3
Requisites for Successful Precompetitive Collaboration
|
Key Points Raised by Speakers
|
REQUISITES FROM THE PHARMACEUTICAL INDUSTRY
Over the last five years, Pfizer has become involved with a number of precompetitive consortia, said Aidan Power, vice president and global head of Molecular Medicine for Pfizer. “We’re building on a repertoire of experience within the industry of consortia that have been developed over the past four or five years. The questions for us now are, what have we learned from that, and what can we do better?”
Several issues need to be resolved in order to establish a collaborative consortia from the pharmaceutical company perspective. A major challenge is defining the domain of precompetitive research. The basic biology, the understanding of disease, biomarkers of prognosis, and even drug responses
all can be areas of precompetitive R&D, Power said. Pharmaceutical companies have recognized that they cannot develop a full understanding of these different facets of drug development on their own. Instead, related Power, they need to leverage the capabilities of many organizations, including government and academia. A few years ago, Pfizer would have considered the chemistry, the execution, and the quality of products to fall into the competitive arena, but even these areas may not be inviolate. Proposals to establish consortia that cover at least part of this territory have generated interest from pharmaceutical companies. However, some stakeholders may still view this research as strictly competitive. The line may be drawn differently between academia, diagnostic companies, and pharmaceutical companies, said Power.
Setting up consortia can also be labor intensive. As Power pointed out, “The political science precedes the real science.” Consortia such as the Biomarkers Consortium and the Serious Adverse Events Consortium took at least 18 months to get off the ground. To establish a contract, the views of multiple parties need to be reconciled.
Internal issues can illustrate inconsistencies regarding, for example, intellectual property. The legal departments of pharmaceutical companies are changing their definitions of what needs to be patented, but only under pressure. “It used to be the case that we patented everything. But establishing patents and continuing to uphold them is a very expensive business, and we can’t afford to do that anymore…. We are beginning to operate in a space that would have been inconceivable to us a few years ago.”
Establishing consortia also raises issues about decision-making and participation criteria. For example, asked Power, what happens when new members come into a consortium? What do they receive? What if their support is not equivalent to the other members? How can rules governing such events be established in advance?
A good example of the potential for collaboration involves the samples collected in clinical trials. If these samples and their related data could be pooled across companies and with academia, intellectual capabilities could be increased and very good research could be done. Another possibility for collaborative research, said Power, would be to look at whether compounds developed for one purpose have valuable uses with other diseases or disorders, since the pharmaceutical industry needs to improve its ability to identify effective compounds that target the appropriate biological mechanisms.
Regarding the future, the precompetitive space is likely to continue to expand at the cost of internal development, Power said, as Pfizer has decided to reduce internal infrastructure costs to free up funds to invest in these ventures. Pharmaceutical companies remain under great pressure to come up with new products, and fewer are becoming available from biotechnology companies than had been anticipated. Pharmaceutical com-
panies will continue to emphasize the establishment of consortia, concluded Power, developing ways to allow access to data, sending their best people to participate, driving to publish findings from precompetitive collaborations, developing new provisions for intellectual property, and taking advantage of government incentives for precompetitive research.
REQUISITES FROM DIAGNOSTIC COMPANIES
LabCorp is one of the two large diagnostic companies in the United States, said Marcia Eisenberg, a senior vice president in the company. It has more than 27,000 employees and more than 1,500 patient service centers. It reports more than 1.2 million results daily from 400,000 specimens collected from 220,000 clients. Its mission is to provide a broad range of clinical and anatomic pathology services to aid clinicians in the diagnosis, monitoring, prediction, and prevention of disease. About 70 percent of its tests are ordered electronically, and 90 percent of results are delivered electronically.
Diagnostic companies interact with many different groups and organizations—physicians, insurance companies, pharmaceutical companies, and government agencies. However, they tend not to interact much with each other. The most prominent form of interaction in the past few years has been the buying and selling of diagnostic companies, with the total number of companies dwindling in the current economic downturn.
Diagnostic labs are required to cooperate in a number of areas to ensure that analysis and reporting are standardized, Eisenberg pointed out. They use many of the same positive and negative controls, share reporting elements and formats, have standards for adverse events and corrective actions, use the same nomenclature, participate in health information exchange systems within the labs and within the systems connected to the labs, and so on. In many of these cases, cooperation is required by the government, done to enhance patient care, or expected as part of the ethos of scientific work. LabCorp, however, has rarely, if ever, engaged in collaborative initiatives of its own accord.
Several areas could easily be collaborative between diagnostic companies, according to Eisenberg. Companies could more widely share the details of their internal quality systems, enhance standards for reporting adverse events and corrective actions, standardize the reporting and handling of incidental findings, and publicize best and less-than-best practices. These could serve as easily achievable first steps to enhance collaborative efforts.
However, more difficult challenges would be posed by those practices that are “the heart of what makes us competitively different,” said Eisenberg. These include standard operating procedures, interpretation
formats, the formatting and standards for orders and requisitions, and the formatting and procedures for notifications. “Those are things that are held quite confidentially.” Eisenberg noted that she personally saw no problem with sharing much information related to standard operating procedures, but her “legal and compliance group would see it otherwise.” It may also be difficult for diagnostics companies to be fully transparent on their assays “because this is how we’re competitive and [how] we [can] separate from each other.” The technologies used by companies are often the same, making the procedures the distinguishing factor among companies.
In general, tasks and technologies that make these companies money may be difficult to share, according to Eisenberg, while regulatory actions and the involvement of certification organizations could be a force for greater industry-industry collaboration.
REQUISITES FROM ACADEMIA
The academic environment is very heterogeneous, said Neal Cohen, professor of anesthesia and medicine and vice dean at the University of California, San Francisco (UCSF), School of Medicine. It encompasses undergraduate and graduate education and a diverse faculty with disparate goals and measures of success. The basic scientist has to publish, the clinician is promoted based on doing good clinical work and disseminating that knowledge, and the clinician scientist is trying to expand the translation of science to patients and expand patient care beyond the institution. As an example of the tensions that these different success measures can create, Cohen cited the merger of the UCSF and Stanford hospitals in the 1990s. “That merger lasted six years: two years for the engagement, two years for the marriage, [and] two years for the divorce.”
The traditional model is no longer adequate, said Cohen. The government and the public believe that science can move faster and provide cures for diseases that have largely defied intervention in the past. Moreover, federal funding for research comes at a cost, related Cohen. Most faculty who have NIH funding, when they submit a proposal, are actually working on their next proposal and have already well articulated the science they are espousing, “because the only way to be successful is to demonstrate that you’ve been successful.” The result is that creativity and innovation are undermined. “These models [for success] aren’t sufficient any longer. We clearly need more complex methodology and clinically important questions answered. We need to think about ways to move forward,” urged Cohen.
Collaborations between academia and industry are thus critical to the success of the academic community, Cohen said, and he expressed confidence that it is possible to cooperate while dealing with the conflicts that arise. “When we’re talking about collaborations … we shouldn’t be think-
ing in the same terms, or with the same definition of precompetitive as we mean within industry, because precompetitive has a very different meaning [in academia]. We need to move beyond it and recognize how we can work collaboratively in a way that fulfills the goals and needs and incentives for academicians and the incentives for industry.”
Several issues within the culture of academia are currently limiting the value that is placed on industry relationships. Intellectual autonomy is a very significant issue in academia, according to Cohen. “Compartmentalization of knowledge [and] the merit and promotion processes need to be reevaluated. If we, as a group of institutions, got together and defined ways that the promotions committees could evaluate interdisciplinary, multidisciplinary activities, it would be very helpful.” The 37th of 47 authors on a paper may have been critical to its success or may simply have provided some patients. “Unless the individual is able to articulate [his or her] role, validate [that] role, and talk about how important [it] is, it will be difficult to move the agenda.” Cohen suggested that databases of participation could be used to evaluate performance.
University policies and procedures need to be devised in such a way as to enable the development of relationships rather than placing restrictions on academic faculty who want to enter into these collaborations, according to Cohen. Contract negotiations, technology transfer, and economic autonomy need to be reexamined. Interdisciplinary research programs within or among institutions also need to be valued appropriately because currently, said Cohen, “even though many of our faculty have very strong relationships, they’re competing for first authorship rather than competing for the best science.”
Strategic visions and scientific strategies need to be developed to allow individual scientific collaborations to advance more rapidly, according to Cohen. Potential collaborators throughout the academic community need to be identified, including among basic and clinician scientists, he continued. Clinicians can provide keys to breakthrough technologies by understanding the mechanisms of disease, monitoring individual responses to and compliance with therapies, and serving as a source for patient cohorts and biological specimens. To facilitate this type of collaboration, UCSF, for example, has developed a searchable website that points to research projects and collaborations by disease. This enables people to talk together from an epidemiological basis, a basic science basis, and a clinical basis. “Getting those people to talk together and understand where there are opportunities to work with industry will be critical,” said Cohen. Such a database could also provide the opportunity and infrastructure for students and residents to identify potential mentors, concluded Cohen, since the training of the next generation of researchers and clinicians also needs to emphasize the importance of these relationships.
The Clinical and Translation Science Awards program has provided a better sense of what people are doing. This model also provides a way of thinking differently about engaging the community at large and building relationships among community providers, industry, and clinicians, Cohen pointed out. “Academia needs to emphasize that relationships are critical, that we are managing them responsibly, that we are addressing the fundamental scientific basis of the relationship, and that we are evaluating the outcomes appropriately.”
An Industry-Academic Framework
Cohen suggested a potential framework for establishing industry-academic collaborations. An oversight structure needs to be defined that promotes exchange of knowledge, he related. For example, a strategic planning board could clearly define goals and objectives. A coordinating committee could identify potential collaborative partnerships and coordinate their activities, identify and leverage campus and investigator expertise, and manage databases and specimen banks. An advisory board could evaluate strategies, provide oversight, and manage conflict-of-interest issues (Figure 3-1).
Industry is also diverse, Cohen emphasized. There are opportunities to develop nonexclusive consortia and networks with academics in what industry defines as precompetitive areas and to create incubators within academic health systems. These relationships need to be managed as a port-
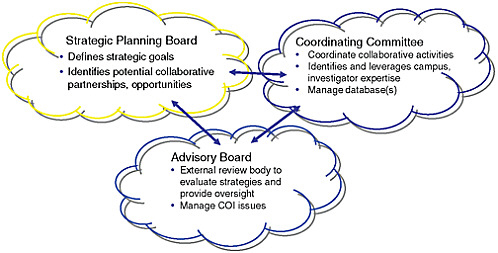
FIGURE 3-1 Model for industry-academic collaboration.
SOURCE: Cohen, IOM workshop presentation on July 22, 2010.
folio, said Cohen, with key partners identified. Master agreements need to be negotiated, so that delays can be minimized for individual agreements.
Risk and the Academic Enterprise
Stephen Eck, vice president for Translational Medicine and Pharmacogenomics at Eli Lilly and Company, observed that if academic investigators, and those who review their grants, are risk averse, industry is much more risk averse, since more is at stake. “How does precompetitive collaboration get those two sectors to actually work on the risky stuff that has potentially very high payoff?”
Investigators are not risk averse, replied Cohen, though they want publications. Rather, the universities are risk averse, especially regarding their public image. “They are very careful to control their imprints, their name, and their position in society.”
Individual investigators vary in the amount of risk they are willing to assume. Some very entrepreneurial basic scientists are always on the cutting edge, despite the difficulties this can raise in getting grants. For example, Stanley Prusiner, who won a Nobel Prize, had great difficulties getting government funding and was supported by industry and donors to advance his science, said Cohen. “He followed his pursuit because he had passion and he was willing to take the risks, and he found people who would help support him, but he could have fallen off the edge of a cliff easily and never completed his research.”








