2
Profile of Rare Diseases
After going from doctor to doctor, I tried to think of how the doctors must have felt…. This is what I think: “This woman is presenting this odd disease [lymphangioleiomyomatosis] that no one knows how to treat, obviously no cure for, and she and her husband are sitting here looking at me all moon-eyed desperate for help with this dilemma they have been blind-sided with. What am I to do? … There simply are no set standards for this, I’m as helpless as she is, yet she has come to me asking for my help.”
Nutt, 2007
To have a rare disease is often to have a condition that goes undiagnosed for years while concerned physicians who have never seen the condition before may offer one diagnosis and then search for another when new or advancing symptoms belie the original diagnosis. Once accurately diagnosed, patients with rare conditions may be treated by physicians who have little evidence or guidance to help them—physicians who may experience the frustration imagined by the patient quoted above. Particularly when a condition is extremely rare, patients and families frequently have to travel long distances to consult with the few experts who have experience in treating and studying their rare diseases; patients and their families may even relocate to make access easier. Although the features of specific rare diseases can differ in myriad ways, the effects on life and functioning are often similar and are emotionally and financially devastating for the affected individuals and their families. Patients and family members may
feel isolated and alone as they face the challenges of finding helpful information, learning a new medical language, and generally charting their way in a daunting new world.
As described in Chapter 1, some rare conditions are extremely rare, found in only a few or a few dozen people. Others occur in hundreds, thousands, or as many as 200,000 people in the United States. Many are genetic in origin or have a genetic component. Others arise from exposure to infections or toxins, from faulty immune responses, or occasionally from adverse responses to therapeutic interventions for other conditions. For many rare conditions, the causes are frustratingly elusive.
Although people may think of a rare disease as something that happens to someone else, rare diseases can afflict anyone, at any age. They can be acute or chronic. Many are debilitating and present an ongoing risk of death. Some are inevitably fatal given current medical options. Approved therapies are available to treat several hundred of these conditions, but most currently have no therapy that cures or modifies the disease itself.
For the rarest conditions, the literature may consist of a single published report describing a few individuals with a previously unidentified genetic syndrome. For other conditions, including a number of the relatively more common conditions such as cystic fibrosis, sickle cell disease, and some cancers, publicly and privately sponsored research has generated a knowledge base that may encompass epidemiology (including natural history studies), genetics, disease mechanisms, diagnostic tests and standards, biomarkers and outcome measures, effective treatments, and evidence-based guidelines for clinical services.
Faced with these realities, many patients and families turn to advocacy groups concerned with specific diseases or to umbrella organizations such as the National Organization for Rare Disorders (NORD) and the Genetic Alliance for support and for information about their condition and available resources. As discussed at the end of this chapter, they may also join together to create new organizations.
This chapter begins with a general overview of what is known about the epidemiology of rare diseases based on data and analyses from the United States and Europe. Epidemiologic studies can provide clues and directions for basic and clinical research to determine the causes and mechanisms of rare diseases and develop methods to prevent, diagnose, and treat these conditions. Subsequent sections of this chapter discuss the varied causes of rare diseases and examine in broad terms the range of available preventive, diagnostic, and treatment strategies for diverse rare diseases. The last section considers the impact of a rare condition on patients, families, and the broader community and recognizes the efforts by patients, families, and advocacy groups to try, in turn, to have an impact on the disease and those affected by it. Reflecting the large number of rare diseases, their great vari-
ability, and the scarcity of systematic information about the spectrum of rare diseases collectively, the chapter makes frequent use of examples.
EPIDEMIOLOGY OF RARE DISEASES
Defining and counting rare diseases is not straightforward. Difficulties in obtaining definitive diagnoses contribute, as do limitations in systems for reporting and tracking such diagnoses. In addition, as described in Chapter 1, countries have adopted different definitions of a rare disease, and researchers are continuously identifying new diseases or disease variants. Therefore, the epidemiology of rare diseases—including the determination of prevalence (the number of people affected at any one time), incidence (the number of new cases in a given year), and patterns of disease (e.g., age distribution) in the population—is inexact.
Moreover, some conditions that initially are classified as rare eventually outgrow that categorization. For example, when AIDS emerged in the United States, it fit the legislative definition of a rare disease—affecting fewer than 200,000 individuals. As the infection spread, as diagnostic capabilities and data collection systems improved, and as researchers developed effective treatments that reduced mortality without curing the disease, the total number of individuals with AIDS grew to nearly 470,000 by 2007 and the number of individuals with HIV infection exceeded 1.1 million (CDC, 2009c).1
If effective but not curative treatment can turn a rare disease into a common one, effective prevention can, conversely, turn a common condition into a rare disease. This is the case with many once common childhood infections such as mumps and measles. Public health officials are concerned, however, that factors such as the development of drug-resistant infectious agents and the opposition of some parents to childhood vaccinations could reverse the situation for some now rare diseases. The former concern—drug resistance—is partly a significant scientific challenge (i.e., developing new anti-infectives) and partly a public health and clinical practice challenge (i.e., discouraging overuse of antibiotics). Preventing negative health consequences from anti-vaccination sentiment involves public health expertise, social science research, clinician communication skills, and public policy responses.
|
1 |
Under the Orphan Drug Act as described in Chapters 1 and 3, once a drug is designated as an orphan and undergoes further development, it then can be approved and qualify for 7 years of marketing protection even if the prevalence of the disease or condition at the time of approval exceeds the rare disease threshold. |
Objectives, Types, and Uses of Epidemiologic Studies of Rare Diseases
The objectives of epidemiologic research in rare diseases include determining the extent, distribution, and burden of these diseases at the population level and helping identify factors that may cause or contribute to their development. Basic epidemiologic studies generate estimates of incidence and prevalence. For congenital disorders, the statistic often reported is the proportion of births (e.g., 1 in 5,000) affected by the condition. Estimates may include breakdowns by age, gender, race or ethnicity, place of residence, and other factors that may offer clues to causation for further investigation.
Epidemiologic data have a variety of policy uses, including providing the prevalence data to support an “orphan” designation for an investigational or already approved drug. Companies seeking this designation must provide the Food and Drug Administration (FDA) with documentation that the proposed indication or use for the drug involves fewer than 200,000 people in the United States.2 For manufacturers seeking a Humanitarian Device Exemption, the FDA must document that the device is intended to treat or diagnose a disease or condition that affects fewer than 4,000 people in the United States per year.
Policy makers may also consider epidemiologic information on prevalence and disease burden—in combination with scientific, political, economic, ethical, and other factors—in making decisions about the allocation of resources for biomedical research. Decisions about research spending, for example, sometimes favor the relatively more common rare conditions such as ovarian cancer, neurofibromatosis, and sickle cell disease, but decision makers also have directed resources to extremely rare diseases, consistent with the value judgments underlying the adoption of special policies to encourage research on rare diseases. (See the analysis of National Institutes of Health [NIH] funding in Chapter 4.)
Natural history studies are another pillar of epidemiologic research on rare conditions. These studies track the course of a disease over time, identifying demographic, genetic, environmental, and other variables that correlate with its development and outcomes in the absence of treatment. Natural history studies have also generated important information about clinical (phenotypic) variation and have helped to identify subtypes of rare disorders that may be produced by different genes or by epigenetic factors that influence the effects of a gene. Such longitudinal studies are often a high priority for a rare disease organization or others interested in a poorly
|
2 |
Rarely, as discussed in Chapter 3, a sponsor will ask for designation based on another option provided by the Orphan Drug Act: that a condition affects more than 200,000 in the United States but there is no reasonable expectation that the cost of developing a drug for that condition will be recovered from sales in the United States. |
understood condition. Longitudinal studies of various sorts may also illuminate treatment effects.
Although natural history studies are not the primary focus of government- or industry-funded research, NIH and pharmaceutical companies as well as other entities do sponsor natural history studies of varying scope and complexity.3 For example, members of the NIH Rare Diseases Clinical Research Network (see Chapter 5 and Appendix E) are undertaking such studies for a number of rare conditions, including several neurological disorders and several forms of vasculitis. Understanding the natural history of a disease is an important step in the development of therapies. As discussed in Chapter 3, FDA staff have identified the lack of such studies as a problem with some applications for approval of orphan drugs.
In 2008, participants in a workshop sponsored by the National Heart, Lung, and Blood Institute and the Office of Rare Diseases Research at the NIH discussed models for analyzing genotype-phenotype associations in rare diseases and made recommendations for more longitudinal studies and also for refinements in study protocols and better tools to evaluate the resulting data (NHLBI, 2008a). It is too early to judge whether these recommendations will yield more high-quality proposals, an improved infrastructure, and more funding for such studies, which are challenging even for common conditions. Recommendations in Chapters 4 and 5 address problems with tissue banking practices and arrangements that limit or complicate their use for natural history and other studies.
Many epidemiologic data for rare diseases come from studies of single diseases. These studies are sponsored by a multitude of different sources and employ a range of methods and data. Data for prevalence or incidence calculations may come from birth certificates or death certificates; hospital discharge, insurance claims, and other administrative databases; patient registries; special surveillance studies; and newborn and other screening programs.
National data collection programs tend to focus on more common conditions, but information about the prevalence and incidence of some
rare conditions is generated through systematic disease tracking systems.4 The Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) collects data on a number of cancers, including some that are relatively uncommon. At the Centers for Disease Control and Prevention (CDC), programs on infectious diseases and birth defects track and report data on several rare conditions. The Agency for Toxic Substances and Disease Registry (ATSDR) tracks data on exposures to toxic substances with a focus on hazardous waste sites. The American Association of Poison Control Centers aggregates surveillance data from regional poison control centers, which report information on a broad range of poisonings, including those resulting from prescription and over-the-counter drugs, household products, and insect bites.
As newborn screening programs become more consistent in the United States, they may provide firmer data on the birth incidence of a number of genetic conditions. Work is continuing to develop a standard framework for reporting the results of newborn screening tests as part of electronic health records and also for analysis of trends by public health agencies (see description at http://newbornscreeningcodes.nlm.nih.gov).
For many rare conditions, one difficulty confronting epidemiologic studies involves the lack of condition-specific codes in the World Health Organization’s (WHO) International Classification of Diseases (ICD). The ICD provides the international standard diagnostic classification that is used for epidemiologic studies as well as for key health system management functions. To cite an example of the problem with lack of specific codes, a single ICD code (E75.2) covers Fabry disease, Gaucher disease, Krabbe disease, Niemann-Pick disease, Farber’s syndrome, metachromatic leukodystrophy, and sulfatase deficiency. (Codes for endocrine, nutritional, and metabolic diseases can be viewed at http://thcc.or.th/ICD-10TM/ge70.htm.) At the urging of a European rare diseases task force, WHO has created an advisory group to make recommendations about coding improvements for rare diseases. That group has been circulating draft materials for comment, which will be followed by field testing; implementation of coding changes is not expected until after 2015 (Aymé, 2009; Tejada, 2009). This project is complex, but its recommendations, if implemented, should strengthen the foundation for epidemiologic and other research on rare diseases. Much of the preparatory work on rare disease coding has been conducted by Orphanet, a European information consortium (originally established by the French Ministry of Health) (Aymé, 2009). Orphanet is also the source of the prevalence data discussed below.
Prevalence Data on Rare Diseases
It is estimated that FOP [Fibrodysplasia Ossificans Progressiva] affects about 3,300 people worldwide, or approximately one in two million people. Such statistics may be better grasped by the following example: if a large football stadium holds 100,000 fans, one would need to fill nearly 20 football stadiums to find one person who has FOP. At the present time, researchers are aware of approximately 700 people throughout the world who have FOP.
IFOPA, 2009, p. 3
“Who else has this rare disease? How many of us are there? What can I expect now? What is known or not known about this disease?” These are among the questions that patients and family members ask as they become, out of necessity, advocates for themselves or others. One step in learning about a rare disease is to determine its prevalence.
The prevalence of a disease in an area or jurisdiction may be expressed as the number, percentage, or proportion of people alive on a certain day who have been diagnosed with the disease. As described in Chapter 1, the European Union defines a rare disease as one with a prevalence of no more than 50 people per 100,000 population, whereas the United States sets a numerical maximum of fewer than 200,000 people in this country.
Prevalence is a function of both the incidence of disease (number of new cases reported in a given period) and the survival (duration of illness for self-limiting or curable diseases such as many infections). Table 2-1 displays NCI data that highlight how differences in survival affect the prevalence of three types of cancers with similar incidence rates but very different survival rates: poor for pancreatic cancer, intermediate for leuke-
TABLE 2-1 Differences in Prevalence for Three Cancers with Similar Numbers of New Cases per Year but Different Survival Rates, 2006
|
|
Estimated New Cases (2009 estimate in parentheses) |
Prevalence (complete)a |
|
Thyroid |
30,180 (37,200) |
410,404 |
|
Leukemia (all types) |
35,070 (44,790) |
231,857 |
|
Pancreas |
33,730 (42,470) |
31,180 (invasive) |
|
aAs defined by SEER, complete prevalence represents the number or proportion of people alive on a certain day who have been diagnosed with a disease, regardless of when the condition was diagnosed. SOURCES: Incidence: http://seer.cancer.gov/csr/1975_2003/results_single/sect_01_table.01.pdf; http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.01.pdf. Prevalence: http://seer.cancer.gov/csr/1975_2006/results_merged/topic_prevalence.pdf. |
||
mia (all types considered together), and good for thyroid cancer.5 Survival for pancreatic cancer is so poor that the estimated number of new cases per year can be higher than the estimated number of people surviving at a given time during the year.6
The committee found no broad compilation of data on the prevalence or incidence of rare diseases in the United States. It did, however, locate a recent report from Orphanet that lists estimated European prevalence for almost 2,000 rare diseases (out of an estimated 5,000 to 8,000 such conditions) (Orphanet, 2009). The list has much in common with the NIH list of rare conditions cited in Chapter 1. The demography, living conditions, and other characteristics of Europe and the United States likewise have much in common. Thus, despite the limitations discussed below, the committee believes that the overall portrait of rare diseases prevalence in the Orphanet report is likely to approximate that in this country.
Figures 2-1A-D show the distribution of rare conditions according to prevalence as presented in the Orphanet report. They reveal an overall distribution that is highly skewed to very rare conditions. In fact, data for approximately 1,400 of the approximately 2,000 conditions (about 70 percent) consist only of case reports for individuals or families. For the conditions not included in the study, the distribution may be even more skewed given that the project began with what were thought to be the more common rare conditions (Eurodis, 2005).
In general, the limitations of the data in the Orphanet report include the use of single numbers for conditions with widely varying estimates of prevalence in the literature7 and the lack of bibliographic citations and explanatory details.8 The committee did not systematically check the data presented in the report, but it did note that a few of the listed conditions
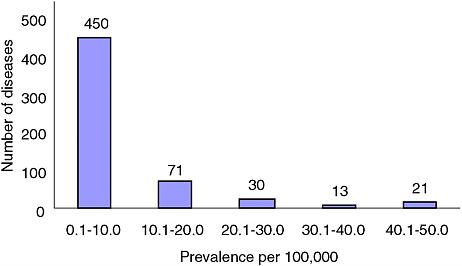
FIGURE 2-1A Number of rare diseases by prevalence up to 50/100,000.
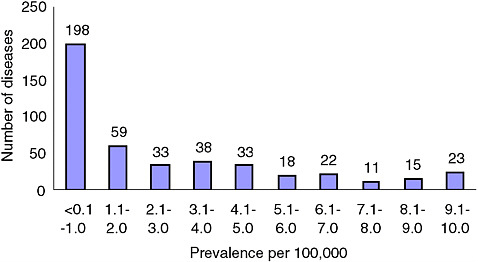
FIGURE 2-1B Number of rare diseases by prevalence of 10/100,000 or less.
(e.g., autism, pulmonary fibrosis) are not rare in the United States. The introduction to the report explicity notes (Orphanet, 2009, p. 2)
a low level of consistency between studies, a poor documentation of methods used, confusion between incidence and prevalence, and/or confusion between incidence at birth and life-long incidence. The validity of the published studies is taken for granted and not assessed. It is likely that there
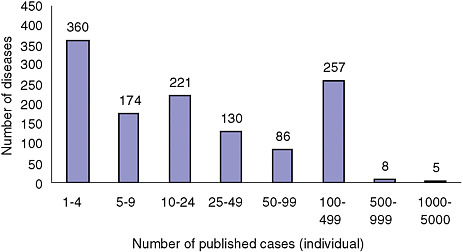
FIGURE 2-1C Number of rare diseases by number of individual cases in literature.
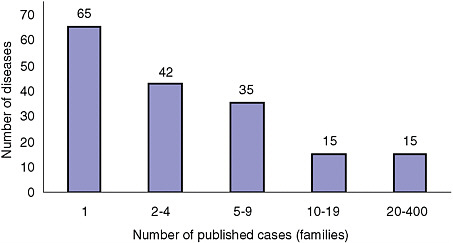
FIGURE 2-1D Number of rare diseases by number of family cases in literature.
SOURCE: Orphanet, 2009.
is an overestimation for most diseases as the few published prevalence surveys are usually done in regions of higher prevalence and are usually based on hospital data. Therefore, these estimates are an indication of the assumed prevalence but may not be accurate.
The factors cited illustrate problems inherent in trying to develop reliable prevalence estimates for rare conditions—individually and collectively. Again, notwithstanding these limitations, the committee expects that the
data provide a rough approximation of the overall distribution of rare conditions, at least for the conditions included.
In part because data on many conditions are limited to case reports or special population studies, no well-supported estimate exists for the number of people collectively affected by rare diseases. A 1989 government report stated that 10 million to 20 million Americans had a rare condition (NCOD, 1989); the corresponding estimates in 2009 range from 25 million to 30 million (see, e.g., ORDR, 2009). The estimates were not accompanied by analyses or substantive citation of sources.
CAUSES OF RARE DISEASES
“How did this happen? Why did this happen to me? What can I do?” Individuals and families struggle with these questions as they try their best to grasp the meaning and impact of a rare disease diagnosis. In the past two decades, epidemiologic, molecular, and other research that takes advantage of scientific and technological advances in the biological sciences has greatly increased the number of rare diseases that have an identified cause—usually, although not invariably, genetic. The Orphan Drug Act, the Rare Diseases Act, and other policy initiatives discussed in this report have contributed to this knowledge by focusing attention, resources, and incentives on the study of rare conditions and products to treat them.
Knowing the genetic, infectious, or other cause of a disease does not necessarily mean that researchers understand the mechanism of the disease. For example, much remains to be learned about Von Hippel-Lindau syndrome, even though mutations in the VHL gene have been identified as the cause and another gene has been implicated in phenotypic variations (Woodward and Maher, 2006). Moreover, a number of more common rare diseases such as cystic fibrosis and sickle cell disease have known causes and reasonably well understood mechanisms but lack cures, satisfactory treatments, or preventive strategies. Nonetheless, identifying the cause of a condition is usually an important step in building the knowledge base for prevention or effective treatment.
Some rare conditions have multiple possible types of causes. For example, some forms of aplastic anemia, which is caused by damage to stem cells in the bone marrow and is diagnosed in about 500 to 1,000 people each year in the United States, are inherited (e.g., Fanconi anemia). More often, though, the condition is acquired as a result of a toxic exposure (e.g., benzene, chloramphenicol), an infection (e.g., hepatitis, herpes virus), radiation or chemotherapy, or another disease (e.g., rheumatoid arthritis) (NHLBI, 2009). Doctors sometimes cannot determine the cause for a specific patient.
For certain rare diseases that have been named and characterized for decades, investigators still have not determined the cause. For example, although the disease was identified decades ago, no cause is known for Gorham’s disease, an extremely rare bone disorder that has been described under more than a dozen different names (LGDA, 2009). To cite other examples, the Vasculitis Research Consortium, which is part of the NIH-funded Rare Diseases Clinical Research Network, is investigating six forms of vasculitis (a group of rare conditions affecting blood vessels) for which the causes are not known (VCRC, 2010).
Genetic Causes
Notwithstanding the imprecision in the count of rare diseases and the difficulty of characterizing thousands of conditions, experts on rare diseases generally agree that the great majority of rare diseases—perhaps 80 percent or more—are genetic in origin (see, e.g., NORD, 2007; NIH, 2008). Many if not most are caused by defects in a single gene, for example, alpha1-antitrypsin deficiency (which may cause serious lung or liver disease) and Friedreich’s ataxia (a neurological disorder that may also be accompanied by cardiac and other problems). Multiple different mutations in that single gene may result in disease of varying features or severity. Other diseases, such as Fanconi anemia, have several named variants, each caused by a defect in a different gene (D’Andrea, 2010). Muscular dystrophy, which was once viewed as a single disease, now is described as having nine major forms, of which Duchenne muscular dystrophy may be the best known.
In some rare conditions, multiple genes may contribute collectively to manifestations of the disorder (Dale and Link, 2009). For example, a recent examination of Williams-Beuren syndrome describes one gene clearly identified as producing the condition’s cardiovascular problems and seven others with suspected roles in producing other common features of the disease (Pober, 2010).9 Continuing research on a number of “single-gene” conditions may suggest or identify additional (modifier) genes that influence the course of the disease, for example, the age at onset, severity, or organ system affected.
Rare genetic conditions are often inherited but may also arise as a result of sporadic or chance mutations. For example, about one-quarter of cases
of Marfan syndrome (a disorder of the body’s connective tissues) may be caused by sporadic mutations that occur by chance in a sperm or egg cell of an unaffected parent (Dietz, 2009).
In addition, some diseases such as sarcoidosis are known or suspected to be heritable, but the specific genetic mutation or mutations have not yet been identified. For other diseases, known genetic causes do not explain all cases and other genes are suspected to play a role. Organizations supporting research on inherited conditions typically make gene identification a top priority as illustrated by the example of the Progeria Research Foundation in Chapter 1 and the examples in Appendix F. Fortunately, the scientific and technical advances cited in Chapter 4 are making gene identification easier, faster, and less expensive.
Infectious Agents
A number of rare diseases have infectious causes. Despite their rarity, some infections such as rabies, botulism, and Rocky Mountain spotted fever are relatively well publicized and feared. Others are truly obscure, for example, Naegleria fowleri. Newspapers and medical journals occasionally highlight cases of extremely rare infections such as Lemierre’s syndrome, an often lethal disease (caused by Fusobacterium necrophorum) that was so nearly eliminated by the advent of antibiotics that it has been termed the “forgotten disease” (Boodman, 2009; Lu et al., 2009).
Some infections (e.g., those caused by Balamuthia mandrillaris and Chromobacterium violaceum) are thought to be rare worldwide (de Siqueira et al., 2005; Glaser et al., 2008). Others, however, are rare in wealthy countries but common in less economically developed countries. Some of these, for example, tuberculosis, were common in wealthy countries such as the United States before effective preventive measures or treatments were discovered and widely applied. One anxiety is that the development and spread of extremely drug resistant strains of tuberculosis and certain other diseases could—absent effective countermeasures—lead to their resurgence in areas where they are now rare. For example, the late 1980s and early 1990s saw a resurgence of tuberculosis in the United States when the number of cases reported rose by 20 percent and several outbreaks in hospitals affected both patients and staff (IOM, 2001).
As discussed in Chapter 1, public health experts and global nonprofit funders have highlighted several infectious diseases as neglected and have promoted international efforts to increase knowledge of these conditions, undertake intensive prevention campaigns, and develop affordable treatments. They also seek to make existing treatments affordable for poor patients and nations.
Research suggests that genetic factors may affect susceptibility to infectious agents, either increasing susceptibility or having a protective effect.
For example, research indicates that sickle cell trait contributes to resistance against malaria. Other genes are likely to affect susceptibility to malaria (Faik et al., 2009) and leprosy (Zhang et al., 2009).
Toxic Agents
Some rare diseases or conditions result from exposure to natural or manufactured toxic substances, including substances that appear as product contaminants. In the United States, examples include arsenic and mercury poisoning, mesothelioma (a cancer caused by exposure to asbestos), and eosinophilia-myalgia syndrome, which is associated with contaminated (or overused) tryptophan, a dietary supplement.10
It is likely that far more types of poisoning could be listed as rare conditions than are included in the list maintained by the Office of Rare Diseases Research (ORDR) at NIH. For example, the committee found newspaper reports of rare cadmium, chromium, phosphine, and other poisonings in the United States, but none is listed as a rare disease. These toxic substances are a concern of ATSDR.
Also not listed are rare poisonings caused by a variety of marine toxins that may contaminate seafood and that are tracked by the CDC (CDC, 2005). Likewise, Amatoxin poisoning, a rare and often fatal illness caused by Amanita phalloides—the “death cap” mushroom—is also not listed. Approximately 50 cases are diagnosed each year in the United States. Doctors who treat patients with this poisoning sometimes obtain FDA approval for emergency use of a milk thistle extract that is manufactured in Europe but that has not clinically evaluated or approved for marketing as a drug in the United States (Coombs, 2009).11
Some drugs have received orphan designation and approval for treatment of rare poisonings. For example, FDA has approved an orphan drug for the treatment of acute cyanide poisoning (hydroxocobalamin [Cyanokit]). Several agents have received orphan designations for treatment of snakebites, but only one has been approved (Crotalidae polyvalent immune fab [ovine] [CroFab] for certain rattlesnake and other snake bites).
Other Causes
Rare conditions may have a variety of other causes. Examples include conditions caused by nutritional deficiencies (e.g., beriberi, which results from thiamine deficiency and is rare in the United States [Medline Plus, 2008]) and injuries (e.g., commotio cordis, in which ventricular fibrillation and sudden death is associated with a nonpenetrating blow to the chest [Maron and Estes, 2010]).
Certain rare conditions are caused by the persistent adverse or toxic effects of treatment for another disease. For example, the ORDR list of rare diseases includes radiation-induced meningioma, which is a rare central nervous system tumor. Secondary cancers are a well-understood risk of radiation therapy and also chemotherapy. FDA has approved a few orphan drugs for the treatment of adverse effects of certain therapies for cancer and other conditions (e.g., dexrazoxane [Totect] for leakage of intravenous anthracycline into surrounding tissue and deferasirox [Exjade] for treatment of chronic iron overload from frequent blood transfusions).12
As is the case with illness caused by poisons, treatment-related illness is not a primary focus of rare diseases policy as such. Drug toxicity and safety in general are, however, major concerns of FDA and an array of other government efforts to protect patient safety. For example, progressive multifocal leukoencephalopathy (a rare brain infection that has been diagnosed in multiple sclerosis patients who have taken the drug Tysabri) has been the subject of several FDA safety notices (see, e.g., FDA, 2010a).
PREVENTION, DIAGNOSIS, AND TREATMENT
For rare diseases collectively, possible preventive, diagnostic, and treatment options and outcomes span a huge range. Some rare diseases are now preventable, many are not. Diagnosis is sometimes straightforward but often frustratingly slow. Cures exist for a few conditions but are a distant hope for most. For some conditions, disease-modifying therapies may allow a nearly normal life, whereas for others, the impact on morbidity and mortality may be very modest. Treatment of symptoms is the mainstay in many cases.
This section and the next offer a broad, descriptive perspective on the range of preventive, diagnostic, and treatment measures; these topics could form the subject for a report in themselves. The discussion is intended to illustrate public health and clinical practices, rather than to evaluate them or provide recommendations.
Prevention
The prevention of rare diseases may take different approaches. Some preventive strategies are relatively simple but striking in effect, while others are complex and demanding. Some raise ethical questions. The discussion below considers primary and secondary prevention. Tertiary prevention, which involves treatment of evident disease to avoid further progression or suffering or to restore health or function, is considered here as treatment. (Other frameworks for prevention policies and research have been developed, particularly for mental disorders [see, e.g., IOM, 2004, 2009a].)
Primary Prevention
Primary prevention seeks to eliminate or reduce risk factors that cause disease. Prevention is a mainstay of the infection control programs of public health agencies. Common primary prevention measures include immunizations (which are usually aimed at conditions that are or have been relatively common) and hand washing and other basic sanitation measures that are employed to control both common and rare infections. These measures, particularly immunization, have made a number of once-common infections, such as chicken pox and measles, rare. Other public and private programs seek to reduce population exposure to toxic agents such as asbestos and mercury. Measures include bans or strict controls on the use of toxic agents and programs to clean up contaminated locations, including buildings in which asbestos is present and abandoned industrial or military sites that are multiply contaminated.
A different type of primary prevention is exemplified in the promotion of folic acid supplementation for women of childbearing age to prevent neural tube defects in their children. Neural tube defects that are listed as rare by ORDR include spina bifida and anencephaly. To prevent fetal exposure to harmful agents, many medications come with prominent warnings advising against use of the drug for pregnant women. Other drugs are approved with special precautions to limit the chance of fetal exposure. For example, thalidomide, a drug best known from the late 1950s for causing birth defects in children of mothers who were prescribed the drug for morning sickness, is now FDA-approved for treatment of two rare conditions (multiple myeloma and erythema nodosum leprosum). FDA required a
restricted distribution program that is limited to registered physicians and pharmacists and to patients who agree to actions to minimize the risk of fetal exposure (Celgene, 2010).
Preventive measures for certain rare diseases sometimes involve very personal and intimate decisions about marriage and childbearing, and some measures may raise ethical questions. For a few serious genetic conditions, such as Tay-Sachs disease, thalassemia, cystic fibrosis, fragile X syndrome, and familial dysautonomia, screening and counseling programs have been developed to identify and advise individuals who carry the gene for the condition (Kaback et al., 1993; Gessen, 2008; Lerner, 2009; Zlotogora, 2009). High-risk couples may be advised about a range of options, including avoiding marriage to another person who is a carrier for the same disease, using contraceptive methods to avoid pregnancy, undergoing in vitro fertilization with embryonic screening, or obtaining prenatal screening with the possibility of pregnancy termination or planning for the birth of an affected child. After genetic testing and community-organized information counseling programs became available, the incidence of Tay-Sachs disease in the United States and Canada dropped by 90 percent from 1970 to 1992 for the Jewish population most at risk (Kaback et al., 1993). Individual or population genetic testing has also been linked to a significant decline in familial dysautonomia for which the incidence is the United States has reached as low as a single case in recent years compared to 10 to 12 cases in many of the years before testing became available in 2001 (Lerner, 2009). Some fear that the result will be less attention to treatment research and assistance for people who have an already rare disease of diminishing prevalence.
Secondary Prevention
Secondary prevention strategies involve screening or testing to identify a condition so that effective treatments can be provided to people before the onset of debilitating symptoms or complications. (Diagnosis when symptoms are evident is discussed below.) Newborn screening programs, which use biochemical or genetic blood tests, are prominent examples. In 2005, the American College of Medical Genetics (ACMG) recommended screening for 29 mostly inherited, serious, rare conditions (Watson et al., 2006). These recommendations were endorsed by a U.S. Department of Health and Human Services advisory panel on heritable disorders and genetic diseases in infants and children (Howell, 2005). As described by the ACMG, the conditions fall in five broad categories: organic acid metabolism disorders, fatty acid oxidation disorders, amino acid metabolism disorders, hemoglobinopathies, and other disorders.
According to a recent study, the “estimated number of cases of disorders that would have been identified in 2006 using the ACMG panel was
6,439, [which is] 32 percent more than the 4,370 that would have been identified otherwise” (Therrell et al., 2008, p. 1012). Four conditions (three hemoglobin disorders and congenital hypothyroidism) accounted for approximately 60 percent of this total, whereas nine of the screened conditions accounted for an estimated 15 or fewer cases.13
All states have newborn screening programs, although they vary in the conditions screened, particularly those outside the recommended core tests (NNSGRC, 2010). Technologies to expand the number of disorders screened are available. The CDC has created the Newborn Screening Translation Research Initiative (CDC, 2009d), and NIH has created the Newborn Screening Translational Research Network (NICHD, 2009). Aside from the availability of new screening technologies, the expansion of screening panels will be influenced by affordability (and possibly cost-effectiveness, taking into account the rate of false positive results), political considerations, and the continued emergence of new therapies that are beneficial when instituted early in life.14
Newborn screening may also trigger genetic testing and counseling of family members. It thereby offers a further opportunity for prevention when monitoring or early treatment can be effective in delaying or limiting the consequences of the condition for an affected sibling or other family member. Parents may first learn of their membership in a rare disease population after a child’s screening.
In addition to allowing treatment at the outset to help prevent damage, the early identification of children with rare disorders can facilitate research by (1) providing a pool of potential research participants who have not developed advanced disease or serious disease-related complications and (2) allowing segmentation of potential participants by genotype (when genetic testing is available) to create more homogeneous study groups, which are important in complex conditions to differentiate the effects of treatment from the effects of other factors. Research use of retained samples from newborn screening programs has generated controversy about whether
such samples should be used in the absence of informed parental permission (Maschke, 2009).
Diagnosis
For many patients, diagnosis comes a frustratingly long time after symptoms first become evident. It follows countless tests and visits to different specialists and centers with multiple diagnoses considered and initially or eventually rejected. This kind of diagnostic odyssey for a rare condition is often described in television shows and newspaper stories about diagnostic mysteries.
A survey of 801 patients conducted in the late 1980s for the National Commission on Orphan Diseases found that approximately one in three reported that obtaining a diagnosis took from 1 to 5 years and one in seven reported that it took 6 years or more (NCOD, 1989). A European survey that focused on eight rare diseases (including cystic fibrosis, Duchenne muscular dystrophy, and Marfan syndrome) received nearly 6,000 completed surveys (Faurisson, 2004). Forty percent of respondents reported their first diagnosis was wrong, and 25 percent reported waiting between 5 and 30 years for a correct diagnosis. Accurate and timely diagnosis is especially important when early diagnosis can significantly affect the course of the disease.
For a few patients whose conditions have defied diagnosis, the NIH Undiagnosed Diseases Program, which was established in 2008, may help. From May 2008 to approximately December 2009, the program received more than 2,300 inquiries and more than 900 medical records, and it accepted 190 patients for evaluation (Garnett, 2010; see also Henig, 2009).
The diagnosis of many rare diseases has been limited historically by imprecise, cumbersome, or expensive testing and by limitations on physician and patient access to the most up-to-date information about rare diseases (including diagnostic criteria) and other diagnostic resources. Clinical specialization and subspecialization also contribute to the extent that specialists focus on their piece of a patient’s complex of symptoms. For example, because of multiorgan involvement, patients with cystic fibrosis may be diagnosed by pulmonologists, gastroenterologists, allergists, or general pediatricians.
When patients or families seek medical help, the initial stages of diagnosis usually still depend on classic clinical practices—the physical examination and taking of a patient history, the use of blood and other laboratory tests, and the application of clinical knowledge and reasoning skills. One dilemma for clinicians and patients is that many rare diseases have neurological, digestive, or other symptoms that accompany a number of common and rare conditions, and depending on the disease and the individual patient, laboratory results may or may not be definitive. Physicians normally will consider common conditions that are consistent with the available
information before considering rare conditions. The widely used clinical aphorism is, “When you hear hoofbeats behind you, don’t expect to see a zebra.”15 Another diagnostic complexity is that a patient may have an atypical presentation of a common disease. Finally, a patient may have an atypical presentation of a rare disease, which may make it almost impossible to arrive at a diagnosis.
Although genetic tests are crucial to the diagnosis of many rare genetic conditions, the ordering of a specific test or set of tests typically depends on a clinician’s evaluation and ability to recognize clues pointing to conditions for which genetic testing may be warranted. According to a unit of the publicly funded National Center for Biotechnology Information, approximately 1,500 tests are now available to assess whether a person carries a gene mutation associated with a specific disease (NCBI, 2009). That number, although growing, still falls considerably short of the number of rare diseases that are thought to be genetic. Examples of genetic conditions for which genetic tests are not commercially available include sitosterolemia (Steiner, 2009) and KBG syndrome (Brancati et al., 2006). NIH recently announced the creation of a registry of genetic tests (NCBI, 2010), and another database GeneTests, which is operated by the National Center for Biotechnology Information, has been available for several years (http://www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests).
Physicians and patients may, however, be frustrated that some genetic tests are only available from a few laboratories based on decisions by patent holders (Cook-Deegan, 2008; Kesselheim and Mello, 2010). Moreover, testing may be very expensive and may not be covered by health plans. Some tests or genetic testing services are marketed not to physicians but to consumers, a development that has provoked considerable controversy (see, e.g., Schickedanz and Herdman, 2009; GAO, 2010a) as well as FDA and Congressional scrutiny (Carmichael, 2010). As discussed in Chapter 7, FDA regulates genetic tests that are packaged as test kits for laboratories that do genetic tests, but it generally does not regulate tests that are both produced and performed by laboratories. It is now reconsidering that approach.
Genetic counseling is recommended for individuals and families following the diagnosis of a rare genetic disorder to help them better understand the disorder, consider their options, and plan for the future. Family members may be advised about their options to be tested.
Many organizations that educate, assist, and advocate for patients with rare conditions seek to educate physicians about the disease. One goal is to increase the likelihood that physicians will recognize certain symptoms
or constellations of symptoms as associated with a particular rare disease and will consider that disease among diagnostic possibilities that should be evaluated further. For example, the Cystic Fibrosis Foundation developed criteria to help standardize approaches to diagnosis (Rosenstein and Cutting, 1998).
Treatment
With the current state of medical science, most people here [with GIST] will never be “cured.” … Thus, considering the alternative, I look forward to pills, surgeries and scans for decades to come (well, really, I am hoping for a cure or more effective treatments to arise sooner).
Patient with GIST (gastrointestinal stromal tumor) Quoted at http://www.gistsupport.org/voices-of-gist/essays/brendas-war-on-gist.php
Discussions of treatment for rare diseases tend to focus on care for a single condition (e.g., Niemann-Pick disease) or a set of related conditions (e.g., lysosomal storage disorders). The NORD Guide to Rare Diseases (2003, with updated and expanded information available online) includes brief overviews of treatment for several hundred rare conditions, but the committee is not aware of reviews of treatment practices and options over the spectrum of rare diseases.16 Various textbooks, online sites, and other resources advise on treatment for a broad range of infections, including some that are rare; other resources advise on treatments for a broad range of poisonings, again including some rare poisonings. Many rare diseases have been discovered relatively recently, so researchers have had limited time to work on identifying their causes and mechanisms of disease as the basis for investigating treatment targets or preventive strategies.
Some reviews have discussed treatment of genetic diseases with varying degrees of breadth. For example, a 1999 review of 372 genetic diseases reported that 34 percent had no effective treatment, 54 percent had treatments that produced partial responses, and 12 percent had treatments that produced complete responses (Scriver and Treacy, 1999). These numbers undoubtedly have changed in the past decade. For example, one of the authors of the 1999 study coauthored a 2008 review of treatment over a 25-year period for 65 conditions involving inborn errors of metabolism. This review reported that “the number of conditions for which there is no
|
16 |
The Cochrane Collaboration has a cystic fibrosis and genetic disorders group that has developed 108 systematic reviews (or plans for reviews), including 61 reviews for various aspects of cystic fibrosis, 26 reviews for sickle cell disease, and 12 reviews for several conditions involving inborn errors of metabolism (see the list at http://www2.cochrane.org/reviews/en/subtopics/55.html). |
response to treatment has progressively decreased; from 31 in 1983, to 20 in 1993, to 17 in 2008” (Campeau et al., 2008, p. 11). It also reported that the number of conditions that fully responded to treatment increased from 8 in 1983 and 1993 to 20 in 2008. As reasons for this progress, the authors cited “new small molecules, new enzyme replacement therapies, more conditions that can be treated by organ and cell transplantation, and new experimental approaches” (p. 11). These analyses involve small and highly selected subsets of all genetic diseases and are likely biased toward those that are well studied and for which there are treatments.
A 2004 textbook review of treatment for genetic diseases observed that treatments judged to be successful initially may later show their limitations (Nussbaum et al., 2004). This pattern may reflect the recognition over time of subtler manifestations of the disease, long-term adverse effects of treatment, and manifestations of the disease not recognized until treatments allowed longer survival. Because drugs are approved on the basis of relatively short-term clinical data involving unrepresentative patient populations, FDA often requires drug sponsors to undertake additional studies following the approval of a drug for marketing. The 2004 review linked the “unsatisfactory” state of treatment for genetic conditions to lack of identification of the causal gene; inadequate understanding of pathophysiology; and irreversible damage at the fetal stage before diagnosis.
Dietz (2010) recently reviewed therapeutic approaches to Mendelian disorders, focusing on approaches that use detailed knowledge of disease pathogenesis. This review, which is cited further in Chapter 4, explores how such understanding is contributing to investigations involving, for example, the replacement of deficient gene products (gene therapy, enzyme replacement therapy); the use of FDA-approved drugs in novel ways; the design of new small-molecule compounds; and the manipulation of gene expression. To repeat a theme of this report, research resources for rare diseases are limited, both collectively and individually. Nonetheless, basic and clinical research have yielded disease-modifying therapies for many conditions.
Table 2-2 illustrates the range of treatments—from surgery to diet and from stem cell therapy to environmental adaptation—that may be deployed for specific rare conditions. Some of these therapies have been used for decades, while others have emerged through technological advances. Many of the procedures cited are accompanied by complex pharmaceutical regimens—some short-term, others indefinite (e.g., use of immunosuppressive drugs following an organ transplant). As with any therapy, expected benefits are often accompanied by risks that may include significant harms. It is important for patients and families to understand and weigh both potential benefits and potential harms of treatment options.
Another way of looking at treatments for rare diseases is to consider
TABLE 2-2 Examples of Currently Available Treatments or Treatments in Development for Rare Diseases
|
Therapeutic Category |
Treatment Example |
Rare Condition |
|
Small-molecule compounds |
Imatinib |
Chronic myelogenous leukemia |
|
Protein therapies |
Enzyme replacement therapy |
Gaucher disease |
|
Metabolic therapies |
Sodium phenylbutyrate |
Urea cycle disorders |
|
Nutritional therapies |
Phenylalanine-restricted diet |
Phenylketonuria |
|
Environmental modification or adaptation |
Avoidance of sunlight |
Xeroderma pigmentosa |
|
Medical procedures |
Phlebotomy |
Hemochromatosis |
|
Surgical procedures |
Open heart surgery |
Tetralogy of Fallot |
|
Medical devices |
Orthopedic implant |
Thoracic insufficiency (e.g., Jeune syndrome) |
|
Organ transplants |
Combined liver-kidney transplant |
Primary hyperoxaluria |
|
Bone marrow or cord blood transplants |
Bone marrow or cord blood transplant |
Hurler syndrome |
|
Stem cell transplants (investigational) |
Neural stem cell transplant |
Neuronal ceroid lipofuscinosis |
|
Genetic therapies (investigational) |
Exon skipping |
Duchenne muscular dystrophy |
|
SOURCE: This table draws from Nussbaum et al., 2004; Dietz, 2010; Maegawa and Steiner, in press. |
||
the range of effectiveness of treatments or the variability in what is anticipated from the use of different therapies. Treatments may be
-
curative,
-
disease modifying, or
-
symptom or function modifying.
Curative Treatments
Truly curative treatments for rare conditions are themselves rare. Immediate treatment may be completely successful for all or most cases of certain rare infections (e.g., Tropheryma whipplei) or certain rare poisonings (e.g., from snakebites or cyanide). Vitamin D supplementation generally cures rickets, although for one form (X-linked hypophosphatemic rickets), a combination of phosphate and a form of vitamin D will treat but not cure the condition (Imel et al., 2010).
Some rare anatomical defects can be corrected (essentially cured) with
surgery, for example, coarctation of the aorta. Certain conditions that can be treated effectively with surgery, such as transposition of the great arteries or tetralogy of Fallot, have features beyond the intrinsic anatomical anomaly that require continued medical attention.
Organ transplantation is considered curative for a few rare conditions, for example, heart transplantation for hypoplastic left heart syndrome. For carefully selected subsets of patients, bone marrow transplantation or transplantation of stem cells from umbilical cord blood is, if successfully performed, considered a cure for Diamond-Blackfan anemia, Wiskott-Aldrich syndrome, and paroxysmal nocturnal hemoglobinuria as well as some cancers (Filopovich et al., 2007; Brodsky, 2009; Clinton and Gazda, 2009). Although they may be considered cures, such procedures come). with significant short- and long-term health risks from the procedure itself and the necessary follow-up care (e.g., use of immunosuppressive drugs). Moreover, transplants are sometimes lifesaving but not curative. For example, umbilical stem cell transplant can save some children with infantile Krabbe disease from death, but they will still have major neurologic deficits (Duffner et al., 2009).
Disease-Modifying Treatment
Disease-modifying therapies are targeted to the underlying pathology of a disease in order to prevent its progression or otherwise limit the harm it creates. For example, with galactosemia, a potentially fatal disorder of galactose metabolism, the restriction of milk products immediately upon diagnosis through newborn screening will interfere with the pathology of the disease and prevent its severe manifestations. Children may still, however, experience various problems such as speech and language difficulties (Lai et al., 2008). Kidney transplantation is lifesaving but not curative for individuals who have nephropathic cystinosis; early initiation of disease-modifying treatment with cysteamine can significantly delay complications (Kleta and Gahl, 2004)
For many disease-modifying therapies, the treatment effect is short-lived and must be repeated indefinitely. Examples include enzyme replacement therapies for conditions such as Gaucher disease, which involves the ongoing use of a biologically created product to act in place of the enzyme that is missing or deficient as a result of a genetic defect. Depending on the condition, such therapy may be effective for some manifestations of the disease but not others (e.g., liver- and bone-related but not brain-related aspects of Gaucher disease) (Schmitz et al., 2007).
In some cases, the mechanism of action of a disease-modifying drug may not be clear. An example is riluzole, which is associated with a modest survival benefit for amyotrophic lateral sclerosis (Bellingham, 2010). An-
other example is hydroxyurea, which is the only disease-modifying therapy identified for sickle cell disease (Segal et al., 2008).
Rational drug design specifically aims to develop new drugs based on knowledge of disease biology. This strategy holds promise for many rare conditions for which no disease-modifying therapies are known. Current treatment for these conditions still emphasizes treatment of symptoms and prevention of complications.
Symptomatic and Functional Therapies
Symptomatic treatments are vital to patient well-being for many chronic rare conditions, especially when more definitive therapies are not available. Painful and distressing symptoms of many rare as well as common diseases include pain, nausea, bladder or bowel dysfunction, itching, dizziness, movement limitations, and speech dysfunction to name a few. Treatments also seek to treat or prevent other disease- or treatment-related complications, for example, infections (such as the bronchitis or pneumonia caused by cystic fibrosis or primary ciliary dyskinesia), anemia (such as that associated with hereditary spherocytosis), and delayed growth (such as that associated with X-linked hypophosphatemic rickets).
To temper symptoms and preserve or improve physical, intellectual, and emotional functioning, clinicians may use a wide variety of therapeutic methods. These include medications, nutritional agents, surgical procedures, psychotherapy, physical and occupational therapy, complex medical devices (e.g., sophisticated communication devices), and less complex devices (e.g., braces).
The above discussion emphasizes the physical dimensions of treatment. Care-giving extends well beyond the physical to include psychological, spiritual, and practical support. These dimensions of care may be especially significant for individuals and families facing serious illness. Genetic counseling is important for individuals and families facing the new diagnosis of a genetic disorder. Also, because many rare disorders are fatal, end-of-life care is important to help patients (to the extent they are able to participate) and families plan for an expected but not necessarily predictable death and to make difficult decisions about the site and nature of care. After a death, continued support can help families and others cope with grief and other consequences of loss.
Delivering Preventive, Diagnostic, and Treatment Services
A variety of factors—including lack of knowledge, lack of resources, or failure to follow recommendations—may interfere with a physician’s or patient’s use of effective diagnostic techniques, preventive measures, and
treatments. Although this report focuses on research and development and not the movement of effective treatments or preventive or diagnostic measures into practice, that movement is crucial if the benefits of research are to be realized in the lives of patients and their families.
One common mission of advocacy organizations is to educate clinicians about rare conditions as a means of improving the provision of care, including the appropriate consideration of new diagnostic and therapeutic options. Depending on the condition and the organization, other strategies may include the development of clinical practice guidelines, quality improvement and assessment programs (including incentives for meeting quality standards), and continuing medical education and consumer education activities.
This section briefly discusses just a few issues in health care delivery that may affect the availability or quality of care provided to people with rare conditions. It does not examine the development and use of clinical practice guidelines, the challenges of emergency care, the role of electronic health records or information systems, or the cost or financing of services. Chapter 6, however, examines health plan coverage and reimbursement of orphan drugs, and Chapter 7 examines coverage and reimbursement of devices marketed for small populations under a Humanitarian Device Exemption.
Specialized Centers for Rare Diseases
For both common and rare diseases, the creation of medical centers or medical practices specializing in the diagnosis and treatment of a disease is a frequent strategy to improve the quality and consistency of care. For rare diseases, specialized centers can offer consultations to outside clinicians, develop care guidelines based on available evidence and experience, and serve as an established referral site in emergencies or other situations in which local resources are insufficient. These centers can also provide a base for research.
One of the early priorities of the Cystic Fibrosis Foundation (CFF) was the establishment of a network of accredited care centers. From two centers at the outset in 1961, CFF now accredits 115 care centers as well as 95 adult care programs (CFF, 2008, undatedb). The foundation has also designated 10 centers as basic research centers and more than 70 as sites for its Therapeutics Development Network (CFF, undatedb).
In 1972, Congress authorized the creation of comprehensive research and treatment centers for sickle cell disease. These centers were subsequently established by what is now the National Heart, Lung, and Blood Institute. In 2007, the American Society of Hematology recommended a number of revisions in the program “to ensure that clinical research is conducted in
a milieu where federally funded comprehensive care programs include a much larger proportion of children and adults with sickle cell disease than is currently served by existing centers, networks, and other governmental support programs” (ASH, 2007). In addition, the organization has recommended changes in the program to promote multidisciplinary, multicenter, collaborative research and more resources for translational research.
Among other examples of specialized centers to improve care delivery, the Children’s Tumor Foundation has created a Neurofibromatosis Clinic Network of affiliated clinics that meet operational principles established by an advisory board. The organization had recognized 38 such clinics in the United States by the end of 2008 (CTF, 2009). The CDC funds comprehensive treatment centers, including more than 130 for hemophilia, 8 for thrombosis and hemostasis, and 6 for thalassemia (CDC, 2009b).
In addition to bringing together comprehensive expertise and resources to address an array of patient needs, specialized care centers make it easier for sponsoring organizations and others to establish and monitor the quality of care and other standards. For rare diseases, however, the evidence base to establish standards may be limited, and the number of patients may be too small for some statistical tracking tools to be very useful.
For extremely rare diseases, networks of comprehensive care centers are the exception, although individual medical centers may still be recognized as loci of clinical expertise. Examples include many of the institutions participating in the NIH Rare Diseases Clinical Research Network (see Chapter 5).
In addition to a focus on systems of care, a priority for many advocacy organizations has been to help patients and families identify individual physicians with some experience and expertise with extremely rare conditions. Organizations may provide a list of physician contacts, as exemplified by the website of the International Fibrodysplasia Ossificans Progressiva (FOP) Association, which lists physician contacts, including clinical researchers at the FOP Research Laboratory at the University of Pennsylvania (who are also cited as emergency contacts). In addition, as noted earlier, NIH has created the Undiagnosed Diseases Program, which sees patients through the NIH Clinical Center.
Pediatric-Adult Care Transition
Children form a substantial part of the population with rare conditions. Although many rare diseases are fatal in infancy or childhood, early diagnosis and improved treatment for a number of conditions have increased the number of infants and children who survive to adulthood. For this group, the transition from pediatric or adolescent to adult care is often a matter of acute concern to the young people themselves, their families, and the professionals who care for them. One review of the importance of managing this
transition noted that even in situations when the need was long anticipated, for example, for children with phenylketonuria (PKU), the response has still fallen short (Scriver and Lee, 2004).
Table 2-3 highlights characteristics of child and adolescent health that may affect the transition from pediatric to adult care for children with serious chronic conditions. To the extent that young people in transition lose health insurance through a parent’s work-based coverage or under Medicaid, the shift from pediatric to adult care may create additional complications and risks. Medicaid covers a range of special services for children that are not usually covered for adults and that may be particularly important for children with severely debilitating rare conditions.
For many serious chronic conditions that begin at birth or in childhood, children’s hospitals usually have a depth of expertise and multidisciplinary inpatient and outpatient care coordination that will not be matched by other medical centers that treat adults (IOM, 2007). Treatment of serious, chronic, rare conditions often involves multiple specialties such as medical genetics, neurology, gastroenterology, psychiatry, endocrinology, and physical therapy. Particularly for conditions that still often result in death in early adulthood, the adult center may have no specialists with experience treating those conditions.
Recognizing the complexities of and deficiencies in chronic care coordination generally, the American College of Physicians (ACP, 2004) has followed the American Academy of Pediatrics (AAP, 2002) in endorsing the concept of the medical home as a centerpiece for medical care and other coordination. In principle, the implementation of this concept would sup-
TABLE 2-3 Characteristics of Child and Adolescent Health That May Affect the Complexity of Health Care Transitions
|
|
|
|
Simpler Transition |
More Complex Transition |
|
Single health condition |
Multiple health conditions |
|
Low risk of future health problems |
High risk of future health problems |
|
No dependence on medical equipment |
Reliance on life-sustaining medical equipment |
|
Rare acute illness, medically stable |
Frequent acute episodes, medically unstable |
|
Few medications |
Multiple medications, medication problems |
|
No cognitive impairments |
Profound mental retardation |
|
No physical impairments |
Serious physical impairments |
|
Mentally healthy |
Mentally ill |
|
No behavioral concerns |
Serious behavioral concerns |
|
SOURCE: IOM, 2007 (adapted from Kelly et al., 2002). |
|
port smooth transitions from pediatric to adult care for children with rare conditions.
IMPACT OF RARE DISEASES ON PATIENTS, FAMILIES, AND COMMUNITIES
I found that families don’t have feelings…. Individuals do. My feelings about this were different from my wife’s, and those are different from my daughter’s. Everyone has their own, very individual experience. That has had an important impact on how our family has dealt with all of it. It’s something that all families need to recognize when they are going through a shared experience like this. Just because you feel or react one way, doesn’t mean your wife or children are experiencing the same thing in the same way. It was quite a thing to realize.
Hollaway, 2007
Rare diseases take their toll on all involved, from affected individuals and their families and friends, to the health professionals who care for them, to their communities, and the larger society. Many rare diseases result in premature death of infants and young children or are fatal in early adulthood. Such premature deaths can have lifelong effects on parents, siblings, grandparents, and others close to a family. Frequently, rare conditions produce devastating long-term functional, physical, and mental disabilities that strain families’ emotional and economic resources. Even for rare conditions that are less severe, the isolation, the uncertainty about the course of the disease, and the frequent lack of effective treatments can have a significant impact.
Just as rare conditions vary, individual and family experiences with debilitating or life-threatening illness clearly vary—as do their responses. The effects of rare conditions on patients and families and their responses are often shaped by socioeconomic status, including differences in income and education. Better outcomes may be linked to medical and nonmedical actions that take such differences in financial and nonfinancial resources into account. Patient and family values also vary. Advocacy groups and educators encourage health care professionals to respect these values as they help patients and families understand what they are facing and make decisions about care and treatment.
High and burdensome costs are not unique to rare diseases, but a number of factors can push patient, family, and societal costs higher for rare conditions than for more common ones. The search for an accurate diagnosis can be not only time-consuming but also expensive. Medications developed specifically for rare conditions can be extraordinarily expensive, costing tens or even hundreds of thousands of dollars per year. Many rare
conditions are diagnosed in childhood and then affect individuals for decades. Many individuals require extensive, long-term supportive care that is not covered by Medicare or private health plans, although Medicaid may cover such services for those who qualify. Even for relatively well-off individuals and families, the expenses associated with life with a rare disease can be a significant burden.
For both individuals and family members, the economic impact of rare diseases extends to lost productivity, lost wages, or the inability to find manageable work with flexible leave, health insurance, and other key benefits. Notwithstanding laws against discrimination based on disability or genetic information (notably the Genetic Information Nondiscrimination Act of 2008, P.L. 110-233), employers may fear the consequences of hiring a person with evident health problems and may take health (including the health of an employee’s family members) into account when making hiring or layoff decisions. For small employers, a single health plan member with extraordinary medical costs can lead to unaffordable premiums for the entire group of employees. As described in Chapter 6, if it survives calls for its repeal, the Patient Protection and Affordable Care Act of 2010 (P.L. 111-148) should make access to insurance easier for many people with rare conditions and should limit certain restrictions on coverage, for example, a lifetime cap on benefits.
I did not choose this work as my career; the vocation was bestowed on me more than 14 years ago when my two children were diagnosed with a genetic disease called pseudoxanthoma elasticum.
Terry, 2009
The physical, emotional, and financial impact of a rare disease on individuals and families has motivated many of them to try, in turn, to have an impact on the disease and others affected by it. They have joined together to form support and advocacy organizations—some focused on individual conditions, others encompassing a number of related conditions, and yet others such as NORD and the Genetic Alliance acting as umbrella organizations and advocates. Although not focused solely on rare conditions, the Genetic Alliance convenes a range of activities to help rare disease and other groups develop, function effectively, and collaborate. NORD likewise provides assistance to rare disease groups, including newly organized groups.
Some groups (e.g., the Vasculitis Foundations, which was founded in 1986 as the Wegener’s Granulomatosis Association) have moved from a concentration on a single condition to a focus on a group of related conditions, some of which previously had not had an organized voice. Such movement reflects both the biological reality that knowledge about one condition may be more generally relevant and the organizational reality
that consolidation can bring operational efficiencies and greater public recognition (see, e.g., Hoffman, 2006).
Many advocacy organizations take on multiple roles, including providing information, supporting patients and families in obtaining needed clinical and other services, offering emotional support, educating clinicians, shaping public policy, and promoting research. Patients, families, and advocacy groups have been a driving force in public policy. Notably, they pressed for the passage of the Orphan Drug Act and the creation of the Office of Rare Diseases Research at NIH. They likewise were active in working for passage of the Genetic Information Nondiscrimination Act and creation of the compassionate allowances program that allows people with a number of rare conditions to qualify quickly for Social Security Disability Insurance (see Chapter 6). Some rare disease groups and their umbrella organizations argue that efforts to influence public policy should take this broad approach rather than focus on funding or other policies aimed at individual rare diseases (see, e.g., Farmer, 2009; Terry, 2010).
As is true for organizations associated with common diseases such as breast cancer or cancer generally, rare disease groups often aim to engage and have an impact on the broader community through public awareness and fundraising efforts. Walks, runs, bike races, telethons, celebrity appearances, and other events involve people in highly visible activities that draw attention to rare conditions and the toll they take. In addition, NORD and other groups promote awareness of rare diseases generally, including through activities associated with Rare Diseases Day.
Of particular relevance for this report, individuals, families, and advocacy groups have also mobilized to promote the study of rare diseases and the development of products to treat these diseases. In some cases, research is the primary focus of advocacy organizations. Although groups may focus mainly on raising money for research and advocating for more public funding for research, some take more active roles. For example, they may work with researchers by organizing group members to participate in clinical studies, provide personal data for natural history studies, contribute tissue samples, and volunteer in other ways (see, e.g., Farmer, 2009; Frohnmayer and Frohnmayer, 2009; Terry, 2009). They also can direct research to issues of most concern to patients and families (Nijsten and Bergstresser, 2010). The Office of Rare Diseases Research at NIH has made involvement of advocacy groups an important feature of the Rare Diseases Clinical Research Network.
Organizational strategies and agendas for supporting research vary depending on the state of knowledge, the organization’s financial resources, the concerns of organizational founders, and other factors. As discussed further in Chapters 4 and 5 and Appendix F, some patient organizations have promoted partnerships with industry and public agencies and devised
new models of “venture philanthropy” to bridge the gulf between basic research findings and approved therapies (see, e.g., IOM, 2008, 2009b; Kelley, 2009; Ashlock, 2010).
In sum, rare diseases have a profound impact on patients and families, but patients and families, in turn, have an impact on the world around them when they organize with others to inform their communities, influence public policy, and stimulate research. Sometimes separately but also in concert, rare disease organizations and their umbrella organizations have worked together on a broad agenda that includes funding for research and technological innovations that will identify the mechanisms of rare diseases and translate these findings into studies that ultimately lead to better ways to prevent, diagnose, and treat these diseases. As this report illustrates, the confluence of scientific advances and policy initiatives provides new opportunities to accelerate this progress.
































