Summary
In 2008, the Institute of Medicine (IOM) published the report Preparing for an Influenza Pandemic: Personal Protective Equipment for Healthcare Workers. At the time of that report, the major influenza-related concern was avian influenza (H5N1). As novel H1N1 influenza A became a reality in 2009, the many unknowns about the virulence, spread, and nature of the virus raised to the forefront issues regarding personal protective equipment (PPE) for healthcare personnel. A major issue was the nature of respiratory protection required because much remains to be learned about the mechanisms of influenza transmission. This report comes at a time when controversies continue on issues related to PPE for healthcare personnel, while at the same time, new horizons in PPE research and attention to PPE innovations offer promise of improvements in healthcare worker safety. Keeping the research momentum going is critical, because between pandemics the focus of research efforts often moves to other issues and the nation remains underprepared.
SCOPE OF THE REPORT
The 2009–2010 H1N1 experience and its accompanying unanswered research questions provided the impetus for the National Personal Protective Technology Laboratory (NPPTL) at the National Institute for Occupational Safety and Health (NIOSH) to ask the IOM to conduct a study
that would update progress on research and identify future directions regarding PPE for healthcare personnel.1
This report is the result of a 12-month study conducted by an ad hoc IOM committee composed of experts in the fields of infectious disease, infection control, public health, occupational safety and health, pulmonary medicine, health promotion, microbiology, emergency response and preparedness, epidemiology, nursing, community health, industrial hygiene, and materials engineering. The IOM committee was charged with identifying new research directions, certification2 and standards-setting issues, and risk assessment issues specific to PPE for healthcare personnel to prevent transmission of pandemic influenza and other viral respiratory infections. The committee was specifically asked to focus on the following issues:
-
research needed to understand and improve the efficacy and effectiveness of PPE, particularly face masks and respirators, for preventing transmission of pandemic influenza or other viral respiratory infections. Specific attention was sought on issues related to the research needed to determine the type of respiratory protection needed for the given exposure, to determine the requirements for protective ensembles to provide an appropriate level of protection based on work tasks, and to improve functionality and address human factor issues such as wearability, compliance, and communications;
-
necessary certification, testing, and standards development issues; and
-
priorities and resources for research and certification efforts.
To accomplish its charge, the committee held three meetings and gathered information through a scientific workshop that included a public comment session, through discussions with numerous individuals in the infection control and occupational safety and health fields, and through a review of the relevant literature. As mentioned above, this report builds on the work of the IOM committee that released the 2008 report, and
throughout this report, the prior work is summarized. In large part, this committee’s task was to examine research conducted since the 2008 report, to assess where research stands on issues key to improving PPE for healthcare personnel exposed to infectious respiratory diseases, and to make recommendations to address current research gaps. Many PPE issues relevant to healthcare personnel are also directly relevant to the PPE needs of workers in other occupations, as well as the general public.
PPE FOR HEALTHCARE PERSONNEL
The term “personal protective equipment” encompasses the specialized clothing or equipment worn by workers for protection against health and safety hazards. For healthcare personnel, PPE may include respirators, face masks, gloves,3 eye protection, face shields, gowns, and head and shoe coverings. Integrating the various types of protective equipment to ensure that they work together as ensembles (e.g., eye protection with a respirator) is an ongoing concern. Infection prevention and control in healthcare workplaces involve, among many other measures, the use of PPE. Infection control precautions follow a tiered approach that considers the possible routes of transmission among patients and healthcare personnel.
One of the challenges for the healthcare field is to clearly understand the differences between respirators and face masks as well as their appropriate uses. Respirators are specifically designed as respiratory protection. They work either by purifying the air inhaled by the wearer through filtering materials or by independently supplying breathable air. For air-purifying respirators (often the type used by healthcare personnel) the major issues are the filtration and the fit—the effectiveness of the filter and the extent to which the respirator has a tight seal with the wearer’s face to restrict inward leakage.
Face masks, including surgical masks and procedure masks, are loose-fitting coverings that are designed to protect the patient from secretions from the nose and mouth of the physician, nurse, or other healthcare professional. Face masks are not designed or certified to protect the wearer from exposure to respiratory hazards; the role of face masks as PPE requires further research.
Measures to prevent influenza transmission to healthcare personnel include all levels of hazard controls. PPE along with vaccination and antiviral medications are components of an overall infection prevention and control program that uses engineering, administrative, and work practice controls. Although all levels of this hierarchy are important, this report is focused on opportunities to improve PPE and the correct use of PPE in healthcare settings.
In discussing the issues relevant to the use of PPE by healthcare personnel, the committee identified a set of criteria as a starting point for decisions on PPE selection and use. PPE for healthcare personnel should
-
effectively reduce risks of disease or injury to healthcare personnel;
-
minimize negative interactions with or effects on patients, their families, and caregivers;
-
be acceptable and usable by healthcare personnel in their daily tasks, including ease of communication and comfort;
-
be practical regarding issues of cost, time, and training; and
-
be appropriate to the occupational risk being encountered.
Having recently been through the 2009–2010 experience with H1N1 influenza, the committee is well aware of the ongoing challenges and controversies surrounding PPE for healthcare personnel. At this time, it is particularly important to build on that experience and take the actions needed to address the research and policy questions that will allow the healthcare community to be better prepared for the next epidemic or pandemic. Experience has shown that relevant research on these issues wanes between pandemics, and not permitting that to happen this time is crucial to resolving the research questions and setting evidence-based policies in place.
UPDATE ON PROGRESS: 2007 TO 2010
Transmission of Influenza and Other Viral Respiratory Diseases
Animal studies have found that the ferret and guinea pig models appear to be highly representative of humans in terms of their susceptibility to infection, the influenza viral strains that display a transmissible phenotype, and the kinetics with which transmission occurs. Experiments per-
formed in both of these animal models suggest that transmission of influenza viruses can proceed by both droplet spray and aerosol routes, which would include respirable particles. Animal studies have also pointed to a number of environmental factors, including relative humidity and temperature, that may influence transmission. Recent studies that employed environmental monitoring of air for influenza as well as others that examined the contamination of fomites and hands with H1N1 have provided insights on the potential for influenza virus contamination of the healthcare environment. Nonetheless, data on the viability of influenza and other respiratory viruses in air samples and on fomites in these settings are limited. Mathematical models have been developed to better characterize the relative contribution of influenza transmission modes. Available, well-specified parameters for these models are limited because information is lacking on the viability of influenza in aerosols, salivary virus concentrations, the amount of virus in respirable and inspirable particles, and the quantity and persistence of viability on various fomites in the healthcare setting. Taken together, progress has been made in understanding the modes of transmission, but the relative contribution of the modes are still unclear. Much remains to be learned about the effectiveness of control measures to prevent transmission.
Observational and controlled studies relevant to PPE use and transmission of influenza or other viral respiratory diseases are limited because study protocols were not usually in place for 2009 H1N1 or for recent seasonal flu periods, and studies have not provided adequate power to answer questions regarding the effectiveness of using PPE in reducing or preventing disease transmission.
Designing and Engineering Effective PPE
PPE is a critical component in the hierarchy of controls used to protect healthcare personnel from influenza and other viral respiratory diseases. Understanding the functional issues related to the design of PPE, as well as the factors that impact use, is critical to ensuring that healthcare personnel are adequately protected and comfortable and can perform their jobs. Important advances have been made in some areas since the last report, but other areas, particularly regarding improvements in gowns, gloves, face masks, and face shields, need to be more fully addressed. Much research has been done regarding filtration of respirator media, but ways to improve fit, including new technologies specifically
for filtering facepiece respirators, need more research because face seal leakage greatly exceeds filter penetration in the overall total inward leakage of respirators. The physiological impact of respirators has been studied in depth, but research in this area is lacking regarding other types of PPE. Integration issues concerning PPE and medical equipment and the impact on operational performance have not been adequately studied. Effective decontamination methods that do not impact the physical characteristics of respirators have been studied for some types of respirators, but with inconclusive results. Finally, the characteristics of a respirator that would specifically address the needs of healthcare personnel (e.g., patient–provider interaction, comfort, reduced physiological burden) have been identified. Addressing these issues is important for developing PPE for healthcare personnel that is safe, effective, and comfortable.
Using PPE: Individual and Organizational Issues
Research during the past several years reveals modest gains in understanding that self-protective behavior in the healthcare settings involves a constellation of interacting and independent components. At a minimum, consideration should be given to the user, the device, the task, and the general work and organizational context. The growing acknowledgment of contextual and organizational factors means that research on PPE and healthcare personnel is closing in on the larger body of occupational safety research, which increasingly emphasizes those factors in understanding occupational safety performance.
Although there are clear gaps and deficiencies in our knowledge base about PPE usage in health care, existing knowledge is sufficient to recommend a four-pronged strategy for immediate implementation. The four elements are: (1) deliberate planning and preparation at the leadership and organizational levels; (2) comprehensive training, including supervisors and managers; (3) widespread and convenient availability of appropriate PPE devices; and (4) accountability at all levels of the organization.
In essence, there should be universal acknowledgement that PPE use is an integral component of providing quality health care. As with other priorities, this aspect of healthcare delivery needs to be carefully planned at the organizational/institutional level. Furthermore, managers and frontline workers alike need to understand and accept their roles and responsibilities, and PPE use needs to be as easy and convenient as poss-
ible. PPE should be factored into all decisions involving task design, staffing, and work assignments. Input from frontline workers should be used to facilitate planning and decision making and to maximize acceptance. Environmental/engineering controls should be utilized wherever possible to control exposures, with PPE used as a supplement or alternative when environmental/engineering controls are not sufficient or feasible. The overall implementation of the PPE program should be monitored regularly, with the goals of continuous improvement, adoption of best practices, and accountability of both supervisor and worker.
Policy Research and Implementation
Preparations and implementation of infection control plans for 2009 H1N1 influenza brought into sharp focus the efforts by healthcare professionals, emergency planners, professional associations, healthcare facilities, policy makers, government agencies, labor unions, and others to address PPE policies and logistics. Articles continue to be published on the recent experience and the challenges and successes in providing face masks, respirators, and other PPE to healthcare personnel. As lessons learned during that experience continue to add to the body of knowledge, incorporating this information into research, policy, and practice efforts will be important. In the initial phases of an epidemic or pandemic—when there are many unknowns about the virus or agent—one of the challenges is to determine PPE policy and then to adapt those policies as information is gained on the severity, transmission, and nature of the disease, with an emphasis on communicating the changes. Standards-setting, regulatory, training, and research efforts continue to move toward improved respiratory protection, and recent work has begun to focus on the specifics of how to tailor PPE devices and PPE training to address the specific needs of healthcare personnel.
RECOMMENDATIONS: A SYSTEMS APPROACH TO PPE RESEARCH
Providing care to ill or injured patients involves a range of potentially hazardous exposures for healthcare personnel. Current infection control precautions address this challenge by providing guidance on PPE and other precautions that varies depending on the mode of transmission of
the pathogen. The ultimate goal would be to have the definitive information that would match the appropriate type of PPE with the pathogen, its mode of transmission, the infectious dose, and its risk to healthcare personnel. In many cases in industrial settings, this level of specificity is available for chemical exposures, although other industrial settings with unknown or mixed exposures continue to pose challenges. Reaching that point for protecting healthcare personnel will require concerted research efforts.
This report explores an integrated approach that addresses the full spectrum of research (from basic research to policy research) and translates research findings into improvements in the standards of healthcare practice (Figure S-1). This approach ensures that basic science initiatives are fully explored while also addressing clinical needs and testing the results in real-world settings, with the expectation that adaptations along the way will be made and tested. Feedback loops to prior stages are also critically important. Such an integrated approach calls for active collaboration and discourse among scientists and clinicians who may not have had previous interactions.
Throughout this report, the committee has highlighted a number of areas in which research is needed. This research has the potential to quickly translate new understandings of disease transmission or PPE engineering into more effective PPE products. Fully implementing these research directions and realizing breakthroughs for improved safety and health for healthcare personnel will require commitments from multiple
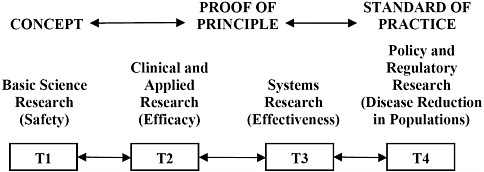
FIGURE S-1 An integrated system moving research into practice, depicting the translation of research from basic science research (T1) through policy and regulatory research (T4). See Chapter 1 for further discussion.
federal agencies as well as ongoing, innovative efforts by PPE designers and manufacturers. The report makes the following recommendations to advance research and transfer these into practice across the spectrum of research opportunities.
Across the Spectrum of Research
Recommendation: Develop Standardized Terms and Definitions
The Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA), in partnership with other relevant agencies and organizations, should work to develop standardized terms, definitions, and appropriate classifications to describe transmission routes and aerodynamic diameter of particles associated with respiratory disease transmission. This effort should involve a consensus from the industrial hygiene, infectious disease, and healthcare communities.
Recommendation: Develop and Implement a Comprehensive Research Strategy to Understand Viral Respiratory Disease Transmission
The National Institutes of Health, in collaboration with other research agencies and organizations, should develop and fund a comprehensive research strategy to improve the understanding of viral respiratory disease transmission, including, but not limited to, examining the characteristics of influenza transmission, animal models, human challenge studies, and intervention trials. This strategy should include
-
an expedited mechanism for funding these types of studies and
-
clinical research centers of excellence for studying influenza and other respiratory virus transmission.
Safety to Efficacy: Basic Science Research (T1) to Clinical and Applied Research (T2)
Recommendation: Continue and Expand Research on PPE for Healthcare Personnel
NPPTL and other agencies, private-sector companies, and other organizations should continue to advance research in designing and evaluating the effectiveness of respirator protection for healthcare personnel and expand its research efforts to improve and evaluate the effectiveness of gloves, gowns, eye protection, face shields, and face masks in preventing the transmission of influenza or other viral respiratory diseases. Areas of focused research needs include
-
effectiveness in preventing fomite, droplet spray, or aerosol transmission;
-
decontamination and reusability;
-
comfort, fit, and usability;
-
impact on task performance; and
-
development of technologies specifically for healthcare personnel.
Recommendation: Examine the Effectiveness of Face Masks and Face Shields as PPE
NPPTL should investigate the effectiveness of face masks and face shields in preventing transmission of viral respiratory diseases.
Recommendation: Improve Fit Test Methods and Evaluate User Seal Checks
NPPTL should develop novel, simpler fit test methods and evaluate the effectiveness of performing user seal checks on N95 respirators.
Efficacy to Effectiveness: Clinical and Applied Research (T2) to Systems Research (T3)
Recommendation: Explore Healthcare Safety Culture and Work Organization
NIOSH and other relevant agencies, such as the Agency for Healthcare Research and Quality, and professional organizations should conduct research to better understand the role of safety culture and other behavioral and organizational factors on PPE usage in healthcare settings. These efforts should include
-
conducting human factors and ergonomics research relevant to the design and organization of healthcare work tasks to improve worker safety by reducing hazardous exposures and effectively using PPE (e.g., reduce unnecessary PPE donning and doffing),
-
exploring the links between patient safety and healthcare worker safety and health that are relevant to the use of PPE, and
-
identifying and evaluating strategies to mitigate organizational barriers that limit the use of PPE by healthcare personnel.
Recommendation: Identify and Disseminate Effective Leadership and Training Strategies and Other Interventions to Improve PPE Use
NIOSH and other relevant agencies and professional organizations should support intervention effectiveness research to assess strategies, including innovative participatory approaches to training, for healthcare and supervisory staff at all levels to improve PPE usage and other related outcomes across the range of healthcare settings. To identify best practices, efforts should be made to
-
conduct observational studies of PPE use by healthcare personnel in different types of work settings;
-
develop, implement, and evaluate comprehensive leadership and training strategies and interventions that go beyond simple knowledge-based training;
-
design training interventions specifically for supervisory and managerial personnel in different types of healthcare settings;
-
examine long-term practice change and safety culture implementation related to educational interventions;
-
improve use and understanding of PPE by home and community healthcare personnel;
-
develop assessment tools and metrics that take a broader approach to PPE and acknowledge the interaction of worker, task, and environmental factors; and
-
be informed by a lessons-learned summit on PPE use by healthcare personnel during the 2009 H1N1 experience.
Effectiveness to Disease Reduction in Populations: Systems Research (T3) to Policy and Regulatory Research (T4)
Recommendation: Develop and Certify Powered Air-Purifying Respirators (PAPRs) for Healthcare Personnel
NPPTL should develop certification requirements for a low-noise, loose-fitting PAPR for healthcare personnel.
Recommendation: Move Forward on Better Fitting Respirators
NPPTL should continue rulemaking processes for total inward leakage regulations that require respirators to meet fit criteria. To improve consumer and purchaser information on fit capabilities, NIOSH should establish a website to disseminate fit test results for specific respirator models on an anthropometric (NIOSH) test panel, where such data exist.
Recommendation: Clarify PPE Guidelines for Outbreaks of Novel Viral Respiratory Infections
NIOSH, other divisions of CDC, OSHA, and other public health agencies should develop a coordinated process to make, announce, and revise consistent guidelines regarding the use of PPE to be worn by healthcare personnel during a verified sustained national/international outbreak of a novel viral respiratory infection. The agencies should tailor their guidance in a timely and coordinated manner as the virulence, contagiousness, and affected populations are further characterized.
Recommendation: Standards and Certification for Face Masks and Face Shields
NIOSH, OSHA, and standards-development organizations should develop the standards and certification processes needed to assess the performance of face masks and face shields as PPE. The development of standards and certification processes should be guided by research regarding their efficacy as PPE:
-
OSHA and CDC should clarify that face masks are governed by the general PPE standard (29 CFR 1910.132) and not by the respiratory protection standard (29 CFR 1910.134).
-
NIOSH should work with other agencies and standards-setting organizations to develop voluntary consensus standards and independent third-party testing and certification processes for face shields and face masks with specific tests for assessing prevention of transmission of viral respiratory diseases.
Recommendation: Establish PPE Regulations for Healthcare Personnel
CDC, including NIOSH, and OSHA should develop and promulgate guidelines and regulations that are consistent regarding the use of PPE by healthcare personnel for influenza and other viral respiratory diseases:
-
To assist employers in complying with the OSHA PPE standard, OSHA should specify the voluntary consensus standards that are required to be met for non-respirator PPE (e.g., gowns, gloves, face shields, face masks) in the event of influenza and other viral respiratory diseases.
-
OSHA, with input from CDC and other agencies and organizations, should work toward promulgating an aerosol-transmissible diseases standard that would include prevention of the transmission of influenza and other viral respiratory diseases.
While the optimist can seek comfort in the fact that progress has been made in the past several years, the pragmatist must wonder about the rate of progress (or lack thereof) on such a critical issue that potentially threatens the security and economic well-being of the nation and the world. The committee hopes that this review will jumpstart and strengthen improvements in PPE for healthcare personnel that could be relevant to a range of viral respiratory diseases.














