5
Development of New Therapeutic Drugs and Biologics for Rare Diseases
When it is obvious that the goals cannot be reached, don’t adjust the goals, adjust the action steps.
Confucius
Once a potential therapeutic drug or biologic has been discovered, the process of developing the therapeutic for a particular disease, whether rare or not, begins with preclinical development and continues through increasingly complex and demanding phases of clinical testing to support approval for marketing. Much of what is done throughout the process of drug development is driven by necessary regulations that require the sponsor of a new drug to demonstrate its safety and efficacy. (Figure 5-1 depicts the process, in simplified form, from the earliest basic investigations through studies undertaken after a product has been approved for marketing.) Although public and nonprofit organizations have sometimes taken a product through this process, this work, which is expensive and risky, has traditionally been done within pharmaceutical and biotechnology companies. Approximately 10 percent of potential therapeutics that effectively pass preclinical development reach the market, and the cost for each is estimated to average from $100 million to more than $1 billion, depending on the disease and other factors and taking the cost of failed drugs into account (see, e.g., DiMasi et al., 2003; PhRMA, 2007; Gassman et al., 2008). According to one study of the 50 largest pharmaceutical firms, about one in six new drugs that entered clinical testing eventually received approval for marketing, but this rate varied widely by therapeutic class and was slightly higher for drugs licensed into a company than for drugs originated by the company (27 percent
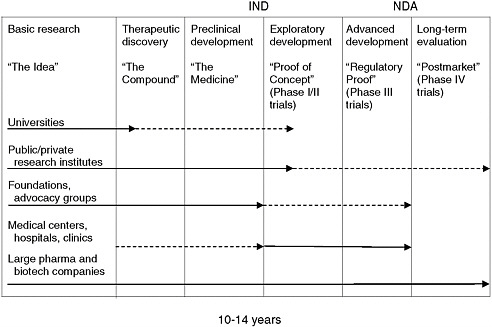
FIGURE 5-1 Drug development: from idea to market and beyond.
NOTES: IND = Investigational New Drug application; NDA = New Drug Application; Major emphasis = ![]() ; Secondary emphasis =
; Secondary emphasis = ![]() .
.
SOURCE: Adapted from Corr, 2008.
versus 16 percent) (DiMasi et al., 2010). The proportion of orphan drug approvals accounted for by large pharmaceutical companies has grown in recent years (Tufts Center, 2010), but the committee found no analysis of the success rate specific to orphan drugs.
Given the relatively low odds of success and the high costs of drug development, pharmaceutical and biotechnology companies usually focus on potential therapies with the highest likelihood of generating a good financial return—as is the case with virtually all companies in any field. This has meant that potential therapies for rare diseases, including therapies for life-threatening conditions, have often languished in the early development pipeline. Moreover, conventional approaches to drug development are often not feasible for rare diseases, which offer not only small markets but also small populations for participation in clinical trials. To paraphrase the adage of Confucius, to achieve the goals of developing effective treatments for rare diseases calls for an adjustment of the action steps.
As described in Chapter 3, the Orphan Drug Act has provided incentives for the development of drugs for rare diseases, and the Food and Drug Administration (FDA) has approved more than 350 applications for
the marketing of such drugs. Today, those incentives combined with the increasing expense and difficulty of developing blockbuster drugs have led some major pharmaceutical companies and biotechnology firms to announce that they are launching or considering orphan drug development (Anand, 2005; Dimond, 2009; Pollock, 2009; Whalen, 2009). In addition, charitable foundations linked to advocacy groups have made significant progress during the past 15 years in strategically filling the investment gap for orphan products and in pushing therapies for rare diseases through the development pipeline. The National Institutes of Health (NIH) is also supporting programs to help translate research discoveries into successful products, and innovative strategies for the conduct and analysis of studies involving small populations are allowing sound research when conventional trials designs are not possible or not feasible. At FDA, the recently created position of Associate Director for Rare Diseases at the Center for Drug Evaluation and Research (CDER) is a positive step (see Chapter 3).
This chapter begins with a description of the traditional approach to preclinical and clinical development as it applies to drug therapies for common or rare diseases. Later sections of this chapter examine the infrastructure for drug development (including biomarkers, patient registries, and clinical research training) and adjusted action steps such as alternative models of organizing and funding orphan product development. Some of these models build on public-private partnerships and other innovative strategies that have emerged from initiatives to speed the development of products for neglected tropical diseases.
PRECLINICAL DEVELOPMENT
Once a single promising compound is selected based on the kinds of basic research and therapeutic discovery reviewed in Chapter 4, companies initiate preclinical studies both in vitro and in animals to evaluate a drug’s safety and potential toxicity. These preclinical studies are also used to assess potential effectiveness. Sponsors design additional studies to provide convincing evidence that a drug is not mutagenic (i.e., it does not cause genetic alterations) or teratogenic (i.e., it does not cause fetal malformations). Because a patient’s ability to excrete a drug can be just as important as the patient’s ability to absorb the drug, other preclinical studies focus in detail on those factors.
The following discussion of therapeutics focuses on drugs but also notes certain special features of preclinical studies for biologics. As described earlier in this report, drugs are chemicals—small-molecule medicines that can be taken orally or that may be administered in various other forms, such as injection, infusion, transdermal patch, or dermal application. Biologics are
proteins, antibodies, peptides, and some vaccines that are usually injected or infused because they cannot be absorbed orally. For purposes of this discussion, they are usually encompassed under the term drug.
The safety and other data from preclinical studies are crucial in determining whether a drug will move on to studies in humans. Preclinical studies also guide researchers in designing phase I clinical trials. For example, preclinical studies with animals help determine the range of dosing of a test drug to be evaluated in a phase I clinical trial. They also help to identify criteria for evaluating safety in humans, including signs and symptoms that should be monitored closely during early clinical trials. Unfortunately, preclinical studies in animals are not precise predictors of what will happen with humans.
In addition, companies often must undertake carcinogenicity studies in animals to help assess whether a potential therapy might cause tumors. Because carcinogenicity studies require considerable time and resources, FDA guidance advises that “they should be performed only when human exposure warrants the need for lifetime studies in animals” (FDA, 1996, p. 1; see also CDER, 2002). The guidance therefore recommends carcinogenicity studies for any pharmaceutical for which clinical use is expected to be continuous over at least 6 months or to involve intermittent but frequent use in the treatment of chronic or recurring conditions (FDA, 1996). Long-term carcinogenicity studies might not be required when the potential therapy is intended for patient populations for whom life expectancy is predicted to be short (e.g., 2 to 3 years, as for some cancer therapies).
FDA’s guidance on carcinogenicity studies also states that rodent carcinogenicity studies usually are not required to be completed prior to conducting large clinical trials in humans, unless a special concern is identified. If studies are required, they ordinarily must be completed before a sponsor applies for marketing approval. However, for drugs intended to treat life-threatening or debilitating diseases, the guidance advises that carcinogenicity testing can be conducted after rather than before a drug is approved for marketing. Thus, FDA required a postmarketing carcinogenicity study for the orphan drug carglumic acid (Carbaglu), a drug that must be used long term (Beitz, 2010) (see also Box 3-3). In addition, the agency generally will not require carcinogenicity studies for endogenous substances such as enzymes that are given as replacement therapy, particularly if previous clinical experience exists with similar products. Thus, FDA has not required pre- or postmarketing carcinogenicity studies for such orphan biologics as galsulfase (Naglazyme) (Weiss, 2005). In at least one case (pentosan polysulfate sodium [Elmiron], approved in 1996), FDA nominated an orphan drug for carcinogenicity testing by the National Toxicology Program (NTP, 2004). The test results allowed the drug’s label to be revised in 2006 to report no clear evidence of carcinogenic risk.
One focus of the analysis of CDER reviews as recommended in Chapter 3 would be how the carcinogenicity guidance is being implemented for orphan drugs across FDA review divisions. Depending on the results of that analysis, CDER might develop additional guidance on this topic.
Preclinical work generates a pharmacologic profile of a drug that will be beneficial long into the drug’s future. For example, researchers can use the profile to develop the initial manufacturing process and the pharmaceutical formulation to be used for testing with humans. Industry has particular strengths in these areas. Researchers can also use specifications assigned in the preclinical stage to evaluate the chemical quality and purity of the drug, its stability, and the reproducibility of the quality and purity during repeat manufacturing procedures. (This is sometimes referred to as “chemistry, manufacturing, and controls [CMC] information.” CMC requirements and CMC activities evolve during the entire development process.) The FDA repurposing initiative described below emphasizes the value of this preclinical work if sponsors see a possible new use of an already-approved drug for a rare disease.
Preclinical studies, as well as manufacture of the drug at small scale, can be very expensive (several million dollars) and time-consuming (1 to 2 years). These studies also require specific expertise, both in the proper design and execution of the studies and in the proper interpretation of the results. Most studies need to be done under good laboratory practice (GLP) conditions to qualify for regulatory submission (21 CFR Part 58). GLP conditions apply not only to specified instrumentation, record keeping, and analysis, but also to specific laboratory conditions that, in most cases, require special facilities. More generally, meeting regulatory requirements for the approval of a drug requires expert knowledge and meticulous documentation. At the stage of drug production, companies must conform to what FDA refers to as current good manufacturing practice, or cGMP, requirements (21 CFR 210, 211).
For biologics (excluding vaccines) the development pathway is similar in many respects to that for small-molecule drugs. Two major differences stand out. First, the production of sufficient quantities of a biologic for preclinical and clinical development studies requires unique approaches for expression of the proteins and their purification to regulatory standards. As is the case for injected drugs, extensive studies are done to formulate the protein for injection under sterile conditions. Second, biologics can potentially elicit an immune response in the recipient. This response must be monitored very closely because it is not always predictable. Thus, biologics may present special issues to be addressed in preclinical studies, such as immunogenicity (i.e., induction of an antibody response) and immunotoxicity (agents intended to stimulate or suppress the immune system may cause cell-mediated changes) (CDER-CBER, 1997).
Preclinical development of biologics is also quite unpredictable. For example, experimental animals are very likely to develop endogenous antibodies against the human wild-type protein that could complicate interpretation of the results related to toxicology, distribution, and metabolism. In many cases, the ease of development of a biologic depends on whether it is a true “wild-type” (i.e., normal) human protein or whether it is a variant protein. As a general rule, the use of wild-type protein as a replacement therapy for a particular rare disease simplifies preclinical as well as clinical development, but there are exceptions. For example, a patient who produces none of a normal human protein because of an underlying genetic defect might easily produce antibodies against replacement wild-type protein, whereas a second patient, who has a different genetic defect and produces low levels of the normal protein, would not recognize the biologic as “foreign” and usually would not mount an immune response to the protein.
By their very nature, studies in preclinical development are major hurdles in the development of therapeutics for all diseases—but especially those that are rare. A later section of this chapter discusses ways in which these hurdles are being or might be addressed by NIH, FDA, companies, and advocacy groups.
The next several sections of this chapter are organized around the clinical trial phases that are conventionally used to develop evidence of safety and efficacy for drugs intended for common conditions. For drugs intended for quite rare diseases, the delineation among phases I, II, and III trials is often not as clear. As discussed in Chapter 3, FDA may not require the usual sequence of trials. The agency strongly encourages sponsors of drugs for rare diseases to seek meetings with FDA to discuss development strategy prior to submission of an Investigational New Drug (IND) application (Pariser, 2010).
PHASE I
CLINICAL TRIALS: SAFETY
Before clinical studies can begin, sponsors must submit an IND application to FDA. This application must include the results of the preclinical studies discussed above. Given the generally small numbers of patients available for the study of rare diseases, sponsors benefit particularly from regulatory guidance on the extent of phase I analysis that CDER considers sufficient prior to the start of phase II clinical trials.
Phase I trials initiate the testing of drugs in humans. They often involve small numbers (20 to 100) of healthy volunteers but sometimes include research participants with a rare or other specific condition for which targeted pathways have been identified as potentially relevant to disease
pathogenesis.1 A phase I study may last for several months. Drug doses usually start at very low levels, and research participants are monitored very carefully as the dose is escalated. In some circumstances and depending on the study protocol, individual participants may receive only one dose.
Phase I studies focus on the evaluation of a new drug’s safety, the determination of a safe dosage range, the understanding of the drug’s clinical pharmacology, the identification of side effects, and sometimes the detection of early evidence of effectiveness if the drug is studied in patients with the target disease. From phase I clinical trials, researchers gain important information about
-
the drug’s effect;
-
the drug’s pharmacokinetics (absorption, distribution, metabolism, and excretion) to better understand a drug’s properties in the body;a drug’s properties in the body;
-
the acceptability of the drug’s balance of potency, pharmacokinetiche acceptability of the drug’s balance of potency, pharmacokinetic properties, and toxicity or the specificity of the drug (i.e., its ability to hit its desired target without altering another biological process); and
-
the tolerated dose range of the drug.
In January 2006, CDER issued guidance on exploratory IND studies (CDER, 2006). It defined such studies (which some refer to as phase 0 clinical studies) as occurring early in the initial phase of clinical studies, having no diagnostic or therapeutic purposes, and involving very limited exposure of humans to the investigational drug. The guidance urged sponsors to consider exploratory IND studies, in particular, for drugs intended for patients with serious, life-threatening diseases, which is often the case for rare diseases. Such studies involve fewer resources than conventional approaches and thus allow sponsors to “move ahead efficiently with the development of promising candidates” (p. 2). For example, during such an exploratory study, sponsors can test one or more related compounds at very low doses that are sufficient to determine the half-life, absorption, metabolism, and excretion of a drug. Such testing is particularly useful in guiding the selection of one compound among several to take to a full phase I study and in providing more early information when concerns exist about the predictive value of preclinical data from animals. It may be particularly helpful in studies of rare diseases.
|
1 |
Among other provisions, government rules on the conduct of research involving human participants require that studies including children either involve no more than minimal risk or have expected benefits that justify the risk involved (OHRP, 2008). Thus, healthy children would generally not be included in a phase I safety study. |
The guidance on exploratory IND studies is relatively recent. Such studies will occur long before an application for approval reaches FDA, so it will take time before the effect of this approach on product development for rare conditions can be assessed. In any case, exploratory studies appear a useful option for a company or other sponsor that is nearing the initiation of clinical research for a drug to treat a rare condition.
PHASE II
CLINICAL TRIALS: PROOF OF CONCEPT OR EFFICACY
In conventional clinical trials for drugs for common conditions, phase II studies provide an investigational drug’s first test of efficacy in research participants who have the disease or the condition targeted by the medication. Even if combined phase I-II trials are performed to obtain initial findings of safety and efficacy, larger phase II trials will normally be needed to determine optimal dosing to maximize efficacy and minimize adverse events. These studies may include up to several hundred participants and may last from several months to a few years.
As described in Chapter 3, for drugs intended for rare conditions, FDA may accept studies involving smaller numbers of research participants than are required for more common conditions. It may also allow the use of historical controls (or possibly no controls) if the rare disease has a defined course in the absence of treatment that will permit comparisons with results for an investigational drug.
Phase II studies help determine the correct dosage, identify common short-term side effects, and define the best regimen to be used in pivotal clinical trials. Conventionally, the initial step is usually a phase IIa clinical trial that is focused on an initial proof of concept. This step is to demonstrate that the drug did what it was intended to do: that is, it interacted correctly with its molecular target and, in turn, altered the disease. Phases I and IIa are sometimes referred to as “exploratory development.” Phase IIb trials are larger and may use comparator agents and broader dosages to obtain a much more robust proof of concept and additional guidance on dose selection. They are often done at a regulatory standard that requires conformance with good clinical practice principles and guidelines (see, e.g., CDER-CBER, 1996, and documents at http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/default.htm).
PHASE III
CLINICAL TRIALS: REGULATORY PROOF
Conventional phase III clinical trials are designed to evaluate a candidate drug’s benefit in a carefully selected patient population with the disease. These trials are to confirm efficacy, further evaluate safety and
monitor side effects, and sometimes compare the candidate drug to commonly used treatments. They provide crucial evidence needed to satisfy regulators that the drug meets the legal requirements for marketing approval and to provide necessary information for product labeling after approval of the drug.
For common conditions, phase III studies are usually conducted with large populations consisting of several hundred to several thousand participants who have the disease or the condition of interest. Specific decisions about the size of the study group will depend on such factors as the magnitude of the effect of interest, characteristics of the study population, and study design. Phase III trials typically take place over several years and at multiple clinical centers around the world. The study drug may be compared with existing treatments or a placebo. Phase III trials are, ideally, double blinded; that is, neither the patient nor the investigator knows which participants are receiving the drug and which are receiving existing treatment or placebo during the course of the trial.
FDA typically requires two phase III clinical trials for approval of a drug, but the law authorizes FDA to approve a drug based on one multicenter study in appropriate circumstances. Because the number of patients available to participate in a clinical trial involving a rare disease is often very small, FDA frequently approves orphan drugs with less extensive requirements for clinical studies (see Chapter 3).
If clinical trials are successful, a New Drug Application (NDA) is submitted to FDA for review. The review process usually takes 10 to 12 months and may include, at the discretion of FDA, an advisory committee review. Drugs for rare conditions may qualify for one of several options for speeding the path to approval (see Chapter 3).
Phase II, and sometimes phase III, trials may fail due to the large heterogeneity of the patient population being studied. As a result of genetic heterogeneity, some research participants may respond well and others may not respond at all to an investigational product. Increasingly, research is subdividing common diseases such as breast or lung cancer into many heterogeneous subtypes that may differ in their responsiveness to different treatments and that may qualify as rare in terms of the number of people who fit a particular subtype.
Because most rare diseases have a more homogeneous genetic pattern than do common diseases and because they are often characterized by similar or identical genetic or epigenetic defects, patients with these diseases could be expected to have a more uniform response to a drug. This should reduce the size of phase II and III studies required to demonstrate efficacy. Indeed, in recognition of this relative homogeneity, CDER has accepted the use of historical controls in phase II trials for extremely rare diseases (see Chapter 3).
PHASE IV
POSTMARKETING STUDIES
FDA will frequently specify postmarketing study (phase IV) requirements to further evaluate an approved drug and obtain more information about safety or effectiveness or both. As described in Chapter 3, such studies are required if the accelerated approval process is used. Approval for one drug (not for a rare disease) was recently rescinded based on postmarketing study results that indicated no benefit. Many of the approvals of drugs for rare diseases reviewed by the committee included provisions for various kinds of postmarketing studies.
Responding to evidence of the agency’s lax monitoring of company fulfillment of postmarketing study requirements, the FDA Modernization Act of 1997 (P.L. 105-115) required FDA to establish a system for monitoring and publicly reporting sponsor progress in fulfilling postmarketing study commitments and requirements. The agency published rules implementing the legislation in 2000 (65 Fed. Reg. 64607). Although study fulfillment is important, the committee was not able to investigate this outcome for orphan drugs.
INFRASTRUCTURE FOR DRUG DEVELOPMENT
The process of drug development, whether it involves a small molecule or a biologic, is expensive and time-consuming. Invariably, it takes not only expertise, but robust infrastructure and significant funds to bring a therapy to market. Almost 70 percent of the total spent in drug development is for failures at various stages of the drug development process.
Although there are several streams of funding for drug development, the total amount is inadequate to support investigation of the thousands of rare diseases profiled earlier in this report—with significant consequences for affected patients, their families, and their communities. Clearly, innovation—on every level and by all stakeholders—is needed. This section expands on the discussion begun in Chapter 4 by describing elements of the infrastructure that are needed for clinical development of therapies, including biomarkers for use as surrogate endpoints in clinical trials, patient registries, clinical trial consortia, and clinical research training.
Not included in the discussions below are many other infrastructure elements, information sharing initiatives, and collaborations. To cite one example, the Clinical Data Interchange Standards Consortium is a nonprofit organization to establish standards for acquiring, exchanging, submitting, and archiving clinical research data (see http://www.cdisc.org/). To cite another example, Tox21 is a new collaboration involving NIH, FDA, and the Environmental Protection Agency. It is intended to develop innovative methods to predict the toxicity of drugs and other chemicals in humans,
speed the testing process by using robotic and informatics technologies to test compounds in cells, and establish priorities for chemicals that require further evaluation (Jones, 2010).
Biomarkers
One important avenue for speeding clinical studies of rare diseases involves the identification of biomarkers to monitor responses to therapy and guide dosing. Biomarkers have multiple uses. As described in a recent Institute of Medicine (IOM) report, they are used “to describe risk, exposures, intermediate effects of treatment, and biologic mechanisms; as surrogate endpoints, biomarkers are used to predict health outcomes” (IOM, 2010a, p. 3). Biomarkers figure significantly in several of the innovative approaches to developing drugs for rare diseases as discussed below.
Developing and validating biomarkers is not a trivial undertaking even for common conditions, but it is highly relevant for rare diseases and warrants concerted attention. Box 5-1 summarizes the recommendations on biomarker evaluation in the IOM report (IOM, 2010a). The IOM report emphasized the importance of context—including disease prevalence and severity—in evaluating biomarkers. It observed that “an intervention meant to treat a rare but life-threatening disease may permit more tolerance of risk than an intervention meant to treat a more common but less serious disease” and that “it may be easier to defend use of a surrogate endpoint for trials of rare and life-threatening diseases than for trials of primary prevention interventions for common but less serious or life-threatening diseases” (IOM, 2010a, p. 113). For biomarkers as well as clinical trial strategies generally, it will be important to consider what constitutes reasonable flexibility in FDA assessments of biomarkers for rare conditions.
Because a validated biomarker can serve as a surrogate endpoint in a clinical trial, this may allow sponsors to reduce the number of research participants and the time required for clinical trials. In addition, the accelerated approval pathway described in Chapter 3 allows FDA to approve a drug based on evidence involving surrogate endpoints that are not considered well established but that are determined to be reasonably likely to predict clinical benefit. FDA then requires postapproval studies to develop further evidence about benefits and risks based on clinical outcomes. (As discussed in Chapter 3, the Government Accountability Office recently expressed concern that FDA did not have an adequate process for monitoring the progress of these studies [GAO, 2009b].)
The Biomarkers Consortium is a public-private partnership that is managed by the Foundation for the National Institutes of Health. It aims to develop biomarkers for use in research, therapeutic and diagnostic development, regulatory approval, and clinical practice (http://www.biomarkersconsortium.org/).
|
BOX 5-1 Summary of Earlier IOM Report Recommendations for Effective Biomarker Evaluation The Evaluation Framework 1. The biomarker evaluation process should consist of the following three steps: 1a. Analytical validation: analyses of available evidence on the analytical performance of an assay; 1b. Qualification: assessment of available evidence on associations between the biomarker and disease states, including data showing effects of interventions on both the biomarker and clinical outcomes; and 1c. Utilization: contextual analysis based on the specific use proposed and the applicability of available evidence to this use. This includes a determination of whether the validation and qualification conducted provide sufficient support for the use proposed. 2a. For biomarkers with regulatory impact, the Food and Drug Administration (FDA) should convene expert panels to evaluate biomarkers and biomarker tests. 2b. Initial evaluation of analytical validation and qualification should be conducted separately from a particular context of use. 2c. The expert panels should reevaluate analytical validation, qualification, and utilization on a continual and a case-by-case basis. SOURCE: IOM, 2010a. |
The consortium has several active and approved projects, none of which currently involve rare diseases. However, one of the specific topics mentioned in the recent solicitation of NIH Challenge Grants was the validation of biomarkers for functional outcomes in rare diseases (03-OD(ORDR)-101).
Several research initiatives are investigating biomarkers for rare diseases, for example, Huntington disease (Aylward, 2007) and pulmonary arterial hypertension (Heresi and Dweik, 2010). An example of a generally accepted biomarker for a rare condition is blood phenylalanine level for the rare disease phenylketonuria. Another example is forced expiratory volume (FEV1), which FDA has accepted as an endpoint to support approval of drugs for cystic fibrosis and other lung disorders (CDER, 2007; Laessig, 2009). The committee heard of at least one example of a CDER review unit’s questioning the use of FEV1 in a clinical area in which its use as a
surrogate has been accepted. This is an example of the kind of inconsistency across divisions at FDA that would be evaluated in the analysis proposed in Recommendation 3-1.
Patient Registries
Chapter 4 discusses the importance of patient registries and biorepositories to support basic research on rare conditions. Patient registries are also essential elements in the process of drug development. A patient registry is more than a list of patients with a particular condition, although that is a first step. It involves the systematic collection of uniform information for a specific purpose(s) (see, e.g., Gliklich and Dreyer, 2007; Forrest et al., 2010). Examples include registries created to help describe the natural history of a disease in the absence of treatment and registries to collect additional information about a drug’s safety or efficacy after the end of pivotal clinical trials (often as required by FDA as a condition for approving a drug).
The conduct of clinical trials for rare disorders is inherently difficult because of the small number of patients. The problem of small numbers is further complicated when the consequences of a rare condition or its treatment reveal themselves slowly. Patient registries can help with the limitations of small numbers in many ways. First, a comprehensive knowledge of the natural history of disease (e.g., that 80 percent of affected individuals die by 5 years of age) can provide a historic benchmark for judgments about the efficacy of a therapeutic intervention. Such knowledge may then allow the use of a single-arm clinical study without a placebo or other concurrent control group. To cite an example, the evidence submitted to support FDA approval of alglucosidase alfa (Myozyme) for infantile-onset Pompe disease included a comparison of outcomes for 18 pivotal trial participants with outcomes for infants followed in a natural history study (Beitz, 2006). If research participants do not have to be divided into test and control groups, this can significantly increase the number of individuals with a rare condition who can participate in the treatment arm, which may have several advantages (e.g., in allowing informative, planned comparisons of study participant subgroups). Also, patients may be more willing to participate in a trial when they are assured they will receive the test drug, particularly when no standard therapy exists.
A second way that registries can help is when the information collected includes biological specimens or links to specimen data. Such information has the potential to reveal biochemical, histologic, or other markers that may be found to be suitable as surrogate endpoints in clinical trials and that, in turn, can reduce the time required for clinical trials used to support FDA approval, particularly for long-term chronic conditions. In addition,
patient registry studies can reveal clinical outcomes that occur prior to but that rigorously predict catastrophic events (e.g., brain damage or death) and so can be used as surrogate measures for clinically relevant outcomes. Ideally, these surrogate clinical markers of disease would occur invariably in advance of the most important clinical outcomes, but their presence (at some reliably measurable level) would not obligate progression to these outcomes, which would allow the productive substitution or modification of experimental therapies in time to make a difference.
Today, an uncounted number of organizations and researchers in this country and around the world maintain rare diseases registries in some form, sometimes for the same condition. No uniform, accepted standards govern the collection, organization, or availability of these data. Organizations and researchers may closely guard their data or may face legal limits (related to patient consent and privacy) on data sharing. At the same time, one estimate is that registries exist for only 20 percent of rare diseases (Wrobel, cited in Forrest et al., 2010). Thus, calls are increasing both for the expanded use of registries and for a more systematic and standardized approach to their creation, maintenance, and accessibility on a national and global basis.
In 2010, the Office of Rare Diseases Research at NIH sponsored a workshop on the intersection of patient registries, biospecimen repositories, and clinical data (see Rubenstein et al., 2010). In 2009, the European organization EPPOSI sponsored a similar workshop on patient registries for rare disorders. As outlined in those workshops and other discussions, features of a systematic, coordinated approach to patient registries for rare diseases would include agreement on minimum common data elements, definitions, and coding protocols and easy access to a common central resource or platform for creating or reconfiguring registries. Not only would these features make the creation or revision of existing registries easier (especially for groups or researchers with limited funds), but also they would facilitate data sharing and pooling. Another feature of a common resource would be the fee-based provision of data management or curation functions. To make the data more widely available for research purposes and to safeguard patient privacy, the common resource would provide for de-identified patient data from registries to be included in an aggregated database. Given the limited resources of many organizations and researchers working on rare diseases, the goal would be for the system to evolve into a self-sustaining, public-private partnership. The specific features of such a system are beyond the scope of this report, but various individuals and groups are working on the elements just described.
A related issue is the absence of standard methods for collecting and categorizing comprehensive patient information and samples during clinical trials for rare disorders, which limits the opportunity for results from
one trial to inform another. Given the inherently small sample size and the often restricted time frame available for evaluating the therapeutic effect of an intervention, opportunities to launch or modify clinical trials for rare disorders based upon new insights or hypotheses are limited. Thus, testing all reasonable hypotheses in parallel for rare disorders is wise stewardship of the limited resource of patient information and material. For example, does a therapy for aortic disease in Marfan syndrome also affect musculoskeletal or ocular manifestations? Are there genetic or other biomarkers that predict response to therapy? With standards in place, it would be possible to establish a dedicated and, to an extent, non-hypothesis-constrained effort to establish a rich dataset and sample repository during clinical trials that could be used to test future hypotheses in a retrospective manner.
One potentially comprehensive approach would be for funding agencies to widely advertise the anticipated launch of clinical trials for rare disorders, to request proposals for and prioritize ancillary studies, and to mandate the establishment of or participation by grantees in standardized patient registries and biorepositories. This would depend on a coordinated intellectual, financial, and physical infrastructure (probably including international acceptance and participation) to support such initiatives.
Clinical Research Consortia
Clinical research consortia can be instrumental components of the infrastructure necessary to advance rare diseases research. In general, such consortia provide the underlying infrastructure to conduct clinical trials in a timely manner, including
-
supporting research sites that have access to both specialized clinical investigators and relevant patient populations;
-
creating a data-coordinating center;
-
establishing a protocol development office;
-
instituting a data safety monitoring board; and
-
providing other components required for clinical investigations.
Consortia can provide a clear pathway for rapidly translating basic research and other discoveries into key trials to evaluate safety and efficacy consistent with FDA expectations. Moreover, they can potentially lower the costs associated with initiating and conducting a clinical trial, which may attract industry partners into the rare diseases clinical research arena.
A spectrum of clinical research consortia currently exists. They include NIH-funded groups, the largest of which is the Children’s Oncology Group (COG); a number of very successful, primarily philanthropically supported
programs such as the Cystic Fibrosis Foundations’ Therapeutics Development Program and the Multiple Myeloma Research Consortium; and a variety of smaller consortia funded by government sources or private foundations. COG is perhaps the preeminent collaborative research organization and was the first to recognize the crucial importance of collaboration in pediatric research. Even common childhood cancers are rare enough that no one center treats the number of children required for large-scale clinical trials. Today, more than 90 percent of the 12,500 children diagnosed with cancer each year in the United States are treated at COG institutions (Liu et al., 2003). COG is thus well positioned to explore adaptive clinical trial designs that have the potential to undergo modification—including imbalancing randomization to favor better-performing treatment arms—while the study is being conducted.
NIH currently funds the Rare Diseases Clinical Research Network (RDCRN), which is in its second cycle of 5-year funding. In addition to their research objectives, another valuable objective of the network consortia is to provide information to patients and families and help patients connect with advocacy groups, clinical experts, and clinical study opportunities. The consortia have to include the following:
-
clinical research projects for observational or longitudinal studies and/or clinical trials (at least two projects are required, one of which must be a longitudinal study);
-
pilot or demonstration projects (at least one project is required); and
-
a training (career development) component.
The RDCRN supports important studies, but disease focus areas studied are quite limited in number—19 consortia that cover more than 135 diseases (see Appendix E). The RDCRN cannot carry out clinical trials for diseases that fall outside the scope of the funded consortia.
Rare diseases investigators do, however, work through other networks to pursue the development of therapeutics. For example, the Pediatric Heart Network (PHN), funded in 2001 by the National Heart, Lung, and Blood Institute (NHLBI) to improve outcomes and quality of life in children with heart disease, allows its eight core sites the flexibility to identify, prioritize, and launch initiatives within their broad domains of expertise in a discretional manner. In 2006, investigators published preclinical findings demonstrating that losartan, an FDA-approved drug for hypertension, prevented aortic aneurysms in a mouse model of Marfan syndrome (Habashi et al., 2006). The network was able to respond to these findings in a timely manner and launched a clinical trial of losartan by February 2007. Network structure and policies allowed the recruitment of 21 auxiliary sites with
sizable Marfan patient populations and specific clinical expertise in the management of this disorder.
The committee believes that a similarly flexible network structure for rare diseases research has value as an addition to the RDCRN. Such a structure could utilize NIH U01 or U10 cooperative agreements. Because clinical expertise and relevant patient populations may vary based on the disease, the network would be designed with the flexibility to engage or partner with specific sites or relevant existing networks.
Other components might be considered to support the productivity of an augmented rare diseases clinical research capacity with an overarching goal of maintaining flexibility to meet the diverse research needs of patients with rare diseases. For example, taking into account its experience to date, NIH might consider alternative models for institutional review board (IRB) review of rare diseases research, for example, a central IRB for rare diseases that would assemble the requisite expertise to review protocols while minimizing the duplication and costs associated with multisite reviews by separate IRBs.2 In addition, it will be important for new rare diseases research to continue the emphasis of the current Rare Disease Clinical Trial Network on active partnerships with relevant advocacy groups and other organizations that are currently committed to rare diseases research.
Innovative Clinical Trial and Data Analysis Strategies
This report has stressed the importance of clinical trial designs and data analysis strategies suited to the challenges of evaluating the safety and efficacy of products for small populations. Chapter 3 discusses the joint education programs of FDA and NIH to familiarize both agency staff and others with these designs. It recommends that the agencies evaluate the extent to which studies submitted in support of orphan drugs are consistent with advances in the science of small clinical trials and associated analytic methods and develop responses based on the findings. It also recommends that the agencies support further work to develop and test innovative clinical research and data analysis strategies for small populations.
An underlying goal of novel clinical trial methodologies is to make better use of available data. To that end, Bayesian statistical methodologies are increasingly being applied in clinical research, which offers the prospect of smaller but more informative trials (see, generally, Berry, 2006). At a basic level, all statistical methods used in clinical research address how to
deal with uncertainty in research data. Bayesian methodologies define that uncertainty in terms of a probability as opposed to a fixed parameter. Calculations can then ensue at any time during the trial, affording potential advantages of “real-time” modifications in trials. For example, modifications might include imbalancing randomization to favor the better-performing arm of a trial or altering the subpopulation being studied to focus on a better-responding group. It is this continual learning feature that underlies the term “adaptive trial design.” Importantly, Bayesian approaches are gaining wider acceptance not only in the medical research community but also in regulatory agencies. (See also the discussion of Bayesian design in medical device trials in Chapter 7.)
Clinical Research Training
Another important foundation for rare diseases research and product development is the training of clinical-translational investigators who understand innovative trial designs that can be applied to drug development-related studies of small populations of patients with rare diseases and who know when they need methodological consultants to give them more expert guidance. In addition, it is important for clinical subspecialists who work with both children and adults with rare diseases to be trained to collect data that will allow standardized and detailed phenotyping and the elucidation of clinical natural histories, two important contributions to research progress in many rare diseases.
A more comprehensive discussion of investigator training is found in Chapter 4. That chapter includes a recommendation for a comprehensive NIH action plan on rare diseases, one element of which would cover research training.
Access to Information on Negative Findings and Decisions
Traditionally, information about failed clinical trials and negative FDA decisions has been limited. With few exceptions, FDA regulations generally prohibit the release of information from or about an IND, NDA, or Biologic Licensing Application (BLA) that does not result in an approval. As a result, FDA does not announce its negative decisions on NDAs, and it does not release information about negative clinical trial findings related to INDs or NDAs that sponsors have withdrawn or abandoned. As part of legal requirements for publicly owned companies, drug and biotechnology companies typically make summary announcements of negative FDA decisions, failed late-stage trials, or company decisions to stop a major drug development effort. These announcements usually do not provide scientific details.
Negative findings are sometimes published in medical journals, but
such results are often not submitted for publication or not published if submitted (Fanelli, 2010). Those that are published may not attract as much media and other attention as reports of successful trials.
Although research sponsors may learn from a negative trial, clinical trial results that are not public represent a waste of potentially valuable information. They also are a disservice to the trial’s research participants who may have put themselves at risk or forgone participation in more promising research. Withheld findings can be a barrier to progress in product development, which is especially troublesome when a rare disease is involved because research on such diseases is so limited. As observed in Chapter 3, the provision of more information about the reasons that drugs that are not approved by FDA or are withdrawn before approval could be particularly valuable for drugs being considered for the treatment of rare diseases.
In recent years, government, medical journals, advocacy groups, trade associations, and others have taken steps to increase the availability of information about successful and unsuccessful clinical trials. Box 5-2 summarizes some of these.
INNOVATION PLATFORMS FOR DRUG DEVELOPMENT
As discussed in Chapter 4, innovation platforms for research often involve the sharing of resources. Companies, federal agencies, and nonprofit patient groups are taking the initiative to build such new models for drug development for both common and rare diseases. This section highlights such models. More extensive discussions are included in summaries of IOM workshops on innovative business models for drug development for rare and neglected diseases (IOM, 2008), venture philanthropy strategies for translational research (IOM, 2009b), and precompetitive collaboration in oncology research (IOM, 2010b).
Industry
Companies have experimented with different models to achieve greater productivity through a higher success rate for drug approvals or lower costs or both. One approach has been to outsource aspects of drug development as in the case of Eli Lilly’s Chorus program. This program, which was developed as a pilot project, has evolved into an alternative research and development unit that focuses on early-stage drug development. It looks for “the most likely winners in a portfolio of molecules (most of which are destined to fail), recommending only the strongest candidates for costly late-stage development” (Bonabeau, 2008, p. 1).
Another industry approach to innovation has been to outsource problem
|
BOX 5-2 Examples of Initiatives to Increase Information About Clinical Trials and FDA or Company Decisions About Products Registration of Clinical Trials The FDA Modernization Act of 1997 directed the Department of Health and Human Services (DHHS) to create a registry of both federally and privately funded trials, now called ClinicalTrials.gov. It requires sponsors to submit information to a databank about clinical trials conducted under an IND application if the trial is to assess efficacy for a drug to treat a serious or life-threatening disease or condition. Registration information includes basic details about the trial protocol, primary clinical endpoints, and the data analysis plan. The FDA Amendments Act of 2007 expanded the reporting requirements to expressly require the registration of device clinical trials and the reporting of clinical trial results. (See generally 42 USC 282(j).) The reported results are to include basic demographic and baseline information on enrolled participants, findings for primary and secondary outcomes, and a point of contact. In addition, the uniform publication requirements of the International Committee of Medical Journal Editors now specify as a condition of consideration for publication of an article on a clinical trial that the trial be registered in a public trials registry (ICMJE, 2009). Information About FDA Evaluations and Actions In recent years, FDA has made more information available from the reviews associated with the approval of an NDA. It has also posted industry and agency presentations made to advisory committees when they are consulted on an application. These presentations thus can be a source of considerable information if FDA does not approve an NDA. Recently, the agency released for public comment a set of 21 “transparency” proposals (FDA, 2010b). If implemented, these proposals would significantly increase access to information about applications submitted |
solving, as in the case of InnoCentive, also an initiative of Eli Lilly. It was born of frustration over certain seemingly intractable aspects of drug synthesis and development (Travis, 2008). Now independent, the company offers public, prize-based challenges to attract a “virtual workforce” to the solution of difficult problems. One example is a set of challenges it organized for a patient group for amyotrophic lateral sclerosis that included a $1 million prize for discovering a validated biomarker to track progression of the disease.
A third approach focuses on precompetitive collaborations across industry. As discussed in Chapter 4 in the context of discovery research, such collaboration might involve several aspects, including that competitors share the costs of early-stage research in rare diseases and also share expertise and findings. Another element might involve cooperation on the development of biomarkers that could be used to monitor therapies for specific diseases and that might ultimately be used as surrogate endpoints
|
to the agency, for example, whether an IND has been placed on hold or terminated, whether an unapproved NDA or BLA has been withdrawn or abandoned, or whether safety concerns have been identified. With respect to designated orphan drugs specifically, one proposal is that FDA would disclose its determination that a certain product may represent a significant therapeutic advance for a rare disease if an application is withdrawn, terminated, or abandoned for other than a safety reason (e.g., withdrawn solely for business reasons). Coalition Against Major Diseases Several major pharmaceutical companies have announced that they will share pooled data from failed clinical trials of drugs for Alzheimer disease. All participating companies will have access to the pooled data as will outside researchers with a valid question. The objective is to identify why the studies fail and use the conclusions to design studies that will be successful. The coalition has similar plans for pooling clinical trials data on Parkinson disease and tuberculosis (Wang, 2010). Pharmaceutical Research and Manufacturers of America (PhRMA) For FDA-approved drugs, PhRMA has created an online clearinghouse of results of industry phase III and IV clinical studies completed since October 1, 2002. It includes a bibliography of and links to published articles and results summaries for unpublished clinical studies. References to scientific papers will be posted when they are published. Consistent with FDA regulations on annual reports, companies are encouraged to post summaries of unpublished studies within a year of study completion. The primary audience is physicians. Submissions to the database are voluntary, but the website says that “PhRMA’s board of directors … agreed that member companies will participate in and support the database and, as with PhRMA’s 2002 Principles, communicate all meaningful results, both positive and negative” (http://www.clinicalstudyresults.org/primers/faq.php). |
for regulatory approval of a therapeutic. The public-private Biomarkers Consortium administered by the Foundation for NIH was described earlier. Another example of cross-industry collaboration is the Coalition Against Major Diseases, which is cited in Box 5-2. It has announced a public-private initiative to support the development of better treatments for Alzheimer disease and Parkinson disease. In addition to the sharing of information on failed and successful trials, this collaboration among pharmaceutical companies, patient advocacy groups, voluntary health associations, and government agencies will include the development of disease progression models to improve the design of clinical trials to meet FDA standards (C-Path, 2009a). Although drug development research on rare conditions may yield fewer trials than development efforts focused on Alzheimer disease and Parkinson disease, the designations and approvals listed in FDA’s orphan drug database illustrate that multiple companies may pursue product
development research on a specific rare disease (see listings at http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm).
Competing companies may also combine insights and work together to solve a particular regulatory problem. A recent example is from the Critical Path Institute (C-Path), an independent, nonprofit organization that brings together FDA, pharmaceutical companies, and others to focus on drug development issues and to support FDA’s Critical Path Initiative (see Chapter 3). C-Path’s Predictive Safety Testing Consortium involves 16 companies. Recently, as a result of joint efforts across these companies, FDA and its European counterpart, the European Medicines Agency, have both agreed to a new standard for preclinical testing of drugs entering development to predict renal toxicity (C-Path, 2009b). The consortium is now working to qualify and validate new biomarkers in other areas. C-Path was also instrumental in developing the Coalition Against Major Diseases.
Advocacy Groups
Given the challenges and expense that beset the traditional model of pharmaceutical research and development in bringing new drugs to market, developing treatments for rare diseases represents an opportunity to test new paradigms. Led by patients and families, disease-specific foundations have begun to do just that (IOM, 2008). As described in Chapter 4, the strategy includes “de-risking” early-stage research and development for promising products by providing philanthropic capital as well as research tools and access to patients.
Although advocacy groups have traditionally provided support for basic discovery research, many of them have recently assumed a more active role in shepherding the drug development process in their areas of focus. Again, one objective is to minimize the risks associated with the early phases of therapeutic development. For example, building on the promising results of its basic research program, the Muscular Dystrophy Association now supports the preclinical work necessary for an IND application, as well as funding a national patient database, early clinical trials, and associated research infrastructure costs (see information at http://www.mdausa.org/research). Appendix F includes other examples. These novel approaches have begun to bear fruit, increasing the number of promising therapies that proceed to later-stage clinical trials (IOM, 2008).
Another Model: Public-Private Partnerships for Neglected Diseases
Public-private partnerships have played an important role in advancing therapeutics for neglected diseases of the developing world. For example, as mentioned in Chapter 4, the Medicines for Malaria Venture is working with
pharmaceutical companies and academic centers to discover promising new molecules; the Special Programme for Research and Training in Tropical Diseases is another example of a public-private partnership. At the end of 2004, about half of the public-private partnerships engaged in research and development for neglected diseases projects involved multinational corporations doing so on a “no-profit, no-loss basis”; the other half involved often smaller firms that found commercial opportunity in these resource-limited markets (Moran, 2005).
Similar kinds of partnerships could be used more effectively to develop therapies for rare diseases. For example, companies could undertake preclinical development activities for compounds entering development for a rare disease from NIH or academic institutions. Alternatively, a partnership could, through sheer volume, coordinate these preclinical development activities using specific contract research companies to complete the work at a regulatory standard and at a reduced price. One example is the International Partnership for Microbicides. This approach uses royalty-free licenses for specific compounds from pharmaceutical and biotechnology companies to develop and distribute vaginal gels and other microbicide products to prevent HIV infection (Brooks et al., 2010).
In the realm of clinical development, an example of public-private partnership is the recently announced Critical Path to TB Drug Regimens (Fox, 2010). With C-Path coordinating, this entity will test promising new treatment regimens in collaboration with FDA scientists and 10 pharmaceutical companies. The same approach could be applied to specific rare diseases.
National Institutes of Health
Just as pharmaceutical companies have had reasons to innovate, NIH has, in recent years, been called upon to complement its support for basic biomedical discovery by facilitating the translation of discoveries into therapies for both common and rare diseases. It too is building “innovation platforms” to support such translation.
As part of its Roadmap initiative, NIH launched the Rapid Access to Interventional Development (RAID) program as a pilot activity in 2004. Not a grant program, RAID supports selected aspects of preclinical development, providing expertise and performing required studies at a regulatory level using existing NIH facilities and contract resources (http://nihroadmap.nih.gov/raid/). Academic investigators as well as qualified small businesses are eligible to use the resource. Although not explicitly targeted to rare diseases, the program is meant to facilitate access to preclinical resources for projects that are unlikely to attract private-sector investment. Approved projects have targeted some rare conditions, including beta-thalassemia and Friedreich’s ataxia. The online program description notes that several
individual NIH institutes offer similar support services. However, the diversity of opportunities and the relatively decentralized structure of NIH may make it difficult for potential grantees to identify the opportunities that best fit their circumstances (Cornetta and Carter, 2010).
In 2006, in recognition of the need to integrate the translational research infrastructure within academic health centers, NIH launched its Clinical and Translational Science Awards (CTSA) program (http://www.ctsaweb.org/). The program now includes 55 institutions; ultimately, NIH plans approximately 60 CTSAs, with total funding of around $500 million per year. Individual CTSA programs are to coordinate clinical research resources within an institution to facilitate involvement in translational research by a greater number of investigators than have traditionally been engaged. The programs are also meant to provide a range of training opportunities and integrate academic medical research with community health. Within the CTSA Child Health Consortium Oversight Committee, a rare diseases work group is seeking to identify gaps in rare diseases research and ways in which the consortium might help fill those gaps.
The CTSA program is also intended to facilitate resource sharing and consortium-wide collaborations, including shared biorepositories and other resources. One example is the Pharmaceutical Assets Portal, which is sponsored by the NIH National Center for Research Resources and Pfizer (http://www.ctsapharmaportal.org/). It allows investigators to learn about compounds that have already been evaluated for specific diseases and might be developed for other conditions.
The networked structure of CTSA institutions would seem to be ideal for facilitating rare diseases research, in which multicenter clinical trials are the rule and investigators are scattered across several institutions. The CTSA program provides a coordinated infrastructure, but funding is still quite limited for the innovative projects it is meant to facilitate.
In 2010, as part of the Patient Protection and Affordable Care Act (Section 10409 of P.L. 111-148), Congress took a significant step to fill the translational research funding gap when it authorized a new Cures Acceleration Network (CAN) to provide up to $500 million annually for conducting and supporting research to develop “high-need cures.” These are cures that the Director of NIH determines to be a priority and for which market incentives are not likely to support timely or sufficient development. The program would cover development of drugs, biologics, medical devices, diagnostics, and behavior therapies. One key feature is the provision of assistance to award recipients in devising research protocols so that they will comply with FDA standards throughout all stages of product development.
The program can fund projects through three types of competitive awards, one of which requires the grantee to provide matching funds. Both
public and private organizations (including pharmaceutical and biotechnology companies) are eligible for funding.
Although not limited to rare diseases, the program, if funded to its full appropriation, will represent an unprecedented resource for the development of therapies for rare diseases and will offer an important complement to the infrastructure provided by the CTSA program. It is not, however, clear to what extent it will subsume or complement the existing Therapeutics for Rare and Neglected Diseases (discussed in Chapter 4) and RAID programs or whether its activities will be integrated with those of the NIH Office of Rare Diseases Research and the Rare Diseases Clinical Research Network. The existing infrastructure of rare diseases and translational research, although slight in relation to the need, is an important resource. Thus, a recommendation at the end of this chapter emphasizes the importance of coordinating new and existing programs to speed the translation of research discoveries into safe and effective therapies, diagnostics, and preventive interventions for people with rare diseases.
Food and Drug Administration
Critical Path Initiative
In addition to collaborating in some of the initiatives described above, FDA launched the Critical Path Initiative in 2004 to “to find fundamentally faster, more predictable, and less costly ways to turn good biomedical ideas into safe and effective treatments” (FDA, 2004, p. 30). The initiative is intended to help build partnerships involving industry, advocacy groups, and others to share information and expertise and to promote problem solving and innovation in a broad range of areas, including biomarker development, information technology, streamlining clinical trials, and clinical investigator training. The predictive safety effort described above is an example of one such collaborative effort.
The 2009 report on the Critical Path Initiative does not cite any activities focused specifically on orphan products. Nonetheless, a number of the activities should help improve the quality and efficiency of drug trials for rare as well as common conditions. For example, one of the collaborations seeks a better understanding of the genetics of drug-induced liver injury, including Stevens-Johnson syndrome, a serious rare disorder (CPI, 2010).
Repurposing Existing Drugs
In parallel to the concept of precompetitive sharing of compounds or data as discussed in Chapter 4, another avenue for innovation involves repurposing old drugs for potential treatments of rare diseases. That dis-
cussion noted that the National Chemical Genome Center is developing a library of approved drugs so that they can be more easily screened for possible repurposing.
Without the need to repeat toxicological or pharmacokinetic assessments, a considerable portion of the costs of bringing a drug through the research and development pipeline can be saved (Chong and Sullivan, 2007). Furthermore, population safety, dosing, and adverse events are already known. In addition, for drugs to treat rare diseases, the marketing protections offered by the Orphan Drug Act provide an incentive to companies that might otherwise not be interested in further work on an old drug for which patent protection had expired.
The Office of Orphan Products Development at FDA recently posted a database of products that already have an orphan drug designation for a rare disease and have been approved for the treatment of some other rare disease, for treatment of a common disease, or both. Such products have already gone through preclinical testing and been judged to be pharmacologically active, safe, and effective for some clinical condition (OOPD, 2010).
The repurposing of existing drugs for rare diseases treatments may lead to higher pricing for existing, more common use of the drug. Although the example of colchicine discussed in Chapters 3 and 6 involves a previously unapproved but widely available drug, it may still be suggestive of one consequence of repurposing if patients with the common condition have limited alternatives.3
Use of Public and Philanthropic Funding to Reduce Overall Development Costs
Public and philanthropic funding for drug development and clinical trials for rare diseases, particularly if directed toward nonprofit, patient-led consortia, reduces the need for a high rate of return for the commercial firms that ultimately manufacture and market a new drug. Such funding potentially could attract more industry investment in these therapies. For drugs whose profit margins might be slim or initially nil, public funding such as that proposed in the previously mentioned Cures Acceleration Net-
work initiative may provide the necessary resources to bridge the “valley of death” from preclinical to clinical phases of testing and then fund pivotal clinical trials. One difference between this program and the NIH Small Business Innovation Research program is that the latter excludes nonprofit entities whereas the former extends eligibility to nonprofit research enterprises, such as patient groups that may be particularly effective in recruiting participants for clinical trials.
Examples involving resource sharing arrangements and public and voluntary funding for the development of treatments for neglected diseases offer possible models for rare diseases. One approach involves humanitarian access licensing by universities that offer publicly funded inventions royalty-free in exchange for commitments from companies to produce the drug at no profit or close to marginal cost for those in need in the developing world. For example, the University of California, Berkeley, struck such an arrangement with the Institute for OneWorld Health and Amyris Biotechnologies (see IOM, 2008; and, generally, So and Stewart, 2009). In exchange for a co-exclusive, royalty-free license from the university, Amyris Biotechnologies pledged to use the microbial process of synthesizing artemisinin and to produce the antimalarial at no profit for the developing world. With the support of a $42 million Gates Foundation grant, all three parties benefited (IOWH, 2004). Notably, Amyris Biotechnologies was able to pursue proof-of-concept testing of this technology without diluting shareholder equity. When a company involved in this kind of arrangement seeks to raise second-round venture capital, equity in the firm will be more valuable with this kind of groundwork in establishing proof of concept of the technology.
RECOMMENDATIONS
Chapter 3 includes a number of recommendations for actions by FDA to identify and reduce problems related either to its own performance or to the performance of sponsors of new drugs that may slow or discourage the development of drugs for rare diseases. These recommendations call on FDA to identify areas of inappropriate inconsistency across CDER units in their review of orphan drug applications, develop related guidance on criteria for approval of orphan drugs based on differences in candidate drugs or the associated rare diseases, continue work to expand understanding and appropriate use of small clinical trial designs, and collaborate with NIH to ensure that NIH-funded product development research meets regulatory standards.
The recommendations in this chapter focus primarily on steps that NIH can take in collaboration with industry and advocacy groups to further accelerate development of safe and effective products for people with rare
diseases. The first recommendation focuses on the preclinical stage of drug development. The objective is to expand the resources and options for accelerating drug development, including the options available to investigators funded by rare diseases advocacy groups.
RECOMMENDATION 5-1: NIH should create a centralized preclinical development service that is dedicated to rare diseases and available to all nonprofit entities.
The creation of this service could be accomplished through several different models. Within NIH, one possibility would be to expand the capacity of the RAID program, which, although not dedicated to rare diseases, does include them in its project portfolio. Similarly, the TRND program currently overseen by the Office of Rare Diseases Research could be expanded not only in terms of the number of awards but also to provide coverage of preclinical development projects such as the selection and arranging of testing of promising compounds.
Alternatively, to leverage involvement and additional funding from companies and philanthropic organizations, a preclinical development service could be based in an entity such as the Foundation for NIH. This foundation was established specifically to support NIH collaboration with academic institutions, industry, and nonprofit groups without certain constraints that apply to NIH itself (FNIH, 2010). The Biomarkers Consortium is an example of this kind of collaboration. A different and possibly complementary approach would be to establish a consortium of pharmaceutical and biotechnology companies through which selected preclinical development projects would be carried out using the resources provided by consortium members or by individual companies.
As emphasized in this chapter, the development and validation of biomarkers for use as surrogate endpoints in clinical studies of drugs for rare diseases will speed such studies and should reduce their costs. Another IOM committee has recommended a Department of Health and Human Services-wide effort to encourage the collection and sharing of data about biomarkers for drugs, biologics, devices, and foods (IOM, 2010a). In addition, the establishment of clearly defined standards for biomarker validation and application in clinical trials for rare disorders will reduce the possibility that FDA will reject applications for the approval of an orphan drug based on inadequate biomarker validation, a problem noted in Chapter 3.
RECOMMENDATION 5-2: In collaboration with industry, academic researchers, NIH and FDA scientists, and patient organizations, FDA should expand its Critical Path Initiative to define criteria for the evaluation of surrogate endpoints for use in trials of products for rare conditions.
In addition to agreement on criteria for the evaluation of surrogate endpoints for clinical trials, the expansion and improvement of patient registries and biorepositories are other important elements in a strategy to accelerate rare diseases research and product development. Today, an uncounted number of organizations and researchers in this country and around the world maintain rare diseases registries and specimen collections in some form, sometimes for the same condition. No uniform, accepted standards govern the collection, organization, or availability of these resources. An increase in the use of registries and biorepositories and a more systematic approach to their creation, maintenance, and accessibility are needed on a national and global basis. Building on work already begun, NIH can take a lead role in working with industry and private partners to make the creation and maintenance of registries and biorepositories easier and less expensive, to expand information sharing, and to promote standards and processes that yield high-quality data and specimens and protect patients or research participants.
RECOMMENDATION 5-3: NIH should support a collaborative public-private partnership to develop and manage a freely available platform for creating or restructuring patient registries and biorepositories for rare diseases and for sharing de-identified data. The platform should include mechanisms to create standards for data collection, specimen storage, and informed consent by patients or research participants.
For example, features of a systematic, coordinated approach to patient registries for rare diseases would include agreement on minimum common data elements, definitions, and coding protocols and also uniform and widely accepted mechanisms for patient or research participant consent. Partners would have easy access to a common central resource or platform for creating or reconfiguring registries. In clinical trials, the latter might involve a biomarker substudy protocol available with the main study protocol. Study participants would then be asked for consent related to the larger clinical trial and for consent related to future biomarker studies. These features would not only make the creation or revision of existing registries easier (especially for groups or researchers with limited funds), but also facilitate data sharing and pooling. Given the limited resources of many organizations and researchers working on rare diseases, the goal would be for the system to evolve into a self-sustaining, public-private partnership. The committee understands that this would be a complicated undertaking at all stages.
In the realm of clinical research, the Rare Diseases Clinical Research Network is a valuable resource but one with a relatively limited and predetermined scope that constrains its ability to take advantage of unantici-
pated opportunities presented by scientific discoveries. In some cases, other research networks have greater flexibility as in the Marfan example cited above. These other networks, however, lack a specific focus on rare diseases. The committee believes that it is desirable to enhance existing clinical research activities focused on rare diseases. This enhancement should include a program or programs that are not strictly organized around specific disease areas but rather have the flexibility to partner with or recruit other existing networks or sites to rapidly capitalize on research advances and to achieve common and broadly defined goals in rare diseases research.
RECOMMENDATION 5-4: NIH should increase its capacity and flexibility to support all phases of clinical research related to rare diseases, including clinical trials of new and repurposed therapeutic agents. Opportunities to be explored include
-
expanding the Rare Diseases Clinical Research Network to address opportunities for diagnostic and therapeutic advances for a greater number of rare diseases;
-
setting priorities for rare diseases research within other NIH clinical trials networks;
-
creating a study group approach to rare diseases, modeled after the Children's Oncology Group; and
-
building additional capability for rare diseases clinical research within the Clinical and Translational Science Awards program.
In addition, although the Cures Acceleration Network will not focus exclusively on rare diseases research, such research falls well within the program’s intended scope and should benefit from it if appropriations for the network support the goals set for it. For the program to target resources effectively, it is important that it be coordinated with the Office of Rare Diseases Research and that the selection process for network projects include individuals with expertise in rare diseases and the science of small clinical trials.
RECOMMENDATION 5-5: NIH should establish procedures to ensure coordination of the activities of the Cures Acceleration Network with those of the Office of Rare Diseases Research, FDA’s orphan products grants program, and other existing initiatives to promote and facilitate the translation of basic science discoveries into effective treatments for rare diseases. It should build on existing resources when appropriate, avoid creating duplicative research infrastructure, and engage advocacy groups in its work.
A precondition for the network to achieve its goals is the appropriation by Congress of adequate resources. In addition, requiring clinical studies funded through the Cures Acceleration Network to disclose both positive and negative results will underscore the importance of sharing data in accelerating progress toward high-need cures.
The recommendations in this chapter and the preceding one focus on strategies that may directly expand and improve the quantity, quality, and efficiency of rare diseases research and orphan product development. The next chapter turns to a quite different set of considerations that may influence company decisions about research and development activities, that is, health plan policies and practices related to drugs and biologics for rare diseases.
































