2
The Future Impact of Current Decisions
|
Key Findings
|
While policy makers’ decisions can have only a small effect on the magnitude of the HIV/AIDS epidemic and its burden1 by 2020, decision makers do have time to set a new course for the epidemic so that the prospects for 2020 onward look more optimistic than is the case today. Depending on decisions outlined in this chapter, morbidity, mortality, and resource and financial burdens faced in 2020 by the U.S. government and African countries for the ensuing decades could differ by a factor of 10 or more. Available policy choices include not only the rate at which HIV/AIDS treatment is scaled up, but also how it is scaled up and how scale-up is linked to the effectiveness of prevention. This chapter examines in turn the potential for reducing HIV incidence, the potential for reducing treatment need, and the key policy choices and associated trade-offs that decision makers must consider in formulating long-term strategies for responding to the epidemic.
POTENTIAL FOR REDUCING HIV INCIDENCE
As noted in Chapter 1, a guiding principle of this study is the need to reduce the incidence of HIV (defined as the number of new infections during a given period of time). The number of people needing treatment in the short term is driven mainly by those currently infected; over time, however, those acquiring infection will require treatment. Reductions in the incidence of HIV infection will take some time to alter the burdens of treatment and mortality but are necessary if these burdens are eventually to decline. Currently, people are becoming in need of treatment more rapidly than they are being placed on treatment. This means that, although the death rate from AIDS has fallen, deaths among those in need of treatment will continue. To improve this situation, two things must happen: treatment coverage must increase, and incidence must fall. The pattern of incidence of new HIV infections will determine the treatment need or, if treatment is not available, the death rate.
The ability to measure and monitor HIV incidence is critical to the capacity to monitor epidemiological trends within selected populations; assess the effectiveness of intervention programs; and appropriately direct limited resources for treatment, prevention, and care. Knowledge of HIV incidence is necessary both to understand transmission patterns and to project the burden of HIV infection in different demographic and at-risk populations. Reliable information on HIV incidence is especially important to support prevention programs in resource-
|
1 |
The burden of HIV/AIDS for a population in a given year can be defined to include five components: its impact on health, on national income, on donor and domestic spending, on the workload of health care providers and institutions, and on the dependence of patients on a daily dose of medication for their survival. The committee’s quantitative estimates of burden in this chapter and Appendix A focus on HIV prevalence as an indicator of the total health burden, on the total number of patients on treatment, on the total spending required to sustain treatment, and on spending as a share of projected national health expenditures. Chapter 4 considers in more depth the burden of HIV prevalence on the health sector in African states. |
limited African countries that continue to bear a disproportionate share of the global burden of HIV/AIDS. Improved estimates of HIV incidence are essential for evaluating ongoing HIV/AIDS prevention and treatment programs in these settings and for guiding the most effective use of the billions of dollars that will be spent on combating the epidemic in the coming years (Busch et al., 2010; Fiamma et al., 2010; Mastro et al., 2010; Welte et al., 2010).
In the past, several methods, including prospective follow-up of cohorts of HIV-negative individuals and mathematical models based on epidemiological information about HIV prevalence and AIDS diagnoses or death rates, were used to estimate incidence over time. Unfortunately, these methods were subject to bias with respect to being unrepresentative of larger populations; were costly; and were directly impacted by the introduction of antiretroviral therapy (ART), which affected the calculation of incidence based on prevalence estimates. As a result of these complexities and limitations of epidemiological and modeling approaches to measuring HIV incidence, there has long been an effort to develop laboratory methods that can distinguish recent from established or long-term HIV infection as a means of estimating HIV incidence. Several assays and algorithms have been developed that have been partially successful in estimating incidence in cross-sectional surveys. However, there is an urgent need to make these assays more sensitive and specific so that accurate estimates of incidence will be available to medical and policy leaders.
Evidence of What Works from Randomized Controlled Trials
Experimental evidence on successful HIV prevention interventions is limited. The randomized controlled trial (RCT), which ensures internal validity in testing and intervention, relies on a clear, ethical, and replicable intervention that can be randomly allocated to a treatment and a control group (Susser, 1996). An effect will be more apparent when the effect size is large against a control providing no effect, and is more readily achieved for biomedical procedures for which adherence is ensured. In addition, the sample size is dependent upon the number of events that can be measured in the control arm. Such favorable conditions are not found when an intense, culturally specific intervention is appropriately implemented. Often because of a low incidence of HIV infection, intermediate behavioral endpoints have been used as surrogates for HIV incidence. However, the complex, context-specific relationship between risk behaviors and acquisition of HIV makes such intermediate endpoints inadequate without further validation. When HIV incidence has been used as an endpoint, many interventions have shown no effect (Padian et al., 2010).
Individual RCTs test the efficacy of interventions in preventing the acquisition of HIV in individuals. In such trials in the mid-1990s, prevention of mother-to-child transmission was confirmed as effective in reducing transmission to the baby by almost 70 percent (Connor et al., 1994). More recently, adult male cir-
cumcision has repeatedly been shown to reduce acquisition in men by 60 percent (Auvert et al., 2005; Bailey et al., 2007; Gray et al., 2007). Recently, hope for an effective vaccine has been raised by marginally significant results of a trial of a combined vaccine (Rerks-Ngarm et al., 2009). And further evidence of efficacy has been found for a vaginal microbicide containing tenofovir (Karim et al., 2010). While these results suggest the possibility of the eventual development of new prevention technologies, much work will be required before these early results can be translated into widely used products.
Moreover, the efficacy of an intervention in an individual does not imply its effect on the spread of HIV in a population. The population-level impact of an efficacious approach can be estimated through models or tested in community RCTs. Community RCTs make it possible to test interventions that can be implemented only at the population level (such as media campaigns) or programs that combine multiple approaches. They are often large and logistically complex, however, and therefore are few in number and provide little evidence of impact. See Box 2-1 for a summary of available results of such trials for sexually transmitted infections (STIs), a potential cofactor of HIV.
|
BOX 2-1 Results of Community Randomized Controlled Trials of Interventions for Sexually Transmitted Infections In Mwanza, Tanzania, syndromic management of STIs led to significantly (42 percent over 2 years) lower HIV incidence in intervention compared with control communities (Grosskurth et al., 1995). In Rakai, Uganda, mass antibiotic administration to control STIs and thereby HIV led to significant reductions in syphilis (relative risk [RR] = 0.8; 95 percent confidence interval [CI] 0.71–0.89) and trichomonas (RR = 0.59; 95 percent CI 0.38–0.91) but no reductions in gonorrhoea, chlamydia, or HIV (Wawer et al., 1999). Reductions in STI prevalence were observed in Masaka, Uganda, where the prevalence of active syphilis (RR = 0.52; 95 percent CI 0.27–0.98) and gonorrhoea (RR = 0.25; 95 percent CI 0.10–0.64) was lower in the syndromic management and information, education, and communication (IEC) arm (but not in the IEC-alone arm) compared with the control arm. However, there was no concomitant reduction in chlamydia prevalence and HIV incidence (Kamali et al., 2003). A trial of syndromic STI management and a peer-led targeted behavior change and condom promotion intervention in Manicaland, rural Zimbabwe, showed no impact on HIV incidence and no difference in reported STI syndromes (Gregson et al., 2007). A community RCT of adolescent sexual health interventions in Tanzania showed no impact on biological outcomes (Ross et al., 2007). STI control programs can decrease HIV incidence under certain programmatic settings and epidemic circumstances. Many negative trials following the promising original Mwanza study have suggested that the intervention in isolation is not robust in diminishing HIV incidence. Nevertheless, the role of STI control remains relevant for multicomponent interventions and should be studied further (Grosskurth et al., 2000; Tanton et al., 2010). |
Evidence of What Works from National Trends
While the results of RCTs are disappointing or inconclusive, observations of population trends in HIV incidence and associated risk behaviors tell a different story, revealing that substantial reductions in risk behaviors have led to detectable reductions in HIV prevalence. Sentinel surveillance data, along with cohort studies in Uganda, provided the first African example of such national successes (Kilian et al., 1999; Stoneburner and Low-Beer, 2004). Subsequently, similar reductions were observed in Zimbabwe (Gregson et al., 2006) and Kenya (Hallett et al., 2006). More recently, a review of trends in prevalence among 15- to 24-year-olds either attending antenatal clinics or participating in household-based surveys used as a marker of recent incidence rates2 found reductions in Botswana, Côte d’Ivoire, Ethiopia, Kenya, Malawi, Namibia, Zimbabwe, Zambia, South Africa, and Tanzania (International Group on Analysis of Trends in HIV Prevalence and Behaviours in Young People in Countries Most Affected by HIV, 2010). These findings demonstrate that HIV/AIDS prevention is possible. In other countries, there may have been reductions in incidence that cannot be distinguished from the expected saturation and stabilization in prevalence.
Unfortunately, identifying the sufficient or necessary conditions for national programs to attain such measurable success has not been possible. What distinguishes countries with and without declines in incidence is not certain. Retrospective analysis suggests that major shifts in the attitudes of populations are required, with information coming from trusted sources in a social and cultural environment rather than specific programmed activity. An analysis linking the intensity of intervention activities and declines in prevalence is not possible since the effort or expenditure preceding the changes has not been documented. If we take a more recent measure of expenditure to be representative of past expenditure, we might expect a relationship. Even when controlling for initial prevalence, however, there appears to be no relationship between expenditure and the scale of reductions in prevalence. Figure 2-1 shows that there is no discernible association between HIV/AIDS prevention spending per person and the degree of improvement in HIV prevalence among young people.
Is There Scope for Greater Effort?
Despite the substantial observed reductions in risk achieved in African countries with generalized epidemics, the levels of HIV incidence in Africa remain high compared with those in other regions. In Zimbabwe, for example, it has been estimated that incidence dropped by 40 percent in urban residents between 1999 and 2004—from around 5 percent per year to 3 percent per year (Hallett et al., 2009). In no African country has prevalence in 15- to 24-year-olds fallen
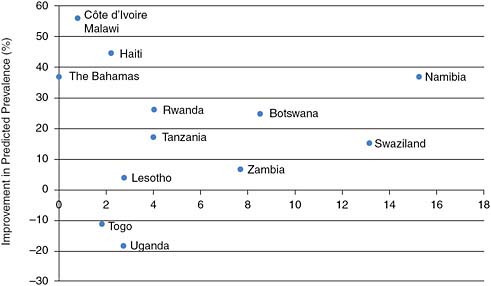
FIGURE 2-1 No obvious association is discernable between aggregate expenditure on prevention of HIV and degree of prevalence decline in countries where decline has been observed.
SOURCE: Committee’s calculations using prevalence data from International Group on Analysis of Trends in HIV Prevalence and Behaviours in Young People in Countries Most Affected by HIV (2010) and expenditure data from UNAIDS epidemic update (UNAIDS, 2008).
below 1 percent. Thus, even where prevention effects reduce the long-term burden of HIV/AIDS, the rate of new infections is still likely to outstrip even modestly ambitious rates of treatment uptake. Prospects for reducing incidence over the long term are more promising, however.
Figure 2-2 shows the results of a model constructed by the committee that assumes optimistically that the uptake rate of 30 percent of unmet need for treatment attained in 2007–2009 is sustained at WHO’s new definition of need,3 but with no additional prevention success, while donor support for treatment increases. For 2020, this model projects about 20 million people on treatment (Panel a) and treatment costs alone of about $15 billion (Panel c), with both measures of burden continuing to rise thereafter until reaching 50 million on treatment and $40 billion per year for treatment by 2050. In the absence of improved prevention, the number of new infections rises from 2 million per year currently to
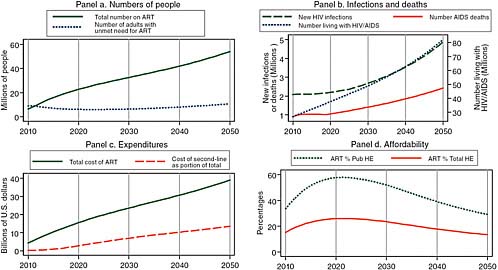
FIGURE 2-2 Assuming increased donor support for treatment without additional prevention, the committee projects for 2020 about 20 million people on treatment and treatment costs alone of about $15 billion.
SOURCE: Committee projections using data from UNAIDS (2008).
NOTE: Pub HE stands for public health expenditure and Total HE stands for total health expenditure.
2.3 million per year in 2020 and almost 5 million per year in 2050 (Panel b, left axis). While treatment expansion succeeds in holding AIDS mortality down to its current level of about 1 million per year through 2020, mortality then begins to rise as a result of the combined effects of accumulating new infections and the eventual deaths of those begun on treatment through 2020, so that annual deaths double to more than 2 million in 2050. With the annual number of new cases being twice as large as the number of deaths through 2020 and a growing multiple of deaths thereafter, the total number of people living with HIV/AIDS grows to 40 million in 2020 and then doubles to 80 million by 2050 (Panel b, right axis). Panel d of Figure 2-2 provides another perspective on the same total cost of HIV/AIDS treatment shown in Panel c, charting its evolution as a percentage of two measures of total African resource availability—total public health expenditure and total public and private health expenditure. Using World Bank estimates of future African economic growth rates and assuming that these expenditures grow at the same rate as the overall economies, the model predicts that after reaching peaks of about 60 percent of public and 25 percent of total health spending in 2020, these measures of burden will begin to improve as economic growth begins to outstrip the growth in treatment cost.
Figure 2-3 uses the same model as Figure 2-2 with the new WHO definition of need for treatment and 30 percent uptake of unmet need, but it assumes that male circumcision is successfully expanded to cover 90 percent of African men by 2025. Under these assumptions, increased donor support for treatment leads to 22 million on treatment at an annual cost of about $15 billion in 2020, about the same burden as in Figure 2-2 without the success of male circumcision, but the longer-term prospect is improved. As can be seen in Panel b of Figure 2-3, the impact of enhanced prevention is to slowly reduce the annual number of new infections until in 2030, it is less than the number of deaths from AIDS, and the number of people living with HIV/AIDS begins to decline.4 As a result of the momentum of the 27 million people surviving on treatment in 2030, the cost of HIV/AIDS treatment only levels off in absolute terms, but it does begin to decline after 2030 relative to projected African governments’ health expenditures.
Figure 2-4 shows the impact of adding other extremely effective prevention interventions to the successful male circumcision modeled in Figure 2-3. Assuming that by 2025 these other interventions reduce the HIV incidence rate to only 30 percent of what it would otherwise have been has little effect on the projected burden of the epidemic in 2020, but it has a dramatic effect by 2050. Because the number of people living with HIV/AIDS begins to decline in 2020, the number of people on treatment begins to decline in 2028, and by 2050, the annual cost of HIV/AIDS treatment has declined from its peak to about one-third less, with an annual cost of around $15 billion per year by 2050 compared with
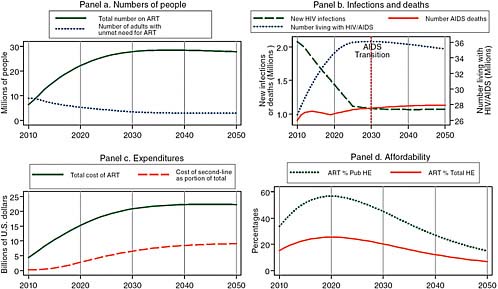
FIGURE 2-3 Even with male circumcision and the transmission-reducing effects of treatment, increased donor support for treatment leads to 22 million on treatment at an annual cost of about $15 billion in 2020, but the numbers living with HIV/AIDS begin to decline in 2030.
SOURCE: Committee calculations using data from UNAIDS (2008).
NOTE: Pub HE stands for public health expenditure and Total HE stands for total health expenditure.
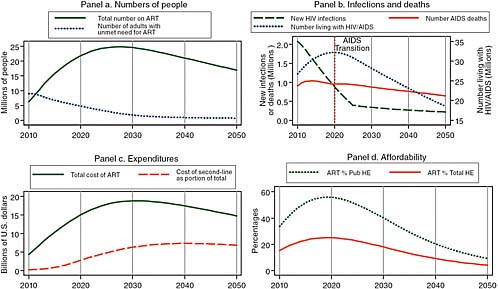
FIGURE 2-4 Given increased donor support and major prevention efforts, the committee projects reduced treatment need and reduced costs in 2050.
SOURCE: Committee calculations using data from UNAIDS (2008).
NOTE: Pub HE stands for public health expenditure and Total HE stands for total health expenditure.
about $23 billion per year at its peak around 2028. Furthermore, after peaking at almost 60 percent of health spending in 2020, the total cost of ART declines to about 10 percent of government health spending in 2050.
Given current trends to slow the recruitment of new patients to treatment and to proceed cautiously in recruiting patients before they have symptoms, treatment costs may be substantially reduced by 2020. Figure 2-5 presents one of the most plausible, if less optimistic, scenarios for 2020. In response to its statement of task, the committee designates this the “best” projection of the HIV/AIDS incidence and burden in Africa. It assumes that recent rates of uptake of about 10 percent of those with unmet need are sustained each year. This 10 percent uptake rate is less than the 30 percent rate achieved between 2007 and 2009, but roughly consonant with PEPFAR’s current goal of 4 million on treatment by 2014. This scenario also assumes that the median threshold CD4 count for treatment initiation remains as it is today, at about 130, and that prevention efforts remain modest, with circumcision reaching only 10 percent of African men by 2025. With this parsimonious approach to treatment and in the absence of improved prevention, the total number of patients on treatment in 2020 reaches 7 million in Africa, and annual expenditures for treatment total approximately $7 billion. The number of people living with HIV/AIDS continues to rise in the coming decades, reaching 70 million by 2050, when annual costs for HIV/AIDS treatment are more than $12 billion per year. The only positive note is that total costs are likely to decline as a proportion of total government health spending, on the assumption that African economies grow at the 4 to 5 percent rates that are projected for the next several years (The World Bank, 2010).
Thus, by 2050, there is little difference in annual treatment costs between the Figure 2-5 scenario of parsimonious treatment without prevention and the scenario of Figure 2-4 with increased donor support and strong HIV/AIDS prevention. The difference between the two is in the intervening cost profile (continuously falling as a percentage of government health spending in Figure 2-5 versus a sharp rise followed by a fall to the equivalent level in Figure 2-4), and in the numbers of and trend in deaths (in 2050, 3 million and rising in Figure 2-5 versus 700,000 and falling in Figure 2-4). Thus, donors resolve to bear a larger proportion of treatment costs, if accompanied by a sufficiently effective prevention effort, can improve the situation in Africa by 2050 relative to a more parsimonious strategy.
Under several plausible scenarios, then, while total HIV/AIDS treatment costs will continue to rise (because a larger share of people will be on more expensive second-line medications), they will eventually decline as a proportion of the health budgets of African countries. This decline will occur immediately if scale-up is parsimonious. Even with more generous scale-up, however, continued growth of the African economies at rates similar to those seen in the last decade, together with improved HIV/AIDS prevention, can produce a decline in HIV/AIDS treatment costs as a percentage of domestic health care spending after 2020
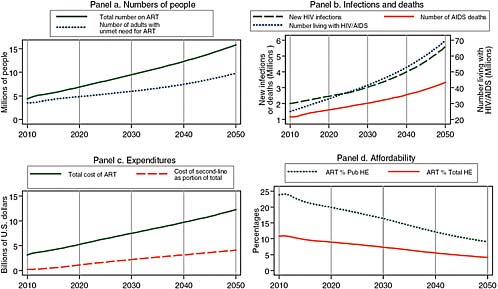
FIGURE 2-5 A plausible “best” projection for the future, assuming a parsimonious approach to treatment scale-up and no improvement in prevention efficacy, showing 7 million on treatment at an annual cost of $7 billion in 2020.
SOURCE: Committee projections using data from UNAIDS (2008).
NOTE: Pub HE stands for public health expenditure and Total HE stands for total health expenditure.
or 2025. Should this decline occur, African governments will be able to move toward greater ownership of their countries’ HIV/AIDS prevention, treatment, and care objectives in the foreseeable future.
Beyond the impact of behavior changes already observed, there might be benefits of expanding prevention efforts. Using interventions with observed efficacy in improving either HIV incidence or intermediate outcomes, we can calculate the predicted impact of prevention interventions, including high coverage of counseling and testing and adult male circumcision. Again the impact is substantial, but falls short of what might be described as controlling the spread of HIV/AIDS. Further reductions are possible, however, if infectiousness is reduced by expanding treatment and if we assume that this expansion leads to no increase in risk behavior (see the next section). The efficacy of a tenofovir vaginal microbicide and developments in vaccines (including the identification of broadly neutralizing antibodies) hold promise for providing additional prevention tools that would further reduce HIV incidence. In addition, structural interventions may be able to play a role. The success of individually oriented interventions in reducing risk behavior is substantially improved when prevention addresses the broader structural factors that shape or constrain individual behavior, such as poverty and wealth, gender, age, policy, and power (Gupta et al., 2008). In combination, these interventions may be able to dramatically reduce HIV incidence.
POTENTIAL FOR REDUCING TREATMENT NEED
If HIV incidence can be dramatically reduced so that more individuals are receiving treatment than are becoming newly infected, the need to initiate treatment will eventually start to decline. Given that treatment is a form of prevention, the earlier during the course of their infection individuals are initiated on treatment, the sooner this reduction in need for treatment will be realized.
It has been argued that a dramatic expansion of testing and immediate initiation of treatment in a “test and treat” strategy could reduce incidence to less than 0.1 percent per year (Burns et al., 2010; Dieffenbach and Fauci, 2009; Granich et al., 2009). The strategy modeled involved annual testing and assumed a 50 percent reduction in risk behavior to generate this optimistic scenario. The observed reduction in transmission in discordant couples in the context of an RCT—93 percent—will likely be less in normal treatment programs, where adherence may be lower and treatment failure more common. Nonetheless, observational studies suggest that the viral load associated with treatment generates the reduced transmissibility expected from studies of transmission as a function of viral load (Quinn et al., 2000; Semaye Fideli et al., 2001). Subsequent modeling work incorporating heterogeneities in risk behavior and greater transmissibility associated with primary viraemia suggests that reductions in incidence may be less than those in an initial model but would still be important.
Unfortunately, the effort required to ensure such regular testing and imme-
diate treatment would be great and beyond anything that has been achieved even in developed countries. Furthermore, there is a concern that increases in risk behavior seen in some developed countries among those receiving treatment and others in their communities (Fisher et al., 2004) could also occur in the heterosexual populations of Africa. To counter this concern, data from South Africa suggest dramatic reductions in risk behavior associated with receiving ART (Venkatesh et al., 2010). Unfortunately, excluding biases in reporting is difficult in such clinical settings. In addition, the behavior of those with a diagnosed HIV infection may be less relevant than the behavior of susceptible individuals and those whose infection is undiagnosed who may continue to spread the virus.
Despite these concerns, there may be a role for treatment as prevention, especially if future regimens can be made to be more easily administered and monitored, with fewer toxicities and simpler dosing. This is an aspiration of UNAIDS laid out in Treatment 2.0 (UNAIDS, 2010). Exploring the relationship among viral load and transmissibility, CD4 count, and period from infection to different CD4 counts, we can calculate the expected proportion of new HIV infections generated by infectious individuals before they start treatment. The earlier treatment begins, the greater the proportion of these infections we would expect to be averted. With approximately a third of new infections being associated with contact with those with a CD4 count below 200, we can expect good treatment coverage to impact the spread of HIV/AIDS, adding to the effect of other prevention interventions.
It should be noted that the optimism surrounding the prevention impact of treatment should be tempered by the distinct lack of population-level empirical evidence. One recent study linked the current diagnosis of HIV infections (presumably a delayed measure of incidence) to the current coverage of treatment (Montaner et al., 2010), but the interpretation of these data is controversial.
It should also be noted that, if earlier treatment is expanded, this obviously means that many more people will need to be on treatment in the short term. The decline in numbers requiring treatment will be gradual, with a pattern reflecting the distribution of periods from infection to treatment need. Using the median of approximately 9 years from HIV acquisition to a CD4 count of 200, with variation depending on age, prior health condition, and other factors (Aalen et al., 1997; Van Der Paal et al., 2007), the lag will be on the order of a decade. Thus any reduction in incidence achieved in 2010 will be only partially realized by 2020, and the benefits of reduced incidence need to be considered over a period of decades.
KEY POLICY CHOICES AND ASSOCIATED TRADE-OFFS
The burden of HIV/AIDS is extremely sensitive to alternative policies. Competing activities can consume available resources; the effects of these various
activities need to be compared and the trade-offs considered to guide decisions about optimum strategies.
Early Versus Late Treatment Initiation
Treatment initiation at a higher CD4 count leads to a better prognosis for those receiving treatment, and as noted above, the WHO guidelines have shifted to recommend earlier treatment. This means that each person treated receives the treatment for a longer period, potentially reducing the treatment available to others—presumably the thinking behind many countries not implementing the WHO guidelines.
Over the short term, allocating a fixed number of treatment slots to sicker patients should save more lives since those being treated would otherwise be near death. Over the longer term, the reduced treatment failure seen with early treatment could reduce the numbers of deaths and increase years of life saved. The optimum time for treatment initiation will therefore depend on the horizon over which benefits are calculated and the discounting that is used to weigh future costs and benefits against current costs and benefits.
In addition to changing the criteria for initiation, the new WHO treatment guidelines recommend the more expensive, less toxic tenofovir. The trade-off between this clinically preferred, more expensive regimen and a poorer, cheaper regimen is one of greater benefit for fewer treated patients.
Coverage with Second-Line Versus First-Line Regimens
Those failing first-line treatment require second-line regimens if they are to continue on treatment. Given that these second-line regimens are more expensive than first-line regimens, more people can be put on the latter for the same cost. Thus, the improved survival of treated patients if a second-line regimen is available should be weighed against the number of people that could benefit from first-line regimens.
Quality Versus Quantity of Programs: Follow-Up, Adherence, and Virological Tests
Similar trade-offs must be considered with respect to expensive programs that invest in following up to ensure that patients are maintained within the program, in promoting and ensuring adherence of patients, and in using laboratory tests to identify treatment failure. Virological measures allow treatment failure to be identified sooner than is possible through observation of symptoms, enabling second-line regimens to replace failing first-line regimens. In addition to improving survival, this approach has the benefit of preventing a failing regimen
from leading to greater drug resistance. An alternative to second-line regimens is to withdraw treatment. This approach would likely reduce survival, but would concomitantly reduce the expense of wasted treatment and the selective pressures on the virus.
Investment in Prevention Versus Treatment
Whether to invest in prevention or treatment has been a recurrent theme in debates about priorities in controlling HIV/AIDS. This trade-off was explicit in the earmarking of funds in the original President’s Emergency Plan for AIDS Relief (PEPFAR) authorization. To explore a trade-off between the benefits of treatment and prevention assumes that they achieve different results. As discussed in the preceding section, however, there is consensus that the two can have a synergistic effect. ART can reduce HIV transmission both directly (by reducing viraemia and thereby HIV transmissibility) and indirectly (by reducing risk behavior among those diagnosed, counseled, and treated and by diffusing attitudes and norms among the population that facilitate a general acceptance of the problem of HIV/AIDS and changes in risk behavior). However, resources and effort are required to realize the prevention benefits of treatment and to avoid unintended consequences of treatment, such as increased risk behavior. Resources are required to build on the hope created by treatment to change behavioral norms among patients and in the larger community. A further reason to link these programs is that advocates of faster treatment uptake can use evidence of prevention success to lobby for more treatment resources.
These potential complementarities between prevention and treatment suggest the need for a strategy that builds on both rather than trading them off. However, separation of the resources available and responsibility for the two means they are likely to be traded off and to compete for resources.
RECOMMENDATIONS
Given the enormous momentum of the HIV/AIDS epidemic, even a large increase in prevention effort and effectiveness over the next few years can have little impact on the mortality or financial burden of HIV/AIDS before 2020. By changing the future course of the epidemic, however, greatly improved HIV/AIDS prevention can dramatically reduce the financial burden of donor and recipient governments beyond 2020. Donors and governments that experience success in reducing new infections to the extent that the total number living with HIV/AIDS begins to fall can reasonably expect treatment expenditures to decline beyond 2020 and therefore can afford to invest some of those future savings in a greater rate of patient enrollment today.
Recommendation 2-15: Measure incidence. African countries, with the support of donors, should develop and implement cost-effective methods for accurately measuring the level of and change in HIV/AIDS incidence to enable better planning and evaluation of HIV/AIDS prevention programs.6
Recommendation 2-2: Analyze trade-offs. The U.S. government should support countries in developing projections of the future burden of their HIV/AIDS epidemics and in assessing the implications of alternative national HIV/AIDS policies for human welfare, capacity, and resources so policy makers can make informed decisions on HIV/AIDS-related trade-offs.
REFERENCES
Aalen, O. O., V. T. Farewell, D. De Angelis, N. E. Day, and O. N. Gill. 1997. A Markov model for HIV disease progression including the effect of HIV diagnosis and treatment: Application to AIDS prediction in England and Wales. Statistics in Medicine 16(19):2191-2210.
Auvert, B., D. Taljaard, E. Lagarde, J. Sobngwi-Tambekou, R. Sitta, and A. Puren. 2005. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 trial. PLoS Medicine 2(11):e298.
Bailey, R. C., S. Moses, C. B. Parker, K. Agot, I. Maclean, J. N. Krieger, C. F. Williams, R. T. Campbell, and J. O. Ndinya-Achola. 2007. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. The Lancet 369(9562):643-656.
Burns, D. N., C. W. Dieffenbach, and S. H. Vermund. 2010. Rethinking prevention of HIV type 1 infection. Clinical Infectious Diseases 51(6):725-731.
Busch, M., C. Pilcher, T. Mastro, J. Kaldor, G. Vercauteren, W. Rodriguez, C. Rousseau, T. Rehle, A. Welte, M. Averill, and J. Calleja. 2010. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24(18):2763-2771.
Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O’Sullivan, R. Vandyke, M. Bey, W. Shearer, R. L. Jacobson, E. Jimenez, E. O’Neill, B. Bazin, J. F. Delfraissy, M. Culnane, R. Coombs, M. Elkins, J. Moye, P. Stratton, and J. Balsley. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. The New England Journal of Medicine 331(18):1173-1180.
Dieffenbach, C. W., and A. S. Fauci. 2009. Universal voluntary testing and treatment for prevention of HIV transmission. Journal of the American Medical Association 301(22):2380-2382.
Fiamma, A., P. Lissouba, O. E. Amy, B. Singh, O. Laeyendecker, T. C. Quinn, D. Taljaard, and B. Auvert. 2010. Can HIV incidence testing be used for evaluating HIV intervention programs? A reanalysis of the Orange Farm male circumcision trial (ANRS-1265). BMC Infectious Diseases 10:137.
|
5 |
The committee’s recommendations are numbered according to the chapters of the report in which they appear. Thus, for example, recommendation 2-1 is the first recommendation in Chapter 2. |
|
6 |
As described in Chapter 2, laboratory-based assays for the calculation of incidence estimates are in development and are urgently needed to provide important epidemiological data on trends of the epidemic and the effectiveness of interventions so that limited resources can be directed most efficiently to limit the epidemic’s further spread. |
Fisher, J. D., D. H. Cornman, C. Y. Osborn, K. R. Amico, W. A. Fisher, and G. A. Friedland. 2004. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: Formative research, acceptability, and fidelity of the Options Project. Journal of Acquired Immune Deficiency Syndromes (1999) 37(Suppl. 2):S78-S87.
Granich, R. M., C. F. Gilks, C. Dye, K. M. De Cock, and B. G. Williams. 2009. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. The Lancet 373(9657):48-57.
Gray, R. H., G. Kigozi, D. Serwadda, F. Makumbi, S. Watya, F. Nalugoda, N. Kiwanuka, L. H. Moulton, M. A. Chaudhary, M. Z. Chen, N. K. Sewankambo, F. Wabwire-Mangen, M. C. Bacon, C. F. Williams, P. Opendi, S. J. Reynolds, O. Laeyendecker, T. C. Quinn, and M. J. Wawer. 2007. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. The Lancet 369(9562):657-666.
Gregson, S., S. Adamson, S. Papaya, J. Mundondo, C. A. Nyamukapa, P. R. Mason, G. P. Garnett, S. K. Chandiwana, G. Foster, and R. M. Anderson. 2007. Impact and process evaluation of integrated community and clinic-based HIV-1 control: A cluster-randomised trial in eastern Zimbabwe. PLoS Medicine 4(3):e102.
Gregson, S., G. P. Garnett, C. A. Nyamukapa, T. B. Hallett, J. J. Lewis, P. R. Mason, S. K. Chandiwana, and R. M. Anderson. 2006. HIV decline associated with behavior change in eastern Zimbabwe. Science 311(5761):664-666.
Grosskurth, H., F. Mosha, J. Todd, E. Mwijarubi, A. Klokke, K. Senkoro, P. Mayaud, J. Changalucha, A. Nicoll, G. ka-Gina, J. Newell, K. Mugeye, D. Mabey, and R. Hayes. 1995. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: Randomised controlled trial. The Lancet 346(8974):530-536.
Grosskurth, H., E. Mwijarubi, J. Todd, M. Rwakatare, K. Orroth, P. Mayaud, B. Cleophas, A. Buvé, R. Mkanje, L. Ndeki, A. Gavyole, R. Hayes, and D. Mabey. 2000. Operational performance of an STD control programme in Mwanza Region, Tanzania. Sexually Transmitted Infections 76(6):426-436.
Gupta, G. R., J. O. Parkhurst, J. A. Ogden, P. Aggleton, and A. Mahal. 2008. Structural approaches to HIV prevention. The Lancet 372(9640):764-775.
Hallett, T. B., J. Aberle-Grasse, G. Bello, L. M. Boulos, M. P. Cayemittes, B. Cheluget, J. Chipeta, R. Dorrington, S. Dube, A. K. Ekra, J. M. Garcia-Calleja, G. P. Garnett, S. Greby, S. Gregson, J. T. Grove, S. Hader, J. Hanson, W. Hladik, S. Ismail, S. Kassim, W. Kirungi, L. Kouassi, A. Mahomva, L. Marum, C. Maurice, M. Nolan, T. Rehle, J. Stover, and N. Walker. 2006. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sexually Transmitted Infections 82(Suppl. 1):i1-i8.
Hallett, T. B., S. Gregson, E. Gonese, O. Mugurungi, and G. P. Garnett. 2009. Assessing evidence for behaviour change affecting the course of HIV epidemics: A new mathematical modelling approach and application to data from Zimbabwe. Epidemics 1:108-117.
International Group on Analysis of Trends in HIV Prevalence and Behaviours in Young People in Countries Most Affected by HIV. 2010. Trends in HIV prevalence and sexual behaviour among young people aged 15-24 years in countries most affected by HIV. Journal of Sexually Transmitted Infections 86(Suppl 2):ii72-ii83.
Kamali, A., M. Quigley, J. Nakiyingi, J. Kinsman, J. Kengeya-Kayondo, R. Gopal, A. Ojwiya, P. Hughes, L. M. Carpenter, and J. Whitworth. 2003. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: A community randomised trial. The Lancet 361(9358):645-652.
Karim, Q. A., S. S. Karim, J. A. Frohlich, A. C. Grobler, C. Baxter, L. E. Mansoor, A. B. Kharsany, S. Sibeko, K. P. Mlisana, Z. Omar, T. N. Gengiah, S. Maarschalk, N. Arulappan, M. Mlotshwa, L. Morris, and D. Taylor. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329(5996)1168-1174.
Kilian, A. H., S. Gregson, B. Ndyanabangi, K. Walusaga, W. Kipp, G. Sahlmuller, G. P. Garnett, G. Asiimwe-Okiror, G. Kabagambe, P. Weis, and F. von Sonnenburg. 1999. Reductions in risk behaviour provide the most consistent explanation for declining HIV-1 prevalence in Uganda. AIDS 13(3):391-398.
Mastro, T. D., A. A. Kim, T. Hallett, T. Rehle, A. Welte, O. Laeyendecker, T. Oluoch, and J. M. Garcia-Calleja. 2010. Estimating HIV incidence in populations using tests for recent infection: Issues, challenges and the way forward. Journal of HIV/AIDS Surveillance & Epidemiology 2(1).
Montaner, J. S., V. D. Lima, R. Barrios, B. Yip, E. Wood, T. Kerr, K. Shannon, P. R. Harrigan, R. S. Hogg, P. Daly, and P. Kendall. 2010. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. The Lancet 376(9740):532-539.
Over, M. 2010. The global AIDS transition: A feasible objective for AIDS policy. Washington, DC: Center for Global Development.
Padian, N. S., S. I. McCoy, J. E. Balkus, and J. N. Wasserheit. 2010. Weighing the gold in the gold standard: Challenges in HIV prevention research. AIDS 24(5):621-635.
Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. The New England Journal of Medicine 342(13):921-929.
Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England Journal of Medicine 361(23):2209-2220.
Ross, D. A., J. Changalucha, A. I. Obasi, J. Todd, M. L. Plummer, B. Cleophas-Mazige, A. Anemona, D. Everett, H. A. Weiss, D. C. Mabey, H. Grosskurth, and R. J. Hayes. 2007. Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: A community-randomized trial. AIDS 21(14):1943-1955.
Semaye Fideli, U., S. A. Allen, R. Musonda, S. Trask, B. H. Hahn, H. Weiss, J. Mulenga, F. Kasolo, S. H. Vermund, and G. M. Aldrovand. 2001. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Research and Human Retroviruses 17(10):901-910.
Stoneburner, R. L., and D. Low-Beer. 2004. Population-level HIV declines and behavioral risk avoidance in Uganda. Science 304(5671):714-718.
Susser, M. 1996. Some principles in study design for preventing HIV transmission: Rigor or reality. American Journal of Public Health 86(12):1713-1716.
Tanton, C., H. A. Weiss, M. Rusizoka, J. LeGoff, J. Changalucha, K. Baisley, K. Mugeye, D. Everett, L. Belec, T. C. Clayton, D. A. Ross, R. J. Hayes, and D. Watson-Jones. 2010. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: A randomized controlled trial. The Journal of Infectious Diseases 201(9):1285-1297.
UNAIDS (The Joint United Nations Programme on HIV/AIDS). 2008. Report on the global AIDS epidemic. Geneva: UNAIDS.
———. 2010. Fact sheet: Treatment 2.0. Geneva: UNAIDS.
Van Der Paal, L., L. A. Shafer, J. Todd, B. N. Mayanja, J. A. Whitworth, and H. Grosskurth. 2007. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS 21(Suppl. 6):S21-S29.
Venkatesh, K. K., G. de Bruyn, M. N. Lurie, L. Mohapi, P. Pronyk, M. Moshabela, E. Marinda, G. E. Gray, E. W. Triche, and N. A. Martinson. 2010. Decreased sexual risk behavior in the era of HAART among HIV-infected urban and rural South Africans attending primary care clinics. Journal of Acquired Immune Deficiency Syndromes 24.
Wawer, M. J., N. K. Sewankambo, D. Serwadda, T. C. Quinn, L. A. Paxton, N. Kiwanuka, F. Wabwire-Mangen, C. Li, T. Lutalo, F. Nalugoda, C. A. Gaydos, L. H. Moulton, M. O. Meehan, S. Ahmed, and R. H. Gray. 1999. Control of sexually transmitted diseases for AIDS prevention in Uganda: A randomised community trial. Rakai Project Study Group. The Lancet 353(9152):525-535.
Welte, A., T. A. McWalter, O. Laeyendecker, and T. B. Hallett. 2010. Using tests for recent infection to estimate incidence: Problems and prospects for HIV. Eurosurveillance 15(24).
The World Bank. 2010. Global economic prospects: Fiscal headwinds and recovery. Washington, DC: The World Bank.




















