2
Effects of
Ocean Acidification
on the
Chemistry of Seawater

As atmospheric carbon dioxide (CO2) increases and dissolves into the ocean, it modifies the chemistry of seawater. This chapter reviews the current knowledge regarding the chemical changes brought about by the increasing CO2—labeled collectively as ocean acidification—in the past, the present, and the future. It first discusses the principal processes that control the acid-base chemistry of seawater and the cycling of carbon in the ocean. The chapter then examines how these processes are modified by increasing CO2 concentrations. Most of these processes are well understood and the uncertainties have to do chiefly with the extent and the timing of the chemical changes, not their nature. Next, previous instances of acidification in the distant past are reviewed and their relevance to the current situation are discussed. Finally, the chapter briefly touches on efforts to mitigate or geoengineer solutions to climate change, and how these efforts are related to ocean acidification. Additional detailed discussions of chemical changes related to acidification can be found in Zeebe and Wolf-Gladrow (2001) and Millero (2006).
2.1 SEAWATER CHEMISTRY
The principal weak acids and bases that can exchange hydrogen ion in seawater and are thus responsible for controlling its pH are inorganic carbon species and, to a lesser extent, borate. Inorganic carbon dissolved in the ocean occurs in three principal forms: dissolved carbon dioxide
(CO2.aq),1 bicarbonate ion (HCO3–), and carbonate ion (CO32–) (see Box 2.1 for definitions.). CO2 dissolved in seawater acts as an acid and provides hydrogen ions (H+) to any added base to form bicarbonate:
![]()
CO32– acts as a base and takes up H+ from any added acid to also form bicarbonate:
![]()
Borate [B(OH)4–] also acts as a base to take up H+ from any acid to form boric acid [B(OH)3]:
![]()
As seen in reactions 1 and 2, bicarbonate can act as an acid or a base (i.e., donate or accept hydrogen ions) depending on conditions.
Under present-day conditions, these reactions buffer the pH of surface seawater at a slightly basic value of about 8.1 (above the neutral value around 7.0). At this pH, the total dissolved inorganic carbon (DIC ~ 2 mM) consists of approximately 1% CO2, 90% HCO3–, and 9% CO32– (Figure 2.1). The total boric acid concentration (B(OH)4– + B(OH)3)) is about 1/5 that of DIC. As discussed in section 2.2, increases in CO2 will increase the H+ concentration, thus decreasing pH; the opposite occurs when CO2 decreases. We note that isotope fractionation between B(OH)3 and B(OH)4– is used for estimating past pH values (Box 2.2).
Life in the oceans modifies the amount and forms (or species) of inorganic carbon and hence the acid-base chemistry of seawater. In the sunlit surface layer, phytoplankton convert, or “fix,” CO2 into organic matter during the day—a process also known as photosynthesis or primary production. This process simultaneously decreases DIC and increases the pH. The reverse occurs at night, when a portion of this organic matter is decomposed by a variety of organisms that regenerate CO2, resulting in a daily cycle of pH in surface waters. A fraction of the particulate organic matter sinks below the surface where it is also decomposed, causing vertical variations in the concentrations of inorganic carbon species and pH. The net result is a characteristic maximum in CO2 concentration and minima in pH and CO32– concentration around 500 to 1,000 meters depth
![]()
1 The proper notation for carbon dioxide gas is CO2.g; carbon dioxide dissolved in water is CO2.aq. However, for simplicity, these notations are not carried through the report; the text provides adequate context to determine which form of CO2 is being discussed.
BOX 2.1
Parameters of the Ocean Acid-base System
DIC = Dissolved Inorganic Carbon concentration
DIC = [CO2] + [HCO3–] + [CO32–]
Where the brackets indicate concentrations in mol/Kg.
pCO2 = partial pressure of CO2 (in ppm or μatm)
pCO2 = [CO2]/KH
Where KH is the solubility constant for CO2 in seawater (which varies with temperature, pressure and salinity)
Total Boric Acid = [B(OH)3]+ [B(OH)4–]
TA = Total Alkalinity
TA = [HCO3–] + 2[CO32–] + [B(OH)4–] + other minor bases
pH ≈ –log10 [H+]
More formally, oceanographers use two different pH scales, the total and the seawater pH scales:
pHT = –log{[H+] + [HSO4–]}
pHSWS = –log{[H+] + [HSO4–] + [HF]}
These two scales differ by about 0.01 units for a salinity S = 35 and temperature T= 25°C.
in many areas of the open ocean as illustrated in Figure 2.2a. Because the intensities of biological processes vary with season and the solubility of CO2 varies with temperature, the pH and the concentrations of inorganic carbon species exhibit cyclical seasonal variations. For reasons discussed below, the vertical distribution of pH in the ocean varies with geographical location, particularly as a function of latitude; this is illustrated in the North-South transect for the Pacific Ocean in Figure 2.2b.
Another important process affecting the acid-base chemistry of seawater is the production of calcium carbonate (CaCO3). Marine life produces the vast majority of CaCO3 in the ocean; mostly in the form of the minerals calcite and aragonite (see Box 2.3). Even though these minerals are supersaturated in surface seawater, they do not normally precipitate spontaneously, but are formed by various organisms to serve as skeletons or hard protective structures. The degree of supersaturation of these minerals, quantified by the parameter Ω (see Box 2.3), varies with temperature, depth and seawater inorganic carbon chemistry; Ω is generally highest in shallow, warm waters and lowest in cold waters and at depth (Feely et al., 2004). When calcium carbonate sinks in the water column, it
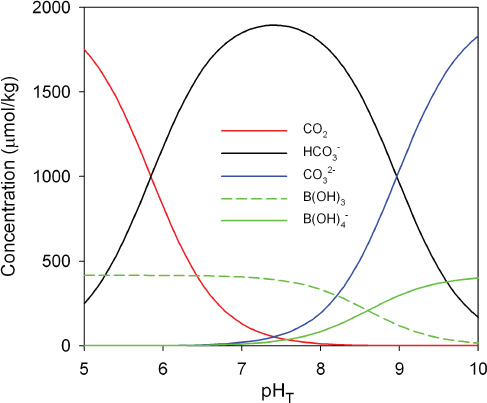
FIGURE 2.1 Typical concentrations of the major weak acids and weak bases in seawater as a function of pH. This diagram is calculated for constant dissolved inorganic carbon (DIC) and constant total boric acid using constants from Dickson et al. (2007) and Lueker et al. (2000).
BOX 2.2
Boron Isotopes as a Paleo-proxy for Seawater pH
Changes in ocean pH can be documented beyond the instrumental period of direct measurements using a proxy based on the incorporation into CaCO3 of the borate ion, B(OH) 4– which has a lighter isotope composition than boric acid, B(OH)3 (Spivack et al., 1993; Sanyal et al., 1995). For time scales shorter than the residence time of boron in the ocean—5-10 million years—measured values in sedimentary carbonates appear to accurately reflect the pH of the growth medium for several calcifying taxa. Results from glacial-interglacial times generally reflect the pH-buffering effect of the CaCO3 cycle (Hönisch, 2005), while records from more recent time intervals reflect acidification of the ocean from rising CO2 concentrations over the past centuries (Liu, 2009).
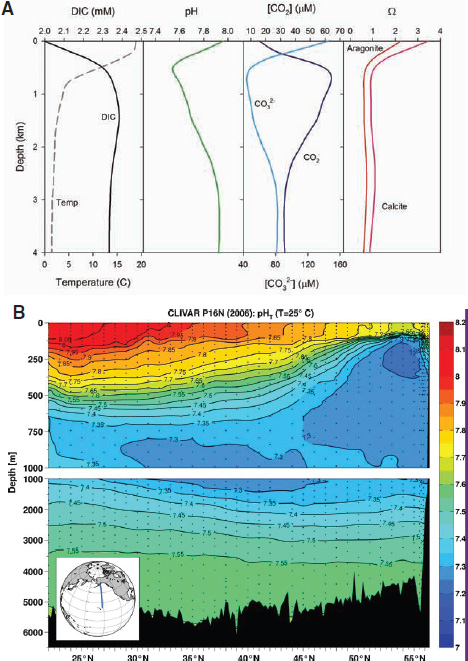
FIGURE 2.2 Inorganic carbon and pH vary as a function of depth and latitude. (a) Vertical profiles typical of the mid-North Pacific showing variations of several seawater chemical parameters with depth. Adapted from Morel and Hering (1993) with calculations using constants from Dickson et al. (2007) and Lueker et al. (2000). (b) Typical distribution of pH with depth along a North-South transect for the Pacific Ocean. (Byrne et al., 2010a).
BOX 2.3
Calcium Carbonate Solubility
Many marine organisms deposit calcareous shells and skeletons made of calcium carbonate (CaCO3), which is a soluble mineral (Sanyal et al., 1995). The solubility of minerals such as CaCO3 varies depending upon the physical properties of the seawater (e.g., temperature, salinity, and pressure) and also the crystal form of the mineral. The solubility is often expressed as the saturation state (Ω) of a mineral: when Ω>1, seawater is supersaturated with respect to CaCO3 and it will remain solid; when Ω<1, seawater is undersaturated and CaCO3 structures may begin to dissolve, unless they are protected from dissolution (e.g., with an organic coating). The saturation state is defined as follows:
![]()
The denominator refers to the stoichiometric solubility product (often designated as Ksp) of the Ca2+ and CO32– concentrations in a solution saturated with respect to the given mineral, and the numerator is the product of the in situ concentrations. Under current pH conditions, CaCO3 is supersaturated in most surface ocean waters. Calcium ion concentration varies little in the open ocean, but ocean acidification decreases the concentration of CO32– and the degree of supersaturation. In estuarine waters both Ca2+ and CO32– concentrations vary widely and can frequently be below saturation.
Most calcium carbonate is precipitated by organisms in one of two forms: calcite (which has a rhombohedral crystal structure) and aragonite (which is orthorhombic). Vaterite, a third form, is rare but of interest because it is involved in the early stages of calcite precipitation in some organisms and is highly soluble. Normally, aragonite is about 1.5 times more soluble in seawater than calcite. However, the calcite crystal structure allows some ionic substitution of magnesium (Mg) for calcium: calcite with > 4 mol% MgCO3 is called “high-Mg calcite” and is usually more soluble than regular calcite.
FROM: Morse and Mackenzie, 1990 and Morse et al., 2006.
becomes less stable (Ω decreases) as a result of the decrease in CO32– concentration and the increase in the solubility of the minerals caused by the higher pressure and the lower temperature. The depth at which CaCO3 becomes undersaturated and begins to dissolve depends on its crystalline form; this “saturation horizon” for calcite is deeper than that for aragonite (see Box 2.3). Precipitation of CaCO3 at the surface lowers the ambient pH, while its dissolution at depth increases it, partially compensating for the inverse effects of the photosynthetic reduction of CO2 that raises pH in surface waters and lowers pH in deeper waters as CO2 is regenerated by metabolic oxidation.
As illustrated in Figure 2.2b, the vertical distribution of pH is not uniform throughout the oceans. The principal cause of these geographical pH variations is the non-uniform distribution of the CO2 concentration resulting from the lower solubility of CO2 gas at higher temperatures, basin-wide patterns of subsurface biological oxidation of organic matter and dissolution of carbonate minerals, and upwelling of CO2-rich deep-water or downwelling of CO2-poor surface water (Sarmiento and Gruber, 2006). This is illustrated in Figure 2.3 (Part A) which shows CO2 concentration as a function of depth in a North-South transect across the North Pacific Ocean. Upwelling around the equator increases CO2 concentration near the surface at low latitudes compared to values in mid latitudes. An increase in surface CO2 is also seen at high latitudes caused by the high solubility of CO2 in cold water. High concentrations in deeper water result from oxidation of organic matter. These geographical patterns in CO2 con-
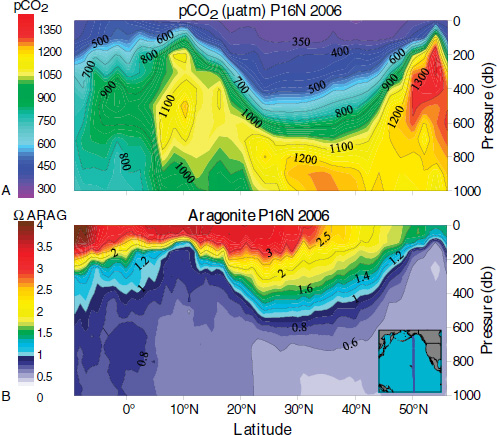
FIGURE 2.3 The distribution of (a) pCO2 and (b) aragonite saturation in the North Pacific Ocean during a transect in March 2006. A pressure of 1 decibar (1 db on the y axis) corresponds approximately to a depth of 1 meter (m). (Fabry et al., 2008b)
centration are reflected in consistent patterns of CO32– concentrations and thus also in the degree of saturation (Ω) of CaCO3 minerals (see Figure 2.3, Part B) and in the buffering capacity of the water (Egleston et al., 2010).
2.2 ANTHROPOGENIC CARBON DIOXIDE EMISSIONS AND OCEAN ACIDIFICATION
The exchange of CO2 at the air-water interface is relatively fast, taking place on a time scale of months to a year so that, on average, the concentration of CO2 in surface seawater remains approximately at equilibrium with that of the atmosphere. As the concentration of atmospheric CO2 gas increases year after year, some of it dissolves into the ocean such that about a third of the total CO2 added to the atmosphere from anthropogenic sources—including fossil fuel emissions, cement production and deforestation—over the past 150 years is now dissolved in the oceans (Sabine et al., 2004; Khatiwala et al., 2009). The increase in dissolved CO2 concentration decreases the pH and shifts the equilibrium of inorganic carbon species in seawater, resulting in an increase in CO2 and HCO3– concentrations and a decrease in CO32– concentration (Figure 2.4). For example, under present conditions in the mid North Pacific, for every 100 molecules of CO2 dissolved from the atmosphere, about 7 remain as CO2, 15 react with B(OH)4–, and 78 react with CO32–, resulting in an increase of HCO3– by 171 molecules. The buffering capacity of seawater—the ability to resist changes in acid-base chemistry upon addition of an acid such as CO2—depends on the concentration of bases, principally CO32– and B(OH) 4–, to neutralize the acid (Figures 2.1 and 2.4). Upon acidification of the oceans, the buffering capacity of seawater will decrease along with pH. Also, ocean water masses that are presently already high in CO2 for any reason are less buffered against further increases in CO2 than those with lower CO2 (Egleston et al., 2010).
The decrease in carbonate ion concentration, CO32–, that results from ocean acidification will lead to reduced rates of calcification, along with the a shoaling of the saturation horizons for calcium carbonate minerals to shallower depths, and a change in the marine calcium carbonate cycle. The resulting overall decrease in CaCO3 precipitation and burial will tend to raise seawater pH, favoring the oceanic uptake of CO2, and providing a small negative feedback on rising atmospheric CO2 and global warming (Heinze, 2004). The extent of this feedback depends in part on the relative contributions of calcite and aragonite, and hence of the organisms that produce them, to the CaCO3 cycle. Model simulations (Gehlen et al., 2007) show that an approximately 30% reduction in CaCO3 production (which was hypothesized to occur when atmospheric CO2 reached 4x pre-industrial values) leads to an additional cumulative oceanic uptake
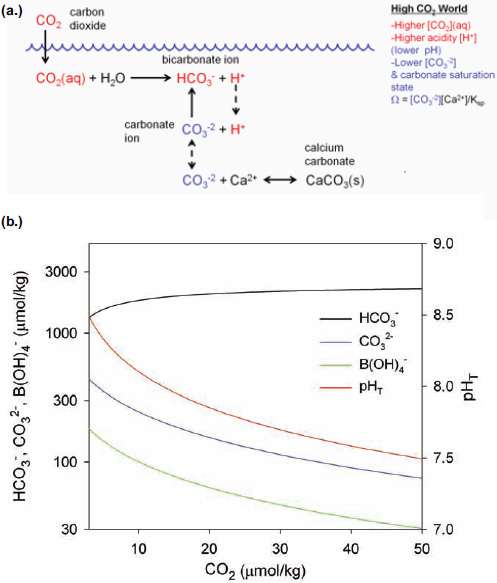
FIGURE 2.4 Schematic (a) and calculations (b) showing the effect of increasing CO2 concentration on acid-base species in seawater. Calculations are made for constant alkalinity using constants from Dickson et al. (2007) and Lueker et al. (2000). Note that the y-axis is on log scale.
of ~6 petagrams (Pg) C, small relative to anthropogenic emissions and other potential climate-carbon cycle feedbacks (Friedlingstein et al., 2006). The reduction in carbonate production and its faster dissolution rate in the water column could also decrease the ballasting of organic carbon by CaCO3 that increases the sinking of organic carbon to the deep ocean (e.g., Armstrong et al., 2002; Klaas and Archer, 2002). This would cause more organic carbon to decompose in shallow water and partially offset the negative CO2 feedback resulting from lower calcification rates (Heinze, 2004). This effect could be enhanced by an increase in phytoplankton production of extracellular organic carbon (see chapters 3 and 4) and by the accelerated bacterial decomposition of organic matter at higher temperature.
A decrease in seawater pH results in a readjustment of all minor acid-base species, in addition to inorganic carbon and borate. These include a myriad of trace organic compounds, inorganic species such as the hydroxyl ion, phosphate and ammonium, and trace metals bound to inorganic or organic compounds. The effect of pH on these chemical species is of interest because several are important nutrients for phytoplankton growth and the chemical forms affect availability for phytoplankton use. For example, iron (Fe) is the most important trace nutrient for marine phytoplankton and inorganic Fe compounds are more biologically available than organically-bound Fe; acidification may cause Fe to become less bioavailable because as the pH decreases, more Fe will become organically bound (Shi et al., 2010). The effect of decreasing pH on Fe bioavailability in surface water is further complicated by the light-induced cycle between oxidized and reduced Fe species, in which a key process—oxidation of reduced Fe—slows down at lower pH. Such effects of acidification on the chemistry and bioavailability of trace metals and other compounds in the ocean have barely been studied at all and, unlike the changes in inorganic carbon species, cannot be predicted with confidence.
In addition, recent studies have shown that ocean acidification can affect the physical properties of seawater. At low frequencies, sound transmission in the ocean is attenuated by volume changes related to acid-base equilibrium of some chemical species. Change in the proportions of such systems, notably the boric acid and borate ion acid-base pair, may thus result in a “noisier ocean” (Hester et al., 2008; Duda, 2009).
2.2.1 Projections for Surface Waters
Because the relationship between atmospheric CO2 and seawater carbonate chemistry is well understood, it is a simple matter to calculate the variations in average pH and inorganic carbon species concentrations in
the surface waters of the open ocean based on the known variations in atmospheric CO2 over the past 150 years (from actual measurements or from ice core data). Independent estimation of past seawater pH have been made using boron isotopes as well (see Box 2.2). Similarly, projections for changes in seawater chemistry can be made for the future on the basis of any future CO2 emission scenario such as those published by the IPCC. Such calculations are shown in Figure 2.5 for the Pacific Ocean; models show that, based on a “business-as-usual” scenario of CO2 emissions, the surface ocean pH will decrease by about 0.3 units within the next 100-150 years (e.g., Wolf-Gladrow et al., 1999; Caldeira and Wickett, 2003; Feely et al., 2004).
Figure 2.6 shows the results of actual measurements of surface seawater chemistry at a station near Hawaii between 1998 and 2008. These data confirm the validity of the calculations and demonstrate the predicted trend of a decrease of about 0.0015 pH units per year. The data also illustrate the seasonal cycle in pH and inorganic carbon species caused
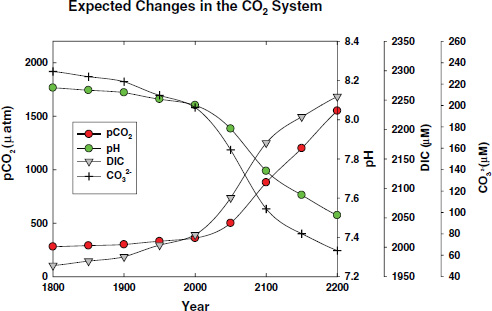
FIGURE 2.5 Projected changes in the pH, and the concentrations of CO2 and CO32– in surface seawater under a business as usual scenario for CO2 emissions over the next two centuries. Calculations were made for a salinity of 35 and temperature of 25°C assuming constant alkalinity using the CO2sys program (Lewis and Wallace, 1998). The projected future values of pCO2 in the atmosphere are based on the estimates of Caldeira and Wickett (2003).
by variations in biological activity discussed above. Because the buffering capacity of seawater decreases with decreasing pH, it is expected that these seasonal variations will amplify in the future.
2.2.2 Projections for Deeper Waters
While the CO2 concentration in the surface ocean tracks the increasing values in the atmosphere, the penetration of that CO2 into deep water depends on the slow vertical mixing of the water column and the transport of water masses in the complex wind-driven circulation and overturning of the oceans (Sarmiento and Gruber, 2006). About half of the anthropogenic CO2 is now found in the upper 400 meters, while the other half has penetrated to deeper water, as illustrated in Figure 2.7 (Feely et al., 2004). This slow penetration of CO2 into the deep ocean is reflected in a slower decrease in pH at depth than at the surface. An illustration of the time lag between surface and deep ocean acidification is shown in Figure 2.8; according to these simple calculations, under a “business-as-usual” scenario of CO2 emissions, it will take about 500 years longer for a 0.3 unit decrease to occur in deep waters compared to surface waters (Caldeira and Wickett, 2003). However, in some regions where the vertical movement of water is relatively fast, the time scale for deep penetration of anthropogenic CO2 will be on the order of decades instead of centuries (Sabine et al., 2004).
FIGURE 2.6 Time-series of mean carbonic acid system measurements within selected depth layers at Station ALOHA, 1988–2007. (First image) Partial pressure of CO2 in seawater calculated from DIC and TA (blue symbols) and in water-saturated air at in situ seawater temperature (red symbols). Linear regressions of the sea and air pCO2 values are represented by solid and dashed lines, respectively. (Second, third, and fourth images) In situ pH, based on direct measurements (orange symbols) or as calculated from DIC and TA (green symbols), in the surface layer and within layers centered at 250 and 1,000 m. Linear regressions of the calculated and measured pH values are represented by solid and dashed lines, respectively. (Dore et al., 2009)
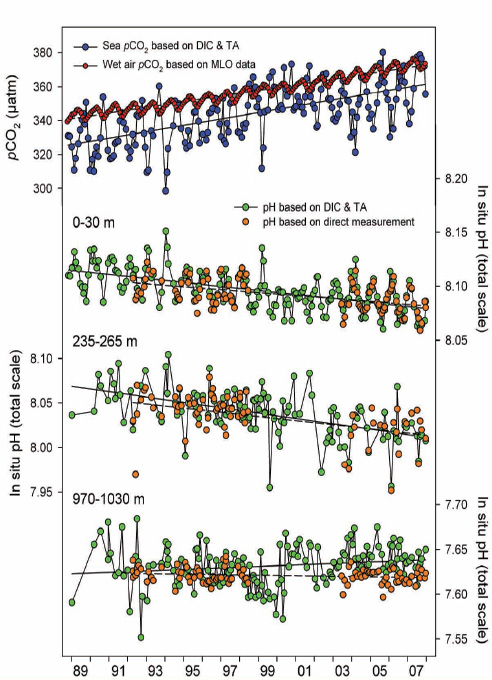
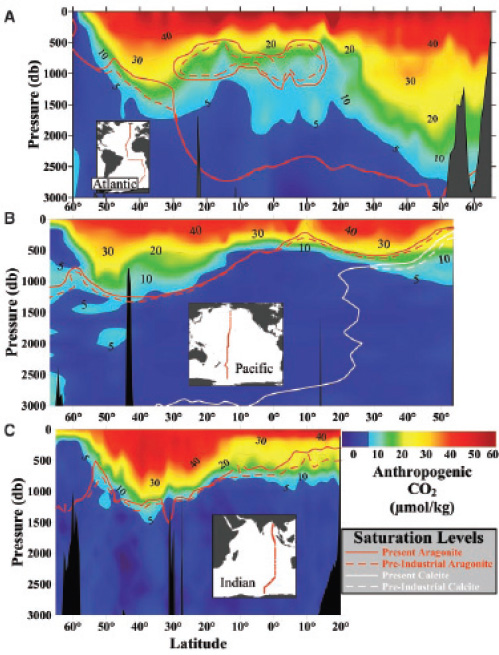
FIGURE 2.7 Vertical distributions of anthropogenic CO2 concentrations (μmol kg–1) and the saturation horizons for aragonite and calcite along north-south transects in the (A) Atlantic, (B) Pacific, and (C) Indian Oceans. A pressure of 1 decibar (1 db on the y-axis) corresponds approximately to a depth of 1 meter (m). (Feely et al., 2004)
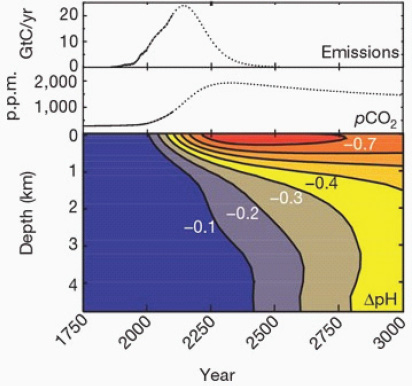
FIGURE 2.8 Atmospheric CO2 emissions, historical atmospheric CO2 levels and predicted CO2 concentrations from this emissions scenario, together with changes in ocean pH based on horizontally averaged chemistry. (Caldeira and Wickett, 2003)
As anthropogenic CO2 penetrates down in the water column, it decreases the CO32– concentration and hence the degree of CaCO3 supersaturation. The result is a slow upward migration, or shoaling, of the saturation horizons for calcite and aragonite. This effect can already be measured (Figure 2.7; Feely et al., 2004). As can be seen on Figure 2.7, the extent of shoaling of the saturation horizons is uneven across ocean basins, reflecting the differences in CO2 penetration caused by the complex movements of water masses.
2.2.3 Projections for Coastal Waters
The acid-base chemistry of coastal waters is much more complex than that of open ocean surface and deep waters. It is affected by freshwater and atmospheric inputs, the supply of both organic matter and algal nutrients from land, and processes in the underlying sediments.
Fresh water runoff tends to have higher dissolved CO2 concentrations and lower pH than ocean water (Salisbury et al., 2008). In surface coastal waters, high photosynthetic activity fueled by nutrient inputs can result in low seasonal CO2 concentrations and high pH. In bottom waters, the decomposition of organic matter, contributed either from land or from local production, increases CO2 and decreases pH. A number of anthropogenic activities can exacerbate coastal acidification, principally those that result in inputs of organic waste or algal nutrients, or that lead to the formation of acid rain (Doney et al., 2007).
Many coastal areas also experience seasonal upwelling of CO2-rich deep water. In general, deep old waters in the ocean tend to have the least invasion of fossil fuel CO2, but some upwelled waters are from shallower waters that are already subject to acidification by anthropogenic CO2. This phenomenon has been shown to occur on the Pacific coast of North America (Figure 2.9; Feely et al., 2008). On that coast, the seasonal upwelling results in a natural seasonal cycle in pH and seawater carbonate chemistry; the extent and degree to which this has been amplified by acidification, resulting in the breaching of corrosive, aragonite dissolving water all the way to the surface, is an important research question. In both river dominated and upwelling dominated coastal regions, future trends in seawater carbon chemistry may also depend strongly on climate change that influences wind patterns, upwelling and river flow. In shallow waters, sediment dissolution can partly buffer acid inputs (Andersson et al., 2003; Thomas et al., 2009).
2.2.4 Projections for High Latitudes
As seen in Figure 2.3, the cold waters of high latitude regions are naturally low in carbonate ion concentration, owing to the increased solubility of CO2 at low temperature and ocean mixing patterns. As a result, surface waters of these areas naturally have a lower degree of supersaturation of carbonate minerals and their acid-base chemistry is less buffered than temperate and tropical surface waters. As the atmospheric CO2 concentration increases, the pH and CO32– concentration in these regions will decrease, and the saturation horizons of aragonite and calcite will move rapidly toward the surface (Olafsson et al., 2009). Seasonal aragonite undersaturation in surface waters has already been observed in the Canada Basin of the Arctic Ocean (Bates et al., 2009; Yamamoto-Kawai et al., 2009). Persistent undersaturation of surface waters with respect to aragonite is projected to occur in high latitude regions by 2100, while in lower latitude surface waters the degree or extent of supersaturation will be reduced (Orr et al., 2005; Steinacher et al., 2009). This is illustrated in Figure 2.10, which shows the projected changes in the aragonite satura-
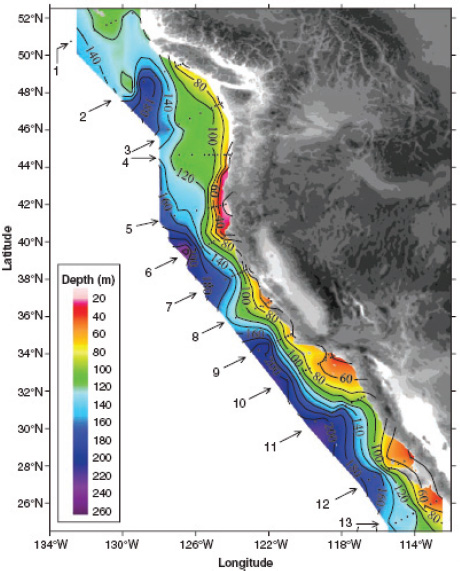
FIGURE 2.9 Distribution of the depths of the undersaturated water (aragonite saturation < 1.0; pH < 7.75) on the continental shelf of western North America from Queen Charlotte Sound, Canada, to San Gregorio Baja California Sur, Mexico. On transect line 5, the corrosive water reaches all the way to the surface in the inshore waters near the coast. The black dots represent station locations. (Feely et al., 2008)
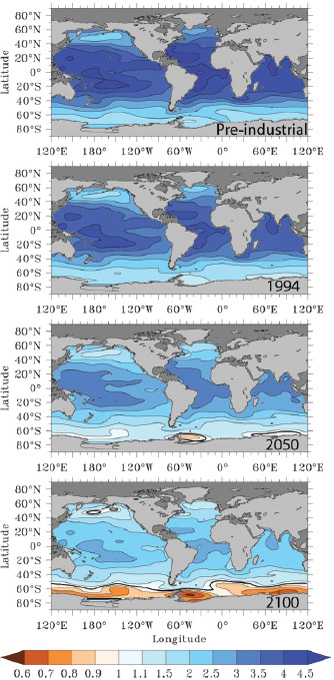
FIGURE 2.10 Surface water aragonite saturation state for the pre-industrial ocean (nominal year 1765), and years 1994, 2050, and 2100. Values for years 1765 and 1994 were computed from the global gridded data product GLODAP (Key et al., 2004), whereas the saturation state for years 2050 and 2100 are the median of 13 ocean general circulation models forced under the IPCC’s IS92a “business-as-usual” CO2 emission scenario (Orr et al., 2005). (Fabry et al., 2008b)
tion state of surface oceans under the “business-as-usual” (i.e., IPCC’s IS92a) emissions scenario through the year 2100 (Orr et al., 2005; Fabry et al., 2008b). Under current rates of CO2 emissions, models project that surface waters of the Southern Ocean, the Arctic Ocean, and parts of the subarctic Pacific will become undersaturated with respect to aragonite by the end of this century, and, in some regions, as early as 2023 (Orr et al., 2005; Steinacher et al., 2009).
2.3 CONTEXT AND CONSTRAINTS FROM THE GEOLOGIC PAST
Information about past changes could be helpful for understanding ongoing changes and their consequences. On time scales of thousands of years and longer, the pH of the ocean is determined primarily by the cycling of CaCO3 (and some silicate) minerals which are dissolved on land, carried by rivers to the ocean where they are reprecipitated, and eventually buried in sediments. Ocean acidification results from the fact that this natural oceanic CaCO3 cycle cannot keep up with the rapid rise in CO2. But eventually, over thousands of years, changes in CaCO3 cycling will neutralize most of the excess acidity and restore the pH of the ocean to near-present-day value. Natural glacial-interglacial changes in atmospheric CO2 over the past 800,000 years, which are recorded in ice cores, occurred over thousands of years, thus reducing the magnitude of change in ocean pH for a given increase in atmospheric CO2 and allowing time for the CaCO3 cycle to keep up (Ridgwell and Zeebe, 2005).
In the deeper geologic past, millions of years ago, atmospheric CO2 concentrations were much higher than today, giving the Earth a warm climate similar to the present-day tropics all the way to the high latitudes. This is often referred to as “hot house” conditions as compared to present-day “ice house” conditions. Again, in these hot-house cycles, because the CO2 concentration changed over millions of years, the CaCO3 cycle stabilized the pH of the ocean to these CO2 changes, as evidence by massive CaCO3 deposits from those periods. While glacial-interglacial cycles and hot house-ice house cycles provide information regarding the response of the ocean carbon cycle to changes in ocean pCO2 over thousands and millions of years, they are not good analogs to current acidification of the ocean by anthropogenic CO2.
2.4 MITIGATION AND GEOENGINEERING
There is currently a great deal of international interest in mitigating the impacts of climate change. However, this leads to the question of how these mitigation strategies will affect ocean acidification and how ocean acidification itself can be mitigated. Clearly, all mitigation strategies for
climate change that reduce CO2 inputs to the atmosphere will also reduce ocean acidification. These include increasing energy efficiency, shifting energy sources from fossil fuels to nuclear and renewables, and implementing carbon capture and storage technologies (Pacala and Socolow, 2004). Similarly beneficial would be carbon management approaches that remove CO2 from the atmosphere through biological sequestration on land (e.g., afforestation, soil conservation) or industrial-scale geochemical approaches (Stephens and Keith, 2008). But geoengineering solutions designed to slow climate warming without reducing atmospheric CO2 concentration, such as injection of sulfate aerosol precursors into the stratosphere (Crutzen, 2006), will not reduce ocean acidification (Wigley, 2006; Boyd, 2008). On a regional scale, in coastal and estuarine waters where acidification in surface waters may result partly from pollution such as acid rain or in bottom waters from eutrophication induced by excessive nutrient inputs, limiting emissions of air or water pollutants may be effective as a mitigation strategy.
Management strategies designed to sequester CO2 in the ocean could potentially exacerbate ocean acidification in intermediate or deep waters. Iron fertilization of surface waters has been suggested as a potential approach for boosting primary production in regions that are iron-limited, thus increasing the export of organic carbon to the subsurface as discussed in the next chapter (Boyd et al., 2007). Critics of iron fertilization have questioned its efficiency at sequestering CO2 and pointed out the difficulty in predicting its ecological consequences. Ocean acidification could affect the efficiency of iron fertilization and its potential consequences by modifying the biological availability of iron in surface seawater (see section 2.2). If effective, iron fertilization would increase the rate of penetration of CO2 into intermediate waters, thus accelerating acidification in those water masses. A similar effect would result from direct injection of CO2 into intermediate or deep ocean waters. Enhanced deep sea acidification could also occur as a result of leakage from sub-seabed CO2 sequestration (Blackford et al., 2009) either in sediments (House et al., 2006) or bedrock (e.g., oil and gas fields, salt domes, etc.) (Caldeira and Wickett, 2005).
The effectiveness of direct ocean CO2 injection techniques could theoretically be enhanced and the resulting acidification minimized by first reacting CO2 with a base, neutralizing the carbonic acid, and producing primarily bicarbonate. Alternatively a base could be added directly to seawater. Most proposed schemes use carbonate rock (e.g., limestone) as the base and differ mostly in the techniques used to accelerate CaCO3 dissolution (Rau and Caldeira, 1999; Caldeira and Rau, 2000; Golomb et al., 2007; Harvey, 2008; Rau, 2008). Sodium hydroxide (NaOH) could also be produced from water electrochemically with the co-produced hydro-
chloric acid (HCl) being neutralized by silicate rocks (House et al., 2007). Given that neutralization of CO2 requires an equivalent amount of base (1:1 molar ratio), the logistics and resource demands for neutralizing a significant fraction of the 2 Pg C (~1014 moles CO2) per year taken up by the ocean are likely to be prohibitive. But such mitigation strategies might be feasible on a local or regional scale.
This page intentionally left blank.






















