1
Introduction
Advances in biomedical research have produced significant opportunities to improve cancer prevention, detection, and treatment. Insights about the genomic and molecular mechanisms of disease have enabled basic scientists to identify new therapeutic targets and develop new agents that are changing the paradigm of cancer research from the development of nonspecific, broadly toxic chemotherapies to the development of highly targeted combinations of therapies. However, the ability to translate biomedical discoveries into advances in cancer care remains dependent on the clinical trials system. Clinical trials provide an essential link between scientific discovery and clinical practice. These trials are crucial to the translation of new knowledge into tangible benefits for patients, and the knowledge gained in a clinical trial can also inform and guide further research into the biology of the disease.
Since its inception in the 1950s, the Clinical Trials Cooperative Group Program has been instrumental in establishing the standards for the care of patients with cancer and for clinical research methods. Research undertaken by the Cooperative Groups has contributed to significant advances in cancer treatment and prevention, including the introduction of new treatments and the use of established treatments for new indications that have led to improved survival and increased cure rates, particularly for pediatric cancers and some early-stage cancers in adults. Furthermore, the importance of the Cooperative Group Program is growing as industry trials are increasingly being conducted outside of the United States. The Cooperative Group Program provides a primary mechanism by which the value of therapeutic agents can be assessed within the medical milieu of the U.S. health care system. However, despite these important contributions and a long record
of accomplishments, the Cooperative Group Program is facing numerous challenges that threaten its ability to continue to undertake large-scale, innovative trials that benefit patient care. Confronting these challenges is essential. A national cancer clinical trials enterprise is necessary “to ensure that the advances in understanding the biological basis of cancer, generated by the past 40 years of research, are harnessed effectively to bring measurable, meaningful benefits to patients” (NCI, 2005).
IMPORTANCE OF CANCER CLINICAL TRIALS
Clinical trials are essential for establishing the evidence base that the oncology community uses to make treatment decisions and to determine the direction of future clinical research. By evaluating the safety and efficacy of new therapies, comparing the effectiveness of existing therapies, and assessing different prevention, screening, and detection strategies, clinical trials are responsible for setting the standard of patient care. In fact, today’s most effective therapies began as hypotheses tested within the clinical trials environment (C-Change and Coalition of Cancer Cooperative Groups, 2006). Clinical trials also provide fundamental information about the biology of cancer, which investigators leverage to advance preclinical research and drug development.
Numerous stakeholders conduct clinical trials with various goals across the spectrum of research. While industry trials primarily focus on drug discovery and development activities with the potential for a substantial return on investment, publicly sponsored trials have a more diverse portfolio, from small, proof-of-concept Phase I and II studies that typically enroll patients with metastatic disease who have already had one or more lines of therapy to large Phase III studies that may focus on adjuvant or neoadjuvant therapy, first-line therapy for metastatic disease, or prevention strategies. Publicly sponsored trials are also more likely to study less common cancers that are not often a focus of industry research and development.
The National Cancer Institute (NCI) supports the largest U.S. network for clinical trials of any type through the use of several different funding mechanisms. NCI supports individual trials through grant mechanisms and research contracts, funds programs that use clinical trial data to advance preclinical research, and also partially funds cancer centers that conduct clinical trials as a component of their overall research and patient care activities. In addition, NCI supports trials through cooperative agreements, such as the Clinical Trials Cooperative Group Program. The various recipients of the funds provided by the use of these different funding mechanisms bring different strengths to the research portfolio.
The largest component of the NCI-supported clinical trials portfolio is the Clinical Trials Cooperative Group Program. The Cooperative Group
Program is the major mechanism through which large-scale cancer clinical trials are conducted in the public interest. The expansive, multi-institutional clinical trials infrastructure maintained by the Cooperative Group Program is recognized for its fundamental importance in reaching a large, diverse community-based patient population, acquiring high-quality data and biospecimens that advance preclinical research, and incorporating a broad range of expertise into trial design, implementation, and statistical analyses. Within the portfolio of NCI-supported clinical trials, the Cooperative Group Program primarily focuses on late-stage translation activities, such as large Phase II and Phase III clinical trials that may have implications for changing treatment practices directly relevant to patient care. Individual institutions can rarely undertake such trials because it would take too long to accrue a sufficient number of patients to achieve timely results.
THE CLINICAL TRIALS COOPERATIVE GROUP PROGRAM
The Clinical Trials Cooperative Group Program began in 1955. At that time, the U.S. Congress was interested in developing a more systematic and planned program for the study of chemotherapy in cancer treatment because studies had shown that the treatment of leukemia and lymphoma with alkylating agents, steroids, antifolate, and mercaptopurine could occasionally result in complete remission of these cancers. Congress appropriated $5 million to establish the Cancer Chemotherapy National Service Center, and NCI initiated the Cooperative Group model to test chemotherapeutic agents in clinical trials. By 1958, 17 Cooperative Groups had been organized and operated under research grants from NCI. Federal funding for chemotherapy research continued to climb: in 1958 alone, Congress appropriated $25 million (Zubrod, 1984).
In the beginning, the primary objective of the Clinical Trials Cooperative Group Program was to test new anticancer agents from NCI’s drug development program. However, between 1955 and 1966, NCI underwent an internal reorganization. In recognition of the importance of clinical trials as an independent research activity, the Cooperative Group Program was separated administratively from the drug screening program and transferred to the Cancer Therapy and Evaluation Branch of the Chemotherapy Program (Keating and Cambrosio, 2002).
During the following decades, NCI implemented some organizational changes to the Program. In 1980–1981, the mechanism of support for the Cooperative Group Program was converted from a grant to a cooperative agreement, which had a profound effect on the Cooperative Group Program. A cooperative agreement enabled NCI to have a considerable role in Cooperative Group activities, including trial concept selection, protocol development, and trial operations (CTEP, 2006) (these are described fur-
|
BOX 1-1 NCI Cooperative Group Program 2010 The NCI Cooperative Group Program is composed of 10 Groups:
|
ther in Chapter 3). In 1983, the Community Clinical Oncology Program (CCOP) was established to ensure that community physicians and cancer patients not treated in academic medical centers had access to cancer clinical trials and to boost the rates of accrual to clinical trial protocols. NCI established the Minority-Based CCOP in 1990 to increase the involvement of racial and ethnic minority patients in clinical trials research and to improve access to the latest advances in cancer treatment, prevention, and control (NCI, 2003).
Cooperative Group membership has evolved over time (Hoogstraten, 1980), and the Program currently includes 10 Cooperative Groups (the names of the 10 Groups and the abbreviations for the groups used throughout the remainder of this chapter are presented in Box 1-1). The focus of each Group varies, but there are four main types of groups: (1) disease-oriented Groups (e.g., GOG); (2) Groups that focus on high-technology, single-modality studies (e.g., RTOG); (3) Groups in which investigators focus on a particular patient population (e.g., COG); and (4) multimodality Groups. Over time the Cooperative Groups have expanded their research mission beyond testing new anticancer agents from NCI’s drug development program to include cancer treatment, prevention, early detection, quality of life issues, rehabilitation, and comparative effectiveness. Each year more than 25,000 patients participate in multi-institutional clinical trials involving more than 3,100 institutions and 14,000 investigators within the 10 Cooperative Groups.1
ACHIEVEMENTS OF THE COOPERATIVE GROUP PROGRAM
The Cooperative Groups have been responsible for numerous advances in cancer research, treatment, and prevention and in the training of investigators. Over the five decades since its inception in the 1950s, the high-quality research conducted by the Cooperative Groups has been instrumental in establishing the standards of cancer patient care and clinical research methods (Mauer et al., 2007), and research undertaken by the Cooperative Groups leads to more than 200 peer-reviewed publications annually. Cooperative Group accomplishments can be organized by influential trials that have, over the 50 years of the Groups’ existence, incrementally provided practitioners with evidence to guide the treatment of patients with cancer (see Box 1-2 for a list of some of these accomplishments). Likewise, Cooperative Group accomplishments can be organized thematically by clinical objective and type of disease. Cooperative Group research has led to the
-
development of new standards for the management of patients with cancer;
-
development of sophisticated investigative techniques;
-
collection of data to obtain regulatory approval for new drugs or new drug indications;
-
refinement in diagnosis and treatment of cancer based on the identification of histologic subtypes of tumors and the recognition of prognostic variables;
-
development of adjuvant and neoadjuvant chemotherapy and concurrent chemoradiotherapy for solid tumors through studies that combine modalities;
-
refinement of the use of chemotherapy through the study of new agents and different dosing schedules;
-
comparison of new cancer treatments against the best available treatments; and
-
development of novel therapeutic agents in Phase I and II trials (Mauer et al., 2007).
Significant advances derived from Cooperative Group research include improvements in the treatment of childhood cancer, the treatment of solid tumors and hematologic malignancies in adults, adjuvant therapy, and combined-modality treatment. Additionally, trials of cancer prevention and the publication of negative findings from Cooperative Group research greatly contribute to ensuring the use of evidence-based treatment and prevention strategies.
|
BOX 1-2 A Sampling of Cooperative Group Accomplishments Pediatric cancers
Hematologic malignancies
|
Breast cancer
|
Lung cancer
Gastrointestinal cancer
|
Genitourinary cancer
|
|
Brain cancer
Gynecologic cancer
|
Childhood Cancer
One of the major accomplishments in research on and the treatment of pediatric cancer is the high rate of participation of children in Cooperative Group clinical trials. In the United States, 90 to 95 percent of all children with a newly diagnosed malignancy are seen at an institution that participates in COG (O’Leary et al., 2008). If a clinical trial is available, more than half of these children participate; for young children (less than 5 years of age), the rates of participation in a clinical trial are closer to 90 percent. The collective achievements of Cooperative Group research over the past four decades have led to effective treatments for childhood cancers and improved cure rates (Mauer et al., 2007). The age-adjusted mortality rate for
Head and neck cancer
Skin cancer
SOURCES: Coltman, 2008; Dignam, 2004; Giantonio et al., 2008; Green et al., 2008; Grothey et al., 2008; Hillman and Gatsonis, 2008; Mauer et al., 2007; O’Leary et al., 2008; Omura, 2008; RTOG, 2009; and Wickerham et al., 2008. For further information on additional Cooperative Group achievements, see CTEP, 2002. |
children with cancer has decreased since 1950 (Figure 1-1), and cure rates have increased from less than 10 percent when the Cooperative Groups were founded to nearly 80 percent at present (O’Leary et al., 2008).
Adult Solid Tumors and Hematologic Malignancies
Cooperative Group research has been instrumental in providing data on the treatment of specific tumors and hematologic malignancies. For example, landmark trials from NSABP first demonstrated equivalent rates of survival between patients undergoing a radical mastectomy and patients undergoing a total mastectomy and then between patients undergoing a
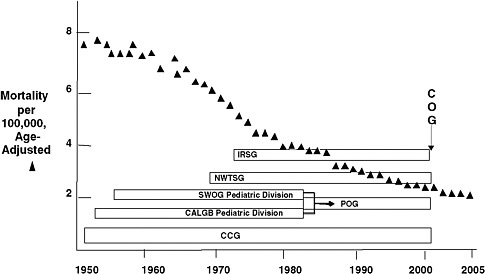
FIGURE 1-1 National rate of mortality from cancer among children younger than 15 years of age and the pediatric Cooperative Groups.
NOTE: CALGB = Cancer and Leukemia Group B, CCG = Children’s Cancer Group, COG = Children’s Oncology Group, IRSG = Intergroup Rhabdomysosarcoma Study Group, NWTSG = National Wilms’ Tumor Study Group, POG = Pediatric Oncology Group, SWOG = Southwest Oncology Group. SWOG and CALGB previously had pediatric divisions within their cooperative group structures. In 2001, the four pediatric groups (IRSG, NWTSG, POG, and CCG) merged to form COG.
SOURCE: Reprinted, with permission, from Reaman, 2009. Copyright 2009 by Children’s Oncology Group.
mastectomy and patients undergoing a lumpectomy followed by radiation therapy, ushering in an era of breast-conserving therapy (Fisher et al., 1977, 1985, 2002a,b). NCCTG demonstrated that lower-dose radiation therapy is as effective as and less toxic than higher-dose radiation therapy for patients with low-grade glioma (Shaw et al., 2002). An ECOG trial demonstrated that bevacizumab (a vascular endothelial growth factor [VEGF] inhibitor) significantly improved the overall rate of survival when it was used in combination with a regimen of oxaliplatin, 5-FU, and leucovorin in patients with advanced colorectal cancer, expanding the FDA-approved indication for the use of bevacizumab (Giantonio et al., 2007, 2008). GOG set the standards for multiagent chemotherapy at all gynecologic sites; for example, the GOG paclitaxel-cisplatin trial demonstrated that paclitaxel adds further benefits when it is used for the treatment of ovarian cancer (reviewed by Omura, 2008).
In terms of hematologic malignancies, CALGB developed the framework for the current therapy of adult patients with AML, refining classifica-
tion of leukemia to include cytogenetic and molecular genetic characteristics (reviewed by Green et al., 2008). In addition, ECOG’s Multiple Myeloma Committee developed trials that established treatment with thalidomide plus dexamethasone as a standard of care for newly diagnosed myeloma (Rajkumar et al., 2006).
Adjuvant Therapy for Solid Tumors
The Cooperative Groups are uniquely positioned to undertake trials of adjuvant therapies because such trials require large numbers of patients, significant data management and statistical support, and the collaboration of multiple oncology specialists (Mauer et al., 2007). Cooperative Group research has demonstrated the benefit of adjuvant therapy for breast, lung, colon, and gastric cancer, as well as melanoma (Haller et al., 2005; Kirkwood et al., 2004; MacDonald et al., 2001; Mamounas et al., 1999; Moertel et al., 1995; Strauss et al., 2004; Winton et al., 2005). For example, CALGB defined the role of adjuvant paclitaxel as part of adjuvant therapy for breast cancer, leading to FDA approval of the use of paclitaxel for this indication (Green et al., 2008; Henderson et al., 2003). NSABP/NCCTG trials demonstrated a significant survival benefit for adjuvant treatment with trastuzumab, a monoclonal antibody, in women with HER-2-positive breast cancer (Romond et al., 2005). NCCTG first demonstrated the value of adjuvant chemotherapy in early-stage colon cancer almost 20 years ago, when patients treated with 5-FU and levamisole after curative surgery were found to have significantly improved outcomes compared with the outcomes for patients treated with surgery alone (Moertel et al., 1990).
Combined-Modality Therapy
Advances in combined-modality therapy are attributable to the multidisciplinary organization and expertise of the Cooperative Groups (Mauer et al., 2007). Five Cooperative Group trials conducted by GOG, RTOG, and SWOG defined the value of chemoradiation for the treatment of cervical cancer. Radiation therapy combined with platinum-based chemotherapy conferred reductions in mortality rates of 30 to 50 percent compared with the mortality rate after treatment with radiation alone for women with locally or regionally advanced cervical cancer or localized cervical cancer with poor prognostic indicators (Keys et al., 1990; Morris et al., 1999; Peters et al., 2000; Rose et al., 1999; Whitney et al., 1999). The results of a CALGB trial (Trial 8433) established the use of induction chemotherapy before definitive radiation as the new benchmark for the management of fit patients with locally advanced non-small-cell lung cancer (Dillman et al., 1996). RTOG found that patients with high-risk head and neck cancer
who received chemotherapy together with radiotherapy after surgery were far less likely to have a recurrence of cancer (RTOG, 2009).
Cancer Prevention and Detection
Prevention efforts have also been a focus of Cooperative Group research. The NSABP Breast Cancer Prevention Trial demonstrated that tamoxifen treatment reduced the incidence of breast cancer by nearly 50 percent in women with an increased risk of developing breast cancer (Fisher et al., 1998). Additionally, the Study of Tamoxifen and Raloxifene demonstrated that tamoxifen and raloxifene are equally effective in reducing the risk of invasive breast cancer (Vogel et al., 2006). A CALGB chemoprevention study demonstrated that aspirin can reduce the risk of colorectal adenoma in patients with a history of colon cancer (Sandler et al., 2003). SWOG’s Prostate Cancer Prevention Trial demonstrated that finasteride treatment resulted in a 24 percent reduction in the prevalence of prostate cancer at 7 years compared with the prevalence in those treated with a placebo (Thompson et al., 2003).
ACRIN focuses on the evaluation of imaging techniques for the screening, diagnosis, and treatment of cancer. One trial, ACRIN Trial 6652, found that digital mammography is superior to film-screen mammography for a subset of women (i.e., women with dense breasts, such as those who are younger and pre- or perimenopausal) (Pisano et al., 2005). These findings have refined the use of digital mammography for women who can benefit from its application (Hillman and Gatsonis, 2008). Also noteworthy are ACRIN trials that are unlikely to be conducted in industry settings but that may provide practice-changing information in the future. Examples include trials that are evaluating colorectal cancer screening using computed tomography (CT) colonography, breast cancer screening using ultrasound and magnetic resonance imaging, and lung cancer screening using CT.
Negative Findings and Previously Unobserved Treatment Risks
Negative research findings are underreported in the published medical literature but are essential in setting the standard of care. A recent study evaluated the proportion of trials listed in a public trials registry that have been published in the peer-reviewed literature. Between 1999 and 2007, the results of less than 6 percent of all industry-sponsored studies had been published, and 75 percent of those had reached a positive conclusion. In contrast, 59 percent of the clinical trials performed by NCI-supported clinical trials networks had been published over the same period of time, and half of those trials reported a positive result (Ramsey and Scoggins, 2008). The latter figure is consistent with an ongoing evaluation of the publication rate
of the findings of more than 1,500 Phase II and III clinical trials performed from 2000 to 2005 by the Cooperative Groups (Doroshow, 2008).
Published Cooperative Group research has demonstrated, for example, that there is no clear benefit of high-dose chemotherapy with stem cell support (a more aggressive therapy with high rates of morbidity and mortality) for the treatment of breast cancer, as well as results from the Selenium and Vitamin E Cancer Prevention Trial, which found that treatment with 200 micrograms of selenium and 400 international units of vitamin E daily does not prevent prostate cancer (reviewed by Coltman [2008] and Dignam [2004]). Large Cooperative Group trials have also revealed important secondary effects of therapy, including both adverse events and previously unobserved treatment risks. For example, an increased incidence of leukemia was observed after treatment with chemotherapy, as was an increase in the incidence of endometrial cancer following tamoxifen treatment (reviewed by Dignam, 2004).
STRENGTHS OF THE COOPERATIVE GROUP PROGRAM
The pharmaceutical and biotechnology industries play a critical role in developing new therapeutic agents for the treatment of cancer. Oncology has become one of the most active areas of drug development by industry, with more new cancer drugs entering the market in recent years than for any other disease category (Woodcock, 2009). Between July 2005 and December 2007, FDA approved 53 new indications in oncology, with 18 new molecular entity approvals and 35 supplemental applications. In comparison, FDA currently approves around 18 new molecular entities annually for all disease areas, which means that oncology has been taking the lion’s share (Woodcock, 2009). The research and development efforts undertaken by industry entail enormous costs and are critical to the progress in cancer treatment.
Publicly funded clinical trials also play a vital, complementary role in advancing science and patient care, particularly by addressing questions that are important to patients but are less likely to be top priorities of industry. With many new therapies already in clinical use, and more than 800 cancer therapeutics in development (PhRMA, 2009), it can be difficult for physicians to assess which treatment is best for an individual patient, especially considering that some drugs may confer only weeks or months of extra benefit, on average.2 Publicly sponsored trials fill an important information void by conducting head-to-head comparisons of different therapeutics from different companies that are already approved for clinical use. The
pharmaceutical industry has less incentive to undertake these comparative effectiveness trials because doing so may negatively affect a company’s bottom line if that company’s drug is found to be inferior. Companies may also have less incentive to combine novel therapies developed by different sponsors, but this may more readily occur in a publicly funded clinical trials environment. Clinical trials evaluating therapies in rare diseases may not be top priorities for industry, since the research and development costs may not be recouped by the small number of patients who receive the therapy. Likewise, trials that assess multimodality therapies, such as radiation therapy, surgery, or devices in combinations with drugs provide data that inform clinical practice, but are usually not high priorities of industry. Clinical trials that determine the optimal duration and dose of treatment with drugs in clinical use may also be lower priorities for industry. In addition, screening and prevention strategies and rehabilitation and quality of life studies are less likely to be top priorities of industry. In these cases, the Cooperative Group Program provides an important setting to conduct clinical trials. Some current examples of Cooperative Group trials that probably would not have been conducted by industry alone include:
-
C80405, a head-to-head trial in first-line treatment for metastatic colorectal cancer that directly compares chemotherapy plus bevacizumab to chemotherapy plus cetuximab. Bevacizumab and cetuximab are both monoclonal antibodies with different specificities. Both have been approved for the treatment of metastatic colorectal cancer, but it is unclear which strategy of combining chemotherapy with a monoclonal antibody is superior, i.e., whether targeting the epidermal growth factor receptor (EGFR) pathway (cetuximab) or VEGF pathway (bevacizumab) increases overall survival. The results of this clinical trial will likely influence treatment decisions, but the companies who developed bevacizumab and cetuximab (Genentech and Bristol-Myers Squibb/ImClone Systems, respectively) are not incentivized to conduct this trial. In addition to the expense of the trial, it is possible that the results of the trial may demonstrate one drug is inferior to the other, impacting one company’s revenue negatively.
-
S0307, a trial that compares adjuvant zoledronate, clodronate, and ibandronate in women with primary breast cancer. One of the agents under evaluation, ibandronate, is off-patent, making it is less likely that this study would be undertaken by industry, although it may have important benefits for the selected patient population.
-
S0521, a trial assessing maintenance therapy versus observation for patients with previously untreated low and intermediate risk
-
acute promyleocytic leukemia. This study involves a very rare disease with already approved agents. The research question, whether favorable outcomes can be achieved with more limited therapy, is also unlikely to be addressed by industry because it may constrict the use of drugs already approved.
-
RTOG 0525, a trial that compares conventional adjuvant temozolomide with dose-intensive temozolomide for newly diagnosed glioblastoma. Pharmaceutical companies may have less incentive to study dose scheduling questions, especially for drugs already approved.
-
GOG 0238, a trial that evaluates radiation therapy versus radiation therapy and chemotherapy with cisplatin in women with pelvic only recurrence of endometrial cancer. Cisplatin is an already approved therapy, and industry is unlikely to investigate this multimodal research question.
Table 1-1 provides additional examples of Cooperative Group trials that the pharmaceutical and biotechnology industries have less incentive to conduct, but have implications that will likely affect clinical practice. It is important to note that industry does help support some of these trials (typically by supplying a drug) but it is unlikely that these trials would have been initiated with industry-only support.
The Cooperative Group Program provides a unique environment for investigators to conduct clinical trials. The public and academic nature of the Groups enables the pooling of public resources to conduct studies in the public interest. The Cooperative Group Program supports trials that explore new methods of cancer treatment and prevention, including studies of combination therapies and proof-of-concept studies, as well as trials that focus on early detection, quality of life, rehabilitation, and comparative effectiveness. In doing so, the Groups often engage the patient advocacy community in the selection and design of their trials (Collyar, 2008). For example, after patient advocates successfully pushed for testing of a lower dose of a standard therapy for multiple myeloma in a Cooperative Group study, the trial results demonstrated improved survival and fewer side effects in the low-dose arm, altering the standard of care (International Myeloma Foundation, 2007). Cooperative Group trial databases and clinically annotated biospecimen repositories have also enabled researchers to conduct correlative science and analyses of cancer prognosis and survivorship that have delineated specific subpopulations, defined cancer staging, and aided in validating prognosis indicators (see also Chapter 2). Cooperative Group trials also extend participation beyond research-oriented facilities—such as academic medical centers and cancer centers—to community hospitals and individual physician practices, some of which participate through
TABLE 1-1 Current Examples of Cooperative Group Phase II and III Trials That Probably Would Not Have Been Conducted by Industry Alone
|
Trial |
Title |
|
Brain and Central Nervous System Cancers |
|
|
N0577 |
Intergroup Study of Radiotherapy vs. Temozolomide Alone vs. Radiotherapy with Concomitant and Adjuvant Temozolomide for Patients with 1p/19q Codeleted Anaplastic Glioma |
|
RTOG-0525 |
Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide in Patients with Newly Diagnosed Glioblastoma |
|
Breast Cancer |
|
|
ACOSOG-Z1031 |
Randomized Trial Comparing 16 to 18 Weeks of Neoadjuvant Exemestane (25 mg daily), Letrozole (2.5 mg), or Anastrozole (1 mg) in Postmenopausal Women with Clinical Stage II and III Estrogen Receptor Positive Breast Cancer |
|
ACOSOG-Z1041 |
Randomized Trial Comparing a Neoadjuvant Regimen of FEC-75 Followed by Paclitaxel + Trastuzumab with a Neoadjuvant Regimen of Paclitaxel + Trastuzumab Followed by FEC-75 Plus Trastuzumab in Patients with HER-2 Positive Operable Breast Cancer |
|
E5103 |
Double-Blind Trial of Doxorubicin + Cyclophosphamide Followed by Paclitaxel + Bevacizumab or Placebo in Patients with Lymph Node Positive and High-Risk Lymph Node Negative Breast Cancer |
|
PACCT-1 |
Program for the Assessment of Clinical Cancer Tests (PACCT-1): Trial Assigning IndividuaLized Options for Treatment: The TAILORx Trial |
|
S0307 |
Trial of Bisphosphonates as Adjuvant Therapy for Primary Breast Cancer |
|
Gastrointestinal and Neuroendocrine Cancers |
|
|
C80405 |
Trial of Irinotecan/5-FU/Leucovorin or Oxaliplatin/5-FU/Leucovorin with Bevacizumab, or Cetuximab (C225), or with the Combination of Bevacizumab and Cetuximab for Patients with Untreated Metastatic Adenocarcinoma of the Colon or Rectum |
|
CALGB-80702 |
Trial of 6 vs. 12 Treatments of Adjuvant FOLFOX Plus Celecoxib or Placebo for Patients with Resected Stage III Colon Cancer |
|
RTOG-1010 |
Trial Evaluating the Addition of Trastuzumab to Trimodality Treatment of HER2 Overexpressing Esophageal Adenocarcinoma |
|
RTOG-0436 |
Trial Evaluating the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation for Patients with Esophageal Cancer Who Are Treated Without Surgery |
|
Brief Rationale for Selection |
Phase |
|
Study of radiation and temozolomide in patients with 1p/19q co-deleted anaplastic gliomas in a rare disease with unique biology. |
Phase III |
|
Study of 2 doses of temozolomide (conventional dose vs. dose-intensive dose) in patients with glioblastoma multiforme. |
Phase III |
|
Neoadjuvant hormonal study in breast cancer. |
Phase III |
|
Neoadjuvant study in breast cancer. |
Phase III |
|
Study includes 3 arms in order to test a duration question relative to bevacizumab-based therapy. |
Phase III |
|
Evaluates the potential benefit of chemotherapy in a patient population selected by a diagnostic test. |
Phase III |
|
Evaluates an agent (ibandronate) that is off-patent but may have important benefits for the selected patient population. |
Phase III |
|
Involves a direct head-to-head comparison of 2 types of monoclonal antibody-based therapy combined with chemotherapy with overall survival as the primary endpoint. |
Phase III |
|
Uses a 2 × 2 factorial design. The duration question regarding adjuvant chemotherapy has clear public health implications and is part of the International Duration Evaluation of Adjuvant Chemotherapy meta-analysis, which leverages other international trials with compatible endpoints. |
Phase III |
|
Study in a very rare subset of a rare disease, in a clinical setting using a specific therapeutic approach (trimodality therapy). |
Phase III |
|
Evaluates an agent in combination with radiation therapy in a rare disease and for a very select therapeutic approach in a specific patient population (nonoperative therapy). |
Phase III |
|
S0518 |
Prospective Randomized Comparison of Depot Octreotide Plus Interferon Alpha vs. Depot Octreotide Plus Bevacizumab (NSC #704865) in Advanced, Poor Prognosis Carcinoid Patients |
|
Genitourinary Cancers |
|
|
E2805 |
ASSURE: Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma |
|
Gynecologic Cancers |
|
|
GOG-0218 |
Trial of Carboplatin and Paclitaxel + Placebo vs. Carboplatin and Paclitaxel + Concurrent Bevacizumab (NSC #704865, IND #7921) Followed by Placebo, vs. Carboplatin and Paclitaxel + Concurrent and Extended Bevacizumab, in Women with Newly Diagnosed, Previously Untreated, Stage III or IV Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer |
|
GOG-0249 |
Trial of Pelvic Radiation Therapy vs. Vaginal Cuff Brachytherapy Followed By Paclitaxel/Carboplatin Chemotherapy in Patients with High-Risk, Early-Stage Endometrial Carcinoma |
|
GOG-0250 |
Randomized Trial of Docetaxel (NSC #628503) and Gemcitabine (NSC #613327) + G-CSF with Bevacizumab (NSC #704865, IND #7921) vs. Docetaxel (NSC #628503) and Gemcitabine (NSC #613327) + G-CSF with Placebo in the Treatment of Recurrent or Advanced Leiomyosarcoma of the Uterus |
|
GOG-0252 |
Trial of Bevacizumab with IV vs. IP Chemotherapy in Ovarian, Fallopian Tube, and Primary Peritoneal Carcinoma NCI-Supplied Agent(s): Bevacizumab (NSC #704865, IND #7921) |
|
GOG-0255 |
Randomized, Double-Blind Trial of a Polyvalent Vaccine-KLH Conjugate (NSC 748933) + OPT-821 vs. OPT-821 in Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer Who Are in Second or Third Complete Remission |
|
GOG-0258 |
Randomized Trial of Cisplatin and Tumor Volume Directed Irradiation Followed by Carboplatin and Paclitaxel vs. Carboplatin and Paclitaxel for Optimally Debulked, Advanced Endometrial Carcinoma |
|
GOG-0261 |
Randomized Trial of Paclitaxel Plus Carboplatin vs. Ifosfamide Plus Paclitaxel in Chemotherapy-Naive Patients with Newly Diagnosed Stage I-IV, Persistent or Recurrent Carcinosarcoma (Mixed Mesodermal Tumors) of the Uterus |
|
RTOG-0724 |
Randomized Study of Concurrent Chemotherapy and Pelvic Radiation Therapy with or without Adjuvant Chemotherapy in High-Risk Patients with Early-Stage Cervical Carcinoma Following Radical Hysterectomy |
|
GOG-0238 |
Randomized Trial of Pelvic Irradiation with or without Concurrent Weekly Cisplatin in Patients with Pelvic-Only Recurrence of Carcinoma of the Uterine Corpus |
|
GOG-0248 |
Randomized Trial of Temsirolimus (NCI-Supplied Agent, NSC #683864, IND #61010) or the Combination of Hormonal Therapy Plus Temsirolimus in Women with Advanced, Persistent, or Recurrent Endometrial Carcinoma |
|
Study evaluates a new agent in a very rare tumor directly against a very different therapy (not against placebo or a combination involving the new agent). |
Phase III |
|
Adjuvant study comparing 2 drugs from different companies to observation. |
Phase III |
|
Included 3 arms in order to test a duration question relative to bevacizumab-based therapy. |
Phase III |
|
A relatively rare clinical scenario in which chemotherapy is being tested with standard agents. |
Phase III |
|
A study in a very rare tumor type—leiomyosarcoma of the uterus. |
Phase III |
|
Study of intravenous (IV) vs. intra-peritoneal (IP) chemotherapy. |
Phase III |
|
Evaluates polyvalent vaccine + adjuvant therapy vs. adjuvant therapy alone in women in 3rd remission ovarian cancer. Involves an academic vaccine without any company support. |
Phase III |
|
A study in a relatively rare clinical scenario evaluating standard agents. |
Phase III |
|
A study in a rare tumor type evaluating standard agents. |
Phase III |
|
Evaluating chemoradiation with or without adjuvant chemotherapy in women with high-risk early-stage cervical cancer after hysterectomy with involving standard agents. |
Phase III |
|
A study of radiation therapy vs. chemoradiation in women with pelvic only recurrence of endometrial cancer and the agents being evaluated are standard. |
Phase II |
|
A study of temsirolimus with or without hormonal therapy in women with recurrent endometrial cancer in a very rare clinical scenario. |
Phase II |
|
Hematologic Cancers |
|
|
CALGB-10603 |
Randomized, Double-Blind Study of Induction (Daunorubicin/Cytarabine) and Consolidation (High-Dose Cytarabine) Chemotherapy + Midostaurin (PKC412) (IND # 101261) or Placebo in Newly Diagnosed Patients < 60 Years of Age with FLT3 Mutated Acute Myeloid Leukemia |
|
CALGB-50303 |
Randomized Study of R-CHOP vs. Dose-Adjusted Epoch-R with Molecular Profiling in Untreated De Novo Diffuse Large B-Cell Lymphomas |
|
S0521 |
Randomized Trial of Maintenance vs. Observation for Patients with Previously Untreated Low and Intermediate Risk Acute Promyelocytic Leukemia |
|
S0777 |
Randomized Trial of CC-5013 (Lenalidomide, NSC-703813) and Low Dose Dexamethasone vs. Bortezomib (PS-341, NSC-681239), Lenalidomide and Low Dose Dexamethasone for Induction, in Patients with Previously Untreated Multiple Myeloma without an Intent for Immediate Autologous Stem Cell Transplant |
|
S0816 |
Trial of Response-Adapted Therapy of Stage III-IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) Imaging |
|
Lung Cancers |
|
|
CALGB-30506 |
Randomized Trial of Adjuvant Therapy in Early-Stage Non-Small Cell Lung Cancer Evaluating the Potential Utility of a Genomic Prognostic Model to Identify Patients as Candidates for Adjuvant Chemotherapy |
|
S0819 |
Randomized Study Comparing Carboplatin/Paclitaxel or Carboplatin/Paclitaxel/Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer |
|
E1508 |
Randomized Study of Cisplatin and Etoposide in Combination with Either Hedgehog Inhibitor GDC-0449 or IGF-1R MOAB IMC-A12 for Patients with Extensive Stage Small Cell Lung Cancer |
|
Pediatric Cancers |
|
|
AALL0232 |
High-Risk B-Precursor Acute Lymphoblastic Leukemia |
|
AALL0331 |
Standard-Risk B-Precursor Acute Lymphoblastic Leukemia |
|
AALL0434 |
Intensified Methotrexate, Nelarabine (Compound 506U78; IND# 52611) and Augmented BFM Therapy for Children and Young Adults with Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia |
|
AEWS0331 |
European Ewing Tumor Working Initiative of National Groups Ewing Tumour Studies 1999 (EURO-E.W.I.N.G. 99) |
|
A rare disease subset that required significant collaboration. |
Phase III |
|
Evaluation of standard agents that involves molecular profiling. |
Phase III |
|
Evaluating whether favorable outcomes can be achieved in a very rare disease with more limited therapy with approved agents. |
Phase III |
|
Evaluating competing therapies involving agents from 2 different companies. |
Phase III |
|
Evaluating the utility of FDG-PET as a biomarker for use in risk stratification and treatment determination for approved therapies in Hodgkin lymphoma. |
Phase II |
|
Evaluating a risk model for relapse in the adjuvant setting for patients with Stage 1 NSCLC and involves comparing standard chemotherapy to observation. |
Phase III |
|
A complex study with 4-drug combinations using 2 targeted therapies to evaluate outcomes and to validate epidermal growth factor receptor (EGFR) markers for EGFR-targeted therapy. |
Phase III |
|
A study in small cell lung cancer with 2 new investigational agent-based therapies from different companies. |
Phase II |
|
Evaluating both induction and maintenance therapy in patients who are less than 10 years of age. |
Phase III |
|
Evaluating the benefit of augmented intensity consolidation as a therapeutic approach. |
Phase III |
|
Involves multiple research questions in a 2 × 2 randomization design evaluating nelarabine therapy and interim maintenance therapy with Capizzi methotrexate or high-dose methotrexate. |
Phase III |
|
Evaluating the schedule/timing of therapy in Ewing sarcoma (every 3 weeks vs. every 2 weeks). |
Phase III |
|
ANBL0032 |
Randomized Study of Chimeric Antibody 14.18 (Ch14.18) in High-Risk Neuroblastoma Following Myeloablative Therapy and Autologous Stem Cell Rescue |
|
SOURCE: Personal communication, Jeffery Abrams, National Cancer Institute, March 2, 2010. |
|
the CCOPs.3 This allows the evaluation of therapies in settings more representative of current medical practice, because the majority of cancer treatment in the United States occurs in the community (Dignam, 2004), and ensures patient access to innovative therapies in settings other than academic medical centers or cancer centers. Additionally, the Cooperative Group Program enables international collaboration, fostering multiple joint protocols involving the Cooperative Groups, the European Organisation for Research and Treatment of Cancer, and other international trial sites (Corn et al., 2008; EORTC, 2009). The Cooperative Groups also provide a training ground for investigators, offering opportunities for mentorship, collaboration, and career advancement through participation in scientific steering committees.
CHALLENGES CONFRONTING THE COOPERATIVE GROUP PROGRAM
Despite the unique mission and history of accomplishments of the Cooperative Groups, the Program is facing numerous challenges that threaten its ability to undertake large-scale, innovative clinical trials that benefit patient care (these challenges are described briefly below; Chapters 2, 3, and 4 explore these challenges in greater detail). Many of these challenges stem from systems problems rather than scientific difficulties. Fundamental to these challenges is a clinical trials infrastructure that has not evolved to accommodate the rapid pace of biomedical discovery. Stagnant funding, inefficient processes, extensive and complex government oversight, and a growing trend toward the conduct of industry trials overseas have contributed to inadequate physician and patient participation in clinical trials, threatening the Cooperative Group Program’s ability to efficiently translate discoveries into clinical applications.
Problems with the current cancer clinical trials system are readily acknowledged by a number of stakeholders: clinical investigators, patient
|
3 |
CCOP enables community physicians to participate in and enroll patients in Cooperative Group Program clinical trials. See http://prevention.cancer.gov/programs-resources/programs/ccop. |
|
Evaluating maintenance therapy in high-risk neuroblastoma; no pharmaceutical company was involved in the development or production of the agent (ch14.18). |
Phase III |
advocates, Cooperative Group leadership, industry participants, as well as NCI. Multiple expert committees have been convened to study these issues and recommend paths forward. Evaluations of the Cooperative Group Program over the past decade, including the Armitage report and implementation committee (NCI, 1997), the Clinical Trials Working Group report (NCI, 2005), and the Translational Research Working Group report (NCI, 2007), acknowledge the many challenges that limit the ability of the Cooperative Groups to rapidly translate biomedical discoveries into clinical applications and have recommended changes to the Program (see Appendix A). However, for the most part, these reports have not yet resulted in transformative program changes. Current NCI activities include the implementation of several recommendations from the Clinical Trials Working Group and Translational Research Working Group reports that have the potential to address some of the recognized challenges.
Clinical Trial Costs Outstrip Program Funding
In recent years, NCI funding for the Cooperative Group Program has been flat and has been declining when the funding is adjusted for inflation. Proponents argue that the $145 million that NCI provides annually to the Program is far below the amount needed to sustain the clinical trials research infrastructure and to support the enrollment and follow-up of patients at clinical trial sites (IOM, 2009). In addition, clinical trials are becoming increasingly expensive as researchers design trials reflective of biomedical innovations, including imaging studies and correlative studies that require the collection, annotation, and storage of biospecimens, as well as biomarker analyses. With growing financial pressures, there are concerns that, as it currently operates, the Cooperative Group Program is unsustainable (see Chapter 3 for a more detailed analysis).
Inefficient Group Processes and Burdensome Oversight
Recent studies demonstrate that protocol activation in Cooperative Group trials is riddled with inefficiencies, leading to median activation
times of 600 to 800 days (Dilts and Sandler, 2006; Dilts et al., 2006, 2008, 2009). In addition to inefficiencies within the concept development process, extensive and overlapping oversight by NCI, institutional review boards, and FDA contributes to delays in activating trials. Collaborations among Cooperative Groups and industry sponsors largely remain nonstandardized, which also increases the time and complexity of clinical trial planning. Because science can change rapidly, the time that it takes to activate a new trial may render obsolete the research question that the clinical trial was designed to answer and threatens the relevance of Cooperative Group trials (see Chapter 3).
Inadequate Patient and Physician Involvement in Cancer Clinical Trials
Few patients and physicians participate in clinical trials for adult cancers. Of the 1.4 million patients with a new diagnosis of cancer in 2008 (ACS, 2008), it is estimated that no more than 5 percent of patients enrolled in clinical trials,4 with some estimates suggesting that less than 3 percent of patients enrolled in clinical trials (reviewed by ENACCT-CCPH, 2008). Likewise, reimbursement concerns, a lack of awareness of clinical trials, physician or patient preference for standard therapies, excessive regulatory burdens, and time constraints prevent many physicians from enrolling patients in clinical trials (C-Change and Coalition of Cancer Cooperative Groups, 2006; Mansour, 1994; Somkin et al., 2005). Because of the trend toward the use of targeted therapy and personalized medicine, clinical trials increasingly rely on stratified populations (see Chapter 2), which require large numbers of patients willing to participate. The low rate of involvement of physicians and patients in clinical trials slows accrual and prevents the Cooperative Groups from efficiently translating new knowledge into better patient care. Many trials never reach their accrual goals and thus generate no meaningful results to guide treatment (see Chapter 4 for more details).
Movement of Industry Trials Overseas
In part because of the difficulty of activating and conducting clinical trials in the United States, there is a growing trend for industry to conduct clinical trials overseas (Getz, 2007; Glickman et al., 2009; IOM, 2009). Cost savings and recruitment efficiencies are cited as the primary drivers of the globalization of clinical trials (Agres, 2005; Normile, 2008). With the movement of clinical trials, clinical investigators, and resources away from the United States, the ability of the United States to maintain a critical mass
of expertise to conduct clinical trials is questionable. Without the conduct of clinical trials in the United States, patients could lose access to promising new therapies as they develop, and in some cases, the results of clinical trials may have less relevance to U.S. patient populations (see Chapter 4).
ORIGIN OF THE STUDY
Recognizing the value of publicly sponsored cancer clinical trials and the challenges that currently confront the U.S. clinical trials system, the Institute of Medicine’s (IOM’s) National Cancer Policy Forum held two workshops to examine these issues and to obtain input from a diverse group of stakeholders. The first workshop, Improving the Quality of Cancer Clinical Trials, held on October 4 and 5, 2007, focused on the science underpinning clinical trials; collaborations among Cooperative Groups, industry, and academia; and the regulatory issues affecting clinical trial development, especially the early stages of development. The second workshop, Multi-Center Phase III Clinical Trials and NCI Cooperative Groups, held on July 1 and 2, 2008, explored the organization and operations of the Cooperative Group Program, patient and physician involvement in Cooperative Group research, and data collection requirements, as well as clinical trial cost and reimbursement issues. The proceedings of both workshops were published by the IOM as workshop summaries (IOM, 2008, 2009).
Invited speakers represented a diverse group of stakeholders, including NCI, FDA, the Centers for Medicare & Medicaid Services, Cooperative Group leadership, clinical investigators from academia and community practice, preclinical research scientists, biostatisticians, bioimaging and biomarker experts, industry participants, insurers, patient advocates, and cancer center administrators. Throughout the workshops, speakers conveyed the importance of Cooperative Group clinical trials in setting the standard of care for cancer treatment, prevention, and detection. However, speakers voiced a number of concerns over the current system, prompting the workshop chair, John Mendelsohn, to note that there was general agreement among workshop participants that the Cooperative Group Program is approaching a state of crisis (IOM, 2009). Other presenters discussed ways in which innovative trial designs, therapeutic combinations, drug-diagnostic codevelopment, molecular imaging, and correlative science have the potential to significantly improve cancer care if the barriers are appropriately addressed.
Based on the input received from these workshops, the director of NCI, John Niederhuber, requested that the IOM conduct a consensus study of cancer clinical trials and the Cooperative Group Program. Funding was obtained from NCI, the Centers for Disease Control and Prevention, the
American Cancer Society, the American Society of Clinical Oncology, the Association of American Cancer Institutes, and C-Change.
COMMITTEE APPOINTMENT AND CHARGE
The NCI asked the IOM to examine a broad a number of topics relevant to cancer clinical trials and the organization and operation of the Cooperative Group Program and to make recommendations that could improve the quality of cancer clinical trials conducted through the program (Box 1-3). To address the charge, the IOM appointed a 17-member committee whose members had a broad range of expertise and experience. Among these individuals were experts in biomedical research, clinical investigation in academia and community practice, statistics, radiology, research and development in the biotechnology and pharmaceutical industries, management research, systems engineering, the health insurance industry, and patient advocacy.
|
BOX 1-3 Committee Statement of Task An IOM committee will review the organization and operation of the National Cancer Institute (NCI) Clinical Trials Cooperative Group Program and recommend ways to improve the quality of cancer clinical trials conducted by the groups. Attention will be focused on how to improve, modernize, and streamline the process, with special consideration given to the recent emphasis on targeted therapies due to an improved understanding of the biology of cancer. Given the limits on funding for cancer clinical trials, there is a particular need to improve efficiency and make efficient use of time, effort, and resources. Specifically, the committee will recommend ways to
|
THE COMMITTEE’S VISION FOR CANCER CLINICAL TRIALS IN 2015
The committee recognized that the numerous reviews of the Cooperative Group Program have not resulted in transformative programmatic change. Indeed, a recently published commentary stated that “[i]ts awkward present form evolved because of decades of tinkering with administrative structures at NCI and the National Institutes of Health, reactions to specific events or perceived risks, and changing needs of various governmental and nongovernmental stakeholders” (Steensma, 2009).
With the goal of providing recommendations that result in systemic change, the committee took a broad view of the clinical trials process rather than simply focusing on NCI’s role. The committee defined the current system’s inadequacies in terms of missed opportunities, misaligned incentives, and collective challenges. Many aspects of the clinical trials infrastructure have not changed dramatically since the 1950s, whereas biomedical discoveries and technology development have been advancing rapidly in recent years. The collective environment in which clinical trials are conducted influences the pace of clinical advances.
The committee then described the needs of an ideal cancer clinical trials system of the near future, circa 2015 (see Box 1-4). The committee envisions a dynamic system that could efficiently respond to emerging scientific knowledge, involve the broad cooperation of stakeholders, and leverage evolving technologies that could provide high-quality, practice-changing research. Clinical trial participation would be the preferred option for patients and physicians because it would provide access to innovative therapies that reflect patient preferences and that are appropriately reimbursed.
This list of ideal characteristics laid the groundwork for the committee deliberations to develop goals and specific recommendations to achieve them. The committee concluded that the academic, governmental, and commercial sectors must join with the public to develop a 21st-century clinical trials system to more effectively leverage scientific advancements and translate them into public health benefits by improving the science, technology, efficiency, and timely completion of the very best cancer clinical trials.
THE COMMITTEE’S CONCLUSIONS AND RECOMMENDATIONS
The committee reviewed the available published literature and obtained input from experts in the field, interested individuals, and institutions to formulate its recommendations. The committee’s recommendations support four main goals for achieving the ideal vision of cancer clinical trials: (1) improve the speed and efficiency of clinical trial design, launch, and conduct, (2) incorporate innovative science and trial design in cancer clini-
|
BOX 1-4 Needs for Cancer Clinical Trials in 2015 Rapid translation of scientific discoveries into public health benefit
A strong publicly supported clinical trials system in the United States that complements commercial trials to develop drugs and devices
A robust, standardized, and accessible clinical trials infrastructure
|
cal trials, (3) improve selection, support, and completion of cancer clinical trials, and (4) incentivize participation of patients and physicians in clinical trials.
ORGANIZATION OF THE REPORT
Chapter 2 provides an overview of the science underpinning the development of cancer therapies and the challenges that must be overcome to achieve the goals of personalized medicine for cancer.
Chapter 3 provides an overview of the structure, organization, and funding of cancer clinical trials and the Cooperative Group Program. It also delineates the inefficiencies in the current system and discusses the collaborative nature of cancer clinical trials.
Harmonized and synchronized rules and guidelines across federal regulatory agencies
Support for clinical investigators
Broad patient involvement in clinical trials
|
Chapter 4 examines the incentives and disincentives for participation in cancer clinical trials, for both patients and clinicians.
Appendix A reviews the recommendations from past evaluations of the Cooperative Group Program and ongoing changes in response to those recommendations. It also includes a summary of the recommendations made in March 2010 by the NCI-appointed Operational Efficiency Working Group.
REFERENCES
ACS (American Cancer Society). 2008. Cancer Facts & Figures 2008. Atlanta, GA: American Cancer Society.
Agres, T. 2005. Clinical trials trickling away. Drug Discovery and Development 2005 Quarter 3(7), http://www.dddmag.com/clinical-trials-trickling-away.aspx (accessed January 22, 2010).
C-Change and Coalition of Cancer Cooperative Groups. 2006. Enhancing Cancer Treatment Through Improved Understanding of the Critical Components, Economics and Barriers of Cancer Clinical Trials. Washington, DC: C-Change; Philadelphia, PA: Coalition of Cancer Cooperative Groups.
Collyar, D. 2008. An essential partnership: Patient advocates and cooperative groups. Seminars in Oncology 35(5):553–555.
Coltman, C. A. 2008. The Southwest Oncology Group: Progress in cancer research. Seminars in Oncology 35(5):545–552.
Corn, B. W., I. D. Wexler, M. Suntharalingam, M. Inbar, W. J. Curran, Jr. 2008. Globalization of the Radiation Therapy Oncology Group: Implementation of a model for service expansion and public health improvement. Journal of Clinical Oncology 26(7):1160–1166.
CTEP (Cancer Therapy Evaluation Program). 2002. Clinical Trials Cooperative Groups: Accomplishments. Bethesda, MD: National Cancer Institute.
CTEP. 2006. National Cancer Institute Clinical Trials Cooperative Group Program guidelines. Bethesda, MD: National Cancer Institute.
Dignam, J. J. 2004. The role of cancer Cooperative Groups within the spectrum of cancer care. Cancer Control 11(1):55–63.
Dillman, R. O., J. Herndon, S. L. Seagren, W. L. Eaton, Jr., and M. R. Green. 1996. Improved survival in stage III non-small-cell lung cancer: Seven year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. Journal of the National Cancer Institute 88(17):1210–1215.
Dilts, D. M., and A. B. Sandler. 2006. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. Journal of Clinical Oncology 24(28):4545–4552.
Dilts, D. M., A. B. Sandler, M. Baker, S. K. Cheung, S. L. George, K. S. Karas, S. McGuire, G. S. Menon, J. Reusch, D. Sawyer, M. Scoggins, A. Wu, K. Zhou, and R. L. Schilsky. 2006. Processes to activate Phase III clinical trials in a cooperative oncology group: The case of Cancer and Leukemia Group B. Journal of Clinical Oncology 24(28):4553–4557.
Dilts, D. M., A. B. Sandler, S. Cheng, J. Crites, L. Ferranti, A. Wu, R. Gray, J. MacDonald, D. Marinucci, and R. Comis. 2008. Development of clinical trials in a cooperative group setting: The Eastern Cooperative Oncology Group. Clinical Cancer Research 14:3427–3433.
Dilts, D. M., A. B. Sandler, S. K. Cheng, J. S. Crites, L. B. Ferranti, A. Y. Wu, S. Finnigan, S. Friedman, M. Mooney, and J. Abrams. 2009. Steps and time to process clinical trials at the Cancer Therapy Evaluation Program. Journal of Clinical Oncology 27(11):1761–1766.
Doroshow, J. H. 2008. Commentary. Publishing cancer clinical trial results: A scientific and ethical imperative. The Oncologist 13(9):930–932.
ENACCT-CCPH (Education Network to Advance Cancer Clinical Trials-Community-Campus Partnerships for Health). 2008. Communities as Partners in Cancer Clinical Trials: Changing Research, Practice, and Policy. Seattle, WA: Community-Campus Partnerships for Health; Bethesda, MD: Education Network to Advance Cancer Clinical Trials.
EORTC (European Organisation for Research and Treatment of Cancer). 2009. EORTC-US National Cancer Institute (NCI) Collaboration. http://www.eortc.be/about/Directory2009-2010/13EORTC-NCICollaboration.htm (accessed March 10, 2010).
Fisher, B., E. Montague, C. Redmond, B. Barton, D. Borland, and E. R. Fisher. 1977. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer 39(Suppl. 6): 2827–2839.
Fisher, B., M. Bauer, R. Margolese, R. Poisson, Y. Pilch, C. Redmond, E. R. Fisher, N. Wolmark, M. Deutsch, and E. Montague. 1985. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. New England Journal of Medicine 312(11): 665–673.
Fisher, B., J. P. Costantino, D. L. Wickerham, C. Redmond, M. Kavanah, M. Cronin, V. Vogel, A. Robidoux, N. Dimitrov, J. Atkins, M. Daly, S. Wieand, E. Tan-Chiu, L. Ford, and N. Wolmark. 1998. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute 90(18):1371–1388.
Fisher, B., S. Anderson, J. Bryant, R. Margolese, M. Deutsch, E. R. Fisher, J. Jeong, and N. Wolmark. 2002a. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine 347(16):1233–1241.
Fisher, B., J. Jeong, S. Anderson, J. Bryant, E. R. Fisher, and N. Wolmark. 2002b. Twenty-five year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. New England Journal of Medicine 347(16):567–575.
Getz, K. A. 2007. Global clinical trials activity in the details. Applied Clinical Trials September 1, 2007:42–44. http://appliedclinicaltrialsonline.findpharma.com/appliedclinicaltrials/Regulatory/Global-Clinical-Trials-Activity-in-the-Details/ArticleStandard/Article/detail/453243 (accessed April 8, 2009).
Giantonio, B. J., P. J. Catalano, N. J. Meropol, P. J. O’Dwyer, P. H. Mitchell, S. R. Alberts, M. A. Schwartz, and A. B. Benson. 2007. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 25(12):1539–1544.
Giantonio, B. J., A. A. Forastiere, and R. Comis. 2008. The role of the Eastern Cooperative Oncology Group in establishing standards of cancer care: Over 50 years of progress through clinical research. Seminars in Oncology 35(5):494–506.
Glickman, S. W., J. G. McHutchison, E. D. Peterson, C. B. Cairns, R. A. Harrington, R. M. Califf, and K. A. Schulman. 2009. Ethical and scientific implications of the globalization of clinical research. New England Journal of Medicine 360(8):816–823.
Green, M. R., S. L. George, and R. L. Schilsky. 2008. Tomorrow’s cancer treatments today: The first 50 years of the Cancer and Leukemia Group B. Seminars in Oncology 35(5):470–483.
Grothey, A., A. A. Adjei, S. R. Alberts, E. A. Perez, K. A. Jaeckle, C. L. Loprinzi, D. J. Sargent, J. A. Sloan, and J. C. Buckner. 2008. North Central Cancer Treatment Group—Achievements and perspectives. Seminars in Oncology 35(5):530–544.
Haller, D. G., P. J. Catalano, J. MacDonald, M. A. O’Rourke, M. S. Frontiera, D. V. Jackson, and R. J. Mayer. 2005. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. Journal of Clinical Oncology 23(34):8671–8678.
Henderson, I. C., D. A. Berry, G. D. Demetri, C. T. Cirrincione, L. J. Goldstein, S. Martino, J. N. Ingle, M. R. Cooper, D. F. Hayes, K. H. Tkaczuk, G. Fleming, J. F. Holland, D. B. Duggan, J. T. Carpenter, E. Frei III, R. L. Schilsky, W. C. Wood, H. B. Muss, and L. Norton. 2003. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. Journal of Clinical Oncology 21(6):976–983.
Hillman, B. J., and C. Gatsonis. 2008. The American College of Radiology Imaging Network—Clinical trials of diagnostic imaging and image-guided treatment. Seminars in Oncology 35(5):460–469.
Hoogstraten, B. 1980. Preface. In Cancer Research: Impact of the Cooperative Groups, edited by B. Hoogstraten, D. P. Carbone, J. R. Durant, B. Fisher, G. D. Hammond, J. F. Holland, and S. Kramer. New York: Masson Publishing USA, Inc.
International Myeloma Foundation. 2007. The International Myeloma Foundation Says ECOG Results Could Improve Survival While Reducing Side Effects for Many Patients. http://myeloma.org/ArticlePage.action?articleId=2056 (accessed November 30, 2009).
IOM (Institute of Medicine). 2008. Improving the Quality of Cancer Clinical Trials: Workshop Summary. Washington, DC: The National Academies Press.
IOM. 2009. Multi-Center Phase III Clinical Trials and NCI Cooperative Groups: Workshop Summary. Washington, DC: The National Academies Press.
Keating, P., and A. Cambrosio. 2002. From screening to clinical research: The cure of leukemia and the early development of the Cooperative Oncology Groups, 1955–1966. Bulletin of the History of Medicine 76(2):299–334.
Keys, H. M., B. Bundy, F. B. Stehman, L. I. Muderspach, W. E. Chafe, C. L. Suggs, J. L. Walker, and D. Gersell. 1990. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine 340(15):1154–1161.
Kirkwood, J. M., J. Manola, J. Ibrahim, V. Sondak, M. S. Ernstoff, and U. Rao. 2004. A pooled analysis of Eastern Cooperative Oncology Group and intergroup trials of adjuvant high-dose interferon for melanoma. Clinical Cancer Research 10(5):1670–1677.
MacDonald, J., S. R. Smalley, J. Benedetti, S. A. Hundahl, N. C. Estes, G. N. Stemmermann, D. G. Haller, J. A. Ajani, L. L. Gunderson, J. M. Jessup, and J. A. Martenson. 2001. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach gastroesophageal junction. New England Journal of Medicine 345(10):725–730.
Mamounas, E. P., S. Weiand, N. Wolmark, H. D. Bear, J. Atkins, K. Song, J. Jones, and H. Rockette. 1999. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: Results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, C-04). Journal of Clinical Oncology 17(5):1349–1355.
Mansour, E. G. 1994. Barriers to clinical trials. Part III. Knowledge and attitudes of health care providers. Cancer 74(Suppl. 9):2672–2675.
Mauer, A. M., E. S. Rich, and R. L. Schilsky. 2007. The role of cooperative groups in cancer clinical trials. Cancer Treatment and Research 132:111–129.
Moertel, C. G., T. R. Fleming, J. S. Macdonald, D. G. Haller, J. A. Laurie, P. J. Goodman, J. S. Ungerleider, W. A. Emerson, D. C. Tormey, and J. H. Glick. 1990. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. New England Journal of Medicine 322(6):352–358.
Moertel, C. G., T. R. Fleming, J. MacDonald, D. G. Haller, J. A. Laurie, C. M. Tangen, J. S. Ungerleider, W. A. Emerson, D. C. Tormey, and J. H. Glick. 1995. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. Journal of Clinical Oncology 13(12):2936–2943.
Morris, M., P. J. Eifel, J. Lu, P. W. Grigsby, C. Levenback, R. E. Stevens, M. Rotman, D. M. Gershenson, and D. G. Mutch. 1999. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New England Journal of Medicine 340(15):1137–1143.
NCI (National Cancer Institute). 1997. Report of the National Cancer Institute Clinical Trials Program Review Group. Bethesda, MD: National Cancer Institute. http://deainfo.nci.nih.gov/ADVISORY/bsa/bsa_program/bsactprgmin.htm#8a (accessed November 19, 2008).
NCI. 2003. Decades of Progress 1983 to 2003: Community Clinical Oncology Program. Bethesda, MD: National Cancer Institute.
NCI. 2005. Restructuring the National Cancer Clinical Trials Enterprise. Report of the Clinical Trials Working Group of the National Cancer Advisory Board. Bethesda, MD: National Cancer Institute.
NCI. 2007. Transforming Translation—Harnessing Discovery for Patient And Public Benefit. Report of the Translational Research Working Group of the National Cancer Advisory Board. Bethesda, MD: National Cancer Institute.
Normile, D. 2008. The promise and pitfalls of clinical trials overseas. Science 322(5899): 214–216.
O’Leary, M., M. Krailo, J. R. Anderson, and G. H. Reaman. 2008. Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Seminars in Oncology 35(5):484–493.
Omura, G. A. 2008. Progress in gynecologic cancer research: The Gynecologic Oncology Group experience. Seminars in Oncology 35(5):507–521.
Peters, W. A., III, P. Y. Liu, R. J. Barrett, II, R. J. Stock, B. J. Monk, J. S. Berek, L. Souhami, P. Grigsby, W. Gordon, Jr., and D. S. Alberts. 2000. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of Clinical Oncology 18(8):1606–1613.
PhRMA (Pharmaceutical Research and Manufacturers of America). 2009. Medicines in Development for Cancer 2009. Washington, DC: Pharmaceutical Research and Manufacturers of America.
Pisano, E., C. Gatsonis, R. E. Hendrick, M. Yaffe, J. Baum, S. Archayya, E. F. Conant, L. L. Fajardo, L. Bassett, C. D’Orsi, R. Jong, and M. Rebner. 2005. Diagnostic performance of digital vs. film mammography for breast cancer screening. New England Journal of Medicine 353(17):1773–1783.
Rajkumar, S. V., E. Blood, D. Vesole, R. Fonseca, and P. R. Greipp. 2006. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 24(3):431–436.
Ramsey, S., and J. Scoggins. 2008. Practicing on the tip of an information iceberg? Evidence of underpublication of registered clinical trials in oncology. The Oncologist 13(9):925–929.
Reaman, G. 2009. Children’s Oncology Group: A national/international infrastructure for pediatric cancer clinical translational research. Presentation to the Institute of Medicine Committee on Cancer Clinical Trials and the NCI Cooperative Group Program, April 23, 2009, Washington, DC.
Romond, E. H., E. A. Perez, J. Bryant, V. J. Suman, C. E. Geyer, N. E. Davidson, E. Tan-Chiu, S. Martino, S. Paik, P. A. Kaufman, S. M. Swain, T. M. Pisansky, L. Fehrenbacher, L. A. Kutteh, V. G. Vogel, D. W. Visscher, G. Yothers, R. B. Jenkins, A. M. Brown, S. R. Dakhil, E. P. Mamounas, W. L. Lingle, P. M. Klein, J. N. Ingle, and N. Wolmark. 2005. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine 353(16):1673–1684.
Rose, P. G., B. Bundy, E. B. Watkins, J. T. Thigpen, G. Deppe, M. A. Maiman, D. L. Clarke-Pearson, and S. Insalaco. 1999. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine 340(15):1144–1153.
RTOG (Radiation Therapy Oncology Group). 2009. A Short History of RTOG. Philadelphia, PA: Radiation Therapy Oncology Group. http://www.rtog.org/history.html (accessed November 9, 2009).
Sandler, R. S., S. Halabi, J. A. Baron, S. Budinger, E. Paskett, R. Keresztes, N. Petrelli, M. Pipas, D. D. Karp, C. L. Loprinzi, G. Steinbach, and R. Schilsky. 2003. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine 348(10):883–890.
Shaw, E., R. Arusell, B. Scheithauer, J. O’Fallon, B. O’Neill, R. Dinapoli, D. Nelson, J. Earle, C. Jones, T. Cascino, D. Nichols, R. Ivnik, R. Hellman, W. Curran, and R. Abrams. 2002. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 20(9):2267–2276.
Somkin, C. P., A. Altschuler, L. Ackerson, A. M. Geiger, S. M. Greene, J. Mouchawar, J. Holup, L. Fehrenbacher, A. Nelson, A. Glass, J. Polikoff, S. Tishler, C. Schmidt, T. Field, and E. Wagner. 2005. Organizational barriers to physician participation in cancer clinical trials. The American Journal of Managed Care 11(7):413–421.
Steensma, D. P. 2009. The ordinary miracle of cancer clinical trials. Journal of Clinical Oncology 27(11):1737–1739.
Strauss, G. M., J. Herndon, M. A. Maddaus, D. W. Johnstone, E. A. Johnson, D. M. Watson, D. J. Sugarbaker, R. L. Schilsky, and M. R. Green. 2004. Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage 1B non-small cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) Protocol 9633. Journal of Clinical Oncology 22(14 Suppl.):7019.
Thompson, I. M., P. J. Goodman, C. M. Tangen, M. S. Lucia, G. J. Miller, L. G. Ford, M. M. Lieber, R. D. Cespedes, J. Atkins, S. M. Lippman, S. M. Carlin, A. Ryan, C. M. Szczepanek, J. Crowley, and C. A. Coltman. 2003. The influence of finasteride on the development of prostate cancer. New England Journal of Medicine 349(3):215–224.
Vogel, V., J. P. Costantino, D. L. Wickerham, M. Cronin, R. S. Cecchini, J. Atkins, T. B. Bevers, L. Fehrenbacher, E. R. Pajon, J. L. Wade III, A. Robidoux, R. Margolese, J. James, S. M. Lippman, C. D. Runowicz, P. A. Ganz, S. E. Reis, W. McCaskill-Stevens, L. G. Ford, V. C. Jordan, and N. Wolmark. 2006. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. Journal of the American Medical Association 295:2727–2741.
Whitney, C., W. Sause, B. Bundy, J. H. Malfetano, E. V. Hannigan, W. C. Fowler, Jr., D. L. Clarke-Pearson, and S. Y. Liao. 1999. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. Journal of Clinical Oncology 17(5):1339–1348.
Wickerham, D. L., M. J. O’Connell, J. P. Costantino, W. M. Cronin, S. Paik, C. E. Geyer, P. A. Ganz, N. Petrelli, E. P. Mamounas, T. B. Julian, and N. Wolmark. 2008. The half century of clinical trials of the National Surgical Adjuvant Breast and Bowel Project. Seminars in Oncology 35(5):522–529.
Winton, T., R. B. Livingston, D. Johnson, J. Rigas, M. Johnston, C. Butts, Y. Cormier, G. Goss, R. Inculet, E. Vallieres, W. Fry, D. Bethune, J. Ayoub, K. Ding, L. Seymour, B. Graham, M.-S. Tsao, D. Gandara, K. Kesler, T. Demmy, and F. Shepherd. 2005. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. New England Journal of Medicine 352(25):2589–2597.
Woodcock, J. 2009. FDA Perspective on Evidence for Regulatory Approval in Cancer. Presented at the National Cancer Policy Forum workshop on Assessing and Improving the Value in Cancer Care, February 9, 2009, Washington, DC.
Zubrod, C. G. 1984. Origins and development of chemotherapy research at the National Cancer Institute. Cancer Treatment Reports 68(1):9–19.




































