6
Challenges in Engaging the Public Policy Community
Over the course of the workshop, several participants stressed the importance of a rapid response to the need for an enhanced regulatory science discipline and infrastructure at FDA. Speakers credited the recent interest in regulatory science to new leadership at the health agencies and a President who is focused on revitalizing science. In early 2010, President Barack Obama requested funding in FDA’s fiscal year 2011 budget specifically to support the advancement of regulatory science.1 William Corr, Deputy Secretary, Department of Health and Human Services (HHS), noted in his presentation, “It is not a huge amount. It’s $25 million, and in the HHS budget, sometimes $25 million seems small. But it is a great beginning.” Hamburg agreed with the importance of expanding regulatory science to open up possibilities for new diagnostics and safer and more effective treatments: “It’s essential,” she said, “that we have a regulatory agency that is scientifically robust and trusted by policy makers and the American people.”
Proper support for scientific capacity and sustainable resources can provide the autonomy FDA needs to pursue its mission free of the influence of political tides or funding mandates, said Hamburg.
Speakers from the patient community, such as Ellen Sigal of Friends of Cancer Research and Margaret Anderson of FasterCures, highlighted the importance of public policy advocates as the ultimate catalysts for political reform. The final workshop session examined ways to interact with the public policy community, gain its support, and mitigate the unique challenges faced in the process.
CHALLENGES IN ENGAGING THE PUBLIC POLICY COMMUNITY
Steven Grossman, founder of Alliance for a Stronger FDA, highlighted three principal challenges that hinder engagement of the public policy community in support for enhanced regulatory science at FDA: funding, policy development, and communication.
-
Funding—Fully 80 percent of FDA’s budget goes to personnel costs. Grossman expressed concern that—despite the increase in FDA’s appropriated budget for 2011—the agency faces an unprecedented need for scientific research combined with increased expectations. Given the fixed expenses required to run the agency, little funding will remain for new initiatives.
-
Policy development—In policy development, there is often a demand for fast results; however, building a regulatory science infrastructure will require a significant investment of resources and time. Strong leadership and a clearly articulated implementation process must be communicated to policy makers at the outset to prevent a loss of support, said Grossman. He also suggested that the move to build a regulatory science infrastructure at FDA should remain independent of user fees and other potential funding sources that could be perceived as posing a conflict of interest.
-
Communication—A common theme underlying public policy challenges is the importance of communication and education. FDA will need to build an understanding of regulatory science among the public and policy makers, remarked Grossman, as well as those directly partnering with the agency.
PUBLIC OPINION POLL DATA ON FDA
Mary Woolley, President, Research!America, presented the results of a survey conducted by her organization (Research!America, 2010) as context for the actions needed to energize the public policy community to support the development of a regulatory science infrastructure at FDA. Woolley noted that public sentiment is dynamic and is driven by emotion,
the media, and high-profile leadership initiatives, among other influences. Figure 6-1, for example, illustrates shifts over the last 6 years in public sentiment on the most important health issues. Woolley predicted that concern about obesity will continue to increase as a result of First Lady Michelle Obama’s “Let’s Move” campaign, aimed at reducing childhood obesity (White House, 2010).
FDA is currently on the public radar, particularly due to recent food and drug recalls. As Figure 6-2 indicates, the majority of those surveyed selected “somewhat confident” when asked about their confidence in current systems for monitoring the effectiveness and safety of new medicines and medical devices. Figure 6-3 shows that respondents cited “protecting public safety” as FDA’s most important role. A subsequent survey question in the same series showed respondents were evenly divided when
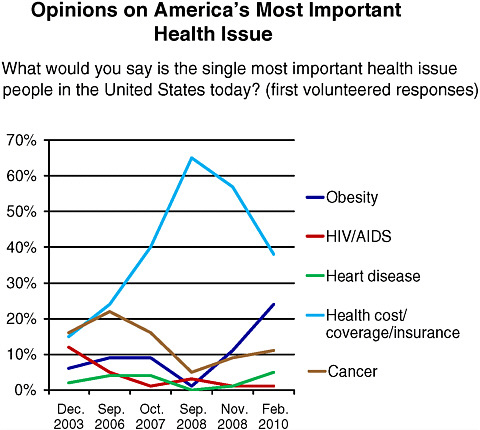
FIGURE 6-1 Shifting opinions on America’s most important health issue, December 2003–February 2010.
SOURCE: National Public Opinion Polls, 2003–2010, Charlton Research Company for Research!America.
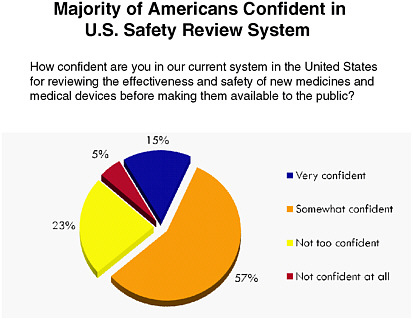
FIGURE 6-2 Americans’ level of confidence in systems for monitoring the effectiveness and safety of new medicines and medical devices.
SOURCE: National Public Opinion Polls, 2003–2010, Charlton Research Company for Research!America.
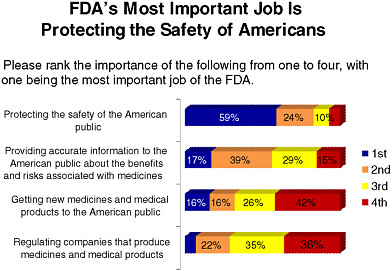
FIGURE 6-3 Americans’ views on FDA’s most important role.
SOURCE: National Public Opinion Polls, 2003–2010, Charlton Research Company for Research!America.
asked whether the FDA should speed up the approval process at the risk of compromising safety (Research!America, 2010). This latter opinion poll reflects the dichotomy in the public’s understanding of the regulatory processes required to deliver the results expected from FDA. Some of the speakers identified improved communication and public outreach as a means to help mitigate this knowledge gap. Workshop participants also expressed desire for detailed surveys that focus specifically on drug development in order to understand the roots of the public’s attitudes toward benefits and risks of FDA-regulated products.
THE POWER OF PATIENT ADVOCACY
Perhaps the most effective way to reach policy makers is through those they are supposed to represent: the public and patients. Newly tested positive for HIV in the mid-1980s, Michael Manganiello, Partner, HCM Strategists, and a patient advocate, recalled his initial layperson’s view of FDA: “I knew about as much about FDA as I did about quantum physics, which was nothing, except I quickly became aware that FDA was the place that was standing in the way of me getting better drugs.” Manganiello said he revised his opinion after learning that many more barriers stood in his way: lack of political will, lack of resources, and lack of support from the American people.
Today, the movement that followed to bring awareness to the HIV/AIDS epidemic is touted as a major success—in rallying public and government support, intensifying scientific innovations, and producing therapeutics. Manganiello credits the grassroots movement of patient groups as the driving force behind this success. Patient groups have grown much more sophisticated since then and generally can exercise considerable political influence over the allocation of scientific resources and expertise.
Patient advocacy groups and disease-based foundations have the potential to aid in the building of regulatory science through media-savvy communication and access to patient populations. They also appreciate the importance of involving FDA to produce results, as well as the challenges that face regulatory agencies. Thus, patient advocates can prove to be valuable partners in future regulatory science initiatives at FDA. Box 6-1 presents an example of the impact of patient advocacy, in this case with respect to cancer therapeutics.
|
BOX 6-1 The Impact of Patient Advocacy for Cancer Therapeutics at FDA Until recently, patient communities were concerned principally with improving research capacity at NIH in their efforts to advance cancer care, according to Ellen Sigal. The role of FDA, on the other hand, remained unclear and thus ignored. The cancer research community has since come to understand the critical functions of FDA in the emergence of new therapeutics for cancer. The community also observed that more could be done to promote cancer care at FDA—beginning with the elevation of oncology to a new office within the agency infrastructure. In July 2005, the cancer community, including professional groups, cancer centers, scientists, the American Society of Clinical Oncology, and the American College of Radiology, succeeded in bringing about the agency’s Office of Oncology Drug Products (OODP). OODP uniquely encompasses both small-molecule drugs and biologics within one office in an effort to consolidate oncology regulations and improve consistency in review standards and policies, and serves as a small-scale example of flourishing regulatory science. In going forward, Sigal observed that OOPD could further advance regulatory science by enhancing collaboration among the different FDA centers, increasing interactions with other health-related federal agencies, expanding external advisory capacity, and harmonizing with international regulatory bodies. SOURCE: Sigal, 2009. |






