4
Effects of
Ocean Acidification
on Marine Ecosystems

Ecosystems are defined by a complex suite of interactions among organisms and also between organisms and their physical environment; a disturbance to any part may lead to cascading effects throughout the system. Ocean acidification has the potential to disturb marine ecosystems through a variety of pathways. Differential sensitivities will result in ecological winners and losers, as well as temporal and spatial shifts in interactions between species (e.g., shifts in the timing of zooplankton development relative to food availability; Pörtner and Farell, 2008), leading to changes in predator-prey, competitive, and other food web interactions. There may also be changes in habitat quality and effects on other ecological processes such as nutrient cycling. Many of the physiological changes from ocean acidification are expected to affect key functional groups –species or groups of organisms that play a disproportionately important role in ecosystems. These include expected effects on phytoplankton, which serve as the base of marine food webs, and on ecosystem engineers, which create or modify habitat (e.g., corals, oysters, and seagrasses). Such changes may lead to wholesale shifts in the composition, structure, and function of these systems and ultimately affect the goods and services provided to society (see Chapter 5). While it is important to understand how ocean acidification will change ocean chemistry and the physiology of marine organisms, as reviewed in chapters 2 and 3, what is equally critical is to understand how these effects may scale up to populations, communities, and entire marine ecosystems. Such changes are likely to be difficult to predict, particularly where more than one species or
functional group will be affected by ocean acidification. In general, higher trophic levels, including most finfish, will likely be sensitive to ocean acidification through changes in the quantity or composition of the food available, although there may be direct physiological effects on some fish species at high pCO2 (see Chapter 3). The difficulty in predicting ecosystem change is compounded by other simultaneous stressors occurring in the oceans now (e.g., pollution, overfishing, and nutrient eutrophication) and in association with climate change. For example, it is projected that surface waters will become warmer, the upper water column will become more stratified, and the supply of nutrients from deep waters and from the atmosphere will change as a result of climate change. Whether these changes, in combination with the effects of ocean acidification, will have synergistic, antagonistic, or additive effects is unknown, but multiple stressors are likely to affect marine ecosystems at multiple scales.
Several previous reports have identified marine ecosystems that are most likely to be at risk from ocean acidification (e.g., Raven et al., 2005; Fabry et al., 2008b). This chapter begins by describing what is known and not known about ecosystem effects of ocean acidification for five vulnerable ecosystems: tropical coral reef, open ocean plankton, coastal, deep sea, and high latitude ecosystems. This is not an exhaustive review of all possible ecological effects, but is instead an overview of the ecosystems that have been identified as most vulnerable to acidification. The chapter looks at examples of high-CO2 periods in the geologic past for possible information on the ecological response to current acidification. It also examines general principles regarding biodiversity, possible thresholds in ecological systems, and managing ecosystems for change.
4.1 TROPICAL CORAL REEFS
Some of the most convincing evidence that ocean acidification will affect marine ecosystems comes from warm water coral reefs. Coral reef ecosystems are defined by the large, wave-resistant calcium carbonate structures, or reefs, that are built by reef calcifiers. The structures they build provide food and shelter for a wide variety of marine organisms (Figure 4.1). There are hundreds of reef-building species; the predominant calcifiers on coral reefs are zooxanthellate corals, which produce hard aragonite skeletons, and calcifying macroalgae,1 which produce high-Mg calcite and aragonite. These groups produce the bulk of the calcium carbonate that make up the reef structures, which in turn support the high biodiversity of coral reef ecosystems. Recent analyses illustrate that
![]()
1 There are two types of calcifying macroalgae that are important to reef formation in tropical coral reef ecosystems: crustose coralline red algae (coralline algae) from the family Corallinaceae and calcifying green algae (genus Halimeda)
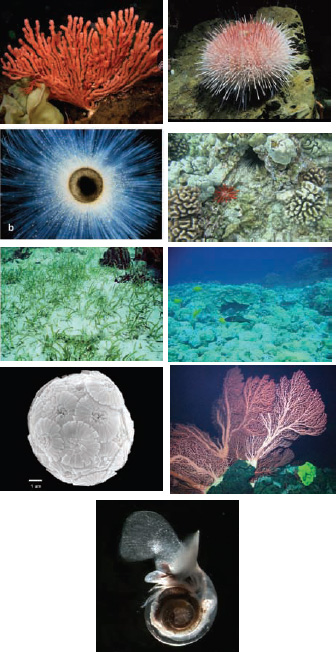
FIGURE 4.1 Some examples of organisms affected by ocean acidification. Red coral (photo courtesy of Jim Barry, MBARI); Sea urchin (photo courtesy of Jim Barry, MBARI); Foramaniferan (photo courtesy of Howard Spero, University of California, Davis); Coral and sea urchins (photo courtesy of Susan Roberts, NRC); Sea grass (photo courtesy of Richard Zimmerman, Old Dominion University); Tropical coral reef and fish (photo courtesy of Susan Roberts, NRC); Coccolithophores (photo courtesy of Mitch Covington, BugWare Inc.); Deep-sea Gorgonian bubblegum coral (photo courtesy of MBARI); and Pteropod (photo courtesy of Russ Hopkroft, University of Alaska, Fairbanks).
reef ecosystems have served as “cradles of evolution” throughout Earth’s biological history (Kiessling et al., 2010); that is, more marine species have originated in reef ecosystems than in any other. As a consequence, a decrease in the resilience of coral reefs or loss of coral reef habitat may adversely affect marine biodiversity in the short and long term. These ecosystems also provide a variety of services to humans, including recreation, fisheries, and coastal protection.
Ocean acidification poses a variety of risks to coral reef ecosystems. A critical vulnerability is the potential for ocean acidification to affect the reef structure itself. Acidification may decrease reef growth by reducing calcification rates, reproduction, and recruitment. It may also increase the dissolution or erosion of existing reef structures. Finally, acidification may indirectly result in the mortality of reef-builders.
The most obvious and best documented effect of ocean acidification is the depression of calcification rates, which will affect skeletal growth of the reef-building organisms. Decreased coral calcification rates are evident on the Great Barrier Reef, where records from massive corals show that calcification rates decreased by about 14% between 1990 and 2005 (De’ath et al., 2009), although the relative roles of increased temperature and ocean acidification could not be determined. Decreased skeletal growth in tropical reef-building corals and coralline algae has been well documented in high CO2 conditions that result in ocean acidification (see Appendix C for a summary; see also reviews in Doney et al., 2009; Kleypas et al., 2006; Langdon and Atkinson, 2005). In stony corals, most studies indicate a 10-60% reduction in calcification rate for a doubling of preindustrial atmospheric CO2 concentration. Differences among studies may reflect different species or experimental setups. Calcification rates in stony corals are affected by factors other than seawater carbonate chemistry, including light, nutrients, and particularly temperature. For example, studies on the effects of temperature show that calcification rates in corals peak near some optimal temperature (usually near the average summertime maximum), then decline at higher values (Clausen and Roth, 1975; Jokiel and Coles, 1977). As a result, increasing temperature from global climate change may initially offset the negative effect of acidification on calcification, but will eventually (and in some cases may already) work synergistically with acidification to decrease calcification. Calcification rates in tropical calcifying macroalgae may decrease even more strongly due to increasing CO2. Several laboratory studies indicate that reef-building crustose coralline algae will calcify more slowly (e.g., 50% reduction; Reynaud et al., 2003; Anthony et al., 2008). Field studies seem to agree with these findings. In one study, coralline algae showed a higher calcification rate that correlated with the natural pH change from the photosynthetic drawdown of CO2 when the algae grew in proximity to
seagrasses (Semesi et al., 2009b). By comparison, in a study of a temperate benthic community, the abundance of crustose coralline algae decreased rapidly with proximity to a shallow submarine CO2 vent, suggesting that coralline algae in this system could not survive at low pH (< 7.7) (Hall-Spencer et al., 2008; Martin et al., 2008). Similar to tropical reef corals, calcification rates of reef-building crustose coralline algae are affected more strongly by ocean acidification at elevated temperature (Anthony et al., 2008). There is little evidence that reef-building corals can adapt to decreased calcification under future ocean conditions.
Growth of reef structures relies not only on the calcification of adult corals, but also on successful recruitment of reef organisms, which is determined by gamete production, fertilization rates, larval development and settlement, and post-settlement growth. Theoretically, acidification could affect recruitment success but there is limited evidence of this and no consistent trends. In one study, ocean acidification did not affect either gamete production in one coral species or larval recruitment in another species (Jokiel et al., 2008). Another study also showed no effect on larval settlement, but did show significant decrease in post-settlement growth (> 50%; Albright et al., 2008). In general, there are few data on any of these aspects for reef-building species, making extrapolation to ecosystem effects difficult. Recruitment success may also be decreased through indirect effects on substrate. The presence of microbial biofilms or crustose coralline algae is important in coral recruitment success (Heyward and Negri, 1999; Negri et al., 2001; Webster et al., 2004; Williams et al., 2008). Reduction in the surface cover of newly recruited reef-building crustose coralline algae under future CO2 conditions (Kuffner et al., 2008) could therefore affect recruitment of coral larvae.
While ocean acidification does not appear to cause direct mortality in corals, several studies suggest that the survival of both major calcifying groups will be indirectly affected by ocean acidification, mainly because of its effects on skeletal growth. Several reviews (Kleypas et al., 2006; Kleypas and Langdon, 2006) list multiple ways that reduced skeletal growth may impact coral survival rates, including the ability to withstand hydrodynamic and erosional forces, age of sexual maturity, rate of fragmentation, skeletal light-gathering properties (Enriquez, 2004), and recruitment success. In addition, there is some evidence that ocean acidification has contributed to bleaching, which can ultimately lead to coral mortality (Anthony et al., 2008).2 Competition for space may also
![]()
2 Most reef-building zooxanthellate coral species depend on photosynthetic endosymbionts—zooxanthallae—to provide energy. Bleaching refers to the loss of these zooxanthallae due to stress, resulting in a loss of color. While corals can regain their endosymbionts and recover from bleaching events, extended bleaching can also result in coral death (Glynn, 1996).
lead to loss of corals as they become more vulnerable to displacement by other organisms, including those that may benefit from ocean acidification, such as non-calcifying macroalgae. Macroalgae compete with corals by taking up suitable surface area, blocking sunlight, and through the sweeping action of algae in waves and currents that can abrade corals or prevent larval settlement on hard substrates. Conditions that favor macroalgal growth (e.g., high nutrients, elimination of herbivores) and/or slow coral growth (e.g., bleaching, disease, ocean acidification) lower the resilience of coral-dominated systems to disturbance and thus increase the likelihood of a regime shift. The density of several invasive macroalgae increased near natural CO2 vents in the Mediterranean (Hall-Spencer et al., 2008), but little is known about the response of this or other groups that compete directly with corals for space. In some cases, an increase in non-calcifying primary producers on reefs (seagrasses and macroalgae) may counter the effects of ocean acidification, by drawing down CO2 directly from the water column during photosynthesis (Palacios and Zimmerman, 2007; Semesi et al., 2009a). While many of these hypothesized effects seem logical, most have not yet been explicitly tested.
The overall calcium carbonate budget and reef-building capacity of a reef depend not only on carbonate production rates, but also on dissolution rates and carbonate removal rates due to erosion and sediment transport. Acidification has been shown to increase dissolution rates of coral reefs; in one extreme example, the skeletons of corals placed in seawater with pH of 7.3–7.6 dissolved completely (Fine and Tchernov, 2007). The combination of decreased calcification rates with increased dissolution rates will shift coral reefs from net production/accretion to net dissolution/erosion at some CO2 threshold (Leclercq et al., 2000; Andersson et al., 2007; Yates and Halley, 2006; Silverman et al., 2009). Several studies indicate that crustose coralline algae will experience accelerated dissolution rates as ocean acidification proceeds and will experience net dissolution as pCO2 levels approach 700 ppm, expected by the end of the century (Jokiel et al., 2008; Kuffner et al., 2008; Martin and Gattuso, 2009). This directly threatens the existence of this key functional group on coral reefs and in coralline algal-based ecosystems. One projection of reef building estimates that, due to reduced coral cover from bleaching and due to ocean acidification, all coral reefs will be in a state of net dissolution once atmospheric CO2 concentration reaches 560 ppm (Silverman et al., 2009). The rapid loss of reef structure in the Galápagos following a severe bleaching event provides some evidence for this; the erosion rates of the Galápagos reefs were the highest recorded on any reef, which appears to be due in part to the naturally high CO2 waters (400-700 ppm) in this region (Manzello et al., 2008).
The combination of potential effects of acidification on the ecosystem engineers of coral reefs—decreased calcification, increased dissolution,
changes in recruitment and survivorship—will ultimately lead to changes in the reef structure. The function of calcium carbonate in reef ecosystems is widely recognized as important, but few studies have addressed what will happen as reef-building slows down. The dramatic loss of coral cover on many reefs has already resulted in “reef flattening” (a reduction in architectural complexity) that reduces the diversity of habitats and thus lowers the ability of the reef to support biodiversity (Alvarez-Filip et al., 2009). Ocean acidification is likely to exacerbate reef flattening. Loss of architectural complexity on reefs has been associated with changes in fish communities (Gratwicke and Speight, 2005; Pratchett et al., 2008), including the overall decline on Caribbean reefs (Paddack et al., 2009). Densities of important commercial species such as lobster have been linked to habitat complexity (Wynne and Côté, 2007), as well as recruitment of larval fish (Feary et al., 2007; Graham et al., 2007). Loss of structural complexity may also affect the recruitment of corals and other invertebrates, but this has not been examined. Finally, if reef structures suffer net erosion, then they lose their breakwater role, leaving coastlines and quiet-water habitats like mangroves more exposed to storm waves. The projected changes on reef structure are thus likely to have major consequences throughout tropical coral reef ecosystems.
4.2 OPEN OCEAN PLANKTONIC ECOSYSTEMS
The open ocean is not a uniform ecosystem; the components vary greatly by location. In open ocean systems, microscopic photosynthetic organisms—phytoplankton—which grow in the sunlit surface waters, serve as the base of diverse and complex food webs including zooplankton and larger free-swimming animals such as fish and marine mammals. Phytoplankton and bacteria also play an important role in cycling nutrients in open ocean ecosystems. Ocean acidification has been found to affect several key processes in open ocean planktonic ecosystems, including calcification, photosynthesis, and nitrogen-fixation. These changes affect the community composition of phytoplankton and zooplankton at the base of open ocean pelagic food webs; effects on these key functional groups may have cascading effects throughout the ecosystem. There may also be changes to the cycles of organic and inorganic carbon, oxygen, nutrients, and trace elements in the sea. In addition, the exchange of carbon dioxide and other climatically relevant trace gas species with the atmosphere may be modified, thus inducing feedbacks on the climate system.
The effect of acidification on calcification rates has been a major area of study because a number of the phytoplankton and zooplankton near the base of the food chain are calcifiers. Of the three major groups of
planktonic calcifiers—coccolithophores, foraminifera, and pteropods (a planktonic snail) (Figure 4.1)—coccolithophores have been studied most widely. While experiments using monospecific cultures of Coccolithophores revealed considerable species- and strain-specific differences in CO2 responses (Rost et al., 2008; Langer et al., 2009), a consistent trend of decreasing calcification with increasing CO2 has been seen in shipboard and mesocosm studies using mixed assemblages (Ridgwell et al., 2009). Studies on planktonic foraminifera and pteropods also indicate reduced calcification and increased calcium carbonate dissolution at elevated CO2 (see Fabry et al., 2008b for review; Moy et al., 2009; see also section 4.5). It is presently unknown to what extent these responses affect the competitive abilities, susceptibility to viral attack, predator-prey interactions, or the fitness of calcifying plankton.
Reduced rates of calcification, along with the shoaling of the saturation horizons for calcium carbonate minerals to shallower depths will also affect the marine calcium carbonate cycle (see Chapter 2) through decreased CaCO3 burial in sediments, additional carbon storage from increased production of extracellular organic carbon by phytoplankton (see below), and by the accelerated bacterial decomposition of organic matter at higher temperature. Ocean acidification can also affect processes related to photosynthetic activity, including increased rates of phytoplankton growth, primary production, and release of extracellular organic matter, as well as shifts in cellular carbon to nitrogen to phosphorus (C:N:P) ratios (e.g., Riebesell et al., 2007; Bellerby et al., 2007; Fu et al., 2007; Hutchins et al., 2009; see also Chapter 3). A shift in the ratio towards higher C:N and C:P at elevated pCO2 was observed during a mesocosm study with a natural plankton community (Riebesell et al., 2007). Changes in the C:N and C:P ratios alter the nutritional value of phytoplankton and may adversely affect growth and reproduction of their consumers (e.g., as seen in copepods and daphnids; Sterner and Elser, 2002). A change in the composition of the biomass is one of the few mechanisms by which biology can alter ocean carbon storage (Boyd and Doney, 2003; Riebesell et al., 2009). If phytoplankton growing at high CO2 produce and export biomass with a higher C:N ratio, it would make the ocean biological pump more efficient in exporting carbon to depth. In a mesocosm experiment, the net effect of this phenomenon was estimated to increase the carbon consumption by 27% in response to a doubling in present day CO2 (Riebesell et al., 2007). The evidence from experiments on natural plankton communities is equivocal, with examples of both increasing and decreasing C:N ratios (Hutchins et al., 2009). In a model study, the hypothesized effect of enhanced organic carbon export due to elevated C:N ratio resulted in a moderate increase in oceanic CO2 uptake (a cumulative value of 35 Pg C by 2100) and a fifty percent increase in
the extent of subsurface low-oxygen zones in the tropical ocean (Oschlies et al., 2008). In addition, increased production of extracellular organic matter under high CO2 levels (Engel, 2002) may enhance the formation of particle aggregates (Engel et al., 2004; Schartau et al., 2008) and thereby increase the vertical flux of organic matter (Riebesell et al., 2007; Arrigo, 2007), which may also affect nutrient availability for phytoplankton in surface waters.
Ocean acidification has the potential to alter the marine nitrogen cycle which controls much of primary production in the sea. Laboratory experiments with the nitrogen-fixing cyanobacterium Trichodesmium revealed an increase in both carbon and nitrogen fixation with increasing pCO2 (Barcelos e Ramos et al., 2007; Hutchins et al., 2007; Levitan et al., 2007; Kranz et al., 2009). Since Trichodesmium is a dominant species in large parts of the nutrient-poor tropical and subtropical oceans, this response has the potential to increase the reservoir of bioavailable nitrogen in the surface layer of these areas. These areas of the ocean are predominantly nitrogen-limited; therefore, an increase in nitrogen fixation would provide additional new nitrogen in low-nutrient subtropical regions and would lead to increased primary production and carbon fixation. The actual increase in nitrogen fixation, however, could be limited by phosphorus or iron supplies. A strong positive relationship between nitrogen fixation and rising CO2 has also been observed for cultured Crocosphaera, a nitrogen-fixing unicellular cyanobacterium, under iron-replete conditions but not under iron limited conditions (Fu et al., 2008), but another nitrogen-fixing cyanobacterium, Nodularia spumigena, showed the opposite response (i.e., reduced growth rate and nitrogen fixation rate at elevated CO2; Czerny et al., 2009).
These effects on calcification, photosynthesis, nitrogen fixation, and other processes will likely lead to shifts in the planktonic community as some species fare better than others under acidification. However, no consistent responses have been obtained in experiments concerning the effect of ocean acidification on plankton community composition. In one experiment with a phytoplankton community dominated by microflagellates, cryptomonads, and diatoms, only the diatom Skeletonema costatum responded to elevated CO2 by increased growth rate (Kim et al., 2006). A similar shift in phytoplankton species composition from Phaeocystis to diatom dominance occurred in another shipboard incubation experiment (Tortell et al., 2002). In contrast, a remarkable resilience of the enclosed plankton communities to seawater acidification was observed in a series of mesocosm CO2 enrichment experiments: no significant differences between CO2 treatments were observed for phytoplankton composition and cell cycle, inorganic nutrient utilization and nutrient turnover, bacterial abundance and diversity, microzooplankton grazing and copepod feeding and egg production (Riebesell
et al., 2008). While shifts in planktonic community composition could theoretically affect higher trophic levels, no experimental results exist to confirm these predictions.
Another important consideration is the possible interactive effects of climate change and acidification such as the warming of surface waters and reduced nutrient availability. Similarly, ocean microbes produce and destroy a number of trace gases that are important for atmospheric chemistry and climate besides CO2 and O2. For example, nitrous oxide (N2O), a powerful greenhouse gas, is a by-product of both nitrification and denitrification and its marine production might thus be affected by acidification. Another important trace gas produced in the oceans is dimethylsulfide (DMS), which serves as a precursor for atmospheric sulfate aerosols that nucleate cloud droplets and cool surface temperatures. Mesocosm experiments at elevated CO2 (Vogt et al., 2008; Wingenter et al., 2007; Hopkins et al., 2010) have shown both positive and negative responses in dissolved DMS responses with both small decreases. In this way, changes in the microbial community composition and activity triggered by ocean acidification may act as a feedback on climate change.
4.3 COASTAL ECOSYSTEMS
Coastal ocean ecosystems include a variety of benthic habitat types, including seagrass beds, kelp forests, tidal wetlands, mangroves, and others. They represent some of the most productive marine ecosystems that support numerous finfish and shellfish fisheries, both managed and cultured. Humans rely on coastal ecosystems for commerce, recreation, protection from storm surges, and a suite of other services; however, there is also a great deal of anthropogenic impact on coastal habitats. This section does not attempt to review all of the possible impacts of acidification on the various types of coastal ecosystems. Rather it highlights some general concerns, particularly for important coastal species and functions such as commercially-important fishery species and ecosystem engineers. Ocean acidification may affect coastal ecosystems in a variety of ways. It can directly impact the growth and survival of coastal organisms, particularly in sensitive reproductive and early developmental stages. It can also affect growth and survival indirectly by altering food web dynamics and nutrient cycling. It is also likely to affect important coastal ecosystem engineers that create habitat.
A major focus of recent studies has been on the potential effects of ocean acidification on the early life history of various species. For many coastal benthic calcifiers, including commercially-important species, reproduction and early development appear to be particularly sensitive to acidification (Kurihara, 2008). Reduced growth and calcification rates, and in
some cases even shell dissolution and mortality, have been reported for larval and juvenile stages in a number of bivalve species: the bay scallop Argopecten irradians (Talmage and Gobler, 2009), the hard clam Mercenaria mercenaria (exposed to sediments that were undersaturated with respect to aragonite; Green et al., 2004, 2009), the soft-shell clam Mya arenaria (Salisbury et al., 2008), the Mediterranean mussel Mytilus galloprovincialis (Kurihara et al., 2008a), the Sydney rock oyster Saccostrea glomerata (Watson et al., 2009), the Pacific oyster Crassostrea gigas (Kurihara et al., 2007), and the Eastern oyster Crassostrea virginica (Miller et al., 2009). Interestingly, Miller et al. (2009) did not see similar effects on the Suminoe oyster, Crassostrea ariakensis, indicating a species-specific response that could lead to shifts in community composition. Hence, these comparative studies did find that some species were more tolerant of high CO2 conditions. Negative effects of acidification have also been seen in the early development of non-bivalve species such as the European lobster Homarus gammarus (Arnold et al., 2009), the Pacific shrimp Palaemon pacificus (Kurihara, 2008), and the sea urchin Echinometra mathaei (Kurihara and Shirayama, 2004). In contrast, juveniles of American lobster (H. gammarus) and the blue crab (Callinectes sapidus) showed elevated rates of calcification at very high pCO2 levels (Ries et al., 2009).
Many studies have also shown negative effects on adult growth and survivorship of these and other coastal benthic species (e.g., Gazeau et al., 2007; Kurihara et al., 2008b; Ries et al. 2009). There were mixed responses—increasing, decreasing, parabolic, and no change in calcification rates—to decreasing saturation state in the eighteen benthic coastal species studied by Ries et al. (2009). It is not known whether positive or negative changes in calcification in these organisms would affect their lifelong productivity, growth, and fitness. Impacts on many other species not yet studied are likely.
Indirectly, acidification may affect the productivity and composition of some coastal ecosystems by affecting the key species at the base of coastal food webs. As noted previously, several calcifying planktonic species are sensitive to seawater pH and carbonate chemistry changes and can be important prey species in coastal ecosystems (e.g., pteropods may be important prey in salmon diets, Armstrong et al., 2008). In addition, the planktonic larvae of many species are also prey items and, as previously discussed, may be negatively affected by acidification. Therefore, coastal organisms that are not directly susceptible to the effects of acidification may indirectly be affected through trophic interactions.
Many coastal habitats depend on ecosystem engineers to build and maintain structures that provide critical habitat for other organisms, including oyster reefs, kelp forests, and seagrass beds. Oysters have already been discussed as species that will likely be negatively affected
by acidification. On the other hand, research has shown increased growth of seagrass (Figure 4.1) with increased CO2 (Zimmerman et al., 1997). It is probable that an increase in total seagrass area will lead to more favorable habitat and conditions for associated invertebrate and fish species (Guinotte and Fabry, 2009).
Coastal ecosystems exhibit naturally high variability in pH and seawater chemistry due to biological activity, freshwater input, upwelling, atmospheric deposition, and other factors. They are also subject to a diversity of stresses caused by human activities, such as organic matter and nutrient inputs, pollution by toxic organic compounds and metals, acid rain, sea level rise and other climate change effects, and overfishing. The effects of ocean acidification on coastal ecosystems may be small relative to the effects of these natural and human-induced stresses. But in some instances, acidification may act synergistically with other factors (Figure 4.2). For example, coastal upwelling is a natural phenomenon that brings deep water to the surface; this water is often undersaturated with respect to calcium carbonate. However, further acidification of these upwelled waters by anthropogenic CO2 uptake may be increasing the intensity and areal extent of these “corrosive” events (Feely et al., 2008). Increased temperature due to climate change is another stressor that is likely to interact with acidification; for example, temperature has been shown to act synergistically with acidification in the development of the Sydney rock oyster (Parker et al., 2009). Another likely interaction is that of increased nutrients and acidification. For example, in kelp forests, it is predicted that local nutrient pollution and increased CO2 will enhance the growth of filamentous algae species while simultaneously decreasing calcifying macroalgae that serve as the understory of kelp forests, thus allowing for a shift from kelp forests to filamentous turf mats (Russell et al., 2009).
Another example of a potential synergism is the interaction between acidification and low oxygen (i.e., hypoxic) or no oxygen (i.e., anoxic) “dead zones.” The decomposition of organic matter near the bottom in shallow coastal waters increases the ambient CO2 concentration and decreases the oxygen concentration and pH. This natural phenomenon can be exacerbated by anthropogenic inputs of organic waste and algal nutrients, resulting in dead zones. But in regions that are only hypoxic, the low oxygen and the high CO2 tend to act in concert to make respiration difficult for a number of aerobic organisms. It is possible that a further increase in CO2 caused directly or indirectly by acidification could increase the intensity or spatial extent of the hypoxic and anoxic events. Examples of ecosystems where this could occur is along many highly productive coastal upwelling zones around the world, such as the eastern Pacific, the Arabian Sea, and along northern and southern west Africa.
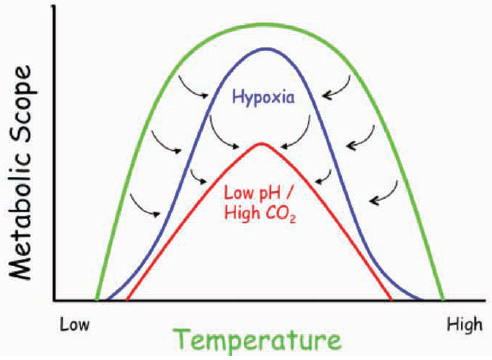
FIGURE 4.2 Specific combinations of environmental factors affect animal performance in ways that can narrow the range of performance for any given factor. These windows of performance (modified from Pörtner and Farrell, 2008) for organisms can be measured along environmental gradients such as temperature. In the example illustrated in the graph, an organism may have a relatively broad temperature tolerance (green line, from low to high), but this tolerance may only be observed under oxygenated conditions and normal seawater pH. Both low oxygen (hypoxia) and lower pH/high CO2 conditions could not only reduce the overall organismal performance, but also could narrow the temperature range under which this organism could survive. Hence, for some organisms, ocean acidification would restrict the habitable range of temperature and reduce the performance range (the metabolic scope which represents the maximium minus the minimum metabolic rate).
There, as previously discussed, the natural cycle in acid-base chemistry resulting from seasonal upwelling is amplified by penetration of anthropogenic CO2 into the upwelled water. The ambient flora and fauna, particularly benthic organisms, may well be affected by annual exposure to acidic and, in some cases, corrosive hypoxic water.
Depending on the differential tolerances of organisms to changes imposed by acidification, there are likely to be shifts in community composition or productivity of the various ecosystems. However, existing
research in coastal ecosystems, as is the case with other ecosystems, has been focused on individual organisms, not on the population, community, or ecosystem levels. Consequently, it is unknown whether populations sensitive to changes in ocean chemistry will be able to adapt through behavioral or physiological changes. For example, populations with individuals possessing genetic variations that tolerate the expected changes in ocean chemistry may result in higher survival or reproductive success because of more-rapid-than-expected adaptation to the new conditions.
It is not known whether coastal ecosystems that do not currently experience natural hypoxic and low pH events are less susceptible to incremental shifts in regional ocean chemistry due to ocean acidification. Areas along the U.S. eastern seaboard, the Gulf of Maine, and others have weaker oxygen minimum zones and higher pH waters along coastal zones. Organisms inhabiting these ecosystems may tolerate larger shifts in ocean chemistry caused by ocean acidification than those in ecosystems overlying more hypoxic upwelling waters, but this hypothesis requires study. Hypoxic dead zones caused by anthropogenic sources have been observed in most urbanized coastlines of the world, regardless of regional oceanography. These events, also accompanied by low pH, may indicate that most coastal ecosystems are sensitive to extreme eutrophication events.
4.4 DEEP SEA, INCLUDING COLD-WATER CORALS
Acidification of the deep ocean will occur more slowly than in surface seawater. But its ecological effects may nonetheless be severe because of the assumed greater sensitivity of the deep biota. Deep-sea organisms live in a cold, dark environment with low nutrient inputs and reduced reliance on visual interactions between predator and prey. These organisms generally grow slowly and have lower metabolic rates than comparable taxa living in warmer surface waters (Seibel and Walsh, 2001, 2003; Goffredi and Childress, 2001; Seibel et al., 1997; Gage and Tyler, 1991; Pörtner et al., 2004). In animals, slow metabolism typically corresponds to a low capacity for gas exchange (i.e., oxygen transport and CO2 release) and reduced enzyme function, including those linked to acid-base regulation (Seibel and Drazen, 2007; Melzner et al., 2009). For example, a logarithmic decrease in passive pH buffering ability with depth has been measured in highly active pelagic predatory cephalopods (Seibel and Walsh, 2003), indicating increasing vulnerability to acid-base disturbance with depth. The environmental stability of the deep sea over long time scales is also postulated to have reduced the tolerance of deep-sea species to environmental extremes through the loss of more tolerant genotypes (Dahlhoff, 2004), thereby decreasing the potential for adaptation to future ocean acidification.
Experimental studies with deep-sea organisms are obviously difficult and very few provide direct information on their sensitivity to acidification. In experiments performed on the abyssal floor off central California, low rates of survival of deep benthic organisms were observed after exposure to a modest decrease in pH (-0.2 units) near pools of liquid CO2 (Barry et al., 2003, 2005; Thistle et al., 2005; Fleeger et al., 2006). In contrast, deep-sea fish and cephalopods survived month-long exposure to mildly acidic waters during these experiments (Barry and Drazen, 2007), although related physiological studies indicate that respiratory stress (impaired oxygen transport) is likely for deep-living cephalopods exposed to low pH waters (Seibel and Walsh, 2003). In other experiments, deep sea crabs were much less able to recover from short-term exposure to very high CO2 than shallow dwelling crabs and this effect was amplified at low oxygen concentrations (Pane and Barry, 2007).
Some likely consequences of future ocean acidification in deep-sea waters can be inferred from organisms inhabiting hydrothermal vent and cold seep environments, which often (but not always) have low pH levels. Echinoderms and some other calcifying taxa are generally absent from hydrothermal vents (Grassle, 1986) and cold seeps (Sibuet and Olu, 1998), presumably as a result of the low ambient pH or other stressful environmental factors. For example, high concentrations of toxic metals (e.g., cadmium, silver, strontium, barium, and others) in vent effluent at some sites (Van Dover, 2000) may limit distribution of some fauna. Other vent and seep taxa thrive, in spite of high CO2 levels, and in some cases exploit the energy-rich conditions in these environments to sustain anomalously high rates of growth (Barry et al., 2007; Urcuyo et al., 2007). Adaptations promoting success for some animals at vent and seep habitats are likely to have evolved over long periods; it remains unknown whether more typical deep-sea animals are capable of adapting to future changes in deep ocean chemistry caused by acidification.
A unique habitat type in the deep sea that deserves particular attention is cold-water coral communities. Cold-water corals, also known as deep-water or deep-sea corals, form ecosystems that are in some ways the deep-water counterparts of tropical coral reefs. Cold-water coral reefs (or bioherms) are also founded on the accumulation of calcium carbonate, providing the structural framework for these biodiverse ecosystems that serve as habitat for a range of organisms, including commercially important fish species (Freiwald et al., 2004; Roberts et al., 2006). The primary reef-building species are stony corals that lack zooxanthellae, the symbiotic algae common in shallow, tropical species. Cold-water coral ecosystems occur globally in darker, colder waters than their tropical counterparts, from depths as shallow as 40 m to greater than 1,000 m (Freiwald, 2002; Freiwald et al., 2004).
As with tropical coral reefs, the main concern for cold-water corals with respect to ocean acidification is the effect on calcification rates for key reef-builders. The geographic distribution of cold-water coral communities suggests that they are limited to waters supersaturated with respect to their predominant skeletal mineralogy aragonite (Guinotte et al., 2006). With expected shoaling of the aragonite saturation horizon, many of these communities may become exposed to waters corrosive to coral skeletons. However, it is unclear whether it is the species or the structures they construct (or both) that are limited by the saturation horizon. Calcification rates in the cold-water species Lophelia pertusa were reduced by an average of 30 and 56% when pH was lowered by 0.15 and 0.3 units relative to ambient conditions, respectively (Maier et al., 2009), but despite this response, calcification rates in this species did not stop completely even in aragonite-undersaturated conditions. It must be noted that this is the only study on the response of a cold-water coral species to ocean acidification.
Deep-sea coral communities are also abundant and ecologically significant on thousands of seamounts throughout the world ocean that could be affected by ocean acidification. Seamounts—undersea mountains that rise from the abyssal plain but do not breach the surface—number about 100,000 worldwide (Figure 4.3). The coral- and sponge-dominated assemblages found near the peaks of seamounts depend nutritionally on suspended organic debris sinking from sunlit surface waters and form important habitat for deep-sea fisheries, including orange roughy, alfonsino, roundnose grenadier and Patagonian toothfish (Clark et al., 2006). Corals that dominate seamount assemblages include stony corals (scleractinians), black corals (Antipatharians), and octocorallians, including sea fans (gorgonians). Waters around seamounts and throughout the deep-sea are naturally more acidic than those found in shallower depths because of the accumulation of carbon dioxide from the respiration of deep-sea organisms. This effect is greatest in areas such as the Northeast Pacific Ocean. Mixing of anthropogenic carbon dioxide into the deep-sea will make these waters even more acidic. Aragonitic corals are much less abundant in the more acidic waters of the Pacific Basin (Roberts et al., 2006), and most species appear to be limited in distribution by the depth of the existing saturation horizon for aragonite, as shown by the strong reduction in the abundance and diversity of scleractinians below this boundary (Guinotte et al., 2006; Cairns, 2007). For seamounts with summits that are more than a few hundred meters below the surface, especially in the Pacific basin where waters are corrosive or nearly so to aragonite, the most common corals are calcitic, including the gorgonians, which often dominate as habitat-forming species. For example, the bubblegum coral (Paragorgia sp.; Figure 4.1) is a common coral found worldwide
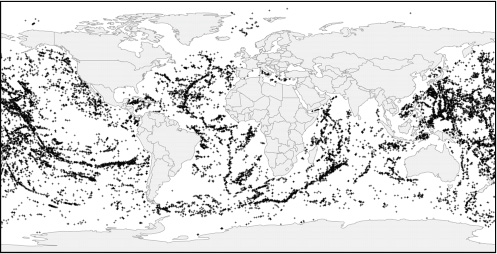
FIGURE 4.3 Global dataset of more than 30,000 potential seamounts (Kitchingman and Lai, 2004; www.seaaroundus.org). Estimates of the total number of seamounts in the world ocean varies greatly depending upon the resolution of bathymetric data available and analytic methods used. The abundances of deep-sea corals on seamounts are correlated closely with the aragonite and calcite saturation horizons (Guinotte et al., 2006).
on seamounts, and can reach at least 3 m in height (Mortensen and Buhl-Mortensen, 2005). Like aragonitic corals, gorgonians and other calcitic corals are likely to be affected by changes in calcite saturation with depth, though protective coverings and tissues may provide some protection from carbonate dissolution.
Seamount coral communities are highly vulnerable to anthropogenic disturbances. Growth rates of deep-sea corals are known to be low, with longevity estimates ranging from at least decades to centuries (e.g., Andrews et al., 2002; Clark et al., 2006), with at least some species living more than 1,000 years. Longevity estimates of some corals from ~500 m depth off the Hawaiian Islands were estimated at 2,742 y (Gerardia sp.) and 4,265 years (Leiopathes sp.) (Roark et al., 2009). The slow growth and long recovery time of seamount coral communities put them at greater risk for damage from human activities, including ocean acidification. Considering the expected rapid shoaling of the calcite and aragonite saturation horizons with future ocean acidification and the observed relationship between coral distributions and existing saturation horizons, deep-sea coral communities on seamounts or bioherms are likely to be impacted.
4.5 HIGH LATITUDES
High latitude waters of the Arctic and Southern oceans are very productive and support diverse pelagic and benthic communities. Some of the richest and most heavily exploited fishing areas in the world are located in high latitude waters, including the northern Bering, Chukchi, and Barents Seas in the Arctic and a krill fishery in the Southern Ocean (Dayton et al., 1994). About half of the U.S. domestic fish catch by biomass tonnage is landed in Alaska (Fisheries Economics of the U.S., 20083). Many protected and endangered marine mammals and seabirds also roam high latitude waters. High biodiversity cold-water coral habitats can be found in the high latitudes, including the “coral gardens” off the Aleutian Islands (discussed in further detail in section 4.4). Yet high latitude organisms are not as well studied as those in lower latitudes and the effects of ocean acidification on polar and subpolar marine life and ecosystems are largely unknown.
Like many other ecosystems, the most likely threat that acidification poses in the high latitudes is to planktonic calcifiers. In the subarctic Pacific, pteropods can be important prey of juvenile pink salmon, accounting in some years for >60% by weight of their diet (Armstrong et al., 2005). When exposed to the level of aragonite undersaturation expected to occur by the year 2100 (see Figure 2.10), thecosomatous pteropods showed visual evidence of reduced calcification (Comeau et al., 2009; Orr et al., 2005). If thecosomatous pteropods cannot adapt to living continuously in seawater that is undersaturated with respect to aragonite, their ranges will contract to shallower depths and lower latitudes that have higher carbonate ion concentrations. The possible exclusion of pteropods from high latitude regions would impact the downward organic carbon flux associated with pteropod fecal pellets (Thibault et al., 1999; Collier et al., 2000) and remove a major source of calcium carbonate in such regions (e.g., Bathmann et al., 1991; Honjo et al., 2000; Gardner et al., 2000; Accornero et al., 2003; Tsurumi et al., 2005). Similarly, if foraminifera densities decrease in some high latitude areas where they are currently abundant (e.g., subarctic Pacific), calcium carbonate export to the ocean interior will be reduced, which would in turn decrease the potential of foraminiferal tests to act as ballast in the transport of organic carbon to the deep sea (Schiebel, 2002; Moy et al., 2009). As in other regions, ocean acidification could also alter the species composition of primary producers and rates of photosynthesis through pH-dependent speciation of nutrients and metals (Zeebe and Wolf-Gladrow, 2001; Byrne et al., 1988; Shi et al., 2009; Millero et al., 2009).
![]()
3 http://www.st.nmfs.noaa.gov/st5/publication/fisheries_economics_2008.html
Polar benthic communities may also be affected by acidification. Although there are major differences in the modern biota and structure of benthic communities in the Arctic and Southern Ocean that reflect the distinct topography and evolutionary history of the polar habitats, there may be similar vulnerabilities in the two systems. Polar invertebrates tend to have low metabolic rates and slow growth rates. In addition, high latitude benthic (and some planktonic) invertebrates can have long generation times compared to warmer water taxa, providing them fewer opportunities to evolve effective adaptations to cope with seawater that will be progressively depleted in carbonate ion concentration and corrosive to calcium carbonate minerals in the coming decades (Orr et al., 2005; Bates et al., 2009; Olafsson et al., 2009). Calcifying macroalgae and marine invertebrates, including cold-water corals, sea urchins, and molluscs, make up significant components of the rich benthic communities in high latitudes, and these are thought to be at risk with increasing ocean acidification.
The aragonite saturation state of seawater provides a clear geochemical threshold when seawater becomes undersaturated with respect to aragonite. While many studies indicate that calcification correlates with the calcium carbonate saturation state of seawater, biological thresholds of the calcification response to ocean acidity may be species-specific. Such differential responses of species to rising ocean acidity may result in competitive advantages that could drive the reorganization of planktonic and benthic ecosystems, thereby affecting food webs, fisheries, and many ecological processes. The high latitudes will be the first ocean regions to become persistently undersaturated with respect to aragonite as a result of anthropogenic-induced acidification (Figure 2.10). Thus, these ecosystems are natural laboratories in which to test many hypotheses on the impacts of ocean acidification and other stressors, particularly those induced by global warming.
Many polar and subpolar ecosystems are undergoing rapid change owing to global warming. The reduction in sea ice, freshening of seawater, and increasing ocean and air temperatures are forcing major ecological shifts in polar regions of both hemispheres. The western shelf of the Antarctic Peninsula is the fastest warming region on earth, with rates of temperature increase nearly five times the global average rate over the past century (Ducklow et al., 2007). Warming sea temperatures may allow shell-crushing crabs to invade the shelf benthos surrounding Antarctica, with significant consequences for benthic organisms that have evolved in the absence of such predators (Aronson et al., 2007). Since the Eocene, cold temperatures have prevented crabs from invading Antarctic shelves; however, king crabs are moving up the western Antarctic continental slope (Thatje et al., 2005) and should they arrive on the continental shelves, the
weakly calcified shells of Antarctic echinoderms and molluscs—further stressed by acidification—would provide little defense from these predators. A change from arctic to subarctic conditions is underway in the northern Bering Sea, and poleward displacement of marine mammals has coincided with a reduction in benthic prey, an increase in pelagic fish, and reduced sea ice (Grebmeier et al., 2006). Again, acidification impacts on prey species could further exacerbate food web changes caused by changing climate conditions. In both hemispheres, the observed regional changes are expected to affect broader areas of the Arctic and Southern Oceans, respectively, in future decades. In addition to warming temperatures, retreat of sea ice and increasing species invasions, high latitude regions, particularly in the north, are subject to heavy fishing pressure which is an additional stressor for these ecosystems.
4.6 LESSONS FROM THE GEOLOGIC PAST
Evidence from the geologic record indicates that the Earth previously experienced periods of high atmospheric CO2 which also changed ocean chemistry. Studies of past ocean chemistry and coincident changes in marine ecosystems may provide insight into the potential impacts of ocean acidification today and in the future.
Approximately 55 million years ago, a large release of carbon into the oceans changed the Earth’s climate and ocean chemistry, an event called the Paleocene-Eocene thermal maximum (PETM). Atmospheric CO2 and global temperature spiked upward and then slowly recovered over a period of more than 100,000 years (Kennett and Stott, 1991; Pagani et al., 2006; Zachos et al., 2001). The evidence from the isotopic compositions of carbon (δ13C) and oxygen (δ18O) in CaCO3 in deep ocean sediments indicate that the release of carbon was relatively rapid (~10,000 years) though the exact duration of the release event is not well constrained by the sedimentary data. The δ13C of surface-dwelling plankton appeared to change instantaneously, while benthic foraminifera recorded transitional δ13C values, as if the atmospheric CO2 changed on a time scale shorter than the circulation time of the ocean (Thomas et al., 2002), which today takes about 1,000 years. However, a longer CO2 release time of 10,000 years is suggested by the sedimentary time scale based on orbital variations (Lourens et al., 2005). The oxygen isotopic composition of the CaCO3 indicates that intermediate-depth ocean, and presumably the Earth’s surface, warmed in concert with the carbon release. Both temperature and CO2 gradually returned to their initial, steady values (Lourens et al., 2005). The recovery to initial conditions of carbon and oxygen occurred on a time scale, over 100,000 years, comparable to the silicate weathering thermostat mechanism for regulating atmospheric CO2 (Berner and
Kothavala, 2001), a further indication that CO2 played a role in the spike in global temperature.
Deep sea sediments from the PETM show extensive dissolution of CaCO3 (Zachos et al., 2005), consistent with an elevation in atmospheric CO2. Somewhat puzzlingly, the extent of CaCO3 dissolution differs greatly between the Atlantic and Pacific basins during that time (Zeebe and Zachos, 2007), possibly the result of regional anoxia events that would reduce mixing of surface sediments. Nonetheless, a number of factors limit the utility of the PETM as an analog for the detailed effects of acidification on the biota and carbon cycle of the ocean. First, the amount of carbon released is not well constrained because the exact source is unknown, and the magnitude of carbon isotope excursions in different carbon isotopic records vary by roughly a factor or two, with larger excursions typically found in soil carbon records than in deep sea sediments. Second, the magnitude of the ocean pH excursion is also unclear because it is dependent on whether the CO2 release was faster or slower than the CaCO3 neutralization time scale.
The PETM was marked by the extinction of CaCO3-producing foraminifera that live on the sea floor, perhaps in response to acidification or alternatively as a result of anoxia in the deep sea. There was not a comparable extinction in shallow-water species such as mollusks, but the occurrence of weakly calcified planktonic foraminifera may indicate changes in carbonate ion concentration in surface waters. A decrease in productivity or diversity, which would be relevant to humankind in the future, is difficult to gauge from the fossil record.
The impact of a comet or asteroid at the boundary between the Cretaceous and the Tertiary periods (also known as the K/T boundary), which occurred 65 million years ago and is responsible for the extinction of the dinosaurs, may have also perturbed the pH of the ocean. In this event, the impact fireball caused the oxidation of atmospheric nitrogen to nitric acid (D’Hondt and Keller, 1991) and produced sulfuric acid from the calcium sulfate enriched carbonate structures at the point of impact (D’Hondt et al., 1994). The atmospheric deposition of nitric and sulfuric acids likely only affected the pH of surface waters which would have recovered ambient pH relatively quickly as they mixed with deeper water. The impact also released large quantities of dust and aerosols that would have darkened the skies and cooled Earth’s atmosphere. As in the PETM, calcifying organisms suffered greater extinction rates than organisms that do not produce CaCO3, but the ecological responses that can be reconstructed could have been the result of the collapse of photosynthesis from the darkened skies, or disruption of other geochemical factors, in addition to or instead of changes in ocean pH.
The largest extinction event in Earth’s history took place 251 million
years ago at the boundary between the Permian and Triassic periods (Knoll et al., 1996). The cause of this event is speculative; possibilities include the impact of a large object (such as a meteor), extensive volcanism, ocean anoxia, or release of methane from methane hydrates. Analysis of the correlations between extinction patterns and physiology suggest that elevated CO2 levels might have played a role, but the duration over which this extinction occurred is unknown.
These three geological events give general support to current concerns about ocean acidification, particularly related to the possibility that calcifying organisms may decrease or even disappear as a result of increasing CO2. However, the severity of the perturbations and their durations are not known with enough accuracy to determine their similarity to conditions resulting from anthropogenic CO2 emissions. As a consequence, responses of marine ecosystems to the ongoing increase in CO2 may not be analogous to the changes in biological diversity associated with events in the deep past. Further development of proxy measurements, such as the use of boron isotopes to estimate ocean pH changes, could provide additional information on the rate and extent of changes in ocean CO2 and pH during these past climatic events.
4.7 BIODIVERSITY, THRESHOLDS, AND MANAGING FOR CHANGE
Regardless of the ecosystem, there is a concern that ocean acidification, along with other stressors, will reduce the biodiversity (i.e., species richness) of marine ecosystems through species extinctions, with potentially important consequences. Changes in species’ abundances, either directly due to the tolerance or intolerance of species to ocean acidification, or indirectly through changes in competitive interactions and trophic linkages, are very likely in the future.
Depending on the sensitivities of species, ocean acidification may result in extinctions that reduce the biodiversity of marine communities. Very little information is available on the effects of ocean acidification on biodiversity, but studies in areas where the water is naturally high in CO2 may provide some indication of the types of changes that could occur with global ocean acidification. For example, studies of species composition in the vicinity of CO2-rich volcanic vents in the Mediterranean Sea suggest that acidification will reduce the biodiversity of shallow, marine benthic communities (Hall-Spencer et al., 2008). High biodiversity in marine ecosystems is generally considered to enhance the stability of ecosystems through “functional redundancy” or “species complementarity.” In other words, when biodiversity is high, there are many species serving similar ecological roles. Reduced ecosystem biodiversity due to
the loss of species increases the dependence of the ecosystem on the services (e.g., prey or predatory rates) provided by the remaining similar species. If key trophic linkages are lost (e.g., an intermediate consumer guild is reduced severely), food web integrity may be compromised, energy flow may be impaired, and significant changes in ecosystem structure and function become likely—an ecological tipping point or threshold has been broached that can lead to a catastrophic change in an ecosystem. These “regime shifts” can move an ecosystem from one stable state to an entirely different state.
Many ecosystems have been demonstrated to undergo regime shifts to alternative ecological states (Scheffer et al., 2001). Analyses of previous regime shifts in both terrestrial and marine ecosystems (e.g., rangelands (Briske et al., 2005), lakes (Carpenter et al., 1999), coral reefs (Norström et al., 2009), open ocean (Overland et al., 2008)) show that they were rarely predicted, and many appeared to be triggered by relatively small events (van Nes and Scheffer, 2004). The growing body of literature now illustrates that the underlying cause for regime shifts is a decrease in ecosystem resilience (Folke et al., 2004; Scheffer et al., 2001). Resilience can be defined as “the amount of change or disturbance that a system can absorb before it undergoes a fundamental shift to a different set of processes and structures” (West et al., 2009). In many regime shifts, once an ecological threshold has been passed, the driver of the change must be reversed to levels far beyond where the shift occurred before the system shifts back to its original state. Regime shifts are likely within those marine ecosystems that experience stress from ocean acidification, either directly (e.g., through elimination of one or more species) or indirectly (e.g., alteration of the physical environment, such as dissolution of substrate), and particularly in combination with other stressors. Ecosystems degraded by acidification also may become more sensitive to other human and climate change stressors beyond ocean acidification.
As stated by Overland et al. (2008) “our current understanding of regime shifts is not a deterministic one, and while one can discuss amplitudes and mean duration of regimes, we cannot predict their precise timing other than to say that they will be a main feature of future climate and ecosystem states.” Nonetheless, developing methods for detecting, and in some cases even predicting or managing, an ecosystem’s approach toward a tipping point or critical threshold has received increasing attention (e.g., de Young et al., 2008; Scheffer et al., 2009). Multiple techniques for identifying regime shifts are now available, but only after they have occurred (Andersen et al., 2009; Carpenter et al., 2008). Recent evidence, suggests that complex systems (including ecosystems) may exhibit certain “symptoms” prior to a regime shift (Scheffer et al., 2009), such as:
(1) a “critical slowing down” of the dynamics which would be expressed as a slower recovery from small perturbations, increased autocorrelation (Dakos et al., 2008), or a shift of variance power spectra toward lower frequencies (Kleinen et al., 2003; Dakos et al., 2008),
(2) notably increased variance (Carpenter and Brock, 2006),
(3) greater asymmetry in fluctuations (Guttal and Jayaprakash, 2008), and
(4) in benthic communities, a breakdown of scaling rules for spatial patterns (Rietkerk et al., 2004).
Recent progress has been made toward attributing ecological shifts, particularly in terrestrial systems, to climate change (Rosenzweig et al., 2008). A major challenge in ocean acidification research is how to attribute ecological shifts to forcing from ocean acidification. In the field, ocean acidification rarely, if ever, will be the only driver of change. Climate change is simultaneously causing changes in temperature, circulation patterns, and other phenomena, so that attribution of changes (or at least part of the change) to ocean acidification will be difficult. In coral reefs, for example, whether the loss of corals is due to rising temperature or from ocean acidification may have little relevance in the overall impact on the ecosystem (loss of corals impacts the base function of the ecosystem). But systems where species are differentially impacted by temperature and/or ocean acidification may exhibit clear signs as to which factor is likely to cause a major ecological shift. Analyses of changes in food webs supporting fisheries, for example, reveal patterns that indicate whether the drivers of that change lie near the base of the food chain or at the top (Frank et al., 2007).
Management of ecological systems for climate change has focused primarily on adaptations that maintain or increase ecosystem resilience (West et al., 2009). The most common recommendation for maintaining resilience is to limit local to regional stressors such as land-based pollution, coastal development, overharvesting, and invasive species. Ecosystems with high biodiversity and/or redundancy of functional groups (e.g., several species fill the role of algal grazers) tend to be more resilient, and recover more quickly following a perturbation, which suggests that managing for biodiversity is a logical means of sustaining ecosystems (Palumbi et al., 2009). Resilience of some stocks to overfishing, for example, appears to be related to warmer regions with greater species richness (Frank et al., 2006; Frank et al., 2007). This suggests that different strategies may be necessary for maintaining resilience across different ecosystems.
























