3
Conceptual Model of Sea-Turtle Abundance and Demography
Demographic information is critical for interpreting abundance trends. Demography refers to the key vital rates or parameters, such as breeding, survival, and dispersal rates. As a concrete example, consider the common practice of assessing population status by counting nests. Setting aside sampling issues (discussed in Chapter 4), a central question in estimating the number of nests on a beach concerns the connection between variations over time in nest numbers and in population abundance. First, the number of nests on a beach in a particular year is the product of clutch frequency (the number of clutches deposited by an individual female turtle in a nesting season) and the number of females that nest on the beach in that year. To provide an index of the number of nesting females that is comparable from year to year, it is necessary either to know or have an estimate of clutch frequency or to assume that it remains constant. Otherwise, it is not possible to separate the effects on nest numbers of variations in the number of nesting females from the effects of variations in clutch frequency.
Second, the connection between the number of nesting females in a year and the number of adult females in the population is complicated by the fact that adult female sea turtles generally do not nest every year. Thus, the number of adult females in a population in a year consists of the ones that nest in that year and the ones that remain at sea. The latter number, which typically is not measured, depends on the numbers nesting in previous years, their remigration intervals (the interval between successive nesting seasons), and the survival rate of at-sea adult females. The issue is complicated by variations over time in the distribution of
the remigration interval and the at-sea survival rate. Without information about remigration intervals and adult survival, it is not possible to relate the number of nesting females in a year to the total number of adult females in that year. Third, adult females make up a small part of the overall population. Their number is an index of population abundance only if their proportion in the population remains stable. Taken together, those complications in the use of nest counts as an index of population abundance underscore the importance of demographic and other information in drawing robust conclusions about a sea-turtle population from observations limited to one part of the population at one stage of the lifecycle. Hence, a conceptual model that links population abundance with the key demographic processes in a single coherent framework is needed.
CONCEPTUAL BACKGROUND
The six species of sea turtles that inhabit U.S. waters share the basic lifecycle characteristics of nesting on land with breaks of a year or more between nesting seasons and varied degrees of site fidelity (see Chapter 2), variable egg survival with an incubation period of about two months and temperature-dependent sex determination, a phase of rapid growth in the open sea, and a protracted juvenile stage of several years. The species then fall into two primary life-history groups that are based on habitat use through their lifecycle. Loggerhead (Caretta caretta), green (Chelonia mydas), hawksbill (Eretmochelys imbricata), and Kemp’s ridley (Lepidochelys kempii) turtles make a developmental shift from pelagic (open ocean) to neritic (coastal, nearshore) habitat as juveniles; the discreteness of the shift may vary (McClellan et al., 2010). Leatherback (Dermochelys coriacea) and olive ridley (Lepidochelys olivacea) turtles, in contrast, remain pelagic throughout their lives. The number of years spent in preadult life stages varies among species, and lifecycle models have had some variability in the number and definition of life stages. All sea turtles undergo extensive migrations during their lives in response to changes in temperature and forage opportunities, and adult males and females migrate for mating and egg laying. With the exception of basking green turtles in Hawaii, only adult females return to land.
A simple but informative conceptual model of loggerhead abundance and demography is shown in Figure 3.1. The representation was developed for causal-loop modeling (Puccia and Levins, 1985), but it provides a generic description of sea-turtle population dynamics (Chaloupka, 2002a, 2003a, 2004) and is not tied to a particular modeling approach. This conceptual model is meant to remind the reader of the big picture and is an effective graphic device to capture in a coherent and integrated framework the key demographic processes and anthropogenic hazards facing
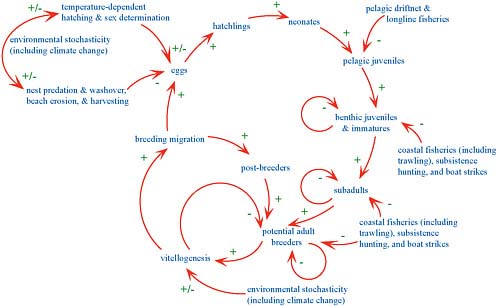
FIGURE 3.1 A conceptual or causal-loop diagram summarizing the ageclass structure and key demographic processes of the model accounting for the population dynamics of Pacific loggerhead sea-turtle populations exposed to various ageclass-specific anthropogenic hazards. +/- = causal-loop polarity: + means two components move in same direction, and - means they move in opposite directions. For instance, as more turtles breed and migrate, the number of potential breeders decreases inasmuch as females do not breed each year because of reproductive constraints. See Puccia and Levins (1985) for details on causal-loop modeling and Chaloupka (2002a, 2003a, 2004) for application to sea-turtle population modeling.
sea turtles—in this specific case, the two Pacific loggerhead populations (Bowen et al., 1994). The causal-loop model not only helps in identifying knowledge gaps but provides a blueprint for simulation models of Pacific loggerhead population dynamics and for the development of population-assessment models and risk-analysis tools. The committee presents the Pacific loggerhead model as an example of what could be developed for U.S. sea-turtle populations.
Causal-loop modeling is a special class of signed directed graph theory and is read as follows in reference to Figure 3.1. Arrowed links between variables (ageclasses and hazards) are negative if the variables change in opposite directions. For instance, as nesting-beach temperature increases above thermal maximum of embryos, egg production (the number of eggs laid in the nest) decreases because of reduced hatching rates. A positive link means that the two variables respond in the same direction. For instance, as egg production increases, the abundance of hatchlings increases. Similarly, if oceanic juvenile abundance decreases, benthic or neritic immature abundance decreases (eventually). Increasing neritic immature abundance will eventually lead to decreasing abundance as a consequence of compensatory density-dependent processes affected by per capita food supply.
Causal-loop modeling is a robust and widely used structured graphic procedure for developing conceptual models that are then used in qualitative modeling of complex biological systems (Puccia and Levins, 1985), ecosystem modeling (Loiselle et al., 2000), epidemiology (Dinno, 2007), and ecosystem-based fishery management (Dambacher et al., 2009). Causal-loop modeling also provides the basis of development of simultaneous equations or simulation modeling that is based on coupled systems of differential equations to explore ecosystem or population dynamics (Hulot et al., 2000; Chaloupka, 2003a). The qualitative conceptual models can also be embedded in probability-network models, such as Bayesian belief networks that are useful in data-poor and knowledge-vague settings (Hosak et al., 2008). A Bayesian belief network modeling approach based on the conceptual model shown in Figure 3.1 has been proposed for assessment of the relative risk posed by exposure for sea-turtle populations in Southeast Asian waters to multiple anthropogenic hazards (Chaloupka, 2007).
CONCEPTUAL MODEL FOR POPULATION ASSESSMENT
Ageclass Structure
The conceptual model of loggerhead sea-turtle population abundance and demography shown in Figure 3.1 comprises the following developmental phases or ageclasses and the abundance associated with those ageclasses (Chaloupka, 2003a):
-
Eggs are laid during the summer on sandy beaches (Kamezaki et al., 2003; Limpus and Limpus, 2003a).
-
Hatchlings emerge from the nests around two months later and escape to the sea during middle to late summer (Salmon et al., 1995).
-
Coastal hatchlings and then neonates recruit during the first year of life after escapement to the oceanic habitat (Witherington, 2002; Whelan and Wyneken, 2007).
-
Juveniles and immatures (more than one year but less than 15 years old; Chaloupka, 1998; Bjorndal et al., 2000a, 2001) inhabit productive oceanic frontal zones (Polovina et al., 2000).
-
Subadults (more than 10 years but less than 25 years old; Chaloupka and Limpus, 2001; Chaloupka, 2003a) recruit from oceanic habitat to coastal habitats and then develop into potential breeding adults.
-
Potential breeding adults (physically and physiologically mature, more than 25 years old; Chaloupka, 2003a) undergo long-distance breeding migrations (Limpus et al., 1992) to population-specific regional rookeries and culminate as courting males and nesting females.
Neritic immatures and adults are assumed to be subject to compensatory density-dependent functions; as the population density increases, the neritic component of the population is regulated by per capita food supply.
Major Demographic Processes
The major demographic processes included in the conceptual model are (1) ageclass-specific reproduction driven by environmental stochasticity,1 (2) temperature-dependent hatching and sex determination, (3) ageclass-specific growth, and (4) ageclass-specific survival.
Reproductive Behavior
Each summer, a highly variable proportion of mature male and female Pacific loggerheads migrate from widely dispersed foraging grounds to regional rookeries in southern Japan (Kamezaki et al., 2003) or the southern Great Barrier Reef region to mate (Limpus et al., 1992). Not all females or males breed each season; a substantial fraction of the potential breeders skip one or more nesting years (Limpus et al., 1994), presumably because of variable food supply (Figure 3.2a), which can be affected by climate (Chaloupka et al., 2008a). That is one reason for the interannual fluctua-
![FIGURE 3.2 (a) Estimated proportion of females (curve) and males (dot) breeding each year in two loggerhead populations that make up the southwestern Pacific genetic stock (data from Limpus et al., 1994; Limpus and Limpus, 2003a; courtesy of M. Chaloupka). (b) Long-term nesting abundance (individually marked turtles) recorded at the Mon Repos rookery on the Woongarra coast, in the southern Great Barrier Reef region (from Chaloupka et al., 2008a; with permission from Elsevier). (c) Temperature-dependent loggerhead hatching probability and (d) temperature-dependent hatchling sex-determination function in Pacific loggerheads. Curve in (c) shows a Thornley type of model fit for hatching probabilities (dots); curve in (d) shows a generalized logistic-function fit for hatchling sex-determination probabilities (dots) (data for plots c and d derived from Limpus et al. [1983, 1985] for the southwestern Pacific loggerhead population; from Chaloupka, 2002b; courtesy of M. Chaloupka). (e) Pelagic loggerhead size (curved carapace length [CCL]) as a function of estimated age (from Zug et al., 1995) with the addition of estimated hatchling size. Curve shows the polyphasic logistic growth function fitted to the growth data indicated by dots (from Chaloupka, 1998; with permission from American Society of Ichthyologists and Herpetologists). (f) Expected group-specific Kaplan–Meier–Turnbull survival functions for the 40 satellite-tracked deep- and light-hooked loggerheads only without the visual clutter of the confidence intervals (from Chaloupka et al., 2004a; with permission from Inter-Research Science Center). (g) Size-at-age growth functions (open circles) for three female southwestern Pacific loggerheads recorded over a 15-year sampling period (reprinted from Chaloupka, 2003a; with permission from Smithsonian Books). Age equals years since recruitment to neritic habitat. Curve shows fitted Weibull type of growth model with AR(2) error derived in Chaloupka (2001a). Solid dots shows age at first breeding event derived from Limpus (1992, 1994). (h) Immature sex-specific Horwitz–Thompson type of abundance estimates for the loggerhead population resident on Heron reef (southern Great Barrier Reef) (from Chaloupka and Limpus, 2001; with permission from Elsevier).](/openbook/12889/xhtml/images/p2001b958g48001.jpg)
FIGURE 3.2 (a) Estimated proportion of females (curve) and males (dot) breeding each year in two loggerhead populations that make up the southwestern Pacific genetic stock (data from Limpus et al., 1994; Limpus and Limpus, 2003a; courtesy of M. Chaloupka). (b) Long-term nesting abundance (individually marked turtles) recorded at the Mon Repos rookery on the Woongarra coast, in the southern Great Barrier Reef region (from Chaloupka et al., 2008a; with permission from Elsevier). (c) Temperature-dependent loggerhead hatching probability and (d) temperature-dependent hatchling sex-determination function in Pacific loggerheads. Curve in (c) shows a Thornley type of model fit for hatching probabilities (dots); curve in (d) shows a generalized logistic-function fit for hatchling sex-determination probabilities (dots) (data for plots c and d derived from Limpus et al. [1983, 1985] for the southwestern Pacific loggerhead population; from Chaloupka, 2002b; courtesy of M. Chaloupka). (e) Pelagic loggerhead size (curved carapace length [CCL]) as a function of estimated age (from Zug et al., 1995) with the addition of estimated hatchling size. Curve shows the polyphasic logistic growth function fitted to the growth data indicated by dots (from Chaloupka, 1998; with permission from American Society of Ichthyologists and Herpetologists). (f) Expected group-specific Kaplan–Meier–Turnbull survival functions for the 40 satellite-tracked deep- and light-hooked loggerheads only without the visual clutter of the confidence intervals (from Chaloupka et al., 2004a; with permission from Inter-Research Science Center). (g) Size-at-age growth functions (open circles) for three female southwestern Pacific loggerheads recorded over a 15-year sampling period (reprinted from Chaloupka, 2003a; with permission from Smithsonian Books). Age equals years since recruitment to neritic habitat. Curve shows fitted Weibull type of growth model with AR(2) error derived in Chaloupka (2001a). Solid dots shows age at first breeding event derived from Limpus (1992, 1994). (h) Immature sex-specific Horwitz–Thompson type of abundance estimates for the loggerhead population resident on Heron reef (southern Great Barrier Reef) (from Chaloupka and Limpus, 2001; with permission from Elsevier).
tion in the number of female loggerheads nesting each year (Figure 3.2b). It is assumed that this function is also density dependent. Assuming successful mating, the female loggerheads then lay a variable number of clutches of eggs on the sandy beaches at the rookeries over the summer nesting season.
Temperature-Dependent Hatching and Sex Determination
The probability of eggs hatching (Figure 3.2c) and the proportion of female hatchings produced are dependent on the nest temperature (Limpus et al., 1985; Matsuzawa et al., 2002). Female hatchlings are produced at higher temperatures and males predominantly at lower temperatures (Figure 3.2d), assuming that nest temperature is within the nonlethal limits for hatching (Figure 3.2c). Many southwestern Pacific loggerhead populations are female-biased because of the high summer beach temperatures at most loggerhead rookeries in the Southern Hemisphere (Limpus et al., 1994), but this is not necessarily the case with foraging-ground populations close to the regional rookery (see Figure 3.2h; Chaloupka and Limpus, 2001).
Somatic Growth and Maturity
Relevant size-at-age data on Pacific loggerheads were summarized by Chaloupka (1998) for northwestern Pacific pelagic loggerheads (Figure 3.2e) that are exposed to the various hazards (Figure 3.2f). Somatic (body) growth functions have been developed by Chaloupka (2003a) for southwestern Pacific neritic female loggerheads (see Figure 3.2g). Limpus et al. (1994) and Limpus and Limpus (2003a) have shown that pelagic loggerheads recruit to a coastal or neritic habitat from the Pacific Ocean at curved carapace length (CCL) of around 70–80 cm. The pelagic phase is estimated at 10–15 years given size-at-age polyphasic growth functions (consisting of two or more phases) derived for northwestern Pacific loggerheads by Chaloupka (1998) and mark–recapture of notched southwestern Pacific loggerhead hatchlings (Limpus et al., 1994). That is a longer duration than the 6–11 years estimated for pelagic-phase duration for Atlantic loggerheads that recruit at a CCL of around 46–64 cm (Bjorndal et al., 2000a, 2003a). Once recruited to a neritic foraging ground in western Pacific coastal waters, there is apparently little evidence of either ageclass- or sex-specific dispersal behavior (Limpus and Limpus, 2001).
Size-at-age data on neritic female loggerheads were analyzed by Chaloupka (2003a) with the Weibull type of growth models (Chaloupka, 2001a) that reflect an accelerated growth phase and with longitudinal data derived for southwest Pacific loggerheads (Limpus, 1992, 1994). Some individual-based growth functions are summarized in Figure 3.2g, which indicates a neritic phase before maturity of around 10–15 years (see Chaloupka, 2003a); this is shorter than for Atlantic loggerheads that recruit at lower sizes and take longer (20 years) to mature (Bjorndal et al., 2001). The polyphasic pelagic juvenile growth function (Chaloupka, 1998) and the Weibull type of neritic-phase growth functions (Chaloupka, 2003a) suggest that Pacific female loggerheads are more than about 25–30 years old at maturity, which is consistent with estimates of the age-specific maturation period for Atlantic loggerheads (Bjorndal et al., 2000a, 2001) despite lower recruitment size for Atlantic loggerheads.
Somatic growth is negligible after the onset of maturity at a CCL of more than 90 cm (Chaloupka, 2003a). There is some evidence of sex-specific growth behavior in Pacific loggerheads (Chaloupka and Limpus, unpublished data) that is known to occur in other Pacific turtle species, such as green turtles along the Great Barrier Reef (Chaloupka et al., 2004b). Male loggerheads grow slightly faster than females at all comparable sizes in the Moreton Bay population resident in warm temperate waters. Although Pacific males might grow slightly faster than females at similar sizes, it seems that age-at-maturity is similar between the sexes inasmuch as males are also larger at maturity (Limpus and Limpus, 2003a). Somatic growth and onset of maturity may well be density dependent for logger-
heads, but such an effect has been demonstrated only in a green turtle population so far (Bjorndal et al., 2000b).
Ageclass-Specific Survival
There are few reliable ageclass-specific survival probability estimates for loggerheads (see review in Chaloupka and Limpus, 2002). Loggerhead egg survival and hatching probabilities in the Pacific were based on estimates given in Limpus et al. (1985) and Matsuzawa et al. (2002). Clutch loss to tidal inundation, extreme rainfall, or beach erosion is low in the loggerhead populations in the Pacific (Limpus et al., 1985; Limpus and Limpus, 2003a). Egg predation—for example, by lizards or pigs—can be high in some southwestern Pacific loggerhead rookeries (Limpus and Limpus, 2003a) but is not a current source of egg mortality in the northwestern Pacific population. Pacific loggerhead eggs and hatchlings are also (or have historically been) exposed to numerous beach-roaming predators, such as foxes and weasels (Chaloupka, 2003a; Kamezaki et al., 2003). There are no estimates of hatchling or neonate survival after escapement to open water for the Pacific populations—some estimates of survival of about 95% during the first few hours after escapement to the open ocean have been derived for a Florida loggerhead population (Whelan and Wyneken, 2007). Bjorndal et al. (2003b) used a catch-curve approach, and Sasso and Epperly (2007) used satellite telemetry to derive estimates of oceanic juvenile annual survival probabilities of 64–81%. No such oceanic ageclass annual-survival probability estimates exist for Pacific loggerhead populations. Comprehensive estimates of ageclass-specific annual-survival probabilities for neritic immatures and adults have been derived from long-term capture–mark–recapture programs for populations from the southwestern Pacific loggerhead population, which range from around 88% to 92%, depending on whether transient behavior is accounted for (Chaloupka and Limpus, 2001, 2002). Although loggerhead annual survival probabilities are ageclass-specific, no sex-specific survival probability differences are apparent in any loggerhead population (Chaloupka and Limpus, 2002).
Anthropogenic Hazards
The conceptual model used here also provides a basis for a structured approach to risk-chain analysis, which comprises the following four major components (Merkhofer, 1987):
-
Hazard (e.g., coastal trawl fisheries)
-
Exposure (e.g., during the nesting season of major loggerhead rookeries along the Atlantic coast of Florida or the Carolinas)
-
Effect (e.g., drowning from entanglement, failure of egg production, recruitment)
-
Judgment (e.g., if exposure is extensive, loggerhead populations decline; this is considered unacceptable and warrants some mitigation strategy)
The major anthropogenic hazards to loggerhead sea turtles in general included in the conceptual demographic model are as follows (Bolten et al., 2010):
-
Climate change that affects sea level and leads to beach washover and inundation of nests (Daniels et al., 2006); nesting-beach erosion (Fish et al., 2005) and temperature, which affect hatching rates (Matsuzawa et al., 2002); and hatchling sex determination (Limpus et al., 1985; Marcovaldi et al., 1997)
-
Nest or emerging-hatchling predation by feral animals or natural predators attracted by human activity (Chaloupka, 2003a; Kamezaki et al., 2003; Engeman et al., 2005)
-
Compaction of nesting beaches due to human activity (Kudo et al., 2003)
-
Egg harvesting or poaching on mainland rookeries (Kamezaki and Matsui, 1997; Kamezaki et al., 2003)
-
Nesting-female and emergent-hatchling exposure to artificial night lighting (Salmon et al., 1995)
-
Hunting of nesting females or foraging-ground matures and immatures (Kamezaki and Matsui, 1997; Gardner and Nichols, 2001)
-
Coastal infrastructure that affects nesting behavior and nesting-beach access (Kamezaki et al., 2003; Mazaris et al., 2009)
-
Coastal development activities in foraging habitat and nesting beaches (Kamezaki et al., 2003; Limpus and Limpus, 2003b)
-
Coastal fisheries (Poiner and Harris, 1996; Cheng and Chen, 1997; Julian and Beeson, 1998; Chaloupka, 2003a; Peckham et al., 2007)
-
Pelagic driftnet (Wetherall et al., 1993) and longline fisheries (Polovina et al., 2000; Chaloupka et al., 2004a; Lewison et al., 2004)
-
Climate change that affects food supply and hence reproductive rates (Chaloupka et al., 2008a)
-
Boat strike in coastal habitats reported as a major cause of sea-turtle strandings in U.S. waters (Boulon, 2000; Chaloupka et al., 2008b)
It is assumed in the model (Figure 3.1) that neonates are not exposed to major anthropogenic hazards inasmuch as they do not appear to be caught in pelagic fisheries (Wetherall et al., 1993; Chaloupka et al., 2004a) and are not known to be caught in subsistence hunting (Gardner and
Nichols, 2001). However, ingestion of anthropogenic debris is a serious issue in this ageclass. Tar or debris was found in 20–63% and 15–17% of neonates, respectively, off the coast of Florida (Witherington, 2002). Those hazards have a direct effect on the long-term viability of a loggerhead sea-turtle population on the basis of the following key demographic metrics (Chaloupka and Limpus, 2001; Matsuzawa et al., 2002; Bjorndal et al., 2003b; Chaloupka, 2003a; Heppell et al., 2003; Limpus and Limpus, 2003a; Mazaris et al., 2005, 2006):
-
Ageclass- and sex-specific foraging-ground abundance
-
Nester abundance
-
Ageclass- and sex-specific survival probabilities
-
Ageclass- and sex-specific dispersal probabilities
-
Sex-specific breeding probabilities
-
Hatchling sex ratio
-
Hatchling production












