1
Introduction
Evidence that climate is changing—including increasing global temperatures, melting glaciers, rising sea level, increasingly severe weather, and shifting seasons and animal migration patterns—is driving national and international discussions on reducing anthropogenic greenhouse gas emissions, the primary cause of climate change. The principal international framework for greenhouse gas reductions is the United Nations Framework Convention on Climate Change (UNFCCC), which is aimed at “stabilization of greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system” (United Nations, 1992, p. 4). The greenhouse gases covered by the UNFCCC include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), sulfur hexafluoride (SF6), perfluorocarbons (PFCs), and hydrofluorocarbons (HFCs).1 In 1997, the parties to the UNFCCC approved the Kyoto Protocol, which contains binding emissions targets for developed countries (United Nations, 1998). The United States is not a party to the Kyoto Protocol, but it is considering a variety of proposals for reducing emissions to mitigate adverse effects of climate change, including an international climate treaty.2
For any international agreement to limit greenhouse gas emissions, monitoring and verification of emissions will be essential to assess the effectiveness of emissions reductions and overall compliance with the terms of the treaty and to give nations confidence that their neighbors are also living up to their commitments. As former president Ronald Reagan said: “Trust but verify.” Emissions verification will also be important for correcting errors in reporting.
DOMAIN OF THE REPORT
This report examines methods for estimating anthropogenic greenhouse gas emissions and for observing their changes over time (see committee charge in Box 1.1). The report asks: How accurate is each method for estimating greenhouse gas emissions? How well can emissions reductions required under a climate treaty be monitored? What new measurement
|
BOX 1.1 Committee Charge The study will review current methods and propose improved methods for estimating and verifying greenhouse gas emissions at different spatial (e.g., national, regional, global) and temporal (e.g., annual, decadal) scales. The greenhouse gases to be considered are carbon dioxide, chlorofluorocarbons (CFCs), hydrofluorocarbons (HFCs), nitrous oxide, methane, and perfluorinated hydrocarbons (PFCs). Emissions of soot and sulfur compounds along with precursors of tropospheric ozone may also be considered. The results would be useful for a variety of applications, including carbon trading, setting emissions reduction targets, and monitoring and verifying international treaties on climate change. |
|
1 |
A separate treaty, the Montreal Protocol on Substances That Deplete the Ozone Layer, covers chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). |
|
2 |
International negotiations are intended to culminate in an agreement at a future Conference of the Parties to the UNFCCC, see <http://unfccc.int/2860.php>. |
methods could be developed within a few years to independently verify emissions estimates?
The focus of this report is on monitoring and verification of the emissions themselves (see definitions in Box 1.2), rather than on implementation of policies designed to control them. The scales of interest range from national to global and from annual to decades. Although some of the methods described in this report have sufficiently high resolution to be used to audit individual emissions sources, which may be of inter-
|
BOX 1.2 Definitions of Terms Used in the Report Activity data—Data on the magnitude of a human activity resulting in emissions or removals during a given period of time. Examples include data on energy use, metal production, management systems, forest clearing, and fertilizer use. Annex I countries—The 41 countries included in Annex I (as amended in 1998) to the UNFCCC, including industrialized countries that were members of the Organisation for Economic Co-operation and Development in 1992 and many countries with economies in transition. Under the convention, Annex I countries committed to returning individually or jointly to their 1990 levels of greenhouse gas emissions by 2000. By default, the other countries are referred to as non-Annex I countries. Anthropogenic emissions—Emissions of greenhouse gases, precursors of greenhouse gases, and aerosols resulting from human activities. Because it is difficult to disentangle anthropogenic and natural components of emissions and removals from land use, the UNFCCC considers emissions and removals on managed lands as anthropogenic. CO2equivalent—The amount of carbon dioxide emission that would cause the same integrated radiative forcing, over a given time horizon, as an emitted amount of a well-mixed greenhouse gas. It is a standard metric for comparing emissions of different greenhouse gases, but does not imply exact equivalence of the corresponding climate change responses. The 100-year global warming potential is used to calculate CO2 equivalents. Emission factor—The rate of emission per unit of activity, output, or input. For example, a particular fossil-fuel power plant may have a CO2 emission factor of 0.765 kg CO2 kWh–1 generated. Inventory—An accounting of an item of interest at a specified date.
Inverse model—A model in which observations are used to infer the values of the parameters characterizing the system under investigation. In this report, inverse models are used to infer sources and sinks for a greenhouse gas from measurements of the atmospheric or oceanic abundance of that gas. Monitoring—The observation of emissions or variables correlated with emissions for the purpose of detecting any changes that may occur over time. Sector—An emission-producing segment of the economy. The Intergovernmental Panel on Climate Change (IPCC) currently specifies four sectors for greenhouse gas reporting: energy; industrial processes and product use; agriculture, forestry, and other land use; and waste. Sink—Any process, activity, or mechanism that removes a greenhouse gas, an aerosol, or a precursor of a greenhouse gas or aerosol from the atmosphere. Removals of greenhouse gases by a sink are conventionally shown as negative emissions. Source—Any process, activity, or mechanism that releases a greenhouse gas, an aerosol, or a precursor of a greenhouse gas or aerosol into the atmosphere. Certain activities, such as forestry, can be both a source and a sink of greenhouse gas emissions. Survey data—Data from a statistically representative sample. Tracer-transport model—A model used to predict the movement of greenhouse gases in the atmosphere or dissolved substances in the oceans. Verification—An independent examination of monitoring data to help establish whether or not a country’s actual emissions are consistent with its obligations under a climate treaty. SOURCES: Adapted from IPCC glossaries (<http://www.ipcc.ch/>) and UNFCCC resources (<http://unfccc.int/2860.php>). |
est for trading schemes or offset projects, the report focuses on the national emission totals that include these activities. Only public domain data (not classified or commercial data) are considered because confidence in a treaty relies on open data for transparency and scientific scrutiny.
Greenhouse Gases
This report considers the anthropogenic greenhouse gases required by the committee charge—CO2, CH4, N2O, CFCs, HFCs, and PFCs—and SF6, but not the optional soot or precursors of tropospheric ozone. The greenhouse gases required by the committee charge, along with SF6, are currently covered by international agreements (CFCs under the Montreal Protocol and the others under the UNFCCC) and were the targets of negotiations at the 2009 United Nations Climate Change Conference (COP 15) in Copenhagen. Thus, there is an immediate practical need to verify emissions of the gases included in this report, which does not extend to the greenhouse agents that were omitted. The short-lived greenhouse agents (soot and other aerosols, aerosol precursors, and precursors to tropospheric ozone) are not covered by international agreements, although many countries have a highly developed capability to monitor them to support air pollution regulations. A comparable capability for the greenhouse gases discussed in this report does not exist.
The focus of international agreements on CO2, CH4, N2O, CFCs, HFCs, PFCs, and SF6 is likely to continue for three reasons. First, these gases are collectively more important greenhouse agents than soot, sulfur compounds, and precursors of tropospheric ozone (Figure 1.1). Commonly cited mitigation targets, such as a maximum of 2C of warming or a maximum concentration of 450 parts per million (ppm) CO2 equivalent, cannot be achieved without large reductions in emissions of CO2, CH4, N2O, CFCs, HFCs, PFCs, and SF6. Second, the gases included in the report are long-lived in the atmosphere (decades to millennia or more), whereas the omitted gases and soot are short-lived (less than a year). Longevity in the atmosphere means that delayed mitigation is costly—CO2 emissions today will add to global climate change for centuries. In contrast, short-lived greenhouse agents
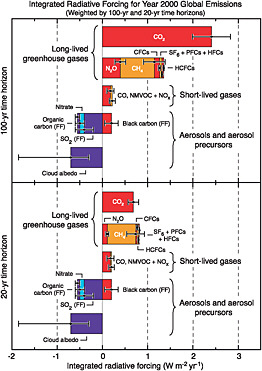
FIGURE 1.1 The relative importance of emissions of anthropogenic greenhouse gases and soot (black carbon) and other aerosols. The bars show the 20- (lower panel) and 100-year (upper panel) radiative forcing of emissions in 2000. SOURCE: Figure 2.22 from IPCC (2007a), Cambridge University Press.
do not entail the same penalties for delay. Because they are removed from the atmosphere in less than a year, today’s emissions will have a smaller impact on global warming in coming decades when the problem becomes most acute. Third, the net radiative forcing from the emission of short-lived gases and aerosols depends greatly on the location and timing of emissions. The time required for air to mix globally is on the order of 2 weeks in the east-west direction and 1 year in the north-south direction across the equator, which is less than the lifetime of short-lived greenhouse agents. For this and other reasons, the greenhouse impact of the ozone precursor NOx (nitrogen oxide) can vary by a factor of 10, depending on whether it is emitted in northern Europe or in the tropics (Wild et al., 2001; see also Table 2.15 of Forster et al., 2007). This makes
it difficult to design a practical international agreement to monitor them as greenhouse agents.
Of the long-lived greenhouse gases included under the UNFCCC, CO2 is responsible for 77 percent of the greenhouse forcing on a 100-year time horizon (Figure 1.2). For this reason, the report devotes considerably more space to CO2 than to the other gases. Note, however, that if we include the short-lived gases and soot and adopt a 20-year horizon, the contribution of CO2 falls to less than 40 percent.
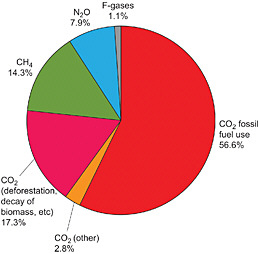
FIGURE 1.2 Global anthropogenic greenhouse gas emissions in 2004. The F-gases include HFCs, PFCs, and SF6. The area in the pie diagram shows 2004 emissions of the gases covered by the UNFCCC, weighted by their 100-year radiative forcing. SOURCE: Figure 1.1b from IPCC (2007b), Cambridge University Press.
Methods of Monitoring
This report evaluates three categories of monitoring approaches: national inventories, satellite measurements of land use, and atmospheric methods.
National Inventories. Under the UNFCCC, Annex I (developed) countries are required to report annual anthropogenic emissions and removals of greenhouse gases. Developing countries also report national inventories, but less frequently and in far less detail than developed countries. The emissions estimates are based on measurements of human activities (i.e., data such as cement or coal-fired electricity production) and corresponding emission factors (see definitions in Box 1.2). Because future international agreements are likely to build on this foundation, the committee evaluates UNFCCC inventory methods and extensions of them that would improve their comprehensiveness and accuracy and increase the rigor of self-reporting. The committee also discusses the capacity building necessary to procure regular inventories from developing countries.
Remote Sensing of Land Use. Greenhouse gas emissions and sequestration from land use are difficult to estimate because they have the same chemical signature as much larger background sources and sinks in the natural biosphere and because they are thinly spread over an enormous area. The dominant sources of land-use emissions are from forestry (primarily tropical deforestation and forest degradation) and agriculture. Land-use emissions in parts of the temperate zone are negative (i.e., net removals by sinks) due to net forest regrowth and other processes (Pacala et al., 2007). Because deforestation is the second largest source of anthropogenic CO2 (the first is fossil-fuel combustion) and because forest conservation and planting are likely to be important mitigation activities in the future, this report devotes considerable attention to methods for monitoring forest cover and structure by satellites. A comparable understanding of N2O and CH4 emissions from crop-lands and grasslands does not exist, both because of the diversity of agricultural practices and because we lack the technology to measure the dominant emissions sources remotely. For these reasons, it remains difficult to provide a useful check on self-reporting of emissions from agriculture, except in specific instances.
Atmospheric Methods. A global network of surface monitoring stations, aircraft, balloons, and satellites routinely measures greenhouse gas abundances in the atmosphere and oceans. Models of the atmosphere and/or oceans are used to estimate greenhouse gas emissions from the abundance data, a method known as tracer-transport inversion. An emissions source located between two monitoring stations will cause the concentration of the gas to be higher at the downwind station than the upwind station. How much higher depends on
both the strength of the source and the pattern of air flow, including wind speed, direction, and turbulence. Thus, to produce emissions estimates from abundance data, one needs an atmospheric model to reconstruct the three-dimensional pattern of air and water flow and mixing around the globe. For this reason, the report devotes considerable space to uncertainties in atmospheric transport models.
The report also evaluates extensions of the atmospheric sampling network that could significantly improve our ability to estimate national emissions and emissions trends. These include measurements of concentrations that would fill spatial gaps in the current sampling grid—for example, samples taken near large sources such as power plants and municipalities that were avoided when the current sampling network was established.
Uncertainty
This report evaluates uncertainties in annual emissions estimates derived from the three monitoring methods described above. In some cases, standard statistical methods can be used to evaluate the uncertainties, but in others, standard methods cannot be applied because our underlying scientific understanding is too incomplete or our measurement capabilities are insufficient. In such cases, we rely on other methods, including expert judgment, that are specified in tables of uncertainty estimates. Uncertainties are categorized in five bins—0-10 percent, 10-25 percent, 25-50 percent, 50-100 percent, and >100 percent (for the last category, it is unclear whether the activity is a source or a sink)—to facilitate cross-comparison between estimates from different methods. An uncertainty of 10 percent means that measurements are accurate to within 10 percent of the true value. Unless indicated otherwise, uncertainties are reported for two standard deviations of the mean (2 or 95 percent confidence interval).
Uncertainties in decadal changes can be computed from the values for annual emissions using standard time-series methods, including simple regression, for calculating the uncertainty of regression slopes. A reasonable expectation is that uncertainties in the decadal change of emissions will be lower than the annual uncertainty.
OVERVIEW OF GREENHOUSE GAS EMISSIONS
Relative Contribution to Climate Change
A greenhouse gas’s instantaneous tendency to change the climate is measured by its radiative forcing, which multiplies the increased abundance of the gas caused by anthropogenic emissions and the gas’s potency as a greenhouse agent. Of the four groups of gases considered in this report, CO2 has the largest radiative forcing (1.66 W m–2 for emissions up to the end of 2005), followed by CH4 (0.48 W m–2), the HCFCs and CFCs (collectively 0.32 W m–2), N2O (0.16 W m–2), and the HFCs, PFCs, and SF6 (collectively 0.02 W m–2; see Forster et al., 2007).
Longevity in the Atmosphere
The longevity of a greenhouse gas in the atmosphere is important because it determines the number of years that today’s emissions will affect climate. Short-lived gases, such as the precursors to tropospheric ozone, are rapidly cleared from the atmosphere; thus, the perturbation caused by emissions appears to adjust rapidly to a change in emissions. However, short-lived chemically reactive gases are coupled with the longer-lived greenhouse gases and thus produce long-lived perturbations to radiative forcing that take decades to reach a steady state (Wild et al., 2001). For even small levels of anthropogenic emissions, the atmospheric abundances of very long-lived gases, such as the PFCs, will continue to rise in proportion to emissions and remain well below the steady-state value at which annual emissions are balanced by annual removals. To eventually halt climate changes caused by greenhouse gases, their abundances in the atmosphere must be stabilized.
The lifetime of CO2 in the atmosphere cannot be ascribed a single value because the carbon cycle consists of a series of interacting reservoirs, each with a different time scale (see Figure 1.3). For example, although land ecosystems or the oceans take up approximately one-sixth of the CO2 in the atmosphere every year, they also return almost the same amount (IPCC, 2007a). Thus, the lifetime of a pulse increase in the atmospheric abundance of CO2 is set not by the short stay of an
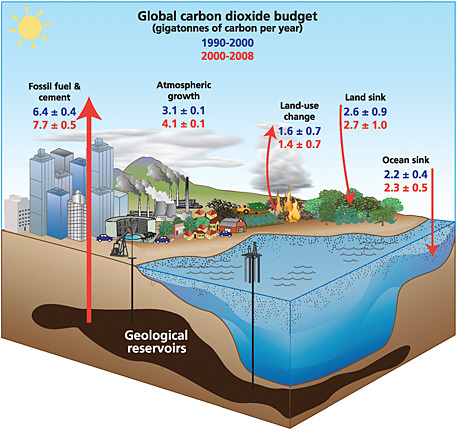
FIGURE 1.3 The global carbon cycle and changes in the sizes of CO2 reservoirs over the last two decades. All values are billions of metric tons of carbon. Arrows show annual fluxes. Values for the 1990s are in blue; those for 2000-2008 are in red. SOURCE: Le Quéré (2009), International Geosphere-Biosphere Programme/Global Carbon Project. Data from Le Quéré et al. (2009).
individual molecule in the atmosphere, but by the small imbalance that the pulse creates between the uptake and removal rates. For example, CO2 molecules spend thousands of years in the oceans once they have been transported into the abyss. Consequently, dissolved CO2 in the deep oceans reflects the atmospheric abundance before the industrial revolution, rather than the increased abundance caused by fossil-fuel burning in the last 200 years. The upshot is that a fraction of the fossil-fuel CO2 emitted is taken up rapidly by the upper ocean and biosphere, but the remainder of the perturbation acts like a very long-lived gas, requiring thousands of years to decay away (Denman et al., 2007).
Methane is short-lived in the atmosphere relative to CO2. A molecule emitted into the atmosphere is oxidized to CO2 in an average of about 8 years, but chemical feedbacks extend this time scale to 12 years (Prather, 1994). This means that the current abundance of methane is derived from the last several decades of emissions. Nitrous oxide has an average residence time of 114 years in the atmosphere before it is photochemically decomposed in the stratosphere. The average atmospheric lifetimes of the other gases considered in this report range from 45 to 1,700 years for CFCs, 1 to 270 years for HFCs, 3,200 years for SF6, and tens of thousands of years for PFCs (Forster et al., 2007).
Emission Sources
CO2 emissions to the atmosphere are caused primarily by fossil-fuel burning (~74 percent in 2004; IPCC, 2007b) and tropical deforestation (~22 percent), although recent work suggests that the contribution from deforestation has decreased to as little as 12 percent of CO2 emissions in 2008 (van der Werf et al., 2009a). Other contributors include industrial processes such as cement production. The primary sources of anthropogenic methane are energy production, ruminant animals, rice agriculture, landfills, and biomass burning (Denman et al., 2007). Natural sources of methane are dominated by wetlands and are approximately one-half the size of anthropogenic sources. Although understanding of anthropogenic N2O sources is incomplete, agriculture is likely the largest source because of the oxidation of nitrogen fertilizer and reduction of nitrite (see Table 7.7 in IPCC, 2007a). Natural sources are dominated by soils under natural vegetation and by microbial transformations of nitrogen compounds in the oceans and are thought to be roughly comparable in size to anthropogenic sources (Boumans et al., 2002; Nevison et al., 2004; Hirsch et al., 2006). The presence of CFCs, HFCs, SF6, and PFCs in the atmosphere is due almost entirely to human manufacture for a wide range of industrial applications in the latter half of the twentieth century (IPCC, 2007a).
Atmospheric Concentrations, Emissions, and Trends
Atmospheric abundances of greenhouse gases are best quantified by dry air mole fractions—the number of molecules of the gas in a set volume divided by the total number of molecules of dry air in the same volume (see Box 1.3). The mole fraction of CO2 in the atmosphere is currently 387 ppm (Figure 1.4), which is more than 100 ppm higher than in the pre-industrial period. Annual anthropogenic emissions of CO2 are between 9 billion and 10 billion metric tons of carbon (Gt C yr–1), increased at 1-2 percent per year over the last three decades of the twentieth century, and 3.4 percent per year from 2000 to 2008, and are projected to decline in 2009 by almost 3 percent due to the weak economy (Canadell et al., 2007; Le Quéré et al., 2009). Approximately half of the annual increase expected
|
BOX 1.3 Measurement Units for Greenhouse Gases in the Atmosphere The concentration of a gas, which is defined as the number of molecules per volume, will vary with altitude and weather systems as the density of the air changes, even if there are no sources or sinks. When a parcel of air rises and expands at lower pressure, the concentrations of all species decrease by the same factor. What is conserved is the mole fraction, the relative abundance of each. When water evaporates or condenses, which adds or removes an extra gaseous component, the mole fraction of all other components will decrease or increase, respectively, by the same proportion. Thus, the property that reflects additions and removals of a trace component is its mole fraction in dry air, which changes only when there are sources or sinks. The dry air mole fraction of CO2 is expressed as parts per million. A mole fraction of 385 ppm means that, on average, in every 1 million molecules of dry air there are 385 CO2 molecules. The mole fraction of methane is typically expressed in parts per billion, and that of the HFCs, PFCs, and CFCs in parts per trillion. |
from these emissions (4-5 Gt C yr–1) accumulates in the atmosphere and the rest is taken up by carbon reservoirs in the oceans and on land (see discussions below and Figure 1.3). Because 1 ppm of CO2 in the atmosphere equals 2.12 Gt C, the atmospheric growth rate currently averages ~2 ppm per year.
The abundance of methane in the atmosphere today is much higher than in the millennium before the industrial era (1,774 parts per billion [ppb] in 2005 versus approximately 700 ppb; see IPCC, 2007a). For the 1970s and 1980s, the growth rate of methane was about 1 percent per year; the rate slowed dramatically in the 1990s and dropped to nearly zero from 2000, but began to grow again in 2007 (Rigby et al., 2008b).3 Several reasons for this anomalous growth pattern have been proposed, but no clear explanation is available (Dlugokencky et al., 2001). The literature on emissions of methane is summarized in Table 7.6 of IPCC (2007a). Estimates for anthropogenic sources range from 264 to 428 Tg CH4 yr–1 (1 Tg CH4 equals one million metric tons of methane) and for natural sources from 145 to 260 Tg CH4 yr–1, although total emissions are more tightly constrained (493 to 667 Tg CH4 yr–1).
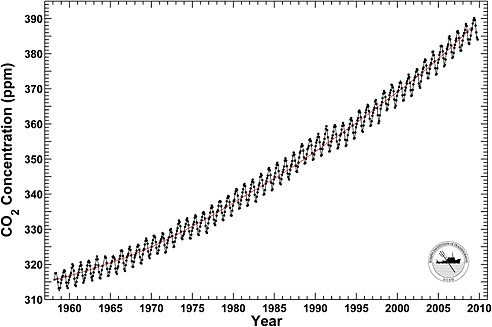
FIGURE 1.4 Monthly mean CO2 concentration at 3,400 m altitude on Mauna Loa, Hawaii. The red curve shows the trend of industrial emissions of CO2 from fossil-fuel combustion and cement production. The annual cycle is driven by the imbalance between seasonal photosynthesis and respiration on the continents. Plants take up CO2 only during the growing season, but plants and animals release it through plant metabolism and the decay of dead organic matter more evenly throughout the year. The long-term increase in atmospheric CO2 is caused by fossil-fuel combustion and land-use change. SOURCE: Courtesy of Ralph Keeling, Scripps Institution of Oceanography. Data are from the Scripps CO2 program.
The abundance of N2O rose from 270 ppb in 1750 to 319 ppb in 2005 (IPCC, 2007a). N2O is now increasing in concentration at an average rate of approximately 0.25 percent per year. Total emissions (13.9 to 18.9 Tg N yr–1) are constrained by the predicted atmospheric lifetime and observed growth rate (Prather et al., 2001), with the growth rate indicating the level of anthropogenic emissions (~6 Tg N yr–1). Inventory estimates of N2O emissions have considerable uncertainties (Prather et al., 2009) as illustrated by the large range in the size of the total global source reported by the Intergovernmental Panel on Climate Change (IPCC): 8.5-27.7 Tg N yr–1 (IPCC, 2007a, Table 7.7).
CFCs are covered by the Montreal Protocol on Substances That Deplete the Ozone Layer, and they are either stabilized or decreasing in concentration. However, HFC, SF6, and PFC abundances are currently increasing (Prinn et al., 2005; Velders et al., 2005; IPCC, 2007a).
Separating Anthropogenic and Natural Components of CO2
Anthropogenic emissions are the emissions of a gas resulting from human activities. Although this definition is easy to apply to fossil-fuel burning, where all emissions are anthropogenic, it becomes problematic for forestry, cropland management, and other land-use sources and sinks, where it is difficult to distinguish emissions and removals due to human influence (e.g., management practices) from those due to natural factors. For example, climate change and fertilization of plants by anthropogenic CO2 and nitrogen deposition probably affect plant growth rates all over the world, but our understanding of these effects is incomplete and will likely remain so for the foreseeable future. To address the problem, the IPCC and UNFCCC have adopted a convention of treating all emissions and removals (sinks) on land that is managed, as anthro-
pogenic.4 Any changes in emissions and removals from these lands are thus considered anthropogenic, regardless of whether natural factors contributed to those changes.
In addition to the definitional ambiguities, monitoring of anthropogenic CO2 emissions is greatly complicated by the natural cycling of CO2 through the terrestrial biosphere and oceans (Figure 1.3). The terrestrial biosphere takes up approximately 120 Gt C yr–1 through photosynthesis and releases almost all of it back to the atmosphere through respiration by plants, animals, and microbes (IPCC, 2007a). Photosynthesis occurs only during daylight hours in the growing season, whereas respiration occurs at all times, albeit at a reduced rate in some seasons (i.e., winter outside the tropics). This diurnal and seasonal imbalance can be quite large; the CO2 sources and sinks that they create are often larger than fossil-fuel fluxes in the same location, except in cities or close to power plants where fossil-fuel emissions are concentrated. Moreover, if we ignore tropical deforestation, terrestrial ecosystems represent a net sink that averaged 2.7± 1.0 Gt C yr–1 over 2000-2008 (Le Quéré et al., 2009). The cause of this sink is not completely understood, although a substantial fraction is due to forest regrowth and other land-use changes in the temperate zone (CCSP, 2007, Chapters 2 and 3) and the remaining fraction may be caused by CO2 fertilization (Friedlingstein et al., 2006). The size of the net terrestrial flux can change from year to year by as much as 5 Gt C (Baker et al., 2006a), in part from anthropogenic fires in tropical forests associated with El Niño events (Randerson et al., 2005; van der Werf et al., 2009b), but is usually within a range of ±1 Gt C yr–1.
The oceans are also a sink for carbon averaging 2.3 ± 0.5 Gt C yr–1 from 2000 to 2008 (Le Quéré et al., 2009). By measuring the changing chemical properties (e.g., pH, pCO2) of the surface ocean from research vessels and commercial ships of opportunity, the annual sink assignable to an ocean basin can be estimated to a precision of about ±10 percent (e.g., Watson et al., 2009). These measurements show that variations in the oceanic sink are too small to explain the multi-gigaton fluctuations in the atmospheric increase of CO2 (IPCC 2007a; Le Quéré et al., 2009). Moreover, because of their comparatively high accuracy, estimates of the oceanic sink provide a valuable constraint on estimates of the magnitude of land sinks at regional and global scales (because the land sink equals the fossil-fuel source minus ocean uptake minus the atmospheric increase).
Fluctuations of natural CO2 sources and sinks create a difficult signal-to-noise problem for efforts to estimate anthropogenic emissions with atmospheric measurements. Seasonally fluctuating background sources and sinks that contribute to the CO2 signal may be of the same order as the emission reductions that might be required under a treaty. The signal-to-noise problem is further exacerbated by the fact that annual fossil-fuel and deforestation emissions represent only about 1 percent of the CO2 in the atmosphere (IPCC, 2007a). This means that anthropogenic emissions will change the average CO2 abundance by only a small amount as air moves across a country over a period of hours to a few days. Thus, an effective way to uniquely identify many large emissions sources is to measure the perturbation in air close to the source, before mixing dilutes the added CO2. The plume of increased concentration above a major point source can be of order 1-10 percent above the background concentration (see Chapter 4).
Because of the signal-to-noise problem, the natural carbon cycle would have to be monitored as part of any effort to monitor anthropogenic emissions. Monitoring the carbon cycle would also constrain estimates of “leakage,” in which reduced emissions in one region or sector lead to increased emissions in another (i.e., soil carbon releases from land newly cultivated for biofuels; see Searchinger et al., 2008; Tilman et al., 2009). Further, the effectiveness of any climate treaty is based on the stabilization of greenhouse gas abundances, whether from anthropogenic or natural sources. Current models indicate that climate change feeds back on natural ecosystems and the ocean to produce new sources or reduce sinks of greenhouse gases, with most of the feedbacks amplifying climate change. For example, warming might cause arctic tundra to emit large quantities of CO2 and CH4, causing further climate change, even more releases of CO2 and CH4, and so on in a positive feedback loop (Walter et al., 2006; Zimov et al., 2006; IPCC, 2007a; Schuur et al., 2009). These effects need to be detected early to ensure that
any agreement to limit anthropogenic emissions has the desired outcome.
ORGANIZATION OF THE REPORT
This report examines methods used to estimate greenhouse gas emissions and identifies enhancements or new techniques that could be used to significantly improve emissions estimates over the next few years. Chapter 2 describes the national greenhouse gas inventories reported under the UNFCCC and their limitations. The chapter focuses on the accuracy of estimates for the gases and activities in the sectors responsible for most of the emissions: energy and agriculture, forestry, and other land use. The primary gases in these two sectors are CO2, CH4, and N2O. Corresponding estimates for gases and activities in the industrial processes and waste sectors are summarized in Appendix A. The primary gases in these sectors are CO2, CH4, N2O, and HFCs. Chapter 3 describes remote sensing measurements of land use that could provide independent estimates of deforestation and some types of agricultural production. It also describes observations and research needed to improve UNFCCC inventories of emissions from agriculture, forestry, and other land use. Chapter 4 examines atmospheric-based estimates of greenhouse gas emissions, which could provide independent checks on emissions from fossil-fuel use and industrial processes. Additional information on sources of atmospheric and oceanic data, methods for estimating atmospheric signals, and technologies for measuring emissions from large local sources appears in Appendixes B, C, and D, respectively. Biographical sketches of committee members appear in Appendix E, and a list of acronyms and abbreviations is given in Appendix F.










