3
Adopting a Risk-Based Decision-Making Approach to Food Safety
As described in Chapter 2, the responsibilities of the U.S. Food and Drug Administration’s (FDA’s) new Office of Foods include providing executive leadership and management to all FDA food-related programs; directing the development of integrated strategies, plans, policies, and budgets to build the FDA’s food-related scientific and regulatory capacities and programs, including the recruitment and training of key personnel and the development of information systems (FDA, 2009); and exercising direct line authority over the Center for Food Safety and Applied Nutrition (CFSAN) and the Center for Veterinary Medicine (CVM). Its responsibilities include both short-term decision making in direct response to a food crisis and longer-term initiatives focused on sustained, continued improvement in food safety and public health. The former responsibility requires rapid decision making in cooperation with multiple regulatory partners, while the latter requires long-term strategic planning aimed at proactive activities that are based on data and risk-based prediction and prioritization. For example, the FDA’s responsibility during a foodborne illness outbreak would focus on identification of the source of contamination (product trace-back), initiation of regulatory action, and product recall. More proactive activities might involve conducting research to address crucial unknowns, undertaking formalized quantitative risk assessment, identifying candidate mitigation strategies to prevent repeat incidents, and ensuring the implementation of those strategies. Critical to both long- and short-term initiatives are improvements in cooperation with partners (see Chapters 4 and 7); efficient data collection, sharing, and analysis (Chapter 5); and communication with the public (Chapter 9).
Clearly, short- and long-term responsibilities coexist as the FDA seeks to both manage and prevent foodborne illness. As noted earlier, the FDA has often been criticized as responding reactively to food problems. Sometimes, this type of action is necessary; the FDA has no choice but to react when a problem manifests itself. However, greater proactive efforts by the FDA would enhance food safety. This chapter presents a conceptual approach for the prioritization of activities and allocation of resources to support both short- and long-term FDA responsibilities for food safety. Accordingly, the chapter lays out the foundation for a proactive, risk-based food safety system. Succeeding chapters describe elements of such a system that are dependent on the success of the approach presented here. For instance, application of a risk-based approach at all levels of regulation is a prerequisite for harmonization of federal, state, and local food safety programs (Chapter 7). Similarly, effective cooperation and communication with diverse stakeholders will require that all levels of the FDA embrace a proactive, risk-based approach to food safety management and facilitate its implementation (Chapter 9).
The committee did not conduct a comprehensive review of the details of all the risk-based activities of the FDA, such as the models utilized or factors considered in making individual decisions. The committee was provided with general information with regard to the FDA’s risk-based activities and describes its understanding of those activities in this chapter. In this discussion, the committee uses concrete examples of those activities and identifies gaps with respect to the extent to which they adhere to the attributes and steps of the recommended approach. Although the committee concluded that those activities would have been enhanced by the use of a more extensive risk-based approach, in this and subsequent chapters the committee also recognizes that the FDA will face challenges in this regard. The committee identified challenges and courses of action to overcome them, for example, in hiring the appropriate personnel and coordinating data collection and sharing (Chapter 5), reorganizing the agency’s food safety research portfolio (Chapter 6), integrating FDA programs with those of state and local governments (Chapter 7), carrying out risk communication and education (Chapter 9), and addressing organizational problems (Chapter 11).
There is consensus that food safety programs and any approach to food safety reform must be both science- and risk-based. This view was first articulated in the 1998 Institute of Medicine (IOM)/National Research Council (NRC) report Ensuring Safe Food: From Production to Consumption (IOM/NRC, 1998) and is also addressed by other reports of the IOM/NRC (IOM/NRC, 2003), the U.S. Government Accountability Office (GAO) (GAO, 2004a,b,c, 2005, 2007, 2008, 2009a,b), consumer groups (Consumers Union, 2008; Tucker-Foreman, 2009), and Congress (Becker,
2008, 2009; Brougher and Becker, 2008). These reports have emphasized the importance of using the best available science to understand foodborne illness, including the identification of causative agents (chemicals, toxins, and microbes) and transmission pathways and the development of appropriate surveillance systems. As the science base has developed, attention over the last decade has increasingly turned to its application within a risk-based framework, with the ultimate goal of improving public health. The term “risk-based” implies the existence of an underlying science base; however, it goes a step beyond to encompass use of the tools of risk and decision analysis to create systems that optimize the ability to prevent and control foodborne illness and improve public health. This chapter focuses on how this type of risk-based system might be constructed and implemented to enable the FDA to deal more effectively with food safety problems.
Ensuring Safe Food provides a rough description of the components necessary for the implementation of a risk-based system:
… [It] require[s] identification of the greatest public health needs through surveillance and risk analysis. The state of knowledge and technology defines what is achievable through the application of current science. Public resources can have the greatest favorable effect on public health if they are allocated in accordance with the combined analysis of risk assessment and technical feasibility…. Thus, both the relative risks and benefits must be considered in allocating resources. (IOM/NRC, 1998, p. 93)
Other documents have furthered the concept of risk-based food safety management. For example, a 2002 discussion paper issued by Resources for the Future1 states:
If the primary objective of the food safety system is to reduce the burden of disease, success requires risk-based resource allocation. The food safety system must make the best possible use of its resources to reduce the disease burden. This means focusing government effort on the greatest risks and the greatest opportunities to reduce risk, wherever they may arise. It means adopting the interventions—presumably some combination of research, regulation, and education that will yield the greatest reduction in illness. (Taylor, 2002, p. 7)
These previous documents go beyond the scope of traditional technical risk assessment by introducing such terms as “risk-based resource allocation” and “relative risk and benefit.” In its deliberations, the committee recognized the need to address risk analysis in the broader context of regu-
|
1 |
See http://www.rff.org/rff/Documents/RFF-IB-02-02.pdf (accessed January 25, 2010). |
latory decision-making processes and risk governance (see, for example, IRGC, 2005, 2009) to manage food safety.
The challenges and best practices for integrating science to support effective risk management decisions are widely recognized, as summarized by a recent NRC study (NRC, 2009a):
The most effective decision support efforts are organized around six principles: begin with users’ needs; give priority to processes over products; link information producers and users; build connections across disciplines and organizations; seek institutional stability; and design processes for learning. Following these principles improves the likelihood of achieving the three main objectives of decision support: increased usefulness of information, improved relationships between knowledge producers and users, and better decisions. (NRC, 2009a, p. 67)
In short, in a society with limited resources, decisions about allocation need to be made in a consistent manner and with the goal of maximizing benefits and reducing risks while considering associated costs. In the area of food safety, a process is needed for allocating resources based on public health data and information. Risk managers must consider a wide variety of factors in their decision-making process, including the needs and values of a diverse set of stakeholders, which may diverge even with respect to public health. These factors might include economic considerations, the controllability of risk, and the population affected. The committee recognizes that such multidimensional comparisons are a highly challenging endeavor. However, the lack of such a systematic approach to risk-based decision making causes problems, from a decrease in public trust to unintended consequences in the marketplace, the environment, and society. In addition, the lack of such an approach may make a regulatory agency more vulnerable to political influences. The need to formally acknowledge the complexity of such decision making and then establish a transparent and systematic way to carry out the decision-making process is the subject of the next section. In addition, in Chapter 4, the committee elaborates further on the issue of how to select interventions. It should be noted that, while the committee concluded that providing the FDA with a stepwise process as a tool for making decisions is appropriate, the development of the FDA’s philosophy, including specific criteria and their weight, is a management decision beyond scope of this study. Thus in Chapter 4 (recommendation 4-2), the committees recommends that the FDA develop its philosophical approach by defining a strategy that delineates factors to consider (e.g., economic factors, public perception, environmental factors) and their weight.
A RISK-BASED APPROACH TO FOOD SAFETY MANAGEMENT
Definitions
Many groups have defined risk and risk characterization. For example, the World Health Organization’s (WHO’s) International Program on Chemical Safety defines risk as “the probability of an adverse effect in an organism, system, or (sub)population caused under specified circumstances by exposure to an agent” (IPCS, 2004). Others have expanded this definition to include the fact that this probability can be expressed quantitatively or qualitatively and that risk characterization includes a discussion of the significant scientific uncertainties in this information. Further, the committee agreed upon the following working definition for a risk-based approach: “a systematic means by which to facilitate decision making to reduce public health risk in light of limited resources and additional factors that may be considered.” The committee identified the following as key attributes of a risk-based food safety system: (1) is proactive based on a strategic management plan; (2) is data driven; (3) is grounded in the principles of risk analysis; (4) employs analytical methods to rank risks based on public health impact; (5) incorporates deliberation with key food safety stakeholders; (6) considers factors such as consumer perception, public acceptance, market impacts, and environmental impacts in decision making when appropriate; (7) employs analytical methods to prioritize the allocation of limited resources to manage risk most effectively; (8) employs measures to evaluate the efficacy of the risk management program on a continuous basis; and (9) performs all of these functions in a systematic and transparent manner with the involvement of stakeholders. These attributes are further described in Box 3-1.
A Conceptual Approach to Risk-Based Food Safety Management
The risk-based system envisioned by the committee will entail analysis and prioritization at several distinct levels:
-
the formulation of a strategic plan that identifies outcomes/goals of the risk-based system,
-
broad-based risk ranking to identify the most important risks based exclusively on public health considerations,
-
the identification of additional data/information needs upon which prioritization of resources may be based,
-
the choice of intervention strategies and allocation of regulatory resources, and
-
the evaluation of outcomes.
|
BOX 3-1 Attributes of a Risk-Based Food Safety System A risk-based system is proactive and based on a strategic management plan. Notwithstanding the need to respond to unforeseeable crises, risk activities should be planned in advance, an exercise that should include various stakeholders and be based on the knowledge gained from past experience with a vision of predicting food contamination problems. Managing a crisis in the short term and implementing a well-developed strategic plan for managing food safety in the long term are equally important; attention to unanticipated outbreaks should not detract from implementation of the strategic plan. A risk-based system is data driven. Although expert opinion is a valuable asset when there are uncertainties or data must be interpreted, a risk-based system should be grounded in science. That is, the collection, analysis, and interpretation of quality data, as well as data management, are essential tasks for the implementation of a risk-based system. A risk-based system is grounded in the principles of risk analysis. A risk-based system should be grounded in risk analysis, with risk assessment, risk communication, and risk management as the essential basis for establishing a sound public health protection capability. If implemented appropriately, the system ideally provides a transparent, data-driven means by which to determine the extent of public health protection achieved as a result of different risk management actions, and therefore it provides a decision-making tool. This concept has worldwide support and has been applied for several decades by regulatory and public health agencies. A risk-based system employs analytical methods to rank risks based on public health impact. A risk-based system systematically ranks risks even if those risks differ in complexity and uncertainty. The development of analytical methods (models) that can assign numerical values to the various risks based on public health impact is the foundation of this activity. A risk-based system employs analytical methods to prioritize the allocation of limited resources to manage risk most effectively. The evaluation of intervention strategies is an essential element of risk management. Risk managers must consider multiple characteristics or |
|
attributes of different risks and integrate these data for the purpose of prioritizing and making effective use of resources. In this manner, decisions are made by considering the food system as a whole, that is, with a systems-based approach. Important decision analysis tools that may be used in this process are feasibility, cost-effectiveness, and cost–benefit analyses. A major element of this activity is a clear statement of regulatory philosophy and the use of a road map showing how decisions will be made regarding the mix of private responsibility, government incentives, and government regulation that will be used to manage different risks. A risk-based system considers other factors, such as consumer perception, cost, controllability, public acceptance, environmental effects, and market impacts, in decision making when appropriate. Risk mitigation strategies and public policy decision making are influenced by factors other than public health risk. These considerations should be formally communicated to stakeholders. A risk-based system employs measures to evaluate the efficacy of the risk management program on a continuous basis. An essential step in a risk-based system is evaluation of the efficacy of the system itself with respect to public health and other factors selected by decision makers. Evaluation of programs, always a daunting process, requires the identification of indicators by which to link interventions to public health outcomes. To collect and integrate food safety data so that attribution models can be built is a critical first step in this process. A risk-based system performs all of these functions in a systematic and transparent manner with the involvement of stakeholders. Risk managers should develop a process for implementing a two-way communication approach whereby stakeholders have an opportunity to engage in the risk-based decision-making process. This approach should include input and access to discussions regarding the basis for decision making, as well as information about the uncertainties and variability of the underlying data. Likewise, a risk-based approach requires disclosure of all sources of information, comprehensive analysis, and transparency regarding the considerations taken into account in the decision-making process. In addition, independent peer review is fundamental to all scientific undertakings and critical for risk-based decision-making processes. |
Figure 3-1 depicts the cycle of risk prioritization and regulatory (intervention) activities that constitutes the basis of a risk-based food safety system. As the figure shows, the system encompasses six basic steps. These steps are outlined below and then discussed in detail, recognizing that they could be ordered differently and are likely to be taken iteratively.
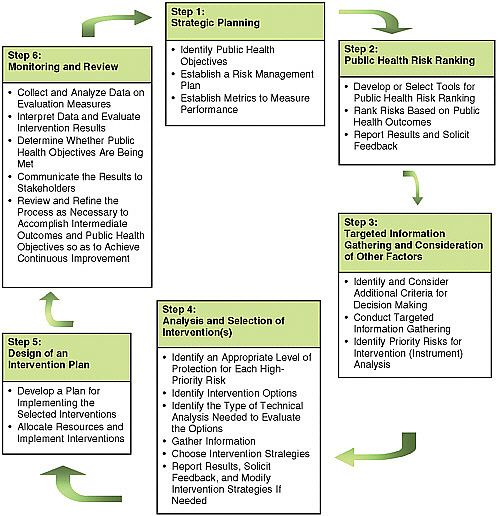
FIGURE 3-1 Steps in a risk-based food safety system (iterative between and within boxes).
Step 1: Strategic Planning
-
Identify public health objectives related to food safety in consultation2 with stakeholders.
-
Establish a risk management plan (general and specific strategic plans for meeting public health objectives and for considering and choosing policy interventions to achieve those objectives).
-
Establish metrics with which to measure performance in consultation with stakeholders.
Step 2: Public Health Risk Ranking (Ranking of Hazards)
-
Develop or select tools (models, measures, or other) for public health risk ranking in consultation with stakeholders.
-
Rank risks based on public health outcomes.
-
Report results to stakeholders and solicit feedback.
Step 3: Targeted Information Gathering on Risks and Consideration of Other Factors That May Influence Decision Making
-
Identify and consider additional criteria upon which risk-based decision making will be based (e.g., public acceptance, cost, controllability, environmental effects, market impacts) in consultation with stakeholders.
-
Conduct targeted information gathering. For each high-priority and/or uncertain risk, determine the need for collection of additional information and implement accordingly:
-
additional data collection (research, surveillance, survey, baseline data), and
-
risk assessment (qualitative, quantitative, semiquantitative).
-
-
Based on that additional information, identify priority risks for which intervention analysis is needed.
Step 4: Analysis and Selection of Intervention(s)
-
Identify an appropriate level of protection for each high-priority risk, based on available data and in consultation with stakeholders.
-
Identify intervention options in consultation with stakeholders.
-
Identify the types of technical analysis, including but not limited
-
to risk assessment, needed to evaluate the options; identify performance measures and the initial design of databases.
-
Gather the information necessary to conduct the technical analysis.
-
Choose intervention strategies for implementation using multicriteria decision analysis.
-
Report results to stakeholders, solicit feedback, and modify intervention strategies if needed.
Step 5: Design of an Intervention Plan
-
Develop a plan for implementing the selected interventions in consultation with stakeholders.
-
Allocate resources and implement interventions.
Step 6: Monitoring and Review
-
Collect and analyze data on evaluation measures selected during strategic planning.
-
Interpret data and evaluate whether the interventions result in the desired intermediate outcomes.
-
Determine whether public health objectives are being met by using performance metrics developed in Step 1 (broad strategic planning).
-
Communicate the results to stakeholders.
-
Review and refine the entire process in an iterative manner as necessary to accomplish both intermediate outcomes and public health objectives so as to achieve continuous improvement over time.
Further Description of the Proposed Approach to Risk-Based Food Safety Management
Step 1:
Strategic Planning
Strategic planning, conducted at several different levels, is an essential element of a successful food safety program. The highest level of strategic planning involves the identification of long-term and broadly stated goals for protecting public health from the threats associated with food contaminants, sometimes referred to as public health objectives. Perhaps the best example of such goals is those proposed for Healthy People (Box 3-2). These goals are considered national in scope and concern the entire food safety system, including components of the system not under FDA jurisdiction. In strategic planning, however, the FDA would also likely include agency-specific intermediate objectives, which might lead only indirectly to
|
BOX 3-2 Food Safety Goals Proposed for Healthy People 2020 Objectives Retained as Is from Healthy People 2010 FS HP2020–1: Reduce severe allergic reactions to food among adults with a food allergy diagnosis. FS HP2020–2: (Developmental) Improve food-employee food preparation practices that directly relate to foodborne illnesses in retail food establishments. Objectives Retained but Modified from Healthy People 2010 FS HP2020–3: Reduce infections caused by key pathogens commonly transmitted through food. FS HP2020–4: Reduce infections associated with foodborne outbreaks due to pathogens commonly transmitted through food. FS HP2020–5: Prevent an increase in the proportion of nontyphoidal Salmonella and Campylobacter jejuni isolates from humans that are resistant to antimicrobial drugs. FS HP2020–6: Increase the proportion of consumers who follow key food safety practices. Objectives New to Healthy People 2020 FS HP2020–7: Reduce the number of outbreak-associated infections caused by food commodity group. FS HP2020–8: Reduce contamination of meat and poultry products by foodborne pathogens. FS HP2020–9: (Developmental) Increase the number of States that have prohibited sale or distribution of unpasteurized dairy products (as defined by FDA, unpasteurized liquid milk and cheeses aged <60 days). SOURCE: http://www.healthypeople.gov/hp2020/Objectives/TopicArea.aspx?id=22&TopicArea=Food+Safety (accessed October 8, 2010). |
improvements in public health. Examples of these sorts of objectives might be improved efficiency of inspections or reorganization of the FDA research function. While accomplishing these objectives might not lead directly to improvements in public health, achieving efficiencies that would ultimately enable improvements in public health would represent measurable movement toward increased safety of the U.S. food supply.
Identification of the specific means by which the goals are to be achieved—for instance, defining the regulatory structures and the nature
and size of the human and technical resources required—is another important component of strategic planning. The strategic planning phase is also when the agency further delineates how scientific research, inspection, and enforcement activities are to be prioritized and deployed. Budgetary issues are central to long-term strategic planning as well. Another important component of strategic planning is describing the metrics that will be used to measure the success of the strategic plan’s implementation, that is, how the program will be evaluated with respect to its success in achieving the stated public health objectives. The issue of measuring success is an important and potentially troublesome one, as will be discussed later in this chapter.
In addition to broadly stated public health objectives, each specific agency function, such as research, inspection, and policy, needs its own strategic plan. Other more narrowly focused strategic planning requirements also arise frequently in conjunction with specific food safety issues. Sometimes these issues can be anticipated, but often they cannot. Therefore, an important aspect of a risk-based food safety management strategy is having the necessary structure and resources in place so the agency can respond rapidly to such emergent situations. Planning for emergencies must therefore be part of the strategic planning process.
The committee believes that all of the risk-based activities discussed in this chapter (e.g., risk assessment, collection of data, research, intervention analysis) should be undertaken only after sufficient strategic planning has been completed. Further, the results of strategic planning should be shared with all constituents involved in each path to decisions. Therefore, risk communication must be carried out during the earliest planning stage. In fact, provisions for the stakeholder contributions expected at all levels of food safety management should be outlined as part of the strategic planning process, to include defining the various stakeholders, the nature of the consultations that will take place with them, the methods to be employed to obtain their feedback and with what frequency, and the process by which the agency will respond to that feedback.
The committee is aware that a balance must be achieved between the time spent in planning and that spent on other, more narrowly focused risk management efforts, but it is convinced that inadequate attention to planning (and an ill-planned initiation of technical analysis) is fatal to an effective risk-based food safety program. Strategic planning is necessary to identify the most efficient path to achieve food safety objectives.
Step 2:
Public Health Risk Ranking (Ranking of Hazards)
The first step in support of the strategic plan is to identify which risks constitute the greatest threat to public health and hence should be a priority
for future analysis. This step is accomplished using tools of public health risk ranking, which itself is a type of risk assessment. Public health risk ranking is a formalized process that involves comparing the relative risk of multiple hazards, including foods, with the purpose of aiding in the establishment of risk management priorities, the allocation of resources, and the identification of critical data and research needs (CAST, 2006; Havelaar et al., 2006; Mangen et al., 2009). At this initial phase of the risk-ranking process, the emphasis is on identifying and comparing hazards and foods with the greatest impact on public health, without consideration of other factors that might also play a role in decision making.
A number of public health risk-ranking models have been produced over the last decade. They differ in their degree of complexity, level of quantification, and approach to model construction. The simplest approach to risk ranking involves the use of personal judgment to create a “risk versus severity” table or matrix to assign rankings. At the other extreme of the spectrum is the joint FDA (CFSAN)–U.S. Department of Agriculture (USDA, Food Safety and Inspection Service [FSIS]) Listeria monocytogenes in Ready-to-Eat Foods Risk Ranking (CFSAN/FSIS, 2003), which ranks foods based on their listeriosis risk and encompasses all the components of a full quantitative risk assessment. Somewhere in the middle are many simpler, semiquantitative public health risk-ranking tools, some of which are summarized in Table 3-1.
Each public health risk-ranking model has been designed with a specific purpose in mind, which then informs its design, scope, and degree of rigor. The general approach involves consideration of the body of scientific evidence on attributes (e.g., potential for amplification of the hazard in the food) that define the risk(s) posed by the various agent–food combinations. These attributes (or criteria) are described qualitatively or semiquantitatively and together are the basis for the risk ranking. Each criterion or attribute is defined by one or more input variables that are described using relevant data sources, usually a combination of personal judgment and scientific evidence. Some of the commonly used criteria are (1) burden of illness (epidemiological attribution), (2) illness severity, (3) population susceptibility, (4) likelihood of contamination, (5) potential for agent amplification, and (6) breadth of exposure. The inputs are combined using a mathematical algorithm that assigns a “rank” based on the values or weights given to each input variable. Although risk ranking can be done at a macro level (such as the entirety of risk associated with a specific food or hazard), it is most often applied to specific hazard–commodity pairs.
A useful way to differentiate risk-ranking approaches is by the features of the data sources used in model construction. In the surveillance-based or “top-down” approach, the level of risk associated with specific foods, hazards, or their combinations is based on information gathered
TABLE 3-1 Semiquantitative Food Safety Risk-Ranking Methods
|
Method |
Brief Description |
Metrics and Design |
Originator(s) |
|
Foodborne Illness Risk-Ranking Modela |
A science-based tool for prioritization of resources in food safety. Consists of three modules: (1) disease incidence, (2) valuation of health outcomes, and (3) attribution. |
Ranks on five measures of social burden. |
Food Safety Research Consortium (U.S.). |
|
Analytical design with user-friendly interface. |
|||
|
iRISK |
Semiquantitatively compares risks of hazard–commodity pairs. |
pDALY calculation for comparative ranking purposes. |
Institute of Food Technologists, Risk Sciences International, and the Food and Drug Administration (U.S.). |
|
Allows for comparison of microbial and chemical hazards. |
|||
|
Analytical platform with web-based user interface. |
|||
|
Closest to the standard risk assessment paradigm. Considers (1) exposure assessment (populations, consumption), (2) hazard characterization (dose–response), (3) process information (effect on prevalence and level of contaminant through stages in continuum), and (4) public health metric pseudo-disability adjusted life years (pDALY). |
|||
|
Risk Rangerb |
Determines relative risks from different product–pathogen–processing combinations. Based on 11 questions posed to the user, which deal with (1) susceptibility and severity, (2) probability of exposure, and (3) probability of the food containing an infectious dose. |
Excel-based mathematical model converts answers to numerical values; values combined to produce a risk-ranking score scaled logarithmically between 0 and 100. |
Australian Food Safety Center of Excellence. |
|
Food Safety Universe Database |
Systematic ranking of food safety risks in three dimensions: food, hazard, and location in chain. Establishes two “axes” upon which are determined (1) probability (consumption, contamination, exposure) and (2) impact (P[illness], severity, difficulty of limiting impact). |
Risk score calculated multiplicatively as a product of six subscores. |
Ontario Ministry of Agriculture and Food. |
|
a See http://www.thefsrc.org/firrm.htm (accessed October 8, 2010). b See http://www.foodsafetycentre.com.au/riskranger.php (accessed October 8, 2010). |
|||
from epidemiological systems such as disease reporting and outbreak databases. It can be argued that these are the best sources of information for public health–based risk ranking because they reflect illness at the point of consumption (NRC, 2009b). However, good epidemiologically based foodborne illness attribution data are not available at this time for the vast majority of hazard–food combinations under FDA jurisdiction, and in most instances do not exist for chronic chemical exposures associated with foods. Another concern with this approach is that it represents disease risk only at the “point of consumption,” which is the net sum of contamination occurring at the preharvest, processing, and final preparation stages (NRC, 2009b). This does not necessarily translate directly to an understanding of the possible source of contamination in the supply chain, including a source at the point of processing, which is the location of the large majority of the FDA’s current activity. The overall role of foodborne illness attribution in a risk-based food safety management system is discussed further at the end of this chapter.
The alternative or “bottom-up” approach to public health risk ranking adheres roughly to the standard microbial risk assessment paradigm and follows the agent through the food chain to produce a prediction of risk to human health relative to other agents and/or foods. This approach is based on research data supplemented by expert judgment, and therefore can be resource-intensive and subjective. It frequently presupposes an understanding of the behavior of microorganisms in complex and changing environments, complexities that may be very difficult to model. It could be argued that some combination of both approaches (bottom-up and top-down) would be better than either one alone.
Many considerations arise in designing a public health risk-ranking model, including model structure, degree of resolution (categorization of foods and agents broadly or narrowly), choice of key risk attributes and their defining criteria, data sources, and weighting approach. Nonetheless, a good risk-ranking model should be fit-for-purpose and be scientifically credible, balanced, transparent, easy to use, and flexible. As such it must provide both the information and the framework necessary to facilitate public health risk ranking in a systematic manner.
As is the case for strategic planning, public health risk ranking can be applied to decision making at various levels. At the uppermost level, identification of the highest-priority risks can be used to support decisions about the balance of resources dedicated to different agency functions. For example, for risk X, what proportion of the agency’s resources should go to research relative to inspection versus risk communication? Or within the inspection function, what proportion of resources should be dedicated to commodity A versus commodity B based on their relative risk ranking? At a lower level, predictive (bottom-up) risk-ranking models with a high
degree of resolution can even function as preliminary risk assessments to determine the need for additional data collection or to predict the efficacy of competing mitigation approaches. In short, public health risk ranking supports the other functions of a risk-based food safety management system in the spirit of the iterative nature of the system.
Step 3:
Targeted Information Gathering on Risks and Consideration of Other Factors That May Influence Decision Making
The committee recognizes that even a risk-ranking process based exclusively on public health aspects and grounded in scientific knowledge requires weighing competing values and objectives. Risk decision making takes place in a broader social context. In its mission to protect the safety of the public food supply, the FDA must usually consider such additional factors as (1) the feasibility of mitigation; (2) economic constraints (both costs and economic consequences); (3) additional public health and welfare concerns of consumers, farmers, the food processing industry, and other stakeholders; and (4) the environmental impacts of proposed mitigation measures. Therefore, it is critical during the information-gathering stage to identify which factors will be considered in the decision-making process.
Risk prioritization, an emerging approach in the food safety arena, uses the combined tools of risk assessment and decision analysis to determine the importance of one risk relative to another. Unlike risk ranking, which the committee has defined as a type of risk assessment exercise, risk prioritization is inherently a risk management tool. In particular, multiple criteria decision analysis (MCDA) shows promise for supporting complex decision making. MCDA allows for the systematic structuring of a decision problem from the perspective of multiple dimensions (not just public health). Implemented as an element of structured decision support (NRC, 2009a), it can assist in decision making by integrating value judgments as well as objective, quantitative measurements within a transparent and systematic framework.
Structured decision making incorporating MCDA consists of three basic phases (compare NRC, 2009a, p. 57). In the first phase, called problem structuring, the agency defines the decision problem with input from key stakeholders. This activity includes (1) bounding the problem and identifying the question to be addressed and the factors to be included or excluded from consideration, (2) identifying the values and objectives of the decision-making process, (3) identifying the specific criteria with which potential actions are to be compared, (4) identifying the attributes with which the performance of a given alternative will be measured, and (5) identifying the potential actions to be compared in the analysis. Examples of criteria that may be used are public health improvements, health risk reductions, eco-
nomic impact, consumer perception, social sensitivity, and environmental effects. In the next phase, called preference modeling, analysts work with all parties to evaluate and represent agency and stakeholder preferences relative to each criterion and to develop an aggregated model that combines preferences across criteria for the purposes of comparing alternative actions (interventions) and assessing trade-offs among the alternatives. Finally, after the ranking of alternatives, sensitivity analysis is performed to identify the most influential criteria and attributes and to evaluate the influence of different preference judgments, an activity that may lead to a change in the ranking of the alternatives (Belton and Stewart, 2002). Recent examples of MCDA approaches applied to food safety include those of Ruzante and colleagues (Henson et al., 2007; Fazil et al., 2008; Ruzante et al., 2009). Ultimately, the outcome of Step 3 is to rerank or reprioritize competing risks.
In some cases, risk prioritization will result in the identification of substantial uncertainties that could well impact the decision-making process. For example, what are the major stakeholder concerns, and how important are they? Are candidate mitigation strategies available, and if so, what is known about their effectiveness? Is the degree of contamination in a product actually known? Is the infectivity/toxicity of a candidate hazard in a population of interest understood? In instances where unknowns are critical to informed decision making, Step 3 helps inform resource allocation with respect to surveillance, research, or further risk assessment efforts. This is not to say that decision making should be placed on hold until every piece of missing information is gathered. When there are sufficient uncertainties that might well impact the choice of a control strategy, however, it is prudent to invest in the collection of information that will improve the ability to make an informed, science-based decision. Alternatively, a risk-ranking/prioritization model (Steps 2 and 3 of the risk-based system) could be designed that would take into account the degree of certainty about public health impact or the need to prioritize based on the potential to cause a particularly serious disease (e.g., bovine spongiform encephalopathy).
Step 4:
Analysis and Selection of Intervention(s)
The next step in a risk-based approach is to identify and select interventions (or instruments) for the highest-priority risks. In economic and policy analysis, the term “instrument” is used to describe the means a government has at its disposal to achieve public policy outcomes—to govern. Instrument types that are often used include laws, economic incentives, self-regulation, standards, contracts, and information and education, all of which establish relationships between the state and its citizens (Treasury Board of Canada Secretariat, 2007). However, the term “instrument” can be interpreted in
many different contexts (e.g., the medical discipline), so to avoid potential misinterpretations, the committee chose to use the term “intervention” instead. For the purposes of this report, the term “intervention” should not be equated exclusively with legislation, but with any means by which policy objectives are pursued. This broad definition includes forms of government action in addition to legislation and encompasses a spectrum from no intervention through reliance on industry self-regulation, use of information and education strategies, coregulation, establishment of incentive-based structures, direct regulation, or a combination of actions (see Chapter 4).
Choosing interventions based on decision analysis is a process that involves multiple tasks. The first is to establish an acceptable level of risk (appropriate level of protection) for each high-priority risk, consistent with the broad goals for protecting public health identified in Step 1 (strategic planning). This task should, of course, be carried out in consultation with stakeholders. Next, it is necessary to identify interventions that could be used—alone or together with other interventions—to address each risk. Candidate interventions can be identified or designed through consultation with stakeholders and based on the scientific analyses performed in Step 3. In point of fact, many candidate interventions will already have been identified in Steps 1, 2, and 3 of the risk-based system during the gathering of information about the risks and the discussion of potential mitigation strategies. Because the objective of the risk-based approach is to allocate limited resources to maximize benefits and minimize risks, decisions about interventions should include an analysis of the value of public health outcomes and uncertainties as well as of the costs and risks of the intervention. This analysis should be undertaken with the understanding that for some interventions (e.g., a new regulatory approach to food inspections), the impact on public health and the cost will be realized only in the long term, and therefore the timing of the analysis is an important consideration. It is important at this stage to consider systematically the full spectrum of interventions (Treasury Board of Canada Secretariat, 2007) to ensure that the alternatives are not prejudged (see also Hammond et al., 1999).
Candidate interventions should then be evaluated by using analytical tools (e.g., risk assessment) that can help identify the types of additional information that might be needed to evaluate the alternatives and the data required. Based on this information and analysis, intervention strategies should be selected and assessed using formal MCDA approaches as described under Step 3. The MCDA approach does not need to be highly sophisticated, but it does need to provide a road map to ensure that the same factors and trade-offs are considered across intervention alternatives for different risk situations. A template (Treasury Board of Canada Secretariat, 2007) can help ensure that more salient aspects of a particular alternative do not dominate the overall choice among interventions (see
Chapter 4). Documenting intervention choices is essential to achieving transparent decision processes.
Step 5:
Design of an Intervention Plan
The fifth step in a risk-based system is to design and implement the selected intervention(s) in consultation with stakeholders. Each intervention will have unique implementation needs, so the details of this step will vary based on the selected intervention and the risk being addressed. For example, this step may involve writing regulations, setting standards, overseeing self-regulation, or designing educational programs and tools, as might be the case for labeling. This step also requires the definition of interim measures (intermediate outcomes) with which to monitor the progress of the intervention’s implementation; these measures, however, should not be a substitute for the ultimate performance measures identified in Step 1 (strategic planning), that is, the measurement of progress in meeting public health objectives. This step also involves systematically choosing the types of resources to be used in carrying out different intervention plans, for example, the mix of federal and state resources. The role of each partner (e.g., federal, state, and local governments; industry) in implementing the intervention needs to be discussed with partners and delineated in the plan.
Step 6:
Monitoring and Review
Integral to any management system is continued monitoring of the system outcomes. In addition to common goals of greater accountability and improvements in performance-based decision making (Cavalluzzo and Ittner, 2004), performance measurement and monitoring can be used for evaluation, control, budgeting, learning, motivation or promotion, and recognition of achievement (Behn, 2003). The committee cautions that, while there is evidence that performance measurement can improve government performance (Bevan and Hood, 2006), it can also be ineffective or even harmful, producing gaming and other unintended consequences (Bird et al., 2005; Johnsen, 2005). For example, the Government Performance and Results Act (GPRA) has been criticized for focusing public managers more on procedural compliance than on performance (Lynn, 1998).
As each intervention is undertaken, it is essential to map appropriate predetermined goals (set during Step 1), such as public health objectives and intermediate objectives, to the actual outcomes of an intervention. Direct metrics of public health might include cases of illness, hospitalizations, deaths, measurements of disease burden (e.g., disability-adjusted life years), or economic costs (e.g., cost of illness). Intermediate metrics are those that,
for example, measure contamination at a point between farm and table. As part of the strategic planning in Step 1, the agency should define one or more agencywide goals, ideally linked with national public health objectives and relating to national reductions in the incidence of key pathogens and their associated diseases or the presence of chemical contaminants. This should be seen as a means of measuring the overall outcome of the risk-based system, providing the agency with a way of assessing whether the selected approach to risk management is effective.
Needless to say, the identification and design of appropriate metrics must be consistent with the data collection system and the means by which the data are interpreted (see also Chapter 5). Foodborne illness attribution data can be particularly relevant in this regard. A prime example of an effort to link human health outcomes with regulatory controls is the creation of the Foodborne Diseases Active Surveillance Network (FoodNet) program in the late 1990s. FoodNet was initiated by the U.S. Centers for Disease Control and Prevention in collaboration with USDA and the FDA, and was intended to assess the effectiveness of the 1996 Hazard Analysis and Critical Control Points (HACCP)/Pathogen Reduction regulations (Scallan, 2007). While FoodNet has produced valuable information (e.g., improved foodborne illness estimates, standardization of methods, identification of risk factors for pathogen-specific illnesses), it does not meet the need for information for effective monitoring of the success of the HACCP/Pathogen Reduction regulations. The overall role of foodborne illness attribution in a risk-based food safety management system is discussed further at the end of this chapter.
The committee discussed and recognized the challenges associated with measuring the success of policy interventions, which have also been cited by others (Havelaar et al., 2006; Charlebois and Yost, 2008). For example, whereas intermediate variables (e.g., pathogen testing in food at the time of processing) may be relatively easier to correlate with the adoption of an intervention, such correlation is, in general, much more difficult for a public health outcome (e.g., measured by FoodNet or national public health trends), even in cases where a link has high face validity (e.g., an intervention that decreases food contamination would be expected to improve public health). In many instances, other factors that are not necessarily controllable confound the identification of such correlations. Although intermediate measures are useful, direct measures of public health impact are essential for truly evaluating the effectiveness of food safety interventions in the long term. Hence, again, the need for accurate and comprehensive foodborne illness attribution data is clear.
Ideally, the monitoring and review step should be performed not by the group planning the intervention but by a different group with expertise in designing the collection, analysis, interpretation, and communication
of appropriate data and results to stakeholders (Chapter 5). As discussed in Chapter 11, this monitoring role could be assumed by an independent, centralized risk-based analysis and data management center. As with other aspects of a risk-based system, this process must be transparent and involve stakeholders meaningfully.
Finally, the entirety of the risk-based approach (Steps 1 through 6) should be seen as an iterative process, with a strong focus on continuous public health improvement. The monitoring and review process should be subject to rigorous quality assurance standards, with periodic quality reviews not only when goals are not being met, but also when goals are being consistently met, which may suggest the need for new standards or new measurement tools.
MOVING TOWARD THE DEVELOPMENT OF A COMPREHENSIVE RISK-BASED APPROACH TO FOOD SAFETY MANAGEMENT
The risk-based system described above is consistent with the principle of evidence-based public health, which has been defined as “the development, implementation, and evaluation of effective programs and policies in public health through application of principles of scientific reasoning, including systematic uses of data and information systems” (Brownson et al., 2003, p. 4). The evidence-based approach includes key characteristics of (1) interventions being based on the best possible science, (2) reliance on multidisciplinary problem solving, (3) systematic program planning, (4) sound evaluation of program efficacy, and (5) information dissemination. The committee advocates application of the evidence-based approach to food safety management.
The risk- and evidence-based food safety management approach described above is meant to be comprehensive in that the general steps are applicable to virtually all FDA food-related decision making. Certainly, the approach is relevant to broad-based prioritization, as might be the case for strategic planning of how best to use agency resources associated with specific functions (e.g., research, inspection, communication, surveillance). However, it is also applicable to decision making within any one unit of the agency, as might be the case for prioritization of the use of resources dedicated to the risk assessment function (e.g., which risk assessments to perform). It is fully applicable as well to specific decision making, such as deciding which of several competing risk reduction strategies to choose for implementation. The committee therefore sees the risk-based approach as providing the underlying structure for all of the FDA’s food safety decisions.
The steps outlined above are not meant to be conducted in a fixed order; rather, the system as a whole should be envisioned as fluid. Further-
more, as noted above, the overall approach, like risk analysis, is intended to be iterative. For example, broad-based public health risk ranking (Step 2) might be applied at the general commodity level (produce versus fish) and considering all agents to identify those of greatest public health concern. This activity would be followed by prioritization and reranking (Step 3) on the basis of additional factors that might affect the decision to intervene. Once high-priority hazards and/or commodities had been identified, the agency might return to Step 2 to place the riskiest specific products and their hazards in a high-risk general commodity category, followed by prioritization (Step 3). Following prioritization would be analysis and selection of intervention(s) (Step 4), during which frequent iteration would occur between Steps 4 and 6 in an effort to establish appropriate levels of protection, identify and evaluate candidate intervention strategies, and collect the information necessary to support the choice of intervention(s), which might or might not include the need for a full quantitative risk assessment. Once the intervention plan had been implemented and monitored (Steps 5 and 6), there would be a need for periodic reevaluation using risk ranking and prioritization (back to Steps 2 and 3) to ensure that resources would continue to be allocated appropriately.
Indeed, given the diversity and inherent dynamics of food safety issues, it is impossible to account for all potential eventualities in advance. As noted earlier, therefore, the risk-based system must be sufficiently flexible to respond to rapidly emerging food safety issues, and it must be reactive enough to facilitate its use in emergency situations, such as the management of foodborne illness outbreaks. Activities within each step, such as data collection, analysis, and modeling, will depend on the type of hazard. During an outbreak, for example, decisions must be made quickly and possibly with an incomplete collection of data. For this reason, it is essential that strategic planning performed in emergency situations be largely standardized so that immediate decisions are based on lessons learned and the likely availability of needed data. As another example, government tools for overseeing the safety of imported foods necessarily differ from those available to ensure the safety of domestically produced food. (Appendix E contains background information on various tools that are used to oversee imported foods here and in other countries.) As discussed in Chapter 4, the lack of jurisdiction over the production of food in other countries is an important differentiating factor for governance purposes. In fact, for imported foods, the data available to make decisions based on risk may be very different from those available for domestic foods, and the analysis will need to take into consideration such factors as the FDA’s knowledge of the foreign country’s food safety system. Still, decision making about prioritizing inspections, allowing importation of a product into the United States, or responding to an emergency situation should be based on the same
attributes listed in Box 3-1 and should follow the same basic risk-based approach. At a different level, flexibility needs to be integrated to allow for the “human element”; for example, an inspector should not be prevented from pursuing a hunch that something might be wrong.
RELATIONSHIP BETWEEN RISK ANALYSIS AND THE RISK-BASED FOOD SAFETY SYSTEM
To date, the term “risk-based” has been interpreted largely in the context of the basic elements of risk analysis. However, there has been some discussion for about a decade regarding the need to expand the meaning of the term. For example, a 2001 discussion paper issued by Resources for the Future3 (Taylor and Hoffman, 2001) suggests that the role of risk analysis be broadened:
There are, however, much broader roles for risk analysis at the level of system design and management…. They include: (1) guiding the allocation of inspection and enforcement resources, and (2) setting priorities for risk reduction initiatives. These are roles for risk analysis that can significantly enhance the effectiveness of the food safety system in reducing risk. (Taylor and Hoffman, 2001, p. 5)
The risk-based food safety management system presented here takes the concepts of risk analysis to an operational level by creating a process that uses analytical methodology to evaluate risk, and then facilitates decision making in light of the myriad factors that need to be considered in the risk management process. This sort of approach is not unlike that of HACCP, which provides the foundation for food safety control at the processing level of the food chain. Like HACCP, this conceptual approach to a risk-based food safety management program provides a road map that clearly defines the course of the process and the types of inputs that need to be considered along the way. This road map is a key component of the transparency of the system, with a focus not just on what has been done, but also on how the system will operate in the future. As envisioned by the committee, such a framework is comprehensive (providing a uniform means of assessing and comparing risk across the food safety system) and transparent (incorporating a clear understanding of how one goes from data to decisions); these and other key attributes of the risk-based system were noted earlier in Box 3-1.
|
3 |
See http://www.rff.org/documents/RFF-DP-01-24.pdf (accessed January 26, 2010). |
THE ROLE OF RISK ANALYSIS IN THE FDA’S CURRENT FOOD SAFETY MANAGEMENT PROGRAM
The FDA has been engaged in risk-based efforts in food safety management for more than a decade now. This section provides a brief synopsis of the committee’s understanding of those efforts, based on a public workshop held March 24, 2009, in Washington, DC, and on follow-up questions and interviews with CFSAN and CVM staff as well as background analysis by the committee. Although the committee has not attempted an in-depth evaluation of the FDA efforts, it has identified some gaps in these efforts. The committee notes that creation of the Office of Foods in 2009 with direct line of authority over CFSAN and CVM will likely impact both the functioning of these units and the ultimate implementation of a risk-based food safety approach.
Risk-Based Activities of CFSAN
Although CFSAN has a long history of conducting safety assessments for food additives and risk assessments for chemicals, it was not until 1999 that the center conducted more complex quantitative risk assessments for pathogens. In 2002, a CFSAN risk analysis working group produced an internal report Initiation and Conduct of All Major Risk Assessments Within a Risk Analysis Framework, which is based on the principles of risk analysis and describes how to prioritize and conduct risk assessments.4 Several offices within CFSAN have a role in developing and coordinating risk-based initiatives5:
-
The Risk Assessment Coordination Team (RACT) in the Office of Food Defense, Communication, and Emergency Response coordinates and manages risk profiles and assessments that require representation from different offices within CFSAN, and sometimes outside of CFSAN or even outside of the FDA. The RACT oversees “virtual” teams that are formed to conduct a project. It also serves as a liaison to appropriate entities—federal, state, and local government; industry; consumer groups; and academia—in the planning of food safety risk analysis activities and related research, and it provides direction for the conduct and coordination of risk analysis activities related to food.
-
In the Office of Food Safety, the Chemical Hazards Assessment Team conducts safety/risk assessments of industrial chemicals, both
-
elemental and organic, including naturally occurring contaminants and allergens.
-
The Economics Team in the Office of Regulations, Policy, and Social Sciences conducts analyses that are integrated with risk assessments, including economic impact analyses of decisions and cost–benefit analyses.
-
The Division of Field Programs and Guidance in the Office of Compliance coordinates and provides oversight for risk-related initiatives that impact field work planning.
-
Other offices at CFSAN that perform food safety assessments are the Office of Food Additive Safety and the Office of Nutrition, Labeling, and Dietary Supplements.
Risk-Based Activities of CVM
CVM also uses tools of risk ranking and risk assessment in its regulatory process (Hartogensis, 2009). CVM representatives stated that their risk management strategy is to prioritize activities aimed at reducing or mitigating risks according to the ranking of the risks and the limits of their authority and resources. However, CVM has not produced a document that delineates a standardized process for conducting risk assessments (or rankings) for potential contaminants in feed or specific guidelines for risk ranking or prioritization (Hartogensis, 2009). Only a few specific examples of CVM’s risk-based activities were provided to the committee. Specifically, the Office of New Drug Evaluation, which reviews information on approvals to manufacture and market animal drugs, is also responsible for evaluating human health impacts that might result from the consumption of drug residues present in the tissues of food animals. To date, the committee is uncertain about the mechanism by which this evaluation is performed. In 2003, a group consisting of CVM officials, along with representatives from the Office of the Commissioner and state regulatory officials, announced the implementation of the Animal Feed Safety System (AFSS). This system represented the first step toward making the agency’s animal feed safety program more comprehensive and risk based. To date, five public meetings to gather stakeholder input have been conducted, and this group is apparently developing a framework document describing the major processes, guidance, regulations, and policy issues entailed in addressing feed safety. As of this writing, however, the significance of the AFSS as applied to risk-based food safety management is unclear (Hartogensis, 2009).
Risk Analysis Products
Over the years, CFSAN and CVM have produced a variety of products related to risk analysis, including safety assessments, risk profiles, qualitative and quantitative risk assessments, and risk–benefit analyses. Perhaps the most comprehensive effort in this regard is the joint FDA (CFSAN)–USDA (FSIS) L. monocytogenes risk-ranking (assessment) model development mentioned earlier. Although the second iteration of this model was completed in 2003 (CFSAN/FSIS, 2003), action plan items are still being developed and implemented (CFSAN, 2008). Another notable risk assessment activity included evaluation of Vibrio parahaemolyticus risks associated with oyster consumption (FDA, 2005). In addition, the agency provides a high level of support to international organizations, such as the Food and Agriculture Organization/WHO and the Codex Alimentarius Commission, which have produced their own risk assessments. The FDA has also used others’ risk assessments in formulating regulations, such as the Shell Egg Rule, for which USDA’s “Risk Assessments for Salmonella Enteritidis in Shell Eggs and Salmonella spp. in Egg Products” was used. Similarly, the U.S. Environmental Protection Agency’s risk assessments have been applied by the FDA for management of chemical contaminants in the food supply.
A complete review of all food safety risk analysis activities with which the FDA has been involved is beyond the scope of this report. However, three new approaches described by FDA representatives during the public workshop held March 24, 2009, are worth discussing.
Public Health Risk Ranking
Under an FDA cooperative agreement, the Institute of Food Technologists convened a panel of experts to develop a risk-ranking prototype designed to analyze data on hazards (both chemical and biological) in food and return an estimate of the resulting health burden at a population level. Termed the iRisk model, this is a bottom-up or predictive modeling approach to risk ranking that requires the application of data and expert judgment to assemble sufficient information with which to predict the ecology of the hazards in the food supply. These results are combined with food intake data and information on hazard virulence or toxicity to produce a prediction of the relative level of risk to human health of the particular hazard–food pair. The model produces a semiquantitative characterization of the disease burden, which can be used for comparison (ranking) purposes and can facilitate evaluation of the impacts of hazard control measures. The model was further developed by Risk Sciences International, a consulting company, into a web-accessible tool. RTI International is currently populat-
ing the iRisk model with data for proof-of-concept testing; Risk Sciences International is revising the model to improve its performance with respect to a variety of features (Wagner, 2009).
Risk-Based Inspection
Much like FSIS, the FDA has been developing models to assist in the allocation of inspectional resources, sometimes referred to as “risk-based inspection” (Engeljohn, 2009; Maczka, 2009). For example, CFSAN’s Division of Field Programs and Guidance, which is responsible for developing tools to assist the Office of Regulatory Affairs (ORA) in resource management, has been working on the identification of high-risk food categories to support the targeting of field inspections and sample collection resources as applied to domestic food products and manufacturers. This effort began in 2002 as a simple document based on expert opinion from CFSAN technical experts. By 2008, a risk-based domestic priorities list had been developed for ranking particular product–hazard combinations and facilities (Wagner, 2009). The model appears to utilize such information as the occurrence of multiple hazards, the potential for fatal illness outbreaks, consumption by all segments of the population, and conditions under which the hazard is likely to occur. For ranking purposes, risk is considered a function of the likelihood of a hazard in a product and the severity of the health effect.
CFSAN also performed a risk-ranking exercise on food manufacturers based on their association with Class I recalls,6 outbreaks, or serious adverse events during 2004, 2005, and 2006. The statistical analysis resulted in a scoring algorithm that was applied to each of the individual food firms. For fiscal year (FY) 2010, the FDA intends to design an updated version of the 2009 algorithm that will be overlaid with compliance history information for specific facilities. In the future, criteria such as the financial viability of the firms and their legal status may also be included as ranking criteria (Givens, 2009; Wagner, 2009).
ORA has reported prioritizing its inspectional resources for FY 2009 based on three categories: category 1, high-risk firm inspections; category 2, inspected plants with compliance issues; and category 3, low-risk industry blitzes. Likewise, CVM is in the process of changing its allocation of inspectional resources so that the resources are allocated in alignment with more objective ranking criteria for the various areas of each program (Hartogensis, 2009). The CVM efforts, which appear to be focused on medicated feeds, are still being developed in conjunction with AFSS; the committee was provided with only limited details.
Risk-Based Management of Imported Foods
The FDA recently embarked on the development of a risk-based approach to managing the safety of food imports, which culminated in the release of the Predictive Risk-Based Evaluation for Dynamic Import Compliance Targeting (PREDICT) model (see also Appendix E). PREDICT is an import screening tool that is intended to automate decisions currently made by import entry reviewers by utilizing intelligence information from numerous sources so as to direct resources to products presenting the greatest risks to public health in a streamlined manner. Criteria such as information about recalls, registration of low-acid canned food processes, agreements with other countries, monitoring of products, and information on certification of facilities and from import certificates are used to calculate a risk score upon which decisions about a food import shipment are based. After a pilot study in June 2007 that the FDA judged to be successful, the agency estimated that PREDICT would be widely implemented by September 2009 (Solomon, 2009).
Food Safety Performance Measures
The Healthy People food safety goals could theoretically serve as the basis for the identification of specific performance measures. However, these goals now use such words as “reduce” and “improve,” which cannot serve as metrics per se. Other targets and indicators for measuring the performance of the federal food safety system have been recently described. For example, the U.S. Department of Health and Human Services (HHS) has identified some food safety–related outcome indicators in its strategic plan (see Box 3-3). Elements of the 2004 FDA Program Assessment Rating Tool (PART) report and progress on implementation of the Food Protection Plan also can be used to identify some performance measures. PART was introduced in 2002 to standardize the measurement of performance in federal agencies, with the intent of linking performance to budgets (Gueorguieva et al., 2009) (see also Chapter 2). Presented by the Office of Management and Budget as a tool for implementing the GPRA, PART assesses GPRA performance strategies and goals, albeit to a limited extent. The GPRA requires that agencies demonstrate accountability and the effectiveness of programs to Congress and the public by establishing performance measures. PART has been described as applying a different level of analysis than the GPRA, conflicting with the GPRA regarding what to measure and how to measure it (GAO, 2005) and having serious limitations and questionable reliability (Radin, 2006; Gueorguieva et al., 2009). More specific to the FDA’s food protection efforts, the agency’s 2010 budget justification for implementation of the Food Protection Plan (FPP) includes several long-term objec-
|
BOX 3-3 Food Safety–Related Outcome Indicators Listed Under “Other Outcome Indicators,” U.S. Department of Health and Human Services’ Strategic Plan (2009)
|
tives and associated measures (see Box 3-4). Overall, these objectives and measures focus on numbers of outputs, voluntary outcomes, and indirect measures of capacity to achieve public health, and hence they might be considered potentially useful metrics of performance. Recent progress reports on the implementation of the FPP also describe a wide variety of outputs (e.g., numbers of public meetings and workshops, technical guidance and rules issued, foreign offices established, memorandums of understanding, cooperative and interagency agreements).
COMPARISON OF THE CURRENT FDA APPROACH TO RISK MANAGEMENT AGAINST THE VISION AND ATTRIBUTES OF A TRUE RISK-BASED DECISION-MAKING APPROACH TO FOOD SAFETY MANAGEMENT
Based on the information presented in public meetings and conversations with FDA staff and other publicly available information, the committee concluded that the agency currently is not practicing some aspects of a systematic risk-based food safety management approach with the attributes identified in Box 3-1. The agency has embraced the tool of risk assessment, and it should be commended for doing so. The development of the risk-ranking/assessment model for L. monocytogenes mentioned above is a notable example of a comprehensive risk assessment produced in cooperation with another food safety agency and with stakeholder involvement.
|
BOX 3-4 Objectives Listed in the U.S. Food and Drug Administration’s (FDA’s) 2010 Congressional Budget Justification for Food Long-Term Objective: Increase access to safe and nutritious new food products. Measure 213301: Complete review and action on the safety evaluation of direct and indirect food and color additive petitions, including petitions for food contact substances, within 360 days of receipt. (Output) Long-Term Objective: Prevent safety problems by modernizing science-based standards and tools to ensure high-quality manufacturing, processing, and distribution. Measure 214101: Number of state, local, and tribal regulatory agencies in the U.S. and its Territories enrolled in the draft Voluntary National Retail Food Regulatory Program Standards. (Outcome) Measure 214102: Percentage of the enrolled jurisdictions which meet 2 or more of the Standards. (Outcome) Long-Term Objective: Provide consumers with clear and timely information to protect them from foodborne illness and promote better nutrition. Measure 212401: Increase by 40 percent the percentage of American consumers who correctly identify that trans fat increases the risk of heart disease. (Outcome) Measure 212402: Increase by 10 percent the percentage of American consumers who correctly identify that saturated fat increases the risk of heart disease. (Outcome) |
It appears that in general, the agency’s microbial risk assessments have been performed in accordance with well-recognized standards (FAO/WHO, 2006; NRC/IOM, 2009).
However, the production of risk assessments and profiles alone does not constitute a risk-based food safety management system. The FDA does not employ the stepwise process outlined above, and it does not appear to have any strategic vision for a risk-based system. The fact that risk-based ranking and inspection models are under development and in various stages of implementation is commendable, but the use of these tools does not imply that a comprehensive risk-based approach is being pursued.
|
Measure 212403: Improve by 10 percent the percentage of American consumers who correctly identify that omega-3 fat is a possible factor in reducing the risk of heart disease. (Outcome) Long-Term Objective: Detect safety problems earlier and better target interventions to prevent harm to consumers. Measure 214201: Number of prior notice import security reviews. (Output) Measure 214202: Number of import food field exams. (Output) Measure 214203: Number of Filer Evaluations. (Output) Measure 214204: Number of examinations of FDA refused entries. (Output) Measure 214205: Number of high-risk food inspections. (Output) Measure 214206: Maintain accreditation for Office of Regulatory Affairs labs. (Outcome) Measure 214303: Convert data from new Electronic Laboratory Exchange Network (eLEXNET) participating laboratories via automated exchange or convert data from existing manual data streams to automated data exchange. (Outcome) Measure 214305: Increase laboratory surge capacity in the event of terrorist attack on the food supply (radiological and chemical samples/week). (Outcome) NOTE: “Output” and “Outcome” designations appear in the Budget Justification. |
The absence of a strategic vision to embrace and implement a risk-based food safety management system is apparent at almost every level. Despite many counterexamples, the relative lack of strategic planning and incorporation of appropriate metrics for evaluating the efficacy of food safety control strategies illustrates the point well. For example, although the latest PART report7 for the FDA, produced in 2003, resulted in a performance rating of “moderately effective,” the report does not mention any direct public health
|
7 |
See http://www.whitehouse.gov/omb/expectmore/detail/10001057.2003.htm (accessed October 8, 2010) |
measures for food safety. Of the ten long-term performance goals8 adopted by the FDA in 2003, the report includes only one that pertains directly to food safety: “increase laboratory surge capacity in the event of a terrorist attack on the food supply.” In this case, it is unclear from the report how the specific targets (i.e., radiation and chemical contamination) for surge capacity were identified. In a similar manner, the recent FPP reports do not appear to map progress to metrics, and the 2010 FDA Congressional Budget Justification for the FPP does not appear to map directly to the plan’s eight goals. Perhaps most notable, the FDA’s 2010 Congressional Budget Justification for Food (Box 3-4) identifies objectives that are not necessarily consistent with the food safety goals proposed for Healthy People 2020 (Box 3-2), and specific metrics for the agency to use in measuring performance in food safety are not identified, with the exception of the few food safety outcome measures stated in the 2009 HHS Strategic Plan (Box 3-3). Briefly, it is not clear that efforts to identify performance measures for food safety or public health are linked to strategic goals as recommended by this committee.
Also, the measures identified in the FPP, the HHS Strategic Plan, and Healthy People 2020 fall short of suggested standards for performance indicators and systems (see, for example, the recommendations in Bird et al., 2005). GAO reports and other assessments suggest that performance indicators have been underutilized to improve agencies’ decision making in the last decade (Cavalluzzo and Ittner, 2004; GAO, 2009a; Taylor, 2009). Among the obstacles to effective use of performance measurement are ambiguous goals and objectives, a lack of commitment to performance measurement on the part of top management, and inadequate measurement and analysis systems (Cavalluzzo and Ittner, 2004; Johnsen, 2005; Taylor, 2009). Overall, the committee found that the FDA and HHS have made limited progress toward establishing and applying performance measures, particularly those related to public health outcomes, as part of a risk-based food safety system.
Most of the other attributes of a risk-based food safety management system (e.g., public health risk ranking, prioritization for resource allocation, transparency in risk management, effective and frequent communication with stakeholders) are all but absent from the FDA’s current approach to food safety. For example, well-articulated management objectives were not delineated when the iRisk model was presented to the committee. Likewise, the committee was not provided with additional details on the PREDICT model, which should undergo extensive peer review before being deployed
in the field. Of interest, it was apparent during the workshop held on March 24, 2009, that stakeholders were unaware of many of the FDA’s more recent risk-ranking/prioritization efforts, including its plans for a risk-based inspection system or the development of PREDICT as a risk-based tool to manage food imports (Bell, 2009; Gombas, 2009; Scott, 2009). Consistent with a recent report (USDA, 2010), the committee concluded that improvement of the agency’s risk-based approach is also needed in the area of preventing risk from chemical contaminants.
The area of risk communication also remains a challenge. In general, the committee found a lack of transparency in the FDA’s food safety activities and insufficient communication with stakeholders. Examples include insufficient description of risk-based initiatives and use of peer-review. Although the FDA’s Risk Communication Advisory Committee (RCAC) was recently created to advise the agency on communication strategies and programs, and the FDA has created an internal Communication Committee to coordinate its communication activities, prioritizing and evaluating risk communication efforts remain a challenge (Chapter 9). During its August 2009 meeting, the RCAC discussed the FDA’s research on consumer knowledge of food recalls and plans for monitoring the effectiveness of communication during recalls. An integrated risk-based management system should enable the FDA to target, design, and evaluate its risk communication more effectively (Morgan et al., 1992).
IMPLEMENTATION OF A RISK-BASED FOOD SAFETY MANAGEMENT SYSTEM
Implementation of a risk-based food safety management system will be successful only if the necessary resources are dedicated to the effort. It should be clear from the preceding discussion that this will be a substantial undertaking. Virtually all of the recommendations in this report can and perhaps should be adopted with the stated purpose of supporting a risk-based approach to food safety management. Nonetheless, the committee has identified a few areas that it considers particularly critical to the successful implementation of a risk-based system, which are discussed below.
Personnel and Analytical Tools
There is a tendency on the part of government agencies, including the FDA, to assume that scientists are interchangeable: that individuals trained to conduct bench experiments in microbiology, for example, can easily be shifted to performing risk assessment or crisis management. It is essential that new scientific staff be acquired to provide the core competencies necessary to create a new, risk-based approach for the agency. The committee
recognizes the difficulty of finding individuals trained in the breadth and depth of food safety problems who are also proficient in epidemiology, mathematical modeling, economics, or other disciplines necessary to support a risk-based approach. Academic institutions do not typically configure their programs with the necessary training to prepare students to be a part of a risk management team. Accordingly, it may be necessary to initiate agencywide recruitment and training efforts to train professionals in the skills necessary to support a risk-based approach. A good example of this sort of initiative is the new FDA Commissioner’s Fellowship Program.
Further, the body of FDA scientists must be able to support both management of crises at the time they occur and prevention of future crises. To ensure that all of the FDA’s responsibilities are met, resources need to be allocated for both routine operations and prevention of long-term food safety problems. While, as recommended here, a risk-based approach is institutionalized over time, the FDA should continue to attend to more immediate issues. The committee found disturbing various testimony confirming that during an emergency, work is redirected, and the FDA’s focus on prevention and long-term efforts receives lower priority. To alleviate this situation and to advance the FDA toward a risk-based regulatory approach, experts will need to be hired in the areas recommended by the committee.
To carry out all its food safety responsibilities and specifically to ensure continuation of everyday operations, then, the FDA’s food programs must include sufficient staff working on food issues to ensure that routine functions will continue even when a crisis emerges. A logical way to address this need is to form functional teams that would work in defined areas identified during the strategic planning process. Many of these teams could support efforts to manage the identified risk-based priorities with a focus on prevention. For example, there could be a Research Team, whose major function would be to support the high-priority research necessary to support the risk-based mission. Likewise, a Surveillance Team would be responsible for interacting with other federal agencies and state and local jurisdictions and for managing centralized epidemiological databases supporting modeling efforts. Similarly, there might be a Risk Assessment Team, a Risk Communication Team, and a Risk Management Team. Recognizing the need for continuous support of the crisis management function, it could be appropriate to have a separate team dedicated to this function. However, crises must not be allowed to preempt a substantial part of the effort allocated to essential noncrisis activities.
Alternatives to the development of agency competency to build and operate a risk-based food safety system are discussed in Chapter 11. One option is to create a centralized risk-based analysis and data management center, which would provide a multiagency and multidisciplinary core of expertise in risk analysis for all agencies with responsibilities for food safety.
This center would mirror the European model, in which such expertise is often housed in quasigovernmental research institutes (such as the National Institute for Public Health and the Environment in the Netherlands) that assist in data collection and provide independent analyses of incoming data for policy makers. Such a center would not subsume an agency’s prerogative to develop food safety policy, and there would remain a need for analytic capacity at the top level of agencies, such as the FDA, with responsibility for food safety. However, the center’s creation would eliminate the need for each agency involved in food safety to develop its own comprehensive expertise in risk analysis independently. As discussed in Chapter 11, a longer-term alternative would be the creation of a unified national food safety agency, which, as part of an overall consolidation of food safety activities (possibly though an intermediate office of food protection) would integrate the risk-based efforts of the multiple agencies currently involved in food safety.
Closely related to the personnel issue is the need for targeted research activities to permit the development of the information infrastructure required to support risk-based food safety management (see Chapter 5). At a basic level, the software needs for these activities are daunting, as most risk-modeling tools are not off-the-shelf software but highly customized. The FDA, alone or in collaboration with other agencies, must commit the resources required for the applied research needed to develop and test software and computer systems that are integral to infrastructure development. Again, the agency may want to support the creation of a risk-based analysis and data management center that would provide these services across all agencies involved in food safety.
Foodborne Disease Attribution Data and Models
The IOM/NRC report Scientific Criteria to Ensure Safe Food states that “science-based food safety criteria must be clearly linked to the public health problem they are designed to address. To accomplish this, a cause/effect relationship needs to be established between contaminants in foods and human disease, that is, to allocate the burden of foodborne disease among foods and food groups” (IOM/NRC, 2003, p. 250). This statement forms the basis of what is now referred to as foodborne disease attribution, defined as “the capacity to attribute cases of foodborne disease to the food vehicle or other source responsible for illness” (Batz et al., 2005, p. 993; EFSA, 2008, p. 5). While the concept is valuable, the committee recognizes the lack of truly reliable attribution data and the somewhat limited scope of this definition. A description of the current sources of foodborne disease attribution data as well as approaches to foodborne disease attribution and their advantages and limitations as reported by a recent NRC committee are summarized in Box 3-5 and Table 3-2, respectively. It is clear that substantially more
|
BOX 3-5 Sources of Foodborne Disease Attribution Data Data on foodborne disease attribution generally come from three major sources: (1) outbreak reports, (2) case control studies, and (3) source tracking. Outbreak Reports: Outbreak investigations have traditionally served as the primary means of identifying food sources for pathogens. When outbreaks are carefully investigated, such data can be extremely valuable. In the United States, however, almost all outbreak investigations are conducted by local health departments, which tend to be overworked and to lack either the laboratory or epidemiologic resources to identify a source. There are significant biases involved in the choice of which outbreaks get investigated (generally those that are large or involve an “interesting” pathogen), and the percentage of outbreaks reported and investigated ranges widely both among and within states. Outbreaks may also not be representative of routine foodborne disease cases: they generally represent a significant breakdown in food practices rather than the endemic pattern of transmission of pathogenic microorganisms. There are issues with timeliness as well: the U.S. Centers for Disease Control and Prevention tends to compile data from outbreak reports only on a sporadic basis, which often results in multiple-year gaps between reporting of national summary data. The United Kingdom has tended to rely on outbreak data in its food attribution/food safety efforts; however, its data collection is more standardized than that of the United States, without the wide variability in reporting from local health department to local health department (Batz et al., 2005). Case Control Studies: When FoodNet was first established, the importance of food attribution in the calculation of food-specific incidence rates was recognized. Consequently, the system was designed to include ongoing case control studies to identify specific foods/food groups that might be consumed more commonly by ill persons infected with a specific |
resources will be needed for further characterization of foodborne disease attribution in support of risk-based food safety management.
Simply knowing the proportion of the occurrence of a particular disease that is associated with a specified hazard is not enough. For example, contamination and agent proliferation (and inactivation) can occur at all stages throughout the food chain. There is a need for attribution estimates across the chain—for example, what proportion of salmonel-
|
pathogen than by well controls. Under the FoodNet program, six case control studies have been conducted. While many have yielded useful epidemiologic data (Friedman et al., 2004; Marcus et al., 2007; Varma et al., 2007), it has become apparent that this is not an effective means to determine attribution percentages: it is expensive and labor intensive, and it yields only crude estimates of the relative contribution of various food categories to disease incidence. Concern has also been raised about possible biases inherent in the selection control process (which has generally involved random digit dialing techniques). Source Tracking: Food safety agencies in the Netherlands and Denmark have pioneered work in the source tracking of pathogens, that is, using molecular markers/typing to link human disease with animal sources. The process requires careful monitoring of isolates from food animals, with appropriate typing, and application of identical typing methods for human isolates. Data are then entered into models that permit real-time calculation of the relative public health impact of various food–pathoge combinations. These data have been used effectively, particularly in the Netherlands, to guide regulatory actions designed to deal with new and emergent problems in the national food safety system. However, this work has dealt almost exclusively with animal sources for pathogens; virtually no work has been done with pathogen contamination of produce, and produce generally has not been included in the source-tracking models. In the United States, some initial efforts were made to develop such a system, focusing primarily on salmonella. However, results have not been impressive, in part because of the incompatibility of data sets (and lack of data sharing). The U.S. Food and Drug Administration has sponsored intramural research on molecular-typing methods that might be utilized in these systems, but to date, efforts to develop appropriate risk models have not led to useful results. |
losis cases attributable to the consumption of contaminated leafy greens is associated with poor personal hygiene practices of food handlers versus preharvest contamination on the farm? Likewise, because agents can be transmitted by multiple routes, more defined data on transmission are needed—for instance, what proportion of human norovirus infections is attributable to foodborne routes as compared with person-to-person transmission? These are simply examples of important questions about
TABLE 3-2 Summary of Approaches to Food Attribution
|
Approach |
Data Needs |
Advantages |
Limitations |
Examples |
|
Foodborne disease surveillance Based on the use of aggregate data from epidemiologic investigation of outbreaks. |
Coordinated surveillance systems with similar data collection efforts throughout a specified location, region, or country. Takes many years to accumulate sufficient data. Must include food vehicle information. |
Usually applied to multiple foods and pathogens. Outbreak data are a measure of attribution at the point of consumption. Able to take into account other routes of transmission (such as travel, contact with animals). Addresses a broad range of microbes and foods. Data are collected routinely on a national basis for a large number of pathogens over many years. |
Assumes equivalence of pathogen-specific contributions of each food type to disease. Adequately classifying multicomponent foods is challenging. Serious gaps in databases exist. |
Batz et al., 2004; Adak et al., 2005; DeWaal et al., 2006 |
|
Case control study Analytic epidemiologic study that compares diseased (cases) with nondiseased (controls) persons with respect to previous exposures; relative role of exposure is determined by comparing frequencies in cases and controls. |
Systematic review of published case control studies and case series to identify relevant risk factors for disease; calculation of population-attributable risk to estimate relative importance of different exposures. |
Identification of sources of sporadic infections. Classic strengths of case control study design, including ability to explore multiple exposures (specific foods, food preparation practices, cross-contamination, travel, other risk factors). |
Classic weaknesses of case control studies, including misclassification, recall bias, limited resolution for commonly consumed foods, establishment of temporality. Many cases required for adequate statistical power. Usually limited to a single microorganism rather than multiple agents. Expensive. |
Multiple examples, but specific applications to food attribution are sparse (see EFSA, 2008) |
|
Microbial subtyping Based on a combination of strain typing collated with epidemiologic surveillance data and mathematical modeling; underlying hypothesis is that control of ultimate reservoir (usually occurring before harvest) will prevent human exposure. |
Integrated, active surveillance of most major sources (food, animals); reliance on extensive collection of representative strains for comparison; information on amount of animal or food product available for consumption. |
Best suited to pathogens that are clonally disseminated through the food chain. Useful in identifying original reservoir and setting priorities for interventions when contamination occurs during production. |
Usually applied to a single pathogen. In current use, but not well suited to organisms that have relatively unstable DNA. Does not take into account effects of other contamination risk factors (such as human handling, cross-contamination). Assumes equivalence of relative pathogen-specific contribution of each food type to disease. Expensive. Requires extensive libraries of isolates from a range of foods or reservoirs. |
van Pelt et al., 2003; Hald et al., 2004, 2007 |
|
Quantitative microbial risk assessment Yields mathematically derived estimates of risk. |
Information on estimates for parameters to use in modeling and uncertainty distributions for estimates; logic model of how parameters are related to each other. |
Allows a high level of detail with respect to specific food commodities. Theoretically, can integrate data obtained from national surveillance programs. |
Serious data gaps. Substantial uncertainty (should be accompanied by uncertainty analysis). Resource intensive. |
CFSAN/FSIS, 2003 (Listeria monocytogenes); Evers et al., 2008 |
|
Expert elicitation Experts combine and weigh data from different sources to estimate attribution. |
More explicit, structured, quantitative methods (including well-calibrated methods and performance-based weighting) are being used and are increasing resolution and transparency. |
Best suited to filling in data gaps. Able to combine information from multiple sources (different experts with different knowledge bases). |
Subjective in nature. Potential for bias (based on respondent background, personal bias), although use of structured approaches decreases bias. Best methodological approaches (calibrated, highly structured) require a high degree of expertise and are resource intensive. |
Hoffmann et al., 2007a,b; Havelaar et al., 2008 |
|
SOURCE: NRC, 2009b. |
||||
attribution that must be answered if food safety risks are to be understood and characterized.
Foodborne disease attribution data and models are essential to support a risk-based food safety management approach. They directly support Steps 1 (strategic planning), 2 (public health risk ranking), and 6 (monitoring and review); they also support the other steps of the process indirectly. From a planning perspective, for example, risk ranking must be based on the hazard–food combinations that generate the greatest burden of disease and/or the most significant negative impact on public health. It is difficult to perform such risk ranking without reliable foodborne disease attribution data. Similarly, it is difficult to evaluate and implement risk-based intervention approaches without knowing the most likely means by which a contaminant enters the food chain or which specific practices contribute to its proliferation and/or inactivation. Finally, in monitoring and reviewing the efficacy of risk management strategies that have already been implemented, it is necessary to determine whether public health objectives are being met. Attribution is a logical metric in this regard, perhaps the most important one, as a reduction in the burden of disease associated with a specific food–hazard combination provides the best evidence that interventions are working. The availability of comprehensive epidemiological attribution data also aids in transparency. In short, solid epidemiological attribution data form the cornerstone of risk-based prioritization, management, and evaluation.
KEY CONCLUSIONS AND RECOMMENDATIONS
The committee defined a risk-based food safety management system as “a systematic means by which to facilitate decision making to reduce public health risk in light of limited resources and additional factors that may be considered.” The committee went on to define the key attributes of such a system and produced a stepwise approach to its design. The committee recognizes that some of the variables to be considered in models used to rank risks from imported foods will be different from those considered for domestic foods. Variables for models used to rank intentional contamination will be different as well. However, the committee believes the recommended risk-based approach is broad enough to apply to all hazards, whether intentionally introduced or not, and to all foods, whether domestically produced or imported. The committee recognizes that this comprehensive risk-based approach is a relatively new concept that will take time and resources to implement.
While the committee commends the FDA for recent steps taken and progress toward risk ranking and prioritization described in this chapter, the FPP falls short of providing a comprehensive vision for a risk-based food safety management system. Much of the agency’s current decision-
making process appears to be based on crisis management rather than a systematic preventive approach. Furthermore, although the FDA states in many of its documents that it operates under a risk-based framework, many of the attributes of a risk-based system that the committee regards as necessary (in particular, strategic planning, comprehensiveness, transparency, external review of risk assessment and intervention analysis programs, and risk communication) are not sufficient in the agency’s current approach. The resources (personnel, data, models) necessary to design and support a risk-based food safety management system are extensive, and the FDA does not have the human capacity, data infrastructure, or organization to support such a function at the present time. The provision of these resources is essential to the success of the FDA’s future food safety risk management activities.
The committee offers the following recommendations to enhance the management of food safety at the FDA.
Recommendation 3-1: The type of risk-based food safety approach outlined by the committee in Box 3-2 should become the operational centerpiece of the FDA’s food safety program. This approach should be embraced by all levels of management and should serve as the basis for food safety decision making, including prioritization of resources dedicated to all agency functions (e.g., inspections, promulgation of regulations, research). This approach should be applied to all domestically produced and imported foods and to all food-related hazards, whether due to unintentional or intentional (i.e., with intent to harm) contamination. The FDA should work with local, state, and national regulatory partners to facilitate the incorporation of these principles into their programs.
Recommendation 3-2: The FDA should develop a comprehensive strategic plan for development and implementation of a risk-based food safety management system. The agency should also develop internal operating guidelines for the conduct of risk ranking, risk assessment, risk prioritization, intervention analysis, and the development of metrics with which to evaluate the performance of the system. The strategic plan and guidelines should include descriptions of data, methodologies, technical analyses, and stakeholder engagement. Further, the strategic plan and all guidelines for the risk-based system should be fully supported by the scientific literature and subjected to peer review. When appropriate, the FDA should adopt guidelines already established by other federal agencies or international organizations.
The following recommendations encompass essential steps that need special attention in the implementation of a risk-based approach.
Recommendation 3-3: The FDA, in collaboration with partners, should identify metrics with which to measure the effectiveness of the food safety system, as well as its interventions. The FDA should include these metrics, and plans for any related data collection, as part of strategic planning. The metrics should have a clearly defined link to public health outcomes.
Recommendation 3-4: The FDA should identify expertise needed to implement a risk-based approach. This includes training current and/or hiring new personnel in the areas of strategic planning; management of data; development of biomathematical models and other tools for risk ranking, prioritization, intervention analysis, and evaluation; and risk communication.
REFERENCES
Adak, G. K., S. M. Meakins, H. Yip, B. A. Lopman, and S. J. O’Brien. 2005. Disease risks from foods, England and Wales: 1996–2000. Emerging Infectious Diseases 11(3):365–372.
Batz, M. B., S. A. Hoffmann, A. J. Krupnick, J. G. Morris, D. M. Sherman, M. R. Taylor, and J. S. Tick. 2004. Identifying the Most Significant Microbiological Foodborne Hazards to Public Health: A New Risk Ranking Model. Food Safety Research Consortium Discussion Paper No. 1. Resources for the Future, Washington, DC. http://www.rff.org/RFF/Documents/FRSC-DP-01.pdf (accessed February 6, 2009).
Batz, M. B., M. P. Doyle, G. Morris, Jr., J. Painter, R. Singh, R. V. Tauxe, M. R. Taylor, and D. M. Lo Fo Wong. 2005. Attributing illness to food. Emerging Infectious Diseases 11(7):993–999.
Becker, G. S. 2008. U.S. Food and Agricultural Imports: Safeguards and Selected Issues. Washington, DC: Congressional Research Service.
Becker, G. S. 2009. Food Safety on the Farm: Federal Programs and Selected Proposals. Washington, DC: Congressional Research Service.
Behn, R. D. 2003. Why measure performance? Different purposes require different measures. Public Administration Review 63(5):586–606.
Bell, J. W. 2009. The USFDA and Ensuring Food Safety Perspective of a Seafood Scientist. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.
Belton, V., and T. J. Stewart. 2002. Multiple Criteria Decision Analysis: An Integrated Approach. Boston, MA: Kluwer Academic Publishers.
Bevan, G., and C. Hood. 2006. Have targets improved performance in the English NHS? British Medical Journal 332(7538):419–422.
Bird, S., C. S. David, V. T. Farewell, H. Goldstein,T. Holt, and P. C. Smith. 2005. Performance indicators: Good, bad, and ugly. Journal of the Royal Statistical Society Series A 168(Part 1):1–27.
Brougher, C., and G. S. Becker. 2008. CRS Report for Congress: The USDA’s Authority to Recall Meat and Poultry Products. Washington, DC: Congressional Research Service.
Brownson, R. C., E. A. Baker, T. L. Leet, and K. N. Gillespie. 2003. Chapter 1: The need for evidence-based public health. In Evidence-Based Public Health, edited by R. C. Brownson, E. A. Baker, T. L. Leet and K. N. Gillespie. Oxford, UK: Oxford University Press.
CAST (Council for Agricultural Science and Technology). 2006. Using Risk Analysis to Inform Microbial Food Safety Decisions. CAST Issue Paper Number 31. Ames, IA: Council for Agricultural Science and Technology.
Cavalluzzo, K. S., and C. D. Ittner. 2004. Implementing performance measurement innovations: Evidence from government. Accounting, Organizations, and Society 29(3–4):243–267.
CFSAN (Center for Food Safety and Applied Nutrition). 2008. Sec. 555.320 Listeria Monocytogenes Draft Guidance. F. C. ORA. Rockville, MD: FDA CFSAN. http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm136694.htm (accessed March 15, 2010).
CFSAN/FSIS (Food Safety Inspection Service). 2003. Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria Monocytogenes Among Selected Categories of Ready-to-Eat Foods. Washington, DC: CFSAN/FSIS. http://www.fda.gov/ohrms/dockets/dailys/03/oct03/103003/99n-1168-ra00001-002-Assessment-vol24.doc (accessed February 9, 2009).
Charlebois, S., and C. Yost. 2008. Food Safety Performance World Ranking 2008. Saskatchewan, Canada: Research Network in Food Systems, University of Regina. http://www.ontraceagrifood.com/admincp/uploadedfiles/Food%20Safety%20Performance%20World%20Ranking%202008.pdf (accessed on March 13, 2010).
Consumers Union. 2008. Increased Inspections Needed for Produce, Processing Plants to Protect Consumers from Unsafe Food. http://www.consumersunion.org/pub/core_food_safety/005732.html (accessed January 25, 2010).
DeWaal, C. S., H. Giselle, K. Barlow, L. Alderton, and L. Vegosen. 2006. Foods associated with foodborne illness outbreaks from 1990 through 2003. Food Protection Trends 26(7):466–473.
EFSA (European Food Safety Authority). 2008. Overview of methods for source attribution for human illness from foodborne microbiological hazards: Scientific opinion of the Panel on Biological Hazards. The EFSA Journal 764:1–43. http://www.efsa.europa.eu/cs/BlobServer/Scientific_Opinion/biohaz_op_ej764_source_attribution_en.pdf?ssbinary=true (accessed February 6, 2009).
Engeljohn, D. 2009. USDA’s Approach to Ensuring Food Safety. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, May 28, 2009.
Evers, E. G., H. J. Van Der Fels-Klerx, M. J. Nauta, J. F. Schijven, and A. H. Havelaar. 2008. Campylobacter source attribution by exposure assessment. International Journal of Risk Assessment and Management 8(1/2):174–190.
FAO/WHO (Food and Agriculture Organization/World Health Organization). 2006. Food. safety risk analysis. A guide for national food safety authorities. FAO Food and Nutrition Series 87. ftp://ftp.fao.org/docrep/fao/009/a0822e/a0822e00.pdf (accessed March 5, 2009).
Fazil, A., A. Rajic, J. Sanchez, and S. McEwen. 2008. Choices, choices: The application of multi-criteria decision analysis to a food safety decision-making problem. Journal of Food Protection 71(11):2323–2333.
FDA (U.S. Food and Drug Administration). 2005. Pathogenic Vibrio Parahaemolyticus in Raw Oysters: Quantitative Risk Assessment on the Public Health Impact of Pathogenic Vibrio Parahaemolyticus in Raw Oysters. http://www.fda.gov/Food/ScienceResearch/ResearchAreas/RiskAssessmentSafetyAssessment/ucm050421.htm (accessed January 26, 2010).
FDA. 2009. Office of the Commissioner reorganization; statement of organizations, functions, and delegations of authority: Action notice. Federal Register 74(158):41713–41734.
Friedman, C. R., R. M. Hoekstra, M. Samuel, R. Marcus, J. Bender, B. Shiferaw, S. Reddy, S. Ahuja, D. L. Helfrick, F. Hardnett, M. Carter, B. Anderson, and R. V. Tauxe. 2004. Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clinical Infectious Diseases 38(Suppl. 3):S285–S296.–S296.296.
GAO (U.S. Government Accountability Office). 2004a. Federal Food Safety and Security System: Fundamental Restructuring Is Needed to Address Fragmentation and Overlap. Washington, DC: GAO.
GAO. 2004b. Food Safety: FDA’s Imported Seafood Safety Program Shows Some Progress, but Further Improvements Are Needed. Washington, DC: GAO.
GAO. 2004c. Food Safety: USDA and FDA Need to Better Ensure Prompt and Complete Recalls of Potentially Unsafe Food. Washington, DC: GAO.
GAO. 2005. Overseeing the U.S. Food Supply: Steps Should Be Taken to Reduce Overlapping Inspections and Related Activities. Washington, DC: GAO.
GAO. 2007. Federal Oversight of Food Safety: High-Risk Designation Can Bring Needed Attention to Fragmented System. Washington, DC: GAO.
GAO. 2008. Food Safety: Improvements Needed in FDA Oversight of Fresh Produce. Washington, DC: GAO.
GAO. 2009a. Agencies Need to Address Gaps in Enforcement and Collaboration to Enhance Safety of Imported Food. Washington, DC: GAO.
GAO. 2009b. Information Technology: FDA Needs to Establish Key Plans and Processes for Guiding Systems Modernization Efforts. Washington, DC: GAO.
Givens, J. M. 2009. Review of FDA’s Role in Ensuring Safe Food (FDA’s Approach to Risk Based Inspections—A Field Perspective). Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.
Gombas, D. E. 2009. FDA’s Role in Food Safety: A Produce Industry Perspective. Paper pre-Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.
Gueorguieva, V., J. Accius, C. Apaza, L. Bennett, C. Brownley, S. Cronin, and P. Preechyanud. 2009. The Program Assessment Rating Tool and the Government Performance and Results Act: Evaluating conflicts and disconnections. American Review of Public Administration 39(3):225–245.
Hald, T., D. Vose, H. C. Wegener, and T. Koupeev. 2004. A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Analsyis 24(1):255–269.
Hald, T., D. M. Lo Fo Wong, and F. M. Aarestrup. 2007. The attribution of human infections with antibiotic resistant Salmonella bacteria in Denmark to sources of animal origin. Foodborne Pathogens and Disease 4(3):313–326.
Hammond, J. S., R. L. Keeney, and H. Raiffa. 1999. Smart Choices: A Practical Guide to Better Decision Making. Boston, MA: Harvard Business Publishing.
Hartogensis, M. 2009. Feed and Pet Food Safety at the Center for Veterinary Medicine. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, May 28, 2009.
Havelaar, A. H., J. Braunig, K. Christiansen, M. Cornu, T. Hald, M. J. Mangen, K. Molbak, A. Pielaat, E. Snary, M. Van Boven, W. Van Pelt, A. Velthuis, and H. Wahlstrom. 2006.2006. Towards an integrated approach in supporting microbiological food safety decisions. Zoonoses Public Health 54(3–4):103–117.
Havelaar, A. H., A. V. Galindo, D. Kurowicka, and R. M. Cooke. 2008. Attribution of food-borne pathogens using structured expert elicitation. Foodborne Pathogens and Disease 5(5):649–659.
Henson, S. J., J. A. Caswell, J. A. Cranfield, A. Fazil, V. J. Davidson, S. M. Anders, and C. Schmidt. 2007. A multi-factorial risk prioritization framework for food-borne pathogens. In Department of Resource Economics: Working Paper 2007-8. http://people.umass.edu/resec/workingpapers/documents/ResEcWorkingPaper2007-8.pdf (accessed January 26, 2010).
Hoffmann, S., P. Fischbeck, A. Krupnick, and M. McWilliams. 2007a. Using expert elicitation to link foodborne illnesses in the United States to foods. Journal of Food Protection 70(5):1220–1229.
Hoffmann, S., P. Fischbeck, A. Krupnick, and M. McWilliams. 2007b. Elicitation from large, heterogeneous expert panels: Using multiple uncertainty measures to characterize information quality for decision analysis. Decision Analysis 4(2):91–109.
IOM/NRC (Institute of Medicine/National Research Council). 1998.. 1998. Ensuring Safe Food: From Production to Consumption. Washington, DC: National Academy Press.
IOM/NRC. 2003. Scientific Criteria to Ensure Safe Food. Washington, DC: The National Academies Press.
IPCS (International Programme on Chemical Safety). 2004. IPCS Risk Assessment Terminology. Geneva, Switzerland: World Health Organization.
IRGC (International Risk Governance Council). 2005. White Paper on Risk Governance: Towards an Integrative Approach. Geneva, Switzerland: IRGC. www.irgc.org (accessed May 14, 2010).
IRGC. 2009. Risk Governance Deficits. An Analysis and Illustration of the Most Common Deficits in Risk Governance. Geneva, Switzerland: IRGC. www.irgc.org (accessed May 14, 2010).
Johnsen, A. 2005. What does 25 years of experience tell us about the state of performance measurement in public policy and management? Public Money and Management 25(1):9–17.
Lynn, L. E. 1998. Requiring Bureaucracies to Perform: What Have We Learned from the U.S. Government Performance and Results Act (GPRA)? Second Revised Draft. The University of Chicago Harris School Working Papers, September 1, 1998. http://harrisschool.uchicago.edu/about/publications/working-papers/ (accessed March 9, 2010).
Maczka, C. 2009. FSIS Data-Driven Inspection. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, May 28, 2009.
Mangen, M. J., M. B. Batz, A. Kasbohrer, T. Hald, J. G. Morris Jr., M. Taylor, and A. H. Havelaar. 2009. Integrated approaches for the public health prioritization of foodborne and zoonotic pathogens. Risk Analysis 30:702–797.
Marcus, R., J. K. Varma, C. Medus, E. J. Boothe, B. J. Anderson, T. Crume, K. E. Fullerton, M. R. Moore, P. L. White, E. Lyszkowicz, A. C. Voetsch, and F. J. Angula. 2007. Re-assessment of risk factors for sporadic Salmonella serotype Enteritidis infections: A case-control study in five FoodNet Sites, 2002–2003. Epidemiology and Infection 135(1):84–92.
Morgan, M. G., B. Fischhoff, A. Bostrom, L. Lave, and C. J. Atman. 1992. Communicating risk to the public. Environmental Science and Technology 26(11):2048–2056.
NRC (National Research Council). 2009a. Informing Decisions in a Changing Climate. Washington, DC: The National Academies Press.
NRC. 2009b. Letter Report on the Review of the Food Safety and Inspection Service Proposed Risk-Based Approach to and Application of Public-Health Attribution. Washington, DC: The National Academies Press.
NRC/IOM (Institute of Medicine). 2009. Letter Report on the Development of a Model for Ranking FDA Product Categories on the Basis of Health Risks. Washington, DC: The National Academies Press.
Radin, B. 2006. Challenging the Performance Movement: Accountability, Complexity and Democratic Values. Washington, DC: Georgetown University Press.
Ruzante, J. M., V. J. Davidson, J. Caswell, A. Fazil, J. A. L. Cranfield, S. J. Henson, S. M. Anders, C. Schmidt, and J. Farber. 2009. A multi-factorial risk prioritization framework for foodborne pathogens. Risk Analysis 30(5):724–742.
Scallan, E. 2007. Activities, achievements, and lessons learned during the first 10 years of the Foodborne Diseases Active Surveillance Network: 1996–2005. Clinical Infectious Diseases 44(5):718–725.
Scott, J. 2009. GMA Perspective on FDA’s Role in Ensuring Safe Food. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.
Solomon, S. 2009. Regulating in the Global Environment. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.
Taylor, J. 2009. Strengthening the link between performance measurement and decision making. Public Administration 87(4):853–871.
Taylor, M. R. 2002. Reforming Food Safety: A Model for the Future. Washington, DC: Resources for the Future. http://www.rff.org/rff/Documents/RFF-IB-02-02.pdf (accessed (accessed January 25, 2010).
Taylor, M. R., and S. A. Hoffman. 2001. Redesigning Food Safety: Using Risk Analysis to Build a Better Food Safety System. Washington, DC: Resources for the Future. http://www.rff.org/documents/RFF-DP-01-24.pdf (accessed January 26, 2010).
Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action. Secretariat: Her Majesty the Queen in Right of Canada. http://www.tbs-sct.gc.ca (accessed January 26, 2010).
Tucker-Foreman, C. L. 2009. Testimony of Carol L. Tucker-Foreman, Distinguished Fellow, The Food Policy Institute, Consumer Federation of America, before the Committee on Agriculture, U.S. House of Representatives. Agriculture. Washington, DC.
USDA (U.S. Department of Agriculture). 2010. FSIS National Residue Program for Cattle. http://www.usda.gov/oig/webdocs/24601-08-KC.pdf (accessed on May 12, 2010).
van Pelt, W., M. A. de Wit, W. J. Wannet, E. J. Ligtvoet, M. A. Widdowson. and Y. T. van Duynhoven. 2003. Laboratory surveillance of bacterial gastroenteric pathogens in the Netherlands, 1991–2001. Epidemiology and Infection 130(3):431–441.
Varma, J. K., M. C. Samuel, R. Marcus, R. M. Hoekstra, C. Medua, S. Segler, B. J. Anderson, T. F. Jones, B. Shiferaw, N. Haubert, M. Megginson, P. V. McCarthy, L. Graves, T. Van Gilder, and F. J. Angula. 2007. Listeria monocytogenes infection from foods prepared in a commercial establishment: A case-control study of potential sources of sporadic illness in the United States. Clinical Infectious Diseases 44(15):521–528.
Wagner, R. 2009. FDA’S Current Approach to Risk Based Domestic Inspection Planning. Paper presented at Institute of Medicine/National Research Council Committee on Review of the FDA’s Role in Ensuring Safe Food Meeting, Washington, DC, March 24, 2009.














































