2
Vaccine Supply
The greatest challenges in vaccine distribution and administration arose because vaccine supply and demand were poorly matched throughout the 2009 H1N1 influenza vaccination campaign, said public health officials and healthcare providers at the workshops. During the early months, demand far outstripped supply, and in the later months, supply far exceeded demand. Participants characterized the supply of vaccine as “trickling in” during fall 2009. They described the challenges of equitably and fairly distributing the limited supplies of vaccine with little information about future vaccine supply. These challenges were exacerbated by raised expectations from overly optimistic projections of vaccine supply and problems with communications about the vaccine supply.
The issues related to vaccine supply are introduced here because many participants said these issues shaped most other aspects of the response. More detailed information about how these issues impacted vaccine distribution and administration as well as communications is included in each of the respective main sections below. Figure 2-1 shows estimates of the number of 2009 H1N1 cases and vaccine doses distributed, from the beginning of the vaccination campaign in October 2009.
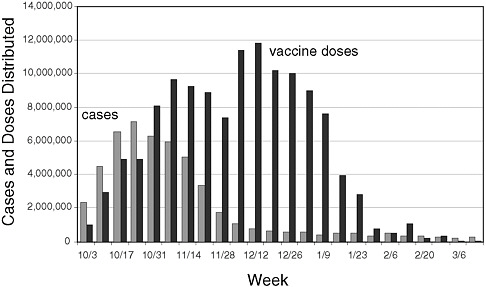
FIGURE 2-1 Estimated 2009 H1N1 cases and vaccine doses distributed, October 2009–March 2010.
SOURCE: Personal communication, Toby Merlin, deputy director of the CDC’s Influenza Coordination Unit, September 17, 2010.
Supply and Demand
Over the summer and through September, expectations for the vaccination campaign grew, fueled partly by wide media coverage and disease incidence. In July 2009, the CDC’s ACIP recommended that initial vaccination efforts focus on five target groups, as mentioned above and discussed in greater detail below (CDC/ACIP, 2009). Providing one dose of vaccine to everyone in these groups would have required 159 million doses. Workshop participants noted that members of these target groups were generally aware that vaccine was recommended for them and expected that vaccine would be available for all members of the target groups once the vaccination campaign began.
Vaccine production delays led to a supply of vaccine that was slower than expected. The first doses were administered to the public on October 5, 2009, but the supply of vaccine during the first 2 months of the program was not sufficient to cover the target groups for whom the ACIP had recommended vaccination. Adding to this difficulty, the majority of the vaccine that was initially available was Live-Attenuated Influenza
Vaccine (LAIV), which was contraindicated for the very young, for those with certain conditions such as asthma, and for pregnant women—among the target groups considered highest priority. Further, many healthcare workers were reluctant to receive LAIV, often because of unfounded concerns about transmission to patients in healthcare settings and vaccine efficacy. This is discussed in more detail below. Unfortunately, this limited supply of vaccine occurred when demand for vaccine was at its highest and around the time of the peak of the fall 2009 wave of influenza. Pediatricians and other healthcare providers at the workshops reported that they were inundated by calls from large numbers of patients and anxious parents of pediatric patients demanding the vaccine or asking where to go for the vaccine.
As vaccine supply started to increase, the incidence of 2009 H1N1 disease declined and the media frenzy quieted. By January 2010, patient demand for vaccine had decreased to the point where many providers were left with vaccine on the shelf. Some public health representatives noted that influenza vaccine demand generally drops at this time of year, even in years in which seasonal influenza activity peaks in January. They said that this likely added to the challenges that were specific to 2009 H1N1. A CDC spokesperson later said that out of the 162 million doses of 2009 H1N1 vaccine produced for the general public, only 90 million doses were used (Moyer, 2010).
Participants noted that vaccine production was accomplished within the desired time frame laid out in the National Strategy for Pandemic Influenza Implementation Plan, issued May 2006. The plan says, “The Federal Government has established two primary vaccine goals: (1) establishment and maintenance of stockpiles of pre-pandemic vaccine adequate to immunize 20 million persons against influenza strains that present a pandemic threat; and (2) expansion of domestic influenza vaccine manufacturing surge capacity for the production of pandemic vaccines for the entire domestic population within 6 months of a pandemic declaration” (Homeland Security Council, 2006, p. 104). In response to 2009 H1N1, vaccine was first available in October (4 months after the WHO pandemic declaration) and widely available in December (6 months after the WHO pandemic declaration). Although the planning parameter was met, workshop participants emphasized that much work is needed to shorten the interval between pandemic declaration and vaccine availability.
Supply Projections
The challenge of dealing with an initially limited supply of vaccine was exacerbated because expectations had been raised by overly optimistic projections of vaccine supply. During summer 2009, state and local public health authorities planned vaccination programs based on robust federal vaccine supply forecasts. When the production schedule fell significantly short of predictions, large-scale changes were needed in planned distribution strategies.
In addition to developing recommendations for five target groups, ACIP also developed recommendations for a subset of these groups to be used in case of limited vaccine availability. This subset of target groups covered approximately 42 million people in the United States. At the workshops, however, state and local public health participants said that during the summer planning phase of the vaccination campaign, the possibility of limited vaccine supply was not emphasized in the communications they received from the federal authorities. Indeed, ACIP’s report said, “Current projections of initial vaccine supply indicate that establishment of a subset of the five initial target groups will not be necessary in most areas” (CDC/ACIP, 2009).
Jay Butler, director of the CDC’s H1N1 Vaccine Task Force, noted, “At many levels throughout the distribution [system], the strategy was not robust for the shortage that we were dealing with…. We prepared for being able to move as much vaccine as quickly as possible and really were concerned that there would be a glut of vaccine. What we had to deal with then was a trickle, and a trickle that really continued for a number of weeks.” David Fleming, director and health officer for Public Health–Seattle & King County, agreed, saying, “We spent months planning for a response where the rate-limiting step was going to be the vaccine distribution system, and that’s not what happened.” For this reason, Fleming continued, “It is important to go back and critically look at … the system that allowed us as a nation to miss for so long that the fundamental problem we were going to be facing was a vaccine shortage.” Many participants agreed with Fleming that there was an opportunity to “figure out how that happened, and to take steps to make sure that that does not happen again in the future.”
Many state and local public health officials and healthcare providers at the workshops also noted that problems with supply projections continued through the initial months of the campaign, when vaccine supply
was still limited. They discussed a lack of information and predictability for future weeks regarding when various locations would receive vaccine and which formulations would be delivered. Participants noted that much of the uncertainty arose because of lack of information about upcoming supply from manufacturers. However, they were also concerned about tracking vaccine through the distribution system, which will be discussed in the next main section. The lack of accurate projections about future supply presented challenges in developing equitable distribution plans, planning clinics, and developing messaging for healthcare providers and the public.
Several participants suggested that federal authorities should develop stronger and more formal partnerships with vaccine producers to ensure they have the most up-to-date information on production and inventory to generate more accurate supply projections. A number of participants also recommended that communications to state and local authorities should include timely, specific information to improve response activities.
2009 H1N1 and Seasonal Influenza
The partial overlap in timing of the 2009 H1N1 and seasonal vaccination campaigns added an extra layer of complexity to planning and response. As a result of the usual production schedule that makes seasonal influenza vaccine available prior to the regular flu season, seasonal vaccine was available prior to the 2009 H1N1 vaccine. This caused some confusion when patients arrived at their healthcare provider’s office expecting to be vaccinated for 2009 H1N1, because that disease was prominent in the media, but often found that only seasonal flu vaccine was available. The situation was further complicated because the two vaccines had different target populations and because later in the fall of 2009, seasonal flu vaccine was no longer available after manufacturers switched to producing 2009 H1N1 vaccine. When 2009 H1N1 vaccine supply increased, participants noted that many patients who had been turned away earlier, or offered the seasonal vaccine, did not return for the 2009 H1N1 vaccine. Participants speculated that these patients had been discouraged by the earlier lack of availability or encountered logistical barriers to making a second trip.
Despite the complications caused by the partially overlapping 2009 H1N1 and seasonal vaccination campaigns in summer and fall 2009, par-
ticipants noted throughout the workshops that seasonal and emergency vaccination campaigns share many common elements. They suggested that improvements to the annual seasonal vaccination campaign would also result in improvements to future emergency vaccination campaigns. To this end, several participants emphasized the importance of continued attention and resources for the seasonal campaign.
Opportunities for Improving Planning Related to Vaccine Supply
Participants acknowledged that the pharmaceutical industry and the CDC overcame enormous challenges to develop, produce, and test the 2009 H1N1 vaccine in just 6 months. However, many also emphasized the importance of developing new technologies and manufacturing techniques to accelerate vaccine production. A concern was that in a more virulent pandemic, 6 months may simply be too long. “We have to redouble the effort to once again give us the science that could put out vaccine quicker, in order to impact a pandemic that’s much bigger,” said Mark Horton, director of the California Department of Public Health.
The need to increase the capacity and speed of vaccine production was discussed in a recent review of the Public Health Emergency Medical Countermeasures Enterprise by the Department of Health and Human Services’ (HHS’s) Office of the Assistant Secretary for Preparedness and Response. The review articulated the following strategic vision: “Our Nation must have the nimble, flexible capacity to produce MCMs [medical countermeasures] rapidly in the face of any attack or threat, known or unknown, including a novel, previously unrecognized, naturally occurring emerging infectious disease” (ASPR, 2010). Among the recommendations for new infrastructure initiatives and enhancements to the current system for developing MCMs from discovery through procurement and stockpiling, the report highlighted immediate needs related to pandemic influenza vaccines. These needs include the development of influenza vaccine candidates that can be manufactured without dependence on virus grown in eggs or cells.
At the workshop, individual suggestions were also made about ways that public health officials can improve planning given the current vaccine supply system. These suggestions are compiled here as part of the factual summary of the workshops and should not be construed as re-
flecting consensus or endorsement by the workshops, the Preparedness Forum, or The National Academies. They are as follows:
-
Federal authorities, state and local public health officials, and other entities involved in planning and communications efforts should “underpromise and overdeliver.”
-
Federal authorities should develop a stronger and more formal partnership with vaccine producers to ensure they have the most up-to-date information on production and inventory and can generate more accurate supply projections.
-
Plan for a range of vaccine supply scenarios when supply is uncertain.








