1
Bromine1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs have been defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million [ppm] or milligrams per cubic meter [mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects, or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGLs represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
The halogen bromine (Br2) is a dark reddish-brown volatile liquid at room temperature. Its oxidizing potential lies between that of chlorine and iodine. Bromine is used as a water disinfectant, for bleaching fibers, and in the manufacture of medicinal bromine compounds, dyestuffs, flame retardants, agricultural chemicals, inorganic bromide drilling fluids, and gasoline additives.
Bromine is a skin, eye, and respiratory-tract irritant. Inhalation causes respiratory-tract irritation and pulmonary edema. Although accidental human exposures have occurred, concentrations were either not reported or were judged unreliable. The data on the inhalation toxicity of bromine are sparse and, at times, conflicting. Aside from old and anecdotal information, the database is limited to one study with human subjects and two lethality studies with the mouse as the test species. One of the lethality studies (Bitron and Aharonson 1978) provided data sufficient for derivation of the relationship between concentrations that result in lethality (LC50 values [concentration with 50% lethality]) and exposure duration: C2.2 × t = k (chemical concentration in air with a chemical-specific exponent applied to a specific end point × exposure time = response).
The AEGL-1 was based on exposures of 20 healthy human subjects to concentrations of 0.1 to 1.0 ppm for at least 30 min (Rupp and Henschler 1967). Eye irritation, but not nose or throat irritation, occurred during a 30-min exposure at 0.1 ppm. At concentrations of ≥0.5 ppm, there was a stinging and burning sensation of the conjunctiva. The 30-min exposure to 0.1 ppm, which caused
mild irritation, was chosen as the basis for the AEGL-1. The 0.1-ppm concentration was divided by an intraspecies uncertainty factor of 3 to protect susceptible individuals. An intraspecies uncertainty factor of 3 was considered sufficient because workers have been occupationally exposed to 1 ppm with no symptoms other than “excess irritation” (Elkins 1959). The resulting 0.033-ppm concentration is 30-fold lower than the 1-ppm concentration that induced excess irritation in healthy workers. Effects at this low concentration appear to be limited to the eyes and upper respiratory tract; there is likely to be little penetration to the lower respiratory tract. Compared with the 0.5-ppm AEGL-1 concentration for chlorine, a chemical that more readily penetrates to the lower respiratory tract, the intraspecies uncertainty factor of 3 for bromine is considered adequate. An intraspecies uncertainty factor of 1 was applied to the 0.5-ppm test value for chlorine because this concentration failed to elicit an asthmatic response in atopic and asthmatic individuals. The resulting 30-min AEGL-1 value of 0.033 ppm was used for all AEGL-1 exposure durations, as adaptation to mild sensory irritation occurs.
The AEGL-2 was based on the exposure to approximately 1 ppm for 30 min, which the subjects in the above study (Rupp and Henschler 1967) found irritating (stinging and burning sensation of the conjunctiva and nose and throat irritation). The 30-min 1-ppm value was divided by an intraspecies uncertainty factor of 3 to protect susceptible individuals and time-scaled to the other AEGL-2 exposure durations by using the concentration-exposure duration relationship of C2.2 × t = k from the mouse lethality study. An intraspecies uncertainty factor of 3 was considered sufficient, as the symptoms may be below those defining the AEGL-2. Furthermore, compared with the 30-min AEGL-2 value of 2.8 ppm for chlorine, this value may be conservative. The 30-min value for the less well-scrubbed chlorine was based on transient changes in pulmonary parameters (without respiratory symptoms) in asthmatic and atopic individuals. No reliable studies with exposures to higher concentrations were located.
Both lethality studies with the mouse described the inhalation toxicity of both chlorine and bromine. However, both studies reported lower LC50 values for chlorine than those reported in more recent well-conducted studies. Nevertheless, the study that reported the lower lethal concentrations for chlorine was used for derivation of the AEGL-3 values for bromine (Schlagbauer and Henschler 1967). The data in this study showed a clear concentration-response relationship; the exposure duration was 30 min. Using probit analysis, a 30-min LC50 value of 204 ppm and a 30-min LC01 of 116 ppm were calculated. The 30-min LC01 of 116 ppm was used as the basis for calculation of AEGL-3 values. The 116-ppm LC01 was divided by a combined uncertainty factor of 10 (3 for interspecies differences [the mouse was the most sensitive species for lethal effects in tests with other halogens] and 3 for intraspecies differences [at high concentrations, bromine is corrosive to the mucous membranes of the respiratory system; effects are not expected to differ greatly among individuals]) and
scaled across time using the relationship C2.2 × t = k, derived from the Bitron and Aharonson (1978) study.
The calculated values are shown in Table 1-1.
1.
INTRODUCTION
Bromine, a halogen, is a dark reddish-brown volatile liquid that vaporizes readily to a red vapor at room temperature. The diatomic state persists in the liquid, gas, and solid phases. Chemically, the electronegativity and oxidizing potential of halogens decrease as the atomic weight increases, thus making bromine intermediate in oxidizing potential between chlorine and iodine. All the halogens form an acid in water, and the reactivity of these acids shows the same relationship as the elemental halogens. The water solubility of bromine is greater than that of chlorine (O’Neil et al. 2001; Teitelbaum 2001). Additional chemical and physical properties are listed in Table 1-2.
The uses of bromine include water disinfection, bleaching fibers and silk, and the manufacture of medicinal bromine compounds and dyestuffs (O’Neil et al. 2001). The global market for bromine-containing compounds includes flame retardants, agricultural chemicals (principally methyl bromide), inorganic bromide drilling fluids such as calcium bromide, and gasoline additives (Glauser 2009). Production of ethylene dibromide, a gasoline antiknock agent for leaded fuels has decreased substantially over the past years. Likewise, the use of brominated fumigants and pesticides, such as ethylene dibromide and methyl bromide, has been restricted in the United States (Teitelbaum 2001). Commercially, bromine is recovered from soluble bromide salts in salt lakes, inland seas, brine wells and seawater. Seawater contains bromine at a concentration of 65 ppm (Teitelbaum 2001).
In 2005, world production was estimated at 587,000 metric tons, most of the bromine being used for brominated flame retardants ((Glauser 2009). Several production plants are located near natural brine sites in Arkansas (Jackisch 1992). Bromine is shipped in bulk quantities in 7,570-liter (L) and 15,140-L lead-lined pressure tank cars or 6,435- to 6,813-L nickel-clad pressure tank trailers filled to at least 92% capacity (Jackisch 1992). Bromine is also shipped in 600-, 1,200-, and 1,800-gallon tank trucks and 2,300- and 4,400-gallon tank cars (Great Lakes Chemical Corporation 1996).
Bromine is a skin, eye, and respiratory tract irritant (Teitelbaum 2001). All of the exposure data on humans and many of the experimental data on animals are extremely old, provide few experimental details, or conflict with more recent information. Therefore, many of the data are considered unreliable. Two inhalation studies with the mouse as the test species and using several concentrations and exposure durations were located. However, both of these studies report values for chlorine that are much lower than those of other researchers.
TABLE 1-1 Summary of AEGL Values for Bromine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
Eye irritation in humans (Rupp and Henschler 1967) |
|
AEGL-2 (Disabling) |
0.55 ppm (3.6 mg/m3) |
0.33 ppm (2.2 mg/m3) |
0.24 ppm (1.6 mg/m3) |
0.13 ppm (0.85 mg/m3) |
0.095 ppm (0.62 mg/m3) |
Conjunctiva and nose and throat irritation in humans (Rupp and Henschler 1967) |
|
AEGL-3 (Lethal) |
19 ppm (124 mg/m3) |
12 ppm (78 mg/m3) |
8.5 ppm (55 mg/m3) |
4.5 ppm (29 mg/m3) |
3.3 ppm (21 mg/m3) |
30 min LC01 in mice (Schlagbauer and Henschler 1967) |
TABLE 1-2 Chemical and Physical Data for Bromine
|
Parameter |
Data |
Reference |
|
Synonyms |
Dibromine |
HSDB 2008 |
|
CAS registry number |
7726-95-6 |
O’Neil et al. 2001 |
|
Chemical formula |
Br2 |
O’Neil et al. 2001 |
|
Structure |
Br-Br |
O’Neil et al. 2001 |
|
Molecular weight |
159.9 (Br2) |
O’Neil et al. 2001 |
|
Physical state |
Dark, reddish-brown fuming liquid, vaporizes rapidly at room temperature |
O’Neil et al. 2001 |
|
Melting and boiling point |
−7.25ºC/59.47ºC |
O’Neil et al. 2001 |
|
Solubility |
17 g/L in water at 20ºC |
Teitelbaum 2001 |
|
Vapor pressure |
175 mmHg at 20ºC |
AIHA 2001 |
|
Vapor density (air = 1) |
3.5 |
AIHA 2001 |
|
Liquid density (water = 1) |
3.1 |
O’Neil et al. 2001 |
|
Flammability |
Not flammable; may cause fire on contact with combustibles |
DOT 1985 |
|
Conversion factors (Br2) |
1 ppm = 6.5 mg/m3 1 mg/m3 = 0.15 ppm |
AIHA 2001 |
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
Champeix et al. (1970) described the case of a worker exposed to an unknown concentration of vapor during an industrial accident. A postmortem examination revealed bromine burns to 20% of the body, extensive pulmonary and
tracheal damage, and effects on the kidneys and liver. In another industrial accident, eight workers were exposed to an unknown concentration of bromine vapor (Suntych 1953). Three workers developed bronchopneumonia, one developed blepharospasm, and the remainder developed laryngitis. One worker died as the result of sudden circulatory failure associated with bronchopneumonia.
Carel et al. (1992) reported a transportation accident involving a semi-trailer truck carrying liquid bromine on an isolated stretch of highway in the Negev Desert, Israel. The driver was pinned in the cabin of the truck and was unable to free himself or reach his protective equipment. He died of bromine intoxication 3 h after the accident occurred.
Using primarily the database on chlorine, the relationship between the toxicity of chlorine and bromine, and the relationship between concentration and time from animal lethality data, Withers and Lees (1986) calculated an LC50 for humans exposed to bromine. Their model incorporates the effects of physical activity, inhalation rate, the effectiveness of medical treatment, and the lethal toxic load function (the relationship between lethality, concentration, and time). Concentrations were based on the estimate that bromine is 1.5 times less toxic than chlorine. The estimated 30-min LC50 at a standard level of activity (inhalation rate of 12 L/min) for the regular, vulnerable, and average (regular + vulnerable) populations were 375, 150, and 315 ppm, respectively. Estimated LC10 values for a 10-min exposure were 325, 130, and 208 ppm, respectively.
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold
The odor threshold for bromine has been variously reported at approximately 0.01 to 3.8 ppm (Rupp and Henschler 1967; Billings and Jonas 1981; Amoore and Hautala 1983; Ruth 1986). Ruth (1986) reported the threshold for irritation at 0.3 ppm, but the source of the data was not stated. Rupp and Henschler (1967) reported that healthy subjects had difficulty distinguishing between the odor of chlorine and the odor of bromine at concentrations up to 1 ppm, the highest concentration tested. The odor has been reported as suffocating by O’Neil et al. (2001) and bleachy and penetrating by Ruth (1986).
2.2.2.
General Toxic Effects
The signs and symptoms associated with human exposure to low concentrations include upper airways irritation, inflammation of the eyelids, lacrimation, coughing, nosebleed, and a feeling of oppression, dizziness, and headache (Flury and Zernik 1931; Alexandrov 1983; Teitelbaum 2001). After several hours these symptoms may be followed by abdominal pain and diarrhea and a measles-like eruption on the trunk and extremities. Inhalation of “larger quantities” results in brown coloration of the eyes, tongue, and mucous membranes of
the mouth, catarrh, salivation, coughing, feeling of suffocation, glottis cramps, hoarseness, bronchitis, and bronchial asthma (Flury and Zernik 1931). Bromine was reported to produce a stinging and burning sensation of the conjunctiva at exposures of ≥0.5 ppm (Rupp and Henschler 1967). Chronic exposure to bromine resulting in excessive tissue levels of bromide ions (bromism) may lead to slowing of cerebration, impaired memory, anorexia, skin rash, headache, slurring of speech, confusion, weakness, disturbed reflexes, drowsiness, and mild conjunctivitis (EPA 1988).
Irritant levels for bromine have been reported in several sources. Many of the data are extremely old and are compromised by inadequate descriptions of vapor-generation methods, analytic-measurement methods, and exposure durations. These data and reviews are cited here for completeness.
Henderson and Haggard (1943), relying on older data including Matt (1889) and Flury and Zernik (1931) who cite Lehmann (1887), stated that 40-60 ppm is dangerous for brief exposures, 4 ppm is the maximum concentration that can be tolerated for 0.5 to 1 h, and 0.1 to 0.15 ppm can be tolerated for prolonged periods of time. Flury and Zernik (1931) and Withers and Lees (1986) cited the data of Matt (1889) who exposed human volunteers to bromine vapor (bromine was poured into a room) for 16 min to 7.67 h. Under this exposure scenario, Matt (1889) stated that 1-2 ppm could be tolerated by workers indefinitely, 3.5 ppm is tolerable for 30 min to 1 h, and 4 ppm is intolerable for work conditions.
Workers regularly exposed to bromine concentrations at approximately 0.3 to 0.6 ppm for 1 year experienced headache; pain in the joints, stomach, and chest; irritability; and loss of appetite (Alexandrov 1983). Long-term exposure can lead to nervous system disorders, myocardial degeneration, and thyroid hyperplasia. The source of the Alexandrov (1983) data was not given. Elkins (1959), citing a personal communication, reported that 1 ppm in a Massachusetts plant handling liquid bromine was judged to be excessively irritating. Flury and Zernik (1931) cited the data of Lehmann (1887) who reported that exposure to 0.75 ppm in a workroom caused no symptoms in 6 h. OSHA (unpublished material, 1997) monitoring data taken from January 1, 1985, to January 1, 1997, and involving 22 samples from 10 area offices, showed that workers are currently exposed to concentrations between 0.00 and 0.18 ppm. “Total times” for the 0.18-ppm exposures ranged from 15 min to 7.5 h.
2.2.3.
Clinical Study
In a clinical study, Rupp and Henschler (1967) determined the odor threshold and subjective irritation concentrations of both chlorine and bromine. These authors subjected 20 healthy students to “low concentrations” of bromine or chlorine in an 8 m3 exposure chamber. Bromine gas was generated directly from a heated 2-L flask containing 50 mL of the liquid; dilutions were made
with fresh air. Analytic determinations were made titrimetrically with thiosulfate solution (higher concentrations) or spectrophotometrically (concentrations below 0.1 ppm). Samples were collected in potassium iodide solution (higher concentrations) or by absorption by o-toluidine hydrochloride (lower concentrations). The odor threshold for bromine was tested at concentrations of 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, and 1.0 ppm over a 30-min duration. Odor intensity was evaluated with the descriptors: minimal, medium strong, strong, and very strong. Subjective eye, nose, and throat irritation was evaluated as concentrations increased from 0 to 0.9 ppm over a 1-h duration.
A total of 20 students were tested, with 3-4 entering the exposure chamber at one time. Upon chamber entry, odor of bromine was noted by all 20 individuals at a concentration of 0.01 ppm, with an intensity of minimal to medium strong. At 0.2 ppm, most subjects rated odor intensity between medium strong and very strong. In the second part of the study, the subjects recorded their irritation every 5 min over a 60-min period. Eye irritation was first noted at a bromine concentration of 0.1 ppm and occurred within the first 30 min of exposure. At concentrations of 0.2 ppm and higher, distinct nose, eye, and throat irritation occurred, with a rapidly increasing concentration response. Between 0.5 and 0.9 ppm, a 5-min exposure was perceived as uncomfortable (concentrations of 0.5 to 0.9 ppm were irritating to the conjunctiva, nose, and throat.); however, the intensity of effect did not increase above 0.5 ppm. Irritation appeared to be limited to the eyes, nose, and throat; a “compelling cough stimulus” was not attained at concentrations up to 0.9 ppm. At similar concentrations, bromine was found to be more irritating than chlorine. In evaluating their own experiment, the authors noted that the actual concentrations were approximately 40% (range 17-57%) less than the nominal concentrations reported above. Measurements were taken in the vicinity of a wall and not in the immediate area of the subjects. In evaluating the experimental results, Henschler considered the threshold for subjective discomfort to be 0.5 ppm (D. Henschler, Institut for Toxicology, Wurzburg, Germany, personal commun., Dec. 21, 1999).
In the same study, Rupp and Henschler (1967) reported sensory irritation for chlorine at concentrations that proved to be nonirritating in later well-conducted studies (reviewed in 54 Fed. Reg. 2455[1989]). For example, Rupp and Henschler (1967) reported conjunctival pain in several subjects after 15 min of exposure to chlorine at 0.5 ppm, whereas, in a study by Rotman et al. (1983), healthy subjects reported no serious subjective symptoms of irritation from chlorine at a concentration of 1 ppm for 8 h. The lack of controls in the Rupp and Henschler (1967) study as well some methodologic shortcomings in the chlorine part of the study are discussed by OSHA (54 Fed. Reg. 2455 [1989]). The more recent studies of odor thresholds reported higher concentrations than those reported in the Rupp and Henschler (1967) study. It should also be noted that concentration and exposure-duration data reported in the text and figures of the Rupp and Henschler study are conflicting.
2.2.4.
Accidents
On the morning of November 8, 1984, an accident at a chemical plant in Geneva, Switzerland, resulted in the release of 550 kg of liquid bromine (Morabia et al. 1988). Bromine in gaseous form was released via the ventilation system with sufficient force to form a dense brown cloud that drifted into the neighborhood. The cloud remained low over the ground and drifted through the center of the town before reaching Lake Geneva where it dissipated. The time elapsed from the release to disappearance of the cloud over the lake was approximately 5 h. An ozone analyzer located at the Ecotoxicological Centre of the Canton of Geneva (location not given) detected an oxidizing substance between 10 and 12 o’clock that morning. At an undefined time, the centre measured bromine concentrations (Draeger tubes equipped with chlorine reactive tubes) to define the outside limits of the potentially contaminated zone. These concentrations were between 0.2 and 0.5 ppm; concentrations were not measured initially or in the vicinity of the plant.
Ninety-one patients were admitted to the casualty, outpatient, and ophthalmology departments of the University Hospital at Geneva (Morabia et al. 1988). These patients reported signs and symptoms of eye irritation (90%), upper airways irritation (68%), cough (47%), expectoration (34%), and headache (46%). One patient, a worker at the plant, was treated for severe acute bronchitis; following hydrocortisone treatment, he rapidly recovered and was discharged the next day. In the remainder of the patients, symptoms were considered moderate and self-limiting. A 1-month follow-up of 62 of the patients indicated that there were no serious late complications.
Following the transportation accident described by Carel et al. (1990, 1992) in Section 2.1 above, several motorists were exposed to bromine vapor when they stopped to assist the driver of the truck. These exposures produced only mild respiratory symptoms and first and second degree burns to exposed areas of the skin. Four persons were treated with steroids because of shortness of breath; one, a heavy smoker, had diffuse lung wheezes. Six to eight weeks after the accident, four of the exposed motorists complained of cough, shortness of breath, chest tightness, eye irritation, headache, dizziness, fatigue, memory disturbances, and sleep and sexual disturbances. Clinical and laboratory examinations, however, revealed no abnormal findings.
2.3.
Developmental and Reproductive Effects
No data concerning developmental and reproductive effects of bromine exposure in humans by the inhalation route were identified in the available literature. Chronic bromism has been associated with two cases of developmental problems (EPA 1988). The bromism was a result of ingestion of bromide salts.
2.4.
Genotoxicity
No data concerning the genotoxicity of bromine in humans were identified in the available literature.
2.5.
Carcinogenicity
No data concerning the carcinogenicity of bromine in humans were identified in the available literature.
2.6.
Summary
No inhalation studies on the developmental and reproductive toxicity, genotoxicity, or carcinogenicity of bromine in humans were located in the available literature. Human exposures may cause eye, skin, and mucous membrane irritation as well as headache, abdominal pain, and dyspnea (Teitelbaum 2001). Incidences of human exposures were found, but few clear concentration-exposure durations were reported. Some of these data indicate that concentrations of ≤1.0 ppm are irritating (Elkins 1959; Rupp and Henschler 1967; Alexandrov 1983; Ruth 1986). Other data are quoted from secondary and tertiary sources. A study using human subjects reported eye irritation at a concentration of 0.1 ppm and additional sensory irritation at concentrations of ≥0.2 ppm (Rupp and Henschler 1967). The results of parts of this study do not agree with data or statements of other, more recent investigators (Rotman et al. 1983; Ruth 1986; 54 Fed. Reg. 2455 [1989]).
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
Several recent sources cited the data of Flury and Zernik (1931), who cited the data of Lehmann (1887). These data are so old that they should be considered unreliable but are reported here for completeness. Lehmann (1887) reported that inhalation exposure of three animal species at 180 ppm (duration not reported) caused severe irritation and corneal clouding, the 7-h LC10 was 140 ppm for both the cat and guinea pig, and exposure at 300 ppm for 3 h caused deaths in rabbits and guinea pigs. Observations at the latter concentration-exposure time revealed pulmonary edema, deposits on the trachea and bronchi, and gastric hemorrhage.
Henderson and Haggard (1943) reported that a concentration of 1,000 ppm is rapidly fatal. Their source of data appears to be Hill (1915) who experimented with guinea pigs. The original data were not located.
3.1.1.
Rats
Ivanov et al. (1976) reported an LC50 of 415 ppm for the rat. Neither the exposure time nor the original citation were stated.
3.1.2.
Mice
Ivanov et al. (1976) reported an LC50 of 4,46 ppm for the mouse. Neither the exposure time nor the original citation was stated.
Two other acute lethality studies, both using the mouse as the test species, provided details of the exposures. Bitron and Aharonson (1978) exposed 1-month-old male albino mice (28 to 126 mice/group) to concentrations of 240 or 750 ppm for four exposure times at each concentration and calculated 50% mortality as a function of exposure time (median lethal exposure time, or Lt50). Bromine vapor was generated from the liquid, collected in an aqueous solution of potassium iodide, and determined by standard iodometry. Mice were restrained in cylindrical glass exposure chambers. Postexposure observations were made over a 30-day period. The data displayed a clear dose-response relationship and Lt50 values for the 240 and 750 ppm exposures were 100 and 9 min, respectively. Mortality at each concentration-exposure duration is listed in Table 1-3. Dose-response curves were presented graphically, and the values listed in Table 1-3 were estimated from the graph. The results of this work were unusual in that many of the deaths were delayed, occurring during the second week of the observation period, rather than during and immediately following exposure. The authors exposed similar groups to chlorine, and it was noted that chlorine is considerably more toxic to mice than bromine.
Using the method of Litchfield and Wilcoxon (1949), Bitron and Aharonson (1978) also computed 0 or 100% mortalities, which they presented graphically. For the 240-ppm concentration, no deaths were calculated to occur following an exposure for 20 min. For the 750-ppm concentration, no deaths were calculated to occur following approximately 5 min of exposure (same as the experimental value).
TABLE 1-3 Mortality in Mice Exposed to Bromine at 240 or 750 ppm
|
Concentration (ppm) |
Exposure Duration (min) |
Mortality (%) |
|
240 |
24 |
7 |
|
|
65 |
27 |
|
|
120 |
50 |
|
|
215 |
90 |
|
750 |
5 |
0 |
|
|
7 |
44 |
|
|
13 |
73 |
|
|
24 |
95 |
|
Source: Bitron and Aharonson 1978. Reprinted with permission; copyright 1978, American Industrial Hygiene Association. |
||
Schlagbauer and Henschler (1967) exposed groups of 10 female NMRI mice (weight 18-23 g) to various concentrations of bromine for 30 min in order to calculate an LC50. Generation and measurement methods were the same as in a companion study that used human subjects (Rupp and Henschler 1967). The data showed a clear dose-response relationship (Table 1-4). The authors calculated a 30-min LC50 of 174 ppm. The 30-min LC01 was 116 ppm.
In a second experiment (Schlagbauer and Henschler 1967), mice were exposed to bromine at concentrations of 22 or 40 ppm for 3 or 6 h, and mortalities were determined after 10 days. No deaths occurred at 22 ppm for 3 h; mortality was 70% for the 6-h exposure. At a concentration of 40 ppm, deaths occurred following the 3-h (3/10) and 6-h (8/10) exposures. Again, it was observed that chlorine was more toxic than bromine (the 30-min LC50 value of bromine was approximately 1.5 times that of chlorine). The present study did not use a control group. In reviewing the data on chlorine and bromine, Withers and Lees (1986) noted that the chlorine 30-min LC50 value of Schlagbauer and Henschler (1967) is lower than values of other researchers.
3.2.
Nonlethal Toxicity
Ivanov et al. (1976) reported on exposures of groups of eight rats (a total of 800 rats) to concentrations of bromine ranging from 0.12 to 77 ppm. The exposure time was 4 h. The authors quoted an earlier source, and no details of the exposure methods or vapor generation or analytic techniques were given. A concentration of 1.5 ppm decreased respiratory frequency by 19% (134 respirations/min in the exposed group compared with 165 respirations/min in the controls). “Olfactory sharpness,” the ability to react to or detect other compounds, appeared to be decreased, and the number of “free cells” in the upper respiratory pathways was increased. The authors considered this concentration the threshold for irritation, which they defined as “Limir.” Respiratory, cardiac, vascular, neural (“SPP” and reflexes), and endocrine system (dynamics of 131I accumulation and release by the thyroid) effects developed at 50 mg/m3 (7.7 ppm), and spermatogenesis was affected at 100 mg/m3 (15 ppm). No further details were reported.
Ivanov et al. (1976) also reported on subchronic (4 month) exposures of rats at three concentrations: 1.9, 0.2, and 0.02 ppm. A 4-month exposure to bromine at 1.9 ppm produced effects on the respiratory, olfactory, and endocrine systems. Exposure at 0.2 ppm for 4 months led to less pronounced changes, which were reversible after a 1-month recovery period. A concentration of 0.02 ppm had no effect.
In a 28-day feeding study with rats, liquid bromine (38%) administered at 20 mg/kg/day induced clinical signs of salivation and decreased activity; increased red-blood-cell count, hemoglobin and packed cell volume (all reversible in 14 days); increased serum glucose; and increased urinary volume with protein (EPA 2005).
TABLE 1-4 Mortality in Mice Exposed to Bromine at 111 to 315 ppm for 30 Min
|
Concentration (ppm) |
Morality |
|
111 |
0/10 |
|
40 |
3/10 |
|
199 |
6/10 |
|
236 |
9/10 |
|
252 |
10/10 |
|
268 |
9/10 |
|
290 |
10/10 |
|
315 |
10/10 |
|
Source: Schlagbauer and Henschler 1967. Reprinted with permission; copyright 1967, International Archives of Occupational and Environmental Health. |
|
3.3.
Developmental and Reproductive Effects
No data concerning developmental effects of bromine in animals were found in the available literature. Ivanov et al. (1976) reported that a 4-h exposure to bromine at 15 ppm affected spermatogenesis in male mice; further details were not reported.
3.4.
Genotoxicity
Liquid bromine, tested at a concentration of 38.0% and a volume of 10 μg/plate, was positive in the Salmonella typhimurium microsome reverse mutation assay with strains TA 1537 and TA 100 in the absence of S9 and with strain TA 1537 in the presence of S9 activation. Bromine was cytotoxic for all strains with and without metabolic activation at more than 3,333 μg/plate (EPA 2002).
3.5.
Chronic Toxicity and Carcinogenicity
No data concerning the chronic toxicity or carcinogenicity of bromine in animals were located in the available literature. Potassium bromate (KBrO3) has been shown to be a renal carcinogen in rats. The mechanism of action has been attributed to the generation of oxygen radicals by KBrO3, a strong oxidizer. KBr is not a carcinogen (Kurokawa et al. 1990).
3.6.
Summary
Data on the acute inhalation toxicity of bromine are sparse. Most of the older experimental data using animal species are of questionable reliability.
Only two studies provide experimental methods and details adequate for consideration in derivation of AEGLs. Bitron and Aharonson (1978) determined Lt50 values for one species, the mouse. The Lt50 at a concentration of 750 ppm was 9 min, and the Lt50 at a concentration of 240 ppm was 100 min. No deaths occurred or were predicted to occur during a 5-min exposure at 750 ppm or during a 20-min exposure at 240 ppm. The study of Schlagbauer and Henschler (1967) reported much lower lethal values for bromine as well as for chlorine. Their 30-min LC50 value for the mouse was 174 ppm. A concentration of 15 ppm for 4 h affected spermatogenesis in male mice (Ivanov et al. 1976). Limited data were located concerning genotoxicity, and no data concerning developmental effects or carcinogenicity were located in the available literature.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
No data on the absorption, distribution, metabolism, or excretion of bromine following inhalation exposures in humans or animals were located in the available literature. Alexandrov (1983) stated that bromine may enter the body following inhalation, ingestion, or skin application, but the source of the data was not provided. Bromine gas reacts at the site of contact and metabolic and kinetic considerations are not relevant regarding the determination of AEGL values.
4.2.
Mechanism of Toxicity
Bromine, a strong oxidizing agent, is a respiratory irritant and can cause pulmonary edema in humans and animals (Teitelbaum 2001). Reaction with water results in the formation of hypobromous acid, HOBr, which slowly decomposes to hydrobromic acid and oxygen (Downs and Adams 1973).
4.3.
Structure-Activity Relationships
The irritating potential of mucus membrane irritants may be related to their water solubility. At 20-25ºC, the water solubility of bromine is 0.214 mol/L, whereas that of chlorine is 0.092 mol/L (Teitelbaum 2001). Water solubility determines the scrubbing capacity or penetration of a gas into the respiratory tract. On the basis of water solubility, bromine would react more intensely in the upper respiratory tract and thus be better scrubbed than chlorine. As a result of being well-scrubbed in the upper nasal passages, bromine may produce a feeling of irritation at a lower concentration than chlorine. Chlorine, on the other hand, would more readily penetrate to the lower respiratory tract, resulting in lethality at concentrations that are lower than those for bromine. This differ-
ence in lethality is substantiated by the study of Rupp and Henschler (1967). Bitron and Aharonson (1978) observed similar results for lethality in studies with the mouse. The 30-min LC50 values for chlorine and bromine are listed in Table 1-5. The 30-min Lt50 (LC50) values for Bitron and Aharonson (1978) were determined using the relationship between concentration and exposure time as explained in Section 7.2; values for the two concentration-exposure durations were averaged. Because of the 30-day postexposure observation period in this study, the Lt50 can be defined as an LC50. For comparison purposes, the 30-min LC50 value for fluorine in the mouse is 225 ppm (Keplinger and Suissa 1968).
Bromine is more water soluble than chlorine (Teitelbaum 2001) and would be scrubbed to a greater extent in the nasal passages than chlorine; relatively smaller amounts would reach the lungs. Because of its reactive potential and decomposition in the presence of water (Teitelbaum 2001), the solubility of fluorine has not been defined. In the respiratory tract of the rat, the relative inhalation toxicities of the hydrohalous acids formed is HF > HCl ≥ HBr (Kusewitt et al. 1989).
4.4.
Other Relevant Information
4.4.1.
Species Differences
No relatively recent data sufficient for comparing differences in species sensitivity for either irritation or lethality were located in the available literature. In the older data, no differences were found in lethality values in two separate exposures between the cat and guinea pig and guinea pig and rabbit (Lehmann 1887). For the halogens fluorine and chlorine, the mouse is the most sensitive tested species (NRC 2004, 2010).
4.4.2.
Susceptible Populations
Individuals with asthma or other respiratory diseases may be more susceptible to the effects of respiratory irritants than healthy individuals. No data on bromine and the asthmatic population were located.
TABLE 1-5 Relative Toxicities of Chlorine and Bromine to the Mouse
|
Chemical |
30-Min LC50 |
Reference |
|
Chlorine |
203 |
Bitron and Aharonson 1978 |
|
127 |
Schlagbauer and Henschler 1967 |
|
|
Bromine |
424 |
Bitron and Aharonson 1978 |
|
174 |
Schlagbauer and Henschler 1967 |
4.4.3.
Concentration-Exposure Duration Relationship
ten Berge et al. (1986) used the two Lt50 data points from the study by Bitron and Aharonson (1978) to determine the concentration and exposure duration relationship of C2.2 × t = k, where C is concentration, t is time, and k is a constant.
5.
DATA ANALYSIS FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
Many of the data on bromine are old, unreferenced, anecdotal, or conflicting (Elkins 1959; Rupp and Henschler 1967; Alexandrov 1983; Ruth 1986; HSDB 2008). Rupp and Henschler (1967) reported that eye irritation occurred at 0.1 ppm. Current monitoring data from the Occupational Safety and Health Administration (OSHA) show that workers are exposed to concentrations up to 0.18 ppm, presumably without irritation. Ruth (1986) reported that the irritation threshold (undefined) was 0.3 ppm; workers exposed to 0.3 to 0.6 ppm for 1 year suffered various symptoms (Alexandrov 1983). Except for the study of Rupp and Henschler (1967), all of these values are either poorly documented or appear unusually low compared with more recent data for other halogens. The only clinical study, Rupp and Henschler (1967), reported eye irritation, but no nose or throat irritation, at a concentration of 0.1 ppm for 30 min. Concentrations of 0.5 to 0.9 ppm were irritating to the conjunctiva, nose, and throat. The intensity of the irritation was not well-described, and their values in related experiments (chlorine) are low in comparison to other researchers.
The more-robust database for chlorine rather than bromine can also be considered when addressing the irritation potential of halogens. In four clinical studies, some with atopic or asthmatic individuals, exposure to chlorine at 0.4 or 0.5 ppm for various periods of time did not produce airway hyper-reactivity or an asthmatic response (Anglen 1981; Rotman et al. 1983; D’Alessandro et al. 1996; Shusterman et al. 1998). Because sensitive individuals were tested, an intraspecies uncertainty factor of 1 was applied to derive an AEGL-1 value for chlorine (NRC 2004).
5.2.
Summary of Animal Data Relevant to AEGL-1
No useful data were available. Ivanov et al. (1976) reported that the threshold of irritation for bromine for rats was 1.5 ppm during a 4-h exposure, but they quoted an earlier source and provided few experimental details.
5.3.
Derivation of AEGL-1
The AEGL-1 was based on exposures of 20 healthy human subjects to
concentrations of 0.1 to 0.9 ppm for up to 60 min (Rupp and Henschler 1967). Eye irritation, but no nose or throat irritation, occurred during a 30-min exposure at 0.1 ppm. At concentrations ≥0.5 ppm, there was a stinging and burning sensation of the conjunctiva. The 30-min 0.1 ppm concentration, which caused mild irritation, was divided by an intraspecies uncertainty factor of 3 to protect susceptible individuals. This adjustment was considered appropriate for acute exposure to chemicals in which the mechanism of action involves surface contact irritation of ocular or respiratory tract tissue or both rather than systemic activity following absorption and distribution of the parent chemical or a biotransformation product to a target tissue (NRC 2001). An intraspecies uncertainty factor of 3 was also considered sufficient because workers have been occupationally exposed to bromine at 1 ppm with no other symptoms than “excess irritation” (Elkins 1959). The resulting 0.033 ppm concentration is 30-fold lower than the 1 ppm concentration that induced excess irritation in healthy workers. Effects at this low concentration appear to be limited to the eyes and upper respiratory tract; there is likely to be little penetration to the lower respiratory tract. Compared with the AEGL-1 value of 0.5 ppm for chlorine, a chemical that more readily penetrates to the lower respiratory tract, the uncertainty factor of 3 is appropriate. The 0.5-ppm concentration of chlorine, with no uncertainty factor applied, was considered protective of the tested atopic and asthmatic individuals. The 30-min AEGL-1 value of 0.033 ppm for bromine was used across all exposure durations because adaptation occurs to mild sensory irritation (Table 1-6). Calculations for AEGL values are in Appendix A. Appendix B is a graph of the toxicity data in relationship to the AEGL values.
The proposed values are far below the 0.3 ppm threshold for irritation in humans reported by Ruth (1986). The values are 27- to 160-fold below the threshold for irritation in rats, as reported by Ivanov et al. (1976), which was 1.5 ppm for 4-h.
6.
DATA ANALYSIS FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
Elkins (1959) quoted 1 ppm as excessively irritating in workers, but these were chronic exposures. Alexandrov (1983) reported severe choking at 1.7-3.5 ppm, but his values were not documented. The healthy subjects in the study of Rupp and Henschler (1967) reported prickling or stinging of the eyes and nose and throat irritation at concentrations of 0.5 to 0.9 ppm (1.0 ppm for 30 min in the odor intensity study). These sensations were reported following exposures of greater than 30 min. The intensity of the symptoms was not well described.
TABLE 1-6 AEGL-1 Values for Bromine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
6.2.
Summary of Animal Data Relevant to AEGL-2
Ivanov et al. (1976) reported that the threshold for acute effects for a 4-h exposure of rats to bromine was 7.7 ppm, but they quoted an earlier source and provided few details.
6.3.
Derivation of AEGL-2
The AEGL-2 was based on the exposure to bromine of 1 ppm (rounded from 0.9 ppm in the odor intensity study) for 30 min, which the subjects in the Rupp and Henschler (1967) study found irritating (stinging and burning sensation of the conjunctiva and nose and throat irritation). The 30-min 1 ppm value was divided by an intraspecies uncertainty factor of 3 to protect susceptible individuals and time-scaled to the other AEGL-2 exposure durations using the concentration-exposure duration relationship of C2.2 × t = k from the mouse lethality study. An intraspecies uncertainty factor of 3 was considered sufficient, as the symptoms may be below those defining an AEGL-2. Furthermore, compared with the 30-min AEGL-2 value of 2.8 ppm for chlorine, this value may be conservative. The 30-min value for the less well-scrubbed chlorine was based on transient changes in pulmonary parameters (without respiratory symptoms) in asthmatic and atopic individuals (Rotman et al. 1983; D’Alessandro et al. 1996). No reliable studies with exposures to higher concentrations were located. The calculated values appear in Table 1-7 and calculations are contained in Appendix A. Appendix B is a category graph of the toxicity data in relation to AEGL values.
7.
DATA ANALYSIS FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
No reliable human data relevant to derivation of an AEGL-3 values were located in the available literature.
TABLE 1-7 AEGL-2 Values for Bromine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.55 ppm (3.6 mg/m3) |
0.33 ppm (2.2 mg/m3) |
0.24 ppm (1.6 mg/m3) |
0.13 ppm (0.85 mg/m3) |
0.095 ppm (0.62 mg/m3) |
7.2.
Summary of Animal Data Relevant to AEGL-3
Two studies using the mouse provided data on lethality. Both studies provided lethality data on bromine and chlorine. Both studies reported lower LC50 values for chlorine than those reported for bromine in more recent well-conducted studies. Bitron and Aharonson (1978) calculated Lt50 values for bromine at a concentration of 750 ppm for 9 min in the mouse and at a concentration of 240 ppm for 100 min. The 240 and 750 ppm concentrations can be considered LC50 values for the tested times. Using the C2.2 × t = k relationship, these two values can be used to calculate 30-min LC50 values. The respective 30-min LC50 values are 415 and 434 ppm (average, 424 ppm). No deaths were calculated to occur at 750 ppm for 5 min or 240 ppm for 20 min.
The study by Schlagbauer and Henschler (1967) provided a lower LC50 for bromine for the mouse. The lethality data in their study showed a good concentration-response relationship and the exposure time was held constant at 30 min. Using probit analysis, a 30-min LC01 of 116 ppm was calculated.
7.3.
Derivation of AEGL-3
Both lethality studies with the mouse described the inhalation toxicity of chlorine and bromine. However, both studies reported lower LC50 values for chlorine than those reported for bromine in more recent well-conducted studies. Nevertheless, the study that reported the lower lethal concentrations for chlorine was used for derivation of the AEGL-3 values for bromine (Schlagbauer and Henschler 1967). The data in this study showed a clear concentration-response relationship; furthermore, the exposure duration was longer than it was in the Bitron and Aharonson (1978) study. Using probit analysis, a 30-min LC50 value of 204 ppm and a 30-min LC01 of 116 ppm were calculated. The 30-min LC01 of 116 ppm was used as the basis for calculation of AEGL-3 values for bromine. The 116 ppm LC01 was divided by a combined uncertainty factor of 10 (3 for interspecies differences [the mouse was the most sensitive species for lethal effects in tests with other halogens] and 3 for intraspecies differences [at high concentrations, bromine is corrosive to the mucous membranes of the respiratory system; effects are not expected to differ greatly among individuals] [NRC 2001]) and scaled across time using the relationship of C2.2 × t = k, which was derived from the Bitron and Aharonson (1978) study. Calculations are provided in Appendix A, and values appear in Table 1-8 below. Appendix B is a category graph of the toxicity data in relation to AEGL values.
The calculated AEGL-3 values for bromine are below those of chlorine (NRC 2004) and fluorine (NRC 2010) (Table 1-9). This result indicates that the values for bromine are protective, as chlorine is 1.5 times more toxic than bromine based on lethality in the mouse. As noted, the database for chlorine is extensive. In addition, the mice used by Bitron and Aharonson (1978) were young
and were restrained in glass enclosures during the exposures. Both of these factors increase the sensitivity of the tested species to chemical toxicity.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
The AEGLs for bromine were derived in the following manner. The AEGL-1 and AEGL-2 values were based on a study with 20 human subjects who were exposed to concentrations of 0.1 to 0.9-1.0 ppm (Rupp and Henschler 1967). Eye irritation noted within a 30- min exposure at 0.1 ppm was used as the basis for the AEGL-1. At 0.5 to 0.9 ppm (1.0 ppm), the nose and throat were also irritated. A 30-min exposure at 1 ppm was used as the basis for the AEGL-2 values. An intraspecies uncertainty factor of 3 was considered sufficient because irritation was confined to the eyes during the 0.1-ppm exposure (precluding an asthmatic response), and workers have been occupationally exposed at 1 ppm with no reported symptoms other than “excess irritation.” The AEGL-1 values were not time-scaled, as adaptation to mild sensory irritation occurs. Time-scaling for the AEGL-2 was based on a lethality study with the mouse (Bitron and Aharonson 1978).
TABLE 1-8 AEGL-3 Values for Bromine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
19 ppm (124 mg/m3) |
12 ppm (78 mg/m3) |
8.5 ppm (55 mg/m3) |
4.5 ppm (29 mg/m3) |
3.3 ppm (21 mg/m3) |
TABLE 1-9 Comparison of AEGL Values for Fluorine, Chlorine, and Bromine
|
Classification |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
|
|
|
|
|
|
Fluorine |
1.7 ppm |
1.7 ppm |
1.7 ppm |
1.7 ppm |
1.7 ppm |
|
Chlorine |
0.5 ppm |
0.5 ppm |
0.5 ppm |
0.5 ppm |
0.5 ppm |
|
Bromine |
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
|
AEGL-2 |
|
|
|
|
|
|
Fluorine |
20 ppm |
11 ppm |
5.0 ppm |
2.3 ppm |
2.3 ppm |
|
Chlorine |
2.8 ppm |
2.8 ppm |
2.0 ppm |
1.0 ppm |
0.71 ppm |
|
Bromine |
0.55 ppm |
0.33 ppm |
0.24 ppm |
0.13 ppm |
0.095 ppm |
|
AEGL-3 |
|
|
|
|
|
|
Fluorine |
36 ppm |
19 ppm |
13 ppm |
5.7 ppm |
5.7 ppm |
|
Chlorine |
50 ppm |
28 ppm |
20 ppm |
10 ppm |
7.1 ppm |
|
Bromine |
19 ppm |
12 ppm |
8.5 ppm |
4.5 ppm |
3.3 ppm |
The AEGL-3 values for bromine were based on a study using the mouse (Schlagbauer and Henschler 1967). Thirty-minute exposures to several concentrations were tested. The data showed a clear concentration-response relationship from which LC50 and LC01values could be calculated. The 30-min LC01 of 116 ppm, derived by probit analysis, was divided by a total uncertainty factor of 10 (3 each for interspecies and intraspecies differences, as the mouse is the most sensitive species in studies with halogens, and at high concentrations, bromine is corrosive to the respiratory tissues, and effects are not expected to differ greatly among species or between individuals) and scaled to the other exposure times using C2.2 × t = k. The resulting values are lower than those for chlorine, which is known to be more toxic than bromine.
The AEGL values for three levels and five exposure periods are summarized in Table 1-10. Data and derivations are summarized in Appendix C.
8.2.
Comparisons with Other Standards and Guidelines
Standards and guidance levels for workplace and community exposures are listed in Table 1-11. The 1-h AEGL-1 and AEGL-2 values are below the respective emergency response planning guideline (ERPG) values. The ERPG values were based on an unobjectionable odor, 0.1 ppm (Rupp and Henschler 1967), and mild and transient health effects at slightly higher concentrations (Rupp and Henschler 1967; Morabia et al. 1986; D. Henschler, Institut for Toxicology, Wurzburg, Germany, personal commun., Dec. 21, 1999). The ERPG-2 was based on the same studies that reported that exposure to concentrations above 0.5 ppm caused coughing, dizziness, and intense irritation to the eyes, nose, and throat. The 1-h ERGP-3 was based on the mouse lethality studies of Bitron and Aharonson (1978) and Schlagbauer and Henschler (1967) and on the concentration- time relationship of Withers and Lees (1986). The 1-h AEGL-3 and ERPG-3 values are similar. The same references were used for derivation of all AEGL values.
TABLE 1-10 Summary of AEGL Values for Bromine
|
Classification |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 (Nondisabling) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
0.033 ppm (0.22 mg/m3) |
|
AEGL-2 (Disabling) |
0.55 ppm (3.6 mg/m3) |
0.33 ppm (2.2 mg/m3) |
0.24 ppm (1.6 mg/m3) |
0.13 ppm (0.85 mg/m3) |
0.095 ppm (0.62 mg/m3) |
|
AEGL-3 (Lethal) |
19 ppm (124 mg/m3) |
12 ppm (78 mg/m3) |
8.5 ppm (55 mg/m3) |
4.5 ppm (29 mg/m3) |
3.3 ppm (21 mg/m3) |
The immediately dangerous to life and health (IDLH) value is based on several reviews and specifically cites the data of Flury and Zernik (1931) and Henderson and Haggard (1943). Full-day workplace standards are all 0.1 ppm, with short-term allowable exposures at 0.2 and 0.3 ppm. The 8-h AEGL-1 and AEGL-2 values are below workplace standards.
TABLE 1-11 Extant Standards and Guidelines for Bromine
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
|
AEGL-2 |
0.55 ppm |
0.33 ppm |
0.24 ppm |
0.13 ppm |
0.095 ppm |
|
AEGL-3 |
19 ppm |
12 ppm |
8.5 ppm |
4.5 ppm |
3.3 ppm |
|
ERPG-1 (AIHA)a |
|
|
0.1 ppm |
|
|
|
ERPG-2 (AIHA) |
|
|
0.5 ppm |
|
|
|
ERPG-3 (AIHA) |
|
|
5 ppm |
|
|
|
IDLH (NIOSH)b |
|
3 ppm |
|
|
|
|
REL-TWA (NIOSH)c |
|
|
|
|
0.1 ppm |
|
PEL-TWA (OSHA)d |
|
|
|
|
0.1 ppm |
|
TLV-TWA (ACGIH)e |
|
|
|
|
0.1 ppm |
|
REL-STEL (NIOSH)f |
|
|
|
|
0.3 ppm |
|
TLV-STEL (ACGIH)g |
|
|
|
|
0.2 ppm |
|
MAK (Germany)h |
|
|
|
|
Withdrawn |
|
MAC (The Netherlands)i |
|
|
|
|
0.1 ppm |
|
aERPG (emergency response planning guidelines, American Industrial Hygiene Association (AIHA 2001): The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. bIDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1996) represents the maximum concentration from which |
|||||
8.3.
Data Adequacy and Research Needs
The data on the toxic effects of bromine are sparse. Because of the sparse-data, lower values were chosen for the AEGL-1 and AEGL-2 than might have been used in the presence of extensive data. No recent reliable human studies were available. Some of the studies that are quoted and requoted in toxicology books were performed as early as the 1880s; vapor generation and analytic techniques have improved since that time. The clinical study by Rupp and Henschler (1967) tested both bromine and chlorine and reported irritant values for chlorine that are lower than those in other studies. The values for bromine may be correspondingly low. Two lethality studies with the mouse as the test species were available for calculation of the AEGL-3 values. Both the key study, Schlagbauer and Henschler (1967), and the study by Bitron and Aharonson (1978) were noted to have lower lethality values for chlorine than those of many other investigators, and their values for bromine may be correspondingly low. Although bromine is less toxic than chlorine, the interim AEGL-3 values for bromine are less than those for chlorine (NRC 2004).
In the absence of reliable studies that address the end point of irritation, a study to determine the exposure concentration producing a 50% decrease in the respiratory rate (RD50) in the mouse would be of value, particularly as it would confirm the irritation potential of bromine relative to that of chlorine.
9.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1996. Supplements to the Sixth Edition Documentation of the Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 2001. The AIHA 2001 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides. Fairfax, VA: AIHA Press.
Alexandrov, D.D. 1983. Bromine and compounds. Pp. 326-329 in Encyclopaedia of Occupational Health and Safety, 3rd. Ed, Vol.1, L. Parmeggiani, ed. Geneva: International Labour Organization.
Amoore, J.E., and E. Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with Threshold Limit Values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6):272-290.
Anglen, D.M. 1981. Sensory response of human subjects to chlorine in air. Ph.D. Dissertation, University of Michigan, Ann Arbor, MI.
Billings, C.E., and L.C. Jonas. 1981. Odor thresholds in air as compared to Threshold Limit Values. Am. Ind. Hyg. Assoc. J. 42:479-480.
Bitron, M.D., and E.F. Aharonson. 1978. Delayed mortality of mice following inhalation of acute doses of CH2O, SO2, Cl2, and Br2. Am. Ind. Hyg. Assoc. J. 39(2):129-138.
Carel, R.S., I. Belmaker, G. Potashnik, M. Levine, R. Blau, and H. Eden. 1990. Late health sequelae of accidental bromine exposure [in Hebrew]. Harefuah 119(9):259-262.
Carel, R.S., I. Belmaker, G. Potashnik, M. Levine, and R. Blau. 1992. Delayed health sequelae of accidental exposure to bromine gas. J. Toxicol. Environ. Health 36(3):273-277.
Champeix, J., P. Catilina, G. Andraud, P. Penel, and N. Lagarde. 1970. Clinical and experimental study of poisoning by bromide vapors [in French]. Pouman Coeur 26(8):895-903.
D’Alessandro, A., W. Kuschner, H. Wong, H.A. Boushey, and P.D. Blanc. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109(2):331-337.
DFG (Deutsche Forschungsgemeinschaft). 2007. List of MAK and BAT Values 2007. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 43. Weinheim, Federal Republic of Germany: Wiley VCH.
DOT (U.S. Department of Transportation). 1985. Chemical Hazard Response Information System (CHRIS): Hazardous Chemical Data. U.S. Department of Transportation, U.S. Coast Guard, Washington, DC.
Downs, A.J., and C.J. Adams. 1973. Chemical properties of the halogens. Pp. 1188-1232 in Comprehensive Inorganic Chemistry, J.C. Bailar, H.J. Emeleus, R. Nyholm, and A.F. Trotman-Dickenson, eds. New York: Pergamon Press.
Elkins, H.B. 1959. Inorganic compounds: Bromine. P. 89 in Chemistry of Industrial Toxicology, 2nd Ed. New York: John Wiley and Sons.
EPA (U.S. Environmental Protection Agency). Office of Pesticide Programs/Health Effects Division Tox One-liners.
EPA (U.S. Environmental Protection Agency). 1988. Reportable Quantity Document for Bromine. Environmental Criteria and Assessment Office, U.S. Environmental Protection Agency, Cincinnati, OH.
EPA (U.S. Environmental Protection Agency). 2005. Bromine/Bromide. Docket EPA-HQ-OPP-2006-0143-0005 Office of Pesticide, U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/pesticides/reregistration/bromine/ accessed Mar. 11, 2010].
Flury, F., and F. Zernik. 1931. Brom. Pp. 121-123 in Schädliche gase dämpfe, nebel, rauch- und staubarten. Berlin: Springer.
Glauser, J. 2009. Bromine. CEH Report No. 719.1000. SRI Consulting’s Chemical Economics Handbook Program [online]. Available: http://www.sriconsulting.com/CE H/Public/Reports/719.1000/ [accessed Feb. 19, 2010].
Great Lakes Chemical Corporation. 1996. Bromine Safety and Handling Guide. Great Lakes Chemical Corporation, West Lafayette, IN.
Henderson, Y., and H.W. Haggard. 1943. Bromine. P. 133 in Noxious Gases, 2nd Ed. New York: Reinhold Publishing Company.
Hill, L. 1915. Gas poisoning. Br. Med. J. (Dec. 4, 1915):801-804.
HSDB (Hazardous Substances Data Bank). 2008. Bromine (CASRN 7726-95-6). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen? HSDB [accessed Feb. 23, 2010].
Ivanov, N.G., A.M. Kliachkina, and A.L. Germanova. 1976. Experimental data for hygienic standardization of bromine and hydrogen bromide content in the air of working areas [in Russian]. Gig. Tr. Prof. Zabol. 20(3):36-39.
Jackisch, P.F. 1992. Bromine. Pp. 536-560 in Kirk-Othmer Encyclopedia of Chemical Technology, 4th Ed., Vol. 4, J.I. Kroschwitz, and M. Howe-Grant, eds. New York: John Wiley & Sons.
Keplinger, M.L., and L.W. Suissa. 1968. Toxicity of fluorine short-term inhalation. Am. Ind. Hyg. Assoc. J. 29(1):10-18.
Kurokawa, Y., A. Maekawa, M. Takahashi, and Y. Hayashi. 1990. Toxicity and carcino-genicity of potassium bromate - a new renal carcinogen. Environ. Health Perspect. 87:309-335.
Kusewitt, D.F., D.M. Stavert, G. Ripple, T. Mundie, and B.E. Lehnert. 1989. Relative acute toxicities in the respiratory tract of inhaled hydrogen fluoride, hydrogen bromide and hydrogen chloride. Toxicologist 9:36 [A 144].
Lehmann, K.B. 1887. Arch. Hyg. 7:335 (as cited in Flury and Zernik 1931).
Litchfield, J.T., and F. Wilcoxon. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96(2):99-113.
Matt, L. 1889. Experimental Contributions to the Theory of the Effects of Poisonous Gases on Human Beings [in German]. Inaugural dissertation. Julius-Maximilliams-Universität, Würzburg.
Morabia, A., C. Selleger, J.C. Landry, P. Conne, P. Urban, and J. Fabre. 1988. Accidental bromine exposure in an urban population: An acute epidemiological assessment. Int. J. Epidemiol. 17(1):148-152.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Broom. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook. net/lasrookNL/maclijst2004.htm [accessed Oct. 24, 2008].
NIOSH (National Institute for Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-Bromine. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. August 1996 [online]. Available: http://www.cdc.gov/niosh/idlh/7726956.html [accessed Feb. 23, 2010].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Pocket Guide to Chemical Hazards: Bromine. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. September 2005 [online]. Available: http://www.cdc.gov/niosh/npg/npgd0064.html [accessed Feb. 23, 2010].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 4 Washington, DC: The National Academies Press.
NRC (National Research Council). 2010. Fluorine. Pp. 230-273 in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 8. Washington, DC: The National Academies Press.
O’Neil, M.J., A. Smith, P.E. Heckelman, J.R. Obenchain, Jr., J. Gallipeau, and M.A. D’Arecca, eds. 2001. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed. Whitehouse Station, NJ: Merck.
Rotman, H.H., M.J. Fliegelman, T. Moore, R.G. Smith, D.M. Anglen, C.J. Kowalski, and J.G. Weg. 1983. Effects of low concentration of chlorine on pulmonary function in humans. J. Appl. Physiol. 54(4):1120-1124.
Rupp, H., and D. Henschler. 1967. Effects of low chlorine and bromine concentrations in man [in German]. Int. Arch. Arbeitsmed. 23(1):79-90.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Schlagbauer, M., and D. Henschler. 1967. Toxicity of chlorine and bromine with single and repeated exposures [in German]. Int. Arch. Arbeitsmed. 23(1):91-98.
Shusterman, D.J., M.A. Murphy, and J.R. Balmes. 1998. Subjects with seasonal allergic rhinitis and nonrhinitic subjects react differentially to nasal provocation with chlorine gas. J. Allergy Clin. Immunol. 101(6 Pt. 1):732-740.
Suntych, F. 1953. Bromine gassing. Prac. Lek. 5:86 (as cited in ACGIH 1996).
Teitelbaum, D.T. 2001. The Halogens. Pp. 731-826 in: Patty's Toxicology, 5th Ed., Vol. 3, E. Bingham, B. Cohrssen, and C.H. Powell, eds. New York: John Wiley & Sons.
ten Berge, W.F., A. Zwart, and L.M. Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13(3):301-309.
Withers, R.M.J., and F.P. Lees. 1986. The assessment of major hazards: The lethal toxicity of bromine. J. Hazard. Mater. 13(3):279-299.
APPENDIX A
DERIVATION OF AEGL VALUES FOR BROMINE
Derivation of AEGL-1
|
Key study: |
Rupp and Henschler 1967 |
|
Toxicity end point: |
Eye irritation in humans at 0.1 ppm for 30 min |
|
Uncertainty factors: |
3 for intraspecies variability |
|
Time-scaling: |
Not applied; adaptation to mild sensory irritation |
|
Modifying factor: |
None |
|
Calculation: |
0.1 ppm/3 = 0.033 ppm |
Derivation of AEGL-2
|
Key study: |
Rupp and Henschler 1967 |
|
Toxicity end point: for 30 min |
Eye, nose, and throat irritation in humans at 1.0 ppm |
|
Time-scaling: |
C2.2 × t = k, based on mouse lethality study (Bitron and Aharonson 1978) |
|
Uncertainty factors: |
3 for intraspecies variability |
|
Modifying factor: |
None |
|
Calculations: |
(Concentration/uncertainty factors)2.2 × t = k (1 ppm/3)2.2 × 30 min = k 2.676 ppm2.2 × min = k |
|
10-min AEGL-2: |
(2.676 ppm2.2 × min/10 min)1/2.2 = 0.55 ppm |
|
30-min AEGL-2: |
0.33 ppm |
|
1-h AEGL-2: |
(2.676 ppm2.2 × min/60 min)1/2.2 = 0.24 ppm |
|
4-h AEGL-2: |
(2.676 ppm2.2 × min/240 min)1/2.2 = 0.13 ppm |
|
8-h AEGL-2: |
(2.676 ppm2.2 × min/480 min)1/2.2 = 0.095 ppm |
Derivation of AEGL-3
|
Key study: |
Schlagbauer and Henschler 1967 |
|
Toxicity end point: probit analysis |
30-min LC01 of 116 ppm in the mouse, calculated by |
|
Time-scaling: |
C2.2 × t = k, based on mouse lethality study (Bitron and Aharonson 1978) |
|
Uncertainty factors: |
3 for intraspecies variability 3 for interspecies variability |
|
Modifying factor: |
None |
|
Calculations: |
(Concentration/uncertainty factors)2.2 × t = k (116 ppm/10)2.2 × 30 min = k 6,590.66 ppm2.2 × min = k |
|
10-min AEGL-3: |
(6,590.66 ppm2.2 × min/10 min)1/2.2 = 19 ppm |
|
30-min AEGL-3: |
116/10 = 12 ppm |
|
1-h AEGL-3: |
(6,590.66 ppm2.2 × min/60 min)1/2.2 = 8.5 ppm |
|
4-h AEGL-3: |
(6,590.66 ppm2.2 × min/240 min)1/2.2 = 4.5 ppm |
|
8-h AEGL-3: |
(6,590.66 ppm2.2 × min/480 min)1/2.2 = 3.3 ppm |
APPENDIX B
CATEGORY GRAPH OF TOXICITY DATA AND AEGL VALUES
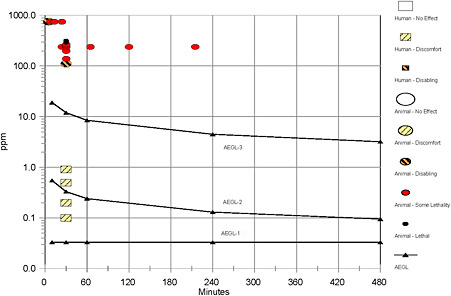
FIGURE B-1 Category graph for bromine.
TABLE B-1 Data Used in Category Graph
|
Source |
Species |
ppm |
Min |
Categorya |
|
AEGL-1 |
|
0.033 |
10 |
AEGL |
|
AEGL-1 |
|
0.033 |
30 |
AEGL |
|
AEGL-1 |
|
0.033 |
60 |
AEGL |
|
AEGL-1 |
|
0.033 |
240 |
AEGL |
|
AEGL-1 |
|
0.033 |
480 |
AEGL |
|
AEGL-2 |
|
0.55 |
10 |
AEGL |
|
AEGL-2 |
|
0.33 |
30 |
AEGL |
|
AEGL-2 |
|
0.24 |
60 |
AEGL |
|
AEGL-2 |
|
0.13 |
240 |
AEGL |
|
AEGL-2 |
|
0.095 |
480 |
AEGL |
|
Source |
Species |
ppm |
Min |
Categorya |
|
AEGL-3 |
|
19 |
10 |
AEGL |
|
AEGL-3 |
|
12 |
30 |
AEGL |
|
AEGL-3 |
|
8.5 |
60 |
AEGL |
|
AEGL-3 |
|
4.5 |
240 |
AEGL |
|
AEGL-3 |
|
3.3 |
480 |
AEGL |
|
Bitron and Aharonson 1978 |
Mouse |
240 |
24 |
SL |
|
|
Mouse |
240 |
65 |
SL |
|
|
Mouse |
240 |
120 |
SL |
|
|
Mouse |
240 |
215 |
SL |
|
|
Mouse |
750 |
5 |
2 |
|
|
Mouse |
750 |
7 |
SL |
|
|
Mouse |
750 |
13 |
SL |
|
|
Mouse |
750 |
24 |
SL |
|
Schlagbauer and Henschler 1967 |
Mouse |
111 |
30 |
2 |
|
|
Mouse |
140 |
30 |
SL |
|
|
Mouse |
199 |
30 |
SL |
|
|
Mouse |
236 |
30 |
SL |
|
|
Mouse |
252 |
30 |
SL |
|
|
Mouse |
268 |
30 |
SL |
|
|
Mouse |
290 |
30 |
3 |
|
|
Mouse |
315 |
30 |
3 |
|
Rupp and Henschler 1967 |
Human |
0.1 |
30 |
1 |
|
|
Human |
0.2 |
30 |
1 |
|
|
Human |
0.5 |
30 |
1 |
|
|
Human |
0.9 |
30 |
1 |
|
aCategory 0, no effect; 1, discomfort; 2, disabling, 3, lethal; SL, some lethality. |
||||
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR BROMINE
Derivation Summary for Bromine
AEGL-1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
0.033 ppm |
|
Key Reference: Rupp, H., and D. Henschler. 1967. Effects of low concentrations of chlorine and bromine on man [in German]. Int. Arch. Arbeitsmed. 23(1):79-90. |
||||
|
Test Species/Strain/Number: 20 human subjects |
||||
|
Exposure Route/Concentrations/Durations: Inhalation, concentrations of 0.1 to 1.0 ppm for at least 30 min |
||||
|
Effects: 0.1 ppm: eye irritation 0.50 to 1.0 ppm: eye, nose, and throat irritation |
||||
|
End Point/Concentration/Rationale: Eye irritation but not nose or throat irritation at 0.1 ppm for 30 min; meets the AEGL-1 definition of notable discomfort. |
||||
|
Uncertainty Factors/Rationale: |
||||
|
Total uncertainty factor: 3 |
||||
|
Interspecies: Not applied, human data used Intraspecies: 3, Workers have been exposed to concentrations up to 1 ppm with irritation being the only reported symptom. Compared with the 0.5 ppm AEGL-1 for the less well-scrubbed chlorine, the value may be conservative. Chlorine at 0.5 ppm for 4 h failed to elicit an asthmatic response in sensitive subjects. |
||||
|
Modifying Factor: Not applied |
||||
|
Animal to Human Dosimetric Adjustment: Not applied. |
||||
|
Time-Scaling: Not applied, adaptation to mild sensory irritation. |
||||
|
Data Adequacy: Compared with the irritancy data on chlorine, these values may be conservative. Based on the small database for bromine, extra protectiveness was considered appropriate. |
||||
AEGL-2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.55 ppm |
0.33 ppm |
0.24 ppm |
0.13 ppm |
0.095 ppm |
|
Key Reference: Rupp, H., and D. Henschler. 1967. Effects of low concentrations of chlorine and bromine on man [in German]. Int. Arch. Arbeitsmed. 23(1):79-90. |
||||
|
Test Species/Strain/Number: 20 human subjects |
||||
|
Exposure Route/Concentrations/Durations: Inhalation, concentrations of 0.1 to 1.0 ppm for at least 30 min |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.55 ppm |
0.33 ppm |
0.24 ppm |
0.13 ppm |
0.095 ppm |
|
Effects: 0.1 ppm: eye irritation 0.5 to 1.0 ppm: eye, nose, and throat irritation |
||||
|
End Point/Concentration/Rationale: Throat irritation at the 1.0 ppm concentration. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 3 |
||||
|
Interspecies: Not applied, human data used. Intraspecies: 3. Symptoms are below those defining an AEGL-2, but no reliable studies with exposures to higher concentrations were located. Irritation appeared to be limited to the upper respiratory tract with likely little penetration to the lower respiratory tract. Compared with the 30-min AEGL-2 value of 2.8 ppm for chlorine (which was protective of sensitive subjects) the uncertainty factor of 3 is adequate. |
||||
|
Modifying Factor: Not applied. |
||||
|
Animal to Human Dosimetric Adjustment: Not applied. |
||||
|
Time-scaling: C2.2 × t = k, based on a mouse lethality study. |
||||
|
Data Adequacy: Compared with the irritancy data on chlorine, these values may be conservative. But, based on the limited data base for bromine, extra protectiveness was considered appropriate. |
||||
AEGL-3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
19 ppm |
12 ppm |
8.5 ppm |
4.5 ppm |
3.3 ppm |
|
Key Reference: Schlagbauer, M., and D. Henschler. 1967. Inhalation toxicity of chlorine and bromine with single and repeated exposures [in German]. Int. Arch. Arbetsmed. 23(1):91-98. |
||||
|
Test Species/Strain/Number: Mouse/NMRI/10 per exposure group |
||||
|
Exposure Route/Concentrations/Durations: Inhalation, 110.5 to 315 ppm for 30 min |
||||
|
Concentration: |
Mortality: |
|||
|
110.5 ppm: |
0/10 |
|||
|
139.7 ppm: |
3/10 |
|||
|
198.9 ppm: |
6/10 |
|||
|
236.0 ppm: |
9/10 |
|||
|
252.1 ppm: |
10/10 |
|||
|
267.6 ppm: |
9/10 |
|||
|
290.3 ppm: |
10/10 |
|||
|
315.0 ppm |
10/10 |
|||
|
End Point/Concentration/Rationale: LC01 calculated by probit analysis |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
19 ppm |
12 ppm |
8.5 ppm |
4.5 ppm |
3.3 ppm |
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 |
||||
|
Interspecies: 3; the mouse was the most sensitive species in other studies with halogens Intraspecies: 3; at high concentrations, the corrosive action of irritants is not expected to differ greatly among individuals. |
||||
|
Modifying Factor: Not applied. |
||||
|
Animal to Human Dosimetric Adjustment: Not applied. |
||||
|
Time-scaling: C2.2 × t = k, based on a mouse lethality study. |
||||
|
Data Adequacy: Compared with the lethality data on chlorine, these values may be conservative, but based on the small database for bromine, extra protectiveness was considered appropriate. |
||||

































