HIV Screening and Access to Care: Exploring Barriers and Facilitators to Expanded HIV Testing
The White House Office of National AIDS Policy (ONAP) is tasked with coordinating government efforts to reduce the number of HIV infections in the United States (ONAP, 2010a). These efforts have included the development of a National HIV/AIDS Strategy (NHAS), released July 13, 2010, the primary objectives of which are to (1) reduce HIV incidence, (2) increase access to care and optimize health outcomes for people living with HIV, and (3) reduce HIV-related health disparities (ONAP, 2010b).1 To supplement other efforts to inform the development and implementation of the NHAS, in the fall of 2009, ONAP commissioned an Institute of Medicine (IOM) committee to evaluate barriers to implementation of an expanded HIV testing and treatment program. Specifically, the committee was asked to examine
-
the extent to which federal and state laws and policies and health insurance policies pose a barrier to expanded HIV testing;
-
the capacity of the health care system to administer a greater number of HIV tests and to accommodate new HIV diagnoses; and
-
federal and state policies that inhibit entry into clinical care or the provision of continuous and sustained clinical care for people with HIV/AIDS.
The statement of task includes more specific questions for the committee to consider within each of these three areas as well (see Box 1).
|
1 |
For further details on the NHAS, visit http://www.whitehouse.gov/administration/eop/onap/nhas. |
|
BOX 1 Statement of Task
|
A 15-member committee, the Committee on HIV Screening and Access to Care, was formed to carry out this study. The committee comprises experts in the areas of HIV/AIDS testing and care policy, HIV/AIDS ethics, epidemiology and biostatistics, HIV/AIDS clinical care and care services research, HIV/AIDS care financing, state HIV/AIDS service programming and implementation, and the behavioral sciences (see Appendix A). ONAP asked the committee to plan and host three public workshops and perform other limited data-gathering activities, such as review of the literature, to address each of the three areas described in the statement of task and prepare three brief reports on those issues. ONAP requested that the reports not include recommendations because it thought that committee summaries of the presentations and discussions at the workshops and other data gathered would be the most helpful way of informing the implementation of the NHAS.
The committee held a planning meeting December 15–16, 2009, at which it discussed the details of the statement of task with representatives from ONAP. From this discussion it was agreed that although the emphasis of the task is the impact of laws and policies on access to HIV testing and HIV/AIDS care, in planning the workshops and other information gathering activities it would be appropriate for the committee to give some consideration to the impact of factors that may fall outside this realm (such as HIV/AIDS stigma, low-self-perceived risk, provider knowledge, etc.) that have been cited as potential barriers to HIV testing and care.
This is the first of three reports that will be prepared by the committee. It focuses on the first part of the committee’s task: the extent to which federal and state laws and policies, private health insurance policies, and other factors pose a barrier to expanded HIV testing.
The committee hosted a public workshop during April 15–16, 2010, in Washington, DC, to explore the extent to which federal, state, and private health insurance policies pose a barrier to expanded HIV testing (see agenda, Appendix B). The committee convened experts from academia, government, the insurance industry, provider groups, and foundations to offer expert testimony (see Appendix C). Also in attendance were more than thirty workshop registrants representing patients, providers of HIV testing and care services, researchers, policy organizations, and others with an interest in this topic (see Appendix D). Invited experts were asked to present their evidence and perspectives. Following each panel, questions were entertained from the committee and the audience.
This report begins with a background section describing the rationale for an expanded HIV testing program. The report then summarizes information from the expert presentations and discussion from the public workshop, as well as information from policy documents and research literature, relevant to the questions posed to the committee in the first part of the statement of task (see number 1 in Box 1).
Between the time that ONAP commissioned the study and the committee’s first workshop, comprehensive health care reform—the Patient Protection and Affordable Care Act (P.L. 111-148)—was enacted into law. Although the effect of health reform on HIV testing was not part of the specific charge to the committee, the committee is aware that health care reform will have significant implications for the health care environment in the United States. To the extent possible, the committee tried to identify places where changes were likely that could impact HIV testing/screening and access to care.2
The Centers for Disease Control and Prevention (CDC) issued revised HIV testing guidelines in 2006 recommending routine testing in health care settings for people ages 13 to 64. Because of their relevance to the discussion of facilitators and barriers to expanded HIV testing, the revised CDC guidelines were frequently brought up by workshop participants and are discussed in several places throughout this report. This is not meant to imply an endorsement by the committee of the CDC’s HIV testing guidelines over those issued by the U.S. Preventive Services Task Force (USPSTF) or others.
BACKGROUND
An estimated 56,300 adolescents and adults are newly infected with HIV in the United States each year (CDC, 2008a). Some populations bear a disproportionate burden of the HIV epidemic. For example, 53 percent (28,700) of the new HIV infections that occurred in 2006 were among men who have sex with men (MSM). Comparing racial and ethnic groups, the rate of new HIV infections among non-Hispanic blacks was seven times the rate among whites (83.7 versus 11.5 new infections per 100,000 population) and the rate of new infections among Hispanics in 2006 was three times the rate among whites (29.3 versus 11.5 per 100,000). African Americans also accounted for the largest share of new infections (45 percent, or 24,900) that occurred in 2006 (CDC, 2008a).
Twenty-one percent of the approximately 1.1 million people in the United States living with HIV/AIDS are unaware that they are HIV infected (CDC, 2008b). People who are unaware of their HIV status may unknowingly transmit the virus to others. One study estimated that unrecognized HIV infection is the source of more than half of new HIV infections (Marks et al., 2006). Behaviors that increase risk for HIV transmission may be
more common among people who are unaware that they are HIV infected (Marks et al., 2005), and there is evidence that people often reduce their risk behaviors following an HIV diagnosis (Eaton and Kalichman, 2009).
According to a 2010 study that used back calculation to estimate prevalence of undiagnosed HIV infection at the end of 2006, whites had the lowest percentage of undiagnosed cases (18.8 percent), compared with Hispanics/Latinos (21.6 percent), blacks/African Americans (22.2 percent), American Indians/Alaska Natives (25.8 percent), and Asians/Pacific Islanders (29.5 percent). In addition, MSM had a significantly greater percentage of undiagnosed HIV infection (23.5 percent) compared with the overall percentage undiagnosed (21 percent), although heterosexual males had the highest overall percentage of undiagnosed HIV infection (26.7 percent) (Table 1). The study also showed that minority MSM and minority heterosexual women had significantly greater percentages of undiagnosed HIV cases than whites in the same categories (Campsmith, 2010).
Developments in the treatment of HIV/AIDS, such as the introduction of highly active antiretroviral therapy (HAART) in the mid-1990s, have
TABLE 1 Estimated Number and Percent of Undiagnosed HIV Cases, by Race/Ethnicity and Transmission Category
|
|
Number |
Percent |
|
Total |
232,700 |
21 |
|
Race/Ethnicity |
|
|
|
White |
72,000 |
18.8 |
|
Black/African American |
113,100 |
22.2 |
|
Hispanic/Latino |
41,900 |
21.6 |
|
Asian/Pacific Islander |
4,500 |
29.5 |
|
American Indian/Alaska Native |
1,200 |
25.8 |
|
Transmission category |
|
|
|
MSM |
124,900 |
23.5 |
|
IDU-male |
19,000 |
14.5 |
|
IDU-female |
10,000 |
13.7 |
|
MSM/IDU |
6,700 |
12.1 |
|
High-risk heterosexual contact-male |
27,900 |
26.7 |
|
High-risk heterosexual contact-female |
42,700 |
21.1 |
|
Other* |
1,600 |
17.6 |
|
*Includes hemophilia, blood transfusion, perinatal exposure, and risk factors not reported or not identified. SOURCE: Campsmith et al., 2010 |
||
resulted in dramatic improvements in HIV-related morbidity and mortality. After increasing steadily during the 1980s, HIV-related mortality rates dropped 70 percent between 1995 and 2005 (KFF, 2009c). People who are unaware of their HIV status cannot reap the benefits of these therapies, however. Many people are tested late in the course of HIV infection after symptoms have already developed and by which time therapy may not be as effective. For example, a 2009 CDC study of 281,421 new HIV diagnoses that occurred in 34 U.S. states between 1996 and 2005 found that 38.3 percent had progressed to an AIDS diagnosis within 1 year of their HIV diagnosis. Progression from HIV infection to AIDS without treatment generally takes about 10 years (CDC, 2009b).
Besides improving health outcomes for the individual, knowledge of one’s HIV status and receipt of care may help to prevent HIV transmission. Viral load or the level of active HIV in the blood and/or genital fluid of someone with HIV appears to be a predictor of HIV transmission.3 There is evidence that timely initiation of antiretroviral therapy (ART), which reduces viral load, can reduce transmission risk. The effectiveness of ART in reducing transmission has been demonstrated by a decrease of perinatally-acquired infection in the United States. This is due in large part to routine screening of pregnant women for HIV infection and the institution of ART as a means of prevention of mother to child transmission (CDC, 2006a).4 Observational studies of sexual transmission of HIV in heterosexual couples and MSM show that individuals who are on and adherent with ART are less likely to transmit the virus to HIV-negative partners (Castilla et al., 2005; Donnell et al., 2010; Porco et al., 2004). The dramatic effect of ART on viral load has led to discussion of the potential benefit of increased HIV testing and earlier initiation of therapy, although many questions remain5 (Dieffenbach and Fauci, 2009; Granich et al., 2009; Holtgrave, 2010; Wagner and Blower, 2009).
In 2006, the CDC issued revised HIV testing guidelines that recommend routine testing in health care settings for individuals between the ages of 13 and 64 (see the following section on the CDC’s revised HIV testing guidelines). However, data from the Kaiser Family Foundation (KFF) show that the percentage of adults aged 18 to 64 who report having been tested for HIV in the last 12 months has remained relatively flat from 2000 to
2009 (Figure 1). Possibly due to risk-based approaches to HIV testing, certain population groups who may be at higher risk for HIV, such as young adults aged 18 to 29, African Americans, and Latinos, have been more likely to report having been tested in the past 12 months (KFF, 2009f).
In a 2009 survey of a nationally representative random sample of more than 2,500 adults, 53 percent of 18 to 64 year olds reported having ever received an HIV test (up from 43 percent in 1997), with African Americans (73 percent) and Latinos (60 percent) being more likely than whites (40 percent) to report having ever been tested.6 Individuals ages 65 and older are much less likely to have been tested; in 2009, just 16 percent of people ages 65 or older reported they had ever been tested for HIV, compared with 54 percent and 61 percent of 28 to 29 year olds and 30 to 49 year olds, respectively (KFF, 2009f).
In 2008, more than half (54.5 percent) of all individuals who were tested for HIV were tested in private doctor or health maintenance organization (HMO) settings, followed by hospital, emergency room and outpatient clinics (16.5 percent), and public health department clinics (5.6 percent). Fifteen percent of individuals were tested in non-clinical settings, such as AIDS clinics or counseling and testing sites. Almost 1 in 5 individu-
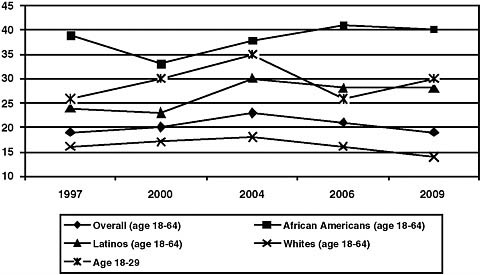
FIGURE 1 Percentage of people who reported being tested for HIV in past 12 months, by year.
SOURCE: Adapted from KFF, 2009f.
als who were tested were tested at primarily public funded sites7 (CDC, 2008c). Although most testing occurs in private doctor and HMO settings, the proportion of individuals who test HIV positive may be higher in hospital settings (such as emergency departments [EDs]) and in public community clinics where patients may be higher-risk or more likely to have already developed symptoms of HIV infection (CDC, 2006b; KFF, 2009b).
CDC Revised HIV Testing Guidelines
The 2006 CDC HIV testing guidelines recommend routine opt-out HIV screening in health care settings of individuals between the ages of 13 and 64, unless prevalence of undiagnosed HIV infection in that setting is documented to be less than .1 percent.8 The guidelines also recommended yearly screening for individuals in high-risk groups,9 and that opt-out HIV screening be included in the routine panel of prenatal screening tests for pregnant women (CDC, 2006c).10 (See Box 2 for key definitions.)
The 2006 CDC guidelines differed from previous guidelines in several ways including recommending that testing be done on an opt-out basis whereby testing is done after notifying the patient unless he or she declines; that general consent for care should be considered sufficient for HIV testing and that separate written consent for testing should not be required; and that pretest counseling should not be a requirement for HIV testing (CDC, 2006c).11 In their rationale for the new guidelines, the CDC cited research showing that testing on the basis of risk behaviors has failed to identify a substantial number of HIV-infected individuals, even among people who visit health care providers (Alpert et al., 1996; Klein et al., 2003; Liddicoat et al., 2004), and the changing demographics of the HIV epidemic to in-
|
7 |
Publicly-funded sites include health care settings (such as public health department clinics, drug treatment facilities, family planning clinics, prenatal clinics, STD clinics, community health clinics) and non-health care settings (AIDS clinic/counseling and testing sites). |
|
8 |
The guidelines go on to state that in the absence of existing data on the prevalence of HIV infection, health care providers should initiate screening until they establish that the diagnostic yield is under 1 per 1,000 individuals screened (CDC, 2006c). |
|
9 |
High-risk groups as defined in the current CDC guidelines are injection drug users and their sex partners, persons who exchange sex for money or drugs, sex partners of HIV infected persons, men who have sex with men, and heterosexual persons who themselves or their sex partners have had >1 sex partner since their last HIV test (CDC, 2006c). |
|
10 |
Separate guidelines are being developed by CDC for non-health care settings. See http://www.cdc.gov/hiv/topics/testing/resources/other/consultation.htm. |
|
11 |
Prior guidelines from the CDC recommended routine counseling and testing for persons at high risk for HIV and for those in acute-care settings where HIV prevalence was ≥1 percent (CDC, 1993, 2001). The current USPSTF guidelines strongly recommend screening of high risk groups and make no recommendation for or against routine screening of those who are not at increased risk for HIV (USPSTF, 2007). |
|
BOX 2 Key Definitions Targeted HIV testing: Performing an HIV test for subpopulations of persons at higher risk, typically defined on the basis of behavior, clinical, or demographic characteristics. Diagnostic HIV testing: Performing an HIV test for persons with clinical signs or symptoms consistent with HIV infection. HIV screening: Performing an HIV test for all persons in a defined population. Opt-out screening: Performing HIV screening after notifying the patient that (1) the test will be performed and (2) the patient may elect to decline or defer testing. Assent is inferred unless the patient declines testing. Informed consent: A process of communication between patient and provider through which an informed patient can choose whether to undergo HIV testing or decline to do so. Elements of informed consent typically include providing oral or written information regarding HIV, the risks and benefits of testing, the implications of HIV test results, how test results will be communicated, and the opportunity to ask questions. HIV-prevention counseling: An interactive process of assessing risk, recognizing specific behaviors that increase the risk for acquiring or transmitting HIV, and developing a plan to take specific steps to reduce risks. SOURCE: Branson et al., 2006. |
clude more individuals such as women, people who live outside of cities, heterosexual men and women, and others who may not be aware they are at risk (CDC, 2006c). The CDC’s HIV testing recommendations differ from the current USPSTF guidelines, which recommend routine screening for pregnant women and high-risk groups, and make no recommendation for or against routine screening for adolescents and adults who are not known to be at increased risk for HIV infection (USPSTF, 2007).
Despite widespread agreement that knowing one’s HIV status is critical and that increased testing is needed, a number of concerns have been expressed about the CDC guidelines and opt-out testing. For example, some have argued that one of the best opportunities to provide prevention counseling and information, which has been found in several intervention studies to prevent HIV infection, is at the HIV testing encounter and cite less counseling before and after testing as a major drawback to the revised CDC guidelines (for example, Holtgrave and McGuire, 2007). In addition,
the costs and consequences of routine testing relative to other prevention strategies are not yet well understood (Holtgrave, 2007). As will be described later in the report, several ethical concerns have also been raised about opt-out HIV testing and the removal of the requirement for specific written consent.
Nevertheless, there is widespread interest in the potential benefits of a more comprehensive testing and treatment strategy. Thus, it is important to understand the facilitators and barriers to the implementation of such a program.
PUBLIC WORKSHOP AND LITERATURE REVIEW
In the following sections, the committee summarizes expert presentations and discussions at the first public workshop and information from policy documents and research literature relevant to the questions posed to the committee. The committee attempted to provide evidence supporting the assertions made by experts, but in many cases there are not studies addressing these issues. Therefore, unless supported by relevant studies, testimony provided by expert witnesses on barriers to HIV testing should be interpreted as opinion by knowledgeable individuals that has not been verified.
Laws, Health Coverage Policies, and Other Policies That Impede HIV Testing
The first specific question posed to the committee was “What are the current federal and state laws, private health coverage policies, and other policies that impede HIV testing?” Laws and policies that can impede HIV testing include
-
state legal requirements for HIV testing;
-
discordant federal HIV testing recommendations;
-
public and private health insurance policies;
-
policies inhibiting use of rapid HIV tests; and
-
policies and practices in corrections settings.
A lack of programs and policies to promote clinician education and training and reduce constraints on practice environments, as well as policies to reduce HIV stigma and discrimination, are also barriers to expanded HIV testing.
State Legal Requirements for HIV Testing
State laws, in particular written informed consent and pretest counseling laws, are often cited in the discussion about barriers to routine HIV testing per the CDC’s revised HIV testing guidelines (GAO, 2009; Mahajan et
al., 2009). Since the release of the CDC’s revised recommendations in 2006, restrictions on HIV testing and informed consent requirements have been removed in a number of states, and other states have proposed legislation to remove these requirements (CDC, 2009d). In the 2 years following the release of the revised guidelines, nine states passed laws that moved them from inconsistent to consistent with CDC’s recommendations (Mahajan et al., 2009).
Laura Bogart of Harvard University described the continuing variation across states with regard to requirements for pretest counseling and written informed consent. Five states require written informed consent for HIV testing, and at least six states require pretest counseling (see Table 2). Additional states require that pretest counseling or written informed consent be provided under certain testing conditions. For example, in Colorado, written informed consent and pretest counseling are required for HIV testing provided at public health testing sites (NCCC, 2010). Carlos del Rio of Emory University pointed out that even when a state’s HIV testing laws are changed, hospitals within the state do not necessarily change their practices. For example, the state of Georgia does not require written informed consent for HIV testing, but does require that a patient consent to an HIV test and that all individuals be counseled before and after testing. For legal protections, hospitals may continue to administer written consent to provide proof that the clinician has consented the patient.
All 50 U.S. states and the District of Columbia allow minors to consent for sexually transmitted disease (STD) services, but several do not explicitly include HIV testing and treatment among these services (Guttmacher Institute, 2010). Some states have specific provisions requiring that minors be at least 14 years old to consent for HIV testing (see Table 2). In South Carolina, minors must be at least 16 years old to consent for HIV testing, and in Montana only emancipated minors can consent for testing. Several states allow, but do not require, physicians to inform a minor’s parent or guardian of HIV test results. In Iowa, a physician must notify the parent or guardian if a minor tests HIV positive (NCCC, 2010). Donna Futterman of the Albert Einstein College of Medicine identified confidentiality and consent concerns as being particularly problematic for adolescents and youth. Although young people have legal rights and protections, many providers and youth may be unaware of them.
Some studies have shown that the availability of anonymous HIV testing, where a code is used rather than an individual’s name, can facilitate HIV testing (Lansky, 2002; Tesoriero et al., 2008). However, several states have legal requirements prohibiting anonymous HIV testing (see Table 2) (KFF and NASTAD, 2009; NCCC, 2010).
States also regulate who can perform HIV testing, including who can order HIV tests and withdraw blood. Rear Admiral Scott Giberson of the Indian Health Service (IHS) described variation in state laws regarding
TABLE 2 Select State HIV Testing Laws
|
States That Require Written Informed Consent |
Massachusetts (HIV-specific written consent required; 16 MGL c.111, §70F),a Michigan (MCLS §333.5133),b Nebraska (HIV-specific written consent required; RRS §71-531),a New York (for HIV testing that will not provide results within 1 hour; PBH §2781 10 NYCRR 63.3),a Pennsylvania (35 PCS §7605)a |
|
|
In Colorado, a written consent form must be used for testing at public health testing sites (6 CCR -1009-9).b |
|
States That Require Pretest Counseling |
Georgia (OCGA § 31-22-9.2),c Michigan (Pretest counseling required, prevention counseling not required; MCLS §333.5133; MCLS §333.5923),b Missouri (12 RSMo §191.653),a New York (PBH §2781 10 NYCRR 63.3),a Pennsylvania (35 PCS §7605), Rhode Island (RIGL §23-6.3-3)b |
|
|
In Colorado, pretest counseling is required for testing at public health testing sites (6 CCR -1009-9.7).b Other states (e.g., Montana, Illinois) require that pretest information be offered. |
|
State Laws on HIV Testing of Minors/Adolescents |
|
|
Must be 14 years old or older to consent to HIV testing: |
Idaho (ID Code §39-3801, IDAPA 16.02.10-015),a New Hampshire (10 RSA 141-C:18),a South Carolina (16 years or older may consent, SCC §20-7-280),b Washington (RCW §70.24.110),a Wisconsin (WS §252.15 (2m) (c))d |
|
Only emancipated minors may consent to testing: |
Montana (MCA §41-1-402)b |
|
Option to notify parent/guardian of HIV test results: |
Colorado (if minor is less than 16 years or unemancipated; CRS §25-4-1405),b Georgia (OCGA §31-17-7),c Illinois (410 ILCS 305/9k),e Kansas (KSA § 65-2892),a Maine (22 MRS §1823),a Massachusetts (16 MGL §12),a Michigan (MCLS §333.5127),b Mississippi (MCA §41-41-13),a Missouri (12 RSMo §191.6562.(1)(f)),a New York (PBH §2782),a Oklahoma (63 OS §2602),a Virginia (VC §32.1-36.1, VC §32.1-69)a |
|
Health care provider or facility must notify legal guardian of an HIV positive result: |
Iowa (4 IC §141A.7)a |
|
aSource last updated January 26, 2010. bSource last updated July 27, 2010. cSource last updated January 25, 2010. dSource last updated May 7, 2010. eSource last updated September 15, 2010. SOURCE: NCCC, 2010. |
|
qualifications needed to perform an HIV test as a barrier to HIV testing within the IHS. A summary of current state regulations on who can perform HIV testing was not available to the committee at the time of this report.12 In 2004, 40 states had regulations on who can perform HIV testing, often limiting testing to trained health care providers and government employees in health departments or corrections settings (Hodge, 2004).
Natalie Cramer of the National Alliance of State and Territorial AIDS Directors (NASTAD) presented data on the concordance of HIV testing offered through state health departments with the CDC’s 2006 revised guidelines for testing of adults, adolescents, and pregnant women in health care settings (Figure 2). In a 2008 survey of health departments, most states reported conducting routine HIV testing in health care settings, but this varied by population with few states reporting routine testing for the general population (adolescents and adults ages 13–64). All states had implemented targeted counseling, testing, and referral programs to reach high risk groups.
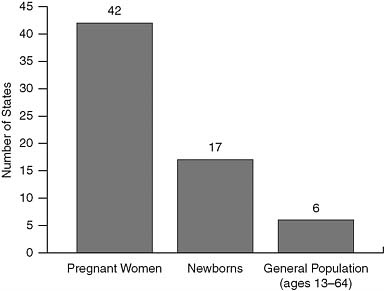
FIGURE 2 Number of states implementing components of the CDC’s 2006 revised HIV testing recommendations.
SOURCE: KFF and NASTAD, 2009.
Research has not consistently established state HIV reporting policies, such as name-based reporting for public health surveillance purposes, as a deterrent to HIV testing. Studies conducted in the late 1980s and early 1990s suggested a deterrent effect of HIV reporting, while more recent studies have found fewer or no deterrent effects (Tesoriero et al., 2008). In an evaluation of New York’s HIV Reporting and Partner Notification law on HIV testing levels and on the HIV testing decisions of high-risk individuals (N = 761), high-risk individuals in all demographic and risk-related subgroups had limited awareness of the state’s reporting and notification law, and few cited concern about named reporting as a reason for avoiding or delaying HIV testing. The law also did not affect HIV testing levels, posttest counseling rates, and anonymous-to-confidential conversion rates among those who tested HIV positive, or Medicaid-related HIV testing rates (Tesoriero et al., 2008). An evaluation of nine states’ HIV reporting policies in the mid-1990s found similar results, although people who lived in states with name-based HIV reporting were more likely to delay testing (CDC, 2004).
In discussing barriers to HIV testing for American Indians and Alaska Natives, Giberson described the problem of state policies and resources (e.g., Ryan White, drug assistance programs, etc.) often being located in areas that are not easily accessible to individuals living in rural parts of the country.
Discordant Federal HIV Testing Recommendations
Public health recommendations issued by CDC and the USPSTF provide guidance to both providers and payors of HIV testing.
Bernard Branson of the CDC described the current recommendations for HIV testing issued by the USPSTF, a federally appointed panel of experts tasked with reviewing evidence regarding the benefits and harms associated with preventive services. The USPSTF assigns an A grade to services for which there is good evidence that the service improves important health outcomes, a B grade to services where there is at least fair evidence to support a recommendation, and a C grade to services where the USPSTF was unable to make a recommendation for or against routine provision of a service because, although at least fair evidence was found that the service can improve health outcomes, the balance of benefits and harms was too close to justify a general recommendation. For HIV testing, the USPSTF has assigned a grade A for screening of high risk groups and pregnant women. However, whereas the CDC guidelines for testing in medical settings recommend routine HIV testing for all individuals age 13 to 64, the USPSTF has assigned a grade of C for routine screening of those who are not at increased risk for HIV (i.e., it does not make a recommendation for or against routine HIV testing) (USPSTF, 2007).
Screening tests covered by Medicare, private health insurers, and the Federal Employee Health Benefit program are linked to recommendations from the USPSTF. The lack of an A or B grade by the USPSTF could pose a barrier to reimbursement for routine HIV testing for these payors. Branson attributed the discordance between the CDC and USPSTF guidelines to differences in the methods used to formulate recommendations as well as the purpose of the recommendations, with the CDC perhaps having a greater emphasis on public health rather than clinical outcomes.
Michael Horberg of Kaiser Permanente described how both the CDC and USPSTF recommendations are used by insurers, providers, and others to inform the development of their own guidelines. Although many professional societies (e.g., American College of Physicians, Infectious Diseases Society of America, American Medical Association, American College of Obstetrics and Gynecology) have adopted the CDC guidelines, others have not (e.g., American Academy of Family Physicians). Cramer also indicated that the discordance between the CDC and USPSTF guidelines has contributed to confusion among providers of HIV testing and has limited insurance coverage for routine HIV testing.
Andrew Baskin of Aetna, Inc., discussed the slow rate of diffusion of changes in practice recommendations, including for routine HIV testing, into practice by health care providers, noting that it can take several years for a clinical recommendation to become implemented as a standard of care.
Public and Private Health Insurance Policies
Workshop participants noted a number of policies related to the financing of HIV testing, including reimbursement for routine testing by public and private health insurers, that are possible barriers to expanded HIV testing. Health care reform will expand access to health insurance coverage for millions of Americans, including many people at risk for and living with HIV/AIDS. Provisions of health care reform that may impact access to HIV testing include
-
the general allowance for dependents up to age 26 to participate on their parent’s or guardian’s health insurance;
-
Medicaid expansions that will provide coverage to many more individuals living in or near poverty by raising the minimum income eligibility criteria and eliminating most categorical eligibility requirements (e.g., disability or parental status);
-
options for states under Medicaid to improve access to preventive services;
-
a requirement that states, as a condition for participation in Medicaid, will establish procedures for conducting outreach to and
-
enrolling vulnerable and underserved populations eligible for medical assistance, including individuals with HIV/AIDS; and
-
the elimination of preexisting conditions exclusions by private insurers.
In addition, under a personal responsibility education provision, states can receive grants to carry out education programs to prevent pregnancy and STDs, including HIV, among adolescents (P.L. 111-148).
Health care reform will not expand coverage to all who need it, however, such as the millions of individuals who are unlawfully present in the United States. This exclusion could potentially diminish the impact of health care reform to control of the HIV epidemic more broadly. In addition, many of the barriers to coverage of HIV testing will remain after it is implemented. There are opportunities to address some of these barriers before full implementation of the reform.
Medicaid and Medicare Medicaid is the major public health insurance program for low-income Americans and others who meet certain eligibility requirements. It finances health care services for over 55 million people in the United States, including families, people with disabilities, and the elderly (KFF, 2009b).
Cindy Mann, Director of the Center for Medicaid and State Operations, provided the committee with a description of the role of Medicaid as an essential provider of HIV-related services. Medicaid is the largest single source of care and coverage for people with HIV with an estimated 40 percent of HIV/AIDS patients receiving services through Medicaid.13 The combined federal/state Medicaid spending related to HIV/AIDS reached $8 billion in FY 2009 (KFF, 2009d). Although Medicaid is an important source of HIV care, many individuals at risk for HIV are currently not eligible for coverage or, if eligible, face barriers to enrollment.
In terms of reimbursement of HIV testing under Medicaid, adults with identified risk factors are covered under a mandatory laboratory benefit. Routine HIV testing is also reimbursable under federal Medicaid law; states have the option of covering routine HIV testing of Medicaid-eligible adults as a preventive or screening benefit under section 1905(a) of the Social Security Act. For children it is a requirement under the Early Periodic Screening, Diagnosis, and Treatment Program, which is the child component of Medicaid. However, although most state Medicaid programs cover HIV testing in some fashion, it has been noted that routine testing
may not be covered at a broad range of sites (Cheever et al., 2007).14 In 2009, a letter was issued to state health officials encouraging provision of routine HIV testing and clarifying the coverage options that are available for federal reimbursement (Mann, 2009). With state budgets in decline, committee member Jennifer Kates of the Kaiser Family Foundation raised concerns about states’ ability to afford optional preventive services for adults. The rates at which HIV tests are reimbursed under Medicaid vary by state. Branson noted that in California, reimbursement only covers the cost of the rapid HIV test ($12.61), while in New York, reimbursement for HIV testing in EDs is set at $130.
Mann also highlighted the important role Medicaid plays in U.S. health reform and the challenging issues that the program will face when reforms are fully implemented. An anticipated 16 million low-income people are expected to enter the Medicaid program by 2019 due to the expansion of income eligibility to a national floor of 133 percent of the national poverty level and elimination of most categorical eligibility requirements, such as disability. This undoubtedly will include a number of people who would benefit from HIV testing who were not eligible for Medicaid under previous law.
Audience member Elaine O’Keefe from Yale University stated that inadequate payment rates and low provider participation are current barriers to access to care for those enrolled in the Medicaid program and that these barriers may be an increasingly important issue as Medicaid enrollment grows under health care reform. Health care reform will remedy this barrier to some extent because, when fully implemented, Medicaid payment rates for primary care services will rise to those used for Medicare physician reimbursement. However, the recently enacted health care legislation will not provide coverage for undocumented aliens in the United States.
Medicare is the largest provider of health insurance in the United States. Most Medicare beneficiaries are 65 or older, but the program also provides health benefits to almost 7 million people younger than 65 who have a disability or chronic condition (California Health Advocates, 2009).
Based on an announcement of authority to cover additional preventive services for beneficiaries and the issuance of new HIV testing guidelines,15 in March 2009 Centers for Medicare & Medicaid Services (CMS) initiated a national coverage analysis to evaluate the existing evidence on HIV screening to determine if the evidence was sufficient for Medicare coverage
of HIV testing (CMS, 2009b). CMS issued a decision memo in September 2009, stating it had determined that the evidence is adequate to conclude that screening for HIV infection, which is recommended with a grade of A by the USPSTF for persons at high risk, is reasonable and necessary for early detection of HIV (CMS, 2009a). CMS would cover annual voluntary HIV screening of Medicare beneficiaries at increased risk for HIV infection per the USPSTF guidelines, and voluntary HIV screening of pregnant Medicare beneficiaries. Medicare would also reimburse testing for beneficiaries who request an HIV test despite reporting no individual risk factors, since this group is likely to include individuals not willing to disclose high-risk behaviors (CMS, 2009a). Coverage for provider-initiated or general population-based routine testing is not mentioned in the decision memo, however.
Branson described how, despite this new coverage policy, it is necessary for the patient to ask for a test and/or for the physician to identify risk factors during the encounter in order to receive reimbursement for HIV testing. Experience suggests that these reimbursement policies may not facilitate routine HIV testing.
Private Insurance Sixty-five percent of people in the United States had private health insurance in 2008 (CDC, 2009a). The committee was not able to assess the extent of reimbursement for routine HIV testing by all private insurers from available resources; however, private insurer reimbursement for routine HIV testing has increased since the release of the CDC’s revised testing guidelines in 2006. According to a 2009 presentation by the CDC, of 11 large healthcare plans, all have established policies to reimburse for targeted HIV screening and six have established reimbursement policies for routine HIV testing (CDC, 2009d). The state of California and the District of Columbia have passed legislation requiring private health insurers to cover routine HIV testing, and similar legislation has been introduced in other states. Despite these developments, a 2009 Government Account-ability Office survey found lack of insurance reimbursement to be a barrier to implementation of routine HIV testing by state and local health departments, provider organizations, and CDC officials (GAO, 2009). As with Medicare, most private insurers use the USPSTF recommendations, which have not recommended routine HIV testing, when developing their reimbursement policies (GAO, 2009; Lubinski, 2008).
With the cost of HIV/AIDS care dwarfing the cost of HIV testing, there may be a financial disincentive for private insurers to promote routine HIV testing. Gary Claxton of the Kaiser Family Foundation provided the committee with an overview of private insurance policies and regulations. Individuals covered by large group insurance plans usually have good coverage for preventive services including HIV tests. However, cost sharing
(e.g., co-pays, deductibles) may represent a significant barrier to testing for many. For those who have health insurance through employers who self-insure under the Employee Retirement Income Security Act (ERISA, P.L. 93-406),16 the extent of coverage of preventive services, including HIV testing, varies. Approximately half of individuals with private health insurance are in self-insured plans.
Claxton described how the distinct segments of the private health insurance industry are regulated: direct purchase coverage (non-group and some associations) is regulated by states; insured employer-sponsored coverage is regulated at both the state and federal level; and self-funded, employer-sponsored insurance is only bound by federal regulation. States generally establish the minimum levels of required benefits for private health insurance, and these vary by state and by market segment. There have been very few federal standards regarding benefits that private insurers must provide, although this will change under health reform.
With the advent of health reform, Claxton described how new private insurance policies will have to cover preventive services with no cost sharing if the USPSTF assigns a grade of A or B to the service. As discussed previously, this includes risk-based HIV testing and routine testing of pregnant women, but excludes routine prevalence-based testing as recommended by the CDC. If a state requires that benefits be covered that are broader than the federal minimum required benefit package (e.g., routine HIV testing with a USPSTF grade of C), the state would have to pay for any cost-sharing subsidies that would be associated with the extra cost of the mandated benefit. Claxton suggested that health reform could actually be an impediment to states requiring more benefits than what the federal government says is the minimum standard developed by the Department of Health and Human Services (HHS).
Horberg pointed out that insurers rarely refuse reimbursement for HIV testing when the testing is prompted by the presence of risk factors, symptoms of AIDS, or an STD. However, the reimbursement may not necessarily include the cost of clinician’s time, counseling, and follow-up. Horberg noted the virtual absence of reimbursement mandates among jurisdictions; only California and the District of Columbia have required private insurers and HMOs to cover HIV screening costs (Horberg, 2010).
A Routine HIV Screening Coverage Act was introduced before Congress in 2009. If passed, the bill would require group health plans, as well as health insurance issuers that offer group health insurance, to provide coverage for routine HIV screening under terms and conditions no less favor-
able than for other routine screenings (H.R. 2137, Routine HIV Screening Coverage Act of 2009). In addition, under the bill insurers could not
-
deny to an individual eligibility, or continued eligibility, to enroll or to renew coverage under the terms of the plan, solely for the purpose of avoiding the requirements of the Act;
-
deny coverage for screening because there are no known risk factors or because it is not medically necessary, or because there was not a referral from a health care provider;
-
require an individual who is a participant or beneficiary to undergo HIV screening, although plans could not provide incentives for not using the coverage;
-
provide incentives to providers to act inconsistent with this Act, or penalize or otherwise reduce or limit the reimbursement of a provider because such provider provided care to an individual participant or beneficiary in accordance with the Act; and
-
deny to an individual participant or beneficiary continued eligibility to enroll or to renew coverage under the terms of the plan, solely because of the results of an HIV test or other HIV screening procedure.
The bill would not preempt state laws that require coverage at least as comprehensive as that required under the bill (H.R. 2137, Routine HIV Screening Coverage Act of 2009).
Baskin discussed another aspect of private insurance, not directly related to reimbursement, that can make it difficult to maintain the confidentiality of test results within the context of private insurance. He described how the explanation of benefits (EOB) that insurers are required by many states to send to subscribers may present such a barrier. EOB’s are routinely sent to the policy holder after the provision of any billable health service. Although the specific test (e.g., an HIV test) or test results would not be identified on the EOB, it would indicate that a generic blood test had been performed. If the subscriber called to find out what blood test had been conducted on his or her spouse or child, the subscriber would be informed of the nature of the test. The extent to which this has posed a problem thus far is not known, but it may potentially increase with the implementation of health care reform, which will enable more dependent children to remain on their parents’ plan by raising the dependent coverage age to 26. Baskin and Claxton felt that this unintended consequence of extending insurance coverage as part of health reform could be remedied through federal regulation.
Special Issues in Financing of HIV Testing in Health Departments, Emergency Departments, and Hospitals Cramer provided an overview of the
role of health departments in the provision of HIV testing. She emphasized how heavily dependent health departments are on public financing for HIV testing programs, with the CDC being the primary funder of HIV testing programs in health departments. In FY 2007, the CDC dispersed approximately $581 million to support HIV prevention programming in health departments in 65 jurisdictions (NASTAD, 2009). However, there is significant instability in the amount of public funding available and uncertainty about levels of private support, making it difficult for programs to expand HIV testing beyond risk-based testing to routine testing. CDC support for HIV testing often does not adequately cover the costs associated with HIV-related health education, risk management, and services provided by non-clinicians working in medical settings. In addition, a reduction in financing of HIV prevention programs by states on the order of $170 million in 2009 was cited by Cramer as an example of a curtailing of support for HIV testing that greatly affects health departments.
Cramer cited the restrictive nature of federal grants as barriers to flexibly meeting the prevention needs of constituents. For example, funding that promotes specific testing strategies, such as opt-out testing in health care settings, often does not take into consideration existing public health programs, infrastructure, and capacity. These focused programs often divert staff and resources from higher impact programs, such as targeted HIV testing, other necessary HIV prevention services, and health department efforts to promote cross-program collaboration and integration with STD, viral hepatitis, and tuberculosis (TB) programs, to name a few. Further, recommendations and guidance associated with cooperative agreements often result in unfunded mandates and further diversion of existing (and limited) resources.
A lack of coordination between multiple federal programs that provide funding for HIV testing (e.g., Health Resources and Services Administration, Substance Abuse and Mental Health Services Administration, and CMS) was also identified by Cramer as a significant barrier to efficient use of support. Health department HIV testing programs are also burdened by the differing federal grant requirements for program implementation, monitoring and evaluation, and data collection.
Based on experience as a physician working within an ED, Jeremy Brown of George Washington University described billing processes in hospital EDs and how they can limit adequate reimbursement for routine HIV testing. Reimbursement rates for services provided in hospital EDs are often bundled, and payment rates are prospectively negotiated between facilities and purchasers. These bundled rates are not necessarily up to date and may not be adequate to cover recently introduced clinical services such as routine HIV tests. In any case, since the reimbursement is a bundled rate there is a disincentive to provide nonessential services. Brown explained
that even though the District of Columbia requires insurers and HMOs to reimburse hospitals for routine HIV testing, the costs associated with HIV testing have not yet been incorporated into his hospitals’ bundled reimbursement rates.
AIDS Drug Assistance Program The AIDS Drug Assistance Program (ADAP) provides medications to treat more than half a million low-income, uninsured and underinsured people living with HIV/AIDS each year (HHS, 2010). The fastest growing component of all Ryan White Programs, ADAP is funded through Part B of the program that provides grants to states and territories. Peter Leone of the University of North Carolina, Chapel Hill noted the problem of ADAP waiting lists, implemented by states to help contain program costs. There were over 4,109 individuals who were on ADAP waiting lists across the country as of November 2010 (NASTAD, 2010). The limitations in availability of drugs may impede efforts to expand HIV testing if people know it will be difficult for them to obtain treatment. While many of the people on ADAP waiting lists are able to receive drugs from pharmaceutical patient assistance programs, such programs are not meant to be a permanent or primary source of treatment access.17
Policies Inhibiting Use of Rapid HIV Tests
Rapid HIV tests make it possible to provide results at the time that testing is done, while conventional tests can take several days to produce results. Thus, rapid testing can help to reduce the number of people who fail to receive their test results (Branson et al., 2006). The Clinical Laboratory Improvement Amendments (CLIA) of 1988 are federal regulatory standards that apply to all clinical laboratory testing. Several of the available rapid HIV tests have received a waiver from CLIA allowing nonlaboratory personnel, including outreach workers and social workers, to conduct rapid HIV tests, provided they receive appropriate training. Because the tests use unprocessed specimens (blood or oral fluid) they are simpler to perform. In addition to clinical settings, waived tests can be performed in HIV counseling and testing sites and in community settings.
Some state and local regulations and statutes have limited the use of rapid tests. For example, until recently, all non-physician providers of rapid tests in California had to have completed extensive phlebotomy training.
del Rio described how some hospital laboratories insist on performing the rapid tests themselves, because of fear of liability, secondary to concerns about the quality of point-of-care HIV testing. The performance of rapid HIV tests by hospital laboratories can slow the availability of results considerably. del Rio also noted the problem of how hospitals may run confirmatory HIV tests (which are usually run on any positive rapid HIV test) on a periodic basis, which may be as infrequently as twice a week. With short hospital stays, a patient could be discharged before results are available. Changes in institutional policies and state and local regulations pertaining to rapid HIV tests would increase rapid testing in hospitals and other settings.
Committee member Liisa Randall of the Michigan Department of Community Health indicated that flexibility in the use of the full range of HIV testing technologies and algorithms across testing sites is needed to appropriately address client/patient needs and preferences, as well as to address provider capacity and resource issues. The balance between the costs and benefits of different testing strategies needs to be weighed for each particular setting. In some situations, conventional laboratory-based testing technologies may be more cost-effective than the rapid HIV test. Providers may find rapid tests desirable in terms of achieving quick turn around times, but laboratory-based testing may improve clinic flow and more easily allow for the bundling of HIV tests with screening for other STDs. Giberson stated that rapid HIV tests are not widely utilized in the IHS, where conventional testing has been instrumental to expansion of testing.
According to a survey conducted by Bogart and colleagues in 2005–2006, rapid tests were used infrequently in nonprofit community settings (Bogart et al., 2008a, 2008b).18 Respondents from community health clinics (CHCs) and community-based organizations (CBOs) were asked if and when they had implemented rapid testing and to identify barriers to their adoption. Although this survey was conducted prior to the issuance of the CDC’s 2006 guidelines, many of the barriers identified by CHCs and CBOs in using rapid tests may still be relevant.
CHCs and CBOs located in areas with higher HIV/AIDS prevalence were more likely to have implemented rapid testing than sites located in lower prevalence communities. Larger sites with more resources (e.g., onsite laboratory, other diagnostic tests provided) were also more likely to be using rapid tests.
Respondents from CHCs and CBOs who were not using rapid tests were significantly more likely than users to agree to the following:
-
Rapid tests are difficult to integrate into my organization.
-
My organization does not have enough space to confidentially conduct rapid tests.
-
Regulations for rapid testing are difficult to understand.
-
Rapid testing does not allow more people to know their HIV status.
-
The procedures for running rapid tests are difficult to learn.
-
My organization is unable to employ dedicated staff members to perform rapid testing.
-
My organization does not have a sufficient number of staff to provide rapid tests.
Because the survey was conducted prior to the issuance of the CDC 2006 guidelines, Bogart suggested that the survey be repeated to provide an updated picture of the diffusion of adoptions of the CDC guideline recommendations.
Policies and Practices in Corrections Settings
Timothy Flanigan of Brown University described the epidemiology of HIV/AIDS within the U.S. correctional system. He reported that 1.5 percent of prison inmates are HIV positive or have confirmed AIDS diagnoses (BJS, 2009b) and that an estimated 15 percent of HIV-infected individuals have contact with the corrections system (Hammett, 2009; Hammett et al., 2002). In addition, Flanigan noted that the majority of individuals in the corrections system are men who do not routinely interact with the primary health care system, and more than half of inmates with sentences over 1 year are black or Hispanic, groups disproportionately affected by HIV/AIDS. Providing HIV testing services in correctional facilities may help to increase the use of HIV prevention services among some high-risk groups for whom the rate of incarceration is higher, and therefore also help to reduce HIV/AIDS health disparities (Macgowan et al., 2009).
Nina Harawa of Charles Drew University described findings from a Bureau of Justice Statistics survey on the status of HIV testing in prisons (BJS, 2009a). According to the survey, as of 2008, a total of 24 states tested all inmates for HIV at admission or sometime during custody. Among these 24 states, six tested upon release from prison, possibly making it difficult to ensure the delivery of confirmatory testing and linkages to care. Twenty-three states tested prisoners at admission, and five tested while in custody.
All 50 states and the federal system tested inmates if they had clinical indication of HIV infection or if they requested an HIV test.
A range of logistical, resource, and policy factors may impact the uptake of routine HIV testing within correctional facilities and populations. Flanigan described how jails are under local jurisdiction, either the county or city, and usually have poor levels of support. The inmates are transient and security is the priority, not health care or public health. There are also severe time pressures in jails, and a high rate of turnover. On average, half of all jail admissions leave within 48 hours. Consequently, if HIV screening is to be conducted among detainees, it needs to be done rapidly. In contrast, most individuals sentenced to prison have a term of at least 1 year and there are structures in place to provide opportunities for HIV testing.
Existing laws may reduce the confidentiality protections for inmates if they are known to have HIV (or for people living with HIV if they are incarcerated). For example, Harawa cited a California law (California HSC. CODE §121070) that requires medical personnel to disclose the HIV status of all inmates to the officer in charge of the detention facility. This officer in charge is then required to notify all employees and volunteers who may have direct contact with the inmate of the inmate’s HIV status. In addition, there may be insufficient controlled space for intake and testing to allow for the sharing of confidential information, and individuals requiring medical care or special diets may be presumed to be HIV infected. Confidentiality is thus very difficult to maintain in correctional settings. Inmates are not afforded the same protections provided to the general population by the Health Insurance Portability and Accountability Act (HIPAA, P.L. 104-191), which sets rules and limits on who can look at and receive health information, such as HIV testing information.
Harawa described how criminal statutes on HIV transmission might be an impediment to testing within corrections settings since a positive HIV diagnosis can increase the sentence or severity attached to specific crimes and because bail amounts may be higher if a convicted person is HIV positive. As of 2008, 28 states had criminal statutes on HIV transmission (KFF, 2008).
To address their medical needs, Harawa described how inmates with HIV may be housed in a limited number of facilities. She suggested that inmates may not want to find out their HIV status if they know that they might be transferred far from family and friends. Some family members must travel great distances to visit their loved ones transferred because of their positive HIV status. Furthermore, work opportunities in prisons may be limited through official or de facto policies. For example, inmates with HIV may be denied work in the kitchen, despite the lack of evidence that this would pose a risk to staff and other inmates. Being unable to work
while incarcerated has implications for sentence length, work release, and halfway house placement.
Lack of Programs and Policies to Promote Clinician Education and Training and Reduce Constraints on Practice Environments
Benjamin Tsoi from the New York City Department of Health and Mental Hygiene discussed findings from a survey of people reporting that they had never been tested for HIV. The primary reasons cited were “You don’t think you’re at risk” (69 percent) and “Your doctor never recommended it” (27 percent) (KFF, 2009a).19 The results of this survey convey how low perceived risk, coupled with a reliance on physicians to prompt testing, has shifted the onus to increase HIV testing onto care providers. Primary care physicians are at the forefront of the HIV/AIDS epidemic, yet a lack of education, training, and resources were cited by several workshop presenters as barriers to routine HIV testing in clinical practice.
del Rio described several areas where there may be a need for primary care provider training and education, as demonstrated in the HIV/AIDS research literature, including
-
awareness of potential risks of HIV infection in patients; u the CDC guidelines and the benefits of early HIV diagnosis with linkage to care;
-
tools for disclosing a positive diagnosis and discussing risk behaviors, such as sexual practices and drug use, with patients;
-
technical training on rapid HIV tests;
-
knowledge of state laws regarding consent and counseling; and
-
availability of HIV care resources in the community (e.g., Ryan White grantees) (Goetz et al., 2009; Jain et al., 2009; Mimiaga et al., 2009).
Giberson stated that many IHS care providers support routine HIV testing, but need more information and training on HIV testing guidelines, implementation strategies, and state HIV regulations. As another example of where training and education might be beneficial, Leone described how many clinicians fail to recognize the signs and symptoms of an acute HIV infection (a condition that can occur 2 to 4 weeks after infection with HIV) and lack an understanding of the window between initial infection and seroconversion and how to make a diagnosis. The possibility of detecting
acute HIV infection and intervening against it is significant because this condition contributes disproportionately to HIV transmission. In addition, detection of acute HIV infection can lead to earlier treatment with ART and linkage with care.
Kevin Cranston from the Massachusetts Department of Public Health stated that clinicians’ variable level of skill and comfort exploring patients’ risk history can contribute to missed opportunities for HIV testing. Health communication research has shown that health care providers are often reluctant to discuss sexual issues with their patients, and may discuss sexual issues differentially based on patient characteristics such as age and sex (Emmers-Sommer et al., 2009; Grant and Ragsdale, 2008). In addition, patients may be reluctant to report risk behaviors to health care providers due to concerns about confidentiality, possible discrimination, or for other reasons. Committee member Randall suggested the need for training to improve providers’ comfort and competence in discussing sexual health issues, disclosing results, and making appropriate referrals. Randall also emphasized that the lack of culturally appropriate health services is a barrier to HIV testing both from a provider and patient/client perspective.
Several workshop participants identified the inability of the current configuration of providers to adequately fulfill the needs of clients imposed by routine testing in medical settings. Staffing changes are needed, specifically the incorporation of dedicated staff to address HIV testing into health care teams. del Rio noted that pilot projects have demonstrated feasibility, but are difficult to sustain without new funding resources (Mehta et al., 2008; Pinkerton et al. 2009). Committee member J. Kevin Carmichael of El Rio Special Immunology Associates described how many of the HIV/AIDS physician specialists who began their careers at the beginning of the HIV/AIDS epidemic are now retiring or moving on to other areas of medicine. Non-physician HIV specialists (e.g., nurses, physician assistants) were identified as potentially being more effective than physicians in facilitating the expansion of testing. Expanded HIV testing leads to increased numbers of individuals who need HIV care services. Giberson and del Rio identified an inability to provide newly diagnosed patients with timely appointments with HIV care providers as a possible barrier to HIV testing.
Several comments were made by workshop participants about the burden of fulfilling data-reporting requirements. Although these requirements often are associated with grants or other types of funding, and not directly related to testing practices, they do require substantial personnel time and thus may limit the ability of staff to engage in other clinical activities, such as expanded HIV testing and treatment programs.
The lack of adequate space within busy medical clinics was also cited as a barrier to testing. Space that ensures privacy is needed when eliciting risk behaviors and providing education, counseling, and HIV testing. For in-
stance, Harawa discussed how the lack of appropriate space poses a serious barrier to HIV testing within the corrections system, particularly in jails.
Lack of Programs and Policies to Reduce HIV Stigma and Discrimination
HIV/AIDS stigma is considered a major barrier to an effective response to the HIV epidemic (Mahajan et al., 2008). In health care settings, stigma attached to behaviors and other aspects of HIV risk may impede patient-provider communication about HIV testing. For example, discomfort discussing sexual risk behaviors has been reported among both patients and health care providers (Bernstein et al., 2008). Experiences of discrimination (e.g., based on sexual orientation, race/ethnicity, drug using status, and other factors) in the health care setting, the belief that discrimination may occur, and distrust of the medical system can also discourage individuals from accessing HIV testing (Bernstein et al., 2008; Malebranche et al., 2004). Ideas about how peers may perceive HIV testing may also play a role in an individual’s choice to be tested for HIV. For instance, a 2009 KFF survey (N = 2,554) of perceptions of testing-related stigma showed that individuals who felt that their peers would think less of them if they were tested for HIV were much less likely to report having been tested in the past 12 months than individuals who felt that their peers would think more of them if they had been tested (9 percent compared with 34 percent, respectively) (KFF, 2009f). Darrell Wheeler of Hunter College identified stigma and racial and sexual discrimination as important barriers to HIV testing for black and African American MSM.
The implications of a positive HIV test may dissuade some individuals from being tested for HIV. Like other stigmatized groups, individuals living with HIV are disadvantaged in a variety of ways, including income, education, housing status, medical treatment, and health (Mahajan et al., 2008). Some individuals with HIV may feel blamed for their HIV infection and encounter fears of contagion by others, including within the medical community (Sayles et al., 2007). Individuals who fear rejection by friends and family, in employment, and the like, may choose not to disclose their HIV status, even to health care providers, potentially resulting in stress from feeling the need to hide their condition and discouraging care and adherence with treatment. Studies of adults with HIV have found a relationship between HIV/AIDS stigma and poorer antiretroviral therapy adherence, health-related quality of life, increased HIV symptoms, and depression (Sayles et al., 2007, 2009).
As discussed later in this report, federal and state disability laws can help to counteract discrimination against individuals with HIV in employment, health care, and other areas. In addition, federal and state privacy laws set limits on who can view an individual’s health information, includ-
ing information about HIV testing. Although the existence of these laws is important, the extent to which they help to prevent discrimination against individuals with or suspected to have HIV is unknown. Many individuals may be unaware of these protections. Furthermore, such policies do little to address possible discrimination from family or community members, which may be a barrier to HIV testing for some individuals.
Conclusions
-
State informed consent and pretest counseling laws are becoming less of a barrier to state implementation of routine testing, although changes and inconsistencies in state HIV testing policies may be a source of confusion for providers. State regulations on who can perform HIV testing can also restrict testing capacity as well as where testing can be offered.
-
In the case of HIV testing in medical settings, recommendations issued by the CDC and the USPSTF are discordant, possibly limiting insurance coverage for routine HIV testing.
-
Barriers to adequate reimbursement for HIV testing vary by payor and setting and stem from policies related to discordant federal HIV testing guidelines (i.e., CDC, USPSTF), limits on the provision of preventive services under Medicaid, cost sharing under private insurance, unstable and insufficient support for services provided within health departments, and issues related to the bundling of costs of services for payments to hospital-based providers. However, there have been a number of changes in support of increased insurer reimbursement for HIV testing.
-
State and local regulations and institutional laboratory policies may inhibit the use of rapid HIV tests in clinical settings. Flexibility in the use of the full range of HIV testing technologies and algorithms (point-of-care and laboratory-based algorithms) across testing sites is needed to appropriately address client/patient needs and preferences as well as to address provider capacity and resource issues.
-
Policies, such as those that compromise confidentiality, limit HIV-positive inmates’ access to work within the facility, and move them away from areas where their families live, and HIV criminalization laws could potentially discourage HIV testing within the corrections system.
-
Barriers to HIV testing by providers include limited education and training and constraints on practice environments. Changes in the legal and regulatory climate surrounding HIV testing have improved opportunities for testing; however, clinicians often lack
-
the resources necessary to incorporate routine HIV testing into their practices.
-
Stigma and discrimination are major barriers to HIV testing, but they have received little attention in programs that manage HIV infections. Programs and policies aimed at the medical community and the public that raise awareness about HIV and HIV-related risks, provide social support, and are culturally sensitive are needed to facilitate expanded testing and improve the quality of life for those affected by the disease.
Methods and Policies to Increase HIV Testing and Identification of HIV Positive Individuals
The second specific question posed to the committee was, “What effective HIV testing methods and/or policies should be implemented by federal, state, or local agencies, federal programs, or private insurance companies that can be used to reach populations with a high HIV prevalence and/or high prevalence of undiagnosed HIV infection.” Methods and policies that might increase HIV testing and identification of HIV positive individuals include
-
wider availability of rapid HIV testing;
-
partner notification and social network strategies;
-
linkage of HIV testing with other care and social services;
-
media and social marketing strategies;
-
strategies that encourage HIV testing by providers;
-
federal and state privacy and discrimination laws; and
-
corrections-specific strategies.
Rapid HIV Testing
Several studies have shown that rapid HIV testing is feasible and is well-accepted by patients, including in settings where services are provided to higher-risk populations, such as corrections settings (Beckwith et al., 2007; Macgowan et al., 2009), STD clinics (Kendrick et al., 2005), and EDs (Brown et al., 2007; Freeman et al., 2009; Merchant and Catanzaro, 2009; Mollen et al., 2008). Whereas conventional tests for HIV can take a few days to produce results, rapid testing can produce results within 30 minutes. Therefore, rapid testing can help to decrease the number of people who fail to learn their test results following testing (Branson et al., 2006; Hutchinson et al., 2006). In one recent study of voluntary counseling and testing in bathhouses involving 1,020 participants, similar percentages of men were found to be HIV positive using rapid and standard testing (2.5
percent and 3.7 percent, respectively), but rapid testing delivered results to more individuals than standard testing (97 percent compared with 71 percent) (Huebner et al., 2010). In another study that enrolled 251 patients with primary/urgent care appointments in two Veteran’s Administration primary care clinics, streamlined counseling coupled with rapid testing significantly increased testing and receipt rates over traditional HIV counseling and testing (testing and receipt rates were 89.3 percent and 79.8 percent under the rapid model, compared with 40.2 percent and 14.6 percent under the traditional testing model) (Anaya et al., 2008).
Rapid tests can be easier to perform than conventional tests. Several rapid HIV tests have waivers to the CLIA quality standards, allowing them to be administered by nonlaboratory personnel including in nonmedical settings. Testing in community settings such as bathhouses, bars, homeless shelters, and churches has in several studies been found to be feasible and effective for reaching populations who are at high risk for HIV and who have a higher prevalence of undiagnosed HIV infection (Aguirre et al., 2007; Bowles et al., 2008; Bucher et al., 2007; Daskalakis et al., 2009; Hatcher et al., 2008; Huebner et al., 2010). In one of the larger studies, for example, 267 (1.1 percent) of 23,000 people who had received rapid HIV testing in community settings such as public parks, homeless shelters, and bars were newly diagnosed as HIV positive. Of those who were diagnosed with HIV, 76 percent were from racial/ethnic minority groups, 58 percent identified themselves as men who have sex with men, and 72 percent reported having multiple sex partners. In addition, most of those who were diagnosed as HIV positive received their confirmatory test result (75 percent) and were referred to care (64 percent) (Bowles et al., 2008). As was described previously in this report, state and local and institutional policies can limit the use of rapid HIV tests at point of care.
Partner Notification and Social Network Strategies
Partner notification has been found to be effective for identification of persons with previously undiagnosed HIV infection (CDC, 2008d, 2010). Partner notification is a key component of partner services20 that involves confidential notification of the sexual and needle sharing partners of HIV infected individuals of possible exposure. A systematic review of studies conducted among a variety of populations for the Task Force on Community Preventive Services showed that between 14 and 26 percent of tested partners of individuals with HIV were found to have undiagnosed HIV (Hogben et
al., 2007). Based on these findings, the Task Force currently classifies the evidence as sufficient to recommend provider referral partner notification (CDC, 2010). Partner services, including partner notification, also have the benefit of providing an opportunity to reach persons who are HIV negative but who are at very high risk for HIV (such as sex and drug-injection partners of persons with HIV) to make them aware of their risk and offer prevention services (Dooley et al., 2007). Partner services are an underused strategy to identify individuals with and at high risk for HIV despite evidence of its effectiveness (CDC, 2008d). In addition to consumer, provider, and community concerns, local policies and procedures may be an impediment to broader implementation. For example, partner services have been found to be routinely provided in publicly funded HIV counseling and testing sites, but less likely to be provided outside of public health sites, unless providers contact health departments for assistance (Dooley et al., 2007; Golden et al., 2004). Further, funding for partner services for HIV is provided through state STD and HIV surveillance program funding, which may be limited.
Similar to partner notification, social network strategies have also been found to be effective for reaching individuals at high risk for HIV. In the CDC’s Social Networks Demonstration Project nine community-based organizations in seven cities received funding to enlist HIV-positive persons to refer others from their social, sexual, or drug-using networks for HIV testing. Over a 2-year period, 422 recruiters referred 3,172 peers for HIV services. Of these, 177 individuals (about 5.6 percent) were determined to be HIV positive, which was significantly higher than the approximately 1 percent that had been identified in other CDC-funded HIV counseling, testing, and referral sites. Sixty-three percent of those diagnosed as HIV positive were linked to medical care and prevention services (Kimbrough et al., 2009). It has been suggested, however, that repeat testing by individuals who are already aware of their HIV status may overestimate the effects of social network strategies. In another study of social network testing in New York City, for example, when the authors linked the identified positive cases to the city’s name-based registry of persons with HIV/AIDS, the HIV prevalence rate for newly reported cases dropped from 5.4 percent (8 of 147) to 3.4 percent (5 of 147). The authors recommended that the evaluation of testing strategies include collaboration with health departments to account for repeat testing (Renaud et al., 2010).21
Manya Magnus of George Washington University cited the use of alternative methods of recruitment, such as peer referral and social net-
work approaches, as possible facilitators of HIV testing for Latino MSM, although strong evidence on best practices and approaches for this population is lacking.
Integration of HIV Testing into Other Care and Social Services
HIV, viral hepatitis, and other STDs share risk factors and modes of transmission. Common risks suggest the need for common intervention strategies (CDC, 2009c). For several years, public health officials and professional associations have been advocating for the integration of services for HIV, STDs, viral hepatitis, and TB.22 An important benefit of service integration is to reduce missed opportunities to offer services to individuals at increased risk for HIV when they do access services (IOM, 2010; Ward and Fenton, 2007). Although further research is needed, there is evidence from studies of HIV testing in STD clinics that HIV testing can be included in the routine battery of tests with high patient acceptance and can help to identify those who are unaware of their HIV-positive status (Campos-Outcalt et al., 2006). Yet, providers may not routinely offer HIV testing to individuals being tested for other STDs (Kushner and Solorio, 2007; Montaño et al., 2008).
Giberson described a new testing policy created within the IHS in conjunction with tribal health authorities that involved bundling HIV and STD testing, as well as community outreach with local media. Health care workers were interviewed after the policy was implemented. The vast majority of the respondents reported that patient acceptance of HIV testing was high or very high under the new policy.
For intravenous drug users, strategies that link HIV testing to receipt of other services such as needle exchange, testing for Hepatitis C, and receipt of drug rehabilitation have been shown to increase rates of HIV testing and counseling (for example, Gunn et al., 2005; Hennessy et al., 2007; Stopka et al., 2007).
Many drug users have co-occurring mental illness. Individuals may take drugs to relieve their symptoms and, in some cases, mental illness may be caused by drug use (DOJ, 2004). Michael Blank of the University of Pennsylvania presented research on HIV among persons with serious mental illness that showed HIV prevalence rates much higher than for the general population (Blank et al., 2002; Rothbard et al., 2009). He concluded that individuals with serious mental illness are an unrecognized high-risk population and suggested that HIV testing be routinely offered in mental health
settings and that HIV risk-reduction interventions be integrated into ongoing mental health treatment.
Media and Social Marketing Strategies
Mass communication campaigns have been used in HIV prevention efforts because of their ability to reach a wide audience in a way that is cost-effective (Cohen et al., 2005). Several quasi-experimental studies have found small but statistically significant effects of HIV/AIDS campaigns on reported behaviors such as condom use and reduced number of sexual partners, among others, as well as on behavioral intentions (Noar, 2009), perhaps suggesting a continued important role for communication campaigns in HIV prevention efforts. Few studies conducted in the United States have assessed the impact of HIV/AIDS campaigns specifically on HIV testing behavior (Noar, 2009). A review of studies conducted in developed countries found that mass media interventions have immediate and overall effects in the promotion of HIV testing, but no long-term effects, implying that there may need to be continuous campaign presence to sustain behavior change (Vidanapathirana et al., 2005).
Social marketing programs have been related to increased condom use and other psychosocial determinants of HIV-related behaviors (Martínez-Donate et al., 2009). Few studies have evaluated the impact of social marketing techniques on HIV testing specifically. One study that used a social marketing campaign (Spanish-language radio, print media, a website, and a toll-free HIV-testing referral hotline) to promote HIV awareness and testing among Latinos living in the U.S.-Mexico border region documented increased HIV testing at partner clinics, with 28 percent of testers reporting exposure to the campaign (Olshefsky et al., 2007). Another study of Latino men who have sex with men and women found that men who were exposed to a social marketing campaign were more likely to report that they intend to be tested for HIV in the next six months than men who had not been exposed to the campaign (Martínez-Donate et al., 2009).
Tsoi mentioned a community outreach program launched by the New York City Department of Health and Mental Hygiene, called The Bronx Knows, to encourage people to undergo voluntary HIV testing. Besides social marketing, such as posters and flyers with information about HIV testing, the campaign involves training of community groups to conduct free testing at a number of sites. Although it is impossible to determine the exact impact of the campaign on HIV testing, the NYC Department of Health and Mental Hygiene’s website reports that HIV testing increased substantially following implementation of the campaign in 2008.23
|
23 |
For more information, see http://www.nyc.gov/html/doh/html/ah/bronx_test.shtml. |
Futterman discussed similar media campaigns to promote HIV testing (Figure 3). Get Screened Oakland, a program of the Office of the Mayor of Oakland, launched a social marketing campaign to raise awareness about HIV and HIV testing among the diverse population of Oakland, California. According to a flyer about the campaign, social marketing materials spurred increases in referrals for testing through the campaign’s 1-800 number.24 A similar initiative called Test Miami was begun in 2009 to increase HIV testing in Miami-Dade County using social marketing strategies as well as efforts to mobilize the health sector and recruit and engage members of the community.25
In a discussion of facilitators of HIV testing among Latino MSM, Magnus noted the importance of adequate translations of social marketing media and related materials, as well as culturally appropriate messages and modes of dissemination. Bogart emphasized that establishing partnerships between providers and communities, such as through the use of health advisors from the communities being targeted, remains key to identifying

FIGURE 3 Municipal HIV/AIDS test scale up campaigns.
SOURCE: Miami-Dade County Health Department, 2010; New York City Department of Health and Mental Hygiene, 2010; City of Oakland, Office of the Mayor, 2010.
|
24 |
Get Screened Oakland, 2007–2009, A Program of the City of Oakland, Office of the Mayor: A Municipal Response to HIV Engaging the Community in Partnership. |
|
25 |
For more information, see http://www.dadehealth.org/hiv/HIVservices.asp. |
appropriate social marketing messages and testing venues (Bucher et al., 2007; Erausquin et al., 2009; Galvan et al., 2006; Olshefsky et al., 2007; Rhodes et al., 2009).
Strategies That Encourage HIV Testing by Providers
Horberg identified two interventions that could greatly facilitate HIV testing by providers: (1) the establishment of a standard of care regarding HIV screening by national credentialing and accreditation bodies and (2) the development and adoption of quality metrics based on HIV testing and/or early detection. At present, there are no such nationally accepted metrics on HIV testing, such as measures in the Healthcare Effectiveness Data and Information Set (HEDIS) or the Physician Quality Reporting Initiative. Horberg described how the Veterans Administration and Kaiser Permanente are recording the stage of disease at time of HIV diagnosis as a quality measure related to HIV testing. He suggested that process measures be developed for monitoring the quality of care, such as retention in care, CD4 cell counts, appropriate Pneumocystis jirovecii pneumonia (PJP) (formerly known as Pneumocystis carinii) prophylaxis and ART, and immunization for influenza, pneumococcus, and Hepatitis B. HIV ribonucleic acid (RNA) control is an outcome quality measure that could be considered. Testing for HIV alone or concurrent with testing for other STDs could also serve as a performance standard. Carmichael suggested that issues pertaining to HIV testing be better integrated into postgraduate and continuing medical education.
Clinicians may fail to perform HIV risk assessments for a variety of reasons, including comfort level with sexual health discussions, time constraints and competing demands, among other reasons. Some research shows that it is possible to change providers’ behavior to increase risk assessment and discussions regarding STD or HIV risk with patients using a systems approach (Bluespruce et al., 2001; Dodge et al., 2001). For instance, a systematized intervention of trainings that addressed knowledge needed for HIV prevention interactions with patients, identified specific roles for care team members, and used reinforcing factors, such as monthly HIV prevention updates, significantly improved how often providers performed HIV-risk assessments and counseling of patients in primary care (Dodge et al., 2001).
Workshop participants also described administrative strategies such as the use of computer aids and other tools to reduce provider-side barriers to HIV testing. Branson mentioned an effective CDC-supported initiative at Jacobi Medical Center in the Bronx, New York, called Project B.R.I.E.F., that used portable kiosks to streamline HIV counseling in a high-volume ED (Calderon et al., 2009). Futterman suggested that a new paradigm is
needed so that HIV testing is viewed in the same manner as the H1N1 vaccine and incorporated into routine medical care. She described a protocol that was developed by the Adolescent AIDS Program at Montefiore Medical Center in New York and the New Jersey AETC to facilitate HIV testing and streamline counseling. The approach, called “Advise, Consent, Test, and Support,” has been found to facilitate improvements in HIV testing in both clinical and community-based settings (AHRQ, 2010) (see Figure 4).26

FIGURE 4 ACTS pocket guide.
SOURCE: Futterman et al., 2004.
|
26 |
For more information see http://www.aids-ed.org/pdf/p03-cf/acts/ACTS-total.pdf. |
Wheeler stated that increased community-based and culturally sensitive care could facilitate HIV testing for some populations, such as black and African American MSM. Randall suggested that patient/client attitudinal barriers could also be addressed through enhanced provider training and education programs. Such programs could improve providers’ cultural competence and communication skills related to risk behaviors and attitudes that limit acceptance of HIV tests. Blank pointed out that individuals coming from the community in which patients reside may be viewed as less threatening and without the “social distance” often seen between patients and their physicians. He went on to describe how community health workers and health care paraprofessionals (e.g., certified nurse assistants, etc.) may be needed to reach and provide care to the estimated 30 million currently uninsured individuals who will have health insurance and better access to care following the implementation of health reform.
Strategies to Increase HIV Testing in Corrections Settings
Flanigan identified corrections, particularly jails, as providing great opportunities for the expansion of HIV/AIDS interventions within high-risk groups. Devon Brown, the head of the District of Columbia Department of Corrections since 2006, characterized the corrections system as the nexus between public safety and public health and as an optimal environment in which to routinely test clients for HIV.
Models for routine jail-based HIV testing are emerging. The committee heard from Flanigan of the experience in Rhode Island where from 2000 to 2007, 169 new cases were identified, representing 15.3 percent of all new diagnoses within the state. Risk assessment conducted as part of this jail-based screening program found that 76 percent of those testing positive did not consider themselves to be at risk for HIV despite reporting risk behaviors. Rapid testing has also been mainstreamed in jails in four CDC-funded states (Florida, Louisiana, New York, Wisconsin). In this project, 269 of 33,211 jail detainees (or .08 percent) tested for HIV in jails during 2004 to 2006 were newly identified with HIV. Almost half (46 percent) of these new diagnoses were among persons with heterosexual risk or no reported risk.
Brown described the automatic HIV testing program that has been instituted in the District of Columbia, which has become an award-winning model that other corrections systems have attempted to replicate. All entries into the corrections system are given the opportunity to be tested for HIV, other STDs, and TB, and are tested unless they refuse. Most clients are African American males, a group with high rates of HIV and often without access to primary care. Brown highlighted the successes of the program—87 percent of clients agree to be tested. Some of those who refuse
testing already know of their HIV-positive status. Any inmate who refuses testing without knowledge of his or her HIV status is counseled and encouraged to be tested. Individuals who test positive also receive counseling. An additional HIV screening is routinely administered as inmates are released. Sexual activity within the jail is acknowledged, and condoms are provided through the facility.
A distinctive aspect of the program is that the same medical providers within the prison are available to inmates following their discharge from prison. A private organization provides health care within the prison and in 34 clinics throughout the city. HIV-positive individuals are provided with a 30-day supply of “bridge” medications to allow them time to get into community-based care. Two case managers are available to encourage appropriate follow-up.
Privacy and Discrimination Laws
Federal and state laws have been established to protect health information, including information about HIV testing. The Privacy Rule under HIPAA, for example, sets rules and limits on who can look at and receive an individual’s health information (HHS, 2010).27 In addition, most states have HIV-specific privacy laws in place, many of which offer greater protections than the HIPAA privacy rule. Under these privacy laws, health information can be disclosed without authorization from the individual only in certain circumstances, including but not limited to health oversight and for public health purposes. The existence of confidentiality protections may help to facilitate HIV testing for some individuals (Chou et al., 2005; Ford et al., 2008).
The Supreme Court affirmed an interpretation of the Americans with Disabilities Act (ADA) that broadly protects persons with disabilities, which includes individuals with HIV. In their first review of a case involving HIV, the court ruled that health care professionals can legally refuse to offer health care services to a patient only if there is objective, scientific data for concluding there is a significant health threat to the safety of the provider (Gostin et al., 1999). Individuals with HIV are protected from discrimination in employment, use of public accommodations (such as use of hospitals, schools, etc.), and by state and local governments under the ADA. The ADA also offers such protections to persons who are discriminated against because they are regarded as being HIV positive and persons who have a
known association or relationship with an individual who is HIV positive. Individuals with both asymptomatic and symptomatic HIV are considered to be disabled under the ADA (DOJ, 2004).
Webber and Gostin (2000) note the argument of some commentators that “despite the apparent protection of federal law, states have a significant role in addressing HIV-based discrimination.” There is, however, a great deal of variation in coverage of HIV in state statutes. A recent review of state disability laws was not available to the committee. A review of state disability laws in 1999 concluded that the states can be divided into three categories, depending on their state level statutes. First, there are states with clearly specified protections for people infected with HIV. Second, there are the states that base their antidiscrimination statutes on the federal definition of disability, allowing a reasonable inference that HIV is covered under disability laws. And third, there are several states that provide little to no protection for HIV-infected residents.
The ADA and state disability laws may help to counteract HIV discrimination, although they clearly cannot prevent all cases of HIV discrimination. Efforts to increase testing highlight the need to better assess and improve the effectiveness of laws and institutions addressing HIV discrimination. Gostin and colleagues note that judicial inquiry should primarily focus not on whether individuals are disabled, but on whether they have experienced discrimination because of their health status (Gostin et al., 1999).
Conclusions
-
Several strategies show promise for increasing identification of individuals with HIV, including rapid HIV testing (including in community settings); partner notification for identifying individuals who are undiagnosed and at high risk for HIV; social network strategies (further research may be needed to account for the potential bias in studies due to repeat testing by individuals already aware of their HIV status); integration of HIV testing with other services, such as testing for other STDs; and HIV/AIDS campaigns.
-
Strategies that might help to promote HIV testing by providers, include the establishment of standards of care and quality metrics based on HIV testing or early detection of HIV; provider education and training related to cultural competency and communication with patients about risk behaviors; and administrative strategies to help streamline counseling and testing in busy practice environments.
-
Corrections settings provide an excellent public health opportunity for HIV testing and successful models can be replicated broadly with appropriate resources and leadership.
-
Efforts to increase HIV testing highlight the need to better assess and improve the effectiveness of laws and institutions in addressing HIV discrimination.
Impact of Opt-Out HIV Testing
The third specific question posed to the committee was “What has been the impact of opt-out HIV testing?” Branson described how the CDC’s 2006 updated guidance on HIV testing attempted to counter some of the barriers to testing experienced by clinicians, such as the administration of prevention counseling and written informed consent, by recommending that HIV testing be conducted routinely and on an opt-out basis. The CDC defines opt-out testing as notifying a patient that testing will be performed unless he or she declines or defers testing. The revised recommendations specify that HIV testing become routine when the expected yield within the facility or community was greater than 1 positive diagnosis per 1,000 tested (Branson et al., 2006). As discussed previously, most states have HIV testing laws that are consistent with the CDC’s opt-out HIV testing guidelines, while a few require written informed consent.
Since the publication of the revised CDC recommendations, several studies conducted in settings such as Veterans’ Affairs Medical Centers, EDs, and community health clinics have demonstrated that patients are accepting of opt-out HIV testing (e.g., Bokhour et al., 2009; Burrage et al., 2008; Freeman et al., 2009; Haukoos et al., 2008; Minniear et al., 2009). One study of routine opt-out HIV testing in an urban community health center found that just 35 percent of 300 patients told they “would be tested for HIV unless they declined testing” agreed to be tested, however (Cunningham et al., 2009).
Fewer studies have evaluated the effects of the presence or absence of informed consent requirements on HIV testing rates or the identification of individuals with HIV. There is some evidence that increased HIV testing has occurred where the written informed consent process was simplified or eliminated. A 2009 study of respondents to the 2004 Behavior Risk Factor Surveillance System survey found that residents in states with written informed consent statutes were somewhat less likely to report having been tested for HIV in the past 12 months (OR = .85; 95 percent CI = .80, .90). The association was significant among respondents who were non-Hispanic white (OR = .77; 95 percent CI = .71, .82) or Asian (OR = .73, 95 percent CI = .53, .99), but not for respondents who were non-Hispanic black (OR = .99; 95 percent CI = .86, 1.14) or Hispanic (OR = 1.14; 95 percent CI = .94, 1.40). In addition, the association of the requirement for written informed consent was greater among respondents who had graduated from college or technical school (OR = .79; 95 percent CI = .72, .88
for college/technical school graduates) than for non-high school graduates (OR = 1.06; 95 percent CI =.86, 1.31) (Ehrenkranz et al., 2009). Bogart and Tsoi noted a study of HIV testing in the San Francisco Department of Health Medical Care System that found the average monthly rate of HIV tests per 1,000 patient-visits increased by 4.38 (or 44 percent) over a 12-month period following elimination of a policy requiring patient written consent for testing. Populations facing the highest barriers to testing (such as men, the homeless, and the uninsured) experienced the highest increases in monthly HIV testing rates. The monthly average number of new positive HIV tests also increased, from 8.9 to 14.9 infections identified per month (Zetola et al., 2007, 2008).
del Rio cited a recent study that found that the probability of diagnosis of HIV-positive individuals in 2006 was 24.9 percent in states with opt-out consent laws compared with 19.9 percent in states with opt-in consent laws (April, 2009). Using these testing rates in a mathematical model,28 the study authors concluded that the mean lifespan of people with HIV was higher in opt-out compared with opt-in states (801.2 months compared with 792.1 months, respectively). Implementation of opt-out testing nationwide was estimated to produce 549,437 life-years saved over the lifetime of the current HIV-positive population (April, 2009).
In one of the largest studies of opt-out HIV testing to date, nontargeted opt-out HIV screening in an ED was associated with a modest increase in the number of patients newly diagnosed with HIV infection, compared with physician-directed diagnostic HIV testing. The prevalence of new HIV diagnoses in the opt-out phase (including those diagnostically tested) and in the diagnostic phase was 15 in 28,043 (.05 percent) and 4 in 29,925 (.01 percent) respectively. Nontargeted opt-out screening did not increase identification of patients earlier in the course of disease, however; the median CD4 cell count for those with new HIV diagnoses in the opt-out phase (including those diagnostically tested) and in the diagnostic phase was 69/µL and 13/µL, respectively, suggesting that patients in both groups were identified late in the course of disease (Haukoos et al., 2010).29
Studies on HIV testing in pregnant women have shown that an opt-out approach can increase testing among pregnant women, increase the number of HIV-infected women who are offered treatment, and reduce mother-to-child transmission (CDC, 2008c).
Cramer described the success of opt-out testing as part of the Expanded Testing Initiative (ETI), implemented in 2007. As part of this program, $111.2 million was allocated to 25 jurisdictions to target HIV testing to
FIGURE 5 Implementation of opt-out testing in health care settings by 65 state, territorial, and local health departments after the Expanded Testing Initiative (as of February 2008).
* = ≥ 80 percent increase.
NOTE: ED = emergency department, OB = obstetrics, STD = sexually transmitted diseases, Sub Abuse TX = substance abuse treatment, TB = tuberculosis.
SOURCE: Adapted from KFF and NASTAD, 2009.
African Americans with the goals of conducting 1.5 million HIV tests to identify 20,000 new HIV positive individuals (NASTAD, 2009). Figure 5 shows implementation of opt-out testing in health care settings by state, territorial, and local health departments as of February 2008, after the ETI was implemented.
Integration of pretest counseling and separate written informed consent for HIV testing into practice may be challenging for providers in terms of time and staffing needs. In his presentation on barriers to HIV testing in emergency departments, Jeremy Brown described the potential challenges of meeting state requirements for pretest counseling and informed consent in hectic emergency department settings, where providers often do not have much time to interface with patients. He noted that it used to be the case under Maryland law, for example, that more than 30 separate issues had to be discussed before an HIV test could be performed (Brown, 2008).30 This law has since been changed to simplify the testing process.
The Ethics of Opt-Out HIV Testing
In the mid-1980s, when HIV antibody tests became widely available, there was debate between those who asserted that the threat of the HIV epidemic justified wide-scale routine testing and those who saw in the new diagnostic assay a grave threat to the privacy of individuals with or at-risk for HIV infection. At that time, when there was little that medicine could offer people with HIV, and discrimination threatened to deny people with HIV the opportunity to work, to go to school, and to obtain life and health insurance, the HIV antibody test was viewed by many in the HIV/AIDS activist community, and by many ethicists, to have substantial risks that were not justified by the benefits of testing (Bayer and Edington, 2009; Gostin, 2006).
It was in this context that exacting standards of consent for HIV testing were developed. Careful pretest counseling was deemed essential to inform those who might be tested about both the risks and the benefits of testing. Individuals, it was argued, should be given the opportunity to provide explicit consent and to assert that they wanted to be tested. Such consent should be documented in a written form. That approach to diagnostic testing was very different from the approach to other clinical encounters where it was assumed that a request for care and treatment by patients entailed a willingness to be subject to appropriate diagnostic testing. But in the mid-1980s, AIDS was widely viewed as different from other medical conditions. The disease was considered “exceptional” and to require policies and practices that were “exceptional” (Bayer and Fairchild, 2006).
Dramatic transformations in therapeutic prospects for people with HIV disease, a less hostile social climate, and the recognition that upward of 21 percent of those with HIV infection remain unaware of their status have radically altered the context of HIV testing. Starting with availability of effective treatment and the fact that the effective clinical management of HIV infection requires early identification, and that the prevention of HIV transmission necessitates a reduction in the number of individuals who are unaware of their HIV status, some began to assert that the standards of consent that defined HIV testing in the earliest stages of the HIV epidemic should be rethought (Bayer and Fairchild, 2006).
The decision on the part of the CDC in 2006 and subsequent action by states to substitute opt-out for written consent to HIV testing sparked an important debate about the ethics of HIV testing and screening in clinical settings. The ethical challenge addressed in this more recent discussion is whether an opt-out approach to testing is compatible with fundamental principles of biomedical and public health ethics.
Proponents of moving to an opt-out approach to consent have argued that such a standard would facilitate testing and identification of indi-
viduals with HIV by reducing the provider burden of administration of HIV-specific written informed consent (Das-Douglas et al., 2008). In addition, by removing the assessment of sexual and drug using risk for HIV, routine testing may help to reduce the stigma associated with HIV testing (Earnshaw and Chaudoir, 2009). Advocates for opt-out HIV testing have also asserted that the ethics of public health warrants an approach to testing that, by increasing the proportion of the infected who know their status, could result in behavioral change and increased timely initiation of care, thus improving health outcomes for individuals diagnosed with HIV and reducing transmission (Gostin, 2006).
Opponents of the shift to opt-out testing have challenged the interpretation of available evidence that suggests that written consent is an impediment to expanded HIV testing. For example, it has been argued that there is insufficient evidence to make the claim that the interests of individuals and the public’s health are compromised by written consent. Furthermore, opponents of opt-out approaches have noted that such approaches begin with an assumption of an “informed right of refusal” where individuals would be told that they would be tested unless they refused, but would ultimately end with situations where individuals would be routinely tested without being told that that was the standard of care. Under those circumstances the “right of refusal” would in fact be dependent on foreknowledge about testing and of the right to say no. Such routinely undertaken testing would, it is argued, be voluntary in name only (ACLU, 2007). Others have questioned the value of an opt-out HIV testing framework unless access to care can be ensured for all individuals who are newly identified as HIV positive (Hanssens, 2007).
Conclusion
While further research is needed, available studies suggest that routine opt-out HIV testing can facilitate HIV testing, probably by reducing some of the administrative barriers to testing experienced by clinicians. Bodies considering adoption of opt-out HIV testing might consider the ethical pros and cons of opt-out testing that have been identified by ethicists and advocates.
CLOSING REMARKS
Expanded HIV testing would help to reduce the number of individuals in the United States who are unaware that they are infected, and thereby facilitate earlier care and better clinical outcomes for those individuals, as well as reduce HIV transmission. Several laws, policies, and procedures in settings where HIV tests are administered may impede expanded HIV test-
ing. The absence of programs and policies to support clinician education and training on HIV testing and to reduce HIV-related stigma and discrimination are also barriers to expanded HIV testing. There are evidence-based approaches to facilitate HIV testing that may be considered as part of an expanded HIV testing strategy. Before implementation of a program of expanded HIV testing, consideration should be given to whether individuals who are diagnosed as HIV positive can be provided timely access to care. Federal and state policies and private health insurance policies/practices that inhibit entry into clinical care or the provision of continuous and sustained clinical care for individuals with HIV will be explored in the committee’s second report.














































