7
Ischemic Heart Disease
This chapter describes the evaluation and management of ischemic heart disease, which has evolved significantly over the past decade. In particular, several clinical trials have documented the benefits of revascularization in patients with acute ischemic syndromes as well as the efficacy of medical therapy, including lifestyle modification in patients with stable coronary disease. A fundamental premise in establishing new listing criteria for ischemic heart disease disability is the linking of anatomic or structural evidence of coronary heart disease (CHD) with both functional impairment and severe anginal symptoms. A flow diagram has been introduced that depicts five pathways to meet listings, including clinical, standard exercise testing, stress imaging, and angiographic anatomic criteria, with one pathway specific for patients with prior coronary artery bypass graft and severe CHD. Because many patients with ischemic heart disease are unable to exercise, standard stress electrocardiographic criteria for ischemia (the sole determinant of objective ischemia assessment in prior cardiovascular disability listings) have been expanded significantly to encompass nonexercise modalities (including nuclear imaging and echocardiography provoked by pharmacologic vasodilator stress) to assess the presence of severe inducible ischemia that, when combined with severe angina (Canadian Cardiovascular Society Class III or IV) would meet a cardiovascular disability listing. Additionally, the criteria by which angiographic CHD meet a listing have been specified, and severe CHD is defined by greater than or equal to 50 percent left main
stenosis and/or greater than or equal to 70 percent proximal/mid stenoses in greater than or equal to two native arteries or bypass grafts. These updated criteria now provide a significantly enhanced and evidence-based approach for making disability determinations based on anatomic and functional criteria in patients with severe angina.
DESCRIPTION
Ischemia is defined as inadequate blood supply (circulation) to a local area due to blockage of the blood vessels supplying the area. Ischemic means that an organ (e.g., the heart) is not getting enough blood and oxygen. Ischemic heart disease, also called coronary heart disease (CHD) or coronary artery disease, is the term given to heart problems caused by narrowed heart (coronary) arteries that supply blood to the heart muscle. Although the narrowing can be caused by a blood clot or by constriction of the blood vessel, most often it is caused by buildup of plaque, called atherosclerosis. When the blood flow to the heart muscle is completely blocked, the heart muscle cells die, which is termed a heart attack or myocardial infarction (MI). Most people with early (less than 50 percent narrowing) CHD do not experience symptoms or limitation of blood flow. However, as the atherosclerosis progresses, especially if left untreated, symptoms may occur. They are most likely to occur during exercise or emotional stress, when the demand for the oxygen carried by the blood increases.
The discomfort experienced when the heart muscle is deprived of adequate oxygen is called angina pectoris. This is a clinical syndrome characterized by discomfort in the chest, jaw, shoulder, back, or arms that is typically aggravated by exertion or emotional stress and relieved promptly with rest or by taking nitroglycerin. Angina usually occurs in patients with CHD, but also can occur in individuals with valvular disease, hypertrophic cardiomyopathy, and uncontrolled hypertension. Infrequently, patients with normal coronary arteries may experience angina related to coronary spasm or endothelial dysfunction (Gibbons et al., 2002a).
Angina is classified using the Canadian Cardiovascular Society (CCS) scheme, which grades angina or an anginal equivalent (e.g., exertional dyspnea) based on a description of the level of activity that causes symptoms (Table 7-1). Class I is defined by angina that occurs with strenuous or rapid or prolonged exertion at work or recreation, but not with ordinary physical activity. Class I activities include chopping wood, climbing hills, cycling, aerobic ballet, ballroom (fast) or square dancing, jogging a 10-minute mile, rope skipping, skating, skiing, playing tennis or squash, and walking 5 miles per hour. Class II is defined by angina that slightly limits ordinary activity, such that angina is precipitated by walking or climbing stairs rapidly, walk-
TABLE 7-1 Canadian Cardiovascular Society Functional Classification of Angina
|
Class |
Definition |
Limitations |
|
I |
No limitation of ordinary activity. Angina occurs with strenuous, rapid, or prolonged exertion at work or recreation. |
Angina may occur with chopping wood, climbing hills, cycling, aerobic ballet, ballroom (fast) or square dancing, jogging a 10-minute mile, rope skipping, skating, skiing, playing tennis or squash, and walking 5 miles per hour. |
|
II |
Slight limitation of ordinary activity. |
Angina may occur with walking or climbing stairs rapidly, walking uphill, walking or climbing stairs after meals; in cold or in wind; under emotional stress; only during the first few hours after awakening; or with walking more than two blocks on level ground and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. |
|
III |
Marked limitation of ordinary physical activity. |
Angina may occur with walking one or two blocks on level ground, and climbing one flight of stairs in normal conditions and at normal pace, playing a musical instrument, performing household chores, gardening, vacuuming, walking a dog, or taking out the trash. |
|
IV |
Inability to perform any physical activity without discomfort. |
Angina may occur at rest. |
|
SOURCE: Adapted from Goldman et al., 1981. |
||
ing uphill, walking or climbing stairs after meals; in cold or in wind; under emotional stress; only during the first few hours after awakening; or with walking more than two blocks on level ground and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. Class III is defined by marked limitation of ordinary physical activity such that angina is precipitated by walking one or two blocks on level ground, climbing one flight of stairs in normal conditions and at normal pace, playing a musical instrument, performing household chores, gardening, vacuuming, walking a dog, or taking out the trash. Class IV is defined by inability to carry on any physical activity without discomfort; anginal syndrome may be present at rest (Campeau, 1976, 2002; Goldman et al., 1981). As many as 3 to 4 million Americans may have silent ischemia, or ischemia without pain, or a heart attack without prior warning. People with angina may also have undiagnosed episodes of silent ischemia. Furthermore, those who have had heart attacks or individuals with diabetes are at risk for developing silent ischemia.
EPIDEMIOLOGY
On the basis of data from the National Health and Nutrition Examination Survey (NHANES) for the period 2003 to 2006, an estimated 17.6 million Americans age 20 or older have CHD, with an overall prevalence of 7.9 percent (9.1 percent in men and 7 percent in women). The overall prevalence of MI is 3.6 percent (4.7 percent in men and 2.6 percent in women). The estimated annual incidence of MI is 935,000, which includes 610,000 new and 325,000 recurrent infarctions. The overall prevalence of angina pectoris is 4.6 percent, with age-adjusted prevalence higher in women than men. CHD accounts for more than half of all cardiovascular events in men and women under age 75. The lifetime risk of developing CHD after age 40 is 49 percent for men and 32 percent for women (Lloyd-Jones et al., 2010).
CHD is the leading cause of death in both men and women. It caused one of every six U.S. deaths in 2006; CHD mortality was 425,425, and MI mortality was 141,462. Approximately every 25 seconds, an American will experience a coronary event, and approximately every minute a death will be attributed to a coronary event. Approximately every 34 seconds, an American will have an MI and 15 percent will die of it (Lloyd-Jones et al., 2010).
In addition, in 2006, 1,115,000 inpatient diagnostic cardiac catheterizations were performed as well as 661,000 inpatient percutaneous coronary interventions (PCIs) and 253,000 coronary artery bypass surgery (CABG) procedures. The estimated direct and indirect cost of coronary heart disease for 2010 is $177.1 billion (Lloyd-Jones et al., 2010).
DIAGNOSTIC CRITERIA AND METHODS
CHD can be diagnosed in several ways. Patients with documented (prior) MI or coronary artery revascularization (either with PCI or CABG) have CHD. Moreover, the presence of typical angina suggests a clinical diagnosis of CHD, but most often requires confirmation by additional diagnostic tests, such as coronary angiography. However, this test is an invasive and relatively costly procedure associated with a low, yet definite, risk of an adverse event. Coronary angiography is most often performed following an abnormal stress test or in the setting of an acute coronary syndrome (unstable angina or heart attack) in individuals who are candidates for revascularization (either by PCI or CABG).
Exercise Stress Tests
Stress testing is usually performed using an exercise tolerance test (ETT) with a treadmill or, occasionally, with bicycle ergometry. The most
commonly applied treadmill protocol is the Bruce protocol, with the modified Bruce, Naughton, Balke (Balke-Ware), Wilson, Taylor, or “ramp” protocols used in some patients. Noting the specific protocol is important because protocols differ by the rate at which the workload increases. The workload achieved during a test for any given protocol can be estimated in units of metabolic equivalents of task (METs) from published nomograms (Fletcher et al., 2001; Thompson et al., 2010). Completion of the first stage of the Bruce protocol is equivalent to 5 METs.
Exercise testing can be performed with electrocardiogram (ECG) monitoring alone or combined with a cardiac imaging test: single photon emission computed tomography (SPECT), positron emission tomography (PET), or with echocardiography imaging. Each modality has specific criteria for an abnormal test. An abnormal exercise ECG is defined by ST-segment displacement, usually an ST-segment depression greater than or equal to 1 mm, measured 0.08 seconds after the J-point, that is horizontal or downsloping (Gibbons et al., 2002b). ST-segment elevation greater than or equal to 1 mm in leads without Q waves occurs infrequently, but this is also considered an abnormal response. An abnormal SPECT or PET study is defined by a perfusion defect (Klocke et al., 2003), with a defect that is the same with rest or exercise (a fixed defect) suggesting infarction. An abnormal exercise echocardiogram is a wall motion abnormality (Pellikka et al., 2007). Usually, such an abnormality that develops or worsens during exercise represents ischemia, whereas a wall motion abnormality that is present at rest and unchanged (fixed) with exercise indicates infarction. The presence of either ischemia or infarction on a stress-imaging study is consistent with the diagnosis of CHD in a patient with angina symptoms.
Stress test results are commonly reported in a dichotomous manner: normal or abnormal, positive or negative for ischemia, and so on. However, for a positive test, the degree of severity of abnormality provides additional information. All stress-testing modalities are limited by their false-positive results (abnormal stress test result, but CHD is not present) and false-negative results (normal stress test result, but CHD is present). Due to variability in image interpretation and imaging artifacts, isolated small mild abnormalities on stress SPECT or stress echocardiogram may be false-positive results, but the more severely abnormal results are more likely to represent a true-positive test (i.e., CHD is present). Additionally, a more severely abnormal test result is associated with an increased likelihood of multivessel CHD and a worse prognosis.
An abnormal test result at a low workload is one of the most reliable indications of a high likelihood of multivessel CHD (McNeer et al., 1978). Other variables associated with multivessel CHD or worse prognosis are shown in Table 7-2 (Dubach, 1988; Gibbons et al., 2002a,b; Klocke et al., 2003; McNeer, 1978; Pellikka et al., 2007). Earlier versions of the CHD
TABLE 7-2 Other Variables Associated with Multivessel CHD or Worse Prognosis
|
ETT |
SPECT |
Echocardiogram |
|
Magnitude ST-segment depression |
Number of perfusion defects |
High WMI score |
|
Number of leads ST-segment depression |
High SSS |
Decrease in LVEF |
|
Duration ST-segment depression in recovery period |
High SRS |
Number of segments with WMA |
|
Angina (especially if limiting symptom) |
TID |
n/a |
|
Decrease in systolic BP below baseline |
n/a |
WMA involving multiple coronary artery territories |
|
Chronotropic incompetence |
Defects involving multiple coronary artery territories |
Rest LVEF < 35 percent |
|
Abnormal heart rate recovery |
Rest LVEF at < 35 percent |
Increase in ESV |
|
High-risk Duke treadmill scorea |
T ℓ 201 lung uptake |
n/a |
|
a The Duke treadmill score can be calculated as follows: Duration (minutes Bruce protocol) – 5 × magnitude ST-segment deflection (mm) – angina index. NOTE: BP = blood pressure; CHD = coronary heart disease; ESV = end systolic volume; ETT = exercise tolerance test; LVEF = left ventricular ejection fraction; SPECT = single-photon emission computerized tomography; SRS = summed reversibility score; SSS = summed stress score; TID = transient ischemic dilatation; WMA = wall motion abnormality; WMI = wall motion index. SOURCES: Dubach et al., 1988; Gibbons et al., 2002a,b; Klocke et al., 2003; McNeer, 1978; Pellikka et al., 2007. |
||
listings included detailed descriptions of interpretation of the exercise ECG. However, most reports in patient records do not provide these descriptors, but rather they simply categorize the exercise ECG as being normal or abnormal, positive or negative. Exercise duration is included in most reports. Similarly, for the stress-imaging procedures, the results can be characterized most accurately by applying the 17-segment model advocated by the American Heart Association (AHA) (Cerqueira et al., 2002). This model can be used to develop a summed stress score or summed reversibility score for SPECT imaging and a wall motion index score for echocardiography. These scoring systems have been validated as accurate tools for prognostic purposes. However, this information is not usually included in reports. Instead, the anatomical location of the defects (reflecting coronary artery distribution) and exercise duration are most often included in exercise imaging reports.
Nuclear and echocardiographic imaging can localize the site of ischemia, although the correlation with angiographic CHD is not perfect. The assignment of coronary artery territories by imaging to anatomical CHD at angiography is as follows: anterior/anteroseptal—left anterior descending artery; inferior/inferoseptal—right coronary artery; lateral—circumflex artery (Cerqueira et al., 2002). Involvement of the lateral territory may be further specified as anterolateral or inferolateral. The artery supplying the apex is variable. For this reason, defects involving the apex alone are not assigned to a coronary artery territory. In addition to multiple coronary artery territories, other markers shown by imaging usually represent extensive ischemia. For nuclear imaging this marker is transient ischemic dilatation, or poststress dilatation of the left ventricle. For echocardiography these markers include a decrease in left ventricular ejection fraction (LVEF) or an increase in end systolic volume between rest and exercise.
In contrast to the imaging modalities, the exercise ECG cannot localize the site of ischemia. Thus, for application of the exercise ECG, there cannot be a requirement for involvement of greater than or equal to two coronary artery territories (for further discussion, refer to the following paragraph, as well as to item 3 in the section on concluding concepts and Recommendation 7-3). Nonetheless, studies have shown that the development of ischemic ECG changes at a low workload is associated with a high likelihood of multivessel CHD (McNeer, 1978). Another variable that can be measured during exercise testing and that occurs less commonly than ECG changes, but also reflects multivessel CHD and a poor prognosis, is a decrease in systolic blood pressure at peak exercise greater than or equal to 10 mm Hg below the baseline blood pressure (Dubach et al., 1988).
To facilitate application of the listings in a uniform manner across the stress-testing modalities, a claimant will meet a listing if exercise capacity is limited (less than or equal to 5 METs), combined with objective evidence of CHD. Given the poor specificity of single mild abnormalities on SPECT or echocardiographic imaging, the presence of defects involving less than or equal to two coronary artery territories is required to increase the likelihood that the claimant has CHD before being granted disability. This requirement for involvement of less than or equal to two-vessel CHD is analogous to the coronary angiogram criteria.
The criteria to meet a listing through the use of an exercise stress test (standard treadmill test, nuclear SPECT or PET, echocardiography) include exercise duration less than or equal to 5 METs and objective evidence for significant ischemia defined in Table 7-3.
Pharmacologic (Nuclear and Echocardiography) Stress Tests
Pharmacologic stress testing using SPECT, PET, or echocardiographic imaging is reserved for patients who are either unable to perform dynamic
TABLE 7-3 Criteria to Meet a Listing Through the Use of an Exercise Stress Test
exercise or unable to achieve at least 85 percent of the age-predicted maximal heart rate with exercise, which is the effort level required to achieve adequate sensitivity to detect coronary artery stenosis capable of causing angina (Klocke et al., 2003; Pellikka et al., 2007). Pharmacologic stress does not consistently cause angina or ECG changes of ischemia, so only the imaging results are diagnostic. Pharmacologic agents are administered intravenously in place of dynamic exercise stress, and the resulting perfusion or wall motion response is compared with the resting state and is interpreted using the same criteria for perfusion defects and wall motion abnormalities listed above for dynamic exercise.
The most frequently used pharmacologic stress agents for SPECT and PET are the vasodilators dipyridamole, adenosine, and regadenoson, which increase blood flow through the coronary arteries, but only modestly increase heart rate in most patients. Many patients experience chest discomfort during the administration of these agents, which should not be interpreted as angina. The agents create differences in blood flow between coronary arteries that have high-grade blockages and normal arteries, which result in perfusion defects that can be detected using radioactive imaging.
The most frequently used pharmacologic agent in stress echocardiography is dobutamine, a positive inotropic agent that increases the force or energy of muscular contractions and increases heart rate and blood pressure. Dobutamine is administered intravenously in increasing doses until the patient reaches 85 percent of the maximal age-predicted heart rate. Atropine may also be required in some patients. If the patient does
not achieve 85 percent of the heart rate response, the resulting images may underestimate the presence of CHD. The positive inotropic effect and increases in heart rate and blood pressure may cause angina and result in abnormal wall motion at peak stress in portions of the heart muscle supplied by coronary arteries with high-grade blockages. Dobutamine may also be used for SPECT imaging.
Coronary CT Angiography
Coronary computed tomography (CT) angiography is an imaging technique during which an iodinated contrast dye is injected through a peripheral vein and images of the coronary arteries are taken using a CT system. It provides images of the coronary arteries similar to those obtained using coronary angiography, during which the dye is injected directly into the coronary arteries using an arterial catheter. It is most useful in patients with an intermediate risk of coronary heart disease. In patients with extensive calcium deposits or prior coronary artery stents, detection of stenosis is difficult (Budoff et al., 2006). Tremendous progress has been made in changing this technique, but lack of standardization and unresolved technical issues do not allow it to be used in place of coronary angiography as a basis for determining disability (Mark et al., 2010; Miller et al., 2008).
TREATMENT
Comprehensive management of angina and stable CHD entails multiple therapeutic approaches, including the following:
-
Identification and treatment of associated diseases that can precipitate or worsen angina and ischemia;
-
Cardiac risk factor identification and intervention;
-
Application of pharmacological and nonpharmacological interventions for secondary prevention;
-
Pharmacological and symptomatic management of angina and ischemia; and
-
Myocardial revascularization with PCI or CABG surgery, when indicated.
A multidimensional management approach integrates all of these considerations, often simultaneously, in each patient. Among pharmacotherapies, three drug classes have been demonstrated to reduce mortality and morbidity in patients with stable CHD and preserved left ventricular (LV) function: aspirin, angiotensin-converting enzyme (ACE) inhibition, and effective lipid lowering. Beta-blockers have been shown to reduce mortal-
ity in patients with prior MI (CAPRICORN Investigators, 2001). Other therapies such as nitrates, beta-blockers, calcium channel blockers, and ranolazine have been shown to improve angina and exercise performance and to reduce ischemia, but have not been proven to reduce mortality in patients with stable CHD.
Clinical practice guidelines for the diagnosis and treatment of chronic stable angina (Fraker et al., 2007; Gibbons et al., 2002a), unstable angina/non-ST-segment elevation myocardial infarction (Anderson et al., 2007), and ST-segment elevation myocardial infarction (Antman et al., 2004; Kushner et al., 2009), have been jointly published by AHA and the American College of Cardiology (ACC). These guidelines detail the indications and timing of medical therapy (including lifestyle modification) and revascularization with PCI and/or CABG (Eagle et al., 2004) and provide guidance for secondary prevention that includes risk factor reduction (Smith et al., 2006). Although revascularization has specific indications, treatment with medical therapy, lifestyle modification, and risk factor reduction is recommended across the spectrum of CHD in both stable and unstable patients and following a coronary event or revascularization.
Recent Advances
The most recent advance in medical therapy consists of the introduction of ranolazine (Chaitman, 2006; Chaitman et al., 2004; Morrow et al., 2007). Nonpharmacologic treatments include spinal cord stimulation (Taylor et al., 2009) and enhanced external counterpulsation (EECP) (Akhtar et al., 2006; Michaels et al., 2004; Soran et al., 2006) for the treatment of angina and ischemia. Advances in revascularization include development of drug-eluting stents (Novack et al., 2009), the introduction of percutaneous support devices in patients undergoing PCI (Goldstein et al., 1998), and increased use of off-pump techniques, as well as minimal access and robotic procedures in patients undergoing CABG (Poston et al., 2008; Sabik et al., 2002). Interest and experience also have been growing in the performance of hybrid revascularization procedures (Stahl et al., 2002) using a collaborative approach between interventional cardiologists and cardiothoracic surgeons.
Ranolazine is the newest antianginal agent approved by the Food and Drug Administration and the first new drug class for angina since calcium channel blockers (CCBs) were introduced 30 years ago. Ranolazine acts by reducing intracellular calcium overload in ischemic myocytes by inhibiting late inward sodium current entry. The net effect of reduced late inward sodium current is a reduction in LV wall tension and myocardial oxygen demand, thereby reducing angina and ischemia. Ranolazine increases exercise tolerance in patients with stable angina, reduces episodes of recurrent
ischemia, and provides additional antianginal benefit in patients who are already on intensive antianginal therapy with beta-blockers and CCBs. While multiple nonspecific side effects of ranolazine have been reported, the drug is well tolerated in clinical practice. The most common side effects are dizziness (6.2 percent), headache (5.5 percent), constipation (4.5 percent), and nausea (4.4 percent), which are more commonly observed at the 1,000 mg twice a day dose. Mean QT prolongation noted in clinical trials ranges from 6 to 8 milliseconds; the clinical relevance of the modest QT prolongation that occurs in a dose-related manner is unclear, but there has been no increased risk of a serious proarrhythmic effect (torsades de pointes) or sudden cardiac death reported in a large, placebo-controlled trial of more than 6,500 patients (MERLIN Trial) (Morrow et al., 2007).
EECP is an alternative treatment for patients with refractory angina. It is generally administered as 35 sequential treatments (1 hour daily; 5 days per week) over 7 weeks. EECP was shown to increase the time to ST-segment depression during exercise testing, reduce angina, and improve health-related quality of life for at least 1 year in a randomized, double-blind study of patients with chronic stable angina (Soran et al., 2006). EECP does not reduce ischemia on myocardial perfusion imaging, and the mechanisms underlying its effects are poorly understood.
Side Effects of Treatments
Nitroglycerin and nitrates can cause vasodilation-induced headache, a decrease in blood pressure, and, more rarely, severe hypotension with bradycardia. The vasodilation by nitroglycerin may be markedly exaggerated and prolonged in the presence of the phosphodiesterase inhibitors sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis), so these agents should not be used concurrently with nitrates.
Most of the adverse effects of beta-blockers occur as a consequence of the known properties of these drugs and include cardiac effects (e.g., severe sinus bradycardia, sinus arrest, reduced LV contractility), bronchoconstriction, fatigue, mental depression, nightmares, gastrointestinal upset, sexual dysfunction, intensification of insulin-induced hypoglycemia, and cutaneous reactions. Lethargy, weakness, and fatigue may be caused by reduced cardiac output or may arise from a direct effect on the central nervous system. Bronchoconstriction results from blockade of beta2 receptors in the tracheobronchial tree. As a consequence, reversible obstructive lung disease (e.g., asthma) may be considered as relative contraindications to beta-blockers, even to beta1-selective agents (Egred et al., 2005).
Calcium channel blockers are potent vasodilators, which may lead to dizziness, hypotension, and reflex tachycardia—particularly with some dihydropyridines. Peripheral edema can occur, usually with the dihydropyri-
dines. Both verapamil and diltiazem can cause bradycardia or conduction disturbances, particularly if coadministered with beta-blockers. Diltiazem and verapamil may exacerbate or precipitate heart failure in patients with reduced LV ejection fraction.
Trends in Morbidity and Mortality
Data from the Framingham Heart Study showed the overall death rates from CHD decreased by 64 percent from 1950 to 1999. From 1996 to 2006, the annual death rate due to CHD declined by 36.4 percent, and the actual number of deaths declined by 21.9 percent (Lloyd-Jones et al., 2010). An analysis of NHANES CHD mortality data between 1980 and 2000 revealed that approximately 47 percent of the decline in mortality could be explained by the use of medical and surgical treatment (including secondary prevention therapies after myocardial infarction or revascularization, initial treatment of MI or unstable angina, treatment of heart failure, revascularization for chronic angina, and other therapies, including antihypertensive and lipid-lowering primary prevention strategies), whereas approximately 44 percent of the mortality decline was attributed to changes in risk factors (including lower total cholesterol, systolic blood pressure, and smoking prevalence, and decreased physical inactivity) (Lloyd-Jones et al., 2010).
According to data from the National Registry of Myocardial Infarction, in-hospital mortality following acute MI declined from 11.2 percent in 1940 to 9.4 percent in 1999 (Lloyd-Jones et al., 2010). Moreover, a recent analysis of the Medicare fee-for-service population revealed that the adjusted hospitalization rate for acute MI declined by 5.8 percent per year from 2002 through 2007, although there was a slower rate of decline in African American patients compared with white patients (Chen et al., 2010).
DISABILITY
Many patients with CHD and chronic stable angina have a reduced functional capacity, particularly those with CCS Class III or IV angina. Revascularization has been shown to be more effective in reducing angina than maximal medical therapy, at least initially (Boden, 2007b), and patients who are not candidates for revascularization may experience disabling symptoms. About 30 percent of patients never return to work after coronary revascularization, and 15 to 20 percent rate their own health as fair or poor despite revascularization (Writing Group for the BARI Investigators, 1997). In addition, health expenditures from lost productivity from morbidity and mortality from CHD in 2010 are estimated to be at $11.3 billion and $69.8 billion, respectively (Lloyd-Jones et al., 2010).
Disability and Work
Only a modest relationship exists between the extent of anatomic CHD or myocardial ischemia and the severity of cardiac symptoms and functional impairment. In patients with a recent MI, the size or location of the MI, a history of previous MI, and poor ventricular function predict functional impairment and return to work after the event (Herlitz et al., 1994; Maeland and Havik, 1986; Rost and Smith, 1992; Smith and O’Rourke, 1988), but these medical factors account for only part of observed disability in these patients. Measures of cardiac functional capacity, such as exercise treadmill performance, correlate well with the ability to participate in strenuous activities, but they correlate only weakly with the ability to perform more routine tasks of daily living and less strenuous work-related activities (Neill et al., 1985).
Angina and fatigue are the most common symptoms limiting sustained work activities (Mital et al., 2004). Both can be significantly improved in most patients using standard medical and surgical treatments. However, a recent review of the literature found that 90 percent of patients experience improvement in symptoms following CABG, but only about 50 percent return to work (Mital et al., 2004). Although treatments are usually successful in decreasing symptoms and improving exercise tolerance, they do not necessarily result in a return to premorbid activities such as work. In short, disability is only partly explained by the severity of CHD and its symptoms.
Higher socioeconomic status (Abbott and Berry, 1991; Maeland and Havik, 1986; Rannevik, 1988), more education (Herlitz et al., 1994; Hlatky et al., 1986; Maeland and Havik, 1986, 1987; Mark et al., 1992; Speziale et al., 1996), younger age (Boudrez et al., 1994; Speziale et al., 1996), and looking forward to returning to work (Boll et al., 1987; Mittag et al., 2001) predict return to work after an acute coronary event. Alternatively, having a job that is physically strenuous or unsatisfying predicts a lower likelihood of returning to work (Boudrez and De Backer, 2000; Drory et al., 2005; Froom et al., 1999; Myrtek et al., 1997; Sellier et al., 2003; Shanfield, 1990; Wenger et al., 1985).
Psychological and social factors also both predict return to work and eventual disability (Cay and Walker, 1988; Maeland and Havik, 1987; Soderman et al., 2003), sometimes even better than the severity of cardiac disease (de Jonge et al., 2006; Sullivan et al., 1997). Depression is one of the most important noncardiac predictors of functional impairment and disability in patients with heart disease (Papakostas, 2009). In 2004, the World Health Organization found that depression was the leading cause of years lost due to disability for both men and women worldwide (WHO, 2008). When both depression and heart disease are present, their effects
on functional status are at least additive. Thus, a claimant who does not quite meet the cardiovascular listings yet is depressed may nevertheless be disabled (Carney and Freeland, 2008).
Work Disability and Patients with CHD
Age, gender, education level, physical work capacity, and type of occupation, as well as psychosocial factors, play a critical role in whether a person returns to work after CHD events (Mital et al., 2004). The determinants of return to work are complex and are not necessarily determined by cardiac findings alone. Persons who have been unemployed for an extended period of time have a more difficult time integrating into the workforce. Cardiac rehabilitation programs can address the physical and psychosocial matters necessary to return to work (Wenger et al., 1995). However, these findings have been challenged, suggesting that this depends on the services that such programs offer with respect to occupational and psychological services.
It has been reported that following CABG, only 30 to 40 percent of patients reported that “heart problems” were the reason for not returning to work (Mital et al., 2004). The factors identified as determining return to work are as follows:
-
Demographic (age, education, predictability of income);
-
Sociological (reaction or attitude to disability, attitude of significant others toward disability);
-
Vocational (predictability of employment status, attitude toward work);
-
Medical (degree of severity of disability, presence of secondary disease); and
-
Psychosocial (motivation to work, adaptability to change, intelligence) (Safilious-Rothchild, 1970).
It has also been documented that return to work depended on educational level attained: 78 percent of those with a college degree, 65 percent of those who completed technical school, 62 percent with a high school diploma, and 40 percent with elementary school education returned to work (Wenger et al., 1985).
One study reported that 85 to 90 percent of patients returned to work within 3 months after an MI (Froelicher et al., 1994). Women who had previously worked, older workers, blue-collar workers with physically strenuous jobs, and people with psychiatric problems had lower rates of returning to work (Shanfield, 1990). Social factors, in addition to medical problems, have been found to be major determinants of return to work.
Occupational work evaluation programs that use simulated work conditions to identify physical and psychological workplace stresses may have a role for disability evaluations, but others suggest that standard clinical testing is sufficient to identify ability to work (Dennis, 1990; Mital et al., 2004). The work simulation consisted mostly of upper arm dynamic work simulations, yet most production and service jobs involve physical movement, postural changes, carrying, and exertion over prolonged periods of time and would therefore require different simulation activities for complete work assessment (Mital et al., 2004).
The most important and decisive factors that influence return to work after a cardiac event are psychological in nature, and availability of disability benefits cannot be ignored (Mital et al., 2004). It has been reported that when patients in the coronary care unit were asked after their MI if they thought they would return to work by 6 months, their responses were highly predictive of actual return, whereas those patients who stated that they did not think they would return to work actually did not (Sivarajan and Newton, 1984). Others have found similar results in patients who were asked prior to CABG about their desire to return to work (Boll et al., 1987).
One approach used in research is the Sickness Impact Profile (SIP), which assesses a number of different domains of how sickness impacts activities today (Bergner et al., 1981). SIP is a 136-item self- or interviewer-administered, behaviorally based, health status questionnaire. Everyday activities in 12 categories are measured. The 12 categories are as follows:
-
Sleep and rest;
-
Emotional behavior;
-
Body care and movement;
-
Home management;
-
Mobility;
-
Social interaction;
-
Ambulation;
-
Alertness behavior;
-
Communication;
-
Work;
-
Recreation and pastimes; and
-
Eating.
Respondents endorse items that describe themselves and are related to their health. The SIP is scored and weighted according to the number and type of items endorsed. Scoring can be done at the level of categories and dimensions as well as at the total SIP level. Both physical and psychosocial domains as well as a total score are provided. This may be worth consid-
ering in research studies of disability to determine how such SIP scores correlate with standard exercise testing to establish capacity and provide a functional measure below which a person would be considered disabled.
In summary, there appears to be no single measure or combination of measures to determine fitness for any work. Given the combination of physical and psychological demands of work, the decision to return to work is likely dependent to a large extent on alternatives available to the patients. The extent to which individuals seek disability insurance after one or more cardiac events is a complex question in need of careful study.
Disability and Functional Limitation
Function with respect to cardiac disease is optimally assessed when the cardiovascular system is subjected to either physical or emotional stress testing; hence, numerous well-known stress-testing methods (both physical and emotional) have been developed, such as the step, bicycle ergometer, and exercise tolerance tests. More recently, the 6-minute walk test has evolved, either because the patient population was considered too frail to embark on a treadmill test or because of the unavailability of appropriate equipment. It is used most frequently in patients with heart failure (ATS, 2002).
Whether a graded exercise test correlates with the patient’s everyday activities such as walking and housework has been evaluated. Patients’ exercise performance was evaluated using a Naughton protocol, which provides a wide range of exercise durations to avoid submaximal exercise in patients with very low exercise tolerance. Exercise was stopped when the symptomatic endpoints—either angina or inability to continue to exercise because of extreme fatigue—were reached. Ventricular tachycardia and hypotension were other endpoints, and each patient achieved maximal effort according to his or her own symptoms (Neill et al., 1985).
Although this study aimed to identify limitations in self-care activities of daily living, it did not address limitations in performance of a minimal amount of work. It was concluded that low-exercise capacity due to angina does not necessarily prevent patients from carrying out their routine avocational activities. Further advancement in the medical management of CHD since 1985 is likely to better control symptoms of ischemia. It was also concluded that interventions to restore exercise capacity to normal might have little or no impact on behavior because there are many nonmedical determinants of adequate function for work (Neill et al., 1985).
Ischemic Heart Disease in Children
Ischemic heart disease in children is often due to structural anomalies of the proximal coronary arteries, coronary fistulae, Kawasaki disease, or injury to the coronary arteries during cardiac surgery. Ischemia may be evaluated using stress or resting echocardiography, radionuclide perfusion studies, or cardiac magnetic resonance imaging, but two abnormalities in two territories are not necessary for diagnosis of ischemia in children. Treatment of ischemia may involve surgical or cardiac interventional approaches.
CURRENT LISTING
The current listing for ischemic heart disease was updated in 2006 (72 FR 2312) when the cardiovascular Final Rule for Revised Medical Criteria for Evaluating Cardiovascular Impairments went into effect, replacing the previous 1994 listing (59 FR 55874). See Boxes 7-1 and 7-2 for the current adult and children ischemic heart disease listings.
CONCLUDING CONCEPTS
-
Treatment of CHD can dramatically improve a claimant’s functional status. For instance, a claimant may have undergone medical testing with results that would meet the listing, but then may undergo coronary revascularization with complete resolution of ischemia and no functional limitations. Therefore, any claimant who has undergone revascularization should be reassessed 3 months or more following the revascularization procedure to determine his or her functional limitations. Similar improvements could also occur with optimal medical therapy, but less frequently than following revascularization.
-
Certain tests more accurately identify the presence of CHD than others. Functional limitation can be more accurately assessed by objective testing than by subjective symptoms. Consequently, there is a hierarchy of preferred tests. Specifically, a claimant may have results from both stress testing and cardiac catheterization (and coronary angiography) that could be discrepant. For instance, an exercise test may show severe ischemia, which alone might meet the listing criteria, but at catheterization minimal disease may be evident. The findings from catheterization should override the findings from a stress test. The same issue applies to the results of stress imaging as opposed to the stress electrocardiogram. In terms of determining functional limitation, objective testing performed on the
|
BOX 7-1 Current Adult Listing for Ischemic Heart Disease 4.04 Ischemic heart disease, with symptoms due to myocardial ischemia, as described in 4.00E3–4.00E7, while on a regimen of prescribed treatment (see 4.00B3 if there is no regimen of prescribed treatment), with one of the following:
OR
OR
SOURCE: SSA, 2008a. |
|
BOX 7-2 Current Childhood Listing for Ischemic Heart Disease What is ischemic heart disease (IHD) and how will we evaluate it in children? IHD results when one or more of your coronary arteries is narrowed or obstructed or, in rare situations, constricted due to vasospasm, interfering with the normal flow of blood to your heart muscle (ischemia). The obstruction may be the result of an embolus, a thrombus, or plaque.When heart muscle tissue dies as a result of the reduced blood supply, it is called a myocardial infarction (heart attack). Ischemia is rare in children, but when it occurs, its effects on children are the same as on adults. If you have IHD, we will evaluate it under 4.00E and 4.04 in part A. SOURCE: SSA, 2008b. |
-
treadmill should override symptom criteria (CCS III) whenever the stress-testing information and symptom criteria are discrepant. For instance, if the records indicate that a claimant has functional Class IV symptoms, but he or she then performs a stress test and exercises for 10 minutes, the results of the stress test should apply.
-
Although there is not a perfect correlation between functional limitation and symptoms and the extent of CAD, the claimant with three-vessel CAD will be much more likely to be limited by his or her CHD than a claimant who may have a 70 percent stenosis in a single branch vessel. Thus, the committee has recommended requiring that a claimant have at least two-vessel CAD by angiography. Recognizing that not all claimants will have undergone coronary angiography, the requirement for two coronary territories by stress imaging makes the application of the noninvasive criteria somewhat analogous to the invasive criteria.
-
Medical therapy is the cornerstone of treatment for patients with CHD no matter whether revascularization with PCI or CABG is performed. Effective lifestyle interventions for CHD include smoking cessation (Critchley and Capewell, 2003), dietary intervention (de Lorgeril et al., 1999; Leren, 1970), and exercise (O’Connor et al., 1989; Oldridge et al., 1988). Effective pharmacologic interventions include aspirin (Antithrombotic Trialists’ Collaboration, 2002), beta-blockers (Lau et al., 1992; Yusuf et al., 1985), ACE inhibitors (Al-Mallah et al., 2006; Khalil et al., 2001), and statins (Baigent et al., 2005), which may reduce mortality among patients with CHD. Calcium channel blockers, long-acting nitrates, and ranolazine decrease angina symptoms and myocardial ischemia, but have not been shown to decrease mortality or MI in CHD patients.
ACC, AHA, and CCS recommend comprehensive lifestyle and pharmacologic interventions with specific risk factor targets (Antman et al., 2008; Gibbons et al., 2002a; Smith et al., 2006), although most cardiovascular clinical trials have tested a single intervention. The multiple simultaneous lifestyle and pharmacologic interventions (referred to as “optimal medical therapy”) tested in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial (Boden et al., 2007b) exemplify the therapeutic model for routine clinical practice (Table 7-4).
FINAL CONCLUSIONS AND RECOMMENDATIONS
We have concluded that the extent of coronary artery disease alone, as assessed by coronary angiography, in the absence of symptoms does not render an applicant disabled at the listings level, and that evidence of functional limitations should also be required to meet the listings (see Figures 7-1 and 7-2).
RECOMMENDATION 7-1. The committee recommends that the definitive diagnosis of coronary heart disease (diagnosed by documented prior myocardial infarction OR prior coronary revascularization OR specific criteria on exercise or stress-imaging tests OR coronary angiography) in patients with Canadian Cardiovascular Society Class III or IV angina or anginal-equivalent symptoms be coupled with a functional limitation to meet a listing.
RECOMMENDATION 7-2. The committee recommends that one of four functional limitations be present to meet a listing:
-
Clinical: Documentation of three separate ischemic episodes requiring unplanned hospitalization (inpatient or observation status), each requiring revascularization (three separate percutaneous coronary intervention [PCI] procedures or two PCI and one coronary artery bypass graft [CABG] procedure) if amenable to revascularization, within a consecutive 12-month period;
OR
-
Exercise stress test (with or without imaging): Ischemic response defined by ST-segment depression of greater than or equal to 1 mm measured 0.08 seconds after the J-point that is horizontal or downsloping in configuration or ST-segment elevation greater than or equal to 1 mm in leads without Q
TABLE 7-4 Optimal Pharmacologic Therapy Based on the COURAGE Triala
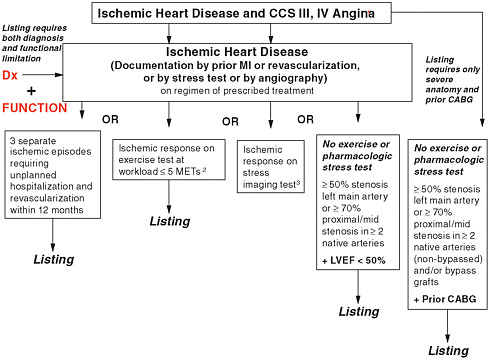
FIGURE 7-1 Coronary heart disease listings.
NOTE: CABG = coronary artery bypass graft; CCS = Canadian Cardiovascular Society; Dx = diagnosis; LVEF = left ventricular ejection fraction; METs = metabolic equivalents of task; MI = myocardial infarction.
1 Defined in report: Canadian Cardiovascular Society Class III or IV.
2 Defined in report: See Recommendation 7-2.
3 Defined in report: See Recommendation 7-2.
-
waves or fall in systolic blood pressure greater than or equal to 10 mm Hg below resting systolic blood pressure at a workload of less than or equal to 5 metabolic equivalents of task;
OR
-
Stress-imaging test: Ischemic response with either exercise or pharmacologic vasodilator stress indicated by greater than or equal to two reversible and/or fixed regional myocardial per-fusion defects during nuclear testing and transient ischemic dilation or resting left ventricular ejection fraction (LVEF) less than 50 percent, OR greater than or equal to two reversible and/or fixed regional wall motion abnormalities and either a fall in LVEF OR resting LVEF less than 50 percent;
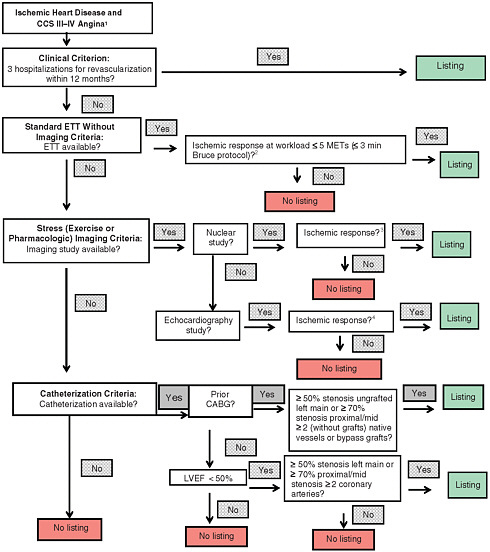
FIGURE 7-2 Coronary heart disease listings: Ischemic heart disease ladder flow diagram.
NOTE: CABG = coronary artery bypass graft; CCS = Canadian Cardiovascular Society; ETT = exercise tolerance test; LVEF = left ventricular ejection fraction; METs = metabolic equivalents of task.
1 Defined: Canadian Cardiovascular Society Class III or IV.
2 Defined in report: See Recommendation 7-2.
3 Defined in report: See Recommendation 7-2.
4 Defined in report: See Recommendation 7-2.
OR
-
Among patients who have not had prior CABG, severe coronary heart disease with either 50 percent stenosis in the left main artery or greater than or equal to 70 percent stenosis in the proximal or midportion of greater than or equal to two major coronary arteries and a LVEF less than 50 percent.
RECOMMENDATION 7-3. The committee recommends that patients with prior coronary artery bypass graft and either severe disease in native coronary arteries that have not been bypassed (greater than or equal to 50 percent stenosis in the left main artery or greater than or equal to 70 percent stenosis in the proximal or midportion of greater than or equal to two major native coronary arteries) and/or greater than or equal to 70 percent stenosis in greater than or equal to two bypass grafts and with Canadian Cardiovascular Society Class III or IV angina (or angina-equivalent symptoms) meet a listing.
RECOMMENDATION 7-4. The committee recommends that children who are disabled prior to interventions are considered as disabled until 6 months following surgery and then reevaluated. The table of pharmacologic interventions for ischemia in adults cannot be applied to children as necessarily appropriate therapies.
REFERENCES
Abbott, J., and N. Berry. 1991. Return to work during the year following first myocardial infarction. British Journal of Clinical Psychology 30(Pt 3):268–270.
Akhtar, M., G. F. Wu, Z. M. Du, Z. S. Zheng, and A. D. Michaels. 2006. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. American Journal of Cardiology 98(1):28–30.
Al-Mallah, M. H., I. M. Tleyjeh, A. A. Abdel-Latif, and W. D. Weaver. 2006. Angiotensinconverting enzyme inhibitors in coronary artery disease and preserved left ventricular systolic function: A systematic review and meta-analysis of randomized controlled trials. Journal of the American College of Cardiology 47(8):1576–1583.
Anderson, J. L., C. D. Adams, E. M. Antman, C. R. Bridges, R. M. Califf, D. E. Casey Jr., W. E. Chavey II, F. M. Fesmire, J. S. Hochman, T. N. Levin, A. M. Lincoff, E. D. Peterson, P. Theroux, N. K. Wenger, R. S. Wright, S. C. Smith Jr., A. K. Jacobs, J. L. Halperin, S. A. Hunt, H. M. Krumholz, F. G. Kushner, B. W. Lytle, R. Nishimura, J. P. Ornato, R. L. Page, and B. Riegel. 2007. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation
and the Society for Academic Emergency Medicine. Journal of the American College of Cardiology 50(7):e1–e157.
Antithrombotic Trialists’ Collaboration. 2002. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. British Medical Journal 324(7329):71–86.
Antman, E. M., D. T. Anbe, P. W. Armstrong, E. R. Bates, L. A. Green, M. Hand, J. S. Hochman, H. M. Krumholz, F. G. Kushner, G. A. Lamas, C. J. Mullany, J. P. Ornato, D. L. Pearle, M. A. Sloan, S. C. Smith Jr., J. S. Alpert, J. L. Anderson, D. P. Faxon, V. Fuster, R. J. Gibbons, G. Gregoratos, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, and A. K. Jacobs. 2004. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 110(9): e82–e293.
Antman, E. M., M. H. Hand, P. W. Armstrong, E. R. Bates, L. A. Green, L. K. Halasyamani, J. S. Hochman, H. M. Krumholz, G. A. Lamas, C. J. Mullany, D. L. Pearle, M. A. Sloan, S. C. Smith Jr.; 2004 Writing Committee Members: E. M. Antman, D. T. Anbe, P. W. Armstrong, E. R. Bates, L. A. Green, M. Hand, J. S. Hochman, H. M. Krumholz, F. G. Kushner, G. A. Lamas, C. J. Mullany, J. P. Ornato, D. L. Pearle, M. A. Sloan, S. C. Smith Jr.; and Task Force Members: S. C. Smith Jr., A. K. Jacobs, C. D. Adams, J. L. Anderson, C. E. Buller, M. A. Creager, S. M. Ettinger, J. L. Halperin, S. A. Hunt, H. M. Krumholz, F. G. Kushner, B. W. Lytle, R. Nishimura, R. L. Page, B. Riegel, L. G. Tarkington, and C. W. Yancy. 2008. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction, writing on behalf of the 2004 writing committee. Circulation 117(2):296–329.
ATS (American Thoracic Society). 2002. ATS statement: Guidelines for the six-minute walk test. American Journal of Respiratory & Critical Care Medicine 166(1):111–117.
Baigent, C., A. Keech, P. M. Kearney, L. Blackwell, G. Buck, C. Pollicino, A. Kirby, T. Sourjina, R. Peto, R. Collins, R. Simes, and Cholesterol Treatment Trialists’ (CTT) Collaborators. 2005. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366(9493):1267–1278.
Bergner, M., R. A. Bobbitt, W. B. Carter, and B. S. Gilson. 1981. The sickness impact profile: Development and final revision of a health status measure. Medical Care 19(8):787–805.
Boden, W. E., R. A. O’Rourke, K. K. Teo, P. M. Hartigan, D. J. Maron, W. Kostuk, M. Knudtson, M. Dada, P. Casperson, C. L. Harris, J. A. Spertus, L. Shaw, B. R. Chaitman, G. B. J. Mancini, D. S. Berman, and W. S. Weintraub. 2006. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial: Veterans Affairs cooperative studies program no. 424. American Heart Journal 151:1173–1179.
Boden, W. E., R. A. O’Rourke, K. K. Teo, P. M. Hartigan, D. J. Maron, W. Kostuk, M. Knudtson, M. Dada, P. Casperson, C. L. Harris, J. A. Spertus, L. Shaw, B. R. Chaitman, G. B. J. Mancini, D. S. Berman, G. Gau, and W. S. Weintraub. 2007a. The evolving pattern of symptomatic coronary artery disease in the United States and Canada: Baseline characteristics of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial. American Journal of Cardiology 99:208–212.
Boden, W. E., R. A. O’Rourke, K. K. Teo, P. M. Hartigan, D. J. Maron, W. J. Kostuk, M. Knudtson, M. Dada, P. Casperson, C. L. Harris, B. R. Chaitman, L. Shaw, G. Gosselin, S. Nawaz, L. M. Title, G. Gau, A. S. Blaustein, D. C. Booth, E. R. Bates, J. A. Spertus, D. S. Berman, G. B. J. Mancini, and W. S. Weintraub, for the COURAGE Trial Research Group. 2007b. Optimal medical therapy with or without PCI for stable coronary disease. The New England Journal of Medicine 356:1503–1516.
Boll, A., L. Klatt, J. Koch, and A. F. Langbehn. 1987. Psychosocial factors influencing return to work after coronary artery bypass surgery (CABS). International Journal of Rehabilitation Research 10:145–154.
Boudrez, H., and G. De Backer. 2000. Recent findings on return to work after an acute myocardial infarction or coronary artery bypass grafting. Acta Cardiologica 55(6):341–349.
Boudrez, H., G. De Backer, and B. Comhaire. 1994. Return to work after myocardial infarction: Results of a longitudinal population based study. European Heart Journal 15(1):32–36.
Budoff, M. J., S. Achenbach, R. S. Blumenthal, J. J. Carr, J. G. Goldin, P. Greenland, A. D. Guerci, J. A. C. Lima, D. J. Rader, G. D. Rubin, L. J. Shaw, and S. E. Wiegers. 2006. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 114(16):1761–1791.
Campeau, L. 1976. Grading of angina pectoris. Circulation 54:5223.
Campeau, L. 2002. The Canadian Cardiovascular Society grading of angina pectoris revisited 30 years later. Canadian Journal of Cardiology 18(4):371–379.
CAPRICORN Investigators. 2001. Capricorn Trial abstract. Lancet 357:1385–1390.
Carney, R. M., and K. E. Freedland. 2008. Depression in patients with coronary heart disease. American Journal of Medicine 121:S20–S27.
Cay, E. L., and D. D. Walker. 1988. Psychological factors and return to work. European Heart Journal 9(Suppl L):74–81.
Cerqueira, M. D., N. J. Weissman, V. Dilsizian, A. K. Jacobs, S. Kaul, W. K. Laskey, D. J. Pennell, J. A. Rumberger, T. Ryan, and M. S. Verani. 2002. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542.
Chaitman, B. R. 2006. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation 113(20):2462–2472.
Chaitman, B. R., C. J. Pepine, J. O. Parker, J. Skopal, G. Chumakova, J. Kuch, W. Wang, S. L. Skettino, and A. A. Wolff. 2004. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: A randomized controlled trial. Journal of the American Medical Association 291(3):309–316.
Chen, J., S.-L. T. Normand, Y. Wang, E. E. Drye, G. C. Schreiner, and H. M. Krumholz. 2010. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: Progress and continuing challenges. Circulation 121(11): 1322–1328.
Critchley, J. A., and S. Capewell. 2003. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: A systematic review. Journal of the American Medical Association 290(1):86–97.
de Jonge, P., T. A. Spijkerman, R. H. S. van den Brink, and J. Ormel. 2006. Depression after myocardial infarction is a risk factor for declining health related quality of life and increased disability and cardiac complaints at 12 months. Heart 92(1):32–39.
de Lorgeril, M., P. Salen, J.-L. Martin, I. Monjaud, J. Delaye, and N. Mamelle. 1999. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complica-
tions after myocardial infarction: Final report of the Lyon diet heart study. Circulation 99(6):779–785.
Dennis, C. 1990. Vocational capacity with cardiac impairment. In Medical perspectives in vocational assessment of impaired workers, edited by S. J. Scheer. Rockville, MD: Aspen Publishers. Pp. 301–334.
Drory, Y., S. Kravetz, N. Koren-Morag, U. Goldbourt, and Israel Study Group on First Acute Myocardial Infarction. 2005. Resumption and maintenance of employment after a first acute myocardial infarction: Sociodemographic, vocational and medical predictors. Cardiology 103(1):37–43.
Dubach, P., V. F. Froelicher, J. Klein, D. Oakes, M. Grover-McKay, and R. Friis. 1988. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 78(6):1380–1387.
Eagle, K. A., R. A. Guyton, R. Davidoff, F. H. Edwards, G. A. Ewy, T. J. Gardner, J. C. Hart, H. C. Herrmann, L. D. Hillis, A. M. Hutter Jr., B. W. Lytle, R. A. Marlow, W. C. Nugent, T. A. Orszulak, E. M. Antman, S. C. Smith Jr., J. S. Alpert, J. L. Anderson, D. P. Faxon, V. Fuster, R. J. Gibbons, G. Gregoratos, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, A. K. Jacobs, and J. P. Ornato. 2004. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 110(9):1168–1176.
Egred, M., S. Shaw, B. Mohammad, P. Waitt, and E. Rodrigues. 2005. Under-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary disease. QJM: An International Journal of Medicine 98(7):493–497.
Fletcher, G. F., G. J. Balady, E. A. Amsterdam, B. Chaitman, R. Eckel, J. Fleg, V. F. Froelicher, A. S. Leon, I. L. Pina, R. Rodney, D. A. Simons-Morton, M. A. Williams, and T. Bazzarre. 2001. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 104(14):1694–1740.
Fraker, T., and S. D. Fihn, writing on behalf of the 2002 Chronic Stable Angina Writing Committee. 2007. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to Develop the Focused Update of the 2002 Guidelines for the Management of Patients with Chronic Stable Angina. Journal of the American College of Cardiology 50:2264–2274.
Froelicher, E. S., L. L. Kee, K. M. Newton, B. Lindskog, and M. Livingston. 1994. Return to work, sexual activity, and other activities after acute myocardial infarction. Heart & Lung 23(5):423–435.
Froom, P., C. Cohen, J. Rashcupkin, E. Kristal-Boneh, S. Melamed, J. Benbassat, and J. Ribak. 1999. Referral to occupational medicine clinics and resumption of employment after myocardial infarction. Journal of Occupational & Environmental Medicine 41(11):943–947.
Gibbons, R. J., J. Abrams, K. Chatterjee, J. Daley, P. C. Deedwania, J. S. Douglas, T. B. Ferguson Jr., S. D. Fihn, T. D. Fraker Jr., J. M. Gardin, R. A. O’Rourke, R. C. Pasternak, and S. V. Williams (members of the Committee to Update the 1999 Guidelines for the Management of Patients with Chronic Stable Angina), and R. J. Gibbons, E. M. Antman, J. S. Alpert, D. P. Faxon, V. Fuster, G. Gregoratos, L. F. Hiratzka, A. K. Jacobs, and S. C. Smith Jr. (members of the Task Force on Practice Guidelines). 2002a. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with
Chronic Stable Angina). http://www.acc.org/clinical/guidelines/stable/stable.pdf (accessed July 8, 2010).
Gibbons, R. J., G. J. Balady, J. T. Bricker, B. R. Chaitman, G. F. Fletcher, V. F. Froelicher, D. B. Mark, B. D. McCallister, A. N. Mooss, M. G. O’Reilly, W. L. Winters Jr., and Task Force members, E. M. Antman, J. S. Alpert, D. P. Faxon, V. Fuster, G. Gregoratos, L. F. Hiratzka, A. K. Jacobs, R. O. Russell, and S. C. Smith Jr. 2002b. ACC/AHA 2002 guideline update for exercise testing: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 106:1883–1892.
Goldman, L., B. Hashimoto, E. F. Cook, and A. Loscalzo. 1981. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: Advantages of a new specific activity scale. Circulation 64(6):1227–1234.
Goldstein, D. J., M. C. Oz, and E. A. Rose. 1998. Implantable left ventricular assist devices. New England Journal of Medicine 339(21):1522–1533.
Herlitz, J., B. W. Karlson, M. Sjölin, H. E. Ekvall, and A. Hjalmarson. 1994. Prognosis during one year of follow-up after acute myocardial infarction with emphasis on morbidity. Clinical Cardiology 17(1):15–20.
Hlatky, M. A., T. Haney, J. C. Barefoot, R. M. Califf, D. B. Mark, D. B. Pryor, and R. B. Williams. 1986. Medical, psychological and social correlates of work disability among men with coronary artery disease. American Journal of Cardiology 58(10):911–915.
Khalil, M. E., A. W. Basher, E. J. Brown Jr., and I. A. Alhaddad. 2001. A remarkable medical story: Benefits of angiotensin-converting enzyme inhibitors in cardiac patients. Journal of the American College of Cardiology 37(7):1757–1764.
Klocke, F. J., M. G. Baird, B. H. Lorell, T. M. Bateman, J. V. Messer, D. S. Berman, P. T. O’Gara, B. A. Carabello, R. O. Russell Jr., M. D. Cerqueira, M. G. St. John Sutton, A. N. DeMaria, J. E. Udelson, J. W. Kennedy, M. S. Verani, K. A. Williams, E. M. Antman, S. C. Smith Jr., J. S. Alpert, G. Gregoratos, J. L. Anderson, L. F. Hiratzka, D. P. Faxon, S. A. Hunt, V. Fuster, A. K. Jacobs, R. J. Gibbons, and R. O. Russell. 2003. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 108:1404–1418.
Kushner, F. G., M. Hand, S. C. Smith Jr., S. B. King III, J. L. Anderson, E. M. Antman, S. R. Bailey, E. R. Bates, J. C. Blankenship, D. E. Casey Jr., L. A. Green, J. S. Hochman, A. K. Jacobs, H. M. Krumholz, D. A. Morrison, J. P. Ornato, D. L. Pearle, E. D. Peterson, M. A. Sloan, P. L. Whitlow, and D. O. Williams. 2009. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 54(23):2205–2241.
Lau, J., E. Antman, J. Jimenez-Silva, B. Kupelnick, F. Mosteller, and T. Chalmers. 1992. Cumulative meta-analysis of therapeutic trials for myocardial infarction. New England Journal of Medicine 327(4):248–254.
Leren, P. 1970. The Oslo diet-heart study: Eleven-year report. Circulation 42(5):935–942.
Lloyd-Jones, D., R. J. Adams, T. M. Brown, M. Carnethon, S. Dai, G. De Simone, T. B. Ferguson, E. Ford, K. Furie, C. Gillespie, A. Go, K. Greenlund, N. Haase, S. Hailpern, P. M. Ho, V. Howard, B. Kissela, S. Kittner, D. Lackland, L. Lisabeth, A. Marelli, M. M. McDermott, J. Meigs, D. Mozaffarian, M. Mussolino, G. Nichol, V. Roger, W. Rosamond, R. Sacco, P. Sorlie, R. Stafford, T. Thom, S. Wasserthiel-Smoller, N. D. Wong, and J. Wylie-Rosett, on behalf of the American Heart Association Statistics Committee
Stroke Statistics Subcommittee. 2010. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 121(7):e46–e215.
Maeland, J. G., and O. E. Havik. 1986. Return to work after a myocardial infarction: The influence of background factors, work characteristics and illness severity. Scandinavian Journal of Social Medicine 14(4):183–195.
Maeland, J. G., and O. E. Havik. 1987. Psychological predictors for return to work after a myocardial infarction. Journal of Psychosomatic Research 31(4):471–481.
Mark, D. B., L. C. Lam, K. L. Lee, N. E. Clapp-Channing, R. B. Williams, D. B. Pryor, R. M. Califf, and M. A. Hlatky. 1992. Identification of patients with coronary disease at high risk for loss of employment. A prospective validation study. Circulation 86(5):1485–1494.
Mark, D. B., D. S. Berman, M. J. Budoff, J. J. Carr, T. C. Gerber, H. S. Hecht, M. A. Hlatky, J. M. Hodgson, M. S. Lauer, J. M. Miller, R. L. Morin, D. Mukherjee, M. Poon, G. D. Rubin, and R. S. Schwartz. 2010. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Journal of the American College of Cardiology 55(23):2663–2699.
Maron, D. J., W. E. Boden, R. A. O’Rourke, P. M. Hartigan, K. J. Calfas, G. B. J. Mancini, J. A. Spertus, M. Dada, W. J. Kostuk, M. Knudtson, C. L. Harris, S. P. Sedlis, R. G. Zoble, L. M. Title, G. Gosselin, S. Nawaz, G. T. Gau, A. S. Blaustein, E. R. Bates, L. J. Shaw, D. S. Berman, B. R. Chaitman, W. S. Weintraub, and K. K. Teo, for the COURAGE Trial Research Group. 2010. Intensive multifactorial intervention for stable coronary artery disease: Optimal medical therapy in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. Journal of the American College of Cardiology 55(13):1348–1358.
McNeer, J. F., J. R. Margolis, K. L. Lee, J. A. Kisslo, R. H. Peter, Y. Kong, V. S. Behar, A. G. Wallace, C. B. McCants, and R. A. Rosati. 1978. The role of the exercise test in the evaluation of patients for ischemic heart disease. Circulation 57(1):64–70.
Michaels, A. D., G. Linnemeier, O. Soran, S. F. Kelsey, and E. D. Kennard. 2004. Two-year outcomes after enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry [IEPR]). American Journal of Cardiology 93:461–464.
Miller, J. M., C. E. Rochitte, M. Dewey, A. Arbab-Zadeh, H. Niinuma, I. Gottlieb, N. Paul, M. E. Clouse, E. P. Shapiro, J. Hoe, A. C. Lardo, D. E. Bush, A. deRoos, C. Cox, J. Brinker, and J. A. C. Lima. 2008. Diagnostic performance of coronary angiography by 64-row CT. New England Journal of Medicine 359:2324–2336.
Mital, A., A. Desai, and A. Mital. 2004. Return to work after a coronary event. Journal of Cardiopulmonary Rehabilitation 24(6):365–373.
Mittag, O., K. D. Kolenda, K. J. Nordman, J. Bernien, and C. Maurischat. 2001. Return to work after myocardial infarction/coronary artery bypass grafting: Patients’ and physicians’ initial viewpoints and outcome 12 months later. Social Science & Medicine 52(9):1441–1450.
Morrow, D. A., B. M. Scirica, E. Karwatowska-Prokopczuk, S. A. Murphy, A. Budaj, S. Varshavsky, A. A. Wolff, A. Skene, C. H. McCabe, and E. Braunwald, for the MERLIN-TIMI 36 Trial Investigators. 2007. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: The MERLIN-TIMI 36 randomized trial. Journal of the American Medical Association 297(16):1775–1783.
Myrtek, M., A. Kaiser, B. Rauch, and G. Jansen. 1997. Factors associated with work resumption: A 5 year follow-up with cardiac patients. International Journal of Cardiology 59(3):291–297.
Neill, W. A., L. G. Branch, G. De Jong, N. E. Smith, C. A. Hogan, P. J. Corcoran, A. M. Jette, E. M. Balasco, and S. Osberg. 1985. Cardiac disability. The impact of coronary heart disease on patients’ daily activities. Archives of Internal Medicine 145(9):1642–1647.
Novack, V., D. Cutlip, N. Kleiman, M. Pencina, L. Mauri, C.-H. Yen, P. Berger, S. Goldberg, M. Kellett, R. Waksman, M. Hong, A. E. Raizner, and D. J. Cohen. 2009. In-hospital and 1-year outcomes among unselected percutaneous coronary intervention patients treated with either sirolimus- or paclitaxel-eluting stents: Results from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) Registry. Journal of the American College of Cardiology: Cardiovascular Interventions 2(8):767–775.
O’Connor, G., J. Buring, S. Yusuf, S. Goldhaber, E. Olmstead, R. Paffenbarger Jr., and C. Hennekens. 1989. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 80(2):234–244.
O’Gara, P. T. 2010. The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial: Can we deliver on its promise? Journal of the American College of Cardiology 55:1359–1361.
Oldridge, N. B., G. H. Guyatt, M. E. Fischer, and A. A. Rimm. 1988. Cardiac rehabilitation after myocardial infarction: Combined experience of randomized clinical trials. Journal of the American Medical Association 260(7):945–950.
Papakostas, G. I. 2009. Major depressive disorder: Psychosocial impairment and key considerations in functional improvement. American Journal of Managed Care 15:S316–S321.
Pellikka, P. A., S. F. Nagueh, A. A. Elhendy, C. A. Kuehl, S. G. Sawada, and the American Society of Echocardiography. 2007. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. Journal of the American Society of Echocardiography 20(9):1021–1241.
Poston, R. S., R. Tran, M. Collins, M. Reynolds, I. Connerney, B. Reicher, D. Zimrin, B. P. Griffith, and S. T. Bartlett. 2008. Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Annals of Surgery 248(4):638–646.
Rannevik, P. K. 1988. Predicting return to work after acute myocardial infarction: Significance of clinical data, exercise test variables and beta-blocker therapy. Cardiology 75:230–236.
Rost, K., and G. R. Smith. 1992. Return to work after an initial myocardial infarction and subsequent emotional distress. Archives of Internal Medicine 152(2):381–385.
Sabik, J. F., A. M. Gillinov, E. H. Blackstone, C. Vacha, P. L. Houghtaling, J. Navia, N. G. Smedira, P. M. McCarthy, D. M. Cosgrove, and B. W. Lytle. 2002. Does off-pump coronary surgery reduce morbidity and mortality? Journal of Thoracic and Cardiovascular Surgery 124(4):698–707.
Safilious-Rothchild, C. 1970. The successful rehabilitant. In The sociology and social psychology of disability and rehabilitation, edited by C. Safilious-Rothchild. New York: Random House. Pp. 216–249.
Sellier, P., P. Varaillac, G. Chatellier, M. C. D’Agrosa-Boiteux, H. Douard, C. Dubois, P. C. Goepfert, C. Monpère, and A. S. Pierre, on behalf of the investigators of the PERISCOP study. 2003. Factors influencing return to work at one year after coronary bypass graft surgery: Results of the PERISCOP study. European Journal of Cardiovascular Prevention & Rehabilitation 10(6):469–475.
Shanfield, S. B. 1990. Return to work after an acute myocardial infarction: A review. Heart & Lung 19(2):109–117.
Sivarajan, E. S., and K. M. Newton. 1984. Exercise, education, and counseling for patients with coronary artery disease. Clinics in Sports Medicine 3(2):349–369.
Smith, G. R., Jr., and D. F. O’Rourke. 1988. Return to work after a first myocardial infarction. A test of multiple hypotheses. Journal of the American Medical Association 259(11):1673–1677.
Smith, S. C., Jr., J. Allen, S. N. Blair, R. O. Bonow, L. M. Brass, G. C. Fonarow, S. M. Grundy, L. Hiratzka, D. Jones, H. M. Krumholz, L. Mosca, R. C. Pasternak, T. Pearson, M. A. Pfeffer, and K. A. Taubert. 2006. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 113(19):2363–2372.
Soderman, E., J. Lisspers, and O. Sundin. 2003. Depression as a predictor of return to work in patients with coronary artery disease. Social Science & Medicine 56(1):193–202.
Soran, O., E. D. Kennard, A. G. Kfoury, and S. F. Kelsey. 2006. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from the International EECP Patient Registry). American Journal of Cardiology 97(1):17–20.
Speziale, G., F. Bilotta, G. Ruvolo, K. Fattouch, and B. Marino. 1996. Return to work and quality of life measurement in coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery 10(10):852–858.
SSA (Social Security Administration). 2008a. Listing of impairments—Adult listings (Part A). Disability evaluation under Social Security (Blue Book). http://www.socialsecurity.gov/disability/professionals/bluebook/AdultListings.htm (accessed July 22, 2010).
SSA. 2008b. Listing of impairments—Childhood listings (Part B). Disability evaluation under Social Security (Blue Book). http://www.socialsecurity.gov/disability/professionals/blue-book/ChildhoodListings.htm (accessed July 22, 2010).
Stahl, K. D., W. D. Boyd, T. A. Vassiliades, and H. L. Karamanoukian. 2002. Hybrid robotic coronary artery surgery and angioplasty in multivessel coronary artery disease. Annals of Thoracic Surgery 74(4):S1358–S1362.
Sullivan, M. D., A. Z. LaCroix, C. Baum, L. C. Grothaus, and W. J. Katon. 1997. Functional status in coronary artery disease: A one-year prospective study of the role of anxiety and depression. American Journal of Medicine 103(5):348–356.
Taylor, R., J. De Vries, E. Buchser, and M. DeJongste. 2009. Spinal cord stimulation in the treatment of refractory angina: Systematic review and meta-analysis of randomised controlled trials. BMC Cardiovascular Disorders 9(1):13.
Thompson, W. R., N. F. Gordon, and L. S. Pescatello. 2010. Clinical exercise testing. In ACSM’s guidelines for exercise testing and prescription, 8th ed., edited by W. R. Thompson, N. F. Gordon, and L. S. Pescatello. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins. Pp. 105–115.
Wenger, N. K., D. Almeida, J. M. Bradford, and S. B. King. 1985. Return to work after coronary artery bypass surgery: Problems and prospects. In Return to work after coronary artery bypass surgery: Psychosocial and economic aspects, edited by P. J. Walter. New York: Springer-Verlag. Pp. 323–331.
Wenger, N. K., E. S. Froelicher, P. A. Ades, K. Berra, J. A. Blumenthal, C. M. E. Certo, A. M. Dattilo, D. Davis, R. F. DeBusk, J. J. P. Drozda, B. J. Fletcher, B. A. Franklin, H. Gaston, P. Greenland, P. E. McBride, C. G. A. McGregor, N. B. Oldridge, J. C. Piscatella, and F. J. Rogers. 1995. Clinical practice guidelines on cardiac rehabilitation. Washington, DC: Agency for Health Care Policy and Research and the National Heart, Lung, and Blood Institute.
WHO (World Health Organization). 2008. The global burden of disease: 2004 update. Geneva, Switzerland: WHO Press.
Writing Group for the BARI (Bypass Angioplasty Revascularization Investigation) Investigators. 1997. Five-year clinical and functional outcome comparing bypass surgery and angioplasty in patients with multivessel coronary disease: A multicenter randomized trial. Journal of the American Medical Association 277(9):715–721.
Yusuf, S., R. Peto, J. Lewis, R. Collins, and P. Sleight. 1985. Beta blockage during and after myocardial infarction: An overview of the randomized trials. Progress in Cardiovascular Diseases 27(5):335–371.
































