4
Barriers to Enhanced Regulatory Science
Progress toward meeting a need for an enhanced regulatory science is impeded by a number of barriers, which are reviewed in this chapter. Many of these barriers are identified in the FDA Science Board report (FDA Science Board, 2007); others relate to deficiencies in information technology (IT), a prerequisite and a foundation for promoting and enhancing regulatory science at FDA, or broader barriers that can be characterized as more systemic in nature.
FINDINGS OF THE FDA SCIENCE BOARD
Cassell summarized the findings of the FDA Science Board report regarding barriers to enhanced regulatory science at FDA. While noting the tremendous advances that FDA has made toward standardizing regulatory science prior to and since the publication of the report, Cassell provided the following overview to describe the need for continual support for the agency.
Gap Between Scope of Responsibilities and Funding Levels
The FDA Science Board calculated that in 2006, the agency regulated $1 trillion in consumer products and oversaw 300,000 sites in 100 different countries (Figure 4-1) with an appropriated budget of just $1.6 million. As of October 2009, FDA reported a total of 11,516 employees and is estimated to regulate $2 trillion across 150 countries worldwide (FDA,
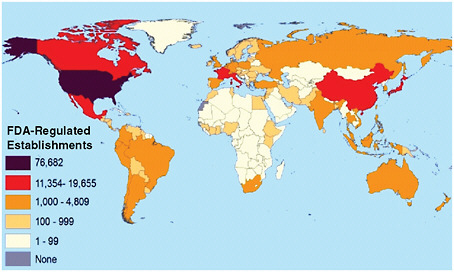
FIGURE 4-1 Breadth of FDA responsibilities by number of establishments as of 2007.
SOURCE: FDA, 2007.
2010e, 2010f). According to FDA, “[i]n the past five years, the number of FDA agreements with its regulatory counterparts throughout the world more than doubled and it continues to grow. FDA has over 100 formal agreements with its counterparts in 29 countries, 18 with the European Commission or its European Union members, and two with the World Health Organization.” In addition, FDA established an office in China as of 2008, and has planned locations in India, Europe, Latin America, and the Middle East (FDA, 2010f). While user fees, such as those allowed by the Prescription Drug User Fee Act, can help support product review, user fees are restrictive, present conflict-of-interest issues, and are viewed with suspicion by the public, according to Cassell.
Since the FDA Science Board report was published, FDA has received limited increases in funding, yet its budget still pales in comparison with the funds allocated to similarly sized agencies. For example, FDA shared a similar budget and workforce as the Centers for Disease Control and Prevention (CDC) approximately 25 years ago (Grossman, 2010). As of 2009, however, CDC’s total budget is more than three times the size of FDA’s, and CDC holds approximately 4,000 more employees (CDC, 2009).
While money is not the sole problem, said Cassell, having sufficient funds is necessary to begin addressing other issues. The agency still needs a sustainable source of funding to gain autonomy and to ensure continuity of its operations.
Workforce Resource Constraints
FDA faces two related issues regarding its workforce: professional development and retention rates. As a science-based agency, FDA is staffed by many of the nation’s best scientists. Because of budgetary and workload issues, however, FDA staff often cannot find time to attend professional workshops and miss opportunities to supplement their knowledge base. FDA also experiences twice the turnover rate among its scientific workforce of other federal agencies; as a result, remaining staff members are stretched thin and overburdened. Cassell added that, in 2006, although it had been given 100 more unfunded mandates since 1981, the agency had fewer full-time employees in 2006 than in 1981.
A large recruitment effort has been under way at FDA to resolve these workforce issues. Programs such as the Commissioner’s Fellowship Program and scholars’ sabbaticals have been created to recruit and train new talent. In 2008, the agency hired 1,200 new employees, 800 of whom filled newly created positions.
Harry Greenberg, Joseph D. Grant Professor of Medicine and Microbiology and Immunology and the Senior Associate Dean for Research at Stanford University School of Medicine, also stressed the importance of enhancing human capital at FDA. Greenberg suggested that trainees could be an important mechanism for improving collaborations within the FDA and between FDA and academia. FDA fellowship programs could be enhanced to allow postdoctoral students to be shared by FDA and academia. Such an arrangement could promote interaction between entities and simultaneously build a pipeline of young talent accustomed to drawing upon the wide-ranging expertise of academic and applying it to FDA’s unique science needs.
Deficient Scientific Base
Cassell observed that, although three of the six FDA centers contain the term “research” in their titles, compounding daily responsibilities limit agency staff opportunities to conduct research. The FDA Science Board recommended that the agency establish a Chief Scientific Officer to establish strong scientific leadership, and the agency has implemented this recommendation. Given the scope and magnitude of the need for an adequate and robust science base at FDA, Cassell suggested, however, that the need must be addressed more systemically and comprehensively than is within the capacity of a single office.
Deficiencies in Information Technology1
Many workshop speakers argued that the greatest barrier to strengthening regulatory science is FDA’s limited ability to adopt and utilize IT. IT is essential as a means to organize FDA’s massive quantities of new and existing information so the agency can make science-based regulatory decisions. Kim cited three distinct but interdependent components of IT that are necessary to support regulatory science, all of which suffer due to IT deficiencies:
-
IT infrastructure—Similar to other infrastructures, such as roads and bridges, IT infrastructure comprises the basic physical and organizational elements needed for the operation of a system. Examples include data centers, networks, computer servers, storage systems, and the organization of an operations panel.
-
Informatics—Informatics, also known as information science, encompasses the practice of information processing and the engineering of information systems, including the structure, algorithms, behavior, and interactions of systems that store, process, access, and communicate information. In the context of the biomedical sciences, genomics and bioinformatics are examples of informatics sciences, involving the establishment of methods for handling vast quantities of data.
-
Scientific computing—Scientific computing consists of the construction of mathematical models and numerical solution techniques and the use of computers to solve scientific problems. In silico2 studies fall in this category.
Kim acknowledged the interdependency and overlap among skill sets and experts in these three areas, but warned that misunderstanding or conflation of the three could lead to misspent funds or underinvestment by stakeholders who view spending on IT infrastructure, informatics, and scientific computing as redundant. He also suggested that, as other organizations and enterprises move forward with advances in IT, FDA will face pressure to conduct its regulatory work at a pace commensurate with the growing demands on the agency. For example, an increasingly data- and informatics-savvy public will have rising expectations of FDA with respect to safety issues, as well as drug supply chains, postmarket surveillance, and adverse event reporting.
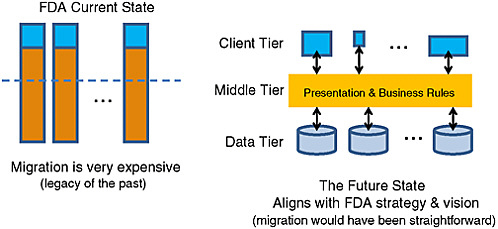
FIGURE 4-2 Flow of data in an Incubator for Innovation in Regulatory and Information Science (IIRIS)/Centers of Excellence (COE) model.
SOURCE: Kim, 2010.
Kim also addressed the question of the timing of investment in IT. He noted that delays in rebuilding infrastructure such as roads and bridges can actually result in lower costs due to improved technology and building materials. In contrast, building or rebuilding an IT infrastructure can become significantly more expensive and time-consuming as investments are delayed. For an information-based organization such as FDA, staff members frequently develop workarounds in the absence of an adequate IT infrastructure. The result is often increased costs and project time to recover lost data and rebuild lost connections into a larger workable foundation.
Kim suggested that, in moving forward, FDA may want to consider adopting the best practices of large enterprises of using data tiers and client tiers, which can be adjusted to the type of user access to information. Figure 4-2 illustrates how the middle tier serves to function as the link between the data layer and the user. Kim referred to an intramural collaboration concept introduced in the FDA Science Board Report, called the Incubator for Innovation and Regulatory Information Science (IIRIS)3 model, which would function as a data sharing mechanism. In addition, if the agency in the future were to adopt a hub (Incubator for Innovation in Regulatory and Information Science [IIRIS]/Centers of Excellence
|
BOX 4-1 The IIRIS Model “[It] would be under the direction of the Chief Scientific Officer and would invest in the recruitment of talented cross-disciplinary scientists to serve as liaisons with groups across the Agency involved in the ‘new science’ programs. The IIRIS team would not do the scientific work, but rather would be the project managers to nurture and track program progress. IIRIS would also be responsible for the creation of the proper computation, technical and biological infrastructures (e.g., measurement, visualization and computational facilities), and work closely with the Director of External Collaborations and Training to create strategic partnerships with academia, industry and governmental laboratories to deliver the competency necessary in science, technology, commerce and policy to support industry innovation and the delivery of safe and efficacious products to the marketplace.” SOURCE: FDA Science Board, 2007, p. 28. |
[COE]) model,4 this data-sharing mechanism would lend itself easily to a collaborative model whereby shared networks of data and information create a whole that is greater than the sum of its parts (see also Chapter 5) (FDA Science Board, 2007, p. 28).
Box 4-1 presents Kim’s description of the IIRIS model to the FDA Science Board.
IT plays a critical role in organizing data for a regulatory science infrastructure. It is also an area of rapid growth and complexity. In the open discussion following his presentation, Kim concluded that dedicated personnel will be needed at FDA for each of the three components of IT listed above. Clear communication by IT experts to the public and stakeholders will be needed as well. Kim noted that, although FDA was excluded from the $1.2 billion granted by the American Recovery and Reinvestment Act of 2009 for the electronic medical records initiative, the agency is now receiving major IT investment from the Information Communication Technology for the 21st Century (ICT-21)5 initiative.
|
4 |
See Chapter 5 for a detailed discussion of the COE model. |
|
5 |
More information on ICT-21 is available at: https://www.fbo.gov/spg/HHS/FDA/DCASC/FDA-SOL-08-00600/listing.html (accessed September 24, 2010). |
SYSTEMIC BARRIERS
Philip A. Pizzo, Dean, School of Medicine, Stanford University, and Chair of the Council of Deans, Association of American Medical Colleges, cited several broader obstacles to the development and promotion of regulatory science, which are encountered not only at FDA but also in academic medical centers. They include attrition of scientific talent due to a lack of financial incentives, driving (and restriction) of research based solely on funding sources and not on science, and reluctance to collaborate because of burdensome legal requirements. According to Pizzo, these more systemic barriers are symptomatic of problems found in the current drug development models.
Greenberg also observed an aversion to regulation found in academic medical settings. He said the nature of academia does not lend itself to a regulatory mindset, and thus, cultural differences will pose an additional challenge to effective collaboration between academic medical centers and FDA.








