Workshop Overview
ANTIBIOTIC RESISTANCE: IMPLICATIONS FOR GLOBAL HEALTH AND NOVEL INTERVENTION STRATEGIES
Infectious diseases remain among the leading causes of morbidity and mortality on our planet. The development of resistance in microbes—bacterial, viral, or parasites—to therapeutics is neither surprising nor new. However, the scope and scale of this phenomenon is an ever-increasing multinational public health crisis as drug resistance accumulates and accelerates over space and time. Today some strains of bacteria and viruses are resistant to all but a single drug, and some may soon have no effective treatments left in the “medicine chest.” The disease burden from multidrug-resistant strains of organisms causing AIDS, tuberculosis, gonorrhea, malaria, influenza, pneumonia, and diarrhea is being felt in both the developed and the developing worlds alike.
The accelerating growth and global expansion of antimicrobial1 resistance (hereinafter referred to as AMR) is a demonstration of evolution in “real time” in response to the chemical warfare waged against microbes through the therapeutic and non-therapeutic uses of antimicrobial agents. After several decades in which it appeared that human ingenuity had outwitted the pathogens, multidrug-resistant “superbugs” have become a global challenge, aided and abetted by the use, misuse, and overuse of once highly effective anti-infective drugs. In the words of the
late Joshua Lederberg, humans and microbes continue to be locked in a contest between “our wits and their genes” (Lederberg, 2000).
It should be noted at the outset of this document that the meaning of the phrase “antimicrobial resistance” is wholly context-dependent. Most commonly, it refers to infectious microbes that have acquired the ability to survive exposures to clinically relevant concentrations of drugs that would kill otherwise sensitive organisms of the same strain. The phrase is also used to describe any pathogen that is less susceptible than its counterparts to a specific antimicrobial compound (or combination thereof). Resistance manifests as a gradient based on genotypic and phenotypic variation within natural microbial populations, and even microbes with low levels of resistance may play a role in propagating resistance within the microbial community as a whole (American Academy of Microbiology, 2009).
Pathogens resistant to multiple antibacterial agents, while initially associated with the clinical treatment of infectious diseases in humans and animals, are increasingly found outside the healthcare setting. Therapeutic options for these so-called community-acquired pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) are extremely limited, as are prospects for the development of the next generation of antimicrobial drugs.
On April 6 and 7, 2010, the Institute of Medicine’s (IOM’s) Forum on Microbial Threats convened a public workshop in Washington, DC, to consider the nature and sources of AMR, its implications for global health, and strategies to mitigate the current and future impacts of AMR. Through invited presentations and discussions, participants explored the evolutionary, genetic, and ecological origins of AMR and its effects on human and animal health worldwide. Participants also discussed host and environmental factors associated with the expansion of AMR, strategies for extending the useful life of antimicrobials, alternative approaches for treating infections, incentives and disincentives for prudent antimicrobial use, and prospects for the discovery and development of ”next generation” antimicrobial therapeutics. While it was the “intent” of the workshop planners and organizers to cover the phenomenon of AMR broadly, workshop presentations and discussions focused almost exclusively on bacterial resistance to antibacterial drugs.
Organization of the Workshop Summary
This workshop summary was prepared by the rapporteurs for the Forum’s members and includes a collection of individually authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on the presentations and discussions at the workshop.
The workshop summary is organized into sections as a topic-by-topic description of the presentations and discussions that took place at the workshop.
Its purpose is to present lessons from relevant experience, to delineate a range of pivotal issues and their respective problems, and to offer potential responses as discussed and described by the workshop participants. Manuscripts and reprinted articles submitted by some but not all of the workshop’s participants may be found, in alphabetical order, in Appendix A.
Although this workshop summary provides a description of the individual presentations, it also reflects an important aspect of the Forum’s philosophy. The workshop functions as a dialogue among representatives from different sectors and allows them to present their beliefs about which areas may merit further attention. These proceedings only summarize the statements of participants in the workshop. They are not intended to be an exhaustive exploration of the subject matter or represent the findings, conclusions, or recommendations of a consensus committee process.
Antimicrobial Drug Resistance in Context
The History of Medicine:
-
2000 B.C.—Here, eat this root.
-
1000 A.D.—That root is heathen. Here, say this prayer.
-
1850 A.D.—That prayer is superstition. Here, drink this potion.
-
1920 A.D.—That potion is snake oil. Here, swallow this pill.
-
1945 A.D.—That pill is ineffective. Here, take this penicillin.
-
1955 A.D.—Oops … bugs mutated. Here, take this tetracycline.
-
1960–1999 A.D.—39 more “oops.”… Here, take this more powerful antibiotic.
-
2000 A.D.—The bugs have won! Here, eat this root.
—Anonymous, as cited by the World Health Organization (WHO, 2000a)
An Inevitable History
The use of antimicrobial drugs, no matter how well controlled, “inevitably leads to the selection of drug-resistant pathogens,” according to workshop speaker Julian Davies, of the University of British Columbia (Davies, 2009). (Dr. Davies’ contribution to the workshop summary report can be found in Appendix A, pages 149-160.) As may be seen in the following illustration (Figure WO-1), there is no man-made defense that cannot be outmaneuvered by microbial evolution and adaptation. As speaker Gerard Wright of McMaster University observed, “there is no such thing as an irresistible antibiotic.” (Dr. Wright’s contribution to the workshop summary report can be found in Appendix A, pages 401-419.)
This characteristic of antimicrobial drugs has been well-known since the dawn of the antibiotic era over seven decades ago, and all too often has been either
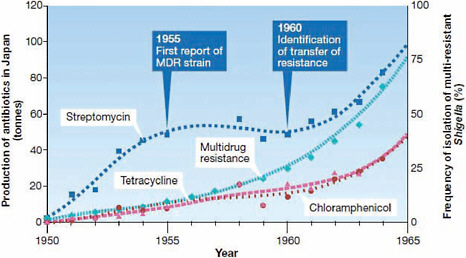
FIGURE WO-1 The relationship between antibiotic resistance development in Shigella dysenteriae isolates in Japan and the introduction of antimicrobial therapy between 1950 and 1965. In 1955, the first case of plasmid determined resistance was characterized. MDR = multidrug resistance. Transferable, multi-antibiotic, resistance was discovered five years later in 1960.
SOURCES: Davies (2007, 2009). Reprinted by permission from Macmillan Publishers Ltd.: EMBO Reports Davies, Copyright 2007.
underestimated or ignored. Hailed as a miracle drug when it was first introduced in 1943, penicillin was eagerly purchased by consumers who initially obtained it without a prescription following the conclusion of World War II (Stolberg, 1998). In a 1945 interview with the New York Times, penicillin’s discoverer Alexander Fleming anticipated the development of drug-resistant bacterial strains. Indeed, penicillin-resistant strains were first isolated from patients in significant numbers a year later, in 1946.
Over the next several decades, researchers discovered and developed a range of antimicrobial agents and classes of compounds with antimicrobial properties, as illustrated in Figure WO-2. Like penicillin, some antimicrobial drugs were directly derived from soil microbes; others were synthesized or modified versions of naturally occurring antimicrobial products (Salmond and Welch, 2008). Beginning in the early 1950s, antimicrobials were also widely adopted for non-human applications, most importantly as livestock feed additives (Davies, 2009).
Despite the warnings of Fleming and others to the contrary, in 1967, the Surgeon General of the United States, Dr. William H. Stewart, claimed that infectious diseases had been conquered through the development and use of antibiotics and vaccines and that therefore it was time to shift the U.S. government’s attention and resources to the “War on Cancer” (Stewart, 1967; Stolberg, 1998).
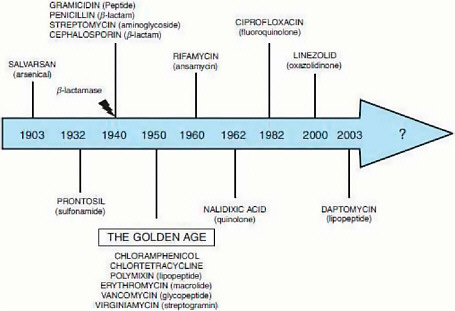
FIGURE WO-2 Major classes of antimicrobials and the year of their discovery.
SOURCE: Davies (2009), IOM (2009b).
The “Antibiotic Era” has been marked by a series of epidemics of resistant organisms (see Box WO-4 [which appears on pages 58-63]), including
-
penicillin-resistant Staphylococcus aureus,
-
methicillin-resistant Staphylococcus aureus (MRSA),
-
vancomycin-intermediate Staphylococcus aureus (VISA),
-
multi-drug-resistant (MDR) Vibrio cholerae,
-
multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis (hereinafter MDR- and XDR-TB),
-
CTX-M2 resistant Escherichia coli and Klebsiella pneumoniae,
-
Clostridium difficile, and many others.
Reports of new outbreaks of these so-called “superbugs” in the popular press are becoming increasingly commonplace events (Davies, 2009).
Numerous studies, reports, and review articles—several of which are cited
throughout this workshop overview—have addressed the phenomenon of AMR from a variety of perspectives. The Forum on Microbial Threats was created in 1996 to provide an ongoing opportunity to explore and discuss a variety of emerging and reemerging infectious disease challenges including the rise of AMR and related issues that were highlighted in the 1992 IOM report, Emerging Infections: Microbial Threats to Health in the United States (IOM, 1992), and further elaborated upon a decade later in the IOM report Microbial Threats to Health: Emergence, Detection, and Response (IOM, 2003). Many Forum workshops have also drawn attention to the significant contribution of AMR to the emergence of infectious diseases as a global public health challenge and have explored the proliferation and distribution of resistant microbes, hosts, vectors, and genes through migration, travel, conflict, trade, and tourism (IOM, 2006, 2008a, 2009a, 2009b, 2010).
The Tragedy of the Commons
The phenomenon of AMR is ultimately both a global public health and environmental catastrophe, a “classic” example of the “tragedy of the commons” illustrated more than 40 years ago in a seminal article by the late ecologist Garrett Hardin (1968). Hardin’s “tragedy of the commons” has proven to be a useful metaphor for understanding how we have come to be at the brink of numerous environmental catastrophes—whether land use, global climate change, access to and availability of uncontaminated and abundant fresh water resources, or antimicrobial resistance. Simply stated, we face a serious dilemma—an instance where individual rational behavior, acting without restraint to maximize personal short-term gain—can cause long-range harm to the environment, others and ultimately to oneself.
Many of the planet’s natural resources are treated as a “commons,” wherein individuals have the right to freely consume its resources and return their wastes to the collective environment. The “logic of the commons” ultimately results in its collapse with the concomitant demise of those who depend upon the commons for survival (Diamond, 2005). Like climate change (IOM, 2008a) and the global water crisis (IOM, 2009a), the emergence of drug-resistant microbes was catalyzed by rational behavior: humans acting without restraint to maximize personal short-term gain.
According to Baquero and Campos (2003), “antibiotics have been considered to be an inexhaustible common, both for prescribers and the general public,” and the resulting over-consumption has produced a “net increase in antibiotic resistance and a likely reduction in the therapeutic efficacy of the drugs.” If one person’s misuse of a drug speeds up the evolution of resistant strains, while simultaneously decreasing his or her chance of being cured, then antimicrobial efficacy can be viewed as a scarce commodity in need of responsible management, on a par with energy, safe food, clean water, and climate stability. As Walker and
coauthors (2009) observed, these and other resources in crisis comprise a nexus of “serious, intertwined global-scale challenges spawned by the accelerating scale of human activity.” Addressing such challenges and their interactive effects, they contend, demands “cooperation in situations where individuals and nations will collectively gain if all cooperate, but each faces the temptation to take a free ride on the cooperation of others.”
Parallels with Pesticides
The rise of AMR closely parallels that of pesticide resistance, as observed by keynote speaker David Pimentel of Cornell University (National Research Council, 2000; Pimentel et al., 1992). (Dr. Pimentel’s contribution to the workshop summary report can be found in Appendix A, pages 294-300.) According to Pimentel, about 550 species of insects and mites are known to be resistant to insecticides, as are 330 species of plant pathogens (fungi, bacteria, and viruses) and 220 weed species in the United States today. Pesticide-resistant organisms represent a serious global problem for agriculture, he observed, with an estimated annual direct cost in the United States alone of $1.5 billion.
Pimentel went on to describe the pesticide “treadmill,” wherein the acquisition of resistance by “pest” organisms through repeated exposures to these toxic chemical compounds forces farmers to use ever-increasing amounts of a given pesticide—or combination of pesticides—to achieve the same level of pest control—until the next generation of effective pesticides becomes available to eradicate the resistant agricultural pests (National Research Council, 2000; Pimentel et al., 1992). This pesticide treadmill is doomed to repeat until either the pest meets a resistance-proof pesticide or the supply of effective new pesticides is exhausted.
Dichlorodiphenyltrichloroethane (DDT) was such a pesticide, Pimentel said, and like penicillin, its introduction after the end of World War II dramatically improved peoples’ lives. Originally used for malaria control, DDT was initially applied only to the insides of houses and huts for vector control, exposing about one mosquito in a million to the pesticide, he explained. Resistance to DDT did not appear until it came into widespread, uncontrolled, agricultural uses, thereby vastly increasing the numbers and types of insects directly or indirectly exposed to the insecticide. As Anopheles mosquito populations became increasingly resistant to DDT, he continued, malaria rates—which greatly declined following DDT’s introduction in the 1940s—began to rise.
While the use of pesticides appear to improve U.S. crop yields by some $40 billion per year, Pimentel observed, these gains must be weighed against the direct and indirect harmful effects associated with pesticide use and abuse to public and environmental health, which he valued at a minimum of $12 billion per year. He noted, moreover, that despite the application of some 6 billion pounds
of pesticides worldwide,3 at a cost of approximately $40 billion, pests continue to consume nearly half of the food produced annually.
Microbial Evolution and the Origins of Resistance
While it is self-evident that the use of antimicrobial drugs has imposed selective pressures on the emergence of resistant microbes, to attribute the development of resistance entirely to imprudent antimicrobial use is, in the words of Spellberg and coauthors, “a fallacy that reflects an alarming lack of respect for the incredible power of microbes” (Spellberg et al., 2008a). In addition to the range of anthropogenic factors that encourage the development of antimicrobial resistance, workshop participants also reflected on the natural systems into which synthetic and mass-produced antibiotics were introduced in the post-World War II era.
Antibiotics in Nature
Humans did not invent antibiotics; we merely observed—often by accident—that bacteria and other microorganisms produced biological compounds capable of killing or suppressing the growth and reproduction of other bacteria (Martinez, 2009). There are a variety of explanations for why microoganisms make antibiotics. A conventional ecological and evolutionary view holds that they enable organisms to kill—or suppress the growth of—competitors and to defend ecological niches (Salmond and Welch, 2008). It is also possible that these products serve other functions, such as signaling or nutrient sequestration (Martinez, 2009).
Some enzymes in the antibiotic biosynthetic pathways appear to have evolved millions to billions of years ago, which suggests that antibiotic-resistance genes and their cognate proteins are also ancient. For example, the bacterial metabolic pathways that produce both β-lactam antibiotics and the enzyme that foils them, β-lactamase, are thought to be more than 10 million years old (Spellberg et al., 2008a). Synthetic antibiotics (most of which are based on naturally-occurring bacterial products) target a variety of bacterial systems, as illustrated in Figure WO-3, including those involved with cell wall synthesis, membrane integrity, transcription, and translation (Salmond and Welch, 2008; Walsh, 2003).
In his workshop presentation, Davies placed antibiotics within the general class of biologically active small molecules, which he referred to as the “parvome.” He observed that members of this “universe of bioactive natural products” share several common attributes, including
-
ancient evolutionary origins, including structural components found in meteorites and “primordial soup” reactions;
-
vast structural diversity;
-
functions that involve many aspects of microbial physiology, behavior, and morphology, including interactions between cells;
-
mechanisms of action involving molecular or macromolecular ligands that subsequently modulate transcription; and
-
presence in all living organisms (best characterized in bacteria, fungi, and plants).
The subset of molecules in the parvome that we have harnessed as antibiotics did not evolve to serve that function, Davies continued. “I believe … that in nature antibiotics are not antibiotics and in nature resistance genes are not resistance genes,” he stated.
Davies noted that antibiotic molecules have been found to promote a great variety of other activities, including recombination, horizontal gene transfer, mutation, metabolism, gene regulation, and signaling, all of which are mediated through cell receptors. Indeed, he added, most of the negative side-effects of antibiotic drugs stem from their interactions with a variety of human cell receptors. Erythromycin and other macrolide drugs, for example, cause stomach upset due to their ability to bind strongly to a receptor for motilin, a peptide that stimulates smooth muscle contraction in the gut. Additional workshop presentations describing the ability of antibiotic compounds to function as mutagens and hormones are discussed in the following section of this overview.
Antibiotics “have amazing effects on bacterial cell physiology,” Davies concluded (Davies et al., 2006). If we knew more about the functions of antibiotic compounds (and resistance genes) in their native environments, he said, “we might get some better ideas on how to control antibiotic resistance and also how to use antibiotics properly.” In particular, Davies suggested studying how small molecules with antibiotic properties influence interactions between and among soil bacteria and single cells.
The Nature of AMR
Soil microbes that produce antibiotics also have mechanisms of resistance, as speaker Gerard Wright, of McMaster University, pointed out. If they did not, he said, “they would produce their antibiotic once and immediately commit suicide.” The variety of mechanisms that microbes use to protect themselves includes altered membrane permeability or binding sites, efflux pumps that export incoming antibiotics, and antibiotic-degrading enzymes, as illustrated in Figure WO-4 (Arias and Murray, 2009; Davies 2009; Salmond and Welch, 2008). Some soil bacteria not only resist clinical antibiotics but can actually subsist on them as a carbon source (Dantas et al., 2008).
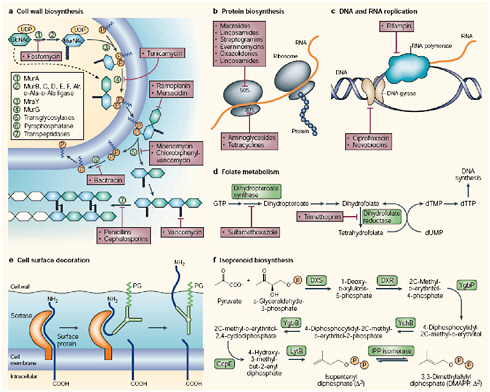
FIGURE WO-3 Principal targets for antibiotic action: a–f depict metabolic pathways in the cell that have been, or are proposed to be, targets for antibiotic action. a | Cell wall biosynthesis: the intracellular steps of murein (peptidoglycan) biosynthesis are catalysed by the enzymes MurA–F and MurG (steps 1–4). Peptidoglycan is a polymer of two hexoses (filled hexagons)—N-acetylglucosamine (GlcNac) and N-acetyl-muramic acid (MurNAc). Peptidoglycan units are transferred to a carrier lipid—bactoprenol-phosphate (orange circles)—which transports precursor molecules across the cell membrane, generating Lipids I and II. Sugars and phosphates are added by transglycosylation and pyrophosphorylation (steps 5 and 6), and finally, a peptide bond between the peptide chains is formed (step 7). Antibiotics that inhibit cell-wall synthesis are indicated. b | Protein biosynthesis: bacterial ribosomes comprise two subunits (30S and 50S) of rRNA and protein. Structural studies have identified the sites at which antibiotics bind (Carter et al., 2000; Hansen et al., 2002; Pioletti et al., 2001; Schlunzen et al., 2001). c | DNA and RNA replication: rifampin binds to RNA polymerase and prevents attachment of the polymerase to DNA, thereby inhibiting transcription. Ciprofloxacin and novobiocin bind to DNA gyrase, thereby preventing the introduction of supercoils in DNA. d | Folate metabolism: folate is necessary for the synthesis of thymine, which, in turn, is an essential component of DNA. The figure shows antibiotics that block steps in folate metabolism and therefore block the synthesis of thymine. e | Cell-surface decoration: during cell-wall synthesis in Gram-positive bacteria, surface proteins are cleaved by sortases—enzymes that are anchored in the membrane by an amino-terminal membrane-
spanning sequence. Sortases covalently attach the amino-terminal cleavage fragment of the surface protein to the peptidoglycan (PG) layer of the cell wall (Pallen et al., 2001). f | Isoprenoid biosynthesis: the enzymes of the non-classical isoprenoid pathway in bacteria are not present in higher organisms (Rohdich et al., 2001), and should therefore be good antibacterial targets. dTMP, thymidylate; dUMP, deoxyuridine monophosphate; DXR, 1-deoxy-D-xylulose 5-phosphate (DX) reductoisomerase; DXS, DX synthase; GcpE, 1-hydroxy-2-methyl-2-(E)-butenyl- 4-diphosphate synthase; GTP, guanosine triphosphate; LytB, Isoprenoid H protein; YchB, 4-diphospho-2C-2-methyl-D-erythritol kinase; YgbB, 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase; YgbP, 4-diphosphocytidyl-2C-methylerythritol synthase.
SOURCE: Walsh (2003). Reprinted by permission from Macmillan Publishers Ltd.: Nature Reviews Microbiology Walsh, Copyright 2003.
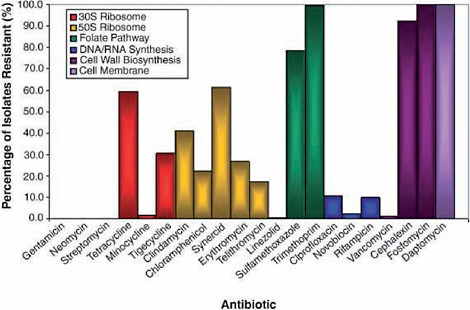
A wealth of antimicrobial-resistant soil bacteria and genes discovered in pristine environments would suggest that a variety of antimicrobial resistance mechanisms exist in nature (Allen et al., 2010; Davies, 2009). Wright described a group of 480 isolates of soil bacteria from the group actinomycetes that his group collected in diverse environments throughout Canada; their drug resistance profiles are presented in Figure WO-5 (D’Costa et al., 2006). Every isolate proved to be resistant to multiple antibiotic drugs.
Wright also reported similar levels of resistance to clinical antibiotics in bacterial samples collected from a Kentucky cave system that has been sealed from the external environment for about 2 million years (Gerard Wright, McMaster University, personal communication, April 6, 2010).
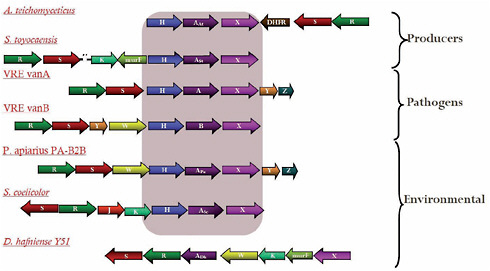
Antibiotic-resistance genes isolated from soil bacteria and those isolated from clinical pathogens share similar structures and functions, Wright noted. He presented a particularly impressive example of this resemblance that occurred among approximately 1 percent of the previously described actinomycete isolates (D’Costa et al., 2006). These microbes were found to possess a suite of genes conferring resistance to vancomycin, once considered an “irresistible” antibiotic because it targets a cell wall polymer rather than an easily mutated protein or nucleic acid. However, not only have clinical cases of resistance to vancomycin been reported, but these findings suggest that the five-gene cluster found to confer resistance in clinical isolates of vancomycin-resistant enterococci (VRE) has existed for thousands of years among bacteria that have never been exposed to vancomycin, as may be seen in Figure WO-6.
AMR is also widespread among commensal organisms, Wright said, referring to a recent study that employed complementary strategies to look for antibiotic-resistance genes and antibiotic-resistant culturable organisms in the microbial flora of healthy humans (Sommer et al., 2009). These investigators
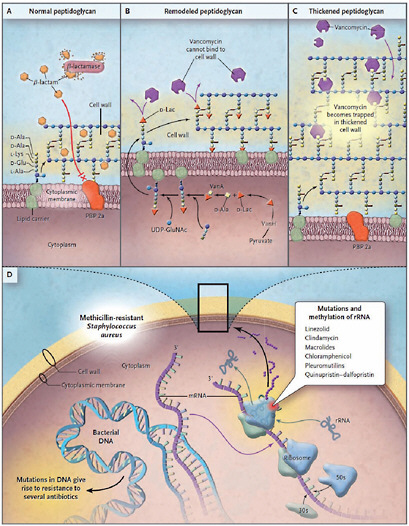
FIGURE WO-4 Common mechanisms of resistance in methicillin-resistant Staphylococccus aureus.
The top three panels depict a schematic magnification of the bacterial cell wall. In Panel A, resistance to β-lactam antibiotics in methicillin-resistant Staphylococcus aureus is caused by the production of a β-lactamase enzyme (penicillinase) and a low-affinity penicillin-binding protein (PBP) 2a. In Panel B, high-level resistance to glycopeptides is caused by the replacement of the last amino acid of peptidoglycan precursors (D-alanine [D-Ala] to D-lactate [D-Lac]). In Panel C, low-level resistance to glycopeptides is associated with increased synthesis of peptidoglycan, “trapping” the antibiotic in outer layers and preventing its interaction with precursors exiting the cytoplasm through the cell membrane. In Panel D, mechanisms of resistance involve mutations or modifications in either [other genomic loci] or [in the] ribosomal RNA (rRNA). D-Glu denotes D-glutamate, L-Lys L-lysine, and UDP-GluNAc uridine diphosphate N-acetylglucosamine.
SOURCE: Arias and Murray (2009).
discovered high levels of both resistant organisms and resistance genes among human commensals. Wright noted that other studies had found a wealth of antibiotic-resistant commensals in the guts of insects (Allen et al., 2009; Kadavy et al., 2000), birds (Bonnedahl et al., 2009), and non-human mammals (Cloud-Hansen et al. 2007; Gilliver et al., 1999; Poeta et al., 2007) that had not been directly exposed to antibiotic drugs.
AMR Genes
Microbes have exchanged genes encoding resistance mechanisms for millennia. Genes conferring resistance to clinical antibiotics (but which also, presumably, provide other selective advantages to their hosts) exist in bacterial populations that have never encountered these compounds (Allen et al., 2010; IOM, 2009b; Salmond and Welch, 2008). The vast majority of antimicrobial resistance genes reside on mobile genetic elements such as insertion sequences,4 integrons,5 transposons,6 and plasmids,7 according to workshop speaker Henry “Chip” Chambers of the University of California, San Francisco. (Dr. Chambers’ contribution to the workshop summary report can be found in Appendix A, pages 83-115.) Bacteria readily acquire these genetic elements from the environment, exchange them through conjugation,8 and receive them via infection by bacterial viruses (bacteriophages, or phages) (Salmond and Welch, 2008). These processes used to acquire “novel” genetic elements are collectively referred to as “horizontal gene transfer.” A mobile genetic element that confers selective advantages upon its host—such as antibiotic resistance—can spread widely, and may be expressed even when the antibiotic it deactivates is not present (O’Brien, 2002).
The collection of all genes that directly or indirectly result in antimicrobial resistance is known as the “resistome.” It includes a subset of genes, dubbed the “subsistome,” that permit microbes to degrade antibiotics and use them as an energy source. Resistance genes are apparently ubiquitous among bacterial genomes, as Davies and Wright noted. The resistome, moreover, includes
|
4 |
Mobile pieces of bacterial DNA (several hundred nucleotide pairs in length) that are capable of inactivating a gene into which they insert small simple transposons (http://www.everythingbio.com/glos/definition.php?word=insertion+sequence+(IS) [accessed June 14, 2010]). |
|
5 |
Mobile DNA elements that can capture and carry genes, particularly those responsible for antibiotic resistance. They do this by site-specific recombination (http://www.medterms.com/script/main/art.asp?articlekey=32273 [accessed June 14, 2010]). |
|
6 |
Mobile pieces of DNA flanked by terminal repeat sequences that can insert into a chromosome, exit, and relocate and typically bear genes coding for these functions (http://www.everythingbio.com/glos/definition.php?word=transposon [accessed June 14, 2010]). |
|
7 |
Small cellular inclusions consisting of a ring of DNA that are not in a chromosome but are capable of autonomous replication (http://wordnetweb.princeton.edu/perl/webwn?s=plasmid [accessed June 14, 2010]). |
|
8 |
A process whereby two cells come in contact and exchange genetic material (http://www.every-thingbio.com/glos/definition.php?word=conjugation [accessed June 14, 2010]). |
“proto-resistance,”9 “quasi-resistance,” and “intrinsic resistance,”10 genes that, under selective pressure, can evolve resistance functions through mutation and/or increased expression (Liu et al., 2010; Tamae et al., 2008).
“We have resistance in clinical [and commensal] organisms … resistance in animals … resistance in the soil, resistance in the water,” Wright observed. “Antibiotic resistance is absolutely everywhere.” This observation raises important questions as to how resistance genes move from the environment into the clinic, and whether barriers to horizontal gene transfer exist or could be created to slow the development of resistance to antimicrobial drugs. To facilitate such research, Wright and colleagues in the United Kingdom are developing a Comprehensive Antibiotic Resistance Database to enable investigators to scan genomes and link resistance gene sequences to molecular, clinical, and surveillance data (McArthur et al., 2010).
Anthropogenic Influences on AMR
Microbial evolution has occurred in two distinct phases, punctuated by the Industrial Revolution, Davies observed. He speculated that, during the current “anthropogenic era,” the pharmaceutical industry has released more antibiotics into the global environment than were ever produced by all the organisms that have ever existed. The selective pressure exerted by manufactured antimicrobials have been amplified by an equally massive onslaught of pesticides, fertilizers, antiseptics, and other industrial products, Davies said, resulting in “intense chemical mutagenesis” that has vastly accelerated the emergence of resistant pathogens.
Several workshop presentations explored the recent evolution of AMR from the perspective of microbes under the selective pressure associated with constant exposures to antibiotics. Others examined the actual and anticipated consequences of AMR for host organisms, as demonstrated in hospital and food animal production settings, and as disseminated locally via wastewater treatment systems and globally through international trade, travel, and tourism.
Selection for Antibiotic Resistance
Selective pressures favoring AMR vary widely among environments, Davies explained. As illustrated in Figure WO-7, he presented a model of three interconnected ecosystems:
-
the “natural” environment, where microbes encounter low concentrations of antimicrobial compounds produced by other microbes, and resistance is low;
-
the “non-clinical” environment, where the presence of man-made antimicrobials raises selective pressure for AMR; and
-
the clinical environment, where the relative concentration of antimicrobials is highest and, consequently, so is AMR.
Individual microbes within each of these three generalized ecosystems also encounter vastly different concentrations of specific antimicrobial compounds. As speaker Patrice Courvalin of the Institut Pasteur pointed out, bacteria can minimize the often considerable energy cost of maintaining AMR in one of two ways: by having resistance genes on mobile genetic elements (since their acquisition can be transient and antibiotic resistance is useful only transiently) and by having antibiotic-inducible resistance mechanisms. (Dr. Courvalin’s contribution to the workshop summary report can be found in Appendix A, pages 141-149.)
Chambers described how Staphylococcus aureus, which has no natural resistance to most standard therapeutic agents, acquired methicillin resistance and eventually emerged first as MRSA in hospitals, and then as community-acquired MRSA (CA-MRSA) outside the healthcare environment (Chambers and DeLeo, 2009). Following some false starts in the form of MRSA clones that failed to expand, successful resistant strains carried a readily transferable resistance element. CA-MRSA strains contain a smaller version of this element that is even easier to mobilize and transfer into a variety of genetic backgrounds, Chambers observed. This characteristic has facilitated the spread of methicillin resistance to S. aureus strains well beyond the healthcare environment.
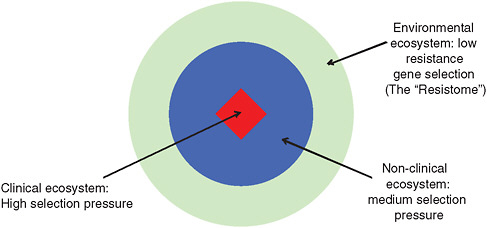
FIGURE WO-7 Three connected antimicrobial ecosystems.
SOURCE: Adapted from Martinez (2009).
The emergence of trimethoprim or sulfamide resistance illustrates the 2-step process through which antibiotic resistance develops, according to Courvalin. In some cases, formerly sensitive strains become resistant following the acquisition of a mobile genetic element; in others, acquisition of these resistance traits are the result of chromosomal mutations. Whether or not a resistant strain persists in a population depends on its fitness relative to other strains (Courvalin, 2008). “Many studies have shown that dissemination of resistance is clearly associated with selective pressure—in other words, the prescription of antibiotics,” Courvalin said. There is nothing we can do to prevent the emergence of resistance, because it occurs by chance, he continued. “The only hope we can have is to delay dissemination of resistance by lowering the selective pressure.”
Antibiotic-Induced Mutation and Transformation
At the lethal concentrations used to treat infections, antibiotics impose a severe selective pressure that can be overcome by several types of resistant pathogens. As several workshop presenters pointed out, however, chronic exposures to lower concentrations of antibiotics—present in growth-promoting animal feed, sewer systems, and in contaminated microenvironments—elicit stress responses in bacteria that induce mutation, thereby increasing opportunities for the evolution of resistance, as discussed below (Kohanski et al., 2010a, 2010b). Bacteria exposed to sublethal concentrations of bactericidal antibiotics—such as the β-lactams, aminoglycosides, or fluoroquinolones—produce increased levels of reactive oxygen species (ROS) that, in turn, cause mutations and increase recombination efficiency in the affected bacterium (see Figure WO-8). ROS also induces the so-called “SOS response,”11 mediated by error-prone DNA polymerases, which creates additional mutations.
Chambers and Courvalin emphasized that, when antibiotics act as mutagens, they do so indiscriminately. Sublethal antibiotic exposures expand the repertoire of genes that undergo selective pressures including, but not limited to, the specific antibiotic causing the stress response. For example, “very low doses of ampicillin will select for resistance to quinolones or to aminoglycosides, and the strain will remain susceptible to the selective agent,” Courvalin reported (Kohanski et al., 2010a). “This is really scary to think about,” Chambers observed, “because now we’re not talking just about antibiotics selecting for drug resistance; we’re talking about antibiotics generating drug resistance and selecting for drug resistance—an amplification, if you will.”
Sublethal antibiotic exposures have also been demonstrated to increase the horizontal transmission of mobile genetic elements among bacteria via the SOS response, Courvalin and Collins noted (Maiques et al., 2006; Ubeda et al., 2005).
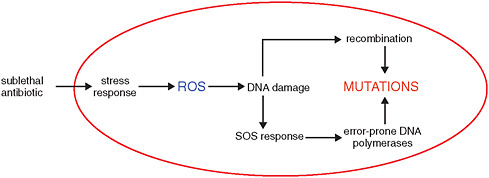
FIGURE WO-8 Antibiotic-induced increase in mutation rate.
SOURCE: Courvalin (2010).
Some bacteria also express mechanisms that increase the dissemination of chromosomal mutations conferring antibiotic resistance, Courvalin explained. In some organisms, low levels of bactericidal antibiotics have been shown to induce competence for transformation,12 leading to increased transfer and distribution of antibiotic resistance genes. The bacterial agent of pneumonia, Streptococcus pneumoniae, which lacks an SOS response, readily integrates genes from other organisms into its chromosome when exposed to sublethal concentrations of bactericidal antibiotics (Prudhomme et al., 2006). As a result, Courvalin said, when people vaccinated against certain S. pneumoniae serotypes are exposed to sublethal levels of bactericidal antibiotics, resistant serotypes may—through antibiotic-induced transformation—acquire the genetic machinery to produce a variant external capsule that the vaccine does not recognize.
Low concentrations of antibiotics also favor the transfer of antibiotic resistance genes (and virulence genes as well) borne on bacterial chromosomes in the form of integrative conjugative elements (ICEs),13 as illustrated in Figure WO-9.
These elements excise from the chromosome by site-specific recombination and are transferred from one bacterium to another, Courvalin explained. Like antibiotic-induced transformation, this is an infectious phenomenon. From one copy of a resistance gene-bearing ICE, two are made: one is inherited vertically, and the other transferred horizontally to another bacterium—perhaps of another
|
12 |
The modification of a genome by the external application of DNA from a cell of different genotype (http://www.everythingbio.com/glos/definition.php?word=transformation [accessed June 16, 2010]). |
|
13 |
Chromosomally located gene clusters that encode phage-linked integrases and conjugation proteins as well as other genes associated with an observable phenotype, such as virulence or symbiosis. They can be transferred between cells and have some phage-like genes, but they do not lyse the cell or form extracellular particles (http://www.nature.com/nrmicro/journal/v3/n9/glossary/nrmicro1235_glossary.html [accessed June 16, 2010]). |
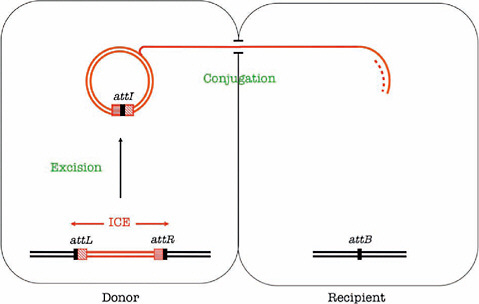
FIGURE WO-9 Transfer of an integrative conjugative element. Integrative and conjugative elements (ICEs) are mobile genetic elements that carry one or several resistance genes. They excise by site-specific recombination between their flanking attachment sites, attR and attL, leading to the formation of an episomal ICE carrying an attI site and an empty attB site in the chromosome. They replicate during their transfer by conjugation and integrate in the chromosome of the recipient. Dissemination of resistance by ICEs is thus infectious and exponential.
SOURCE: Courvalin (2010).
species or even genus—which integrates the resistance gene into its chromosome and continues the process. In this system, Courvalin observed, antibiotics fit the operational definition of hormones because they are synthesized by one cell (an antibiotic-producing organism) and act on a distant cell at low concentration by binding to a specific receptor.
Thus, the critical unit of AMR transmission is the resistance-associated gene or gene cassette, and the “vector” could be viewed alternatively as the microbial genome in which the gene or cassette is found, or the microbial community in which the resistant microbe resides, or the host or broader ecosystem that carries the community.
Hospital-Acquired Infections
Resistance poses a growing threat to the treatment and control of infectious diseases, including those that have long been endemic in human populations
(like malaria) as well as those that have caused recent pandemics such as HIV/AIDS and influenza (WHO, 2010). Resistance to one of the very first antibiotics, penicillin, arose almost immediately upon its introduction to the clinic, as has occurred for every antibiotic developed since. Considering the full spectrum of known antibiotic effects on bacterial evolution, Chambers stated that they represented “the strongest—not the only, but by far the strongest—selective pressure ever encountered by the human microbiome.”
Nowhere is this pressure more critical than in healthcare settings where, as a result of the overuse and misuse of antibiotics, several commensal organisms—otherwise benign bacteria that commonly exist on the skin, throughout the alimentary tract, or in the vagina—have emerged as pathogens (Alekshun and Levy, 2006; Goossens et al., 2005; Riedel et al., 2007). The following six pathogens, denoted by the acronym ESKAPE, cause the majority of hospital-acquired infections in the United States, and frequently prove resistant to antibacterial drugs (Hidron et al., 2008; Rice, 2008):
-
Enterococcus faecium
-
Staphylococcus aureus
-
Klebsiella pneumoniae
-
Acinetobacter baumanii
-
Pseudomonas aeruginosa
-
Enterobacter spp.
It has been estimated that about half of all clinical antibiotics are used inappropriately to “treat” non-bacterial viral infections and other health problems that cannot be cured with these drugs, or the wrong antibiotics are given, or the course of treatment is either too short or too long (Center for Global Development, 2010). The prevalence of AMR is more likely in those situations where antimicrobial use is greatest and exposures to these drugs are highest (Goossens et al., 2005; Riedel et al., 2007). The excessive use and misuse of antibiotics are generally attributed to inappropriate prescribing by physicians, as well as the lack of timely and specific microbiological diagnostic tests. However, the risk for resistance increases when people can easily obtain antibiotics without a prescription, or when they self-medicate with antibiotics left over from previous courses of treatment (Plachouras et al., 2010).
Calculating disease burden Most analyses of the impact of AMR have focused on the developed world, where resistant pathogens rank among the top infectious disease public health threats (ECDC, 2007). Yet AMR in developing countries undoubtedly adds to the already heavy burden of infectious diseases experienced in these countries (Okeke et al., 2005). In the United States, hospital-acquired resistant infections are associated with more than 63,000 deaths per
year (Resources for the Future, 2009). In Europe, the death toll from multi-drug resistant bacterial infections is believed to exceed 25,000 per year (ECDC and EMEA, 2009). Speaker Dominique Monnet, of the European Centre for Disease Prevention and Control (ECDC), presented preliminary results of a recent ECDC-led attempt to calculate the disease burden in Europe associated with the frequently diagnosed types of multi-drug resistant infections (see Table WO-1). (Dr. Monnet’s contribution to the workshop summary report can be found in Appendix A, pages 287-293.)
Because these calculations only considered certain multi-drug resistant infections, an average cost for a hospital day, and productivity losses that were incurred only during hospital stay, the resulting economic burden is certainly an underestimate. Since the calculations were based on parameters from surveillance systems and the published literature, and since infection rates and healthcare costs vary considerably among European countries, the researchers also constructed a nomogram, shown in Figure WO-10, to compare the effect of various parameter estimates on in-hospital costs.
Healthcare-acquired infections of all types (including antimicrobial-resistant infections) increase hospital charges, lengths of stay, and mortality an average of 2-fold for patients in the United States, according to speaker Robert Weinstein of Stroger (Cook County) Hospital and Rush Medical College. (Dr. Weinstein’s contribution to the workshop summary report can be found in Appendix A, pages 379-400.) It is estimated that treatment of antibiotic resistant infections further doubles these costs (Cosgrove, 2006). Weinstein emphasized the many uncertainties inherent in calculating the costs of AMR. For example, he reported that, while investigators can compare antibiotic-resistant hospital-acquired infections to hospital-acquired infections that are non-resistant, some patients are more vulnerable to contracting resistant infections in the first place. Statistical devices known as propensity scores are used to reduce selection bias by equating groups based upon their expression of certain traits within a given set of known conditions (Griswold
TABLE WO-1 Burden of Multidrug-Resistant (MDR) Bacteria in the European Union, Iceland, and Norway, 2007
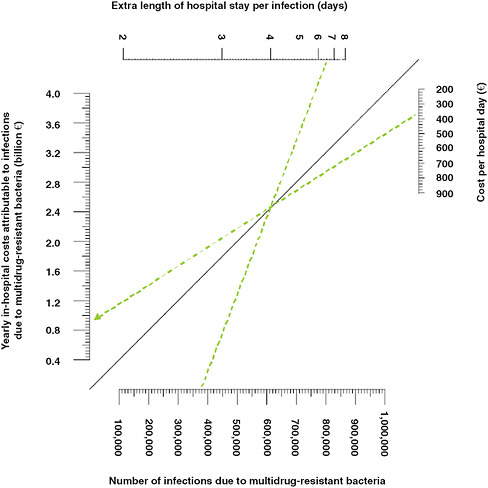
FIGURE WO-10 Economic burden of multidrug-resistant (MDR) bacteria: nomogram for in-hospital costs. This nomogram can be used to calculate yearly in-hospital costs attributable to infections due to multidrug-resistant bacteria with various values for the total number of infections, the average extra length of hospital stay per infection, and the average cost per hospital day.
SOURCE: ECDC and EMEA (2009).
et al., 2010). To control for confounding factors among patient populations that can be measured, researchers use regression or other statistical analyses of randomly sampled populations. Weinstein added, however, that some potential confounding factors cannot be measured.
In an attempt to more precisely estimate the AMR-associated burden of disease, Weinstein and colleagues employed 3 different methods in a study of nearly 1,400 high-risk patients at their Chicago-area teaching hospital (Roberts et al., 2009). Among this group, 13.5 percent had antimicrobial-resistant infec-
tions (ARIs) that resulted in a total cost (medical costs plus societal costs of lost productivity due to death and disability) of more than $13 million.
Five factors accounted for nearly all the excess medical costs among patients with healthcare-acquired infections including ARIs, Weinstein said: use of the intensive care unit, laboratory tests, medications, blood transfusions, and radiology tests. The vast majority of hospital-acquired ARIs are associated with the use of such medical devices as catheters and ventilators, he said.
The rate of hospital-acquired resistant infections may be expected to grow given the increasing numbers of elderly patients in the United States and other developed countries as well as recent increases in the neonatal intensive care unit population, coupled with the concomitant rise in demand for surgery, transplantation, and chemotherapy (Boucher et al., 2009). Weinstein hoped that the future adoption and analysis of electronic medical records would not only provide better estimates of hospital service costs of ARIs but also support efforts to monitor and reduce those costs by controlling infections.
Indirect consequences of AMR for medical care Resistance among the ESKAPE pathogens largely results from antibiotic prescribing practices by hospital physicians, according to speaker Louis Rice from the Louis Stokes Cleveland VA Medical Center and Case Western Reserve University School of Medicine. (Dr. Rice’s contribution to the workshop summary report can be found in Appendix A, pages 301-307.) In addition to the previously described health and economic costs associated with resistance in hospital-acquired infections, Rice noted that AMR negatively affects the ability of hospital physicians to care for patients in the following ways:
-
AMR has rendered formerly beneficial therapies, such as prophylactic antibiotic treatment to reduce neutropenia14 in oncology patients, useless.
-
Regional variations in AMR among various pathogens complicate the interpretation of treatment guidelines for infectious diseases.
-
Physicians use less-effective second-line antibiotics to treat resistant infections, for example, vancomycin or daptomycin for β-lactam-resistant Staphylococcus aureus infections (Fowler et al., 2006; Kim et al., 2008).
-
For severe cases of multidrug-resistant infections, physicians resort to using agents such as polymixin B and colistin that are more toxic and less well characterized than standard antimicrobial therapeutics, and to which resistance can also develop (Antoniadou et al., 2007).
TABLE WO-2 Major Antimicrobial Agent Classes Approved for Non-Therapeutic Use in Animals
|
Antimicrobial Class |
Species |
Prophylaxis |
Growth Promotion |
|
Aminoglycoside |
Beef cattle, goats, poultry, sheep, swine |
Yes |
No |
|
β-Lactam (penicillin) |
Beef cattle, dairy cows, fowl, poultry, sheep, swine |
Yes |
Yes |
|
β-Lactam (cephalosporin) |
Beef cattle, dairy cows, poultry, sheep, swine |
Yes |
No |
|
Ionophore |
Beef cattle, fowl, goats, poultry, rabbits, sheep |
Yes |
Yes |
|
Lincosamide |
Poultry, swine |
Yes |
Yes |
|
Macrolide |
Beef cattle, poultry, swine |
Yes |
Yes |
|
Polypeptide |
Fowl, poultry, swine |
Yes |
Yes |
|
Streptogramin |
Beef cattle, poultry, swine |
Yes |
Yes |
|
Sulfonamide |
Beef cattle, poultry, swine |
Yes |
Yes |
|
Tetracycline |
Beef cattle, dairy cows, fowl, honey bees, poultry, sheep, swine |
Yes |
Yes |
|
Other |
|
|
|
|
Bambermycins |
Beef cattle, poultry, swine |
Yes |
Yes |
|
Carbadox |
Swine |
Yes |
Yes |
|
Novobiocin |
Fowl, poultry |
Yes |
No |
|
Spectinomycin |
Poultry, swine |
Yes |
No |
|
SOURCE: GAO (1999). |
|||
Food Animal Production
Many classes of antimicrobial agents, originally developed to treat human diseases, are also used in food animal production. As illustrated in Table WO-2, the major antimicrobial agent classes approved for non-therapeutic uses in animal agriculture include, but are not limited to, polypeptide antibiotics,15 tetracyclines, macrolides, penicillins, quinolones, and sulfonamides.
The widespread use of these powerful and persistent chemical agents in livestock production operations, aquaculture, and agriculture is associated with the emergence of drug-resistant infections in these settings and has been linked to the establishment and spread of drug-resistant infections in humans (Heuer et al., 2009; IOM, 2003; Silbergeld et al., 2008). The largest non-human use of antimicrobial agents is in food animal production,16 and most of this is in healthy
animals in order to increase growth or prevent diseases (Shea, 2003). Non-therapeutic uses of antimicrobials are believed to promote weight gain, increase the meat yield per pound of feed used, and prevent the spread of infections in feedlots (OTA, 1979), which are a significant risk in the crowded conditions in which livestock and poultry are typically raised (Silbergeld et al., 2008).
According to speaker Jørgen Schlundt, former director of the World Health Organization’s (WHO’s) Department of Food Safety and Zoonoses,17 three key observations suggest that antimicrobial use in animals affects human health. (Dr. Schlundt’s contribution to the workshop summary report can be found in Appendix A, pages 308-326.)
-
Most foodborne diseases are zoonoses (infectious diseases that can be transmitted from vertebrate animals to humans).
-
The use of antimicrobials in food animals selects for zoonotic bacteria that can transfer resistance genes to human pathogens. This observation is based on several instances in which the rise in human infections resistant to a specific antimicrobial compound followed its introduction or expanded use in animals (Threlfall et al., 1997, 1998; Webster, 2009; Wulf and Voss, 2008), and additional instances of decline in resistant human infections following a ban or restriction in the use of an antimicrobial (Dutil et al., 2010). While these consequences have been most directly linked to foodborne diseases, Schlundt acknowledged that antimicrobial use may have a significant indirect impact on human health by expanding reservoirs of resistance (Serrano, 2005; Smith et al., 2009).
-
Foodborne diseases involving resistant bacteria have been associated with an increase in adverse human health consequences. These include more frequent treatment failure, greater severity of infection, prolonged duration of infection, more bloodstream infections, and, as previously noted, longer hospitalizations and increased mortality, Schlundt said.
While some workshop participants suggested that more spacious accommodations for livestock and poultry could provide similar benefits to prophylactic antibiotic treatment, others noted that crowding per se would not be a problem if infection control measures were properly taken and consistently practiced. The choice of foodstuff might also influence the development of AMR in livestock. David Pimentel suggested, for example, that grass-fed cattle tended to be less vulnerable to infection than grain-fed cattle.
Use of antimicrobials as animal growth promotants in the United States As was previously noted in Table WO-2, at least 17 classes of antimicrobial agents are approved for use as animal growth promotants in the United States (Angulo and Nunnery, 2004), including tetracyclines, penicillins, macrolides, and analogs of other antibiotics used to treat human infections. No public health reporting system exists in the United States to track the use of antimicrobial drugs in livestock production operations (Shea, 2003). Government food safety studies, however, routinely detect antimicrobial-resistant bacteria in beef, chicken, and pork sold in supermarkets in the United States and Europe (Mason and Mendoza, 2009; Silbergeld et al., 2008).
Pimentel reported that more than 70 percent of antibiotics used in the United States are consumed by livestock. He contended that the annual U.S. expenditure of between $1.2 and $2.5 billion for these drugs—which on average increase an animal’s weight by 2 to 5 percent—represents a mere fraction of the true cost of their use. Accounting for deleterious effects on the environment and on human health, Pimentel estimated that the use of growth-promoting antibiotics costs the United States at least $20 billion per year.
Danish ban on growth-promoting antibiotics Livestock and poultry producers, feed manufacturers, and other interested parties assert that antimicrobials reduce the cost of raising meat animals, and therefore the price of food, but an impartial investigation and analysis of the true costs and benefits of using growth-promoting antimicrobials has yet to be conducted (Graham et al., 2007). Workshop participants therefore considered an imperfect, but informative, substitute for such a study: the results of a decade-long ban on the non-therapeutic use of antimicrobials in food animals in Denmark. As depicted in Figure WO-11, this phased ban began in 1994 and was completed in 1999.
Schlundt reported that after the ban took effect, Danish pork production increased continuously as antimicrobial consumption per kilogram of pork produced declined (see Box WO-1).
This statistic belies some important details, however, as several workshop participants pointed out. From 1992, the peak year of antibiotic use for the purpose of growth promotion in swine, to 2008, overall antibiotic use declined substantially—by over 50 percent—as a result of the ban in Denmark. Speaker Shelley Hearne, of the Pew Charitable Trusts, pointed out that U.S. industry has expressed alarm over increased treatment of diarrhea and a rise in mortality in piglets in the years immediately following the ban. (Dr. Hearne’s contribution to the workshop summary report can be found in Appendix A, pages 174-190.) The WHO found that diarrhea in young pigs did increase following the ban, creating a short-term need to increase therapeutic antibiotic use. However, levels of diarrhea treatment began to decline after 7 months and were back to the pre-ban levels after 1 year. Moreover, piglet mortality has improved considerably in recent years. According to Danish industry representatives, minor changes in animal husbandry, such as more frequent
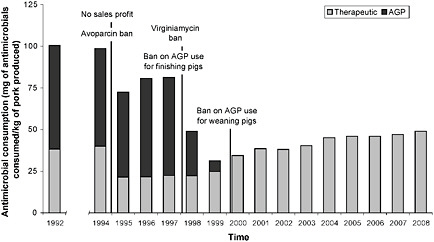
FIGURE WO-11 Danish experience after growth promoter ban. Antimicrobial consumption per kilogram of pork produced. AGP = antimicrobial growth promoters.
SOURCE: Adadpted from Aarestrup et al. (2010); 71(7):726, Fig 2, p. 730) with permission of the AVMA.
cleaning of housing, improved ventilation, later weaning, additional space for animal movement, as well as experimenting with feed quality and additives made up for the lack of routine antibiotics on most farms. The United States has an effective model in Denmark to draw upon when it comes to protecting public health.
The most significant impact of agricultural antimicrobial use may be the expansion of reservoirs of resistance, through the transfer of resistance genes within and across microbial communities (Smith et al., 2009). Ultimately, greater quantities of antimicrobial compounds in the environment are likely to cause greater harm. The extent of this harm remains to be determined, however, along with other potential outcomes of the Danish experiment, such as whether this attempt to reduce the non-therapeutic use of antimicrobials in food animals has resulted in fewer resistant bacterial infections in humans.
Antimicrobial Treatment of Crop Plants
Fungi and viruses pose a greater threat to most crop plants than bacteria (Vidaver, 2002). Selective breeding of plants for disease resistance has reduced, but not eliminated, the economic impacts of several fungal and viral plant pathogens. However, fungicides are increasingly being used to “control” many crop diseases, resulting in the development of fungicide resistance. Strategies for managing fungicide resistance, as with antimicrobial resistance, are aimed at delaying its development (Damicone and Smith, 2009).
Antimicrobials are primarily used for disease prevention in fruit trees, which are typically sprayed with streptomycin or oxytetracycline (Vidaver, 2002). Widespread resistance to streptomycin has been found among bacterial phytopathogens, but no resistance among these bacteria has yet been reported for oxytetracycline. Alternatives such as biocontrol agents, transgenic plants, and novel chemicals are being developed to avoid the high costs and environmental concerns associated with the use of antimicrobials on crop plants.
Antibiotics in Aquaculture
Antibiotics are used in the farming of fish and crustaceans much as they are used for poultry, cattle, and pigs: to prevent disease (and thereby promote growth and increase yield) and to treat infections (Serrano, 2005). No antibiotic has been specifically designed for aquaculture applications. Instead, the fish farming industry employs many of the same drugs used in livestock production (and in veterinary and human medicine), typically combining them with feed.
Research suggests that more than 70 percent of the antibiotics used in aquaculture operations wind up in the environment (Serrano, 2005). Excess feed, along with drug-containing excrement, accumulates in sediments below fish pens in natural waterways and in the bottoms of man-made ponds, exposing bacterial populations present in those hydrosoils to antibiotics. Data strongly suggest
that horizontal transfer of resistance genes on plasmids has been demonstrated between bacteria in the water of fishponds and in marine sediments. Farmed salmon may be raised in open-ocean pens located in bays and fjords, into which antibiotic-laden fish food is added,18 allowing resistant bacteria to flow freely out of these permeable pens to the larger ocean environment.
Resistant bacteria present on live and uncooked fish and shellfish—and on meat and poultry—can infect humans who touch or consume these products. In vitro experiments have demonstrated that plasmids carrying resistance determinants can be transferred from fish pathogens to human pathogens, including Vibrio cholerae and Vibrio parahemolyticus (Angulo, 1999). During the 1991 cholera epidemic in Latin America, V. cholerae isolates from Peru, where the epidemic began among shrimp farm workers, were found to be uniquely multidrug-resistant. Multidrug resistance was also detected in noncholera Vibrio pathogens infecting the shrimp. It has been hypothesized that these microbes may have transferred resistance to the epidemic cholera strain (Serrano, 2005; Weber et al., 1994).
Wastewater Treatment Plants: Resistance Reactors
The flow of water links ecosystems, providing myriad opportunities for the exchange of resistance genes within and among microbial communities (Choi, 2007; Davies, 2009). Davies noted that wastewater treatment plants serve as particularly effective mixing vessels for bacteria and their associated plasmids. Several recent reports described the isolation and sequencing of antibiotic multidrug resistant plasmids from bacteria present in sewage sludges derived from wastewater treatment facilities and in effluents released from the treatment plant into the environment (Szczepanowski et al., 2004, 2005, 2008; Tennstedt et al., 2005). Davies reported that bacterial isolates from a single wastewater treatment plant in Germany contained 140 different antibiotic resistance genes (Szczepanowski et al., 2009).
Figure WO-12 depicts a network of genetic “reactors”—including the microbiota of individual animals, as well as larger ecosystems such as farms, aquaculture facilities, and hospitals—that amplify and distribute antimicrobial resistance genes. Davies observed that resistance genes recycle constantly among these reactors, driven by varying selective pressures imposed by antimicrobials in any given environment.
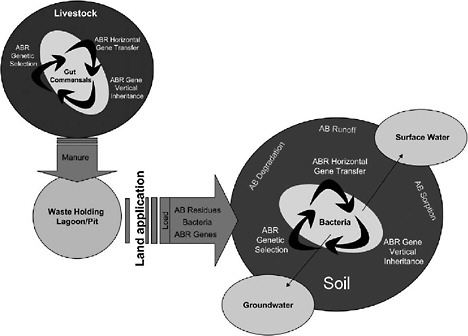
FIGURE WO-12 Conceptualized view showing the possible fates of antibiotic residues and mechanisms of antibiotic resistance gene acquisition and dissemination by bacteria, beginning with land application of animal waste as the source of entry of drugs, bacteria, and resistance genes into the soil environment. AB = antibiotic, ABR = antibiotic resistance.
SOURCE: Reprinted from Chee-Stanford et al. (2009) with permission from the Journal of Environmental Quality.
Population Mobility, Globalization, and AMR
While much of the workshop focused on the ways in which the discovery, development, and widespread use of antimicrobial drugs has accelerated the emergence of resistant pathogens, this trend has also been facilitated by a coincident increase in human mobility coupled with increased globalization (MacPherson et al., 2009). Today, international travel and commerce (most notably the explosive growth of commercial air transportation over the past 50 years) drives the heterogeneous global distribution of microbial pathogens and the organisms that harbor them (IOM, 2003, 2010). Travel is increasingly rapid, more socially widespread, and more ubiquitous, connecting once-remote areas (which serve as both “sources” and “sinks” for emerging infectious diseases) to more developed regions on the planet. International trade in food and other agricultural commodities, as well as in wildlife, has also markedly increased among an ever-widening network of producers and markets.
Concomitant increases in human mobility and AMR have elevated the risk to patient care and public health associated with resistant infections, observed speaker Douglas MacPherson of McMaster University. (Dr. MacPherson’s contribution to the workshop summary report can be found in Appendix A, pages 257-287.) These risks are frequently viewed from the perspective of the pathogen and addressed as specific diseases or syndromes, he said. In MacPherson’s view, current circumstances call for a more integrated and harmonized approach to the hazards to human and animal health presented by resistant pathogens (among other microbial threats) in a world that presents few barriers to the movement of resistant microorganisms. He and coauthors have advised that “a shift in the existing paradigm of pathogen-focused policies and programs to address population mobility, as a part of a multi-factorial approach to the determinants of globalization of threats and risks, will contribute to a healthier future for everyone” (MacPherson et al., 2009).
Future Trends in AMR
Having reviewed a wealth of evidence showing the inevitable development of AMR from a range of perspectives, workshop participants examined the potential impacts of AMR in the immediate future. Economist Ramanan Laxminarayan, of Resources for the Future and Princeton University, observed that such predictions are hampered by problems inherent in directly measuring AMR-associated health and economic costs, as previously described by Weinstein. (Dr. Laxminarayan’s contribution to the workshop summary report can be found in Appendix A, pages 190-221.) Laxminarayan added, however, that it is equally difficult to calculate the direct health and economic benefits of antimicrobial drugs. If penicillin had never been introduced, he mused, might improvements in infection control eventually have reduced infectious disease mortality to current levels? “It’s easy to slack off infection control if you think that the patient can be treated with an antibiotic,” he observed, echoing an argument that other workshop participants raised in favor of limiting prescription access to generic antibiotics. On the other hand, he acknowledged, the availability of antimicrobials largely makes possible medical interventions such as organ transplantation; between 1998 and 2007, 20,000 organ transplantations, on average, were performed per year (American Journal of Transplantation, 2009).
Leaving aside the problems of quantification, Laxminarayan considered several important trends in AMR development and impact, and what they imply for the next 5 to 10 years. In the United States and other developed countries, he predicted, increasing AMR is likely to raise healthcare costs, but not necessarily mortality, as physicians substitute newer, more expensive drugs as first-line therapy for resistant infections (often without evidence that it is needed). Meanwhile, he said, combined increases in wealth and access to antimicrobials in developing
countries will save lives in some places, but result in vastly increasing AMR where antimicrobials are used inappropriately. Subsequent workshop presentations and discussions identified opportunities to shape the more distant future by mitigating the health and economic consequences of AMR through the development of novel therapeutics and by managing these and existing antimicrobials in order to preserve their effectiveness.
Staying Ahead of AMR
Speaker Brad Spellberg of the University of California, Los Angeles, and colleagues have observed that “we will never truly defeat microbial resistance; we can only keep pace with it” (Spellberg et al., 2008a). (Dr. Spellberg’s contribution to the workshop summary report can be found in Appendix A, pages 326-365.) Their publication, along with many other analyses of AMR (American Academy of Microbiology, 2009; Center for Global Development, 2010; Interagency Task Force on Antimicrobial Resistance, 2001; OTA, 1979; Spellberg et al., 2008a; Tenover and Hughes, 1996; WHO, 2001a), recommends preserving the effectiveness of existing antimicrobials as long as possible while encouraging the development of new classes of antimicrobials and alternative therapeutic strategies to address infectious diseases. Strategies to accomplish these goals were discussed throughout the workshop and were the focus of a session entitled “Novel approaches for drug discovery, development, and mitigation of resistance.”
Preserving Antimicrobial Effectiveness
Investigators and policy makers generally agree upon at least three key steps that must be taken in order to prevent the development and spread of resistance to existing and future antimicrobials: (1) limit their use, (2) discourage their misuse, and (3) reduce the burden of infectious disease through preventive hygiene and infection control practices (ICIUM, 2004a, 2004b; Weinstein, 2001). In 2001, the European Union’s (EU’s) ministers of health adopted several measures specifically aimed at containing the spread of resistance by encouraging the prudent use of antimicrobial agents (Monnet and Kristinsson, 2008). In recent years, a series of published reports suggested that significant progress had been made toward this goal through such efforts as:
-
training physicians in good prescribing practice (Jindrak et al., 2008; Molstad et al., 2008),
-
public education campaigns (Goossens et al., 2008; Huttner et al., 2010; Molstad et al., 2008; Prins et al., 2008), and
-
improved infection control in the community and in hospitals (Anonymous, 2008; Goossens et al., 2008).
In the United States, efforts to control antimicrobial resistance have been primarily directed to the hospital environment. These efforts have included (1) improvements in environmental and hand hygiene (Bleasdale et al., 2007; Hayden et al., 2006; Munoz-Price and Weinstein, 2008; Weinstein, 2001), (2) screening programs to identify incoming and outgoing cases of MRSA infection (Harbarth et al., 2008; Robicsek et al., 2008), and (3) interventions to improve and/or restrict antibiotic use, such as the use of computer-based order entry systems to direct prescribing behavior (MacDougall and Polk, 2005; Weinstein, 2001). Some of the strategies that were discussed to preserve the effectiveness of current antimicrobial therapies included the following:
-
developing evidence-based standards for prudent antimicrobial use;
-
the use of susceptibility testing and the development of additional diagnostics to inform therapeutic choices; and
-
prohibiting the non-therapeutic use of antimicrobials in animals and reserving critical antimicrobials for human use.
Prudent use Antimicrobial stewardship—using these drugs to maximize their efficiency while limiting opportunities for resistance to develop—is the best short-term approach to mitigating the impact of AMR, Rice asserted. He went on to suggest that efforts to support the prudent use of antimicrobials had been hampered by the lack of data regarding the effectiveness of specific measures, coupled with the general perception by physicians that antibiotics represent, at worst, a “therapeutically neutral” treatment choice for infectious disease.
Rice dismissed the notion that narrow-spectrum antibiotics would reduce selective pressure and thereby limit resistance, arguing that no existing antibiotic targets a truly narrow group of pathogens. Rather, “the only truly convincing streamlining [of antimicrobial treatment] is stopping [use],” he said, since evidence does suggest that short therapeutic courses reduce the development of resistance. A study comparing 8- and 15-day courses of antibiotic therapy for patients being treated for ventilator-associated pneumonia, for example, found not only no difference in efficacy of treatment, but fewer resistant pathogens in the shorter treatment group among those who developed recurrent pulmonary infections (Chastre et al., 2003). Another study of similar patients reported that those who received a 3-day treatment course of ciprofloxacin were less than half as likely to develop resistant infections, superinfections, or both when compared to patients who received a standard course of therapy that lasted an average of 9 days with antibiotics chosen by their physicians (Singh et al., 2000).
“Let’s just establish a dose and treat for a short period of time,” Rice concluded, “then we should be able to reduce the overall selective pressure that is being exerted by antimicrobial therapy in the hospital by a lot.” Currently, there are limited data upon which to base prescribing decisions regarding the “optimal” length of antimicrobial treatment. Rice noted, however, that several such studies
were recently funded by the National Institutes of Allergy and Infectious Diseases (NIAID) to address this data gap.
Susceptibility testing and diagnostic development Physicians treating resistant cases of infectious disease turn to antimicrobial susceptibility tests performed in hospital laboratories to inform their choice of therapeutic agent (Holland et al., 2009). Speaker Fred Tenover, of Cepheid, noted that hospital laboratories also employ these tests to compile data on local patterns of AMR in individual microbial species, typically on an annual basis. (Dr. Tenover’s contribution to the workshop summary report can be found in Appendix A, pages 365-379.) Physicians regularly consult these test results, known as antibiograms,19 to guide initial treatment decisions for patients who exhibit symptoms of infection.
Tenover reported that a decade-long review of antimicrobial susceptibility testing by the Centers for Disease Control and Prevention (CDC) concluded that most laboratories produce accurate test results for antimicrobial agents against common bacterial species (Chaitram et al., 2003; Tenover et al., 2001). Many of these same laboratories, however, proved less proficient at identifying rapidly emerging organisms—such as vancomycin-resistant Staphylococcus aureus (VRSA), extended-spectrum β-lactamase (ESBL) producers, and Klebsiellas that produce carbapenemase (Steward et al., 2003; Tenover et al., 2004)—and he went on to identify several factors that have contributed to this inaccuracy, including
-
the presence of marginally resistant pathogens that, although difficult to detect, prove clinically significant;
-
the increasing emergence of multiply resistant organisms (e.g., ESBLs containing several β-lactamases); and
-
lags of months to years in adapting standards and automated systems to enable them to identify recently emerged resistant pathogens.
In the United States, guidelines for performing susceptibility testing are established by the Clinical and Laboratory Standards Institute and are revised periodically to reflect emerging resistance trends, Tenover said. He observed that current susceptibility testing protocols generally work well, but they must be updated to reflect every novel AMR strain as it emerges. Tenover also noted that, while susceptibility testing could, in theory, be used to conduct surveillance for the presence of AMR in potential animal reservoirs or in the environment, such applications have been rare, and the data that have been generated appear to have had little impact on medical practices or policy.
Several workshop participants observed that the development and use of susceptibility testing and other diagnostics are crucial to addressing the rise and
expansion of AMR, particularly as antibiotics become increasingly expensive—so much so that it might be cost effective to use susceptibility testing to rule out potential treatments, as well as rule them in. An audience member from Doctors Without Borders suggested that his organization’s experience with treating malaria in developing countries anticipates the benefits of improved diagnostics for AMR pathogens. When susceptibility testing is used to individually tailor antimalarial treatment, he said, they also achieved greater precision, and therefore efficacy, of the treatment.
Regulating antimicrobial use in animals Schlundt described a series of recommendations made by the WHO in response to mounting evidence that non-therapeutic antimicrobial use in livestock encourages the development of AMR in human pathogens. Following an initial call, in 1997, to monitor AMR in food animals and food of animal origin and to manage associated risks, the WHO developed principles for the containment of AMR in food animals (WHO, 2000b). These principles included the termination or rapid phase-out of antimicrobial growth promoters, active surveillance for AMR and antimicrobial use practices to inform national policies on AMR containment, and monitoring bacteria isolated from animals, food of animal origin, and humans for AMR.
An IOM report that looked broadly at microbial threats to health examined the widespread use of antimicrobials in livestock production operations, aquaculture, and agriculture; the emergence of drug-resistant infections in these settings; and the spread of drug-resistant infections to humans (IOM, 2003). Among other recommendations the committee, co-chaired by the late Joshua Lederberg and by Margaret Hamburg, called for the Food and Drug Administration (FDA) to ban the non-therapeutic uses of antimicrobials for animal growth promotion if those classes of drugs were also used in human clinical medicine.
A recent WHO ranking of antimicrobials according to their importance in human medicine could inform efforts to reserve critical classes of antimicrobial drugs exclusively for human use (Collignon et al., 2009). While this strategy has been discussed for some time and should, in theory, be effective, participants considered it impractical in the short term. Davies noted that daptomycin, an antibiotic used exclusively in humans, has been in use for about a decade without the development of transferable drug resistance, despite the known existence of resistance genes.
Developing Novel Antimicrobials
Novel antimicrobial drugs are needed for a number of reasons, including treating chronic infections. However, there has been a gap in the development of new antimicrobials in the past several decades, leading to the search for alternatives to antimicrobial drugs.
FIGURE WO-13 Systemic (i.e., non-topical) antibacterial new molecular entities approved by the FDA, per 5-year period.
SOURCE: Reprinted from Clinical Infectious Diseases, Spellberg et al. (2008a), published by The University of Chicago Press. © 2007 by the Infectious Diseases Society of America. All rights reserved.
Arrested development Despite the rising need for new antimicrobial drugs, as illustrated in Figure WO-13, there has been a several-decades-long gap in new drug development (Boucher et al., 2009; Spellberg et al., 2008a). A recent EU report (ECDC and EMEA, 2009) stated that the current pipeline for antibacterial drugs with the potential to offer a benefit over existing medications consists of just 15 candidates. Of these, only two drugs, both at an early stage of development, feature new targets or mechanisms of action against multidrug-resistant, Gram-negative bacteria.
In his workshop presentation, Spellberg noted several well-known factors that make antimicrobials unattractive development prospects for pharmaceutical companies:
-
the high cost of drug development;
-
the short duration of typical antimicrobial therapy as compared with treatments for chronic conditions, such as high blood pressure; and
-
difficulties with the FDA approval process specific to anti-infective drugs.
Spellberg’s remarks focused on the FDA approval process as, in his opinion, the most significant obstacle to developing novel antimicrobial drugs in the United States. While antibiotics once “sailed through” the FDA approval process, he said, “six of the seven antibacterials that have come up before the FDA have not been approved, which has to be the first time in the history of the agency that that has ever been the case.”
These approval failures reflect the near impossibility of evaluating antibacterial drugs in trials that compare experimental drugs with agents that have previously proven superior to placebo in randomized, placebo-controlled trials since several important antibiotics predate the advent of such trials (see Box WO-2).
Spellberg suggested, instead, that experimental antimicrobials be compared with agents of known effectiveness on the basis of such clinical endpoints as the resolution of the signs or symptoms of infection. The FDA is currently reviewing this and other approaches for antibacterial drug trials, according to Ed Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Spellberg also raised the issue of antimicrobial classification by the FDA as a possible roadblock to development. If the FDA approves antiretrovirals for the treatment of HIV (not HIV in the lung or HIV in the skin) and antifungals for the treatment of invasive organisms (such as candidiasis or aspergillosis), why does the agency not apply the same logic to the approval of antibacterials for the treatment of diseases such as pneumonia and skin infections?20 This system encourages pharmaceutical companies to pursue drugs for diseases such as skin infections that already have plenty of treatment options, Spellberg argued, rather than seek much-needed treatments for pathogens such as highly resistant Gram-negative pathogens.
Chronic infections In addition to the mounting problem of AMR, novel anti-microbials are needed to address the similar but distinct challenge of chronic infections. In these paradoxical cases, exemplified by a range of diseases caused by bacterial biofilms,21 antibiotics have only limited efficacy against susceptible cells, according to speaker Kim Lewis of Northeastern University. (Dr. Lewis’ contribution to the workshop summary report can be found in Appendix A, pages 233-256.) Biofilm diseases include pediatric infections of the middle ear by Haemophilus influenzae, dental diseases caused by Streptococcus and Actinomyces, infection of medical devices such as catheters and prosthetic hips and knees by Staphylococcus aureus and S. epidermidis, endocarditis, and infections in cystic fibrosis caused by Pseudomonas aeruginosa. An estimated 65 percent of all infections in developed countries are caused by biofilms (Lewis, 2007).
When a biofilm is treated with low concentrations of an appropriate antibiotic, the vast majority of cells die, but a small fraction persist and repopulate the biofilm, thereby sustaining infection, Lewis explained. These “persister” cells are not mutants, but phenotypic variants that are dormant, and therefore immune to antibiotic assault (Shah et al., 2006). Unlike resistant cells—which prevent bactericidal antibiotics from binding to their targets—persisters are tolerant of
antibiotics because target molecules are inactive as a result of dormancy. “In order to understand tolerance, we need to appreciate that bactericidal antibiotics kill not by stopping functions, but by creating either corrupted products or toxic products that then kill the cell,” Lewis observed. “If the target is inactive, there will be no corrupted or toxic product, and no death” (Kohanski et al., 2010b; Lewis, 2007). The small proportion of biofilm cells that are persisters, therefore, function as a pathogen refuge in the presence of antibiotic.
What causes persisters to assume this protected but unproductive state? Lewis’s group examined intracellular toxins known to induce dormancy, and they found that these molecules also rendered cells highly tolerant to antibiotics
|
BOX WO-2 FDA Trials for Antimicrobial Drugs: Plugging the Pipeline? The FDA currently uses a model known as noninferiority to evaluate virtually all experimental drugs for indications for which treatments already exist.a In a non-inferiority trial, patients are randomly assigned to one of two groups: one group receives a standard comparator drug already on the market; the other group receives the experimental drug. There is no direct comparison to placebo. If the two drugs prove similarly effective, there are two possible interpretations: either both drugs are better than placebo or neither drug is better than placebo. However, if the comparator drug has been demonstrated to be superior to placebo in previously conducted randomized, controlled studies, and the experimental drug is then shown to be noninferior in efficacy to the comparator drug, then the experimental drug can be inferred to be superior to placebo as well. For this reason, the FDA has come to insist that comparator drugs used in noninferiority trials be previously shown to be superior in efficacy to placebo. This policy has far-reaching implications for the approval of novel antimicrobial drugs, according to Spellberg. Since the first antibiotics predate the advent of randomized, placebo-controlled studies by two decades (unlike most other drug classes, which were subject to placebo-controlled studies from the outset), and these antibiotics are unquestionably effective, it has never been ethical to test antibiotics against a placebo. Similar arguments complicate so-called superiority trials for antibiotics, which determine whether an experimental drug performs better than an approved comparator drug. A new antibiotic would most likely prove superior in patients who are infected with bacteria resistant to the comparator drug, but it would be unethical to enroll such patients in a trial in which some would receive a useless treatment for a resistant infection. “That’s like taking a patient with methicillin-resistant Staphylococcus aureus and giving them a 50 percent chance of being treated with methicillin,” Spellberg said. “You can’t [ethically] do that study.” These dilemmas could be overcome, Spellberg said, by using analyses performed on early antibiotics in lieu of placebo-controlled randomized studies, so that these drugs could serve as comparators in noninferiority trials of novel antibiotics. He stated that between 1936 and 1950 at least 15 studies of antibacterial agents (sulfonamides or penicillin) were conducted on patients with community-acquired pneumonia (primarily but not exclusively pneumococcal in etiology), then a leading cause of mortality in the United States. Although neither randomized |
(Schumacher et al., 2009). One such toxin, called TisB, is activated by the bacterial SOS response, which (as previously described) also increases mutation rates, and, thereby, opportunities for antibiotic resistance to emerge (Dörr et al., 2009, 2010). Lewis observed that, when sublethal antibiotic exposures trigger the SOS response, it can lead to the creation of persisters that are multidrug tolerant.
He and coworkers then analyzed pathogen isolates from patients with chronic infections, whose exposure to periodic high doses of antibiotics would be expected to select for comparatively high levels of persistence (Lafleur et al., 2010; Lewis, 2007; Mulcahy et al., 2010). This is indeed what the researchers found, Lewis said, and these results clearly demonstrate that the ability to make persisters plays
|
in the modern sense nor placebo controlled, these studies were sufficiently controlled to permit valid comparisons between patients who received antibiotics and those who did not, Spellberg asserted. Every study showed a significant decline in pneumonia mortality among patients given antibiotics (Spellberg et al., 2008b). Another point of contention regarding the FDA approval process for antibiotics involves the choice of trial endpoints. Spellberg observed that some consider mortality to be the only acceptable endpoint for trials of potentially lifesaving drugs for syndromes such as pneumonia, although such clinical trial endpoints may be problematic. In theory, the clinical effects of antibiotics must be more significant than their effects on mortality, because “dead people don’t clinically respond,” he said. Practically, antibiotic trials using mortality as an endpoint for pneumonia would require huge enrollments, because mortality rates for that disease are less than 5 percent. “That means you’re going to need 5,000 patients in a study to adequately power the study,” he continued. “You have to do 2 of those studies to get an indication, so you need to enroll 10,000 patients into a Phase III program. That will cost $500 million and will take 5 to 10 years to enroll.” “The critics believe that mortality is the most sensitive endpoint to detect a relatively ineffective drug and that if you don’t use mortality, you increase the risk of approving a relatively ineffective drug,” Spellberg observed. He contended, on the contrary, that relatively ineffective drugs (such as sulfonamides) can have huge mortality benefits, and that noninferiority studies using such clinical endpoints as symptom resolution have demonstrated when drugs are ineffective, as is the case with daptomycin, when partially inactivated by surfactant in the lung, or with tigecycline, when hypermetabolized in patients with ventilator-associated pneumonia. “The FDA simply has to move past radical skeptics and use available data to enable antibiotic noninferiority studies with clinical endpoints,” Spellberg asserted, even if it requires a statutory change recognizing the uniqueness of antibiotics. “Antibiotics are the only class of drugs that loses efficacy over time,” he concluded. “If you do not continually replace them, you will end up not having effective drugs.” SOURCE: Spellberg et al. (2008b).
|
a key role in infection, and one distinct from resistance. “In acute infection, it is very important for the pathogen to be able to have resistance, both intrinsic and acquired,” Lewis explained. He went on to observe that chronic infections favor persister cells and tolerance, both of which are reinforced by selective pressure in the form of repeated high doses of antibiotic.
“Rescue drugs” and novel antibiotics Considerable discussion focused on the question of whether the antibiotic “development gap”—as previously depicted in Figure WO-13 (Boucher et al., 2009; Spellberg et al., 2008a)—represents a natural, and thus insurmountable, barrier. Some workshop participants speculated that the proverbial “low-hanging fruit” of drug-worthy compounds has already been developed and is likely to fail due to AMR. Participants who adopted this view found greater promise in alternatives to antimicrobial drugs, such as vaccines or antibody therapy (as discussed in a subsequent section), or in strategies to rescue existing antibiotics from resistance, such as those described below by speaker James Collins of Boston University. (Dr. Collins’ contribution to the workshop summary report can be found in Appendix A, pages 116-140.) Other participants expressed a more sanguine view of prospects for new antimicrobials, including Michael Fischbach, of the University of California, San Francisco, whose presentation (discussed below) mapped several routes to novel drugs. (Dr. Fischbach’s contribution to the workshop summary report can be found in Appendix A, pages 160-174.)
Turning resistance off Collins’s group was part of the team that deduced that bactericidal antibiotics elicit the SOS response and stimulate oxidative damage associated with programmed cell death at high concentrations (Dwyer et al., 2007) and mutagenesis leading to resistance at sublethal concentrations (Kohanski et al. 2010a). Based on these observations, they screened compound libraries for molecules that might enhance bactericidal action by knocking out the SOS response. One of their “hits,” when combined with gentamicin, increased an antibiotic’s activity by 1,000-fold, Collins reported. Collins’s group also found small molecules that could increase the ability of bactericidal antibiotics to produce oxidative damage in target cells, which also enhanced antibiotic effectiveness.
To deliver the SOS-inhibiting system in conjunction with bactericidal antibiotic, Collins and coworkers developed a lysogenic bacteriophage22 system that proved quite efficient (Lu and Collins, 2009). His group, in collaboration with the Walter Reed Medical Institute and the U.S. Army, is exploring the feasibility of
|
22 |
A lysogenic phage is a “temperate” bacteriophage (such as lambda phage) that integrates its genome into the genome of the host without immediately transcribing and making new virus particles. However, at a later time, the integrated genome can be excised and begin to be actively transcribed, producing virus particles that eventually burst the cell. This is opposite of the “lytic” variety of bacteriophage (T4 phage) that immediately transcribe and make new virus after infecting the host cell, causing rapid lysis (http://wiki.answers.com/Q/What_is_a_lysogenic_bacteriophage [accessed on June 23, 2010]). |
using such adjuvant phage in combination with antibiotics to treat resistant Acinetobacter infections among U.S. soldiers returning from Iraq and Afghanistan.
Another version of this bacteriophage system offers promise for treating biofilm infections, such as those previously described by Lewis. As illustrated in Figure WO-14, these bacteriophage infect cells on the surface of the biofilm, where they launch a two-pronged attack by multiplying and lysing cells in an accelerating cascade, while an enzyme engineered on their surface breaks down the biofilm’s polysaccharide matrix (Lu and Collins, 2007).
New scaffolds increase novelty Fischbach described several potential paths that might lead to new antibiotics in the future (see Appendix A6). He observed that not only are there too few antibiotics available today, but the ones that are available are too similar to one another. Four major classes of antibiotics discovered between 1930 and 1970—the penicillins; the cephalosporins; the quinolones; and the macrolides—command about 80 percent of market share for these drugs. The chemical entity that defines each class of antibiotics, known as a scaffold, has been altered to produce several generations of drugs, each of which has forestalled resistance for ever-shorter periods, as illustrated in Figure WO-15.
Finding or creating antibiotics based on new scaffolds bodes well for eluding resistance for a longer time, Fischbach explained. “Look how important each [existing] scaffold has been for the treatment of bacterial infections,” he said. This approach to new drug discovery could also serve as a basis for generations of improved derivatives. His presentation highlighted several examples of new anti-
FIGURE WO-14 Modified bacteriophage enter and destroy the biofilm matrix.
SOURCE: Collins (2010).
FIGURE WO-15 Synthetic tailoring is widely used to create successive generations of antibiotic classes. Scaffolds are colored black; peripheral chemical modifications are colored red. The quinolone scaffold is synthetic, whereas the other scaffolds are natural products.
SOURCE: Reprinted from Fischbach and Walsh (2009) with permission from AAAS (see Appendix A6, pages 160-174).
biotic scaffolds, some of which were discovered in nature and others in synthetic small-molecule libraries amassed by pharmaceutical and chemical companies.
Lessons learned from previous antibiotic discovery efforts can inform the search for new scaffolds, Fischbach observed. This approach is shown in Figure WO-16.
The identification of novel targets, in the form of essential enzymes, from the genomic sequences of pathogens initially appeared to be a promising strategy, he said. High-throughput screening of synthetic compound libraries for inhibitors of these enzymes, however, has yet to lead to the development of a new antibiotic. In many cases, candidate compounds proved impossible to deliver to their intracellular targets. Fischbach advocated solving the delivery problem first, by screening compounds in whole-cell assays and then using genomic approaches23 to identify the intracellular targets that enable candidate compounds to kill pathogen cells. Taking this idea further, Lewis and coworkers have pioneered an automated “whole-animal” system that uses the roundworm Caenorhabditis elegans to screen libraries of compounds for antibiotic activity (Moy et al., 2006).
As to where to find compounds, Fischbach noted that current antibiotic scaffolds did not originate in antibiotic discovery programs but were serendipitously identified from various industrial synthetic chemical libraries. He advised researchers to look for large, diverse, chemical libraries to screen for antibiotic activity, such as those libraries developed by pharmaceutical companies to address a range of therapeutic areas.
Novel natural products The “low-hanging fruit” of natural antibiotics may already have been harvested, Fischbach acknowledged. Switching metaphors, he nevertheless asserted that the natural product well is far from dry and—employing the tools of genomics—could be plumbed far deeper, as depicted in Figure WO-17.
Because genes that encode for natural products are clustered on chromosomes, they are easy to find and, with the help of bioinformatics, their functions can often be inferred. The microbe that is used to produce commercial erythromycin, for example, contains many similar natural product gene clusters, but only one of them makes erythromycin; the products of the other clusters remain unknown. Perhaps under different growth conditions Fischbach speculated—echoing an earlier observation by Davies—the microbe will produce these mystery molecules. If so, new antibiotic scaffolds may be coaxed from this and other bacteria that have already been screened for antibiotic activity. “Maybe we don’t need to go to the corners of the Earth to find new soils to find new natural products,” he mused. “We just need to go to the backyard and then spend a little more time with each of the microbes that we collect in order to tickle them to make what they can already make.”
Another cryptic but plentiful potential source of novel antibiotics is the
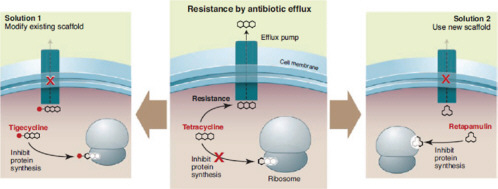
FIGURE WO-16 Surmounting resistance with scaffold alterations. Two ways of overcoming resistance are shown, using tetracycline (center) as an example. First, the tetracycline scaffold can be chemically modified, creating a tetracycline derivative like tigecycline that is no longer a substrate for the efflux pump (left). Second, a new scaffold like retapamulin, which is not a substrate for efflux and binds to a different site in the ribosome, can be used instead of tetracycline (right).
SOURCE: Reprinted from Fischbach and Walsh (2009) with permission from AAAS (see Appendix A6).
FIGURE WO-17 Mining genes for drugs.
SOURCE: Reprinted from Walsh and Fischbach (2009) in Scientific American with permission © 2009 Tolpa Studios, Inc. www.tolpa.com.
vast majority of species present in non-culturable bacterial communities, Lewis said. His group has devised ways to grow some of these elusive microbes, and in so doing has discovered that many are dependent on growth factors supplied by other community members, without which the unculturable cells remain dormant (D’Onofrio et al., 2010). Intrigued by this interesting parallel between unculturable bacteria and persisters, the researchers have launched a collaboration with a small biotech company capable of growing large numbers of previously unculturable cells using their method, which they are screening for antibiotic activity against Actinobacteria.24 “We get lots of very interesting compounds, very distantly related to known antibiotics,” Lewis said, adding that he expects that “from novel biology you will get novel chemistry.”
Indigenous bacterial communities must also produce antibiotics, Fischbach suggested. He noted that genomic analysis from the Human Microbiome Project has identified two bacterial species (one from the gut, the other on skin) with natural product gene clusters similar to those known to express a venerable class of antibiotics known as thiopeptides. “If microbes in our gut and microbes on our skin are producing antibiotics that are almost as potent as vancomycin,” he observed, “it could have a number of effects on the structure of the [microbiome] and on how we think about the antibiotics we take.”
The ideal antibiotic If one could envision an ideal antibiotic, Lewis observed, it would be a prodrug: a molecule that has no activity until it diffuses into a cell and is modified by a specific enzyme into a reactive product—in this case, a product that hits unrelated targets and causes cell death. Prodrug antibiotics would resolve multiple problems, he noted: no persisters could evade prodrugs, no penetration problems would occur with Gram-negative species, and the activity spectrum would be broad. Moreover, this mechanism of action has been tested and found robust in the antimicrobial metronidazole, and in several anti-tuberculosis drugs. Speaker Stuart Levy, of Tufts University and the Alliance for Prudent Use of Antibiotics (APUA), added another feature to the antibiotic wish list: the capacity for self-destruction. (Dr. Levy’s contribution to the workshop summary report can be found in Appendix A, pages 222-232.) “Antibiotics that self-destruct after they do their job in the body would be fantastic,” he said, “because, personally, I think most if not a lot of resistance is occurring outside the body.”
Alternatives to Antimicrobials
To some investigators, the inevitability of AMR represents a fatal short-coming of antimicrobial therapy as it has been practiced since the advent of penicillin and is a reason to seek alternative approaches to treating infectious
diseases (Casadevall, 1996). According to speaker Arturo Casadevall of the Albert Einstein College of Medicine, “a fundamental decision that was made in the mid-20th century is responsible for a lot of the problems that we have, and that is the development of nonspecific therapies.” (Dr. Casadevall’s contribution to the workshop summary report can be found in Appendix A, pages 75-82.) The legacy of cheap, effective, well-tolerated, broad-spectrum antimicrobials is not limited to AMR, he said, but also includes damage to indigenous microbial communities, such as the human intestinal microbiota, and the phenomenon of superinfection (a secondary infection that occurs during treatment for infection by a different pathogen). Casadevall further observed that broad-spectrum anti-microbials have created a culture of empiricism in medicine, in which treatment choices are determined by guesswork rather than by diagnosis. He added that this trend has accelerated the “vicious spiral” of treating ever-increasing AMR with drugs of ever-decreasing specificity, which in turn has discouraged the development of pathogen-specific therapy.
While he acknowledged that broad-spectrum antibiotics have undeniably benefited individuals by providing rapid and effective life-saving therapy for microbial diseases, Casadevall expressed skepticism about their overall benefit to society. He wondered whether antibiotic-induced damage to the human microbiota might underlie four recent and detrimental health trends: increasing rates of obesity, autism, asthma, and atopy, such as peanut allergy. He noted, moreover, the associations between some types of cancer and prior antimicrobial therapy, observing that the common denominator in these associations may be damage to normal microbiota. “If one of those associations is established … society is just not going to allow the use of broad-spectrum antibiotics, and drug discovery is going to have to change,” he predicted. That will create a need for pathogen-specific therapeutics (drugs that could, for example, target virulence factors, or that are able to exploit physiological differences between target and non-target pathogens) and, along with them, improved diagnostics.
Vaccines, which are both pathogen specific and protective, are considered by many to be the best possible defense against infectious disease. Their potential to alleviate the burden of AMR has been demonstrated in the development and deployment of vaccines against Haemophilus influenzae (Hib) (WHO, 2009) and Streptococcus pneumoniae (Kyaw et al., 2006), and also in aquaculture, where vaccine use can reduce reservoirs of drug-resistant bacteria and transferable resistance genes (Heuer et al., 2009). However, few vaccines have appeared in recent decades (Spellberg et al., 2008a), and workshop participants were not optimistic about prospects for a future upsurge. Furthermore, they noted, vaccines will not end the need for antimicrobials to treat conditions such as opportunistic infections.
Immunotherapy, which Casadevall studies, offers a near-term alternative to vaccines as a replacement for antibiotics (Saylor et al., 2009). Therapeutic antibodies are highly specific, do not select for resistance in non-targeted organ-
isms, and can be modified to provide a variety of effects (Casadevall et al., 2004). However, only one monoclonal antibody25 is licensed for use against a microbial disease—the disease caused by respiratory syncytial virus.26 Nevertheless, Casadevall reported, numerous monoclonal antibodies are in clinical trials. His group is currently developing a monoclonal antibody-based therapy for cryptococcal meningitis. Some antibodies can function as antimicrobials simply by binding to target cells, as suggested by the recent finding that antibody binding alters pathogen gene expression and metabolism (McClelland et al., 2010). Antibodies can also be “armed” by radiolabeling, enabling them to bind and kill specific microbial targets (Dadachova and Casadevall, 2009; Dadachova et al., 2006).
The downside to both vaccines and immunotherapy is the cost compared to antimicrobial drugs. Antibiotics could be replaced with immunoglobulins, Casadevall observed, but at a high price—one made even higher by the necessity for diagnostics. Moreover, he said, data from the pre-antibiotic era reveal that the efficiency of anti-body treatment declines after symptoms develop. Patients who are not diagnosed at an early stage of infection are likely to need antimicrobial drug treatment(s).
Before the advent of antibiotics, phage therapy for bacterial infections was widely employed in the West and is still used in Eastern Europe and the former Soviet Union (Hanlon, 2007; Sulakvelidze and Morris, 2001; Sulakvelidze et al., 2001). Had penicillin been invented a decade later, Casedevall speculated, bacteriophages might have achieved far greater prominence in antimicrobial therapy. Promising results from recent animal studies using bacteriophage to treat bacterial infections, including those described previously by Collins (Lu and Collins, 2007, 2009), have renewed interest in phage-based antimicrobials (Hanlon, 2007; Sulakvelidze and Morris, 2001). Recombinant or engineered phage lysins—enzymes used by phage to destroy the bacterial cell wall for release of phage progeny—are also being explored as therapeutics (Daniel et al., 2010; Fischetti, 2008).
Investigators have also proposed various alternatives to classic targets for antimicrobial therapeutics. Virulence factors—such as the adhesins, toxins, and antibiotic-resistance determinants—are considered promising targets because their elimination would disable but not kill the bacterium, keeping the selection pressure for resistance relatively low (Alekshun and Levy, 2004). Strategies that target quorum sensing, a process that regulates the expression of many virulence factors, offer additional possibilities in the search for novel antimicrobial targets (Alekshun and Levy, 2004; Salmond and Welch, 2008).
Casadevall also suggested that greater attention should be paid to host response
|
25 |
Monoclonal antibodies are raised against a single antigen in cells that are clones of a single parent (germ) cell. |
|
26 |
A respiratory virus that infects the lungs and breathing passages. Most otherwise healthy people recover from a respiratory synctial virus infection in 1 to 2 weeks; however, infection can be severe in some people, such as certain infants, young children, and older adults (http://www.cdc.gov/rsv/ [accessed June 18, 2010]). |
factors that influence whether the presence of a microbe constitutes colonization or disease (Casadevall and Pirofski, 2003). Indeed, he observed, diagnostics should be developed to distinguish whether tissue damage results from microbial action or the host’s immune response. At the moment, he said, “we do not have the capacity to distinguish between colonization and disease in the hospital.”
Focusing on the host may provide additional alternatives to antimicrobials, in the form of infectious disease therapeutics targeting host genes that pathogens co-opt in order to reproduce and spread (Cohen, 2009). Such host-oriented therapeutics are expected to be less vulnerable to resistance than traditional antimicrobials, but that remains to be determined. We may also have much to learn from insects, whose response to invading pathogens differs markedly from that of mammals, and whose immune defenses are rarely breached (Haine et al., 2008).
Policy Challenges and Opportunities
The policy challenges presented by AMR are plainly laid out in the title of the seminal 1992 book, The Antibiotic Paradox: How Miracle Drugs Are Destroying the Miracle (Levy, 1992, 2002). Recognition of this growing threat has paralleled that of infectious disease emergence, along with the understanding that both trends are driven by the global dissemination of microbes, hosts, vectors, and genes (American Academy of Microbiology, 2009; IOM, 2003, 2008b, 2010). The spread of resistance is directly or indirectly influenced by the “globalization” of human migration, travel, trade, and tourism (IOM, 2010; MacPherson et al., 2009); by agricultural practices and the food chain (IOM, 2006); and by water, in its diverse forms and uses (IOM, 2009a).
As noted previously, AMR both resembles and intersects with a range of global challenges, including energy, food safety, clean water, and climate stability. In her presentation, which outlined efforts by the Pew Charitable Trusts to support stewardship and development of antimicrobial drugs, Hearne asserted that policy makers should view antimicrobials much as they view energy: a limited, valuable resource to be conserved, and for which new sources need to be identified and exploited. She outlined four basic steps that she felt were needed in order to achieve the interrelated goals of antimicrobial stewardship and development—strategies that were discussed throughout the workshop—and that have long been advocated to address the threat of AMR:
-
limit the use of antimicrobials,
-
discourage their misuse,
-
reduce infection through disease prevention measures, and
-
create incentives for improved treatment and innovation.
The Global Challenge of Antibiotic Stewardship
“It’s the overwhelming culture in a lot of the world that antibiotics solve everything,” an audience member observed. “Antibiotic abuse is worldwide.” While over-the-counter access to antibiotics—illustrated in Figure WO-18—doubtless contributes to this abuse, several participants urged attention to additional risks for AMR associated with the manufacture and agricultural use of counterfeit antibiotics, a trend that appears to be increasing in the developing world. What, they asked, can be done to convince people—indeed, societies—to change these behaviors?
Addressing this challenge is central to the mission of APUA “to control infectious diseases worldwide through appropriate access to, and use of, antimicrobials and the containment of antimicrobial resistance,” according to Levy. This international alliance, comprised of over 60 local chapters representing more than 100 countries, supports both the gathering and the dissemination of scientific information on AMR, in partnership with public health organizations such as the WHO and the Pan American Health Organization. APUA aims to make AMR a public health issue with government ownership and financial support, even in countries where health resources

FIGURE WO-18 Over-the-counter availability of antibiotics in the Cancun (Mexico) airport.
SOURCE: Photo courtesy of David Relman, personal photo (2009).
and expenditures are limited, Levy said. He noted that, by publishing all findings and achievements of APUA-supported programs, no matter how small, the alliance has raised public awareness of AMR and recognition of leaders in each country who are tackling this issue.
Laxminarayan reminded workshop participants that much of the developing world lacks access to antimicrobials, so it would be inappropriate to send a universal message encouraging the world to use less of these drugs. “Many people whose lives could be saved by antibiotics have never seen an effective antibiotic in their life,” he asserted, and he offered instead as a model the Integrated Management of Childhood Illness, a program that has successfully supported the rational use of antibiotics (Black et al., 2003; Okeke et al., 2005).
In response to his own rhetorical question as to whether antibiotic effectiveness can be managed as a global “good,” Laxminarayan went on to describe the Global Fund to Fight AIDS, TB and Malaria27 which he held up as a rare example of an effective global program supported by a robust financing mechanism. Thanks to this initiative, the private-sector price of artemisinin combination therapy has declined from about 8 dollars to about 5 cents, with the remainder paid by donors, wholesalers, and pharmaceutical companies, he reported. There are few additional examples of such programs, Laxminarayan observed, but AMR should be viewed as deserving similar support as malaria, since, in both cases, subsidizing appropriate treatment is certain to save significant numbers of lives.
Opportunities for National Legislation and Regulatory Policies
Hearne identified three legislative proposals—two of which have been introduced to the Congress of the United States (see Box WO-3) and a third more comprehensive synthesis of these and other policy elements—that collectively would advance the twin goals of antibiotic stewardship and development:
-
The Strategies to Address Antimicrobial Resistance (STAAR) Act, aimed at advancing federal plans for AMR research and surveillance.
-
The Preservation of Antibiotics for Medical Treatment Act (PAMTA), designed to withdraw the routine use of seven classes of antibiotics vitally important to human health from food animal production unless animals or herds are sick with disease or unless drug companies can prove that their use does not harm human health.
|
27 |
The Global Fund to Fight AIDS, TB and Malaria (often referred to as the Global Fund or GFATM) was established in January 2002 to dramatically increase global financing for interventions against the two pandemics (malaria is actually endemic). It is the largest international funder of programs to combat malaria and tuberculosis, providing two-thirds of all financing, and it provides 20 percent of all international funding to combat HIV/AIDS. The Global Fund asserts that, as of June 2007, 1.9 million lives have been saved thanks to efforts in 136 countries supported by the Global Fund. |
|
BOX WO-3 Legislation to Address AMR: The STAAR Act and PAMTA In late 2005, legislation promoted by the Infectious Diseases Society of America to encourage investment by pharmaceutical companies in antimicrobial research and development was introduced, but not enacted, in the 109th Congress (Spellberg et al., 2008a). In September 2007, the 110th Congress passed the Food and Drug Administration Amendments Act of 2007 (P.L. 110-85, 2007), which included provisions for gathering data on the extent and spread of antibiotic resistance among bacteria. On May 13, 2009, U.S. Representative Jim Matheson introduced the Strategies to Address Antimicrobial Resistance (STAAR) Act (H.R. 2400), which proposes to build on the federal Action Plan (IDSA, 2009). Specifically, the STAAR Act, if enacted, would create an Antimicrobial Resistance Office within the office of the Assistant Secretary of Health in the Department of Health and Human Services (HHS), establish an expert advisory board, and strengthen research and surveillance efforts toward reducing the threat of antimicrobial resistance.a In a related development, on March 17, 2009, the Preservation of Antibiotics for Medical Treatment Act (PAMTA) of 2009 (H.R. 1549/S. 619) was introduced in the U.S. House of Representativesb and the U.S. Senate.c The passage of this legislation would amend the Federal Food, Drug, and Cosmetic Act to require the Secretary of HHS to deny applications for new animal drugs that fit the definition of a “critical antimicrobial animal drug” unless the applicant demonstrates that there is a reasonable certainty of no harm to human health due to the development of antimicrobial resistance attributable to the non-therapeutic use of the drug. The legislation defines a “critical antimicrobial animal drug” as a drug intended for use in food-producing animals that contains specified antibiotics or other drugs used in humans to treat or prevent disease or infection caused by microorganisms. PAMTA further requires the Secretary to withdraw approval of a non-therapeutic use of such drugs in food-producing animals 2 years after the date of enactment of this Act unless certain safety requirements are met and it directs specified congressional committees to hold hearings on the implementation of such a withdrawal of approval (H.R. 1549/S. 619). It is unlikely that either piece of legislation will be enacted into law by the close of the 111th Congress in 2010.
|
-
A comprehensive bill that could include incentives to the research community and industry to embrace antimicrobial development as well as support for antibiotic stewardship. This legislation could combine the STAAR Act and PAMTA with lessons learned from European bans on the use of antimicrobials for growth-promotion in food animals, according to Hearne. It also could draw upon incentives, such as those in the successful Orphan Drug Act (P.L. 97-414, 1983), which both pushed and pulled the pharmaceutical industry to develop therapeutics for disorders affecting fewer than 200,000 people in the United States.
Hearne stressed that it was imperative that Congress muster the political will required to pass comprehensive legislation to preserve the existing antimicrobial therapies while simultaneously stimulating research and development (R&D) innovation to ensure a steady supply of replacement treatments as the old ones become obsolete. To that end, Hearne said she was heartened by the growing interest among policy makers to address antimicrobial resistance and by the number of supporters in both the House and Senate for PAMTA. Returning to the workshop discussion on the interpretation of the results of the Danish ban on the use of growth-promoting antimicrobials in food animals, she said that a similar “dissection of data” should be taking place on this issue at the U.S. national level. To this point, speaker Jeffrey Levi, of Trust for America’s Health, suggested that, despite polarization on the issue of antimicrobial growth promoters, “there is a consensus that this should be driven by science and public health and not necessarily the interests of the industry … [and] that is an argument for FDA regulation, because FDA knows how to insulate itself from industry, with varying degrees of success.”
Levi focused on the existence of substantial administrative opportunities to advance AMR stewardship and development, noting that the post-healthcare reform climate does not favor the passage of more health-related legislation. Nevertheless, he observed that “almost everything, if not everything, in the STAAR Act can be implemented administratively,” much as plans for influenza pandemic preparedness were. “There are many other issues in the federal government without a legislative mandate where there is a coordinating mechanism and a government-wide strategy and an implementation plan,” he said. “It didn’t take an act of Congress to have a national strategy on pandemic flu or to have very detailed implementation plans” after which it was possible to request appropriate additional resources from Congress.
The passage of healthcare reform has created an important opportunity to advance antimicrobial stewardship, in the form of significant funding ($15 billion over the next 10 years) for preventative public health programs, Levi added. This initiative could include efforts to educate the public and the medical community about AMR, he said, given committed leadership and an actionable plan. Levi offered one such proposal: a public health campaign intended to raise public expectations of protection from AMR in healthcare settings while increasing
public understanding of the prudent use of antimicrobials. “If we do this education campaign right, it will get people to start asking the question, ‘Why are we in this mess in the first place, and why isn’t science able to provide us the new tools that we need?’” he speculated. “That will then generate support for the other parts of the agenda, including developing new products.”
Ultimately, Hearne said, she believes the United States could have comprehensive reform that establishes a new drug category for antimicrobials (discussed below). In Hearne’s view, this would promote conservation of therapeutic resources while encouraging innovation and development in the arena of new drugs and treatments. However, she added, such legislation can only come about with the support of the scientific community, which is viewed by the vast majority of Americans—according to research conducted by the Pew Research Center—as a trusted source of information for decision making (Pew Research Center for the People & the Press, 2009). “The scientific community can speak with great credibility to the American people about the need for us to protect their health,” Levi concurred. If the issue of AMR is framed in that way, he said, the FDA (among other federal agencies) “can move forward wherever the science may take it.”
Promoting the Development of Novel Antimicrobials
Workshop participants discussed a range of regulatory reforms and economic incentives to address AMR in both the near- and far-term by bringing more antimicrobials to market and by refilling the developmental pipeline.
Bringing new drugs to market Spellberg stated that a statutory change might be needed in order for the FDA to establish an approval process for antimicrobial drugs based on noninferiority studies with clinical endpoints. He and Levy, among others, observed that such a change is justified given the uniqueness of antimicrobials as a class of drugs, on the basis of the following factors:
-
Antimicrobial use by individuals, through the subsequent shedding of both active and resistant bacteria, affects every community from the immediate family to the local environment to the global ecosystem.
-
Unlike other drugs (but like pesticides), antimicrobials lose their effectiveness over time as microbes inevitably develop resistance.
-
Antimicrobial effectiveness permits the use and development of other medical advances, such as transplantation and cancer chemotherapy, and of surgery in general.28
|
28 |
This point was also raised by Rice as an existing, but overlooked, incentive to pharmaceutical development: How would the pharmaceutical companies’ bottom line be affected by a significantly reduced use of cancer chemotherapy because it is perceived as too dangerous because of the resistant organisms that you cannot treat? How about reduced transplants? How about reduced joint replacements? |
In addition to providing a framework for regulatory reform, establishing anti-microbials as a unique drug category, Levy observed, would also permit special considerations for these drugs as incentives for development, such as extended patent life and tax relief. Spellberg asserted that only a “Chinese menu” of incentives—including but not limited to basic science and business grants, contracts, tax credits, new funding through agencies such as the Biomedical Advanced Research and Development Authority (BARDA),29 patent extensions, guaranteed markets, and liability protection—would be sufficient to refill the antimicrobial pipeline. Levi stated that many mechanisms already exist to create these incentives through such agencies as BARDA and the FDA. Levi went on to note that, under the leadership of the former Forum co-chair and current Commissioner of the FDA, Margaret A. Hamburg, the FDA is increasingly guided by a public health standard, as evidenced by the creation of the Center for Tobacco Products.
When public health standards underlie regulatory decision making, reform can be achieved more efficiently through administrative channels than through legislation, Levi asserted. Based on his lengthy prior experience with regulatory reform to address HIV/AIDS, he assured advocates of antimicrobial stewardship and development that, armed with scientific evidence and “good, compelling, very emotional cases,” it is possible to reach the regulators and achieve more flexibility in the drug approval process.
Reviving research and development While it is critical to bring new antimicrobials to the market in the near-term, there is an equal need to promote R&D of the next generation of antimicrobials, Spellberg observed. However, he added, such efforts may only be economically viable if conducted by a not-for-profit entity charged with developing antimicrobial therapies for societal use. He noted that a similar initiative has been undertaken to support the R&D of tuberculosis drugs (Global Alliance for TB Drug Development, 2010).
Others suggested that the task of identifying new antimicrobial entities, such as scaffolds, may be so great as to require not only the participation of the pharmaceu-
|
How about overall reduced elective surgery because physicians are afraid of being sued because of a resistant organism that cannot be treated? All of these highly profitable ventures are made possible by effective prophylactic and therapeutic antimicrobial therapy. So my question to the pharmaceutical industry: Whatever happened to protecting the franchise? When I mentioned this to someone who had 16 years of experience in the pharmaceutical industry, he thought it was an interesting idea but said, “Never happen. Everybody is so siloed into their own little area, but nobody cares about what the other area does.” And I think that’s a real shame. I really think, if the pharmaceutical companies could look forward and say, “We need to protect these really, really profitable areas, and one way to do that is to make sure we have therapy to treat the complications that result from them,” I think the world would be a better place. |
|
29 |
BARDA, within the Office of the Assistant Secretary for Preparedness and Response in the Department of Health and Human Services, provides an integrated, systematic approach to the development and purchase of the necessary vaccines, drugs, therapies, and diagnostic tools for public health medical emergencies. See http://www.hhs.gov/aspr/barda/index.html (accessed August 3, 2010). |
tical industry but also unprecedented cooperation among individual firms. Forum member Paul F. Miller, of Pfizer, Inc., observed that recent years have witnessed significant coordination among companies in basic research areas that would mutually benefit the participants, such as developing tools for information technology, drug safety, and pharmacokinetics. Beyond that, however, industry cooperation poses significant problems, as several Forum members pointed out.
Forum member George Poste, of Arizona State University, noted that it would be important to obtain a patent for a novel scaffold or molecule relatively early in the development process; however, antitrust law is still an impediment to companies working together. He instead suggested that jointly discovered entities be shepherded through proof-of-concept by a non-profit organization, which could then sell the experimental compounds to individual companies interested in developing them further.
Then again, Poste mused, from the perspective of a pharmaceutical company, “unless you’ve got a clear way to have a return on your investment, [coupled with] a transparent regulatory approval pathway, you would have to say you are not fulfilling your fiduciary responsibility to your shareholders if you embarked upon that latter journey [toward developing an antimicrobial], which is going to cost you hundreds of millions of dollars.”
Paths Forward
In light of the polarized environment surrounding the issue of antimicrobial use in food animals, workshop participants expressed skepticism that political will can be marshaled anytime soon to address the full spectrum of AMR-associated threats to health. Advocates for antimicrobial stewardship and development must therefore turn popular opinion in their favor, which requires public understanding of the issue. Until then, in the words of Forum member Fred Sparling, lobbying will continue to trump evidence. Unfortunately, as many acknowledged, the threat of AMR is subtle and complex, difficult to grasp and to convey, and lacks a demonstrable connection between cause and effect—in short, it is a tragedy of the commons.
Workshop participants appeared divided as to how best to adapt to this reality. Some insisted that what is needed are more and better data, so as to characterize relationships among patterns of antimicrobial use, the development of (transferable) resistance, and human disease, and to assess the risks inherent in each. Although a variety of statistics illustrate a range of medical and economic impacts associated with AMR, the true scope of the problem remains to be determined. Needed data would include not only the incidence and prevalence of resistant infections, but also accurate measurements of the full spectrum of resistance mechanisms contributing to the ongoing burden of infectious disease and their far-reaching medical and socioeconomic effects (American Academy of Microbiology, 2009).
At an even more basic level, it is nearly impossible to estimate the total amount of antimicrobial drugs used by humans and animals, according to Forum member Michael Osterholm of the Center for Infectious Disease Research and Policy. “We are far, far too confident in how much we know about this topic,” he asserted. Other important unknowns include the relative importance of various routes to AMR. “Where is the biggest impact on antibiotic resistance?” Levy asked. “Is it in the individual getting the drug or is it in all the massive numbers of bacteria outside who are being confronted by low doses of drugs in general?” Without answers to such questions, it will be difficult to know how to address AMR most effectively.
On the other hand, several workshop participants suggested that we have sufficient knowledge in hand already to reduce AMR. The four-pronged strategy outlined by Hearne at the workshop and by others before her provides a framework for acting on that knowledge. If more evidence is needed to move forward, Sparling suggested, it is mainly to push past obstacles created by lobbyists and financial interests. Levy, who once made a study of recommendations to address AMR from reports preceding the WHO Global Strategy for Containment of Antimicrobial Resistance (WHO, 2001a), recalled that each report committee “wanted to say something different, but it always comes down to saying the same thing.”
Nevertheless, he said, he remains an optimist regarding the future of anti-microbial therapy. “I think we can find new drugs, but we have to learn how to use these drugs better,” Levy advised. “If we can use them less, that’s fine. Less people affected, less effect on the environment, less effect on the innocent bystanders: the other bacteria that are sharing that environment.”
WORKSHOP OVERVIEW REFERENCES
Aarestrup, F. M., V. F. Jensen, H. D. Emborg, E. Jacobsen, and H. C. Wegener. 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. American Journal of Veterinary Research 71(7):726–33.
Alekshun, M. N., and S. B. Levy. 2004. Targeting virulence to prevent infection: To kill or not to kill? Drug Discovery Today: Therapeutic Strategies 1(4):483–9.
Alekshun, M. N., and S. B. Levy. 2006. Commensals upon us. Biochemical Pharmacology 71(7):893–900.
Allen, H. K., K. A. Cloud-Hansen, J. M. Wolinski, C. Guan, S. Greene, S. Lu, M. Boeyink, N. A. Broderick, K. F. Raffa, and J. Handelsman. 2009. Resident microbiota of the Gypsy Moth mid-gut harbors antibiotic resistance determinants. DNA and Cell Biology 28(3):109–17.
Allen, H. K., J. Donato, H. H. Wang, K. A. Cloud-Hansen, J. Davies, and J. Handelsman. 2010. Call of the wild: Antibiotic resistance genes in natural environments. Nature Reviews Microbiology 8(4):251–9.
American Academy of Microbiology. 2009. Antibiotic resistance: An ecological perspective on an old problem. Washington, DC: American Academy of Microbiology.
American Journal of Transplantation. 2009. Special issue: The 2008 SRTR report on the state of transplantation. American Journal of Transplantation 9(4p2):869–78. Published online March 26.
|
BOX WO-4 A Gallery of Antibiotic-Resistant Pathogens Methicillin-resistant Staphylococcus aureus (MRSA; Figure WO-4-1), which might be considered the “poster child” of drug-resistant microbes, is on the rise almost everywhere (ECDC, 2007). MRSA causes approximately 20,000 deaths per year in the United States, more than HIV/AIDS (Resources for the Future, 2009; Walsh and Fischbach, 2009). Nearly one in five persons who contract a MRSA-associated disease dies from it, and an increasing number of its victims are young and otherwise healthy (Walsh and Fischbach, 2009). 
FIGURE WO-4-1 Methicillin-resistant Staphylococcus aureus. SOURCE: CDC, Public Health Image Library (PHIL 10046). First identified nearly 50 years ago, MRSA has undergone rapid evolutionary changes and epidemiologic expansion (Deresinski, 2005). It has spread beyond the confines of the hospital setting to emerge in the community, where community-acquired MRSA (CA-MRSA) is rapidly becoming a dominant pathogen. In recent years, previously healthy individuals without either direct or indirect contact with healthcare facilities have become infected with CA-MRSA, and, in some community settings, CA-MRSA strains have become the predominant form of S. aureus isolated from skin infections, especially among children. CA-MRSA clusters and outbreaks have occurred among diverse communities of Native Americans, prison inmates, military recruits, children in child care centers, and competitive athletes, among others. Most CA-MRSA infections have involved skin and skin structures, but lethal invasive infections have also occurred. |
|
Coming full circle, CA-MRSA strains are now invading healthcare facilities, where in some cases they are displacing the dominant hospital-associated strains of S. aureus. Another cyclical pattern is emerging as companion animals and their human handlers pass MRSA infections—mainly CA-MRSA—back and forth to one another and others within their “communities” (Lloyd, 2007; Oehler et al., 2009; Rutland et al., 2009). Vancomycin-resistant Staphylococcus aureus (VRSA; Figure WO-4-2) began emerging in hospitals in 2002 (Walsh and Fischbach, 2009). 
FIGURE WO-4-2 Vancomycin-resistant Staphylococcus aureus. SOURCE: http://www.foogle.biz/mrsa/ (accessed February 22, 2010). Copyright Dennis Kunkel Microscopy, Inc. VRSA arose when a MRSA strain acquired a five-gene plasmid “cassette” conferring resistance to vancomycin which, up to this time, was long considered the antibiotic of last resort for staph infections. VRSA cell walls are modified by the actions of these genes in such a way that vancomycin cannot bind to them. Because it is sensitive to few antibiotics in clinical use, VRSA infections have a correspondingly high mortality rate. Thankfully, it has not spread widely. Multidrug-resistant tuberculosis (MDR-TB; Figure WO-4-3) affects approximately 5 percent of all TB patients treated in 2006, or about 500,000 people worldwide, according to the World Health Organization (WHO, 2008). |
FIGURE WO-4-3 Multidrug-resistant tuberculosis. SOURCE: CDC, Public Health Image Library (PHIL 9997). Many consider this to be a substantial underestimate of the true prevalence of MDR-TB (IOM, 2009c). There are also extensively drug-resistant strains of TB, which defy second-line therapies, and newly emerged TB strains (Loddenkemper and Hauer, 2010) that resist all available drugs. Vancomycin-resistant enterococci (VRE; Figure WO-4-4) include members of two species, Enterococcus faecalis and Enterococcus faecium, which are among the most prevalent causes of hospital-acquired infections worldwide (Werner et al., 2008). 
FIGURE WO-4-4 Vancomycin-resistant enterococci. SOURCE: NIH, courtesy of USDA. |
|
VRE first appeared in a few European countries in the late 1980s. Currently, six types of acquired vancomycin resistance in enterococci are recognized, of which two are widespread. As with VRSA, VRE infections can be treated with a very few recently introduced antibiotics, and even for those, resistant cases have already been reported. VRE have caused hospital outbreaks worldwide, and the vancomycin-resistance gene (vanA) has crossed genus boundaries to MRSA (Willems et al., 2005). Extended-spectrum β-lactamase (ESBL)-producing enterobacteria (Figure WO-4-5) resist both β-lactams and fluoroquinolones, the main therapeutic choices to treat infections caused by these microorganisms (Alekshun and Levy, 2006; Coque et al., 2008). 
FIGURE WO-4-5 Detection of extended-spectrum β-lactamase production by the double disk test on DSM-ES agar. Disks: center, amoxycillin + clavulanate 20 + 10 μg; right, cefepime 30 μg; left, ceftriaxone 30 μg; top, ceftazidime 30 μg; bottom, aztreonam 30 μg. SOURCE: © 2003 Cagatay et al; licensee BioMed Central Ltd. From http://www.biomedcentral.com/1471-2334/3/22/ (accessed February 22, 2010). Enterobacteriaceae have become one of the most important causes of hospital- and community-acquired infections. ESBL-producers have increasingly been found in both hospital and community settings. They apparently colonize some hosts asymptomatically, who then serve as carriers for these commensal-like pathogens, inadvertently increasing its geographic and host range (Alekshun and Levy, 2006). Clostridium difficile (Figure WO-4-6) infections can cause severe, potentially fatal cases of diarrhea when competing members of the intestinal microbiota are killed during treatment with broad-spectrum antibiotics, such as clindamycin, semi-synthetic penicillins, and cephalosporins (Alekshun and Levy, 2006). |
FIGURE WO-4-6 Clostridium difficile. SOURCE: http://www.denniskunkel.com/product_info.php?products_id=9284 (accessed February 22, 2010). Copyright Dennis Kunkel Microscopy, Inc. A recently emerged hypervirulent strain of C. difficile, which has since become epidemic, produces increased levels of several toxins. This hypervirulent strain of C. difficile is resistant to the fluoroquinolone class of antibiotics, the use of which is increasingly linked to outbreaks of C. difficile-associated diarrhea (Blossom and McDonald, 2007). Extensively drug-resistant and pandrug-resistant Gram-negative bacteria include strains resistant to all but one or two classes of antibiotics (extensively resistant) and to all available antibiotic classes (pandrug-resistant) (Souli et al., 2008). Gram-negative bacteria possess a double cell membrane, which shields them from many antibiotics (Walsh and Fischbach, 2009). Resistance to almost all clinically used antibiotics has occurred among strains of Escherichia coli, its relative Klebsiella pneumoniae (Figure WO-4-7), and in two pathogens associated with opportunistic infections, Pseudomonas aeruginosa and Acinetobacter baumannii. During the past three decades, Acinetobacter has emerged as an infectious agent of importance to hospitals worldwide, and it has demonstrated an alarming tendency to accumulate diverse mechanisms of resistance (Munoz-Price and Weinstein, 2008). Several pandrug-resistant strains of Acinetobacter have been noted to have infected members of the U.S. armed services stationed in Iraq and Afghanistan, leading to cases in DoD and VA medical facilities and to concerns about the spread of disease caused by this organism to an ever larger community (CDC, 2004). Gonococci (Figure WO-4-8) are Gram-negative bacteria responsible for the sexually transmitted disease gonorrhea. Rates of gonorrhea vary greatly among countries in the developed and developing world, with South and Southeast Asia, |
FIGURE WO-4-7 Klebsiella pneumoniae. SOURCE: http://www.ciriscience.org/ph_156-Klebsiella_pneumoniae_Copyright_Dennis_Kunkel_Microscopy (accessed February 22, 2010). Copyright Dennis Kunkel Microscopy, Inc. sub-Saharan Africa, and Latin America—the most resource-poor settings—having the highest rates. Antibiotic resistance increasingly compromises the effectiveness of individual case management and disease-control programs; inexpensive treatment regimens are often rendered ineffective and effective ones are often unaffordable. In much of the world, gonococci are resistant to penicillin, tetracycline, spectinomycin, and ciprofloxacin. Currently, the CDC sexually transmitted disease treatment guidelines recommend that cephalosporin antibiotics be used to treat all gonococcal infections in the United States (CDC, 2009; WHO, 2001b). 
FIGURE WO-4-8 False-colored scanning electron micrograph of a human phagocyte and gonococci (green). SOURCE: © Rockefeller University Press, 2004. Originally published in J. Exp. Med. |
Angulo, F. J. 1999. Use of antimicrobial agents in aquaculture: potential for public health impact, edited by P. H. Service. Washington, DC: Food and Drug Administration.
Angulo, F. J., and J. A. Nunnery. 2004. Antimicrobial resistance in zoonotic enteric pathogens. Review Scientifique and Tecchnologique 23(2):485–96.
Anonymous. 2008. Recent trends in antimicrobial resistance among Streptococcus pneumoniae and Staphylococcus aureus isolates: The French experience. Eurosurveillance 13(46).
Antoniadou, A., F. Kontopidou, G. Poulakou, E. Koratzanis, I. Galani, E. Papadomichelakis, P. Kopterides, M. Souli, A. Armaganidis, and H. Giamarellou. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: First report of a multiclonal cluster. Journal of Antimicrobial Chemotherapy 59(4):786–90.
Arias, C. A., and B. E. Murray. 2009. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. New England Journal of Medicine 360(5):439–43.
Baquero, F., and J. Campos. 2003. The tragedy of the commons in antimicrobial chemotherapy. Revista Espanola de Quimioterapia 16(1):11–3.
Black, R. E., S. S. Morris, and J. Bryce. 2003. Where and why are 10 million children dying every year? Lancet 361(9376):2226–34.
Bleasdale, S. C., W. E. Trick, I. M. Gonzalez, R. D. Lyles, M. K. Hayden, and R. A. Weinstein. 2007. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Archives of Internal Medicine 167(19):2073–9.
Blossom, D. B., and L. C. McDonald. 2007. The challenges posed by reemerging Clostridium difficile infection. Clinical Infectious Diseases 45(2):222–7.
Bonnedahl, J., M. Drobni, M. Gauthier-Clerc, J. Hernandez, S. Granholm, Y. Kayser, A. Melhus, G. Kahlmeter, J. Waldenström, A. Johansson, and B. Olsen. 2009. Dissemination of Escherichia coli with CTX-M type ESBL between humans and yellow-legged gulls in the south of France. PLoS One 4(6):e5958.
Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spell-berg, and J. Bartlett. 2009. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases 48(1):1–12.
Cagatay, A. A., T. Kocagoz, and H. Eraksoy. 2003. Dio-sensimedia: A novel culture medium for rapid detection of extended spectrum β-lactamases. BMC Infectious Diseases 3:22.
Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407(6802):340–8.
Casadevall, A. 1996. Crisis in infectious diseases: Time for a new paradigm? Clinical Infectious Diseases 3(4):790–4.
Casadevall, A., and L. A. Pirofski. 2003. The damage-response framework of microbial pathogenesis. Nature Reviews, Microbiology 1(1):17–24.
Casadevall, A., E. Dadachova, and L. A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nature Reviews, Microbiology 2(9):695–703.
CDC (Centers for Disease Control and Prevention). 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. Morbidity and Mortality Weekly Report 53(45):1063–6.
CDC. 2009. Basic information about antibiotic-resistant gonorrhea (ARG), http://www.cdc.gov/STD/gonorrhea/arg/basic.htm (accessed March 2, 2010).
Center for Global Development. 2010. The race against drug resistance. A report of the Center for Global Development’s Drug Resistance Working Group. Washington, DC: Center for Global Development.
Chaitram, J. M., L. A. Jevitt, S. Lary, and F. C. Tenover. 2003. The World Health Organization’s External Quality Assurance System Proficiency Testing Program has improved the accuracy of antimicrobial susceptibility testing and reporting among participating laboratories using NCCLS methods. Journal of Clinical Microbiology 41(6):2372–7.
Chambers, H. F., and F. R. DeLeo. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews, Microbiology 7(9):629–41.
Chastre, J., M. Wolff, J. Y. Fagon, S. Chevret, F. Thomas, D. Wermert, E. Clementi, J. Gonzalez, D. Jusserand, P. Asfar, D. Perrin, F. Fieux, and S. Aubas (PneumA Trial Group). 2003. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. Journal of the American Medical Association 290(19):2588–98.
Chee-Sanford, J. C., R. I. Mackie, S. Koike, I. G. Krapac, Y. F. Lin, A. C. Yannarell, S. Maxwell, and R. I. Aminov. 2009. Fate and transport of antibiotic residues and antibiotic resistance genes fol-lowing land application of manure waste. Journal of Environmental Quality 38(3):1086–108.
Choi, C. Q. 2007. Pollution in solution. Scientific American 296(1):22–3.
Cloud-Hansen, K. A., K. M. Villiard, J. Handelsman, and H. V. Carey. 2007. Thirteen-lined ground squirrels (Spermophilus tridecemlineatus) harbor multiantibiotic-resistant bacteria. Journal of the American Association of Laboratory Animal Science 46(3):21–3.
Cohen, S. N. 2009. Microbial drug resistance: An old problem in need of new solutions. In Microevo-lution and co-adaptation. Washington, DC: The National Academies Press.
Collignon, P., J. H. Powers, T. M. Chiller, A. Aidara-Kane, and F. M. Aarestrup. 2009. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clinical Infectious Diseases 49(1):132–41.
Collins, J. J. 2010. Radical approaches to antibacterials and antibiotic resistance.. Presentation given at the April 6-7, 2010, public workshop, “Antimicrobial Resistance: Implications for Global Health and Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Coque, T. M., F. Baquero, and R. Canton. 2008. Increasing prevalence of ESBL-producing Entero-bacteriaceae in Europe. Eurosurveillance 13(47).
Cosgrove, S. E. 2006. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clinical Infectious Diseases 42(Suppl 2):S82–9.
Courvalin, P. 2010. Antibiotic-induced resistance flow. Presentation given at the April 6-7, 2010, public workshop “Antimicrobial Resistance: Implications for Global Health and Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Courvalin, P. 2008. Predictable and unpredictable evolution of antibiotic resistance. Journal of Internal Medicine 264(1):4–16.
Dadachova, E., and A. Casadevall. 2009. Radioimmunotherapy of infectious diseases. Seminars in Nuclear Medicine 39(2):146–53.
Dadachova, E., M. C. Patel, S. Toussi, C. Apostolidis, A. Morgenstern, M. W. Brechbiel, M. K. Gorny, S. Zolla-Pazner, A. Casadevall, and H. Goldstein. 2006. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Medicine 3(11):e427.
Damicone, J., and D. Smith. 2009. Fungicide resistance management. http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-2317/F-7663web.pdf (accessed March 19, 2010).
Daniel, A., C. Euler, M. Collin, P. Chahales, K. J. Gorelick, and V. A. Fischetti. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 54(4):1603–12.
Dantas, G., M. O. Sommer, R. D. Oluwasegun, and G. M. Church. 2008. Bacteria subsisting on antibiotics. Science 320(5872):100–3.
Davies, J. 2007. Microbes have the last word: A drastic re-evaluation of antimicrobial treatment is needed to overcome the threat of antibiotic-resistant bacteria. EMBO Reports 8(7):616–21.
Davies, J. 2009. Antibiotic resistance and the future of antibiotics. In Microbial evolution and co-adaptation. IOM. Washington, DC: The National Academies Press, pp. 160–72.
Davies, J., G. B. Spiegelman, and G. Yim. 2006. The world of subinhibitory antibiotic concentrations. Current Opinions in Microbiology 9(5):445–53.
D’Costa, V. M., K. M. McGrann, D. W. Hughes, and G. D. Wright. 2006. Sampling the antibiotic resistome. Science 311(5759):374–7.
Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: An evolutionary, epidemiologic, and therapeutic odyssey. Clinical Infectious Diseases 40(4):562–73.
Diamond, J. 2005. Collapse: How societies choose to fail or succeed. New York: Viking Penguin.
D’Onofrio, A., J. M. Crawford, E. J. Stewart, K. Witt, E. Gavrish, S. Epstein, J. Clardy, and K. Lewis. 2010. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chemistry and Biology 17(3):254–64.
Dörr, T., K. Lewis, and M. Vuli. 2009. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genetics 5(12):e1000760.
Dörr, T., M. Vulič, and K. Lewis. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biology 8(2):e1000317.
Dutil, L., R. Irwin, R. Finley, L. K. Ng, B. Avery, P. Boerlin, A. M. Bourgault, L. Cole, D. Daignault, A. Desruisseau, W. Demczuk, L. Hoang, G. B. Horsman, J. Ismail, F. Jamieson, A. Maki, A. Pacagnella, and D. R. Pillai. 2010. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerging Infectious Diseases 16(1):48–54.
Dwyer, D. J., M. A. Kohanski, B. Hayete, and J. J. Collins. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Molecular Systems Biology 3:91.
ECDC (European Centre for Disease Prevention and Control). 2007. Microbes without borders: Key facts on infectious diseases in Europe. Stockholm, Sweden: ECDC.
ECDC and EMEA (European Agency for the Evaluation of Medicial Products). 2009. The bacterial challenge: Time to react. Stockholm, Sweden: ECDC, European Medicines Agency. http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf (accessed on August 24, 2010).
Fischbach, M. A., and C. T. Walsh. 2009. Antibiotics for emerging pathogens. Science 325(5944): 1089–93.
Fischetti, V. A. 2008. Bacteriophage lysins as effective antibacterials. Current Opinion in Microbiology 11(5):393–400.
Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fätkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove (S. aureus Endocarditis and Bacteremia Study Group). 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. New England Journal of Medicine 355(7):653–65.
GAO (General Accounting Office). 1999. The agricultural use of antibiotics and its implications for human health. Washington, DC: GAO.
Gilliver, M. A., M. Bennett, M. Begon, S. M. Hazel, and C. A. Hart. 1999. Antibiotic resistance found in wild rodents. Nature 401(6750):233–4.
Global Alliance for TB Drug Development. 2010. http://www.tballiance.org/home/home.php (accessed June 21, 2010).
Goossens, H., M. Ferech, R. Vander Stichele, and M. Elseviers (ESAC Project Group). 2005. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 365(9459):579–87.
Goossens, H., S. Coenen, M. Costers, S. De Corte, A. De Sutter, B. Gordts, L. Laurier, and M. Struelens. 2008. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC). Eurosurveillance 13(46).
Graham, J. P., J. J. Boland, and E. Silbergeld. 2007. Growth promoting antibiotics in food animal production: An economic analysis. Public Health Reports 122(1):79–87.
Griswold, M. E., A. R. Localio, and C. Mulrow. 2010. Propensity score adjustment with multilevel data: Setting your sites on decreasing selection bias. Annals of Internal Medicine 152(6):393–5.
Haine, E. R., Y. Moret, M. T. Siva-Jothy, and J. Rolff. 2008. Antimicrobial defense and persistent infection in insects. Science 322(5905):1257–9.
Hanlon, G. W. 2007. Bacteriophages: An appraisal of their role in the treatment of bacterial infections. International Journal of Antimicrobial Agents 30(2):118–28.
Hansen, J. L., J. A. Ippolito, N. Ban, P. Nissen, P. B. Moore, and T. A. Steitz. 2002. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Molecular Cell 10(1):117–28.
Harbarth, S., C. Fankhauser, J. Schrenzel, J. Christenson, P. Gervaz, C. Bandiera-Clerc, G. Renzi, N. Vernaz, H. Sax, and D. Pittet. 2008. Universal screening for methicillin-resistant Staphylococ-cus aureus at hospital admission and nosocomial infection in surgical patients. Journal of the American Medical Association 299(10):1149–57.
Hardin, G. 1968. The tragedy of the commons. Science 162(5364):1243–8.
Hayden, M. K., M. J. Bonten, D. W. Blom, E. A. Lyle, D. A. van de Vijver, and R. A. Weinstein. 2006. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clinical Infectious Diseases 42(11):1552–60.
Heuer, O. E., H. Kruse, K. Grave, P. Collignon, I. Karunasagar, and F. J. Angulo. 2009. Human health consequences of use of antimicrobial agents in aquaculture. Clinical Infectious Diseases 49(8):1248–53.
Hidron, A. I., J. R. Edwards, J. Patel, T. C. Horan, D. M. Sievert, D. A. Pollock, S. K. Fridkin, National Healthcare Safety Network Team, and Participating National Healthcare Safety Network Facilities. 2008. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infection Control and Hospital Epidemiology 29(11):996–1011.
Holland, T. L., C. W. Woods, and M. Joyce. 2009. Antibacterial susceptibility testing in the clinical laboratory. Infectious Disease Clinics of North America 23(4):vii, 757–90.
Huttner, B., H. Goossens, T. Verheij, and S. Harbarth (CHAMP Consortium). 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infectious Diseases 10(1):17–31.
ICIUM (International Conference on Improving Use of Medicines). 2004a. ICIUM theme summary: Antimicrobial resistance. International Conference on Improving Use of Medicines. Chiang Mai, Thailand: ICIUM.
______. 2004b. Policies and programmes to improve use of medicines: Recommendations from ICIUM 2004 International Conference on Improving Use of Medicines. Chiang Mai, Thailand: ICIUM.
IDSA (Infectious Diseases Society of America). 2009. The strategies to address antimicrobial resistance Act H.R. 2400. Arlington, VA: IDSA.
Interagency Task Force on Antimicrobial Resistance. 2001. A public health action plan to combat antimicrobial resistance. Part I: Domestic issues. Washington, DC.
IOM (Institute of Medicine). 1992. Emerging infections: Microbial threats to health in the United States. Washington, DC: National Academy Press.
_____. 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
_____. 2006. Addressing foodborne threats to health: Policies, practices, and global coordination. Washington, DC: The National Academies Press.
_____. 2008a. Global climate change and extreme weather events: Understanding the contributions to infectious disease emergence. Washington, DC: The National Academies Press.
_____. 2008b. Vector-borne diseases: Understanding the environment, human health, and ecological connections. Washington, DC: The National Academies Press.
_____. 2009a. Global issues in water, sanitation, and health. Washington, DC: The National Academies Press.
_____. 2009b. Microbial adaptation and co-evolution: A tribute to the life and scientific legacies of Joshua Lederberg. Washington, DC: The National Academies Press.
_____. 2009c. Addressing the threat of drug-resistant tuberculosis: A realistic assessment of the challenge: Workshop summary. Washington, DC: The National Academies Press.
_____. 2010. Infectious disease movement in a borderless world. Washington, DC: The National Academies Press.
Jindrak, V., J. Marek, V. Vanis, P. Urbaskova, J. Vlcek, L. Janiga, and V. Maresova. 2008. Improvements in antibiotic prescribing by community paediatricians in the Czech Republic. Eurosurveillance 13(46).
Kadavy, D. R., J. M. Hornby, T. Haverkost, and K.W. Nickerson. 2000. Natural antibiotic resistance of bacteria isolated from larvae of the oil fly, Helaeomyia petrolei. Applied and Environmental Microbiology (11):4615–9.
Kim, S. H., K. H. Kim, H. B. Kim, N. J. Kim, E. C. Kim, M. D. Oh, and K.W. Choe. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrobial Agents and Chemotherapy 52(1):192–7.
Kohanski, M. A., M. A. DePristo, and J. J. Collins. 2010a. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Molecular Cell 37(3):311–20.
Kohanski, M. A., D. J. Dwyer, and J. J. Collins. 2010b. How antibiotics kill bacteria: From targets to networks. Nature Reviews, Microbiology 8(6):423–35.
Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, and C. G. Whitney (Active Bacterial Core Surveillance of the Emerging Infections Program Network). 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. New England Journal of Medicine 354(14):1455–63.
Lafleur, M. D., Q. Qi, and K. Lewis. 2010. Patients with long-term oral carriage harbor high-persister mutants of C. albicans. Antimicrobial Agents and Chemotherapy 54:39–44.
Lederberg, J. 2000. Infectious history. Science 288(5464):287–93.
Levy, S. B. 1992. The antibiotic paradox: How miracle drugs are destroying the miracle. New York: Plenum Press.
———. 2002. The antibiotic paradox: How the misuse of antibiotics destroys their curative powers, 2nd Edition. New York: Perseus Publishing.
Lewis, K. 2007. Persister cells, dormancy, and infectious disease. Nature Reviews, Microbiology 5(1):48–56.
Liu, A., L. Tran, E. Becket, K. Lee, L. Chinn, E. Park, K. Tran, and J. H. Miller. 2010. Antibiotic sensitivity profiles determined with an Escherichia coli gene knockout collection: Generating an antibiotic bar code. Antimicrobial Agents and Chemotherapy 54(4):393–403.
Lloyd, D. H. 2007. Reservoirs of antimicrobial resistance in pet animals. Clinical Infectious Diseases 45(Suppl. 2):S148–52.
Loddenkemper, R., and B. Hauer. 2010. Drug-resistant tuberculosis: A worldwide epidemic poses a new challenge. Deutsches Ärzteblatt International 107(1–2):10–19.
Lu, T. K., and J. J. Collins. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proceedings of the National Academy of Sciences USA 104(27):11197–202.
Lu, T. K., and J. J. Collins. 2009. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proceedings of the National Academy of Sciences USA 106(12):4629–34.
MacDougall, C., and R. E. Polk. 2005. Antimicrobial stewardship programs in health care systems. Clinical Microbiology Reviews 18(4):638–56.
MacPherson, D. W., B. D. Gushulak, W. B. Baine, S. Bala, P. O. Gubbins, P. Holtom, and M. Segarra-Newnha. 2009. Population mobility, globalization, and antimicrobial drug resistance. Emerging Infectious Diseases 15(11):1727–32.
Maiques, E., C. Ubeda, S. Campoy, N. Salvador, I. Lasa, R. P. Novick, J. Barbé, and J. R. Penadés. 2006. β-Lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. Journal of Bacteriology 188(7):2726–9.
Martinez, J. L. 2009. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proceedings, Biological Sciences 276(1667):2521–30.
Mason, M., and M. Mendoza. 2009. Pressure rises to stop antibiotics in agriculture. Associated Press. http://abcnews.go.com/Health/wirestory?id=9435333 (accessed October 25, 2010).
McArthur, A. G., F. Nizam, N. Waglechner, and G. D. Wright. 2010. Towards a comprehensive antibiotic resistance database. 2nd ASM Conference on Antimicrobial Resistance in Zoonotic Bacteria and Foodborne Pathogens in Animals, Humans and the Environment, Toronto, Canada.
McClelland, E. E., A. M. Nicola, R. Prados-Rosales, and A. Casadevall. 2010. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. Journal of Clinical Investigation 120(4):1355–61.
Molstad, S., O. Cars, and J. Struwe. 2008. Strama—A Swedish working model for containment of antibiotic resistance. Eurosurveillance 13(46).
Monnet, D., and K. Kristinsson. 2008. Turning the tide of antimicrobial resistance: Europe shows the way. Eurosurveillance 13(46).
Moy, T. I., A. R. Ball, Z. Anklesaria, G. Casadei, K. Lewis, and F. M. Ausubel. 2006. Identification of novel antimicrobials using a live-animal infection model. Proceedings of the National Academy of Sciences USA 103(27):10414–9.
Mulcahy, L. R., J. L. Burns, S. Lory, and K. Lewis. 2010 (in review). Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. Journal of Bacteriology.
Munoz-Price, L. S., and R. A. Weinstein. 2008. Acinetobacter infection. New England Journal of Medicine 358(12):1271–81.
National Research Council. 2000. The future role of pesticides in U.S. agriculture. Washington, DC: National Academy Press.
O’Brien, T. F. 2002. Emergence, spread, and environmental effect of antimicrobial resistance: How use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clinical Infectious Diseases 34(Suppl. 3):S78–84.
Oehler, R. L., A. P. Velez, M. Mizrachi, J. Lamarche, and S. Gompf. 2009. Bite-related and septic syndromes caused by cats and dogs. Lancet Infectious Diseases 9(7):439–47.
Okeke, I. N., K. P. Klugman, Z. A. Bhutta, A. G. Duse, P. Jenkins, T. F. O’Brien, A. Pablos-Mendez, and R. Laxminarayan. 2005. Antimicrobial resistance in developing countries. Part II: Strategies for containment. Lancet Infectious Diseases 5(9):568–80.
OTA (Office of Technology Assessment). 1979. Drugs in livestock feed. U.S. Congress, Washington, DC.
Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases-a richness of substrates? Trends in Microbiology 9(3):97–102.
Pew Research Center for the People & the Press. July 9, 2009. Public praises science; Scientists fault public, media. Scientific achievements less prominent than a decade ago; A survey conducted in collaboration with the American Association for the Advancement of Science. http://www.sciencedaily.com/releases/2009/07/090709124743.htm (accessed October 25, 2010).
Pimentel, D., H. Acquay, M. Acquay, M. Biltonen, P. Rice, M. Silva, J. Nelson, V. Lipner, S. Giordano, A. Horowitz, and M. D’Amore. 1992. Environmental and economic costs of pesticide use. Bioscience 41(10):750–60.
Pioletti, M., F. Schlünzen, J. Harms, R. Zarivach, M. Glühmann, H. Avila, A. Bashan, H. Bartels, T. Auerbach, C. Jacobi, T. Hartsch, A. Yonath, and F. Franceschi. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine, and IF3. EMBO Journal 20(8):1829–39.
Plachouras, D., D. Kavatha, A. Antoniadou, E. Giannitsioti, G. Poulakou, K. Kanellakopoulou, and H. Giamarellou. 2010. Dispensing of antibiotics without prescription in Greece, 2008: Another link in the antibiotic resistance chain. Eurosurveillance 15(7):1–4.
Poeta, P., D. Costa, G. Igrejas, B. Rojo-Bezares, Y. Saenz, M. Zarazaga, F. Ruiz-Larrea, J. Rodrigues, and C. Torres. 2007. Characterization of vanA-containing Enterococcus faecium isolates carrying Tn5397-like and Tn916/Tn1545-like transposons in wild boars (Sus scrofa). Microbial Drug Resistance 13(3):151–6.
Prins, J. M., J. E. Degener, A. J. de Neeling, and I. C. Gyssens (SWAB Board). 2008. Experiences with the Dutch Working Party on antibiotic policy (SWAB). Eurosurveillance 13(46).
Prudhomme, M., L. Attaiech, G. Sanchez, B. Martin, and J. P. Claverys. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313(5783):89–92.
P.L. 97-414. 1983. Orphan Drug Act, as amended. http://www.fda.gov/ForIndustry/DevelopingProd-uctsforRareDiseasesConditions/Overview/ucm119477.htm (accessed May 28, 2010).
P.L. 110-85. 2007. Food and Drug Administration Amendment Act of 2007. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110cong_public_laws&docid=f:pub1085.110.pdf (accessed October 15, 2010).
Resources for the Future. 2009. New study finds MRSA on the rise in hospital outpatients. http://www.rff.org/News/Press_Releases/Pages/New-Study-Finds-MRSA-on-the-Rise-in-Hospital-Outpatients.aspx (accessed February 7, 2010).
Rice, L. B. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. Journal of Infectious Diseases 197(8):1079–81.
Riedel, S., S. E. Beekmann, K. P. Heilmann, S. S. Richter, J. Garcia-de-Lomas, M. Ferech, H. Goosens, and G. V. Doern. 2007. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. European Journal of Clinical Microbiology and Infectious Diseases 26(7):485–90.
Roberts, R. R., B. Hota, I. Ahmad, R. D. Scott 2nd, S. D. Foster, F. Abbasi, S. Schabowski, L. M. Kampe, G. G. Ciavarella, M. Supino, J. Naples, R. Cordell, S. B. Levy, and R. A. Weinstein. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: Implications for antibiotic stewardship. Clinical Infectious Diseases 49(8):1175–84.
Robicsek, A., J. L. Beaumont, S. M. Paule, D. M. Hacek, R. B. Thomson, Jr., K. L. Kaul, P. King, and L. R. Peterson. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Annals of Internal Medicine 148(6):409–18.
Rohdich, F., K. Kis, A. Bacher, and W. Eisenreich. 2001. The nonmevalonate pathway of isoprenoids: Genes, enzymes, and intermediates. Current Opinion in Chemical Biology 5(5):535–40.
Rutland, B. E., J. S. Weese, C. Bolin, J. Au, and A. N. Malani. 2009. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerging Infectious Diseases 15(8):1328–30.
Salmond, G. P., and M. Welch. 2008. Antibiotic resistance: Adaptive evolution. Lancet 372:S97–103.
Saylor, C., E. Dadachova, and A. Casadevall. 2009. Monoclonal antibody-based therapies for microbial diseases. Vaccine 27(Suppl. 6):G38–46.
Schlundt, J. 2010. The contribution of antimicrobial use in food animal production to the emergence of antimicrobial resistance in humans. Presentation given at the April 6-7, 2010, public workshop on “Antimicrobial Resistance: Implications for Global Health and Novel Intervention Strategies” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Schlunzen, F. R., R. Zarivach, J. Harms, A. Bashan, A. Tocilj, R. Albrecht, A. Yonath, and F. Franceschi. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413(6858):814–21.
Schumacher, M. A., K. M. Piro, W. Xu, S. Hansen, K. Lewis, and R. G. Brennan. 2009. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 323(5912):396–401.
Serrano, P. H. 2005. Responsible use of antibiotics in aquaculture. FAO fisheries technical paper. Rome, Italy: Food and Agriculture Organization of the United Nations.
Shah, D., Z. Zhang, A. Khodursky, N. Kaldalu, K. Kurg, and K. Lewis. 2006. Persisters: A distinct physiological state of E. coli. BMC Microbiology 6:53.
Shea, K. M. 2003. Antibiotic resistance: What is the impact of agricultural uses of antibiotics on children’s health? Pediatrics 112(1 Pt. 2):253–8.
Silbergeld, E. K., J. Graham, and L. B. Price. 2008. Industrial food animal production, antimicrobial resistance, and human health. Annual Review of Public Health 29:151–69.
Singh, N., P. Rogers, C. W. Atwood, M. M. Wagener, and V. L. Yu. 2000. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. American Journal of Respiratory and Critical Care Medicine 162(2 Pt. 1):505–11.
Smith, T. C., M. J. Male, A. L. Harper, J. S. Kroeger, G. P. Tinkler, E. D. Moritz, A. W. Capuano, L. A. Herwaldt, and D. J. Diekema. 2009. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One 4(1):e4258.
Sommer, M. O., G. Dantas, and G. M. Church. 2009. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 325(5944):1128–31.
Souli, M., I. Galani, and H. Giamarellou. 2008. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Eurosurveillance 13(47).
Spellberg, B., R. Guidos, D. Gilbert, J. Bradley, H. W. Boucher, W. M. Scheld, J. G. Bartlett, J. Ed-wards, Jr., and the Infectious Diseases Society of America. 2008a. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clinical Infectious Diseases 46(2):155–64.
Spellberg, B., G. H. Talbot, E. P. Brass, J. S. Bradley, H. W. Boucher, D. N. Gilbert, and the Infectious Diseases Society of America. 2008b. Position paper: Recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clinical Infectious Diseases 47(Suppl. 3):S249–65.
Steward, C. D., J. M. Mohammed, J. M. Swenson, S. A. Stocker, P. P. Williams, R. P. Gaynes, J. E. McGowan, Jr., and F. C. Tenover. 2003. Antimicrobial susceptibility testing of carbapenems: Multicenter validity testing and accuracy levels of five antimicrobial test methods for detecting resistance in Enterobacteriaceae and Pseudomonas aeruginosa isolates. Journal of Clinical Microbiology 41(1):351–8.
Stewart, W. H. 1967. A mandate for state action. Washington, DC: Association of State and Territorial Health Officers.
Stolberg, S. G. 1998. Superbugs. New York Times Magazine, August 2, www.nytimes.com/1998/08/02/magazine/superbugs.html (accessed August 24, 2010).
Sulakvelidze, A., and J. G. Morris, Jr. 2001. Bacteriophages as therapeutic agents. Annals of Medicine 33(8):507–9.
Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrobial Agents of Chemotherapy 45(3):649–59.
Szczepanowski, R., I. Krahn, B. Linke, A. Goesmann, A. Pühler, and A. Schlüter. 2004. Antibiotic multiresistance plasmid pRSB101 isolated from a wastewater treatment plant is related to plasmids residing in phytopathogenic bacteria and carries eight different resistance determinants including a multidrug transport system. Microbiology 150(Pt. 11):3613–30.
Szczepanowski, R., S. Braun, V. Riedel, S. Schneiker, I. Krahn, A. Pühler, and A. Schlüter. 2005. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151(Pt. 4):1095–111.
Szczepanowski, R., T. Bekel, A. Goesmann, L. Krause, H. Krömeke, O. Kaiser, W. Eichler, A. Pühler, and A. Schlüter. 2008. Insight into the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to antimicrobial drugs analysed by the 454-pyrosequencing technology. Journal of Biotechnology 136(1–2):54–64.
Szczepanowski, R., L. Burkhard, I. Krahn, K. Gartemann, T. Gützkow, W. Eichler, A. Pühler, and A. Schlüter. 2009. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology 155:2306–19.
Tamae, C., A. Liu, K. Kim, D. Sitz, J. Hong, E. Becket, A. Bui, P. Solaimani, K. P. Tran, H. Yang, and J. H. Miller. 2008. Determination of antibiotic hypersensitivity among 4,000 single-geneknockout mutants of Escherichia coli. Journal of Bacteriology 190(17):5981–8.
Tennstedt, T., R. Szczepanowski, I. Krahn, A. Pühler, and A. Schlüter. 2005. Sequence of the 68,869 bp IncP-1alpha plasmid pTB11 from a waste-water treatment plant reveals a highly conserved backbone, a Tn402-like integron and other transposable elements. Plasmid 53(3):218–38.
Tenover, F. C., and J. M. Hughes. 1996. The challenges of emerging infectious diseases. Development and spread of multiply-resistant bacterial pathogens. Journal of the American Medical Association 275(4):300–4.
Tenover, F. C., M. J. Mohammed, J. Stelling, T. O’Brien, and R. Williams. 2001. Ability of laboratories to detect emerging antimicrobial resistance: Proficiency testing and quality control results from the World Health Organization’s external quality assurance system for antimicrobial susceptibility testing. Journal of Clinical Microbiology 39(1):241–50.
Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O’Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycinresistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrobial Agents and Chemotherapy 48(1):275–80.
Threlfall, E. J., A. Graham, T. Cheasty, L. R. Ward, and B. Rowe. 1997. Resistance to ciprofloxacin in pathogenic Enterobacteriaceae in England and Wales in 1996. Journal of Clinical Pathology 50:1027–8.
Threlfall, E. J., F. J. Angulo, and P. G. Wall. 1998. Ciprofloxacin-resistant Salmonella typhimurium DT 104. Veterinary Record 142:255.
Ubeda, C., E. Maiques, E. Knecht, I. Lasa, R. P. Novick, and J. R. Penadés. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Molecular Microbiology 56(3):836–44.
Vidaver, A. K. 2002. Uses of antimicrobials in plant agriculture. Clinical Infectious Diseases 34(Suppl 3):S107–10.
Walker, B., S. Barrett, S. Polasky, V. Galaz, C. Folke, G. Engstrom, F. Ackerman, K. Arrow, S. Carpenter, K. Chopra, G. Daily, P. Ehrlich, T. Hughes, N. Kautsky, S. Levin, K. G. Maler, J. Shogren, J. Vincent, T. Xepapadeas, and A. de Zeeuw. 2009. Environment. Looming global-scale failures and missing institutions. Science 325(5946):1345–6.
Walsh, C. 2003. Where will new antibiotics come from? Nature Reviews Microbiology 1(1):65–70.
Walsh, C. T., and M. A. Fischbach. 2009. New ways to squash superbugs. Scientific American 301(1):44–51.
Weber, J. T., E. D. Mintz, R. Canizares, A. Semiglia, I. Gomez, R. Sempertegui, A. Davila, K. D. Greene, N. D. Puhr, D. N. Cameron, F. C. Tenover, T. J. Barrett, N. H. Bean, C. Ivey, R. V. Tauxe, and A. P. Blake. 1994. Epidemic cholera in Ecuador: Multidrug-resistance and transmission by water and seafood. Epidemiology and Infection 112(1):1–11.
Webster, P. 2009. Poultry, politics, and antibiotic resistance. Lancet 374(9692):773–4.
Weinstein, R. A. 2001. Controlling antimicrobial resistance in hospitals: Infection control and use of antibiotics. Emerging Infectious Diseases 7(2):188–92.
Werner, G., T. M. Coque, A. M. Hammerum, R. Hope, W. Hryniewicz, A. Johnson, I. Klare, K. G. Kristinsson, R. Leclercq, C. H. Lester, M. Lillie, C. Novais, B. Olsson-Liljequist, L. V. Peixe, E. Sadowy, G. S. Simonsen, J. Top, J. Vuopio-Varkila, R. J. Willems, W. Witte, and N. Woodford. 2008. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance 13(47).
WHO (World Health Organization). 2000a. Overcoming antibiotic resistance. Geneva, Switzerland: WHO.
_____. 2000b. WHO global principles for the containment of antimicrobial resistance in food animals. Geneva, Switzerland: WHO.
_____. 2001a. WHO global strategy for containment of antimicrobial resistance. Geneva, Switzerland: WHO.
_____. 2001b. Antimicrobial resistance in Neisseria gonorrhoeae. http://www.who.int/drugresistance/Antimicrobial_resistance_in_Neisseria_gonorrhoeae.pdf (accessed March 2, 2010).
_____. 2008. Anti-tuberculosis drug resistance in the world. Fourth global report by the WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance. Geneva, Switzerland: WHO.
_____. 2009. Invasive Haemophilus influenzae type B (Hib) disease prevention. http://www.who.int/nuvi/hib/en/ (accessed July 3, 2010).
———. 2010. Tackling antimicrobial resistance: The Third Global Patient Safety Challenge 2010, http://www.who.int/patientsafety/amr/en/ (accessed March 19, 2010).
Willems, R. J., J. Top, M. van Santen, D. A. Robinson, T. M. Coque, F. Baquero, H. Grundmann, and M. J. Bonten. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerging Infectious Diseases 11(6):821–8.
Wright, G. D. 2010. AMR in the environment and the evolution of resistance. Presentation given at the April 6-7, 2010, public workshop “Antimicrobial Resistance: Implications for Global Health and Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Wright, G. D., and M. Morar. 2010 (forthcoming). The genomic enzymology of antibiotic resistance. Annual Review of Genetics.
Wulf, M., and A. Voss. 2008. MRSA in livestock animals—An epidemic waiting to happen? Clinical Microbiology Infections 14(6):519–21.