clinical centers with dedicated time to conduct its trials. In addition, TrialNet draws on a large affiliate network that includes several hundred physician practices across the country. It also uses a professional media group to draw attention to its research efforts. Most recently, the Jonas Brothers and Miss America have served as spokespersons for TrialNet studies. Also, an important attraction of TrialNet studies is the fact that the travel costs of clinical trial participants are paid for by the network. In addition, TrialNet is able to build on its connection with JDRF and ADA. JDRF’s website continues to be an important tool for referring patients to clinical trials.
Navigating the IRB Process
The reality of conducting clinical research today is that multicenter trials, with multiple local IRBs, are required to implement a trial capable of providing robust, informative answers. Greenbaum explained that TrialNet has put a great deal of effort into adapting to this situation. For one thing, it has adopted a proactive approach of providing explicit instructions to IRBs to help guide their decision-making process. For example, TrialNet protocols are drafted with specific language stating that it is permissible to study children in a particular trial and citing the guidelines and rules that apply. Greenbaum said IRBs appreciate the inclusion of this specific language in the protocol because it relieves them of the responsibility for making the decision as to which guidelines or rules apply in the case of a particular research study.
TrialNet also has a protocol template that includes a number of sections designed to facilitate the regulatory approval process. The sections range from substantial additions citing federal regulations regarding research in children to minor variations in the language of informed consent forms for patients. Greenbaum noted that in her experience, the key to creating a successful trial protocol (i.e., reducing the need for protocol amendments and deviations) is the inclusion of open wording. For example, a trial protocol might state that “no more” than a particular amount of serum or plasma will be drawn from each research subject in the trial. Because the amount of serum or plasma needed at a particular time in the study is likely to change, this wording allows for the necessary variation and eliminates the need to submit additional paperwork (i.e., a protocol deviation or resubmittal for a protocol revision). Preparing such carefully written protocols that include deliberate yet open wording has therefore helped TrialNet conduct more efficient clinical trials in terms of the recruitment and retention of patient subjects.
Informed Consent
Although efforts have been made to streamline and improve the informed consent process, it remains a challenge for both investigators and patients. Greenbaum stated that the overlap between the confidentiality language of informed consent forms and federal requirements under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) makes drafting clear, readable consent documents somewhat difficult. Despite the growing tendency in the field to emphasize obtaining the final patient signature on an informed consent document, TrialNet has tried to make informing and educating patients a priority instead of merely obtaining their signature. TrialNet has developed patient participant handbooks and quizzes separate from the consent process to ensure that patients really understand what the trial involves. In addition, TrialNet requires that physicians be actively involved in the informed consent process for patients, a feature not commonly found in other study settings, according to Greenbaum.
During the workshop discussion, Perry Cohen, a Parkinson’s patient advocate, noted that his organization, Parkinson Pipeline Project, has developed a research participant bill of rights and responsibilities. The document lays out the features of clinical research that patients desire if they are to participate in a trial. The declaration includes patient requests and responsibilities related to informed consent issues, as well as rights to post study data (e.g., trial results and options for care after the trial ends).2
|
2 |
More information on the Parkinson Pipeline Project and the Declaration of Clinical Research Rights and Responsibilities for People with Parkinson’s can be found at http://www.pdpipeline.org/advocacy/rights.htm. |
8
Building a Robust Clinical Trials Infrastructure
The first day of the workshop focused on the organization of clinical trials and considered various approaches based on different types of diagnosis, study sponsor, and research entity, as well as other factors. The case studies and discussions highlighted a wide range of concerns about how clinical trials are currently conducted and the potential decline in the nation’s capacity to conduct trials at a time when demand for them is increasing. The absolute number of meaningful inquiries that can be made into new products, services, and ways of delivering health care is limited by cost and the availability of qualified investigators and patients willing to participate. Thus, while the number of research questions is rapidly expanding, there are serious questions about the capacity of the U.S. clinical research enterprise to answer more than a fraction of them.
Drawing on the insights and discussions from the first day of the workshop, day two provided an opportunity for participants to consider current strategies and new approaches for conducting clinical trials in the United States. The need to develop a learning health care system that bridges the gap between clinical research and clinical practice was a key theme throughout the meeting. The goals of comparative effectiveness research (CER) are closely aligned with those of a learning health care system—in CER, clinical research is conducted in settings that are as similar as possible to those in which the intervention will be applied in practice (IOM, 2009d). Various forms of clinical research can support a learning health care system. Randomized controlled trials (RCTs) that take place in an academic setting remain the gold standard for clinical inquiry and will continue to be an important tool for future research. But new approaches, skills, and
capacity will be needed to carry out the range of research necessary to meet the needs of a learning health care system.
This chapter begins with an overview of some current efforts to improve clinical trials in the United States, as well as some international examples. The chapter then turns to the suggestions for improving clinical trials that resulted from the four disease-specific breakout session discussions. Finally, Janet Woodcock’s vision for a stable, continuously funded clinical research network in the United States is described.
CURRENT EFFORTS TO IMPROVE CLINICAL TRIALS
Any effort to effect large-scale improvements in the clinical research enterprise must be informed by an examination of smaller-scale efforts already under way. While a number of individual institutions, companies, and non-profit organizations are engaged in streamlining the clinical trials process, the workshop focused on the efforts of the Clinical and Translational Science Awards (CTSA) program, particularly in the creation of templates for agreements used in the clinical trials process; the Clinical Trials Transformation Initiative (CTTI); the National Institutes of Health’s (NIH’s) Roadmap for Medical Research; and an overview of international efforts.
Efforts of the Clinical and Translational Science Awards (CTSA) Program
Barbara Alving, Director, National Center for Research Resources (NCRR) within NIH, described the CTSA program and its role in improving clinical trials in the United States. Launched in 2006 and directed by NCRR, the program makes grants to institutions that provide an academic home for clinical and translational science throughout the United States, working to accelerate the translation of laboratory discoveries into new treatments for patients. The five strategic goals of the CTSA consortium of institutions are:
-
to build national clinical and translational research capacity;
-
to provide training and career development for clinical and translational scientists;
-
to enhance consortium-wide collaborations;
-
to improve the health of communities and the nation; and
-
to advance T1 translational research to move basic laboratory discoveries and knowledge into clinical testing.1
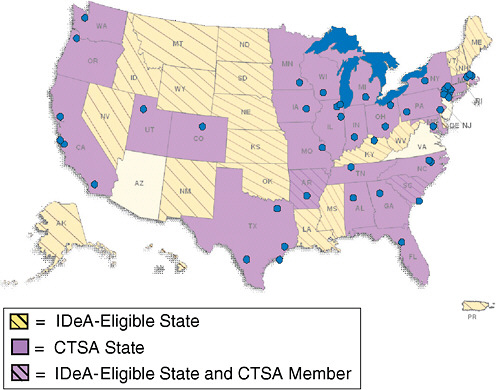
FIGURE 8-1 CTSAs include 46 institutions in 26 states. When the program is fully implemented in 2011, it will include approximately 60 institutions.
NOTE: IDeA = Institutional Development Award.
SOURCE: Alving, 2009. Reprinted from the National Center for Research Resources, NIH.
Currently, 46 academic institutions make up the CTSA consortium, covering 26 states (Figure 8-1). Alving noted that CTSAs are deployed so that their reach is effectively nationwide. In the western United States, the University of Washington works with a number of sites in Idaho, Montana, and Wyoming that do not have medical schools. The IDeA-eligible2 states are funded to create Centers of Biomedical Research Excellence.
CTSA institutions are also engaged in public–private partnerships. For instance, the University of Rochester has created an Intellectual Property
|
2 |
Institutional Development Awards (IDeAs) are funded by NCRR/NIH to foster health-related research and enhance the competitiveness of investigators at institutions located in states in which the aggregate success rate for applications to NIH has historically been low. Additional information on the IDeA program can be found at http://www.ncrr.nih.gov/research_infrastructure/institutional_development_award/. |
Portal3 to aggregate and market technologies from CTSA institutions and NIH. Fifteen CTSA institutions are currently contributing information on their technologies to the site. Alving mentioned another Web-based tool, the CTSA Pharmaceutical Assets Portal,4 which links those with an interest in pharmaceutical products to investigators nationwide, as well as at NIH, who want to study the products.
Alving listed the six areas in which the CTSA program is focusing significant effort to facilitate improvements in the clinical trial process:
-
developing data-driven approaches to process improvement;
-
reviewing steps involved in the initiation of clinical trials;
-
naming “Champions of Change” at academic health centers—individuals with the authority to effect changes;
-
educating academic health centers about uniform templates for clinical trial agreements (CTAs) (see below);
-
developing tools for enrollment of clinical trial participants; and
-
developing Web-based tools for management of clinical trial data.
Alving noted that currently, the performance of CTSA institutions with respect to the length of time it takes for clinical trial contracts to be initiated is similar to that of non-CTSA academic institutions: both experience significant delays from the point at which a clinical trial protocol reaches an Institutional Review Board (IRB) office to the point at which initial ethical review is complete. While CTSA institutions vary greatly in terms of the time frames involved, Alving hopes that as a consortium, they can develop best practices to effect widespread improvement in these time frames across both CTSA and non-CTSA institutions.
As a broad-based network of academic institutions dedicated to clinical and translational research, the CTSA consortium represents a number of key academic stakeholders engaged in clinical trials. Alving pointed out that while CTSA institutions enjoy the benefits of close collaboration with each other, some CTSA initiatives are available to all institutions, CTSA and non-CTSA alike.
Alving stated that it takes anywhere from 4 to 7 months to negotiate a CTA between an academic institution and industry. She noted that, regardless of the disease of focus in a clinical trial, a contracts office is responsible for negotiating the contract, and providing templates (disease-specific as well as general) for that office to choose from can facilitate the negotiation pro-
|
3 |
Additional information on the Intellectual Property Portal can be found at http://www.rochesterctsa.org/ip/. |
|
4 |
Additional information on the CTSA Pharmaceutical Assets Portal can be found at http://www.CTSApharmaportal.org/. |
cess. To streamline the lengthy negotiation process, the IOM Drug Forum commissioned the development of templates for both CTAs and material transfer agreements (MTAs).5 The templates, which are intended for widespread use, incorporate language considered acceptable to key stakeholders. Where companies and universities tend to have significant differences, the templates annotated the standard language to highlight and provide context for those differing positions. Alving described CTSA program efforts to disseminate the CTA and MTA templates to the CTSA consortium and to educate academic health centers on how they can be used effectively. The National Cancer Institute (NCI) also has created template agreements to facilitate contract negotiations. The NCI templates—Standard Terms of Agreement for Research Trial (START) Clauses—are based on the results of a survey of all NCI cancer centers.
Alving also described the following programs supporting CTSA institutions and other clinical research programs:
-
Research Electronic Data Capture (RedCap)6 gives research teams an easy way to collect, disseminate, and protect the privacy of study data. It comprises two secure Web-based applications and provides software and support to partners (CTSA institutions, General Clinical Research Centers, Research Centers in Minority Institutions, and other institutions) at no charge in exchange for participation in the consortium. Alving reported that 3,000 researchers currently use RedCap across 56 institutions and 22 countries.
-
CTSApedia7 will be a comprehensive online resource for those seeking courses in clinical and translational research. This resource will be available to both CTSA and non-CTSA institutions.
-
Researchmatch.org, launched in October 2009, is a Web-based patient recruitment registry connecting willing clinical trial volunteers with researchers. It currently supports the CTSA consortium of institutions.8
|
5 |
The CTA and MTA templates can be found at http://iom.edu/~/media/Files/Activity%20Files/Research/DrugForum/April27-28/TemplateCTA%2042209.ashx and http://iom.edu/~/media/Files/Activity%20Files/Research/DrugForum/April27-28/TemplateMTA%2042209.ashx. |
|
6 |
Additional information on RedCap can be found at http://www.project-redcap.org/. |
|
7 |
Additional information on CTSApedia can be found at http://www.ctspedia.org/do/view/CTSpedia/WebHome. |
|
8 |
Additional information on the Research Match Network can be found at https://www.researchmatch.org/partners/. |








