2
WIC and Birth Outcomes
During this session, moderated by Gail Harrison, the two presenters (Michael Lu and Theodore Joyce) addressed selected topics related to maternal health and nutrition and birth outcomes, and the two discussants (Marianne Bitler and Patrick Catalano) added clarifying information. Each of them recommended topics for the research agenda for the Supplemental Nutrition Program for Women, Infants, and Children (WIC).
THE ROLE OF PERICONCEPTIONAL NUTRITION
Presenter: Michael Lu
The path toward a healthier population begins with improving periconceptional nutrition, emphasized Lu. The periconceptional period extends from preconception through conception, implantation, placentation, embryogenesis, and organogenesis. These are critical stages that affect both immediate birth outcomes and also the long-term health and development of the child.
Research Background
Increasingly, studies have linked periconceptional nutrition to reproductive outcomes. These outcomes include ovulatory infertility (Botto et al., 2004; Chavarro et al., 2006, 2007a,b,c, 2008a,b), birth defects (Groenen et al., 2004; Krapels et al., 2004; Lumley et al., 2001; Smedts et al., 2008; Velie et al., 1999; Verkleij-Hagoort et al., 2006), spontaneous preterm birth
(Bukowski et al., 2009; Vahratian et al., 2004), preeclampsia (Bodnar et al., 2006; Catov et al., 2007), and infants with low birth weight and who are small for their gestational age (Timmermans et al., 2009). For example, the First and Second Trimester Evaluation of Risk (FASTER) trial found that preconceptional folate supplementation for at least 1 year was associated with a lower risk of spontaneous extreme preterm birth (20–28 weeks) and that the risk was inversely proportional to the duration of preconceptional folate supplementation.
Although the biological mechanisms linking periconceptional nutrition with specific reproductive outcomes are not clearly understood, Lu indicated that some evidence makes connections that are biologically plausible. For example, nutrition plays a role in host susceptibility to infection and inflammation, and these in turn may be related to placental complications. In approximately one-third of the cases of preterm birth, the placental vessels show failure of vascular remodeling, and in 15 to 25 percent they show residual vascular pathology characterized by thrombosis and atherosis (see Figure 2-1).
Lu briefly reviewed evidence of the role of the placenta in fetal programming and future disease risk (see Godfrey, 2002) and noted potential effects of periconceptional nutrition on epigenetic modification (Sinclair et al., 2007; Steegers-Theunissen et al., 2009). In particular, he emphasized periconceptional nutrition as it relates to allostasis and allostatic load. Allostasis refers to the maintenance of stability through change, and allostatic load refers to the cumulative physiological toll from chronic stress. Chronic, repeated stress causes the body to lose its ability for self-regulation (McEwen, 1998). Lu postulated that chronically “bombarding” the body with high-sugar and high-fat diets and with high stress will gradually re-
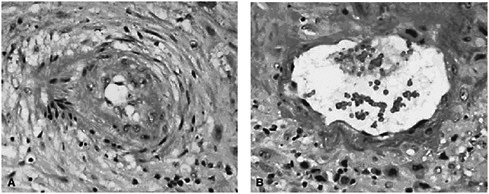
FIGURE 2-1 (A) Spiral artery in a preterm pregnancy; (B) spiral artery in a normal pregnancy.
SOURCE: Salafia and Popek (2008). Reprinted with permission.
duce the body’s ability to self-regulate and will damage regulatory systems to the point that fetal programming will be sub-optimal. Lu stated that the interconception period provides a critical window of opportunity to restore allostasis to optimize the woman’s health before she becomes pregnant.
Research Proposals
Lu said that the research mentioned above makes it clear that the agenda for WIC research should include studies conducted during interconception in order to address the preconceptional period before a subsequent pregnancy. His three research proposals were:
-
Observational studies on the effect of interconceptional nutrition on birth and long-term child health outcomes. These studies should incorporate biomarkers to identify mechanisms and pathways. Consider potential collaboration with the National Children’s Study through adjunct studies. Through the provider-based recruitment strategies being used, WIC sites could potentially be valuable for study participant recruitment.
-
Nutritional intervention studies that begin at interconception. Prioritize women with previous adverse outcomes and communities with marked nutrition and health disparities. Conduct single nutrient, multi-nutrient, and whole foods supplementation studies. Conduct both efficacy and effectiveness research.
-
Nutritional research that focuses on women’s health before, between, and beyond pregnancy. Outcomes to consider include metabolic allostasis and allostatic load, postpartum weight retention, and breastfeeding.
In closing, Lu said, “I think it is really time that we put the ‘W’ back in WIC.”
THE IMPACT OF WIC ON BIRTH OUTCOMES
Presenter: Theodore Joyce
Joyce opened by saying that there is relatively weak evidence that WIC protects against adverse birth outcomes. He agreed with Lu that the focus needs to be on maternal health, beginning at preconception. His presentation briefly addressed the level of prenatal intervention that WIC provides and evidence from early studies of WIC, pointed out challenging sources of methodological biases (especially gestational age bias and postpartum bias), and made suggestions for a change in focus.
Level of the Intervention
Given that the average period a pregnant woman is on WIC is 4.5 months, that the average monthly prenatal food voucher is worth approximately $50, and that the marginal propensity to consume1 is about 20 percent for WIC, Joyce estimated that WIC provides about $45 in extra food consumption for the pregnancy. This amount alone, he argued, cannot explain the very large improvements in birth outcomes associated with WIC participation that have been reported in the literature.
Evidence from Early Experimental Studies
Three early high-quality experimental studies (Klerman et al., 2001; Metcoff et al., 1985; Rush et al., 1980) reported no effect from WIC or nutritional supplementation during pregnancy on birth outcomes, but they are seldom cited. The study by Rush and colleagues even concludes that protein supplementation may be a risk factor for preterm birth.
Sources of Bias
Time-series evidence is inconsistent with large treatment effects. Considering the findings by Devaney et al. (1992) of about a 4 percent difference in low birth weight between WIC participants and nonparticipants on Medicaid, one would expect to see a change in the rate of low birth weight over time. But although WIC enrollment has quadrupled since 1983, there has been no visible effect of WIC enrollment on low birth weight rates (see Figure 2-2). Similarly, there is no visible association between WIC enrollment and singleton preterm birth rates. In a report on preterm birth, the Institute of Medicine (IOM, 2007) concluded that little is known about how to prevent these early births.
A number of studies of low birth weight and preterm birth have used methods that introduce large biases. For example, gestational age bias may arise because women with longer-term pregnancies have more time to enroll in WIC as a prenatal participant. Compared with the women who enroll early, those who enroll late in their pregnancy are more likely to have good birth outcomes simply because the pregnancy went to term (Devaney, 2010). An example of gestational age bias is shown in Figure 2-3. A notable feature of the graph is that the rate of low birth weight decreases dramatically for women who enroll in WIC very late in their pregnancy. Obviously, this improvement in low birth weight rates cannot be attributed to WIC.
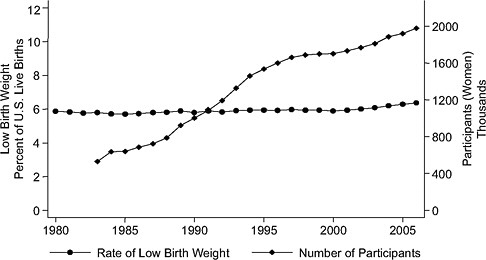
FIGURE 2-2 Rate of low birth weight (singleton births) versus number of WIC participants, United States, 1983–2007.
SOURCE: Joyce (2010), using participation data from Christopher Swann, University of North Carolina at Greensboro (unpublished).
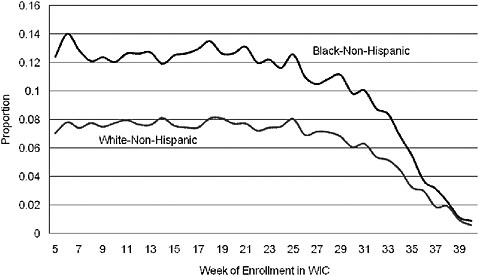
FIGURE 2-3 Rate of low birth weight by race and week of prenatal WIC enrollment (author’s tabulations from North Carolina Pregnancy Nutrition Surveillance System, 1996–2003).
SOURCE: Joyce (2010).
Comparing the rate of preterm births for women who enrolled prenatally with those who enrolled post partum may introduce another bias related to the reason for the late enrollment. Data from the North Carolina Pregnancy Surveillance System suggest that postpartum bias occurs when the outcome is preterm birth but not when the outcome is the number of newborns who are small for their gestational age. When prenatal and postnatal enrollees are compared with regard to age, education, ethnic background, participation in Medicaid and food stamps, and prenatal smoking, postpartum bias is evident. That is, the characteristics of the women who enrolled after giving birth are more favorable to good pregnancy outcomes than are the characteristics of the women who enrolled when pregnant.
Changing the Research Focus
The research agenda needs to focus on maternal health and behaviors, Joyce said. Maternal health provides a mechanism by which to improve infant and child health. Among the key factors are preconceptional smoking (rates of which are extremely large for white, non-Hispanic North Carolina WIC participants), obesity (applicable to a large percentage of women of childbearing age), prenatal weight gain (which exceeds Institute of Medicine recommendations for a large proportion of women), and breastfeeding (for which racial and ethnic differences are extremely large). It would be useful to collect data on body mass index whenever a woman visits the WIC office, as well as on blood pressure and low-density lipoproteins, and on hemoglobin A1c for women with diabetes. In the same way that some entities are working to change unhealthful behaviors such as smoking, consider paying women to breastfeed rather than to bottle feed.
Summarizing Thoughts
Joyce concluded his presentation by suggesting that research should move away from a focus on birth outcomes and toward improving maternal health as the means to improve infant health. Low-cost ways of collecting basic health data are needed.
RESPONSE
Discussant: Marianne Bitler
Methodological Issues
Quality of the Evidence
Standards of evidence have become more rigorous over time. Selection bias may explain some of the positive effects that have previously been re-
ported for WIC participation. As an example of selection bias, women who choose to participate in WIC may be more motivated than WIC-eligible non-participants. However, there appears to be little evidence of positive selection for WIC participation when women are drawn from Medicaid samples.
Bitler questioned whether selection bias was sufficient to account for all the differences discussed by Joyce, giving two examples of studies that addressed selection bias carefully. In particular, Hoynes et al. (2010) looked at the roll out of WIC at the county level and demonstrated statistically significant improvements in average birth weight and reductions in low birth weight among WIC participants. Also, Figlio et al. (2009) used tight participant and non-participant comparison groups (based on income close to the threshold for WIC) and found a reduction in low birth weight associated with WIC participation.
Time Series Interpretation
WIC may have different effects for different subgroups. With the expansion of WIC eligibility over time, some of the added participants may benefit less than the original group. This could partially account, for example, for the lack of change in the rate of low birth weights in the time series graph (Figure 2-2) shown by Joyce.
Channels Through Which WIC Works
Progress is needed in understanding the channels through which WIC works. As Joyce explained earlier, the monetary value of the WIC food package is not likely to be enough to explain the positive effects attributed to WIC; but the WIC food package may change the composition of the food consumed by participants. Within the WIC setting, women may already be predisposed to good behavior, and there may be better opportunities to move participants in the right direction.
Research Proposals
Bitler suggested a version of a randomized controlled trial that could be conducted in WIC settings:
-
Identify the domains through which WIC appears to have an effect (e.g., nutritional advice, smoking cessation).
-
Offer competitive grants to clinics to improve along one of the dimensions.
-
Choose clinics that qualify, and randomly assign some of them to be awarded money and some of them not.
Bitler also made data-related suggestions similar to those Joyce had made: (1) find ways to link up Centers for Disease Control and Prevention surveillance data and make it more publicly available, and (2) collect data beyond simply WIC participation and timing of entry in WIC.
Concluding Thoughts
In summary, Bitler concluded that there is compelling evidence that (1) WIC works on some dimensions, (2) the mechanisms by which WIC works need to be understood, (3) randomized designs are needed to help provide this information, and (4) data need to be linked and made more publicly available.
RESPONSE
Discussant: Patrick M. Catalano2
A 1990 study (USDA/FNS, 1990) showed that women who participated in WIC during their pregnancies had lower Medicaid costs for themselves and their babies than did women who did not participate. WIC participation was also linked with longer gestation periods, higher birth weights, and lower infant mortality. Many changes have occurred since that study was conducted, however. The nation has experienced an obesity epidemic, for example, and diabetes rates are closely tied to obesity rates. Based on proposed criteria for gestational diabetes, about 16 to 20 percent of U.S. women may now be classified as having gestational diabetes.
Although only one time series shown earlier by Joyce is consistent with the growth in WIC participation—namely, the one depicting the rates of infants born who are small for their gestational age (Figure 2-4), data from Catalano’s hospital indicate that the adjusted average birth weight increased by 120 grams from 1975 to 2003. The entire birth weight curve has shifted up, and pregravid maternal obesity is the factor that has the strongest correlation with the change in the birth weight curve.
Although trends in preterm birth have not improved with increased WIC participation (Figure 2-3), a larger proportion of the preterm births are now late preterm births occurring between 34 and 36 weeks of gestation rather than before 34 weeks (Figure 2-5). Much of this change may be related to changes in obstetrical practice (earlier delivery of infants). Furthermore, definitions need to be considered when examining birth weights. For example, the decrease in average birth weight in some studies may be
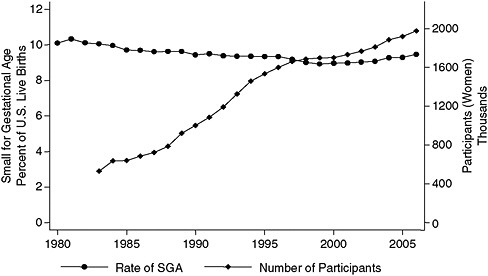
FIGURE 2-4 Rate of small-for-gestational-age births versus number of WIC participants, United States, 1983–2007.
SOURCE: Joyce (2010), using participation data from Christopher Swann, University of North Carolina at Greensboro (unpublished).
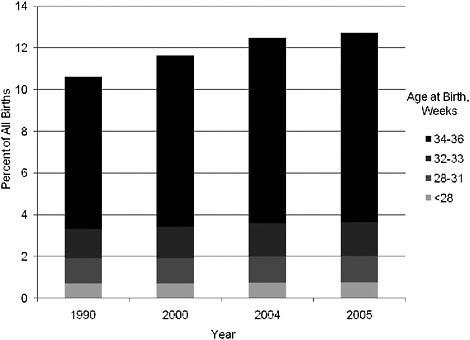
FIGURE 2-5 Distribution of preterm births by gestational age, 1990–2005.
SOURCE: NCHS (2007).
explained by earlier delivery of neonates that are classified as full-term infants.
Catalano said that pregravid weight and weight gain during pregnancy merit attention. Gestational weight gain by underweight women substantially decreases the risk of having an infant that is small for gestational age, but gestational weight gain has little effect on that risk for overweight and obese women. It is worth noting that high gestational weight gain3 by underweight women is accompanied by a 50 percent increase in the newborn’s fat mass, but the long-term significance of that fat gain is unknown. Nonetheless, Catalano said, pregravid body mass index is the strongest predictor of adiposity of the newborn at birth, followed by gestational age and gestational weight gain; it also is the strongest predictor of the percentage of body fat at age 8 years.
Research Proposals
The dollar amount available for WIC research is relatively small. Hence, Catalano believes the research question or questions need to be specific and directed toward the health of the mother. Most importantly, addressing the health of the pregnancy should begin before pregnancy, if possible, but at the very least early in pregnancy, and it should continue through the postpartum (interconceptional) period. Pregnancy offers a teachable moment. Accordingly, Catalano stressed that the research needs to be multispecialty, including pediatricians, obstetricians, and allied health professionals such as nutritionists and physical educators, with emphasis placed on factors related to lifestyle, such as diet, exercise, and smoking cessation.
GROUP DISCUSSION
Moderator: Gail G. Harrison
The session’s presenters, as well as David Paige, participated in the brief discussion and raised the following points:
-
Paige suggested that to understand the impact of WIC participation, we may need new metrics for identifying risks. Low birth weight (≤2,500 g), for example, is not a sensitive tool to measure impact. Preterm birth, about which very little is known, does not indicate either low birth weight or intrauterine growth restriction. One needs to distinguish long-term from short-term fetal growth
-
restriction. By contrast, congenital anomaly indicates a problem very early in pregnancy and is a major cause of death.
-
Joyce used preterm birth as an example in his presentation because it was a key measure of effect in early reports of the effectiveness of WIC and also because preterm birth is closely linked with low birth weight and extremely closely linked with very low birth weight.
-
Paige and Lu emphasized that WIC needs to be better integrated with the health care system to achieve a more comprehensive, effective, and targeted delivery system.
-
Catalano reemphasized the need to determine appropriate sizes for neonates born to women of widely different body mass index and to identify the gestational weight gain associated with appropriate fetal growth.
SUMMARY OF SUGGESTED RESEARCH TOPICS
The research suggestions made during this session focused on the mother’s health and behaviors rather than on the infant. These suggestions included observational studies and nutritional interventions especially during the interconceptual period and an approach for conducting randomized controlled trials in the clinic setting to test carefully selected interventions. It was pointed out that there is a need for data collection that goes beyond WIC participation status and time of enrollment and for improved data linkages and public access to the data. Also, it was suggested that collaboration with the National Children’s Study may be possible through adjunct studies.
REFERENCES
Bodnar, L. M., G. Tang, R. B. Ness, G. Harger, and J. M. Roberts. 2006. Periconceptional multivitamin use reduces the risk of preeclampsia. American Journal of Epidemiology 164(5):470–477.
Botto, L. D., R. S. Olney, and J. D. Erickson. 2004. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 125C(1):12–21.
Bukowski, R., F. D. Malone, F. T. Porter, D. A. Nyberg, C. H. Comstock, G. D. Hankins, K. Eddleman, S. J. Gross, L. Dugoff, S. D. Craigo, I. E. Timor-Tritsch, S. R. Carr, H. M. Wolfe, and M. E. D’Alton. 2009. Preconceptional folate supplementation and the risk of spontaneous preterm birth: A cohort study. PLoS Medicine 6(5):e1000061.
Catov, J. M., L. M. Bodnar, R. B. Ness, N. Markovic, and J. M. Roberts. 2007. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. American Journal of Epidemiology 166(3):296–303.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2006. Iron intake and risk of ovulatory infertility. Obstetrics and Gynecology 108(5):1145–1152.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2007a. A prospective study of dairy foods intake and anovulatory infertility. Human Reproduction 22(5):1340–1347.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2007b. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstetrics and Gynecology 110(5):1050–1058.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2007c. Dietary fatty acid intakes and the risk of ovulatory infertility. American Journal of Clinical Nutrition 85(1):231–237.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2008a. Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertility and Sterility 89(3):668–676.
Chavarro, J. E., J. W. Rich-Edwards, B. A. Rosner, and W. C. Willett. 2008b. Protein intake and ovulatory infertility. American Journal of Obstetrics and Gynecology 198(2): 210.e1–210.e7.
Devaney, B. 2010. WIC Turns 35: Program Effectiveness and Future Directions. In Cost Effective Programs in Children’s First Decade: A Human Capital Integration, edited by A. J. Reynolds, A. Rolnick, M. M. Englund, and J. Temple. New York: Cambridge University Press.
Devaney, B., L. Bilheimer, and J. Schore. 1992. Medicaid costs and birth outcomes: The effects of prenatal WIC participation and the use of prenatal care. Journal of Policy Analysis and Management 11(4):573–592.
Figlio, D., S. Hamersma, and J. Roth. 2009. Does prenatal WIC participation improve birth outcomes? New evidence from Florida. Journal of Public Economics 93(1-2):235–245.
Godfrey, K. M. 2002. The role of the placenta in fetal programming––A review. Placenta 23(Suppl. A):S20–27.
Groenen, P. M., I. A. van Rooij, P. G. Peer, M. C. Ocke, G. A. Zielhuis, and R. P. Steegers-Theunissen. 2004. Low maternal dietary intakes of iron, magnesium, and niacin are associated with spina bifida in the offspring. Journal of Nutrition 134(6):1516–1522.
Hoynes, H., M. Page, and A.H. Stevens (article under review). 2010. Can targeted transfers improve birth outcomes? Evidence from the introduction of the WIC program.
IOM (Institute of Medicine). 2007. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press.
Joyce, T. 2010. The impact of WIC on birth outcomes. Presentation at the IOM workshop, Health Impacts—Planning a WIC Research Agenda. Washington, DC, July 20–21.
Klerman, L. V., S. L. Ramey, R. L. Goldenberg, S. Marbury, J. Hou, and S. P. Cliver. 2001. A randomized trial of augmented prenatal care for multiple-risk, Medicaid-eligible African American women. American Journal of Public Health 91(1):105–111.
Krapels, I. P., I. A. van Rooij, M. C. Ocke, C. E. West, C. M. van der Horst, and R. P. Steegers-Theunissen. 2004. Maternal nutritional status and the risk for orofacial cleft offspring in humans. Journal of Nutrition 134(11):3106–3113.
Lumley, J., L. Watson, M. Watson, and C. Bower. 2001. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database of Systematic Reviews (3):CD001056.
McEwen, B. S. 1998. Protective and damaging effects of stress mediators. New England Journal of Medicine 338(3):171–179.
Metcoff, J., P. Costiloe, W. M. Crosby, S. Dutta, H. H. Sandstead, D. Milne, C. E. Bodwell, and S. H. Majors. 1985. Effect of food supplementation (WIC) during pregnancy on birth weight. American Journal of Clinical Nutrition 41(5):933–947.
NCHS (National Center for Health Statistics). 2007. Births: Final data for 2005. National Vital Statistics Report 56(6):1–104. http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_06.pdf (accessed September 16, 2010).
Rush, D., Z. Stein, and M. Susser. 1980. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 65(4):683–697.
Salafia, C. M., and E. J. Popek. 2008. Plancental development and early pregnancy pathology. In Global Library of Women’s Medicine, edited by J. J. Sciarra. http://www.glowm.com/index.html?p=glowm.cml/section_view&articleid=150 (accessed September 16, 2010).
Sinclair, K. D., C. Allegrucci, R. Singh, D. S. Gardner, S. Sebastian, J. Bispham, A. Thurston, J. F. Huntley, W. D. Rees, C. A. Maloney, R. G. Lea, J. Craigon, T. G. McEvoy, and L. E. Young. 2007. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proceedings of the National Academy of Sciences USA 104(49):19351–19356.
Smedts, H. P., M. Rakhshandehroo, A. C. Verkleij-Hagoort, J. H. de Vries, J. Ottenkamp, E. A. Steegers, and R. P. Steegers-Theunissen. 2008. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. European Journal of Nutrition 47(7):357–365.
Steegers-Theunissen, R. P., S. A. Obermann-Borst, D. Kremer, J. Lindemans, C. Siebel, E. A. Steegers, P. E. Slagboom, and B. T. Heijmans. 2009. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 4(11):e7845.
Timmermans, S., V. W. Jaddoe, A. Hofman, R. P. Steegers-Theunissen, and E. A. Steegers. 2009. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: The Generation R Study. British Journal of Nutrition 102(5):777–785.
USDA/FNS (U.S. Department of Agriculture/Food and Nutrition Service). 1990. The Savings in Medicaid Costs for Newborns and Their Mothers from Prenatal Participation in the WIC Program, Volume 1. Alexandria, Virginia: USDA/FNS.
Vahratian, A., A. M. Siega-Riz, D. A. Savitz, and J. M. Thorp, Jr. 2004. Multivitamin use and the risk of preterm birth. American Journal of Epidemiology 160(9):886–892.
Velie, E. M., G. Block, G. M. Shaw, S. J. Samuels, D. M. Schaffer, and M. Kulldorff. 1999. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. American Journal of Epidemiology 150(6):605–616.
Verkleij-Hagoort, A. C., J. H. de Vries, N. T. Ursem, R. de Jonge, W. C. Hop, and R. P. Steegers-Theunissen. 2006. Dietary intake of B-vitamins in mothers born a child with a congenital heart defect. European Journal of Nutrition 45(8):478–486.














