CHAPTER TWO
Coalbed Methane Produced Water in Western U.S. Basins: Hydrogeological and Geochemical Foundations
A fundamental challenge regarding management of coalbed methane (CBM) produced water is determining to what degree surface water and groundwater resources may be depleted, supplemented, degraded, or enhanced and over what time periods as consequences of CBM extraction and management of produced water. To understand these consequences this chapter reviews the features of western CBM basins including (1) the hydrogeological characteristics of the basins; (2) the nature of connections between water in methane-bearing coal deposits and surface water and groundwater systems in the basins; and (3) the chemistry and age of the waters in the coalbeds.
The chapter focuses primarily on two basins—the San Juan Basin in Colorado and New Mexico and the Powder River Basin in Wyoming and Montana. These basins capture and contrast the currently known range of CBM produced water quality and quantity and produced water management approaches throughout the western CBM basins. The Uinta, Piceance, and Raton basins of Utah, Colorado, and New Mexico are also briefly discussed (see Figure 2.1). At present, no CBM production occurs in North Dakota.
HYDROGEOLOGICAL FOUNDATIONS
Origins of CBM and Associated Water
Coal is formed from plant matter (organic material) that has undergone burial, consolidation, and heating over millions of years under younger sediments. In the western United States, the wetland areas that provided the organic material for present-day coal basins existed between about 145 million and 56 million years ago. The plant matter formed either within alluvial systems of streams, lakes, and peat swamps, all of non-marine origin (northern Rocky Mountain area of the United States), or behind barrier islands and in back bays, lagoons, and deltas along the midcontinental seaway with waters of marine or
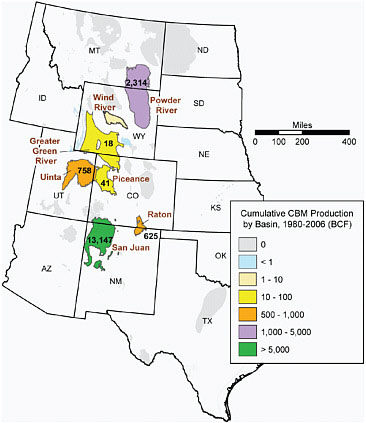
FIGURE 2.1 Map of western CBM basins within the six states that are the subject of this study. Only those basins with cumulative production to date greater than 40 billion cubic feet (BCF) are included in the discussion in this report. SOURCE: Adapted from EIA (2007).
brackish origin (southern reaches of the Rocky Mountains; see Figure 2.2). Because of the naturally discontinuous distribution of these wetland settings and the tectonic processes that affected buried coals during and after their formation, most of the coal deposits now in these western basins, although regionally pervasive, are also discontinuous. The coal deposits occur as seams or beds that are often distributed as discrete “lenses” or layers that pinch out, terminate, or branch (see descriptions of individual basins below). Discontinuities within these coalbeds or seams (hereafter referred to as “coalbeds”) are important in that they affect the way in which water in the coalbeds and surrounding sedimentary formations migrates and is replenished.
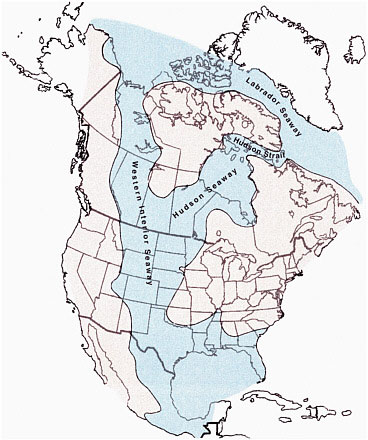
FIGURE 2.2 Illustration of the Cretaceous interior seaways, including the Western, Hudson, and Labrador seaways. The Cretaceous Period lasted from about 145 million to 65 million years ago. Coal-bearing basins in the western United States that are the subject of this report formed from organic-rich sediments (plant material) deposited in and along the wetlands of the Western Interior Seaway. The organic-rich sediments were deposited through Cretaceous and Paleocene (ca. 65 million to 56 million years ago) times during the rise and fall of intercontinental sea levels. SOURCE: W.A. Cobban and K.C. McKinney, USGS. Available at esp.cr.usgs.gov/research/fossils/ammonites.html.
Coalbeds can serve as aquifers or subsurface rock layers that are sufficiently permeable1 to conduct groundwater and can provide sufficient water for human use. Other less permeable materials (e.g., siltstones, shales, clays) above and below the coal seams—sometimes
called “aquitards”2—can inhibit upward or downward water flow from coalbeds. This inhibited flow essentially causes a water-saturated coalbed to be “confined” with respect to contained water. When water in a confined coalbed connects to the water table at an elevation above the elevation of the coal seam, the water in the coalbed may become overpressured with respect to the pressure exerted by a static column of water in the overlying rock (termed “hydrostatic pressure” or “hydrostatic head”). When a well is placed in a confined coal seam, the water level will rise to an elevation above the seam.
Because of the discontinuous nature of coalbeds, not all groundwater flow in coal-bearing basins can be described by simple hydrogeological models. These models usually describe water as moving from higher elevation “recharge” areas into lower elevation discharge areas from which the water may flow out as streams and springs. Groundwater “recharge” is a process by which water moves downward from the surface to groundwater and can occur naturally (e.g., rainwater percolation) or through artificial (human-induced) means. In some basins where natural recharge areas are located far from downgradient portions of a coalbed or other aquifers, replenishment of these aquifers by infiltrating precipitation may not occur within human lifetimes or even thousands to millions of years when water is removed from the aquifer. In essence this “old” or “fossil” water in a coalbed aquifer can be considered a “nonrenewable” resource once removed from the coalbed. The “age” of the water, or its residence time in the coalbed, thus also indicates the degree to which the CBM water is connected to surface water and shallow groundwater. “Old” water would suggest slow or inhibited connections to surface water or shallow groundwater that otherwise might serve as a source of “new” water to a coalbed.
Determining the connections between coalbeds and surrounding aquifers (hydraulic connections) and the renewability of the water resource in the coalbed is important to understanding the consequences of water removal from the coalbed during CBM production (described in detail later in the chapter). The age of the water in coalbeds from which CBM is being extracted thus can become an important factor in determining how produced water is managed. The next section outlines the development of methane and associated water in coalbeds. Subsequently, geological and hydrogeological characteristics of each basin are briefly described because of the role they play in determining both the volume and quality of water produced during CBM extraction.
Production of Methane and Water from Coalbeds
Methane associated with buried coalbeds is originally formed from one of two processes: thermogenesis or microbial methanogenesis. Thermogenesis involves the degradation of organic matter by temperatures usually greater than 120°C (248°F) associated with pressure from burial at depths greater than about 1,000 feet. The San Juan Basin contains coals with themogenic methane. Microbial methanogenesis is the decay of organic matter through microbial activity at relatively shallower depths and lower temperatures than those related to thermogenesis; the Powder River Basin coals contain methane generated in this way. In addition to genesis of methane during compaction and heating of organic material, coals develop systematic fractures or “cleats,” roughly analogous to cleavage planes in minerals (Riese et al., 2005). The presence of water in the coalbeds keeps the methane adsorbed on the surfaces of the coal and within the cleats and adsorbed to walls in the micropore structure of the coal matrix (see Figure 1.1). Water in the coalbeds may derive from (1) original water (“connate” water) associated with freshwater or marine settings in which the organic material was originally deposited, and/or (2) water (e.g., rainfall) that later percolated from the surface through to the coals as they were progressively buried.
To extract methane adsorbed to the coal, water must be pumped out of coal seams to lower the water pressure (head) and allow the methane to desorb, coalesce, and bubble into the pumped water, analogous to the formation of bubbles of carbon dioxide in a bottle of carbonated beverage when the cap is removed (see Figure 1.1). The amount of water that must be removed from the coalbeds in order to release methane depends on the original water pressure in the coal, the physical capacity of the coal to hold and release water, and the extent to which coals may be hydraulically connected to adjacent geological formations. Water production records show that the volume of water pumped from individual CBM wells generally decreases exponentially with time, with a corresponding increase in the rate of methane production (see Figure 2.3). In many cases, water pumping may discontinue within 10 to 20 years of initial pumping, while methane production may continue.
In contrast to conventional oil and gas fields where produced water is sometimes reinjected into the producing formation to enhance oil and gas recovery, CBM produced water is not returned to the coal seams from which it was extracted because doing so would hinder additional methane recovery. Thus, other options are considered with respect to storage, disposal, or use of the CBM produced water.
The generalized trend shown in Figure 2.3 for water and methane production related to CBM extraction is useful for discussion of long-term predictions for water and gas volumes from a particular basin. However, the volume of water produced per year, the ratio of water to gas extracted from a well, and lifetime water production within and between the western CBM basins do not follow a common trend. Hydrogeological properties and operational practices affect the volume of water produced. For example, the rate at which
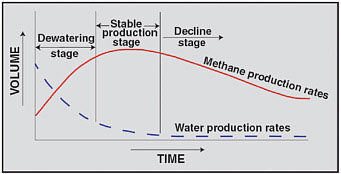
FIGURE 2.3 Schematic production curves for typical CBM wells show that operator-controlled water production rates decrease exponentially over time while methane production increases before moving into a stage of decline. Water production is a function of initial, operator-controlled pumping rates that aim to reduce pressure and stimulate flow of water and gas to the well. Once gas flow has been achieved, over time, the operator will gradually reduce the water production rate until the gas production rate is maximized. SOURCE: Nuccio (2000).
pumped water enters wells during production depends on the natural hydraulic properties and water-filled porosity of the coal seam containing the methane and the operator-controlled water-pumping rate. Shallow, weakly-consolidated coalbeds may have extensive internal fractures and interconnection of fractures that produce a porous and permeable formation capable of releasing large amounts of water during methane production (e.g., the Powder River Basin). In other areas where the methane-bearing coalbeds lie at much greater depths, the amount of water that must be pumped from the coal and the rate at which that water can be pumped to facilitate the release of methane are often limited by the effective water-filled porosity and permeability of the coal (e.g., the San Juan Basin). The limited interconnectivity between fractures and cleats in these deeper coals often requires use of hydraulic fracturing to stimulate release of the methane (Box 2.1; see also Chapter 5).
CBM production and associated produced water volumes are also a function of economic conditions. Generally, if the price of natural gas goes below a certain price point, the CBM operator will begin to “shut in” (cease to produce from) wells, which will reduce the quantity of produced water generated by the industry. When natural gas prices are above a certain level, CBM operators will generally increase production to generate more income and profit. The total volume of CBM produced water generated by a CBM operator will thus vary as a result. Other factors such as contract deliverables, reservoir management requirements, and reservoir energy may also affect the decision to shut in a well or keep it in production.
|
BOX 2.1 Hydraulic Fracturing Hydraulic fracturing is a technique used in many oil and gas production settings to help release oil or gas from the geological formation and allow the hydrocarbon to flow more freely and consistently to the well bore. The technique injects fluids and sand under pressure into the formation of interest to open and stimulate the growth of new fractures, thereby increasing the number of pathways through which oil or gas can reach the well. Among the CBM basins examined in this study, hydraulic fracturing is used to enhance the flow of methane gas in the San Juan, Raton, Piceance, and Uinta basins. Hydraulic fracturing is used very infrequently in CBM operations of the Powder River Basin due to the high natural permeability of the shallow, methane-bearing coal seams. The standard industry practice for oil and gas operators to fracture a formation hydraulically is to fill the space between the outside of the steel casing of the well pipe and drill hole with cement along some or all of the well bore to the top of the target rock unit from which oil or gas (including methane from coalbeds) is going to be recovered. Holes or perforations are then blasted through the well casing opposite the target rock formation (for CBM production, the target is the coal seam). Fracturing fluids, if used, are pumped under high pressure through these holes into the target formation and are then pumped, together with the oil and gas and/or any produced water, back to the surface for recovery and disposal. In the Montana portion of the Powder River Basin, water, rather than other fluids, may be injected into CBM wells by some operators to improve conductivity around the well bore. The well casing and encasing concrete in a CBM well are designed to maximize recovery of all types of fluids from the target formation and to minimize loss of fluid, whether hydraulic fracture fluid, oil, gas, or water, to other geological formations along any part of the well bore. |
Western CBM Basins
This section provides an overview of the variations in regional geological and hydro-geological histories for the western CBM basins. These variations have direct bearing on the subsurface depth from which methane is extracted and the volume and chemistry of associated produced water. In discussing the chemistry of CBM produced water, the committee sometimes uses the qualifying word “relatively” to denote differences in the total dissolved solids (TDS)3, salinities, and sodicities of CBM produced waters as they vary across the western basins. For example, CBM produced water from the Powder River Basin is sometimes described as “relatively fresh,” whereas CBM produced water from the San Juan Basin may be described as having “relatively high salinity.” The section on “Geochemical Foundations”
later in this chapter provides the background for the use of these terms throughout the report.
POWDER RIVER BASIN
The Powder River Basin of Wyoming and Montana covers approximately 25,800 square miles (see Figure 2.4). CBM in the basin is derived from coals in the Tongue River and
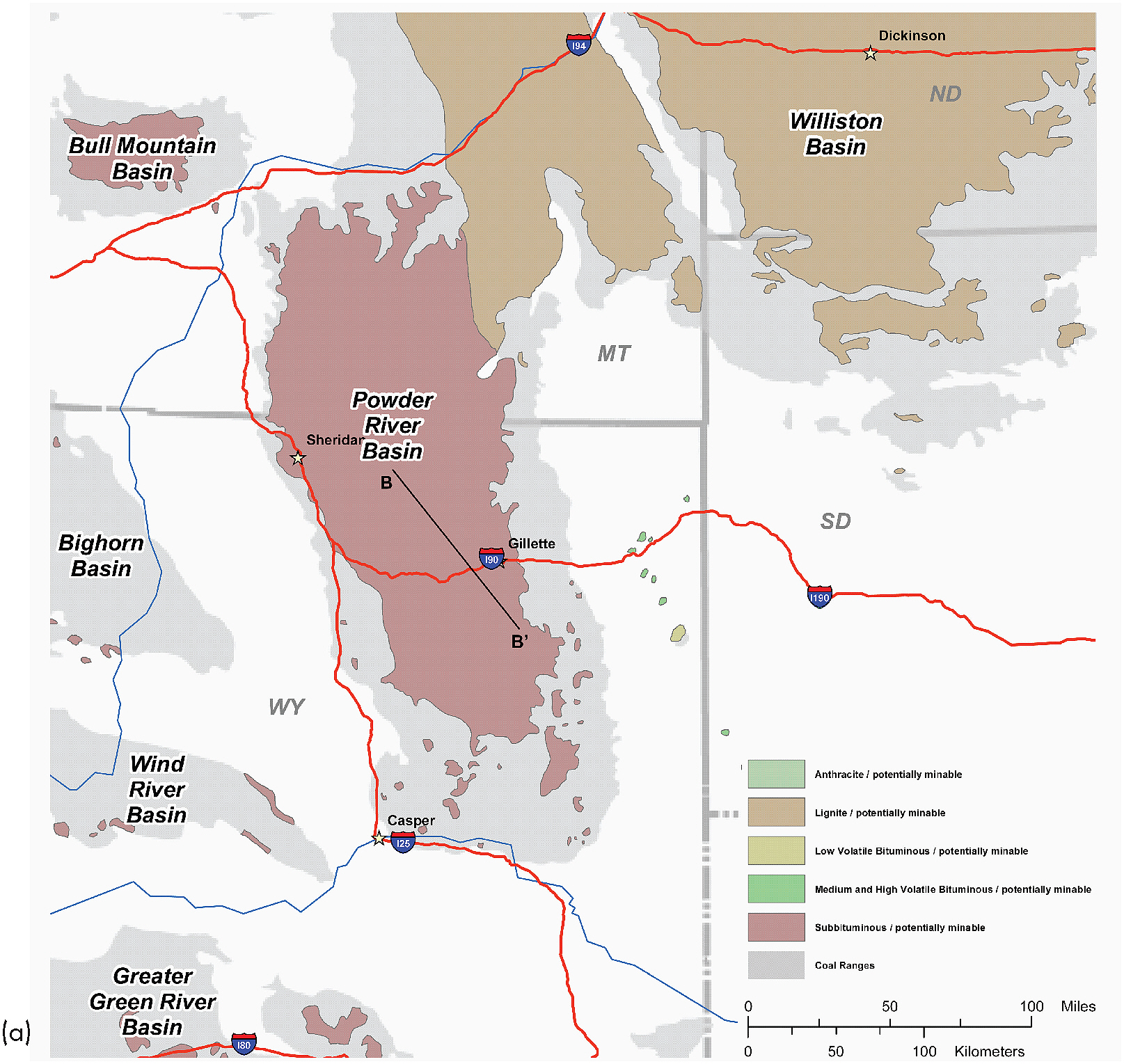
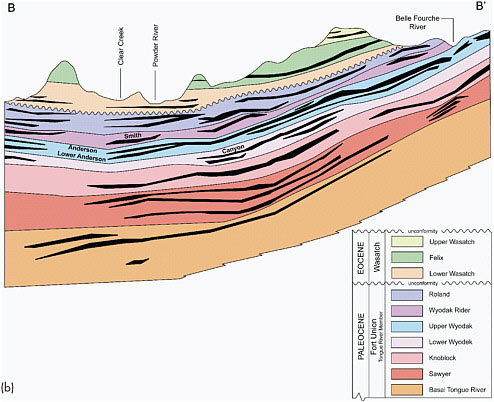
FIGURE 2.4 (a) Powder River Basin of northeastern Wyoming and southeastern Montana. Major drainages flow north or east into the Missouri river system. Location of cross-section B-B’ in Figure 2.4b is shown within the purple-brown shading that indicates coal of subbituminous grade. (b) Northwest-southeast geological cross section through the basin depicts a major coal-bearing and CBM-producing rock unit, the Fort Union Formation, and the overlying Wasatch Formation. Although oil and gas production began in the Powder River Basin in the 1920s, the first CBM well was not drilled there until the late 1980s (in the Wyoming portion of the basin). By the end of 2008, approximately 18,000 CBM wells were extracting methane from the Tongue River and Lebo Shale members of the Fort Union Formation, mostly at shallow depths ranging from approximately 450 to 4,500 feet (USGS, 2005). Wells shallower than 450 feet have produced methane from coalbeds in some localized areas. The Wyodak coal zone, including the Lower and Upper Wyodak and Wyodak Rider zones, contains the Canyon, Anderson, Smith, and Big George coals, which are CBM production targets. Vertical and lateral correlations in the Fort Union Formation show successive splitting of thick coal beds resulting in overlapping coal zones (Flores et al., 2010). This effect has played a role in the use of slightly different nomenclature to identify coal horizons in the basin. For example, the Anderson coal is sometimes referred to as the Wyodak coal; in the northwestern part of the basin and in Montana, the Anderson and Canyon coals are interleaved with the Dietz coal (sometimes referred to as the “Anderson-Dietz coal”). The Smith and Big George coals are not easily differentiated in every part of the basin and are sometimes referred to as the Smith/Big George coal, or as in the case of this cross section, only as the Smith coal. Elsewhere in the basin, the Big George coal occurs in the same part of the Wyodak Rider zone and is identified as such (Copeland and Ewald, 2008). The Lebo Shale Member is not depicted on this cross section but lies below the Tongue River Member. SOURCES: (a) ALL Consulting (2003); (b) Adapted from Copeland and Ewald (2008).
Lebo Shale members of the Fort Union Formation, which formed about 65 million to 56 million years ago (Paleocene time). The Fort Union Formation, a heterogeneous geological unit of sandstone, shale, and coal deposits, is overlain by the Wasatch Formation in many locations. These formations outcrop extensively around the east-central margin of the Powder River Basin near Gillette, Wyoming, and around the west central margin of the basin near Sheridan, Wyoming. Open-pit and strip-mining commercial coal operations are common in the outcrop areas.
Thickness of individual coalbeds in the Fort Union Formation ranges from a few inches to over 200 feet, with an average thickness of 25 feet. The Fort Union Formation originally was deposited on the margins of an ancient interior seaway as part of river (freshwater, fluvial) systems with braided, meandering, and dissected streams in the center of the basin and alluvial plains along the basin margins (USGS, 1999; Copeland and Ewald, 2008). The irregular spatial and vertical distributions of coalbeds (laterally and vertically discontinuous) reflect shifts of these fluvial and alluvial systems through time. The Tongue River Member of the Forth Union Formation contains thick, laterally extensive coalbeds that vary unpredictably in thickness and geometry, terminating and merging abruptly. The Tongue River Member, including the Wyodak coal zone4 and the Canyon and Anderson coals within this zone, contains most of the recoverable CBM in the Wyoming portion of the Powder River Basin (Figure 2.4b; Copeland and Ewald, 2008).
In the eastern part of the basin, regional groundwater flow moves from the south and east toward the northwest and into the central part of the basin (Daddow, 1986; Martin et al., 1988). In the southeastern part of the basin, regional groundwater flow is to the north, although local flow often varies from this overall pattern (BLM, 2003; USGS, 2005). The generally northward regional groundwater flow in the basin moves slowly because of pinching out of sandstone units, which are the principal water-conducting deposits contributing to groundwater flow. Water in sandstone aquifers associated with the coalbeds can be hydraulically confined, particularly in deeper, isolated beds far from recharge areas. Individual coalbeds in the Wasatch, Fort Union, and Lance formations (e.g., the Anderson coal) can also constitute important aquifers.
The Wyodak and Wyodak Rider coal zone of the Fort Union Formation is the most hydrologically continuous unit in the Powder River Basin and, together with its related coalbeds (the Anderson, Canyon, Big George, and Smith coals; Figure 2.4b), constitutes a regional aquifer. Limited recharge to the Wyodak and Wyodak Rider coal zone occurs at outcrops along the eastern margin of the Powder River Basin (e.g., Daddow, 1986). Recharge water flows downgradient within the coalbeds that outcrop at the surface. These
coalbeds then act as independent isolated aquifers. Flow into or out of the coalbeds along fault and fracture lines takes place to a limited extent (Frost et al., 2010).
Because the origin of the coals in the Wyodak and Wyodak Rider coal zone was in a freshwater setting, as opposed to a marine setting—which was the case for the coal deposits of the San Juan, Raton, Uinta, and Piceance basins—the connate waters associated with the Powder River coals were probably fresher from the outset compared to the connate coalbed waters in the other basins. In cases where the Powder River Basin coals are also connected hydraulically to natural recharge areas, the higher relative permeability of the coals would facilitate flow and contribute further to water chemistry in the coals having relatively few dissolved solids compared to water in coalbeds of other western CBM basins (see section on “Geochemical Foundations” later in this chapter). As a result of some combination of these natural circumstances, relatively fresh connate water and/or higher relative permeability, produced water from the Powder River Basin coalbeds is generally less saline than waters produced from other western CBM basins. The low TDS content and low salinity allow management of the CBM produced water either through direct discharge to ephemeral and perennial streams (either with or without treatment) or storage in surface impoundments (see later in chapter for water chemistry and Chapter 4 for details of water management practices in the basin). The degree to which water in the coals of the Wyodak and Wyodak Rider coal zones represents original (“old” or “fossil”) connate water and/or younger water that percolated into the coal from surface recharge areas is not well constrained with geologic, geophysical, geochemical, or hydrologic data.
SAN JUAN BASIN
The San Juan Basin covers about 7,500 square miles in the Four Corners region of the adjoining states Utah, Colorado, Arizona, and New Mexico (Figure 2.5). The basin strikes west-northwest to east-southeast and is asymmetrical in shape, with the deepest and thickest sedimentary rocks located in the north-central portion of the basin. The major coal-bearing and methane-producing unit is the Cretaceous Fruitland Formation, underlain by the Pictured Cliffs Sandstone. The layered and discontinuous Fruitland coals have three-dimensional complexity, reflecting the original complexity of the back-barrier lagoonal wetland ecosystems from which they originated (Snyder et al., 2003; Riese et al., 2005).
Production of CBM from the San Juan Basin occurs at depths ranging from 550 to 4,000 feet in three distinct and geographically discrete hydraulic pressure and permeability zones: (1) a central, high hydraulic head, high-permeability “fairway” (primarily in the gray shading of the basin in Figure 2.5a); (2) a northern, high hydraulic head, low-permeability area (primarily in the green and purple-brown shading of the northern part of the basin in Figure 2.5a); and (3) a southern, low hydraulic head, low-permeability area (primarily in the purple-brown shading of the southern part of the basin in Figure 2.5a). Although the
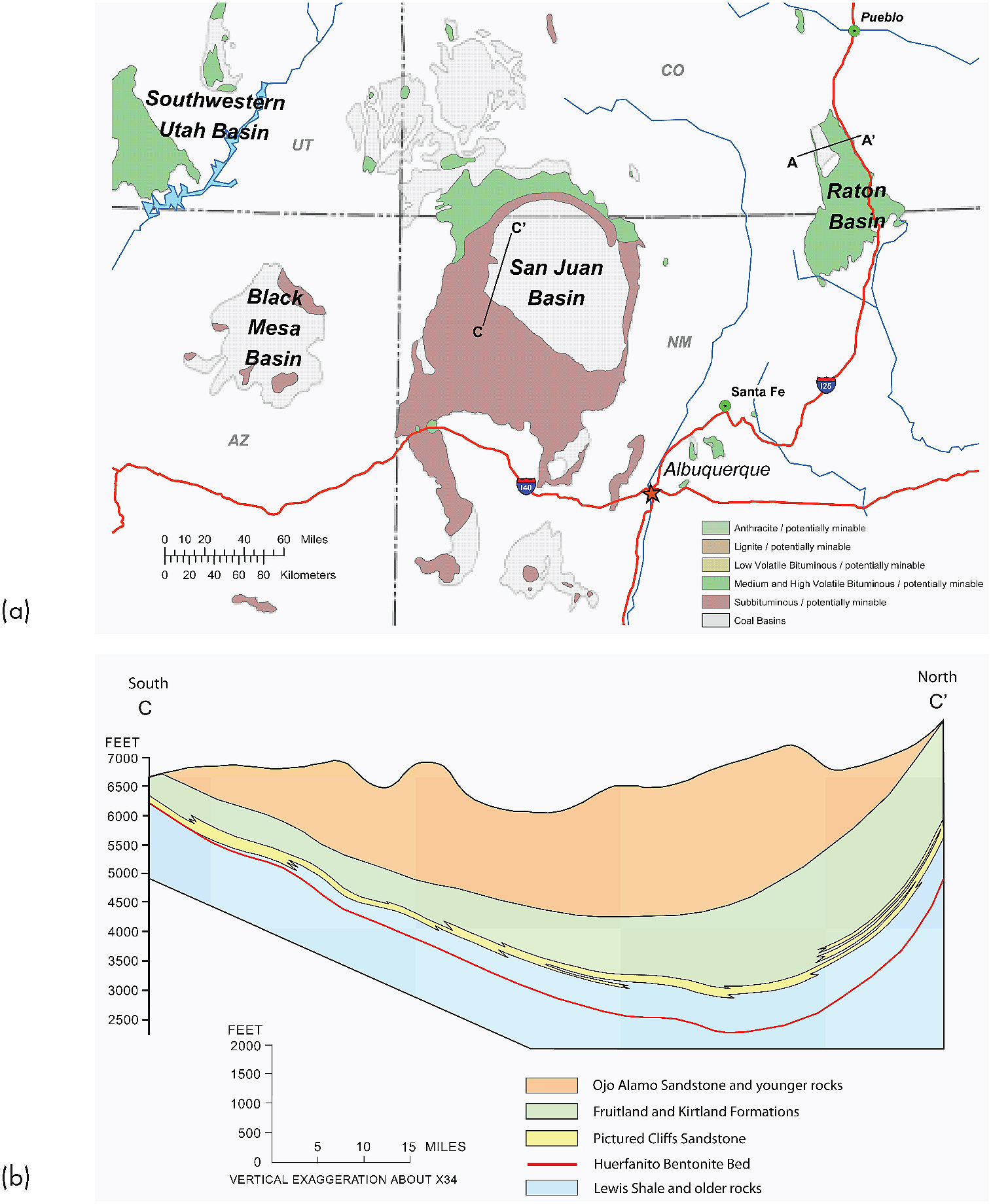
FIGURE 2.5 (a) The San Juan Basin of northeastern New Mexico and southwestern Colorado. The green, purple-brown, and gray shading indicates coal of bituminous through subbituminous grade. Location of cross-section C-C’ in Figure 2.5b is identified. (b) A south-north cross section through the San Juan Basin shows the asymmetry of the basin and its major coal-bearing and methane-producing rock unit, the Fruitland Formation, underlain by the Pictured Cliffs Sandstone and overlain by the Ojo Alamo Sandstone. Although oil and gas production began in the San Juan Basin in the 1920s, CBM development did not flourish until the mid-1980s. By the end of 2008, more than 7,000 CBM wells were active, extracting methane from coal deposits primarily within the Fruitland Formation at depths up to 4,000 feet below the surface. SOURCES: (a) ALL Consulting (2003); (b) Adapted from Fassett (2008).
hydraulic head in two of these zones is considered “high” (with respect to the hydrostatic water column), the reservoir water volumes are relatively low (with respect to the Powder River Basin), while the gas volumes remain relatively high (EPA, 2004; see also Table 2.1). Given this condition and the relatively high salt content of the produced water (refer to section on “Geochemical Foundations” later in this chapter), CBM producers in the San Juan Basin put a large majority of produced water from the coalbeds into temporary storage in above-ground storage tanks for later reinjection into formations below the coal.
RATON BASIN
The Raton Basin of Colorado and New Mexico covers approximately 3,100 square miles (see Figure 2.5a) and is an elongate asymmetric syncline approximately 80 miles long (north-south direction) and 50 miles wide (east-west direction) (see Figure 2.6). Coal
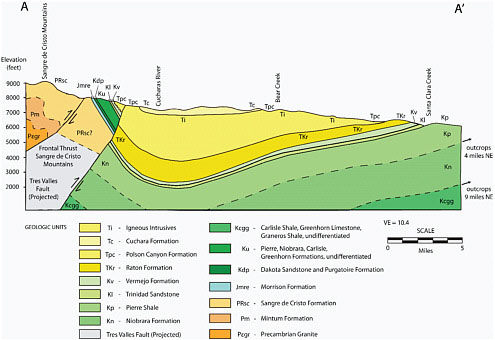
FIGURE 2.6 West-to-east cross section (see location A–A′ in Figure 2.5a) through the Raton Basin shows an asymmetry similar to the San Juan Basin. The primary coal-bearing and CBM-producing units are the Vermejo and Raton formations. Depth to the methane-bearing Vermejo Formation coal zone is about 2,400 feet (Johnson and Finn, 2001). SOURCE: Adapted from Stevens et al. (1992). Reproduced by permission of the Gas Technology Institute.
and associated methane in the Raton Basin derive from the late Cretaceous Vermejo and Raton formations, which overlie the Trinidad Sandstone, a basinwide marine sandstone (Figure 2.6). The Vermejo Formation was deposited as a collection of channel, lagoon, coastal swamp and delta plain deposits and the Raton Formation was deposited on a continental alluvial plain as a collection of channel, overbank, and swamp deposits. Numerous thin coalbeds in the Vermejo and Raton formations cannot be correlated over more than a few miles (Haley, 2004). Magmatic intrusions into the sediments also disrupt the sedimentary rock succession, including the coals. As with the other CBM basins in the West, water quality as a function of TDS varies widely across the basin, ranging from 900 to 3,500 ppm TDS on the western side of the basin, to 15,000 to 30,000 ppm TDS closer to the eastern outcrop.
PICEANCE AND UINTA BASINS
The Piceance Basin is located in the northwest corner of Colorado (see Figure 2.7a). Commercially recoverable amounts of methane occur in the Upper Cretaceous Mesaverde Group, which covers about 7,225 square miles of the basin. The Mesaverde Group ranges in thickness from about 2,000 feet on the west to about 6,500 feet on the east side of the basin (Johnson, 1989). Depth to the methane-bearing Cameo-Wheeler-Fairfield coal zone is about 6,000 feet, making methane extraction somewhat more technically challenging than in other areas where the formations are shallower (Figure 2.7b). In general, potable water wells in the Piceance Basin extend a few hundred feet below the ground surface, vertically a mile from the methane-producing zone. Below a depth of about 200 feet, the salinity of produced water can be as much as half the salt concentration of seawater (EPA, 2004).
The Uinta Basin of east-central Utah and northwestern Colorado covers approximately 14,400 square miles and is similar in its composition and history to the Piceance Basin. The Uinta Basin is separated structurally from the Piceance Basin near the Utah and Colorado border (Figure 2.7a). Similar to the Piceance Basin, coal occurs in the Cretaceous Mancos Shale and the overlying Mesaverde Group at depths of about 1,000 to over 7,000 feet below ground surface (Garrison et al., 1997). Coals from which CBM can be commercially recovered occur 4,200 to 4,400 feet below the surface (Gloyn and Sommer, 1993). Coalbeds are present within Cretaceous sedimentary rocks throughout much of the Uinta Basin. However, CBM exploration has targeted coalbeds in a sandstone member within the Mancos Shale and coalbeds in the Mesaverde Group. The sandstone in the Mancos shale was deposited in a fluvial-deltaic environment. The coalbeds in the Mesaverde Group consist of coal interbedded with sandstone and a combination of shale and siltstone. As with the Piceance Basin, water quality associated with the Uinta Basin CBM can be very saline, and salinity of produced water may be as much as that of seawater.
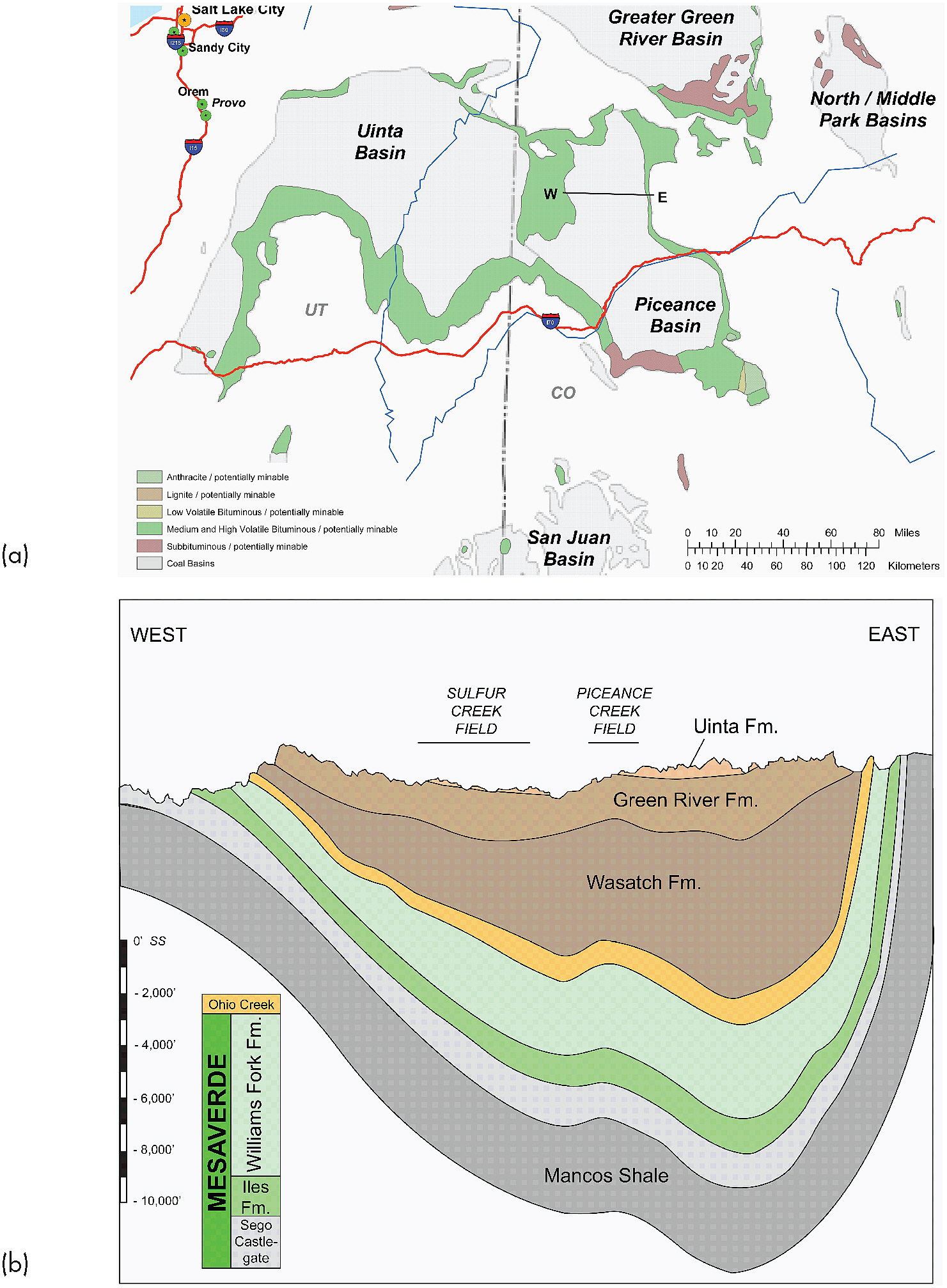
FIGURE 2.7 (a) Geological map of the Piceance and Uinta basins. Cross-section for the Piceance Basin in Figure 2.8b is identified. (b) Generalized west-east geological cross section across the Piceance Basin of Colorado. The CBM is found in the Mesaverde Group. Fm = Formation. SOURCES: (a) ALL Consulting (2003); (b) RMAG Special Publication by Yurewicz, D.A., et al. Copyright 2003 by Rocky Mountain Association of Geologists. Reproduced with permission of Rocky Mountain Association of Geologists.
Variations in CBM Produced Water Volumes
The Powder River and San Juan basins have seen the most CBM development, followed by the Raton Basin, as illustrated by the number of wells operating in each basin (Table 2.1). The Piceance and Uinta basins have seen significantly less CBM development. Water and gas production curves from the Powder River, San Juan, Piceance, and Raton basins illustrate variations in CBM produced water volumes. These variations illustrate the difficulty in predicting water and gas production volumes for a basin through time due to interplay of natural hydrogeological characteristics of the basins, the number of existing and new wells, and operator pumping rates.
The greatest volume of water production occurs in the Powder River Basin (Figure 2.8a, b), where the methane-producing coalbeds are water filled, relatively porous and permeable,
TABLE 2.1 Water and gas production information for CBM in the Powder River, San Juan, and Raton Basins in 2008 (data for the Piceance and Uinta basins are from 2006 and 2000, respectively)
|
Basin |
State |
Start Date |
Depth (feet) to Coalbeds for Methane Production |
Estimated Water Production (million barrels) |
Estimated Gas Production (million MCF) |
Approx. No. of CBM Wells |
Estimated Water-to-Gas Ratio (barrels/MCF) |
|
Powder River |
WY, MT |
1989, 1998 |
450–4,500 |
718 |
435.2 |
18,000 |
1.65 |
|
San Juan |
CO, NM |
1985 |
Up to 4,000 |
46 |
1,210.5 |
7,500 |
0.038 |
|
Raton |
CO, NM |
Early 1980s |
Up to 2,400 |
131 |
147.2 |
3,400 |
0.89 |
|
Piceance |
CO |
1989 |
Up to 6,000 |
0.30 |
0.25 |
110 |
1.2a |
|
Uinta |
UT |
Early 1990s |
4,200–4,400 |
31 |
73.8 |
1,255 |
0.42 |
|
aRelatively high water-to-gas ratio in 2006 does not reflect long-term CBM production trends in the Piceance Basin. Refer to Figure 2.8d. SOURCES: Powder River Basin data adapted from Meredith et al. (2010); wogcc.state.wy.us (accessed March 5, 2010); and C.D. Frost, presentation to the committee, June 2, 2009. Raton Basin data adapted from Hemborg (1998); Topper (2009); and M. Fesmire, presentation to the committee, June 2, 2009. San Juan Basin data adapted from S.S. Papadopulos & Associates, Inc. (2006); Topper (2009); M. Fesmire, presentation to the committee, June 2, 2009; and D. Mankiewicz, presentation to the committee, June 2, 2009. Piceance Basin data adapted from S.S. Papadopulos & Associates, Inc. (2007), and Topper (2009). Uinta Basin data adapted from Rice and Nuccio (2000) and EPA (2004). |
|||||||
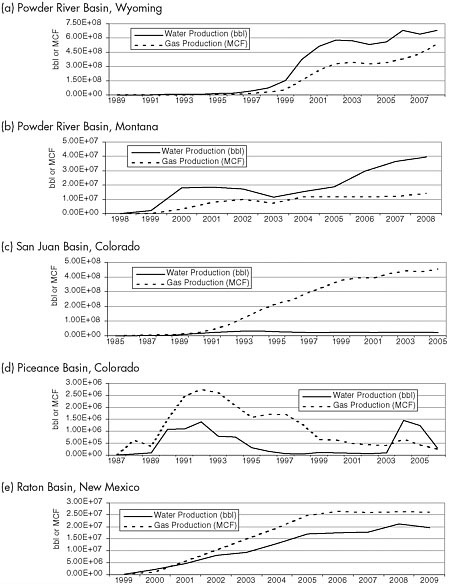
FIGURE 2.8 Annual water and gas production curves for CBM activities in the Powder River, San Juan, Piceance, and Raton Basins. “bbl” = barrel. SOURCES: Powder River Basin, Wyoming, data adapted from wogcc.state.wy.us/ (accessed March 5, 2010); Powder River Basin, Montana, data adapted from Meredith et al. (2010); San Juan Basin, Colorado, data adapted from S.S. Papadopulos & Associates, Inc. (2006); Piceance Basin, Colorado, data adapted from S.S. Papadopulos & Associates, Inc. (2007); and Raton Basin, New Mexico data adapted from octane.nmt.edu/gotech/Main.aspx (accessed June 21, 2010).
and located at relatively shallow depths compared to the other western CBM basins. In both the Wyoming and Montana portions of the basin, water and gas volumes have increased from the late 1990s until the present, as have the number of water- and CBM-producing wells, yielding an average water-to-gas ratio (in barrels of water produced per thousand cubic feet [MCF] of gas produced) greater than 1 (Table 2.1).5 Attempts at methane extraction in one-sixth of the Powder River Basin fail because water-to-gas ratios are excessively high, compared to other parts of the basin (Table 2.1).6
CBM production in the San Juan Basin yields a much lower water-to-gas ratio than in the Powder River Basin (Figure 2.8c). Low ratios are typical of basins with deeper, less permeable coalbeds that have been producing methane for a longer period of time. A graph of production from the San Juan Basin shows a steady increase in gas production since the mid-1990s, while water production from the basin peaked in 1993 and has since followed a steady decline.
In an example from the Piceance Basin, which has the lowest CBM production levels of any of the western CBM basins, two spikes in water production are apparent (Figure 2.8d). The first peak in water production in late 1992 reflects the increase in pumping rates and number of wells as production began in the basin. The second is due to input of a large number of new wells in the 2003 to 2004 period and accounts for the relatively high water-to-gas ratio in 2006 (see Table 2.1).
Acknowledgment of these kinds of variations in water production from basin to basin and within a basin is important when considering CBM produced water management options. Any discussion of “average,” “annual,” or “total” water production values requires clarifying information, including the length of time over which CBM operations have been active in the basin, the total number of operating wells, the number of existing and new wells in a given part of a basin in a given year, how long those wells may be in operation, and the rate of pumping by the operator. Spacing of adjacent wells may also have an effect on how quickly or slowly water production proceeds in a CBM field (see Chapter 5). These types of data are not necessarily available in a single data repository for each state or basin but have to be compiled from numerous information sources (see Figure 2.8 and Table 2.1 for some of these data sources).
|
5 |
A U.S. barrel (bbl) is equivalent to 42 gallons. One thousand MCF is equivalent to 1,000,000 cubic feet (see values in Table 2.1). |
|
6 |
D. Fischer, Wyoming Department of Environmental Quality, presentation to the committee, March 30, 2009. |
CASE STUDIES: REGIONAL HYDROGEOLOGY AND HYDRAULICS OF THE SAN JUAN AND POWDER RIVER BASINS
San Juan Basin
Fine-grained rocks (shale) confine the Fruitland coal and sandstone aquifers in the San Juan Basin. These aquifers become unconfined at outcrops at the basin margins. The situation is similar for the coal-bearing units of the Raton Basin. Because the coal-bearing beds outcrop at the surface at elevations higher than where they occur in the interior of the basin and the coalbeds are confined, the coalbeds had been previously considered a case of a classic “confined aquifer” that recharges at the outcrops along basin margins. Regionally, this model would predict groundwater to move in a southerly direction, from topographically high recharge areas in the north to the central part of the basin and to the lower basin margins, where the groundwater would discharge.
However, data indicate that this classic confined aquifer model for the San Juan Basin is too simplistic to adequately describe the complex hydrological process that governs confined coalbed water recharge, drawdown, and discharge. Isotopic analyses, including iodine, chloride, carbon, oxygen, and hydrogen in groundwater of multiple geochemical systems, independently document that the residence time (age) of CBM water in the San Juan Basin is on the order of thousands to tens of millions of years (e.g., Phillips et al., 1986, 1989; Snyder et al., 2003; Riese et al., 2005; see Box 2.2). Within the uncertainties of isotopic analysis, these data indicate that meaningful recharge of groundwater to all coalbed aquifers with the exception of some of the peripheries of these basins, in close proximity to the outcrop areas, has not occurred within the scale of human time.
Recent data from multiple lines of geological, geochemical, geophysical, biological, and ecological investigation have further demonstrated that the last major recharge of water to the San Juan Basin coal systems occurred during Eocene time, approximately 35 million to 40 million years ago (Riese et al., 2005). The Riese et al. (2005) study sampled waters from over 100 CBM wells and examined chemical and isotopic differences across the basin. The geochemical results showed the areal distributions of different water geochemical types (“fingerprints”) and a lack of coherent geochemical development along previously assumed regional flow paths from basin edges to the center of the basin (anticipated in the classical “confined aquifer” model). The geochemical patterns were consistent with compartmentalization of the basin into discrete hydrogeological zones with different water qualities.
The data also support the idea that Eocene and post-Eocene (younger than about 34 million years) uplift of the basin may have caused hydraulic conductivities in the coalbeds to decrease even further due to gas desorption from the coals and to effectively isolate those aquifers. Geochemical and geological evidence suggests that later (Miocene to Holocene, or about 23 million years ago through more recent time) geological events changed the stress
|
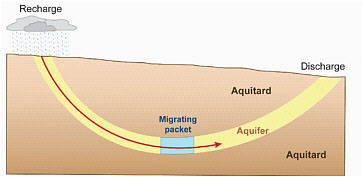
BOX 2.2 Age of Groundwater Hydrogeologists can test the validity of their conceptual models of groundwater flow systems, including predictions of where groundwater may recharge and discharge, by determining the approximate age of groundwater since it entered the subsurface as precipitation recharge. Fundamentally, an answer to the question “How old is the water?” can be determined geochemically (Bethke and Johnson, 2008). Knowing the age of groundwater, even within an order of magnitude, bears greatly on whether the water extracted from aquifers can be replenished by precipitation within human time frames, or can be considered “fossil” or ancient, nonrenewable water much like solid mineral resources such as coal and metals. Furthermore, directly determining the age of water sampled along subsurface flow paths also can be used to evaluate regional hydraulic properties used to calculate extractable water volumes. For decades, hydrogeologists have used various isotopes and other tracers to estimate groundwater age (Clark and Fritz, 1997). In groundwater “age dating,” hydrogeologists assume simple plug flow (see figure below) and then correct age dates with respect to chemical processes (e.g., methane formation affecting carbon-14 dates) and hydrodynamic processes (e.g., diffusion of isotopes into fine-grained rocks or sediments surrounding the aquifer in question). Because of these complexities and others (see Bethke and Johnson, 2008), isotopic dating of groundwater usually can reliably be done at order-of-magnitude accuracy. In other words, analysis of a full suite of isotopes in groundwater can determine if the water is a few years old, tens of years old, hundreds of years old, thousands of years old, or millions of years old. Isotopic age dating of water is based on elements that have multiple possible masses because of variable numbers of neutrons in their nucleii. For example, the most common forms of isotopically stable carbon have atomic weights of 12 and 13 atomic mass units (written as 12C, 13C), in order of decreasing frequency. Isotopically stable hydrogen in water can have atomic weights of 1 or 2 atomic mass units (written as 1H, 2H). Oxygen can have atomic weights of 16, 17, and 18 atomic mass units (written as 16O, 17O, and 18O). Some heavy isotopes of individual elements (those with highest atomic weights), particularly those of hydrogen and oxygen in water and carbon in organic and inorganic materials are sorted (fractionated) from their lighter isotopes as they move through the hydrological cycle and become involved in certain biochemical processes. For example, the fractionation of lighter from heavier hydrogen and oxygen in water causes water from precipitation |
field and allowed deep fracture networks to propagate up through the rock units. These fractures enhanced the connectivity of otherwise isolated portions of the reservoir and now allow the CBM wells to effectively “mine” the connate water of the sedimentary formation. These connate waters were trapped at the time the original sediments were deposited tens of millions of years ago and are not being recharged.
The various datasets show that outcrops of the Fruitland Formation are not a significant mechanism for recharge of the coal aquifers. Rather, groundwater discharges to the surface at these outcrops in seeps that may have been active for millennia. Moreover, methane in the deep coal does not hydraulically connect to methane gas seeps at outcrops,
further documenting the hydrogeological compartmentalization of the San Juan Basin (Riese et al., 2005).
Oldaker and Fehn (2005) report that surface waters and shallow groundwater are less than 60 years old in the Raton Basin but that produced water from CBM wells more than 1,800 feet deep could be at least 1.2 million years old, based on tritium (3H), carbon-14, and chlorine isotope analyses. Although comprehensive isotopic studies similar to those in the San Juan Basin have not yet been conducted in the Raton Basin, these results suggest a conceptual model for CBM water in the Raton Basin similar to the San Juan Basin, particularly given the common depositional environments for the coals in the two basins.
If proven more regionally correct, Raton Basin CBM water may also consist primarily of fossil water, which occurs in compartmentalized methane-bearing coalbeds.
Today, the Raton and San Juan basins receive minimal recharge to their groundwater systems as a result of arid climate conditions. Although the groundwater systems and their sources of recharge are complex and not fully understood, the presence of high TDS waters supports the lack of recharge, as does the discontinuity or compartmentalization of all coal deposits that would further minimize recharge to coalbeds. Although removal of water from coalbeds during CBM operations may induce some degree of leakage of water over time into the coalbeds from surrounding finer-grained rocks and potentially from other aquifers through deep fracture zones, CBM water at depth does not appear to be a “renewable” resource in the San Juan and Raton basins, based on the suite of data available.
Powder River Basin
Few studies have been conducted specifically to date the age of groundwater in the Powder River Basin, but existing results indicate that some of the CBM produced water may be thousands of years old. Geochemical analysis from the eastern portion of the basin has shown that much of the deeper groundwater associated with CBM has no tritium (3H), implying the water is at least 50 years old (Bartos and Ogle, 2002). Preliminary carbon-14 dating of dissolved inorganic carbon in CBM water appears to show water that is radiocarbon “dead,” implying it is at least 14,000 years old.7 These results are significant since the samples were taken only a few miles from presumed coalbed recharge areas at the land surface. Brinck et al. (2008) and Frost et al. (2010) concluded from evaluation of geochemical evolution of CBM produced water in proximity to recharge areas that the influence of recharge at outcrop sites likely does not extend more than 2 to 4 kilometers (1.2 to 2.4 miles) beyond the recharge sites. Correspondingly, the water in relatively close proximity to recharge sites is likely recent in geological perspective, whereas CBM water toward the center of the basin may represent “older” water. These results provide some constraints on recharge rates being slow or relatively inhibited in the studied areas of the Powder River Basin.
Sharma and Frost (2008) further determined that the isotopic composition of dissolved inorganic carbon associated with CBM produced water can be readily distinguished from the isotopic composition of dissolved inorganic carbon found in surface water and groundwater of the Powder River watershed. This type of distinction allows for easy long-term monitoring to determine the extent to which in situ and surface-discharged CBM produced water moves within the subsurface and in receiving streams. Campbell et al. (2008) determined from strontium isotopic analyses in groundwater and produced water that some
coal aquifers were hydraulically confined while others were not and that faults may provide some, although limited, connectivity for fluid migration between coal formations. Sharma and Frost (2008) suggested the same, based on carbon isotope analysis. These study results are the first of their kind to attempt to describe movement of CBM produced water with these types of isotopes. These studies are explored further in Chapter 5.
Powder River Basin coalbeds also appear to contain some younger water than do the coalbeds from the San Juan or Raton basins, particularly near the outcrop areas at basin margins, which serve as recharge avenues. Additionally, the CBM produced water of the Powder River Basin has lower concentrations of solutes due to some combination of the origins of the coals in freshwater settings and subsequent interaction of the water in the coals with percolating surface water (see also “Geochemical Foundations” below).
Case Study Summary
With respect to CBM basins, isotopic and other data in the San Juan Basin demonstrate that much of the produced water may not be a renewable resource because of its great age compared to human lifetimes. Water extracted from the San Juan Basin is fossil water that has not been renewed for tens of millions of years. For the Raton Basin and at least some portions of the Powder River Basin, away from outcrop recharge areas, the data are fewer and not comprehensive, but similar results are suggested. Although regionally pervasive, the discontinuous nature of the coalbeds has led to limited ability for water to pass through the coals under gravity flow, even in cases where permeability within a coalbed may be relatively high.
GEOCHEMICAL FOUNDATIONS
In addition to geological and hydrogeological constraints on the volume of CBM produced water, analysis and understanding of the various management approaches to CBM produced water also require an introduction to CBM produced water chemistry. The two primary constituents of CBM water are sodium bicarbonate (NaHCO3, or baking soda) and sodium chloride (NaCl, or table salt; Rice and Nuccio, 2000). Constituents appearing in smaller quantities in produced water include calcium, magnesium, potassium, and barium, whereas elements such as aluminum, ammonia, selenium, arsenic, iron, manganese, boron, copper, and zinc are sometimes present in trace amounts (McBeth et al., 2003). Typically, CBM produced water contains only minimal amounts of fine, inorganic particulate matter, otherwise known as coal fines. In some instances, facultative8 iron-oxidizing bacteria and degraded methanogenic bacteria may also be present in small amounts.
A series of geochemical processes remove sulfate through oxidation/volatilization and calcium and magnesium by precipitation or ion exchange/adsorption from CBM water; these processes leave the CBM water with substantially more bicarbonate and sodium (see Box 2.3). However, the TDS concentration of CBM produced water ranges from fresh to saline (i.e., 200 milligrams per liter [mg/L] to 170,000 mg/L) because of variable amounts of sodium, bicarbonate, and chloride (see Table 2.2). The recommended TDS limit for drinking water is 500 mg/L; for beneficial use, such as irrigation in Wyoming, the limit is 2,000 mg/L; and for wildlife and livestock watering in Wyoming, the limit is 5,000 mg/L (EPA, 2009; Wyoming DEQ, 2005). For comparison, seawater has a TDS of approximately 35,000 mg/L. In addition to TDS, sodium in CBM produced water is of interest as it relates to the consideration of CBM produced water for irrigation. A measure that is used to determine the influence of sodium on soils and plants is related to the sodium adsorption
|
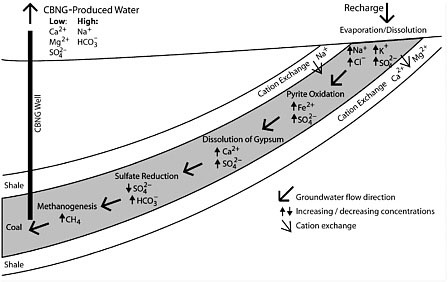
BOX 2.3 Geochemical Processes and Their Control on CBM Water Composition Three geochemical processes control the fact that CBM water contains substantially more bicarbonate and sodium than magnesium, calcium, and sulfate: (1) microbial reduction of sulfate contributed by dissolving the mineral gypsum, or reduction of sulfate at depth, which may release bicarbonate; (2) removal of calcium and magnesium by ion exchange, which releases sodium, and by precipitation of calcite (CaCO3); and (3) enhanced dissolution of sulfide minerals and organic matter oxidation in water recharge areas, both of which generate acid. Brinck et al. (2008) schematically show how these processes generically occur along groundwater flow paths in the Powder River Basin (see figure below). The geochemical processes governing the evolution of sodium bicarbonate-dominated waters have been known for an extensive period of time because of the occurrence of such waters, absent methane, along the Atlantic and Gulf coastal plains (Foster, 1950; Chapelle and Knobel, 1983). In the case of the CBM water in the Powder River Basin, where coalbeds are relatively shallow in comparison to the other western CBM basins, much of the methane formed is related to geologically recent microbial processes. However, in basins where CBM is produced during lithification (the transformation of buried sediments to rock like material) of much deeper strata, the CBM is related to the thermogenic chemical, physical, or biological change experienced by the sediment and organic debris after its initial deposition and during and after the lithification processes that formed the coal. CBM produced waters of the San Juan, Raton, Piceance, and Uinta Basins also have a chloride signature which is not shown in the conceptual diagram of Brinck et al. (2008). See also Figure 2.9. |
ratio (SAR; a numeric expression of the concentration of sodium, relative to the concentration of calcium and magnesium in produced water; see also Chapter 5 for discussion of issues related to TDS and sodium in CBM produced water).
A comparison of representative principal salt constituent concentrations of water produced from major CBM basins in North America illustrates consistently elevated concentrations of sodium and bicarbonate and relatively (in most cases substantially) lower concentrations of calcium, magnesium, and sulfate (see Figure 2.9). Also evident are the substantially lower chloride and TDS concentrations in waters produced from the Powder River Basin, compared to waters from the other basins (see earlier sections of this chapter). The Powder River Basin contains primarily sodium bicarbonate-type formation water, whereas waters from the Piceance, Uinta, Raton, and San Juan basins contain sodium bicarbonate/chloride-type water.
TABLE 2.2 Range of Total Dissolved Solids (TDS) in mg/L for CBM Produced Water from each Western Basin Compared to TDS for General Water Types and Water Quality Standards
|
|
TDS in mg/L |
|
Western CBM basins |
|
|
Powder River |
250 to greater than 3,000 |
|
San Juan |
10,000 to greater than 100,000 |
|
Raton |
900 to 30,000 |
|
Uinta |
6,350 to 42,700 |
|
Piceance |
Greater than 10,000 |
|
General water types |
|
|
Fresh water |
Less than 1,000 |
|
Saline water |
Greater than 1,000 |
|
Seawater |
35,000 |
|
Water quality standards |
|
|
U.S. Safe Drinking Water Act (secondary standard) |
500 |
|
Wyoming agriculture standards (Class II) |
2,000 |
|
Wyoming livestock standards (Class III) |
5,000 |
|
NOTE: Both TDS and salinity can be used to measure water quality. TDS is a quantitative measure of all residual dissolved minerals after evaporation and is generally expressed as mg/L. Salinity measures the concentrations of dissolved salts in the water volume. Salinity may be measured by TDS, electrical conductivity, or osmotic pressure. Where sodium chloride and sodium bicarbonate are known to be the dominant minerals in a sample of water, such as in waters of the western basins, high TDS will often indicate high salinity. SOURCES: ALL Consulting (2003); S.S. Papadopulos & Associates (2007); water.usgs.gov/watuse/wu-glossary.html (accessed July 6, 2010); www.watereuse.org/information-resources/about-desalination/glossaryd (accessed July 6, 2010); Wyoming DEQ (2005). |
|
Generally, TDS concentration ranges from the hundreds to thousands of milligrams per liter in produced water in the basins where sodium bicarbonate dominates, such as in the Powder River Basin, whereas TDS concentrations can exceed tens of thousands to more than 100,000 mg/L where sodium chloride dominates the chemistry of the CBM water, such as in the San Juan Basin (Table 2.2). In the Powder River Basin, much of the geochemical signature of the produced water is derived from water-rock interactions (Box 2.3).
Jackson and Reddy (2007) report concentrations of trace elements and volatile organic compounds (Gas Research Institute, 1995; Rice et al., 2000) for produced water in the Powder River Basin (see also Jackson and Reddy, 2010). Some metals, such as barium, appear to have concentrations close to the solubility of controlling minerals (e.g., barite).
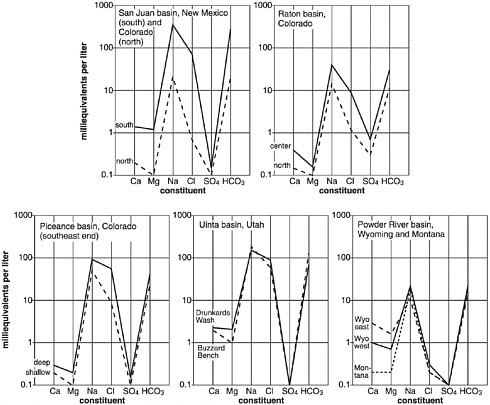
FIGURE 2.9 Predominant dissolved chemical constituents of typical CBM produced water from major methane-producing basins. Note the much higher concentrations of chloride associated with the San Juan, Raton—Colorado portion, Piceance, and Uinta Basins compared to the Powder River Basin. Note also the difference in concentrations in sodium and bicarbonate within the San Juan Basin where concentrations decrease from south (New Mexico) to north (Colorado). Data sets examined from the New Mexico portion of the Raton Basin indicated values similar to those in the Colorado portion (Haley, 2004). For ease of plotting the data, millequivalents per liter on the vertical axis normalizes milligrams per liter to both the number of atoms present per solute per liter and the valence, or charge, of the ions. SOURCE: Van Voast (2003). © 2003 by American Association of Petroleum Geologists. Reproduced by permission of AAPG whose permission is required for further use.
NOTE: Milliequivalent is a unit of measure denoting one-thousandth of a molar equivalent of a substance. An “equivalent,” in the case of Figure 2.9, refers to the amount of a substance (e.g., calcium) that supplies one mole of charge (positive or negative). Because calcium has a charge of +2, one mole of calcium provides two equivalents of charge. Therefore, a 1 milliequivalent/L solution of calcium is equal to a 0.5 millimole/L solution. For ions with a single charge, such as sodium and bicarbonate, one milliequivent/L is equal to one millimole/L. To convert millimole/L to mg/L, multiply by the molecular weight of the substance.
Potentially toxic metals, such as arsenic, lead, and chromium, are generally found at concentrations less than most water quality standards in certain locations (Jackson and Reddy, 2007; see Table 2.3).
Once at the surface, CBM produced water also undergoes chemical changes associated with atmospheric equilibration and mixing with in-stream and soil-adsorbed elements. Aquifer mineral and coal composition, oxidation state, pH, sorption to aquifer mineral surfaces, and the extent to which solids precipitate along water flow paths in the aquifer all control macro- and trace element concentrations. Patz et al. (2006) documented changes in concentrations of trace metals in surface water in the Powder River Basin as such waters moved downgradient, below produced water discharge points. McBeth et al. (2003) determined that soluble salt and trace metal concentrations in surface storage ponds may increase or decrease depending on time, the underlying soil and rock material, and the
TABLE 2.3 Concentrations of trace elements (g/L) in CBM waters in outfalls and surface impoundments in the Powder River Basin: Little Powder, Powder, and Tongue River watersheds.
|
|
Little Powder River |
Powder River |
Tongue River |
|||
|
|
Outfalls |
Disposal Ponds |
Outfalls |
Disposal Ponds |
Outfalls |
Disposal Ponds |
|
Aluminum |
304±132 |
573±266 |
181±80.7 |
251±95.0 |
1,817±1,174 |
361±162 |
|
Arsenic |
0.75±0.75 |
9.74±6.74 |
0.75±0.75 |
3.75±0.75 |
0.75±0.75 |
1.50±0.75 |
|
Barium |
614±49.4 |
334±35.7 |
514±98.9 |
284±61.8 |
271±26.1 |
130±23.3 |
|
Boron |
99.3±4.76 |
126±19.2 |
141±21.5 |
164±12.3 |
109±6.81 |
124±6.81 |
|
Cadmium |
<1.12±1.12 |
<1.12±1.12 |
<1.12±1.12 |
<1.12±1.12 |
<1.12±1.12 |
<1.12±1.12 |
|
Chromium |
8.84±1.04 |
8.84±1.56 |
12.0±2.60 |
11.4±1.56 |
8.32±1.56 |
9.36±1.56 |
|
Copper |
10.8±1.27 |
19.1±4.45 |
7.63±2.54 |
19.7±1.91 |
10.8±1.91 |
17.2±1.91 |
|
Iron |
124±11.2 |
217±107 |
81.0±13.4 |
203±89.9 |
71.5±34.1 |
145±46.4 |
|
Lead |
<2.07±2.07 |
<2.07±2.07 |
<2.07±2.07 |
<2.07±2.07 |
<2.07±2.07 |
<2.07±2.07 |
|
Manganese |
12.6±3.85 |
11.5±8.24 |
8.24±2.20 |
3.30±1.10 |
7.69±2.75 |
7.14±2.20 |
|
Molybdenum |
<0.96±0.96 |
2.88±1.92 |
0.96±0.29 |
1.92±0.96 |
<0.96±0.96 |
1.92±0.96 |
|
Selenium |
1.58±0.79 |
1.58±0.79 |
1.58±0.79 |
2.37±0.79 |
0.79±0.79 |
0.79±0.79 |
|
Zinc |
7.85±1.96 |
9.15±1.96 |
7.19±1.96 |
10.5±3.27 |
10.5±3.92 |
18.3±5.23 |
|
NOTE: Outfalls refer to direct discharges of from water separated from individual methane wells. Disposal ponds are containment structures that store the discharge water from multiple outflows (see Chapter 4). Mean values (g/L) plus one standard deviation are shown for each constituent. SOURCE: Jackson and Reddy (2007). Figures converted from micromoles per liter in the original data source to micrograms per liter in this table. |
||||||
degree to which mixing occurs in holding ponds (see also Chapters 4 and 5). However, in many circumstances—particularly in Colorado and Wyoming where produced water is present on the landscape—the spatial distributions, concentrations, and fate of trace elements in the water remain uncertain given the minimal sampling and analysis available (see also Chapter 5).9
In contrast to the studies outlined above that examined inorganic carbon, trace concentrations of dissolved organic substances may also be present in some CBM produced waters, although these substances in CBM produced waters are neither well documented nor researched. Phenols, biphenyls, heterocyclic compounds, polycyclic aromatic hydrocarbons (PAHs), and other organic constituents have been measured in some produced waters, with PAHs being the most common organic substance detected or measured. Orem et al. (2007) report microgram per liter (µg/L) concentrations of organic compounds in CBM produced waters in the Powder River Basin, with PAH values up to 23 µg/L. The committee was unable to find other data regarding organic substances dissolved in CBM produced waters of the other western basins.
GROUND- AND SURFACE WATER CONNECTIVITY AND GROUNDWATER MODELING: DATA GAPS AND UNCERTAINTIES
Concern over management of CBM-produced water stems largely from two factors: water quantity and quality on local and regional watershed scales. Litigation during the past decade has been extensive, with plaintiffs registering concerns over numerous water quality and quantity issues and their effects (see also Chapters 3 and 5). Additionally, a number of research projects have involved either monitoring and data gathering or modeling in an attempt to define the extent of local or regional water resource responses to CBM produced water withdrawals and discharges. However, for the purposes of planning CBM produced water management, questions remain with regard to the effects of large-scale, localized, regional, and/or basin-wide withdrawals; deep-well reinjection; discharge for disposal through infiltration or evaporation; and release of treated or untreated CBM water to ephemeral and perennial streams. For purposes of evaluating these various management options on water quality and quantity (discussed in detail in Chapters 4 and 5), data to determine the connectivity of groundwater and surface water and groundwater modeling are necessary. The gaps and uncertainties related to connectivity and modeling conclude the discussion in this chapter.
Data Gap to Establish Surface Water and Groundwater Connectivity
Establishing quantitatively the extent to which CBM-producing formations hydraulically connect to surface waters and major aquifers is necessary to predict the effects of CBM water withdrawal and management on surface water and groundwater quantity and quality. As discussed above, the only study that included sufficient geochemical, geological, geophysical, hydrological, and other data to establish the degree of hydraulic connectivity between methane-bearing coalbeds and surface and shallow groundwater was conducted in the San Juan Basin (Riese et al., 2005). Such data are needed to assess fundamental aspects of the groundwater flow system, the water level (potentiometric surface) and how it changes, surface water and groundwater interaction, calculations or quantitative assessments of recharge rates, and discharge areas for major streams flowing into and across CBM basins. Because comprehensive data and analyses of this nature are lacking for other western CBM basins, the committee considers this a significant information gap.
Gaps with Modeling Groundwater Flow
Although natural systems are complex, numerical models of groundwater flow in CBM basins have used fairly simple approaches in which water is modeled to move uniformly within relatively homogeneous aquifers. Thus, interactions that might occur between local, shallow streams and groundwater and deep CBM-associated waters may not be adequately represented by the model parameters. Independent and comprehensive data are needed to test and confirm the validity of the results of groundwater models for CBM basins beyond calibration to water level (the potentiometric surface). In some cases where groundwater models are used to characterize groundwater flow, the model results have not been rigorously examined through a combination of sensitivity analysis, history matching, and using multiple lines of calibration (e.g. Anderson and Woessner, 1992; ASTM, 2000). Understanding model limitations and uncertainties becomes particularly important when results of models may be used to assess the longer-term consequences to groundwater levels from CBM-related water-pumping activities.
In the Powder River Basin, for example, one modeling study indicated effects from CBM pumping that included depression of the potentiometric surface of coal aquifers, which serve as local water sources, and potential loss of stream flow for as long as 50 years (Meyers, 2009). Simple mathematical models (e.g., the Glover-Balmer method) related to the effects of regional CBM withdrawals in the San Juan Basin have also been employed and model results interpreted to suggest stream depletion and drawdown of the potentiometric surface of coal-bearing formations within 20 miles of their outcrop area (e.g., S.S. Papadopulos & Associates, Inc., 2006; Hathaway et al., 2006). In the case of the San Juan Basin where other studies yielded results with sufficient isotopic age dating (Box 2.2), the
data show CBM produced water is primarily fossil groundwater that has not been recharged for thousands to tens of millions of years, which contradicts the model results for stream depletion and drawdown. Similarly comprehensive data to test the results from the Powder River Basin modeling study are not currently available.
The Glover-Balmer method incorporates assumptions based on pumping water from a well constructed in an artesian aquifer. Many of these assumptions are violated when applied to pumping from multiple wells in a complicated watershed (e.g. Spalding and Khaleel, 1991; Sophocleous et al, 1995). For example, the method assumes that the aquifer is “isotropic” (permeability the same horizontally and vertically) and “homogeneous” (the same material everywhere). In CBM basins, interlayered coarse- and fine-grained rocks occur and generate notable heterogeneous and non-isotropic conditions. The method also assumes that the aquifer extends to infinity. This assumption can be valid locally when a well is pumped, but does not apply to a basin where geologic units pinch out or disappear over short distances as they do in the western CBM basins. The Glover-Balmer method is only useful as a first approximation, at best, if at a watershed scale.
Although modeling may be useful for broad assessment of possible hydraulic relationships in CBM basins, numerical models of hydrogeological systems currently do not yield unique results. Different, multiple combinations of input parameters can produce the same overall results for measurements of water levels and other hydrological data typically used to calibrate the model (e.g., Oreskes et al., 1994; McDonnell et al., 2007). Furthermore, current models cannot yet characterize complex water-rock interactions, differences in hydraulic properties, or boundary conditions in CBM basins. Thus, testing the results and assumptions of numerical and other groundwater models against data from the field or area being modeled is important in order to establish a level of reliability that is suitable for making management decisions. For example, if a model predicts decreasing flow in streams because of CBM production, then low-flow measurements in the rivers presumed to be affected are necessary to test the model results. Similarly, if modeling suggests that streams receive water from coalbeds to maintain baseflow, then chemical measurements in the streams are necessary to determine if CBM “fingerprints” (chemical constituents typical of CBM formations) are present in the water.
Despite these limitations, groundwater models of basins can predict general travel time of groundwater along flow paths, and these predictions can be tested by age dating the water. Until the gap is filled between the results of groundwater models and the necessary data to test them, care is urged with regard to using model results alone to make regulatory or other determinations regarding produced water management. The ability to place more reliance in the future on outputs of models that more closely resemble natural complexities of the hydraulic conditions of CBM basins necessitates demonstrating better convergence between existing model results and data collected and analyzed from the basins.
CHAPTER SUMMARY
Quantitative understanding of the degree and extent of connectivity between surface water and shallow groundwater systems and methane-producing coalbeds is important when evaluating the potential effects of CBM extraction, coproduction of water, and subsequent management of the produced water. The degree of connectivity bears on the groundwater flow system, surface water and groundwater interaction, calculations or quantitative assessments of recharge rates, and discharge areas for major streams flowing into and across CBM basins. Effective management of water produced during CBM extraction is contingent on establishing to what degree surface water and groundwater resources may be depleted, degraded, supplemented, or enhanced and over what time periods.
For the western CBM basins, methane developed together with coal over millions of years from different fluvial, lagoonal, and nearshore freshwater and marine settings that contained organic material, which was progressively buried. Although these coals are regionally pervasive, individual coalbeds are discontinuous, reflecting the original meandering and discontinuous environmental setting in which plant matter was deposited and subsequent tectonic activity. Methane in the coal is held adsorbed to the coal surfaces by surrounding water pressure; water in the coal may represent original (connate) water from the environment in which the organic material was initially deposited and/or some “younger” water that has percolated from the surface or shallow groundwater into the coalbeds. Technology used to extract methane from coalbeds relies on pumping the water from the coalbed to the surface to reduce the water pressure and allow the methane to be released from the coal and up the well bore.
Variations in regional geological and hydrogeological histories for the western CBM basins have had direct bearing on the subsurface depth of the coalbeds and the differences in the volumes of methane and the volume and chemistry of the associated produced water. In the Powder River Basin of Montana and Wyoming, relatively high CBM produced water volumes with generally low dissolved salt concentration in comparison to other western CBM basins are due to the occurrence of methane-bearing coalbeds with relatively high permeability and water-filled porosity. CBM-produced water volumes are lower in the San Juan and other western CBM basins, where the methane-producing coalbeds typically occur at greater depths than in the Powder River Basin and have correspondingly lower permeabilities. The deeper coalbeds yield lower water-to-gas ratios and produced water with higher dissolved salt concentrations.
Because many of the coal seams and beds in these western basins are discontinuous, the way in which water in the coal and surrounding sedimentary rocks migrates and is replenished is more complicated than what simple hydrological systems predict. Where discontinuities and/or low permeability exist in the coalbeds, groundwater may move very slowly and natural replenishment of coalbeds after water is withdrawn may not occur in
human lifetimes or even in thousands to millions of years. Such “old” or “fossil” water is considered a nonrenewable resource once it is withdrawn.
Several studies using geological, geochemical, geophysical and hydrological data indicate that the water in the San Juan Basin is probably thousands to tens of millions of years old, except at recharge areas—in other words, produced water from CBM extraction in the San Juan Basin is fossil water that will not be renewable over human lifetimes. Preliminary data from the Raton Basin indicate that some of the produced water from CBM extraction may also be fossil water. Although a few isotopic studies have suggested some of the CBM produced water in the Powder River Basin is fossil water, more detailed analyses incorporating water chemistry, isotope study, and geophysical data collection—such as those done in the San Juan Basin—would clarify the extent to which fossil water and/or recharge with younger water occurs in the Powder River Basin. Using a full suite of geological, geochemical, hydrological, and geophysical data, and particularly using isotopic analyses to approximate the age of the water, will help determine whether the produced water is a resource that will be depleted by CBM production or replenished over shorter timescales.
Lack of renewability of the water resource that is extracted during CBM production is an important variable to consider in determining produced water management strategies. The renewability of water has implications for the degree of hydraulic connectivity between methane-bearing coalbeds and surrounding groundwater systems and surface waters and also the intended management of the water subsequent to extraction.
Chemical constituents in the produced CBM waters from the basins vary between and within basins and reflect variability in hydrological systems. The two primary constituents of produced water are sodium bicarbonate and, to a lesser extent, sodium chloride. TDS concentrations in the western basins range from fresh to saline (200 to 170,000 mg/L). The Powder River Basin contains primarily sodium bicarbonate-type formation water and low TDS, whereas the Piceance, Uinta, Raton, and San Juan basins contain sodium bicarbonate chloride-type water at higher concentrations than in the Powder River Basin and generally high TDS. Once at the surface, water produced with methane extraction may undergo further chemical changes associated with atmospheric equilibration and mixing with instream and soil-adsorbed elements. Aquifer mineral and coal composition, oxidation state, pH, sorption to aquifer mineral surfaces, and the extent to which solids precipitate along water flow paths in the aquifer all control trace element concentrations.
Although groundwater modeling may be useful for broad assessment of possible hydraulic relationships in CBM basins, current models cannot yet characterize complex water-rock interactions, differences in hydraulic properties or boundary conditions present in CBM basins. As with connectivity issues, testing the results and assumptions of groundwater models for CBM basins against complete suites of data from the basins is important to provide an appropriate level of reliability of the model results.
REFERENCES
ALL Consulting. 2003. Handbook on Coal Bed Methane Produced Water: Management and Beneficial Use Alternatives. Prepared for Groundwater Protection Research Foundation; U.S. Department of Energy; and National Petroleum Technology Office, Bureau of Land Management. Tulsa, OK. Available at gwpc.org/e-library/documents/general/Coalbed%20Methane%20Produced%20Water%20Management%20and%20Beneficial%20Use%20Alternatives.pdf (accessed March 4, 2010).
Anderson, M.P., and W.W. Woessner. 1992. Applied Groundwater Modeling. Academic Press, San Diego.
ASTM (American Society of Testing Materials). 2000. Designation: D 5718—95 Standard Guide for Documenting a Ground-Water Flow Model Application.
Bartos, T.T., and K.M. Ogle. 2002. Water Quality and Environmental Isotopic Analyses of Ground-Water Samples Collected from the Wasatch and Fort Union Formations in Areas of Coalbed Methane Development: Implications to Recharge and Ground-Water Flow, Eastern Powder River Basin, Wyoming. U.S. Geological Survey Water-Resources Investigations Report 2002-4045. Cheyenne, WY: U.S. Geological Survey. Available at pubs.usgs.gov/wri/wri024045/ (accessed March 26, 2010).
Bates, R.L., and J.A. Jackson (eds.). 1987. Glossary of Geology. American Geological Institute: Alexandria, VA. 788 pp.
Bethke, C.M., and T.M. Johnson. 2008. Groundwater age and groundwater age dating. Annual Review of Earth and Planetary Science 36:121-152.
BLM (U.S. Bureau of Land Management). 2003. Montana Final Statewide Oil and Gas Environmental Impact Statement and Proposed Amendment of the Powder River and Billings Resource Management Plans. BLM/MT/PL-03/005. Miles City, MT: Bureau of Land Management. Available at www.archive.org/details/montanafinalstat02unitrich (accessed March 10, 2010).
Brinck, E.L., J.I. Drever, and C.D. Frost. 2008. The geochemical evolution of water co-produced with coal bed natural gas in the Powder River Basin, Wyoming. Environmental Geosciences 15(4):153-171.
Campbell, C.E., B.N. Pearson, and C.D. Frost. 2008. Strontium isotopes as indicators of aquifer communication in an area of coal bed natural gas production, Powder River Basin, Wyoming and Montana. Rocky Mountain Geology 43(2):149-175.
Chapelle, F.H., and L.L. Knobel. 1983. Aqueous geochemistry and exchangeable cation composition of glauconite in the Aquia aquifer, Maryland. Ground Water 21(3):343-352.
Clark, I.D., and P. Fritz. 1997. Environmental Isotopes in Hydrogeology. New York: Lewis Publishers.
Copeland, D.A., and M.L. Ewald (eds.). 2008. Water Associated with Coal Beds in Wyoming’s Powder River Basin: Geology, Hydrology, and Water Quality. Wyoming State Geological Survey Exploraton Memoir No. 2. Fort Collins, CO: Citizen Printing.
Daddow, P.B. 1986. Potentiometric-surface map of the Wyodak-Anderson coal bed, Powder River Structural Basin, Wyoming, 1973-84. U.S. Geological Survey Water-Resources Investigations Report 85-4305. Washington, DC: U.S. Geological Survey.
EIA (Energy Information Agency). 2007. U.S. Coalbed Methane: Past, Present, and Future. Panel 2 of 2. Washington, DC:
EIA. Available at www.eia.doe.gov/oil_gas/rpd/cbmusa2.pdf (accessed March 4, 2010).
EPA (U.S. Environmental Protection Agency). 2004. Summary of coalbed methane descriptions. Chapter 5 in Evaluation of Impacts to Underground Sources of Drinking Water by Hydraulic Fracturing of Coalbed Methane Reservoirs. Washington, DC: EPA. EPA 816-R-04-003. Available at www.epa.gov/ogwdw000/uic/wells_coalbedmethanestudy.html (accessed March 10, 2010).
EPA. 2009. Drinking water contaminants: List of Contaminants & their MCLs. Available at www.epa.gov/safewater/contaminants/index.html (accessed July 27, 2010).
Fassett, J.E. 2008. Geology and Coal Resources of the Upper Cretaceous Fruitland Formation, San Juan Basin, New Mexico and Colorado. Chapter Q in Geologic Assessment of Coal in the Colorado Plateau: Arizona, Colorado, New Mexico, and Utah, M.A. Kirschbaum, L.N.R. Roberts, and L.R.H. Biewick, eds. U.S. Geological Survey Professional Paper 1625-B. Washington, DC: U.S. Department of the Interior.
Flores, R.M., B.D. Spear, P.A. Purchase, and C.M. Gallagher. 2010. Revised Subsurface Stratigraphic Framework of the Fort Union and Wasatch Formations, Powder River Basin, Wyoming and Montana. USGS Open-File Report 2010-1061. Available at pubs.usgs.gov/of/2010/1061/ (accessed July 8, 2010).
Foster, M.D. 1950. The origin of high sodium bicarbonate waters in the Atlantic and Gulf Coastal Plains. Geochimica et Cosmochimica Acta 1:33-48.
Frost, C.D., E.L. Brinck, J. Mailloux, S. Sharma, C.E. Campbell, S.A. Carter, and B.N. Pearson. 2010. Innovative approaches for tracing water co-produced with coalbed natural gas: Applications of strontium and carbon isotopes of produced water in the Powder River Basin, Wyoming and Montana. In Coalbed Natural Gas: Energy and Environment, K.J. Reddy, ed. Hauppauge, NY: Nova Science Publishers.
Garrison, J.R., Jr., T.C.V. van den Bergh, C.E. Barker, and D.E. Tabet. 1997. Depositional sequence stratigraphy and architecture of the Cretaceous Ferron Sandstone; implications for coal and coalbed methane resources; a field excursion. P.K. Link (non-survey editor), B.J. Kowallis (non-survey editor), Mesozoic to Recent Geology of Utah. Geology Studies 42(2):155-202.
Gas Research Institute. 1995. Atlas of Gas-Related Produced Water for 1990. Gas Research Institute Topical Report GRI-95/0016.
Gloyn, R.W., and S.N. Sommer. 1993. Exploration for coalbed methane gains momentum in Uinta Basin. Oil and Gas Journal 5:73-76.
Haley, C. 2004. Hydrologic and geologic characteristics of the coalbed methane resource, Raton Basin, New Mexico. Unpublished master’s thesis, New Mexico Institute of Mining and Technology. 261 p.
Hathaway, D., G. Barth, F.B. Grigsby, and K.L. MacClune. 2006. MODFLOW….NOT: A Simple But Effective Solution to a Regulatory Question. Available at www.sspa.com/Publications/Hathaway2006MODFLOWvol1.pdf (accessed April 5, 2010).
Hemborg, H.T. 1998. Spanish Peak Field, Las Animas County, Colorado: Geologic setting and early development of a coalbed methane reservoir in the Central Raton Basin. Colorado Geological Survey, Department of Natural Resources, Denver, CO, Resource Series 33.
Jackson, R.E., and K.J. Reddy. 2007. Trace element chemistry of coal bed natural gas produced water in the Powder River Basin, Wyoming. Environmental Science and Technology 41(17):5953-5959.
Jackson, R.E., and K.J. Reddy. 2010. Coalbed Natural Gas Product Water: Geochemical Transformations from Outfalls to Disposal Ponds. In Coalbed Natural Gas: Energy and Environment, K.J. Reddy, ed. Hauppauge, NY: Nova Science Publishers.
Johnson, R.C. 1989. Geologic History and Hydrocarbon Potential of Late Cretaceous-Age, Low-Permeability Reservoirs, Piceance Basin Western Colorado. U.S. Geological Survey Bulletin 1787-E. Washington, DC: U.S. Department of the Interior.
Johnson, R.C., and T.M. Finn. 2001. Potential for a basin-centered gas accumulation in the Raton Basin, Colorado and New Mexico. In Geologic Studies of Basin-Centered Gas Systems, V.F. Nuccio and T.S. Dyman, eds. U.S. Geological Survey Bulletin 2184-B.
Kazemi, G.A., J.H. Lehr, and P. Perrochet. 2006. Groundwater Age. New York: Wiley-Interscience.
Martin, L.J., D.L. Naftz, H.W. Lowham, and J.C. Rankl. 1988. Cumulative Potential Hydrologic Impacts of Surface Mining in the Eastern Powder River Structural Basin, Northeastern Wyoming. U.S. Geological Survey Water-Resources Investigations Report 88-4046.
McBeth, I., K.J. Reddy, and Q.D. Skinner. 2003. Chemistry of trace elements in coalbed methane product water. Water Research 37:884-890.
McDonnell, J.J., M. Sivapalan, K. Vaché, S. Dunn, G. Grant, R. Haggerty, C. Hinz, R. Hooper, J. Kirchner, M.L. Roderick, J. Selker, and M. Weiler. 2007. Moving beyond heterogeneity and process complexity: A new vision for watershed hydrology. Water Resources Research 43.
Meredith, E.L., J.R. Wheaton, S. Kuzara, T.A. Donato, S. Bierbach, and C. Schwarz. 2010. 2009 Annual Coalbed Methane Regional Ground-Water Monitoring Report: Northern Portion of the Powder River Basin, Montana. Open-file report 591. Butte: Montana Bureau of Mines and Geology.
Meyers, T. 2009. Groundwater management and coal bed methane development in the Powder River Basin of Montana. Journal of Hydrology 368:178-193.
Nuccio, V.F. 2000. Coal-Bed Methane: Potential and Concerns. U.S. Geological Survey Fact Sheet FS-123-00. Washington, DC: U.S. Department of the Interior. Available at pubs.usgs.gov/fs/fs123-00/fs123-00.pdf (accessed March 23, 2010).
Oldaker, P.R. and U. Fehn. 2005. Dating of coal bed methane reservoir and surface waters in the Raton Basin, Colorado, and the Susitna Basin, Alaska. American Association of Petroleum Geologists Meeting, Rocky Mountain Section, September 24-26, Jackson, WY.
Orem, W.H., C.A. Tatu, H.E. Lerch, C.A. Rice, T.T. Bartos, A.L. Bates, S. Tewalt, and M.D. Corum. 2007. Organic compounds in produced waters from coalbed natural gas wells in the Powder River Basin, Wyoming. Applied Geochemistry 22(10):2240-2256.
Oreskes, N., K. Shrader-Frechette, and K. Belitz. 1994. Verification, validation, and confirmation of numerical models in the earth sciences. Science 263:641-646.
Patz, M.J., K.J. Reddy, and Q.D. Skinner. 2006. Trace elements in coalbed methane produced water interacting with semiarid ephemeral stream channels. Water, Air, and Soil Pollution 170:55-67.
Phillips, F.M., L.A. Peeters, M.K. Tansey, and S.N. Davis. 1986. Paleoclimatic inferences from an isotopic investigation of groundwater in the central San Juan Basin, New Mexico. Quaternary Research 26:179-193.
Phillips, F.M., M.K. Tansey, L.A. Peeters, S. Cheng, and A. Long. 1989. An isotopic investigation of groundwater in the San Juan Basin New Mexico: Carbon 14 dating as a basis for numerical flow modeling. Water Resources Research 25:2259-2273.
Rice, C.A., and V.F. Nuccio. 2000. Water Produced with Coalbed Methane. U.S. Geological Survey Fact Sheet FS-156-00. Washington, DC: U.S. Department of the Interior. Available at pubs.usgs.gov/fs/fs-0156-00/fs-0156-00.pdf (accessed February 23, 2010).
Rice, C.A., M.S. Ellis, and J.H. Bullock, Jr. 2000. Water Co-Produced with Coalbed Methane in the Powder River Basin, Wyoming. Preliminary compositional data. U.S. Geological Survey Open-File Report 00-372. Washington, DC: U.S. Department of the Interior.
Riese, W.C., W.L. Pelzmann, and G.T. Snyder. 2005. New insights on the hydrocarbon system of the Fruitland Formation coal beds, northern San Juan Basin, Colorado and New Mexico, USA. GSA Special Papers 387:73-111.
Sharma, S., and C.D. Frost. 2008. Tracing coal bed natural gas co-produced water using stable isotopes of carbon. Ground Water 46(2):329-334.
Snyder, G.T., W.C. Riese, S. Franks, U. Fehn, W.L. Pelzmann, A.W. Gorody, and J.E. Moran. 2003. Origin and history of waters associated with coal-bed methane: 129I, 36Cl, and stable isotope results from the Fruitland Formation, Colorado and New Mexico, USA. Geochimica et Cosmochimica Acta 67(23):4529-4544.
Spalding, C.P. and R. Khaleel. 1991. An evaluation of analytical solutions to estimate drawdowns and stream depletion by wells. Water Resource Research 27(4): 597-609.
Sophocleous, M., A. Koussis, J.L. Martin, and S.P. Perkins. 1995. Evaluation of simplified stream aquifer depletion models for water rights administration. Ground Water 33(4): 579-588.
S.S. Papadopulos & Associates, Inc. 2006. Coalbed Methane Stream Depletion Assessment Study: Northern San Juan Basin, Colorado. Prepared in conjunction with the Colorado Geological Survey for the State of Colorado Department of Natural Resources and the Colorado Oil and Gas Conservation Commission. Boulder, CO. Available at water.state.co.us/pubs/pdf/CMSDA_Study.pdf (accessed August 30, 2010).
S.S. Papadopulos & Associates, Inc. 2007. Coalbed Methane Stream Depletion Assessment Study: Piceance Basin, Colorado. Prepared in conjunction with the Colorado Geological Survey for the State of Colorado Department of Natural Resources and the Colorado Oil and Gas Conservation Commission. Boulder, CO. Available at water.state.co.us/groundwater/cbm/piceance/PICEANCE_DRAFT_FINAL.pdf (accessed February 26, 2010).
Stevens, S., T.E. Lombardi, B.S. Kelso, and J.M. Coates. 1992. A geologic assessment of natural gas from coal seams in the Raton and Vermejo Formations, Raton Basin. GRI Topical Report 92/0345. Available at www.vallevidal.org/Downloads/EPA_Raton_Basin.pdf. (accessed May 4, 2010).
Topper, R. 2009. Coalbed methane and ground water: Competing resources? Presentation to American Water Resources Association Colorado, January 27, Colorado Geological Survey. Available at www.awracolorado.havoclite.com/sites/191/pics/CBM-Produced Water.pdf (accessed March 4, 2010).
USGS (U.S. Geological Survey). 1999. 1999 Resource Assessment of Selected Tertiary Coal Beds and Zones in the Northern Rocky Mountains and Great Plains Region. Professional Paper 1625A. Denver, CO: USGS. Available at pubs.usgs.gov/pp/p1625a/ (accessed March 5, 2010).
USGS. 2005. Total Petroleum System and Assessment of Coalbed Gas in the Powder River Basin Province, Wyoming and Montana. Washington, DC: USGS. Available at pubs.usgs.gov/dds/dds-069/dds-069-c/chapters.html (accessed March 23, 2010).
Van Voast, W.A. 2003. Geochemical signature of formation waters associated with coalbed methane. AAPG Bulletin 87(4):667-676.
Water Systems Council. 2007. Wellcare information on Total Dissolved Solids. Developed under Assistance Agreement No. X-83256101-0 awarded by the U.S. Environmental Protection Agency. Washington, DC. Available at www.watersystemscouncil.org/VAiWebDocs/WSCDocs/2010920TDS_FINAL.pdf (accessed July 7, 2010).
Wyoming DEQ (Department of Environmental Quality). 2005. Chapter 8 in Quality Standards for Wyoming Groundwaters. Water Quality Rules and Regulations. Available at deq.state.wy.us/wqd/wqdrules/ (accessed February 26, 2010).
Yurewicz, D.A., K.M. Bohacs, J.D. Yeakel, and K. Kronmueller. 2003. Source rock analysis and hydrocarbon generation, Mesaverde Group and Mancos Shale, northern Piceance Basin, Colorado. In Piceance Basin 2003 Guidebook: Rocky Mountain Association of Geologists, K.M. Peterson, T.M. Olson, and D.S. Anderson, eds. Pp. 130-153.