Workshop Summary
INTRODUCTION
Despite spending more time and money in developing novel therapeutics, the success rate for new pharmacologic treatments has been poor. Although the research and development (R&D) expenditures have grown 13 percent each year since 1970 (a 50-fold increase), the number of new drugs approved annually is no greater now than it was 50 years ago (Booth and Zemmel, 2004; Munos, 2009). Over the past decade, skyrocketing costs and the complexity of the scientific knowledge upon which to develop new agents have provided incentives for alternative approaches to drug development, if we are to continue to improve clinical care and reduce mortality. These challenges create opportunities for improved collaboration between industry, academia, government, and philanthropic organizations at each stage in new drug development, marketing, and implementation. Perhaps the most appropriate initial step in addressing the need for collaboration is to consider more precompetitive relationships that allow sharing of scientific information to foster drug development.
While these collaborative relationships in basic and preclinical research on drug targets and the early stages of clinical testing are acknowledged to be potentially important drivers for innovation and more rapid marketing of new agents, they also raise a number of concerns that must be addressed. For example, acknowledgment of academic productivity and independence and economic competitiveness must be considered and these
challenges managed to foster a culture of collaboration. At the same time, regulatory issues, the need for standardization, and intellectual property (IP) concerns must be confronted if the current models for drug development are to be refined to encourage robust participation in precompetitive collaborations.
Recognizing the growing importance of precompetitive collaborations in oncology drug development, as well as the challenges these innovative collaborations pose, the National Cancer Policy Forum of the Institute of Medicine (IOM) held a workshop titled Extending the Spectrum of Precompetitive Collaboration in Oncology Research on February 9 and 10, 2010, in Washington, DC. At the workshop, speakers addressed:
-
Current driving forces for precompetitive collaborations;
-
Benefits of such collaborations;
-
Challenges to collaborating;
-
Types of precompetitive collaborations and what can be shared;
-
Precompetitive collaboration examples;
-
Lessons learned and best practices formulated from these examples of collaboration; and
-
Next steps that could facilitate more precompetitive collaborations in oncology drug development.
This document is a summary of the workshop proceedings. The views expressed in this summary are those of the speakers and discussants, as attributed to them, and are not the consensus views of the workshop participants or members of the National Cancer Policy Forum.
Building on the National Cancer Policy Forum’s workshop, the IOM’s Roundtable on Translating Genomic-Based Research for Health held a related workshop on precompetitive collaboration July 22, 2010, titled Establishing Precompetitive Collaborations to Stimulate Genomics Driven Drug Development. A published summary of that workshop is also planned.
CURRENT DRIVING FORCES FOR COLLABORATION
John Wagner, vice president of clinical pharmacology at Merck & Co., began the workshop by pointing out that the notion of precompetitive collaboration is not new, nor is it limited to biomedical applications. A precompetitive collaboration launched by the semiconductor industry
in the 1980s (SEMATECH)1 boosted the global competitiveness of U.S. companies within this industry (see Box 1). The software industry is also known for its precompetitive collaborations, which Stephen Weber defined as “competitors sharing early stages of research that benefit all,” in his book The Success of Open Source (Weber, 2004).
But Wagner said a number of factors are currently driving precompetitive collaborations in biomedicine, most notably the standard drug development model does not appear to be working very effectively. He presented a slide showing that the new molecular entity output per dollar spent on research and development has been declining since 1970 (see Figure 1). In addition, he cited a 2004 analysis of the success rates of compounds making it from first-in-human trials to registration during a 10-year period (1991–2000) for 10 large pharmaceutical companies. The average success rate for all therapeutic areas is approximately 11 percent; in oncology, the probability of graduating from the drug development pipeline and making it to market is only 5 percent (Kola and Landis, 2004). “This tees up the issue of the need for different models of doing research and development, including precompetitive collaborations,” Wagner said.
Several speakers expanded on the shortcomings of current approaches to drug development, suggesting that alternative approaches will be required. Many of the speakers proposed precompetitive collaborations as an approach worthy of careful consideration. Many factors have made the standard model for developing drugs inadequate, they pointed out, including the growing complexity of research and far-ranging and uneven distribution of knowledge, patient variability that contributes to the uncertainty and low success rate, increasing emphasis on comparative effectiveness and evidence-based medicine, the increasingly long and expensive time lines of drug development, and declining research and development budgets.
Increasing Complexity and Data
Many speakers noted the growing complexity of basic and clinical research in oncology, much of which hinges on deciphering the intricate networks of molecular pathways involved in the formation and progression of various cancers, as well as predicting patients’ likely responses to treatments aimed at the targets within those networks. The increasing need to integrate
|
1 |
SEMATECH stands for SEmiconductor MAnufacturing TECHnology (http://www.sematech.org/). |
|
BOX 1 SEMATECH SEMATECH (SEmiconductor MAnufacturing TECHnology) is a collaboration of semiconductor manufacturers that was established in 1987 with the goal of improving U.S. competitiveness of the semi-conductor industry in the global market. William Spencer, chair emeritus of SEMATECH, noted that semiconductors are the backbone of computing power that extends not just to personal computers, but to the microprocessors that are in most appliances, automobiles, and communication and entertainment devices. “The technology is important from the standpoint of [the semiconductor] business, but more so because it drives these other businesses by increasing productivity each year,” said Spencer. In the 1970s, the United States owned 70 percent of the semi-conductor market, but by the 1980s it was rapidly losing market share to other countries, including Japan. Recognizing this, SEMATECH was established as a research and development collaboration among the major U.S. manufacturers of semiconductors. Congress also hoped that improved semiconductor manufacturing would bolster the defense technology base and matched industrial funding through the Defense Advanced Research Projects Agency. Industry members initially were required to contribute 1 percent of their semiconductor sales revenue, with a minimum contribution of $1 million and maximum contribution of $15 million. By 1994, the United States had regained market leadership and SEMATECH was funded solely by the contributions of its members. Over time, SEMATECH’s membership grew to include international companies. A significant accomplishment that contributed to SEMATECH’s success, according to Spencer, was the creation of a long-term semi-conductor technology roadmap. This roadmap laid out the goals of the |
genetics, genomics, and proteomics into new drug development requires data repositories and much more sophisticated information technology (IT) to analyze data. The magnitude of these challenges, speakers noted, may necessitate greater collaboration to ensure access to broader expertise than is often available within a single company or academic institution.
Stephen Friend, president of Sage Bionetworks, illustrated how advances in molecular biology have fueled an explosion of data in the past decade (see Figure 2). “We are going to be swimming in data until models
|
industry, the science barriers that were hampering their achievement, and ways to overcome those barriers. In addition, the financial success of SEMATECH hinged in part on its creation of a piece of equipment that is essential for the manufacture of semiconductors and that is still in use today. Under the umbrella of SEMATECH were about a dozen individual projects, each with a limited focus, such as lithography. There was an oversight committee composed of chief technical officers or the heads of manufacturing from participating companies. Innovations by participants in SEMATECH could only be patented by the specific originators if they shared these innovations royalty-free with all consortium members. Spencer remarked on the surprising willingness of semiconductor companies, which at the time were engaged in cut-throat competition with each other, to work cooperatively to do the research and development needed to propel the semiconductor industry in the United States forward. He attributed part of this willingness to leadership. The founding chief executive officer of SEMATECH, Robert Noyce, brought instant credibility to SEMATECH because of his technical contributions to the semiconductor industry and his success as an entrepreneur. Spencer noted that “three things—crisis, competitive companies coming together, and industry leadership, were essential to getting SEMATECH started.” Spencer concluded his talk by saying, “I am a strong believer that cooperation and collaboration, whatever it is, between government and industry can work … and has had an impact on how research and development in the semiconductor industry is done everywhere in the world today.” SOURCES: Spencer presentation (February 9, 2010) and IOM, 2007. |
can be made” that make sense of the data, Friend said. Bryn Williams-Jones, associate research fellow and head of eBiology at Pfizer, concurred, noting that “in spite of knowing a lot more and having a lot more data to go on, we are actually getting worse at finding out anything, and are not much more productive.” He called for more data analysis standards so that more valid conclusions can be drawn from the data acquired. Developing such standards will require a collaborative effort.
Williams-Jones suggested that companies should not develop their
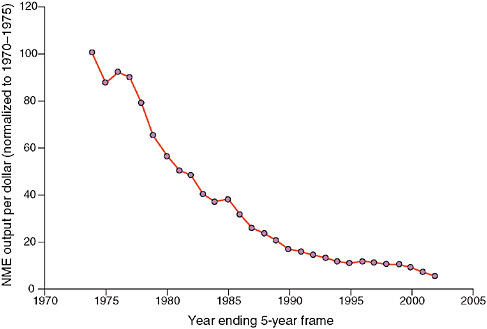
FIGURE 1 30-year decline in new molecular entities per dollar spent on research and development (R&D). There has been a 30-year decline in pharmaceutical industry productivity, as measured by new molecular entities per dollar spent on R&D, normalized to 5-year rolling average of 1970 to 1975. While research and development costs have increased 50-fold during this time period, the output of investigational new drug candidates and new drug application products has stayed flat.
NOTE: NME = new molecular entity.
SOURCES: Wagner presentation (February 9, 2010) and Booth and Zemmel (2004). Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Drug Discovery, Booth, B., and R. Zemmel. 2004. Prospects for productivity. 3(5):451–456, copyright 2004.
own costly information technology infrastructures, but rather join a collaborative endeavor that provides that infrastructure, ideally in the virtual public domain. “We are a drug discovery industry, and none of us can afford to reinvent and source an entirely proprietary software system that is going to be able to help us deal with this. We should be doing that in the public domain,” he said. “Even for Pfizer, which has one of the world’s largest R&D budgets, it would be naïve to expect that we have wide enough domain expertise. We should focus our time thinking about what is and what isn’t competitive…. As we stand at a crossroad, expecting lots more data to come with not much more money to spend on it, we ought to think about whether we are going to continue internalizing or move into
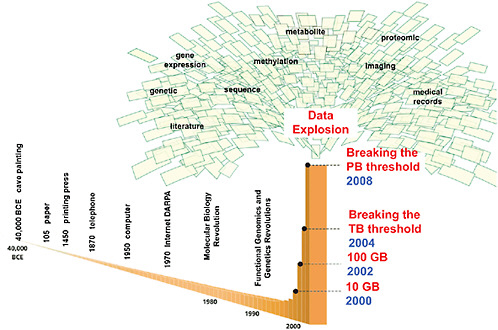
FIGURE 2 Advances inmolecular biology, functional genomics, and genetics have fueled an explosion of data. Computational methods for integrating massive molecular and clinical datasets are needed to create predictive disease models that can recapitulate complex biological systems, according to Friend. Models can inform understanding of disease causality and can generate new mechanisms, targets, diagnostics, and knowledge.
NOTE: BCE = before common era, DARPA = Defense Advanced Research Projects Agency, GB = gigabyte, PB = petabyte, TB = terabyte.
SOURCE: Friend presentation (February 9, 2010). Reprinted, with permission, from Eric Schadt and Stephen Friend.
the virtualization phase. Given that we have all built these overlapping very similar IT systems, if we did this once properly in the public domain, that might actually cause a rising tide that floats all the [pharmaceutical industry] boats,” Williams-Jones added.
A collaborative effort is also needed to create complex models of the causes and treatment targets of cancers, Friend said. Drug development models that depend on simple pathway approaches are no longer appropriate, he pointed out, because studies indicate that when one pathway that fuels cancer growth is blocked, a redundant pathway will enable the cancer to thrive. He showed one slide that illustrated the complex transcriptional networks involved in the growth of brain tumors (see Figure 3) and stressed that “people are recognizing that these cells and disease states are intricately
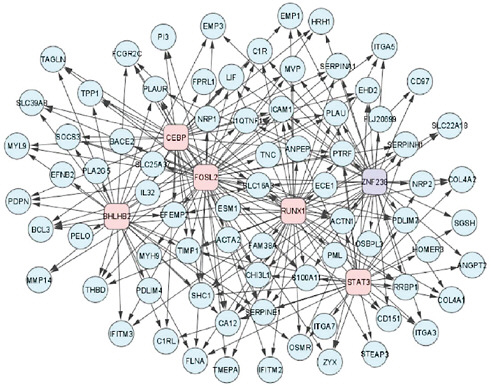
FIGURE 3 A network of transcription factors (boxes) and their mesenchymal gene expression signature targets (circles) involved in high-grade glioma.
SOURCES: Friend presentation (February 9, 2010) and Carro et al. (2010). Reprinted by permission from MacMillan Publishers Ltd: Nature, Carro, M. S., W. K. Lim, M. J. Alvarez, R. J. Bollo, X. Zhao, E. Y. Snyder, E. P. Sulman, S. L. Anne, F. Doetsch, H. Colman, A. Lasorella, K. Aldape, A. Califano, and A. Iavarone. 2010. The transcriptional network for mesenchymal transformation of brain tumours. 463(7279):318–325, copyright 2010.
wired networks that are brilliantly built through evolution with redundancy, which is why so often a drug does not work the way you thought it would, and does all those things you hadn’t expected.” He added that given their complexity, “no one company is going to be able to afford to have the best map of those networks for very long, even if they invest heavily.”
David Wholley, director of the Biomarkers Consortium, agreed with Friend that the bigger picture required to understand and treat cancer is causing a paradigm shift in biomedical research and drug development that requires new approaches. “The increasing complexity, amount of data, and downstream effects on regulatory science are leading to the dawning realization that nobody is smarter than everybody else,” Wholley said.
Neal Cohen, vice dean and professor at the University of California–San Francisco (UCSF) School of Medicine, added that biomedical research is increasingly a multidisciplinary venture dependent on much more difficult research methodology, both of which fuel the need for more collaboration. To be successful, he noted, individual investigators increasingly rely on collaborators to gain expertise outside an individual investigator’s discipline.
Karim Lakhani, an assistant professor at Harvard Business School, concurred, pointing out how knowledge is unevenly and widely distributed so that “no one organization or set of actors can monopolize knowledge…. This is the fundamental problem we face in our pharma business now. If you think about the explosion in research, the specialization that happens across disciplines, there is no way we can just be in our little silo and innovate, especially when diseases are multicategorical, multisymptomatic, and multicausal. We need to think of new ways to access this type of knowledge. We have reached the limits and we have to work together because you can’t do it alone.”
Lakhani noted that the widely distributed nature of knowledge is also evident within Joy’s law,2 which states: “No matter who you are, most of the smartest people work for someone else.” To illustrate the distributed nature of knowledge, he gave the example of Robert Langer, an expert in tissue engineering at Massachusetts Institute of Technology (MIT). Langer collaborated with about 40 percent of the most prolific authors of journal articles on the topic during 2004–2006. Although Langer is central in the domain of his field, his publications were only a fraction of the 6,000 articles published on the topic in that 2-year period by 17,000 authors in
the field, a network map constructed by Lakhani showed (see Figure 4). “There is no way that Langer or his team can know exactly what is going on in the entire domain,” Lakhani said.
Another of Lakhani’s network maps showed that the number of publications by a science team at a large pharmaceutical company was dwarfed by the multitude of publications by other research teams in the field. “They thought that they were the cat’s meow in this area of neuroscience, but they were really quite marginal,” said Lakhani. “This is the problem faced by most organizations—most of the smartest people don’t work for them.”
Patient Variability
Friend stressed that most standard-of-care cancer treatments available today are effective in only a minority of patients, in part because of the tremendous variability in the molecular abnormalities driving tumor formation (IOM, 2007; PCAST, 2008; Spear et al., 2001), which standard drug trials do not consider. Those clinical trials that do try to account for such variability with standard trial designs often need thousands of volunteers, which make the clinical trials costly, risky, and lengthy, noted Laura Esserman, director of the Carol Franc Buck Breast Care Center at UCSF. She pointed out that breast cancer has several different subsets of the disease that respond differently to the same breast cancer drugs. “If you are not able to use a biomarker to tell you how to subset that patient population or to target [their specific disease], you are going to need 10 times as many patients to get an answer, and you are more likely to miss the benefits of certain drugs. We have turned breast cancer into a group of orphan diseases, and that is really going to be the step forward for every disease,” Esserman said.
Cohen added that “there is great interest in comparative effectiveness studies to assess which therapies are most appropriate for individual patients, and to define personalized approaches to clinical management. All of that is very different from what we have done in the past, and necessitates a different model.”
Mark McClellan, director of the Engelberg Center for Health Care Reform at the Brookings Institution, concurred that an absence of validated markers for patient subsets makes trials longer and less predictable, as does an absence of validated disease models. This hampers the development of innovative therapies and leads to more company efforts being spent devel-
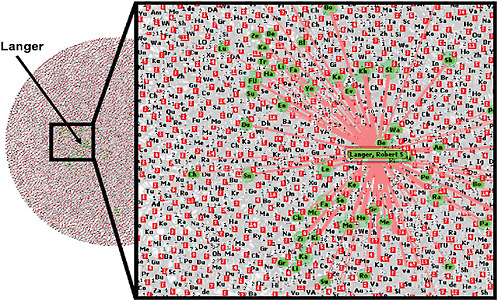
FIGURE 4 Distributed nature of knowledge. A network map of all authors who published 2 or more articles on tissue engineering from 2004 to 2006 included more than 17,000 authors and 6,000 articles. Authors highlighted in green have more than half of their articles coauthored by Robert Langer, an expert in tissue engineering. Central in the domain of his field, Langer collaborated with 40 percent of the most prolific authors; however, his publications represent only a fraction of the articles published during this 2-year time period.
SOURCE: Lakhani presentation (February 9, 2010).
oping reliable “me-too” drugs, Cohen noted. “There are not a lot of breakthrough therapies on the front lines that change how we manage patients, and how patients will respond to the therapies,” he said. Thinh Nguyen, counsel for Science Commons, added that there is also an increasing reliance on a few blockbuster drugs, rather than diverse sources of revenue by drug companies.
Precompetitive collaborations that combine datasets could provide enough data to validate both disease models and biomarkers, thereby reducing the uncertainty in current drug development, and improving the success rate and the willingness of drug companies to undertake the development of innovative therapies, according to McClellan.
Raymond Woosley, president and CEO of the Critical Path Institute (C-Path), added, “there is a clear need for innovative drug development. The long cycle time we think is at the core of why companies are willing to share, to meet regularly when they all have day jobs, to define the common data elements that they will all use to obtain qualification of biomarkers for use as an indication by the regulators.”
Declining Research and Development Budgets
Many drug companies’ research and development budgets have been declining. Cohen noted that Pfizer plans to reduce its R&D spending by $3 billion by 2012, AstraZeneca has cut its research staff by 3,500 employees, and Sanofi-aventis is cutting its R&D spending by 20 percent (Rockoff, 2010). At the same time, academic institutions are facing flat or declining funding from federal sources, which remain the largest contributor to academic R&D. Eighty-four percent of federal R&D funding is granted by the National Institutes of Health (NIH); however, inflation-adjusted NIH funding went from doubling during 1994 to 2003 to a 2.2 percent decline in 2008 (Boat, 2010; Dorsey et al., 2010). Although non-federal funding sources have mitigated this decline somewhat, Boat (2010) notes that this trend is unlikely to continue due to economy-driven shrinkage of endowments, philanthropy, business profits, and tax revenue. “Clearly the downward pressure on R&D budgets has driven folks to play together more than to compete,” said Stephen Eck, vice president of translational medicine and pharmacogenetics at Eli Lilly and Company. “That is probably a good thing because budgetary pressures do drive efficiencies, and I think it makes us realize that we do not need to do and own everything.”
Collaboration-Enabling Technology
More positive forces are also propelling collaboration, including having the technology available to develop personalized medicine, having more quality information in the public domain, and having lower barriers to information sharing, noted several speakers.
The quantity of data in the public domain has grown dramatically, and the quality of the data has also improved vastly, Nguyen said. In addition, those data have become more useful and accessible with the increase in open-source and other public tools for data analysis and exploitation. “What is available in the commons is starting to be almost as good as what companies can develop themselves internally,” he said.
Nguyen and Jill Altshuler, founder and principal of AltshulerGray, stressed that there also seems to be greater consensus among scientists that data should be shared virtually and in the public domain. They pointed out that the Internet is enabling new kinds of distributed collaborations that involve virtual communities, which can easily network. “There’s been a democratization of science and health care with more information online,” Altshuler said.
Eck summarized the forces driving collaborations by saying “timing is everything, and right now we have a very good environment to try to encourage companies to find common platforms.”
BENEFITS OF COLLABORATING
Speakers pointed out numerous benefits of precompetitive collaborations. In addition to making drug development quicker and less redundant, risky, and expensive, precompetitive collaborations can foster a productive synergy that promotes thinking outside the box, brings in researchers with diverse expertise, and sparks innovation. By combining datasets and having more reviewers of the data analyses, research collaborations also have more statistical power and less bias, which makes their conclusions more reliable and amenable to regulation. Research collaborations also have the potential to more rapidly close the knowledge gap to further progress in the biomedical field. In addition, by creating a bigger value pie, each participating company, individual, or institution can be rewarded with a slice of a bigger value that can result in more profits downstream from the collaboration, and a competitive advantage over those who do not participate in the collaboration.
Synergy of Cross-discipline/Cross-institution Collaborations
Cohen pointed out that newly developing technologies and products benefit from integrating knowledge and expertise from multiple sources. Bernard Munos, advisor in corporate strategy at Eli Lilly and Company, agreed. Munos suggested that some of the big breakthroughs of the twentieth century, such as the development of antibiotics, radiotherapy, the purification of insulin, and even some advances in molecular biology, occurred because pharmaceutical companies were able to harness science that was tangential—or even alien—to pharmacology (Munos, 2010). For example, when Eli Lilly licensed insulin in the 1920s, there was no technology to extract and purify proteins, nor was there a supply network to collect large amounts of glands from slaughterhouses (Munos, 2010). By taking on these challenges, Munos noted that the company’s work resulted in the introduction of Iletin, the first commercially available insulin product (Lilly, 2010), because Lilly was able to reach outside its core competencies. Munos noted that this is in sharp contrast to the tightly scripted target-based drug discovery process that has emerged in the pharmaceutical industry over the past couple of decades: “Sciences that lie outside the field of what can typically be encountered in pharmaceutical companies have and have had significant contribution to the development of therapies, but you don’t find them very often in drug companies today,” Munos said. “How many physicists are working in pharmaceutical research?” He added that many drug companies are not harnessing new technologies such as nanotechnology or stem cells because it falls outside their domain. In addition, Munos cited an analysis that found that breakthroughs in biomedical science typically emerge from scientists with considerable scientific diversity who tend to be boundary-crossers and can communicate well with scientists in multiple fields (Hollingsworth, 2007).
Another way to harness diverse expertise is to do “insourcing.” Altshuler gave the example of Biogen’s Innovation Insourcing Initiative. With this initiative, Biogen actively seeks academics with late-stage biology ideas who are having difficulty attracting additional basic research funding from NIH, but are not yet advanced far enough to attract venture capital. Biogen brings in these scientists working on something relevant to one of their therapeutic areas and provides them with access to all of Biogen’s resources for drug discovery and development. “The idea is that Biogen recognizes it doesn’t have all the great ideas resident within its own organization, so it leverages ideas from the outside to which it wouldn’t otherwise have access,” said Altshuler.
Prizes awarded to those who solve scientific problems is another way to tap a broad array of expertise. Usually the prize winner is someone whose expertise is tangential to the field in which the problem emerged, Lakhani noted. For example, the winner of the prize for developing a device that could accurately determine longitude was given to an unknown clockmaker, despite Isaac Newton’s proclamation that only an astronomer could succeed at the task. “Newton believed this because he did a local search based on his knowledge domain. We always do a local search when we are trying to solve a problem—we apply our methods, experience, and training to the problem. Newton was an astronomer, so of course he said the only way to get the solution is with astronomy,” Lakhani said.
A more current example of the productive synergy of collaboration is that of the contests held by Innocentive.3 This organization takes unsolved problems from R&D laboratories and firms, and challenges scientists around the world to solve those problems. The winner receives a financial award. Lakhani’s analysis of these contests (Jeppesen and Lakhani, in press) demonstrated that winners often have technical expertise outside of or marginal to the problem field, and that the more heterogeneous the solver population—that is, the competitors—the more likely the problem will be solved. According to Lakhani, about 30 percent of problems taken on by Innocentive are solved, but they usually aren’t solvable in most organizations.
The MATLAB Programming Contest4 is another example of a semi-annual contest that invites individuals to submit their code to a virtual platform, shared by contestants, to solve computer programming problems. In one MATLAB contest, called peg solitaire, the goal was to develop the most optimized algorithm and central processing unit time. However, the MATLAB contests are unique because they have three distinct phases: darkness, twilight, and daylight. During the darkness phase, individuals submit their code and receive a score based on performance. In the twilight phase, contestants submit the code, receive a score, and are able to see who is on the leader board. During the daylight phase, contestants can see codes submitted by others, and can modify and resubmit those codes. Once the contest enters the daylight phase, Lakhani noted that there is a dramatic improvement in performance as individuals build on other competitors’ codes. Speaking to the synergy of collaborations, the winning code in the peg solitaire contest contained only 40 percent of code written by the winning contestant; the remaining 60 percent of the code was borrowed from 30 other contestants.
To more definitively test whether collaborative contests are more productive than non-collaborative ones, Lakhani conducted a 2-week contest to solve a genomics problem. During the first week the contest was fully competitive, akin to the Innocentive contest, but during the second week there was information sharing akin to what was done in the MATLAB contest. Lakhani’s analysis of the contest’s results reveals the power of collaboration. The top 34 entries exceeded the state of the art in computational biology by a factor of 100 to 1,000. Ten different approaches were used, only two of which were found in genomics literature. Most contestants entered during the competition phase, but the collaborative phase performed better and was more efficient. “This is exactly how science gets done,” Lakhani said. “In most scientific endeavors we have many labs chasing [the same problem], trying to find the magic answer. We have lots of inefficiency going on. There is a race that people are trying to win. The collaborative phase is where we change the rules and say we want collective outcomes and people to share the rewards together.”
William Spencer, chair emeritus of SEMATECH, concurred about the productive value of collaborations and the synergy they can create. “There is a logical inconsistency in the notion that every company or organization has the best people. In general, what happens is one company or one organization may have the best organic chemists, another one the best microbiologists. If you can find a way to bring these people together in an environment where they can cooperate, the returns you get from that are much greater than those from individuals who are working solely inside their own companies,” Spencer said.
Improved Validity
Cohen called for collaboration, not only in early stages of research, but in the clinical testing of potential drugs. He noted that testing in large and diverse populations is needed to assess patient variability in response to drugs, and this may be best accomplished by doing collaborative clinical research that employs community physicians. Such research could be aided by electronic health records. “We need to engage the broader clinical science community and the community providers,” he said.
Another action that may help in this regard is to engage a broader group of patients via patient websites and networks. James Heywood, co-founder and chair of PatientsLikeMe, said that his studies have heightened statistical power, as well as an enhanced ability to do cohort
matching, because of the voluminous and detailed personal medical data that patients submit to his website, PatientsLikeMe.com, which can be considered a collaborative effort on the part of patients. The website has developed tools that enable the sharing and prospective analysis of data, particularly personal health information about rare diseases because those data are difficult to amass at one institution. “We measure variables prospectively in communities using what we believe will ultimately become a personalized discovery platform that has the ability for one person to use this platform alone, or instantly form collaborations with others, to identify novel and new disease biomarkers, and treatments that work,” Heywood said.
Heywood asserted that the data gathered in this manner can be of better quality than data gathered in typical clinical trials, as exemplified by the data the site gathered on whether lithium forestalled progression of amyotrophic lateral sclerosis (ALS). A PatientsLikeMe analysis determined that lithium was not effectively improving symptoms or delaying disease progression in patients who took the drug 18 months after preliminary evidence in the medical literature suggested it might effectively treat ALS. Heywood said his study had four times the power of the original preliminary study and used patient volunteers from the real world (IOM, 2010a). PatientsLikeMe disseminated their findings about lithium to their ALS members a year before a large-scale clinical trial testing lithium as an ALS treatment was stopped because of futility.
Joseph Vockley, director of The Cancer Genome Atlas (TCGA), pointed out the advantage of collaborative research in helping to eliminate bias and providing additional checks and balances that improve the validity of research results. TCGA is a large collaborative effort among government, academia, and industry aimed at sequencing the genomes of various types of tumors to aid the discovery of new drug targets (see Box 2). Not only has this collaboration generated a high level of statistical power in their analyses, he said, but because the participating investigators are constantly challenging the quality of data collected and each others’ interpretations of the data, valuable checks and balances are in place. “Frequently, if you have a single principal investigator being funded to do research in an area, they are imparting some of themselves, some of their thinking into the data that they are generating and its interpretation, so you could look at this as reflecting an individual bias in the data. When you have a large group of people meeting to discuss the data, you end up getting a consensus result,” Vockley said.
|
BOX 2 The Cancer Genome Atlas The Cancer Genome Atlas (TCGA) is a large collaborative project whose participants include representatives from government, academia, and industry. The project is designed to facilitate future discovery of pharmaceutical and diagnostic targets in cancer by generating genome characterization data on 20 tumor types for a statistically significant number of tumors (500 cases per tumor type) and matched normal tissue. TCGA began as a pilot project to develop infrastructure needed to systematically characterize genomic changes in hundreds of tumors. To date, the pilot project has achieved comprehensive sequencing, characterization, and analysis of genomic changes in glioblastoma multiforme and ovarian cancer. According to the TCGA website, the success of the pilot project, as exemplified by the broad use of the publicly accessible datasets, provided rationale to expand the project. The National Institutes of Health announced in September 2009 that it is investing $275 million in TCGA over the next 2 years to sequence and characterize genomic changes in 20 types of cancer. This project is expected to take 5 years to complete. TCGA is cofunded and comanaged by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI). “The advantage of this collaboration is that we at the NCI are learning a lot of new ways to get to the same goals, but by different methods, and the NHGRI I hope is learning the same thing,” said Joseph Vockley, director of TGCA. “We both have our ways of doing things, so bringing our two methods of management together strengthens the project and gives us a much more diverse set of expertise in the management staff.” TCGA has four types of centers, three of which are funded by NCI, and the fourth by the NHGRI:
|
Hundreds of tissue accrual sites are providing retrospective samples for TCGA, as well as other sites that are providing tissues prospectively. All the samples initially go into two biospecimen core resources, which are funded by NCI, for processing before they are sent to the genome characterization and sequencing centers. All data generated at these centers are entered into the data coordinating centers, with no transmission of data among centers within the project. That way, everyone in both the community and the network gets access to all data at the same time. Data are provided in an open-access format on the web so that researchers in the community can download the data and run their own analyses. Eventually data analysis software tools will be posted to the website. Nineteen centers across the United States are involved in TCGA, not including centers who are participating in tissue accrual (see the following table for a list of centers involved in various aspects of TCGA). A single steering committee composed of the many principal investigators from all the centers make decisions for TCGA on monthly calls. Decision making among such a large group can be difficult, Vockley noted. Therefore, the much smaller executive committee composed of five of the principal investigators (which he called a kick squad) is instrumental to this decision making. “They go after the important issues that are threatening the project” and make related recommendations to the steering committee, Vockley said. “This group of five can help to facilitate getting these decisions made, or at least bringing the subjects of concern up to the entire group.” “TCGA is there to bring a large dataset of information to all the various communities—academia and industry—so that everybody can start their discovery process from a platform or from a single level that will hopefully accelerate discovery,” Vockley explained. |
|
NOTES: Baylor = Baylor College of Medicine; BCCA = British Columbia Cancer Agency; Brigham/Harvard = Brigham & Women’s Hospital and Harvard Medical School; Broad/Harvard = Broad Institute of the Massachusetts Institute of Technology and Harvard University; Harvard = Harvard Medical School; Hopkins = Johns Hopkins University; HudsonAlpha = HudsonAlpha Institute for Biotechnology; IGC = International Genomics Consortium; ISB = Institute for Systems Biology; Lawrence Berkeley = Lawrence Berkeley National Laboratory; MD Anderson = MD Anderson Cancer Center; Nationwide Children’s = Nationwide Children’s Hospital; NCICB = National Cancer Institute Center for Bioinformatics; Sloan-Kettering = Sloan-Kettering Institute; SRA = SRA International; UC Santa Cruz = University of California Santa Cruz; UNC = University of North Carolina; USC = University of Southern California; WUSM = Washington University School of Medicine. SOURCES: Vockley presentation (February 10, 2010) and NCI and NHGRI, 2010a,b,c. |
McClellan agreed. “For broad-based collaborations, the opportunity for consensus building [by] bringing different viewpoints together … provides an ability to not only make sure the right questions are being asked and addressed effectively,” but also builds consensus for more confident regulation, he said. He added that the Food and Drug Administration (FDA) is more likely to approve a treatment faster, via accelerated approval, priority review, or other mechanisms, if there is consensus that treatment will be beneficial and that the studies showing the treatment’s safety and effectiveness have been adequately done with many checks and balances.
Spencer added that having the best minds from several fields collaborate can reduce the risk involved in developing a new product, which is another benefit of collaboration. These experts, who understand the capabilities and shortcomings of their specific niches, are more likely to make the right decisions and avoid costly mistakes if they work together, he said.
Closing Knowledge Gaps and Exploiting Unused Data
Munos pointed out that large gaps in our knowledge of cell biology exist. He noted that about 40 percent of human genes (or around 8,000) are unannotated (Munos, 2010). “It’s very difficult to build a model when it rests on a system, 40 percent of the makeup of which is unknown,” he said. Munos called for open innovation and collaboration to close the genome knowledge gap that is hampering progress in developing biomarkers. McClellan added, “There clearly are some major gaps in the ability to target treatments effectively related to these underlying development science uncertainties.” Friend agreed: “In biomedical research, we need an open-access platform to figure out what is going on because the data and models are much more complex than people had anticipated” (see Box 3).
Eck noted that opportunities are also missed if collaborations do not occur—not from the standpoint of getting a drug approved, he said, but because “there is a huge amount of very good science that does not get done because it is not needed for an FDA approval, yet those tools and information are there in the background and they never surface in an interesting way. I stumbled across a project where an academic investigator had a very good science question and Lilly and another pharmaceutical company had the raw data that could be used to answer it. We are missing out on many of these opportunities if we do not find these precompetitive spaces to work in.”
Altshuler added that collaborations can focus people’s efforts on rare diseases or problems that may otherwise receive less attention (see Box 4).
Foundations for research on various diseases, for example, have spurred (and funded) research collaborations on disorders that large pharmaceutical companies tend to ignore because they have limited market potential. The publicity generated by large-scale collaborations can also focus the public, FDA, and other policy makers’ attention on issues that need to be addressed. McClellan agreed, saying, “It is a great way to highlight outstanding issues in a high-profile way through conferences, reports, and other activities that the collaborations can produce.”
Increase the Size of the Value Pie
An important benefit of precompetitive collaboration is that it increases the size of the value “pie” by enabling innovation that would not have occurred otherwise, and by reducing the cost and risks of that innovation for each of the collaborators, Altshuler stressed. “Precompetitive collaborations can’t be exploited as a near-term, stand-alone, profit-making opportunity, but often they are a crucial step to get to a downstream profit-making opportunity,” she said. “What we have seen is that businesses can cooperate and collaborate to increase the size of the pie, while they continue to compete around how they are going to divide up the pie.” Williams-Jones added that the rising tide of collaborations and the information and standards it generates “is going to raise all of us, whether it is me in my juggernaut, or people in their smaller boats.”
Altshuler added that prize mechanisms that induce collaboration are especially cost-effective because, although the prizes are large sums of money, they are being spent only on successes, not failures. “Much of the cost of paying for a new drug is paying for failure, so a prize can have a very nice return on investment versus in-house research and development,” she said.
Pearl Huang, vice president of oncology at Merck & Co., who forged the Merck–AstraZeneca collaboration on a combination therapy for cancer, stressed that she does not view this collaboration as precompetitive. “I see it as something that, if it works, will give us a huge competitive advantage,” she said (see Box 5).
Shorten Drug Development Time Lines and Improve Efficiency and Cost-Effectiveness
Several speakers pointed out that economies of scale and scope generated by collaborations should speed up the pace of drug development simply
|
BOX 3 Sage Bionetworks Sage Bionetworks is a nonprofit foundation created in 2009 to provide a commons for the creation of disease models based on the assembly of coherent biomedical data into probabilistic and integrative bionetwork models. These models evolve via modifications made by contributor scientists. The ultimate mission of Sage is to accelerate the elimination of human diseases. Sage has several active partners, including the National Cancer Institute’s Clinical Trials Cooperative Group Program, universities, government agencies, foundations, pharmaceutical companies, information technology and tool providers, and patient advocacy groups. These partners contribute datasets or information technology and tools to Sage’s commons that should enable researchers to build integrative network models to describe various disease processes. Sage’s main strategic priorities are to:
According to Stephen Friend, president of Sage Bionetworks, achieving these goals requires establishing the rules and governance |
because more resources will be devoted to the task. “It will accelerate discovery if by no other reason than the sheer mass of action,” Vockley said.
In addition, by reducing redundancies, the resources of all of the collaborators can be devoted to the collaborative enterprise. As Williams-Jones noted, when collaborations do not occur, “every company has got their own more or less same pipeline, more or less same data integrated more or less the same way, and we are wasting large piles of money.” Cohen added that by reducing the redundancies involved in creating infrastructure and the technologies needed to conduct research, collaborations will reduce costs “and our best efforts will be put into the collaboration, which will result in improved benefit to the public.”
Progress will also be quicker because there will be a strong foundation
|
of the commons. In that regard, Sage has held several meetings and congresses with stakeholders to create and ratify governance rules for how data and models will be shared in the commons, and how models created will be cited, as well as to determine the standards and tools that will be used to enable data integration. Friend expects that researchers, companies, government agencies, and foundations to want to contribute to Sage’s repository of data and models because they will benefit from the “neighboring zones of information that will be developed by others. As that builds out soon, their own research will be much more informed by what others have done.” Friend views Sage Bionetworks as a foundation that “has a job to nurture something which is bigger than it is—the commons and the platform.” He pointed out that all the data, as well as the models built from them, will be put in the public domain. “We cannot work with a group unless—after an interval of time in which we are generating a model—that model, the data, and the meta-data are all dropped into the public domain,” Friend said. “Biomedical research and an understanding of disease models are going to be driven by open access to data and models, and a platform where that can be done. That’s what we need in order to move forward.” SOURCE: Friend presentation (February 9, 2010). |
from which to build. “If you are living in a world where your competitive advantage begins from a base of knowledge of the disease biology, I guarantee it will shorten the time to develop a drug,” said Friend. Esserman noted that the I-SPY 2 TRIAL,5 a public–private collaboration, is trying to do away with the current drug development model that takes 10 years, $1 billion, and thousands of volunteers to take one drug to market. She estimated that her collaborative clinical trial of biomarkers and treatments for breast cancer could cut the amount of clinical testing time for a new drug in half,
|
5 |
I-SPY 2 TRIAL (Inspection of Serial studies to Predict Your Therapeutic Response with Imaging And moLecular analysis 2) is a Phase II multisite clinical trial testing multiple experimental drugs while simultaneously assessing the effectiveness of various biomarkers to predict response to the investigational agents (see section on what to share). |
|
BOX 4 Open Source Drug Discovery This global consortium led by the government of India, which provided $38 million of seed funding for it, aims to provide a global virtual platform where researchers can collaborate and collectively discover drug therapies for malaria, tuberculosis, and other diseases that cause major health care problems in the developing world. Launched in 2008, Open Source Drug Discovery (OSDD) has more than 2,000 participants, including students, scientists, academic institutions, and companies. Membership is open to anyone and members can commit to giving funds or sharing resources. OSDD structures its online forum by breaking down large, complex problems, such as how to develop effective therapies for tuberculosis, into smaller work packages, such as annotating the tuberculosis bacterium’s genome, identifying drug targets and their expression, screening compounds to see if they inhibit targets, optimizing non-toxic compounds found to hit the targets, and pre-clinical and clinical testing of the inhibitors. OSDD will accept any idea, software data, article, or molecule that might aid such drug discovery, and will provide funding for clinical testing of promising potential drugs. Each activity or solving of a defined problem on the platform is linked to a predetermined set of credit points. Based on the points accrued, contributors are awarded four levels of membership, each with certain sets of rights, privileges, and responsibilities. OSDD is committed to releasing any eventual drug whose discovery and/or development it fosters free of intellectual property encumbrances. According to Bernard Munos, advisor in corporate strategy at Eli Lilly and Company, OSDD’s Connect2Decode initiative to annotate the genome of the tuberculosis bacterium recruited more than 800 scientists with appropriate expertise within weeks of being launched, and the entire project to annotate more than 1,000 genes is expected to take only 4 months. “This provides a model of how to eliminate the knowledge gap,” said Munos. SOURCES: Munos presentation (February 9, 2010) and OSDD, 2010. |
|
BOX 5 Merck–AstraZeneca Preclinical and Clinical Testing Collaboration AstraZeneca and Merck have established an innovative collaboration in which each company is contributing one of their own investigational compounds for a two-drug combination therapy that is expected to be more effective than either compound used alone. Although combination therapy for cancer is standard, such combinations are usually tested late in clinical development or after registration, or a new potential treatment is tested in combination with standard therapy. In addition, most combination therapies involve two or more drugs aimed at the same target. In contrast, the AstraZeneca compound had gone no further than Phase II clinical testing, and the Merck compound had only been tested in 100 people when the two companies decided to do preclinical testing of both compounds together. In addition, each compound hits a different target. Together, the compounds are expected to have greater effects on tumors than individually because of their complementary action in an oncogene growth factor signaling network. As Pearl Huang, vice president of oncology of Merck & Co. explained, there are two divergent signaling pathways, called MEK (mitogen-activated protein kinase 1) and PI3K (phosphatidylinositol-3 kinase), downstream from a tyrosine kinase signaling pathway known to foster cancer growth. When the PI3K pathway is inhibited, it triggers the MEK backup pathway to become more active via a growth factor signaling loop. Merck had developed a compound that blocks the MEK pathway, and AstraZeneca had developed a compound that blocks Akt (protein kinase B), a component of the PI3K pathway (see figure in this box). So combination therapy with both compounds is likely to be much more effective than either compound alone. “It was a scientific argument that was irresistible,” said Huang. “If you are going to break a barrier for the first time, it is critical to find that sweet example where people just cannot say no.” Before they decided to collaborate, both Merck and AstraZeneca had shown that their compounds could selectively and effectively block their targets in preclinical studies, and that they were safe in early human trials. The two companies forged an agreement that enabled joint preclinical testing of combination therapy with them. When that testing showed promising results, the two companies agreed to do a |
|
joint Phase I testing of the combination, which began in December 2009. “That was record time for both organizations in terms of moving forward from intent to first-in-human testing for something as complex as this,” Huang noted. Together the companies designed a testing plan that would assess the dose, sequence, and context of the combination, including subpopulations in which to test their combination of compounds. The collaboration agreement between Merck and AstraZeneca was staged so that initially it was just an agreement that covered preclinical rights and preclinical scope, and then expanded to include the clinical testing agreement. Decision rights and costs are shared under joint governance in the collaboration. In case conflicts arose, the agreement also included a conflict resolution roadmap, “but so far the team has not had to use it because there is a very compelling argument for doing these experiments, and there is a compulsion within the team to do what is right,” Huang said. The agreement includes a freedom-of-operation clause for both parties that enable each to undertake multiple combination studies with similar agents that can occur independently and in parallel. “Both parties have other compounds hitting, if not the same targets, the complementary targets in the same pathways, and both companies have the intention of fully testing those possibilities because we cannot presume that the first experiment we do is the correct experiment,” Huang said. “We felt very strongly, as we were putting these experiments together, that we maintain freedom of collaboration because the goal is still to get the best combination for the patients, and if this Akt inhibitor does not work, and another one does, we believe we should have the freedom to do that experiment.” The intellectual property that results from the collaboration will be shared by the inventors, while the intellectual property that was brought to the collaboration at its onset remains intact. What is still a big unknown is how the Food and Drug Administration will regulate the combination therapy. “No one has coregistered two unregistered drugs,” Huang observed. SOURCE: Huang presentation (February 10, 2010). |
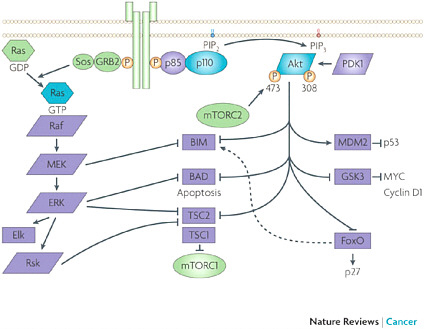 The Merck–AstraZeneca drug combination targets two pathways: the PI3K-Akt signaling pathway and the MEK signaling pathway, both known to foster cancer growth. SOURCES: Huang presentation (February 10, 2010) and Engelman, 2009. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Cancer, Engelman, J. A. 2009. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. 9(7):550–562, copyright 2009. |
get five times more products for one-fifth the cost, with half the number of volunteers. Esserman added that by shortening clinical trial times, not only is efficiency improved, but enthusiasm for the trial is sustained.
Win–Win Situation
Several speakers concluded their talks by noting that when precompetitive collaborations work, they can provide a win–win situation for drug companies, patients, academic researchers, and insurers by speeding up and by lowering the costs of drug development, and by reducing the risk and uncertainty of drug development. For example, the Myelin Repair Foundation’s Accelerated Research Collaboration, described by Lakhani, aims to address systemic problems in medical research and commercial drug development through “a radical new process that recognizes the incentives and limitations of academic scientists, commercial biopharma, government regulators, and patients and their families, and fosters behavioral changes by adding tangible value to everyone” (MRF, 2010) (see Box 6). Altshuler went further by claiming that successful collaborations are beneficial to society because “it benefits all of us to improve quality, to increase innovation, and to reduce costs. I think precompetitive collaboration is a particularly relevant topic to the health care debate.” In discussing precompetitive collaborations to develop predictive cancer biomarkers, Esserman added, “the whole point is that it does not hurt anyone, and it helps everyone.”
CHALLENGES TO COLLABORATING
Although precompetitive collaborations have many advantages, they also have several challenges that need to be overcome to be successful, speakers noted. These challenges include
-
Technical issues such as the need for standards and interoperability of information technology;
-
Legal issues such as ownership of intellectual property, antitrust law, and conflict of interest;
-
Regulatory issues, such as the willingness of regulators to accept new models of drug development based on collaborative efforts;
-
The need for incentives and rewards, and changing the surrounding culture so it supports sharing; and
-
Time constraints faced by leaders needed to participate and make collaborations successful.
Standards and Interoperability
“We have lots of different data in different buckets,” Nguyen explained. “The data are in different formats. When you use the word ‘gene’ in one database, it doesn’t mean the same thing as the word ‘gene’ in another database. How do you make them talk to each other or link up to each other so you can do data integration, bioinformatics? This technical challenge has to be solved if you are going to do large-scale bioinformatics.” McClellan added that addressing inconsistent or otherwise non-comparable data requires standards and infrastructure that can be costly to develop.
Williams-Jones agreed, adding, “we are really good at building these big databases that won’t talk to each other.” He pointed out that even a big database with everyone’s data interoperable within it is not sufficient without standard ways to analyze and interpret that data. “We are not very good at building standards on top of that database so we can start to make assertions based on the data—Is the gene linked to this disease? Is this compound linked to this target or other targets?—moving up from the basic data at the bottom end to application knowledge at the top end. We have got lots at the bottom, but not very much at the top,” Williams-Jones said.
Friend noted that the current way of doing research will have to change because the lack of standardization limits the usefulness of the data and prevents investigators from building on new knowledge. Currently, he said, “the person who gets funded to generate the data is the person who is funded to make the analysis, and the conclusion comes out in a way where no one else is in a position to use it afterward. This isn’t a sustainable way to do research. Imagine instead a world where you assume the data you were going to generate was going to be coupled to data other people have generated, and people are going to take that data and those models and use them later. To do that, many of the ways we do our experiments are absurd. We don’t keep track of conditions. We don’t annotate in a standard way. How are we going to get really good quality data to pull this off? We have to solve this issue because if we can get to the point where the data are in a standard format, then we will start an n+1 engine, which means that the addition of another small piece of information to whatever data I had before makes everything significantly better.”
Heywood added that the standard way of coding patients’ symptoms often is not adequate and does not capture important nuances. “The reality is that what people are coded and diagnosed with, and the terms used, are being driven much more by reimbursement than they are by health,” he said.
Heywood called for standard terminology that is understandable by
|
BOX 6 Myelin Repair Foundation’s Accelerated Research Collaboration The mission of the nonprofit Myelin Repair Foundation (MRF) is to stimulate the rapid discovery and delivery of myelin repair treatments to multiple sclerosis patients by building relationships and managing every step in the therapeutic development process from basic science to Food and Drug Administration approval. Karim Lakhani, assistant professor at Harvard Business School, pointed out that the MRF has an infrastructure that forces collaboration across disciplines at five academic laboratories. The laboratories represent expertise in neurobiology, genetics, cellular models, animal models, proteomics, and immunology. The MRF provides half the funding for the research on myelin done at these laboratories, and in return, the investigators commit to sharing their results immediately across all the participating laboratories prior to publication. With coordination from the MRF, this team developed a research plan and designed experiments to carry out that plan, which focuses on identifying therapeutic targets for myelin repair that will lead to patient treatments. The MRF research consortium of five laboratories was launched in 2005, and since then has:
|
physicians, researchers, and patients. Friend suggested having terminology that uses the common terms patients use to describe their symptoms. “Let the community of patients define the terms because then they will be used in a common way. There will be biorepositories hanging out all over the world, and electronic medical records, and neither are going to do any good until you have a common vocabulary and ways to query those,” Friend said. Heywood added that PatientsLikeMe requires any companies it partners with for developing measures of disease to commit to putting those measures out in the public domain. “We don’t want to be proprietary in the definitions of how we measure disease and ultimately want to make that a public resource,” he said.
Lakhani noted that one of the big issues the MRF needed to confront was intellectual property, since the five laboratories were located in different universities with different technology transfer offices and different rules. The MRF developed a framework for establishing membership and technology transfer agreements with each participating university. Through these agreements, the MRF files patent applications protecting the intellectual property developed in its funded laboratories, which cover discoveries that may contribute to potential treatments. According to the MRF website, patent protection can reduce the financial risks to pharmaceutical companies, which may increase the industry’s interest in undertaking new drug development and clinical trials for myelin repair treatments. Income generated from patents is used to fund future research, with the aim to create a self-sustaining research model. SOURCES: Lakhani presentation (February 9, 2010) and MRF, 2010. |
Legal Issues
Concerns over privacy, conflict of interest, antitrust law, and the sharing of international data can inhibit precompetitive collaborations. Cohen said that conflict of interest and conflict of commitment for academics and for society as a whole are big issues. He pointed out that faculty who are the world’s experts in specific areas are often asked to consult with a company on whether they should pursue a line of research or develop a product. But as soon as the academics do such consulting, they are banned from being a principal investigator for evaluating the product. Huang said that academics are often willing to collaborate with industry in biomedical research, but there is an inability to collaborate due to conflict-of-interest rules and
policies. “There is an increasing stranglehold on individuals in universities, either from the tech transfer office that is slower than molasses, or from their rules over conflict of interest and disclosures, etc. So it becomes much more difficult now to collaborate than in the past,” she said.
“We won’t eliminate these conflicts, but we need to figure out how to manage them,” Cohen said. “We need to be transparent about them, and we are trying to figure out how to do that in a way that is meaningful for the public. Do I disclose that I have a $3 million NIH grant, most of which is subcontracts that have little to do with my scientific pursuits?”
Intellectual property issues in general often have hampered multi-institutional relationships, in part by the institutions, and in part by the investigators who want to protect their own intellectual property. The university protects that investigator’s right to that information, often more vigorously than the individual investigators. “So to ask them to share it is to ask them to share their soul,” Cohen said. “We need to get over that. We need to recognize that there is value in sharing that information, and it won’t compromise academic productivity.”
Other contract issues pose legal challenges to collaboration, including limitations that corporations have on what they can give away, as well as liability concerns, Nguyen pointed out. Friend raised the issue of sharing data internationally, and whether the Patriot Act puts restrictions on such sharing. Vockley noted that the TCGA data are shared internationally unless restricted for patient privacy reasons. But he added that discussions on international data sharing are ongoing and a “work in progress.” Another looming issue Cohen said needs to be resolved is who owns electronic medical records and can limit access to them by researchers.
FDA Regulatory Issues
Some speakers and attendees expressed hesitancy over their willingness to participate in new collaborative drug development models without knowing how well received those efforts will be by FDA. Munos pointed out what he called regulatory gaps. “A pharmaceutical company can say that the reason why they have embraced target-based drug discovery is that this is the only system that exists for which there is regulatory clarity,” he said. “They are understandably reluctant to launch a batch of studies to then find out later that they have to redo them all because the studies they did weren’t designed to meet the regulatory requirements that did not exist at the time the studies were launched. There needs to be a coevolution
between scientific innovation and regulation, and that coevolution doesn’t exist or happen very effectively today.” Munos called for a greater engagement from the broad scientific community about how to regulate innovative collaborative studies, perhaps using a “Wiki-like” open virtual platform for brainstorming and developing consensus on this issue.
Huang noted that regulatory uncertainty was one of the biggest challenges in the Merck–AstraZeneca collaboration. To date, no one has coregistered two previously unregistered drugs, she said. “This raises all kinds of questions going forward. Are you obligated to show that monotherapy does not work before you can demonstrate that a combination therapy will?” An adaptive trial6 might be a way to deal with this question, Huang added. Esserman said that FDA’s reaction to adaptive trial designs, such as the I-SPY 2 TRIAL, is still in flux, although FDA recently released a draft guidance on the use of adaptive Bayesian designs for trials of drugs and biologics, and issued a final guidance on the use of adaptive Bayesian designs for device trials (FDA, 2010b,c,d).
In addition, Esserman noted that prior to the I-SPY 1 TRIAL,7 “FDA was not willing to think about the neoadjuvant setting or complete pathological response as a potential registration path, but now it is something they might consider…. They are definitely willing to accept the idea that you can use this trial as a way to indicate what biomarker you pick to do a more targeted Phase III trial. Now that the data from I-SPY 1 are maturing, they clearly show that complete pathological response can be a valid endpoint if you know how to analyze the data and include the right subsets of patients. If we work toward that as an industry, this will truly change drug development in the oncology world forever because this is not unique to breast cancer. Everything that we have built into I-SPY can be adapted to any other disease.”
John Mendelsohn, president of MD Anderson Cancer Center, raised the question of whether FDA would accept the biomarker results in the I-SPY 2 TRIAL to approve the biomarkers being tested. Esserman responded that the trial is only using biomarkers that already have FDA approval or clearance,
|
6 |
An adaptive trial is one that incorporates one or more decision points into the design. How a trial proceeds following each decision point depends on the data observed up to that point. |
|
7 |
The I-SPY 1 TRIAL, a Phase II trial not intended for product registration, preceded the I-SPY 2 TRIAL. I-SPY 1 was designed to evaluate neoadjuvant chemotherapy in patients with locally advanced breast cancer and to identify indicators of response to therapy using pathological complete response. |
or have an investigational device exemption for stratification of patients. Esserman noted that all of the biomarker tests used in the trial, which are being evaluated for their ability to predict efficacy of a drug, are being performed in Clinical Laboratory Improvement Amendments of 1988 (CLIA)-certified laboratories, which means that the data from I-SPY 2 could be used to get a biomarker registered or approved. However, the sojourn time for any drug in the trial will be too short to assess the validity of the biomarker, she added. But the positive results could lead to FDA approval to use the biomarker in a follow-up registration trial, Esserman said.
Gregory Curt, chair of the CEO Roundtable on Cancer’s Life Sciences Consortium Task Force and U.S. medical science lead of emerging products at AstraZeneca-Oncology added that it is also important to understand what trial designs FDA would be willing to consider for the registration of combination therapies in development. Martin Murphy, chief executive officer of the CEO Roundtable on Cancer, noted that this was also a topic of discussion at the 2009 Conference on Clinical Cancer Research (see Box 7). He added that conclusions from that meeting will be published in an upcoming article in The Oncologist (Clark et al., 2010).
FDA has been involved in collaborative activities aimed at improving regulation and product development. The objective of precompetitive sharing, said Woosley, is to develop a scientific consensus on which methods are qualified for use both among those who will use the methods (e.g., industry) and those who will accept the methods (e.g., FDA). Described in Box 8, Woosley provided an overview of the Critical Path Institute, a collaboration that has forged key partnerships, created collaborations, and helped build new working relationships among federal regulators and the industries they regulate (Critical Path Institute, 2010a). Additionally, McClellan discussed the Reagan–Udall Foundation, whose goal is to advance FDA’s mission to modernize product development, accelerate innovation, and enhance product safety (see Box 9).
Culture Change That Encourages Sharing
For precompetitive collaborations to thrive, several speakers noted the need to change cultures and environments in the field that encourage competition rather than collaboration. Competing companies often compel their employees to keep silent about their endeavors, and the sharing of information is often frowned on lest information be divulged that might compromise the company’s competitive advantage. Competition is rife in
academia as well, where investigators compete with each other to get grants and promotions and to be the first to answer scientific questions and publish their findings. Even universities compete with each other for high-quality students and researchers and the status that comes with these investigators. Rewards and incentives in many of these institutions are set up to encourage competition, and do not recognize collaborative efforts.
In addition to the traditional academic notions of academic freedom and intellectual autonomy, “merit, tenure, and promotion processes in the university undermine everything we are trying to accomplish here,” Cohen said. “People are promoted for their independent research directions. We need to get over that and think about how being a member of a collaborative group allows you to succeed, and get academic rewards for it.”
Esserman agreed, saying, “we need to think very hard about how we give credit for group science and for people participating in group science. I think it is much easier for the senior people to do it and much harder for the junior faculty.” Lakhani pointed out that “promotion, tenure, Nobel prizes, [and] research grants are all geared toward competition and being proprietary, and that works against collaboration.” University policies and procedures regarding technology transfer and linked royalty streams, economic autonomy, and contract negotiations also hamper collaborations, Cohen added.
However, there is diversity in the sharing practices within the sciences, suggesting that it is possible to foster collaborations with the right environment and cultural expectations, Nguyen pointed out. Some fields, such as astronomy, have systems in place to share preliminary data. One study (Stodden, 2010) found that one of the best predictors of whether a scientist will want to share his or her data is whether others in their field share data.
“Some of the norms in science of how you share data have to change,” Nguyen said. But he noted that scientists in both academia and industry sometimes have legitimate reasons for not sharing their data if they believe the data provide their core competitive advantage. Problems arise, however, when they err on the side of protectiveness for fear of giving away what someday might contribute to an important company trade secret or academic paper. To counter the excessive protectiveness that hampers collaboration, “there has to be leadership at the top that says, ‘it is okay to share, and we will tolerate some mistakes in pursuit of these higher goals,’” Nguyen said.
Curt added that “as people get more experienced about the risks versus the benefits of early sharing, that paradigm [of not sharing] could change.”
|
BOX 7 Conference on Clinical Cancer Research Mark McClellan, director of the Engelberg Center for Health Care Reform at the Brookings Institution, discussed the Conference on Clinical Cancer Research, held in both 2008 and 2009 by Friends of Cancer Research and the Engelberg Center for Health Care Reform. The objective of these all-day meetings was to identify and develop recommendations for specific barriers to clinical cancer research. The conference facilitated dialogue among different stakeholders, including academia, industry, the Food and Drug Administration (FDA), the National Cancer Institute (NCI), and patient advocacy groups. The 2009 conference addressed:
As an outcome of the 2009 conference, FDA has agreed to issue industry guidances on two of the topics—data submission standards and evidence requirements, as well as the development of rational drug combinations with investigational targeted agents. The first guidance will discuss the type and extent of data collection required for supplemental indication trials and the second guidance will explore situations in which a large-scale four-arm Phase III trial (Drug A vs. Drug B vs. Drug A+B vs. standard of care) may be modified. In the session on data submission standards and evidence requirements, the panel discussed recommendations from an American Society for Clinical Oncology–led collaborative effort to optimize data collection requirements when supplemental indications are sought for previously |
He noted that when Merck decided to publish the structure of the protease protein they used as the basis of an anti-HIV drug, “many people in the company thought they were giving away the keys to the kingdom, but what experience showed us was that it had no effect on the Merck HIV program. The only effect it had was to advance the field.”
|
approved drugs. The panel noted the excessive and frequently unnecessary amount of data collected for supplemental new drug applications and supplemental biologic agents that add to the cost of acquiring additional indications on a label for a drug or biologic. This collaborative effort collected data from eight trials run by four companies and one NCI Cooperative Group to determine whether important safety information would be omitted by only gathering toxicity data on a subsample of patients enrolled in a supplemental indication clinical trial using a drug for which a substantial toxicity profile already exists. The results of the analysis suggested that data subsampling would not lose important information about the safety profile. The panel on the development of rational drug combinations with investigational targeted agents proposed strategies for simultaneous development of targeted cancer therapeutics used in combination. Three feasible codevelopment scenarios were described: synthetic lethality, co-enhancement, and uni-enhancement. Stakeholders from FDA, NCI, clinical oncologists at Washington University and Mayo Clinic, and the Lance Armstrong Foundation participated in this panel’s activities. A unique aspect of the Conference on Clinical Cancer Research is that subcommittee meetings of the panelists were convened before the conference and chaired by a moderator from an academic institution. Each subcommittee was staffed appropriately for pre-meetings and authoring of issue briefs that contained topic background, work-to-date of the subcommittee, and specific recommendations to address the problem. In addition, this facilitated conference discussions and post-conference follow-up activities, including the publication of the panels’ findings in an issue of The Oncologist. SOURCES: McClellan presentation (February 10, 2010) and Abrams et al., 2010; Clark et al., 2010; Schilsky et al., 2008. |
Munos cautioned that more attention should be paid to cultural influences and what he called “status quo police” that try to protect large organizations from change. “There is a misconception in the pharmaceutical industry that innovation is a byproduct of organization, and if we can somehow organize right, innovation will follow. This is a fallacy. Innovation is a by-product
|
BOX 8 Critical Path Institute The “critical path” is an engineering term for the most efficient and direct route to a final product. With the release of the 2004 report, Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products, the Food and Drug Administration (FDA) launched the Critical Path Initiative, the agency’s strategy to drive innovation in the scientific processes through which FDA-regulated products are developed, evaluated, manufactured, and used. In support of the Critical Path Initiative, the Critical Path Institute (C-Path) was founded by FDA and the University of Arizona in 2005 as an independent, nonprofit organization dedicated to bringing scientists from FDA, industry, and academia together to improve the path for innovative new drugs, diagnostic tests, and devices to reach patients in need. C-Path manages industrial consortia of companies willing to share precompetitive knowledge and work in support of projects that are identified as high priority by FDA, and are in the interest of public health. C-Path’s mission has been to serve as a “trusted third party” to enable innovative collaborations among government regulators, the academic community, and regulated businesses. To enforce its neutral status, C-Path does not accept monies from organizations that develop products regulated by FDA, or that would create a real or perceived conflict of interest. Sources of support include contributions from both the public sector and private foundations in the state of Arizona, as well as grant awards from FDA and the Agency for Healthcare Research and Quality. The research done to generate the data used by C-Path, however, is funded by the regulated industry as well as by the Foundation for the National Institutes of Health (FNIH) and the Innovative Medicines Initiative. Participants in C-Path’s consortia include 28 major pharmaceutical companies, FDA and the European Medicines Agency (EMEA),a 4 National Institutes of Health (NIH) institutes, 6 patient advocacy organizations, and more than 600 scientists. “We felt like we had to have the regulators present, not just sitting in occasionally, but actually working as advisors on a day-to-day basis with the teams. We also needed the scientists from the NIH and academia there to bring in the new science that can make this process more advanced. Then we found very quickly that you really have to have the patients, not as window dressing, but with a voice and give them a vote at the table,” said Raymond Woosley, president and CEO of C-Path. C-Path’s consortia include one aimed at improving safety testing of new drugs that involves sharing testing methods for drug safety, one aimed at improving drug efficacy by developing qualified patient-reported outcomes, and one whose goal is to foster sharing of placebo |
|
and control clinical data. The consortia are international endeavors, with members from across the globe. Each consortia has four or five working groups, each composed of about 20 scientists who meet every month by telephone and every quarter in person. C-Path’s accomplishments include
Participants in C-Path consortia sign a legal agreement indicating that all the consensus data they produce will be made public, although C-Path does provide companies with the opportunity to initially limit the disclosure to encourage sharing. SOURCES: Woosley presentation (February 9, 2010); Critical Path Institute, 2010a,b; FDA, 2004, 2008, 2010a.
|
|
BOX 9 Reagan–Udall Foundation Mark McClellan, director of the Engelberg Center for Health Care Reform at the Brookings Institution, cited the Reagan–Udall Foundation as a potential source of support for collaborations in development and regulatory science. Created in 2007 by the Food and Drug Administration Amendments Act of 2007, the Foundation was designed and given statutory authority to collaborate closely with the Food and Drug Administration (FDA) on scientific priorities to advance the agency’s mission to modernize medical veterinary, food, and cosmetic product development, thereby accelerating innovation and enhancing the safety of medical products. Due to the absence of congressional appropriations, the Foundation has been slow to get off the ground, according to McClellan. But the Foundation plans to collaborate or contract with stakeholders, such as FDA, university consortia, public–private partnerships, academia, nonprofits, and industry, to efficiently and effectively advance its goals and priorities. SOURCE: McClellan presentation (February 10, 2010). |
of culture, not organization. The need to change culture is an issue that is not addressed enough by the pharmaceutical industry,” he said.
Culture also becomes an issue when it fosters different managing styles in collaborating institutions. Vockley noted that the difference in cultures among the various government agencies participating in the TGCA project is a challenge. The National Cancer Institutte (NCI), for example, is much more hands-on with their funded investigators, he said, whereas the National Human Genome Research Institute (NHGRI) is less involved. “This brings about different administrative policies within different organizations, and we have found that we were able to come to common ground, but it does take some time to do so,” Vockley said.
Time and Commitment
Several speakers pointed out the need to have the leaders of institutions participating in collaborations actively involved in the process. But these
leaders in industry and government agencies have limited time which can hamper their ability to meet in person or by phone on a monthly or even more frequent basis, so as to forge and maintain collaborations. “The most difficult thing about consortia is not money, but getting the right people to invest their time,” said Wholley. Woosley agreed, adding, “everybody has got a day job,” and there have to be rewards and incentives to overcome the “consortium fatigue” that many executives experience. “We do have too many meetings,” Vockley acknowledged. “Frequently we have people stop participating in the project in a meaningful way simply because they just have too many demands and they cannot do it all.” McClellan added that the management and coordination costs for collaboration may be significant, and developing consensus for action may be difficult.
WHAT TO SHARE
Nguyen prefaced his discussion of what can be shared in collaborations, that is, what is considered precompetitive, by noting that the different infrastructures of academia and industry foster differing notions of what can be shared and when. “In industry, the dividing line is what is precompetitive and what is competitive, and in academia it is what is competitive for me so that I can get my grants or my research done, but then everything else is postcompetitive once I publish. There is a gap between what [academic] scientists consider to be postcompetitive and what industry considers to be precompetitive,” Nguyen said, with industry often waiting until patents are filed before publicly disclosing the information those patents are based on, while academic scientists are often eager to publish their data before that stage.
But this gap in what is considered precompetitive is closing, Nguyen noted, because companies are increasingly focusing more on data exploitation than data generation as their core competency that should be protected, as well as shrinking research and development budgets. Likewise, on the academic side, there is greater availability of high-quality data in the public domain. “The quality of data [in the public domain] has improved drastically over time, and the public tools—like some of the open-source tools that you have heard about—have grown much more sophisticated,” Nguyen said. “These changes mean that what is available in the commons is starting to be almost as good as what companies can develop themselves internally.” Nguyen also suggested that some of the norms regarding how people share data in the commons have started to solidify. He pointed to a
recent opinion piece from genomic scientists who recommended that post-competitive data and tools be shared openly in the public domain without legal restrictions (Schofield et al., 2009).
Due to this expanding public domain, Williams-Jones observed, companies “are all reading the same literature and taking the same data in, so it shouldn’t be that much of a surprise that we are all running the same programs in the same disease areas, and pursuing the same targets. Precompetitive is not what you’ve got, but what you do with it.” Consequently, the methods, standards, and tools used in biomedical research and the early stages of development are not as proprietary. How that information is used by individual companies in the later stages of drug development is more important.
This was evident in several suggestions by conferees for what is considered precompetitive and sharable, such as:
-
Standards for common data elements, data analyses, and information technology infrastructure;
-
Biomarker data and standards;
-
Control data, and data and insights from failed trials;
-
Compound toxicity information;
-
Disease model knowledge, including pathway networks and the molecular basis of disease;
-
Clinical methods; and
-
Contract language used to build collaborations.
Bioinformatics Resources and Standards
Nguyen and Williams-Jones stressed sharing bioinformatics resources and tools, as well as common data standards, formats, and tools, to integrate data. “Companies spend large amounts of time and money on internally developing or licensing data integration and analysis tools that analyze public domain data. This reinventing the wheel in bioinformatics is costly and no single company can create and enforce industry-wide data integration standards. This is an area where, without sacrificing some of your competitive advantages, you can develop some common tools,” Nguyen said. Williams-Jones described several collaborations with the goal of improving bioinformatics in medical product development (see Box 10).
Common data elements and standards are also critical, noted Friend, Woosley, Heywood, and other speakers and attendees. “We have to be referring to and recording things in the same way,” Woosley said. He also
suggested sharing methods for safety and efficacy testing. “Companies are willing to share how they test drugs, and we have also found that they are willing to share their knowledge of diseases when they are running into brick walls and not making progress,” he noted.
Biomarker Data and Standards
Some speakers, such as McClellan, Wagner, Curt, Eck, and Nguyen, suggested that biomarker standards, or even the biomarkers themselves, should be shared. “Even in the critical area of biomarkers, there is more appreciation that we are overlapping and reduplicating in each of our company programs, and that could be done better if they were done to a certain standard,” said Curt. Eck added that the “tools that we all build internally at great expense, such as PET [positron emission tomography] ligands and biomarkers, really would be better served if we just made them available for free because they would become validated more quickly, or their warts would become known more quickly.”
Speakers discussed several precompetitive collaborations involving biomarker data and biomarker standards, including the Biomarkers Consortium and its associated projects.
The Biomarkers Consortium8
Wholley elaborated on the Biomarkers Consortium, a project of the Foundation for the NIH (FNIH).9 This consortium, whose founding partners included FDA, NIH, the Centers for Medicare & Medicaid Services (CMS), the Biotechnology Industry Organization, and the Pharmaceutical Research and Manufacturers of America, was prompted by the growing awareness of the importance of biomarkers. Many view biomarkers as key
|
8 |
Information about the Biomarkers Consortium is from David Wholley’s presentation on February 9, 2010. |
|
9 |
The FNIH is a nonprofit, nongovernmental organization formed in 1996, when it was authorized by the U.S. Congress to support the mission of the NIH by creating and managing public–private partnerships. The Foundation provides a neutral forum that can engage and forge partnerships among often traditionally competitive participants. These participants include industry, academia, federal agencies, and the philanthropic community. With its grants, contracts, and project management capabilities, the Foundation supports major research partnerships, as well as scientific education, training, conferences, and program facilitation. |
|
BOX 10 Collaborations Aimed at Improving Bioinformatics and Information Technology Bryn Williams-Jones, associate research fellow and head of eBiology at Pfizer, elaborated on several initiatives and collaborations aimed at improving bioinformatics and information technology, including the European Bioinformatics Institute (EBI) industry program, the Pistoia Alliance, and the Innovative Medicines Initiative (IMI). European Bioinformatics Institute The EBI industry program hosts precompetitive quarterly meetings with 16 member drug companies and agribusinesses working in the fields of pharmaceutical and biotechnology research and development informatics. Members work together to organize intensive workshops that focus on key informatics issues encountered during drug discovery and development. The program also initiates special bioinformatics research projects with targeted collaborative funding. The program provides a neutral meeting place for intercompany interactions on bioinformatics, Williams-Jones pointed out. Members pay a subscription fee to join the program. That subscription helps pay for member-deemed priority pilot projects that involve all participating companies. For example, one such pilot project entailed research on text mining. Pistoia Alliance The Pistoia Alliance is an initiative to streamline precompetitive elements of the pharmaceutical drug discovery workflow, such as chemistry, biological screening, and logistics, by developing open standards for common business terms, relationships, and processes. More specifically, this collaboration of more than 20 mostly pharmaceutical companies aims to: |
to reducing the time and expense required to bring new drugs to market (Park et al., 2004; PCAST, 2008; Zerhouni et al., 2007). The development and validation of cancer biomarkers is also critical to the success of targeted therapies, as evidenced by Herceptin and other drugs.
But biomarker development and validation lag far behind the development and clinical testing of the innovative treatments that depend on them for their success. Wholley pointed out that out of more than 1,000 putative cancer protein or peptide biomarkers described in the literature
The Pistoia Alliance’s board of directors develops a roadmap and gives final approval for the technical standards developed by the technical committee. Pharmaceutical and biotechnology companies are the only voting members. (Participating vendors and academics do not vote.) Innovative Medicines Initiative The IMI is a unique public–private partnership between the pharmaceutical industry (represented by the European Federation of Pharmaceutical Industries and Associations) and the European Communities (represented by the European Commission). The initiative’s overall goal is to make Europe the world leader in pharmaceutical research for the benefit of the economy and society, by removing research bottlenecks in the current drug development process. The world’s largest public–private partnership, IMI receives funding from the European Union and industry in-kind resources. A major focus of IMI is knowledge management, which includes translating various standards in European electronic health records to extract common data. IMI also provides a drug–disease modeling library and clinical pharmacokinetic and pharmacodynamic models that are in the public domain. In addition, IMI has created a publicly accessible database containing data on target biology. SOURCE: Williams-Jones presentation (February 9, 2010). |
(Polanski and Anderson, 2006), only 9 are FDA approved as “tumor-associated antigens” and fewer than 1 per year have been approved by the FDA since 1998. There is a lack of biomarker validation for other diseases as well as cancer. “A lot of people feel something needs to be done to improve this rather low rate of success and get over this biomarker barrier,” Wholley said.
Consequently, the Biomarkers Consortium was launched in 2007 to facilitate the development and validation of biomarkers using new and
existing technologies in a precompetitive context. The Consortium aims to qualify biomarkers and validate the underlying analytical technologies for specific applications in diagnosing disease, predicting therapeutic response, or improving clinical practice. In the spirit of precompetitiveness, however, the Consortium will not qualify or validate biomarkers in areas that directly intersect with certain compounds being developed by a specific company.
The Consortium is expected to generate information that can inform regulatory decision making, and its results are broadly available to the entire scientific community, not just its participants. “The whole goal of the Consortium is to drive significant public health benefit,” said Wholley.
The Consortium has more than 50 contributing members, including 12 of the largest pharmaceutical companies, academic researchers, and numerous nonprofit organizations. The Executive Committee of the Consortium has senior representatives from NIH, FDA, the pharmaceutical industry, the FNIH, CMS, and patient advocacy groups. Steering committees for four major types of diseases also have equal representation from NIH, FDA, industry, and academia and are composed of 20 to 30 individuals. These committees, along with the Executive Committee, decide what biomarker projects to pursue, and direct smaller project teams of 8 to 10 people, which also have balanced representation across all sectors, to carry out the project. Projects are approved based on their scientific merit, precompetitive quality, and feasibility.
The project plan, which is developed by both the steering committee and project team, includes governing policies for intellectual property and data sharing, confidentiality, conflict of interest, selection and award of grants and contracts, and antitrust issues, which are posted on the Internet (FNIH, 2010c).
There is core funding for the Consortium that enables it to run its basic infrastructure, but projects are funded on a case-by-case basis, which can be challenging. “Hopefully, we have the involvement of companies in the steering committees and project teams that are really interested in working on a project because when it comes to the end of the day, a company can choose to fund a given project or not as they wish,” said Wholley. He added that “a lot of the projects also rely on in-kind basic investment by NIH, which helps, because industry is looking at this and saying, ‘we are leveraging a large preexisting investment that has already been made by the NIH.’”
The Consortium already has funded and launched seven projects, including the I-SPY 2 TRIAL, discussed below. Two imaging studies, ini-
tially developed under the Oncology Biomarker Qualification Initiative, have also been approved and funded (see Box 11). Five additional projects are fully planned and in the funding process, with two more in the near-term development pipeline, including one on renal toxicity biomarkers that is being done with another consortium (C-Path). One project has been completed; this project pooled deidentified and blinded biomarker data from four companies to assess the performance of adiponectin as a potential marker of glycemic efficiency. This analysis found that in patients with type 2 diabetes mellitus, adiponectin level is a robust predictor of glycemic response to peroxisome proliferator-activated receptors. Additionally, the investigators concluded that “cross-company precompetitive collaboration is a feasible and powerful approach to biomarker qualification” (Wagner et al., 2009).
I-SPY 2 TRIAL10
I-SPY 2 is a Phase II multisite clinical trial that was launched March 17, 2010, to test multiple experimental drugs while simultaneously assessing the ability of various biomarkers to predict response to the investigational agents. I-SPY 2 builds on I-SPY 1,11 which was designed to evaluate neoadjuvant chemotherapy in patients with locally advanced breast cancer, bringing together data from multiple molecular biomarker studies and biomedical imaging (Barker et al., 2009).
In I-SPY 2, 800 patients with locally advanced breast cancer will have their tumor biopsies characterized by a panel of biomarkers, some of which are established and approved and some of which are exploratory or need to be qualified. Biomarkers that have FDA approval or clearance, or have an investigational device exemption will be used to stratify patients to treatments, while exploratory biomarkers will be tested to determine if they predict response to a drug. The results from biomarker tests on these biopsy samples will be used to assign the patients to different groups that will receive 1 of up to 12 experimental drugs and/or standard drug therapy prior to surgery. Using biomedical imaging, the effect on the tumor will be
|
10 |
Information on I-SPY 2 is from Laura Esserman’s presentation on February 10, 2010, and David Wholley’s presentation on February 9, 2010. |
|
11 |
I-SPY 1 was a collaboration involving the NCI’s Specialized Programs of Research Excellence, the American College of Radiology Imaging Network, Cancer and Leukemia Group B, and the NCI Center for Biomedical Informatics and Information Technology. |
|
BOX 11 Oncology Biomarker Qualification Initiative The Oncology Biomarker Qualification Initiative (OBQI) is an agreement between the Food and Drug Administration (FDA), the National Cancer Institute (NCI), and Centers for Medicare & Medicaid Services (CMS) to collaborate on improving the development of cancer therapies and the outcomes for cancer patients through biomarker development and evaluation. Established in 2006, the goal of the OBQI is to validate particular biomarkers so they can be used to evaluate promising technologies in a manner that will shorten clinical trials, reduce the time and resources spent during the drug development process, improve the linkage between drug approval and drug coverage, and increase the safety and appropriateness of drug choices for cancer patients. Two imaging studies, initially developed under the OBQI, have been approved and funded by the Biomarkers Consortium. These clinical trials assess the use of fluorodeoxyglucose-positron emission tomography (FDG-PET) imaging in non-Hodgkin’s lymphoma and in non-small-cell lung cancer. The goal of these trials is to determine the linkage of FDG-PET imaging to the effect of conventional cytotoxic drugs in clinical outcome and survival. By establishing a role for FDG-PET in assessing response to treatment and predicting outcome, it is thought that FDG-PET has the potential to be validated as a surrogate endpoint for clinical benefit in these cancers. According to Gary Kelloff, special advisor to NCI’s Cancer Imaging Program, with a few additional studies, FDG-PET could facilitate drug development and patient care by enabling faster Phase II studies that evaluate new treatments, accelerated approval in Phase III trials, with full approval contingent on evidence of clinical benefit after longer term follow up, and better patient care by ceasing ineffective therapies earlier. The data from these clinical trials will inform both regulatory review processes and CMS decision making regarding reimbursement for these imaging tests. SOURCES: Kelloff presentation slides provided in the workshop briefing book, FNIH, 2010a; Kelloff et al., 2005; NCI, 2006, 2010. |
measured at four points during the 6 months the patients receive treatment, and when the tumor is removed. The patients will then be monitored for 5 years.
This innovative study uses an adaptive trial design to enable researchers to use early data from one set of patients to guide decisions about which treatments might be more useful for patients later in the trial. The study design also enables drugs to be dropped quickly from the trial if they are ineffective or harmful (FNIH, 2010b). “We are categorizing patients by biomarker category, so if we see the drugs are not improving the standard response, maybe in particular biomarker classes, we will restrict it and stop assigning to those groups where it is not working, and give it only to the groups where it is working,” said Esserman, who is one of two principal investigators of the I-SPY 2 TRIAL.
In addition, the study design allows drugs to be graduated to Phase III trials sooner if they are shown to be beneficial. Once drugs graduate to Phase III testing or are dropped, new drugs will seamlessly be entered into the trial to take their place.
Promising data on biomarkers in I-SPY 2 can be used to support a Pre-market Approval (PMA) application at FDA or to request to use a biomarker to stratify patients in a Phase III validation study, according to Esserman.
Wholley was enthusiastic about the I-SPY 2 TRIAL. “This will potentially revolutionize the design of clinical trials,” he said. “It will help identify which patients benefit most from which therapies. And from the industry perspective, if the trial is successful, it will mean that some of these companies that put their drugs through this trial can do Phase III trials with hundreds rather than thousands of patients. So it is better, faster, and cheaper moving forward.”
The I-SPY 2 TRIAL focuses on patients with locally advanced cancers, who have a higher likelihood of less favorable long-term outcomes and a higher risk of relapse (Valero et al., 1996). By incorporating adaptive features in the clinical trial design of I-SPY 2, it is possible that these patients, particularly those who enter after much learning and adapting has taken place, will get a better chance of being put on a regimen that will effectively treat their cancer, Wholley explained.
The trial is testing promising drugs by class from many companies, each of which is contributing the experimental agents. “We are not testing one company’s IGFR [insulin-like growth factor receptor] inhibitors as well as another company’s IGFR inhibitors. Whoever’s drug is farthest along
the pipeline goes in first, the whole industry learns, and we move forward,” Esserman said.
The unique structure of the trial and the multiple companies involved in it, however, create numerous challenges, especially in the regulatory arena. Usually multiple drugs and biomarkers require multiple trials, each with its own investigational new drug (IND) application. Even when a drug is successful in the first phase of testing, the trial has to be stopped and a new one created to continue testing in the next phase. This is extremely time consuming and inefficient, Esserman noted. To speed up the process, the Biomarkers Consortium, trial organizers, and FDA worked together to develop a plan in which the master IND being used by the trial is being held by the FNIH, who manages the Biomarkers Consortium along with several other large biomedical partnerships. The FNIH was chosen because it was seen as a trusted, neutral third party that can sponsor and manage the trial fairly and effectively.
In addition, the initial five experimental agents that will be used in the trial were approved for testing purposes by FDA and the relevant institutional review boards (IRBs) before the trial started. Other agents that will be evaluated in I-SPY 2 TRIAL (there will be as many as 12) will be submitted to FDA and IRBs for approval for testing purposes as the trial progresses, so that by the time investigators are ready to add new agents to the trial, they will be ready to enter new patients. Each time a new agent is added to the trial, an appendix is added rather than changing the protocol. Esserman stressed that an effort was made to involve all the stakeholders from all the sites as early as possible. For example, in preparation for IRB approval, 45 key stakeholders were brought together for education and feedback. This changed a traditionally long linear process, with consecutive approvals by various participants and inefficient reapproval loops, to a more streamlined team effort.
No single company stands to be the sole beneficiary of the I-SPY 2 TRIAL. The IP resulting from the trial will be handled according to existing policies of the Biomarkers Consortium (see also Figure 5):
-
Preexisting IP related to agents contributed by companies will remain with the company owning that IP.
-
Preexisting IP related to biomarkers and platforms will remain with the inventing companies, and be licensed for use in the project. In some cases, the tests have been published and are available commercially.
-
New IP will be managed by the FNIH, acting as a trusted third party
FIGURE 5 The approach to new intellectual property (IP) in the I-SPY 2 TRIAL. In I-SPY 2, inventing organizations grant exclusive licenses to new IP to the Foundation for the National Institutes of Health (FNIH), with the FNIH prosecuting and managing resulting patents. FNIH will then market and license IP to interested parties and return royalties to the inventing organizations, less expenses.
SOURCE: Wholley presentation (February 9, 2010).
-
to hold and license the new inventions. The FNIH will return a fair share of royalties (less expenses) to inventing organizations.
-
The FNIH prosecutes and manages resulting patents.
-
Data are expected to be broadly applicable, and will be made broadly available.
Participating institutions in the I-SPY 2 TRIAL use common data elements and a shared information technology infrastructure, which employs tools provided by caBIG.12 Within the caGRID, the underlying architecture of caBIG, the I-SPY 2 TRIAL is leveraging several bioinformatics platforms, including caTISSUE, caARRAY, and caIntegrator. Access to the data is democratized and credit is shared. “Everything about the I-SPY trials is about taking the team approach,” said Esserman. At the same time, she pointed out, each participating company is made to feel like they have their own trial within a trial. “It is a way to share and still give people a
|
12 |
caBIG stands for the cancer Biomedical Informatics Grid, an information network that enables members of the cancer community to share data and knowledge (https://cabig.nci.nih.gov). |
sense that they still have some control over what is happening,” she said. In addition, to give more people credit for their work, rather than the trial having a single principal investigator, two chaperones are assigned for every agent and every biomarker.
The I-SPY 2 TRIAL is expected to cost approximately $26 million over 5 years (FNIH, 2010b). Some funding secured for the trial includes contributions from Safeway, Inc., Johnson & Johnson, Genentech, and Eli Lilly and Company. The FNIH is working to raise the remaining funding from pharmaceutical and other companies, nonprofit cancer organizations, and philanthropic foundations and individuals. Only some pharmaceutical companies who have funded I-SPY 2 are participating in the trial. “We really wanted to separate the financial support from the drug supply,” said Esserman.
Esserman summarized the numerous benefits the trial is expected to have for a wide range of stakeholders, including patients, FDA, pharmaceutical and device industries, academia, NCI, and CMS (Table 1).
Disease Characterization and Models
Most speakers also suggested that disease characterization and disease models, including target identification, and other basic science information can be shared without hampering competitiveness. “Most companies would not regard biomarker data, toxicity data, biological pathways, and basic target information as competitive, particularly if they are far away from identifying a particular active drug or compound,” Nguyen said. Friend added that a significant difference exists between sharing information about diseases and sharing information about compounds and their effects on the diseases, and companies will be more likely to share the former than the latter.
Clinical Methods and Contracts
On the clinical side, McClellan suggested sharing endpoints and data collection standards and methods, including clinical designs and statistical analysis methods. For optimum regulation, McClellan also suggested sharing evidentiary standards for markers, tests, and therapies; guidance for trial designs and endpoints; and codevelopment of treatments. Curt suggested sharing standard contract language for clinical trials to expedite negotiations required between industry and publicly-funded investigators before the launch of a collaborative trial. He spoke about the CEO Roundtable on
TABLE 1 Value Proposition and Benefit for Partners Involved in the I-SPY 2 TRIAL
|
Stakeholder |
Value Proposition and Benefit |
|
Patients |
Opportunity to drive path to personalized treatment Potentially more effective treatment/management |
|
Food and Drug Administration |
Provides for evidence-based regulatory policy |
|
Pharmaceutical industry |
More efficient drug development and approval path Better early response criteria |
|
Device industry |
Larger markets Less risk |
|
Centers for Medicare & Medicaid Services |
Helps define reasonableness and need |
|
Academia/National Cancer Institute |
Better clinical data More effective treatment/management |
|
SOURCE: Esserman presentation (February 10, 2010) and adapted from NIH, 2008. |
|
Cancer Life Sciences Consortium, which recognized that a lot of time and effort could be saved if industry–academic collaborations shared the same basic contract language when negotiating collaborations, and developed such contract language, which has been favorably reviewed by the U.S. Department of Justice (see Box 12). Nguyen added that Science Commons, which is an offshoot of Creative Commons (see Box 13), has a suite of standard forms for sharing biological materials, and recently released a legal tool developed by Creative Commons called CC0 1.0 Universal13 that allows people to mark data as being in the public domain so that anyone can use them.
Information on Failed Compounds or Those on the Market
Munos suggested sharing everything that is known about a compound once it is approved for marketing so that physicians can use that knowledge
|
BOX 12 CEO Roundtable on Cancer Life Sciences Consortium This roundtable was established in 2001 and consists of 17 representatives from 11 pharmaceutical companies and 26 representatives from National Cancer Institute- (NCI-) Designated Comprehensive Cancer Centers. The Life Sciences Consortium is a task force of the Roundtable and brings together Roundtable members to further its goals, which are to:
To help achieve its first goal of expediting the R&D process, the Life Sciences Consortium acted on research that found the most rate-limiting step in the development of clinical trials was contracting and budgeting. To expedite the contract and budget negotiations required between industry and publicly funded investigators before the launch of a collaborative trial, the Consortium and NCI reviewed copies of 78 redacted clinical trial agreements and identified 45 key concepts related to intellectual property, study data, subject injury, indemnification, confidentiality, and publication rights. From these agreements, they then gleaned the exact language that embodied the key concepts and used it to create standardized and harmonized clauses for clinical trial agreements that are designed to serve as a starting point for contract negotiations. The analysis found that several key concepts showed greater than 67 percent similarity across the agreements, suggesting that negotiations frequently reach common results for these concepts. The U.S. Department of Justice gave the proposed clauses a favorable review and indicated that it had no intention to challenge the initiative. Nine out of eleven of the Life Sciences Consortium companies have adopted the START (Standard Terms of Agreement for Research Trial) |
to deliver the best possible care using the compound. He also suggested sharing information about compounds that fail in preclinical or clinical development due to toxicity or lack of efficacy, so that others do not waste time pursuing the same dead-end pathway. “There is a lot of duplication of errors in industry. When companies typically abandon a compound—they
|
clauses for their oncology programs, with one making it their standard operating procedure, and another using the clauses for all therapeutic areas. The Consortium plans to use the same process to write material transfer agreements for academic collaborations in the laboratory to expedite the process of preclinical development. The Life Sciences Consortium recently began addressing its second goal of developing a pool of precompetitive intellectual property for biomarkers. It plans to use NCI as a “safe harbor” for this effort because NCI currently has a robust biomarker program, according to Gregory Curt, chair of the CEO Roundtable on Cancer’s Life Sciences Consortium Task Force and U.S. medical science lead of emerging products at AstraZeneca-Oncology. Consortium companies will present their biomarker programs under confidentiality to NCI, which will select the most promising markers for coinvestment and collaboration. This will prevent the duplicative and expensive research the individual companies and NCI are spending on biomarker development and should, along with clinical and preclinical START clauses, significantly reduce the amount of time used to validate biomarkers. To reduce the regulatory burden of new cancer drug approvals, the Consortium participated in a collaboration convened by the Brookings Institution and Friends of Cancer Research (described in Box 7) that analyzed cancer clinical trial data voluntarily submitted by the Consortium’s members to discern the optimal types and amounts of data that should be collected in Phase III trials for supplemental approvals of cancer drugs. The Food and Drug Administration is currently considering the recommendations and plans to develop a guidance for industry on this topic. SOURCES: Curt presentation (February 10, 2010); Abrams et al., 2010; CEO Life Sciences Consortium, 2010; CEO Roundtable on Cancer and NCI, 2008; Dilts and Sandler, 2006; DOJ, 2008; IOM, 2010b. |
cease to research it—that compound is quietly buried and no one ever knows why the compound was abandoned. Companies frequently look over their shoulders and tend to work in similar areas on similar targets. So if one company finds early that there is a problem with a target and they
|
BOX 13 Science Commons Launched in 2005, Science Commons designs strategies and tools for faster, more efficient web-enabled scientific research by crafting policy guidelines and legal agreements as well as developing technology to make research, data, and materials easier to find and use. The Science Commons offers:
|
keep that knowledge secret, then a lot of money is being spent by the other companies to rediscover what is already known,” Munos said.
Curt added that results from failed trials often are not published or referenced, and that putting those results on the company website does not make it searchable by Medline, MedLab, or Google. Munos then pointed out that the Journal of Field Experiments will publish the results of failed trials, and Murphy, editor of The Oncologist, added that his jour-
SOURCES: Nguyen presentation (February 9, 2010) and Science Commons (2010a,b,c,d,e). |
nal has a policy of publishing the negative results of clinical trials so they will surface in PubMed searches. “The Oncologist has taken that stand because we firmly believe that it is in the best interests of cancer research and ultimately better cancer patient care to have this information out,” Murphy said.
More controversial, but still important, is the sharing of raw data, particularly in control arms, said Eck. “It is one thing to do a meta-analysis
of analyzed data, but it becomes much more powerful if you can aggregate together raw data because your statistical power is much greater. But I think this will probably raise even more eyebrows,” he said.
Despite the acknowledgment that much can be shared, speakers also recognized that there will still be some things that are too proprietary to share, such as candidate compounds and novel chemistry design. Some speakers also recognized that despite what can be shared without hampering competitiveness, a lot still goes unshared. “The biggest gap is in really understanding the molecular basis of disease and matching these compounds with the patients, and that is one for which there is very little sharing,” said Huang.
TYPES OF PRECOMPETITIVE COLLABORATIONS
Several different precompetitive collaboration types have been developed to date. Altshuler examined about 50 of these collaborations and classified them as to whether they have open or restricted participation and open or restricted outputs. They also vary according to their goals. Altshuler identified two broad collaboration goals: to build enabling platforms and to conduct research. These goals can be further subdivided by the four different types of outputs they produce, including
-
Development of standards and tools;
-
Generation and aggregation of data;
-
Knowledge creation; and
-
Product development.
Collaborations aimed at building enabling platforms focus on developing standards and tools or generating and aggregating data to achieve a necessary scale for research. Collaborations that conduct research seek to create new knowledge or to turn that knowledge into a product by accessing resources and capabilities across organizations.
Collaborations are more likely to have open participation, such as web-based contests open to anyone with a laptop, if novel perspectives are sought from diverse fields and the need for quantity of input outweighs the need for quality control of that input. In contrast, collaborations are more likely to have restricted participation if the opposite is true or if the cost of equipment (e.g., genetic sequencing equipment) needed to participate or other factors create barriers to entry.
Collaborations that enable open access to the outputs of the collaboration are those for which no one can directly profit from the outputs, and for which the problem tackled by the collaboration would benefit “from having many eyes on it,” said Altshuler. In contrast, collaborations with outputs closer to commercialization, especially those involving intellectual property, are likely to be more restricted, as are collaborations that require funding by participants, so as to avoid the free-rider problem.
The online encyclopedia Wikipedia is an example of a collaboration in which there are both open participation and open access to outputs. In contrast, with collaborative agreements between companies, such as the Merck–AstraZeneca collaboration to jointly develop a treatment from which only they will profit, there is highly restricted access to both participation and outputs.
Some collaborations have restricted participation, but open outputs, or vice-versa. An example of a collaboration with restricted participants, but open access to its outputs, is the Human Genome Project. This international government-sponsored consortium of laboratories was formed to sequence the human genome and map its genes. Outputs of this project are in the public domain. In contrast, prizes and contests are collaborations with open participation but restricted access to outputs, which are only used by the sponsors of the prize or contest.
Altshuler charted the four types of collaborations, according to how open access is to participation and outputs, along with the four main goals of collaborations, to define eight basic models of precompetitive collaborations (see Box 14).
The eight models of collaboration, also listed in Figure 6, include
-
Open-source initiatives, which mainly occur earlier in the research and development spectrum, have open access to both participation and outputs, and can have any of the four goals listed previously. Altshuler noted that it is possible to build a successful profit model based on such initiatives. Many successful businesses, for example, have been built using Linux’s open-source software.
-
Industry consortia for process innovation, which are consortia of industry members aimed at improving the noncompetitive aspects of the research and development process, including technology development. These consortia, including SEMATECH and C-Path, have restricted participation and can have both open or restricted outputs.
|
BOX 14 Models of Collaborative Relationships
SOURCE: Altshuler presentation (February 9, 2010). |
-
Discovery-enabling consortia, which are consortia of academia and/or academia plus industry where the goal is to provide a critical mass of data for innovation that cannot be achieved by the individual participants alone. By compiling these data warehouses, future research is enabled, but the warehouses themselves are not monetizable, Altshuler pointed out. Examples of discovery-
FIGURE 6 (Facing page) The eight models of precompetitive collaboration, and examples of each, as analyzed by Altshuler: open-source initiatives, industry consortia for process innovation, discovery-enabling consortia, public–private consortia for knowledge creation, prizes, innovation incubators, industry complementors, and virtual pharma companies.
NOTE: Alliance for Cell Sig = Alliance for Cellular Signaling; AZ = AstraZeneca; Biogen bi3 = Biogen Idec Innovation Incubator; C-Path = Critical Path Institute; CCMX = Competence Centre for Materials Science and Technology; CDISC = Clinical Data Interchange Standards Consortium; CERN = European Organization for Nuclear Research; CHDI = CHDI Foundation; GSK = GlaxoSmithKline; HapMap = International HapMap Project; HGP = Human Genome Project; MMRF = Multiple Myeloma Research Foundation; NLP = Natural Language Processing; OD = Open Database; OSDD = Open Source Drug Discovery; P&G = Procter and Gamble; RNAi = RNAi Consortium; SAEC = International Serious Adverse Event Consortium; SLAC = SLAC National Accelerator Laboratory; SNP = Single Nucleotide Polymorphism; Tech to Bus = Technology-to-Business.
SOURCE: Altshuler presentation (February 9, 2010)
-
enabling consortia include the Human Genome Project and CERN (European Organization for Nuclear Research). Discovery-enabling consortia have restricted participation; however, the outputs may either be open or restricted.
-
Public–private consortia for knowledge creation, which include the Biomarkers Consortium and the Innovative Medicines Initiative. Such partnerships provide industry and academia with the opportunity to work more closely together within a framework other than the traditional sponsored research relationship, Altshuler said. These partnerships are typified by restricted participation and open outputs. They have critical downstream value, even though they offer no immediate market potential.
-
Prizes, such as the Innocentive prize that Lakhani described previously (see section on synergy of cross-discipline/cross-institution collaborations). In this model, there is open participation, but restricted access to outputs, and the aim is to develop a product. Prizes can be given for small incremental, but critical, steps in research. Alternatively, prizes can also address big game-changing problems, such as the X PRIZE, which, for example, has been given for the innovative development of private spaceships. Because sponsors do not have to pay for failed efforts, prizes generally give a good return on the investment of prize money compared to in-house research and development, Altshuler pointed out, and the large prizes generate a tremendous amount of publicity and bring more potential innovators to the table.
-
Innovation incubators, which entail sponsored research that is brought in-house, such as Biogen’s Innovation Insourcing Initiative program described previously in the section on synergy of cross-discipline/cross-institution collaborations. These incubators can have restricted or open participation, but their outputs are restricted to the company that is doing the insourcing. These collaborations fill the gap between publicly sponsored research, which often is focused on basic research, and venture capital-funded research, which occurs at a much later stage in the research and development process. These collaborations reduce the risk for the sponsoring company, which can pick from projects already showing promise, and also benefit the sponsored investigator, who can tap the resources of an already established company, according to Altshuler.
|
BOX 15 Multiple Myeloma Research Foundation The Multiple Myeloma Research Foundation (MMRF) integrates and funds the research efforts of its participating institutions in order to achieve its mission to accelerate the development of novel and combination treatments for multiple myeloma. As the leading funder of multiple myeloma research, the MMRF has facilitated basic research, clinical trials, and correlative studies, including collaborative studies. For example, the Multiple Myeloma Research Consortium (a sister organization to the MMRF) Genomics Initiative, involving a collaboration with the Broad Institute and Translational Genomics Research Institute, completed sequencing of the first multiple myeloma whole genomes, which will be used to identify targets for new therapeutics. Genome data were posted in an open-access web portal prior to publication and in near real-time. According to its website, the Foundation’s endeavors have resulted in bringing multiple myeloma patients four new treatments that are extending lives around the globe. SOURCES: Altshuler presentation (February 9, 2010) and MMRF, 2009, 2010. |
-
Industry complementors are focused collaborations among a small number of competitors for mutual benefit, such as the Merck– AstraZeneca collaboration described in Box 5. These collaborations have both restricted participation and restricted access to outputs.
-
Virtual pharmaceutical companies, which are typically driven by foundations to develop drugs for their disease of interest. The Multiple Myeloma Research Foundation pioneered these types of collaborations (see Box 15). The foundation funds the research and requires the immediate sharing of information generated from that research with fellow grantees. “They drive progress by forcing collaboration among their collaborators,” said Altshuler. These collaborations also have both restricted participation and restricted access to outputs, and because they are virtual, they enable founda-
-
tions to direct funding toward the best researchers, without being encumbered by the assets and history of a large pharmaceutical firm.
LESSONS LEARNED
Workshop participants discussed many lessons they had learned about how to foster successful collaborations, including
-
Start the collaboration by defining its goal and then develop a pre-project and project plan, as well as a longer roadmap of what needs to be accomplished and how best to accomplish it. Establish realistic goals and be flexible to new ways of operating to accomplish those goals.
-
Involve all stakeholders early.
-
Include the leadership of a participating company or institution and commit a sufficient amount of time and resources to effectively carry out the collaboration.
-
Make sure there is a critical mass of support for the collaboration and that there are internal champions for it.
-
Actively manage the collaboration; consider using a trusted third party in the management of the collaboration.
-
Make sure to address important legal details of collaborations, such as antitrust issues, intellectual property, and contracts.
-
Establish standards, quality improvement design, and optimization, and use common data elements.
Set Goals and Devise Game Plan
Planning is critical to the success of a collaboration, several speakers emphasized. “Starting the plan early is key,” Vockley said. He suggested creating both a preproject and a project plan. Such planning could define the management and governance of the collaboration, how it will be funded, and a research plan that eliminates rate-limiting steps. For example, with TCGA, acquiring adequate tissue was identified as rate limiting. Research criteria could also be part of the plan, but criteria need to be flexible and realistic enough that they do not impede the collaboration’s progress, Vockley said. McClellan also suggested flexibility in planning how participants in a research collaboration can use the data and information resulting from the collaboration.
Cohen suggested that when setting up collaborations, one needs to define the roles of all the participating parties, what values they bring to the project, and ways to engage each of them in the collaboration process. With the strengths of each participant in mind, one then can assess what aspects of a project certain participants are best at addressing internally. Cohen also suggested defining the goal of the relationships created in a collaboration, and the scientific synergies that will result from it, and then identifying opportunities to establish cross-disciplinary relationships beyond those that are traditionally pursued.
“We need to define what it is we are trying to accomplish—is it purely information sharing by academic and industry researchers so that each party can proceed on their own path? Or is the goal to identify products that might be developed by the company with the intent for the agreement to move beyond preclinical collaboration to a clinical environment? Each of these goals requires a different master agreement,” Cohen said. Altshuler added that the reason for having a specific collaboration needs to be clear from the outset. She suggested making sure there is an alignment upfront in participants’ goals in a collaboration to facilitate coordination and progress. Lakhani added that a collaboration plan should include resource-sharing agreements so it is clear how resources and staff will be shared, as well as the conditions for entry, exit, and ending the collaboration.
Wholley stressed that the success of consortia hinges on their governing policies, which must be established at the start of a collaboration. Such “ground rules” include intellectual property, data sharing, antitrust, selection and award of grants and contracts, confidentiality, and conflict of interest.
Spencer suggested going even further with planning by having collaboration participants craft a long-term roadmap for the collaboration that identifies what barriers need to be overcome. Such a road map was crucial to the success of SEMATECH, Spencer said. He also suggested planning for globalization because drug development is an international effort.
Involve All Stakeholders Early
Several speakers suggested that all stakeholders become involved in the collaboration as early as possible, especially FDA and patient advocacy groups, when appropriate, so that everyone’s goals align and everyone has some ownership in project planning. Vockley stressed the importance of communication among parties to “make sure that everyone is on the same page.” Esserman added, “You have to get people to the table at the begin-
ning, otherwise when you present them with something that has changed, they are not likely to accept it.”
According to Esserman, a phenomenal effort was made to bring in all the stakeholders early in the planning process of the I-SPY 2 TRIAL. Esserman set up weekly calls that included the clinical team lead investigators, senior leadership of the companies, legal representatives, drug suppliers, and the FNIH.
In addition, all the participating IRB chairs and other representatives from the initial 10 research sites in the trial were invited to a meeting hosted by Esserman and the UCSF IRB chair. At that meeting, participants received information about the new trial process and were given the opportunity to hammer out the trial specifics that would meet each site’s IRB criteria before the trial protocol was established. “The lesson is to work ahead,” Esserman said. “When we put the trial through our IRB, it got through without being sent back for anything.”
Similarly, Esserman and some other study investigators met early on with FDA to ask them for suggestions on how to devise their innovative trial protocol to best achieve the study goals. “If you let the usual way of doing business persist, you will not be successful,” Esserman said. “If we had just designed the study protocol and handed it to FDA [without soliciting their prior input], they would have rejected it because it did not follow the rules.” Woosley concurred that “you have to have the regulators at the table as advisors and participants in this process.”
Cohen added that when developing master agreements for collaborations (specifically for industry–academia collaborations), one must work with all the participating IRBs and with the contracts and grants and technology transfer offices of the university to understand potential barriers and ways to overcome them.
Involve the Right People
Several people at the workshop stressed that the leadership of a participating organization, company, or institution needs to actively participate in and be committed to the collaboration. Curt pointed out that because drug company CEOs were involved in the Life Sciences Consortium, they could use their authority to dictate that other divisions of their companies, such as the contracts and legal departments, collaborate with NCI and the other participants of the Life Sciences Consortium. These CEOs also were able to ensure their employees could allocate the time needed to participate in
the collaborative venture. “In industry, and to a lesser extent in academia, [we do have] a sort of paramilitary, top-down society where the leadership really has to set the command, and give the troops the wherewithal to follow,” Curt said.
Spencer pointed out that industry leaders played a key role in SEMATECH, but added that additional participants lower in the hierarchy, who can commit more time to a collaborative endeavor, were also needed. He also warned against companies using consortia as a “dumping grounds” for their less useful employees, and stressed that it was important to get the best people involved in collaborations. Curt agreed that involving the right people in collaborative efforts is necessary. He pointed out that participants in the Life Sciences Consortium’s effort to develop standard clauses for research contracts included individuals who do the actual contracting for their institutions and could indicate what type of contract language would be acceptable for their institutions. Altshuler added that “if you are trying to establish a new standard for your industry, you need a critical mass of players behind it—that is quite important.”
Friend was critical of many consortia, “which often end up being driven by consensus and bring together the lowest common denominator for the longest period of time,” he said. “We need to figure out a way where the gathering together outperforms, and it isn’t necessarily by the rule of consensus,” he added. McClellan concurred, noting that, “by focusing on consensus, it may be more difficult to take on key innovative issues around the edges and push the envelope.”
Mendelsohn noted that successful collaborations usually have champions that are supported by the leadership. For example, he noted the leadership at UCSF had to be willing to let Esserman devote a substantial percentage of her time to crafting and supporting the collaborative I-SPY 2 TRIAL. “You need a champion, and you need a boss for that champion who is willing to support them and give them the freedom to run,” Mendelsohn said. Cohen added that “any time these [academic–industry] collaborations come to the fore, they are often based on person-to-person relationships that require that someone step up to the plate and be the academic champion within an institution.”
Nguyen also stressed the need for adequate leadership and internal champions to promote collaborative efforts. He pointed out that the NIH has several policies about how publicly funded researchers share certain resources and their findings. These policies have provided the guidance needed to institute the Science Commons. In addition, Nguyen noted that
within individual institutions, internal champions “who are passionate about sharing in the commons and can then be evangelists for everybody else in the organization [are needed].”
Workshop participants also stressed the importance of having a stable source of funding for collaborations. Spencer said a stable source of funding for 6 or 7 years was key to SEMATECH’s success. Woosley noted that sharing has a cost, and that FDA needs the funding to support its participation in consortia and other collaborations. He added that often collaborations are never hatched or fail because of insufficient funding. “People have tried to share and pool placebo data for many years, and have actually gotten the data from companies, but they have not been able to get the funding to actually use it,” he said. “I think our current granting system does not really acknowledge the kinds of infrastructure that are needed, and our Congress has not given FDA the funding it needs to participate in these kinds of precompetitive discussions.”
Nguyen added that many resources for supporting collaborative efforts, particularly those that involve creating and maintaining a public domain (commons) where data or other information is shared, are ephemeral. Without some steady source of funding for the effort it takes to create and maintain collaborations, the commons will fail, he said. “Many funders of the research will not provide funds to maintain the data or to share the data. So what happens is you do the research and then you throw away the data. That is a wasteful way for us to use limited funding dollars and not leverage the potential for this stuff to be in the commons,” he said. Both Nguyen and Woosley called for innovative business models to support collaborative endeavors.
Actively Manage Collaborations
Many speakers at the conference stressed the need for active management of collaborations. “You need a coordinator or project manager who understands the science, and is good at linking people together,” said Lakhani. He thought the active management of the researchers involved in the Myelin Repair Foundation by its chief operating officer has been key to its success. “The coordination role that is needed is not a manager saying, ‘tomorrow you better make this happen,’ but rather a coordination of knowledge across the organization. That is critical. You can’t rely on scientists to self-organize,” Lakhani said.
McClellan concurred, adding, “do not underestimate the manage-
ment support needed to make these collaborations work. As you get to a significant number of partners, it does require experienced, full-time management, and an explicit governance structure.” Altshuler added that “project management is really important. For some of these very big efforts that need to be coordinated across a number of sites, it can be crucial to have dedicated professional project management mapping out exactly what needs to happen and when.” She also pointed out that even the most open-sourced enterprises seem to have a need for some coordination.
Invoke a Trusted Third Party
Speakers noted that having a trusted third party to broker and/or manage a collaboration can often facilitate cooperation. The FNIH, for example, acts as a trusted third party for the I-SPY 2 TRIAL. The FNIH holds the master IND for the project, and it also manages the trial through the Biomarkers Consortium. “Having the Foundation for NIH be the trusted broker here is key because it meant that everyone had to give up their stake and agree to trust the FNIH as the honest broker,” Esserman noted.
Curt noted that the success of the Life Sciences Consortium project to develop START clauses for legal collaboration contracts (see Box 9) was in large part due to engaging NCI as a trusted third party in the initiative. Without NCI’s participation, “the threat of potential litigation due to anti-competitiveness would have probably stopped what we were trying to do because one lawyer from one company saying ‘you are at risk’ would have been enough to spook the whole herd,” said Curt.
McClellan added that “it helps to have a neutral convener—not a stakeholder with a particular interest—but one that could be trusted by the broad base of stakeholders we think help make these efforts a success.” Such a neutral convener can “create the safe, legal harbor needed for collaborations to occur,” he said. Woosley concurred, and said “that honest broker component to precompetitive sharing is an essential element because if you are asking people to share, but you are in it for yourself, or you are trying to make money or want to hold the IP, it is just not going to work.”
Address Legal Issues
Speakers described a number of legal issues that have to be addressed for collaborations to be successful. These legal issues include antitrust law,
conflict of interest, and intellectual property rights. Both McClellan and Eck recommended addressing potential antitrust issues in collaborations, with Eck recommending consultation with the U.S. Department of Justice when devising collaborations if there are concerns with antitrust issues. Curt noted that the Justice Department approved the Life Sciences Consortium’s START clauses for academic and industry collaborations.
Discerning how intellectual property will be shared in collaborations can be especially challenging, but several speakers suggested that these issues need to be addressed at the start of a collaboration. “Don’t let the lawyers tell you it can’t be done—force them to do some creative problem solving,” said Lakhani. He suggested having the lawyers of the collaborators work together to devise innovative new models for sharing intellectual property, adding, “you want to spend a lot of time to get this right.”
A lot of work went into crafting the IP agreement used in the I-SPY 2 TRIAL, Wholley noted. This agreement recognizes that the significant preexisting IP that is being brought to the trial by the companies and academic researchers will stay with their originators, but that new IP discovered by the trial, such as a new genetic signature that predicts therapy response, will be equitably shared by the collaborators. “The only way we felt we could do this would be to have the FNIH sit in the middle and act as the honest broker for managing this IP,” said Wholley. For any new IP created by the collaboration, the inventing organizations will grant exclusive license to the FNIH, which will prosecute and manage the resulting patents, fairly market and license the IP to interested parties, and return a fair share of the royalties less expenses to the inventing organizations.
Who has access to data generated by a collaboration and who has publication rights are other issues for collaborative agreements to address, speakers suggested. In the I-SPY 2 TRIAL, when a given biomarker and therapeutic combination graduates, the company that created the tested therapeutic will be given access to the data on that therapeutic and biomarker combination 6 months before the rest of the collaborators view the data. But every collaborator will have equal access to the data on exploratory biomarkers at the same time.
Provide Rewards and Incentives
Many speakers stressed the importance of providing rewards and incentives for collaboration as well as countering the general culture in both aca-
demia and industry that promotes competition rather than collaboration. They pointed out that tenure and other reward systems in academia are based on individual, not collaborative, research projects and publications, which provides a disincentive for faculty, especially junior faculty, to participate in collaborative ventures.
“There is tremendous pressure for people to not work together,” said Woosley. “There is a lot of criticism when academic scientists work with industry—they are then denied being on advisory committees [for FDA]. The universities train the stars who get the R01s, who get the Nobel Prizes and the papers in Nature. They don’t train the people who can work effectively in teams.” Lakhani added, “scientists in their own labs are very open, but across labs they are extremely competitive and very closed. You have to create a culture of transparency and sharing and create norms around it. Institutions and practices can enable this. We need to think about how we recognize [collaborative] achievement instead of individual lab achievement.”
In the I-SPY 2 TRIAL, rather than rely on the traditional system that only gives recognition to the principal investigator in a clinical trial, the investigators created a new system that recognizes the contributions from more of the collaborating researchers: two investigators were assigned as “chaperones” for every compound and for every biomarker being tested in the trial. “That way people can own some piece of the research and move forward. Because if you do not get people feeling in academia like they have credit, then they are not going to collaborate either,” said Esserman.
Other speakers suggested that industry also has a culture of competition and secrecy, even though most pharmaceutical companies have the same information on basic biology and thus are pursuing the same targets for their drugs. Friend called for breaking down the illusion that there are some very powerful unique advantages that sit within different companies and cannot be shared. Spencer added, “One of the things that the people who came to SEMATECH were told was ‘listen but don’t talk and lock your file cabinet.’ Each company believed it had the secrets to making semiconductor devices better than anybody else. What their engineers and scientists found after being there for a short time was that there were very few secrets, and that everybody was rediscovering the same thing over and over again. They immediately learned that if they could share this information, they were saving everybody a lot of money.”
Standards and Quality Control
Some speakers suggested developing and using data standards and data elements to facilitate collaborations. Woosley suggested using the data standards of the Clinical Data Interchange Standards Consortium. Esserman added that for the I-SPY 2 TRIAL, “it is really critical to have common data elements for easier integration of clinical imaging and molecular results so you can send stuff around.” Standard research methods and procedures also must be followed. “If you are really going to do team science, everyone has to adhere to standards, even the people who think they are the most expert,” said Esserman. “We learned to send people around and certify every site to make sure they are doing everything right.” She suggested making sure there is a quality improvement design built into research collaborations. Heywood also stressed the importance of optimizing collaborative research to ensure data quality.
NEXT STEPS
Participants at the conference suggested several next steps to take to foster precompetitive collaborations, including
-
Seeking public support for collaborations and advocating for funding;
-
Holding a meeting with key constituents in oncology to determine how to move the field forward;
-
Having an appropriate authoritative body establish a set of standards for the sharing of data, material, and tools, and/or general standards for collaboration;
-
Publicizing collaboration success stories and management plans; and
-
Developing innovative business models for collaborations.
Seek Public Support for Collaborations
Some speakers offered specific suggestions for fostering more public support and funding for collaborative ventures. McClellan suggested that policies financially reward the development of shared data repositories and infrastructure for effective collaboration. Such incentives could include direct payments (i.e., government funding) for infrastructure or for participation and reporting of data, as well as payments for achievement of well-defined outcomes. McClellan echoed Woosley’s request that more funding
be given to FDA so it can participate in more collaborations and that more public funding be available, in general, to support collaborative ventures. “These issues are fundamentally important to making better treatments available and should be much higher on the list of public health concerns of the nation than they actually are,” said McClellan.
McClellan also suggested that patients could give more momentum to collaborative efforts by advocating for them and framing the issues in a way that could potentially garner more public support and funding. Woosley concurred, pointing out that “there is a huge opportunity now to get the disease groups to speak with one voice, and to talk about precompetitive sharing for all diseases. A lot of disease foundations are setting up venture philanthropy organizations to fund the kind of business initiatives that they want, and they are very concerned that the basic research has not been translated into business opportunities, which are how the patients really get the final benefit.”
Friend added, “I am struck by the emerging role of disease foundations as engines for therapies. They have more of a voice now. We need to highlight patient advocates and disease foundations and the roles they can play.” Friend added that patients can play an important advocacy role, especially in fostering the collaborations needed to further personalized medicines. “The patients can say ‘why isn’t this drug working in me?’ and can collect the data if others aren’t collecting the data.”
Establish Collaboration Standards and Incentives
Attendee Richard Bookman, vice provost for research at the University of Miami Leonard M. Miller School of Medicine, suggested that an appropriate authoritative body devise a set of standards on the sharing of data, materials, tools, and collaboration that federal, state, and other funding agencies of biomedical research could use as guidance when shaping their grant programs. Woosley suggested advocating that the implementation of electronic medical records that will soon be supported by the federal government stimulus bill include common data elements that could be useful in research and would help ease research collaborations.
Publicize Collaboration Success Stories and Management Plans
McClellan suggested publicizing collaborations that have been done successfully and others that show promise, as well as the specific pathways for doing collaborations effectively. Several participants suggested putting the
management plans that have been drafted for some of the collaborations discussed at the workshop into the public domain, if possible, so that others can learn from them. Wholley noted that the project management plan template for the Biomarkers Consortium is posted on the Web,14 and Williams-Jones said that the model grant agreement for the Innovative Medicines Initiative can be accessed from the Internet.15 Esserman agreed with Friend’s suggestion that the IP and data-sharing collaborative agreements that have been forged by the I-SPY 2 TRIAL be distributed more widely and combined with efforts by Science Commons and others to build similar standard agreements, noting that “everything we built in this trial can be reused.”
As an incentive for industry and academia to use the START clauses when collaborating, Murphy said the CEOs of the Roundtable on Cancer are trying to develop some sort of public acknowledgment of those companies and institutions that use the clauses and how the clauses have accelerated the clinical trial development process.
Develop Innovative Business Models
Some speakers suggested devising innovative business models that support collaborations. Woosley suggested creating “innovative ways for companies to come together to pool their diagnostics and drugs, and to develop more comprehensive strategies, rather than just a single agent.” Esserman noted that for the I-SPY 2 TRIAL she had to come up with a new business model to support the trial because she did not want to adhere to the old model of having a drug company, whose drug was being tested, financially back the study. Instead, the goal was to have broader based support, which is likely to benefit many stakeholders, including the drug industry. Although the funding for the trial is still being worked out, some drug companies are opting to help fund the trial even though they are not contributing molecules to be tested.
Lakhani discussed what type of business model is needed for precompetitive research and compared that to the traditional business model. Lakhani said the traditional innovation model that most pharmaceutical firms use is the private innovation model, in which firms make private investments to solve technical problems and expect monopoly returns for
their successful innovations. IP is jealously guarded and patented in this model, and sharing of knowledge only happens through accidental spillovers, that is, from employees with the knowledge switching companies. In contrast, in a collective innovation model, which typically involves government funding of research, individuals get external subsidies to solve technical problems and the knowledge they acquire in the process is given to a common pool for reuse and creative recombination. In this model, parties self-regulate through norms such as reciprocity, recognition, and peer esteem. But free riding is a central concern and can occur.
What is beginning to emerge, according to Lakhani, is a private–collective hybrid model, in which firms and individuals exert private effort but disclose their work to others in a common pool. Innovators get selective benefits through participation that outweigh the cost of investment. These benefits include access to new knowledge and new materials, access to people, and shared risk. Participants can combine the knowledge from the common pool with their own specific and proprietary assets to create value, and free riders cannot share in the selective benefits. SEMATECH is a successful example of the private–collective innovation model, according to Lakhani (see Table 2).
There was some debate at the workshop about whether a large-scale SEMATECH-like umbrella effort should be made to support collaborations in biomedicine or more specifically in oncology, or whether support should be focused on individual collaborative projects and consortia. Spencer noted that a SEMATECH-like umbrella organization and source of funding could reduce the significant amount of time they spend trying to garner funding for and supporting their collaborative efforts. “You have got a whole series of projects where if you shared that activity you could cut down the time, the administrative overhead, that each of these principal investigators or heads of projects has to allow them to get on with the real work of getting something done,” he said. “Oncology could take the lead in looking at something that was funded by the government and by private industry, and you have got a CEO Roundtable already, so you are light years ahead of where we were in the semiconductor industry in 1985 when we were trying to get SEMAT-ECH rolling.”
Cohen pointed out that a centralized support organization for collaborations in oncology or biomedicine could not only support scientific pursuits, but also, or instead, efforts to develop new regulatory pathways for collaboratively developed drugs and other public policy advances needed to support collaborative research.
TABLE 2 Innovation Models
|
Private Innovation Model |
Collective Innovation Model |
Private–Collective Hybrid Model |
|
Firms make private investments to solve technical problems—is often risky |
Individuals get external subsidies to solve technical problems |
Firms/Individuals exert private effort, but disclose work to others in a common pool |
|
Expect monopoly rents for their successful innovations |
Knowledge is given to a common pool for reuse and creative recombination |
Innovators get “selective benefits” through participation that outweigh the cost of investment:
|
|
Innovation outcomes are rival and excludable |
Parties self-regulate through norms like reciprocity, recognition, and peer esteem |
Participants can combine knowledge from common pool with own specific and proprietary assets to create value |
|
Sharing of knowledge only happens through “accidental” spillovers |
Free riding becomes a central concern |
Free riders cannot share in the selective benefits |
|
SOURCE: Lakhani presentation (February 9, 2010). |
||
Munos countered that a centralized approach to fostering collaborations in biomedicine has the danger of squelching the diverse, creative collaborative approaches that are currently undertaken and surviving on shoestring budgets. “Rather than feeding a lot of money in the system, I would feed some money into those people who are coming up with experimental models. Most of them will fail, but those that succeed could prove to be very disruptive of the traditional pharma R&D model, and might be able to renew it in ways that would be pretty healthy,” Munos said.
Altshuler said there is a false dichotomy of centralized support versus support for more entrepreneurial ventures. “Certain types of problems can be handled one way, and certain ones can be handled another way. Is there
a role going forward for some central funding that can set priorities and not necessarily encompass that which is already going on?” she asked.
Woosley noted the current significant consortium fatigue among those participating in collaborations and stressed that “any efforts that we come up with should really focus on coordinating and getting maximum benefit from all the dollars that are already in place before we ask for more, because I think we are going to get a lot of resistance from an industry that is really downsizing its research efforts right now.” Williams-Jones concurred, and suggested seeing beyond the spectrum of oncology, and considering other disease areas as a way to tap into funding for collaborative research and relieve consortium fatigue. “We really need a global solution that will allow us to spend the little cash that we do have to spread across this,” he said.
Mendelsohn pointed out that different collaborative efforts may require different approaches to tackle them, and that the SEMATECH approach may not be appropriate for all of them. “We need to attack the issues of regulat[ion], infrastructure, and interoperable datasets, and we have to attack the issue of how to make biomarker-driven drug selection trials work. Those are three different problems that may require different approaches,” he said.
SUMMARY
After 2 days of presentations and lively discussion, during which Washington, DC, was blanketed in a crippling snowstorm, it became apparent that a number of factors are currently driving precompetitive collaborations, including declining R&D budgets combined with the growing complexity of biomedical research. Several participants viewed precompetitive collaboration as a means to solve some of the problems that currently plague the drug development process both in oncology and in other therapeutic areas.
Speakers also noted that precompetitive collaborations have to be crafted carefully to provide incentives and rewards to participants while avoiding legal, cultural, technical, and other obstacles. Innovative regulations and business plans may foster precompetitive collaborations and enable their products to seamlessly enter the market.
Speakers discussed many lessons that can be learned from the precompetitive collaborations that have been attempted and/or accomplished successfully, including the importance of starting a collaboration by defining its goals and planning for how those goals can be accomplished, bring-
ing together the right stakeholders early in the planning process, actively managing the collaboration, and using a trusted third party to foster collaborations. Speakers also suggested that critical legal issues be addressed, such as intellectual property and patents, the sharing of data, conflict of interest, and antitrust issues.
To further precompetitive collaboration, speakers suggested several next steps. Some ideas included: seeking more public support and funding for collaborations, publicizing collaboration success stories and management plans, and having an appropriate authoritative body establish a set of standards for sharing precompetitive materials.
REFERENCES
Abrams, J., R. Erwin, G. Fyfe, and R. L. Schilsky. 2010. Data submission standards and evidence requirements. The Oncologist 15(5):488–491.
Barker, A., C. C. Sigman, G. J. Kelloff, N. M. Hylton, D. A. Berry, and L. J. Esserman. 2009. I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clinical Pharmacology & Therapeutics 86(1):97–100.
Boat, T. F. 2010. Insights from trends in biomedical research funding. Journal of the American Medical Association 303(2):170–171.
Booth, B., and R. Zemmel. 2004. Prospects for productivity. Nature Reviews Drug Discovery 3(5):451–456.
Carro, M. S., W. K. Lim, M. J. Alvarez, R. J. Bollo, X. Zhao, E. Y. Snyder, E. P. Sulman, S. L. Anne, F. Doetsch, H. Colman, A. Lasorella, K. Aldape, A. Califano, and A. Iavarone. 2010. The transcriptional network for mesenchymal transformation of brain tumours. Nature 463(7279):318–325.
CEO Life Sciences Consortium. 2010. START clauses. http://ceo-lsc.org/TaskForceScrape.aspx (accessed April 19, 2010).
CEO Roundtable on Cancer and NCI (National Cancer Institute). 2008. Proposed standardized/harmonized clauses for clinical trial agreements. Rockville, MD: CEO Roundtable on Cancer and NCI.
Clark, A., M. Ellis, C. Erlichman, S. Lutzker, and J. Zwiebel. 2010. Development of rational drug combinations with investigational targeted agents. The Oncologist 15(5):496–499.
Critical Path Institute. 2010a. About us. http://www.c-path.org/about.cfm (accessed April 20, 2010).
Critical Path Institute. 2010b. Financial status. http://www.c-path.org/funding.cfm (accessed April 20, 2010).
Dilts, D. M., and A. B. Sandler. 2006. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. Journal of Clinical Oncology 24(28):4545–4552.
DOJ (Department of Justice). 2008. Response to the CEO Roundtable on Cancer’s request for business review letter. http://www.justice.gov/atr/public/busreview/237311.htm (accessed April 19, 2010).
Dorsey, E. R., J. de Roulet, J. P. Thompson, J. I. Reminick, A. Thai, Z. White-Stellato, C. A. Beck, B. P. George, and H. Moses III. 2010. Funding of US biomedical research, 2003–2008. Journal of the American Medical Association 303(2):137–143.
Engelman, J. A. 2009. Targeting PI3K signalling in cancer: Opportunities, challenges, and limitations. Nature Reviews Cancer 9(7):550–562.
FDA (Food and Drug Administration). 2004. Innovation/Stagnation: Challenge and opportunity on the critical path to new medical products. Silver Spring, MD: FDA.
FDA. 2008. FDA, European Medicines Agency to consider additional test results when assessing new drug safety. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116911.htm (accessed April 20, 2010).
FDA. 2010a. FDA’s Critical Path Initiative. http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm (accessed May 25, 2010).
FDA. 2010b. Guidance for the use of bayesian statistics in medical device clinical trials. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071072.htm (accessed June 1, 2010).
FDA. 2010c. Draft guidance for industry: Adaptive design clinical trials for drugs and biologics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm201790.pdf (accessed June 1, 2010).
FDA. 2010d. FDA issues guidance to help streamline medical device clinical trials. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm199993.htm (accessed June 1, 2010).
FNIH (Foundation for the National Institutes of Health). 2010a. The Biomarkers Consortium: Approved and funded. http://www.biomarkersconsortium.org/index.php?option=com_content&task=view&id=134&Itemid=187 (accessed May 25, 2010).
FNIH. 2010b. The Biomarkers Consortium launches I-SPY 2 breast cancer clinical trial. http://ispy2.org/docs/ISPY2_Press%20Release_3-15-10.pdf (accessed April 20, 2010).
FNIH. 2010c. The Biomarkers Consortium policies and procedures. http://www.biomarkersconsortium.org/index.php?option=com_content&task=section&id=6&Itemid=40 (accessed April 22, 2010).
Hollingsworth, J. R. 2007. High cognitive complexity and the making of major scientific discoveries. In Knowledge, Communication, and Creativity, edited by A. Sales and M. Fournier. London and Thousand Oaks, CA: Sage Publications.
IOM (Institute of Medicine). 2007. Cancer biomarkers: The promises and challenges of improving detection and treatment. Washington, DC: The National Academies Press.
IOM. 2010a. A foundation for evidence-driven practice: A rapid learning system for cancer care workshop summary. Washington, DC: The National Academies Press.
IOM. 2010b. A national cancer clinical trials system for the 21st century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press.
Jeppesen, L. B., and K. R. Lakhani. In press. Marginality and problem-solving effectiveness in broadcast search. Organization Science.
Kelloff, G. J., J. M. Hoffman, B. Johnson, H. I. Scher, B. A. Siegel, E. Y. Cheng, B. D. Cheson, J. O’Shaughnessy, K. Z. Guyton, D. A. Mankoff, L. Shankar, S. M. Larson, C. C. Sigman, R. L. Schilsky, and D. C. Sullivan. 2005. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clinical Cancer Research 11(8):2785–2808.
Kola, I., and J. Landis. 2004. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery 3(8):711–716.
Lilly. 2010. History: Milestones in medical research. http://www.lilly.com/about/history/ (accessed May 24, 2010).
MMRF (Multiple Myeloma Research Foundation). 2009. MMRC Multiple Myeloma Genomics Initiative first to sequence complete myeloma genomes http://www.themmrf.org/about-the-mmrf/powerful-news/press-releases/mmrc-multiple-myeloma-genomics-initiative-first-to-sequence-complete-myeloma-genomes.html (accessed April 20, 2010).
MMRF. 2010. About the MMRF: Results. http://www.themmrf.org/about-the-mmrf/results/ (accessed May 3, 2010).
MRF (Myelin Repair Foundation). 2010. About us. http://www.myelinrepair.org/about (accessed April 21, 2010).
Munos, B. 2009. Lessons from 60 years of pharmaceutical innovation. Nature Reviews Drug Discovery 8(12):959–968.
Munos, B. 2010. Can open-source drug R&D repower pharmaceutical innovation? Clinical Pharmacology and Therapeutics 87(5):534–536.
NCI (National Cancer Institute). 2006. New federal health initiative to improve cancer therapy: Patients will benefit from rapid development and delivery of new cancer treatments. http://www.cancer.gov/newscenter/pressreleases/OBQI (accessed April 22, 2010).
NCI. 2010. NCI Office of Technology and Industrial Relations. Partnerships: Food and Drug Administration. http://otir.cancer.gov/programs/partnerships_fda.asp (accessed April 22, 2010).
NCI and NHGRI (National Human Genome Research Institute). 2010a. The Cancer Genome Atlas: About Us. http://cancergenome.nih.gov/about/index.asp (accessed May 2, 2010).
NCI and NHGRI. 2010b. The Cancer Genome Atlas: Expanded program.http://cancergenome.nih.gov/wwd/program/ (accessed May 24, 2010).
NCI and NHGRI. 2010c. The Cancer Genome Atlas: Cancers selected for study. http://cancergenome.nih.gov/wwd/cancers_studied_by_tcga.asp (accessed May 24, 2010).
NIH (National Institutes of Health). 2008. NIH Public–Private Partnership Program: Improving the nation’s health by facilitating collaborations between the NIH and other public and private partners. http://ppp.od.nih.gov/pppinfo/value.asp (accessed May 25, 2010).
OSDD (Open Source Drug Discovery). 2010. Home Open Source Drug Discovery. http://www.osdd.net/ (accessed May 2, 2010).
Park, J. W., R. S. Kerbel, G. J. Kelloff, J. C. Barrett, B. A. Chabner, D. R. Parkinson, J. Peck, R. W. Ruddon, C. C. Sigman, and D. J. Slamon. 2004. Rationale for biomarkers and surrogate end points in mechanism-driven oncology drug development. Clinical Cancer Research 10(11):3885–3896.
PCAST (President’s Council of Advisors on Science and Technology). 2008. Priorities for personalized medicine. Washington, DC: Office of Science and Technology Policy.
Polanski, M., and N. L. Anderson. 2006. A list of candidate cancer biomarkers for targeted proteomics. Biomarker Insights 2:1–48.
Rockoff, J. D. 2010. Pfizer plans to cut R&D by up to $3 billion. Wall Street Journal, February 4, p. B3.
Schilsky, R. L., J. Abrams, J. Woodcock, G. Fyfe, and R. Irwin. 2008. The need for data collection and reporting standards. Paper presented at the Conference on Clinical Cancer Research, Washington, DC.
Schofield, P. N., T. Bubela, T. Weaver, L. Portilla, S. D. Brown, J. M. Hancock, D. Einhorn, G. Tocchini-Valentini, M. Hrabe de Angelis, N. Rosenthal, and CASIMIR Rome Meeting participants. 2009. Post-publication sharing of data and tools. Nature 461(7261):171–173.
Science Commons. 2010a. Patent Licenses. http://sciencecommons.org/projects/patent-licenses (accessed April 20, 2010).
Science Commons. 2010b. Biological Materials Transfer Project. http://sciencecommons.org/projects/licensing/ (accessed April 20, 2010).
Science Commons. 2010c. The Health Commons. http://sciencecommons.org/projects/healthcommons/ (accessed April 20, 2010).
Science Commons. 2010d. The Neurocommons. http://sciencecommons.org/projects/data/ (accessed April 20, 2010).
Science Commons. 2010e. Science Commons: Building on the Creative Commons model to further scientific success. http://sciencecommons.org/about/details/ (accessed April 20, 2010).
Spear, B. B., M. Heath-Chiozzi, and J. Huff. 2001. Clinical application of pharmacogenetics. Trends in Molecular Medicine 7:201–204.
Stodden, V. 2010. The scientific method in practice: Reproducibility in the computational sciences. MIT Sloan Research Paper No. 4883-10. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=1550193 (accessed April 20, 2010).
Valero, V., A. U. Buzdar, and G. N. Hortobagyi. 1996. Locally advanced breast cancer. The Oncologist 1(1):8–17.
Wagner, J. A., E. C. Wright, M. M. Ennis, M. Prince, J. Kochan, D. J. Nunez, B. Schneider, M. D. Wang, Y. Chen, S. Ghosh, B. J. Musser, M. T. Vassileva. 2009. Utility of adiponectin as a biomarker predictive of glycemic efficacy is demonstrated by collaborative pooling of data from clinical trials conducted by multiple sponsors. Clinical Pharmacology and Therapeutics 86(6):619–625.
Weber, S. 2004. The success of open source. Cambridge, MA: Harvard University Press.
Zerhouni, E. A., C. A. Sanders, and A. C. von Eschenbach. 2007. Biomarkers Consortium: Public and private sectors working in partnership to improve public health. The Oncologist 12(3):250–252.