4
Hydrogen Sulfide1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs have been defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million [ppm] or milligrams per cubic meter [mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGLs represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Hydrogen sulfide (H2S) is a colorless, flammable gas at ambient temperature and pressure. It has an odor similar to that of rotten eggs and is both an irritant and an asphyxiant. The air odor threshold ranges between 0.008 and 0.13 ppm, and olfactory fatigue may occur at 100 ppm. Paralysis of the olfactory nerve has been reported at 150 ppm (Beauchamp et al. 1984). Mean ambient air concentrations for H2S range between 0.00071 and 0.066 ppm.
Controlled human data were used to derive AEGL-1 values. Three of 10 volunteers with asthma exposed to H2S at 2 ppm for 30 min complained of headache and 8 of 10 experienced nonsignificant increased airway resistance (Jappinen et al. 1990). As there were no clinical symptoms of respiratory difficulty and there were no significant changes in forced vital capacity (FVC) or forced expiratory volume in 1 second (FEV1), the AEGL-1 was based exclusively on increased complaints of headache in the three volunteers (Jappinen et al. 1990). A modifying factor of 3 was applied to account for the wide variability in complaints associated with the foul odor of H2S and the shallow concentration response at the relatively low concentrations that are consistent with definition of the AEGL-1. The 30-min experimental value was scaled to the 10-min and 1-, 4-, and 8-h time points by using the concentration-exposure duration relationship, C4.4 × t = k, where C is concentration, t is time, and k is a constant. The exponent 4.4 was derived from rat lethality data ranging from 10-min to 6-h exposures.
The level of distinct odor awareness (LOA) for H2S is 0.01 ppm (see Appendix C for LOA derivation). The LOA represents the concentration above which it is predicted that more than half the exposed population will experience at least a distinct odor intensity, and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of the exposure due to odor perception. Thus, the derived AEGL-1 values are considered to have warning properties.
The AEGL-2 was based on the induction of perivascular edema in rats exposed to H2S at 200 ppm for 4 h (Green et al. 1991; Khan et al. 1991). An uncertainty factor of 3 was applied as rat and mouse data suggest little interspecies variability. An intraspecies uncertainty factor of 3 was applied to account for sensitive individuals. The intraspecies uncertainty factor of 3 is considered sufficient because applying the default uncertainty factor of 10 would result in a total uncertainty factor of 30, which would yield AEGL-2 values inconsistent with the total database for H2S. AEGL-2 values derived with larger uncertainty factors are essentially identical to or below the 10-ppm concentration causing no adverse health effects in humans exercising to exhaustion for up to 30 min (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Therefore, the total uncertainty factor applied in the derivation of AEGL-2 is 10. The 4-h experimental value was then scaled to the 10- and 30-min and 1- and 8-h time points, using C4.4 × t = k. The exponent 4.4 was derived from empirical rat lethality data ranging from 10-min to 6-h exposures.
The AEGL-3 was based on the highest concentration causing no mortality in the rat after a 1-h exposure (504 ppm) (MacEwen and Vernot 1972). An uncertainty factor of 3 was used to extrapolate from animals to humans as rat and mouse data suggest little interspecies variability. An uncertainty factor of 3 was applied to account for sensitive individuals. The intraspecies uncertainty factor of 3 is considered sufficient because applying the default uncertainty factor results in AEGL-3 values inconsistent with the data. AEGL-3 values derived with larger uncertainty factors were equal to or less than twice the concentration that failed to produce adverse health effects in humans exercising to exhaustion for up to 30 min (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Increased mortality or irreversible medical conditions consistent with the definition of AEGL-3 are unlikely at such concentrations. Therefore, the total uncertainty factor is 10. The value was then scaled to the 10- and 30 min and 1-, 4-, and 8-h time points, using C4.4 × t = k. The exponent 4.4 was derived from rat lethality data ranging from 10-min to 6-h exposures.
The AEGL values are listed in Table 4-1.
1.
INTRODUCTION
Hydrogen sulfide is a colorless, flammable gas at ambient temperature and pressure (NIOSH 1977). The National Fire Protection Association (NFPA 1974) placed H2S in the highest flammability classification. Precautions against fire
and explosion must be exercised to maintain airborne H2S below 0.43%. It has an odor similar to that of rotten eggs and it is both an irritant and an asphyxiant. The odor threshold is between 0.008 and 0.13 ppm, and olfactory fatigue, resulting in a lack of detection of odor, may occur at 100 ppm. Paralysis of the olfactory nerve has been reported at 150 ppm (Beauchamp et al. 1984). Mean ambient air concentrations in the United States range between 0.00071 and 0.066 ppm (NRC 1977; Graedel et al. 1986; Warneck 1988).
Approximately 90% of the H2S in the atmosphere occurs from natural sources. Hydrogen sulfide arises through bacterial reduction of sulfates and organic sulfur-containing compounds. It is emitted from crude oil, stagnant or polluted water, sewers, and manure or coal pits with low oxygen content. A small amount of H2S is emitted from volcanoes, vents, mudpots, and similar geologic formations (ATSDR 2006).
Hydrogen sulfide is synthesized commercially for use in rayon manufacturing, as an agricultural disinfectant, and as an additive in lubricants. It is also used as an intermediate in sulfuric acid and inorganic sulfide manufacturing and it is a by-product of pulp and paper manufacturing (Jaakkola et al. 1990) and geothermal operations (Kage et al. 1998); it is present in “sour” crude petroleum (NIOSH 1977; Guidotti 1994), roofing tar (Hoidal et al. 1986), natural gas, and shale oil (Ahlborg 1951; Kilburn 1993). Hydrogen sulfide has been manufactured in ton quantities for use in production of heavy water and as a moderator in nuclear reactors (NRC 1977). In 1997, it was manufactured in the United States by three companies at five sites (ATSDR 1999). Most H2S is made and used captively or transported by pipeline. As of 2007, total domestic commercial production in the United States exceeded 1.1 × 106 tons/year (Kroshwitz and Seidel 2007).
The physicochemical properties of H2S are presented in Table 4-2.
TABLE 4-1 Summary of AEGL Values for Hydrogen Sulfide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
0.75 ppm (1.05 mg/m3) |
0.60 ppm (0.84 mg/m3) |
0.51 ppm (0.71 mg/m3) |
0.36 ppm (0.50 mg/m3) |
0.33 ppm (0.46 mg/m3) |
Headache in humans with asthma (Jappinen et al. 1990) |
|
AEGL-2 (Disabling) |
41 ppm (59 mg/m3) |
32 ppm (45 mg/m3) |
27 ppm (39 mg/m3) |
20 ppm (28 mg/m3) |
17 ppm (24 mg/m3) |
Perivascular edema in rats (Green et al. 1991; Khan et al. 1991) |
|
AEGL-3 (Lethality) |
76 ppm (106 mg/m3) |
59 ppm (85 mg/m3) |
50 ppm (71 mg/m3) |
37 ppm (52 mg/m3) |
31 ppm (44 mg/m3) |
Highest concentration causing no mortality in the rat after a 1-h exposure (MacEwen and Vernot 1972) |
TABLE 4-2 Chemical and Physical Data for Hydrogen Sulfide
|
Parameter |
Data |
Reference |
|
Common name |
Hydrogen sulfide |
ATSDR 2006 |
|
Synonyms |
Hydrosulfuric acid, stink damp, sulfur hydride, sulfurated hydrogen, dihydrogen monosulfide, sewer gas, swamp gas, rotten-egg gas |
ATSDR 2006s |
|
CAS registry number |
7783-06-4 |
ATSDR 2006 |
|
Chemical formula |
H2S |
ATSDR 2006 |
|
Molecular weight |
34.08 |
ATSDR 2006 |
|
Physical state |
Colorless gas |
ATSDR 2006 |
|
Melting, boiling, and flash points |
−85.49ºC, −60.33ºC, and 26ºC |
ATSDR 2006 |
|
Density |
1.5392 grams/liter at 0ºC |
ATSDR 2006 |
|
Density in air |
1.192 |
ATSDR 2006 |
|
Solubility |
1 gram in 242 milliliters of water at 20ºC; soluble in alcohol, ether, glycerol, gasoline, kerosene, crude oil, carbon disulfide |
ATSDR 2006 |
|
Vapor pressure |
15,600 mmHg at 25ºC |
ATSDR 2006 |
|
Conversion factors in air |
1 ppm = 1.4 mg/m3 1 mg/m3 = 0.7 ppm |
AIHA 2000 |
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
According to U.S. Occupational Safety and Health Administration records, there were 80 fatalities in 57 H2S incidents from 1984 to 1994 (Fuller and Suruda 2000). Nineteen deaths and 36 H2S-induced injuries occurred among people attempting to rescue victims overcome by the gas.
The clinical toxicology of H2S has been reviewed (Smith and Gosselin 1979; Gosselin et al. 1984; Reiffenstein et al. 1992). Literature accounts of human fatalities after inhalation of H2S are abundant; however, exposure concentrations and durations in these accidents are generally not rigorously defined. Vapor concentrations on the order of 500 to 1,000 ppm or more are usually fatal within minutes (API 1948; Ahlborg 1951; Reiffenstein et al. 1992). Most fatalities occur in confined spaces (sewers, animal processing plants, manure tanks) and result from respiratory failure, initially presenting with respiratory insufficiency, noncardiogenic pulmonary edema, coma, or cyanosis. In many cases,
people lose consciousness after only one or two breaths, termed “slaughterhouse sledgehammer” (ATSDR 2006). Osbern and Crapo (1981) reported a typical, unfortunate accident involving an underground liquid manure storage pit. A farmer drained the liquid manure to a depth of 45 cm and entered the pit to retrieve a lid that a cow had kicked into the tank. The farmer was overcome within a few minutes, as were three men who attempted to rescue him. Three of the men lapsed into unconsciousness and died before reaching the hospital. Autopsy showed massive liquid manure pulmonary aspiration in two individuals and fulminant pulmonary edema without manure aspiration in the third. “Increased heart-blood sulfide levels” indicated significant H2S exposure. The clinical course of the surviving patient was complicated by hemodynamic instability, respiratory distress syndrome, and pulmonary infection. Air samples taken a week after the accident detected H2S at 76 ppm; however, pit air concentrations were likely higher at the time of exposure because of temperature and manure concentration.
Two workers collapsed and died within 45 min after entering a sewer manhole (NIOSH 1991). A concentration of 200 ppm was measured in sewer air 6 days after the accident. In another accident, a worker at a poultry-processing plant died after exposure to an estimated H2S concentration of 2,000 to 4,000 ppm for an estimated 15 to 20 min (Breysse 1961). Pulmonary, intracranial, and cerebral edema and cyanosis were observed at autopsy.
Hsu et al. (1987) reported 10 cases of accidental H2S poisoning. The H2S concentration was 429 ppm 4 h after the accident. Five victims died at the site of exposure. Four lost consciousness within 2 to 20 min of the accident and fell into a deep coma for approximately 48 h, regaining consciousness only after extensive hyperbaric oxygen therapy. Electrocardiograms indicated T-wave changes in all five survivors and changes in the P-wave in the patient remaining in the coma for 2 days. By day 9 after the accident, the electroencephalograms (EEGs) were essentially normal in four victims, while the P-wave returned to normal on day 21 and the T-wave returned to normal on day 36 in the most severely poisoned patient. On day 3, blood urea nitrogen increased to 39.2 milligrams per deciliter and remained above normal through day 13, while serum glutamic pyruvic transaminase activity remained increased through day 8. No pulmonary edema or long-term neurologic abnormalities were identified.
Autopsy of H2S victims often reveals pulmonary edema (Adelson and Sunshine 1966; Winek et al. 1968) and petechial hemorrhage into the lungs and brain along with gray-green cyanosis or a purple-to-green cast to the cerebral cortex, viscera, and blood (Freireich 1946; Breysse 1961; Adelson and Sunshine 1966). Postmortem formation of sulfhemoglobin contributes to these discolorations. Pulmonary edema is not always associated with death in H2S-induced central respiratory arrest inasmuch as very high (1,000 ppm) exposures induce prompt unconsciousness, apnea, and anoxic convulsions with risus sardonicus and opisthotonos (Hurwitz and Taylor 1954). If victims are promptly evacuated, recovery can be rapid. It is at lower “but nevertheless fatal” exposure concentra-
tions where the development of pulmonary and other systemic signs of H2S intoxication are permitted (Gosselin et al. 1984).
2.2.
Nonlethal Toxicity
2.2.1.
Case Reports
Albuminuria and hematuria (Osbern and Crapo 1981), brain stem and cortical damage (Hurwitz and Taylor 1954), neurasthenia, amnesia, and other psychic disorders and difficulties with equilibrium to frank tremor can afflict survivors of acute H2S intoxication. People acutely exposed to lower but nonfatal concentrations commonly experience lacrimation, photophobia, corneal opacity, tachypnea, dyspnea, tracheobronchitis (with elevated risk for bronchopneumonia), gastrointestinal distress (nausea, vomiting, diarrhea), arrhythmias, and palpitations, but these changes generally resolve promptly upon evacuation to fresh air. Patients can be left with a residual cough, hyposmia, dysosmia, or phantosmia (Kilburn and Warshaw 1995; Hirsch and Zavala 1999). At much higher concentrations, recovery from coma can be relatively rapid, and the clinical course is usually complete but slow in those patients who do not die. Artificial respiration is appropriate in victims with depressed or absent breathing, as are supportive steps to combat development of pulmonary edema (Gosselin et al. 1984). Oxygen is indicated in those patients with acute respiratory distress syndrome (Smith 1996).
Case reports concerning nonlethal H2S effects in humans are abundant; however, exposure parameters, concentration, and duration are often either unreported or only estimated. Symptoms of acute H2S exposure include ocular and respiratory tract irritation, nausea, headaches, loss of equilibrium, memory loss, olfactory paralysis, loss of consciousness, tremors, and convulsions (ATSDR 2006). Among tunnel, rayon, and sewer workers exposed for several hours to days, keratoconjunctivitis (“gas eye”) is commonplace (Vanhoorne et al. 1995). This condition is characterized by tearing, burning, and scratchy irritation of the cornea and conjunctivae, and the symptoms generally resolve without intervention or sequelae after cessation of exposure (Grant 1974). The threshold for ocular irritation by H2S alone has been reported as 10 to 20 ppm (WHO 1981), but accounts of eye pain, burning, and photophobia in the presence of related sulfides put the threshold at no more than 6 ppm (Vanhoorne et al. 1995).
Parra et al. (1991) reported cases of 14 workers likely poisoned with H2S from toilet facilities. The toilets were connected to a manure pit without a siphon. Workers complained of eye, nose, and throat irritation; nausea; dizziness; vomiting; and dyspnea. (One worker died a few hours after hospital admission. Hemorrhagic bronchitis and asphyxia were identified at autopsy.) Most workers recovered uneventfully; however, after a symptom-free period of 3 weeks, one worker reported dyspnea, chest tightness, and hemoptysis. A mild, bilateral, interstitial fibrosis was found on a chest X-ray and pulmonary function tests
showed mild restrictive pulmonary disease. Five months after the accident, the patient was asymptomatic except for residual exertion dyspnea.
Six patients were examined 5 to 10 years after accidental exposures to unknown concentrations of H2S (Tvedt et al. 1991a,b). They had been unconscious for 5 to 20 min in the H2S atmospheres. Persistent neurologic symptoms included impaired vision, memory loss, decreased motor function, tremors, ataxia, abnormal learning and retention, and slight cerebral atrophy. One patient was severely demented.
In another report, 37 workers (ages 24 to 50 years) were accidentally exposed to an undetermined concentration of H2S while drilling a pit to lay the foundation for a municipal sewage pumping station (Snyder et al. 1995). Symptoms included headache, dizziness, breathlessness, cough, burning discomfort in the chest, throat and eye irritation, nausea, and vomiting. Most workers recovered uneventfully; however, one worker died and another remained in a coma for 5 days. The comatose patient was aggressively treated with hyperbaric oxygen. He was discharged from the hospital on day 15 with slow speech, impaired attention span, easy distractibility, isolated retrograde amnesia, decreased ability to communicate, impaired visual memory, and poor retention of new information. His condition was unchanged at 12 and 18 months after exposure. Numerous other reports of permanent or persistent neurologic effects after exposure to H2S have been published (Wasch et al. 1989; Kilburn 1993; Kilburn and Warshaw 1995; Kilburn 1997). As with the other case studies, these reports lack definitive exposure parameters.
In May and June 1964, a H2S emission from an industrial landfill in Terre Haute, Indiana, resulted in nearby residents complaining about odor and nausea, loss of sleep, shortness of breath, and headache (HEW 1964). Samples collected from five sites around the city indicated H2S concentrations ranging from <2 to >300 parts per billion (ppb); however, the observations are confounded by concurrent exposure to other malodorous pollutants such as smoke from burning garbage and sulfurous coal tar. Data summarized and experiments carried out by the State of California Department of Health Services showed that the geometric mean of the threshold odor concentration for H2S was approximately 0.008 ppm (Amoore 1985). It was also stated that, as a provisional rule, it appears that when an unpleasant odor reaches approximately 5 times its odor threshold concentration, the mean concentration for complaints of odor annoyance is attained. Factors responsible for odor annoyance were categorized as the unpleasant odor sensation itself, effects on social life, and instigation of headache or nausea (Amoore 1985).
In another report, Ruth (1986) reported an odor threshold range of 0.0005 to 0.01 ppm and listed an irritating H2S concentration of 10 ppm.
Members of the Mobile Monitoring Team, Source Sampling Team, Technical Support Team, and Systems Planning and Implementation Team of the Texas Natural Resource Conservation Commission (TNRCC) conducted a mobile laboratory sampling trip to the Corpus Christi, Texas, area from January 31 to February 6, 1998 (TNRCC 1998). The mean H2S concentration downwind
from an oil refinery was 0.09 ppm (30-min downwind average). The six staff members complained of persistent objectionable odors, eye and throat irritation, headache, and nausea. In most cases, the symptoms subsided within a few hours after leaving the sampling site; throat irritation persisted in two staff members through the following day. The exposure duration was about 5 h. Sulfur dioxide, benzene, methyl t-butyl ether, and toluene were also detected, and it is possible that these chemicals contributed to the complaints.
Some authors hypothesize that odors, such as the rotten egg smell associated with H2S, may trigger asthma attacks; however, quantitative data supporting this premise are limited, and it is uncertain whether a toxicologic mechanism or stress-induced anxiety is involved. It is clear that objectionable odor can affect behavior; individuals detecting odors may stay indoors, temporarily leave the neighborhood or area, complain to officials, or consider a change of residence (Shusterman 1992). Many objectionable odor sources have been implicated in asthma attacks, annoyance, and behavioral modifications and include municipal odors (landfills, sewage treatment plants), agricultural odors (composting, feed lots), industrial odors (pulp mills, refineries, hazardous waste sites) (Shusterman 1992), and household (perfumes, flowers, cleaning products, food/cooking odors) and bodily odors (Stein and Ottenberg 1958; Herbert et al. 1967). Bruvold et al. (1983) used a survey questionnaire to determine whether people living downwind of two sewage treatment plants in California (Pacifica and Novato) detected H2Sodor and experienced odor annoyance more frequently than people living in two control communities. Hydrogen sulfide concentrations in the test communities ranged from 1 to 6 ppb. Odor was reported by 49 of 54 respondents in Pacifica compared with 4 of 54 respondents in the Pacifica control community and 19 of 50 respondents in Novato compared with 1 of 48 respondents from the Novato control community. When respondents were asked to rate odor annoyance on a scale of 0 (no annoyance) to 10 (extreme annoyance), the following median annoyance scores were obtained: Pacifica affected = 7.9, Pacifica control = 5.5, Novato affected = 4.3, Novato control = 1.0. One in nine respondents in the “exposed” neighborhoods also reported that they or a family member had been made sick by the odors; however, only 1% of this group sought medical attention and no odor-induced asthma was reported in these communities.
Rossi et al. (1993) examined the association between emergency room visits for asthma attacks and weather (temperature, humidity, barometric pressure, rainfall), levels of air pollutants (nitrogen dioxide, sulfur dioxide, H2S, total suspended particles), and pollen counts. No association was found between pollen counts and weather conditions, except for an inverse correlation with temperature. The most significant correlation was found for nitrogen dioxide, with lesser correlations for sulfur dioxide, H2S, and total suspended particles. The daily mean H2S concentration was 0.0022 ppm (range 0 to 0.02 ppm) and the daily maximum was 0.01 ppm (range 0 to 0.12 ppm). Only the relationship between nitrogen dioxide and asthma attacks held after controlling for temperature.
Stein and Ottenberg (1958) interviewed 25 hospitalized patients with asthma to determine whether odors precipitated asthma attacks. Initially, the patients were asked what would precipitate an asthma attack. If odors were not mentioned, they were then specifically asked about odors. The responses were then analyzed for the “character of the odorous substance.” Twenty-two of the 25 subjects stated that odors precipitated attacks; of these 22, about half initially specified odor as a precipitating factor, whereas the others included odor only when prompted by the interviewer. The most common (74%) “precipitating odors” were categorized as “cleanliness/uncleanliness and included urine, sweat, feces, disinfectant, bleach, camphor, dirty/musty, smoke, sulfur, chemicals, paint, horses, and barn; followed by “romantic odors” (21%) including perfume, spring, and flowers; and foods (5%) such as bacon, onion, and garlic. In another report, Herbert et al. (1967) administered questionnaires to two groups of patients with asthma, one from a psychiatric hospital and one from a general hospital, to determine whether odors precipitated asthma attacks. Approximately 80% of patients reported that odor triggered asthma attacks, with the most common triggers being paint, tobacco fumes, wood smoke, household odors, and paraffin. The authors concluded that emotional distress could precipitate an asthmatic attack. There was no clear differentiation between the two groups of patients. In both the Stein and Ottenberg (1958) and Herbert et al. (1967) reports, the results are compromised by possible undefined concurrent exposures, no measure of repeatability, and the fact that neither the patient nor the interviewer was blinded to the inquiry.
2.2.2.
Epidemiologic Studies
Jappinen et al. (1990) studied a cohort of 26 male pulp mill workers (mean age 40.3 years, range 22 to 60 years) to assess the possible effects of H2S on respiratory function. The workers experienced daily exposure to H2S “usually below the maximum permitted concentration of 10 ppm.” Bronchial responsiveness, FVC, and FEV1 were measured after at least 1 day off work and at the end of a workday. No significant changes in respiratory function or bronchial responsiveness were observed at the end of the workday compared with control values.
Studies of communities located near pulp mills have reported increased incidences of respiratory system symptoms (irritation and cough) and central nervous system symptoms (headaches, migraine) (Partti-Pellinin et al. 1996). Although H2S concentrations have been reported in these studies, the populations were also exposed to relatively high concentrations of other malodorous sulfur compounds such as sulfur dioxide and mercaptans. Thus, it is difficult to define a concentration-response relationship for H2S from these reports.
Hessel et al. (1997) studied a group of 175 oil and gas workers (mean age 35 years) in Alberta, Canada. Hydrogen sulfide exposure concentrations were not available; therefore, exposure groups were determined by questioning the
workers about “exposures strong enough to cause symptoms,” “exposures that resulted in loss of consciousness (knockdown),” or no exposure. Exposures strong enough to cause symptoms were reported by 51 workers, “knockdown” was reported by 14 workers, and 110 workers reported no exposure. Exposures strong enough to cause symptoms were not associated with lower spirometric values. Knockdowns were not associated with lower spirometric values but were associated with shortness of breath while hurrying on the level or up a slight hill, wheezing with chest tightness, and wheezing attacks.
In another study, 21 swine confinement facility owner-operators were tested by spirometry immediately before and after a 4-h work period (Donham et al. 1984). The confinement workers had statistically significant (p < 0.05) reductions in pulmonary flow rates ranging from 3.3% to 11.9% mean forced expiratory flow (FEF) after the 4-h work period. The report states that the work environment was sampled for particulates and gases during the exposure period and that evidence suggested a concentration-response relationship between carbon dioxide and H2S exposure and lung function decrements. However, these monitoring data were not presented in the study report.
2.2.3.
Experimental Studies
Jappinen et al. (1990) exposed a group of 10 people with asthma (3 men age 33 to 50 years and 7 women age 31 to 61 years) to H2S at 2 ppm for 30 min. The subjects had been diagnosed with bronchial asthma for 1 to 13 years and were under medical supervision. Severe asthma patients were excluded from the protocol. Two volunteers were exposed simultaneously in a 10-m3 sealed tile-walled exposure chamber with an oxygen flow of 2 liters (L)/min. Hydrogen sulfide concentration was monitored continuously with a sulfur dioxide analyzer connected to a converter that transformed H2S into sulfur dioxide at 840ºC. The H2S was supplied to the chamber from laminated plastic bags through plastic tubing. All asthma subjects complained of an unpleasant odor and nasal and pharyngeal dryness at the initiation of exposure. Three of the 10 complained of headache after exposure. There were no significant effects on FVC, FEV1, or FEF values after exposure to H2S. Airway resistance (Raw) value was slightly decreased in two and increased in eight subjects. The range of Raw differences was –5.95% to +137.78%, with an average increase of 26.3%; no accompanying clinical symptoms were observed. The range of specific airway conductance (SGaw) differences was –57.7% to +30%, with an average decrease of 8.4%. These effects were not statistically significant; however, in two subjects, changes were greater than 30% in both Raw and SGaw.
Bhambhani and Singh (1991) exposed 16 healthy male volunteers (age 25.2 ± 5.5 years) to H2S at 0, 0.5, 2.0, or 5.0 ppm during graded cycle exercise performed to exhaustion (up to 16 min). Filtered air from a pressurized cylinder was passed through a stainless steel humidifying chamber at a flow rate of 50 L/min. After humidification, the air was mixed with H2S at 1,000 ppm in nitro-
gen regulated from a pressurized cylinder to obtain the desired concentration of H2S in the test atmosphere. The H2S was then collected in three polyethylene bags connected in a series and suspended from the ceiling. The flow of gas into and out of each bag was controlled with a two-way stopcock and the H2S concentration was continuously monitored with a sulfur dioxide analyzer connected to a converter that transformed H2S into sulfur dioxide. Each subject completed a preliminary graded exercise test (H2S at 0 ppm) designed to determine maximum oxygen uptake (VO2max) and to identify the anaerobic and respiratory compensation thresholds by use of respiratory gas-exchange criteria. The subject began pedaling an electronically braked cycle ergonometer at zero load at a speed of 60 revolutions/min for 4 min. The power output was then increased by 200 kilopond-meters (kpm)/min every 2 min. Until volitional fatigue was achieved, there was no further increase in oxygen uptake with increasing power output (when VO2max was achieved). After the preliminary test, each subject completed four additional tests, one every 2 weeks, while breathing H2S at 0, 0.5, 2.0, or 5.0 ppm in a random order. After a 4-min warmup period at zero load, the power output was increased so that it was midway between zero load and ventilatory threshold 1 (VT1) from the preliminary test. The subject pedaled at this power output for 3 min, after which the power output was increased midway between VT1 and VT2 from the preliminary test. Pedaling was continued for an additional 3 min and then raised to the VT2 level. After 3 min of pedaling, power output was increased 200 kpm/min every minute until the VO2max was achieved. There was no effect on heart rate or expired ventilation as a result of H2S exposure during submaximal or maximal exercise. There was a tendency for oxygen uptake to increase and for carbon dioxide output to decrease with increasing H2Sconcentration; however, these effects were significant (p < 0.05) only at 5.0 ppm. Blood lactate levels also increased significantly (p < 0.05) as a result of exposure to H2S at 5.0 ppm. Maximal power output was not affected, however, thus questioning the biologic and toxicologic significance of the measured effects.
In a followup study, Bhambhani et al. (1994, 1996a) exposed 25 healthy volunteers (13 men, age 24.7 ± 4.6 years and 12 women, age 22 ± 2.1 years) to H2S at 0 or 5 ppm for 30 min while exercising on a cycle ergonometer at 50% of their VO2max. The exposure protocol is essentially identical to that described by Bhambhani and Singh (1991). There were no treatment-related effects on oxygen uptake, carbon dioxide production, respiratory exchange ratio, heart rate, blood pressure, arterial blood oxygen and carbon dioxide tensions or pH, or perceived exertion ratings (Bhambhani et al. 1994). In men, the muscle citrate synthetase decreased 19% (p < 0.05) after H2Sexposure compared with controls. Muscle lactate and lactic acid dehydrogenase increased 24% (not significant [n.s.]) and 6% (n.s.), respectively, and cytochrome oxidase decreased 9% (n.s.) after H2Sexposure compared with the control condition. In women, the muscle citrate synthetase decreased 19% (n.s.) after H2S exposure compared with controls. Muscle lactate and lactic acid dehydrogenase were not affected in women and cytochrome oxidase increased 23% (n.s.) after H2S exposure compared with
the control condition (Bambhani et al. 1996a). None of the subjects reported adverse health effects after H2S exposure.
In another followup study, Bhambhani et al. (1996b) exposed 19 healthy volunteers (9 men, age 27.4 ± 6.4 years and 10 women, age 21.8 ± 3.0 years) to H2S at 0 or 10 ppm for 15 min while exercising on a cycle ergonometer at 50% of their VO2max. The exposure protocol is essentially identical to that described by Bhambhani and Singh (1991). There were no treatment-related effects on FVC, FEV1, peak expiratory flow rate, FEF rate, or maximal ventilation volume in either sex.
In another study, Bambhani et al. (1996b), exposed 28 healthy volunteers (15 men, age 23.4 ± 5.2 years and 13 women, age 21.8 ± 3.0 years) to H2S at 0 or 10 ppm for 30 min while exercising on a cycle ergonometer at 50% of their VO2max. The exposure protocol is essentially identical to that described by Bhambhani and Singh (1991). There were no treatment-related effects on oxygen uptake, carbon dioxide production, respiratory exchange ratio, heart rate, blood pressure, arterial blood oxygen, and carbon dioxide tensions. Muscle lactate increased 33% (n.s.) in men and 16% (n.s.) in women after exposure to H2S. Muscle cytochrome oxidase decreased by 16% in men, whereas it increased by 11% in women after exposure to H2S. None of the subjects reported adverse health effects after H2S exposure.
2.3.
Developmental and Reproductive Toxicity
Xu et al. (1998) conducted a retrospective epidemiologic study of female workers in a large petrochemical facility in Beijing, China. The study was designed to investigate the association between petrochemical exposure and spontaneous abortion. The facility consisted of 17 major production plants, which are divided into separate workshops, allowing for the assessment of exposure to specific chemicals. Married women (n = 2,853) between the ages of 20 and 44 years who had never smoked and did not consume ethanol participated in the study. These women reported at least one pregnancy during their employment at the plant. The association between occupational exposures and spontaneous abortion were adjusted for age, education level, plant shift work, standing and kneeling hours at work, noise level, dust concentration, passive smoke at home and work, and diet. There were 106 women (3.7% of the study population) exposed to only H2S and the data showed a significant association between occupational H2S exposure and increased frequency of spontaneous abortion. The spontaneous abortion rate was 12.3% (odds ratio 2.3, 95% confidence interval 1.2 to 4.4) in workers exposed to H2S compared with 2.9% in the control group. No H2S exposure concentrations were reported.
2.4.
Genotoxicity
Genotoxic studies on acute human exposure to H2S were not available.
2.5.
Carcinogenicity
Carcinogenicity studies on human exposure to H2S were not available. However, no increase in cancer incidence was found in a cohort living downwind from natural gas refineries in Alberta, Canada, from 1970 to 1984 (ATSDR 2006).
2.6.
Summary
Hydrogen sulfide is both an irritant and an asphyxiant. At relatively low concentrations (<10 ppm), minor ocular and respiratory irritation occur; at higher concentrations (hundreds to thousands of ppm), the central nervous system is affected and paralysis of the respiratory center may lead to rapid death. Ocular effects described after inhalation of H2S include acute conjunctivitis (gas eye) at concentrations of 50 to 100 ppm and are believed to be result from direct contact of H2S with the eye. The threshold for eye irritation is reportedly 6 to 20 ppm (WHO 1981; Vanhoorne et al. 1995; ACGIH 1996). There are numerous reports of accidental poisonings with H2S; however, neither reliable concentrations nor duration parameters are described for lethal or nonlethal end points. Several reports suggest that neurologic effects may persist in survivors of severe H2S poisonings. Some reports hypothesize that odors, such as the rotten egg smell associated with H2S, may trigger asthma attacks and other health effects; however, it is uncertain whether a toxicologic mechanism or a nontoxicologic odor-related mechanism is involved. No adverse effects were observed in male or female volunteers exposed to H2S at 5 or 10 ppm for up to 30 min while exercising to exhaustion (Bambhani and Singh 1991; Bambhani et al. 1994, 1996a,b). Headache in 30% and clinically insignificant increased airway resistance were observed in asthma patients exposed to H2S at 2 ppm for 30 min (Jappinen et al. 1990). An association between spontaneous abortion and occupational exposure to H2S was identified in a cohort of female petroleum plant workers; however, no H2S concentrations were provided (Xu et al. 1998). No information on potential human genotoxicity or carcinogenicity was located.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
3.1.1.
Rats
Groups of five male and five female Wistar rats were exposed to different concentrations of H2S for 10, 30, or 60 min (Arts et al. 1989; Zwart et al. 1990). The test atmospheres were monitored at the inlet and outlet of the glass exposure chambers (0.90 m long and 0.15 m diameter; flow rate 25 to 40 L/min). The 10-min LC50 (concentration with 50% lethality) value was 835 ppm, whereas the 30- and 50-min LC50 values were 726 and 683 ppm, respectively.
MacEwen and Vernot (1972) exposed groups of 10 male Sprague-Dawley rats to H2S at 400, 504, 635, or 800 ppm for 1 h. The exposures were conducted in a 30-L glass bell jar at an air flow rate of 30 L/min and chamber concentrations were continuously monitored with an ion-specific sulfide electrode. Gasping was observed during exposure. Rats surviving the 14-day observation period exhibited normal weight gains; however, congestion and mottling of the kidney and liver accompanied by moderate to severe fatty changes in the liver were observed at necropsy. A 1-h LC50 value of 712 ppm was calculated. Mortality data are summarized in Table 4-3.
Tansy et al. (1981) exposed groups of five male and five female Sprague-Dawley rats to H2S at 0 to 600 ppm for 4 h, followed by a 14-day observation period. Animals were exposed simultaneously in a 75-L glass chamber. A 4-h LC50 of 444 ppm was calculated.
Prior et al. (1988) exposed groups of male and female Long Evans, Sprague-Dawley, and Fischer 344 (F344) rats to different concentrations of H2S for 2, 4, or 6 h, followed by a 14-day observation. Exposure chambers were clear, acrylic horizontal flow chambers with two removable stainless steel cones with a total volume of 69 L. Hydrogen sulfide concentrations were monitored by gas chromatography four times per hour. LC50 values of 587, 501, and 335 ppm were calculated for the 2-, 4-, and 6-h time points, respectively. No strain differences were observed.
In another study, Khan et al. (1990) exposed groups of 12 male F344 rats to H2S at 0, 10, 50, 200, 400, or 500 to 700 ppm for 4 h. Animals exposed to H2S at 500 to 700 ppm died from the exposure. This study, including nonlethal effects observed at lower concentrations, is described in Section 3.2.1.
Groups of five male Sprague-Dawley rats were exposed to H2S at 0 or 1,655.4 ± 390.9 ppm (Lopez et al. 1989). The H2S was passed sequentially through a regulator and flowmeter and then mixed with a supply of conditioned air. The H2S flow was adjusted to maintain the target concentration and the test atmosphere was analyzed by gas chromatography. All test rats died within 3 min, but none of the control rats died from exposure. Rats exposed to H2S exhibited dyspnea characterized by exaggerated abnormal, audible respiration and had frothy fluid from the nose and mouth during exposure. Pulmonary edema was evident in exposed rats at necropsy. No abnormalities were observed in control animals.
TABLE 4-3 Mortality of Rats Exposed to Hydrogen Sulfide for 1 Hour
|
Concentration (ppm) |
Mortality |
|
400 |
0/10 |
|
504 |
0/10 |
|
635 |
1/10 |
|
800 |
9/10 |
|
Source: MacEwen and Vernot 1972. |
|
3.1.2.
Mice
Groups of five male and five female Swiss mice were exposed to different concentrations of H2S for 10, 30, or 50 min (Arts et al. 1989; Zwart et al. 1990). The test atmospheres were monitored at the inlet and outlet of the glass exposure chambers (0.90 m long and 0.15-m diameter; flow rate 25 to 40 L/min). The 10-min LC50 value was 1,160 ppm, and the 30- and 50-min LC50 values were 800 and 676 ppm, respectively.
MacEwen and Vernot (1972) exposed groups of 10 male CF1 mice to H2S at 400, 504, 635, or 800 ppm for 1 h. The exposures were conducted in a 30-L glass bell jar at an air flow rate of 30 L/min and chamber concentrations were continuously monitored with an ion-specific sulfide electrode. Gasping and convulsions were observed during exposure. Mice surviving the 14-day observation exhibited normal weight gains; however, one mouse each from the 635- and 800-ppm groups had a blocked urethral opening due to encrustation of the external orifice, and consequently the bladders were distended upon examination at 14 days. A 1-h LC50 value of 634 ppm was calculated. Mortality data from this study are summarized in Table 4-4, and rat and mouse LC50 values are summarized in Table 4-5.
TABLE 4-4 Mortality of Mice Exposed to Hydrogen Sulfide for 1 Hour
|
Concentration (ppm) |
Mortality |
|
400 |
2/10 |
|
504 |
0/10 |
|
635 |
5/10 |
|
800 |
8/10 |
|
Source: MacEwen and Vernot 1972. |
|
TABLE 4-5 Summary of LC50 Values of Rats and Mice Exposed to Hydrogen Sulfide
|
Exposure Duration |
LC50 (ppm) |
Reference |
|
|
Rat |
Mouse |
||
|
10 min |
835 |
1,160 |
Zwart et al. 1990 |
|
30 min |
726 |
800 |
Zwart et al. 1990 |
|
60 mina |
683 |
676 |
Zwart et al. 1990 |
|
1 h |
712 |
634 |
MacEwen and Vernot 1972 |
|
2 h |
587 |
ND |
Prior et al. 1988 |
|
4 h |
501 |
ND |
Prior et al. 1988 |
|
4 h |
444 |
ND |
Tansy et al. 1981 |
|
6 h |
335 |
ND |
Prior et al. 1988 |
|
aZwart et al. (1990) reported a 50-min LC50 value. However, because the exposure was 60 min, it is assumed that this value is a 60-min LC50 value. Abbreviation: LC50, concentration with 50% lethality; ND, no data. |
|||
3.2.
Nonlethal Toxicity
3.2.1.
Rats
Groups of 12 male F344 rats were exposed to H2S at 0, 10, 200, or 400 ppm for 4 h (Lopez et al. 1987, 1988). Exposure chambers were clear, acrylic horizontal flow chambers with two removable stainless steel cones with a total volume of 69 L. Hydrogen sulfide was passed sequentially through a regulator and flowmeter and was then mixed with a supply of filtered air. The flow of H2S and air was adjusted to maintain the target concentration in the exposure chambers. Gas flow volume was 17 L/min. Hydrogen sulfide concentrations were monitored by gas chromatography three times per hour. Actual concentrations were within 4% of nominal. Four rats from each group were sacrificed at 1, 20, and 44 h postexposure. No animals died during exposure. No effects were noted at 10 ppm. At the end of the 4-h exposure, rats in the 400-ppm group were lethargic but rapidly recovered. Lactate dehydrogenase (LDH) and protein in nasal passages were initially increased in rats administered 400 ppm but had returned to baseline levels 20 h after exposure (Lopez et al. 1987). Bronchoalveolar cell counts were decreased only in the 400-ppm group. Alkaline phosphatase (400 ppm) and LDH (200 and 400 ppm) activities in lung lavage fluid were increased up to 90%. Lung protein concentrations were increased 3,000% and remained elevated through 44 h postexposure at 400 ppm. Treatment-related nasal cavity lesions were observed only at 400 ppm (Lopez et al. 1988). Necrosis and exfoliation of respiratory and olfactory mucosal cells were observed; however, squamous epithelial cells were not affected. Lesions were distributed midway along the nasal passages involving nasal and maxillary conchae but not ethmoidal conchae. Injured respiratory mucosa was undergoing repair at 44 h; however, olfactory mucosa continued to exfoliate after 44 h.
Green et al. (1991) exposed groups of six male F344 rats to H2S at 0, 200, or 300 ppm for 4 h utilizing the exposure system described by Lopez et al. (1987, 1988). Animals were sacrificed 1 h after exposure. Actual exposure concentrations were 0, 194.1 ± 3.7, and 290 ± 3 ppm. No adverse clinical signs were observed in animals exposed to 200 ppm; however, there was a significant (p < 0.001) increase in protein and LDH in lavage fluid from these animals compared with controls. Focal areas of perivascular edema and occasional collections of proteinaceous material in the alveoli were observed in animals from the 200-ppm group. Rats exposed to H2S at 300 ppm were visibly stressed during exposure and their lungs showed focal areas of red atelectasis and patchy alveolar edema with perivascular and peribronchial interstitial edema. Significantly (p < 0.001) increased protein concentration and LDH activity were found in lung lavage fluid from animals exposed to 300 ppm. Exposure to H2S at 300 ppm produced significant (p < 0.01) abnormalities of surfactant activity parameters.
Khan et al. (1990) exposed groups of 12 male F344 rats to H2S at 0, 10, 50, 200, 400, or 500 to 700 ppm for 4 h utilizing the exposure system described by Lopez et al. (1987, 1988). Four rats from each group were sacrificed at 1, 24,
or 48 h postexposure. No effects were observed at 10 ppm. Cytochrome c oxidase activity in lung mitochondria was significantly (p < 0.05) decreased at 50 ppm (15%), 200 ppm (43%), and 400 ppm (68%) at 1 h postexposure compared with controls. Cytochrome c oxidase activity returned to normal 24 h postexposure in the 50-ppm animals, was about 89% of normal at 24 and 48 h postexposure at 200 ppm, and remained at 70% of normal 24 and 48 h postexposure in the 400-ppm group. Animals inhaling H2S at 500 to 700 ppm died and had >90% inhibition of cytochrome c oxidase activity. Succinate oxidase was significantly (p < 0.001) decreased at 200 ppm (40%) and 400 ppm (63%) at the 1-h time point. Succinate-cytochrome c reductase and NADH-cytochrome c reductase were unaffected by exposure to H2S.
Kahn et al. (1991) studied groups of six male F344 rats inhaling H2S at 0, 50, 200, or 400 ppm for 4 h using an exposure system identical to that described by Lopez et al. (1987). All rats were sacrificed immediately after cessation of exposure, and lungs were lavaged. Greater than 90% of the cells lavaged were pulmonary alveolar macrophages. Exposure to H2S at 50 and 200 ppm did not affect the number of cells recovered; however, cell viability was significantly (p < 0.05) reduced in animals exposed to 400 ppm. This experiment also showed complete inhibition of zymosan-induced stimulation of respiratory rates of the macrophages from animals exposed to H2S at 200 and 400 ppm.
In a third study, Kahn et al. (1998) exposed groups of male F344 rats to H2S at 0, 1, 10, or 100 ppm, 8 h/day, 6 days/week, for 5 weeks. Lung, brain, and liver mitochondria were analyzed for cytochrome oxidase activities, and erythrocytes were analyzed for superoxide dismutase and glutathione peroxidase activities. No effects were noted at 1 ppm. Rats exposed to H2S at 10 and 100 ppm exhibited concentration-related reductions in cytochrome c oxidase activity in lung (p < 0.05) mitochondria. Cytochrome c oxidase activity in liver mitochondria was not affected by exposure. A small (8%) decrease in superoxide dismutase activity was noted in 100-ppm rats compared with controls.
Male F344 rats (number per group not stated) were exposed to H2S at 0 or 400 ppm for 4 h or 1,500 ppm for 4 min (Lefebvre et al. 1991). The exposure system was similar to that described by Lopez et al. (1987, 1988). Immediately after exposure, the eyes were washed with 0.4 milliliter of saline and the lavage fluid was collected for exfoliative cytology. The number of exfoliated cells was increased in animals exposed to H2S (44 cells/microliter [μL] at 400 ppm; 35 cells/μL at 1,500 ppm) compared with control (19 cells/μL). Exposure to H2S also increased the proportion of conjunctival to corneal epithelial cells recovered compared with controls.
Kohno et al. (1991) exposed groups of male Wistar rats to H2S at 75 ppm for 20 to 60 min. Heart rates were decreased 10% to 27% during exposure and up to 1 h postexposure compared with controls, which was thought to be a possible compensatory response. Necropsy showed slight lung congestion in exposed animals.
Dorman et al. (2002) exposed groups of male CD rats to H2S at 10, 30, 80, 200, or 400 ppm for 3 h and examined hindbrain, lung, liver, and nasal cyto-
chrome oxidase activity and sulfide concentrations immediately after exposure. Lung sulfide and sulfide metabolite concentrations were also analyzed at 0, 1.5, 3, 3.25, 3.5, 4, 5, and 7 h postexposure to 400 ppm. Lung sulfide concentrations increased during exposure and returned to baseline levels within 15 min after exposure to 400 ppm, and lung sulfide metabolite concentrations transiently increased immediately after the end of exposure. Decreased cytochrome oxidase activities were noted in the olfactory epithelium at 30, 80, 200, and 400 ppm. Increased olfactory sulfide concentrations were noted after exposure to 400 ppm. No effects were noted for hindbrain and nasal respiratory sulfide concentrations. Nasal respiratory epithelial cytochrome oxidase activity decreased in animals exposed to H2S at 30, 80, 200, and 400 ppm. Liver sulfide concentrations were increased at 200 and 400 ppm, and liver cytochrome oxidase activity was increased in all treatment groups.
Brenneman et al. (2000) exposed groups of male Sprague-Dawley rats to H2S at 0, 10, 30, or 800 ppm for 6 h/day, 7 days/week, for 10 weeks. Multifocal, bilaterally symmetrical olfactory neuron loss and basal cell hyperplasia, limited to the olfactory mucosa, were observed in rats exposed to 30 or 80 ppm. Lesions were observed in the dorsal medial meatus and dorsal and medial areas of the ethmoid recess. No treatment-related effects were noted at 10 ppm.
Brenneman et al. (2002) then exposed groups of male Sprague-Dawley rats to H2S at 0, 30, 80, 200, or 400 ppm for 3 h/day for 1 or 5 consecutive days. Nasal passages were examined histologically 24 h postexposure and lesion recovery was assessed 2 and 6 weeks after the 5-day exposure. Regeneration of the respiratory mucosa and full thickness necrosis of the olfactory mucosa localized to the ventral and dorsal medial meatus were noted after the single 3-h exposure to H2S at 80, 200, or 400 ppm. A single exposure to 400 ppm resulted in severe mitochondrial swelling in sustentacular cells and olfactory neurons, progressing to olfactory epithelial necrosis and sloughing. Repeated exposure to 80, 200, or 400 ppm resulted in necrosis of the olfactory mucosa with early mucosal regeneration extending from the dorsomedial meatus to the caudal regions of the ethmoid recess.
In a repeated exposure study, groups of five male Sprague-Dawley rats were exposed to H2S at 0 (nitrogen air mixture), 25, 50, 75, or 100 ppm for 3 h/day for 5 days (Skrajny et al. 1996). The rats were implanted with hippocampal electrodes in the dentate gyrus or CA1 region to determine the effects of H2S on EEG activity in the hippocampus and neocortex. Exposures occurred in a 33-L acrylic chamber designed to allow observation of the animal during recording of the EEG during exposure. Filtered room air was drawn through the chamber with a vacuum pump and mixed with H2S in nitrogen. The mixture was passed through a diffuser in the top of the chamber at an airflow of 11 L/min. The H2S concentration was continuously monitored by gas chromatography. Exposure to H2S at 100 ppm resulted in increased hippocampal theta activity but did not change the basic behavior-EEG correlation. Total hippocampal theta activity increased in a cumulative manner in both the dentate gyrus and CA1 regions during exposure to H2S at 25, 50, 75, and 100 ppm. The increase was significant
(p < 0.05) after exposure on days 3, 4, and 5 and did not return to control levels during the 24-h period between exposures. Complete recovery of the 100-ppm animals required about 2 weeks.
In a subchronic study, groups of 15 male and 15 female Sprague-Dawley rats were exposed to H2S at 0, 10.1, 30.5, or 80.0 ppm for 6 h/day, 5 days/week, for 90 days (CIIT 1983a). No animals died during the study. Clinical signs included crustiness around the ear tags; crusty noses, eyes, and muzzles; red-stained fur; swollen red ears; rales; lacrimation; and swollen muzzles and eyes. Decreased body weight gain and decreased brain weights were observed in high-concentration animals of both sexes. No treatment-related effects were observed with regard to food consumption, ophthalmology, neurologic function, clinical pathology, gross pathology, histopathology, and neuropathology.
3.2.2.
Mice
In a subchronic study, groups of 10 male and 12 female B6C3F1 mice were exposed to H2S at 0, 10.1, 30.5, or 80.0 ppm for 6 h/day, 5 days/week, for 90 days (CIIT 1983b). Two control mice were found dead, and one 30.5-ppm mouse was accidentally killed during the study. Two 80-ppm mice exhibiting prostration or hypoactivity were sacrificed in extremis. Clinical observations at 80 ppm included alopecia, missing anterior appendage, and loss of use of an anterior appendage. Decreased body weight gain was observed in high-concentration animals. Neurologic function studies revealed two 80-ppm animals not responding to an artificial light stimulus and two others with an irregular gait due to missing appendages. At sacrifice, 89% of high-concentration males and 78% of high-concentration females displayed inflammation of the anterior nasal mucosa in the anterior segments of the nose. No effects were noted in controls or in the low- or midconcentration animals. No treatment-related effects were observed with regard to ophthalmology, hematology, serum chemistry, and urinalyses.
3.2.3.
Rabbits
Mixed-breed rabbits exposed to H2S at 72 ppm for 1.5 h developed ventricular repolarization (Kosmider et al. 1966). Rabbits exposed to 72 ppm for 0.5 h/day for 5 days exhibited cardiac arrhythmia. Histochemical staining of myocardial cells from exposed rabbits showed a decrease in ATP phosphohydrolase and NADPH2 oxidoreductase.
3.2.4.
Monkeys
Rhesus monkeys exposed to H2S at 500 ppm for 22 min developed ataxia, anorexia, and parenchymal necrosis in the brain, while exposure to 500 ppm for
35 min resulted in conjunctival irritation, sudden loss of consciousness, and respiratory and cardiac arrest (Lund and Wieland 1966). Monkeys exposed to H2S at 500 ppm for 25 min, followed by a 17-min exposure to 500 ppm 3 days later had extensive changes in gray matter and moderate liver hyperemia upon necropsy (Lund and Wieland 1966). No other details on tissue pathology results were provided. No other experimental details were presented.
3.3.
Developmental and Reproductive Toxicity
Dorman et al. (2000) exposed groups of 12 male and 12 female Sprague-Dawley rats to H2S at 0, 10, 30, or 80 ppm for 6 h/day, 7 days/week, for 2 weeks before breeding. Exposures continued during a 2-week mating period and then from gestation days 0 to 19. Exposure of the dams and pups (eight rats per litter after culling) resumed between postnatal day 5 and 18. Adult male rats were exposed for 70 consecutive days. Offspring were evaluated for motor activity, passive avoidance, functional observational battery, acoustic startle response, and neuropathology. A significant (p < 0.05) decrease in food consumption was observed in F0 males only in the 80-ppm group during the first week of exposure. There were no deaths and no treatment-related adverse clinical signs in F0 males or females. There were no significant effects on reproductive performance of the F0 rats as assessed by the number of females with live pups, average gestation length, and average number of implants per pregnant female. No treatment-related effects in pups were noted with regard to growth, development, and behavioral tests. No other effects were noted at any concentration.
Saillenfait et al. (1989) exposed groups of eight pregnant Sprague-Dawley rats to H2S at 0, 50, 100, or 150 ppm for 6 h/day on days 6 to 20 of gestation. Hydrogen sulfide (10% in nitrogen) was delivered from a compressed gas cylinder by a pressure-regulating valve into a 200-L stainless steel dynamic flow inhalation chamber. Prenatal exposure to H2S at 100 and 150 ppm resulted in a slight (−4%) decrease (p < 0.01) in fetal body weight. However, the biologic significance of this finding is questionable as the dams also lost weight and there were a greater numbers of live fetuses per litter at these concentrations. No maternal or fetal effects were noted at 50 ppm.
Groups of 17 to 24 pregnant Sprague-Dawley rats (and offspring) were exposed to H2S at 0, 20, 50, or 75 ppm for 7 h/day from gestation day 1 through delivery and up to postnatal day 21 (Hayden et al. 1990a). Each group was culled to litters with 12 pups each at parturition. Animals were exposed in a 90-L acrylic dome-shaped chamber. Air supply to the chamber was room air drawn through a HEPA (high-efficiency particulate air) filter with a vacuum blower and mixed with H2S in nitrogen. The mixture was passed through an orifice plate through a diffuser at the top of the chamber. Actual H2S concentrations were within 5% of nominal concentrations. Maternal blood glucose increased about 50% (p < 0.05) on day 21 postpartum in all exposure groups. This increase was accompanied by a possible decrease in serum triglyceride in dams and pups
on day 21. No treatment-related effects were noted for serum alkaline phosphatase, LDH, or serum glutamic-oxaloacetic transaminase.
In a similar study, groups of five or six pregnant Sprague-Dawley rats were exposed to H2S at 0, 20, 50, or 75 ppm for 7 h/day from gestation day 6 through postpartum day 21 (Hayden et al. 1990b). There was a concentration-dependent increase in parturition time (measured in minutes) of about 10%, 20%, and 40% over matched controls at 20, 50, and 75 ppm, respectively. The biologic significance of this effect is questionable because parturition time was quite variable among control groups. Maternal liver cholesterol increased (p < 0.05) in the 75-ppm group. No other treatment-related effects were noted in the dams or their offspring.
Hannah and Roth (1991) exposed Sprague-Dawley rats to H2S at 0, 20, or 50 ppm for 7 h/day from gestation day 5 through postpartum day 21. The mean Purkinje cell terminal path length significantly increased in the 20- and 50-ppm groups compared with controls; however, the biological significance of this finding is unclear as no concentration response was observed. The study neither mentioned whether there were any adverse effects on maternal health nor provided neurobehavioral parameters in followup studies of H2S-exposed offspring.
Skrajny et al. (1992) exposed groups of 20 pregnant Sprague-Dawley rats to H2S at 0, 20, or 75 ppm for 7 h/day from day 5 postcoitus through postpartum day 21. Animals were exposed in a 90-L chamber designed to allow continuous observation. Air flow was 30 L/min and H2S concentrations were verified by gas chromatography. Increased (p < 0.05) serotonin levels were observed in the frontal cortex on day 21 postpartum of pups exposed to H2S at 20 ppm, and increased (p < 0.01) serotonin levels were observed in the cerebellum and frontal cortex on postpartum days 14 and 21 in pups exposed to H2S at 75 ppm. Norepinephrine concentrations increased (p < 0.05) at 75 ppm in the cerebellum at postpartum days 7, 14, and 21 but significantly increased in the frontal cortex only at postpartum day 21. At 20 ppm, frontal cortex norepinephrine levels decreased compared with controls on days 14 and 21. No neurobehavioral correlates were provided in preliminary or followup investigations to assist in determining the biologic significance of those findings.
3.4.
Genotoxicity
Studies on the genotoxic potential of H2S are limited. Hydrogen sulfide was negative in an Ames reverse mutation assay in Salmonella typhimurium strains TA97, TA98, and TA100 with and without hepatic S9 from male Sprague-Dawley rat and Syrian hamster liver (ATSDR 2006). No other genotoxicity studies were located.
3.5.
Carcinogenicity
No information on the carcinogenic potential of H2S was located in the available literature.
3.6.
Summary
Animal lethality studies from various laboratories have produced similar results. All F344 rats died when exposed to H2S at 500 to 700 ppm for 4 h (Khan et al. 1990), whereas no F344 rats died when similarly exposed to H2S at up to 400 ppm for 4 h (Lopez et al. 1987, 1988; Khan et al. 1990, 1991; Green et al. 1991; Lefebvre et al. 1991). Rat LC50 values range from 683 to 712 ppm for periods of 10 min to 1 h (MacEwen and Vernot 1972; Zwart et al. 1990) and from 335 to 587 ppm for periods of 2 to 6 h in Sprague-Dawley, Long Evans, and F344 rats (Tansy et al. 1981; Prior et al. 1988). Mouse LC50 values range from 634 to 1,160 ppm for periods of 10 min to 1 h (MacEwen and Vernot 1972; Zwart et al. 1990).
Toxicity studies identified the respiratory and nervous systems as the primary targets for H2S, with some cardiac involvement. Nasal pathology and decreased bronchiolar macrophage counts have been observed in rats exposed to 400 ppm for 4 h (Lopez et al. 1988; Kahn et al. 1991), and enzyme activity changes (LDH, cytochrome c oxidase, succinate oxidase) have been observed in rats exposed to H2S at 50 to 400 ppm for 4 h (Lopez et al. 1987, 1988; Khan et al. 1990; Green et al. 1991). Pulmonary edema and atelectasis developed in rats exposed to H2S at 300 ppm for 4 h (Green et al. 1991). Changes in EEG activity were identified in rats repeatedly exposed to H2S at 25 to 100 ppm (Skrajny et al. 1996), and biochemical brain changes were identified in mice exposed to H2S at 100 ppm for 2 h.
Data suggesting that H2S exposure can impair prenatal neurologic development in rats are equivocal; inconsistencies in some developmental studies make the data difficult to interpret. Genotoxicity data are extremely limited and no carcinogenicity data were located.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
Hydrogen sulfide is present as an endogenous substance in normal mammalian (Warenycia et al. 1990; Kage et al. 1992; Mitchell et al. 1993) tissues. Normal tissues contain relatively high (μg/g) concentrations of endogenous sulfide ion (HS-) (Dorman et al. 2002). Endogenous sulfide also arises from bacterial activity in the lower bowel (NRC 1977) and there is some evidence that H2S participates in normal nerve transmission (Kimura 2000).
The major metabolic and excretory pathway of H2S involves oxidation of the sulfide to sulfate. The exact mechanism of the oxidation is unknown; however, both enzymatic (sulfide oxidase) and nonenzymatic catalytic systems have been proposed. Glutathione stimulates mitochondrial oxidation of thiosulfate to sulfite in vitro, and it is possible that a sulfite intermediate may be converted to
sulfate by sulfite oxidase. After oxidation, H2S is excreted either as free sulfate or as conjugated sulfate in the urine (Beauchamp et al. 1984).
Two other metabolic pathways for H2S have been identified as follows: methylation of H2S to produce methanethiol and dimethylsulfide and reaction of hydrosulfide with metallo- or disulfide-containing enzymes (Dorman et al. 2002). Data suggest that thiol S-methyltransferase catalyzes the methylation of H2S to yield less toxic methanethiol and dimethylsulfide (Beauchamp et al. 1984). The reaction of H2S with metalloenzymes, such as cytochrome oxidase, is a mechanism of toxicity and is described in Section 4.2.
Hydrogen sulfide may also reduce disulfide bridges in proteins and this reaction is likely responsible for H2S-induced inhibition of succinic dehydrogenase. Oxidized (but not reduced) glutathione protects against H2S poisoning. This protective mechanism is likely due to the scavenging of hydrosulfide by the oxidized glutathione disulfide linkage, thus preventing the reaction of the sulfide with other enzymatic sites (Beauchamp et al. 1984).
4.2.
Mechanism of Toxicity
Hydrogen sulfide acts similarly to cyanide by interrupting the electron transport chain through inhibition of cytochrome oxidase. Tissues with high oxygen demand (e.g., cardiac muscle, brain) are particularly sensitive to sulfide inhibition of electron transport (Ammann 1986). At physiologic pH, the dissociated hydrosulfide anion and undissociated H2S (K1 = 1.0 × 10-7) (Williams and Williams 1967) are in equilibrium. At high, nonphysiologic concentrations, H2S (as HS-) acts similarly to cyanide (as CN-) by direct, reversible inhibition of mitochondrial cytochrome c oxidase (ferrocytochrome c-oxygen oxidoreductase) (Nicholls 1975; Smith et al. 1977; Kahn et al. 1990), the principal terminal oxidase of aerobic metabolism. The sulfide anion forms a reversible complex with the heme A prosthetic groups of cytochromes a and a3 (electrons received from cytochrome c by cytochrome a heme are normally transferred to the cytochrome a3 heme). Spectral data demonstrate that sulfide and cyanide anions bind to the trivalent cytochrome a3 iron (Nicholls 1975); interestingly, the affinities of cyanide and hydrosulfide for cytochrome oxidase binding sites are of the same order of magnitude (Wever et al. 1975). As a result of the electron transfer blockage, oxidative phosphorylation and aerobic metabolism are compromised, peripheral tissue partial pressure of oxygen increases, and the unloading gradient for oxyhemoglobin decreases. High concentrations of oxyhemoglobin are thus found in the venous return, resulting in flushed skin and mucous membranes. Lactic acidemia occurs as a result of the increased demand placed on glycolysis. Although the signs of H2S poisoning are essentially identical to those of cyanide poisoning, H2S has a greater tendency to produce conjunctivitis and pulmonary edema (Smith 1996).
Hydrogen sulfide can also directly stimulate the chemoreceptors of the carotid and aortic bodies to produce hyperpnea and cardiac irregularity. However,
death is due to respiratory arrest that may occur within minutes at high H2S concentrations (Smith 1996).
4.3.
Concurrent Exposure Issues
Data suggest that ethanol consumption may potentiate the toxic effects of H2S. Poda (1966) reported that “it took very little” H2S to overcome six persons who had been drinking heavily during a 16- to 24-h period before reporting to work. Rats pretreated with ethanol (0.33 or 0.66 gram per kilogram of body weight, intraperitoneally) 0.5 h before exposure to H2S at 790 to 800 ppm for a maximum of 20 min lost consciousness in 35% less time than rats exposed only to H2S (Beck et al 1979). However, the results of the rodent study are equivocal because all rats receiving only the 20-min H2S exposure died but some rats pretreated with ethanol survived.
Increased chronic and recurrent headaches were observed in Finnish pulp workers exposed simultaneously to H2S (peak up to 20 ppm), methyl mercaptans, and sulfur dioxide (ATSDR 2006). In another study, no increase in respiratory symptoms or pulmonary function abnormalities were noted in Canadian pulp and paper mill workers exposed to H2S at 0.05 ppm, sulfur dioxide at 0.3 ppm, carbon monoxide at 8.3 ppm, total particulates at 0.8 ppm, and chlorine at <0.05 ppm (ATSDR 2006). No other details were reported.
4.4.
Structure-Activity Relationships
As previously stated, H2S and cyanide are potent inhibitors of the cytochrome oxidase system and produce similar effects. Both compounds selectively react with cytochrome aa3. Also, similar to cyanide, H2S may inhibit other metalloproteins containing alkali metals such as horseradish peroxidase, potato polyphenol oxidase, and catalase. Inhibition of the latter enzymes occurs only at concentrations much greater than those required to inhibit cytochrome oxidase. The toxicologic significance of these enzyme inhibitions is unclear as the critical position of cytochrome c oxidase in oxidative metabolism makes its inhibition felt earliest and strongest (Smith 1996).
The hydrosulfide ion complexes with methemoglobin to form sulfmethemoglobin, which is analogous to cyanmethemoglobin. The dissociation constant for cyanmethemoglobin is 2 × 10-8 mole (mol)/L, whereas the dissociation constant for sulfmethemoglobin is about 6 × 10-6 mol/L. In both cases, nitrite-induced methemoglobinemia provides protection and had antidotal effects against H2S poisoning (Smith 1996).
4.5.
Temporal Extrapolation
The concentration-exposure time relationship for many irritant and systemically acting vapors and gases can be described by the relationship Cn × t =
k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). However, mortality data suggest that the value of n for H2S does not fall within this range (see Figure 4-1). For rat lethality data, the derived values of n approach 8 for times up to 1 h. As longer time points are included, the value of n decreases, suggesting that at short time points (and relatively low concentrations) a rather broad change in exposure duration (not concentration) is associated with a relatively small change in response. It is likely that at some point (between 1 and 6 h) there is a steep threshold (providing for an all-or-none response); however, given the available data, it is not possible to quantitate this point on the concentration-response curve. Because rat data were used to derive AEGL-2 and AEGL-3 values and because AEGL values are derived for periods of 10 min to 8 h, it is most appropriate to select an n value from this species for the period of time closest to the time periods encompassed by the AEGL values. Furthermore, use of default time-scaling parameters yields AEGL values less consistent with the overall data set. Thus, the value n = of 4.4, derived from rat lethality data from 10 min to 6 h (r2 = 0.92), was selected for extrapolation of H2S response across time.
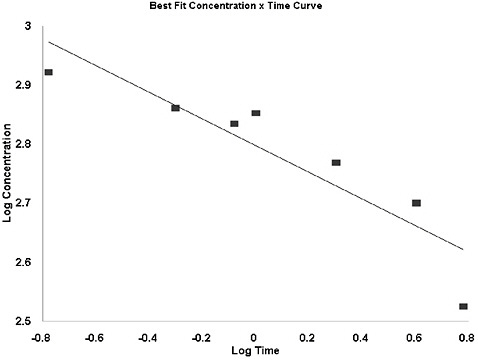
FIGURE 4-1 Log concentration vs. log time of rat LC50 values.
5.
RATIONALE AND PROPOSED AEGL-1
5.1.
Human Data Relevant to AEGL-1
Complaints of headache were recorded in 3 of 10 adult volunteers with asthma exposed by controlled inhalation of H2S at 2 ppm for 30 min (Jappinen et al. 1990). Bhambhani et al. (1991; 1994; 1996a,b) exposed 88 exercising healthy male and female volunteers (ranging in age from 18 to 61 years) to H2S at 0.5 to 10 ppm for up to 30 min. No treatment-related respiratory or cardiac effects or complaints of headache were observed.
5.2.
Animal Data Relevant to AEGL-1
Effects observed from inhalation exposure of experimental animals to H2S are generally no-effect levels or more severe than those defined by AEGL-1.
5.3.
Derivation of AEGL-1
Controlled human data were used to derive AEGL-1 values. Three of 10 volunteers with asthma exposed to H2S at 2 ppm for 30 min complained of headache and 8 of 10 experienced nonsignificant increased airway resistance (Jappinen et al. 1990). As there were no clinical symptoms of respiratory difficulty and no significant changes in FVC or FEV1, the AEGL-1 was based exclusively on increased complaints of headache in the three volunteers (Jappinen et al. 1990). A modifying factor of 3 was applied to account for the wide variability in complaints associated with the foul odor of H2S and the shallow concentration response at the relatively low concentrations that are consistent with definition of the AEGL-1. The concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k (ten Berge et al. 1986). For scaling the AEGL values for H2S across time, the empirically derived chemical-specific value of 4.4 (derived from rat lethality data ranging from 10-min to 6-h exposure durations; Figure 4-1) was used as the exponent n. The AEGL-1 values for H2S are presented in Table 4-6, and the calculations for these AEGL-1 values are presented in Appendix A.
The level of distinct odor awareness (LOA) for H2S is 0.01 ppm (see Appendix C for LOA derivation). The LOA represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of the exposure due to odor perception. Thus, the derived AEGL-1 values are considered to have warning properties.
TABLE 4-6 AEGL-1 Values for Hydrogen Sulfide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
0.75 ppm (1.05 mg/m3) |
0.60 ppm (0.84 mg/m3) |
0.51 ppm (0.71 mg/m3) |
0.36 ppm (0.50 mg/m3) |
0.33 ppm (0.46 mg/m3) |
6.
RATIONALE AND PROPOSED AEGL-2
6.1.
Human Data Relevant to AEGL-2
Case reports describing human poisonings with H2S leading to effects consistent with the definition of AEGL-2 are abundant. However, because of a lack of reliable concentration and duration parameters, these data are not appropriate for derivation of AEGL-2 values.
6.2.
Animal Data Relevant to AEGL-2
Nasal passage necrosis and exfoliation and increased lung lavage fluid bronchiolar cell counts and enzyme activities were observed in rats exposed to H2S at 400 ppm for 4 h (Lopez et al. 1988). Decreased cytochrome c oxidase activity (Khan et al. 1990) and decreased pulmonary alveolar cell macrophage activity (Kahn et al. 1991) were also observed in rats exposed to H2S at 400 ppm for 4 h. Focal areas of red atelectasis and patchy alveolar edema with perivascular and peribronchial interstitial edema, increased protein concentration and LDH activity in lung lavage fluid, and abnormal surfactant activity were observed in rats exposed to H2S at 300 ppm for 4 h (Green et al. 1991). Rats exposed to H2S at 200 ppm for 4 h exhibited minor perivascular edema and a significant increase in protein and LDH in lung lavage fluid (Green et al. 1991). Khan et al. (1991) observed no change in pulmonary alveolar macrophage counts in rats exposed to 200 ppm for 4 h.
6.3.
Derivation of AEGL-2
The focal areas of perivascular edema in rats exposed to H2S at 200 ppm for 4 h (Green et al. 1991; Khan et al. 1991) are used as the basis for AEGL-2 values. An uncertainty factor of 3 is used to extrapolate from animals to humans as rat and mouse data suggest little interspecies variability. An uncertainty factor of 3 will also be applied to account for sensitive individuals. The intraspecies uncertainty factor of 3 is sufficient because application of 10 yields AEGL-2 values inconsistent with the total database. AEGL-2 values derived with a total default uncertainty factor were less than the10-ppm concentration causing no effects in humans exercising to exhaustion (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Thus, the total uncertainty factor is 10.
The concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k (ten Berge et al. 1986). For scaling the AEGL values for H2S across time, the empirically derived chemical-specific value of 4.4 (derived from rat lethality data ranging from 10 min to 6 h exposure duration, Figure 4-1) was used as the exponent n. The AEGL-2 values for H2S are presented in Table 4-7, and the calculations for these AEGL-2 values are presented in Appendix A.
7.
RATIONALE AND PROPOSED AEGL-3
7.1.
Human Data Relevant to AEGL-3
Human lethality data were anecdotal and lacked reliable concentration and time parameters. Thus, those reports were not appropriate for establishing AEGL-3 values.
7.2.
Animal Data Relevant to AEGL-3
Well-conducted LC50 studies are available for rats (MacEwen and Vernot 1972; Tansy et al. 1981; Prior et al. 1988; Zwart et al. 1990) and mice (MacEwen and Vernot 1972; Zwart et al. 1990).
7.3.
Derivation of AEGL-3
The highest H2S concentration causing no mortality in the rat after a 1-h exposure is 504 ppm (MacEwen and Vernot 1972). This value is used as the basis for deriving AEGL-3 values. An uncertainty factor of 3 is used to extrapolate from animals to humans as rat and mouse data suggest little interspecies variability. An uncertainty factor of 3 will also be applied to account for sensitive individuals. The intraspecies uncertainty factor of 3 is sufficient because application of the default 10 yields AEGL-3 values inconsistent with the total database. AEGL-3 values derived with a total default uncertainty factor were less than twice the concentration causing no effects in humans exercising to exhaustion (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Effects consistent with the definition of AEGL-3 are unlikely at such concentrations. Therefore, the total uncertainty factor is 10. The concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k (ten Berge et al. 1986). For scaling the AEGL values for H2S across time, the empirically derived chemical-specific value of 4.4 (derived from rat lethality data ranging from 10 min to 6 h exposure duration; Figure 4-1) was used as the exponent n. The AEGL-3 values for H2S are presented in Table 4-8, and the calculations for these AEGL-3 values are presented in Appendix A.
These values are supported by the fact that no deaths were observed in rats exposed to H2S at 80 ppm for 6 h/day, 5 days/week, for 90 days (CIIT 1983a).
8.
SUMMARY OF PROPOSED AEGLS
8.1.
AEGL Values and Toxicity End Points
The derived AEGL values for various levels of effects and durations of exposure are summarized in Table 4-9. Headache in humans with asthma was used as the basis for AEGL-1, and focal areas of perivascular edema in rats were used as the basis for AEGL-2. The highest concentration causing no mortality in rats after a 1-h exposure was used to calculate the AEGL-3.
The odor threshold ranges between 0.008 and 0.13 ppm, olfactory fatigue may occur at 100 ppm, and paralysis of the olfactory nerve has been reported at 150 ppm (Beauchamp et al. 1984). Furthermore, the LOA for H2S is 0.01 ppm (see Appendix C for LOA derivation). The LOA represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of the exposure due to odor perception. Thus, the derived AEGL-1 values are considered to have warning properties, and the characteristic odor will also be present at AEGL-2 and AEGL-3 concentrations.
TABLE 4-7 AEGL-2 Values for Hydrogen Sulfide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-2 |
41 ppm (59 mg/m3) |
32 ppm (45 mg/m3) |
27 ppm (39 mg/m3) |
20 ppm (28 mg/m3) |
17 ppm (24 mg/m3) |
TABLE 4-8 AEGL-3 Values for Hydrogen Sulfide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-3 |
76 ppm (106 mg/m3) |
59 ppm (85 mg/m3) |
50 ppm (71 mg/m3) |
37 ppm (52 mg/m3) |
31 ppm (44 mg/m3) |
TABLE 4-9 Summary of AEGL Values for Hydrogen Sulfide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (Nondisabling) |
0.75 ppm (1.05 mg/m3) |
0.60 ppm (0.84 mg/m3) |
0.51 ppm (0.71 mg/m3) |
0.36 ppm (0.50 mg/m3) |
0.33 ppm (0.46 mg/m3) |
|
AEGL-2 (Disabling) |
41 ppm (59 mg/m3) |
32 ppm (45 mg/m3) |
27 ppm (39 mg/m3) |
20 ppm (28 mg/m3) |
17 ppm (24 mg/m3) |
|
AEGL-3 (Lethality) |
76 ppm (106 mg/m3) |
59 ppm (85 mg/m3) |
50 ppm (71 mg/m3) |
37 ppm (52 mg/m3) |
31 ppm (44 mg/m3) |
8.2.
Other Exposure Criteria
The criteria for H2S exposure have been established and are shown in Table 4-10.
TABLE 4-10 Extant Standards and Guidelines for Hydrogen Sulfide
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.75 ppm |
0.60 ppm |
0.51 ppm |
0.36 ppm |
0.33 ppm |
|
AEGL-2 |
41 ppm |
32 ppm |
27 ppm |
20 ppm |
17 ppm |
|
AEGL-3 |
76 ppm |
59 ppm |
50 ppm |
37 ppm |
31 ppm |
|
ERPG-1a |
0.1 ppm |
|
|
|
|
|
ERPG-2a |
30 ppm |
|
|
|
|
|
ERPG-3a |
100 ppm |
|
|
|
|
|
Acute MRL (ATSDR)b |
0.07 ppm |
|
|
|
|
|
EEGL (NRC)c |
50 ppm (10 min) |
|
|
|
10 ppm (24 h) |
|
|
|
|
|
15 ppm (10 d) 30 ppm (24 h) |
|
|
IDLH (NIOSH)e |
100 ppm |
|
|
|
|
|
TLV-TWA (ACGIH)f |
|
|
|
|
10 ppm |
|
PEL-TWA (OSHA)g |
|
|
|
|
20 ppm |
|
PEL-acceptable maximum peak, 10 min, once only per 8-hr workshift (OSHA)g |
50 ppm |
|
|
|
|
|
TLV-STEL (ACGIH)h |
15 ppm |
|
|
|
|
|
REL-STEL (NIOSH)i |
10 ppm |
|
|
|
|
|
MAK (Germany)j |
|
|
|
|
10 ppm |
|
MAC (The Netherlands)k |
|
|
|
|
10 ppm |
|
OELV- LLV (Sweden)l |
|
|
|
|
10 ppm |
|
OELV-CLV (Sweden)l |
15 ppm (15min) |
|
|
|
|
|
aERPG (emergency response planning guidelines, American Industrial Hygiene Association) (AIHA 2001): The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-3 is the maximum airborne con |
|||||
8.3.
Data Adequacy and Research Needs
The database for human exposure for effects defined by AEGL-1 is relatively good as controlled chamber studies with asthma patients and otherwise healthy volunteers are available. These studies, when considered together, provide good concentration-response information and are appropriate for derivation of AEGL-1 values. Case reports of accidental human exposure to H2S leading to effects consistent with the definitions of AEGL-2 and AEGL-3 did not include concentration or duration parameters adequate for derivation of AEGL-2 or AEGL-3 values. However, numerous animal studies were available for derivation of AEGL-2 and AEGL-3 values. The animal studies were well-conducted in a number of species over a broad range of exposure durations and were adequate for derivation of AEGL-2 and AEGL-3 values for H2S.
9.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1996. Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2000. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
Adelson, L., and I. Sunshine. 1966. Fatal hydrogen sulfide intoxication. Report of three cases occurring in a sewer. Arch. Pathol. 81(5):375-380.
Ahlborg, G.G. 1951. Hydrogen sulfide poisoning in shale oil industry. AMA Arch. Ind. Hyg. Occup. Med. 3(3):247-266.
AIHA (American Industrial Hygiene Association). 2000. Emergency Response Planning Guidelines for Hydrogen Sulfide. Akron, OH: AIHA Press.
Ammann, H.M. 1986. A new look at physiologic respiratory response to H2S poisoning. J. Hazard. Mater. 13(3):369-374.
Amoore, J.E. 1985. The Perception of Hydrogen Sulfide Odor in Relation to Setting an Ambient Standard. Prepared for California Air Resources Board ARB Contract A4-046-33. April 10, 1985 [online]. Available: http://www.arb.ca.gov/research/ apr/past/a4-046-33.pdf [accessed Apr. 2, 2010].
API (American Petroleum Institute). 1948. API Toxicological Review (Hydrogen Sulfide). New York: API.
Arts, J.H., A. Zwart, E.D. Schoen, and J.M. Klokman-Houweling. 1989. Determination of concentration-time-mortality relationships versus LC50s according to OECD guideline 403. Exp. Pathol. 37(1-4):62-66.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Hydrogen Sulfide. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA.
ATSDR (Agency for Toxic Substances and Disease Registry). 2006. Toxicological Profile for Hydrogen Sulfide. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA.
July 2006 [online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp114.pdf [accessed Apr. 1, 2010].
ATSDR (Agency for Toxic Substances and Disease Registry). 2009. Minimal Risk Level (MRLs) for Hazardous Substances, December 2009. Agency for Toxic Substances and Disease Registry [online]. Available: http://www.atsdr.cdc.gov/mrls/mrllist.asp [accessed Apr. 21, 2010].
Beauchamp, R.O., Jr., J.S. Bus, J.A. Popp, C.J. Boreiko, and D.A. Andjelkovich. 1984. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 13(1):25-97.
Beck, J.F., F. Cormier, and J.C. Donini. 1979. The combined toxicity of ethanol and hydrogen sulfide. Toxicol. Lett. 3(5):311-313.
Bhambhani, Y., and M. Singh. 1991. Physiological effects of hydrogen sulfide inhalation during exercise in healthy men. J. Appl. Physiol. 71(5):1872-1877.
Bhambhani, Y., R. Burnham, G. Snydmiller, I. MacLean, and T. Martin. 1994. Comparative physiological responses of exercising men and women to 5 ppm hydrogen sulfide exposure. Am. Ind. Hyg. Assoc. J. 55(11):1030-1035.
Bhambhani, Y., R. Burnham, G. Snydmiller, I. MacLean, and T. Martin. 1996a. Effects of 5 ppm hydrogen sulfide inhalation on biochemical properties of skeletal muscle in exercising men and women. Am. Ind. Hyg. Assoc. J. 57(5):464-468.
Bhambhani, Y., R. Burnham, G. Snydmiller, I. MacLean, and R. Lovlin. 1996b. Effects of 10-ppm hydrogen sulfide inhalation on pulmonary function in healthy men and women. J. Occup. Environ. Med. 38(10):1012-1017.
Bhambhani, Y., R. Burnham, G. Snydmiller, and I. MacLean. 1997. Effects of 10-ppm hydrogen sulfide inhalation in exercising men and women: Cardiovascular, metabolic, and biochemical responses. J. Occup. Environ. Med. 39(2):122-129.
Brenneman, K.A., J.A. James, E.A. Gross, and D.C. Dorman. 2000. Olfactory neuron loss in adult male CD rats following subchronic inhalation exposure to hydrogen sulfide. Toxicol. Pathol. 28(2):326-333.
Brenneman, K.A., D.F. Meleason, M. Sar, M.W. Marshall, R.A. James, E.A. Gross, J.T. Martin and D.C. Dorman. 2002. Olfactory mucosal necrosis in male CD rats following acute inhalation exposure to hydrogen sulfide: Reversibility and possible role of regional metabolism. Toxicol. Pathol. 30(2):200-208.
Breysse, P.A. 1961. Hydrogen sulfide fatality in a poultry feather fertilizer plant. Am. Ind. Hyg. Assoc. J. 22(3):220-222.
Bruvold, W.H., S.M. Rappaport, T.C. Wu, B.E. Bulmer, C.E. DeGrange, and J.M. Kooler. 1983. Determination of nuisance odor in a community. Water Pollut. Control 55(3 Part 1):229-233.
CIIT (Chemical Industry Institute of Toxicology). 1983a. 90-Day Vapor Inhalation Toxicity Study of Hydrogen Sulfide in Sprague-Dawley Rats. EPA/OTS-0883-0255. Chemical Industry Institute of Toxicology, Research Triangle Park, NC.
CIIT (Chemical Industry Institute of Toxicology). 1983b. 90-Day Vapor Inhalation Toxicity Study of Hydrogen Sulfide in B6C3F1 Mice. EPA/OTS-0883-0255. Chemical Industry Institute of Toxicology, Research Triangle Park, NC.
DFG (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values 2000. Maximum Concentration and Biological Tolerance Values at the Workplace Report No. 36. Weinheim, Federal Republic of Germany: Wiley VCH.
Donham, K.J., D.C. Zavala, and J. Merchant. 1984. Acute effects of the work environment on pulmonary functions of swine confinement workers. Am. J. Ind. Med. 5(5):367-375.
Dorman, D.C., K.A. Brenneman, M.F. Struve, K.L. Miller, R.A. James, M.W. Marshall, and P.M. Foster. 2000. Fertility and developmental neurotoxicity effects of inhaled hydrogen sulfide in Sprague-Dawley rats. Neurotoxicol. Teratol. 22(1):71-84.
Dorman, D.C., F.J. Moulin, B.E. McManus, K.C. Mahle, R.A. James, and M.F. Struve. 2002. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: Correlations with tissue sulfide concentrations in the rat brain, liver, lung and nasal epithelium. Toxicol. Sci. 65(1):18-25.
Freireich, A.W. 1946. Hydrogen sulfide poisoning: Report of two cases, one with fatal outcome from associated mechanical asphyxia. Am. J. Pathol. 22(1):147-155.
Fuller, D.C., and A.J. Suruda. 2000. Occupationally related hydrogen sulfide deaths in the United States from 1984 to 1994. J. Occup. Med. 42(9):939-942.
Gosselin, R.E., R.P. Smith, and H.C. Hodge. 1984. Section III. Therapeutics index. Pp. 198- 202 in Clinical Toxicology of Commercial Products, 5th Ed. Baltimore, MD: Williams and Wilkins.
Graedel, T.E., D.T. Hawkins and L.D. Claxon. 1986. Atmospheric Chemical Compounds: Sources, Occurrence and Bioassay. Orlando: Academic Press.
Grant, W.M. 1974. Toxicology of the Eye: Drug, Chemicals, Plants, Venoms, 2nd Ed. Springfield, IL: Charles C. Thomas.
Green, F.H., S. Schurch, G.T. DeSanctis, J.A. Wallace, S. Cheng, and M. Prior. 1991. Effects of hydrogen sulfide exposure on surface properties of lung surfactant. J. Appl. Physiol. 70(5):1943-1949.
Guidotti, T.L. 1994. Occupational exposure to hydrogen sulfide in the sour gas industry: Some unresolved issues. Int. Arch. Occup. Environ. Health 66(3):153-160.
Hannah, R.S., and S.H. Roth. 1991. Chronic exposure to low concentrations of hydrogen sulfide produces abnormal growth in developing cerebellar Purkinje cells. Neurosci. Lett. 122(2):225-228.
Hayden, L.J., H. Goeden, and S.H. Roth. 1990a. Exposure to low levels of hydrogen sulfide elevates circulating glucose in maternal rats. J. Toxicol. Environ. Health 31(1):45-52.
Hayden, L.J., H. Goeden, and S.H. Roth. 1990b. Growth and development in the rat during sub-chronic exposure to low levels of hydrogen sulfide. Toxicol. Ind. Health 6(3-4):389-401.
Herbert, M., R. Glick, and H. Black. 1967. Olfactory precipitants of bronchial asthma. J. Psychosom. Res. 11(2):195-202.
Hessel, P.A., F.A. Herbert, L.S. Melenka, K. Yoshida, and M. Nakaza. 1997. Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. Am. J. Ind. Med. 31(5):554-557.
HEW (U.S. Department of Health, Education, and Welfare). 1964. The Air Pollution Situation in Terre Haute, Indiana with Special Reference to the Hydrogen Sulfide Incident of May-June, 1964. PB-227 486. U.S. Department of Health, Education, and Welfare, Public Health Service, Division of Air Pollution, Washington, DC.
Hirsch, A.R., and G. Zavala. 1999. Long-term effects on the olfactory system of exposure to hydrogen sulphide. Occup. Environ. Med. 56(4):284-287.
Hoidal, C.R., A.H. Hall, M.D. Robinson, K. Kulig, and B. Rumack. 1986. Hydrogen sulfide poisoning from toxic inhalations of roofing asphalt fumes. Ann. Emerg. Med. 15(7):826-830.
Hsu, P., H.W. Li, and Y.T. Lin. 1987. Acute hydrogen sulfide poisoning treated with hyperbaric oxygen. J. Hyperbaric Med. 2(4):215-221.
Hurwitz, L.J., and G.L. Taylor. 1954. Poisoning by sewer gas with unusual sequelae. Lancet 266(6822):1110-1112.
Jaakkola, J.J., V. Vilkka, O. Marttila, P. Jappinen, and T. Haahtela. 1990. The South Karelia Air Pollution Study. The effects of malodorous sulfur compounds from pulp mills on respiratory and other symptoms. Am. Rev. Respir. Dis. 142(6 Pt 1):1344-1350.
Jappinen, P., V. Vilkka, O. Marttila, and T. Haahtela. 1990. Exposure to hydrogen sulfide and respiratory function. Br. J. Ind. Med. 47(12):824-828.
Kage, S., T. Nagata, K. Takekawa, K. Kimura, K. Kudo, and T. Imamura. 1992. The usefulness of thiosulfate as an indicator of hydrogen sulfide poisoning in forensic toxicological examination: A study with animal experiments. Jpn. J. Forensic Toxicol. 10(3):223-227.
Kage, S., S. Ito, T. Kishida, K. Kudo, and N. Ikeda. 1998. A fatal case of hydrogen sulfide poisoning in a geothermal power plant. J. Forensic Sci. 43(4):908-910.
Khan, A.A., M.M. Schuler, M.G. Prior, S. Yong, R.W. Coppock, L.Z. Florence, and L.E. Lillie. 1990. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol. Appl. Pharmacol. 103(3):482-490.
Khan, A.A., S. Yong, M.G. Prior, and L.E. Lillie. 1991. Cytotoxic effects of hydrogen sulfide on pulmonary alveolar macrophages in rats. J. Toxicol. Environ. Health 33(1):57-64.
Khan, A.A., R.W. Coppock, M.M. Schuler, and M.G. Prior. 1998. Biochemical effects of repeated exposures to low and moderate concentrations of hydrogen sulfide in Fischer 344 rats. Inhal. Toxicol. 10(11):1037-1044.
Kilburn, K.H. 1993. Case report: Profound neurobehavioral deficits in an oil field worker overcome by hydrogen sulfide. Am. J. Med. Sci. 306(5):301-305.
Kilburn, K.H. 1997. Exposure to reduced sulfur gases impairs neurobehavioral function. South. Med. J. 90(10):997-1006.
Kilburn, K.H., and R.H. Warshaw. 1995. Hydrogen sulfide and reduced-sulfur gases adversely affect neurophysiological functions. Toxicol. Ind. Health 11(2):185-197.
Kimura, H. 2000. Hydrogen sulfide induces cyclic AMP and modulates NMDA receptor. Biochem. Biophys. Res. Commun. 267(1):129-133.
Kohno, M., E. Tanaka, T. Nakamura, N. Shimojo, and S. Misawa. 1991. Influence of the short-term inhalation of hydrogen sulfide in rats [in Japanese]. Eisei Kagaku 37(2):103-106.
Kośmider, S., E. Rogala, and A. Pacholek. 1966. Studies on the toxic action mechanism of hydrogen sulfide [in German]. Int. Arch. Arbeitsmed. 22(1):60-76.
Kroshwitz, J.I., and A. Seidel. 2007. Sulfur compounds. Pp. 621-701 in Kirk-Othmer Encyclopedia of Chemical Technology, 5th Ed., Vol. 23, A. Seidel, ed. Hoboken, NJ: Wiley-Interscience.
Lefebvre, M., D. Yee, D. Fritz, and M.G. Prior. 1991. Objective measures of ocular irritation as a consequence of hydrogen sulfide exposure. Vet. Hum. Toxicol. 33(6):564-566.
Lopez, A., M. Prior, S. Yong, M. Albassam, and L.E. Lillie. 1987. Biochemical and cytologic alterations in the respiratory tract of rats exposed for 4 hours to hydrogen sulfide. Fundam. Appl. Toxicol. 9(4):753-762.
Lopez, A., M. Prior, S. Yong, L. Lillie, and M. Lefebvre. 1988. Nasal lesions in rats exposed to hydrogen sulfide for 4 hours. Am. J. Vet. Res. 49(7):1107-1111.
Lopez, A., M.G. Prior, R.J. Reiffenstein, and L.R. Goodwin. 1989. Peracute toxic effects of inhaled hydrogen sulfide and injected sodium hydrosulfide on the lungs of rats. Fundam. Appl. Toxicol. 12(2):367-373.
Lund, O.E., and H. Wieland. 1966. Patologic-anatomic findings an experimental hydrogen sulfide poisoning. A study on rhesus monkeys [in German]. Int. Arch. Arbeitsmed. 22(1):46-54.
MacEwen, J.D., and E.H. Vernot. 1972. Acute toxicity of hydrogen sulfide. Pp. 66-70 in Toxic Hazards Research Unit Annual Technical Report: 1972. Report No. ARML-TR-72-62. Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, OH. August 1972.
Mitchell, T.W., J.C. Savage, and D.H. Gould. 1993. High-performance liquid chromatography detection of sulfide in tissues from sulfide-treated mice. J. Appl. Toxicol. 13(6):389-394.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Zwavelwaterstof. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed April 12, 2010].
NFPA (National Fire Protection Association). 1974. Pp. 49-160 to 49-161 in National Fire Codes: A Compilation of NFPA Codes, Standards, Recommended Practices and Manuals, Vol. 3. Combustible Solids, Dusts and Explosives. Boston: NFPA.
Nicholls, P. 1975. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts on the reduced alpha-peak. Biochim. Biophys. Acta 396(1):24-35.
NIOSH (National Institute for Occupational Safety and Health). 1977. Criteria for a Recommended Standard: Occupational Exposure to Hydrogen Sulfide. HEW(NIOSH) 77-158. U.S. Department of Health, Education and Welfare, Public Health Service. Center for Disease Control and Prevention, Public Health Service, National Institute for Occupational Safety and Health [online]. Available: http://www.cdc. gov/niosh/pdfs/77-158a.pdf [accessed April 9, 2010].
NIOSH (National Institute for Occupational Safety and Health). 1991. Two Maintenance Workers Die after Inhaling Hydrogen Sulfide in Manhole, January 31, 1989. Fatality Assessment Control Evaluation In House Report FACE 8928. National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/ face/in-house/full8928.html [accessed April 9, 2010].
NIOSH (National Institute for Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-Hydrogen Sulfide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. August 1996 [online]. Available: http://www.cdc.gov/niosh/idlh/77830 64.html [accessed April 9, 2010].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Pocket Guide to Chemical Hazards: Hydrogen Sulfide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. September 2005 [online]. Available: http://www.cdc.gov/niosh/npg/npgd0337.html [accessed Apr. 21, 2010].
NRC (National Research Council). 1977. Hydrogen Sulfide. Baltimore: University Park Press.
NRC (National Research Council). 1985. Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 4. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2002. Review of Submarine Escape Action Levels for Selected Chemicals. Washington, DC: National Academy Press.
Osbern, L.N., and R.O. Crapo. 1981. Dung lung: A report of toxic exposure to liquid manure. Ann. Intern. Med. 95(3):312-314.
Parra, O., E. Monso, M. Gallego, and J. Morera. 1991. Inhalation of hydrogen sulfide: A case of subacute manifestations and long term sequelae. Br. J. Ind. Med. 48(4):286-287.
Partti-Pellinen, K., O. Marttilla, V. Vilkka, J.J. Jaakkola, P. Jäppinen, and T. Haahtela. 1996. The South Karelia Air Pollution Study: Effects of low-level exposure to malodorous sulfur compounds on symptoms. Arch. Environ. Health 51(4):315-320.
Poda, G.A. 1966. Hydrogen sulfide can be handled safely. Arch. Environ. Health 12(6):795-800.
Prior, M.G., A.K. Sharma, S. Yong, and A. Lopez. 1988. Concentration-time interactions in hydrogen sulfide toxicity in rats. Can. J. Vet. Res. 52(3):375-379.
Reiffenstein, R.J., W.C. Hulbert, and S.H. Roth. 1992. Toxicology of hydrogen sulfide. Ann. Rev. Pharmacol. Toxicol. 32:109-134.
Rossi, O.V., V.L. Kinnula, J. Tienari, and E. Huhti. 1993. Association of severe asthma attacks with weather, pollen, and air pollutants. Thorax 48(3):244-248.
Ruijten, M.W.M.M., R. van Doorn, and A.P. van Harreveld. 2009. Assessment of Odour Annoyance in Chemical Emergency Management. RIVM Report 609200001/2009. RIVM, Bithoven, The Netherlands: RIVM [online]. Available: http://www.rivm.nl/ bibliotheek/rapporten/609200001.pdf [accessed April 12, 2010].
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Saillenfait, A.M., P. Bonnet, and J. de Ceaurriz. 1989. Effects of inhalation exposure to carbon disulfide and its combination with hydrogen sulfide on embryonal and fetal development in rats. Toxicol. Lett. 48(1):57-66.
Shusterman, D. 1992. Critical review: The health significance of environmental odor pollution. Arch. Environ. Health 47(1):76-87.
Skrajny, B., R.S. Hannah, and S.H. Roth. 1992. Low concentrations of hydrogen sulfide alter monoamine levels in the developing rat central nervous system. Can. J. Physiol. Pharmacol. 70(11):1515-1518.
Skrajny, B., R.J. Reiffenstein, R.S. Sainsbury, and S.H. Roth. 1996. Effects of repeated exposures of hydrogen sulfide on rat hippocampal EEG. Toxicol. Lett. 84(1):43-53.
Smith, L., H. Kruszyna, and R.P. Smith. 1977. The effect of methemoglobin on the inhibition of cytochrome c oxidase by cyanide, sulfide or azide. Biochem. Pharmacol. 26(23):2247-2250.
Smith, R.P. 1996. Toxic responses of the blood. Pp. 335-354 in Cassarett and Doull’s Toxicology: The Basic Science of Poisons, 5th Ed., C.D. Klaassen, ed. New York: Macmillan.
Smith, R.P., and R.E. Gosselin. 1979. Hydrogen sulfide poisoning. J. Occup. Med. 21(2):93-97.
Snyder, J.W., E.F. Safir, G.P. Summerville, and R.A. Middleberg. 1995. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am. J. Emerg. Med. 13(2):199-203.
Stein, M., and P. Ottenberg. 1958. Role of odors in asthma. Psychosom. Med. 20(1):60-65.
Swedish Work Environment Authority. 2005. Occupational Exposure Limit Value and Measures against Air Contaminants. AFS 2005:17 [online]. Available: http://ww w.av.se/dokument/inenglish/legislations/eng0517.pdf [accessed Apr. 6, 2010].
Tansy, M.F., F.M. Kendall, J. Fantasia, W.E. Landin, R. Oberly, and W. Sherman. 1981. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced-sulfur compounds. J. Toxicol. Environ. Health 8(1-2):71-88.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13(3):301-309.
TNRCC (Texas Natural Resources Conservation Commission). 1998. Real-Time Gas Chromatography and Composite Sampling, Sulfur Dioxide, Hydrogen Sulfide, and Impinger Sampling, Corpus Christi Mobile Laboratory Trip, January 31- February 6, 1998. Memorandum from Tim Doty to JoAnn Wiersma. April 20, 1998.
Tvedt, B., K. Skyberg, O. Aaserud, A. Hobbesland, and T. Mathiesen. 1991a. Brain damage caused by hydrogen sulfide: A follow-up study of six patients. Am. J. Ind. Med. 20(1):91-101.
Tvedt, B., A. Edlund, K. Skyberg, and O. Forberg. 1991b. Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: Affection of motor function, memory, vision, and hearing. Acta. Neurol. Scand. 84(4):348-351.
Vanhoorne, M., A. de Rouck, and D. de Bacquer. 1995. Epidemiological study of eye irritation by hydrogen sulfide and/or carbon disulphide exposure in viscose rayon workers. Ann. Occup. Hyg. 39(3):307-315.
Warenycia, M.W., L.R. Goodwin, D.M. Francom, F.P. Dieken, S.B. Kombian, and R.J. Reiffenstein. 1990. Dithiothreitol liberates non-acid labile sulfide from brain tissue of H2S-poisoned animals. Arch. Toxicol. 64(8):650-655.
Warneck, P. 1988. Chemistry of the Natural Atmosphere. San Diego: Academic Press.
Wasch, H.H., W.J. Estrin, P. Yip, R. Bowler, and J.E. Cone. 1989. Prolongation of the P-300 latency associated with hydrogen sulfide exposure. Arch. Neurol. 46(8):902-904.
Wever, R., B.F. van Gelder, and D.V. Dervartanian. 1975. Biochemical and biophysical studies on cytochrome c oxidase. XX. Reaction with sulphide. Biochim. Biophys. Acta 387(2):189-193.
WHO (World Health Organization). 1981. Hydrogen Sulfide. Environmental Health Criteria 19. Geneva: WHO [online]. Available: http://www.inchem.org/documents/eh c/ehc/ehc019.htm [accessed Apr. 15, 2010].
Williams, V.R., and H.B. Williams. 1967. Pp. 138 in Basic Physical Chemistry for the Life Sciences. San Francisco: W.H. Freeman.
Winek, C.L., W.D. Collom, and C.H. Wecht. 1968. Death from hydrogen sulfide fumes. Lancet 1(7551):1096.
Xu, X., S.I. Cho, M. Sammel, L. You, S. Cui, Y. Huang, G. Ma, C. Padungtod, L. Pothier, T. Niu, D. Christiani, T. Smith, L. Ryan, and L. Wang. 1998. Association of petrochemical exposure with spontaneous abortion. Occup. Environ. Med. 55(1): 31-36.
Zwart, A., J.H.E. Arts, J.M. Klokman-Houweling, and E.D. Schoen. 1990. Determination of concentration-time-mortality relationships to replace LC50 values. Inhal. Toxicol. 2(2):105-117.
APPENDIX A
TIME-SCALING CALCULATIONS FOR HYDROGEN SULFIDE
Derivation of AEGL-1
|
Key study: |
Jappinen et al. 1990 |
|
Toxicity end point: |
Headache in human asthma subjects |
|
Scaling: |
C4.4 × t = k (2 ppm)4.4 × 0.5 h = 10.27 ppm-h |
|
Modifying factor: foul odor of H2S |
3, wide variability in complaints associated with the and the shallow concentration response at the relatively low concentrations that are consistent with definition of the AEGL-1 |
|
Calculations: |
|
|
10-min AEGL-1: |
C4.4 × 0.167 h = 10.27 ppm-h C4.4 = 61.5 ppm C = 2.6 ppm 10-min AEGL-1 = 2.6/3 = 0.75 ppm |
|
30-min AEGL-1: |
C4.4 × 0.5 h = 10.27 ppm-h C4.4 = 20.54 ppm C = 2.00 ppm 30-min AEGL-1 = 2.0/3 = 0.60 ppm |
|
1-h AEGL-1: |
C4.4 × 1 h = 10.27 ppm-h C4.4 = 10.27 ppm C = 1.71 ppm 1-h AEGL-1 = 1.7/3 = 0.51 ppm |
|
4-h AEGL-1: |
C4.4 × 4 h = 10.27 ppm-h C4.4 = 2.57 ppm C = 1.28 ppm 4-h AEGL-1 =1.28/3 = 0.36 ppm |
|
8-h AEGL-1: |
C4.4 × 8 h = 10.27 ppm-h C4.4 = 1.28 ppm C = 1.06 ppm 8-h AEGL-1 = 1.06/3 = 0.33 ppm |
Derivation of AEGL-2
|
Key studies: |
Green et al. 1991; Khan et al. 1991 |
|
Toxicity end points: |
Minor perivascular edema present and significant increase in protein and LDH in lung lavage fluid. |
|
Scaling: |
C4.4 × t = k (200 ppm)4.4 × 4 h =4.31 × 1010 ppm-h |
|
Uncertainty factors: |
3 for interspecies variability 3 for intraspecies variability |
|
Calculations: |
|
|
10-min AEGL-2: |
C4.4 × 0.167 h = 4.31 × 1010 ppm-h C4.4 = 2.58 × 1011 ppm C = 414.4 ppm 10-min AEGL-2 = 414.4/10 = 41.4 ppm |
|
30-min AEGL-2: |
C4.4 × 0.5 h = 4.31 × 1010 ppm-h C4.4 = 8.62 × 1010 ppm C = 322.2 ppm 30-min AEGL-2 = 322.2/10 = 32.2 ppm |
|
1-h AEGL-2: |
C4.4 × 1 hr = 4.31 ×1010 ppm-h C4.4 =4.31 × 1010 ppm C = 274 ppm 1-h AEGL-2 =274/10 = 27.4 ppm |
|
4-h AEGL-2: |
C4.4 × 4 hr = 4.31 × 1010 ppm-h C4.4 = 1.08 ×1010 ppm C = 199.9 ppm 4-h AEGL-2 =199.9/10 = 19.9 ppm |
|
8-h AEGL-2: |
C4.4 × 8 h =4.31 × 1010 ppm-h C4.4 = 5.39 × 109 ppm C = 170.6 ppm 8-h AEGL-2 =170.6/10 = 17.1 ppm |
Derivation of AEGL-3
|
Key study: |
MacEwen and Vernot 1972 |
|
Toxicity end point: exposure in rats |
Highest concentration causing no death after a 1-h |
|
Scaling: |
C4.4 × t = k (504 ppm)4.4 × 1 h = 6.06 × 1011 ppm-h |
|
Uncertainty factors: |
3 for interspecies variability 3 for intraspecies variability |
|
Calculations: |
|
|
10-min AEGL-3: |
C4.4 × 0.167 h = 6.06 × 1011 ppm-h C4.4 = 3.63 × 1012 ppm C = 759.8 ppm 10-min AEGL-3 = 759.8/10 = 75.9 ppm |
|
30-min AEGL-3: |
C4.4 × 0.5 h = 6.06 × 1011 ppm-h C4.4 = 1.21 × 1012 ppm C = 590.8 ppm 30-min AEGL-3 = 590.8/10 = 59.1 ppm |
|
1-h AEGL-3: |
C4.4 × 1 h = 6.06 × 1011 ppm-h C4.4 = 6.06 × 1011 ppm C = 503.9 ppm 1-h AEGL-3 =503.9/10 = 50.4 ppm |
|
4-h AEGL-3: |
C4.4 × 4 h = 6.06 × 1011 ppm-h C4.4 = 1.52 × 1011 ppm C = 366.7 ppm 4-h AEGL-3 =366.7/10 = 36.7 ppm |
|
8-h AEGL-3: |
C4.4 × 8 hr = 6.06 × 1011 ppm-h C4.4 = 7.58 × 1010 ppm C = 312.8 ppm 8-h AEGL-3 = 312.8/10 = 31.3 ppm |
APPENDIX B
ACUTE EXPOSURE GUIDELINES FOR HYDROGEN SULFIDE
Derivation Summary for Hydrogen Sulfide
AEGL-1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.75 ppm |
0.60 ppm |
0.51 ppm |
0.36 ppm |
0.33 ppm |
|
Key Reference: Jappinen, P., V. Vilkka, O. Marttila, P. Jappinen, and T. Haahtela. 1990. Exposure to hydrogen sulfide and respiratory function. Br. J. Ind. Med. 47(12):824-828. |
||||
|
Test Species/Strain/Number: Human/10 asthma patients |
||||
|
Exposure Route/Concentrations/Durations: Inhalation/2 ppm /30 min |
||||
|
Effects: Odor and pharyngeal dryness at the beginning of exposure; headache (3/10); increased Raw (significant in 2/10) with no accompanying clinical signs or lung function effects |
||||
|
End Point/Concentration/Rationale: headache/2 ppm |
||||
|
Uncertainty Factors/Rationale: Interspecies 1: subjects were human |
||||
|
Modifying Factor: 3 to account for the wide variability in complaints associated with the foul odor of H2S and the shallow concentration response at the relatively low concentrations that are consistent with definition of the AEGL-1 |
||||
|
Animal to Human Dosimetric Adjustment: NA |
||||
|
Time-Scaling: Cn × t = k, where n = 4.4; value derived from rat lethality data ranging from 10 min to 6 h. Data point used for AEGL-1 derivation was 30 min. Other time points were based on extrapolation. |
||||
|
Data Quality and Research Needs: These values are supported by the fact that no adverse effects were observed in healthy humans exposed to H2S at 5 ppm for 30 min or 10 ppm for 15 min while exercising to exhaustion (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b). Using these concentrations and applying an uncertainty factor of 10 for sensitive human subpopulations, the following AEGL-1 values would be obtained: 0.64, 0.50, 0.43, 0.31, and 0.26 ppm for the 10- and 30-min and 1-, 4-, and 8-h time points, respectively, for the 5-ppm exposure for 30 min; and 1.1, 0.85, 0.73, 0.53, and 0.45 ppm for the 10- and 30-min and 1-, 4-, and 8-h time points, respectively, for the 10-ppm exposure for 15 min. These values suggest that the proposed AEGL-1 values are protective. |
||||
AEGL-2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
41 ppm |
32 ppm |
27 ppm |
20 ppm |
17 ppm |
|
Key References: Green, F.H., S. Schurch, G.T. DeSanctis, J.A. Wallace, S. Cheng, and M. Prior. 1991. Effects of hydrogen sulfide exposure on surface properties of lung surfactant. J. Appl. Physiol. 70(5):1943-1949. Khan, A.A., S. Yong, M.G. Prior, and L.E. Lillie. 1991. Cytotoxic effects of hydrogen sulfide on pulmonary alveolar macrophages in rats. J. Toxicol. Environ. Health 33(1):57-64. |
||||
|
Test Species/Strain/Number: (1) Rat/F344/6 males; (2) Rat/F344/6 males |
||||
|
Exposure Route/Concentrations/Durations: Inhalation/0, 200, or 300 ppm/4 h; Inhalation/0, 50, 200, or 400 ppm/4 h |
||||
|
Effects: 200 ppm: No adverse clinical signs or gross lung pathology, increased protein and LDH in lavage fluid 300 ppm: Clinical signs during exposure, increased protein and LDH in lavage fluid, lung atelectasis, and edema 50 and 200 ppm: No effect on viability of pulmonary alveolar macrophages 300 ppm: Decreased viability of pulmonary alveolar macrophages (200 ppm for 4 h was determinant for AEGL-2) |
||||
|
End Point/Concentration/Rationale: (1) No-effect level for gross lung pathology, minor perivascular edema, increased protein and LDH in lung lavage fluid. (2) No-effect level for pulmonary alveolar macrophage viability/200 ppm |
||||
|
Uncertainty Factors/Rationale: Interspecies 3: rat and mouse data suggest little interspecies variability Intraspecies 3: The intraspecies uncertainty factor of 3 is considered sufficient because application of the default uncertainty factor of 10 would result in a total uncertainty factor of 30, which would yield AEGL-2 values inconsistent with the total database. AEGL-2 values derived with a total uncertainty factor of 30 would be 14 ppm for 10 min, 11 ppm for 30 min, 9.0 ppm for 1 h, 6.7 ppm for 4 h, and 5.7 ppm for 8 h, values essentially identical to or below the 10-ppm concentration causing no effects in humans exercising to exhaustion (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Total Uncertainty Factor: 10. The total adjustment is 10 because each of the factors of 3 represents a logarithmic mean (3.16) of 10; therefore, 3.16 × 3.16 = 10. |
||||
|
Modifying Factor: NA |
||||
|
Animal to Human Dosimetric Adjustment: NA |
||||
|
Time-Scaling: Cn × t = k, where n = 4.4; value derived from rat lethality data ranging from 10 min to 6 h. Data point used for AEGL-2 derivation was 4 h. Other time points were based on extrapolation. |
||||
|
Data quality and research needs: Two well-conducted studies in rats support one another. |
||||
AEGL-3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
76 ppm |
59 ppm |
50 ppm |
37 ppm |
31 ppm |
|
Key Reference: MacEwen, J.D., and E.H. Vernot. 1972. Acute toxicity of hydrogen sulfide. Pp. 66-70 in Toxic Hazards Research Unit Annual Report: 1972. Report No. ARML-TR-72-62. Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, OH. |
||||
|
Test Species/Strain/Sex/Number: Sprague-Dawley rats/10 males/concentration |
||||
|
Exposure Route/Concentrations/Durations: Rats/inhalation: 400, 504, 635, or 800 ppm/1 h (Highest concentration causing no death in rats after a 1-h exposure (504 ppm) was determinant for AEGL-3.) |
||||
|
End Point/Concentration/Rationale: Highest concentration causing no death in rats after a 1 h-exposure/504 ppm/threshold for death for 1-h exposure in rats. |
||||
|
Effects: Concentration Mortality 400 ppm 0/10 504 ppm 0/10 635 ppm 1/10 800 ppm 9/10 |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 for rat and mouse data suggest little interspecies variability Intraspecies: 3 is considered sufficient because application of the default uncertainty factor of 10 would result in a total uncertainty factor of 30, which would yield AEGL-3 values inconsistent with the total database. AEGL-3 values derived with a total uncertainty factor of 30 would be 25 ppm for 10 min, 20 ppm for 30 min, 17 ppm for 1 h, 12 ppm for 4 h, and 10 ppm for 8 h, values equal to or less than 2-fold the concentration causing no effects in humans exercising to exhaustion (Bhambhani and Singh 1991; Bhambhani et al. 1994, 1996a,b, 1997). Effects consistent with the definition of AEGL-3 would be unlikely to occur at such concentrations. Total Uncertainty Factor: 10. The total adjustment is 10 because each of the factors of 3 represents a logarithmic mean (3.16) of 10; therefore, 3.16 × 3.16 = 10. |
||||
|
Modifying Factor: NA |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data |
||||
|
Time-Scaling: Cn × t = k, where n = 4.4, value derived from rat lethality data ranging from 10 min to 6 h. Data point used for AEGL-3 derivation was 1 h. Other time points were based on extrapolation. |
||||
|
Data Quality and Research Needs: Well-conducted study with appropriate end point for AEGL-3. |
||||
APPENDIX C
Derivation of the Level of Distinct Odor Awareness for Hydrogen Sulfide
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by Ruijten et al. (2009).
The odor detection threshold (concentration at which 50% of people detect an odor; OT50) for H2S was calculated to be 0.0006 ppm (Ruijten et al. 2009).
The concentration (C) leading to an odor intensity (I) of distinct odor detection (I = 3) is derived with the Fechner function:
For the Fechner coefficient, the default of kw = 2.33 is used because of a lack of chemical-specific data:
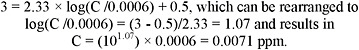
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in everyday life factors such as sex, age, sleep, smoking, upper airway infections, and allergy as well as distraction increase the OT50 by a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 seconds) which leads to the perception of concentration peaks. On the basis of current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustment for distraction and peak exposure lead to a correction factor of 4/3 = 1.33.















































