11
How Grandmother Effects Plus Individual Variation in Frailty Shape Fertility and Mortality: Guidance from Human-Chimpanzee Comparisons
KRISTEN HAWKES
In the first paper to present formal theory explaining that senescence is a consequence of natural selection, W. D. Hamilton concluded that human postmenopausal longevity results from the contributions of ancestral grandmothers to the reproduction of their relatives. A grandmother hypothesis, subsequently elaborated with additional lines of evidence, helps explain both exceptional longevity and additional features of life history that distinguish humans from the other great apes. However, some of the variation observed in aging rates seems inconsistent with the tradeoffs between current and future reproduction identified by theory. In humans and chimpanzees, our nearest living relatives, individuals who bear offspring at faster rates do not cease bearing sooner. They continue to be fertile longer instead. Furthermore, within both species, groups with lower overall mortality rates have faster rates of increase in death risk with advancing age. These apparent contradictions to the expected life history tradeoffs likely result from heterogeneity in frailty among individuals. Whereas robust and frail alike must allocate investments between current and future reproduction, the more robust can afford more of both. This heterogeneity, combined with evolutionary tradeoffs and the key role of ancestral grandmothers they identify, helps explain aspects of human aging that increasingly concern us all.
Department of Anthropology, University of Utah, Salt Lake City, UT 84112. E-mail: hawkes@anthro.utah.edu.
Long postmenopausal survival is a characteristic of our species. The use of life expectancy to compare human populations can obscure this fact because high infant and juvenile mortality kept all national life expectancies below 50 until the 20th century (Oeppen and Vaupel, 2002). As historical demography shows, girls that survived childhood usually lived long past menopause in previous centuries (Keyfitz and Fleiger, 1968). Hunter-gatherer survival curves are especially instructive (Howell, 1979; Hill and Hurtado, 1996; Early and Headland, 1998; Blurton Jones et al., 2002; Hill et al., 2007). They document characteristic human longevity in the absence of agriculture, public health institutions, and scientific medicine, all of which emerged long after the initial evolution of our species (Hawkes and Blurton Jones, 2005; Gurven and Kaplan, 2007). Distinctive and at first puzzling human postmenopausal survival was addressed in classic papers that used evolutionary theory to explain why living things grow old.
G. C. Williams (1957) laid out demographic reasons why declines in adaptive performance with increasing adult age emerge from the forces of natural selection. Because life is risky, cohorts inevitably diminish across adulthood. Consequently, the forces of selection weaken with age as fewer remain to be affected by it at older ages. Williams explained how the same forces result in different rates of senescence among species that reproduce more than once depending on two aspects of life history. First, when background mortality risk is lower, more individuals survive to older ages and selection against senescence is stronger. Second, selection against senescence is also stronger when the potential fitness-related payoffs to survivors increase with age. He illustrated the latter effect with the slow senescence of indeterminate growers that continue to increase in size and rate of egg production throughout adulthood.
Concluding that evolutionary life history theory predicts no post-reproductive period in normal life spans, Williams then addressed the apparent contradiction posed by survival past menopause in our own species by observing that older women still investing in descendants are not literally postreproductive. Hamilton (1966) mathematically modeled the tradeoffs nominated by Williams and demonstrated that the forces of selection shape mortality schedules to converge asymptotically with the age when reproduction ends. This process leaves, as Williams had surmised, few if any postreproductives. Because “much the best” (Hamilton, 1966, p. 27) demographic data are available on humans, Hamilton used a human population to explore the fit of observation with theory. This required him to explicitly confront the apparent discrepancy in the case of humans (Hamilton, 1966, p. 37):
[T]he rather definite age of menopause seems conspicuously ignored by the as yet gently rising curve of the force of mortality. It is, moreover, a matter of common knowledge that the post menopausal woman normally remains a useful and healthy member of the community for some time… . [This] can be attributed to the beneficial effects of continued survival on the survival and reproduction of descendants…. In fact … the comparatively healthy life of the postreproductive woman … inevitably suggests a special value of the old woman as a mother or grandmother during a long ancestral period….
Such a grandmother hypothesis, subsequently elaborated with comparative and phylogenetic evidence not available when the classic papers appeared, can explain not only the evolution of human longevity but other similarities and differences in life history between humans and the other great apes. We live longer; we take longer to mature but have shorter birth intervals; and we share common ages of terminal female fertility with the other great apes (Hawkes et al., 1998; Robson et al., 2006). The hypothesis focuses on females because as noted by both Williams and Hamilton our mid-life menopause is a central clue to human life history evolution and because the hypothesis employs E. L. Charnov’s (1991, 1993) model of tradeoffs faced by females to explain mammalian life history variation. The forces of selection explored by Williams (1957, 1966), Hamilton (1966), Charnov (1993), and many other students of life history evolution (Stearns, 1992; Charlesworth, 1994) attend to fitness effects and not to proximate mechanisms, but T. B. L. Kirkwood’s disposable soma model (Kirkwood and Rose, 1991) based on the same evolutionary tradeoffs between current and future reproduction has directed attention to processes of cellular maintenance and repair that affect somatic aging rates (Kirkwood and Holliday, 1979; Finch, 2007). Such processes likely have similar effects in both sexes, because longer-lived mothers pass on their cellular maintenance mechanisms to both sons and daughters.
I briefly summarize this elaborated grandmother hypothesis, then turn to patterns that initially seem inconsistent with the tradeoffs between current and future reproduction identified in evolutionary explanations for senescence. I focus on two apparent inconsistencies between theoretical expectations and empirical observations. First, theory predicts that current reproductive output should subtract from effort invested in maintenance for survival and reproduction in the future, yet individuals with higher fertility rates tend to continue bearing offspring to older ages; and in humans, women with later last births then survive longer afterward (Perls et al., 1997; Jacobsen et al., 2003; Emery Thompson et al., 2007; Gagnon et al., 2009). Second, theory predicts that lower adult mortality should slow rates of senescence, yet when populations of the same species are compared, the groups with lower mortality have steeper increases in death risk
with advancing age (Strehler and Mildvan, 1960; Gavrilov and Gavrilova, 2001). More survival to older ages makes senescence—measured as the pace of increase in age-specific mortality—appear to be faster. Heterogeneity of frailty within populations may explain these apparent contradictions (Hawkes et al., 2009).
J. W. Vaupel and colleagues (1979, 1998) proposed that heterogeneity in frailty might explain why the increase in mortality rates across adulthood begins to slow and even cease at advanced ages in humans and many other taxa. If individuals vary in their vulnerabilities to death, the more frail will usually die younger. Survivors to the oldest ages will therefore be a subset of the population enriched with individuals that had lower vulnerability all along. L. D. Mueller, M. R. Rose, C. L. Rauser, and colleagues (Mueller and Rose, 1996; Rauser et al., 2006; Rose et al., 2007) judged Vaupel’s hypothesis to be in conflict with Hamilton’s forces and found those forces themselves sufficient to explain the mortality plateaus. I argue here that rather than being mutually exclusive alternatives, heterogeneity of frailty and tradeoffs between current and future reproduction explain different things. Both are needed to account for salient aspects of fertility and mortality schedules in general, and those of humans and chimpanzees in particular. As Williams and Hamilton recognized, women usually outlive their fertility. This is not true of chimpanzees. Although childbearing ends at the same age in both species, only humans regularly survive for decades longer. Heterogeneity within populations can explain why this divergence in life history results in fertility schedules with different shapes.
A GRANDMOTHER HYPOTHESIS
Anthropologists continue to debate the phylogenetic relationships among fossil taxa representing our ancestors and cousins (Wood, 2010), but genetic evidence unequivocally corroborates Darwin’s hypothesis about our African ape ancestry (Glazko and Nei, 2003). The genera ancestral to our own are often characterized as bipedal apes (Wood and Collard, 1999), and chimpanzees are commonly used as a living model for the ancestors of our genus because they are genetically closest to us and similar in body and brain size to these extinct taxa (Robson and Wood, 2008). Correlations between life history traits and adult size across the living primates (Charnov, 1993) support the relevance of a chimpanzee model for the early members of our lineage.
Like other primates, chimpanzees feed themselves after weaning (Goodall, 1986). Systematic observations among modern hunter-gatherers show that human youngsters can be remarkably efficient foragers, acquiring large fractions of their own requirements at young ages (Blurton Jones
et al., 1997; Bliege-Bird and Bird, 2002; Bird and Bliege-Bird, 2005); but unlike chimpanzees, humans still depend on provisioning by others after weaning. Help is especially crucial for certain kinds of foods (Hawkes et al., 1995). Reliance on resources that young juveniles cannot handle effectively requires mothers to provision weaned offspring, but mothers nursing new infants provide less for their weaned children who receive subsidies from grandmothers (Hawkes et al., 1997).
The productivity of Hadza hunter-gatherer grandmothers especially in gathering hard-to-acquire staples, and the importance of their subsidies to weaned children with infant siblings (Hawkes et al., 1997), suggests a scenario about the ancestral past. An ecological change that reduced the availability of foods juveniles could handle independently would have opened a novel fitness window to older females without nursing infants of their own (Hawkes et al., 1997). By helping to feed weanling grandchildren, elder females would have allowed their daughters to bear the next baby sooner without affecting the survival of previous offspring. More vigorous elders, through greater reproductive success of their daughters, would have spread their slower somatic aging to more descendants. Longer adult life spans then reduced the cost of waiting longer to mature, delaying age at maturity and increasing adult body size (Hawkes et al., 2003). Because later births would interfere with grandmothering, selection would not have favored delaying ages of fertility decline. Increased allocation to somatic maintenance would have left less for current reproduction through the childbearing years, but subsidies from elders would have more than compensated, raising the fertility of childbearers (Hawkes, 2003).
We hypothesized that such a shift might have given rise to genus Homo (O’Connell et al., 1999; Hawkes, 2003) when drying environments and increased seasonality altered foraging opportunities for ancestral populations between 2 and 3 million years ago as forests shrank and grasslands spread across Africa (deMenocal, 1995; Bromage and Schrenk, 1999). Changes in body size and form are consistent with such a shift, as is the colonization of new habitats about that time. The hypothesis also helps explain the location of early archaeological sites and the composition of the faunal assemblages associated with them (O’Connell et al., 2002).
A formal model of the verbal grandmother scenario outlined here remains to be developed, but others have formalized links between the evolution of human longevity and the economic productivity of elders. H. S. Kaplan and A. J. Robson (2009) have shown that aging rates can be connected to the contribution adults make to juvenile survival. R. D. Lee (2003) has demonstrated that when intergenerational transfers of assistance are incorporated into a formal theory of senescence, it is the transfers instead of fertility that determine equilibrium aging rates. His simulations show that when elders transfer resources to close kin, mortality schedules
very like those observed in hunter-gatherers are maintained by selection against deleterious mutations (Lee, 2008).
AGE STRUCTURES
Our grandmother hypothesis relies on Charnov’s model of life history evolution (Charnov, 1991, 1993) to explain how correlated allometries in mammalian life history features apply to humans (Hawkes et al., 1998; Alvarez, 2000). Comparisons between other great apes and humans (Robson et al., 2006) have been essential in highlighting distinctive human life history features. As noted, chimpanzees are an especially important comparative model for phylogenetic, ecological, and morphological reasons. Fig. 11.1 shows the female side of the age structure for a human hunter-gatherer population and wild chimpanzees modeled from life tables.
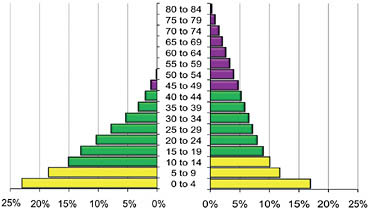
FIGURE 11.1 Female age structures modeled from life tables. Each bar shows the percentage of the population in the 5-year age class indicated in the vertical axis. Lightest bars, juvenile years; medium-gray bars, childbearing years; darkest bars, post-fertile years. Humans are on the right, represented by Hadza huntergatherers with Blurton Jones’s (2002) data. In this population, life expectancy at birth is 33 years. With growth rate 1.3%/year, 32% of the women (those over 15) are past the age of 45. Growing populations are younger because more are born than die. If this population was stationary, the percentage of adult women past the age of 45 would be 39% (Hawkes and Blurton Jones, 2005). The left side of the figure represents the synthetic wild chimpanzee population constructed by Hill and colleagues (2000) using data from five wild study sites. Average age at first birth is 13 in wild chimpanzees so the 10- to 14-year age class is included in the childbearing years. Fertility ends by ~45 in both species. Less than 3% of the adult chimpanzees (counted as those over 10 years) are past the age of 45. The chimpanzee model assumes a stationary population.
The human example on the right in Fig. 11.1, the Hadza (Hawkes and Blurton Jones, 2005), is similar to other hunter-gatherers. Life expectancy at birth is <40 years, but a substantial fraction of adults are past the child-bearing years. This is not true of chimpanzees, modeled on the left of Fig. 11.1 from the wild population synthesized from five wild study sites (Hill et al., 2001). Lower mortality in humans as compared to the other great apes has long been attributed to our propensity for cooperation and resource sharing (Sahlins, 1959), patterns that must surely affect death risks. The grandmother hypothesis highlights sharing by grandmothers in particular because, as noted by Hamilton, evidence that women remain healthy and productive past their fertility provides a clear link between human longevity and fitness payoffs to ancestral grandmothering. Sometimes elders survive with help from younger kin, but an evolutionary perspective predicts help to generally flow from older to younger relatives (Kaplan, 1994). Measures of strength and productivity among post-menopausal hunter-gatherers demonstrate their provisioning capacities (Blurton Jones and Marlowe, 2002; Walker and Hill, 2003). High fractions of maximum function through and beyond the childbearing years in humans contrast with the earlier geriatric declines of chimpanzees (Goodall, 1986; Finch and Stanford, 2004).
DEMOGRAPHIC AGING RATES BETWEEN AND WITHIN SPECIES
As expected from Hamilton’s model, age-specific mortality curves increase exponentially across adulthood (Mueller and Rose, 1996). This exponential increase was identified in human actuarial data by B. Gompertz in the early 19th century (Gompertz, 1825). A model bearing his name gives a fair fit to mortality data across a wide range of species (Finch, 1990):
Here m is the mortality hazard rate, G describes the rate of increase in adult mortality with increasing age (t), and A represents age-independent adult mortality. Building on previous work by G. A. Sacher (1977), C. E. Finch (1990) labeled A the initial mortality rate (IMR). Taking the natural log, the equation yields a line representing the logarithm of the hazard of death across adulthood with the log of the IMR as its intercept and G as its slope. In the Gompertz model, differences in longevity between populations of the same species or between species can be due to differences in the initial mortality rate (A), differences in G [or its transformed value, ln2/G, the mortality rate doubling time (MRDT)], or both. The slope (G), or the MRDT, is the demographic aging rate (Sacher, 1977). Across spe-
cies, lower initial mortality rates are correlated with shallower slopes and longer doubling times (Sacher, 1977; Finch, 1990; Ricklefs, 1998; Pletcher and Neuhauser, 2000).
Some have suggested that an MRDT of 7–9 years characterizes humans [e.g., Finch (2007, p. 12)], but MRDTs vary at least twofold across human populations (Hawkes et al., 2009). That variation among populations is correlated with variation in the initial mortality rate. However, the correlation is in the direction opposite from that predicted by a current vs. future reproduction tradeoff. Instead of the cross-species pattern identified by Sacher (Sacher, 1977; Finch, 1990; Ricklefs, 1998; Pletcher and Neuhauser, 2000), human populations with lower mortality levels (A) have faster rates of demographic aging (G). The age-specific mortality rate doubles more quickly, MRDT is shorter, when the age-independent risk of death (A) is lower.
This relationship, named for B. L. Strehler and A. S. Mildvan (1960), who first identified it across human populations, is robust and well described (Gavrilov and Gavrilova, 2001). Fig. 11.2 shows this Strehler–Mildvan correlation across a convenience sample of human populations chosen to represent a wide range of socioecologies and initial mortality rates [from Hawkes et al. (2009), with two Pygmy populations (Migliano, 2005) added here]. The figure is constructed from Gompertz models that were fitted to life tables for each population. Following Finch (1990) the models consider age-specific mortality risk from ages 30 to 80 [see discussion in Hawkes et al. (2009)]. The log of A, the hazard of death at age 30 (representing the IMR) is on the horizontal axis, and G, the slope of the log of the Gompertz curve is on the vertical axis. This correlation between the two variables across populations of the same species has also been found in widely diverse taxa where suitable data are available (Pletcher and Neuhauser, 2000; Gavrilov and Gavrilova, 2001). The limited data for chimpanzees are also plotted in Fig. 11.2. The synthetic chimpanzee population in the wild (Hill et al., 2001) used in Fig. 11.1 and the synthetic population from captivity (Dyke et al., 1995) represent variation in IMRs and demographic aging rates in that species. The same Strehler–Mildvan relationship found across human populations holds for chimpanzees.
A HETEROGENEITY HYPOTHESIS
As noted, Strehler–Mildvan correlations across populations of the same species are opposite to those generally found in cross-species comparisons. Williams’ (1957) verbal arguments, Hamilton’s (1966) formal treatment, and Kirkwood’s disposable soma model (Kirkwood and Rose, 1991) link lower mortality to stronger selection against senescence, and so slower rates of aging. Fig. 11.2 shows the opposite pattern. Within-species
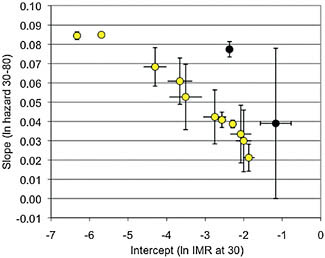
FIGURE 11.2 The slope of the log of the hazard of death from ages 30–80 by the log of the intercept at age 30 (IMR) taken from the values of A and G in Gompertz models calculated from life tables for a convenience sample of 11 human (open circles) and two synthetic chimpanzee populations (filled circles). See table 1 of Hawkes et al. (2009) for the values plotted here. The sample includes five hunter-gatherer populations, the United States and Japan to represent lower mortality levels falling in the upper left corner (the lowest IMRs and the steepest slopes), and two other cases to represent high-mortality populations depending on agriculture. Here, two pygmy populations from Migliano (2005) are added, the Aeta and the Batak. The chimpanzees are the synthetic wild population from Hill et al. (2001) and the synthetic captive population from Dyke et al. (1995). All life tables are female except for the !Kung and Agta, for which sexes were not distinguished in the original sources. Parameters were calculated on 5-year age classes, conditional on survival to the beginning of the age class preceding age 30. For the 11 human populations (yellow circles), the correlation between these estimates is –0.955
lower mortality (lMR) is associated with a steeper increase in death risk across adulthood—faster rates of demographic aging. The evolutionary models all assume that more energy allocated to somatic maintenance pays off in future reproduction but leaves less for current reproductive effort. Life history variation among individuals of the same population often seems to go in the opposite direction as well. Women with higher fertility rates and later ages at last birth also have higher subsequent survival rates (Perls et al., 1997; Müller et al., 2002; Smith et al., 2002, 2009; Jacobsen et al., 2003; Gagnon et al., 2009). Such apparent absence of the expected tradeoffs within populations is a regular finding in field studies in animal behavior (van Noordwijk and de Jong, 1986; Pettifor et al., 1988; Lessels, 1991). A common explanation is that individuals differ in their resources.
When these differences are ignored (or unobservable) and subjects are pooled, the resource differences obscure the tradeoff because those with more resources can have more of everything. Like houses and cars (van Noordwijk and de Jong, 1986), more into mortgage payments leaves less for auto loans, but those with bigger budgets can put more into both.
If there is such heterogeneity, so that health and otherwise unobserved differences in frailty vary within the populations shown in Fig. 11.2, that heterogeneity could account for the Strehler–Mildvan correlations in the following way (Hawkes et al., 2009). Frail individuals die earlier. They die even earlier under more severe conditions. Such mortality selection (Manton and Stallard, 1984), or culling (Wachter, 2003), changes the relative representation of subpopulations among the survivors. Older age classes are a biased subset of younger ones and that bias affects their average mortality risk. In higher mortality populations of both humans and chimpanzees, older age classes are more strongly culled, leaving proportionately fewer frail survivors. Conversely, when background mortality is low, mortality selection is weaker and more of the frail survive longer. Although absolute risk of death is lower, the relative risk in each age class increases more steeply with advancing age because later age classes include more individuals with relatively greater vulnerability.
Heterogeneity could take many forms (Vaupel and Yashin, 1985). One simple possibility is that populations are composed of two (unobserved) subpopulations, each with a Gompertz schedule of risk. The frailer sub-population has higher mortality risk at each age and steeper increasing risk. The log of the risk of death at each age has both a higher intercept and higher slope in the frailer subpopulation (Hawkes et al., 2009; Wilmoth and Horiuchi, 1999). Fig. 11.3 displays the age-specific mortality curves for simulated populations with such heterogeneity facing two different background conditions of mortality risk. In each condition there are two subpopulations, with exactly the same relative differences in age-specific risk of death. Gompertz demographic aging is linear with age on this semilog plot. The simulation uses observed ranges of variation in initial mortality rates and slopes across the sample of human populations in Fig. 11.2 to estimate realistic ranges. Fig. 11.3 shows the age-specific risk for the subpopulations and for the whole population when the subpopulations are pooled. More of the frail die at each age, and older age classes are increasingly biased toward the more robust in both conditions; but when overall mortality is low—the lower set of lines—more of the frail survive to older ages and so their higher and steeper risk has a larger effect on the relative risk of later age classes. The difference between the two subpopulations is identical in both environments, but the increase in mortality with age is about twice as steep when background mortality is lower. This is the same difference seen across empirical populations in Fig. 11.2.
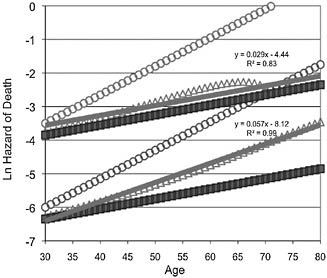
FIGURE 11.3 Two model subpopulations, one frail (open circles) and the other robust (filled squares), exposed to two conditions of age-independent mortality. Initial mortality rates are low (similar to the United States and Japan) for the lower set of lines and high (similar to Hadza hunter-gatherers) for the upper set of lines. Both subpopulations face Gompertz age-specific risk. Initial mortality rates for the two subpopulations differ by 0.35/year in both conditions with slopes of 0.03/year and 0.085/year, respectively. The simulations plot the age-specific mortality rate for the population pooled from these two subpopulations (open diamonds). The trendlines (single line described by the equations and correlation coefficients) measure how well the population mortality curves fit Gompertz models. The slope of the trendline is the demographic aging rate. For the population in the high-mortality condition, that slope is about half as steep as in the low-mortality condition. Relative size of the subpopulations at the initial adult age makes a difference. Here it is assumed to be the same at both high and low background mortalities because background risk is assumed to affect the frail proportion in two opposing ways. When age-independent mortality is high, so is the risk of early life tradeoffs that leave survivors more frail (see the discussion of early origins in the text). However, higher mortality also strengthens mortality selection across juvenile years, leaving a smaller fraction of the frail juveniles alive at maturity. On the other hand, when background mortality is low, fewer have faced early survival tradeoffs that increase frailty, making the frailer subpopulation smaller initially. Yet, weaker mortality selection across the juvenile years leaves more of the frail subpopulation surviving to adulthood.
HETEROGENEITY AND FERTILITY
The same kind of differential frailty proposed to underlie the Strehler–Mildvan correlations in Fig. 11.2, and modeled in Fig. 11.3, is relevant to age-specific fertility. As shown in Fig. 11.1, the childbearing years end at the age of ~45 in both humans and chimpanzees. Like other female mammals, humans and chimpanzees build initial oocyte stocks in early life that then deplete with age (vom Saal et al., 1994). Most of the initial stock is lost to atresia, a continuing process of cell death that begins near birth. In women, stocks decline from ~7 million oocytes at 5 months after conception to <2 million at birth and ~400,000 at puberty (Baker, 1963). Only one in a thousand of those remaining when ovarian cycling begins actually ovulate. Numbers continue to fall across young and middle adulthood, reaching thresholds associated first with reduced fecundability, then secondary sterility, and finally menopause ~10 years after last birth. Average ages at these thresholds differ some across populations (Bentley and Muttukrishna, 2007) with substantial variation around the averages (Faddy and Gosden, 1996; O’Connor et al., 2001; Sievert, 2006; Broekmans et al., 2009). The classic counts of human ovarian follicle stocks show that among females of the same age, remaining primordial follicle stocks can vary by two orders of magnitude (Block, 1952; Richardson et al., 1987; Gougeon et al., 1994).
Chimpanzee follicle stocks also vary among individuals of similar age (Jones et al., 2007). Archived ovarian sections taken at necropsy from captive chimpanzees of ages 0–47 years index this variation and the declining numbers with age (Jones et al., 2007). Exponential regressions fit to the age-specific primordial follicle counts on those sections and also to the whole ovary counts across that 0- to 47-year range in the classic human datasets provide a quantitative comparison of follicular loss rate in the two species. The intercepts—the heights—of the two regression lines are necessarily different because the human data represent whole ovaries and only single sections were available for the chimpanzees. [An average section is ~1/2,000 of a human ovary (Block, 1952; Richardson et al., 1987)—likely the same for chimpanzees.] However, the rate of depletion with age measured this way, on these samples, across this age range, is indistinguishable between the two species (Jones et al., 2007). This similarity is consistent with a wider body of findings, including hormone and cycling data from captive chimpanzees (Graham, 1979; Gould et al., 1981; Lacreuse et al., 2008), suggesting they would reach menopause at about the same ages humans do—if they lived long enough (Walker and Herndon, 2008).
As implied by these similarities and noted above, humans and chimpanzees can give birth into their mid-forties but not beyond. However, in spite of this similarity in the end of the childbearing years (Fig. 11.1), the shapes of age-specific fertility curves in the two species are strikingly
different. Fig. 11.4 displays the average age-specific fertilities for three hunter-gatherer populations and the conservative age-specific fertility schedule synthesized from six wild chimpanzee populations by M. Emery Thompson and others (2007). Human populations can differ widely in fertility levels, but among them—hunter-gatherers included—the change in the rate of babies born to women of each age has a familiar peaked shape. “[I]n all populations where reliable records have been kept, fertility is zero until about age 15, rises smoothly to a single peak, and falls smoothly to zero by age 45–50” (Coale and Demeny, 1983, p. 27). The fertility schedule for wild chimpanzees is flat-topped instead. The rate reached before the age of 20 continues with little change for two more decades.
The percentages running along the horizontal axis in Fig. 11.4 show the relative size of each age class compared to the first age class of
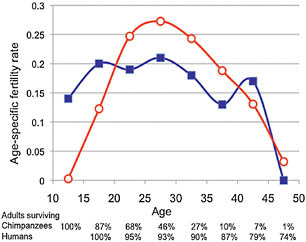
FIGURE 11.4 Age-specific fertility rates (ASFR) for humans and chimpanzees. Humans (open circles) are represented by the average of three hunter-gatherer populations: !Kung Bushmen of Botswana (Hill and Hurtado, 1996), Ache of Paraguay (Hill and Hurtado, 1996), and Hadza of Tanzania (Blurton Jones et al., 2002). Estimates for chimpanzees in the wild (closed squares) come from the conservative fertility schedule synthesized from six study sites by Emery Thompson et al. (2007). The bumps reflect small sample size (627 risk years in the initial chimpanzee adult age class declining to 8 risk years in the 45- to 49-year interval (Emery Thompson et al., 2007, supplementary table 2). The percentages along the horizontal axis indicate the proportion of those reaching adulthood that survive to the age class. The top row of percentages comprises estimates for chimpanzees from the number of risk years in each age class (Emery Thompson et al., 2007, supplementary table 2). They are just slightly lower than the model in Fig. 11.1 from the life table (Hill et al., 2001). The bottom row comprises human estimates from the female life table for Hadza hunter-gatherers (Blurton Jones et al., 2002).
adulthood. The chimpanzee figures come from the number of risk years observed in each age class in Emery Thompson and colleagues’ (2007) supplementary table 2. For human hunter-gatherers the figures come from the female life table for Hadza foragers (Blurton Jones et al., 2002). As the percentages show, almost all of the chimpanzees that survive to adulthood then die during the childbearing years; only 1% do not. By contrast, 24% of the hunter-gatherer women die during the childbearing years; 76% do not.
Emery Thompson and colleagues (2007) demonstrated heterogeneity in chimpanzee fertility in their six-site sample by looking for associations between fertility rates and survival in females over the age of 25. They divided their observations into healthy and unhealthy years. An observation year for a given chimpanzee was considered healthy if she survived an additional 5 years or more, and unhealthy if she did not. Their figure 2 (Emery Thompson et al., 2007, p. 2152) shows that fertility in the thirties was about twice as high in females who would survive at least 5 more years than in those who would not. The finding indicates that mortality selection across the childbearing years culls the females with lower fertility. As the age classes shrink to almost nothing, they are increasingly biased to the less frail, more fertile females. Consequently, average fertility changes little even if the fertility of the survivors is declining relative to their own earlier rate.
We found similar heterogeneity in fertility in 19th century Utah women [the Utah Population Data Base (UPDB) (Bean et al., 1990)]. Although not hunter-gatherers, these women practiced natural fertility (Henry, 1961), so potential for continued childbearing is reflected by actual births. Individual records make it possible to investigate links between variation in fertility rate and age at last birth. Of 42,493 parous UPDB women born between 1849 and 1890, the 10,440 whose fertility ended before the age of 35 had fertility rates in the preceding years about half as high as the 2,695 women who would have last births after 45 (Hawkes and Smith, 2010). This parallels the chimpanzee variation with an important difference: all the women in the Utah sample, whatever their age of last birth, survived at least to the age of 50. The sample was restricted to women who lived at least to that age to avoid the confound of early last births due to early death (Hawkes and Smith, 2009). Subjects were also restricted to those married once and neither widowed nor divorced to reduce effects these characteristics may have on fertility.
Assuming that heterogeneity in fertility is similar in the hunter-gatherer women, this variation combined with the different survival schedules of humans and chimpanzees can explain the different shapes of the fertility schedules shown in Fig. 11.4. Most women, whatever their frailty, survive the childbearing years, whereas across those years mortality culls chim-
panzee females down to a least frail few. The human schedule is peaked because women with both high and low risk of fertility failure outlive the childbearing years. Beginning about the age of 30, each subsequent age interval contains more women who are past their last parturition. This drives down the average rate of baby production for later age intervals (Wood, 1989, 1994; Hawkes and Smith, 2010). The chimpanzee schedule is flat because heterogeneity in ovarian aging is culled away by mortality selection. Most chimpanzees die during the childbearing years and the survivors are females whose fertility rate has been high all along (Emery Thompson et al., 2007; Hawkes and Smith, 2010). In captive chimpanzees, lower mortality allows more frail individuals to survive longer so that captive chimpanzee fertility slopes down from a peak, more like the human pattern (Littleton, 2005; Roof et al., 2005).
ORIGINS OF HETEROGENEITY IN EARLY LIFE
Human age structures looked much like the hunter-gatherer example shown in Fig. 11.1 until the 20th century when life expectancies at birth began to increase in some populations (Keyfitz and Fleiger, 1968; Oeppen and Vaupel, 2002). Until the mid-20th century, these increases were largely a consequence of decreasing numbers of dying infants and children: lower juvenile mortality is strongly associated with lower fertility. Fig. 11.5 shows number of births for UPDB women who survived at least to 50 by their own birth year across the 19th century (Hawkes and Smith, 2009). After the middle of the 19th century, fertility began a steady decline—falling to half of its earlier level by 1900. Fig. 11.5 also shows a concurrent change in adult mortality. The average age at death for women who had survived at least to 50 increased from ~75 at mid-century to ~80 at its end. These decreases in mortality and fertility typify changes in some other populations at about the same time, likely due to improvements in nutrition, sanitation, and medicine (Fogel, 2004; Finch, 2007). By the end of the 20th century, continuing decreases in fertility and increases in juvenile and adult survival resulted in life expectancies double those of most historical and ethnographic populations (Oeppen and Vaupel, 2002; Gurven and Kaplan, 2007; Finch, 2010). The increases in survival allowed increased heterogeneity at older ages. Other effects on heterogeneity are likely as well.
Associations between regional infant mortality rates and late life morbidities led D. J. Barker to propose his infant and fetal origins of adult disease hypothesis (Barker and Osmond, 1986; Barker et al., 1989; Gluckman et al., 2008). Those who survive nutritional and disease insults in early life are predisposed to metabolic and cardiovascular disease in adulthood. Pursuit of Barker’s hypothesis has revealed that when historical cohorts
![FIGURE 11.5 Number of births (filled circles) and age at death (open diamonds) in cohorts of UPDB women by their birth year across the 19th century, redrawn from Hawkes and Smith (2009)]. Only women who survived past the age of 50 are included.](/openbook/12931/xhtml/images/p2001c3c8g226001.jpg)
FIGURE 11.5 Number of births (filled circles) and age at death (open diamonds) in cohorts of UPDB women by their birth year across the 19th century, redrawn from Hawkes and Smith (2009)]. Only women who survived past the age of 50 are included.
have been exposed to famines and epidemics during fetal life they have higher rates of disease and mortality in later adulthood than do adjacent cohorts (Finch and Crimmins, 2004; Mazumder et al., 2009). Analyses of adult morbidity and mortality by birth month and season have yielded similar evidence of heterogeneity in frailty stemming from nutritional constraints and disease exposure in early life (Moore et al., 1997; Doblhammer and Vaupel, 2001).
Differences between cohorts are necessarily an underestimate of the likely heterogeneity. B. Mazunder and colleagues (2009) compared morbidities in later adulthood between Americans who were likely exposed in fetal life to the 1918 influenza pandemic and those in adjacent cohorts. As they noted, “Maternal health during the pandemic peak … varied widely from no clinical infection, mild uncomplicated flu or flu with severe secondary pneumonia that still permitted normal birth” (p. 4). Those whose birth dates indicate probable fetal flu exposure must include some unexposed individuals. In the same way, adjacent cohorts must include some individuals whose mothers experienced infection. Nutrition, energy expenditure, and stress may also impact the effects of disease (Finch, 2007; Kuzawa and Quinn, 2009; Kuzawa and Sweet, 2009), and recent disease history of groups in the sample may also influence responses to early life conditions (Costa et al., 2007; Pennington et al., 2009). Even with the imperfect association between exposure and birth date and effects of these unmeasured covariates, rates of cardiovascular disease after the age of 60 were >20% higher in those whose fetal development coincided with pan-
demic (Mazumder et al., 2009). That this is a minimum estimate of early life effects on heterogeneity in aging rates is underscored by longitudinal datasets documenting within-cohort associations between early growth and both ovarian aging and mid-life physical performance (Hardy and Kuh, 2002; Kuh et al., 2002).
The early origins hypothesis predicts that declines in mortality in the Utah women during the second half of the 19th century would affect the next generation. Longer survival likely indicates better nourishment, less illness, and reduced hardship. If so, the children of those surviving longer would have been less exposed to nutritional limits and infection in early life, and so have lower risks of various later morbidities. Improvements in nutrition, general public health, and, subsequently, medical interventions should mitigate early life insults and reduce consequent heterogeneity. However, lowered mortality also reduces mortality selection, allowing greater heterogeneity to persist to older ages. This heterogeneity hypothesis (Fig. 11.3) to explain population variation in demographic aging (Fig. 11.2) applies to chimpanzees (and other taxa) as well as humans. Life expectancy at birth for female chimpanzees in the wild is 15 years (Hill et al., 2001). In captivity it is 29 years (Dyke et al., 1995). This doubling of chimpanzee life expectancy is associated with reductions in rates of infection and nutritional stress (Goodall, 1986; Williams et al., 2008). In both chimpanzees and humans, improvements in nutrition and hygiene combined with medical interventions can double life expectancy. And in both, longer life expectancies are associated with faster rates of demographic aging (Fig. 11.2)—due perhaps, as argued here, to increased heterogeneity of frailty at older ages (Fig. 11.3).
BACK TO GRANDMOTHERS
This heterogeneity hypothesis may explain why humans, chimpanzees, and other taxa display Strehler–Mildvan correlations. The similarities cannot explain why humans usually outlive the childbearing years and chimpanzees do not (Figs. 11.1 and 11.2). Physiological mechanisms, let alone genetic differences that underlie the survival differences, remain elusive (de Magalhães and Church, 2007; Finch, 2010), although mitochondrial mutation rates may be involved (Kujoth et al., 2007; Nabholz et al., 2007). Hamilton’s forces (Hamilton, 1966; Rose et al., 2007) do not specify particular mechanisms of aging, but their incorporation in an analysis of human survival curves points to a deep history of reproductive benefits accruing to postmenopausal women in our lineage. Mueller, Rose, and Rauser have focused attention on the period of life in many species when mortality rates slow from an exponential increase and may become constant at succeeding age intervals. They reject Vaupel’s heterogeneity of
frailty hypothesis (Vaupel et al., 1998) as a general explanation for these mortality plateaus, finding evidence more consistent with expectations from evolutionary theory about late life (Mueller and Rose, 1996; Rauser et al., 2006; Rose et al., 2007). When individuals survive past normal life spans, they are beyond the ages where senescence has been molded by ancestral forces of selection. “Hamiltonian theory predicts that late-life mortality rates should plateau and evolve according to the last age of reproduction in a population’s evolutionary history” (Rauser et al., 2006, p. 26). Because human mortality rates begin to decelerate and depart from a Gompertz curve only around the ninetieth year (Vaupel et al., 1998), the mortality plateau criterion implies that contributions to reproduction from ancestral grandmothers continued through their eighties.
This demographic evidence of grandmaternal effects on reproduction in our lineage has other implications that can barely be touched on here. S. B. Hrdy (Hrdy, 1999, 2005, 2009; Burkart et al., 2009) has hypothesized that selection pressures for distinctively human cognitive and emotional capacities arose from our evolution as cooperatively breeding apes. Unlike our nearest living relatives, human mothers accept help with babies right from parturition. Depending on help, they can bear a new baby while previous offspring still need provisioning. This has consequences for selection pressures on both mothers and infants. Unlike chimpanzee mothers, humans must also consider the occupation and whereabouts of potential helpers as well as the needs of still dependent weaned children. Abilities to juggle these additional concerns supersede the more single-minded focus on the newborn of other ape mothers. The novel maternal sensitivities create problems in turn for human infants that do not arise for other infant apes. Human babies cannot count on mother’s undivided commitment, so capacities to actively engage her and also to evaluate and engage other helpers are crucial. In high infant mortality environments, selection on those capacities would have been especially strong. Hrdy (2009) links those circumstance to the evolution in our lineage of motivations and capacities for intersubjective engagement that M. Tomasello and colleagues (Tomasello and Rakoczy, 2003; Tomasello et al., 2005) identify as the foundation for human prosociality.
Ethnographers have documented the ubiquity and importance of allomothering from many kinds of kin in living human communities (Sear and Mace, 2008), but grandmothers in particular are implicated in the hypothesis about the evolution of human life history entertained here. If ancestral grandmothers provided the help that initially allowed mothers in our lineage to move on to the next baby before the previous one could feed itself, propelling the evolution of human postmenopausal longevity, that initiated cooperative breeding in a previously independently breeding ancestral ape. These arguments link distinctive human cognitive and
emotional capacities to selection pressures that arose as a consequence of ancestral grandmothering.
Ovarian aging appears to differ little between modern humans and chimpanzees, making it likely the same pattern characterized our ancestors. Before the shifts to greater longevity in our lineage, heterogeneity in ovarian and somatic aging would have been strongly culled by mortality selection across the childbearing years. If grandmother effects reduced mortality across those years, heterogeneity in ovarian aging would have expanded as more and more women outlived their fertility. Subsidies for relatives’ reproduction would have begun well before the average age at last birth, let alone the average age of menopause. By this argument, heterogeneity in ovarian aging is an ancient legacy of grandmothering in our lineage; but now such heterogeneity poses unprecedented concerns in the human populations where childbearing is delayed and nuclear families are isolated as never before. Many women find they have missed their own windows of fertility (Broekmans et al., 2009). Although aging is often seen as a process that befalls the old, evolutionary theories of aging predict that function begins to decline in early adulthood. Such declines have been documented not only in fertility, but in muscle strength and cognitive performance (Hunter et al., 2000; Salthouse, 2009); and where mortality levels have dropped to evolutionarily unprecedented lows, heterogeneity in somatic competence is increasingly well documented in those past mid-life (Mitnitski et al., 2005; Rockwood and Mitnitski, 2007). Just as grandmothering may have expanded heterogeneity in ovarian aging by lowering mortality across the childbearing years, recently dropping mortality rates at older ages expand heterogeneity well beyond them. As continuing innovations in medical and daily living technologies interact with mortality selection to produce complex dynamics in the health and welfare of elders (Manton, 2008), the heterogeneity in ovarian and somatic aging that is an aspect of our evolved life history becomes an increasing medical as well as social, economic, and political concern of our time.
ACKNOWLEDGMENTS
I am grateful for valuable insights and advice from J. F. O’Connell, J. E. Coxworth, J. K. Blevins, C. W. Kuzawa, and S. B. Hrdy, and for research support from the National Science Foundation.




















