B
Innovation and the Orphan Drug Act, 1983-2009: Regulatory and Clinical Characteristics of Approved Orphan Drugs
Aaron S. Kesselheim1
INTRODUCTION
Pharmaceutical research in the United States relies on both government funding for the basic science behind drug development and private investment, which finances the majority of clinical research and manufacturing process.2 The revenue potential of a drug in treating a particular disease can influence for-profit manufacturers’ willingness to devote necessary resources to its development. If a disease affects a limited number of patients and does not allow recovery of private research investment, then therapeutic products for that condition may be developed slowly or not at all. In the United
States, Congress passed the Orphan Drug Act in 1983 to provide incentives for industry investment in treatments for such rare conditions.3
The Orphan Drug Act provided manufacturers with three primary incentives: (1) federal funding of grants and contracts to perform clinical trials of orphan products; (2) a tax credit of 50 percent of clinical testing costs; and (3) an exclusive right to market the orphan drug for 7 years from the date of marketing approval. The market exclusivity incentive protects orphan drug manufacturers from competition for 7 years, which allows greater discretion in pricing.4 Additional benefits available to sponsors of orphan-designated products include close coordination with the Food and Drug Administration (FDA) throughout the drug’s development, priority FDA review, and a waiver of drug application fees. (The first two benefits may also be available to sponsors of nonorphan drugs for serious or life-threatening conditions and unmet needs.) The legislation initially targeted drugs for which there was “no reasonable expectation” that sales in the United States could support development of the drug. Because that criterion was difficult to assess and manufacturers were wary of showing the government their internal financial projections,5 an amendment in 1984 defined a rare disease as a condition affecting fewer than 200,000 people in the United States.
The act empowered the FDA to review and approve requests for orphan drug status, coordinate drug development, and award research grants. The FDA created the Office of Orphan Product Development (OOPD) to help manage this regulatory function. Although the initial legislation permitted manufacturers to apply for orphan product designation at any time, a 1988 amendment required sponsors to apply for orphan designation before submitting applications for marketing approval.
From 1983 through 2009, a total of 2,112 orphan designations were assigned by the OOPD. Of those designations, 347 (16 percent) had been approved by the FDA as of the end of 2009. In contrast, 34 drugs that were approved from 1967 to 1983 would have qualified under the Orphan Drug Act based on their approval for a rare condition. Some authors have regarded the act as crucial in the development of certain important products. For example, an effective treatment for infant botulism, a rare neurological disease affecting about 100 U.S. children per year, was described as being developed due to concerted efforts of the California Department of Health
|
3 |
21 USC 360bb(a)(2) (2008). |
|
4 |
Prices for orphan drugs can reach more than $400,000 per year. Health plans may cover these drugs, but many require substantial patient cost sharing. See Appendix C. See also Walsh B. The tier IV phenomenon—shifting the high cost of drugs to consumers. March 9, 2009. Available at http://assets.aarp.org/rgcenter/health/tierfour.pdf. |
|
5 |
Asbury CH. The Orphan Drug Act: the first 7 years. JAMA 1991;265(7):893-897. |
Services, supported by OOPD grants and close coordination with the FDA.6 As pharmaceutical manufacturers are cited as focusing more attention on developing orphan products,7 policy makers are considering whether to offer orphan-like incentives to basic and translational research aimed at other conditions.8 Congress recently passed a law that directs the Commissioner of the FDA to “convene a public meeting regarding which serious and life threatening infectious diseases potentially qualify for available grants and contracts under the Orphan Drug Act or other incentives for development,” thereby opening the door to providing orphan drug-like incentives for new antibiotics to treat multidrug-resistant infections in the United States.9
This appendix was developed to provide some background data on the implementation of the Orphan Drug Act. Data from publicly available FDA files were collected to provide a comprehensive overview of drugs approved with orphan designations, with attention paid to the drugs’ innovativeness as well as their scientific and regulatory characteristics. In addition, characteristics of the clinical trial development process of orphan-designated drugs were analyzed.
PRIOR ORPHAN DRUG ACT RESEARCH
Prior research has been done on various aspects of the Orphan Drug Act. A few studies provided perspectives on the early implementation of the incentive. One analysis by Asbury of the first 42 orphan-designated products approved from 1983 to 1989 found that among the 33 nonbiologic drugs, 21 (64 percent) were New Molecular Entities (NMEs). The FDA ranked 38 percent of these NMEs as “important” therapeutic gains and 48 percent as “moderate” therapeutic gains.10 Asbury reported that annual sales of 25 of 40 orphan drugs were less than $1 million, while annual sales of 3 were for greater than $100 million. A study by Shulman and Manocchia analyzed 121 orphan drug approvals from 1983 to 1995 (involving 102 different drugs).11 Fifteen drugs were approved for more than one orphan indication. They found that the drugs averaged about 8 years in clinical development
(from Investigational New Drug [IND] to New Drug Application [NDA]) and approximately 1.8 years in FDA review.
Both Asbury and Shulman and Manocchia provide some data on the types of manufacturers sponsoring orphan-designated drugs. Asbury notes that 39 of the 42 drugs she analyzed were sponsored by members of the biopharmaceutical industry, from a total of 30 different firms. Shulman and Manocchia report that small-sized firms (categorized by annual worldwide sales) made up more than half of all drug sponsors, and small- and mid-sized firms together made up approximately three-quarters of the sample.
Three more recent studies have examined trends in orphan drug approvals. For the period 1983-2007, Seoane-Vazquez and colleagues studied 322 orphan-designated drug approvals, including 72 biologicals (22.4 percent) and 250 nonbiological drugs (77.6 percent).12 The most common group of diseases targeted was cancer (25.5 percent). The approved drugs emerged from 155 different sponsors but were concentrated in 83 companies (54 percent of the total) that accounted for 67.7 percent of the total number of orphan approvals. During 1983-2007, the FDA approved 635 NMEs, and the authors reported that the first NDAs for 115 (18.1 percent) of these NMEs were approved by the FDA for an orphan indication. Seoane-Vazquez and colleagues also examined the market exclusivity period for orphan drugs. Orphan-designated drugs had a shorter FDA review time on average than nonorphan NMEs (1.6 years versus 2.2 years). The authors found that the minimum effective market exclusivity life (including orphan drug market exclusivity) was 9.9 ± 3.7 years for orphan NMEs and 10.5 ± 4.1 years for other NMEs (no statistically significant difference), while the maximum effective patent and market exclusivity life (including orphan drug market exclusivity) was 11.7 ± 5.0 years for orphan NMEs and 13.9 ± 5.5 years for other NMEs (p < 0.001). They concluded that the orphan drug market exclusivity incentive had a positive yet relatively modest overall effect on the market exclusivity life.
Another review of orphan drugs conducted by Wellman-Labadie and Zhou included drugs approved from 1983 through May 2009.13 Charting the number of orphan approvals as a function of time, the authors found that an average of about eight orphan-designated drugs per year were approved, although the annual approval rates included a number of peaks (in the mid-1990s and mid-2000s) and valleys (early 1990s and early 2000s). Wellman-Labadie and Zhou concluded that the highest rate of orphan drug
approvals overall was in the field of oncology (27 percent), followed by endocrine-metabolic, hematology, infectious diseases, and neurological disorders. They found that the “top 10” pharmaceutical and biological companies (by U.S. revenue) accounted for about 75 percent of the approvals.
Finally, Coté and colleagues compared trends in orphan drug approval from 1983 through July 2009 with trends for all drug approvals during the same time.14 They found that while there has been a peak and more recent decline in the number of new drugs approved overall, the number of new orphan drugs remained relatively constant from 1984 through 2008. As a result, the number of orphan drug approvals as a percentage of all drug approvals increased from 17 percent (1984-1988) to 31 percent (2004-2008) and was 35 percent in 2008. They concluded that orphan products now represent about one-third of FDA-approved drugs and biologics. In 2009, they reported that 11 of the 29 new molecular entities approved were orphan-designated products.
These studies report trends in absolute numbers of approvals, as well as of other characteristics of orphan drugs, including the characteristics of their sponsors, lengths of market exclusivity, and fields of use. This appendix provides further data about additional regulatory and clinical features of U.S. orphan drug approvals through a comprehensive review of orphan drugs (1983-2009), as well as a detailed analysis of smaller subsets of more recently approved orphan drugs.
METHODS
The primary source for this analysis was a public domain master list of orphan product designations and approvals published by the FDA OOPD.15 From this source, a list of all drugs with orphan product designations between January 1, 1983, and December 31, 2009, was extracted. The OOPD database records all brand or generic names, date of orphan designation, date of approval, proposed indication, specific indication, and sponsoring company. To avoid double counting specific products, the list was manually searched for drugs with multiple orphan designations, which were then combined into single entries if they had the same generic name and were marketed by the same manufacturer. This process allowed identification of the number of total orphan designations attached to each approved product, as well as the number of those designations that were approved by the FDA.
|
14 |
Coté T, Kelkar A, Xu k, Braun MM, Phillips MI. Orphan products: an emerging trend in drug approvals. Nature Rev: Drug Discovery 2010;9(1):84-85. |
|
15 |
http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. |
Once a full list of separate products was completed, it was supplemented with additional data obtained from a variety of sources. First, the FDA website was employed for individual product searches.16 From this source, a number of regulatory characteristics with respect to each drug were identified:
-
whether it was approved under a New Drug Application or a Biologics Licensing Application;
-
the original FDA approval date for a drug that was already on the market at the time of its orphan designation;
-
the review classification as priority (P), defined by the FDA as a drug that appears to represent an advance over available therapy; standard (S), defined as a drug that appears to have therapeutic qualities similar to those of an already-marketed drug; and/or orphan (O), defined as a product that treats a rare disease affecting fewer than 200,000;
-
the route of administration of the drug;
-
whether generic versions of the product are available and the date these generics were first made available; and
-
whether the drug product has been discontinued or removed from the market.
Further information was sought from the current product label for each orphan drug identified in the initial search. The product label is a formal, FDA-approved document describing the product, its approved indications, and pertinent safety and efficacy information. From the orphan-designated drug product labels, the following items were identified:
-
the chemical description of the product;
-
whether the product was intended to be a drug or a diagnostic tool;
-
the existence of nonorphan indications for the product; and
-
the existence of any “black box warnings” (the most severe product safety warning recommended by the FDA).
Finally, more in-depth information was obtained about a subset of drugs with orphan designations. For some approved drugs, individual product searches on the FDA website can also provide links to digital copies of the full FDA review packets. The review packets typically include the regulatory reviews by different FDA officers (medical, statistical, pharmacologic, etc.) and other formal regulatory documents associated with the agency evaluation of the drug. The medical officer review contains the
final statement from the medical officer, including a detailed description of the regulatory history, product development, and trials performed to prove efficacy and safety. Through this search process, copies of the full medical officer review were obtained for 81 approved orphan drugs from 2000 to 2009. From these data, the following items were identified:
-
the innovativeness of each drug, including whether it was (1) completely new, defined as a drug with a unique molecular structure that was unrelated to any drug previously approved by the FDA (i.e., “first in class”); (2) a variation of a prior drug, defined as a drugs with similar chemical structure that differed either by method of administration or peripheral chemical components;17 or (3) an old drug, defined as a drug that had already been available in U.S. and/or overseas markets;
-
comparative regulatory information about whether the drug was previously approved for its orphan indication in another similar market or whether the drug had been approved for any nonorphan indications; and
-
whether other treatments had been approved for the indication being sought.
For medical officer reviews obtained in the past 3 years (N = 30), further details about the clinical trial development process were extracted for the drug leading to its orphan designation and FDA approval. The goal was to describe the length and rigor of the clinical trial development process. During development, drugs undergo a number of trials intended to measure their effect on a certain disease. FDA medical officers designate the particular trials used to support a drug’s efficacy for a particular condition as either pivotal efficacy trials or supportive efficacy trials.
Apart from efficacy trials, drugs may also undergo a number of other human trials that impact knowledge about the safety of the drug; such trials could include early-stage Phase I trials on healthy volunteers, as well as open-label continuations of efficacy trials for drugs intended to treat chronic diseases. Efficacy trials, whether pivotal or supportive, also provide evidence of safety. The FDA judges the safety of a product for a particular indication based on all studies done on that product at the time of its review.
In this part of the analysis, the following items were identified:
-
the dates of IND application, orphan drug designation, NDA or Biologics License Application (BLA) submission, and approval;
-
the number of pivotal and supportive efficacy trials conducted on which the approval was based, including the number of comparator arms in those trials;
-
whether the efficacy trials were controlled and, if so, whether the drug was compared against an active comparator or a placebo;
-
whether efficacy trials were blinded;
-
whether the efficacy trials were classified as Phase I, II, III, or IV by the FDA medical officer;
-
whether the efficacy trials were multicenter or single-center studies;
-
the average time of exposure during the efficacy trials;
-
whether the end points of the efficacy trials were surrogate (hematologic markers, interval response rate, etc.) or final (i.e., mortality, disease cure, etc.);
-
the number of patients enrolled in efficacy trials;
-
the existence of a data safety monitoring board or independent review committee organized by the manufacturer to assist in evaluation of the efficacy trials;
-
the total number of human trials conducted by the manufacturer;
-
the total number of human subjects in whom the drug was tested;
-
whether the FDA identified methodological concerns about the clinical development trials;
-
whether published data were used to support the application;
-
whether the FDA convened an Advisory Committee to evaluate the drug prior to approval and, if so, whether the vote was unanimous; and
-
whether the FDA imposed postmarketing commitments on the manufacturer, and the nature of those requirements (i.e., additional trials, a patient registry, a Risk Evaluation and Mitigation Strategy [REMS]).
RESULTS
All Approved Drugs
From 1983 through 2009, the FDA approved 347 total drugs with orphan designations. However, a single drug can be approved for multiple orphan indications. For example, while somatropin (human growth hormone) accounted for 16 approvals overall, these approvals involved 9 brand-name drugs (some with multiple orphan approvals); the criteria used in this study—drugs having the same active ingredients and manufacturers—identified 6 “separate” products for further study (see Table B-1). Novartis’ imatinib (Gleevec) was approved for the treatment of chronic myelogenous leukemia (2001), gastrointestinal stromal tumors (2002), eosinophilic leukemia (2006), mastocytosis (2006), myeloproliferative disease
(2006), acute lymphoblastic leukemia (2006), and dermatofibrosarcoma (2006). Imatinib is therefore a single orphan drug with seven different disease-based approvals. To cite a different situation, the combination product benzoate-phenylacetate was initially approved in 1987 as Ucephan (Immunex Corp.), an oral formulation for management of hyperammonemia, but the manufacturer later withdrew it from the market. Another company then sought new approval as an orphan product for the same indication (but in an intravenous formulation) under the name Ammonul (Ucyclyd, a subsidiary of Medicis Pharmaceutical Corp.) in 2005. For the purposes of analyzing the impact of the Orphan Drug Act in this study, benzoate-phenylacetate counted as two separate products because it appears to have originated from two separate manufacturers and also to differ in formulation.
This process identified a subset of 279 separate orphan products among the original sample that were approved for 347 designations or indications. Within this subset, 233 products had a single approved orphan designation, 36 products had two designations, 5 products had three designations, 3 drugs had four designations, and 2 products had seven designations each
TABLE B-1 Orphan Approvals for Somatropin Products (human growth hormone, hGH)
|
Brand Name |
Year Approved |
Manufacturer(s) |
Comments |
Separate Product? |
|
Nutropin |
1985 |
Genentech |
|
Y |
|
Protropin |
1985 |
Genentech |
Identical to Nutropin, except for single amino acid on the N-terminus of the molecule |
N |
|
Humatrope |
1987 |
Lilly |
|
Y |
|
Serostim |
1996 |
Serono |
|
Y |
|
Saizen |
1996 |
Serono |
Designated as an orphan but not granted market exclusivity. Structurally equivalent to Serostim, but given a different brand name for a different indication |
N |
|
Genotropin |
1997 |
Pharmacia and Upjohn |
|
Y |
|
Nutropin Depot |
1999 |
Genentech |
New delivery system and slightly different formulation |
Y |
|
Zorbtive |
2003 |
Serono |
Same structure as Serostim and same manufacturer, although given different brand name for different orphan indication |
N |
|
Norditropin |
2007 |
Novo Nordisk |
Originally approved in 1995 |
Y |
(including imatinib). The sample included 275 therapeutic products and 4 drugs used as diagnostic agents. The FDA-defined regulatory classification of orphan-designated products could be identified for 208 products. Among that group, there were 133 products (64 percent) classified as NMEs.18 Information about review status was assessed for drugs approved after 1992, when the priority review classification was created; among orphan products, 144 (70 percent) were listed as Priority drugs, whereas 61 (30 percent) were classified as Standard.
Among the 279 products, small molecules (183, 65 percent) outnumbered biologic-based orphan products (96, 35 percent), although the ratio has changed in recent years as the number of new biologic products overall has increased. From 1990 to 1999, 27 orphan products were approved under a BLA (21 percent) and 101 were approved under an NDA (79 percent), while from 2000 to 2009, 32 products were approved under a BLA (29 percent) and 78 were approved under an NDA (71 percent). In the full product group, there were 214 (77 percent) products approved under an NDA and 65 (23 percent) approved under a BLA.19 Among the biologic-based drugs were 24 hormones, 18 clotting factors, 12 enzymes, 11 monoclonal antibodies, 9 antibodies, 9 protein conjugates, 7 cytokines, 4 proteins, and 2 biological mixtures.
The greatest number of the 279 orphan products was approved primarily for use in oncology-related conditions (79, 28 percent), predominantly chemotherapy, but also management of cancer-related conditions such as electrolyte disturbances and adverse effects of drug management. In the second-largest group, 43 products (15 percent) were approved for various infectious diseases, including HIV/AIDS-related conditions. The next largest clinical indications were neurological or psychiatric conditions (31, 11 percent) and enzyme deficiencies (28, 10 percent).20 Renal, cardiovascular, rheumatologic, dermatological, gastroenterological, and pulmonary conditions each made up less than 10 percent of the approvals. Thirty-six (13 percent) different products had indications specifically for pediatric
patients. Of the approved products, 83 were intended to be taken orally (33 percent); 136 through intramuscular, subcutaneous, or intravenous injection (54 percent); 6 directly applied to the eye (2 percent); 5 topical preparations (2 percent); 4 inhalants (2 percent); and 16 other miscellaneous preparations (6 percent).21
The data show that compared to nonorphan drugs, relatively few drugs approved with orphan designations are exposed to generic competition. Focusing on just those drugs approved before the year 2000 provides a fair assessment of the rate of generic competition, because the 7-year market exclusivity period is now over for that entire group. This analysis excluded
-
a number of orphan drugs because they were approved as biologic drugs under a BLA, for which no generic approval pathway existed;22
-
some of the more complex hormones approved under NDAs (e.g., somatropin) that the FDA has determined are not appropriate to approve as generics based on bioequivalence data under the current guidelines; and
-
25 drugs that have been removed from the market—no further explanation was provided on the FDA website for these removals, except in the case of two products, where the removal was listed as being “unrelated to safety issues.” Additional details about withdrawals could not be identified from the FDA website or online archives.
Among 108 qualifying products with orphan designation approved under an NDA from 1984 to 1999 that are still available, 49 (45 percent) had A-rated generic alternatives that were manufactured by a competitor.
Regulatory and Scientific Characteristics of Orphan Drugs
In the entire group of approved orphan drugs, among the 248 orphan products for which data could be found on the FDA website, there were 164 (66 percent) products for which the original FDA approval date coincided with the initiation of their orphan drug market exclusivity, meaning that their original approval in the United States was for an orphan indication. The remaining 84 (34 percent) had all been approved by the FDA for another indication prior to their orphan drug designation. To cull more information about the inventiveness, regulatory histories, and other clinical uses of drugs approved with orphan designations, full medical reviews from the NDA were available from the FDA website for 81 of the 101 drugs ap-
proved with orphan designations from 2000 to 2009.23 Additional information, including the current drug label, was available from the FDA website for all products, except nine clotting factors, approved during this time.
Among the drugs in this subset, 34 met the study definition of “new” (34 percent). Such drugs included those approved as a New Molecular Entity that had not previously been available in any other form anywhere in the world before the current regulatory submission.24
Another 36 (36 percent) were adaptations of or related to prior-approved drugs. Seventeen (47 percent) of these drugs involved changes in method of administration. For example, the intravenous orphan drug So-Aqueous (sotalol IV), approved in 2009 for ventricular tachyarrhythmias, was a variation based on method of administration of the oral orphan drug Betapace (sotalol) approved in 1992 for the same indication. The remaining 19 (53 percent) drugs were members of the same class as previously approved products. For example, ambrisentan (Letairis), approved in 2007 for pulmonary artery hypertension, is in the same drug class as the orphan drug bosentan (Tracleer), approved in 2001 for the same indication.25
Finally, there were 31 drugs (31 percent) that had previously been approved in the United States or elsewhere.26 Thirteen of the 31 old drugs (42 percent) were available in the United States at the time of their orphan drug approval.27 For example, raloxifene (Evista), approved in 2007 to reduce the risk of invasive breast cancer in certain high-risk post-menopausal women, was approved in 1997 for the prevention of osteoporosis in post-menopausal women. Twenty-seven of the 31 drugs (87 percent) were previously available overseas or in Canada. For example, Tindamax (tinidazole), approved by the FDA in 2004 for treatment of intestinal giardiasis, had
been approved for such use since 1975 in Australia and 1982 in the United Kingdom.
As seen in Figure B-1, numbers of approvals for these different categories show considerable variability from year to year. In 2000, three new drugs, two variations, and one old drug were approved as orphans. In 2009, one new drug, six variations, and seven old drugs were approved as orphans.
The data also show that a number of drugs from this sample were approved for orphan indications where approved therapy already existed for some aspect of the disease. Fifty-seven (56 percent) orphan drugs approved during this period were approved for diseases or conditions that had other approved therapeutic alternatives. For example, two antiepileptic drugs—topiramate (Topamax) and rufinamide (Banzel)—were approved during the period to manage Lennox-Gastaut syndrome, an extremely rare childhood form of epilepsy that occurs in about 0.2-2.8 per 10,000 live births. At the time that rufinamide was approved, felbamate (Felbatol) and lamotrigine (Lamictal) had also already been approved as orphan drugs for the condition.28 Similarly, five orphan products have been approved to treat pulmonary artery hypertension.29 This measure does not address whether different drugs were more or less effective for the particular condition, but in nearly all cases, head-to-head studies comparing two drugs approved for the same indication have not been conducted.
Orphan Drug Clinical Trials Development Process
The FDA lists 47 unique drugs approved for orphan designations between 2007 and 2009.30 In the final step of the analysis, the clinical trial development of these drugs was investigated in depth. For these drugs, the full medical officer reviews for 30 (64 percent) were located. The 17 drugs
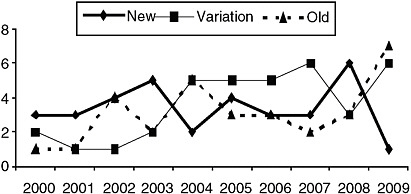
FIGURE B-1 Annual orphan drug approvals by “newness.” The thick line represents number of orphan drugs approved each year where the molecular structure is completely new. The dotted line represents the number of orphan approvals each year for drugs previously available on the market in the United States or elsewhere. The thin line represents the number of orphan drugs approved each year that were variations or members of the same class of previously approved drugs.
for which the details were not located included 9 clotting factors or immune globulins, 7 already-marketed drugs,31 and 1 other product.32
Of the 30 drugs for which the full medical officer reviews were analyzed, the NDA and IND dates were obtained for 17 of the products. An average of 3.8 years lapsed from the date of the IND to the date of the orphan drug designation, while an average of 5.9 years lapsed from the date of the IND to the date of the NDA. Approximately 0.7 years passed from NDA submission to approval.
The 30 drugs collectively underwent a total of 71 trials evaluating their efficacy. These efficacy trials enrolled a median of 75 participants (inter-
quartile range [IQR]: 34-157) and took a median of 8.5 weeks (IQR: 2-20 weeks). Fifty-five of those trials were considered “pivotal” efficacy trials for the approval of the product, while the remaining 16 were considered supportive. In total, 13 orphan drugs in this sample were approved based on a single efficacy trial, including 8 based on a single Phase III trial, 4 based on a single Phase II trial, and 1 based on a single Phase I trial. The sample as a whole was approved on the basis of a median of two efficacy trials (IQR: 1-2) per drug.33
Among the 55 pivotal trials, 27 were conducted in a double-blind fashion (49 percent), 5 were single-blinded (9 percent), and 23 were not blinded at all (42 percent). Thirteen of the trials were single-arm (24 percent). Thirty-eight of the trials were randomized (69 percent). Twenty-six of the pivotal trials were placebo-controlled (47 percent), while 11 used active comparators (20 percent) (these do not include historical controls or different doses of the drug itself). There were 30 pivotal Phase III studies (55 percent), 17 pivotal Phase II studies (31 percent), 1 pivotal Phase I study (2 percent), and 4 pivotal Phase IV studies (7 percent).34 There were 39 (71 percent) multicenter trials and 16 (29 percent) single-center trials.
Thirty-two of the pivotal trials (58 percent) used final end points, while 23 used surrogate end points (42 percent). For example, nilotinib (Tasigna), a drug approved in 2007 for “chronic phase (CP) and accelerated phase (AP) Philadelphia chromosome positive chronic myelogenous leukemia (CML) in adult patients resistant to or intolerant to prior therapy that included Gleevec (imatinib),” was approved on the basis of one pivotal efficacy trial measuring cytogenetic and hematologic response rates, not overall survival. FDA medical officers pointed out methodological concerns with efficacy trials relating to 7 of the 30 drugs in the sample (23 percent), although all drugs were approved. Notably, for four of the pivotal trials, the primary efficacy end point was not achieved or the improvement was not statistically significant (7 percent).
For the safety analysis, which included all efficacy trials as well as Phase I and Phase II trials and open-label extension studies, the sample of drugs approved for orphan designations underwent a median of 11.5 trials (IQR: 4.5-15), involving a median of 502 participants (IQR: 263-980). Among all 389 safety trials, 28 percent were Phase I trials (individuals without the disease in question), 45 percent were Phase II trials, and 13 percent
were Phase III trials.35 Fifteen drugs (50 percent) supplemented their safety records with references to already-published data, including experiences with the drug in other settings such as Europe and Canada. For 6 of the 30 drugs (20 percent), an independent data safety monitoring board or review committee was used during the clinical development process.
FDA medical officers identified life-threatening adverse events with 8 of the 30 drugs in the sample (27 percent). Formal expert FDA Advisory Committees were organized to provide opinions regarding the approval of 9 drugs (30 percent), voting their approval each time (although they were unanimous only 3 times). Postmarketing commitments were required for 12 products (40 percent), 5 (17 percent) manufacturers were required to conduct specific trials, 8 (27 percent) were required to set up an official REMS program, and 3 (10 percent) were required to initiate a formal data safety registry of patients.
ANALYSIS
This review of the regulatory and scientific characteristics of drugs developed under the Orphan Drug Act involved three different subsets of orphan drugs. The first subset was the full list of orphan drugs approved from 1983 to 2009. While there were a total of 347 approvals, those approvals included 279 separate drugs. Among that sample, the vast majority of drugs were approved only for a single orphan condition (most likely in the field of oncology).
The second subset was the 101 orphan drugs approved from 2000 to 2009; in this sample, more details of the drugs’ regulatory history and scientific context of their approval were assessed. The sample was roughly evenly divided among “new” drugs, drugs already available in the United States or abroad, and variations of previous drugs, although the numbers of old drugs and drug variations approved as orphan drugs increased over the time period.
The final subset consisted of the 30 products approved from 2007 to 2009 where full FDA medical officer reviews were available; in this sample, the clinical trial development process was analyzed. The results showed that clinical trial development took about 5.9 years, with official orphan drug designation occurring toward the end of the development process. Orphan drugs were generally approved on the basis of efficacy studies conducted in small numbers of participants. The efficacy studies varied in their complexity. Some pivotal studies were large, multicenter randomized trials where the product was tested against an active comparator. Others
lacked randomization and blindedness.36 Use of surrogate end points was common, although we do not know how the extent of use compares to trials of nonorphan drugs. A substantial minority of approved orphan drugs demonstrated important adverse events in their premarketing trials, and nearly half included postmarketing commitments intended to better assess their safety.
The study has a number of limitations. The data were obtained from freely available material on the FDA website, so to the extent that errors were made in posting that information, they may be reflected in these data as well. The medical officer reviews used for the in-depth analysis of trials followed a standard pattern but were composed by different authors. Though these reviews were often more than a hundred pages long, there may have been some details about the trials that were not mentioned in the final posted review. In addition, 14 medical officer reviews could not be obtained through the publicly accessible FDA website, a sample that included many replacement clotting factors and orphan drugs approved via a supplemental NDA pathway. Inclusion of these cases may have affected the proportions of efficacy and/or safety trials reported in this study.
The results from the clinical trial process suggest that the length of drug development for orphan drugs, on average, approximates similar estimates for nonorphan drugs and may even be slightly less. Orphan designation can be granted at any time during the development of a drug, even before IND designation. Although this analysis only included the IND and NDA dates for 17 products approved from 2007 to 2009, for these drugs, on average, orphan drug designation did not occur until well into the clinical testing phase. If a product was initially designed solely for a particular orphan disease, one might predict that orphan product designation would occur more frequently in close temporal proximity to the filing of the IND application that initiates manufacturers’ clinical trials. On the other hand, it may be that a new drug demonstrates a potential application to an orphan disease once it reaches Phase I or II trials. These results show that orphan product designation can occur closer to the final step in drug development (the NDA).
A substantial number of pivotal efficacy trials for orphan drugs were open label, non-randomized, and placebo-controlled. Surrogate end points were also common. Surrogate end points, which must be considered “reasonably likely … to predict clinical benefit,”37 can be well-suited to studies
of orphan drugs in cases where trials evaluating long-term clinical outcomes is not feasible and in cases where premarketing trials can be completed with fewer patients and with less cost. Mortality and other clinical outcomes can be rare and hard to measure, particularly in trials with a limited population of patients. However, because of their small numbers and shortened time frames, trials that assess surrogate end points may provide a limited view into a drug’s safety. Drugs approved on the basis of surrogate end points must be followed up with Phase IV verification studies, although the GAO has pointed out that for a substantial number of drugs approved on the basis of surrogate end points, including midodrine (ProAmantine), approved as an orphan drug in 1996,38 the required Phase IV studies have not been completed even after years of experience.39
From a safety standpoint, new orphan drugs were generally studied in fewer than 1,000 participant prior to approval, and nearly a third of those patients were young and healthy volunteers in Phase I trials. Therefore, the safety record for these products, as with all new drugs, is incomplete at the time of FDA approval. Monitoring the postapproval use of orphan drugs to evaluate potential safety concerns is important, especially for drugs approved despite serious methodological concerns expressed by FDA medical officer reviews. In the past, there have been cases where methodological concerns raised at the FDA level have not been translated adequately onto the label or in communications about an approved drug.40
|
38 |
Harris G. F.D.A. backtracks and returns drug to market. NY Times. 3 Sept 2010, at A11. |
|
39 |
General Accounting Office. FDA needs to enhance its oversight of drugs approved on the basis of surrogate endpoints (GAO-09-866). 23 Sept 2009. Available at: http://www.gao.gov/new.items/d09866.pdf. |
|
40 |
Schwartz LM, Woloshin S. Lost in transmission—FDA drug information that never reaches clinicians. N Engl J Med 2009;361(18):1717-1720. |


















