5
Clinical Sciences Research
Reseach in the clinical sciences helps put into practice the discoveries that arise from the research in the fields described in the three previous chapters. Because the term “clinical research” is used to cover such a broad and diverse array of activities, its definition has proved to be controversial, primarily over the issue of whether the research does or does not require direct interaction with living patients or other human research subjects. The most expansive definition of clinical research is that agreed upon in 1998 at the Graylyn Clinical Research Consensus Development Conference, organized by the Association of American Medical Colleges (AAMC), the American Medical Association (AMA), and the Wake Forest University Medical Center. The Graylyn conferees defined
clinical research as a component of medical and health research intended to produce knowledge valuable for the understanding of human disease, preventing and treating illness, and promoting health. Clinical research involves interactions with patients, diagnostic clinical materials or data, or populations in any of the following areas: (1) disease mechanisms (etiopathogenesis); (2) bi-directional integrative (translational) research; (3) clinical knowledge, detection, diagnosis and natural history of disease; (4) therapeutic interventions including clinical trials of drugs, biologics, devices and instruments; (5) prevention (primary and secondary) and health promotion; (6) behavioral research; (7) health services research, including outcomes, and cost-effectiveness; (8) epidemiology; and (9) community-based trials.1
This definition was adopted by the U.S. Congress in the Clinical Research Enhancement Act (P.L. 110-148) of November 2000.
In response to this definition, those determined to carve out and distinguish research requiring direct interaction with living patients coined the term patient-oriented research. Another distinction is commonly made for translational research, which describes research that explores the applicability of the results of basic research to clinical care (for example, in clinical trials, especially early Phase 1 or 2 trials). In addition, translational research may also include studies of how to facilitate the introduction of newly established clinical knowledge into broad clinical practice and the obstacles thereto, or it may describe studies of the clinical effectiveness or cost effectiveness of new knowledge applied in clinical practice across very large and diverse populations. A publication authored by members of the Institute of Medicine’s Clinical Research Roundtable2 proposed that the first two of these different kinds of translational research, with their different strategies, technologies, time scales, training, and resource requirements, be distinguished as T1 and T2 and that the obstacles encountered be referred to as T1 blocks and T2 blocks, respectively. Subsequently, others have carried this terminology further by designating T3 blocks and even T4 blocks. This terminology has become widely accepted.
Despite the critical role that clinical research in all its forms plays in achieving the nation’s health goals, the clinical research enterprise has for years been underdeveloped. Recent scientific advances have begun to set the stage for a dramatic transformation of our capacity to diagnose, prevent, and treat disease and disability. But accomplishing this transformation will not only require the translation and wide-scale application of these increasingly remarkable basic research advances into health care practice, but will also demand profound changes in individual and group behaviors. The latter will not be achievable without substantially enhancing our understanding of individual and population behaviors, which in turn will require significantly greater investment in the
|
1 |
Summary of Report of the Graylyn Development Consensus Conference, November 1998, from Report 13 of the Council on Scientific Affairs (I-99), Update on Clinical Research. Available online at: http://www.ama-assn.org/ama/pub/article/2036-2392.html. |
|
2 |
Sung, N.S., W.F. Crowley, Jr., M. Genel, P. Salber, L. Sandy, L.M. Sherwood, S.B. Johnson, V. Catanese, H. Tilson, K. Getz, E.L. Larson, D. Scheinberg, E.A. Reece, H. Slavkin, A. Dobs, J. Grebb, R.A. Martinez, A. Korn, and D. Rimoin. 2003. Central challenges facing the national clinical research enterprise. JAMA 289:1278-1287. |
social and behavioral sciences to accomplish the transformation of our health-care system from its primary focus on individual health care to a concentration on individual and population health maintenance.
Health services research, which involves the study of the efficiency, effectiveness, and costs of health care practices and systems, has become indispensable to understanding and informing the future of health care. Despite the promises of a more rational and equitable health care marketplace envisioned in the Health Care Reform Act, health care costs have been rising steadily for decades and consuming an increasing fraction of the nation’s gross domestic product. Expenditures in the United States on health care surpassed $2.3 trillion in 2008, more than three times the $714 billion spent in 1990, and over eight times the $253 billion spent in 1980. This relentless growth in costs, coupled with the aging of the American population, the severe economic recession, and the sharply rising federal deficit, is placing great strains on the private-sector, state, and federal systems used to finance health care, including private employer-sponsored health insurance coverage and public insurance programs such as Medicare and Medicaid.
The quality of the nation’s health care system has been an issue for many years. In 2001, the IOM, launched an effort to examine and recommend improvements in the nation’s quality of care. Successive IOM reports have highlighted the unacceptably poor status of our health care system as a whole, the high frequency and costs of medical errors resulting as much from systemic as individual failures, the almost unique failure of the health care industry in comparison with other sectors of the U.S. economy to adopt and exploit powerful new information technologies, and the shameful and adverse consequences of the continuing problem of the uninsured. Another effort to highlight the quality of health care began in 2003 with the publication of a series of reports by the Agency for Healthcare Research and Quality (AHRQ) that address the state of health care from the perspective of quality and disparity. These reports describe in great detail the impact of the organizational, administrative, financing, safety, access and other deficits of our cobbled-together health-care “system” on individuals, communities, businesses, and the entire nation. The need to address these major problems makes it imperative that “clinical research” be broadly conceived to encompass the assessment of health outcomes, cost-effectiveness, finance, access, information strategies, and other research related to the organization, deployment, utilization and quality of the nation’s health-care systems and services. At this time it is difficult to estimate the impact of the 2010 Patient Protection and Affordable Care Health Care Reform Act on the opportunities and challenges in clinical research.
There are many factors contributing to the continued underdevelopment of the clinical research enterprise. These include: (a) the extra time and expense required for clinical research training along with the inherent complexity, difficulty, and costs of patient-oriented clinical research, and the challenges these pose in competing successfully for sponsored research support, especially from National Institutes of Health,3 (b) the sharply declining ability to cross-subsidize clinical research from hospital and faculty clinical practice income as a result of the major changes wrought in health care financing over the past 20 years, (c) the debt burden that inclines many physicians in training to forgo clinical research careers for the more likely rewards of clinical practice, and finally, (d) the still uncertain status of the full spectrum of clinical research within the culture of the academic health center, where traditionally, basic science and clinical prowess have often been valued more highly than clinical research. Notwithstanding this formidable array of deterrents, abundant anecdotal evidence indicates that physician-scientists who leave research careers often do so because of insufficient institutional support, a perceived lack of available mentors, licensure regulations, and role models and the attendant discouragement.4
The need for increased investment in clinical research has been increasingly recognized in diverse funding programs—public, private, and philanthropic—as well as in academic medical and health centers.5 These issues were addressed by Task Force II, a group assembled by the AAMC to analyze the problems posed by the need to develop the full potential of clinical research. A number of the recommendations of Task Force II have been realized, including the requirement by the accrediting bodies of medical schools (ACME) and residency programs Accreditation Council for Graduate Medical Education (ACGME), respectively, that all medical students and all residents be exposed to the principles of clinical research; having medical schools assume central oversight of clinical research training programs in order to ensure the “protected time” of trainee; and that academic medical centers invest in shared core facilities to support translational and clinical research.
Nevertheless, that this underinvestment continues is indicated by the remarkably small fraction of the total annual expenditures directed to health care that is invested in clinical research. The NIH is the single largest public-sector source of funding for clinical research, and its commitment to clinical research has increased substantially since the late 1990s, driven in part by the recommendations of the highly influential report of the NIH Director’s Panel on Clinical Research, chaired by David G. Nathan and released in December 1997. Although NIH support of clinical research awards during
|
BOX 5-1 Recommendations from the Association of American Medical Colleges Task Force II Report, Promoting Translational and Clinical Science: The Critical Role of Medical Schools and Teaching Hospitals Recommendation 1: Every future physician should receive a thorough education in the basic principles of translational and clinical research, both in medical school and during residency training. Recommendation 2: The Liaison Committee on Medical Education (LCME) should add education in translational and clinical research to the requirements for medical school accreditation, and the Accreditation Council for Graduate Medical Education (ACGME) should embed understanding of translational and clinical research within its required core competencies. Recommendation 3: Training for translational and clinical investigators should comprise completion of an advanced degree with a thesis project (or an equivalent educational experience), tutelage by an appropriate mentor, and a substantive postdoctoral training experience. Recommendation 4: Sufficient support should be given to new junior faculty who are translational and clinical investigators to maximize their probability of success. Recommendation 5: Training in translational and clinical research should be accelerated through comprehensive re-structuring so that these scientists can become independent clinicians and investigators at the earliest possible time. Recommendation 6: Institutions, journals, the NIH, and other research sponsors should take steps to facilitate appropriate academic recognition of translational and clinical scientists for their contributions to collaborative research. Recommendation 7: The NIH should modify the K23 and K24 awards to enhance their value in supporting clinical and translational research training and mentoring. |
the proceeding two decades had remained largely constant at about 34 percent of total extramural research dollars, the NIH has now launched several well-received training awards for junior and mid-career physician scientists. There are also other support mechanisms, most notably the Clinical and Translational Science Awards (CTSAs), directed by the National Center for Research Resources (NCRR) and launched in 2006, all of which are transforming the quality and quantity of support of physician-scientists in universities and academic health centers. Much of the NIH funding for the CTSA has been recovered from closing down the General Clinical Research Centers (GCRC) program, begun in the 1960s to create a national network of such centers, situated primarily in academic health centers, and targeted initially to support what was then cutting-edge studies of metabolic diseases in human research subjects.
As of July 2010, 55 CTSAs had been funded in universities and academic health centers across the country, creating local, regional, and national systems to increase the efficiency and productivity of clinical and translational research and to develop ways to reduce the time it takes for clinical research to become available for use in treatments for patients. The NIH intends that there will be 60 centers when this program becomes fully implemented in 2012, although that number may increase. The CTSA—which require partnerships not only among academic medical institutions and health centers with other components of universities, but also with community hospitals, clinics, and health care practices—are truly creating increasing interest and excitement in clinical research across universities and their community partners, as well as attracting non-biomedical investigators from across universities into multidisciplinary clinical research programs. However, it is too early to predict the ultimate success of this program or whether it will achieve its ambitious goals.
Notwithstanding these positive steps to enhance training and support for physician-researchers in the clinical sciences, the past two decades have been particularly challenging for the funding of all academic health professionals and especially for the support of research activities in the clinical environment that are not clearly tied to specified funding streams. Clinical research, broadly defined, has yet to achieve the breadth and depth of currency it deserves.
To develop the nation’s clinical research capacity will require a sufficient workforce of highly trained clinician investigators in the several health research professions as well as Ph.D.s in the diverse areas of knowledge that are encompassed in the expansive definition of “clinical research.” Building this workforce will require enhanced support across the clinical research disciplines and will especially require supporting clinician-scientists, who must be accomplished in both their clinical and their scientific disciplines.
DEFINING THE CLINICAL RESEARCH WORKFORCE
The clinical research workforce is as varied as the definition of the field. It consists of individuals with doctorates in the basic sciences, graduates of professional degree programs (mostly M.D.s), graduates of health sciences and public
health programs, and dual- or multiple-degree holders. These scientists play an important role in improving the capabilities and the delivery of the nation’s health care, because their research spans the spectrum from discovery to delivery to critical assessment of delivery and the functioning of the health care enterprise. Some areas of research, however, are purely clinical, such as health services, oral health, and nursing, and they will be addressed in later chapters of this report. We also address individuals who fit the expansive Graylyn definition, which embraces research in health services and in the social and behavioral sciences; these topics likewise will similarly be addressed in later chapters of this report.
With this definition in place, it has proved difficult to analyze the specific number of individuals in the clinical research workforce because current workforce databases focus on their current research areas. Therefore, the basic workforce analysis for this report will include Ph.D.s with degrees in the health fields listed in Appendix C, as well as that fraction of the M.D. population in medical school clinical departments that conduct NIH-supported clinical research, along with doctorates with a degree from a foreign institution that are in some way identified as clinical researchers. A major shortcoming of this approach is that does not capture the complete workforce, especially M.D.s who are involved in the design and oversight of clinical trials and as well as those conducting research in non-medical areas of an academic institution or in industrial laboratories.
EDUCATIONAL BACKGROUND OF THE CLINICAL RESEARCH WORKFORCE
The problems discussed in identifying those currently engaged in clinical research make it difficult to assess the educational background of clinical researchers in the same detail as is done for researchers in the biomedical and behavioral and social sciences, because such studies can only be done for those individuals who are currently participating in or have completed graduate programs that offer a Ph.D. in the clinical fields. The difficulty of such an approach to computing the overall workforce is underscored by the increasing numbers of Ph.D.s (both postdoctoral workers and faculty) from the basic biomedical sciences who are pursuing careers in clinical departments of medical schools and at major teaching hospitals. There are presently more of these Ph.D.s employed in the clinical departments than in basic sciences departments.
Many of these Ph.D.s, however, are likely to be involved in basic biomedical research, which happens to be performed in the labs of M.D. or M.D./Ph.D. scientists involved in biomedical research, albeit in a clinical department environment. At present there is no way of distinguishing between Ph.D.s conducting basic biomedical research from those involved in clinical research (see Figure 5-1).
GRADUATE STUDENTS
The following discussion draws on data from the National Science Foundation Survey of Graduate Students and Postdoctorates in Science and Engineering and records individuals who are studying in clinical departments (as defined in Appendix C). The graduate student population in these clinical departments at doctoral-granting institutions grew by 67 percent from 2000 to 2008 (see Figure 5-2). The growth in the number of graduate students is greater than that in the other broad fields in this study where the size of the graduate population has increased more slowly. It should also be noted that the robust growth is primarily reflects an increase in the number of female graduate students (see Figure 5-2). (Nursing graduate students were excluded from the data, because many of these students will not receive a doctorate, and the pool of students pursuing a doctorate is discussed in the nursing chapter.)
However, one has to be very cautious in interpreting the data of Figure 5-2. Given the fact reported below that only about 2,000 students graduated with a Ph.D. and that the best available evidence suggests that the time to degree was not much more than six years, we have to assume that 40 percent of the students listed in Figure 5-2 either quit or graduated with an M.S. degree. This is supported by the observation (see Figure 5-3) that typically 30 to 40 percent of these students were self-supporting, a circumstance more characteristic of master’s students than of those pursuing the Ph.D. The type of financial support the students in the clinical sciences receive is quite different from that in the other fields (Figure 5-3).
GRADUATE SUPPORT AND THE ROLE OF THE NRSA IN TRAINING
Figure 5-3 shows the mechanisms of support for full-time graduate students in the clinical sciences. The number of traineeships and fellowships for graduate support in the clinical sciences has held relatively constant, at about 4,000 students each year over the past decade. Support for the increased number of students has largely come from increased teaching assistantships, research assistantships, and, especially, from self funding. The sources of external support have also changed over time with NIH support growing from 10 percent in 1979 to 25 percent in 2008, and non-federal support (excluding self-support) growing from 25 percent to 60 percent over the same time period (see Figure 5-4).
NIH data for traineeships and fellowships shows a smaller number of National Research Service Awards (NRSAs) slots ranging from 823 in 2005 to 1,035 in 2008 (see Table 5-1). Like the other two broad fields in this study, support was rather constant in the 1990s. The decline in 2000 might be the result of higher stipend levels and the fixed NRSA budgets for the training programs. The difference among the numbers shown in Tables 5-1, 5-2, and Figure 5-4 is
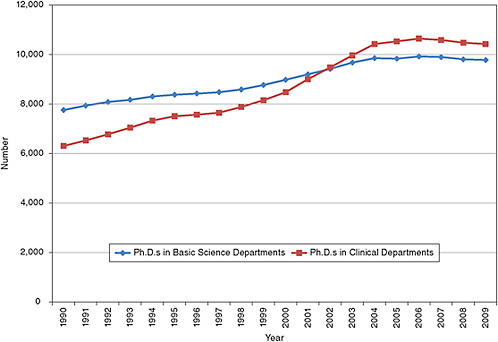
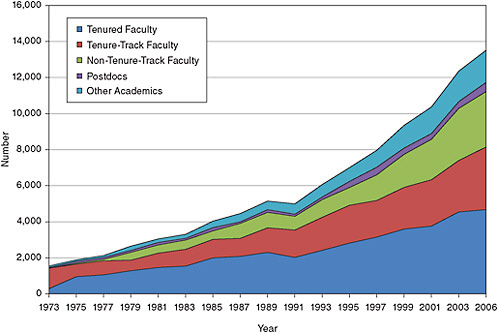
FIGURE 5-1 Tenured and tenure-track faculty by type of medical school department, 1990-2009.
SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
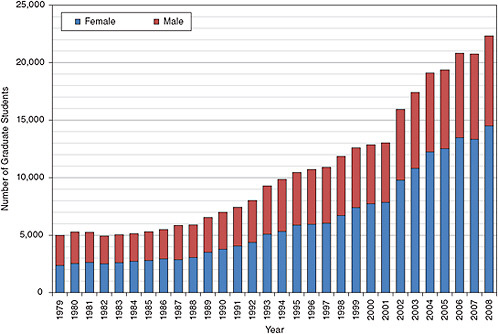
FIGURE 5-2 Full-time graduate enrollment in the clinical sciences.
SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
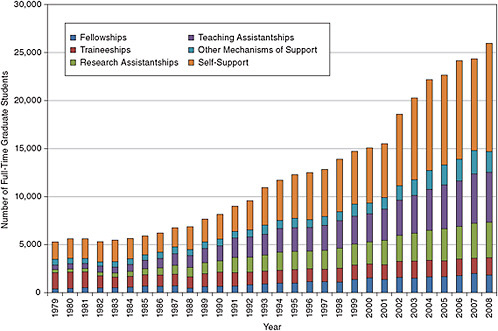
FIGURE 5-3 Mechanisms of support for full-time graduate students in the clinical sciences.
SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
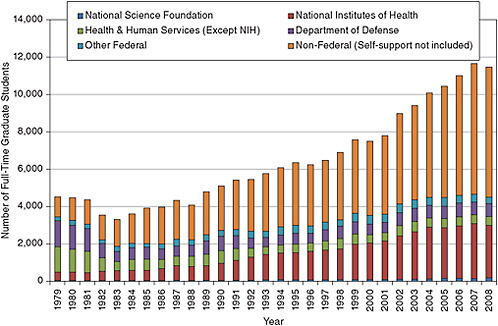
FIGURE 5-4 Sources of internal and external support of full-time graduate students in the clinical sciences.
SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
TABLE 5-1 NRSA Predoctoral Trainee and Fellowship Support in the Clinical Sciences (Excluding Health Services)
|
|
Trainees (T32) |
Fellowship (F30, F31) |
Total |
|
1990 |
385 |
153 |
538 |
|
1995 |
830 |
108 |
938 |
|
2000 |
558 |
123 |
681 |
|
2005 |
633 |
190 |
823 |
|
2006 |
602 |
209 |
811 |
|
2007 |
711 |
222 |
933 |
|
2008 |
807 |
228 |
1035 |
|
SOURCE: NIH database. |
|||
the result of NRSA support through other HHS agencies, primarily AHRQ.
The growth in the graduate population is naturally reflected in the number of doctoral degrees in the clinical sciences fields, with more than a six-fold increase from the early 1970s, and much of the increase involves an increased participation by women. The modest increase in male Ph.D.s is similar to what has happened in the graduate population more generally (Figure 5-5). The citizenship of doctorates in the clinical sciences differ from those in the biomedical sciences with about 16 percent awarded to temporary residents and 6 percent to permanent residents. However, minority participation accounted for about 12 percent of the degrees in 2008.
POSTDOCTORAL FELLOWS
Among Ph.D.s in the three fields, reviewed in this report, those in the clinical sciences are the least likely to have postdoctoral training, because less than 20 percent have traditionally planned such study versus the 30 percent and nearly 70 percent in the behavioral and biomedical sciences, respectively. It is likely that this small number of individuals specifically educated in research in the clinical sciences represents a minimum estimate of those involved in this type of research. One might add two additional categories to this postdoctoral pool, namely (a) individuals educated in basic biomedical research who have shifted to clinical research (and who may be expected to reside in clinical departments) and (b) international postdoctoral researchers trained in clinical research. One might be tempted to compute these numbers from the number of postdoctoral fellows in clinical departments. However it is clear that over the past two decades many Ph.D. postdoctorates and faculty in clinical departments have in fact conducted basic biomedical research, although the exact fraction of the total pool involved in clinical research is impossible to determine from the available data sources. Reflecting this point is the fact that the fraction of all postdoctoral fellows with medical degrees (not resident fellows) in clinical departments decreased from 61 percent in 1983 to 22 percent in 2008, while the number
TABLE 5-2 NRSA Postdoctoral Trainee and Fellowship Support in the Clinical Sciences (Excluding Health Services)
|
|
Trainees (T32) |
Fellowship (F32) |
Total |
|
1990 |
1287 |
99 |
1386 |
|
1995 |
1553 |
75 |
1628 |
|
2000 |
1467 |
93 |
1560 |
|
2005 |
1893 |
140 |
2033 |
|
2006 |
1930 |
131 |
2061 |
|
2007 |
1872 |
137 |
2009 |
|
2008 |
1968 |
143 |
2111 |
|
SOURCE: NIH database. |
|||
of foreign-educated postdoctoral fellows increased from 25 percent in 1983 to 45 percent in 2008.
Detailed data are not collected on the source of clinical research training support at the postdoctoral level by individual federal agency, but the type of training support, at least in academic institutions is available (Figure 5-6). The traineeships and fellowships portion has been increasing at a slow rate, while in contrast the number of individuals on research grants has increased five-fold since the late 1970s. The NRSA contribution to postdoctoral training support mirrors the general trend for fellows and trainees, but at a lower level, because support is available from sources other than NRSA (see Table 5-2).
THE CLINICIAL RESEARCH WORKFORCE
It is extremely difficulty to determine the number of individuals contributing to the clinical research workforce from the available data. The primary sources of data are the NSF Survey of Doctorate Recipients and the AAMC Faculty Roster. In the former dataset Ph.D.s are classified by the area in which they receive their degree as defined according to the fields listed in Appendix C. Since these are considered to be clinical fields, we surmise that they are likely to be conducting clinical research. The AAMC dataset is comprehensive with regard to Ph.D.s in clinical departments in medical schools, but as mentioned earlier conducting research in a clinical department does not imply that the research is clinical. Indeed, it is quite likely that individuals with Ph.D.s in either basic sciences or clinical departments are conducting biomedical research. With this in mind, because individuals with different degrees conduct clinical research and no data source comprehensively captures their activities, it is best to look at the workforce from the perspective of the different degrees that lead to a clinical researcher. The basic clinical workforce, as described by the NSF data, is composed of those 23,282 individuals in 2006 with a Ph.D. in those clinical fields characterized in Appendix C. This number is the potential workforce of individuals employed or seeking employment. Those employed in S&E number 22,229. More
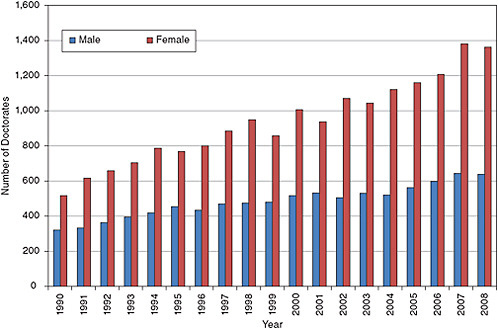
FIGURE 5-5 Doctoral degrees awarded in the clinical sciences.
SOURCE: NSF. 2008. Survey of Earned Doctorates. Washington, DC: NSF.
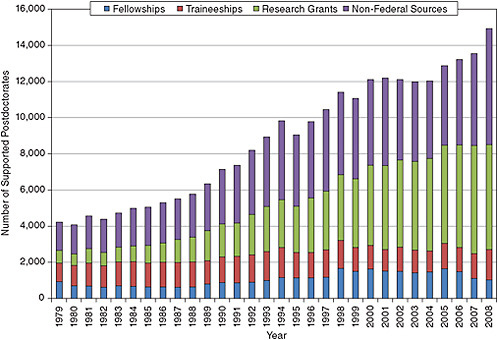
FIGURE 5-6 Academic postdoctoral support in the clinical sciences, 1979-2008.
SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
current data on the Ph.D.s in the workforce are not available, but the AAMC roster of medical school faculty has data through 2009. The number of M.D.s conducting clinical research in medical schools can be estimated from the number with R01 support in 2006 at about 2,950 and 2,850 in 2009. In addition there were about 1,450 M.D.s in 2006 and 1,550 in 2009 with other non-R01 forms of grant support from the NIH. A longitudinal examination of NIH data6 over a 40-year time span shows that the number of M.D.s applying for a first R01 grant has remained remarkably flat over most of that interval, and that in 2004 (the last year for which data were available) the number of M.D./Ph.D.s and M.D.s applying for a first R01 had become almost identical. Of course, neither of these counts captures the clinical researchers in the M.D. population that have support from non-NIH sources. As has been stressed repeatedly, even if we can ascertain the total number of M.D.s with R01 support it is still difficult to determine how many of these grants are for basic science alone.
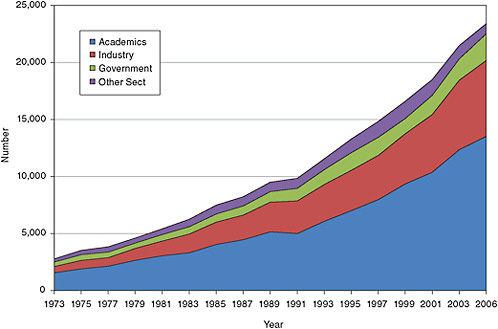
Thus the overall workforce is composed of approximately 12,000 graduate students, 5,000 postdoctoral fellows, some 23,000 Ph.D.s beyond the postdoctoral stage, a number of M.D.s that is poorly defined but probably not more than 1,000, and an unknown number of foreign-born scientists working in this area. The total number then is at least 41,000, of which 24,000 completed their graduate and postdoctoral education (see Figure 5-7). The overall clinical workforce, including postdoctoral fellows, has grown significantly from about 2,850 in the early 1970s to the current level. Much of this growth has been in the academic sector, but the industrial sector has also shown a significant increase as is shown in Figure 5-6. As was the case with the educational characteristics of clinical Ph.D.s, data on their career progression and employment characteristics are only well known for Ph.D.s from U.S. institutions. The steady growth in the aca-demic sector in the past decade has been due in part to the employment of non-tenure-track faculty and other academics (usually research associates) who jointly made up about 40 percent of the faculty in 2006 (see Figure 5-8).
Tenured and tenure-track faculty hold the majority of the positions, but their percentage has fallen from around 80 percent in the mid-1980s to 60 percent in 2006. This decline is not surprising, because there has been a movement by institutions toward temporary or soft money positions by institutions in many fields in recent years. This change in the composition of the faculty is confirmed in the AAMC data for medical schools, which show that from 1980 to 2009 the percentage of Ph.D. faculty in non-tenure-track positions in clinical departments increased from about 35 percent to near 60 percent (see Figure 5-9).
A concern for the clinical research workforce is the increase in the age at which individuals receive their doctor-ate. From 1986 to 2006 the median age of the workforce has increased from being in the 41 to 43 age cohort in 1986 to the 51 to 52 cohort in 2006 (Figure 5-10). The aging of the clinical workforce is also seen in the data from the AAMC Faculty Roster where the median age of the medical school faculty has increased from about 46 years to 52 years from 1989 to 2009 (see Figure 5-11).
The lower level of interest among postdoctoral training among those Ph.D. holders in fields listed in Appendix C is shown in Appendix Table F-5 and is reflected in the portion of the workforce that is working in postdoctoral positions. Only about 2 percent of the clinical U.S. Ph.D.s have held postdoctoral positions in recent years and almost all are in academic institutions. If the faculty in clinical departments is examined, the picture is somewhat different. There are about 8,000 U.S. citizens or permanent residents in these positions. The difference between in Appendix Table F-6 and Figure 5-12 is probably the result of Ph.D.s with biomedical degrees getting their training in clinical departments.
Table F-5 also shows that minorities only represented 8.6 percent of the clinical research population in 2006, even though their numbers grew from about 100 in 1973 to a little more than 1,100 in 2006. This is better than in the biomedical sciences and about the same as in the behavioral and social sciences. The data show, as they did for the other fields, a small number of temporary residents in the research population, but since the data reflect only those individuals who were trained in U.S. institutions, there may be a larger percentage of temporary residents in the workforce with foreign doctorates.
Although the number of M.D. clinical researchers is not known exactly it appears that in recent years individuals with a Ph.D. have dominated the field. In the 1970s only 2,600 Ph.D.s made up the workforce, and only a few hundred degrees were awarded each year. There did not appear to be a change in the number of M.D.s in clinical research since the 1970s, even though the Ph.D. workforce grew by a factor of seven during that time. There may be several reasons for this change, but a primary one is probably the increased educational debt of medical school graduates. Except for graduates of dual-degree (e.g., M.D./Ph.D. or D.D.S.//Ph.D.) programs, most physicians and dentists today begin their professional careers with sizable educational debts.
In 2009, the AAMC reported that the average educational debt of current graduates was $156,456, with 79 percent of the graduates having a debt of at least $100,000 and 58 percent having a debt of at least $150,000. The level of educational debt for dental students is comparable to that of medical students. In 2006, the average it was more than $130,571, and 72 percent had an educational debt of more than $100,000. The increased debt results from the practice in dental schools that requires students to purchase their dental instruments during their clinical training. Although health care professionals are permitted to postpone payments on their student loans during NRSA or other authorized research
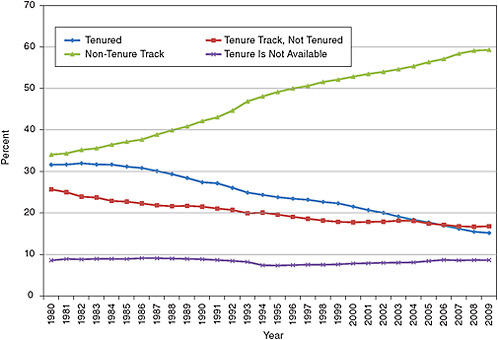
FIGURE 5-9 Tenure status of Ph.D.s in clinical departments in medical schools, 1980-2009.
SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
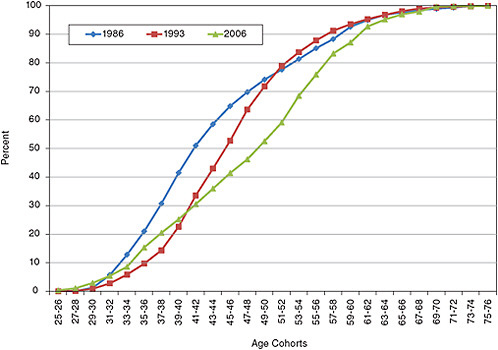
FIGURE 5-10 Cumulative age distribution for the clinical workforce.
SOURCE: NSF. Survey of Doctorate Recipients, 1973-2006. Washington, DC: NSF.
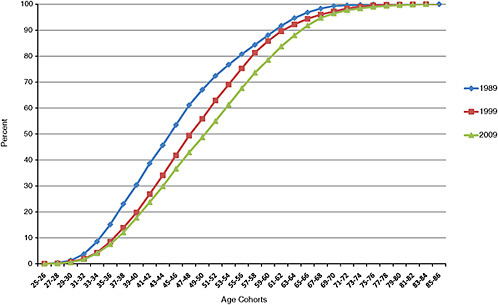
FIGURE 5-11 Age distribution of Ph.D.s on medical school faculty in clinical departments in 1989, 1999, and 2009.
SOURCE: AAMC. Association of American Medical Colleges Faculty Roster, 2009.
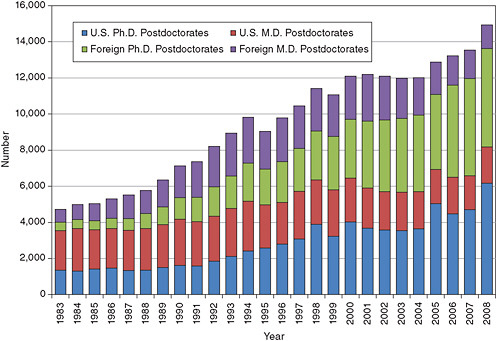
FIGURE 5-12 Clinical postdoctoral fellows by degree type.
SOURCE: NSF. 2008. Survey of Graduate Students and Postdoctorates in Science and Engineering. Washington, DC: NSF.
training programs, this option may not be widely used, and even if it were used, additional training places financial and other burdens on a young physician.
Congress has authorized several educational loan repayment programs for M.D.s who enter clinical research training programs, for minority M.D.s who pursue clinical training, and for others pursuing designated career paths. There are perhaps a half-dozen different programs authorized, and the NIH has been vigorous in making these programs known to successful candidates. The explicit purpose of these programs is to mitigate educational debt burdens for M.D.s pursuing clinical research training. M.D. graduates from clinical research training programs (e.g., those receiving one of the several K awards) must have protected time to develop their independent research careers, an increasingly difficult situation in today’s increasingly competitive health care markets. Another obstacle is the limitation on salaries for NIH-funded physician-investigators. The cap is set annually set by Congress to be no more than that of an executive grade; this grade has varied in recent years between level II and level I. It is now set at $199,700, and although that is not an insignificant amount, it is below what many practicing clinicians or medical faculty can earn.
Dual-Degree Training
In addition to predoctoral and postdoctoral program support in the clinical sciences through the NRSA mechanism, dual-degree programs are another attractive option for health care professionals seeking clinical research training. The NIH currently, has three dual-degree training programs: (1) the Medical Scientist Training Program (MSTP), (2) individual M.D./Ph.D. fellowships, and (3) the Dental Scientist Training Program (DSTP).
These dual-degree programs are very attractive, because they provide students with several career options, and the level of educational debt that students are left with is much lower than that for regular M.D. students. The MSTP in the National Institute of General Medical Sciences (NIGMS) is the largest and oldest programs, dating back to 1964, and today it funds 880 students training at 35 medical schools and universities. An additional 31 MSTP trainees are supported by other institutes. Offering fellowships for M.D./Ph.D. training is more recent; they were instituted in 1989 by the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse to encourage dual-degree training in the areas of mental health, behavior, and neuroscience. The fellowship program is much smaller in scale, supporting about 140 new students each year. The latest type of dual-degree training to be introduced is the DSTP, which was created following the recommendations from the 1994 study of the NRSA program. The National Institute of Dental and Craniofacial Research supports about 90 dual-degree dental students through the T32 and F30 DSTP—in 16 different dental schools (only 2 of these schools do not have T32 DSTP trainees).
A student in a typical M.D./Ph.D. program begins intensive research training after the second year of medical school. At this point in their training, the students have had little exposure to clinical medicine and the challenges and research opportunities that are inherent therein. After three-plus years completing work required for the Ph.D. degree, the students return to the medical curriculum for the third and fourth years. For dual-degree graduates who elect to pursue full clinical specialty training, an additional three to five, or more, years typically ensue before the individuals can turn their attention fully to research. At that point, to begin an additional formal program of clinical research training is unappealing.
The M.D./Ph.D. programs were envisioned as a way to bring more M.D.s into clinical research, but in practice relatively few participants receive research training in clinical research methods, and only about 20 percent of the M.D./Ph.D.s actually go on to pursue clinical research careers. Educational debt does not appear to be the reason, because their debt averaged about $15,000 in 2006. Many have argued that these programs are not effective in training clinical researchers because of their structure. An analysis in 1996 of the fields of study chosen by MSTP participants found that nearly 60 percent of graduates from the late 1980s and early 1990s had their Ph.D.s in five basic science fields: biochemistry, neuroscience, molecular biology, cell biology, and pharmacology. As a consequence the work they were exposed to in their Ph.D. program was focused on basic research, and this attracted them to a research career in the biomedical sciences. As a result in their subsequent research careers, MSTP graduates focused almost entirely on laboratory-oriented research, albeit typically in clinical departments and in areas of relevance to that clinical discipline, and they sought NIH funding for such research projects at the same rate as Ph.D.s.
Recognizing this problem NIGMS has recommended that institutions provide broader opportunities within the M.D./Ph.D. training mechanism. The institute issued new guidelines for the MSTP that urged medical schools with such training grants to extend their programs in order to give students “a breadth of doctoral research training opportunities” in fields including computer science, the social and behavioral sciences, economics, epidemiology, public health, bioengineering, biostatistics, and bioethics. However, most M.D./Ph.D. programs have been slow to respond, and there has been little change in the descriptions of the programs. And in most cases, the basic structure of two years/three years/two years persists.
In addition to formal dual M.D./Ph.D. programs, other approaches are being tried to attract M.D.s to clinical research. Examples include master’s level programs in specific clinical areas, which are becoming popular in some research-oriented medical schools and which may be designed to provide academic formal training in such areas as quantitative and methodological principles of medical
genomics, epidemiology, biostatistics, clinical trial design and analysis, etc. These programs appear to be very attractive to medical students and may encourage them to pursue careers as physicians in clinical research.
Clearly, identifying optimal training mechanisms for attracting medical students to clinical research, and then structuring effective training programs to prepare the students and graduates for successful clinical research careers, remains a large challenge for the biomedical community and the funding agencies. Toward this end, the recent adoption by the ACME and the ACGME of recommendations from the AAMC’s Task Force II on Translational and Clinical Research, viz., that medical students and residents should be exposed to the basic principles of translational and clinical research and to the research challenges and opportunities therein, may over time increase the population of medical graduates with a keen interest in pursuing clinical research careers. Finding mechanisms that will encourage students in these dual-degree programs to conduct clinical research continues to be a challenge.
RECOMMENDATIONS
Recommendation 5–1: The total number of NRSA positions awarded should remain at least at the 2008 level. Furthermore, training levels after 2008 should be commensurate with the rise in the total extramural research funding in the biomedical, clinical, and behavioral and social sciences. A decline in extramural research would also call for a decline in training.
Recommendation 5–2: The NIH, in consultation with academic medical leadership, should exercise leadership in identifying better training mechanisms for attracting medical students into translational and clinical research, and the NIH should fund pilot programs designed to implement promising new approaches to accomplishing that objective.