that clinical trial participants incur substantially higher costs than those receiving the standard-of-care therapy (Goldman et al., 2003). However, several studies have suggested that clinical trial participation is associated with only modest increases in costs (Bennett et al., 2000, 2001; Goldman et al., 2001, 2003).
Some insurers have altered policies to cover care related to participation in clinical trials (Bennett et al., 2001; Kolata and Eichenwald, 1999). In a recent IOM workshop, one insurer noted the value of clinical trial participation because it provides a standardized protocol and defined treatment plan for patients, whereas the treatment received through usual clinical practice may be highly variable (IOM, 2009b). In January 2010, the major insurers of Florida, representing about 90 percent of Florida’s group health insurance market, signed the Florida Clinical Trial Compact, agreeing to cover the costs of routine care for those participating in Phase II to IV cancer clinical trials that are approved by NIH, NCI, FDA, the Department of Defense, or accredited Florida medical schools and specialty hospitals (Colavecchio, 2010; Florida Clinical Trial Compact, 2010). Likewise, four other states, Georgia, Michigan, New Jersey, and Ohio, have special agreements to voluntarily provide coverage for clinical trials (see Table 4-5).
In addition, some insurers acknowledge that they pay for care related to participation in a clinical trial by unknowingly authorizing services that are part of a clinical research protocol (IOM, 2000; Mechanic and Dobson, 1996). Participants in an IOM workshop suggested that payment for care related to clinical trial participation without prior authorization by the insurance plan carries risks. If the insurer were to discover that the person was participating in a clinical trial, the potentially denied care costs could be significant (IOM, 2009b).
TABLE 4-5 States with Special Agreements to Cover Routine Care Costs Associated with Clinical Trial Participation
|
State (Year Became Effective) |
Who Is Required to Pay? |
What Services or Benefits Are Covered? |
Other Criteria |
|
Florida (2010) |
All major insurers |
Routine patient care costs associated with Phase II through IV clinical trials. |
Trials include those that involve a drug that is exempt under federal regulations from a new drug application or those that are approved by one of the following bodies: a Cooperative Group of the National Institutes of Health, the Food and Drug Administration (in the form of an investigational new drug application), the U.S. Department of Defense, the U.S. Department of Veterans Affairs, or the National Cancer Institute. |
|
Georgia (2002) |
All major insurers |
Routine patient care costs associated with Phase I through IV cancer clinical trials. |
Trials include those that involve a drug that is currently exempt under federal regulations from a new drug application or those that are approved by specified federal agencies or a local institutional review board. |
|
|
|
|
Provides for the coverage of cancer screens and examinations in accordance with the most recently published guidelines and recommendations established by any nationally recognized health care organization. |
|
Michigan (2002) |
Private insurance plans, health maintenance organizations, and Medicaid |
Routine patient care costs associated with Phase II and III cancer clinical trials. |
Coverage for Phase I trials is under consideration. |
|
New Jersey (1999) |
All insurers |
Routine patient care costs associated with all phases of cancer clinical trials. |
|
|
Ohio (1999) |
State employees on Ohio Med Plan |
Routine patient care costs associated with Phase II and III cancer treatment clinical trials. |
Preauthorization is required for clinical trial participation. |
|
SOURCES: Colavecchio, 2010; Florida Clinical Trial Compact, 2010; NCSL, 2009. |
|||
Employer-Sponsored Plans Subject to ERISA
Laws in 23 states have mandated coverage of routine care costs related to participation in a clinical trial (NCSL, 2009). However, state laws do not affect the majority of individuals covered by employer-sponsored health plans. Plans affecting about 131 million individuals are primarily regulated by federal law through the provisions of the Employee Retirement Income Security Act of 1974 (ERISA) (Chaikind, 2003). ERISA provides federal jurisdiction over the regulation of employee benefits plans (such as private-sector, employer-sponsored health plans) and preempts state laws mandating expanded access to health care through workplace coverage for some plans (Butler, 2000). ERISA plans are not required to cover the costs of routine care for patients on clinical trials.
The interpretation of ERISA has generally divided employer-sponsored health plans into two different types: self-insured plans, in which the employer rather than the insurer assumes the risk for paying for covered services, and insured employer plans, or purchased insurance. About 67 million people are covered by self-insured plans, which are preempted from state law and are covered only by ERISA. For the 64 million people who are covered by insured employer plans, federal law preempts state laws that “relate to” employee benefits plans but state laws apply for issues involving the business of insurance. Various interpretations of insured employee plans have blurred what issues are applicable to state laws. Traditionally, the courts have favored preempting state law for most employee benefits situations, but this may be changing (Chaikind, 2003). Because state laws mandating routine coverage for the care associated with participation in a clinical trial will not result in universal coverage, federal law is needed. In March 2009, Senator Edward Kennedy and 21 cosponsors introduced the 21st Century Cancer Access to Life-Saving Early Detection, Research, and Treatment (ALERT) Act.9 Among other actions, the bill would amend ERISA to expand access to cancer clinical trials by requiring health plans governed by the requirements of ERISA to continue providing coverage for routine care, regardless of enrollment in a clinical trial. Likewise, an amendment to the U.S. Senate version of the health care reform bill would require insurers to cover routine costs of care for approved clinical trials for patients with cancer or other life-threatening diseases.10 The Access to Cancer Clinical Trials Act of 2009 (H.R. 716/S. 488) would amend ERISA to prohibit a group health plan from (1) denying eligible participant or beneficiary participation in cancer clinical trials that are federally funded or
conducted under an investigational new drug application reviewed by FDA; (2) denying, limiting, or imposing additional conditions on the coverage of routine patient costs related to participation in a clinical trial; and (3) discriminating against an individual on the basis of participation in a clinical trial.11 This legislation has also been introduced in previous legislative sessions, including those in 2008, 2007, 2006, 2003, 2001, and 1999.12 Reflecting the language of these bills, the committee recommends that the U.S. Congress amend ERISA to prohibit health plans from denying (or from limiting or imposing additional conditions on) coverage for routine care associated with clinical trial participation.13
Medicare and Medicaid Coverage
Before 2000, Medicare and Medicaid beneficiaries were not reimbursed for expenses related to their participation in a clinical trial because the Health Care Financing Administration (the prior name of CMS) believed that the original Medicare legislation did not give the Health Care Financing Administration the authority to provide reimbursement for costs associated with clinical trials (Arnold and Vastag, 2000; IOM, 2009b). In 2000,
an IOM committee recommended that Medicare provide reimbursement for the costs of routine care for patients in clinical trials (IOM, 2000), and President Bill Clinton signed an executive order directing the Medicare program to provide reimbursement for the costs of routine care associated with participation in a clinical trial (Arnold and Vastag, 2000). Medicare began reimbursing routine care costs for beneficiaries enrolled in qualified clinical trials through a National Coverage Decision (NCD). In 2007, CMS reconsidered the 2000 NCD, clarified the policy, and also introduced the Coverage with Evidence Development program, which enables CMS to cover a medical intervention with the condition that the agency may concurrently collect data on the intervention while reimbursing it (CMS, 2009).
In an effort to extend reimbursement for cancer therapy, in 2005 Medicare made an NCD that covered the off-label use of four anticancer drugs, but coverage was restricted to nine trials sponsored by NCI (NCI, 2009a). To reduce the uncertainty over what Medicare would cover, NCI and CMS developed billing instructions and explicit information about what costs Medicare would cover and what costs the sponsor of the clinical trial would cover (Table 4-6) (IOM, 2009b). An initial analysis of individuals enrolled in these nine trials found that Medicare-eligible subjects comprised between one-fifth and one-third of the participants currently enrolled, whereas traditionally, only about 13 percent of people enrolled in clinical trials are aged 65 years and older (IOM, 2009b).
However, beyond those nine trials, inconsistencies in Medicare coverage continue because each CMS contractor is allowed to determine whether a particular item of service is considered standard of care, or if procedures in a clinical trial fall within the “reasonable and necessary” standard (IOM, 2009b). Likewise, beneficiaries who participate in Medicare Advantage
TABLE 4-6 Comparison of Medicare Policies
|
Question |
2000 Clinical Trials Policy |
2005 Anticancer Drug National Coverage Decision |
|
What kind of costs are covered? |
Routine costs associated with the patient’s medical care in the clinical trial would be covered. |
Both routine and nonroutine costs associated with the patient’s care in any of nine designated trials are covered. An example would be an additional laboratory or imaging test required by the study protocol for data analysis. |
|
Does the policy pay for off-label use of anticancer drugs? |
Coverage for off-label use varies depending on whether the trial in question meets the policy’s requirements. |
Yes. In the nine designated trials, off-label use is covered of anticancer drugs. |
|
SOURCE: NCI, 2009a. |
||
plans14 face 20 percent coinsurance for drugs and may be unable to afford the expensive new drugs often used in clinical trials.15 Because of the high rate of coinsurance, Medicare Advantage beneficiaries are underrepresented in clinical trials (Fitterman, 2008; Lin et al., 2008). In addition, Medicare Advantage copayments make it difficult to mask participants and providers to their treatment if the copayments differ between the investigational items and services.
Medicare Part D plans may also be limiting access to cancer therapeutics. According to Avalere Health and the American Cancer Society, cancer patients enrolled in Medicare Part D plans spend more on copayments and face increased restrictions on access to orally administered cancer drugs. From 2006 to 2009, Medicare stand-alone prescription drug plans have been shifting name-brand orally administered cancer drugs to higher formulary tiers, requiring beneficiaries to pay from 26 to 35 percent of the cost (Murphy et al., 2008). Plans are also increasing the number of drugs requiring prior authorization for coverage to control access to orally administered cancer therapeutics. Geography and the prescription drug plan that a person chooses can influence how much a beneficiary will pay out of pocket for orally administered cancer therapeutics. According to modeling simulations conducted by Avalere Health and ACS CAN, hypothetical drug regimens for a woman with breast cancer could vary from $1,985 for the American Association of Retired Persons MedicareRX Saver in Florida to $2,551 for the Humana Part D Plans Standard in California (Co-payments to rise as access to drugs tightens for patients on Medicare Part D, 2008; Murphy et al., 2008).
Third-Party Payment Policies and Clinical Trial Participation
In 2009, the Agency for Healthcare Research and Quality (AHRQ) issued a technology assessment report that examined the impact that third-party payment policies have on clinical trials and evidence-based medicine. Overall, the AHRQ report found that the lack of a national consensus regarding the financial responsibility for clinical trial-related health care
costs results in uneven reimbursement policies, which may hamper patient recruitment and negatively affect evidence-based medicine (AHRQ, 2009). Specifically, the report found, “[v]arious plausible scenarios are supported by anecdotal evidence, though data do not exist to describe and quantify the actual impact of payment policy on clinical trial participation” (AHRQ, 2009). These scenarios include the following:
-
when third-party payors provide reimbursement for a diagnostic or therapeutic treatment outside a clinical trial setting, patients may be less likely to participate in a trial assessing that intervention;
-
if third-party payors initiate coverage for an intervention under study while clinical trials are still accruing patients, enrollment may slow down or stop because patients may lose the financial incentive to participate and may opt to avoid the extra time or demands of clinical trial participation (such as filling out questionnaires);
-
if an intervention becomes covered outside of a trial but is not reimbursed for patients enrolled in the trial, patients may be less willing to enroll or continue participation;
-
when two arms of a trial with two different interventions have distinct payment structures, patients may take financial factors into account in their decisions to participate or not; and
-
third-party payment structures may contribute or may be perceived to contribute additional financial and time burdens to people who participate in trials (AHRQ, 2009).
Uncertainty over insurance coverage has the potential to decrease provider and patient participation in clinical trials. However, third-party payment policies aligned with participation in high-quality clinical trials have the potential to improve treatment and sometimes reduce the costs of care associated with less-effective treatments: “A paradox exists in reimbursement policies in which insurers may refuse to cover a promising new therapy because it is available only through clinical trials while covering what is considered standard treatment even though it may often be ineffective and sometimes more expensive” (Bennett et al., 2001).
If cancer care is to be evidence based and relevant to the diverse cancer patient population, it is important for coverage policies to encourage rather than deter patient enrollment in clinical trials. In recognition of these issues, the committee recommends that health care payment policies value the care provided to patients in clinical trials and adequately compensate that care. For example, CMS (via a national coverage decision), federal and state health benefits plans, and private health insurers should establish consistent payment policies to cover all patient care costs (except for study-related costs, such as study drugs, devices, and tests, which should be paid for by
the manufacturer) in clinical trials approved through the NCI prioritization mechanism, without having to pay for experimental therapies administered to patients outside of a clinical trial. Any such limitation in coverage should not affect off-label use that is backed by evidence from clinical trials published in the scientific literature, as evidence-based off-label use constitutes standard of care for many cancer therapies and is therefore not experimental.
As a quid pro quo for improved coverage of the care received as a part of participation in clinical trials, insurers should be able to eliminate coverage of experimental therapies delivered outside of the clinical trial setting. Currently, many patients who are not enrolled in trials receive experimental therapy and expect coverage for it. The committee’s approach is analogous to the “coverage with evidence development” mechanism that CMS has occasionally used, in which coverage is provided only within the context of a clinical trial. However, any such limitation in coverage should not affect off-label indications backed by evidence from clinical trials published in the scientific literature, as off-label use constitutes the standard of care for many cancer therapies and is therefore not experimental.
SUMMARY
The recommendations in this chapter support two of the committee’s goals: (1) incentivize participation of patients and physicians in clinical trials, and (2) improve the prioritization, selection, support, and completion of cancer clinical trials. On the basis of the review of the information described in this chapter, the committee agreed that incentives must be realigned to increase participation by clinical investigators and community physicians in publicly sponsored clinical trials and to increase access to clinical trials by patients. Likewise, the committee recognized that additional steps need to be taken to facilitate the implementation, conduct, and completion of the very best clinical trials.
A robust clinical trials infrastructure is largely dependent on a critical mass of patients and physicians willing to participate in clinical trials. However, current indications suggest that participation in clinical trials is the exception rather than the rule, both for patients and physicians. For clinical investigators, concerns about reimbursement, extensive regulatory burdens, and academic procedures regarding tenure, promotion, and career development can all deter participation in trials. Patient access to clinical trials is also an import issue to consider. Even if patients are eligible for trials and are informed about the option by their physicians, they may decline due to financial concerns, as coverage of patient care costs in clinical trials by health insurers is not consistent.
In terms of physicians, multiple stakeholders need to take steps to support the recruitment and retention of clinical investigators in both com-
munity practices and academia. An important first step is ensuring that physicians receive reimbursement commensurate with the level of time and resources involved in conducting clinical trial research. The many duties required of physicians and other key research staff, such as research nurses and clinical research associates, to participate in clinical trials are costly in terms of both time and resources. Even in cases where routine patient care in a clinical trial is covered by health insurers, the current payment policies do not reflect the additional time needed to enroll and follow patients in a trial. Before a trial can be opened at a particular site, much work must be done to ensure compliance with federal regulations governing human subjects research. Once a trial is opened, a significant amount of time is spent discussing potential trial options with patients. If a patient enrolls, the data collection and documentation requirements are substantially more onerous than for patients receiving standard therapy outside of a trial.
Therefore, NCI should increase the per case reimbursement rate. A substantial increase in the NCI per case reimbursement would constitute a major step toward aligning the incentives of physicians with those of their patients who wish to participate in clinical trials. The per case reimbursement has been set at $2,000 since 1999, even though the median costs are estimated at $3,500 to $6,000 per patient. When the discrepancy between the per case reimbursement and the actual cost of participation is excessive, as it is now, it becomes a major disincentive to participation. NCI should also provide funding to site and trial principal investigators to cover the time they need to develop and oversee approved clinical trials. The provision of funds for principal investigators to cover the time needed to develop and oversee approved trials could improve the speed and quality of trials and encourage greater participation. In addition, the American Medical Association should establish new CPT codes, reimbursed by CMS, private insurers, and other third-party payors, to pay an enhanced reimbursement for offering, enrolling, managing, and following a patient in a clinical trial. New CPT codes, with a higher reimbursement rate that acknowledges the additional time and resources needed to counsel and care for a patient in a clinical trial, would address an important deterrent to physician participation in clinical trials. With a proper definition of eligible trials, use of such a code could be easily audited.
Ensuring that physicians are recognized, rewarded, and appropriately trained in clinical trial research is also essential to encouraging participation. All stakeholders, including academic medical centers, community practices, professional societies, and NCI, should work to ensure that clinical investigators have adequate training and mentoring, paid protected research time, the necessary resources, and recognition. Ultimately, the inability to recruit, train, and retain a sufficient number of talented clinical investigators will compromise the ability to conduct clinical trials in the
United States, to the detriment of the U.S. biomedical research enterprise and to patients, those who participate in clinical trials as well as those who do not. Clinical trials help to raise the standard of care in the community by setting examples, and they have educational and training value for the oncologists involved, as physicians gain early knowledge of new drugs and gain experience with delivering complex therapies. One way to recognize investigators is through a certification program. NCI should work with a nonprofit foundation to develop a certification program and registry, as recommended by the CTWG. A certification program for all research staff (including physicians, nurses, clinical research associates, pharmacists, etc.) would recognize the valuable contributions these professionals make to improving patient care.
In addition, academic medical centers should develop policies and evaluation metrics that recognize and reward clinical and team research in promotion and tenure decisions. The large-scale, multi-institutional trials that are the hallmark of the Cooperative Group Program require a team approach to research. However, career advancement in the field has traditionally focused on individual accomplishment. Collaborative work is not adequately recognized, rewarded, or supported in the current system. Furthermore, clinical investigation is often accorded less value than either basic research or patient care. This must change if we wish to have talented individuals embark on a career that entails active participation in clinical investigation, in cancer as well as other diseases. NCI should recognize and reward Cooperative Group efforts in Cancer Center Support Grant (CCSG; P30) site visits, and allow the CCSG research base to include the federal per case funding required by cancer centers that participate in support of Cooperative Group trials. CCSG review criteria do not adequately incentivize participation in multi-institutional Cooperative Group trials, and instead favor individual investigator-initiated trials emerging from basic discoveries within a cancer center’s own institution. Recognizing the per case reimbursements for Cooperative Group Trials in the CCSG assessment of a cancer center’s funding base would acknowledge the importance of patient accrual in these trials and encourage broader participation at those centers. Clinical research is a complex endeavor that requires training, mentoring, and paid time set aside for research to master and apply the skills needed to undertake innovative trials.
If cancer care is to be evidence-based and relevant to the diverse patient population, it is important for coverage policies to encourage rather than deter patient enrollment in trials. Therefore, the committee recommends that health care payment policies value the care provided to patients in clinical trials and adequately compensate that care. Inadequate health care coverage is a major deterrent to participation in trials, for patients as well as physicians. Health care insurers traditionally have not paid for “experi-
mental therapy.” However, much of the care provided to cancer patients is similar regardless of whether the patient is receiving a standard or experimental drug. Some insurers and states acknowledge this and reimburse the routine clinical care of patients enrolled in trials, while others do not. Policies at CMS regarding coverage of care in clinical trials have been in flux recently, and, absent National Coverage Decisions, may not be nationally uniform because fiscal intermediaries and carriers have some discretion on coverage, which can cause regional variation and inconsistency. Furthermore, the provisions of the ERISA, which places the regulation of employee benefit plans (including health plans) primarily under federal jurisdiction for about 131 million people, preempts state laws governing such things as access to care and mandated coverage.
Thus, coverage of care in clinical trials is variable and may be uncertain. Patients who are interested and willing to enroll in a trial may decline due to an inability to pay for care that is not, or may not be, covered. Others might still enroll, but then may experience significant financial hardship as a result. If such patients drop out of the trial, the scientific integrity of the trial can be compromised due to inferential problems that result from missing data. In order to facilitate patient and physician participation in clinical trials, more consistent policies regarding patient care costs are needed. The committee recommends that CMS (via a national coverage decision), federal and state health benefits plans, and private health insurers establish consistent payment policies to cover all patient care costs (except for study-related costs, such as study drugs, devices, and tests, which should be paid for by the manufacturer) in clinical trials approved through the NCI prioritization mechanism, without having to pay for experimental therapies administered to patients outside of a clinical trial. Currently, many patients who are not enrolled in trials receive experimental therapy and expect coverage for it. Any such limitation in coverage should not affect off-label use that is backed by evidence from clinical trials published in the scientific literature, as evidence-based off-label use constitutes standard of care for many cancer therapies and is therefore not experimental.
In addition, the U.S. Congress should amend ERISA to prohibit health plans from denying (or from limiting or imposing additional conditions on) coverage for routine care associated with clinical trial participation. The committee’s recommended approach is analogous to the “coverage with evidence development” mechanism that has occasionally been used by CMS, in which coverage is only provided within the context of a trial.
However, taking steps to align the incentives of patients and providers to participate in clinical trials may not be effective unless more is done to educate patients about the availability and value of clinical trials. The committee recommends that CMS, federal and state health benefit plans, and private health insurers work with health care providers to educate patients
more effectively about the availability, payment coverage, and value of clinical trials. Educational efforts should focus on making the general population more aware of clinical trials. One reason is that it can be difficult for patients to sort through a large volume of new information and make complex decisions having just received a diagnosis of a life-threatening illness. Patients often lack comprehensive and reliable information about clinical trials and may not be able to identify trials they might be eligible for. Patients value reliable information from trusted sources, including family members, so appropriate education efforts could provide useful information that would allow patients to make informed choices about trial participation. In addition, as noted in more detail below, user-friendly electronic tools would increase awareness of trials and make it easier for physicians and patients to enroll in the most appropriate studies.
To ensure the rapid conduct and completion of clinical trials, the enrollment of patients onto clinical trials must be improved. At the same time, it is essential that clinical trials conducted by the Cooperative Groups maintain high quality standards. Thus, NCI, Cooperative Groups, and physicians should take steps to increase the speed, volume, and diversity of patient accrual and to ensure high-quality performance at all sites participating in Cooperative Group trials. In addition, they should encourage greater enrollment in high-priority trials, regardless of where the trial originated. Currently, the majority of patients who participate in clinical trials are enrolled by a small percentage of participating sites. Many sites enroll only a few patients in Cooperative Group clinical trials—enough to maintain their status as investigators. These circumstances can contribute to the underrepresentation in clinical trials of minority and underserved populations. Given the importance of trials in generating the evidence needed for making the best treatment decisions, more physicians should be encouraged to include trial participation in their clinical practice. As mentioned previously, providing adequate case reimbursement would help to align physician and patient incentives and facilitate higher accrual at participating sites. However, another obstacle to increasing patient enrollment is that physicians may lack timely and easy-to-access information about clinical trials that would be appropriate for their patients. Some public databases with information about clinical trials exist, but in current form, may not adequately serve the information needs of physicians and patients as they are not in the normal workflow of a busy clinical practice. Therefore, the committee recommends that NCI, Cooperative Groups, and physicians develop electronic tools that cue physicians practicing oncology via EMR systems about trials for which a particular patient is eligible. User-friendly electronic tools, available with the right features for physician workflow, would increase awareness of trials and make it easier for physicians and patients to enroll in the most appropriate studies. Complementing increased
awareness, the committee encourages physicians to incorporate clinical trial involvement into their practices. Physicians should strive to make participation in clinical trials a key component of clinical practice and to achieve the ASCO exemplary attributes for academic and community clinical trial sites, including high accrual rates of 10 percent or more.
Eligibility criteria present another challenge to increasing enrollment. Historically, stringent eligibility criteria have excluded many patients, including, for example, those with prior cancers or certain prior treatments. However, it has been argued that adoption of less-restrictive eligibility criteria for most studies would permit more rapid accrual and also allow broader generalizations, better mimic medical practice, and reduce complexity and costs, without compromising patient safety or requiring major increases in sample size. To facilitate accrual speed, volume, and diversity, NCI, Cooperative Groups, and physicians should encourage the development of patient eligibility criteria that allow the broadest participation possible. Greater involvement by patient advocates could help to facilitate this change. Thus, the committee encourages greater participation of patient advocates in trial concept development and accrual planning, and partnerships with patient advocacy organizations to support accrual efforts. Advocates also provide valuable input to study design and procedures, safety and confidentiality issues, feasibility, informed consent processes, and other factors important to potential research participants to help facilitate the development, implementation, and recruitment processes.
Ensuring consistent quality at participating trial sites is also important. Site credentialing requirements vary among the Cooperative Groups, making it difficult for sites that wish to engage with multiple groups. Therefore, the committee recommends that NCI, Cooperative Groups, and physicians establish a centralized credentialing system for participating sites and eliminate investigators and sites with low rates of accrual or inadequate data management skills or quality. A centralized credentialing system, perhaps outsourced to an independent entity, would increase consistency and quality across sites, and eliminate the burden of re-credentialing. Such a system would also facilitate greater enrollment in high-priority trials, regardless of where the trial originated, because sites would be credentialed to participate in any Cooperative Group Trial. Moreover, elimination of low accruing sites would reduce costs and improve the efficiency of the trials system.
REFERENCES
ACS CAN (American Cancer Society Cancer Action Network). 2009. Barriers to Provider Participation in Clinical Cancer Trials: Potential Policy Solutions, Draft. Washington, DC: American Cancer Society Cancer Action Network.
Agres, T. 2005. Clinical trials trickling away. Drug Discovery and Development, July 5.
AHRQ (Agency for Healthcare Research and Quality). 2009. Horizon Scan: To What Extent Do Changes in Third-Party Payment Affect Clinical Trials and the Evidence Base? Rockville, MD: Agency for Healthcare Research and Quality.
Albrecht, T. L., S. S. Eggly, M. E. J. Gleason, F. W. K. Harper, T. S. Foster, A. M. Peterson, H. Orom, L. A. Penner, and J. C. Ruckdeschel. 2008. The influence of clinical communication on patients’ decision making about clinical trials. Journal of Clinical Oncology 26(16):2666–2673.
Altshuler, J. S., and D. Altshuler. 2004. Organizational challenges in clinical genomic research. Nature 429(6990):478–481.
Andrews, N., J. E. Burris, T. R. Cech, B. S. Coller, W. F. Crowley, E. K. Gallin, K. L. Kelner, D. G. Kirch, A. I. Leshner, C. D. Morris, F. T. Nguyen, J. Oates, and N. S. Sung. 2009. Translational careers. Science 324(5929):855.
Arnold, K., and B. Vastag. 2000. Medicare to cover routine care costs in clinical trials. Journal of the National Cancer Institute 92(13):1032.
ASCO (American Society of Clinical Oncology). 2008. Community Oncology Research Grant. http://www.ascocancerfoundation.org/TACF/Grants/Grant+Opportunities/Community+Oncology+Research+Grant (accessed September 24, 2009).
ASCO certification program emphasizes quality of care. 2009. The Cancer Letter 35(22):6.
Bennett, C. L., T. J. Stinson, V. Vogel, L. Robertson, D. Leedy, P. O’Brien, J. Hobbs, T. Sutton, J. C. Ruckdeschel, T. N. Chirikos, R. S. Weiner, M. M. Ramsey, and M. S. Wicha. 2000. Evaluating the financial impact of clinical trials in oncology: Results from a pilot study from the Association of American Cancer Institutes/Northwestern University Clinical Trials Costs and Charges Project. Journal of Clinical Oncology 18(15):2805–2810.
Bennett, C. L., J. R. Adams, K. S. Knox, A. M. Kelahan, S. M. Silver, and J. S. Bailes. 2001. Clinical trials: Are they a good buy? Journal of Clinical Oncology 19(23):4330–4339.
Blayney, D. W. 2009. Results of Cooperative Group Site Poll. Presented at the NCI National Cancer Advisory Board Meeting, September, Bethesda, MD.
Braunholtz, D. A., S. J. L. Edwards, and R. J. Lilford. 2001. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.” Journal of Clinical Epidemiology 54(3):217–224.
Butler, P. A. 2000. ERISA and state health care access initiatives: Opportunities and obstacles. New York: The Commonwealth Fund.
CALGB (Cancer and Leukemia Group B). 2009. Cancer and Leukemia Group B: Tomorrow’s cancer treatments today. CALGB Membership Information. http://www.calgb.org/Public/membership/membership.php (accessed September 29, 2009).
C-Change. 2005. A Guidance Document for Implementing Effective Cancer Clinical Trials: Version 1.2. Washington, DC: C-Change.
C-Change and Coalition of Cancer Cooperative Groups. 2006. Enhancing Cancer Treatment Through Improved Understanding of the Critical Components, Economics, and Barriers of Cancer Clinical Trials. Washington, DC: C-Change and Philadelphia, PA: Coalition of Cancer Cooperative Groups.
Chaikind, H. R. 2003. ERISA Regulation of Health Plans: Fact Sheet. Washington, DC: Congressional Research Service.
CMS (Centers for Medicare & Medicaid Services). 2009. NCD for Routine Costs in Clinical Trials (310.1). http://www.cms.hhs.gov/mcd/viewncd.asp?ncd_id=310.1&ncd_version=2&basket=ncd%3A310.1%3A2%3ARoutine+Costs+in+Clinical+Trials (accessed October 25, 2009).
Coalition of Cancer Cooperative Groups. 2006. Baseline Study of Patient Accrual onto Publicly Sponsored Trials: An Analysis Conducted for the Global Access Project of the National Patient Advocate Foundation. Philadelphia, PA: Coalition of Cancer Cooperative Groups (http://www.CancerTrialsHelp.org).
Colavecchio, S. 2010. Insurers Agree to Cover Patients in Clinical Trials. Insurance Companies Will Have to Continue Routine Coverage for Cancer Patients Who Participate in Clinical Trials, Under a New State Agreement. The Miami Herald, January 14. http://www.miamiherald.com/516/story/1424009.html (accessed February 1, 2010).
Collyar, D. 2008. An essential partnership: Patient advocates and cooperative groups. Seminars in Oncology 35(5):553–555.
Colon-Otero, G., R. C. Smallridge, L. J. Solberg, T. D. Keith, T. A. Woodward, F. B. Willis, and A. N. Dunn. 2007. Disparities in participation in cancer clinical trials in the United States: A symptom of a healthcare system in crisis. Cancer 112(3):447–454.
Comis, R. 2003. A brief history of the research and treatment of lung cancer from 1970–2003. International Journal of Clinical Oncology 8(4):230–233.
Comis, R. 2007. The Coalition of Cancer Cooperative Groups. Journal of Oncology Practice 3(5):280.
Comis, R. L., J. D. Miller, C. R. Aldige, L. Kregs, and E. Stoval. 2003. Public attitudes toward participation in cancer clinical trials. Journal of Clinical Oncology 21(5):830–835.
Comis, R. L., J. D. Miller, D. D. Colaizzi, and L. G. Kimmel. 2009. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. Journal of Oncology Practice 5(2):50–56.
Co-payments to rise as access to drugs tightens for patients on Medicare Part D. 2008. The Cancer Letter 23(11):1, 4.
Cox, K., and J. McGarry. 2003. Why patients don’t take part in cancer clinical trials: An overview of the literature. European Journal of Cancer Care 12:114–122.
CTEP (Cancer Therapy Evaluation Program). 2006. National Cancer Institute Clinical Trials Cooperative Group Program Guidelines. Bethesda, MD: National Cancer Institute.
Damon Runyon Cancer Research Foundation. 2009. Clinical Investigator Award Overview. http://www.damonrunyon.org/for_scientists/more/clinical_investigator_award_overview (accessed December 17, 2009).
Davis, S., S. F. Schulman, L. D. Hill, R. D. Pinkham, L. P. Johnson, T. W. Jones, H. B. J. Kellogg, H. M. Radke, W. W. Sikkema, P. C. Jolly, and S. P. Hammar. 1985. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer 56(7):1710–1718.
De Angelis, C., J. M. Drazen, F. A. Frizelle, C. Huag, J. Hoey, R. Horton, S. Kotzin, C. Laine, A. Marusic, A. J. P. M. Overbeke, T. V. Schroeder, H. C. Sox, and M. B. Van der Weyden. 2004. Trial registration: A statement from the International Committee of Medical Journal Editors. New England Journal of Medicine 351(12):1250–1251.
Demmy, T. L., J. M. Yasko, D. E. Collyar, and M. L. Katz. 2004. Managing accrual in cooperative group clinical trials. Journal of Clinical Oncology 22(15):2997–3002.
Doroshow, J. H., and G. Hortobagyi. 2010. Operation Efficiency Working Group: Compressing the Timeline for Cancer Clinical Trial Activation. Final report. Presented at the Clinical Trials and Translational Research Advisory Committee Meeting, March 2010, Bethesda, MD.
EDICT. 2008. The EDICT Project: Policy Recommendations to Eliminate Disparities in Clinical Trials. Houston, TX: EDICT Project.
Ellis, P. M. 2000. Attitudes towards and participation in randomised clinical trials in oncology: A review of the literature. Annals of Oncology 11:939–945.
EmergingMed. 2009. EmergingMed: About Us. http://www.emergingmed.com/pub_aboutus.asp (accessed October 27, 2009).
ENACCT-CCPH (Education Network to Advance Cancer Clinical Trials-Community-Campus Partnerships for Health). 2008. Communities as Partners in Cancer Clinical Trials: Changing Research, Practice, and Policy. Bethesda, MD: Education Network to Advance Cancer Clinical Trials; Seattle, WA: Community Campus Partnerships for Health.
Fitterman, L. K. 2008. Clinical Trial Policy. Presented at the NCPF Workshop on Multi-Center Phase III Clinical Trials and NCI Cooperative Groups, Washington, DC.
Florida Clinical Trial Compact. 2010. Agreement to Reduce Cancer Morbidity and Mortality in Florida. http://www.efrwc.org/_Temp/Clinical%20Trial%20Agreement%20Final.pdf (accessed February 1, 2010).
GAO (Government Accounting Office). 1999. NIH Clinical Trials: Various Factors Affect Patient Participation. Washington, DC: Government Accounting Office.
George, S. L. 1996. Reducing patient eligibility criteria in cancer clinical trials. Journal of Clinical Oncology 14(4):1364–1370.
Getz, K. A. 2007. Global clinical trials activity in the details. Applied Clinical Trials, September 1, pp. 42–44. http://appliedclinicaltrialsonline.findpharma.com/applied-clinicaltrials/Regulatory/Global-Clinical-Trials-Activity-in-the-Details/ArticleStandard/Article/detail/453243 (accessed April 8, 2009).
Glickman, S. W., J. G. McHutchison, E. D. Peterson, C. B. Cairns, R. A. Harrington, R. M. Califf, and K. A. Schulman. 2009. Ethical and scientific implications of the globalization of clinical research. New England Journal of Medicine 360(8):816–823.
Goldman, D. P., M. L. Schoenbaum, A. L. Potosky, J. C. Weeks, S. H. Berry, J. J. Escarce, B. A. Weidmer, M. L. Kilgore, N. Wagle, J. L. Adams, R. A. Figlin, J. H. Lewis, J. Cohen, R. Kaplan, and M. S. McCabe. 2001. Measuring the incremental cost of clinical cancer research. Journal of Clinical Oncology 19(1):105–110.
Goldman, D. P., S. H. Berry, M. S. McCabe, M. L. Kilgore, A. L. Potosky, M. L. Schoenbaum, M. Schonlau, J. C. Weeks, R. Kaplan, and J. J. Escarce. 2003. Incremental treatment costs in National Cancer Institute-sponsored clinical trials. Journal of the American Medical Association 289(22):2970–2977.
HarrisInteractive. 2001. Misconceptions and lack of awareness greatly reduce recruitment for cancer clinical trials. Health Care News 1(3):1–3.
HHS (U.S. Department of Health and Human Services). 2009. Health Information Technology for the Future of Health and Care: Meaningful Use. http://healthit.hhs.gov/portal/server.pt?open=512&objID=1325&parentname=CommunityPage&parentid=1&mode=2 (accessed October 27, 2009).
HHS. 2010. Types of Grant Programs. http://grants.nih.gov/grants/funding/funding_program.htm (accessed February 1, 2010).
IOM (Institute of Medicine). 1999. The Unequal Burden of Cancer: An Assessment of NIH Research Programs for Ethnic Minorities and the Medically Underserved. Washington, DC: National Academy Press.
IOM. 2000. Extending Medicare Reimbursement in Clinical Trials. Washington, DC: National Academy Press.
IOM. 2006. Developing a National Registry of Pharmacologic and Biologic Clinical Trials: Workshop Report. Washington, DC: The National Academies Press.
IOM. 2009a. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington, DC: The National Academies Press.
IOM. 2009b. Multi-Center Phase III Clinical Trials and NCI Cooperative Groups: Workshop Summary. Washington, DC: The National Academies Press.
Jha, A. K., T. G. Ferris, K. Donelan, C. DesRoches, A. Shields, S. Rosenbaum, and D. Blumenthal. 2006. How common are electronic health records in the United States? A summary of the evidence. Health Affairs 25(6):w496–w507.
KFF (Kaiser Family Foundation). 2009. Medicare Fact Sheet: Medicare Advantage. Menlo Park, CA: Kaiser Family Foundation.
Kolata, G., and K. Eichenwald. 1999. Group of insurers will pay for experimental cancer therapy. The New York Times, December 16, p. C1.
Ley, T. J., and L. E. Rosenberg. 2005. The physician-scientist career pipeline in 2005: Build it, and they will come. Journal of the American Medical Association 294(11):1343–1351.
Lin, C. J., D. E. Heron, and K. Connelly. 2008. Does Medicare HMO reimbursement policy hinder clinical trial participation. International Journal of Radiation Oncology*Biology*Physics 72(1 Suppl. 1):S138.
Malakoff, D. 2008. Spiraling costs threaten gridlock. Science 322(5899):210–213.
Mansour, E. G. 1994. Barriers to clinical trials. Part III. Knowledge and attitudes of health care providers. Cancer 74(Suppl. 9):2672–2675.
Masys, D. 2009. Electronic Data Capture and Analysis by NCI Cooperative Groups. Presented at the NCPF workshop on Cancer Clinical Trials and the NCI Cooperative Group Program, Washington, DC.
Mathieu, S., I. Boutron, D. Moher, D. G. Altman, and P. Ravaud. 2009. Comparison of registered and published primary outcomes in randomized control trials. Journal of the American Medical Association 302(9):977–984.
Mauer, A. M., E. S. Rich, and R. L. Schilsky. 2007. The role of cooperative groups in cancer clinical trials. Cancer Treatment and Research 132:111–129.
Mechanic, R. E., and A. Dobson. 1996. The impact of managed care on clinical research: A preliminary investigation. Health Affairs 15(3):72–89.
Melisko, M. E., F. Hassin, L. Metzroth, D. H. Moore, B. Brown, K. Patel, H. S. Rugo, and D. Tripathy. 2005. Patient and physician attitudes toward breast cancer clinical trials: Developing interventions based on understanding barriers. Clinical Breast Cancer 6(1):45–54.
Mooney, M. 2008. Cooperative Group Clinical Trials Complexity Funding: Model Development and Trial Selection Process. Presented at the Clinical Trials and Translational Research Advisory Committee Meeting, December 8, 2008, Bethesda, MD.
Murphy, L., S. Sadownik, C. Ford, V. Barton, C. Schmidt, and S. Barber. 2008. Cost Sharing for Cancer Patients in Medicare, 2009. Washington, DC: Avalere Health, American Cancer Society Cancer Action Network.
NCCN (National Comprehensive Cancer Network). 2009. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed December 4, 2009).
NCI (National Cancer Institute). 1997. Report of the National Cancer Institute Clinical Trials Program Review Group. http://deainfo.nci.nih.gov/ADVISORY/bsa/bsa_program/bsactprgmin.htm#8a (accessed November 19, 2008).
NCI. 2001. Cancer Clinical Trials: The Basic Workbook. Bethesda, MD: National Cancer Institute.
NCI. 2005a. President’s Cancer Panel 2004-2005 Annual Report: Translating Research into Cancer Care: Delivering on the Promise. Bethesda, MD: National Cancer Institute.
NCI. 2005b. Report of the Clinical Trials Working Group of the National Cancer Advisory Board: Restructuring the National Cancer Clinical Trials Enterprise. Bethesda, MD: National Cancer Institute.
NCI. 2009a. Clinical Trials Covered Under the Medicare Anti-Cancer Drugs National Coverage Decision. http://www.cancer.gov/clinicaltrials/developments/NCD179N (accessed October 25, 2009).
NCI. 2009b. Help Using the NCI Clinical Trials Search Form. http://www.nci.nih.gov/clinicaltrials/search-form-help/page1 (accessed October 27, 2009).
NCI-TACF clinical investigator award. 2009. The Cancer Letter 35(26):8.
NCSL (National Conference of State Legislatures). 2009. Clinical Trials: What Are States Doing? August 2009 Update. http://www.ncsl.org/default.aspx?tabid=14331 (accessed October 22, 2009).
NIH (National Institutes of Health). 2008. About ClinicalTrials.gov. http://clinicaltrials.gov/ct2/info/about (accessed October 27, 2009).
Normile, D. 2008. The promise and pitfalls of clinical trials overseas. Science 322(5899): 214–216.
Peppercorn, J., J. C. Weeks, E. F. Cook, and S. Joffe. 2004. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. The Lancet 363(9405):263–270.
Reaman, G. 2009. Children’s Oncology Group: A National/International Infrastructure for Pediatric Cancer Clinical Translational Research. Presented to the Institute of Medicine Committee on Cancer Clinical Trials and the NCI Cooperative Group Program, April 23, 2009, Washington, DC.
Robinson, W. R., J. Ritter, A. S. Rogers, S. Tedjarati, and C. Lieberenz. 2009. Clinical trial participation is associated with improved outcome in women with ovarian cancer. International Journal of Gynecological Cancer 19(1):124–128.
Roy, P., G. V. Hudson, B. V. Hudson, J. Esteve, and A. J. Swerdlow. 2000. Long-term survival in Hodgkin’s disease patients: A comparison of relative survival in patients in trials and those recorded in population-based cancer registries. European Journal of Cancer 36(3):384–389.
Sateren, W. B., E. L. Trimble, J. Abrams, O. W. Brawley, N. Breen, L. Ford, M. S. McCabe, R. Kaplan, M. Smith, R. Ungerleider, and M. Christian. 2002. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of Clinical Oncology 20(8):2109–2017.
Schain, W. S. 1994. Barriers to clinical trials. Part II. Knowledge and attitudes of potential participants. Cancer 74(Suppl. 9):2666–2671.
Schrier, R. W. 1997. Ensuring the survival of the clinician-scientist. Academic Medicine 72(7):589–594.
Siminoff, L. A., A. Zhang, N. Colabianchi, C. M. S. Sturm, and Q. Shen. 2000. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. Journal of Clinical Oncology 18(6):1203–1211.
Somkin, C. P., A. Altschuler, L. Ackerson, A. M. Geiger, S. M. Greene, J. Mouchawar, J. Holup, L. Fehrenbacher, A. Nelson, A. Glass, J. Polikoff, S. Tishler, C. Schmidt, T. Field, and E. Wagner. 2005. Organizational barriers to physician participation in cancer clinical trials. The American Journal of Managed Care 11(7):413–421.
Stiller, C. A. 1994. Centralised treatment, entry to trials and survival. British Journal of Cancer 70(2):352–362.
Taylor, H. 2004. Most physicians do not participate in clinical trials because of lack of opportunity, time, personnel support and resources. Harris Interactive: Health Care News 4(9):1–8.
Underwood, S. M. 2000. Minorities, women, and clinical cancer research: The charge, promise, and challenge. Association of Educational Psychologists Journal 10(Suppl. 8):s4–s12.
Varki, A., and L. E. Rosenberg. 2003. Emerging opportunities and career paths for the young physician-scientist. Nature Medicine 8(5):437–439.
Verheggen, F. W. S. M., F. Nieman, and R. Jonkers. 1998. Determinants of patient participation in clinical studies requiring informed consent: Why patients enter a clinical trial. Patient Education and Counselling 35:111–125.
Vist, G. E., K. B. Hagen, P. J. Devereaux, D. Bryant, D. T. Kristoffersen, and A. D. Oxman. 2005. Systematic review to determine whether participation in a trial influences outcome. British Medical Journal 330(7501):1175–1181.
Vist, G. E. D. Bryant, L. Somerville, T. Birminghem, A. D. Oxman. 2008. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database of Systematic Reviews Jul 16 (3):MR000009.
Wallace, P. 2009. Kaiser Permanente Oncology-Specific Care Management Systems. Presented at the NCPF Workshop on A Foundation for Evidence-Driven Practice: A Rapid Learning System for Cancer Care, Washington, DC.
Zarin, D. A., and T. Tse. 2008. Moving toward transparency of clinical trials. Science 319 (5868):1340–1342.
Zarin, D. A., T. Tse, and N. C. Ide. 2005. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine 353(26):2779–2787.
Zon, R., N. J. Meropol, R. B. Catalano, and R. L. Schilsky. 2008. American Society of Clinical Oncology statement on minimum standards and exemplary attributes of clinical trial sites. Journal of Clinical Oncology 26(15):2562–2567.












































