Clinical Trial Methodologies and Placebo Response
The effect of placebo response rates in clinical trials for depression has added a layer of complexity and difficulty to the process of designing trials and interpreting the trial results. Placebo response refers to a patient’s clinical improvement in response to an inactive substance (e.g., sugar pill). Most clinical trials in depression are placebo-controlled. The ethical issue of whether placebo-controlled trials should be conducted when effective therapies are available remains contentious. McNulty noted that while placebo-controlled studies are not required by the FDA, it is difficult to design a trial the FDA will accept without including a placebo study arm.
Placebo response rates in clinical trials for depression have been increasing, but variable, over time. The paper cited by Potter (Walsh et al., 2002) reports that the response to placebo across trials varied significantly—from approximately 10 percent to more than 50 percent—and was frequently substantial: in approximately half of the studies, 30 percent or more of patients assigned to placebo exhibited a clinically significant improvement. In addition, over the course of 2 decades (1980–2000), the proportion of patients responding to placebo increased at the rate of approximately 7 percent per decade (Walsh et al., 2002). The proportion of patients responding to active medication over this time period showed a similar increase. It should be noted that placebo response is a significant issue in the design of trials in many different disease areas.
According to Potter, the variability in placebo response rates over time has made it difficult to plan large clinical trials in depression. In designing clinical trials, it is customary for researchers to look to prior experiences with similar trials in the medical literature to determine how to power the study statistically and develop a target sample size. When there is such dramatic variability in placebo response rates across studies, researchers are left with little information with which to construct an informative, adequately powered study.
DEVELOPING INFORMATIVE CLINICAL TRIALS FOR DEPRESSION
A number of workshop participants noted that the current state of research in depression is marked by an inability to effectively distinguish one antidepressant treatment from another and identify the patient populations best served by a particular drug. In discussing how best to advance clinical research in depression, presenters and audience members raised a number of issues and possibilities.
Combination Therapy: Antidepressants and Psychotherapy
Deborah Zarin, Director of clinicaltrials.gov, National Library of Medicine, highlighted nonpharmacologic interventions for depression (e.g., psychotherapy) and studies that have shown such interventions to be at least as effective or sometimes more so than pharmacologic treatments. She suggested that future depression research further explore such interventions or combinations either alone or in combination with pharmacologic interventions to develop the best treatments. Potter questioned whether it would be better to focus new research efforts on identifying a meaningful distinction between two antidepressants before trying to develop the optimal combination of psychotherapy and antidepressant. Kalali referred to Quintiles’ efforts in conducting two of the largest trials testing the combination of psychotherapy and medication but cited the limited availability of psychotherapy in the United States as a major barrier to its widespread use. In addition, he pointed out that psychotherapy has been shown to be successful in treating mild to moderate depression, but that many individuals with more severe forms of depression would not be candidates for psychotherapy.
Trivedi suggested that depression-focused psychotherapy should be included in research efforts to understand treatment effectiveness. However, many of the same issues affecting medication research also plague psychotherapy research. In response, Zarin suggested that studies of antidepressants could be improved by successfully characterizing the intensity of the patient visits that occur in a clinical trial; that is, there is an impact from the half-hour visits that take place during a clinical trial that goes beyond the effects of the drug being studied. This type of clinical management should be characterized further and could explain some of the variability in clinical trial outcomes for antidepressants, according to Zarin. Potter agreed that more sophisticated tools for measuring what happens in patient visits are necessary. He also mentioned Eli Lilly’s effort to design tools for measuring the impact of clinical trial visits on depression treatments. In the end, the measurement tools varied significantly based on individual trial site characteristics and were determined to be too imperfect for practical use.
Accelerating Depression Research
Potter suggested that, despite the importance of creating new therapies for depression, investment in this area is no longer a top priority for some in the pharmaceutical industry because no path forward exists for obtaining clear, interpretable answers to essential research questions. The challenges facing depression research go beyond simply improving the efficiency of clinical trials and include gaining a deeper understanding of what consti-
tutes useful clinical trial information, as well as how signal detection can be improved. Potter explained that moving beyond a single rating instrument (HAM-D) for depression will probably be necessary.
Also reflecting on current challenges, Trivedi suggested that the field of neuroscience has made a number of breakthroughs in recent years, but the area of depression research has yet to combine these breakthroughs in a meaningful way. Thus large investments will be required to combine clinical moderators with genetic, serological, and other biomarkers to map depression and develop more comprehensive markers for disease severity. Further research into various markers for depression could help distinguish which treatments work for which patients. In addition, Trivedi explained that the vast majority of clinical trials in depression are short term and focus on the first 8 to 10 weeks of treatment. Studying the long-term effects of treating depression, a chronic disease, could help accelerate the development of new therapies.
Trivedi also mentioned the importance of developing new animal models for studying depression subtypes. For instance, no animal model for treatment-resistant depression exists. Thus, if a new therapy for treatment-resistant depression were developed today, it would be studied with the same animal models used for other conditions.
In addition to developing new animal models to advance drug development in psychiatry, Kalali noted that it is important to increase collaboration among industry, academia, and government to advance the interests of patients and move the field of psychiatry forward at a time when the high rate of failure in drug development is driving investment away from psychiatry and toward easier targets and diseases. Kalali suggested that more pharmaceutical companies should share their data regarding the rate at which the placebo response diverges from the response to active medication (i.e., placebo separation data) in clinical trials. These data could improve overall understanding of placebo response in depression trials and help in developing new, more effective trial methodologies. As an example of a large collaborative effort, Kalali highlighted his work as Chair of the Evidence Based Methodology Initiative (EMI) of the International Society for CNS (central nervous system) Drug Development. The EMI is currently conducting a Cochrane-like review of the literature (published and unpublished) in clinical trial methodologies in CNS and assigning a level of evidence to each example.1 The goal is to create a better understanding of the gaps that exist in current methodologies and the areas that require
|
1 |
Cochrane reviews explore the evidence for and against the effectiveness and appropriateness of treatments in specific circumstances to facilitate the choices of doctors, patients, policy makers, and others in health care (http://www.cochrane.org/reviews/clibintro.htm). |
more evidence. The EMI hopes to create a pathway for improving CNS clinical trial methodologies.
To accelerate the development of new drugs to better serve patients, McNulty believes there should be greater integration of clinical and basic science. For example, if neurologists discover interesting differences in the brain potentially linked to placebo response rates, they should have some way to work with a basic scientist to research the meaning of these differences and explore them for the benefit of patients. Drawing on a wide range of scientific expertise could be useful in accelerating drug development for depression. Even in the promising area of genomic research, answers have been limited, according to McNulty. For instance, the gene for Huntington’s disease has been known for a number of years, yet no new therapies to treat or cure the disease have been developed. Huntington’s is a single-gene disorder, whereas depression is likely a polygenic disease in which environmental factors and genes interact in a way that is even more complex than in Huntington’s. Thus, significant challenges exist in the application of advances in genomics to depression research.
The appropriate study size for depression research was debated during the workshop discussions. Potter mentioned that placebo-controlled inpatient trials of early antidepressant medications were small studies—approximately 50 patients per study arm. According to Potter, these small trials were predictive of the benefit many patients receive from the medications. Potter suggested that large trials are not necessary for signal detection as long as the trial includes the right patient population. Paul Hébert expressed surprise that sample sizes for depression trials are so small, considering depression is a disease affecting a large portion of society. He suggested that depression researchers embrace the idea of larger trials to distinguish among different treatment effects. Califf also expressed surprise at the extent to which the field focuses on designing smaller, more precise trials. He suggested that if the same data were examined in his field of research, the conclusion would be to conduct trials 10–20 times larger than they are today.
In addition to study size, workshop participants considered the appropriate length of a clinical trial in depression. Trivedi noted that in a chronic, long-term illness such as depression or diabetes, clinical trials that follow patients for a significant length of time are important. Long-term studies for chronic diseases require successful patient retention strategies to be effective since significant dropout rates over time can jeopardize the validity of trial results. To illustrate the power of large, multicenter, long-term clinical trials for evaluating depression therapies, Trivedi described the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (see Box 5-1).
|
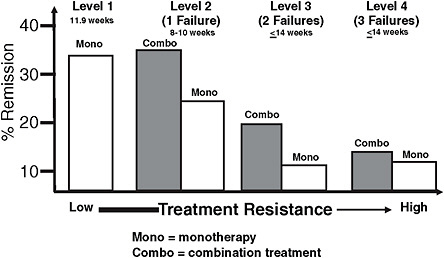
BOX 5-1 Case Study: STAR*D The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study was a large, federally funded clinical trial that tested the effectiveness of antidepressants in a population diagnosed with major depressive disorder. The trial enrolled more than 4,000 patients and included 7 years of patient follow-up. To generate results applicable to the traditional clinical care setting, the diverse study population included individuals seeking treatment from a physician in a specialty or primary care setting, as well as patients with other psychiatric or medical comorbidities. STAR*D was by far the largest and longest trial to date comparing treatments for depression; the next largest trial in this area included approximately 700 participants. STAR*D used a sequential treatment method to treat depression and did not include a placebo arm. After the first treatment step (monotherapy with an SSRI), one-third of patients achieved remission (i.e., were symptom-free). Those patients who did not achieve remission in the first treatment step proceeded to the second, third, and fourth steps depending on their response at each step. As patients progressed from the first step, the number who achieved remission declined (see the figure below). The decline in remission following initial treatment failures is similar to that seen with other chronic diseases. However, Trivedi noted his concern that after the first two treatment failures for depression, the number of treatments currently available becomes limited. With each new treatment level, relapse rates increase for patients, and remission becomes more difficult. Because STAR*D was a large, long-term trial and not placebo-controlled, comparing its results with those of the more typical, placebo-controlled depression trials has been useful. For instance, in a placebo-controlled study, patients with a wide range of disorders would be accepted into the trial (e.g., both anxious and nonanxious depression patients) and randomized to receive the active treatment or placebo. In contrast, patients in the STAR*D study chose a treatment acceptable to them, and randomization was limited to their acceptable treatment range; |
Finally, Potter explained that intention-to-treat analysis is useful and can be especially effective in depression research.2 According to Potter, this methodology allows outcome measurements to be continued at the end of the study even if the intervention does not continue.
6
Clinical Trials in Cancer
Cancer rates increase as the population ages. Diagnoses of cancer are increasing worldwide, including in developing countries. Renzo Canetta, Vice President, Oncology Global Clinical Research, Bristol-Myers Squibb Company, noted that for the most part, cancer is an equal opportunity disease—throughout the world there are no dramatic differences in its biology and paths of treatment. Despite significant progress in cancer prevention, early diagnosis, and treatment, there is a large unmet medical need for treatments for major cancers (e.g., lung, prostate, breast, colon).
Canetta cited three main factors that are currently affecting the clinical trial enterprise in oncology: cost, time, and motivation. He contended that the cost of conducting clinical trials in cancer, the length of time required, and the commitment of investigators and patients to participating are interconnected. According to PhRMA, there are currently more than 800 new anticancer drugs in the development pipeline. At the same time, the participation rate in trials among adult cancer patients is extremely low. Questions arise, then, as to who will study all of these drugs, who will prioritize their study, and how enough patients will be identified to study them, especially given limitations in the infrastructure necessary to conduct clinical trials, including investigators and patients, discussed in Chapter 3.
This chapter begins with presenting a patient’s perspective on clinical trials in cancer. Next, the National Institutes of Health’s (NIH’s) National Cancer Institute (NCI) Clinical Trials Cooperative Group Program is dis-
cussed as one of the major sponsors of clinical research in cancer. The chapter ends with a discussion of industry-sponsored clinical trials in cancer.
CLINICAL TRIALS IN CANCER: A PATIENT PERSPECTIVE
According to data presented by Margaret Mooney, Chief, Clinical Investigations Branch, Cancer Therapy Evaluation Program within NCI, and Musa Mayer, breast cancer advocate of AdvancedBC.org, approximately 3 percent of adult cancer patients participate in clinical trials. An analysis of more than 500 NCI Cancer Therapy Evaluation Program (CTEP) trials by Steven Cheng revealed that 40 percent of trials failed to achieve minimum patient enrollment, and more than three of five phase III trials failed to do so. As discussed in Chapter 3, the failure of clinical trials to enroll enough patients moves health care further away from evidence-based practice and represents a tremendous amount of wasted effort.
Patient Perceptions of Clinical Trials
In an analysis of 23 oncology studies and 6,000 patients, some of the barriers to participating in clinical trials cited most frequently by patients were (1) fear of a reduced quality of life, (2) concern about receiving a placebo, (3) potential side effects, and (4) concern that the experimental drug might not be the best option (Mills et al., 2006). In addition, patients also cited barriers such as inconvenience of participation, dislike of randomization, wanting one’s own doctor to make decisions, feeling coerced, and loss of control over treatment decisions. The single most influential factor in enrolling patients in clinical trials is physician influence. As discussed in Chapter 3, however, a number of barriers affect a physician’s willingness to refer patients to clinical trials.
As a breast cancer advocate and a 20-year cancer survivor, Mayer has focused her work on metastatic breast cancer, the most advanced form of the disease. Over the last 10 years she has participated in an online community (BCMets.org) of women with metastatic breast cancer and their families. Mayer described the 1,100-person community as relatively typical Internet users seeking health care information; they tend to be younger, better educated, less diverse, and more affluent (i.e., of higher socioeconomic status) than the general population. As metastatic breast cancer patients, they are keenly aware that their treatment options are limited and thus are profoundly vested in the search for the next drug to treat their disease. Mayer noted that because of these factors, women with metastatic breast cancer should be good candidates for clinical trials of new drugs.
Mayer conducted an informal, qualitative survey of 49 women from the BCMets.org community to explore the level of physician and patient
involvement in clinical trials, attitudes about participating in trials, eligibility criteria issues, motivation for participation or nonparticipation, and the overall clinical trial experience. The survey results revealed the following barriers to patient participation in clinical trials:
-
Lack of encouragement (or active discouragement) from treating physicians to participate. More than half of the women surveyed said their oncologists never mentioned clinical trials to them or actively discouraged them from participating. Oncologists who did recommend trials to patients were usually investigators themselves or recommending a trial at their own institution.
-
Inconvenience of trial participation (travel, cost, missing work and/or time with family).
-
Misinformation in that women fear getting “no treatment” (placebo), even though providing the best standard of care is the ethical requirement in cancer trials. Equipoise1 is poorly understood by patients; some believe that the control arm of the trial will offer them no treatment and that the experimental arm is inherently better.
-
The misconception that clinical trials are a last-ditch effort, and one should participate only after failing to respond to approved, conventional treatments.
-
Difficulty with eligibility criteria. Some women reported that in trying to enter a clinical trial, they were disqualified because of:
-
past treatment regimens (i.e., “extensively pretreated” or too much chemotherapy);
-
stage of disease (i.e., not recently diagnosed) or the presence of brain metastases; or
-
the presence of advanced disease when many drug trials test first-, second-, or third-line treatments.
-
Advanced-disease patients and those who have had extensive pretreatment are the most motivated to participate in clinical trials, according to Mayer, but, as indicated by the survey results, are frequently disqualified because of a trial’s eligibility criteria. Conversely, recently diagnosed patients who are frequently sought for clinical trials are attempting to cope with the news of their diagnosis and tend to follow the standard treatment protocols prescribed by their doctor rather than participating in clinical trials. On the other hand, as noted in Chapter 3, patients who participate in trials
|
1 |
As noted in Chapter 3, equipoise is the point at which a rational, informed person has no preference between two (or more) available treatments (Lilford and Jackson, 1995). In clinical research, the ethical concept of equipoise is satisfied when genuine uncertainty exists as to the comparative therapeutic benefits of the therapies in each arm of a clinical trial. |
generally report having an overwhelmingly positive experience. Access to emerging therapies and a desire to help other women through advances in research were two of the reasons reported by the women in Mayer’s survey for participating in a clinical trial.
Public Education on Clinical Trials
Well-designed clinical trials play a key role in medical advances and the development of evidence-based health care. However, Mayer noted that public education on the true value of clinical research and the reality of participating in a clinical trial is seriously lacking. As described above, a large number of myths and misconceptions about the experience of participating in a trial exist. Therefore, public education on the link between improvements in health care and clinical research—specifically, clinical trials—is needed. Mayer suggested that involving trained patient advocates at each step of the clinical research process, even in preclinical phases, could provide significant benefit in helping to design informative trials, as well as recruit patients to participate.
Melvyn Greberman, President of Public Health Resources, LLC, referred to the collaboration of the Cancer Biomedical Informatics Grid (caBIG), the Dr. Susan Love Research Foundation, and the Avon Foundation for Women in creating the Army of Women—an initiative designed to enhance consumer participation in clinical research. According to Greberman, the Army of Women has reached 400,000 of the 1 million women it has committed to signing up as potential participants in cancer research studies. The Army of Women collaboration is interested in working with industry and provides a good format for addressing patient and public education issues.
THE NATIONAL CANCER INSTITUTE’S CLINICAL TRIALS COOPERATIVE GROUP PROGRAM
The federal government plays a large and important role in funding and organizing clinical research in oncology. NCI funds approximately half of all cancer trials in the United States. Mooney described the unique structure of NCI’s Clinical Trials Cooperative Group Program.
NCI coordinates a large number of clinical research networks through the Clinical Trials Cooperative Group Program. The program includes nine groups focused on cancers that affect adults and one that focuses on pediatric cancers. Some of the adult groups look at multiple diseases, while some, such as the Gynecologic Oncology Group, specialize by focusing on one area of cancer. Since the 1960s, the NCI cooperative groups have grown from primarily regional sites to large, nationwide networks that encompass a range of different sites and participants. The successes of the program
have resulted in improved care and outcomes for cancer patients. Mooney explained that the program’s accomplishments over the past 50 years have been a result of the direct involvement of clinical investigators, patients, and their families in designing, conducting, and monitoring clinical trials. The early and ongoing involvement of patient advocacy communities in designing and conducting clinical trials has resulted in some of the most robust improvements in cancer treatment to date.
Because the NCI cooperative groups are not oriented to gaining regulatory approval for a new drug, as is the case in industry, they can take a broader, public focus and examine multiple types of research questions in one trial. This breadth of focus is significant with respect to the amount of useful research data that has been generated by going beyond the traditional primary endpoint of a trial. Mooney explained that the large amount of data collected in response to multiple research questions in NCI-sponsored trials can be extremely helpful in learning more about cancer and how to manage it effectively, but also makes some of the trials less pristine in terms of efficiency.
As the field of oncology has progressed over the last 50 years, the true diversity of the set of cancer diseases has been uncovered. Mooney explained that the new understanding of the molecular classification of cancer diseases has allowed a greater focus on particular treatments and patient populations. Thus, new sets of challenges in cancer clinical research have been created. Screening patients for particular molecular characteristics using tissue samples has introduced a new level of scientific and logistic complexity to clinical trials. Mooney explained that the search for rare patient subsets is one reason why clinical trials have become increasingly global—enough patients cannot be found in the United States.
Streamlining NCI’s Clinical Trials Process
Despite differences in research focus, the NCI system shares with industry and all medical disciplines the growing pressure to reduce research costs in the face of declining budgets. In response to this challenge, the NCI Clinical Trials Working Group was launched in 2004. This group is charged with developing recommendations and an implementation plan to optimize the NCI clinical trials system in five critical areas (Box 6-1).
INDUSTRY-SPONSORED CANCER CLINICAL TRIALS
Many issues and obstacles encountered in the clinical trial process are common across organizations that sponsor the research, whether government or industry. In terms of cost, Canetta noted six major drivers for industry-sponsored clinical trials in cancer: