3
Studying Sex Differences in Translational Research: Examples from Four Major Disease Areas
Following the introductory presentations on the challenges and opportunities for studying sex differences in neuroscience research, four specific disease areas within neuroscience were discussed in greater detail: depression, pain and pain perception, sleep medicine, and multiple sclerosis and neuroinflammation. These areas were identified by the planning committee as particularly relevant to the discussion with known sex differences and therefore areas with potential for critical advances. In addition, these diseases have very different etiologies and thus allowed a broad overview of many different mechanisms. (Key points of the presentations in each disease area are provided in boxes at the end of each set of panel presentations, Boxes 3-1 through 3-4.)
DEPRESSION
Characterization of Sex Differences in Depression
In science, we seek to define variables on which populations are similar to and differ from one another, said Katherine Wisner, director of Women’s Behavioral HealthCARE at the University of Pittsburgh Medical Center. Two sexes provide a source of “variable partitioning” that creates a natural opportunity for comparative investigation. Disease states have been studied for a long time, considering a variety of different impacting factors (e.g., environment). The task at hand is to look at disease states by sex or gender across the lifecycle, and harness that information for treatment. The
ultimate question is whether treatments will be optimized by incorporating sex and gender principles into interventions.
Women are 1.5 to 2.5 times more likely to experience major depression than men, from puberty onward. Women have the highest prevalence of depression during the childbearing years and are also at increased risk during the perimenopausal period (the 5-year period before the cessation of menses). According to a World Health Organization study, depression is the leading cause of days lost to disability for women worldwide. Depression is often discussed as if it was a homogeneous illness, but further study is needed into the different subtypes of depression and how they vary by sex and gender.
Wisner cited one recent study that included sex-specific analyses is the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study, a large-scale clinical trial of multiple depression treatments (Marcus et al., 2005). The investigators found differences in symptoms between the two sexes. Depressed women experienced more anxiety, physical somatoform symptoms, and bulimia. For men, the symptoms concurrent with depression tended to be obsessive-compulsive symptoms and substance use. Depressive episodes were longer in women than men, and suicide attempts occurred more frequently. Interestingly, women were more likely than men to have remission (loss of all symptoms) in response to the drugs under investigation (30 percent of women compared to 24 percent of men), and half of women responded (had symptom reduction) compared to 44 percent of men. The question facing clinicians now is how to apply this information.
Individualized, personalized treatment for depression and other psychiatric illnesses is a primary goal of translational research. In addition to sex and gender, individuals vary with regard to symptoms, comorbidities, clinical factors, personal history, family features, social background, genetic polymorphisms, developmental stage, and characteristics identified from brain imaging or other technologies. Differences in the longitudinal development of males and females also naturally provide a variety of hormonal conditions under which to study sex differences as well as the hormonal changes that are unique to females: in utero differentiation, menarche, the premenstruum, pregnancy, postpartum, and menopause.
When considering a disease state or a process, there is a broad biological-to-societal spectrum of distal health determinants that fluctuate throughout an individual’s lifetime; from basic genetics, to gene–environment interactions, to the physical and social environments (e.g., which pollutants or other stressors an individual is subjected to often vary by gender) (Misra et al., 2003). Proximal determinants, including biomedical responses (e.g., nutritional status, inflammatory response) and behavioral responses (e.g., alcohol use, actively practicing a religion) impact the disease process acutely. Health outcomes are influenced by these distal and proximal determinants, as well as by inputs and processes such as health care.
Wisner closed noting that the so-called “valleys of death” in clinical and translational research are, in fact, valleys of opportunity. Mechanisms such as the Specialized Centers of Research and Building Interdisciplinary Research Careers in Women’s Health programs are bringing people together to eliminate these valleys. Questions to be addressed when translating neuroscience research are whether there is enough of a sex difference to merit changing the way medicine is practiced to accommodate those differences, and if so, how to train individual practitioners to consider these differences in practice.
Fetal Antecedents to Sex Differences in Depression
Jill Goldstein, director of research at the Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital and professor of psychiatry and medicine at Harvard Medical School, discussed fetal hormonal programming of sex differences in the brain, and its role in understanding sex differences in depression.
The incidence of major depressive disorder has an approximately 2:1, female-to-male ratio. Furthermore, depression is comorbid with several chronic diseases, including the fact that the comorbidity of depression and cardiovascular disease is the fourth leading cause of morbidity and mortality worldwide. Goldstein and colleagues are currently testing the hypothesis that there are shared etiologies associated with understanding sex differences in depression and cardiovascular disease; that they are initiated during the sexual differentiation of the brain; and that they involve disruption of the fetal hormonal programming of the brain, which leads to endocrine disruptions throughout life, and sex differences in adulthood in these chronic diseases.
Throughout life windows of opportunity are available for studying sex differences in these disorders, Goldstein said. These occur when the brain and the body are flooded differentially with hormones: fetal development, puberty, pregnancy, perimenopause, and menopause. Although depression and cardiovascular disease are, for the most part, adult-onset disorders, they have developmental precursors, and considering this lifespan perspective is important.
Some risk factors for depression that have been identified from population-level studies include small for gestational age; low birthweight; obstetric complications (e.g., preeclampsia, oxygen deprivation); second trimester influenza; and second to third trimester famine. Population-level studies in the field of cardiovascular risk and hypertension have identified some of the same fetal risk factors for cardiovascular disease: small for gestational age; low birthweight; preeclampsia; and maternal prenatal famine.
Studies on the fetal programming of cardiovascular disease have focused on prenatal and early life stress and the disruption of the hypothalamic pi-
tuitary adrenal (HPA) axis in development. The HPA axis is also the focus of studies on the fetal programming of sex differences in depression.
Timing is critical for understanding sex effects, Goldstein said. Population-level studies that have looked at first trimester factors have found fewer sex differences in the incidence of these disorders than those that looked at second and third trimester factors. This may, in part, reflect the fact that hormonal regulation of the sexual differentiation of the brain starts at the beginning of second trimester, when testes begin to secrete testosterone, which has direct and indirect effects (through aromatization into estradiol) on brain sexual differentiation. In addition, as described by Arnold (see Chapter 2), prior to gonad differentiation, genetics play a critical role in sex differentiation.
Estrogen and testosterone have major effects on neuronal growth and development. These effects are not all over the brain, but are region specific, in areas such as the hypothalamic and amygdala nuclei, the hippocampus, medial dorsal thalamus, and areas of the cortex. Although much of the previous work on the sexual differentiation of the brain has been conducted in animals, magnetic resonance imaging (MRI) of healthy human brains shows that brain regions affected by sex hormones during development are highly sexually dimorphic (i.e., exhibit sex differences in brain volumes relative to the size of the cerebrum) (Goldstein et al., 2001).
Brain imaging studies of depression show crossover between those brain regions that are normally highly sexually dimorphic and those that are implicated as abnormal in depression, including the paraventricular nucleus, lateral hypothalamic area, hippocampus, and areas of the amygdala. Imaging studies of central nervous system (CNS) control of the autonomic nervous system show that some of those same brain regions are also important for regulation of the heart. This, Goldstein said, is the basis for her studies on shared etiologies for depression and heart disease.
Studying the stress response circuitry is a model system for the study of hormonal regulation of the brain and of the impact on major depressive disorder, Goldstein explained. Stress response circuitry crosses over with mood regulation, control of the HPA axis, and brain regions that regulate heart and blood pressure through autonomic nervous system function, such as the hypothalamic paraventricular nucleus and hippocampus. To characterize the hormonal phenotype in response to stress using this model, blood was collected and heart rate was monitored while an individual was lying in the MRI scanner, viewing a visual stress response challenge. The responses of the brain to low- and high-arousal pictures (e.g., a cow in a green field versus a serious car crash) were compared. Results showed that the stress response circuitry in the healthy brain activates differently at different points in the menstrual cycle, and those hormonal differences contribute to explaining sex differences in stress response circuitry activation (Goldstein
et al., 2005, 2010). The physiology of male and female healthy brains is different, and findings show that male and female brains act differently to maintain homeostasis with regard to one’s response to stress.
Stress response circuitry function is abnormal in women with recurrent depression. Furthermore, in depression, there is a lower parasympathetic control, which can be operationalized as the high-frequency component of heart rate variability, and which has been found to be significantly associated with estradiol in women. Thus, brain activity deficits, hormonal deficits, heart rate and autonomic nervous system function, and sex differences in the brain are all highly related to each other and increased understanding will contribute important new knowledge regarding depression and its comorbidity with major general medical diseases, such as cardiovascular disease.
Goldstein is now looking at the shared fetal antecedents to sex differences in depression and risk for cardiovascular disease using the National Collaborative Perinatal Cohort developed in the 1960s. This study initially followed a New England cohort of 17,000 women in Boston and Providence through their pregnancies, and their children for 7 years after birth, until study funding ran out. Over the past 20 years, study participants (who are now adults) have been re-recruited and interviewed, and a subsample have been brought to the brain imaging center, facilitating human studies of fetal antecedents to brain, hormone, and heart regulation phenomenology and risk for different psychiatric and general medical diseases.
In a separate study, Goldstein and colleagues are following 300 discordant sibling pairs, one of whom has been exposed to fetal growth restriction or to preeclampsia, and the other as the unaffected control. As an example, one initial finding shows that healthy adult males who were exposed to fetal growth restriction or preeclampsia have significantly less parasympathetic control of the heart than females.
In conclusion, Goldstein stressed that understanding sex differences in depression and its comorbid conditions is absolutely critical for sex-specific drug discovery and development. One must take a life-course perspective for understanding the medical implications of sex differences for both the healthy brain and for models of disease. Taking a brain–body approach for understanding the impact of sex differences in the brain will be fruitful for new sex-specific drug discovery and other treatment modalities. Finally, clinical and population-level research is critical for informing the development of basic animal models and vice versa.
Sex Differences in Translational Studies of Major Depression
Etienne Sibille, associate professor in the Translational Neuroscience Program at the University of Pittsburgh, explained that major depression
is a heterogeneous syndrome that is characterized by chronic low mood. Mechanistically, depression is a chronic, recurrent disease known to be influenced by genes and environment. The higher prevalence of depression in women is cross-cultural, and is probably one of the most robust findings in all of psychiatric epidemiology, Sibille said, and yet this finding often is not considered in basic studies.
Perspectives differ on the origin of sexual dimorphism in depression. A societal perspective focuses on the interaction between increased victimization and female character traits. In the Darwinian perspective on the adaptive role of mood, mood is defined as emotion over time that is less dependent on immediate triggers. Low or high mood is a source of information about goal achievement and serves as a regulator of effort and energy allocation. For example, behavior inhibition associated with low mood is an adaptive response that saves resources in the face of unachievable goals or potential negative outcomes. Low mood, under normal conditions, is critical in strategy reassessment. Under this definition, based on sexual selection theory, women allocate more effort and energy in long-term reproductive goals and are more sensitive than men to negative outcomes about lifetime strategies in the context of normal mood regulation. In the Darwinian perspective, depression is a chronic maladaptive state of mood dysregulation. For reasons as yet unknown, the female system is evolutionarily more at risk of a maladaptive state. The biological perspective seeks to determine if increased female vulnerability to develop depression is due to sex hormones in early development (organizational) or in adulthood (activational).
For translational studies, mood states (e.g., anxiety-like and antidepressant-like behaviors) can be modeled in animals, including rodents. Mood regulation neural networks are conserved across mammalian species. Still, the primary pathology of depression is poorly characterized because there are numerous limits with current animal models. The models are often oversimplified; there is poor conceptualization of baseline traits versus induced depressive-like states; conceptualization of syndrome versus single behavior is poor; and little consideration is given to sex differences. Specific concerns include differences between behavioral tests, genetic models designed to characterize a trait, and animal models that induce depressive states. The forced swim test as a behavioral animal model of depression, for example, is not a really a model at all, but rather a single behavioral response. Its only value is predictive validity for short-term response to antidepressants. Genetic models are generally very good, but we must recognize that often, what is reported is the impact of the lack of a specific gene on traits. These are not multisystem models, but a single entry into complex disease.
Sibille described her work with the unpredictable chronic mild stress (UCMS) model, which induces a depressive-like state in mice that mimics, in a naturalistic way, both the role of stress in precipitating depressive
pathology and the time frame of therapeutic response to antidepressive treatment. Mice are subjected to an unpredictable regimen of mild psychosocial stressors such as forced bath, predator’s song or smell, tilted cage, or social stress. Over 4 to 6 weeks, they develop a syndrome, or a collection of symptoms, including measurable outcome of behaviors that relate to emotions (e.g., increased anxiety, increased depressive-like behavior), increased anhedonia-like behavior). Physiological changes also occur, such as decreased weight gain, reduced quality of coat, and neuroendocrine changes. One study using this model has also shown cardiovascular changes. After onset, this syndrome can be blocked by chronic application of antidepressants. Using the UCMS model, Sibille and colleagues have shown that emotionality (expressed as Z score) is much higher after stress in female subjects than male. In another test, female mice genetically altered to express low levels of serotonin transporters are more vulnerable to chronic stress than male counterparts (Joeyen-Waldorf et al., 2009).
This model has also been used to test the translational hypothesis that the molecular pathology of altered mood regulation will manifest as conserved gene changes across species. Using large-scale gene expression data from human postmortem brain analysis, researchers have shown that changes in the amygdala of depressed human subjects actually predict what is observed in the amygdala of chronically stressed mice, and vice versa (Sibille et al., 2009). A set of 32 core genes has been identified that form a tight gene network, which is structurally conserved across species. This, Sibille said, suggests that in the context of depression or chronic stress, existing cellular pathways are abnormally recruited.
In summary, Sibille said, in the evolutionary context of mood regulation, these findings suggest that sexual dimorphism in biological mechanisms of depression should be expected. Animal models are associated with considerable limitations, at the levels of both concept and interpretation. UCMS could serve as an appropriate model of the human syndrome, and rodent findings parallel sex differences of human depression, setting the basis for development of realistic studies of sexual dimorphism. Ultimately, evidence shows sexual dimorphism in the primary pathology of depression in humans.
Industry Perspective on the Implications of Sex Differences for Translational Research
Carla Canuso, senior director of external innovation, Neuroscience Therapeutic Area at Johnson & Johnson, provided an overview of how and when industry considers sex differences, particularly in antidepressant drug development, during each phase of development, from preclinical through postmarketing.
Industry is necessarily concerned with regulations and guidance from the Food and Drug Administration (FDA) and regulatory bodies around the world. The FDA issued guidance in 1993 about the inclusion of women in the clinical evaluation of drugs, lifting the restriction on the participation of women of childbearing potential in Phase I and early Phase II trials (see http://www.nlm.nih.gov/services/ctphases.html), even before the completion of all animal toxicology studies. This placed greater onus on investigators to employ strict inclusion criteria regarding the use of birth control or abstinence, as well as strict guidance for pregnancy monitoring, and put more responsibility on institutional review boards to monitor clinical protocols. The intent was to have fair balance and representation of both sexes in the study so the data could be analyzed to detect any clinically significant differences. The guidance also addressed the assessment of demographic differences in pharmacodynamics in Phase I and II studies. Interestingly, Canuso said, the guidance specifically noted that the effects of menstrual cycle on pharmacokinetics should be evaluated when feasible, but this is not routinely done.
The European Medicines Agency (EMEA) does not have specific guidance on the inclusion of women, but EMEA did conduct a recent review of International Conference on Harmonisation (ICH) guidelines to determine whether special guidance for inclusion of women was needed. EMEA concluded that the current ICH guidance is sufficient to address the special needs of women and, in a review of recent clinical trials, found that women were adequately represented.
Canuso concurred with the previous speakers regarding the limitations of animal models, which are necessary for drug development. Animal models show sex differences in depression and stress, and that these differences in stress response are related to differences in corticotropin-releasing factor and serotonin neurotransmission. These are core regulators of mood and the coping response. The vast majority of preclinical studies done in the pharmaceutical industry are done in males, partly because of variation across the estrous cycle in laboratory animals. Nonetheless, studies are rarely replicated in females of the species, Canuso said. Preclinical studies have poor predictability of sex differences with respect to clinical response and toxicology, including reproductive toxicology, teratogenicity, and carcinogenicity.
Despite the 1993 FDA guidance, the vast number of participants in Phase I clinical trials are male, Canuso said. Reasons include the logistical challenges of birth control for women participants (e.g., double-barrier methods, the need to be on oral contraceptives for 3 months prior to entry into the study) and the lengthy informed consent process for early phase studies of drugs in women. The pharmacokinetics of drugs differ between women and men, not just because of body weight or volume of distribution, but also hormonal interplay. Also, drug–drug interaction studies must be
done for coadministration of antidepressant drugs, which are substrates or inhibitors of the cytochrome P-450 system, and drugs women commonly take that are metabolized by the P-450 system (e.g., oral contraceptives, tamoxifen).
Throughout all phases of clinical development, the consideration of sex differences should include designing studies to be appropriately enriched for women; requiring birth control or abstinence; pregnancy reporting; data analysis using sex-specific laboratory ranges; and studying sex-specific pharmacodynamic responses and adverse drug reactions.
Other sex-specific factors are considered for proof of concept and pivotal trials conducted for product registration. Products may have sex-specific indications (e.g., premenstrual dysphoric disorder; vasomotor symptoms associated with menopause; postpartum depression), or have been developed for use in only one sex (e.g., a safety concern in the opposite sex). As a result of the 1993 FDA guidance, inclusion of women in Phase II and III studies is generally adequate, and subgroup analyses by sex is included in labeling.
Finally, sex differences also come into play in Phase IV and postmarketing studies. Populations of interest are studied further, such as those with comorbidities. Investigator-initiated studies are conducted by academic researchers. Epidemiological studies are used to revise labels as new information comes to light following widespread use. Pregnancy registries are also used more often.
In closing, Canuso offered several ways industry can foster translational research in neuroscience, as follows:
-
Partner with academia to advance the science of personalized medicine, while considering sex and gender in every phase of drug development so that differential responses in dosing, efficacy, and safety can be fully appreciated.
-
Partner with academia to develop and validate better preclinical animal models that are truly predictive of the diseases, and then study both sexes of the species in those models.
-
Identify and evaluate sex-specific endophenotypes and other biomarkers, such as increased stress sensitivity.
-
Identify moderators and predictors of disease, specifically those that may confer resilience.
-
Establish multisector collaborations across industry, academia, and the National Institutes of Health (NIH) and create data-sharing mechanisms (e.g., the Psychiatric Genome-wide Association Study [GWAS] Consortium and the North American Antiepileptic Pregnancy Registry) so that once viable drug targets are identified, there will be large datasets that can be used to assess and validate them.
|
BOX 3-1 Key Points: Depression
|
PAIN AND PAIN PERCEPTION
Sex Differences in Pain and Pain Perception
Studies in humans have shown that females generally experience more clinical pain and often show greater experimental pain responses (i.e., have lower thresholds and less tolerance for pain) than males, said Karen J. Berkley, professor of psychology and neuroscience at Florida State University. That difference, however, can be manipulated by a variety of experimental factors (e.g., stimulus type, pain scale used, testing paradigms, endpoints selected) and impacted by individual factors (e.g., age, reproductive status, general health, blood pressure, food intake, odors, social and cultural factors).
Although individuals show significant variability when it comes to alleviating pain, some generally accepted sex differences in pain are worth considering. First, more painful conditions have a higher prevalence in females than males. In other words, women are more likely to have painful chronic conditions than men. The underlying basis for this disparity is not known, but probably has multiple causes, Berkley said, and is an opportunity for further research. Second, hundreds of therapies are available to
alleviate pain, and women use more of them (e.g., drugs, herbal products, complementary alternative medicine) than men. Yet little attention has been paid to how this usage difference affects the efficacy and side effects of various treatments.
One of the key questions considered in a 2007 consensus report on studying sex differences in pain and analgesia was “is there enough evidence to warrant sex-specific pain interventions?” The authors concluded that “the findings are mixed” and that “the evidence does not appear strong enough to warrant sex-specific pain interventions in most situations” and noted that more studies are required, including clinical trials that should take sex into consideration and report any differences in outcomes (Greenspan et al., 2007, p. 14).
The consensus group also expressed concern about the “translation hindering” effects of the “disconnects” among specialties, and between basic and clinical researchers. Berkley also noted that translational research is not unidirectional from animal research to clinical practice, but is really circular, and evidence from human and clinical research should inform animal models.
In conclusion, Berkley said that knowledge of statistical sex differences is already beginning to save lives and improve the health of both females and males, but dissemination of this knowledge is key. Better understanding of the interplay between social roles and health is needed. These issues are complex, but seemingly small increments in knowledge can have large lifetime impacts.
Dissecting Pain and Pain Perception into Sex-Related Endophenotypes
Emeran Mayer, director of the University of California–Los Angeles (UCLA) Center for Neurobiology of Stress, studies persistent pain syndromes, with a focus on visceral pain from the gastrointestinal and urinary tracts. Based on reported spontaneous symptoms, persistent pain syndromes are more common in women (including irritable bowel syndrome [IBS] and interstitial cystitis). Awareness is growing that persistent pain syndromes (e.g., fibromyalgia, temporomandibular joint disorder, vulvodynia, interstitial cystitis, IBS) are not distinct diseases, but rather, they significantly overlap with each other, and with disorders of affect and mood, particularly anxiety, depression, and somatization. Most of these disorders are studied using experimental pain assays to determine if an individual has a high or a normal pain threshold or pain sensitivity. Such measurement would be simple if the relationship between pain perception and a stimulus was linear. But pain perception is a highly complex, modulated system (Figure 3-1). These networks prepare the system and modulate perception of a stimulus
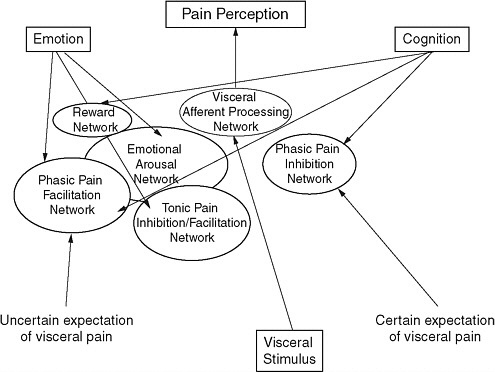
FIGURE 3-1 Pain perception is not a linear pathway from stimulus to pain but is a highly complex, modulated system, and each component potentially could be sex specific.
whether it is painful or not. In the case of a distending gastric stimulus, there are sex differences in the expectation of pain (certain and uncertain) much more than physical responses to the actual stimulus. Therefore, differences in one of these modulated systems may result in a sex-specific bias for pain perception and spontaneous pain.
Mayer proposed a reverse translational approach that takes into account sex-related differences in the endophenotypes. An endophenotype is a physiological or biological abnormality that cuts across categorical disease definitions, and may be shared by several disorders. In deconstructing complex symptom-based syndromes into biological endophenotypes, the goal is to work backward from the syndrome through the symptoms; to the cognitive phenotype; to the underlying neural networks, cellular systems, and component cells; and ultimately, to the genes or network of genes that correlate with endophenotypes.
Sometimes sex and gender differences can be studied only in humans, as in the clinical phenotype of IBS, a common persistent pain disorder affecting the gastrointestinal tract. Because no animal models exist that
can report the painful symptoms, investigators have relied on measuring reflexive and behavioral responses to noxious stimuli. However, there are neurobiological endophenotypes that can be studied in animals, such as visceral sensitivity, descending pain inhibition, emotional arousal, and associated brain responses.
Mayer provided several examples of how endophenotypes have been used to deconstruct complex syndromes in pain conditions. In closing, Mayer said that studying endophenotypes in humans and in animals can be productive in understanding sex differences in pain.
Sex and Gender Differences in Pain Across the Lifespan
Although many painful conditions are more prevalent in females than males overall, the prevalence of different painful conditions varies across the lifespan, resulting in variations in sex ratios across the lifespan, explained Linda LeResche, professor at the University of Washington School of Dentistry. Neck and shoulder pain as well as joint pain, which occur more frequently in females, tend to increase with age in both sexes (Hasvold and Johnsen, 1993). Abdominal pain (excluding menstrual pain) decreases with age in both sexes (Agréus et al., 1994). Migraine exhibits a large sex difference after puberty, but the curve is more bell shaped and the prevalence disparity between females and males lessens with age (Stewart et al., 1992).
LeResche described her work on temporomandibular joint and muscle disorders (TMD), which cause musculoskeletal pain in the region of the jaw joint and associated facial muscles. TMD is more common in women than in men, and peak prevalence occurs during the reproductive years. Her initial studies suggested that women taking hormone replacement therapy (HRT) were at increased risk of being treated for pain from TMD (LeResche et al., 1997). As a follow-up, subsequent studies are focusing on the association of endogenous hormone patterns and pain.
Whether sex differences in pain begin in adolescence and are associated with pubertal development was a question LeResche and colleagues explored (LeResche et al., 2005). Looking at TMD pain, headache, stomach pain, and back pain, she found that prevalence of pain in boys varied by painful condition (some increasing, some decreasing with pubertal development). For girls, pain increased across pubertal development for all four conditions. Another study assessed whether cyclic changes in levels of reproductive hormones (i.e., the menstrual cycle and pregnancy) are related to pain in female TMD patients. The results indicate that when estrogen levels are predicted to be high, pain is actually lowest, and correspondingly, when estrogen and progesterone levels are low, pain is the highest. In other words, TMD pain was highest for all
women during the menstrual period. Similar patterns in clinical facial pain were observed across pregnancy (pain was lower during the later months of pregnancy, when estradiol and progesterone levels are high). Note that this pain is not in the reproductive system, but is potentially influenced by hormone levels.
In summary, the presence and intensity of pain in women are related to hormone (especially estradiol) levels. Questions remain as to whether these relationships are strong enough to be taken into account in research and treatment. Large individual differences exist in the correlation between pain and hormone levels, suggesting avenues for further research, including further lifecycle studies (e.g., menopause); relationship of gender-related factors (e.g., social role expectations, coping) to pain in non-Western cultures; common mechanisms underlying negative affective states (pain, depression, somatic symptoms) in women; and differential pain mechanisms by sex (even if pain outcomes are the same).
Open Discussion: Pain and Pain Perception
During the open discussion, panelists and participants delved further into the research aspect of sex differences in pain, including animal models and endophenotypes. Mayer reemphasized that more focus is needed to understand the mechanisms underlying the generation of chronic spontaneous pain. Current thinking is that the main stimulus produces a pain, but another perspective is that these disorders may have a developmental aspect. At an early stage, there may have been a nociceptive input to the brain, and later in life a pain memory may be recalled based on mood states, affective states, or other stressors. Berkley concurred with the need to understand basic mechanisms, but noted that studying stimulus-induced changes also has a role.
A participant agreed that spontaneous pain is a significant clinical problem. He noted that animal models are studying allodynia, which is a real symptom of chronic pain, but is not a particularly important symptom or one that patients often complain about. He also expressed concern about studying endophenotypes, suggesting that perhaps it was more of a step backward than forward. Panelists responded that proxies are useful when the painful experience cannot be quantified. Current research suggests that male and female brains issue the subjective report of chronic pain by different mechanisms. A rational approach to drug development would be to target these sex-specific mechanisms that together generate the same symptom. In terms of drug development, the participant responded, the critical factor is predictability; do the existing models in the field have predictability or not? If a disorder has a high sex prevalence, and that sex prevalence cannot be shown in the animal model, it does not have predictability.
|
BOX 3-2 Key Points: Pain and Pain Perception
|
SLEEP MEDICINE
Sleep Regulation
Although sleep medicine has become a huge field, our understanding of basic sleep regulatory processes and their consequences for disease are still lacking, said Roseanne Armitage, director of the Sleep and Chronophysiology Laboratory at the University of Michigan. Understanding sex differences in any clinical disorder requires knowing about those differences in healthy individuals, and the developmental time course of when those differences emerge. During sensitive periods in development, the magnitude of the sex differences is more likely to increase or decrease, depending on what modifying conditions are in play.
The time course of slow-wave activity is a proxy for sleep homeostasis, the recovery function that occurs during sleep. In healthy adults (women and men, ages 18 to 40), a mild challenge to sleep (e.g., extending wakefulness by a couple of hours) results in only subtle sex differences in the amount of slow-wave activity across the night. However, as the magnitude of the sleep challenge increases, the sex differences become larger with women displaying significantly greater accumulation and dissipation of slow-wave activity. After 40 hours of sleep deprivation, the magnitude of the sex difference is nearly doubled. There is an extraordinarily large response in the initial accumulation of slow-wave activity in women and a
very rapid decay across the night in women compared to men (Armitage, 2007). This suggests a greater adaptive response in homeostasis in females than in males, Armitage said, but it takes a challenge to the regulatory system to elicit that difference.
Important factors to understand are the conditions under which sex differences increase in magnitude, what creates a greater adaptive response in females than in males, and what other factors interact with those. Although baseline sleep studies of the organization of sleep are important, they do not provide information on the sleep regulatory mechanism the way evoking a response in brain regulation through challenge does. Challenge studies are necessary for understanding risk factors for certain diseases that are sex specific, either in their prevalence or in a variety of conditions within the disorder.
The magnitude of the sex difference in slow-wave activity in depressed individuals of the same age range is nearly three times greater than it is in healthy control individuals with no personal or family history of psychopathology. Following a mild challenge to sleep regulation, there is a significant difference between depressed women and depressed men, suggesting that depression itself is a challenge to sleep and brain regulation. There appears to be a sex-dependent propensity to move outside normal homeostasis—an overresponse in depressed women and an underresponse in depressed men.
Age-related sex differences in slow-wave activity occur within healthy control groups. Most studies are not powered sufficiently to consider age subgroups within sex subgroups, and such studies can become very expensive if they include longitudinal designs. But this area needs additional focus, Armitage said, to truly appreciate sex differences in sleep regulation and their consequences for disease. Depression is just one model of conditions under which larger sex differences in sleep regulation are observed than in healthy individuals.
Interaction of Sleep and Circadian Rhythmicity
Prominent sex differences have been observed in slow-wave sleep and slow-wave activity, concurred Jeanne Duffy of the Division of Sleep Medicine at Harvard Medical School and Brigham and Women’s Hospital. As the amount of slow-wave sleep declines with age, sex differences become more pronounced. When an individual is deprived of sleep, he or she responds with increased slow-wave sleep. Individuals differ in the amount of slow-wave sleep they have, and what those differences imply for actual sleep need or sleep deprivation.
Profound sex differences are observed when comparing objective and subjective measures of sleep quality. In one study of adults, for example,
the number of awakenings that were (objectively) recorded on a polysomnogram was greater in women than in men, but (subjective) self-reported awakening from the same individuals on the same nights was greater from men (O’Donnell et al., 2009). The basis of this disconnect is not understood.
The ability to sleep depends, in part, on sleep/wake homeostasis, which depends not only on how long a person has been awake, but also on the time of day they are attempting to sleep which is influenced by the circadian timing system. This system also influences most aspects of human physiology, producing rhythmic daily variations to time events optimally (e.g., body temperature is lower at night and higher during the day).
Growth hormone release is an example of a sex-specific rhythmic variation where the hormone is release in large amounts at the beginning of the night in men, while women tend to have more pulses of release during the day. Early studies exploring the role of sleep on hormonal changes and circadian rhythms were largely done in men resulting in some misleading generalizations for both sexes.
The effect of sleep on circadian rhythms is not well understood, nor whether there are sex differences, but data suggest there may be differential impacts on sleep deprivation. The body’s response to attempts to sleep at the wrong time of day may have a sex difference as well. As noted earlier, sleep changes profoundly with aging, a change also associated with sex differences.
Males and females differ in their tolerance for staying awake for extended periods. During the usual nighttime hours, women unintentionally fall asleep more frequently than males when they are asked to stay awake for a 36-hour period (Buysse et al., 1993). However, women have faster reaction times after being awake for extended periods (Duffy et al., 2009).
Coregulation by the circadian timing system and the “sleep–wake homeostat” allows humans to keep a stable level of alertness and performance across a normal 16-hour waking episode. This is different from most animal species, which have polyphasic sleep–wake patterns. In addition, most current model systems involve nocturnal animals.
Misalignment between the timing of sleep and the underlying circadian rhythm timing has both health and safety consequences. These are exacerbated by the associated reduction in sleep. For example, there are large differences in the way hormones related to metabolic and cardiovascular functions are regulated under circadian misalignment versus alignment. Studies suggest important differences in how circadian and sleep misalignment may affect the two sexes, but data are currently limited.
Duffy closed by describing some of the challenges to understanding sex differences in sleep regulation. Similar to other areas of research, many basic studies on sleep and circadian rhythms are done in male animals. Con-
ducting sleep studies is technically challenging because sleep feeds back on circadian rhythms. Although women are now included in clinical studies, many studies are not powered to test whether sex differences exist.
Sex differences occur in sleep, both in slow-wave activity and self-reported sleep need and sleep duration. Reductions in sleep have important metabolic consequences, and influences depression and subjective reports of pain. A better understanding of sex differences in sleep has implications for many of the other sex-related health disorders.
Sex Differences in Subjective and Objective Measures of Sleep
Rachel Manber, director of the Stanford Sleep Medicine Clinic at Stanford University, expanded on the discussion of sex differences in the objective and subjective measures of sleep. Objectively, compared to men, women seem to have shorter sleep-onset latency, spend less time awake in bed, have fewer awakenings, and ultimately get more total sleep time and have higher sleep efficiency. Sleep efficiency is the ratio between time spent asleep and time spent in bed, Manber explained. Women also have less slow-wave sleep during the second half of the night. Subjectively, however, across all ages, women report more disturbed sleep than men. A National Sleep Foundation poll found that women report that they need more sleep, or their sleep is insufficient and more disturbed, compared to men. These differences persist even after controlling for psychiatric conditions. Given less than 7 hours of sleep, men are more likely than women to report better functioning during the day. However, on objective vigilance tests or performance tests, not much difference is apparent.
Subjective/objective differences in sleep exist across the board. There are subjective, but not objective, differences in the consequence of menstrual phase and menopausal status on sleep. Menstrual phase effects also cause large individual differences in sleep. About 15 percent of women experience a clinically meaningful disturbance in sleep when they are premenstrual compared to when they are in their follicular phase (Manber and Bootzin, 1997).
Manber stressed the need to better understand subjective–objective sleep discrepancies. Subjective sleep is extremely relevant because the perceived lack of sleep causes people to seek help. Subjective sleep is also easier to study, she noted.
The sexes show differences in sleep disorders in terms of both prevalence and presentation. Insomnia, for example, is more prevalent in women, at a ratio of 2:1. Restless leg syndrome is also more prevalent in women, but a related disorder, periodic limb movement disorder, shows no sex difference. Narcolepsy, rapid eye movement (REM) behavioral sleep disorder,
and obstructive sleep apnea are all more prevalent in men (Krishnan and Collop, 2006).
Differences in presentation can be important for diagnosis and treatment. Obstructive sleep apnea, for example, presents differently in men and women (Valipour et al., 2007; Wahner-Roedler et al., 2007). Women with obstructive sleep apnea tend to be more obese and report more fatigue and less energy. However, they are less likely to report witnessed apnea or heavy snoring, considered the most important clinical presentations warranting a sleep study to confirm or rule out sleep apnea. Women are less likely to respond to sleep apnea treatment and their apnea is less likely to abate or decrease in severity following weight loss than men.
Sleep is very sensitive to perturbations in stress and emotional and physical well-being. Sleep, or the lack of it, also influences a person’s ability to regulate emotions. Therefore, Manber said, sex differences in sleep in psychiatric disorders are not surprising. Not much is known, but as Armitage discussed, studies have shown that men are more likely to experience slow-wave sleep deficiencies in depression, which may indicate impaired homeostatic regulation of sleep. Another area in which little is known about sex differences is sleep and posttraumatic stress disorder (PTSD). A metaanalysis found that by restricting the sample to studies of only men, PTSD-specific sleep disturbances had a very strong effect compared to controls, but looking at studies that included both sexes, or female-only samples, the effect was not as strong (Kobayashi et al., 2007).
Manber described her current work on moderators of treatment response in depressed individuals with comorbid insomnia as part of the TRIAD (Treatment of Insomnia And Depression) study. Both sexes are represented, she said, and the sample is large enough to facilitate analysis of the interaction between moderators of treatment response and sex.
In summary, Manber reiterated the need to better understand sex differences in the discrepancy between objective and subjective sleep; the need to understand sex differences in the presentation, course, and treatment of specific sleep disorders; and the need to have sufficiently large samples to allow examination of the interaction between moderators and sex in treatment outcomes research.
Health Consequences of Sex Differences in Sleep and Circadian Rhythms
Although one can argue that sleep is of, by, and for the brain, the brain sits in a corporeal body, and the body is essential for the brain’s continued adventure throughout life, said Martica Hall, associate professor of psychiatry, psychology, and clinical and translational studies at the University of Pittsburgh School of Medicine. Every dimension of health
and functioning is effected by circadian rhythms, and vice versa. As already discussed, normative sleep and normative circadian rhythms have marked sex differences, and those differences affect mental health. But do these sex differences matter to the sleep–health relationship with regard to physical health and corporeal health disorders?
Compelling evidence shows that sex differences in sleep bear on both resilience and vulnerability to health and disease. In studies of sleep and health, the three current areas of emphasis are obesity, cardiovascular disease, and metabolic disease. The common focus in these studies is sleep apnea, and the usual subjects are males, including male rats or mice. However, until menopause, men and women have different susceptibility and vulnerability to sleep apnea syndrome.
One example is sex differences in the cardiovascular response to hypoxia during the sleep period. Differences in mean arterial pressure have been demonstrated between male and female rodents in an induced hypoxic condition. Mean arterial pressure of ovariectomized females was similar to that of males.
In addition to apnea, other factors to consider include subjective sleep quality, sleep duration, fragmented sleep, sleep depth, and cortical arousal during sleep. Hall cited a recent study showing that short sleep was a risk factor for incident hypertension in women, but men showed no relationship between sleep duration and incident hypertension.
Women who have sleep disorders also have more hyperaroused brains during sleep. Fast frequency electroencephalography (EEG) activity was shown to be increased during non-REM sleep in women with insomnia compared to controls, but men with insomnia showed no difference from controls. Women with increased fast frequency EEG activity during sleep also have been shown to be at greater risk for metabolic syndrome.
In conclusion, Hall said she and others are now looking at real-time evaluation of sleep and physiology (the pathways through which sleep may affect health outcomes, such as hearing rate variability, autonomic imbalance, or inflammation), and studying people in their habitual environments (i.e., in participants’ homes rather than in sleep laboratories). Interdisciplinary collaborations are also important, and Hall noted the need for emphasis on sleep and accelerated aging, both at the cellular and molecular levels.
Open Discussion: Sleep Medicine
Animal models were once again a topic of much interest during the open discussion. Panelists and participants also discussed sleep patterns in adolescents, and how subjective and objective measures factor into the drug development process.
Animal Models
A fundamental issue with animal models is that rodents have polyphasic sleep, while human adults are monophasic. Big differences occur in sleep regulation across the lifecycle, so animal models should be developmentally appropriate, Armitage said. Although animal neurophysiologists have worked on sleep studies for years, few have included both sexes of rodents in their studies.
A participant raised the issue of the disconnect between objective and subjective measures of sleep in humans and pointed out that there are no animal models that can take this into consideration. Animal research has been useful, he said, in telling us what we should look for in humans. For example, early studies of rodents established a sex difference in circadian rhythms by demonstrating that the estrous cycle can influence the rhythm.
Subjective Versus Objective Measures in Drug Development
The subjective versus objective sleep measure conundrum is particularly relevant to the development of new drugs for sleep, a participant observed. Is it more important that the subject feels subjectively better? Or is more important that the subject is objectively better? Which measure should be used in developing a new drug?
Hall responded that going forward, researchers are using more quantitative and sophisticated techniques to measure what is happening in the brain during sleep. This is one area where animal models are helpful and directive, she added.
A methodological issue is whether the objective measures being used are just not good enough to capture the subjective experience, Manber said. Which method to use really depends what disorder is being studied. In insomnia, for example, subjective measures may be more important.
A participant from a pharmaceutical company concurred that in insomnia research, subjective measures are important to patients. But objective measures are important in drug development, he said, and can be useful in translation. In early drug development, researchers are looking for outcome measures that can facilitate smaller studies. Polysomnography, for example, is much preferred to the subjective measures, which have a smaller effect size and larger variability.
|
BOX 3-3 Key Points: Sleep Medicine
|
MULTIPLE SCLEROSIS AND NEUROINFLAMMATION
Sex Differences in Multiple Sclerosis: Clinical, Imaging, and Pathology
Multiple sclerosis (MS) is a multifocal inflammatory disorder that affects the CNS, including the brain, spinal cord, and optic nerve, explained Robert Fox, associate staff and medical director of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. MS is typically diagnosed between ages 25 and 45, and is the most common non-traumatic cause of disability among young adults, affecting one in 1,000 persons in the United States. By 15 years from onset, about 75 percent of individuals have a progressive course of disease, and moderate to severe disability. Multiple sclerosis lesions can be seen on axial brain and spinal cord MRI, and are apparent pathologically postmortem.
Disease Course
About 85 percent of MS patients start with a relapsing–remitting form of disease, with episodes of neurological dysfunction that go into remis-
sion, but may leave them with some residual disability. Of those, about 75 to 80 percent will eventually, over about 15 years or more, transition into a gradually progressive course of disease with few, if any relapses. This is the secondary progressive phase of MS, when there is gradual progression of disability.
About 10 percent of new patients will present initially with a primary progressive course, a gradual worsening over time. This is similar to secondary progressive MS, just without the preceding relapsing–remitting phase. This subset is probably a mixture of both secondary progressive patients who had non-clinical events or subclinical relapses, as well as patients with a true primary neurodegenerative disorder without much active inflammation.
A smaller group of patients, about 5 percent, have what is called progressive relapsing MS. They appear to have primary progressive MS, and then they have a remission.
Finally, a rare variant of MS called neuromyelitis optica is sometimes considered a completely different disease. Inflammation targets primarily the optic nerve and the spinal cord. When it affects the brain, it causes large atypical brain lesions that do not look like typical MS.
Two categories are used for measuring disease: active inflammation and accumulated injury. To assess active inflammation, clinicians consider the frequency of relapses and measure gadolinium-enhancing lesions. Accumulated injury assessments factor in disability progression, MRI lesion burden, brain atrophy, and information obtained from advanced imaging technologies.
Sex Differences in Multiple Sclerosis
Fox reviewed the current knowledge about sex differences in MS based on clinical observations, imaging, histopathology, and clinical trials. Overall, MS is two to three times more common in women than in men (Alonso and Hernán, 2008; Orton et al., 2006). This ratio decreases with age (Marrie et al., 2010). Note that the sex ratio among primary progressive MS patients, who have a worse prognosis because they are progressing from the beginning, is equal. Also of note, neuromyelitis optica is at least 3 times more common in women than in men (and some surveys suggest up to 10 or 20 times). Peak incidence of MS is earlier in females, occurring around ages 35 to 39 in women, and 45 to 49 in men. Peak prevalence is also earlier in women compared to men (ages 45 to 49 for women and 55 to 69 for men). This difference is important to keep in mind when interpreting studies on prognosis, Fox said, as many studies have not properly controlled for differential age, and differential age at diagnosis.
Over time, the prevalence and peak age of MS have been increasing. The incidence has remained fairly stable, and the ratio between men and
women in terms of the incidence is roughly the same. This may be due to less diagnostic delay in more recent times (which will increase the prevalence without changing the incidence) and greater survival (which does not necessarily mean greater quality of life).
In terms of prognosis relative to relapses and progressive disability, observations vary. A large number of earlier studies suggest that females have a favorable prognosis. Quite a few other studies have shown no sex difference in the prognosis of MS. Although a sex difference is prominent in the incidence in neuromyelitis optica, no sex differences have been observed in progression.
With regard to the role of sex hormones in MS, from the clinical point of view it is clear and widely accepted that relapse rate decreases during pregnancy, particularly the third trimester. Then, in the months after delivery, a rebound brings the relapse rate back to the prepregnancy rate or perhaps even higher. The effect of oral contraceptives is less clear. A lower incidence of MS is seen among women using oral contraceptives, although some studies have found no effect. One study, interestingly, found an increased risk in long-term users of oral contraceptives, suggesting the need for further research in this area.
Earlier and smaller MRI studies (n = 50 to 413) of active inflammation found gadolinium-enhancing lesions were more common in women, but they did not control for the differential age and the disease course of patients. Later studies that did control for some of these covariates, enrolling 700 and 1,300 patients, found no sex difference in gadolinium-enhancing lesions.
MRI studies of cumulative injury found no sex difference in T2 lesions after covariate adjustment. Studies of T1 lesions observed no sex difference in relapsing–remitting MS, and greater T1 lesion volume in male primary progressive MS. No sex differences in atrophy have been demonstrated in cross-sectional or longitudinal studies. Results are conflicting about gray-matter atrophy; some studies suggest it is greater in men, and others indicate it is equal in both sexes. Studies using advanced imaging metrics of MS tissue injury, including diffusion-weighted imaging and magnetization transfer ratio, have not demonstrated any sex differences.
Only a few histopathologic studies of MS have evaluated sex differences. No differences have been observed in studies of active inflammation (equal number of microglial cells and T-cells in MS lesions and normal-appearing white matter in men versus women), tissue damage (equal number of amyloid-precursor, protein-positive spheroids; equal reduction in axonal density; no difference in number, type, or distribution of cortical lesions), or repair (no difference in the pathologic evidence of repair, oligodendrocyte precursor cells, and other related lineages; no difference in remyelinating lesions between men and women). The only pathologic sex
difference available in the literature, Fox said, was a low fiber density in spinal pyramidal tracts in men. All together, evidence showing a sex difference is limited in the histopathology of MS.
The vast majority of clinical trials have found no effect of sex on outcome. Many of these trials had a preplanned covariate analysis of sex, and while some reported no effect of sex, others did not report any sex-based results. However, notable exceptions include two studies of interferon in secondary progressive MS, which found that only women had slowed progression of disability. A third interferon study did not find any effect of sex. Whether these studies adjusted for covariates such as age of patient and age at diagnosis was unclear, Fox said.
A post-hoc analysis of data from a study of glatiramer acetate and primary progressive MS showed that only men had a slowed progression of disability. This difference persisted after covariate adjustment. Several other glatiramer acetate studies (in relapsing–remitting MS and primary progressive MS) did not identify a sex difference. No clinical trial has shown a differential effect of sex on any MRI measure.
In summary, Fox said, MS is two to three times more common in women. Onset is later in men and is more likely to be primary progressive MS, making interpretation of studies challenging. Women may have a better prognosis, but the differences decrease after adjusting for age at diagnosis and disease course. Pregnancy decreases disease activity, but whether oral contraceptives have an impact is unclear.
Despite the observations of smaller studies, the larger MRI studies fail to suggest an effect of sex on MRI measures of either inflammation or tissue injury. Histopathology shows virtually no effect of sex, nor did most clinical trials. A few post-hoc analyses suggest some sex-based effect, but how to interpret these findings is unclear in light of all the other studies that found no impact.
Altogether, while a sex difference is clear in the incidence of MS, little evidence is available on sex differences in the clinical course of MS. That does not mean, however, that consideration of sex differences should not be included in planning future studies.
MULTIPLE SCLEROSIS AND NEUROINFLAMMATION: CONSIDERING SEX DIFFERENCES IN DESIGNING THERAPEUTIC AGENTS
Multiple sclerosis is just one of a list of autoimmune diseases that show sex differences. Halina Offner, professor of neurology and anesthesiology and perioperative medicine at Oregon Health and Science University (see ohsu.edu), said 78 percent of people affected with autoimmune diseases are women.
Experimental autoimmune encephalomyelitis (EAE) is the prevailing animal model for MS. Disease can be induced in genetically susceptible rodents by immunization with spinal cord homogenates, myelin, or specific myelin peptides in combination with adjuvant (active EAE), and by adoptive transfer of encephalitogenic cells (passive EAE). Typically, EAE is thought to be a Th1/Th17-mediated disease. However, other cellular players such as antigen-presenting cells also play a role in disease progression. Increased numbers of antigen-presenting cells in the CNS increase susceptibility to and severity of EAE in female mice. T-cell response to foreign antigen increases the numbers of activated T-cells in female versus male CNS. This could be influenced by sex steroids, Offner said. CNS T-cells recruit higher numbers of antigen-presenting cells to the CNS, which enhance the encephalitogenic activity of myelin-specific T-cells, thus producing the “high susceptibility” phenotype found in female mice.
MS does have sex-based immune differences. Females mount a stronger immune response, with higher levels of antigen-presenting cells, CD4+ Th2 cells, and immunoglobulin responses. Males have severe inflammation and enhanced CD4+ Th1 and CD8+ T-cell activity. The immune functions of males and females have fundamental differences, which may require different immunomodulatory strategies in MS. The regulatory balance between the detrimental and beneficial effects of immune cells in MS is very complex, Offner explained.
In MS, the initiating factors are susceptibility genes and environmental factors. Sex hormones and neuroendocrine factors are very important modulatory factors in the immune and autoimmune responses, Offner said.
Some data on MS are rather controversial. Offner cited a report on the recent increase in incidence rates of MS in females compared to males (Debouverie, 2009) and several studies that showed increased ratios of females to males (Eikelenboom et al., 2009; Maghzi et al., 2010; Sadovnick, 2009). One study connected the increase in the sex differences to vitamin D, suggesting that higher levels of vitamin D are associated with a lower incidence of multiple sclerosis only in women (Kragt et al., 2009).
MS cases are well known for increasing with geographic distance from the equator, and vitamin D levels decrease with distance from the equator. Decreasing MS cases with increasing ultraviolet (UV) light also has been documented in several countries. UV light catalyzes the first step of vitamin D3 synthesis (the inactive form of the vitamin), and serum D3 levels correlate with exposure to the UV light.
To evaluate this further, researchers looked at the effects of vitamin D3 in the EAE model. The severity of disease was much lower in females who were fed a vitamin diet, but there was no difference in males in clinical disease (Spach and Hayes, 2005). Further studies demonstrated that estrogen was needed for vitamin D3 to inhibit EAE in female mice. Ovari-
ectomy eliminated the vitamin D3-mediated inhibition of EAE, and estrogen replacement in these animals restored vitamin D3-mediated inhibition of EAE.
If humans have similar gender differences in vitamin D metabolism, Offner said, then sunlight deprivation would increase the MS risk more significantly in women than in men, which could explain higher incidence of MS in females.
The current hypothesis is that there may be a female bias in the protective effects of vitamin D3 in MS, and that insufficiency in vitamin D3 may contribute to the higher female/male sex ratio. Lifestyle changes could be linked to insufficient sunlight exposure in recent years (women in the workforce, indoor lifestyle, sunscreen use). Also, declining ovarian function and limited vitamin D3 supplies may be driving the transition of relapsing– remitting to chronic progressive MS.
In contrast, Offner described a study in which male and female animals with EAE were treated with a recombinant T-cell receptor ligand (RTL) construct at the onset of disease. The results showed a significant reduction in disease activity in mice treated with RTL, which was comparable in both sexes. Spinal cord imaging of mice at the onset of disease and at 3 days posttreatment with RTL showed reversal of T-cell infiltration, again with no difference between females and males. Based on these preclinical studies with RTL in the EAE model, a Phase I safety study in MS patients was designed. Thirty-four adult subjects (male and female) who received varying doses of RTL injections or placebo were followed for more than 90 days. The primary outcome measure was whether the RTL construct increased MS disease activity, and the conclusion was that the RTL did not increase MS disease activity by any measure in either females or males.
In conclusion, Offner said that sex differences matter in many clinical diseases and animal disease models. Disease models such as EAE often have used males, with the assumption that this decreases experimental variability caused by female hormone cycling. There are also misconceptions that disease mechanisms or treatment effects will be the same for both sexes. This is not the case, as demonstrated in the two examples provided, showing that response to vitamin D is sex dependent, while response to RTL is not. Therefore, preclinical studies should always include both sexes.
Studying Sex Differences from Bedside to Bench to Bedside
When discussing sex-based disparities in health, separating incidence from progression is important. Incidence is akin to susceptibility, or the immune system’s overactivation in autoimmune diseases like MS and lupus, whereas progression or disability accumulation can be a reflection of not only the immune system, but the overlay of the CNS reaction to that im-
mune attack. These are very different situations, said Rhonda Voskuhl, director of the Multiple Sclerosis Research and Treatment Program at UCLA. Although sex differences in the progression of MS generally have not been observed, there is a clear difference in incidence.
Sex differences in MS could be from the basic differences between males and females, including sex hormones (estrogen and testosterone) or sex chromosomes (XX or XY gene effects). Conditions that occur in one sex, but not in another, such as pregnancy, could also be major factors. In the case of MS, pregnancy reduces relapses by 80 percent, significantly more than most of the currently available drug therapies, which reduce relapses by about 33 percent. (Some reduce relapses by half to two thirds, but these carry with them risks of significant adverse events.) Understanding this effect of pregnancy on MS pathogenesis could aid the discovery and development of better therapies for MS.
Voskuhl described a “bedside-to-bench-to-bedside” approach to considering MS. The classic bench-to-bedside approach is somewhat risky, she said, starting with a molecule or a pathway, studying it in vitro and in vivo and then in human trials, often finding that the treatment does not work as predicted in people. Sex differences are known to be clinically important as major disease modifiers. A better approach in the case of MS is to go from what is known clinically, to characterize the cellular and molecular mechanisms, then to go back to the patients with a clinical trial.
One example of this approach is the bedside observation of reduced relapses during pregnancy. As discussed, this could be related to any number of things, including hormones or vitamin D. Several laboratories have now shown that estrogens, particularly estriol, are protective in the animal model of MS. Based on this clinical observation, and the subsequent results from animal models, estriol was then administered in pill form to people with MS in a Phase I trial, and reduction in the number and size of lesions was observed. A multicenter Phase II trial is under way.
A second example stems from the clinical observation of the sex difference in incidence of MS, and the fact that men are older than women at disease onset. Based on this decreased susceptibility of younger men (when testosterone levels are high), studies of the potentially protective role of testosterone in MS were conducted in the animal model. Results supported the protective role of testosterone, and a pilot study of men with MS found that treatment with testosterone gel slowed brain atrophy and caused immune shifts that are biomarkers for improvement. A Phase II trial of testosterone in men with MS is being planned.
As discussed by Arnold (Chapter 2), sex differences are not all about adult hormones. Developmental hormones and sex chromosomes also have effects. Using the four core genotype mouse model describe by Arnold to study EAE and lupus, Voskuhl found sex chromosome clearly had an effect,
with the XX genotype promoting disease development in both EAE and lupus (Smith-Bouvier et al., 2008). Voskuhl is also studying developmental hormone effects using the same system.
In summary, Voskuhl stressed that clinical observations can lead to promising potential treatments for MS. Research on sex hormones has reached clinical trials, and research on sex chromosome effects are still in the early stages, but may have potential as well. Whether the sex chromosome effect is an X dosage effect or a Y gene effect—and what that gene is—remains to be seen.
Animal studies to elucidate mechanisms, and pilot trials of potential products in humans, are possible, but funding is a major obstacle, Voskuhl said. The small pilot trials described by Voskuhl were funded by the MS Society and the NIH. But the challenge is funding a potentially $30 million Phase III trial, especially when the drug under study is not a patent-protected product (e.g., estriol). Although estriol is already broadly used and well characterized with regard to safety, the fact that it is not patent protected means that a pharmaceutical company that might otherwise invest in developing a new product may not be able to recoup the significant product development costs through future product sales.
Open Discussion: Multiple Sclerosis and Neuroinflammation
In the open discussion, panelists further considered the implications of sex differences in MS, and how MS is different from the other diseases discussed so far. Participants were interested in further information on the studies discussed, and raised issues about approaches to research.
Fox noted that for the other diseases discussed at the workshop, sex differences are apparent in the disease course. However, for MS, although the incidence is different for men and women, the disease course appears similar. Voskuhl noted that although progression may not be different, the immune response is more aggressive in women. The question is why, if immune response is different, is progression not different? A difference in the brain must be counter to the difference in the immune system.
A question was raised as to whether estriol had been tested only in women, and if there are any obstacles to trying it in both sexes. Voskuhl responded that estriol has been shown to have an effect in male mice with EAE as well. This has not been done in clinical trials. The adverse events could be different, she noted; there would be no concern about endometrial or uterine cancers in men. Precedents have been set for giving estrogens to men with other diseases.
A participant suggested that another way to study sex differences is to look at the factors at play when, for example, a male develops a disorder that is common in women. Another participant cautioned that care must
be taken when looking at clinic populations versus the larger community. People with the most severe conditions are the ones who come to tertiary care.
|
BOX 3-4 Key Points: Multiple Sclerosis (MS) and Neuroinflammation
|
In the community, there are large sex differences in pain, but within the tertiary care setting there are not many differences between males and females.
OVERARCHING DISCUSSION
Following the disease-specific panel discussions of issues related to sex differences in translational research, the four panel moderators and the workshop cochairs assembled to consider overarching issues across major disease areas.
Making the Decision to Study Sex Differences
The panel first considered when, during development of products to treat disorders of the nervous system, consideration of sex differences would be most appropriate, taking into account financial, time, and material resource constraints.
Richard Nakamura, director of the Division of Intramural Research Programs at the National Institute of Mental Health,1 said that ideally, information should be collected on any sex differences that may emerge, but sex differences are unlikely to be identified in relatively small proof-of-concept trials. The larger studies, such as STAR*D, are the ones that need to focus on sex differences. Investigators should publish any supplementary data on sex differences that emerge from trials, even when the data are not statistically significant.
He also noted that large databases, such as the NIH’s GWAS databases, could provide a valuable resource in trying to understand sex differences. In addition, the Veterans Administration and other large healthcare entities that have large electronic health records systems should be encouraged to develop common features and nomenclature so that these systems could be analyzed for information on sex differences. Progress toward a system of universal medical records in the United States would aid this effort as well.
From an industrial perspective, Chi-Ming Lee, executive director of Translational Science at AstraZeneca Pharmaceuticals, noted that unexpected failures in later phases of product development (Phase II or III) are extremely costly. A company usually has a very strong rationale, and has met certain criteria, before moving a drug from early phase into the later phases of development. Data from animal models are considered in these decisions, but experience has shown that some animal models that were relied on failed, in the sense that they did not predict the outcomes later observed in humans.
Theoretically, sex differences should be addressed early, Lee said, but there are many considerations. Should every animal model study include both male and female? Simply including females in studies will not necessarily provide the answer, and can provide a false sense of security. If either preclinical or clinical evidence suggests that sex makes a difference, then further research on these differences should be conducted. Many factors must be considered, including, but not limited to, hormonal effects, sex chromosome effects, lifespan, psychosocial factors, or species differences.
Paul Hoffman, associate chief of staff for Research and Program De-
velopment at North Florida/South Georgia Veterans Health System, concurred that evidence of an increased prevalence in one sex versus another merits further attention. The question is where the funding is best applied. In clinical trials, sex should be treated as a variable, and studies should be designed with enough power to allow for analyses by sex (rather than sex being a post-hoc analysis).
Zorn emphasized the need for a business case before a pharmaceutical company expends significant resources. The current costs of bringing a drug from the bench to the patient are in the range of $1.8 billion. To include sex as a variable in preclinical studies and clinical trial design, a hypothesis based on data is needed. While much of the data presented at the workshop have been very good and could be used to generate hypotheses about sex differences, some of those data are not yet clear enough to justify the significant investment required. A stronger investment is needed in basic research, he said, looking at differences in the sexes all the way down to the molecular level, so that drug developers have a solid base on which to test these differences in humans. The responsibility for obtaining this evidence falls to basic, preclinical, and clinical researchers of all kinds, communicating and working together.
A participant pointed out that beyond the costs of development, a company must also bear the costs of educating physicians and providers about the use of the product.
Nakamura added that over the past few years, there has been discussion that the business model for CNS disorder medication development is basically failing, and that many pharmaceutical firms are leaving this therapeutic area. Studying sex differences, to the extent that they are a complicating factor, adds costs when developing a profitable drug. On the other hand, there is a limited business case for developing reasonable molecules that are inexpensive. Perhaps the NIH could help share the risk and leverage the strengths of federally funded research and industry by performing proof-of-concept studies while providing industry with the opportunity to develop distributable drugs.
A participant said that in recent years, charities such as the Bill and Melinda Gates Foundation have funded the development of compounds that are either not patentable, or are for a rare disease in countries where people cannot pay for products. Perhaps this alternative funding approach should be considered for sex differences.
Lee noted that many companies are now involved in various consortiums, such as the Foundation for NIH’s Biomarker Consortium, where money is pooled and risk is shared. Lee also said that the blockbuster business model is really in the past. Moving forward, there is definitely consideration of personalized medicine, but the investment has to be justified.
Another industry participant concurred that industry is moving away
from big blockbuster drugs and is much more comfortable with smaller markets. In personalized healthcare, it is implicit that not only is the target population restricted, but the efficacy of the drug is potentially increased and thus has a higher chance. A product may be approved for the 20 percent of the people who have a particular mutation or amplification of a gene, with payers willing to reimburse the manufacturer for it because it works.
Another participant wondered if a sex difference is something that a drug developer would simply rather not know because that information prevents them from marketing the product to everyone, potentially cutting the marketing in half.
An industry participant responded that he was not aware of many drugs in development that were so much more efficacious for one sex than the other that they merited pursuing a separate, sex-based efficacy claim. However, safety problems detected in animals can often be restricted to one sex, or the therapeutic index may be different. The company is then faced with the difficult decision of whether to continue development of the drug for one sex, or stop and try to switch to another molecule in the family (assuming it is not a mechanism-based toxicology or adverse event). Risk/benefit also needs to be considered. If toxicology in one sex is bad, should the drug still be developed for the opposite sex? If the other sex takes it inadvertently or doctors do not read the label or prescribe it off-label for the other sex anyway, that is a significant risk.
Encouraging Basic Research into Sex Differences
Many participants asserted that if basic researchers were somehow required to study both males and females, the amount of information available about sex differences could be rapidly expanded, but such a mandate would be too expensive, and there would be a huge pushback from the research community. Other, more practical options could be to require study sections to rate grants on their comparison of sexes, or to put greater power in the hands of program officers, asking them to commit a certain amount of their funding to basic research grants that consider sex differences. There could be a study section, or similar alternative mechanism, for vetting which sex differences in human conditions and diseases should be funded.
A participant added that, as a previous recipient of NIH funding, he believed that forcing researchers to include both sexes in animal research was a bit unpalatable, but that it would be reasonable to ask them to justify why they use one sex or another, or both.
Interdisciplinary Collaboration
As the panel presentations demonstrated, there are commonalities and shared factors across different clinical conditions with respect to sex differences. Given limited funds, participants suggested that one way to have a greater impact is to target funding toward those commonalities. One way is to encourage interdisciplinary cooperation through Requests for Applications. This would not need to be a large multicenter effort, but could be two researchers from different disciplines who are working in collaboration. A participant noted that the NIH interdisciplinary programs are intended to do this.


































