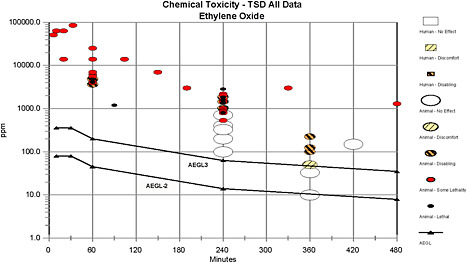
2
Ethylene Oxide1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. AEGL-2 and AEGL-3, and AEGL-1 levels, as appropriate, will be developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and will be distinguished by varying degrees of severity of toxic effects. It is believed that the recommended exposure levels are applicable to the general population, including infants and children and other individuals who may be susceptible. The three AEGLs have been defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million [ppm] or milligrams per cubic meter [mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could
experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects, or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGLs represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Ethylene oxide is a highly flammable gas produced in very large quantities in the United States (5.3 to 6.3 billion pounds). It is very reactive with nucleophilic substances such as water, alcohols, halides, amines, and sulfhydryl compounds. Ethylene oxide is used as an intermediate in the production of ethylene glycol and nonionic surfactants; a small amount is used as a fumigant for sterilizing foods and heat-sensitive medical equipment.
The database on the toxicity of ethylene oxide vapor in humans and experimental animals is extensive, including data on all aspects of toxicity except lethality in humans. Pharmacokinetics data show that ethylene oxide is readily absorbed from the respiratory tract in humans and other animals. Ethylene oxide alkylates proteins and DNA, and it is metabolized primarily by nonenzymatic hydrolysis, enzymatic hydrolysis, and glutathione conjugation.
The odor detection threshold for ethylene oxide was reported to be 260 ppm by one investigator and 700 ppm by another. In humans, ethylene oxide vapors affect the eyes, respiratory tract, central and peripheral nervous systems, gastrointestinal tract (probably secondary effects to nervous system toxicity), hematopoietic system, and possibly the reproductive system and fetus. Acute exposure to ethylene oxide at the odor detection level (≥260 ppm) causes eye and upper respiratory tract irritation and signs and symptoms of effects on the central and peripheral nervous systems. Acute exposure to a calculated concen-
tration of at least 500 ppm for 2 to 3 min caused hematologic effects and more severe effects on the central nervous system than those noted at the odor detection level. Most effects observed after acute exposure are reversible, including effects on the nervous system. Repeated exposures exacerbate peripheral nerve damage. Human studies have provided evidence suggestive of reproductive toxicity, some evidence of an association between exposure to ethylene oxide and genetic damage to somatic cells, and limited evidence of carcinogenicity.
Acute lethality studies in experimental animals showed that mice are the most sensitive species (4-h LC50 [concentration with 50% lethality] = 660 to 835 ppm), followed by the dog (4-h LC50 = 960 ppm) and rat (4-h LC50 = 1,537 to 1,972 ppm; 1-h LC50 = 4,439 to 5,748 ppm). Immediate deaths were likely due to respiratory failure and delayed deaths were due to secondary respiratory infections. Experimental animals exposed to lethal and nonlethal concentrations of ethylene oxide showed evidence of eye and respiratory tract irritation and effects on the central and peripheral nervous systems. Additional studies in animals exposed to ethylene oxide for up to 6 h/day provided evidence of reproductive toxicity (subchronic exposure), developmental toxicity, neurotoxicity, genetic toxicity in germ cells, and carcinogenicity.
Data were available for deriving AEGL-2 and -3 values. Values for AEGL-1 were not derived because concentrations causing mild sensory irritation are ≥260 ppm, which is above the AEGL-2 values and would not serve as a warning of potential exposure. Therefore, AEGL-1 values are not recommended. The absence of AEGL-1 values does not imply that exposure below the AEGL-2 is without adverse effects.
The AEGL-2 values were based on an acute neurotoxicity study in rats exposed to 0, 100, 300, or 500 ppm for 6 h (Mandella 1997a) and a developmental toxicity study with pregnant rats exposed to ethylene oxide at 10, 33, or 100 ppm for 6 h/day during organogenesis (Snellings et al. 1982a). The point of departure is 100 ppm, the no-observed-adverse-effect level (NOAEL) for neurotoxicity and developmental toxicity. The decrease in fetal body weight and the increase in litter incidence of delayed ossification of the vertebrae at 100 ppm were not toxicologically significant, and 100 ppm is the NOAEL for the collective neurotoxicity end points (droopy, half-closed eyelids; impaired locomotion; low arousal; and no response to approach). A total uncertainty factor of 10 was applied to the point of departure: 3 for interspecies sensitivity and 3 for intraspecies variability. An uncertainty factor of 3 was selected for interspecies sensitivity because similar neurotoxicity effects (distal axonal degeneration) have been observed in rats and humans. Direct alkylation of DNA, proteins, and other macromolecules—one potential mechanism of toxicity—is not expected to differ across species. Physiologically based pharmacokinetic (PBPK) models have shown that the area under the curve, peak blood levels, internal dose in milligram per kilogram of body weight (mg/kg), and hemoglobin adduct levels (measure of internal exposure) in humans are similar to or lower than the corresponding values for rats. In addition, the hemoglobin adduct level in rats and humans is proportional to exposure concentration. A factor of 3 was selected for
intraspecies variability because glutathione-S-transferase polymorphism in humans modulates systemic exposure as measured by hemoglobin adducts. Ethylene oxide exposure measured by hemoglobin adduct levels is within a factor of 3 in individuals with the GSTT1 genotype (conjugator) and the GSTT1-null genotype (nonconjugator). There is no evidence that individuals with respiratory diseases, including asthma, would respond differently to ethylene oxide concentrations far below odor detection or irritation levels. The time-scaling approach used ten Berge’s equation in which Cn × t = k (chemical concentration in air with a chemical-specific exponent applied to a specific end point × exposure time = response), where n = 1.2, based on analysis of rat lethality data. The AEGL value for a 10-min exposure is the same as the 30-min value because of the uncertainty of extrapolating from a 6-h exposure to 10 min.
AEGL-3 values were derived from a lethality study with rats (Jacobson et al. 1956). An LC01 (concentration with 1% lethality) value (628 ppm), which is considered an approximation of the lethality threshold, was estimated from a 4-h acute inhalation study with rats. Uncertainty factors of 3 for interspecies sensitivity and 3 for intraspecies variability (total uncertainty factor of 10) were applied to the LC01. The rationale for the interspecies uncertainty factor was the same as that described for AEGL-2 as a rat study was used to derive the AEGL values and the exposure concentration was within range for the PBPK model simulations showing linearity of systemic uptake. An intraspecies uncertainty factor of 3 was selected because glutathione-S-transferase polymorphism can modulate systemic exposure as measured by hemoglobin adduct levels, and individuals with asthma are not expected to be affected differently by ethylene oxide exposure. An interspecies or intraspecies uncertainty factor of 10 would lower the 10- and 30-min AEGL values below the odor detection or irritation thresholds and exposure concentrations associated with life-threatening events. Scaling to the different timeframes was based on ten Berge’s equation (Cn × t = k), where n = 1.2. The AEGL value for a 10-min exposures is the same as the 30-min value because of the uncertainty of extrapolating from a 4-h exposure to 10 min.
Assessment of carcinogenicity data (alveolar or bronchiolar adenomas or carcinomas in the lungs of female mice) showed that extrapolating the total cumulative exposure over 2 years to a single exposure and estimating a 10–4 risk resulted in AEGL-3 values of 1,300, 1,300, 640, 160, and 80 ppm for 10- and 30-min and 1-, 4-, and 8-h exposures, respectively. These values exceed those derived for AEGL-2 and AEGL-3.
AEGL values derived for ethylene oxide are summarized in Table 2-1.
1.
INTRODUCTION
Ethylene oxide (a monoepoxide) is a gas at room temperature and normal atmospheric pressure; the vapor density is 1.49. The vapor is highly flammable at concentrations ranging from 3% to 100%, and it may undergo explosive de-
composition (WHO 1985; Gardiner et al. 1993). Ethylene oxide is very reactive with nucleophiles such as water, alcohols, halides, amines, and sulfhydryl compounds (EPA 1985, WHO 1985). Physicochemical properties of ethylene oxide are presented in Table 2-2.
TABLE 2-1 Summary of AEGL Values for Ethylene Oxide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a (Nondisabling) |
Not Recommended |
|||||
|
AEGL-2 (Disabling) |
80 ppm (144 mg/m3) |
80 ppm (144 mg/m3) |
45 ppm (81 mg/m3) |
14 ppm (25 mg/m3) |
7.9 ppm (14 mg/m3) |
NOAEL for neurotoxicity and developmental toxicity (Snellings et al. 1982; Mandella 1997a) |
|
AEGL-3 (Lethal) |
360 ppm (648 mg/m3) |
360 ppm (648 mg/m3) |
200 ppm (360 mg/m3) |
63 ppm (113 mg/m3) |
35 ppm (63 mg/m3) |
Lethality (Jacobson et al. 1956) |
|
aThe absence of AEGL-1 values does not imply that exposure below the AEGL-2 is without adverse effects. |
||||||
TABLE 2-2 Physical and Chemical Data for Ethylene Oxide
|
Parameter |
Value |
Reference |
|
Chemical name |
Ethylene oxide |
|
|
Synonyms |
1,2-epoxyethane, oxirane, dimethylene oxide, ethene oxide |
|
|
CAS registry no. |
75-21-8 |
|
|
Chemical formula |
C2H4O |
|
|
Molecular weight |
44.05 |
Budavari et al. 1996 |
|
Physical state |
Colorless, flammable gas |
Budavari et al. 1996 |
|
Boiling and freezing points |
10.4ºC and –112.5ºC |
Gardiner et al. 1993 |
|
Specific gravity |
0.8966 at 0/4ºC; 0.8711 at 20/20ºC |
Gardiner et al. 1993 |
|
Solubility |
Soluble in water, acetone, acetone, benzene, ethanol, and diethyl ether |
IARC 1994 |
|
Vapor pressurea |
1.50 atm; 152 kPa, 1.52 bar at 21ºC |
Braker and Mossman 1980 |
|
Vapor density |
1.49 at 40ºC |
Gardiner et al. 1993 |
|
Liquid density |
0.8824 at 10/10ºC |
IARC 1994 |
|
Critical temperature |
468.95 K, 195.8ºC, 384.4ºF |
Braker and Mossman 1980 |
|
Autoignition temperature |
702 K, 429ºC, 804ºF |
Braker and Mossman 1980 |
|
Flammability limit |
3.0-100% |
Braker and Mossman 1980 |
|
Conversion factor |
1 ppm = 1.8 mg/m3 at 25ºC, 1 atm |
Gardiner et al. 1993 |
|
aatm, atmosphere; kPa, kilopascal. |
||
Ethylene oxide is produced in very large quantities in the United States and in other countries. Estimated U.S. production was 5.3 to 6.2 billion pounds in 1990 (Gardiner et al. 1993; IARC 1994) and 5.6 billion pounds in 1992 (IARC 1994). Worldwide production exceeded 12 billion pounds (IARC 1994) and may be as high as 16.5 billion pounds (Gardiner et al. 1993). Ethylene oxide is used as an intermediate in the production of ethylene glycol (antifreeze), which accounts for about 60% of its use; nonionic surfactants, which account for about 16%; ethanolamines, which account for about 8.5%; and glycol ethers, diethylene glycol, triethylene glycol, and other chemicals, which account for the remaining 16% (IARC 1994). A small amount of ethylene oxide is used as a fumigant for sterilizing heat-sensitive medical and dental equipment and foods, such as spices and nuts (Gardiner et al. 1993; IARC 1994).
Ethylene oxide is not persistent in the environment; the estimated degradation rate in the atmosphere is 37% in 5.8 days. The half-life is 12 to 14 days in fresh water and 4 days in salt water (EPA 1985; IARC 1994).
The database for ethylene oxide is very large; humans and experimental animal studies on acute toxicity, developmental and reproductive toxicity, genetic toxicity (somatic and germ cells), carcinogenicity, and pharmacokinetics and metabolism were available. These data were used to derive the AEGL values.
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
No studies were available on lethality attributed to ethylene oxide exposure in humans. Marchand et al. (1957) reported the accidental death of three workers involved in the manufacture of ethylene oxide. They experienced vomiting, abdominal pain, diarrhea, headache, and severe nervous system effects that progressed to coma, circulatory collapse, and respiratory failure. Pulmonary edema and congestion of the meninges and brain were observed at the postmortem examination of one of them. The workers were exposed to glycol chlorohydrin, dichloroethane, and ethylene oxide; the deaths were attributed to glycol chlorohydrin and dichloroethane exposure and not to ethylene oxide.
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold
Several human studies on ethylene oxide exposure were available in the literature. In one study, human volunteers sniffed ethylene oxide from an osmoscope (an apparatus attached to the nose) to determine the detection level and description of the odor (Jacobson et al. 1956). The ethylene oxide atmospheres were generated in a 0.7-m3 chamber and drawn into the osmoscope. The concen-
tration of ethylene oxide in the chamber was analyzed by collecting the chamber air into a solution of calcium chloride (CaCl2) and hydrochloric acid (HCl) or a 50% solution of magnesium bromide (MgBr2) containing 0.1 N sulfuric acid (H2SO4) and titrating with sodium hydroxide (NaOH). The subjects described the odor as pleasantly to sickeningly sweet, fruity, alcoholic, or acetone- or etherlike. The median detectable concentration was 700 ppm (1,260 mg/m3) with a 95% confidence interval of 317 to 1,540 ppm (571 to 2,772 mg/m3).
Hellman and Small (1974) conducted a study in which a trained panel of subjects (“trained odor panel”) characterized the sensory odor properties of 101 petrochemicals, one of which was ethylene oxide. The properties were defined as (1) absolute odor threshold, the concentration at which 50% of the panel detected an odor; (2) 50% odor recognition threshold, the concentration at which 50% of the panel defined the odor as being representative of the odorant; (3) 100% odor recognition threshold, the concentration at which 100% of the panel defined the odor as being representative of the odorant; and (4) hedonic tone, the pleasure or displeasure associated with the odor quality as judged by the panel. They also derived an “odor index”, which is the vapor pressure (ppm)/100% odor recognition threshold (ppm). The absolute odor threshold for ethylene oxide was 260 ppm (468 mg/m3), and the 50% and 100% odor recognition thresholds were both 500 ppm (900 mg/m3). The odor index was 2,000 ppm, which placed ethylene oxide in a category of low odor potential. The odor was considered to be sweet or olefinic and was judged as neutral with respect to odor pleasantness or unpleasantness. Hellman and Small (1974) did not report the number of subjects involved in this study or provide additional information on the “training” the subjects received. Cawse et al. (1980) reported that olfactory fatigue occurs upon repeated exposure to ethylene oxide, thus rendering ineffective the warning properties of odor.
The level of distinct odor awareness (LOA) for ethylene oxide calculated based on an odor threshold of 260 ppm and using the guidance provided by van Doorn et al. (2002) is 1,625 ppm. This value is similar to the 95% upper confidence limit on the median odor threshold reported by Jacobson et al. (1956). The derivation of the LOA is presented in Appendix C.
2.2.2.
Case Reports and Anecdotal Data
The following case studies describe signs and symptoms of ethylene oxide intoxication and the concentrations and exposure durations at which they occurred.
Salinas et al. (1981) reported that a female nurse was exposed to ethylene oxide vapor while disposing of an ampule she accidentally dropped. Her exposure lasted 2 to 3 min and she showed immediate signs and symptoms of intoxication, including repeated episodes of nausea, stomach spasms, paleness, light-headedness, short periods of unconsciousness, convulsive movements of her arms and legs, and periods of apnea (cessation of breathing). Muscle twitching,
nausea, and malaise continued for 24 h after exposure; malaise and an inability to perform minor motor tasks continued for up to 1 week after exposure. Chest X-rays, laboratory studies, and arterial blood gases were normal. The patient was asymptomatic 2 months after exposure. The authors estimated maximum exposure as 500 ppm based on the release of 17 g of ethylene oxide into the sterilizer bag, resulting in a minimal peak concentration of 500 micrograms per milliliter (μg/mL) in the bag. Her exposure may have been considerably greater than the calculated concentration of 500 ppm.
Five hospital workers were exposed for 30 min to ethylene oxide vapors emitted from a leaky sterilizer at concentrations high enough to be detected by odor (≥260 ppm) (Deleixhe et al. 1986; Laurent 1988). The sterilizing gas consisted of a mixture of ethylene oxide and carbon dioxide (15/85, v/v). The equipment was operated under 6 atmospheres of pressure, and the concentration of ethylene oxide in the equipment was 1,200 mg/L. The investigators did not specify the method for monitoring the air concentrations after this accident, but a colorimetric method and flame-ionization detection had been used previously. The sterilizer workers experienced ethylene oxide concentrations at the odor threshold of 260 ppm, but it could have been higher. Measured concentrations were 15 to 50 ppm 2.5 h after the accident and about 5 ppm the next day. Two workers experienced only headache and diarrhea, which disappeared within 70 h after exposure; the other three workers experienced more serious signs of toxicity, which included irritation of the upper respiratory tract, dry mouth and thirst, conjunctival irritation, severe headache, and intense generalized pruritus, along with muscular weakness in one worker and dizziness in another. Muscular weakness may have been a sign of toxicity to the peripheral nervous system. Nausea, vomiting, and diarrhea started 20 h after exposure, lasted for 14 days, and cleared up by 21 days. Hemolysis was noted on days 9 to 11 and persisted until day 16.
Garry et al. (1979) described the symptoms experienced by 12 workers exposed to ethylene oxide in the instrument and materials sterilization area. Informed consent was obtained from this study population. Another group of 12 individuals represented an unexposed or incidentally exposed population. Freon gas was used as a carrier with the ethylene oxide to prevent an explosion. Ambient ethylene oxide concentrations were monitored over the entire sterilization cycle by infrared spectroscopy and gas chromatography. Gas chromatography identified the two constituents in the sterilizing gas but could not be used for measuring ethylene oxide because the humidified air resulted in poor absorption of ethylene oxide to the charcoal filter. The frequency of upper respiratory tract irritation indicated that exposure was intermittent, showing a bimonthly cycle over a 5-month period. During a 2-month period, 12 nurses experienced sore throat and dry mouth (most prominent symptoms), diarrhea, conjunctival irritation, headache, nausea, speech difficulty, recent memory loss, weakness, dizziness, and incoordination. The maximum ethylene oxide concentrations ranged from 36 ppm (64.8 mg/m3) in the room about 15 feet from the sterilizer (probably representing the breathing zone) to 1,500 ppm (2,700 mg/m3) in the open
drain leading from the sterilizing unit. Garry et al. (1979) also reported that an investigator was exposed to 1,500 ppm for 5 min; symptoms of intoxication were not described. The signs and symptoms Garry et al. (1979) described cannot be attributed to a single exposure. However, the investigators noted that illnesses were periodic and alleviated by time away from the workplace.
Finelli et al. (1983) described the signs and symptoms experienced by three sterilizer operators accidentally exposed to ethylene oxide over 4-month to 12-year periods. Ambient ethylene oxide concentrations were not determined. Symptoms of intoxication included numbness, tingling, cramps, weakness, and incoordination in the lower extremities and cramps in the hands. In addition, frequent complaints reported by the sterilizer operators included eye irritation, headaches, smelling of fumes, sleeplessness, and nervousness. Neurologic examination showed distal abnormalities in the legs and feet (reflex, vibratory sensation, and flexion) but no abnormalities in cranial nerves. An abnormal gait was noted in one patient and bilateral footdrop was found in two patients. Nerve conduction studies showed abnormalities in motor and sensory conduction potential in the lower extremities in two patients and normal conduction potential in the third. Electromyograms showed abnormal potentials in the lower extremities. The resulting diagnosis was distal axonal neuropathy (peripheral neuropathy). Two patients were fully recovered within 7 months and one was almost fully recovered after 6 months.
The National Institute for Occupational Safety and Health conducted a survey to assess the effects of exposure to ethylene oxide on 10 hospital workers (Zey et al. 1994). The workers complained of headache, dizziness, mucous membrane irritation, nasal bleeding, vomiting, diarrhea, facial flushing and swelling, fatigue, nervousness, and a “sweet”-like odor. The 8-h time-weighted average (TWA) concentration in the breathing zone of the workers ranged from 0.23 to 0.56 ppm, with short-term excursions reaching 77 ppm in one area of the breathing zone and 11 ppm in another. The authors believed the concentrations were higher than those measured in the present investigation, because the employers noticed the ethylene oxide odor, which has a detection threshold higher than the measured concentration. The clinical signs also suggest exposure to higher concentrations.
Deschamps et al. (1992) described a case of persistent nonimmunologic asthma and slight peripheral neuropathy that developed in a worker exposed to ethylene oxide 4 h/day for 4 days. The worker was about 18 m from an ethylene oxide leak and he wore no protective equipment. The worker noticed an odor, suggesting that the concentration was ≥260 ppm. Signs and symptoms after the 4-day exposure included coughing, shortness of breath, and wheezing. Respiratory symptoms persisted and 1 year after the accident, pulmonary function tests showed bronchial obstruction and bronchial hyperreactivity. The forced vital capacity was 93% of the predicted value, forced expiratory volume in 1 s (FEV1) was 74% of the predicted value, midexpiratory flow rate (forced expiratory flow 25% to 75%) was 44% of the predicted value, and the FEV1 after 600 μg of acetylcholine showed a 20% decrease. The respiratory effects persisted for at least 3
years after exposure. Immunologic tests showed no formation of immunoglobulin E antibodies to ethylene oxide. The investigators proposed that the time of onset of symptoms was too short to be explained by a sensitizing mechanism. They further suggested that the alkylating properties of ethylene oxide probably explained why the onset of symptoms occurred after the fourth day of exposure, because alkylating injuries take longer to appear than direct irritation or caustic injuries. A neurologic examination showed signs of proprioceptive axonal neuropathy. An additional five workers, including one with asthma, were exposed because of the leak; none of them experienced respiratory symptoms.
Gross et al. (1979) reported on three workers accidentally exposed for 2 weeks to 2 months to ethylene oxide vapor from a leaky sterilizer. Symptoms they experienced included irritation of the conjunctiva and mucous membranes, decreased sense of smell and taste, headaches, nausea, vomiting, and lethargy. One patient had recurrent major motor seizures, but there was no evidence of peripheral neuropathy. A second worker experienced muscle weakness and increased fatigue and showed evidence of peripheral neuropathy. A third worker had problems with memory and thinking, difficulty swallowing, cramps, numbness, and weakness in the arms and legs, along with clinical signs that included slurred speech, confusion, weakness of facial and distal muscles, and muscular incoordination. A neurologic test also showed evidence of peripheral neuropathy. The exposure concentrations for these workers were not monitored; however, intermittent odor detection of ethylene oxide suggested excursions greater than 260 ppm during work shifts.
2.2.3.
Epidemiologic Studies
Bryant et al. (1989) surveyed sterilizer workers from 27 hospitals who were potentially exposed to ethylene oxide. Short-term symptoms were identified by means of a questionnaire sent to 241 workers; 182 responded, 165 of whom worked with ethylene oxide. The age of the cohort ranged from less than 20 years (1%) to greater than 60 years (9%). The sterilizers used in the hospitals included table top or portable sterilizers and built-in sterilizers with and without ventilation hoods. The portable sterilizers used cartridges containing 100% ethylene oxide, and the other sterilizers used a mixture containing ethylene oxide and an inert carrier gas. The investigators did not describe the analytic procedure for determining ethylene oxide concentrations. Ethylene oxide concentrations ranged from peaks of 11 to 23.5 ppm, decreasing to <1 ppm within 60 seconds (s) or from 8.5 ppm decreasing to 1 ppm within 160 s depending on the type of sterilizer used. The total exposure concentration per sterilizer cycle ranged from undetectable to 10.7 ppm with exposure durations per cycle ranging from 166 s (2.77 min) to 705 s (11.75 min). The mean concentration per cycle was 3.4 ppm. The detection of the ethylene oxide odor suggests that the concentrations exceeded 260 ppm, at least briefly. The most prevalent symptoms other than the odor of ethylene oxide included headaches, skin and eye irritation, dry mouth,
and sore throat. Other symptoms included skin rash, runny nose, loss of sense of smell, shortness of breath, nausea, numbness in fingers, and drowsiness. A larger number of workers exposed to concentrations above the mean concentration reported more symptoms than workers exposed to concentrations below the mean, suggesting a concentration effect. Some symptoms may have been due to daily peak exposures and some were likely due to repeated exposures over a prolonged period.
2.3.
Developmental and Reproductive Toxicity
Hemminki et al. (1982) conducted a cross-sectional study on the spontaneous abortion rate (number of spontaneous abortions per number of pregnancies) among the staff of 80 Finnish hospitals who used ethylene oxide to sterilize heat-sensitive equipment. Control groups exposed to ethylene oxide were identified by hospital nursing staff, who also distributed the questionnaires to the subjects. The return rate for the questionnaires was about 91% for both groups. Specific exposure data were not reported in this study, but the mean 8-h TWA ranged from 0.1 to 0.5 ppm, with the peak concentration reaching 250 ppm at Finnish hospitals. Data from about 24 hospitals showed that concentrations varied between 5 and 10 ppm for about 20 min when the sterilizer door was open (Hemminki et al. 1983). The data as summarized in Table 2-3 are presented as crude and adjusted rates (age, parity, decade of reported pregnancy, coffee and alcohol consumption, and smoking habits). Crude and adjusted spontaneous abortion rates were significantly elevated in female staff exposed to ethylene oxide compared with the unexposed control group. Data obtained from hospital discharge records produced similar results for the spontaneous abortion rates: 22.5% (p < 0.05, compared with controls) for the staff exposed to ethylene oxide and 9.2% for the controls. The abortion ratio (number of spontaneous abortions per number of births) based on hospital records was also higher in workers exposed to ethylene oxide (33.3% compared with 11.8% for controls, p < 0.05). The findings of this study are not conclusive; several weaknesses are evident. Both the exposed and control populations were identified by the nursing staff without corroborating exposure data. Hospital discharge records confirmed the results for only about one-third of the respondents. There are inherent recall biases when results are based on respondents’ memories. The number of sterilizing staff exposed only to ethylene oxide during pregnancy was very small compared with the other groups.
Rowland et al. (1996) conducted a cross-sectional epidemiologic study on the reproductive outcome among California dental assistants potentially exposed to ethylene oxide and showed an increased risk of adverse reproductive outcome associated with exposure. The exposed population consisted of respondents who listed ethylene oxide as the method used to sterilize instruments at the last menstrual date of their last pregnancy. Adverse pregnancy outcomes included spontaneous abortion (<21 weeks), preterm delivery (21 to 36 weeks), and post-term
delivery (≥42 weeks). Thirty-two women reported exposure to ethylene oxide; spontaneous abortion occurred in five, preterm birth occurred in three, and post-term birth occurred in five. Of the 1,288 unexposed women in the study; 88 reported a spontaneous abortion, 56 reported a preterm birth, and 141 reported a post-term birth. The adjusted relative risks of adverse outcomes are presented in Table 2-4. The small number of respondents exposed to ethylene oxide reduces the statistical power of the study and limits the analysis of confounding factors (unscavenged nitrous oxide, high use of amalgam, and cigarette smoking). However, the study authors conducted a sensitivity analysis and concluded that the missing nitrous oxide and smoking data did not bias their results on reproductive outcome. This study has a number of limitations and weaknesses. Exposure measures were not reported, but the authors noted that high concentrations were likely because of the type of sterilization system dental technicians use. The exposure status of the respondents was not confirmed, and the reproductive outcome of the respondents was not verified through hospital records. Although this study suggested that exposure to ethylene oxide can adversely affect the outcome of pregnancy and that the effect can occur at any stage of pregnancy, the results are not conclusive.
TABLE 2-3 Spontaneous Abortion Rates Among Hospital Sterilizing Staff and Controls
|
Group |
Total Number of Pregnancies |
Crude Rate (%) |
Adjusted Ratea (%) |
|
Sterilizing staffb |
1,443 |
11.3 |
9.7 |
|
Exposed during pregnancy |
545 |
16.7c |
15.1c |
|
Not exposed during pregnancy |
605 |
6.0 |
4.6 |
|
Uncertain |
293 |
123c |
11.3c |
|
Ethylene oxide alone |
|
|
|
|
Exposed during pregnancy |
82 |
20.7d |
16.1c |
|
Not exposed during pregnancy |
1,068 |
10.3 |
7.8 |
|
Control |
1,179 |
10.6 |
10.5 |
|
aAdjusted for age, parity, decade of reported pregnancy, coffee and alcohol consumption, and cigarette smoking. bIncludes staff exposed to ethylene oxide, glutaraldehyde, and formaldehyde sterilants. cp < 0.05 for exposed versus nonexposed pregnancies. dp < 0.01 for exposed versus nonexposed pregnancies. Source: Hemminki et al. 1982. Reprinted with permission; copyright 1982, British Medical Journal. |
|||
TABLE 2-4 Adverse Pregnancy Outcomes Among Female Dental Assistants Exposed to Ethylene Oxide
|
Reproductive Outcome |
Number Exposed |
Relative Risk |
95% Confidence Interval |
|
Spontaneous abortiona |
32 |
2.5 |
1.0-6.3 |
|
Preterm birtha |
21 |
2.7 |
0.8-8.8 |
|
Post-term birtha |
17 |
2.1 |
0.7-5.9 |
|
Spontaneous abortion, preterm birtha |
32 |
2.6 |
1.3-5.4 |
|
Spontaneous abortion, preterm birthb |
26 |
2.3 |
1.0-5.4 |
|
Spontaneous abortion, preterm or post-term birtha |
25 |
2.7 |
1.2-6.1 |
|
Spontaneous abortion, preterm or post-term birthb |
20 |
2.5 |
1.0-6.1 |
|
aAdjusted for age only. bAdjusted for age, unscavenged nitrous oxide, and high use of amalgam. Source: Rowland et al. 1996. Reprinted with permission; copyright 1996, Epidemiology. |
|||
2.4.
Carcinogenicity
2.4.1.
Epidemiologic Studies
Several epidemiologic studies have been conducted on the mortality experience of workers potentially exposed to ethylene oxide. Types of cancer that are of concern among workers exposed to ethylene oxide include lymphohematopoietic cancers (combined), leukemia, non-Hodgkin’s lymphoma, and cancer of the brain, stomach, and pancreas.
Hogstedt et al. (1979a) reported three leukemia cases among 230 workers potentially exposed to ethylene oxide in a factory where hospital equipment was sterilized, whereas only 0.2 case was expected based on a rough estimate of the person-years of observation and sex- and age-specific rates in Sweden. Exposure concentrations ranged from 2 to 70 ppm with 8-h TWA concentrations of 20 ± 10 ppm in the breathing zone and 150 ppm at floor level. Hogstedt and coworkers followed these workers and two additional cohorts engaged in the production of ethylene oxide; one group produced ethylene oxide by the chlorohydrin method and another used direct oxidation of ethylene. The three cohorts composed a total of 709 Swedish workers with total followup extending from 1961 to 1985 for mortality and to 1983 for cancer (Hogstedt et al. 1979a,b; Hogstedt 1988). Ethylene oxide exposures varied over the years (Hogstedt et al. 1979b), ranging from a high of 1,300 mg/m3 (~260 ppm, odor detection) to averages of <25 mg/m3 (14 ppm) during the 1940s, 10 to 50 mg/m3 (6 to 28 ppm) during the 1950s and early 1960s, and 1 to 10 mg/m3 (0.6 to 6 ppm) during the 1970s. Con-
founding exposures included ethylene chlorohydrin, ethylene dichloride, bis(2-chloroethyl)ether, other chlorinated chemicals, and ethylene glycol. The risk of all cancers combined, stomach cancer, and blood and lymphatic cancer— particularly the risk of stomach cancer and leukemia among male workers—was increased. The risk of cerebrovascular diseases among male workers exposed to ethylene oxide was also increased (Hogstedt 1988). Because of confounding exposures to other chemicals, the observed effects cannot be attributed to ethylene oxide alone.
Steenland et al. (1991) conducted a retrospective mortality study on 18,254 U.S. workers (55% female and 45% male) employed for at least 3 months at 14 facilities that produced sterilized medical supplies and spices. The average followup was 16 years. The average 8-h TWA concentration was 4.3 ppm (7.7 mg/m3) for sterilizer operators and 2.0 ppm (3.6 mg/m3) for other exposed workers. No statistically significant increases were observed for the number of deaths due to all causes, all cancers, all hematopoietic cancers, leukemia-aleukemia, non-Hodgkin’s lymphoma, or stomach cancer compared with mortality rates for the general U.S. population. However, deaths due to hematopoietic cancers showed a significant positive trend (p = 0.03) with increasing time since first exposure (latency), and deaths due to kidney cancer were significantly increased (p < 0.05) when the latency was >20 years. Significant increases in the mortality rates for all hematopoietic cancers and lymphosarcoma and reticulosarcoma were noted for male workers. Steenland et al. (1991) noted that their study was limited by the small number of cases and short followup time.
Wong and Trent (1993) analyzed the data on the same cohort consisting of 18,728 workers. They also showed no statistically significant increases in mortality rates except for deaths due to non-Hodgkin’s lymphoma among male workers; this increase did not show a trend associated with duration of employment or latency. However, the number of cases was very small. This study also was reported by UCCPC (1991).
Bisanti et al. (1993) conducted a study on 1,971 male Italian chemical workers: 637 were licensed for at least 1 year to handle only ethylene oxide and 1,334 were licensed for at least 1 year to handle ethylene oxide and other chemicals. The license was a qualitative indication of exposure. No quantitative exposure estimates were available. Followup of the entire cohort from 1940 to 1984 showed six deaths due to hematopoietic cancer and four due to lymphosarcoma or reticulosarcoma (p < 0.05). Five cases of hematopoietic cancers (p < 0.05) and three cases of lymphosarcomas and reticulosarcomas (p < 0.001) occurred in the subcohort licensed to handle only ethylene oxide.
Teta et al. (1993) followed the mortality of 1,896 chemical workers potentially exposed to ethylene oxide from 1940 to 1988. These investigators did not find a statistically significant increase in mortality due to all malignant neoplasms or lymphohematopoietic, stomach, brain, or pancreatic cancer.
Hagmar et al. (1995) analyzed the mortality experience of a cohort consisting of 2,170 workers (1,309 women and 861 men) employed for at least 1 year in facilities producing medical supplies sterilized with ethylene oxide. Eth-
ylene oxide exposure was initially about 40 ppm at one facility and 75 ppm at the other; it decreased over the years so that only sterilizer operators were exposed to concentrations greater than 0.2 ppm in later years. These investigators failed to find statistically significant increases in the risks of malignant neoplasms, lymphohematopoietic neoplasms, and leukemia.
Shore et al. (1993) evaluated available epidemiologic studies and conducted a meta-analysis of 10 cohorts that included 29,800 workers with potential exposure to ethylene oxide. A total of 2,540 deaths were recorded. No association was found between ethylene oxide exposure and risk of leukemia, pancreatic cancer, brain and nervous system cancer, and total cancer. A suggested increased risk was observed for non-Hodgkin’s lymphoma and stomach cancer; however, evaluations of intensity, frequency, and duration of exposure and latency did not support the conclusion. This study was also reported by UCCPC (1993).
2.4.2.
Risk Assessment
In 1984, the Occupational Safety and Health Administration (OSHA) reported the results of a quantitative cancer risk assessment on occupational exposure to ethylene oxide. For a 45-year working lifetime exposure to 1 ppm, OSHA estimated 12 to 23 excess deaths due to cancer per 10,000 workers. OSHA (49 Fed. Reg. 25734[1984]) reported that Crump (no date provided) estimated 3.7 to 23 deaths per 10,000 workers, the Ethylene Oxide Industry Council estimated 18 to 79 deaths per 10,000 workers, and Sielken (no date provided) estimated 1 to 6 deaths per 10,000 workers.
The Environmental Protection Agency (EPA) reported a 95% upper bound on slope or q1* of 1 × 10–4 μg/m3 based on the total incidence of leukemia and brain gliomas in female Fischer 344 (F344) rats (from data reported by Snellings et al. 1981) (EPA 1985). Current estimates for single exposures to ethylene oxide are presented in Appendix B.
2.5.
Genetic Toxicity
2.5.1.
Epidemiologic and Case Studies
Various end points of genetic toxicity have been studied extensively in humans receiving accidental acute high-level exposures and long-term low-level exposures to ethylene oxide. The populations receiving the most attention are sterilizer operators and chemical manufacturing workers. The literature has been reviewed recently by Rhomberg et al. (1990), Dellarco et al. (1990), and IARC (1994). These reviews described both positive and negative associations between exposure to ethylene oxide and increased frequencies of sister chromatid
exchanges (SCEs) and chromosome aberrations in peripheral lymphocytes. Because the literature is quite extensive, only a few studies are described in this report.
Although most studies involve long-term exposure to ethylene, two acute exposure studies with mixed results were located in the literature. Laurent (1988) reported increased SCE frequencies in peripheral lymphocytes of three sterilizer workers accidentally exposed for 30 min to ethylene oxide concentrations exceeding the odor detection level (260 ppm or 1,260 mg/m3). Clinical symptoms are described in Section 2.2 of this document. SCE frequencies analyzed in the peripheral lymphocytes 5 days and 2 years after the accident were compared with a group of control or chronically exposed workers. Five days after the accident, the mean SCE frequency was significantly elevated, by 160% compared with the control group and by 144% compared with the chronically exposed group. The mean SCE frequency in the chronically exposed group was significantly elevated (112%) compared with the control group. A significant increase in the proportion of high-frequency cells (cells with more than 15 SCEs per cell) was observed in the exposed subjects 5 days after the accident; this increase accounted for the increased frequency of SCEs. By 2 years after the accident, SCE frequencies had returned to the preaccident level.
Tates et al. (1995) compared several end points of genetic damage in seven chemical workers incidentally exposed to ethylene oxide at concentrations ranging from 52 to 785 mg/m3 (29 to 436 ppm, 8-h TWA concentrations) with a group of seven unexposed controls. Frequencies of SCEs, hprt mutants, and micronuclei were evaluated in peripheral lymphocytes harvested 89 to 180 days after exposure. Although the level of hemoglobin adducts also indicated very high exposures to ethylene oxide, the various genetic tests showed no positive results compared with the control group. These results differ from those obtained by Laurent (1988). However, Tates et al. (1995) did not conduct their genetic tests until 89 to 180 days (3 to 6 months) after exposure, and it is possible that any genetic lesions formed were repaired before that time or the lymphocytes were replaced by natural turnover of the cells. Tates et al. (1995) also did not see increases in the same genetic parameters in workers chronically exposed to ethylene oxide at average concentrations ranging from <0.006 ppm (0.01 mg/m3) to <0.1 ppm (0.18 mg/m3), which may have been too low to induce measurable genetic damage.
Garry et al. (1979) reported that four sterilizer operators exposed for 2 months to ethylene oxide at concentrations high enough to cause respiratory and neurologic symptoms had elevated SCE frequencies 3 and 8 weeks after the last exposure. A concentration of 36 ppm (64.8 mg/m3) was measured 15 feet from the sterilizer and 1,500 ppm (2,700 mg/m3) was found at floor level. The mean SCE frequency was 9.75 ± 0.75 per metaphase cell 3 weeks after exposure and 10.34 ± 2.55 per metaphase cell 8 weeks after exposure, compared with 5.98 ± 0.31 in the control group (eight subjects). In four asymptomatic workers inci-
dentally exposed to ethylene oxide, including one subject exposed to 1,500 ppm (2,700 mg/m3) for 5 min, the frequency of SCEs for the group was elevated (mean = 9.73 ± 0.98 SCEs per metaphase cell) 7 to 9 weeks after the last known exposure to ethylene oxide.
In a recent study, Major et al. (1996) compared genetic damage in two groups of nurses exposed to ethylene oxide with control groups. One group comprised 9 nurses exposed to ethylene oxide at 5 to 20 mg/m3 (2.8 to 11 ppm) and 14 controls, and the other group comprised 10 nurses exposed to ethylene oxide at 5 to 100 mg/m3 (2.8 to 55.6 ppm) and 27controls. A group of 48 “historic” controls was also used for comparison. Compared with their respective hospital controls, both groups of nurses showed increased frequency of SCEs, chromosome aberrations, or lectin-stimulated labeling index. Aberrations in exposed nurses included deletions, dicentrics, chromatid exchanges, and rings. The background rates in the two control populations varied, indicating differences in confounding factors (alcohol consumption, smoking, age). Overall, this study showed genetic damage in both exposed groups.
2.5.2.
Risk Assessment
Rhomberg et al. (1990) calculated risk estimates for heritable translocations in offspring of fathers exposed to ethylene oxide; they used data reported by Generoso et al. (1990) for their estimates. For an exposure of 10 ppm for 8 h/day for 3 weeks or 15 days of exposure (1,200 ppm × hours), 16 translocation carriers are expected among 10,000 live offspring. Preston et al. (1995) reviewed the Rhomberg et al. (1990) assessment and concluded that the genetic risk for induction of reciprocal translocations would be negligible at low doses. They further noted that Rhomberg overestimated the risk by a factor of 10. Natarajan et al. (1995) used a parallelogram approach to assess genetic risk in humans based on dominant mutations. Their assessment considered genetic end points in germ cells and somatic cells in animals, including humans. They estimated a risk of 4 × 10−4 above background from occupational exposure to ethylene oxide at 1 ppm for 1 year.
2.6.
Occupational Exposure
Workers have been exposed to ethylene oxide at concentrations ranging from undetectable to peaks at moderately high concentrations. Occasionally, very high concentrations have been experienced during accidental exposures but not in the routine work environment. Data on some occupational exposures to ethylene oxide are presented in Table 2-5. Additional information was presented by IARC (1994).
TABLE 2-5 Occupational Exposure to Ethylene Oxide
|
Industry |
Duration of Exposure |
Concentration (ppm) |
Signs and Symptoms of Exposure |
Reference |
|
Hospital sterilizer operation |
5-min TWA |
62.5 ± 46 (13-160) |
Not reported |
Sarto et al. 1984 |
|
|
1 cycle |
15.8 ± 9.8 (3.7-35.5) |
Not reported |
|
|
|
8-h TWA |
10.7 ± 4.9 (3.7-20) |
Not reported |
|
|
Hospital sterilizer operation |
8-h TWA |
0.1-0.5 |
Not reported |
Hemminki et al.1982 |
|
Hospital sterilizer operation |
Peak |
Up to 250 |
Not reported |
|
|
|
20 min |
5-10 |
Not reported |
Hemminki et al. 1983 |
|
Hospital sterilizer operation |
Purge cycle |
36-1500 |
Upper respiratory and neurologic symptoms |
Garry et al. 1979 |
|
Hospital sterilizer operation |
8-h TWA |
ND to 6.3 |
Not reported |
Elliott et al. 1988 |
|
|
2-30 min |
ND to 103 |
Not reported |
|
|
Hospital folding and packing |
8-h TWA |
ND to 6.7 |
Not reported |
|
|
Abbreviation: TWA, time-weighted average; ND, not detectable. |
||||
2.7.
Summary
No adequate data are available on the lethality of ethylene oxide in humans. Nonlethal effects of ethylene oxide and the exposure concentrations at which the effects occur are summarized in Table 2-6. Primary targets for nonlethal effects include the eyes, respiratory tract, and central and peripheral nervous systems. Experimental studies, case reports, and epidemiologic studies have documented noncancer effects on the respiratory tract, eyes, central and peripheral nervous system, gastrointestinal tract (probably due to nervous system toxicity), hematopoietic system, and possibly the reproductive system and fetus. The absolute odor detection level for ethylene oxide is 260 ppm as reported by one author, and the median odor threshold is 700 ppm as reported by another. The odor recognition level is 500 ppm. As noted in Table 2-6, nonlethal effects occur after exposure to ethylene oxide concentrations approximating the odor threshold (≥260 ppm) for short periods (2 to 30 min) or repeatedly for a few days. Genetic damage to somatic cells occurs at concentrations below 260 ppm. Chronic exposure to low 8-h TWA concentrations is associated with the same effects as acute exposure, possibly due to daily high-level excursions.
TABLE 2-6 Summary of Nonlethal Effects of Ethylene Oxide in Humans
|
Concentration |
Exposure Duration |
Effects |
Reference |
|
|
ppm |
mg/m3 |
|||
|
1,3349 |
2,4028 |
10 s |
Definitely irritating to nasal passages |
Walker and Greeson 1932 |
|
2,670 |
4,806 |
Not reported |
Slightly irritating to nasal passages, acetic acid-like odor |
Walker and Greeson 1932 |
|
3,260 |
1,260 |
30 min |
Odor, headache, gastrointestinal effects, eye and upper respiratory tract irritation, pruritus, muscle weakness, dizziness, hemolysis |
Deleixhe et al. 1986; Laurent 1988 |
|
3,260 |
3,1260 |
4 h/d for 4 d |
Coughing, shortness of breath, wheezing, slight peripheral neuropathy, nonimmunologic asthma |
Deschamps et al. 1992 |
|
Excursions of 3,260 |
3,1260 |
2 wk to 2 mon |
Eye and mucous membrane irritation, difficulty swallowing, headache, gastrointestinal effects, lethargy, fatigue, problems with memory and thinking, major motor seizures, peripheral neuropathy |
Gross et al. 1979 |
|
3,500 |
900 |
2-3 min |
Gastrointestinal effects, unconsciousness, apnea, muscle twitching, malaise, incoordination for up to 1 wk |
Salinas et al. 1981 |
|
Not reported |
Not reported |
4 mon to 12 y |
Eye irritation, headaches, smelling of fumes, distal axonal neuropathy |
Finelli et al. 1983 |
|
36-1,500 |
65-2,700 |
Cyclic for 2-5 mon |
Upper respiratory irritation, eye irritation, sore throat and dry mouth, gastrointestinal effects, headache, speech difficulty, recent memory loss, weakness, dizziness, and incoordination |
Garry et al. 1979 |
|
0.23-0.56 (TWA) |
0.4-1 |
Chronic |
Sweetlike odor, headache, dizziness, irritation of mucous membranes, gastrointestinal effects, fatigue, and nervousness |
Zey et al. 1994 |
|
Excursions of 11 or 77 |
19.8-139.6 |
|
|
|
|
Peak = 23.5 |
42.3 |
Up to 1 min |
Odor, headache, skin and eye irritation, dry mouth, sore throat, runny nose, shortness of breath, nausea, and numbness in fingers |
Bryant et al. 1989 |
|
Total up to 10.7 |
19.3 |
Up to 11.75 min |
|
|
|
Average 3.4 |
6.1 |
Not reported |
Drowsiness |
|
|
0.1-0.5 (8-h TWA) |
0.18-0.9 (8-h TWA) |
During pregnancy |
Increased risk of spontaneous abortion |
Hemminki et al. 1982 |
|
Peak 250 |
450 |
|
|
|
|
5-10 (20 min daily) |
9-18 |
|
|
|
|
Not reported |
Not reported |
Any duration during pregnancy |
Increased risk of spontaneous abortion, preterm birth, or post-term birth |
Rowland et al. 1996 |
|
Abbreviation: TWA, time-weighted average. |
||||
Signs of toxicity occurring after short-term exposure to ethylene oxide include eye and upper respiratory tract irritation, nausea, vomiting, diarrhea, headache, dizziness, malaise, fatigue, muscle weakness, and signs and symptoms of peripheral neuropathy. Other effects noted in some studies include dry mouth, sore throat, runny nose, shortness of breath, apnea, memory loss, and seizures. Nonimmunologic asthma was reported in one study; this effect has not been confirmed and may not be due to ethylene oxide exposure. One worker with asthma exposed to ethylene oxide at the odor detection level experienced no symptoms suggestive of effects on the respiratory tract. Two epidemiologic studies presented evidence suggesting that exposure to ethylene oxide is associated with adverse reproductive outcomes: spontaneous abortions, preterm births, and post-term births. An increase in the rate of spontaneous abortions was reported for a cohort exposed to ethylene oxide at concentrations ranging from 0.1 ppm (8-h TWA), to 5 to 10 ppm for 20-min intervals, to peaks of 250 ppm.
Epidemiologic studies conducted to assess the effect of exposure to ethylene oxide on mortality due to malignant neoplasms in workers in chemical factories or sterilizer facilities have produced mixed results with regard to increased cancer risk. Some studies showed increased risks for lymphohematopoietic cancer in the entire cohort or in male subcohorts, whereas other studies showed no increased risk. IARC (1994) concluded that the evidence of carcinogenicity based on human studies is limited.
Human studies on exposure to ethylene oxide also showed that the frequency of SCEs is increased in peripheral lymphocytes of workers exposed to concentrations approximating the odor threshold (260 ppm) for 30 min, exposed to concentrations high enough to cause respiratory and neurologic symptoms for 2 months, incidentally exposed to concentrations of 36 to 1,500 ppm, or chronically exposed to concentrations of 2.8 to 55.6 ppm. The frequency of chromosome aberration was also increased by chronic exposure. Increased frequency of genetic damage was not associated with exposure to high incidental concentrations ranging from 29 to 436 ppm (8-h TWA) when cells were analyzed 89 to 180 days after exposure, suggesting that repair or cell turnover had likely occurred. No damage was observed after chronic exposures to concentrations less than 0.1 ppm.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
3.1.1.
Rats
Jacobson et al. (1956) exposed groups of 10 male white rats to ethylene oxide vapor at 2,298, 1,992, 1,843, 1,648, 1,343, or 882 ppm (4,140, 3,590, 3,320, 2,970, 2,420, or 1,590 mg/m3) for 4 h and observed the animals for signs of toxicity and death for the next 14 days. Ethylene oxide in air was pumped into a 0.4-m3 inhalation chamber operated under constant-flow conditions. The
chamber atmosphere was analyzed by a colorimetric procedure in which ethylene oxide was collected in a solution of 60% CaCl2 and 0.1 N HCl or a 50% solution of MgBr2 and 0.1 N H2SO4 and titrated with NaOH. Both methods gave similar results. Clinical signs observed in the exposed groups included frequent movement and preening, clear nasal discharge, lacrimation, occasional salivation, diarrhea, gasping that increased in severity during exposure, and death. Mortality occurred in all groups. The mortality data are summarized in Table 2-7. The LC50 was 1,460 ppm (2,630 mg/m3) (confidence interval [C.I.] = 620 to 2,550 ppm). Signs of upper respiratory tract irritation, tracheal congestion and petechial hemorrhages, and mild edema in the lungs and peribronchial region were seen upon gross examination. In addition, a secretion was noted around the eyes and nose, and the stomach was distended.
In another 4-h acute inhalation study, groups of five male and five female Sprague-Dawley rats were exposed to ethylene oxide (99.9%) vapor at 1,850, 1,443, or 1,021 ppm (3,330, 2,597, or 1,838 mg/m3); groups of five males also were exposed to 2,182 or 2,026 ppm (3,928 or 3,647 mg/m3) and five females were exposed to 1,637 ppm (2,947 mg/m3) (Nachreiner 1991). The animals were exposed in a 1,300-L glass and stainless steel dynamic chamber. The chamber atmospheres were analyzed with a gas chromatograph equipped with a flame ionization detector. Surviving animals were observed for 14 days after exposure. This study is summarized in Tables 2-8 and 2-9. The LC50 was 1,972 ppm (C.I. = 1,887 to 2,061) for male rats, 1,537 ppm (C.I. = 1,391 to 1,698 ppm) for female rats, and 1,741 ppm (C.I. = 1,655 to 1,831 ppm) for the combined sexes. During exposure, signs of eye, nasal, and oral irritation (blepharospasm; wetness and encrustation around the eyes, nose, and mouth; swollen eye tissue), hypoactivity, and signs of respiratory distress (audible respiration, mouth breathing, increased or shallow respiration, and gasping) were noted (Table 2-8). Clinical signs immediately after exposure included tremors and an absence of tail and toe
TABLE 2-7 Mortality in Male White Rats Exposed to Ethylene Oxide Vapor for 4 Hours
|
Concentration |
|
|
|
ppm |
mg/m3 |
Mortality (%) |
|
2,298 |
4,140 |
10/10 (100) |
|
1,992 |
3,590 |
10/10 (100) |
|
1,843 |
3,320 |
9/10 (90) |
|
1,648 |
2,970 |
4/10 (40) |
|
1,343 |
2,420 |
2/10 (20) |
|
882 |
1,590 |
2/10 (20) |
|
Source: Jacobson et al. 1956. Reprinted with permission; copyright 1956, American Medical Association. |
||
pinch reflex in some groups. Clinical signs indicative of eye and respiratory tract irritation and neurologic effects were observed during the first 3 or 4 days after exposure. No clinical signs were observed after the day of exposure in the 1,021-ppm group or after day 4 in the other exposure groups. Gross findings included brain hemorrhage, lung discoloration and hyperinflation, crusts and scabbing in the oral cavity and pharynx, and abnormal contents in the nose (see Table 2-9 for details). Microscopic findings consisted primarily of congestion and hemorrhage in males found dead in the 2,182-ppm group and females found dead in the 1,850-ppm group. Other lesions included alveolar histiocytosis, pulmonary edema, interstitial pneumonitis, and emphysema.
TABLE 2-8 Lethality and Clinical Signs in Male and Female Sprague-Dawley Rats Exposed to Ethylene Oxide Vapor for 4 Hours
|
Effects |
Concentration (ppm) |
||||||||
|
Males |
Females |
||||||||
|
2,182 |
2,026 |
1,850 |
1,443 |
1,021 |
1,850 |
1,637 |
1,443 |
1,021 |
|
|
Number of deaths |
4/5 |
4/5 |
0/5 |
0/5 |
0/5 |
5/5 |
4/5 |
1/5 |
0/5 |
|
During Exposure |
|
|
|
|
|
|
|
|
|
|
Blepharospasm |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Wetness around eyes and nose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Hyperactivity |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Mouth breathing |
+ |
|
|
|
|
+ |
|
|
|
|
After Exposure |
|
|
|
|
|
|
|
|
|
|
Unkempt fur |
+ |
+ |
+ |
|
|
+ |
+ |
|
|
|
Wetness or encrustation around eyes, nose, and mouth |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Swollen tissue around eyes |
|
|
|
|
+ |
|
|
|
+ |
|
Mouth breathing |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Audible respiration |
+ |
+ |
+ |
|
|
+ |
+ |
+ |
|
|
Gasping |
+ |
+ |
+ |
|
|
+ |
|
|
|
|
Decreased, increased, or shallow respiration |
+ |
+ |
+ |
|
+a |
+ |
+ |
|
+a |
|
Absence of tail and toe pinch reflex |
|
+ |
|
|
|
|
+ |
|
|
|
Hypoactivity |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
|
Tremors |
|
+ |
|
+ |
|
|
|
+ |
|
|
aIncreased respiration rate and shallow respiration only. Source: Nachreiner 1991. |
|||||||||
TABLE 2-9 Gross Findings in Male and Female Sprague-Dawley Rats Exposed to Ethylene Oxide for 4 Hours
|
Effects |
Concentration (ppm) |
||||||||
|
Males |
Females |
||||||||
|
2,182 |
2,026 |
1,850 |
1,443 |
1,021 |
1,850 |
1,637 |
1,443 |
1,021 |
|
|
Brain: hemorrhage |
3a |
0 |
0 |
0 |
0 |
|
|
|
|
|
Lungs: discoloration, diffuse or focal and multifocal |
3 |
4 |
0 |
3 |
2 |
5 |
4 |
1 |
3 |
|
Lungs: hyperinflated |
3 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Nose: abnormal contents |
|
|
|
|
|
3 |
0 |
0 |
0 |
|
Oral and pharyngeal: crust, scab, scale |
|
|
|
|
|
3 |
0 |
0 |
0 |
|
aNumber of animals with lesions; five animals per group were exposed. Source: Nachreiner 1991. |
|||||||||
In a 1-h acute inhalation study, groups of five male Sprague-Dawley rats were exposed to ethylene oxide at measured concentrations of 6,161, 5,546, or 4,827 ppm and groups of five females were exposed to concentrations of 4,287, 4,202, 4,064, 3,966, or 3,609 ppm (Nachreiner 1992). Ethylene oxide in air (4,000 to 7,000 ppm) was metered into a 120-L glass and stainless steel dynamic exposure chamber. The chamber atmospheres were analyzed with a gas chromatograph equipped with a flame ionization detector, and nominal concentrations were calculated based on the amount of ethylene oxide delivered to the chamber. All surviving animals were observed for 14 days. Mortality and clinical signs are summarized in Table 2-10 and gross findings are shown in Table 2-11. No deaths occurred in the male group exposed to 4,827 ppm or in the female group exposed to 3,609 ppm. The LC50 was 5,748 ppm (95% C.I. = 5,276 to 6,262 ppm,) for males, 4,439 ppm (C.I. = 4,034 to 4,884 ppm) for females, and 5,029 ppm (95% C.I. = 4,634 to 5,459 ppm) for the combined sexes. Because of extreme variations in the analytic concentrations (3,584 to 4,432 ppm), which probably explain the unusual mortality rate, the 4,064-ppm female group was not included in the calculation for the LC50. Clinical signs of toxicity were observed in all groups during and after the 1-h exposure up to day 3 or 4 postexposure. Restlessness was observed in all groups during the first 10 min of exposure. In all groups of males and in the 4,827-ppm female group, only lacrimation was observed on the day of exposure; periocular wetness was observed in the
remaining female groups. These findings suggest that ethylene oxide was irritating to the eyes and the respiratory tract and toxic to the nervous system. Gross examination showed effects in the nose, lungs, and kidneys (Table 2-11). Lung weights were elevated in animals that died before the study ended compared with the lungs of animals that survived until study termination, particularly in the male groups.
TABLE 2-10 Clinical Signs in Male and Female Sprague-Dawley Rats Exposed to Ethylene Oxide for 1 Hour
|
Effects |
Concentration (ppm) |
|||||||
|
Males |
Females |
|||||||
|
6,161 |
5,546 |
4,827 |
4,827 |
4,202 |
4,064 |
3,966 |
3,609 |
|
|
Mortality (%) |
4/5 (80) |
1/5 (20) |
0/5 (0) |
5/5 (100) |
1/5 (20) |
5/5 (100) |
2/5 (40) |
0/5 (0) |
|
During Exposure |
|
|
|
|
|
|
|
|
|
Restlessness |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Wetness around eyes |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Lacrimation |
+ |
+ |
+ |
+ |
|
|
|
|
|
Mouth breathing |
+ |
|
|
|
|
|
|
|
|
Hypoactivity |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
No acoustic startle reflex |
+ |
+ |
+ |
+ |
|
|
|
|
|
After Exposure |
|
|
|
|
|
|
|
|
|
Unkempt fur |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Encrustation or wetness: eyes, mouth, nose |
|
+ |
+ |
+ |
|
|
+ |
|
|
Decreased respiration |
+ |
+ |
|
+ |
+ |
+ |
+ |
|
|
Hypoactivity |
+ |
+ |
|
+ |
|
+ |
+ |
+ |
|
Ataxia |
+ |
|
|
|
+ |
+ |
+ |
+ |
|
Tremors |
+ |
+ |
|
|
+ |
+ |
+ |
|
|
Source: Nachreiner 1992. |
||||||||
TABLE 2-11 Gross Findings in Male and Female Sprague-Dawley Rats Exposed to Ethylene Oxide for 1 Hour
|
Effects |
Concentration (ppm) |
|||||||
|
Male |
Female |
|||||||
|
6,161 |
5,546 |
4,827 |
4,827 |
4,202 |
4,064 |
3,966 |
3,609 |
|
|
Encrustation in the nose |
2a |
1 |
0 |
2 |
1 |
3 |
2 |
B |
|
Lungs: discoloration, diffuse or focal and multifocal |
4 |
1 |
0 |
5 |
4 |
5 |
3 |
B |
|
Lungs: hyperinflated |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
B |
|
Kidneys: diffuse color change |
|
|
|
0 |
0 |
3 |
0 |
B |
|
aNumber of animals with lesions; five animals per group were exposed. Source: Nachreiner 1992. |
||||||||
Hollingsworth et al. (1956) conducted several experiments in which rats and other species were exposed to ethylene oxide (97.0% to 98.6%) vapor for various durations in a 450-L metal chamber. Ethylene oxide concentration in the chamber was determined by a colorimetric procedure using H2SO4 and MgBr2 titrated with NaOH. Controls were included but not described. The investigators reported that all 10 male and 10 female rats died after exposure to ethylene oxide at a concentration of 841 ppm (1,510 mg/m3) for 7 h/day, 5 days/week for eight exposures. Gross and microscopic effects were assessed on rats after two or three exposures to ethylene oxide and killed 1 or 3 days after the last exposure. Microscopic effects occurred in the lungs (interstitial edema, congestion, alveolar hemorrhage), liver (fatty degeneration), kidneys (congestion and cloudy swelling of the convoluted tubules), and adrenal glands (fat vacuoles). Renal effects were more severe 3 days after exposure than on the first day after exposure. Exposure to 357 ppm (640 mg/m3) for 7 h/day, 5 days/week for seven exposures resulted in the death of 2/20 rats (10 males and 10 females exposed). Severe lung irritation and secondary pulmonary effects were observed in these animals. In another experiment, 10 male and 10 female rats were exposed to ethylene oxide vapor at 357 ppm for 33 to 59 exposures for 48 to 85 days. Growth was retarded, and by the 38th exposure 18 rats (90%) had died because of secondary respiratory effects. Near the end of the exposure period, neuromuscular impairment at the lumbar and sacral region manifested as paralysis and muscular atrophy of the hindlimbs was observed. The two surviving rats (males) were allowed to recover after 42 exposures.
Jacobson et al. (1956) exposed 20 male white rats to ethylene oxide vapor at 440 ppm (720 mg/m3) for 6 h/day, 5 days/week for 6 weeks; they included an
equal number of unexposed animals as controls. Chamber description and analytic procedure were the same as described for a single exposure to rats and mice except the chamber size was 0.7 m3. Thirteen deaths (65%) occurred among the 20 exposed rats. Clinical signs observed in the exposed rats included a reddish discharge from the nose, diarrhea, labored breathing, hindlimb weakness followed by hindlimb paralysis during the last 2 weeks of exposure, and progressive weight loss. No significant pathologic effects were noted except for marked hemosiderosis in the spleen of a few animals. The weight loss and paralysis were reversible in five rats observed for several months after terminating exposure.
3.1.2.
Mice
Jacobson et al. (1956) exposed groups of 10 female white mice to ethylene oxide at 1,365, 1,343, 960, 882, 860, or 533 ppm (2,460, 2,420, 1,730, 1,590, 1,550, or 960) for 4 h and observed them for 14 days or until death. Ethylene oxide in air was pumped into a 0.4-m3 chamber operated under constant flow conditions. The chamber atmosphere was monitored by collecting chamber air into a solution of 60% CaCl2 and 0.1 N HCl or a 50% solution of MgBr2 and 0.1 N H2SO4 and titrating the mixture with NaOH. Similar results were obtained by both methods. The mice showed clinical signs similar to those in the rat, which included frequent movement and preening, clear nasal discharge, lacrimation, occasional salivation, gasping followed by severe dyspnea, and death. Mortality data are summarized in Table 2-12. The LC50 for mice was 835 ppm (1,504 mg/m3) (C.I. = 623 to 1,040 ppm). The only gross finding reported for mice was distension of the stomach.
In a National Toxicology Program (NTP) (1987) inhalation study, groups of five male and five female B6C3F1 mice were exposed to ethylene oxide (>99%) vapor at concentrations of 0, 100, 200, 400, 800, or 1,600 ppm (180, 360, 720, 1,440, or 2,880 mg/m3) for 4 h and observed for 14 days. Analytic concentrations were determined with a photoionization detector or gas chromatograph equipped with a flame ionization detector. Analytic concentrations were within 5% of target concentrations. Mortality data are summarized in Table 2-13. No animals of either sex died after exposure to 100 to 400 ppm. All males exposed to 800 ppm died 2 to 6 days after exposure and four females exposed to 800 ppm died 1 to 3 days after exposure. All male and female mice exposed to 1,600 ppm died within 4 h after exposure. Lacrimation and dyspnea were observed at 800 ppm; severe dyspnea, incoordination, semiconsciousness, and diarrhea were observed in animals exposed to 1,600 ppm. No clinical signs were described for the 100- and 400-ppm groups. An LC50 value of 660 ppm (95% C.I. = 509 to 856 ppm) was calculated for female mice; an LC50 value was not calculated for male mice. Postmortem examinations were not conducted on these animals.
TABLE 2-12 Mortality in Female White Mice Exposed to Ethylene Oxide Vapor for 4 Hours
|
Concentration |
Mortality (%) |
|
|
ppm |
mg/m3 |
|
|
1,365 |
2,460 |
10/10 (100) |
|
1,343 |
2,420 |
10/10 (100) |
|
960 |
1,730 |
7/10 (70) |
|
882 |
1,590 |
3/10 (30) |
|
860 |
1,550 |
6/10 (60) |
|
533 |
960 |
1/10 (10) |
|
Source: Jacobson et al. 1956. Source: Jacobson et al. 1956. Reprinted with permission; copyright 1956, American Medical Association. |
||
TABLE 2-13 Mortality in Male and Female B6C3F1 Mice Exposed to Ethylene Oxide Vapor for 4 Hours
|
Concentration |
Mortality (%) |
||
|
ppm |
mg/m3 |
Male |
Female |
|
100 |
180 |
0/5 |
0/5 |
|
200 |
360 |
0/5 |
0/5 |
|
400 |
720 |
0/5 |
0/5 |
|
800 |
1,440 |
5/5 (100%) |
4/5 (80%) |
|
1,600 |
2,880 |
5/5 (100%) |
5/5 (100%) |
|
Source: NTP 1987. |
|||
NTP (1987) also conducted a 14-day study in which male and female B6C3F1 mice were exposed to ethylene oxide at concentrations of 0, 50, 100, 200, 400, or 800 ppm (90, 180, 360, 720, or 1,440 mg/m3), 6 h/day, 5 days/week. Analytic concentrations were determined with a photoionization detector or gas chromatograph equipped with a flame ionization detector. All five male and four of five female mice exposed to 800 ppm died within 1 day of exposure; one female died within 2 days of exposure, thus confirming the lethality of 800 ppm in the single-exposure study. Clinical signs at 800 ppm included hunched posture and listlessness. All animals exposed to 50 to 400 ppm survived except for two females exposed to 200 ppm; their deaths were not related to exposure.
Hollingsworth et al. (1956) reported that all five female mice died after exposure to ethylene oxide at a concentration of 841 ppm (1,510 mg/m3), 7 h/day, 5 days/week for eight exposures. Four of 10 female mice died after seven exposures to ethylene oxide at 357 ppm (640 mg/m3). Moderate loss of body weight and severe lung injury indicative of irritation and secondary pulmonary effects were observed in these animals. Another 10 female mice similarly exposed to 357 ppm for 33 exposures over 48 days showed growth retardation and
all died due to secondary respiratory infection. The concentration of ethylene oxide in the chamber atmosphere was determined by a colorimetric procedure using H2SO4 and MgBr2 titrated with NaOH.
In a 6-week inhalation study, Jacobson et al. (1956) exposed 30 female white mice to ethylene oxide vapor at 400 ppm (720 mg/m3) for 6 h/day, 5 days/week for 6 weeks. An equal number of unexposed animals were included as controls. Chamber description and analytic procedure were the same as described for the single exposure of rats and mice except the chamber size was 0.7 m3. A slight weight loss was observed in exposed animals compared with controls. Twenty-four (80%) mice exposed to ethylene oxide died during the study compared with three (10%) controls. No significant pathologic changes were reported.
3.1.3.
Guinea Pig
Waite et al. (1930) exposed guinea pigs to ethylene oxide (99.5%) vapor at concentrations of 8.5%, 6.3% to 6.4%, 5.1%, 4%, 1.4% to 2.5%, 0.7%, 0.3%, 0.13%, 0.05%, and 0.025% for various durations ranging from 1 to 480 min. The concentrations correspond to ethylene oxide vapor in air at 85,000, 63,000 to 64,000, 51,000, 40,000, 14,000 to 25,000, 7,000, 3,000, 1,300, 500, and 250 ppm. The concentration of ethylene oxide was determined by drawing the vapor into 2 N HCl and titrating the solution with barium hydroxide in the presence of a colorimetric indicator. One to four guinea pigs were exposed to each concentration. Twenty-four guinea pigs from the same colony were used as controls. Deaths occurred during exposure to 8.5% ethylene oxide for 33 min. Deaths also occurred within 24 h after exposure to concentrations of 6.3% to 6.4% for 10 or 20 min, 2.5% for 60 min, 1.4% for 60 or 107 min, 0.7% for 150 min, and 0.3% for 330 min. Deaths occurred between 1 and 8 days in groups exposed to 5.1% for 6 min, 4% for 20 min, 1.4% for 20 min, 0.7% for 60 min, 0.3% for 190 min, and 0.13% for 480 min. No deaths occurred in the groups exposed to 1.4% for 10 min, 0.7% for 20 min, 0.3% for 70 min, 0.13% for up to 290 min, and 0.025% or 0.05% for 480 min. Clinical signs of toxicity were observed at all concentrations. Changes in rate, depth, and amplitude of respiration occurred at all concentrations. Nasal irritation, profuse lacrimation, blinking, and squinting (signs of eye irritation) were observed at all concentrations except 0.025%. The eyes showed reddening of the conjunctiva and prominent vessels in the sclera immediately after exposure but not after 24 h. Bloody and frothy nasal exudate were observed at concentrations ≥ 0.3%; an unsteady gait, staggering, and falling on their sides were noted at concentrations ≥ 0.7%; and dyspnea progressing to gasping occurred at concentrations ≥ 0.3%. The onset and duration of these signs varied depending on the concentration of ethylene oxide. Gross pathologic examination showed lung congestion and edema, frothy serous exudate from the trachea and bronchi, and hyperemia of the liver and kidneys of animals dying during exposure or within 24 h after exposure. Animals killed
immediately after exposure showed evidence of lung congestion, whereas those surviving for 2 to 4 days also showed changes in their kidneys. Animals killed after 4 days showed slight lung congestion and slight changes in their kidneys or no signs of serious injury; no pathology was observed 8 days after exposure.
Hollingsworth et al. (1956) reported that all eight male and eight female guinea pigs died after exposure to ethylene oxide at 841 ppm (1,510 mg/m3) for 7 h/day, 5 days/week for eight exposures. Gross and microscopic effects were assessed in guinea pigs exposed two or three times and killed 1 or 3 days after exposure. Toxic effects occurred in the lungs (interstitial edema, congestion, alveolar hemorrhage), liver (fatty degeneration), kidneys (congestion and cloudy swelling of the convoluted tubules), and adrenal glands (fat vacuoles). The renal effects were more severe after 3 days than after 1 day. A control group was included but not described. The ethylene oxide concentration in the chamber was determined by a colorimetric procedure using H2SO4 and MgBr2 titrated with NaOH and a colorimetric indicator.
3.1.4.
Dogs
Three male beagle dogs per group were exposed to ethylene oxide vapor at concentrations of 2,830, 1,393, 710, or 327 ppm (5,100, 2,510, 1,282, or 590 mg/m3) for 4 h followed by a 14-day observation period (Jacobson et al. 1956). Chamber description and analytic procedure were the same as described for a single exposure of rats and mice except the chamber size was 0.7 m3. Death occurred only in the groups exposed to 2,830 and 1,393 ppm, and all deaths occurred within 24 h after exposure. The LC50 for dogs was 960 ppm (1,730 mg/m3). Mortality data are summarized in Table 2-14. Clinical signs observed at 2,830 ppm included lacrimation; clear nasal discharge; frothy, colorless, mucous vomitus; diarrhea; convulsions; dyspnea; and death. Dogs exposed to 1,393 ppm showed similar signs except for diarrhea, convulsion, and dyspnea. No clinical signs were observed at 710 or 327 ppm. Pathologic changes included moderate lung congestion, dilation of perivascular lymphatic spaces, perivascular edema, and distension of the stomach.
TABLE 2-14 Mortality in Male Beagle Dogs Exposed to Ethylene Oxide Vapor for 4 Hours
|
Concentration |
Mortality (%) |
|
|
ppm |
mg/m3 |
|
|
2,830 |
5,100 |
3/3 (100) |
|
1,393 |
2,510 |
3/3 (100) |
|
710 |
1,280 |
0/3 (0) |
|
327 |
590 |
0/3 (0) |
|
Source: Jacobson et al. 1956. Source: Jacobson et al. 1956. Reprinted with permission; copyright 1956, American Medical Association. |
||
3.1.5.
Other Species
One female rabbit, one male rabbit, and one female monkey died after exposure to ethylene oxide at 841 ppm (1,510 mg/m3), 7 h/day, 5 days/week for eight exposures (Hollingsworth et al. 1956). One rabbit of each sex and one female monkey were similarly exposed to ethylene oxide for 33 to 59 exposures in 48 to 85 days. Ethylene oxide concentration in the chamber was determined by a colorimetric procedure using H2SO4 and MgBr2 titrated with NaOH and a colorimetric indicator. The male rabbit died after 48 exposures. In rabbits and monkeys, neuromuscular impairment of the lumbar and sacral region manifested as paralysis, and muscular atrophy of the hindlimbs occurred during the latter part of the exposure. Complete recovery was attained 100 to 132 days after exposure was terminated.
3.2.
Nonlethal Toxicity
Section 3.1 contains data on effects occurring at concentrations not causing death of the animals under study. These data may be used to further assess nonlethal toxicity in laboratory animals.
3.2.1.
Rats
Embree et al. (1977) reported that 15 Long-Evans male rats exposed by inhalation to ethylene oxide at 1,000 ppm (1,800 mg/m3) for 4 h showed signs of toxicity including “central depression,” diarrhea, and eye and respiratory tract irritation. These animals were used in a dominant-lethal study and were not further investigated for general toxicity.
3.2.2.
Mice
Snellings et al. (1984a) reported the results of a subchronic inhalation study using B6C3F1 mice exposed to ethylene oxide (99.9%) vapor. Groups of 30 male and 30 female mice were exposed to ethylene oxide vapor in a 4,350-L stainless steel and glass chamber at target concentrations of 0, 10, 50, 100, or 250 ppm, 6 h/day, 5 days/week for 10 weeks (males) or 11 weeks (females). Analytic concentrations were determined with a gas chromatograph equipped with a flame ionization detector; concentrations were within 6% of the targets. No treatment-related clinical signs of toxicity or body weight changes were observed in animals exposed to ethylene oxide. Erythrocyte parameters were depressed, suggesting slight anemia in males and females at 250 ppm. Spleen weights were depressed in both sexes and testes weights were depressed in males; no corresponding histopathologic effects were observed, suggesting that the organ weight changes were not toxicologically significant. Neuromuscular
screening tests performed on five females at 6 weeks and on five mice of each sex at study termination showed treatment-related effects for five parameters (toe and tail pinch reflex, righting reflex, gait, and locomotor activity) at 250 ppm and for two parameters (gait and locomotor activity) at 50 and 100 ppm.
Groups of 10 male and 10 female B6C3F1 mice were exposed to ethylene oxide at concentrations of 0, 50, 100, 200, 400, or 600 ppm (90, 180, 360, 720, or 1,080 mg/m3), 6 h/day, 5 days/week for 14 weeks (NTP 1987). Analytic concentrations were determined with a photoionization detector or gas chromatograph equipped with a flame ionization detector. All mice exposed to 400 or 600 ppm died within the first 4 weeks of the study. Clinical signs observed in mice exposed to ethylene oxide at 600 ppm included anorexia, dyspnea, decreased activity, bloatedness, and listlessness. One male in each of the remaining groups died before the end of the experiment. Treatment-related histopathologic effects were observed in the kidneys of males (≥100 ppm) and females ((≥200 ppm), thymus of males and females ((≥200 ppm), nasal cavity of both sexes ((≥200 ppm), and spleen of both sexes (600 ppm). No treatment-related effects occurred at 50 ppm.
3.2.3.
Dogs
Two of three male beagle dogs exposed to ethylene oxide vapor at 290 ppm (523 mg/m3) for 6 h/day, 5 days/week for 6 weeks showed clinical signs of toxicity, including vomiting, occasional tremors, and transient weakness in the hindlimbs (Jacobson et al. 1956). The chamber and the analytic procedure were the same as described for a single exposure of rats and mice except the chamber size was 0.7 m3. A mild anemia developed in all three dogs. Pathologic effects included lung congestion and moderate alveolar collapse, which was probably due to irritant effects of ethylene oxide, and muscular atrophy (fat replaced muscle fibers), which caused weakness in the hindlimbs. No deaths occurred among the exposed or control animals.
3.3.
Neurotoxicity
In an acute neurotoxicity study, groups of 10 male and 10 female Sprague-Dawley rats were exposed by whole body inhalation to ethylene oxide at 0, 100, 300, or 500 ppm for 6 h and observed for 14 days after exposure (Mandella 1997a). The rats were exposed under dynamic conditions with monitoring of chamber atmosphere by gas chromatography four times during the exposure cycle. Nominal concentrations were determined based on the amount of test material consumed during the exposure cycle. The mean analytic concentrations were within 2% of the target concentrations. Neurobehavioral assessments that included the standard functional observational battery (FOB) and motor activity
tests were performed on all animals on day 1 and on days 8 and 15 after exposure. At study termination, five rats of each sex per group were killed and perfused in situ for neuropathologic examination and the brain and peripheral nerves of the control and 500-ppm groups were examined microscopically. The results of the FOB assessment showing exposure-related effects are presented in Table 2-15. The FOB assessment showed increased incidences of the following findings among a total of 20 males and females (combined) exposed to 0, 100, 300, and 500 ppm and assessed on day 1: drooping, half-closed eyelids; slightly impaired locomotion; low level of arousal; and no reaction to approach. The incidences of low arousal and no response to approach were significantly increased in male rats and both sexes combined at 300 and 500 ppm, and the incidence of droopy, half-closed eyelids was significantly increased in both sexes at 500 ppm. The increased incidence of slightly impaired locomotion was not significant but showed a clear exposure-related trend and was considered related to exposure to ethylene oxide. The incidence of low arousal was increased at 100 ppm, but not significantly, compared with controls. According to the investigator, these end points were indicative of a decrease in alertness and reflex response. The low arousal state, which was characterized by a decrease in the normal exploratory activity of the animals, and the slightly droopy or half-closed eyelids were indicative of decreased alertness. The lack of response to approach was indicative of a decreased reflex response. Motor activity also was decreased in both sexes at 500 ppm and in males at 300 ppm and was correlated with the decrease in normal exploratory activity. Overall, this study showed exposure-related acute neurotoxicity in male and female rats on day 1 after a single exposure to ethylene oxide at 300 or 500 ppm. The effects were reversible because no clear exposure-related effects were observed on day 8 or 15. The NOAEL for this study was 100 ppm.
Ohnishi et al. (1985) studied the effect of inhaled ethylene oxide vapor on neuropathy in rats. Five male Wistar rats were exposed to ethylene oxide at a concentration of 500 ppm, 6 h/day, 3 days/week for 13 weeks. Five pair-fed animals exposed to ambient air served as controls. Clinical signs in the exposed rats included an awkward gait at weeks 5 to 8 and slight to moderate hindlimb ataxia starting at week 9 or 10. Light and electron microscopic examination of peripheral nerves showed axonal degeneration of myelinated fibers in the fasciculus gracilis and hindlimb nerves. The degenerative changes accounted for the ataxia observed in these animals.
In a 4-week range-finding study, groups of five male and five female Sprague-Dawley rats were exposed by whole-body inhalation to ethylene oxide vapor at concentrations of 0, 100, 300, 400, or 500 ppm (Mandella 1997b). Chamber atmospheres were within 2% of the target concentrations. No exposure-related effects were observed at 100 ppm. One female rat in the 500-ppm group was found dead on day 18. Clinical signs observed at 500 ppm included irregular gait, decreased fecal volume, lethargy, prostration, emaciation, yellow anogenital staining, moist rales, labored breathing, paleness, and black and
TABLE 2-15 Summary of the Acute Neurotoxicity Study in Rats
|
Observation |
0 ppm |
100 ppm |
300 ppm |
500 ppm |
|
Males (n = 10) |
|
|
|
|
|
Drooping, half-closed eyelids |
1 (10%) |
1 (10%) |
0 |
5 (50%) |
|
Slightly impaired locomotion |
0 |
0 |
2 (20%) |
1 (10%) |
|
Low arousal |
0 |
2 (20%) |
5* (50%) |
9** (90%) |
|
Approach response—no reaction |
1 (10%) |
2 (20%) |
6* (60%) |
6* (60%) |
|
Females (n = 10) |
|
|
|
|
|
Drooping, half-closed eyelids |
1 (10%) |
1 (10%) |
5 (25%) |
3 (30%) |
|
Slightly impaired locomotion |
0 |
0 |
0 |
2 (20%) |
|
Low arousal |
0 |
1 (10%) |
1 (10%) |
6* (60%) |
|
Approach response—no reaction |
1 (10%) |
1 (10%) |
1 (10%) |
4 (20%) |
|
Males + Females (n = 20) |
|
|
|
|
|
Drooping, half-closed eyelids |
2 (10%) |
2 (10%) |
5 (25%) |
8** (40%) |
|
Slightly impaired locomotion |
0 |
0 |
2 (10%) |
3 (15%) |
|
Low arousal |
0 |
3 (15%) |
6** (30%) |
15** (75%) |
|
Approach response—no reaction |
2 (10%) |
3 (15%) |
7* (35%) |
10** (50%) |
|
*p < 0.05, **p < 0.01 compared with controls. Source: Mandella 1997a. |
||||
brown stains on the snout. Body weights of males and females exposed to 300, 400, or 500 ppm decreased by 12% to 42% at study termination and food consumption decreased by 15% and 18% in females and males, respectively, during the first week. The neurologic assessment at weeks 3 and 4 showed that hindlimb grip strength decreased 22% to 36% in both sexes at 300, 400, and 500 ppm; this effect was more severe at 400 and 500 ppm. Landing foot splay decreased 29% to 42% in both sexes at week 3 or 4 at 400 and 500 ppm; this effect was more severe at 500 ppm. The postmortem examination showed decreased absolute brain weight in males with 500-ppm exposure. No exposure-related gross lesions were observed, and only minimal to slight vacuolation of the white matter of the thalamus and medulla oblongata was observed in both sexes at 500 ppm. The NOAEL for the 4-week inhalation study was 100 ppm.
In a subchronic neurotoxicity study, groups of 15 male and 15 female Sprague-Dawley rats were exposed by whole body inhalation to ethylene oxide vapor at concentrations of 0, 25, 50, 100, or 200 ppm for 14 weeks (Mandella 1997c). Chamber atmospheres were analyzed by gas chromatography; mean analytic concentrations were within 1% of target concentration. Neurobehavioral assessments (functional observational battery) were conducted on 10 rats of each sex after exposure for 5, 9, and 14 weeks and after a 13-week recovery period.
Five rats of each sex were assessed for gross and microscopic lesions after exposure for 14 weeks and after the 13-week recovery period. No exposure-related effects were observed at 100 ppm and no exposure-related effects were observed for clinical signs, mortality, or cholinesterase activity at any concentration. Body weight gain decreased 16% to 17% during exposure to 200 ppm with a concomitant decrease in food consumption. The neurobehavioral assessment showed no exposure-related effect except for a 25% decrease in hindlimb grip strength in females exposed to 200 ppm. The level of motor activity did not differ between exposed and control rats. Postmortem examination showed no exposure-related gross or microscopic lesions in nervous system tissue. The NOAEL for this study was 100 ppm.
Groups of 12 cynomolgus monkeys were exposed whole body to ethylene oxide (>99% pure) in 3.5-m3 stainless-steel and glass chambers at concentrations of 0, 50, or 100 ppm for 7 h/day, 5 days/week for 24 months (Setzer et al. 1996). The monkeys obtained from the wild were of unknown age; the mean body weights were 5.26, 5.39, and 5.21 kg for the control, 50-, and 100-ppm groups, respectively. The controls were exposed to room air in the chambers. Chamber atmospheres were analyzed by gas chromatography; the analytic concentrations were within 10% of target and ethylene oxide was absent from the control atmosphere. Body weight was measured weekly followed by monthly measurements after 2 months. Maximum nerve conduction velocity for the sciatic-tibial nerves and electroencephalographic (EEG) measurements were conducted five times during exposure. Two animals from each group were sacrificed at the end of the exposure period for neuropathologic examination. The remaining animals were maintained for an additional 7 years without ethylene oxide exposure, at which time two additional animals per group were subjected to neuropathologic examination.
Mean body weight of monkeys exposed to 50 ppm was similar to that of controls, but the 100-ppm group weighed significantly less than controls from week 25 to the termination of exposure. The maximum nerve conduction velocity of the monkeys exposed to ethylene oxide did not differ significantly from that of controls at any time during exposure, but it was consistently lower in the 100-ppm group than in controls from 12 months to the termination of exposure. The investigators noted that maximum nerve conduction velocity of two animals in the 100-ppm group showed a large decline between 12 months and the termination of exposure. The maximum nerve conduction velocity was not significantly affected in animals exposed to ethylene oxide at the end of the 7-year recovery period. No significant effect was observed on EEG measurements. Neuropathologic examination of two monkeys per group after exposure for 2 years showed lesions indicating axonal dystrophy in the medulla oblongata, restricted to the nucleus gracilis at 50 and 100 ppm. The lesions were negative/trace or negative in the two controls, slight or severe in the two 50-ppm monkeys, and negative or slight in the two 100-ppm monkeys. Demyelination in the extreme distal portion of the fasciculus gracilis was seen in one monkey in each group; the lesion was severe at 100 ppm. Neuropathologic examination of
two monkeys per group maintained for the additional 7 years showed slight or moderate axonal dystrophy in the two monkeys in each group, including controls. This study is inconclusive because it is difficult to draw conclusions about the neuropathologic lesions based on examination of only two animals from each group. The investigators noted that one animal in the 100-ppm group showing the decline in maximum nerve conduction velocity also showed severe demyelination of the fasciculus gracilis.
3.4.
Reproductive and Developmental Toxicity
3.4.1.
Rats
Groups of 17 to 22 pregnant F344 rats were exposed to ethylene oxide (99.9) vapor at concentrations of 0 (two control groups), 10, 33, or 100 ppm (18, 59, or 180 mg/m3) for 6 h/day on gestation day (GD) 6 to 15 inclusive (Dow Chemical Co. 1982; Snellings et al. 1982a). The rats were exposed in a 4,400-L stainless steel and glass chamber, and chamber atmosphere was monitored by a gas chromatograph equipped with a flame ionization detector. The dams were killed on GD 20 for evaluation of maternal, reproductive, and developmental parameters. No effects were noted on maternal body weight gain, preimplantation loss, resorptions, or fetal deaths. The weights of male (3.1 g versus 3.3 or 3.4 g for controls) and female (2.9 g versus 3.0 or 3.1 for controls) fetuses were significantly (p < 0.05) reduced at the 100-ppm exposure; crown-to-rump length was not affected. There were no gross external or visceral malformations. Delays in vertebrae ossification occurred in a higher percentage of litters in the 100-ppm group than in either control group; the increase was not statistically significant. Variations in ossification occurred in the distal thoracic vertebral centra. Although the incidence of delayed ossification at 100 ppm did not achieve statistical significance (p = 0.10), the effect is considered treatment related, because delayed ossification is a definite effect at 125 ppm (see BRRC 1993 below). These effects suggest a mild growth retardation with no corresponding effect on maternal body weight gain. No effects occurred at 10 or 33 ppm.
In another developmental toxicity study, groups of 25 pregnant CD rats (Sprague-Dawley stock) were exposed to ethylene oxide (99.8%) vapor at concentrations of 0 (control), 50, 125, or 225 ppm (90, 225, or 405 mg/m3), 6 h/day on GD 6 to 15 inclusive (BRRC 1993). The dams were exposed in 900-L stainless steel and glass chambers, and the chamber atmosphere was analyzed with a gas chromatograph equipped with a flame ionization detector. The analytic concentrations were within 3% of target concentrations. The dams were killed on GD 21 for evaluation of maternal and developmental parameters. No treatment-related clinical signs of toxicity or maternal mortality occurred. Absolute maternal body weight and body weight gain were significantly decreased at 125 and 225 ppm. Food consumption during the exposure period also was significantly reduced at 225 ppm. Mean fetal weights were significantly reduced in
male (96%, 95%, and 90% of control weights) and female (97%, 95%, and 90% of control weights) fetuses at 50, 125, and 225 ppm, respectively. The incidences of litters with skeletal variations (primarily unossified or poorly ossified areas) in the head region, phalanges, forelimbs and hindlimbs, and sternum were increased at 125 and 225 ppm. Twelve types of variations were observed in the 225-ppm group, and three were observed at 125 ppm. No increase in the incidence of delayed ossification occurred at 50 ppm. Therefore, the minimal effect on fetal weight at 50 ppm and the lack of a statistical effect on the incidence of delayed ossification suggest that the small reduction in fetal weight approximated the threshold for growth retardation.
Saillenfait et al. (1996) conducted a developmental toxicity study with pregnant Sprague-Dawley rats exposed to ethylene oxide on GD 6 to 15 inclusive. Exposure conditions were 0, 400, 800, or 1,200 ppm for 0.5 h once a day and 0, 200, or 400 ppm or 0, 800, or 1,200 ppm for 0.5 h three times a day. The animals were exposed in a 200-L chamber, and the chamber atmosphere was monitored by gas liquid chromatography. Analytic concentrations were within 17% of target concentrations. The animals were observed daily, and body weights were recorded on GD 6, 11, 16, and 21. All surviving dams were killed on GD 21 for evaluation of maternal and developmental parameters. No treatment-related clinical signs of toxicity were observed in any group exposed to any concentration once daily; however, one dam exposed to 1 × 800 ppm died due to causes unrelated to exposure. Maternal weight gain was not significantly affected in groups exposed to any concentration once a day. Maternal body weight gain in the 3 × 1,200-ppm group was 26% (p < 0.01) of the control value from GD 6 to 11, 32% (p < 0.01) of the control value from GD 11 to 16, and 30% of the control value from GD 6 to 15. Absolute maternal weight gain (less gravid uterine weight) in the 3 × 1,200-ppm group declined to 41% (p < 0.01) of control weight, and it declined to 82% (p > 0.05) of control weight in the 3 × 800-ppm group. Body weights of male and female fetuses in the 3 × 1,200-ppm and the 3 × 800-ppm groups significantly (p < 0.01) decreased to 90% and 93% to 94% of the control value, respectively, compared with control weights. Mean fetal weight at 3 × 200 ppm, but not at 3 × 400 ppm, also decreased significantly compared with its control because of the large size of the control fetuses. This study used three control groups, and the mean live litter size of the control group for the 3 × 200-ppm and 3 × 400-ppm exposure groups was small, resulting in larger fetuses compared with the other control groups. This study either showed no fetal weight changes or did not show a clear concentration-response relationship except for the 3 × 800- and the 3 × 1,200-ppm groups.
The Saillenfait et al. (1996) study also showed a significant increase in the incidences of litters with dilated renal pelvis (13/18 versus 4/18 for controls, p < 0.01) and dilated ureter (14/18 versus 7/18 for controls, p < 0.01) in the group exposed to 1 × 1,200 ppm. Considering the wide variations that can occur in renal development (Woo and Hoar 1972), it is doubtful that the variations in the renal pelvis and ureter are of biological significance.
Hackett et al. (1982) (also reported by Hardin et al. 1983) reported on a study in female Sprague-Dawley CD rats exposed to filtered air (control) or 99.7% ethylene oxide vapor at 150 ppm (270 mg/m3) for 7 h/day, 5 days/week for 3 weeks before mating. The rats were exposed in a 2,350-L stainless steel chamber; ethylene oxide concentration in the exposure chamber was monitored by gas chromatography. After the 3-week premating period, rats exposed to filtered air were subdivided: group 1 was exposed to filtered air throughout gestation, group 2 was exposed to filtered air from GD 1 to 6 and to ethylene oxide from GD 7 to 16, and group 3 was exposed to ethylene oxide from GD 1 to 16. This study is summarized in Table 2-16. The rats exposed to ethylene oxide for 3 weeks continued on the same treatment throughout gestation (group 4). There were 41 pregnant rats per group except for group 4, which had 39 rats. The mean body weight of female rats exposed to ethylene oxide vapor before mating and throughout gestation (group 4) was significantly lower (4% to 6%) than that of controls (group 1) during the latter part of the premating period and throughout gestation. The investigators reported no other treatment-related or biologically significant maternal effects. No statistical differences occurred in the pregnancy rate or in the number of live fetuses per litter compared with the control. Fetal parameters showing treatment-related effects included decreases in male and female fetal weights and increases in the incidence of litters with reduced ossification of the skull and sternebrae in all groups exposed for any duration during gestation compared with controls. No treatment-related malformations were observed. These results showed that ethylene oxide exposure produced fetal effects whether administered over a prolonged period or during organogenesis, and the effects were indicative of growth retardation.
In a one-generation reproduction study, groups of 30 male and 30 female F344 rats were exposed to ethylene oxide (99.9%) vapor at concentrations of 0 (control), 10, 33, or 100 ppm (18, 59,or 180 mg/m3) for 6 h/day for 12 weeks before mating (5 days/week), during mating (7 days/week), and during GD 0 to 19 and day 5 to 21 postpartum (7 days/week) (Snellings et al. 1982b). Two control groups were included in the study. The animals were exposed in a 4,400-L stainless steel and glass chamber and ethylene oxide concentrations were monitored with a gas chromatograph equipped with a flame ionization detector. The survival rates and fertility indices of male and female rats were not affected by exposure to ethylene oxide. The length of gestation increased in 7/14 dams at 100 ppm compared with controls. In addition, the median number of pups born (4 versus 10 for controls), median number of implantation sites (6 versus 10 for controls), and median number of fetuses born per implantation site (0.57 versus 0.92 to 1.0 for controls) were significantly lower in dams at 100 ppm. No effect was observed on pup survival. At 100 ppm, mean F1 pup weight on day 4 post-partum was significantly greater than that of controls, and F1 male pup weight in the 33-ppm group was significantly less than that of the control groups on day 21 postpartum. The mixed results suggest no treatment-related effect on pup weight in this study.
TABLE 2-16 Maternal, Reproductive, and Developmental Effects of Exposure to Ethylene Oxide Vapor by Inhalation in Rats
|
Parameter |
Exposure Groupa |
|||
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
|
|
Maternal Body Weight (g) |
|
|
|
|
|
Day 21, premating |
278b |
277 |
280 |
267* |
|
GD 6 |
298 |
298 |
293 |
279* |
|
GD 11 |
315 |
314 |
308 |
295* |
|
GD 16 |
339 |
335 |
328 |
317* |
|
GD 21 |
382 |
381 |
378 |
360* |
|
Reproductive |
|
|
|
|
|
No. live litters/no. pregnant |
41/41 |
41/41 |
41/41 |
38/39 |
|
No. implantation sites/dam |
14.7 |
14.0 |
14.8 |
14.3 |
|
No. resorptions/litter |
0.75 |
0.71 |
0.92 |
1.60* |
|
No. live fetuses/litter |
13.9 |
13.5 |
13.8 |
12.7 |
|
Fetal Parameters |
|
|
|
|
|
Weight of female (g) |
3.56 |
3.35* |
3.23* |
3.12* |
|
Weight of male (g) |
3.73 |
3.53* |
3.47* |
3.34* |
|
Crown-to-rump length (mm), female |
36.1 |
35.3* |
34.7* |
34.8* |
|
Crown-to-rump length (mm), male |
36.5 |
36.1* |
35.8* |
35.6* |
|
Morphologic alterationsc |
|
|
|
|
|
Reduced ossification, skull |
3/2 (4.9) |
16/9 (22.0)* |
10/9 (22.0)* |
14/10 (26.3)* |
|
Reduced ossification, sternebrae |
69/23 (56.1) |
145/36 (87.8)* |
159/36 (87.8)* |
155/33 (85.8)* |
|
aGroup 1, unexposed during gestation; group 2, exposed GD 7-16; group 3, exposed GD 1-16; group 4, exposed from premating through GD 1-16. bMean values except when presented as incidence. cNumber of fetuses per number of litters; numbers in parentheses are percentage of affected litters relative to controls. *p ≤ 0.05, compared with controls. Source: Hackett et al. 1982. |
||||
Mori et al. (1991) studied the effects of inhalation exposure to ethylene oxide on spermatogenesis in Wistar rats. Groups of six male rats were exposed to ethylene oxide vapor at concentrations of 50, 100, or 250 ppm (90, 180, or 450 mg/m3) for 6 h/day, 5 days/week for 13 weeks and killed 40 h after the last exposure. The control group consisted of 12 males rats exposed to clean air. The animals were exposed in a 0.2-m3 inhalation chamber; the chamber atmosphere was monitored with a gas chromatograph equipped with a flame ionization detector (Mori et al. 1989). No treatment-related effects were observed on mean body weight, testicular weight, testicular lactate dehydrogenase activity, or epididymal sperm count. The following treatment-related effects were observed at 250 ppm: degenerative changes in the seminiferous tubules, decrease in epididymal weight (80% or control weight), marked decrease in sperm count in the
epididymis body plus tail, and marked increase in incidence of sperm head abnormalities (immature and teratic types combined and immature types separately). When analyzed separately, the incidence of teratic types was significantly elevated in all exposure groups. No other changes were observed in groups exposed to ethylene oxide at 50 or 100 ppm.
Mori et al. (1989) conducted another study in which groups of six to eight male Wistar rats were exposed to clean air or ethylene vapor at 500 ppm (900 mg/m3) for 6 h/day, 3 days/week for 2, 4, 6, or 13 weeks. Chamber description and the monitoring procedure were as described above. End points evaluated included body weights (controls were pair-fed), weight and histopathology of the testes and epididymides, and testicular enzyme activities. No significant effect was observed on body weight at any time during the study. The testes of exposed rats were atrophic after 13 weeks of treatment and the relative testicular weights showed corresponding decreases (82%, 59%, and 46% of control weight) after 4, 6, and 13 weeks, respectively. Relative epididymal weight decreased (86%, 71%, and 59% of control weight) at the same time points. Histopathologic examination of the testes showed degenerative changes in the seminiferous tubules manifested as mild degeneration of germ cells at 2 weeks, conspicuous degeneration at 4 weeks, exfoliation of germ cells at 6 weeks, and a marked reduction in germ cells in about 50% of seminiferous tubules, which contained only Sertoli cells, at 13 weeks. Plasma testosterone levels were not affected. Testicular glutathione reductase activity was reduced at all time points, glutathione peroxidase activity was decreased at 2 weeks and increased at 6 and 13 weeks, and glutathione-S-transferase activity was increased at 4 to 13 weeks.
3.4.2.
Mice
Generoso et al. (1987) and Rutledge and Generoso (1989) showed that exposure to ethylene oxide before mating or within 24 h of mating can have pronounced effects on mouse fetal development.
In the study to assess the effect of inhaled ethylene oxide on preovulatory oocytes, Generoso et al. (1987) exposed female mice to ethylene oxide at 0 or 1,200 ppm (2160 mg/m3) for 1.5 h/day for 4 consecutive days before mating or to 300 ppm (540 mg/m3) for 6 h/day for 10 exposures over a 14-day premating period. Chamber and analytic procedures were not described. The dams were killed on GD 17 to assess the effect on resorptions, midgestational deaths, and late fetal deaths. The number of implants per female was significantly reduced at 300 ppm but not at 1,200 ppm. However, the percentage of resorptions in both groups of females exposed before mating was significantly elevated by 10.8% (3.0% in controls) and 41.1% (6.4% in controls) at 1,200 and 300 ppm, respectively. Midgestational deaths and late fetal deaths were slightly elevated but not significantly; the induced loss of conceptuses was 15.7% at 1,200 ppm and 58.2% at 300 ppm, showing that exposure to the lower concentration for a longer time was more effective than the high concentration for a short time.
Rutledge and Generoso (1989) exposed groups of female (C3H × C57BL)F1 and (SEC × C57BL)F1 mice to ethylene oxide vapor at 0 (control) or 1,200 ppm (2,160 mg/m3) for 1.5 h beginning 1, 6, 9, or 25 h after a 30-min mating period; the mice were killed on GD 17. The following exposure times correspond to different developmental stages of the zygote: 1 h, sperm entry; 6 h, early pronuclear stage before DNA synthesis; 9 h, pronuclear DNA synthesis stage; and 25 h, early two-cell stage. Additional experimental groups were included to study the effects of ethylene oxide exposure on the preovulatory oocytes. Two additional groups of female mice were exposed similarly to 0 or 1,800 ppm (3,240 mg/m3) 6 h after mating and were killed serially on GD 11 to 15 to determine the effect on midgestational development. A marked reduction was observed in the number of live fetuses from female mice exposed to ethylene oxide vapor 1 h after mating (6 fetuses per dam versus 9.72 for controls) and 6 h after mating (1.81 fetuses per dam versus 10.11 for controls). In addition, the incidence of abnormal fetuses markedly increased when females were exposed 1 h (14.7% versus 0.2% for controls) and 6 h (39.2% versus 1.7% for controls) after mating. The predominant types of abnormalities were hydrops (different degrees of edema ranging from thick neck to a “balloon-like fetus”) and eye defects. Defects in the limbs and tail occurred in females exposed 6 h after mating. Other abnormalities included abdominal wall defect, cleft palate, exencephaly, and small size. Generoso et al. (1987) reported that the percentage of resorptions was significantly elevated at all times, but the greatest effect occurred in females exposed 6 h (52.9%) after mating; the induced loss of conceptus was 82.25%. Significant, but less severe, effects occurred when females were exposed at 9 and 25 h after mating. Analysis of the uterine content of females exposed to 1,800 ppm and killed on GD 11 to 15 showed significant increases in fetal deaths, particularly on GD 15 (late deaths). The number of defective living fetuses per dam significantly increased, whereas the number of living fetuses per dam decreased. Most dead fetuses were hydropic (Rutledge and Generoso 1989).
Ribeiro et al. (1987) evaluated the effect of inhaling ethylene vapor at 0, 200, or 400 ppm on sperm morphology in mice. Male Swiss Webster mice were exposed 6 h/day for 5 days, and killed 1, 3, and 5 weeks after exposure. The results showed that ethylene oxide induced concentration-related increases in the incidences of abnormal spermatozoa, spermatids, and preleptotene spermatogonial cells compared with the incidences in controls. The increases were statistically significant at both doses.
Weller et al. (1999) conducted a developmental toxicity study in mice to ascertain whether Haber’s rule (concentration × time [C × T]) was valid for ethylene oxide toxicity. One to three female C57BL/6J mice were mated with one male of the same strain, and the day a vaginal plug was found was designated as GD 0. The female mice were assigned randomly to groups based on weight gain between GD 0 and GD 4 or 5. On GD 7, groups of female mice were exposed by whole-body inhalation to ethylene oxide vapor at 0, 2,100, or 2,700 ppm-h in a dynamic chamber (45 L/min) equipped for continuous monitoring of ethylene
oxide. Individual concentrations ranged from 350 to 1,800 ppm and exposure times ranged from 1.5 to 6 h (see Table 2-17 for individual exposure conditions). The analytic concentrations were within 5% of the target concentrations. Maternal toxicity was assessed based on survival, clinical signs, and body weight gain. The dams were killed on GD 18, the uteri were removed, and developmental toxicity was assessed based on number of resorptions, viability of fetuses, fetal weight, crown-to-rump length, external and internal abnormalities, and skeletal malformations.
The results are summarized in Table 2-17. Maternal deaths attributed to ethylene oxide exposure occurred at 2,700 ppm-h at concentrations of 1,350 ppm and above and at 2,100 ppm-h at a concentration of 1,400 ppm; maternal weight loss occurred at 2,100 and 2,700 ppm-h, with the higher concentrations being more effective than the lower concentrations. The clinical signs assessed included fur appearance, movement, arousal, eyes (crusty or normal), and breathing (labored or normal). One or more of the clinical signs were observed 30 min after exposure to 2,100 and 2,700 ppm-h, with the incidence showing an increasing trend with increasing exposure concentration at 2,100 ppm-h, and the incidence was 95% or greater at 2,700 ppm-h. The overall incidence of clinical signs at 24 h was lower than that observed 30 min after exposure and showed an increasing trend with exposure concentrations at 2,100 and 2,700 ppm-h. Developmental toxicity was exhibited by increased resorptions, decreased fetal body weight, decreased crown-to-rump length, and increased litter incidences of eye defects after exposure to ethylene oxide. The percentage resorptions at 2,100 ppm-h and reduction in fetal weight and crown-to-rump length at 2,100 and 2,700 ppm-h showed increasing severity with exposure concentration. The number of litters at 1,350 ppm and above was too small to assess an exposure-related trend for eye defects. Nevertheless, the data showed that the litter incidence was greater in groups exposed to 700 and 900 ppm for 3 h than in groups exposed to 350 and 450 ppm for 6 h, which was greater than the total incidence in controls. No treatment-related skeletal defects were observed in fetuses from dams exposed to ethylene oxide. This study showed developmental effects at the lowest concentration tested (Weller et al. 1999).
3.4.3.
Rabbits
Groups of 30 female New Zealand White rabbits were artificially inseminated and exposed to ethylene oxide vapor (99.7% purity) at 150 ppm for 7 h/day from GD 1 to 19 or GD 7 to 19 (Hackett et al. 1982). The rabbits were exposed in a 2,350-L stainless steel chamber; ethylene oxide concentration in the exposure chamber was monitored by gas chromatography. Another group of 30 inseminated rabbits was exposed to filtered air throughout the study. All rabbits were killed on GD 30. No statistically significant effects were observed on mean food consumption, mean body weight, organ weights, histopathologic lesions, or maternal, reproductive, or developmental parameters.
TABLE 2-17 Developmental Toxicity in C57BL6 Mice Exposed Whole Body to Ethylene Oxide on GD 7
|
Concentration (ppm) × Time (h) |
Exposed (sperm +) |
Maternal Effects |
Developmental Effects |
||||||||||
|
Number Deaths (%) |
Weight Lost (%) |
% with Clinical Signs |
Number with Fetuses (%) |
Number Implants |
Number Resorptions (%) |
Number Dead Fetuses (%) |
Fetal Weight (g) |
C-R Length (mm) |
Number Offspring (litters) |
Eye Defects (Offspring/ Litters)a |
|||
|
30 min |
24 h |
||||||||||||
|
0 × 1.5 |
50 |
0 |
1.2 |
2.3 |
0 |
28 |
203 |
28 (13.8) |
0 |
0.92 |
19.22 |
175 (28) |
13 (6) |
|
0 × 1.75 |
8 |
0 |
0.7 |
12.5 |
12.5 |
6 |
50 |
3 (6.0) |
0 |
0.97 |
20.03 |
47 (6) |
5 (3) |
|
0 × 2 |
28 |
1 (3.6) |
0.3 |
0 |
0 |
14 |
95 |
11 (11.6) |
1 (1.1) |
0.99 |
20.70 |
83 (14) |
4 (3) |
|
0 × 3 |
38 |
0 |
3.4 |
2.6 |
0 |
19 |
141 |
15 (10.6) |
1 (0.7) |
0.93 |
19.71 |
125 (19) |
5 (4) |
|
0 × 6 |
30 |
1 (3.3) |
3.8 |
6.7 |
0 |
19 |
150 |
14 (9.3) |
0 |
0.99 |
19.52 |
136 (19) |
12 (6) |
|
Total |
154 |
2 (1.3) |
1.9 |
4.8 |
2.5 |
86 (55.8%) |
639 |
71 (11.1) |
2 (2.1) |
0.96 |
19.84 |
566 (86) |
39 (22) |
|
C × t = 2,100 ppm-h |
|||||||||||||
|
1,400 × 1.5 |
39 |
3 (7.7) |
7.2 |
100.0 |
20.7 |
8 (22.2) |
62 |
24 (38.7) |
17 (27.4) |
0.72 (75) |
16.89 (85) |
21 (8) |
7 (3) |
|
700 × 3 |
41 |
0 |
6.6 |
81.6 |
5.3 |
22 (53.7) |
168 |
27 (16.0) |
3 (1.8) |
0.88 (92) |
19.24 (97) |
139 (22) |
53 (15) |
|
350 × 6 |
33 |
0 |
4.7 |
53.1 |
3.1 |
19 (57.6) |
152 |
13 (8.6) |
1 (0.7) |
0.97 (101) |
19.90 (100) |
138 (19) |
20 (8) |
|
C × t = 2,700 ppm-h |
|||||||||||||
|
1,800 × 1.5 |
73 |
41 (56.2) |
13.0 |
100.0 |
66.2 |
3 (9.4) |
22 |
14 (63.6) |
0 |
0.70 (73) |
16.66 (84) |
8 (3) |
7 (1) |
|
1,543 × 1,.75 |
23 |
15 (65.2) |
13.5 |
95.7 |
72.2 |
1 (12.5) |
7 |
1 (14.3) |
0 |
0.76 (79) |
17.83 (90) |
6 (1) |
6 (1) |
|
1,350 × 2 |
76 |
27 (35.5) |
11.4 |
100.0 |
39.7 |
7 (14.3) |
20 |
9 (45.0) |
1 (5.0) |
0.86 (90) |
18.74 (94) |
10 (7) |
3 (2) |
|
900 × 3 |
50 |
1 (2.0) |
8.8 |
98.0 |
24.0 |
11 (22.5) |
86 |
22 (25.6) |
5 (5.8) |
0.82 (85) |
18.42 (93) |
59 (11) |
34 (9) |
|
450 × 6 |
41 |
0 (0) |
6.2 |
95.1 |
2.4 |
20 (40.1) |
|
28 (18.9) |
0 |
0.97 (101) |
19.32 (97) |
120 (20) |
13 (10) |
|
aIncludes anophthalmia and microthalmia. Abbreviation: C-R, crown-to-rump. Source: Weller et al. 1999. Reprinted with permission; copyright 1999, American Industrial Hygiene Association. |
|||||||||||||
3.5.
Carcinogenicity
Inhalation carcinogenicity studies have been conducted in mice and rats. Adkins et al. (1986) conducted a 6-month lung tumor bioassay in female A/J mice exposed to ethylene oxide at 0, 70, or 200 ppm (126 or 360 mg/m3) and showed an increased incidence of lung adenomas (Adkins et al. 1986). The number of lung tumors per tumor-bearing mouse statistically significantly increased (1.62, 1.53, and 2.47, for 0, 70, and 200 ppm, respectively) in the 200-ppm group.
A study reported by the NTP (1987) with B6C3F1 mice exposed to ethylene oxide vapor at 50 or 100 ppm for 102 weeks showed statistically significant increases in the incidences of alveolar and bronchiolar adenomas or carcinomas and Harderian gland tumors in male and female mice and increases in uterine tumors and malignant lymphomas in female mice. Snellings et al. (1984b) and Garman et al. (1985) reported on a study using groups of 120 male and 120 female F344 rats exposed to ethylene oxide concentrations of 0, 10, 33, or 100 ppm for 2 years. Ten rats per group were killed at 6 and 12 months; 20 rats per group were killed at 18 months and all survivors were killed when the study terminated. The results showed statistically increased incidences in mononuclear cell leukemia in males and females and in subcutis fibromas in males. Garman et al. (1985) specifically analyzed the brain tumors and reported increased incidences at 33 and 100 ppm and a dose-related trend for both male and female rats. The incidence of gliomas showed a statistically significant increase at 100 ppm in male rats. These data are summarized in Table 2-18. Another study with male F344 rats exposed to ethylene oxide at 50 or 100 ppm showed increased incidences of mixed cell gliomas of the brain at 50 ppm and peritoneal mesotheliomas at 100 ppm (Lynch et al. 1984a).
3.6.
Genetic Toxicity
Ethylene oxide readily alkylates DNA and other macromolecules; it has been studied extensively in numerous genetic toxicity systems using both prokaryotic and eukaryotic cells in vitro and in vivo. The results of these tests showed that ethylene oxide is genotoxic in bacteria, yeast and other fungi, Drosophila melanogaster, and rodent and human cells, causing gene mutations, gene conversions, sex-linked lethal mutations, and heritable translocations in the nonmammalian systems and unscheduled DNA synthesis, gene mutations, SCEs, chromosomal aberrations, and micronuclei in cultured mammalian cells (Golberg 1986, Dellarco et al. 1990; IARC 1994). The formation of DNA adducts with ethylene oxide in several mammalian systems shows that ethylene oxide alkylates genetic material. There is also a wealth of information showing that inhalation exposure to ethylene oxide causes genetic damage in somatic and germ cells in rodents, monkeys, and rabbits. A few of the studies are presented in this report; reviews by Golberg (1986), Dellarco et al. (1990), and IARC (1994) are sources for additional information.
TABLE 2-18 Inhalation Exposure to Ethylene Oxide: Summary of Carcinogenicity Studies
|
Animal Description |
Exposure Protocol |
Response |
Reference |
|||
|
Species/ Strain |
Sex |
Number in Group |
Tissue and Tumor Type |
Incidencea |
||
|
Mouse/A/J |
F |
30 |
0, 70, or 200 ppm, 6 h/d, 5 d/wk, for 6 mon |
Lung adenoma |
Adkins et al. 1986 |
|
|
Mouse/ B6C3F1 |
M |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Alveolar or bronchiolar adenoma or carcinoma |
11/50, 19/50, and 26/50* |
NTP 1987 |
|
|
M |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Harderian gland papillary cystadenoma |
NTP 1987 |
|
|
|
F |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Alveolar or bronchiolar adenoma or carcinoma |
2/49, 5/48, and 22/49* |
NTP 1987 |
|
|
F |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Harderian gland papillary cystadenoma |
1/46, 6/46, and 8/47* |
NTP 1987 |
|
|
F |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Uterus, adenoma or adenocarcinoma |
0/49, 4/47, and 5/49* |
NTP 1987 |
|
|
F |
50 |
0, 50, or 100 ppm, 6 h/d, 5 d/wk, for 102 wk |
Malignant lymphoma |
9/49, 6/48, and 22/49* NTP 1987 |
|
|
Rat, F344 |
M |
50 |
0, 0, 10, 33, or 100 ppm, 6 h/d, 5 d/wk, 2 y |
Spleen, mononuclear cell leukemiac |
Snellings et al. 1984b |
|
|
|
M |
100 |
0, 0, 10, 33, or 100 ppm, 6 h/d, 5 d/wk, 2 y |
Brain, gliomasd |
1/181 (both control), 0/92, 3/85, 6/87* |
Garman et al. 1985 |
|
|
M |
50 |
0, 0, 10, 33, or 100 ppm, 6 h/d, 5 d/wk, 2 y |
Skin, subcutis fibromac |
Snellings et al. 1984b |
|
|
|
F |
50 |
0, 0, 10, 33, or 100 ppm, 6 h/d, 5 d/wk, 2 y |
Spleen, mononuclear cell leukemiac |
Snellings et al. 1984b |
|
|
|
F |
100 |
0, 0, 10, 33, or 100 ppm, 6 h/d, 5 d/wk, 2 y |
Brain, gliomasd |
0/187, 1/94, 2/90, and 2/78 |
Garman et al. 1985 |
|
Rat, F344 |
M |
80 |
0, 50, or 100 ppm 7 h/d, 5 d/wk, 104 wk |
Brain, mixed cell glioma |
0/76, 2/77, and 5/79* |
Lynch et al. 1984a |
|
|
M |
80 |
0, 50, or 100 ppm 7 h/d, 5 d/wk, 104 wk |
Body cavity, peritoneal mesothelioma |
3/78, 7/79, and 21/79* |
Lynch et al. 1984a |
|
|
M |
80 |
0, 50, or 100 ppm 7 h/d, 5 d/wk, 104 wk |
Spleen, mononuclear cell leukemia |
24/77, 38/79,* and 30/76 |
Lynch et al. 1984a |
|
aTumor incidence presented in order of exposure groups as shown in “Exposure Protocol” column. bStatistically significant when compared with the combined control groups. cIncidences based on number of rats killed at 24 months. dIncidences based on number of animals at risk. *p < 0.05, test group compared with control. |
||||||
3.6.1.
Germ Cells
Table 2-19 summarizes the data on genetic toxicity in germ cells in rats and mice.
Sega et al. (1988) exposed male (C3H × B/10)F1 mice to ethylene oxide at concentrations of 450 ppm (810 mg/m3) for 4 h, 900 ppm (1620 mg/m3) for 2 h, or 1,800 ppm (3240 mg/m3) for 1 h and showed increased DNA strand breaks and unscheduled DNA synthesis as measured by incorporation of [3H]thymidine into DNA. An exposure-rate effect was observed; 1,800 ppm for 1 h was more effective than 900 ppm for 2 h, which was more effective than 450 ppm for 4 h.
Sega et al. (1991) also examined the effect of exposure rate on DNA alkylation of reproductive targets (sperm and testes) and hemoglobin. Male (C3HRl × B/10Rl)F1 mice were exposed to [3H]ethylene oxide at 75 ppm for 4 h, 150 ppm for 2 h, or 300 ppm for 1 h (300 ppm × hours); alkylation of DNA was measured 90 min and 1, 3, and 6 days after terminating exposure. This study showed that epididymal and vas sperm were alkylated by ethylene oxide; the amount of alkylation was greater in epididymal sperm than in vas sperm, suggesting a greater susceptibility in developing sperm. Alkylation of both epididymal and vas sperm increased with exposure rate. There was no suggestion of repair, as the binding level did not decrease as a function of time after exposure. Alkylation of hemoglobin also showed an exposure rate effect, with no decrease with time after exposure.
The dominant-lethal assay is one test used to screen for mutagenicity in germ cells. Embree et al. (1977) showed that dominant lethality is induced in male Long-Evans rats exposed to ethylene oxide at 1,000 ppm (1,800 mg/m3) for 4 h. The exposed males were mated with female rats each week for 10 consecutive weeks. Significant increases in postimplantation deaths were observed during the first 5 weeks of mating for ethylene oxide–exposed rats compared with controls. Postimplantation deaths are indicated by an increased number of dead implants per female (weeks 2, 3, and 5) and number of dead implants per total implants (mutagenic index) (weeks 1, 2, 3, and 5). There was a significant decrease in the fertility index (number of pregnant females per number of females mated) during weeks 3 and 4 and in the total number of implants per total number of pregnancies during week 2. Preimplantation losses were not affected. The increase in postimplantation deaths during the first 5 weeks suggests that ethylene oxide affected germ cells after meiosis.
Generoso et al. (1983) repeatedly exposed (101 × C3H)F1 male mice to 255 ppm (459 mg/m3) for 6 h/day, 5 days/week for 2 or 11 weeks and evaluated dominant lethality after mating the exposed males with (C3H ×C57BL)F1 females for 3.5 days after the last exposure. Both treatments produced marked increases in the number of dead implants (average = 37% and 50% after 2 and 11 weeks, respectively) and dominant lethality (average = 39% and 55% after 2 and 11 weeks, respectively). The effect after 11 weeks of treatment was slightly greater than that after 2 weeks.
TABLE 2-19 Genotoxic Effects of Inhaled Ethylene Oxide on Germ Cells in Male Rodents
|
Species and Strain |
Assay |
Experimental Protocol |
C × t |
Results |
Reference |
|
Rat, Long-Evans |
Dominant lethalitya |
1,000 ppm for 4 h; mated with females weekly for 10 wk |
4,000 ppm-h |
Positive: increase in dead implants per pregnancy (wk 2, 3, 5) and dead implants per total implants (wk 1, 2, 3, 5) |
Embree et al. 1977 |
|
Mouse, (C3H × B110)F1 |
DNA strand breaks and UDS |
450 ppm for 4 h, 900 ppm for 2 h, or 1,800 ppm for 1 h |
1,800 ppm-h |
Positive: DNA strand breaks and UDS; exposure-rate effect: 1,800 ppm >900 ppm >450 ppm |
Sega et al. 1988 |
|
Mouse, (C3H × B110)F1 |
DNA alkylation of sperm and hemoglobin |
75 ppm for 4 h, 150 ppm for 2 h, or 300 ppm for 1 h |
300 ppm-h |
DNA alkylation of epididymal and vas sperm and hemoglobin |
Sega et al. 1991 |
|
Mouse, (101 × C3HF1) |
Dominant lethalityb |
255 ppm, 6 h/d, 5 d/wk for 2 or 11 wk |
15,300 ppm-h or 84, 150 ppm-h |
Positive: dominant lethals produced after 2 (39%) and 11 (55%) wk |
Generoso et al. 1983 |
|
Mouse, (C3H × 101)F1 |
Dominant lethality |
Control, 300, 400, or 500 ppm, 6 h/d for 4 d |
7,200 ppm-h 9,600 ppm-h, 12,000 ppm-h |
Positive: exposure-related increase; 4%, 27%, and 62% dominant lethality |
Generoso et al. 1986 |
|
Mouse, (C3H × 101)F1 |
Dominant lethality |
Control, 300 ppm for 6 h/d, 600 ppm for 3 h/d, or 1,200 ppm for 1.5 h/d for 4 d |
1,800 ppm-h |
Positive: exposure-rate increase; 11%, 32%, and 64% dominant lethality |
Generoso et al. 1986 |
|
Mouse, (C3H × 101)F1 |
Dominant lethality |
Control, 165, 204, 250, or 300 ppm 6 h/d, 5 d/wk for 6 wk, then 7 d/wk for 2.5 wk |
47,025-85,500 ppm-h |
Positive: dose-related increase; 6%-8%, 13%-14%, 23%-24%, and 45%-60% dominant lethality |
Generoso et al. 1990 |
|
Mouse, (C3H ×101)F1 |
Heritable translocation |
Control, 165, 204, 250, or 300 ppm 6 h/d, 5 d/wk for 6 wk, then 7 d/wk for 2.5 wk |
47,025-85,500 ppm-h |
Positive: dose-related increase; 0.05%, 2.80%, 5.09%, 10.84%, and 25.53% translocation carriers in combined female strains |
Generoso et al. 1990 |
|
aDefined as number of dead implants per total implants. bDefined as average number of living embryos in experimental group per average number for controls. Abbreviation: UDS, unscheduled DNA synthesis. |
|||||
Generoso et al. (1986) also conducted dominant lethality tests with (C3H × 101)F1 male mice exposed to ethylene oxide at 300, 400, or 500 ppm (540, 720, or 900 mg/m3) for 6 h/day for 4 days; the total concentrations were 7,200, 9,600, or 12,000 ppm-h. Each exposure group was accompanied by a control. The treated animals were mated each day with a different female ([SEC × C57BL]F1) starting on the day after exposure ended and continuing for 12 days (500 ppm) or 8 days (300 and 400 ppm). The results showed that the maximum effects occurred during mating days 4.5 to 7.5 at 500 ppm; a marked decrease was observed for the number of living embryos and marked increases were observed in the number of dead implants and the number of females with one or more dead implants. Clear, but less pronounced, effects were seen at 400 ppm and only marginal effects were seen at 300 ppm. The overall dominant lethality showed a clear concentration-response relationship.
In another study, Generoso et al. (1986) examined the effect of exposure rate on dominant lethality. Male mice of the same hybrid strain were exposed to ethylene oxide at 300 ppm for 6 h/day, 600 ppm for 3 h/day, or 1,200 ppm for 1.5 h/day for 4 days (total concentration = 1,800 ppm-h/day). The exposed males were mated with (SEC ×C57BL)F1 females starting 5 days after the last exposure. Each exposure group was accompanied by a control. A clear exposure-related increase in the frequency of dominant lethality was observed.
In 1990, Generoso et al. evaluated the effect of inhaled ethylene oxide on dominant lethality and heritable translocations. Groups of (C3H × 101)F1 male mice were exposed to ethylene oxide at 165, 204, 250, or 300 ppm (297, 367, 450, or 540 mg/m3) for 6 h/day, 5 days/week, for 6 weeks followed by exposure for 7 days/week for 2.5 weeks. During the last 10 days of exposure and 1 day after the last exposure, the male mice were mated with T-stock or (SEC × C57BL)F1 females. No significant dominant lethality was seen at 165 ppm in either strain as assessed by the number of live embryos. At 204 ppm, there was a significant decrease in the number of live embryos in one strain and a significant increase in the number of females with one or more dead implants in both strains; this dose showed an overall marginal effect on dominant lethality. At 250 and 300 ppm, clear effects on dominant lethality were indicated in both strains by decreases in the number of live embryos, increases in the number of dead implants, and increases in the number of females with one or more dead implants. The frequency of dominant lethals showed a concentration-related increase that was not linear.
In the experiment on heritable translocations, Generoso et al. (1990) evaluated the frequency of semisterile and sterile male offspring and analyzed the carriers for translocations. Each female strain had a concentration-related increase in the frequency of translocation carriers; the increases achieved statistical significance (p < 0.01 compared with controls) at all concentrations. The response curves were not linear.
3.6.2.
Somatic Cells
Other genetic toxicity tests including SCE and chromosome aberration have been performed on peripheral lymphocytes and bone marrow cells in laboratory animals exposed to ethylene oxide by inhalation. Kligerman et al. (1983) compared the frequencies of SCEs in peripheral lymphocytes taken from male F344 rats exposed to ethylene oxide at target concentrations of 0, 50, 150, or 450 ppm for 6 h/day for 1 or 3 days. The frequency of SCEs per metaphase significantly increased only at 450 ppm (10.4 versus 7.8 in controls) after a single exposure, whereas the frequencies significantly increased at all concentrations (7.5, 9.1, 10.3, and 13.6 for 0, 50, 150, and 450 ppm, respectively) after three exposures. The frequency of SCEs was similar for a single exposure to 450 ppm (2,700 ppm-h) and repeated exposures to 150 ppm (2,700 ppm-h). In addition, only repeated exposures caused increases in the number of high-frequency cells with ≥20 SCEs per metaphase.
A study conducted by Ong et al. (1993) showed that SCEs are induced in spleen and bone marrow cells of male F344 rats exposed to ethylene oxide at 100 ppm for 6 h/day, 300 ppm for 2 h/day, or 600 ppm for 1 h/day, 5 days/week, for 3, 6, or 9 months. The frequency of SCEs in spleen cells did not show a clear concentration-response relationship at any time point, but a cumulative response was seen as duration of exposure increased. The frequency of SCEs in bone marrow cells was highest at the lowest concentration and there was no clear increase with duration of exposure.
The frequency of SCEs also increased in lymphocytes of New Zealand White rabbits exposed to ethylene oxide at 200 or 400 ppm, 6 h/day or 1,500 ppm for 15 min two times a day, 5 days/week up to a cumulative concentration of about 48,000 ppm-h (Yager 1987). A clear exposure-rate effect was not observed.
Vergnes and Pritts (1994) reported that male F344 rats and male B6C3F1 mice exposed to ethylene oxide at 200 ppm for 6 h/day, 5 days/week, for 4 weeks had significantly elevated frequencies of micronuclei in polychromatic erythrocytes in the bone marrow. The mean percentage of micronucleated cells was 0.79% for rats (0.30% for controls) and 0.72% for mice (0.22% for controls).
Lynch et al. (1984b) reported that the frequency of SCEs and chromosome aberrations significantly increased at both concentrations in lymphocytes of adult male cynomolgus monkeys exposed to ethylene oxide at 50 or 100 ppm for 7 h/day, 5 days/week, for 2 years. Mitotic activity of the lymphocytes was also reduced.
3.6.3.
DNA Alkylation
Ethylene oxide is a reactive epoxide that readily alkylates DNA and proteins without metabolic activation (Golberg 1986). Ehrenberg et al. (1974) de-
tected radioactive binding to the nucleic acid fraction of tissues from mice exposed to radioactive ethylene oxide at 29 ppm for 82 min and analyzed 73 min after exposure. The relative binding activity in the tissues was as follows: kidney > spleen > lung > liver > testes > brain. Ehrenberg et al. (1974) further identified 7-(2-hydroxyethyl)guanine (7-HEG) as one of the DNA adducts formed after exposure to ethylene oxide. Potter et al. (1989) exposed male F344 rats by nose-only inhalation to [14C]ethylene oxide at 1, 10, or 33 ppm for 6 h and isolated DNA from brain, lung, liver, spleen, kidney, and testes. A linear relationship was observed for the formation of 7-HEG and concentration of ethylene oxide in air. Alkylation frequencies ranged from 0.0786 to 0.118, 0.777 to 0.964, and 3.03 to 3.66 nanomoles of 7-HEG per g of DNA at concentrations of 1, 10, and 33 ppm, respectively, for all tissues except testis, which was 60% lower (0.065, 0.466, and 2.00, respectively). Bolt and Leutbecher (1993) exposed male Sprague-Dawley rats to [14C]ethylene oxide in a closed system until the ethylene oxide disappeared from the atmosphere; a linear increase was again observed for the formation of 7-HEG adducts in liver and spleen (exposure concentration in ppm was not provided). The animals were sacrificed immediately after exposure.
In a time-course study, Walker et al. (1990) exposed male F344 rats to ethylene oxide at 300 or 500 ppm (6 h/day, 5 days/weeks) for 1 or 3 days or 1, 2, or 4 (300 ppm only) weeks and measured DNA adducts in brain, kidneys, liver, lungs, spleen, and testes 1 h after cessation of exposure. DNA adducts were detected in all tissues after the first exposure, with adduct levels increasing with repeated exposure time particularly during the second week after exposure to 500 ppm and after the first exposure at 300 ppm. The highest concentrations were found in the lungs and the lowest concentrations were in testes. The disappearance of adducts after a 4-week exposure to 300 ppm was gradual and linear, with some adducts persisting 10 days after cessation of exposure. The half-life of disappearance of adducts was approximately 7 days.
Walker et al. (1992a) conducted a similar experiment in male B6C3F1 mice exposed to ethylene oxide at 100 ppm for 1 or 3 days or 1, 2, or 4 weeks (6 h/day, 5 days/week) and compared the results with those of male F344 rats exposed to 300 ppm for 4 weeks. DNA adducts were measured in lungs, kidneys, liver, spleen, testes, and brain. In control mice, 2 to 6 picomoles (pmol) of 7-HEG per mg of DNA was detected. At the early time points (not further described), formation of 7-HEG adducts was similar to that of controls, but as exposure duration increased adduct formation increased, attaining a steady-state concentration only in lungs by 4 weeks. After exposure for 4 weeks, 7-HEG adducts showed a greater persistence in rat tissues than in the mouse, except for the kidney. The half-life of adduct disappearance was 6.9 days in mouse kidney and 1 to 2.3 days in other tissues in the mouse. In this study, the half-life of disappearance of DNA adducts in rats ranged from about 2.9 to 5.8 days in all tissues. Two minor adducts (O6-(2-hydroxyethyl)guanine [O-HEG] and 3-(2-hydroxyethyl)guanine [3-HEG)]) were also detected in the rats exposed to 300 ppm for 1 to 4 weeks. Steady-state concentrations of 1.0-1.2 pmol of O-HEG per
mg of DNA was achieved by 2 weeks in brain, kidney, lung, and spleen, whereas steady state was achieved after a few days for 3-HEG adducts (1 pmol of adduct per mg of DNA). After a 4-week exposure the concentration of these adducts was 250- to 300-fold lower than the concentration of 7-HEG.
Walker et al. (1992a) conducted another study with male mice and rats exposed to ethylene oxide at 0, 3, 10, 33, or 100 ppm for 6 h/day, 5 days/week, for 4 weeks. Another group of rats was exposed similarly to 300 ppm. After a 4-week exposure to 100 ppm, formation of 7-HEG adducts was similar in all mouse tissues (21 to 38 pmol of 7-HEG per micromole [μmol] of guanine), with testes containing the lowest level. In rats exposed to 100 ppm for 4 weeks, 7-HEG formation was lower in liver, kidney, and testes (44 to 55 pmol of 7-HEG per μmol of guanine) than in other tissues (81 to 105 pmol of 7-HEG per μmol of guanine); the lowest amount was found in testes. The dose-response relationship for 7-HEG formation was nonlinear for rats and mice (lung, brain, and spleen).
3.7.
Summary
Acute lethality data are summarized in Table 2-20. Mice are the most sensitive species, followed by dogs and rats. The LC50 values for 4-h exposures ranged from 660 ppm for female mice to 1,972 ppm for male rats, and 1-h LC50 values ranged from 4,439 ppm for female rats to 5,748 ppm for male rats; a 1-h study was not available for the mouse. The lowest concentration causing death in a 4-h exposure study was 533 ppm (20% mortality) for female mice. A slightly higher concentration of 800 ppm causes 100% mortality in male mice. The lowest concentration causing death in a study of 1-h exposure of female rats was 3,966 ppm (40% mortality). Lethal concentrations of ethylene oxide vapor are irritating to the eyes and upper and lower respiratory tract. Death in all cases did not appear to involve severe respiratory tract irritation but was due to respiratory failure, probably involving the central nervous system (Golberg 1986). Lethal concentrations of ethylene oxide also cause neurologic effects manifested by absence of tail, toe pinch, and startle reflexes; ataxia; semiconsciousness; and convulsions. In addition, vomiting occurs in dogs and diarrhea occurs in rats and dogs, which also may be due to a neurologic mechanism and not to a direct effect on the gastrointestinal tract. Pathologic lesions develop in the liver, kidney, and respiratory tract of animals exposed to lethal concentrations.
Clinical signs observed in animals that survived a single exposure to ethylene oxide vapor were similar to those observed in animals that died. Eye and respiratory tract irritation and neurologic signs were the primary effects observed in animals surviving exposure to ethylene oxide. The effects were usually reversible within a few days after exposure, depending on the concentration of ethylene oxide vapor. Several studies on repeated exposures (6 h/day, 5 days/week) to ethylene oxide vapor were available; durations ranged from
TABLE 2-20 Summary of Lethality Data for Experimental Animals
|
Species and Sex |
LC50a |
Exposure Time (min) |
Comments |
Reference |
|
|
ppm |
mg/m3 |
||||
|
Rat, male |
1,460 |
2,630 |
240 |
Lowest experimental concentration causing death was 882 ppm (20%) |
Jacobson et al. 1956 |
|
Rat, male |
1,972 |
3,550 |
240 |
Lowest experimental concentration causing death was 2,026 ppm (80%); no deaths at 1,850 ppm |
Nachreiner 1991 |
|
Rat, female |
1,537 |
2,767 |
240 |
Lowest experimental concentration causing death was 1,443 ppm (20%); no deaths at 1,021 ppm |
Nachreiner 1991 |
|
Rat, male and female |
1,741 |
3,134 |
240 |
No comments |
Nachreiner 1991 |
|
Rat, male |
5,748 |
10,346 |
60 |
Lowest experimental concentration causing death was 5,546 ppm (20%); no deaths at 4,827 ppm |
Nachreiner 1992 |
|
Rat, female |
4,439 |
7,990 |
60 |
Lowest experimental concentration causing death was 3,966 ppm (40%); no deaths at 3,609 ppm |
Nachreiner 1992 |
|
Rat, male and female |
5,029 |
9,052 |
60 |
No comments |
Nachreiner 1992 |
|
Mouse, female |
835 |
1,504 |
240 |
Lowest experimental concentration causing death was 533 ppm (20%); lowest concentration tested |
Jacobson et al. 1956 |
|
Mouse, male |
ND |
ND |
240 |
LC50 was not calculated; 100% mortality at 800 ppm; no deaths at 400 ppm |
NTP 1987 |
|
Mouse, female |
660 |
1,188 |
240 |
Lowest experimental concentration causing death was 800 ppm (80%); no deaths at 400 ppm |
NTP 1987 |
|
Dog, male |
960 |
1,730 |
240 |
No deaths occurred at 710 pm |
Jacobson et al. 1956 |
|
Guinea pig |
ND |
ND |
480 |
1,300 ppm caused death |
Waite et al. 1930 |
|
Guinea pig |
ND |
ND |
330 |
3,000 ppm caused death |
Waite et al. 1930 |
|
Guinea pig |
ND |
ND |
190 |
3,000 ppm caused death |
Waite et al. 1930 |
|
Guinea pig |
ND |
ND |
150 |
7,000 ppm caused death |
Waite et al. 1930 |
|
Guinea pig |
ND |
ND |
60 |
25,000 ppm caused death |
Waite et al. 1930 |
|
Guinea pig |
ND |
ND |
10 |
63,000 ppm caused death |
Waite et al. 1930 |
|
aLC50 (concentration with 50% lethality) or percentage mortality at the lowest experimental concentration causing death. Abbreviation: ND, not determined. |
|||||
6 weeks with dogs, to 10 to 14 weeks with rats and mice, to 24 months with monkeys. Clinical signs observed after repeated exposures were similar to those observed after a single exposure. However, respiratory tract irritation progressed to secondary effects; neurologic effects progressed to hindlimb weakness, muscle atrophy, and paralysis, depending on the concentration. Growth retardation, mild anemia, and pathologic lesions in adrenal gland, thymus, nasal cavity, kidney, and spleen occurred after repeated exposures to ethylene oxide vapor. Neurologic effects, including hindlimb paralysis, were reversible and resolved several months after exposure was terminated. Neurotoxicity studies showed effects related to locomotion, arousal, approach response, and closed eyes after a single exposure and grip strength after repeated exposure for 4 or 14 weeks. Neuropathologic effects included axonal degeneration of myelinated fibers in the fasciculus gracilis and hindlimb nerves in rats after exposure for 13 weeks and axonal dystrophy in the medulla oblongata and demyelination of the fasciculus gracilis in monkeys after exposure for 24 months.
Developmental and reproductive toxicity studies using rats, mice, and rabbits exposed to ethylene oxide are summarized in Table 2-21. There are some inconsistencies in the developmental toxicity studies. The studies by Snellings et al. (1982a) and BRRC (1993) showed developmental effects at ≥50 ppm in rats exposed for 6 h/day (300 ppm-h), whereas the study by Saillenfait et al. (1996) showed developmental effects only at ≥800 ppm in rats exposed for 0.5 h three times per day (1,200 ppm-h). The difference is not due to strain sensitivity, because the BRRC (1993) and Saillenfait et al. (1996) studies used Sprague-Dawley rats. No developmental effects were observed in rabbits exposed to 150 ppm. Reproductive toxicity studies showed effects at ≥50 ppm in rats and ≥200 ppm in mice after repeated exposures. The study by Weller et al. (1999) in mice, which is summarized in Table 2-17, showed developmental effects manifested as resorptions, growth retardation, and eye defects after a single inhalation exposure to ethylene oxide at ≥350 ppm.
Ethylene oxide is a direct alkylating agent that is genotoxic in numerous in vitro and in vivo test systems. Ethylene oxide vapor is genotoxic in mammalian germ cells as evidenced by induction of dominant lethality, heritable translocations, DNA strand breaks, and unscheduled DNA synthesis (see Table 2-19). It is genotoxic in somatic cells as indicated by induction of SCEs, chromosome aberrations, or micronuclei in peripheral lymphocytes, spleen cells, or bone marrow cells. In addition to its genotoxic activity in somatic cells, ethylene oxide is carcinogenic in mice and rats. Positive results have been obtained with the mouse lung tumor bioassay (70 ppm) and the standard 2-year bioassays in mice and rats at ≥100 ppm. The carcinogenicity results are summarized in Table 2-18. IARC (1994) concluded that the animal data provided sufficient evidence of carcinogenicity of ethylene oxide.
TABLE 2-21 Developmental and Reproductive Effects of Ethylene Oxide Vapor
|
Species |
Exposure |
Effect |
Reference |
|
Rat |
0, 10, 33, 100 ppm, 6 h/d, GD 6-15 |
33 ppm, NOEL 100 ppm, mild growth retardation of fetus |
Snellings et al. 1982a |
|
Rat |
0, 50, 125, 250 ppm, 6 h/d, GD 6-15 |
50 ppm, slight fetal growth retardation 125 ppm, maternal effects and fetal growth retardation 250 ppm, more severe maternal effects and fetal growth retardation |
BRRC 1993 |
|
Rat |
0, 150 ppm, 7 h/d, 5 d/wk, premating, GD 7-16 or 1-16 |
Growth retardation of fetus regardless of stage of exposure |
Hackett et al. 1982 |
|
Rat |
0, 400, 800, 1,200 ppm, 0.5 h/d, GD 6-15 |
No effects on fetus at any concentration |
Saillenfait et al. 1996 |
|
Rat |
0, 200, 400, 800, 1,200 ppm, 0.5 h, 3 times per day, GD 6-15 |
800 ppm, fetal growth retardation 1,200 ppm, maternal effects and fetal growth retardation |
Saillenfait et al. 1996 |
|
Mouse |
0, 1,200 ppm, 12 h, GD 1 |
Fetal deaths, hydrops, and other malformations |
Rutledge and Generoso 1989 |
|
Mouse |
0, 200, 400 ppm, 6 h/d, 5, 15, or 25 exposures |
200 ppm, abnormal spermatozoa 400 ppm, abnormal spermatozoa |
Ribeiro et al. 1987 |
|
Rat |
0, 10, 33, 100 ppm, 6 h/d, one-generation reproduction |
33 ppm, NOEL 100 ppm, reproductive and fetal effects |
Snellings et al. 1982b |
|
Rat, males |
0, 50, 100, 250 ppm, 6 h/d, subchronic |
50 ppm, abnormal sperm, teratic type 100 ppm, abnormal sperm, teratic type 250 ppm, abnormal sperm, testicular degeneration |
Mori et al. 1991 |
|
Rabbits |
0, 150 ppm, 7 h/d, GD 7-19 or 1-19 |
No developmental effects |
Hackett et al. 1982 |
|
Abbreviation: NOEL, no-observed-effect level; GD, gestation day. |
|||
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism, Disposition, and Kinetics
Ethylene oxide is metabolized or biotransformed primarily by two pathways: hydrolysis and glutathione conjugation. According to Golberg (1986), ethylene oxide is not a substrate for epoxide hydrolase but forms ethylene glycol via nonenzymatic hydrolysis and oxalic acid after several additional steps. Martis et al. (1982) showed that ethylene oxide, administered to dogs by intravenous (i.v.) injection, is rapidly hydrolyzed to ethylene glycol. Tardif et al. (1987) identified the following metabolites in the urine of mice administered ethylene oxide by i.v. injection (20 or 60 mg/kg) or after inhalation exposure (200 ppm for 6 h): 2-hydroxyethylmercapturic acid (HMA), S-(2-hydroxyethyl)-L-cysteine, S-carboxymethyl-L-cysteine, and ethylene glycol. Only HMA and ethylene glycol were identified in rat urine and ethylene glycol was found in rabbit urine. The metabolites were qualitatively similar for both routes of exposure for each species but were quantitatively different. The proportion of the total dose excreted was small, with the rat excreting the larger portion followed by the mouse and rabbit, which excreted only 2% to 3% of the i.v. or inhalation dose. Tardif et al. (1987) examined the urine for specific metabolites and may not have identified all metabolites, particularly those of ethylene glycol.
Brown et al. (1996) found no difference in the in vitro production of ethylene glycol by heat-inactivated and active mouse liver cytosol, suggesting that production of ethylene glycol was not due to cytosolic epoxide hydrolase activity. Brown et al. (1996) also noted that the high rate of nonenzymatic hydrolysis relative to enzymatic hydrolysis in rats made it impossible to accurately determine the Michaelis constant for enzymatic hydrolysis. Brown et al. (1996) also compared microsomal and cytosolic ethylene oxide metabolizing activity in liver and kidney in rats and mice and found most of the activity in the cytosolic fraction. Therefore, microsomal metabolism, including microsomal epoxide hydrolase activity, would contribute little to ethylene oxide metabolism.
Ethylene oxide reacts with chloride ions to form 2-chloroethanol followed by glutathione conjugation (glutathione S-transferase) and the formation of S-(2-hydroxyethyl)-L-cysteine (Golberg 1986; Fennell 1996). Metabolism of ethylene oxide via the glutathione-S-transferase pathway is saturable because of the depletion of glutathione. McKelvy and Zemaitis (1986) demonstrated that glutathione in various tissues in the rat and mouse was depleted after 4-h exposures to 1,200 and 900 ppm, respectively. Glutathione was depleted by as much as 82% to 85% in the liver of both species. Glutathione levels returned to control levels within 24 h after exposure in all tissues except bone marrow and testes in the rat and bone marrow, testes, and lungs in the mouse. Physiologically-based pharmacokinetic (PBPK) model simulation of rats exposed to ethylene oxide at 100, 600, and 1,200 ppm for 4 h estimated glutathione depletion to be 10% to 15% in the liver, 60% to 70% in the lungs, and 60% to 80% in the testes (Krishnan et al. 1992). PBPK modeling of mice exposed to ethylene oxide for 4 h es-
timated glutathione depletion to be about 27%, 51%, 79%, and 83% in the liver and 45%, 62%, 78%, and 79% in the lungs at 100, 200, 300, and 400 ppm, respectively. Glutathione was depleted by 41% and 63% in kidney and by 19% and 15% in testes at 300 and 400 ppm, respectively (Brown et al. 1998). In 1996, Fennell reported that PBPK models indicated that glutathione conjugation accounted for about 10% of ethylene oxide metabolism in humans, 50% in rats, and 75% in mice, with most of the remaining ethylene oxide undergoing nonenzymatic hydrolysis. Fennell and Brown (2001) estimated the relative contributions of conjugation, hydrolysis, and exhalation of unchanged ethylene oxide at concentrations up to 600 ppm and showed that at ≤200 ppm about 80% of ethylene oxide is metabolized by glutathione conjugation and <60% is metabolized at concentrations up to 600 ppm in mice. In rats, about 60% of ethylene oxide at concentrations ≤ 400 ppm is metabolized by glutathione conjugation and a slightly lower percentage is metabolized at 600 ppm. In humans, only about 20% of ethylene oxide is metabolized by glutathione conjugation at concentrations ≤ 600 ppm. The remaining ethylene oxide undergoes hydrolysis or is exhaled unchanged. Hydrolysis accounted for about 10% and 20% of ethylene oxide biotransformation in mice exposed to concentrations up to 200 and 600 ppm, respectively, 30% to 35% in rats, and 60% in humans.
Brugnone et al. (1986) studied workers exposed to ethylene oxide at 0.2 to 22.5 mg/m3 (0.11 to 12.3 ppm) and reported that, at steady state, 75% to 80% of inhaled ethylene oxide is absorbed into the body. The concentration of ethylene oxide in alveolar air ranged from 0.05 to 7 mg/m3 (0.03 to 3.8 ppm). The venous blood:alveolar air coefficients ranged from 12 to 17 and the venous blood:environmental air coefficients ranged from 2.5 to 3.3. Brugnone et al. (1986) calculated a mean absorption of 7.2 to 7.7 mg of ethylene oxide for an 8-h exposure to 2 mg/m3 (1.11 ppm) at an alveolar ventilation rate of 10 L/min. Filser et al. (1992) used the data presented by Brugnone et al. (1986) to calculate a mean half-life of 42 min for ethylene oxide in humans.
Maples and Dahl (1993) reported that blood uptake gradually increased during the first 15 min and reached a plateau at about 60 ng per g of blood in male F344 rats exposed to ethylene oxide vapor at 5 ppm for 60 min. Blood concentrations in mice after inhalation exposure to 50, 100, 200, 300, and 400 ppm for 4 h were relatively constant during exposure to ≤200 ppm but continued to rise during exposure to concentrations exceeding 200 ppm (Brown et al. 1998). The terminal blood concentrations were linear up to 200 ppm but showed a definite deviation from linearity above 200 ppm. The data on glutathione depletion explain the nonlinear increase in ethylene oxide concentration in the blood of mice exposed to >200 ppm. On the basis of PBPK modeling, Fennell and Brown (2001) estimated that ethylene oxide concentrations in the blood of mice, rats, and humans would be similar up to 100 ppm, followed by a steep increase in mice at concentrations above 200 ppm and a continued linear increase in humans and rats. Ehrenberg et al. (1974) calculated a biological half-life (t2) of 9 min for male CBA mice exposed to [1,2-3H]ethylene oxide at concentrations ranging from 1.15 to 33 ppm (average concentrations) for 60 to 107
min. These investigators inferred that 2.5 μmol/kg (approximately equal to 2.5 μmol/L) is absorbed after an exposure of 1 ppm-h. Ehrenberg et al. (1974) concluded that the degree of protein alkylation could be used to monitor the tissue dose of alkylating agent. Martis et al. (1982) reported a mean half-life of about 33 min for a dog given ethylene oxide by i.v. injection. They also noted that elimination kinetics was not dose dependent in dogs.
Brown et al. (1996) reported t2 for ethylene oxide clearance from the blood as 13.8 ± 3.0 min for male rats and 10.8 ± 2.4 min for female rats exposed to 100 ppm; similar values were obtained for animals exposed to 330 ppm. For mice, the t2 was 3.12 ± 0.2 and 5.4 ± 0.5 min for male mice and 2.4 ± 0.2 and 5.6 ± 0.2 min for female mice exposed to 100 and 330 ppm, respectively. The authors noted that the increase in t2 in mice exposed to 330 ppm was due to saturation of metabolism in mice probably because of glutathione depletion. They measured ethylene oxide concentrations in blood, muscle, brain, and testes 2 to 10 min after a 4-h exposure. Peak tissue concentrations were similar for all tissues except testes, which were 50% and 20% lower in the mouse and rat, respectively, than in other tissues. Ethylene oxide concentrations were slightly higher in the tissues of the rat (except for testes) exposed to 100 ppm than in mice, whereas at 330 ppm, the concentrations were slightly higher in mice than in rats. The authors also noted that clearance of ethylene oxide from the tissues was similar to that from blood.
In the PBPK model developed by Csanady et al. (2000), systemic uptake of ethylene oxide in blood was predicted to be 58% in rats and 79% in humans, with half-lives of about 19 min and 1 h, respectively. This model also predicted that 92% of ethylene oxide in humans is metabolized and about 8% is exhaled unchanged.
Simulation of tissue distribution showed that uptake into and elimination from tissues (peak ethylene oxide concentration) and blood, except for testes, is similar within each species of mice and rats exposed to 100 or 330 ppm for 4 h (Brown et al. 1996). This study demonstrated that blood concentrations are representative of the concentration of ethylene oxide in other tissues. Fennell and Brown (2001) simulated blood concentration, area under the curve (AUC), and dose in mg/kg by PBPK modeling. The model used pulmonary uptake in mouse obtained from nose-only exposures (40%) and in rats obtained from nose-only (43%) and unrestrained exposures (60%). Pulmonary uptake in humans was set at 78%. The model output underestimated the parameters for restrained and unrestrained rats; the results presented below are for unrestrained rats compared with humans and restrained rats compared with mice. After 6-h exposures to 100 ppm, the model shows that the peak blood concentration was 10% less, the AUC was 14% less, and the dose in mg/kg was 70% less in humans than in rats. The model showed that the peak blood concentration and AUC were almost identical, but the dose in mg/kg is about 70% less in restrained rats than in restrained mice. The AUC and peak blood concentration in restrained rats were below the levels in unrestrained rats. Of the three parameters modeled, dose in mg/kg ap-
peared to best represent the species differences for mice, rats, and humans. The ratio of the dose in mg/kg/day was 8:2:1 for mice:rats:humans. Therefore, the PBPK model showed that (based on pharmacokinetics) humans are not likely to be more sensitive than rodents at concentrations up to 100 ppm and that humans are pharmacokinetically more like rats than mice. Fennell and Brown (2001) did not model concentrations above 100 ppm to compare concentrations that saturate the glutathione-S-transferase pathway in mice. Under saturating concentrations, the difference between rats and humans compared with mice is expected to be greater.
Osterman-Golkar et al. (1976) demonstrated that alkylation of hemoglobin in mice could be used as a measure of exposure to ethylene oxide. Potter et al. (1989) reported that hemoglobin adduct formation showed a linear trend and no evidence of saturation in male F344 rats exposed to 0, 3, 10, or 33 ppm for 6 h. Adduct formation measured as nmol of N1-(2-N-hydroxyethyl)histidine per g of globin was 0.136, 1.03, and 4.64 for 3, 10, and 33 ppm, respectively. Walker et al. (1992b) also showed that hemoglobin adduct formation exhibited a linear trend in mice and rats exposed to ethylene oxide at 3 to 33 ppm (6 h/day, 5 days/week, for 4 weeks) but was nonlinear over the exposure ranges 3 to 100 ppm for mice and 3 to 300 ppm for rats.
Mayer et al. (1991) reported that ethylene oxide concentration in the workplace was significantly associated with N-hydroxyethylvaline (HEV) hemoglobin adducts after adjusting for smoking. In a review of the literature, Kolman et al. (2002) noted that two investigators reported similar levels of HEV hemoglobin adduct formation in individuals when occupational exposure to ethylene oxide was standardized to 1 ppm. Hemoglobin adduct formation also correlated with the smoking status of individuals in the population exposed to ethylene oxide via conversion of ethylene found in cigarette smoke (Kolman et al. 2002). Because ethylene oxide is metabolized by glutathione conjugation, the level of HEV adducts also was correlated with the polymorphic genotype, with higher levels found in homozygous nonconjugators compared with heterozygous or homozygous conjugators. Fennell et al. (2000) studied the level of HEV adducts in smokers and nonsmokers and noted that the GSTT1 genotype but not the GSTM1 genotype had an impact on the HEV adduct levels. Fennell et al. (2000) concluded that HEV adducts (a measure in internal dose levels) were increased by 50% to 70% in smokers who had the GSTT1-null genotype (non-conjugators). Yong et al. (2001) reported that workers with the GSTT1-null genotype (nonconjugators) exposed to ethylene oxide had significantly higher (2-fold) HEV levels than conjugators. Ehrenberg and Tornqvist (1995) concluded that the blood dose in humans would be about the same as that in the rodents. They noted uncertainty in their estimate because of the uncertainty in determining time-weighted occupational exposures for humans. Their estimates of HEV adduct formation per 1 ppm-h were12, 16, and 12 pmol/g/ppm-h for humans, rats, and mice, respectively.
Tavares et al. (1994) studied HEV hemoglobin adducts in blood samples taken from smoking and nonsmoking mothers and their newborn infants (<48 h
old). Cigarette smoke contains ethylene and ethylene oxide, with ethylene occurring in much higher levels than ethylene oxide. The investigators found that the level of hemoglobin adducts in newborns of smokers was significantly higher than in those of nonsmokers, and a linear relationship was found for maternal and newborn HEV levels. The newborn adduct levels, however, were lower than the maternal levels, with the ratio of maternal:newborn ranging from 1.1 to 3.1 (mean = 1.8) for nonsmokers and 1.4 to 4.1 (mean 2.7) for smokers.
Farmer et al. (1996) studied HEV hemoglobin adducts in maternal blood from smokers and nonsmokers along with the adduct levels in umbilical cord (fetal) blood. They found a linear relation between the maternal and fetal HEV hemoglobin adduct levels, with higher levels found in maternal blood at a ratio of 2.7 to 2.8. This study also showed a linear correlation between maternal and newborn HEV adduct levels. The GSTM1 genotype has no statistically significant influence on the adduct levels in smokers or nonsmokers. Both studies showed a linear correlation between maternal and fetal and newborn HEV hemoglobin adducts. The differences between maternal and newborn levels may have been due to differences in the makeup of the polypeptide chains of the maternal and fetal and newborn hemoglobin.
Mayer et al. (1991) noted that the hemoglobin adduct level correlated with the frequency of SCEs in peripheral lymphocytes. Yong et al. (2001) found a significant association between ethylene oxide exposure and frequency of SCEs after adjusting for smoking and other confounders. The frequency of SCEs was unexpectedly lower in nonconjugators (GSTM1-null genotype) than in conjugators (GSTM1 genotype) (Yong et al. 2001). The authors noted that the decreased SCEs may have been due to nonchemical specificity of the end point and the lack of expression of the isozyme in lymphocytes.
In summary, evaluation of the uptake, metabolism, and excretion of ethylene oxide showed qualitative similarities among species (humans, rats, and mice). Uptake of ethylene oxide after inhalation is proportionally similar among species. The database showed that humans are more like rats than mice with regard to metabolism (detoxification) of ethylene oxide. Therefore, the rat is a better surrogate than the mouse for AEGL development. HEV adducts in hemoglobin can be used as a measure of exposure and the level of adduct formation correlates with glutathione-S-transferase polymorphism; nonconjugators have higher HEV adduct levels than conjugators. These results showed that genotypic diversity of glutathione S-transferase can modulate the detoxification of ethylene oxide and should be considered in AEGL development.
4.2.
Mechanism of Toxicity
Ethylene oxide is a direct-acting alkylating agent; it alkylates DNA and proteins. Ethylene oxide is also a mild primary irritant and a central nervous system depressant.
Finelli et al. (1983) noted that distal axonal neuropathy is characterized by primary axonal degeneration with secondary demyelination affecting distal segments of long tract fibers without involving the neuronal bodies. They postulated that ethylene oxide affects the peripheral and central nervous systems by interfering with metabolism of neuronal perikaryon or axonal transport, thus inhibiting delivery of essential metabolites to nerve terminals. Deschamps et al. (1992) noted that workers exposed to ethylene oxide for 4 h/day for 4 days showed signs of proprioceptive axonal neuropathy. Ohnishi et al. (1985) noted axonal degeneration of myelinated nerve fibers in rats after subchronic inhalation exposure to ethylene oxide. Because distal axonal neuropathy (peripheral neuropathy) has been diagnosed in humans exposed to ethylene oxide (Finelli et al. 1983), the mechanism in humans and rats may be similar.
Deschamps et al. (1992) proposed that the alkylating properties of ethylene oxide played a role in respiratory tract irritation. The time of onset of symptoms indicated that direct irritation or caustic injury was not the mechanism causing respiratory tract irritation.
The mechanisms by which ethylene oxide induces developmental and testicular toxicity (not including genetic damage to germ cells) are not known. Protein alkylation may be involved—that is, alkylation of enzymes in testicular toxicity (Mori et al. 1989). It is likely that protein and DNA alkylation also are involved in inducing developmental toxicity.
Genetic toxicity of ethylene oxide is probably mediated by alkylation of DNA or proteins, which can alter the structure and functional activities of genes, chromosomes, and protein. Genetic toxicity indicated by increased frequencies of SCEs, chromosome aberrations, and micronuclei is probably caused by DNA alkylation. Carcinogenicity is probably mediated by genetic toxicity resulting from DNA alkylation.
Generoso et al. (1986) suggested that dominant lethality involves alkylation of chromosomes. In another study, heritable translocations were confirmed by cytogenetic analysis of the offspring, thus showing structural alterations in chromosomes that could be due to DNA alkylation. Although the mechanism by which ethylene oxide induced effects in the zygote leading to fetal deaths, resorptions, and structural fetal defects is not known, Generoso et al. (1987) and Katoh et al. (1989) noted that genetic damage is a likely candidate. However, cytogenetic analysis of zygotes and midgestational fetuses ruled out numerical and structural alterations in chromosomes (Katoh et al. 1989). Russell et al. (1984) also ruled out gene mutations and specific locus mutations. Katoh et al. (1989) proposed that the effects may be mediated by a nonmutagenic process involving changes in gene expression.
4.3.
Structure-Activity Relationships
Ethylene oxide is structurally similar to but more toxic than propylene oxide.
4.4.
Other Relevant Information
4.4.1.
Species Variability
Acute lethality studies showed that mice are the most sensitive species, followed by dogs and rats. The LC50 value was about three times greater for male rats than for female mice exposed for 4 h. It is difficult to compare the toxicity in humans with that of laboratory animals because of the lack of quantitative exposure data, and most human studies involved repeated exposures under occupational conditions.
4.4.2.
Susceptible Populations
Developmental toxicity studies showed that the developing embryo and fetus are more sensitive than the adult to toxic effects of ethylene oxide. No studies have been conducted on preweanling or weanling pups to determine whether the young animals are as sensitive to ethylene oxide exposure as the developing embryo and fetus. AEGL-2 values were derived from a developmental toxicity study, thereby incorporating data for the susceptible population.
4.4.3.
Concentration-Exposure Duration Relationship
The LC50 data for 1- and 4-h inhalation exposures of rats can be used to determine the relationship between exposure concentration and exposure duration for ethylene oxide. The 1-h LC50 value is 5,029 ppm (Nachreiner 1992) and the 4-h LC50 values are 1,460 (Jacobson et al. 1956) and 1,741 (Nachreiner 1991) ppm. The regression line using the three data points representing only two exposure durations is presented in Figure 2-1. Although only two exposure durations were available for calculating the value of n, this method is better than using the default values of n to derive AEGL values for ethylene oxide. The calculated value of n is 1.2.
4.4.4.
Concurrent Exposure Issues
There are no known concurrent exposure issues related to ethylene oxide.
5.
DATA ANALYSIS FOR AEGL-1
5.1.
Human Data Relevant to AEGL-1
There are no human data directly related to AEGL-1 derivation. Humans have been exposed to ethylene oxide at a wide range of concentrations. These studies did not correlate effects with exposure concentrations.
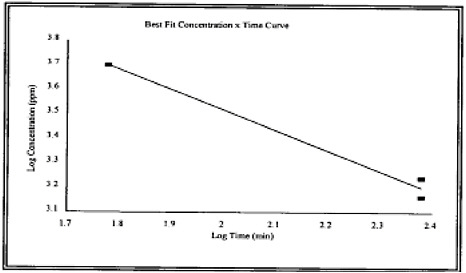
FIGURE 2-1 Rat data: Concentration-time curve for LC50 values for ethylene oxide.
5.2.
Animal Data Relevant to AEGL-1
The same data sets described for AEGL-2 were considered for deriving AEGL-1 values.
5.3.
Derivation of AEGL-1
No AEGL-1 values are recommended (see Table 2-22). The odor threshold and sensory irritation occur at ethylene oxide concentrations higher than those causing systemic effects. In addition, all AEGL-2 values are below the odor threshold. The absence of AEGL-1 values does not imply that exposure below the AEGL-2 level is without adverse effects.
6.
DATA ANALYSIS FOR AEGL-2
6.1.
Human Data Relevant to AEGL-2
Nonlethal effects of ethylene oxide in humans are summarized in Table 2-6. The epidemiologic studies provided evidence suggesting adverse reproductive outcomes (Hemminki et al. 1982; Rowland et al. 1996); however, these studies had a number of limitations and lacked exposure data for quantitative evaluation of AEGL values. Other nonlethal effects were shown to be reversible upon termination of exposure; however, some effects, particularly those occurring in the
TABLE 2-22 AEGL-1 Values for Ethylene Oxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not recommended |
||||
nervous system, are reasons for concern. Several human studies described effects due to ethylene oxide, but only three involved single or very short-term exposures. The study by Deschamps et al. (1992) showed respiratory tract irritation, nonimmunologic asthma, and peripheral neuropathy in one subject accidentally exposed to concentrations at the odor threshold (≥260 ppm) for 4 h/day for 4 days. Respiratory tract irritation could be attributed to each daily exposure, whereas peripheral neuropathy could have been caused by a single exposure and exacerbated upon repeated exposures. Other studies have shown that peripheral neuropathy may be exacerbated by repeated exposures to ethylene oxide (Finelli et al. 1983). The study by Laurent (1988) showed respiratory tract irritation, nervous system effects, and hemolysis in five workers exposed to ethylene oxide at the odor threshold for 30 min. Salinas et al. (1981) described nervous system effects leading to unconsciousness, apnea, and muscle twitching in an individual exposed to a calculated concentration of 500 ppm for 2 to 3 min. There is considerable uncertainty about the exposure concentration reported by Salinas et al. (1981); therefore, this study should not be used to derive AEGL values. In the remaining studies showing nonlethal effects, duration of exposure was not reported or subjects were exposed repeatedly for durations ranging from 2 weeks to more than a year. Genetic lesions in somatic cells were observed in individuals after a single exposure. These genetic lesions are not relevant end points for evaluating AEGL-2 levels, because the only disease state associated with genetic lesions in somatic cells is carcinogenicity.
6.2.
Animal Data Relevant to AEGL-2
The primary animal studies that can be used to derive AEGL-2 values are an acute neurotoxicity study in rats (Mandella 1997a), developmental toxicity studies in rats (Snellings et al. 1982a; BRRC 1993; Saillenfait et al. 1996), a developmental toxicity study in mice (Weller et al., 1999), and a reproductive toxicity study in mice (Ribeiro et al. 1987). Acute neurotoxicity in rats included drooping, closed eyelids; impaired locomotion; low arousal; and no approach response after exposure to concentrations ≥ 300 ppm for 6 h (Mandella 1997a). Decreased hindlimb grip strength was observed in rats exposed to 300 ppm for 4 weeks (Mandella 1997b) or to 200 ppm for 14 weeks (Mandella 1997c). Ohnishi et al. (1985) showed that axonal degeneration of myelinated fibers in the fasciculus gracilis and hindlimb nerves appeared to be related to hindlimb ataxia in rats exposed to 500 ppm for at least 9 weeks. A developmental toxicity study in mice showed effects after exposure to 350 or 450 ppm for 6 h or 700 or 900 ppm for 3 h (Weller et al. 1999). Exposure to ethylene oxide > 900 ppm caused ma-
ternal deaths in mice. The BRRC (1993) and Snellings et al. (1982a) studies showed developmental effects in rats exposed 6 h to 100 ppm and higher during organogenesis. The Saillenfait et al. (1996) study showed developmental toxicity in rats exposed to 800 or 1,200 ppm for 3 h and maternal effects at 1,200 ppm but not at the lower concentrations. The Saillenfait et al. (1996) study had inconsistencies and the results differed considerably from those of other developmental toxicity studies. Although developmental toxicity was observed in the rat after repeated exposures during organogenesis, developmental toxicity was also observed after a single exposure of mice and the critical time for inducing developmental toxicity on GD 7. The critical end point (growth retardation) in the developmental toxicity studies in rats exposed repeatedly also was observed in mice after a single exposure. Therefore, a cumulative effect due to exposure is not necessary to explain growth retardation in rat fetuses. Ribeiro et al. (1987) reported abnormal spermatozoa in mice exposed to 200 or 400 ppm for 6 h/day for only 5 days. The remaining studies on nonlethal toxicity involved repeated exposures of monkeys, rats, mice, and dogs and effects after the first or second exposure were not described.
6.3.
Derivation of AEGL-2
AEGL-2 values were based on two rat studies, a neurotoxicity study by Mandella (1997a) and a developmental toxicity study by Snellings et al. (1982a). Rat studies are more appropriate for deriving AEGL-2 values because the exposure concentrations are below saturating levels, and developmental toxicity and neurotoxicity in the rat are the two most sensitive effects caused by exposure to ethylene oxide. The NOAEL for both studies was 100 ppm for a 6-h exposure. In the developmental toxicity study, pregnant female rats were exposed to 0, 10, 33, or 100 ppm for 6 h/day from GD 6 to 15 (Snellings et al. 1982a). Fetal body weight was slightly, but significantly, decreased and the incidence of delayed ossification was increased but not significantly; therefore, the effect at 100 ppm is not considered adverse. In another study, the increased litter incidence of poorly or unossified skeletal areas was statistically significant at 125 ppm and, taken together with decreased fetal body weight, the effects were considered adverse (BRRC 1993). In the acute neurotoxicity study, rats were exposed to 0, 100, 300, or 500 ppm for 6 h before assessment for neurotoxicity. The incidences of two FOB end points (low arousal and no reaction to approach) were significantly increased and the incidences of two additional FOB end points (droopy or half-closed eyelids and impaired locomotion) were increased but not significantly at 300 ppm, the next concentration above the NOAEL. The FOB was assessed after exposure was terminated, and this slight delay may have reduced the magnitude of the response assessed in the FOB evaluation. Three of the FOB end points (low arousal, no reaction to approach response, and impaired locomotion) suggested that ethylene oxide affected mobility of the animals, which was demonstrated explicitly as decreased mobility at 500 ppm. A
decrease in mobility may affect the ability to escape. More serious neurotoxicity was observed during repeated exposure to ethylene oxide (Ohnishi et al. 1985; Mandella 1997b,c).
The NOAEL of 100 ppm for developmental toxicity and neurotoxicity in the rat is selected as the point of departure (POD) for AEGL-2 development. The benchmark dose approach was not used for AEGL-2 derivation because no single FOB end point was considered more toxicologically significant than another. Each end point showed a clear increasing trend when analyzed separately but not when the data were combined. Therefore, the NOAEL for all the neurotoxic effects was considered appropriate for deriving AEGL-2 values. The rat is considered the most appropriate species for AEGL derivation because pharmacokinetic data and the PBPK models (particularly for glutathione conjugation) show that the rat is more like humans than the mouse. A total uncertainty factor of 10 (3 for interspecies sensitivity and 3 for intraspecies variability) was applied to the POD. An uncertainty factor of 3 was selected for interspecies sensitivity. Very limited information is available on the mechanism of ethylene oxide–induced neurotoxicity in rats or humans; however, similar effects (distal axonal degeneration and neuropathy) have been observed in both species. Ethylene oxide directly alkylates DNA and other macromolecules; this mechanism of toxicity is not expected to differ across species. PBPK models have shown that the AUC, peak blood levels, dose in mg/kg of body weight, and hemoglobin adduct levels (measure of internal exposure) in humans are similar to or lower than those of rats (Fennell and Brown 2001). HEV adduct formation in hemoglobin is proportional to air concentrations across species and adduct formation per ppm-h exposure is similar for rats, mice, and humans (Ehrenberg and Tornqvist 1995). Therefore, the dosimetry is not expected to differ considerably in rats and humans. An uncertainty factor of 3 was selected for intraspecies variability. Glutathione-S-transferase polymorphism accounts for some variation within the population. The level of HEV hemoglobin adducts used as a measure of systemic exposure in humans is affected by glutathione-S-transferase polymorphism expressed by conjugator (GSTM1 or GSTT1) or nonconjugator (GSTM1-null or GSTT1-null) genotypes. Muller et al. (1998) examined the HEV hemoglobin adducts in smoking and nonsmoking individuals with conjugator or nonconjugator genotypes and found that the level of HEV hemoglobin adducts in nonsmoking individuals with the GSTT1-null genotype was at least twice that in the nonsmoking individuals with the GSTT1 genotype (Muller et al. 1998). Fennell et al. (2000) also reported that the level of HEV hemoglobin adducts was significantly higher in smoking individuals with the GSTT1-null genotype than in individuals with the GSTT1 genotype. These results indicate that ethylene oxide levels in individuals with the GSTT1-null genotype would be higher than in those with the GSTT1 genotype after exposure to ethylene oxide. However, the variation in exposure as measured by hemoglobin adducts appears to be within a factor of 3. There is no evidence that individuals with respiratory diseases, including asthma, respond differently to ethylene oxide exposure. One
study showed no effect on an individual with asthma exposed to ethylene oxide at odor detection levels (≥260 ppm) (Deschamps et al. 1992).
Timeframe extrapolation was performed according to ten Berge’s equation (ten Berg et al. 1986), where n = 1.2 was derived from the rat lethality data (described in Section 4.4.3). The AEGL-2 value for a 10-min exposure is the same as that derived for a 30-min exposure because of the uncertainty of extrapolating from a 6-h exposure to a 10-min exposure. The resulting AEGL-2 values are summarized in Table 2-23.
AEGL-2 values were derived from acute neurotoxicity and developmental toxicity studies. The NOAEL for developmental toxicity is supported by a study showing growth retardation at 125 ppm (BRRC 1993). The NOAEL of 100 ppm for neurotoxicity is supported by 4- and 13-week neurotoxicity studies (Mandella 1997b,c) that had effects at 200 and 300 ppm and NOAELs at 100 ppm.
7.
DATA ANALYSIS FOR AEGL-3
7.1.
Human Data Relevant to AEGL-3
No human lethality data are available for deriving AEGL-3 values. Epidemiologic data provided limited evidence that exposure to ethylene oxide is associated with an increased risk of lymphatic and hematopoietic cancer (IARC 1994). Quantitative assessments of human cancer data will not be attempted for data sets providing only limited evidence of carcinogenicity.
7.2.
Animal Data Relevant to AEGL-3
Several lethality studies are available for deriving AEGL-3 values. One-hour inhalation studies have been conducted in male and female rats (Nachreiner 1992), and 4-h inhalation studies have been conducted in male and female rats (Jacobson et al. 1956; Nachreiner 1991), male and female mice (Jacobson et al. 1956; NTP 1987), and male dogs (Jacobson et al. 1956). The LC50 values varied for the three species studied: mice were slightly more sensitive than dogs, which were more sensitive than rats. All studies were well-conducted; the Nachreiner (1991, 1992) studies were more comprehensive with regard to the end points evaluated. There were some intraspecies variations in the rat and mouse studies. The mice and rats used by Jacobson et al. (1956) were of unspecified strain, the Nachreiner study (1991, 1992) used Sprague-Dawley rats, and the NTP (1987) study used B6C3F1 mice. Therefore, strain differences could have accounted for some of the variations in LC50 values. Probit analysis of the dog data consisted of either 100% mortality or 100% survival, thereby producing a highly uncertain LC50 value that should be interpreted with caution.
TABLE 2-23 AEGL-2 Values for Ethylene Oxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
80 ppm (144 mg/m3) |
80 ppm (144 mg/m3) |
45 ppm (81 mg/m3) |
14 ppm (25 mg/m3) |
7.9 ppm (14 mg/m3) |
Long-term exposure studies have shown that inhaled ethylene oxide is carcinogenic to the mouse (Adkins et al. 1986; NTP 1987) and rat (Lynch et al. 1984a; Snellings et al. 1984b; Garman et al. 1985). Concentrations associated with significant increases in tumor incidences are 100 ppm for 6 h/day repeatedly for 2 years or 200 ppm for 6 months.
A study in mice exposed to 1,200 ppm for 1.5 h on the day of mating (Rutledge and Generoso 1989) should be considered when evaluating data pertinent to deriving AEGL-3 values. Rutledge and Generoso (1989) observed a high incidence of late fetal deaths and hydrops. Although the exposure time was critical (the first 24 h after mating) for inducing these effects, any value derived for AEGL-3 should be protective of the zygote.
7.3.
Derivation of AEGL-3
Lethality thresholds (LC01) were derived from the mouse, rat, and dog data (Table 2-24) The LC01 for dogs exposed for 4 h is much lower than the values obtained for the other species; however, the results from the dog data have a higher degree of uncertainty than the results for other species. The LC01 values derived from the mouse and rat data range from 264 to 922 ppm for a 4-h exposure, with the mouse values being lower than those for the rat.
The rat is not the species most sensitive to acute inhalation exposure to ethylene oxide, but it is the more appropriate species for deriving AEGL-3 values, because pharmacokinetic data indicate that the rat is more like humans than the mouse. The rat study by Jacobson et al. (1956) was selected to derive the AEGL-3 values because it presented a better dose-response relationship and a more conservative POD than the Nachreiner (1991, 1992) studies. The LC01 value of 628 ppm is the POD derived from the Jacobson et al. (1956) rat data. The LC01 is an estimate of the lethality threshold and is below the lowest concentration causing death. The uncertainty factors are 3 for interspecies sensitivity and 3 for intraspecies variability (total = 10). Death after ethylene oxide exposure is attributed to respiratory failure, probably involving the central nervous system (Golberg 1986). Although the exact mechanism of these effects is not known, alkylation of macromolecules may be involved and this reaction is not expected to differ considerably among species. In addition, the exposure concentrations were within range for the PBPK model simulations showing linearity of systemic uptake (Fennell and Brown 2001). PBPK models have shown that the AUC, peak blood levels, dose in mg/kg, and hemoglobin adduct levels (measure
TABLE 2-24 Estimates of the Threshold for Lethality (LC01) of Ethylene Oxide
|
Species |
Exposure Duration (h) |
LC50 (ppm) |
LC01 (ppm) |
Reference |
|
Dog |
4 |
960 |
120 |
Jacobson et al. 1956 |
|
Mouse |
4 |
623a |
264 |
NTP 1987 |
|
|
4 |
835 |
406 |
Jacobson et al. 1956 |
|
Rat |
4 |
1460 |
628 |
Jacobson et al. 1956 |
|
|
4 |
1,741a |
922 |
Nachreiner 1991 |
|
Rat |
1 |
5,029a |
2494 |
Nachreiner 1992 |
|
aCombined data sets for males and females. Abbreviations: LC50, concentration with 50% lethality; LC01, concentration with 1% lethality. |
||||
of internal exposure) for humans are similar to or lower than the corresponding values for rats (Fennell and Brown 2001). An intraspecies uncertainty factor of 3 was selected because a glutathione-S-transferase polymorphism expressed by conjugator (GSTM1 or GSTT1) or nonconjugator (GSTM1-null or GSTT1-null) genotypes can modulate systemic exposure as measured by hemoglobin adduct levels (Muller et al. 1998). The variation in exposure as measured by hemoglobin adducts in conjugators and nonconjugators appears to be within a factor of 3. There is no evidence that individuals with respiratory diseases, including asthma, respond differently to ethylene oxide exposure. In addition, an interspecies or intraspecies uncertainty factor of 10 would produce10- and 30-min AEGL-3 values far below concentrations expected to be associated with life-threatening events.
The ten Berge equation (Cn × t = k, where n = 1.2) was used to extrapolate to other time points. The value of n was derived empirically from the 1 and 4-h LC50 values for rats. The AEGL-3 values for 10 and 30 min and for 1, 4, and 8 h are 360, 360, 200, 63, and 35 ppm. The AEGL-3 value for a 10-min exposure is the same as that derived for a 30-min exposure, because the AEGL values were derived from a 4-h exposure, which increases the uncertainty for deriving the 10-min value. The AEGL-3 values are summarized in Table 2-25.
Ethylene oxide is carcinogenic in laboratory animals. Dose-response data were used to estimate the excess lifetime risk associated with a single exposure to ethylene oxide. Calculations for AEGL-3 estimates are presented in Appendix B of this document. EPA’s linearized multistage model (GLOBAL86) (Howe et al. 1986) was used to derive a unit risk for ethylene oxide from incidence data on alveolar and bronchiolar adenomas and carcinomas in female mice exposed to ethylene oxide vapor for 2 years. This site showed the highest incidence and was therefore considered to be the most sensitive target. AEGL-3 values based on carcinogenicity data are 1,300, 1,300, 640, 160, and 80 ppm for a 10–4 risk
TABLE 2-25 AEGL-3 Values for Ethylene Oxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
360 ppm (648 mg/m3) |
360 ppm (648 mg/m3) |
200 ppm (360 mg/m3) |
63 ppm (113 mg/m3) |
35 ppm (63 mg/m3) |
level at exposure durations of 10 and 30 min and 1, 4, and 8 h, respectively. These values are higher than those based on a lethality threshold.
The proposed AEGL-3 value for a 30-min exposure is higher than the odor threshold of 260 ppm as reported by Hellman and Small (1974). Deschamps et al. (1992) reported toxic effects, but no lethality, for 4-h exposures at the odor detection level. Laurent (1988) also reported toxic effects, but no lethality, for 30-min exposures at the odor detection level. The odor detection level has been reported to range from 260 to as high as 1,540 ppm (upper 95% C.I.). Salinas reported very serious neurologic effects after a 2- to 3-min exposure to a reported concentration of 500 ppm (calculated concentration); this concentration is probably too low for the initial exposure.
8.
SUMMARY OF PROPOSED AEGLs
8.1.
Proposed AEGLs
The AEGL values are presented in Table 2-26. No lethality data for humans were available for deriving the AEGL-3 values; the limitations in the epidemiologic studies precluded an unequivocal conclusion on carcinogenicity based on human data. Data from long-term animal studies were used to estimate AEGL-3 values based on carcinogenicity. The calculations showed that the AEGL-3 values based on the excess lifetime cancer risk from a single exposure to ethylene oxide is greater than the concentration associated with the lethality threshold. Therefore, the AEGL-3 values were derived from an estimate of the lethality threshold (LC01) for rats.
AEGL-2 values were derived from rat developmental and neurotoxicity end points; the NOAEL was 100 ppm for a 6-h exposure in both studies.
AEGL-1 values were not derived because the odor threshold and sensory irritation occur at concentrations above those causing systemic toxicity and would not serve as a warning of exposure. In addition, the proposed AEGL-2 and -3 values are below the odor threshold with the exception of AEGL-3 values for 10 and 30 min.
8.2.
Comparison of AEGLs with Other Standards and Criteria
Table 2-27 summarizes standards and guidelines established by various agencies and organizations. The American Conference of Governmental Indus-
trial Hygienists Threshold Limit Value, National Institute for Occupational Safety and Health (NIOSH) recommended exposure limits, NIOSH short-term exposure limit, and Occupational Safety and Health Administration permissible exposure limits are based on cancer risk associated with lifetime occupational exposures and should not be compared with the acute values derived for emergency standards. The NIOSH standard for immediately dangerous to life or health is based on an LC50 value of 800 ppm for a 4-h exposure. This value was adopted for a 30-min exposure. The emergency response planning guideline (ERPG)-3 value of 500 ppm is considerably higher than the AEGL-3 value of 200 ppm for 1 h, and the ERPG-2 value of 50 ppm is similar to the AEGL-2 value of 45 ppm for 1 h (AIHA 2005). The ERPG-3 level for 1 h was based primarily on the acute inhalation study of Jacobson et al. (1956) and the observation that short-term exposure of humans to high concentrations has not been associated with mortality. The ERPG-2 value was based on reproductive and developmental toxicity studies. The NRC (1986b) recommended an emergency exposure guidance level of 20 ppm for a 1-h exposure and 1 ppm for a 24-h exposure.
8.3.
Data Quality and Research Needs
A number of uncertainties are associated with deriving AEGL values for ethylene oxide. The lack of definitive exposure data precluded using human data to derive AEGL-1 and AEGL-2 values. Estimates of exposure were based on the odor detection threshold of 260 ppm. Human studies with ethylene oxide to fill data gaps are precluded because of the potential carcinogenicity of the substance.
TABLE 2-26 AEGL Values for Ethylene Oxide
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
Not recommendeda |
|
|
|
|
|
|
AEGL-2 (Disabling) |
80 ppm (144 mg/m3) |
80 ppm (144 mg/m3) |
45 ppm (81 mg/m3) |
14 ppm (25 mg/m3) |
7.9 ppm (14 mg/m3) |
NOAEL for neurotoxicity and developmental toxicity (Snellings et al. 1982a; Mandella 1997a) |
|
AEGL-3 (Lethal) |
360 ppm (648 mg/m3) |
360 ppm (648 mg/m3) |
200 ppm (360 mg/m3) |
63 ppm (113 mg/m3) |
35 ppm (63 mg/m3) |
Lethality (Jacobson et al. 1956) |
|
aThe absence of AEGL-1 values does not imply that exposure below the AEGL-2 value is without adverse effects. |
||||||
TABLE 2-27 Extant Standards and Guidelines for Ethylene Oxide
|
Guideline |
Exposure Duration |
|||||
|
10 min |
15 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
Not recommended |
|
|
|
|
|
|
AEGL-2 |
80 ppm |
80 ppm |
45 ppm |
14 ppm |
7.9 ppm |
|
|
AEGL-3 |
360 ppm |
360 ppm |
200 ppm |
63 ppm |
35 ppm |
|
|
ERPG-1 (AIHA)a |
|
|
|
|
|
|
|
ERPG-2 (AIHA)a |
|
|
|
50 ppm |
|
|
|
ERPG-3 (AIHA)a |
|
|
|
500 |
|
|
|
EEGL (NRC)b |
|
|
|
20 ppm |
|
1 ppm (24 h) |
|
IDLH (NIOSH)c |
|
|
800 ppm |
|
|
|
|
REL-TWA (NIOSH)d |
|
|
|
|
|
<0.1 ppm |
|
PEL-TWA (OSHA)e |
1 ppm |
|
|
|
|
|
|
TLV-TWA (ACGIH)f |
|
|
|
|
|
1 ppm (A2) |
|
EL (OSHA)g |
5 ppm (15-min excursion limit) |
|
|
|
|
|
|
REL-STEL (NIOSH)h |
5 ppm (ceiling) |
|
|
|
|
|
|
MAC (The Netherlands)i |
|
|
|
|
|
0.5 ppm |
|
MAK (Germany) j |
No value (carcinogenicity category 2, germ cell mutagen category 2) |
|||||
|
aERPG (emergency response planning guidelines of the American Industrial Hygiene Association) (AIHA 2001): The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. bEEGL (emergency exposure guidance levels of the National Research Council) (NRC 1986b) is the concentration of contaminants that can cause discomfort or other evidence of irritation or intoxication in or around the workplace but avoids death, other severe acute effects, and long-term or chronic injury. cIDLH (immediately dangerous to life or health standards of the National Institute for Occupational Safety and Health) (NIOSH 1996) represents the maximum concentration from which escape within 30 min would be possible without any escape-impairing symptoms or irreversible health effects. dREL-TWA (recommended exposure limits–time-weighted average of the National Institute for Occupational Safety and Health) (NIOSH 2005) is analogous to the American |
||||||
The database of animal studies is robust, containing acute inhalation studies in several species, an acute neurotoxicity, developmental and reproductive toxicity studies in several species, in vivo germ cell studies, and carcinogenicity studies in two species. However, a single-day developmental toxicity study would clear up some of the concerns about fetal growth retardation as a single-exposure or repeat-exposure event. In addition, pharmacokinetics and metabolism studies were available. Therefore, the toxicity of ethylene oxide is well characterized in animals.
9.
REFERENCES
ACGIH (American Conference of Governmental Hygienists). 2003. P. 31 in TLVs and BEIs Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental Hygienists, Cincinnati, OH.
Adkins, B., Jr., E.W. Van Stee, J.E. Simmons, and S.L. Eustis. 1986. Oncogenic response of strain A/J mice to inhaled chemicals. J. Toxicol. Environ. Health 17(2-3):311-322.
AIHA (American Industrial Hygiene Association). 2005. The AIHA 2005 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Handbook. Fairfax, VA: AIHA Press.
Bisanti, L., M. Maggini, R. Raschetti, S.S. Alegiani, F.M. Ippolito, B. Caffari, N. Segnan, and A. Ponti. 1993. Cancer mortality in ethylene oxide workers. Br. J. Ind. Med. 50(4):317-324.
Bolt, H.M., and M. Leutbecher. 1993. Dose-DNA adduct relationship for ethylene oxide. Arch. Toxicol. 67(10):712-713.
Braker, W., and A.L. Mossman, eds. 1980. Pp. 322-329 in Matheson Gas Data Book. Lyndhurst, NJ: Matheson.
Brown, C.D., B.A. Wong, and T.R. Fennell. 1996. In vivo and in vitro kinetics of ethylene oxide metabolism in rats and mice. Toxicol. Appl. Pharmacol. 136(1):8-19.
Brown, C.D., B. Asgharian, M.J. Turner, and T.R. Fennell. 1998. Ethylene oxide dosimetry in the mouse. Toxicol. Appl. Pharmacol. 148(2):215-221.
BRRC (Bushy Run Research Center). 1993. Ethylene Oxide: Developmental Toxicity Study of Maternally Inhaled Vapor in CD Rats With Cover Letter Dated 06/01/93. Doc No. 86-930000252. Office of Toxic Substances, U.S. Environmental Protection Agency.
Brugnone, F., L. Perbellini, G.B. Faccini, F. Pasini, G.B. Bartolucci, and E. DeRosa. 1986. Ethylene oxide exposure: Biological monitoring by analysis of alveolar air and blood. Int. Arch. Occup. Environ. Health 58(2):105-112.
Bryant, H.E., N.D. Visser, and K. Yoshida. 1989. Ethylene oxide sterilizer use and short-term symptoms amongst workers. J. Soc. Occup. Med. 39(3):101-106.
Budavari, S., M.J. O’Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 647 in The Merck Index, 11th Ed. Rahway, NJ: Merck.
Cawse, J.N., J.P. Henry, M.W. Swartzlander, and P.H. Wadia. 1980. Ethylene oxide. Pp. 432-471 in Kirk-Othmer Encyclopedia of Chemical Technology, 3rd Ed., Vol. 9. New York: John Wiley and Sons.
Crump, K.S., and R.B. Howe. 1984. The multistage model with a time-dependent dose pattern: Applications to carcinogenic risk assessment. Risk Anal. 4(3):163-176.
Csanady, G.A., B. Benk, C. Putz, P.E. Kreuzer, W. Kessler, C. Baur, M.L. Gargas, and J.G. Filser. 2000. A physiological toxicokinetic model for exogenous and endogenous ethylene and ethylene oxide in rat, mouse, and human: Formation of 2-hydroxyethyl adducts with hemoglobin and DNA. Toxicol. Appl. Pharmacol. 165(1):1-26.
Deleixhe, P.A., A. Balsat, and C. Laurent. 1986. Acute ethylene oxide intoxication; a report of five cases [in French]. Arch. Belg. 44(11-12):478-488.
Dellarco, V.L., W.M. Generoso, G.A. Sega, J.R. Fowle III, and D. Jacobson-Kram. 1990. Review of the mutagenicity of ethylene oxide. Environ. Mol. Mutagen. 16(2):85-103.
Deschamps, D., N. Rosenberg, P. Soler, G. Maillard, E. Fournier, D. Salson, and P. Gervais. 1992. Persistent asthma after accidental exposure to ethylene oxide. Br. J. Ind. Med. 49(7):523-525.
DFG (Deutsche Forschungsgemeinschaft). 2007. P. 69 in List of MAK and BAT Values 2007. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 43. Weinheim, Federal Republic of Germany: Wiley VCH.
Dow Chemical Co. 1982. Ethylene Oxide Teratology Study With Cover Letter. 8DS, OTS 84003A. Office of Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
Ehrenberg, L., and M. Tornqvist. 1995. The research background for risk assessment of ethylene oxide: Aspects of dose. Mutat. Res. 330(1-2):41-54.
Ehrenberg, L., K.D. Hiesche, S. Osterman-Golkar, and I. Wenneberg. 1974. Evaluation of genetic risks of alkylating agents: Tissue doses in the mouse from air contaminated with ethylene oxide. Mutat. Res. 24(2):83-103.
Elliott, L.J., V.L. Ringenburg, P. Morelli-Schroth, W.E. Halperin, and R.F. Herrick. 1988. Ethylene oxide exposures in hospitals. Appl. Ind. Hyg. 3(5):141-145.
Embree, J.W., J.P. Lyon, and C.H. Hine. 1977. The mutagenic potential of ethylene oxide using the dominant-lethal assay in rats. Toxicol. Appl. Pharmacol. 40(2):261-267.
EPA (U.S. Environmental Protection Agency). 1985. Health Assessment Document for Ethylene Oxide. Final Report. EPA-600/8-84-009F. Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC.
Farmer, P.B., R. Cordero, and H. Autrup. 1996. Monitoring human exposure to 2-hydroxyethylating carcinogens. Environ. Health Perspect. 104(Suppl. 3):449-452.
Fennell, T.R. 1996. Biomarkers of exposure and susceptibility: Application to ethylene oxide. CIIT Activities 16:2-6.
Fennell, T.R., and C.D. Brown. 2001. A physiologically based pharmacokinetic model for ethylene oxide in mouse, rat, and human. Toxicol. Appl. Pharmacol. 173(3):161-175.
Fennell, T.R., J.P. MacNeela, R.W. Morris, M. Watson, C.L. Thompson, and D.A. Bell. 2000. Hemoglobin adducts from acrylonitrile and ethylene oxide in cigarette smokers: Effects of glutathione-S-transferase T1-null and M1-null genotypes. Cancer Epidemiol. Biomarkers Prev. 9(7):705-712.
Filser, J.G., B. Denk, M. Tornqvist, W. Kessler, and L. Ehrenberg. 1992. Pharmacokinetics of ethylene in man: Body burden with ethylene oxide and hydroxyethylation of hemoglobin due to endogenous and environmental ethylene. Arch. Toxicol. 66(3):157-163.
Finelli, P.F., T.F. Morgan, I. Yaar, and C.V. Granger. 1983. Ethylene oxide-induced polyneuropathy. A clinical and electrophysiologic study. Arch. Neurol. 40(7):419-421.
Gardiner, T.H., J.M. Waechter, and D.E. Stevenson. 1993. Epoxy compounds. Pp. 329-433 in Patty’s Industrial Hygiene and Toxicology, 4th Ed, G.D. Clayton, and F.E. Clayton, eds., New York: John Wiley and Sons.
Garman, R.H., W.M. Snellings, and R.R. Maronpot. 1985. Brain tumors in F344 rats associated with chronic inhalation exposure to ethylene oxide. Neurotoxicology 6(1):117-137.
Garry, V.F., J. Hozier, D. Jacobs, R.L. Wade, and D.G. Gray. 1979. Ethylene oxide: Evidence of human chromosomal effects. Environ. Mutagen. 1(4):375-382.
Generoso, W.M., R.B. Cumming, J.A. Bandy, and K.T. Cain. 1983. Increased dominant lethal effects due to prolonged exposure of mice to inhaled ethylene oxide. Mutat. Res. 119(3):377-379.
Generoso, W.M., K.T. Cain, L.A. Hughes, G.A. Sega, P.W. Braden, D.G. Gosslee, and M.D. Shelby. 1986. Ethylene oxide dose and dose-rate effects in the mouse dominant-lethal test. Environ. Mutagen. 8(1):1-7.
Generoso, W.M., J.C. Rutledge, K.T. Cain, L.A. Hughes, and P.W. Braden. 1987. Exposure of female mice to ethylene oxide within hours after mating leads to fetal mal-formation and death. Mutat. Res. 176(2):269-274.
Generoso, W.M., K.T. Cain, C.V. Cornett, N.L. Cacheiro, and L.A. Hughes. 1990. Concentration-response curves for ethylene-oxide-induced heritable translocations and dominant lethal mutations. Environ. Mol. Mutagen. 16(2):126-131.
Golberg, L. 1986. Hazard Assessment of Ethylene Oxide. Boca Raton, FL: CRC Press.
Gross, J.A., M.L. Haas, and T.R. Swift. 1979. Ethylene oxide neurotoxicity: Report of four cases and review of the literature. Neurology 29:978-983.
Hackett, P.L., M.G. Brown, R.L. Buschbom, M.L. Clark and R.A. Miller. 1982. Teratogenic Study of Ethylene and Propylene Oxide and n-Butyl Acetate. NTIS
PB83-258038. Prepared by Battelle Pacific Northwest Labs., Richland, WA, for the National Institute for Occupational Safety and Health, Cincinnati, OH.
Hagmar, L., Z. Mikoczy, and H. Welinder. 1995. Cancer incidence in Swedish sterilant workers exposed to ethylene oxide. Occup. Environ. Med. 52(3):154-156.
Hardin, B.D., R.W. Niemeier, M.R. Sikov, and P.L. Hackett. 1983. Reproductive-toxicologic assessment of the epoxides ethylene oxide, propylene oxide, butylene oxide, and styrene oxide. Scand. J. Work Environ. Health 9(2 Spec. No):94-102.
Hellman, T.M., and F.H. Small. 1974. Characterization of the odor properties of 101 petrochemicals using sensory methods. J. Air Pollut. Control Assoc. 24(10):979-982.
Hemminki, K., P. Mutinen, I. Saloniemi, M.L. Niemi, and H. Vainio. 1982. Spontaneous abortions in hospital staff engaged in sterilizing instruments with chemical agents. Br. Med. J. 285(6353):1461-1463.
Hemminki, K., P. Mutinen, and M.L. Niemi. 1983. Spontaneous abortions in hospital sterilising staff [letter]. Br. Med. J. 286(6382):1976-1977.
Hogstedt, C., N. Malmqvist, and B. Wadman. 1979a. Leukemia in workers exposed to ethylene oxide. JAMA 241(11):1132-1133.
Hogstedt, C., O. Rohlen, B.S. Berndtsson, O. Axelson, and L. Ehrenberg. 1979b. A cohort study of mortality and cancer incidence in ethylene oxide production workers. Br. J. Ind. Med. 36(4):276-280.
Hogstedt, L.C. 1988. Epidemiological studies of ethylene oxide and cancer: An updating. Pp. 265-270 in Methods for Detecting DNA Damaging Agents in Humans: Applications in Cancer Epidemiology and Prevention, H. Bartsch, K. Hemminki, and I.K. O’Neill, eds. IARC Sci. Publ. No. 89. Lyon: IARC Press.
Hollingsworth, R.L., V.K. Rowe, F. Oyen, D.D. McCollister, and H.C. Spencer. 1956. Toxicity of ethylene oxide determined on experimental animals. AMA Arch. Ind. Health 13(3):217-227.
Howe, R.B., K.S. Crump, and C. Van Landingham. 1986. GLOBAL86: A Computer Program to Extrapolate Quantal Animal Toxicity Data to Low Doses. Prepared for U.S. Environmental Protection Agency, Washington, DC. Subcontract No. 2-251U-2745.
IARC (International Agency for Research on Cancer). 1994. Ethylene oxide. Pp. 73-159 in Some Industrial Chemicals, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 60. Lyon: IARC Press.
Jacobson, K.H., E.B. Hackley, and L. Feinsliver. 1956. The toxicity of inhaled ethylene oxide and propylene oxide vapors. AMA Arch. Ind. Health 13(3):237-244.
Katoh, M., N.L. Cacheiro, C.V. Cornett, K.T. Cain, J.C. Rutledge, and W.M. Generoso. 1989. Fetal anomalies produced subsequent to treatment of zygotes with ethylene oxide or ethyl methanesulfonate are not likely due to the usual genetic causes. Mutat. Res. 210(2):337-344.
Kligerman, A.D., G.L. Erexson, M.E. Phelps, and J.L. Wilmer. 1983. Sister-chromatid exchange induction in peripheral blood lymphocytes of rats exposed to ethylene oxide by inhalation. Mutat. Res. 120(1):37-44.
Kolman, A., M. Chovanec, and S. Osterman-Golkar. 2002. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: Update review (1990-2001). Mutat. Res. 512(2-3):173-194.
Krishnan, K., M.L. Gargas, T.R. Fennell, and M.E. Andersen. 1992. A physiologically based description of ethylene oxide dosimetry in the rat. Toxicol. Ind. Health 8(3):121-140.
Laurent, C. 1988. SCE increases after an accidental acute inhalation exposure to EtO and recovery to normal after 2 years. Mutat. Res. 204(4):711-717.
Lynch, D.W., T.R. Lewis, W.J. Moorman, J.R. Burg, D.H. Groth, A. Khan, L.J. Ackerman, and B.Y. Cockrell. 1984a. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol. Appl. Pharmacol. 76(1):69-84.
Lynch, D.W., T.R. Lewis, W.J. Moorman, J.R. Burg, D.K. Gulati, P. Kaur, and P.S. Sabharwal. 1984b. Sister-chromatid exchanges and chromosome aberrations in lymphocytes from monkeys exposed to ethylene oxide and propylene oxide by inhalation. Toxicol. Appl. Pharmacol. 76(1):85-95.
Major, J., M.G. Jakab, and A. Tompa. 1996. Genotoxicological investigation of hospital nurses occupationally exposed to ethylene-oxide: I. Chromosome aberrations, sister-chromatid exchanges, cell cycle kinetics, and UV-induced DNA synthesis in peripheral blood lymphocytes. Environ. Mol. Mutagen. 27(2):84-92.
Mandella, R.C. 1997a. An Acute Inhalation Neurotoxicity Study of Ethylene Oxide (498-95-A) in the Rat via Whole-Body Exposure. Final Report. Study No. 95-6097. Prepared by Huntingdon Life Sciences, East Millstone, NJ, for Allied Signal, Inc, Morristown, NJ, and ARC Chemical Division, Balchem Corporation, Slate Hill, NY.
Mandella, R.C. 1997b. A 13-Week Inhalation Neurotoxicity Study of Ethylene Oxide (498-95A) in the Rat via Whole-Body Exposures with Recovery. Study No. 95-6099. Prepared by Huntingdon Life Sciences, East Millstone, NJ, for Allied Signal, Inc, Morristown, NJ, and ARC Chemical Division, Balchem Corporation, Slate Hill, NY.
Mandella, R.C. 1997c. A 4-Week Inhalation Range-Finding Study of Ethylene Oxide (498-95A) in the Rat via Whole-Body Exposures. Study No. 95-6098. Prepared by Huntingdon Life Sciences, East Millstone, NJ, for Allied Signal, Inc, Morristown, NJ and ARC Chemical Division, Balchem Corporation, Slate Hill, NY.
Maples, K.R., and A.R. Dahl. 1993. Levels of epoxides in blood during inhalation of alkenes and alkene oxides. Inhal. Toxicol. 5(1):43-54.
Marchand, M., R. Delesvaux, C. Claeys, and F. Lejeune. 1957. The toxicity of ethylene oxide and a report of three fatal cases of poisoning. Rev. Med. Min.10:5-9 (Abstract in AMA Arch Ind. Health. 18(1):66 [1958]).
Martis, L., R. Kroes, T.D. Darby, and E.F. Woods. 1982. Disposition kinetics of ethylene oxide, ethylene glycol, and 2-chloroethanol in the dog. J. Toxicol. Environ. Health 10(4-5):847-856.
Mayer, J., D. Warburton, A.M. Jeffrey, R. Pero, S. Walles, L. Andrews, M. Toor, L. Latriano, L. Wazneh, D. Tang, W.Y. Tsai, M. Kuroda, and F. Perera. 1991. Biologic markers in ethylene oxide-exposed workers and controls. Mutat. Res. 248(1):163-176.
McKelvy, J.A., and M.A. Zemaitis. 1986. The effects of ethylene oxide (EO) exposure on tissue glutathione levels in rats and mice. Drug Chem. Toxicol. 9(1):51-66.
Mori, K., M. Kaido, K. Fujishiro, and N. Inoue. 1989. Testicular toxicity and alterations of glutathione metabolism resulting from chronic inhalation of ethylene oxide in rats. Toxicol. Appl. Pharmacol. 101(2):299-309.
Mori, K., M. Kaido, K. Fujishiro, N. Inoue, O. Koide, H. Hori, and I. Tanaka. 1991. Dose dependent effects of inhaled ethylene oxide on spermatogenesis in rats. Br. J. Ind. Med. 48(4):270-274.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Ethyleenoxide. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Oct. 24, 2008].
Muller, M., A. Kramer, J. Angerer, and E. Hallier. 1998. Ethylene oxide-protein adduct formation in humans: Influence of glutathione-S-transferase polymorphisms. Int. Arch. Occup. Environ. Health 71(7):499-502.
Nachreiner, D.J. 1991. Ethylene Oxide: Acute Vapor Inhalation Toxicity Test in Rats (Four-Hour Test). Project ID 54-76. Bushy Run Research Center, Export, PA.
Nachreiner, D.J. 1992. Ethylene Oxide: Acute Vapor Inhalation Toxicity Testing According to D.O.T. Regulations (One-Hour Test). Project ID 54-593. Bushy Run Research Center, Export, PA.
Natarajan, A.T., R.J. Preston, V. Dellarco, L. Ehrenberg, W. Generoso, S. Lewis, and A.D. Tates. 1995. Ethylene oxide: Evaluation of genotoxicity data and an exploratory assessment of genetic risk. Mutat. Res. 330(1-2):55-70.
NIOSH (National Institute for Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-Ethylene Oxide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. August 1996 [online]. Available: http://cdc.gov/niosh/idlh/75218.html [accessed Mar. 21, 2010].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Pocket Guide to Chemical Hazards: Ethylene Oxide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. September 2005 [online]. Available: http://www.cdc.gov/niosh/npg/npgd0275.html [accessed Mar. 21, 2010].
NRC (National Research Council). 1986a. EEGLs for carcinogens. Pp. 25-27 in Criteria and Methods for Preparing Emergency Exposure Guidance Level (EEGL), Short-Term Public Emergency Guidance Level (SPEGL), and Continuous Exposure Guidance Level (CEGL) Documents. Washington, DC: National Academy Press.
NRC (National Research Council). 1986b. Pp. 35-71 in Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 6. Benzene and Ethylene Oxide. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 1987. Toxicology and Carcinogenesis Studies of Ethylene Oxide (CAS No. 75-21-8) in B6C3F1 Mice (Inhalation Studies). NTP TR 326. NIH 88-2582. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, Research Triangle Park, NC.
Ong, T., H.K. Bi, S. Xing, S., J. Stewart, and W. Moorman. 1993. Induction of sister chromatid exchange in spleen and bone marrow cells of rats exposed by inhalation to different dose rates of ethylene oxide. Environ. Mol. Mutagen. 22(3):147-151.
Ohnishi, A., N. Inoue, T. Yamamoto, Y. Murai, H. Hori, M. Koga, I. Tanaka, and T. Akiyama. 1985. Ethylene oxide induces central-peripheral distal axonal degeneration of the lumbar primary neurones in rats. Br. J. Ind. Med. 42(6):373-379.
Osterman-Golkar, S., L. Ehrenberg, D. Segerback, and I. Hallstrom. 1976. Evaluation of genetic risk of alkylating agents. II. Haemoglobin as a dose monitor. Mutat. Res. 34(1):1-10.
Potter, D., D. Blair, R. Davies, W.P. Watson, and A.S. Wright. 1989. The relationships between alkylation of haemoglobin and DNA in Fischer 344 rats exposed to [14C]ethylene oxide. Arch. Toxicol. (Suppl. 13):254-257.
Preston, R.J., T.R. Fennell, A.P. Leber, R.L. Sielken, Jr., and J.A. Swenberg. 1995. Reconsideration of the genetic risk assessment for ethylene oxide exposures. Environ. Mol. Mutagen. 26(3):189-202.
Rhomberg, L., V.L. Dellarco, C. Siegel-Scott, K.L. Dearfield, and D. Jacobson-Kram. 1990. Quantitative estimation of the genetic risk associated with the induction of heritable translocations at low-dose exposure: Ethylene oxide as an example. Environ. Mol. Mutagen. 16(2):104-125.
Ribeiro, L.R., D.M. Salvadori, C.A. Perera, and W. Becak. 1987. Activity of ethylene oxide in the mouse sperm morphology test. Arch. Toxicol. 60(4):331-333.
Rowland, A.S., D.D. Baird, D.L. Shore, B. Darden, and A.J. Wilcox. 1996. Ethylene oxide exposure may increase the risk of spontaneous abortion, preterm birth, and postterm birth. Epidemiology 7(4):363-368.
Russell, L.B., R.B. Cumming, and P.R. Hunsicker. 1984. Specific-locus mutation rates in the mouse following inhalation of ethylene oxide, and application of the results to estimation of human genetic risk. Mutat. Res. 129(3):381-388.
Rutledge, J.C., and W.M. Generoso. 1989. Fetal pathology produced by ethylene oxide treatment of the murine zygote. Teratology 39(6):563-572.
Saillenfait, A.M., F. Gallissot, P. Bonnet, and J.C. Protois. 1996. Developmental toxicity of inhaled ethylene oxide in rats following short-duration exposure. Fundam. Appl. Toxicol. 34(2):223-227.
Salinas, E., L. Sasich, D.H. Hall, R.M. Kennedy, and H. Morriss. 1981. Acute ethylene oxide intoxication. Drug Intel. Clin. Phar. 15(5):384-386.
Sarto, F., I. Cominato, A.M. Pinton, P.G. Brovedani, C.M. Faccioli, V. Bianchi, and A.G. Levis. 1984. Workers exposed to ethylene oxide have increased incidence of sister chromatid exchange. Pp. 413-419 in Monitoring Human Exposure to Carcinogenic and Mutagenic Agents. IARC Scientific Publication No. 59. Lyon: IARC Press.
Sega, G.A., E.E. Generoso, and P.A. Brimer. 1988. Inhalation exposure-rate of ethylene oxide affects the level of DNA breakage and unscheduled DNA synthesis in spermiogenic stages of the mouse. Mutat. Res. 209(3-4):177-180.
Sega, G.A., P.A. Brimer, and E.E. Generoso. 1991. Ethylene oxide inhalation at different exposure-rates affects binding levels in mouse germ cells and hemoglobin. Possible explanation for the effect. Mutat. Res. 249(2):339-349.
Setzer, J.V., W.S. Brightwell, J.M. Russo, B.L. Johnson, D.W. Lynch, G. Madden, J.R. Burg, and H. Sprinz. 1996. Neurophysiological and neuropathological evaluation of primates exposed to ethylene oxide and propylene oxide. Toxicol. Ind. Health. 12(5):667-682.
Shore, R.E., M.J. Gardner, and B. Pannett. 1993. Ethylene oxide: An assessment of epidemiologic evidence on carcinogenicity. Br. J. Ind. Med. 50(11):971-977.
Snellings, W.M., C.S. Weil, and R.R. Maronpot. 1981. Ethylene Oxide, Two Years Inhalation Study. Final Report. Prepared for U.S. Environmental Protection Agency, by Bushy Run Research Center, Pittsburg, PA.
Snellings, W.M., R.R. Maronpot, J.P. Zelenak, and C.P. Laffoon. 1982a. Teratology study in Fischer 344 rats exposed to ethylene oxide by inhalation. Toxicol. Appl. Pharmacol. 64(3):476-481.
Snellings, W.M., J.P. Zelenak, and C.S. Weil. 1982b. Effects on reproduction in Fischer 344 rats exposed to ethylene oxide by inhalation for one generation. Toxicol. Appl. Pharmacol. 63(3):382-388.
Snellings, W.M., C.S. Weil, and R.R. Maronpot. 1984a. A subchronic inhalation study on the toxicologic potential of ethylene oxide in B6C3F1 mice. Toxicol. Appl. Pharmacol. 76(3):510-518.
Snellings, W.M., C.S. Weil, and R.R. Maronpot. 1984b. A two-year inhalation study of the carcinogenic potential of ethylene oxide in Fischer 344 rats. Toxicol. Appl. Pharmacol. 75(1):105-117.
Steenland, K., L. Stayner, A. Greife, W. Halperin, R. Hayes, R. Hornung, and S. Nowlin. 1991. Mortality among workers exposed to ethylene oxide. N. Engl. J. Med. 324(20):1402-1407.
Tardif, R., R. Goyal, J. Brodeur, and M. Gerin. 1987. Species differences in the urinary disposition of some metabolites of ethylene oxide. Fundam. Appl. Toxicol. 9(3):448-453.
Tates, A.D., Boogaard, F. Darroudi, A.T. Natarajan, M.E. Caubo, and N.J. van Sittert. 1995. Biological effect monitoring in industrial workers following incidental exposure to high concentrations of ethylene oxide. Mutat. Res. 329(1):63-77.
Tavares, R., P. Ramos, J. Palminha, M.A. Bispo, I. Paz, A. Bras, J. Rueff, P.B. Farmer, and E. Bailey. 1994. Transplacental exposure to genotoxins. Evaluation in haemoglobin of hydroxyethylvaline adduct levels in smoking and non-smoking mothers and their newborns. Carcinogenesis 15(6):1271-1274.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Teta, M.J., L.O. Benson, and J.N. Vitale. 1993. Mortality study of ethylene oxide workers in chemical manufacturing: A 10 year update. Br. J. Ind. Med. 50(8): 704-709.
UCC (Union Carbide Corp.). 1993. Ethylene Oxide: An Assessment of Epidemiologic Evidence on Carcinogenicity with Attachment and Cover Letter Dated 07/22/93. Doc. No. 86-930000340. Office of Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
UCCPC (Union Carbide Chemical and Plastic Co.). 1991. A Cohort Mortality Study of Workers Potentially Exposed to Ethylene Oxide (Final) with Cover Letter Dated 07/25/91. Doc. ID No. 86-910000937. Office of Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
Van Doorn, R., M. Ruijten, and T. van Harreveld. 2002. Guidance for the Application of Odor in Chemical Emergency Response, Version 2.1. August 29, 2002 [Presented at the NAC/AEGL Meeting, September 2002, Washington, DC].
Vergnes, J.S., and I.M. Pritts. 1994. Effects of ethylene on micronucleus formation in the bone marrow of rats and mice following four weeks of inhalation exposure. Mutat. Res. 324(3):87-91.
Waite, C.P., F.A. Patty, and W.P. Yang. 1930. Acute response of guinea pigs to vapors of some new commercial organic compounds. IV. Ethylene oxide. Public Health Rep. 45(32):1832-1844.
Walker, V.E., T.R. Fennell, J.A. Boucheron, N. Fedtke, F. Ciroussel, and J.A. Swenberg. 1990. Macromolecular adducts of ethylene oxide: A literature review and a time-
course study on the formation of 7-(2-hydroxyethyl)guanine following exposure of rats by inhalation. Mutat. Res. 233(1-2):151-164.
Walker, V.E., T.R. Fennell, P.B. Upton, T.R. Skopek, V. Prevost, D.E. Shuker, and J.A. Swenberg. 1992a. Molecular dosimetry of ethylene oxide: Formation of persistence of 7-(2-hydroxyethyl)guanine in DNA following repeated exposure of rats and mice. Cancer Res. 52(16):4328-4334.
Walker, V.E., J.P. MacNeela, J.A. Swenberg, M.J. Turner Jr., and T.R. Fennell. 1992b. Molecular dosimetry of ethylene oxide: Formation and persistence of N-(2-hydroxyethyl)valine in hemoglobin following repeated exposures of rats and mice. Cancer Res. 52(16):4320-4327.
Walker, W.J.G., and C.E. Greeson. 1932. The toxicity of ethylene oxide. J. Hyg. 32(3):409-416.
Weller, E., N. Long, A. Smith, P. Williams, S. Ravi, J. Gill, R. Henessey, W. Skornik, J. Brain, C. Kimmel, G. Kimmel, L. Holmes, and L. Ryan. 1999. Dose-rate effects of ethylene oxide exposure on developmental toxicity. Toxicol. Sci. 50(2):259-270.
WHO (World Health Organization). 1985. Ethylene Oxide. Environmental Health Criteria 55. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc55.htm [accessed Mar. 22, 2010].
Wong, O., and L.S. Trent. 1993. An epidemiological study of workers potentially exposed to ethylene oxide. Br. J. Ind. Med. 50(4):308-316.
Woo, D.C., and R.M. Hoar. 1972. “Apparent hydronephrosis” as a normal aspect of renal development in late gestation of rats: The effect of methyl salicylate. Teratology 6(2):191-196.
Yager, J.W. 1987. Effect of concentration-time parameters on sister-chromatid exchanges induced in rabbit lymphocytes by ethylene oxide inhalation. Mutat. Res. 182(6):343-352.
Yong, L.C., P.A. Schulte, J.K. Wiencke, M.F. Boeniger, L.B. Connally, J.T. Walker, E.A. Whelan, and E.M. Ward. 2001. Hemaglobin adducts and sister chromatid exchanges in hospital workers exposed to ethylene oxide: Effects of glutathione-S-transferase T1 and M1 genotypes. Cancer Epidemiol. Biomarkers Prev. 10(5):539-550.
Zey, J.N., V.D. Mortimer, and L.J. Elliott. 1994. Ethylene oxide exposures to hospital sterilization workers from poor ventilation design. Appl. Occup. Environ. Hyg. 9(9):633-641.
APPENDIX A
DERIVATION OF AEGL VALUES FOR ETHYLENE OXIDE
Derivation of AEGL-2
|
Key studies: |
Snellings et al. 1982a; Mandella 1997a |
|
Toxicity end point: |
NOAEL for neurotoxicity and developmental toxicity in rats, 100 ppm |
|
Time-scaling: |
ten Berge’s equation: Cn × t = k, where n = 1.2 derived from rat data |
|
Uncertainty factors: |
Total = 10 3 for interspecies sensitivity 3 for intraspecies variability |
|
Calculations: |
|
|
6-h exposure (experimental) |
C = 100 ppm/10 (uncertainty factor) = 10 ppm Cn × t = k; n = 1.2 C = 10 ppm, t = 6 h, k = 95.09 ppm-h |
|
10-min AEGL-2 |
80 ppm, same as the 0.5-h value |
|
30-min AEGL-2 |
C = (k/t)1/1.2 = (95.09 ppm-h /0.5 h)1/1.2 = 80 ppm C = 80 ppm |
|
1-h AEGL-2 |
C = (k/t)1/1.2 = (95.09 ppm-h /1 h)1/1.2 = 45 ppm C = 45 ppm |
|
4-h, AEGL-2 |
C = (k/t)1/1.2 = (95.09 ppm-h /4 h)1/1.2 = 14 ppm C = 14 ppm |
|
8-h AEGL-2 |
C = (k/t)1/1.2 = (95.09 ppm-h /8 h)1/1.2 = 7.9 ppm C = 7.9 ppm |
Derivation of AEGL-3
|
Key study: |
Jacobson et al. 1956 |
|
Toxicity end point: |
Lethality; the LC50 for white male rats was 1,460 ppm for a 4-h exposure. The data were extrapolated to a LC01 (628 ppm) to approximate the lethality threshold. |
|
Time-scaling: |
ten Berge’s equation: Cn × t = k, where n = 1.2 derived from rat data |
|
Uncertainty factors: |
Total = 10 3 for interspecies sensitivity 3 for intraspecies variability |
|
Calculations: |
C = 628 ppm/10 (uncertainty factor) = 62.8 ppm Cn × t = k; c = 62.8 ppm, n= 1.2, t = 4 h, k = 574.93 ppm-h |
|
10-min AEGL-3 |
360 ppm (same as the 0.5-h value) |
|
30-min AEGL-3 |
C = (k/t)1/1.2 = (574.93 ppm-h /0.5 h)1/1.2 = 355 ppm C = 360 |
|
1-h AEGL-3 |
C = (k/t)1/1.2 = (574.93 ppm-h /1 h)1/1.2 = 199 ppm C = 200 ppm |
|
4-h AEGL-3 |
C = (k/t)1/2 = (574.93 ppm-h /4 h)1/1.2 = 62.8 ppm C = 63 ppm |
|
8-h AEGL-3 |
C = (k/t)1/1.2 = (574.93 ppm-h /8 h)1/1.2 = 35 ppm C = 35 ppm |
APPENDIX B
PRELIMINARY CANCER ASSESSMENT OF ETHYLENE OXIDE
In 1985, EPA reported a unit risk or q1* for inhalation exposure to ethylene of 1 ×10-4 μg/m3 based on the combined incidences of leukemia and brain gliomas in F344 rats as reported by Snellings et al. (1981) (EPA 1985). A study by NTP (1987) was completed after EPA conducted its risk assessment of ethylene oxide. This study was summarized in Table 2-15 of the text and the data for lung tumors in female mice will be used to calculate another unit risk (q1*). The calculations of the unit risk and the AEGL values for carcinogenicity are presented below.
Data summary (NTP 1987): Groups of 50 male and 50 female B6C3F1 mice were exposed to 0, 50, or 100 ppm for 6 h/day, 5 days/week, for 102 weeks. The incidence of lung adenomas and carcinomas in females was 2/49, 5.48, or 22/49 for 0, 50, or 100 ppm, respectively.
Derivation of the unit risk for ethylene oxide:
Convert exposure concentrations for 6 h/day and 5 days/week to continuous exposure:
The unit risk (q1*) derived from the linearized multistage model is 8.82 × 10–3 (mg/m3)–1.
The calculations for AEGL values following the method presented by NRC (1986a) are presented below.
To calculate a “virtually safe dose” of d at a cancer risk of 10–4:
To calculate the total cumulative dose for a total lifetime exposure of 70 years, which is equivalent to 25,600 days:
In the adjustment to allow for uncertainties in assessing potential cancer risks under short-term exposures under the multistage model (Crump and Howe 1984), the total dose is divided by a factor of 6:
Therefore, a 24-h exposure concentration associated with a 10−4 risk is 26.7 ppm. The 10−4 cancer risk associated with exposures for 10, 30, 60, 240, and 480 min can be calculated from the following equation:
The AEGL values associated with risks of 10−4, 10−5, and 10−6 are presented in Table B-1.
TABLE B-1 AEGL Values Associated with Different Risks
|
Exposure Time |
10−4 |
10−5 |
10−6 |
|
10 min |
1,300 ppm |
130 ppm |
13 ppm |
|
30 min |
1,300 ppm |
130 ppm |
13 ppm |
|
1 h |
640 ppm |
64 ppm |
6.4 ppm |
|
4 h |
160 ppm |
16 ppm |
1.6 ppm |
|
8 h |
80 ppm |
8 ppm |
0.8 ppm |
APPENDIX C
DERIVATION OF THE LEVEL OF DISTINCT ODOR AWARENESS (LOA) FOR ETHYLENE OXIDE
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than one-half of the exposed population will experience at least a distinct odor intensity and about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
The odor detection threshold (OT50) for ethylene oxide is calculated from the odor threshold of 260 ppm reported by Hellman and Small (1974) and adjusted by Van Doorn et al. (2002):
The concentration (C) leading to an odor intensity (I) of distinct odor detection (I = 3) is derived by using the Fechner function:
For the Fechner coefficient, the default kw = 2.33 is used because of the lack of chemical-specific data:
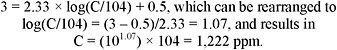
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in everyday life, factors, such as sex, age, sleep, smoking, upper airway infections, and allergy, as well as distraction, increase the odor detection threshold by up to a factor of 4. In addition, it takes into account that odor perception is very fast (about 5 s), which leads to the perception of concentration peaks. Based on the current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustments for distraction and peak exposure lead to a correction factor of 4/3 = 1.33.
Therefore, the LOA for ethylene oxide is 1,625 ppm.
APPENDIX D
ACUTE EXPOSURE GUIDELINE LEVELS FOR ETHYLENE OXIDE
Derivation Summary for Ethylene Oxide
AEGL-1 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not recommended |
||||
|
Key reference: Not applicable |
||||
|
Test species, strain, number: Not applicable |
||||
|
Exposure route, concentration, durations: Not applicable |
||||
|
Effects: Not applicable |
||||
|
End point, concentration, rationale: Not applicable |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: Not applicable |
||||
|
Interspecies: Not applicable Intraspecies: Not applicable |
||||
|
Modifying Factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable |
||||
|
Time-scaling: Not applicable |
||||
AEGL-2 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
80 ppm |
80 ppm |
45 ppm |
14 ppm |
7.9 ppm |
|
Key references: Snellings, W.M., R.R. Maronpot, J.P. Zelenak, and C.P. Laffoon. 1982a. Teratology study in Fischer 344 rats exposed to ethylene oxide by inhalation. Toxicol. Appl. Pharmacol. 64(3):476-481. Mandella, R.C. 1997a. An Acute Inhalation Neurotoxicity Study of Ethylene Oxide (498-95-A) in the Rat Via Whole-Body Exposure. Final Report. Study No. 95-6097. Prepared by Huntingdon Life Sciences, East Millstone, NJ , for Allied Signal, Inc, Morristown, NJ, and ARC Chemical Division, Balchem Corporation, Slate Hill, NY. |
||||
|
Test species, strain, number: Sprague-Dawley rats, 10/sex/group |
||||
|
Exposure route, concentration, durations: Inhalation; 0, 100, 300, or 500 ppm for 6 h |
||||
|
Effects: Developmental toxicity 10 ppm: no effect 33 ppm: no effect |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
80 ppm |
80 ppm |
45 ppm |
14 ppm |
7.9 ppm |
|
Effects: 100 ppm: statistically significant decrease in fetal body weight and non-statistically significant increase in the litter incidence of delayed ossification (not considered toxicologically significant) |
||||
|
Neurotoxicity: |
||||
|
0 ppm: droopy, half-closed eyelids (10%) and no response to approach (10%); other end points (0%) 100 ppm: droopy, half-closed eyelids (10%); low arousal (15%); and no response to approach (15%) 300 and 500 ppm: droopy, half-closed eyelids (25%, 40%**); impaired locomotion (10%, 15%); low arousal (30%,** 75%**); and no response to approach (35%,* 50%**) *p < 0.05; **p < 0.01 |
||||
|
End point, concentration, rationale: NOAEL for neurotoxicity at 100 ppm; low arousal was observed at 100 ppm, but the incidence did not reach statistical significance (p = 0.12, Fisher’s exact test); the next higher concentration of 300 ppm caused significant increases in the incidences of low arousal and no reaction to approach response. |
||||
|
Uncertainty factors and rationale: Total uncertainty factor:10 |
||||
|
Interspecies: 3, one potential mechanism of toxicity, direct alkylation of DNA and proteins, is not expected to differ across species. Neurotoxicity similar in rats and humans (distal axonal degeneration, neuropathy); PBPK models have shown that the AUC, peak blood levels, internal dose in mg/kg of body weight, and hemoglobin adduct level (measure of internal exposure) for humans are similar to or lower than the corresponding values for rats. Intraspecies: 3, An uncertainty factor of 3 was selected for intraspecies variability because glutathione-S-transferase polymorphism can modulate systemic exposure as measured by hemoglobin adducts but appears to be within a factor of 3 within the population. Individuals with asthma are not expected to respond differently to ethylene oxide concentrations below the odor detection and irritation thresholds. |
||||
|
Modifying factor: 1 |
||||
|
Animal to human dosimetric adjustment: 1 |
||||
|
Time-scaling: Cn × t = k, where n = 1.2 as determined from empirical LC50 data for the rat for 1 and 4 h. |
||||
|
Data quality and support for the AEGL values: Human studies to evaluate adverse effects of ethylene oxide on reproduction and development have not been conclusive. However, multiple animal studies showed that ethylene oxide is a developmental toxicant in rats and mice. Humans and rats show similar manifestations of peripheral neurotoxicity; legs and hindlimbs are primary targets, with distal axonal degeneration and peripheral neuropathy developing in humans and rats. The AEGL-2 values are below the concentrations that cause respiratory tract irritation and are below the odor detection threshold. |
||||
AEGL-3 VALUES
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
360 ppm |
360 ppm |
200 ppm |
63 ppm |
35 ppm |
|
Key reference: Jacobson, K.H., E.B. Hackley, and l. Feinsliver. 1956. The toxicity of inhaled ethylene oxide and propylene oxide vapors. AMA Arch. Ind. Health 13(3): 237-244. |
||||
|
Test species, strain, number: White male rats, 10 per group |
||||
|
Exposure route, concentration, durations: Inhalation, 882, 1,343, 1,648, 1,843, 1,992, 2,298 ppm for 4 h. |
||||
|
Effects: Clinical signs: Frequent movement and preening, clear nasal discharge, lacrimation, salivation, diarrhea, gasping, and death. Gross observations: Signs of upper respiratory tract irritation, tracheal congestion, and petechial hemorrhages and mild edema in the lungs and peribronchial region. Mortality: 882 ppm (2/10), 1,343 ppm (2/10), 1,648 ppm (4/10), 1,843 ppm (9/10), 1,992 ppm (10/10), and 2,298 ppm (10/10); LC50 = 1460 ppm. |
||||
|
End point, concentration, rationale: Lethality; LC01 = 628 ppm for 4 h, the estimated threshold for lethality derived by probit analysis of the data. |
||||
|
Uncertainty factors and rationale: Total uncertainty factor: 10 |
||||
|
Interspecies: 3, one potential mechanism of toxicity, direct alkylation of DNA and proteins, is not expected to differ across species. PBPK models have shown that the AUC, peak blood levels, internal dose in mg/kg of body weight, and hemoglobin adduct level (measure of internal exposure) for humans are similar to or lower than the corresponding values for rats. Intraspecies: 3, An uncertainty factor of 3 was selected for intraspecies variability because glutathione-S-transferase polymorphism can modulate systemic exposure as measured by hemoglobin adducts but appears to be within a factor of 3 within the population. Individuals with asthma are not expected be respond differently to ethylene oxide concentrations below or slightly above the odor detection and irritation thresholds. An interspecies or an intraspecies uncertainty factor of 10 would place AEGL-3 values below concentrations likely to be associated with life-threatening events. |
||||
|
Modifying factor: 1 |
||||
|
Animal to human dosimetric adjustment: 1 |
||||
|
Time-scaling: Cn × t = k, where n = 1.2 as determined from empirical LC50 data for the rat for 1 and 4 h. |
||||
|
Data quality and support of AEGL values: AEGL-3 values for ethylene oxide were derived from one of several well-conducted studies. AEGL-3 values are below the estimated 10–4 risk associated with the lifetime risk of developing cancer after a single exposure. The 10- and 30-min values exceed the lower limit on the odor detection threshold. Respiratory tract irritation may occur at these concentrations and reversible neurologic effects may occur at the AEGL-3 concentrations, but life-threatening events are unlikely to occur. |
||||