3
Effects of Ocean
Acidification on the
Physiology of
Marine Organisms

The dissolution of anthropogenic carbon dioxide (CO2) into the ocean is causing a series of changes in ocean water chemistry: an increase in the CO2 concentration, a decrease in the calcium carbonate saturation (Ω) and pH, and a change in the chemistry of many biologically important chemical species, as discussed in Chapter 2. These chemical changes will affect a range of biological processes in marine organisms, including the precipitation of calcium carbonate, fixation and respiration of CO2, regulation of internal pH, and uptake of nutrients for growth. The questions are by what mechanisms will higher CO2 affect an organism’s physiology, to what degree will this affect the fitness of different organisms, and how will high CO2 effects on individual organisms be dampened or amplified at the ecosystem level. This chapter reviews what is known of the effects of ocean acidification on a range of biological processes that have been studied in various organisms. It focuses on processes that are likely to be affected by acidification, both those that are common to many organisms (i.e., calcification and pH control) and those that affect primary production, which provides the principal influx of organic material and energy to marine ecosystems (i.e., photosynthetic carbon fixation, nutrient uptake, and nitrogen fixation). Chapter 4 reviews how these effects on individual organisms may scale up to the ecosystem level.
3.1 CALCIFICATION
Calcium carbonate (CaCO3) is one of the most common building materials used in the formation of skeletons, shells, and other protective
structures in the marine biota. Marine calcifying organisms include many taxonomic groups and occupy diverse ecological niches. Important examples include photosynthetic primary producers (e.g., coccolithophores and coralline algae), zooplankton (e.g., pteropods), mollusks (e.g., clams, mussels, and oysters), crustaceans (e.g., crabs and lobsters), and animals that harbor photosynthetic symbionts (e.g., reef-building corals, some planktonic foraminfera). In most of these organisms, CaCO3 is the principal constituent of the “hard part.” But in some organisms, only a part of the exoskeleton is calcified (e.g., the calcite ossicles of sea stars), while, in others, calcium carbonate is integrated into an organic exoskeleton structure (e.g., lobster and crab shells). These CaCO3 structures are most often in the form of calcite, aragonite, high-magnesium calcite, or a mixture of these mineral forms, and the mineral form may change through the development of the organism (Politi et al., 2004; see also Box 2.3).
Most calcifying organisms studied so far show a decrease in calcification or shell weight (either a slower rate of calcification or a decrease in the mass of CaCO3 per individual) in response to elevated CO2 and reduced pH. This is the best-documented and most widely observed biological effect of the acidification of seawater. It has been reported in a range of organisms, including coccolithophores, foraminifera, mussels, urchins, oysters and other bivalves, corals, and coralline algae (e.g., see Fabry et al. 2008b; Ries et al., 2009). In some organisms, a significant reduction in calcification was observed for a decrease in pH of 0.2-0.4 units, in the range predicted to occur over the next century; in others, a significant effect was only observed under more severe acidification. A few studies have shown that some calcifying organisms are insensitive to seawater acidification, or even increase calcification over the range of pH projected for the next century (Ries et al., 2009; Wood et al., 2008; Miller et al., 2009). In coccolithophores, the effect can be complicated by the increase in growth rate caused by high CO2 (see below), such that the calcification rate per cell may increase while the ratio of inorganic to organic cellular carbon may decrease (Iglesias-Rodriguez et al., 2008). Note that increased calcification is not necessarily an indication of increased health of the organism, and there is some early evidence that other processes can be affected by the strain introduced by accommodation of the increased CO2 (Wood et al., 2008). It is also possible that species that live in environments where pH and CO2 concentrations are variable may be more tolerant of the overall increase in acidity, though this hypothesis has not been tested.
In some groups of organisms, a decrease in calcification is associated with more frequent malformations of the carbonate structures (e.g., coccolithophores; Riebesell et al., 2000; Langer et al., 2006), smaller and thinner shells in foraminifera (Moy et al., 2009) and mollusks (Miller et al., 2009; Talmage and Gobler, 2009), slower shell extension rates (e.g., mollusks;
Miller et al., 2009), and weakened shells (e.g., barnacles, mollusks) (Bibby et al., 2007; Clark et al., 2009; McDonald et al., 2009; Tunnicliffe et al., 2009). In reef-building corals, which have been studied most extensively, a wide range of responses has been observed, but on average, a doubling of preindustrial atmospheric CO2 concentration resulted in about a 10-60% decrease in calcification rates (Langdon and Atkinson, 2005; see also Figure 3.1 as an example, section 4.1, and Appendix C). In some species, seawater acidification led to reduced rates of larval development and increased larval mortality (e.g., echinoderms) as a result of the instability of the nascent calcified structures which are often less well crystallized than the mature form. It must be noted, however, that the physiological role of calcification is not always clear. For example, laboratory cultures of coccolithophores that have lost the ability to calcify grow at normal rates (Rost and Riebesell, 2004). Some species of corals can grow well in cultures without precipitating aragonite, even though the very structure of a coral reef depends on the precipitation of the mineral (e.g., Fine and Tchernov, 2007).
The spontaneous precipitation of CaCO3 in seawater requires a high degree of supersaturation of the mineral (i.e., Q>>1) (which is proportional to the carbonate ion [CO32–] concentration when pressure, temperature, and calcium ion concentration are kept constant). Within organisms, this is achieved by controlling Ω at the site of calcification at a value generally higher than that of seawater (e.g., Al-Horani et al., 2003; Furla et al., 1998; Bentova et al., 2009) through a process that involves pumping of various ions into specialized cellular compartments. Despite the fact that organisms control internal Ω with these processes, calcification has been observed to correlate well with the external value of Ω in many experiments and several taxa (see Figure 3.1); therefore, the external Ω and CO32– concentration may serve as indicators of the calcification response caused by acidification. There are several hypotheses regarding the correlation between external Ω and biological calcification; for example, acidification of the external medium may increase the energetic cost of calcification in some organisms. The energetic cost of calcification should depend on the underlying biochemical mechanisms, which are presently not well understood and are likely to differ widely among taxonomic groups. Marubini and others (2008) addressed multiple hypotheses to explain why acidification causes a decrease in coral calcification rates and suggested that decreases in intracellular or extracellular pH, or shifts in the buffering capacity of the calcifying fluid were likely. A recent study on a temperate coral provides evidence that the calcification response reflects changes in the proton pumping capacity, which is necessary to maintain the high saturation states of the internal calcifying fluid (Cohen et al., 2009). This is supported by results from an analytical survey of calcifica-
tion responses across multiple taxa, which suggests that the calcification response correlates with an organism’s ability to regulate its internal pH, as well as other factors such as the degree of shell protection by organic coatings, shell mineralogy, and whether the organism utilizes photosynthesis (Ries et al., 2009).
As ocean acidification decreases the CO32– concentration, it decreases the degree of supersaturation of CaCO3 in the upper water column, brings the saturation horizons closer to the surface, and may result in some organisms being exposed to undersaturated (corrosive) waters. This may result in the dissolution of previously precipitated minerals at shallower depths. For example, when exposed to the level of aragonite undersaturation expected to occur at high latitudes by the year 2100 (see Chapter 2), shell dissolution was visually evident in a subarctic pteropod species within 48 hours (Orr et al., 2005). Such conditions of undersaturation will happen sooner for the more soluble aragonite, which is formed by corals, some calcifying macroalgae, and some mollusks, than for calcite, produced by coccolithophores, foraminifera, echinoderms, many deep-sea corals, some mollusks, and crustaceans (Pearse et al., 1987). In some species, the calcareous shells and skeletons are protected from dissolution by organic coatings, but in others they are largely exposed to the surrounding seawater (e.g., some bivalve mollusks such as scallops and oysters (Ries et al., 2009). There are apparently large differences among taxa, with some organisms being able to maintain calcified structures in highly corrosive waters (Wood et al., 2008; Tunnicliffe, 2009).
Overall, the acidification of seawater should prove unfavorable for most calcifying organisms, and this is likely to constitute a major negative effect on the marine biota. But it must be emphasized that despite exten-
FIGURE 3.1 Examples of the effect of decreasing CO32– concentration (A) or of increasing pCO2 (B and C) on calcification rates in various taxa. As pCO2 increases, the carbonate ion concentration in the water will decrease and hence the aragonite saturation will also decrease. (A) In the Biosphere 2 coral mesocosm, the system was perturbed by adjusting the carbonate ion concentration (Langdon et al., 2000). In (B), the blue mussel Mytilus edulis and Pacific oyster Crassostrea gigas (Gazeau et al., 2007), and (C), coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica (Riebesell et al., 2000), pCO2 was increased to mimic the effect of higher atmospheric pCO2 on the carbonate saturation state. For cross-comparison, dashed lines have been added to these plots to indicate pCO2 concentrations of 280 ppm (preindustrial concentration), 390 ppm (current-day concentration), 560 ppm (twice preindustrial concentration), and 780 ppm (estimated concentration in the year 2100). In A, the pCO2 concentrations are roughly estimated based upon average aragonite saturation level (Ωarag) across the coral reefs.
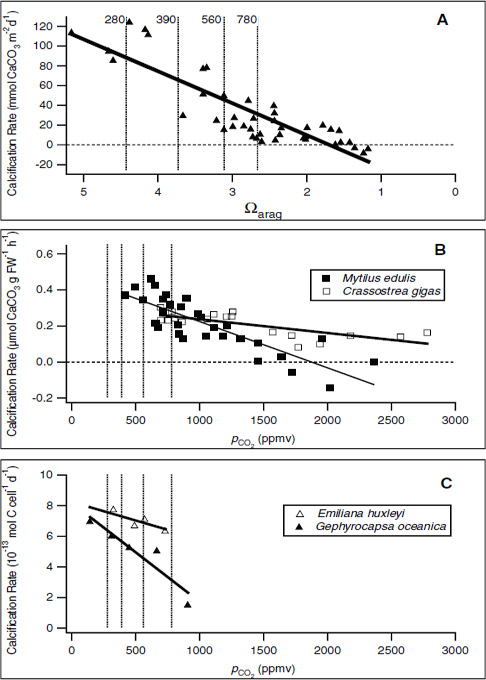
sive research efforts we still have a poor understanding of the mechanisms and regulation of the calcification process in marine organisms.
3.2 INTERNAL PH CONTROL AND OTHER METABOLIC PROCESSES
Biological membranes are generally highly permeable to dissolved CO2, therefore, dissolved CO2 will equilibrate across membranes following the concentration gradient. Dissolved CO2 in internal fluids tends to form bicarbonate and free hydrogen ions, acidifying the medium as it does in seawater. Most heterotrophic organisms excrete CO2, produced as a by-product of metabolic activity, by utilizing a concentration gradient from high internal to the lower, external dissolved CO2. If external dissolved CO2 rises, the efficiency of this mechanism will decrease, potentially affecting acid-base balance in the organism.
Most heterotrophic organisms maintain internal pH lower than normal seawater—(Hochachka and Somero, 2002). Bacteria often have optimal intracellular pH values between 7.4 and 7.8 that they maintain over a fairly wide range in external pH (Booth, 1985; Padan et al., 2005). The internal pH of multicellular marine organisms is also typically lower than seawater, with a progressive decrease in pH from external to internal spaces: extracellular fluids (blood spaces, fluids surrounding cells) have a pH lower than external seawater, and intracellular pH is lower (~0.4 pH units) than that of the extracellular fluids. Intracellular pH is tightly modulated because many metabolic processes are regulated by small shifts in the pH of the medium or depend on a small proton gradient across membranes. Hence, the metabolism of the organism is usually linked to the homeostasis of internal pH as well as the internal to external pH gradient (Pörtner et al., 2004). As a consequence, an increase in the environmental CO2 concentration from ocean acidification could perturb the internal acid-base balance of organisms, potentially affecting a variety of cellular functions ranging from protein synthesis to calcification.
The ability to buffer or control internal pH varies considerably among organisms, in part related to their complexity. In all organisms, partial pH control is achieved through the passive buffering capacity of the internal fluids and the active regulation of various ion pumps (Seibel and Walsh, 2003). Multicellular organisms typically have greater passive buffering capacities, and many can control the pH of their body fluids by secreting or eliminating acid or base through specialized organs (Melzner et al., 2009). These homeostatic mechanisms allow some aquatic organisms to acclimate to a range of external pH/pCO2. But the metabolic cost of this acclimation may slow the growth or decrease the fitness of some organisms and some may not be able to acclimate at all (e.g., Wood et al., 2008).
Many experiments on metabolic costs have been conducted at lower pH and higher CO2 concentrations than expected from ocean acidification over the next century. Nonetheless, this work provides a mechanistic understanding of how animals respond to internal acidosis caused by high environmental CO2 levels. The effects on various metabolic functions may lead to metabolic depression, which decreases all aerobic activities of the organism (Pörtner et al., 2004).
In taxa with respiratory proteins (e.g., hemoglobin, hemocyanin), extracellular acidosis affects oxygen transport and respiratory efficiency due to the reduced oxygen affinity of these proteins at lower pH (Seibel and Walsh, 2003). Animals that actively regulate internal pH will have a greater metabolic demand to meet the high energetic cost of pumping ions across membranes, but the decreased affinity of the respiratory proteins will make it more difficult for the organism to meet that metabolic demand due to the reduction in overall aerobic respiration (Pörtner et al., 2000).
Tolerance for acidification varies greatly among phyla and is linked to metabolic rate and, in turn, to the transport capacities for oxygen and CO2. Because metabolic activity generates by-products that lower pH and increase CO2 in animal tissues, many highly active animals have mechanisms that help them regulate pH and CO2 levels in their internal fluids and tissues. Those animal groups (e.g., mammals, fishes, and some mollusks), have a high capacity for oxygen and CO2 transport and exchange and appear to be tolerant of more acidic environmental conditions, at least over short periods that are similar to the conditions resulting naturally from bouts of high activity (Melzner et al., 2009). In contrast, many marine invertebrate taxa that have been examined have less developed gas exchange and acid-base regulatory capacities, and are expected to have lower tolerance to acid-base disruption caused by ocean acidification (Melzner et al., 2009). Still, there is quite a lot of variation across taxa and little is known about the extent of this variation since some groups, such as gelatinous zooplankton, have not yet been studied. Because of the high energetic demands of acid-base regulation, the ability of organisms to cope with acid-base disturbance also varies among habitats, with those inhabiting energy-poor habitats (e.g., deep-sea environments) exhibiting less tolerance than others.
There are likely to be many other important effects of acidification beyond internal pH control, particularly in higher organisms such as finfish. For example, a recent study showed impaired olfactory discrimination and homing ability in the larvae of the orange clownfish Amphiprion percula (Munday et al., 2009). Currently, these effects are almost completely unknown. To date, the state of knowledge concerning the effects of decreasing pH and increasing CO2 on most marine organisms is sparse. Although many of the underlying physiological mechanisms are under-
stood in some detail, knowledge of the metabolic consequences for individual performance remains weak. Understanding is particularly poor concerning the sensitivities of various life stages of marine organisms, although initial studies suggest vulnerability of early life history phases of several groups such as bivalves and some echinoderms (Talmage and Gobler, 2009; Dupont and Thorndyke, 2009). Even less is known about the cumulative, lifelong effects of a lower pH environment in terms of how it will affect the performance, growth, survival, and fitness of individuals, especially when combined with other likely stressors.
3.3 PHOTOSYNTHETIC CARBON FIXATION
In the oceans, photosynthesis—the formation of organic matter using sunlight energy—is carried out chiefly by microscopic phytoplankton and, to a lesser extent, by macroalgae and seagrasses. For this purpose, photosynthetic organisms must acquire, among other things, inorganic carbon (i.e., CO2) from seawater. Dissolved CO2 is the substrate used in the “carbon fixation” step of photosynthesis, not the more abundant forms of dissolved inorganic carbon. Thus CO2, which is at low concentration in the ambient water, must be concentrated at the site of fixation; this is a difficult and energy-consuming process because the CO2 molecule diffuses readily through biological membranes and continuously leaks out of cells. It is thus expected that an increase in the CO2 concentration of surface seawater would facilitate marine photosynthesis and lead in some cases to an increase in primary production (i.e., the rate of organic matter synthesis per unit time and unit area of the ocean).
Enhancement of photosynthesis under high-CO2 conditions has indeed been observed in a number of experiments with some, but not all, species of marine algae. For example, an enhancement of photosynthesis at high CO2 has been seen in calcifying coccolithophore species that form massive blooms in many oceanic regions (Riebesell et al., 2000; Zondervan et al., 2002), but not in some marine diatoms (Burkhardt et al., 1999) or in the symbiotic dinoflagellates of corals (zooxanthellae) (Schneider and Erez, 2006). In another example, two types of cyanobacteria, Synechococcus and Prochlorococcus—representing the two dominant open ocean cyanobacteria species—responded differently to high CO2 conditions (Figure 3.2). In a few cases a decrease in photosynthesis has been seen at elevated CO2 (Reynaud et al., 2003). Increased photosynthetic rate does not always translate to higher growth; it appears that at high CO2, some phytoplankton species release a sizeable fraction of their photosynthate as extracellular organic matter (Engel, 2002).
The effect of increasing CO2 concentration on photosynthesis depends on the underlying biochemical mechanisms involved in concentrating CO2
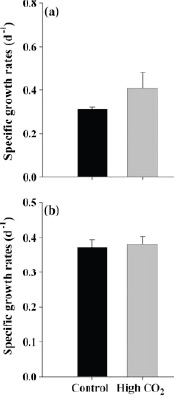
FIGURE 3.2 The effect of CO2 on the growth of (a) Synechococcus, which shows an increase in growth rate in high CO2 and (b) Prochloroccus, which shows no effect of high CO2 on growth rate. (Fu et al., 2007)
at the site of fixation. These mechanisms have not all been elucidated, but are known to differ between different photosynthetic marine organisms. For example, some diatoms concentrate CO2 using a mechanism similar to the one that operates in so-called “C4 plants” such as sugar cane and maize (Reinfelder et al., 2000, 2004; McGinn and Morel, 2008). The difference in the CO2 concentrating mechanisms presumably explains the different responses of phytoplankton species.
Overall, an increase in the CO2 concentration is expected to enhance rather than decrease the growth of photosynthetic organisms and the production of organic matter in the ocean. But this effect is generally modest and appears variable among species; it may thus lead to a shift of dominant species of phytoplankton (see also Chapter 4.2). In most cases, the potential enhancement of primary production by CO2 will be constrained by nutrient limitation. These projections are based on limited data in the marine environment, but they are supported by the analogy with land plants, which possess similar underlying photosynthetic mechanisms. A large number of observations on terrestrial plants exposed to high CO2 show a boost in photosynthesis and a differential response among species.
3.4 NUTRIENT ACQUISITION AND LIMITATION
In different oceanic regions, primary production by phytoplankton can be limited by the availability of various key nutrients, most commonly nitrogen, phosphorus, or iron. Ocean acidification may alter the availability of nutrients in three ways: (1) by changing the chemical forms of nutrients in the water; (2) by changing the activity of enzymes that convert nutrients into useable forms; and (3) by changing the nutrient requirements of the phytoplankton.
The acquisition of nutrients depends on their chemical form—the chemical “species”—present in the water. This is particularly true for trace metals such as zinc, cobalt, nickel, or iron, which are essential for various biochemical processes inside cells. These metals are readily taken up when present as free ions or ions bound to chloride, hydroxide, or other inorganic species, but require specialized uptake machinery when bound in organic complexes (Morel et al., 2003). Because the bulk of most bioactive metals are bound in organic complexes and the extent of such binding is generally sensitive to pH, it is thought that metal bioavailability might be affected by the acidification of surface seawater. The most important case is that of iron, which limits phytoplankton growth in large parts of the equatorial Pacific and high latitude oceanic regions. An increase in organic complexation makes dissolved iron less bioavailable as pH decreases (Shi et al., 2010); however, this effect may be offset by other effects of pH on the cycle of iron in surface seawater including an increase in the solubility of iron oxides and an enhancement in the light-induced redox cycle of iron.
The bioavailability of nutrients may also be affected through the influence of pH on biochemical rather than chemical processes. In some cases, phytoplankton can use enzymes to convert nutrients that are not readily available into a useable form. For example, free phosphate in seawater can be readily taken up by phytoplankton but, when its concentration is very low, some organisms can use phosphate bound in organic compounds. In this case the phosphate must first be cleaved enzymatically from the organic molecule before being utilized. In the range of pH relevant to the surface ocean at present and in the future, the activity of the enzyme responsible for this cleavage (known as alkaline phosphatase) decreases rapidly with decreasing pH (Figure 3.3). Since the enzyme operates outside the cell, it responds directly to acidification of the external medium. Therefore, organisms that depend on organic phosphate for growth will have a more difficult time acquiring phosphate in an acidified ocean, which may negatively affect their growth. Similar changes in bioavailability may occur for some organic forms of nitrogen such as amino acids or amines, which are also acquired by some phytoplankton species through extracellular enzymatic processes (Palenik and Morel, 1990, 1991a, 1991b).
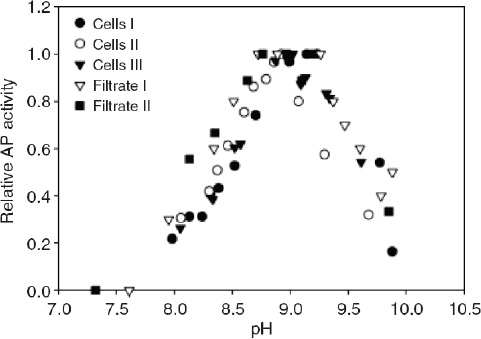
FIGURE 3.3 The activity of alkaline phosphatase (AP) vs. pH in Emiliania huxleyi cultures; three measurements were taken for AP on the cell surface, while two were taken in the culture filtrate. Note that AP activity decreases as pH decreases (in the range expected from acidification). (Xu et al., 2006)
Primary production—the amount of organic matter that is synthesized per area of surface seawater per unit time—generally depends on the rate of supply of the limiting nutrient. A change in primary production requires either a change in nutrient requirement (e.g., an increase in unit of carbon fixed per unit of the limiting nutrient) or in the supply of the limiting nutrient. There is evidence that at high CO2, phytoplankton produce organic matter with a different elemental composition, particularly a higher carbon to nitrogen (C:N) ratio, suggesting that the phytoplankton are able to change their N requirement (e.g., Riebesell et al., 2007; Bellerby et al., 2007; Fu et al., 2007; Hutchins et al., 2009). Such an effect would lead to an increase in the quantity of organic carbon formed per unit of limiting nutrient. It must be noted that a reduction in the nutrient supply may result from the increased stratification of surface seawater caused by increases in global temperatures. In this way the effects of increasing CO2 on climate and on ocean chemistry may compound or partly alleviate each other (see Chapter 2).
3.5 NITROGEN FIXATION
Nitrogen fixation is the process of converting atmospheric nitrogen gas (N2), which cannot be used by organisms as a source of nitrogen for biosynthesis, into ammonium (NH4+), a form readily available to the biota. In the oceans, this process is predominantly carried out by a few specialized cyanobacteria. Nitrogen fixation represents a major input of “new” nitrogen to marine ecosystems and is thus a key in controlling primary production in large regions of the world’s oceans. Nitrogen fixation is an “expensive” biochemical process that requires synthesis of a complex, iron-rich enzyme and uses large amounts of energy. Changes in the availability of iron and, possibly, other nutrients may thus change the rate of N2 fixation in the oceans. In one study, elevated CO2 decreased growth and N2 fixation rates in the heterocystous cyanobacterium Nodularia spumigena (Czerny et al., 2009). But a few experiments with cultures of the dominant marine N2 fixer Trichodesmium have shown a substantial increase in the rate of N2 fixation at elevated CO2 concentrations (Hutchins et al., 2007; Levitan et al., 2007; Barcelos e Ramos et al., 2007; Kranz et al., 2009). Some recent experiments with natural populations of Trichodesmium incubated at high CO2 appear to confirm the laboratory results (Hutchins et al., 2009; see also Chapter 4.2).
3.6 ACCLIMATION AND ADAPTATION
Acclimation is the process by which an organism adjusts to an environmental change that gives individuals the ability to tolerate some range of environmental variability. Although acclimation may allow individual organisms to survive a certain amount of stress, metabolic performance, including growth and reproduction, may be depressed in scale with the magnitude of the environmental perturbation. Therefore, acclimation may have population-level effects even though survival is increased at the level of the individual. The potential for individuals of most species to acclimate to higher CO2 and lower pH is not known, but will become increasingly important as ocean CO2 levels rise.
Adaptation is the ability of a population to evolve over successive generations to become better suited to its habitat. Adaptation to changing ocean chemistry is likely on some level for most taxa that have sufficient genetic diversity to express a range of tolerance for ocean acidification. Rates of adaptation are linked strongly to generation times, which range from days for many microbial and unicellular organisms (e.g., allowing for >30,000 generations by 2100) to as long as decades (e.g., allowing for only ~10 generations by 2100) for some slow-growing, long-lived marine animals. It remains unknown whether populations of most species possess both the genetic diversity and a sufficient population turnover rate
to allow adaptation at the expected rate and magnitude of future pH/pCO2 changes. It is conceivable that some reef calcifiers or cold water corals could adapt to ocean acidification if they evolve a calcification mechanism that allows them to precipitate CaCO3 at normal rates, but this type of adaptation has not been documented in corals. Survival of these organisms depends on their capacity to cope with skeletal loss by a change in life history (i.e., shift to cryptic existence), defenses (e.g., toxin production), or other means. Adaptation to compensate for weaker or smaller skeletons has not been demonstrated, but this topic has barely been investigated (e.g., Bibby et al., 2007).
The persistence of various taxa under increasing ocean acidification will depend on either the capacity for acclimation (plasticity in phenotype within a generation) or adaptation (plasticity in genotype over successive generations) or a combination of both. The relative capabilities of various taxa in terms of both acclimation and adaptation will likely influence the composition of marine communities and therefore result in a range of consequences for marine ecosystems.
This page intentionally left blank.














