3
Designing and Engineering Effective PPE
Understanding workplace hazards is critically important to ensuring that personal protective equipment (PPE) is available to healthcare personnel facing an influenza pandemic or other hazardous working conditions. Research on the transmission and virulence of the influenza virus and other potential infectious agents (Chapter 2) will inform decisions on the design and engineering of healthcare PPE.
As innovative approaches begin to address the PPE challenges of the healthcare workplace, further efforts are needed that focus on how to address the unique or varied issues that healthcare personnel face—easy communications with patients and families, PPE that can be changed or reused between different patients, PPE that is comfortable during long wear times, and PPE that does not interfere with work performance. Healthcare personnel are not alone in having job-specific PPE requirements. Firefighters need PPE that addresses high temperatures, construction workers on roofs and high-rise structures need protection from falls, and both have many other PPE requirements. Innovations in healthcare PPE are starting to be seen in the marketplace, but much more needs to be done to move the design of PPE from an industrial perspective toward the realities of the healthcare workplace.
This chapter focuses on research on designing and engineering effective PPE. The chapter begins with a brief overview of the 2008 report, followed by a synopsis of research that has been conducted in the past several years. The chapter concludes with the committee’s thoughts on research gaps and immediate and long-term research directions.
BACKGROUND AND CONTEXT FROM THE 2008 REPORT
The 2008 Institute of Medicine (IOM) report provided the outline for a lifecycle approach to PPE and emphasized that, in addition to fit and filtration for respirators and functionality requirements for other types of PPE, numerous other factors play a significant role in the design and development of healthcare PPE. These factors include issues involving visibility, comfort and wearability, durability, maintenance and reuse, aesthetics, and cost (Figure 3-1).
In considering a framework for the design and development of PPE, the 2008 committee addressed the three phases of the design and engineering process typically associated with a product’s lifecycle:
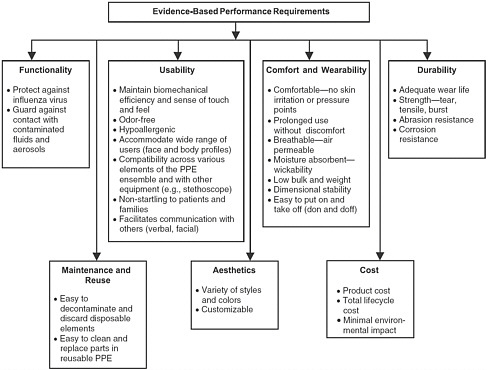
FIGURE 3-1 A structured approach to evidence-based performance requirements for personal protective equipment (PPE).
SOURCE: IOM (2008).
-
User requirements analysis: understanding the work hazards and barriers to PPE use;
-
Design realization: identifying the key characteristics (Figure 3-1) and translating the evidence-based performance requirements into the specific design of the PPE component while making appropriate trade-offs among the factors that drive design, including degree of protection, comfort, and the cost of designing the specific PPE component to meet the regulatory requirements; and
-
Field use and evaluation: requiring that the new PPE be tested in the field in order to provide a realistic assessment of its performance and to identify unintended consequences of use.
Fit and filtration are the major functional issues in the design and engineering of respirators. Most research has focused on filtration. National Institute for Occupational Safety and Health (NIOSH) ratings for respirators of 95, 99, or 100 percent filtration efficiency are based on the percentage of 0.3 μm particles that do not penetrate the test filter (IOM, 2008). Influenza viruses, with estimated sizes ranging from approximately 0.08 to 0.12 μm (although droplets with the virus can vary widely in size), follow standard particle filtration theory, and therefore a number of types of filters are effective. Less is known about issues regarding inward face seal leakage and other aspects of respirator fit. The 2008 report recommended research on a number of respirator issues, including decontamination and reuse methods, comfort and tolerability concerns, powered air-purifying respirators (PAPRs) designed to meet the needs of healthcare personnel, and improved face seals.
The 2008 report also addressed research for gowns, gloves, eye protection, face protection, and other types of PPE that might be needed to protect workers from infectious disease. These types of barrier protection are designed primarily to protect against droplet spray and contact transmission that might occur when particles are transferred to the respiratory mucosa or conjunctiva (of the eyes) of susceptible individuals within close range. Testing of gowns has focused primarily on liquid barrier performance and breathability of the fabric, with four levels of liquid barrier performance defined by the Association for the Advancement of Medical Instrumentation’s (AAMI’s) testing standard, AAMI PB70. The prior report emphasized the need to explore whether specific clinical situations require varying types of gowns or whether other specifications are needed, as well as issues regarding feasibility of reuse, interface with
other types of PPE (especially gloves), and advances in materials technology, including repellant finishes (IOM, 2008). For protective eye-wear, including transparent face shields, issues regarding the interface with respirators were found to be a critical need. In addition, product performance standards for eye protection need to be defined more clearly because they now focus on the thickness and impact resistance of eye protection but do not address issues relevant to influenza transmission (IOM, 2008).
Healthcare personnel’s use of gloves can serve several purposes in infection control—creating a barrier to direct contact with contaminated surfaces, preventing patient-to-patient contamination if gloves are changed between patients and proper hand hygiene is performed, and increasing awareness of the potential for self-inoculation when gloved hands touch the mucosa of the mouth, nose, or eyes. Research needs regarding gloves that were identified in the 2008 report included better barrier protection as well as wearability and improved interfaces with gowns and other PPE. Adherence to hand hygiene and other infection control practices are also important in preventing disease transmission.
The 2008 report provided a list of immediate opportunities and long-term research needs for improving the design and effectiveness of healthcare PPE. The report also provided a set of recommendations in this area, which can be briefly summarized as follows:
-
Define evidence-based performance requirements for PPE.
-
Adopt a systems approach to the design and development of PPE.
-
Increase research on the design and engineering of the next generation of PPE.
-
Establish measures to assess and compare the effectiveness of PPE.
UPDATE ON RECENT RESEARCH
Research efforts since the prior report have continued to address a range of design and engineering issues with the goal of improving the PPE available to healthcare personnel and others. The following section provides an overview of recent research efforts, beginning with the research focused on respirators and face masks.
Respirators and Face Masks: Fit and Filtration
The protection provided by a particular respirator is a function of both the filtration capabilities of the material and how well the device fits the wearer. Total inward leakage (TIL) is the combination of filtration, face seal leakage, and leakage through respirator components, such as the exhalation valve.
Filtration
Several issues concerning filtration have been raised recently. First, the filtration efficiency of face masks is a concern because they are not developed as filtration devices. Second, researchers have been concerned about the penetration of nanoparticles (which includes the size range of influenza and other viruses) through respirator filter media and whether current NIOSH respirator certification methods accurately account for those particles. Third, shortages of respiratory protection may occur during a pandemic, so alternative filter materials and equipment have been investigated.
Filtration efficiency of face masks Two recent studies investigated the filtration efficiency of face masks. Oberg and Brosseau (2008) evaluated filtration performance of nine face masks (cup, flat, duckbill, one and two straps, ear loops, surgical, laser, and procedure). Filter efficiencies ranged from 0 to 84 percent for the latex sphere tests and 4 to 90 percent in the sodium chloride (NaCl) tests. Dental masks showed significantly higher penetration (6 to 75 percent for latex and 53 to 90 percent for salt) than hospital masks (0.02 to 0.7 percent for latex and 4 to 37 percent for salt). Only 1 of the hospital masks (mask H) had less than 5 percent penetration of the salt particles. Lee and colleagues (2008c) investigated the protection factor of face masks and respirators with a challenge of particles representing bacterial and viral size ranges (aerodynamic size: 0.04 to 1.3 μm) and found that none of the masks had protection factors > 10. The protection factors of the tested N95 respirators were an average of 8 to 12 times greater than those of masks. One previous study (Li et al., 2006) reported that face masks provided 95 percent filtration efficiency for potassium chloride. However, Brosseau and Harriman (2007) pointed out that the study did not use a standard method and that the authors did not fully describe the technique. None of the face masks tested by Oberg
and Brosseau (2008) or Lee and colleagues (2008c) provided sufficient protection to be considered respirators. This is not surprising considering the fact that face masks were not intended to be respiratory protective equipment.
Penetration of small particles NIOSH certification tests for N95 respirators use an NaCl aerosol challenge with a 300 nm most penetrating particle size (MPPS).1 However, many electret filter media that use electrostatic charge to capture particles have an MPPS ranging from 30 to 100 nm (Shaffer and Rengasamy, 2009). Concerns have been raised regarding the filtration performance of N95 respirators against smaller viral- and bacterial-sized particles. Eninger and colleagues (2008b) reviewed the NIOSH aerosol particle-size distribution and measurement method. The authors found that, although the salt aerosol does contain a significant fraction of ultrafine (diameters < 100 nm) particles, the method and equipment used cannot accurately measure the contributions of particles below 100 nm. In fact, 68 percent by count and 8 percent by mass of salt particles below 100 nm did not significantly contribute to the filter penetration measurement. Therefore, the existing NIOSH certification protocol may not adequately reflect the penetration of ultrafine particles.
Several groups of researchers have investigated the filtration performance of respirators against nanoparticles. Eninger and colleagues (2008a) investigated the filtration performance of one N95 and two N99 filtering facepiece respirators against one inert particle and three virus aerosols at flow rates of 30, 85, and 150 L/min. The respirators were sealed on a manikin. The most penetrating particle size for challenge aerosols was < 0.1 μm for all three respirators. Mean particle penetration, by count, was increased significantly when the size fraction of particles < 0.1 μm was included compared to particles > 0.1 μm. Penetration of the salt aerosol was greater than that of the tested biological aerosols, suggesting that inert aerosols can be used to assess filter penetration of virions. Inhalation airflow rate had a significant effect on particle penetration. The authors suggested that further research is needed with cyclic flows with high peak inspiratory flows.
A study of the filtration performance of five N95 and two P100 filtering facepiece respirators against monodisperse silver aerosol particles
in the 4 to 30 nm range at 85 L/min found that both types of respirators showed a decrease in percentage of penetration, with a decrease in particle diameter down to 4 nm (Rengasamy et al., 2008). This study supports prior studies that indicate that NIOSH-approved air-purifying respirators provided expected filtration protection against nanoparticles. A follow-up study using a polydisperse NaCl aerosol test with a 238 nm mass median aerodynamic diameter and two monodisperse aerosol tests concluded that the eight filtering facepiece respirator models tested met expected filtration performance against nanoparticles (Rengasamy et al., 2009). The NIOSH-certified respirators have a minimum efficiency of 95 percent for the N95 and 99.97 percent for the P100. The European Norm requires a minimum efficiency of 94 percent for a filtering facepiece respirator class P2 (FFP2) and 99 percent for a filtering facepiece respirator class P3 (FFP3). Penetrations from the polydisperse aerosol test were < 1 percent for the N95 and FFP2 models and < 0.03 percent for the P100 and FFP3 models.
In a study by Eshbaugh and colleagues (2009), the researchers examined the effects of varying flow conditions on aerosol penetration for both N95 and P100 filtering facepiece respirators and cartridges. Challenges were inert solids and oil aerosols with particle sizes in the range of 0.02 to 2.9 μm; three constant flow and four cyclic flow conditions were used. Penetration increased under increasing constant- and cyclic-flow conditions. The MPPS for the P100 filters was 50 to 200 nm and 50 nm for N95 filters. Shaffer and Rengasamy (2009) reviewed research published since 2000 on respirator filtration and leakage data for nano-particles. The MPPS was in the 30 to 100 nm range and was impacted by the filter media and test conditions, particularly flow rate. They found that filtration of monodisperse nanoparticles at the MPPS varied from 1.4 to 10 percent for the N95 filtering facepiece respirator. They identified the greatest need for further research as human laboratory or workplace protection factor studies to measure TIL for respirators used for protection against nanoparticles. Wander and Heimbuch (2009) tested one N95 and one P100 filtering facepiece respirator with aerosolized particles (count mode diameter ~0.8 μm) of H1N1 and inert beads at 85 L/min using the Laboratory-Scale Aerosol Tunnel. The N95 removed > 99 percent of viable H1N1 while the P100 removed > 99.99 percent. They performed the same against the inert beads. The authors concluded that infectious microorganisms and inert particles of the same size have the same impact on the filtering efficiency of filtering facepiece respirators.
Although these studies used different challenge aerosols and different test methods, several common results emerge. Inhalation airflow rate had a significant effect on particle penetration (Eninger et al., 2008a; Eshbaugh et al., 2009). NIOSH- or European Norm–certified filtering facepiece respirators achieved expected filtration performance when challenged with nanoparticle aerosols (Eninger et al., 2008a; Eshbaugh et al., 2009; Rengasamy et al., 2008, 2009). The MPPS is below 100 nm for most electret filter media (Eninger et al., 2008a; Eshbaugh et al., 2009; Rengasamy et al., 2009), though one researcher (Eshbaugh et al., 2009) reported 200 nm for two models of filtering facepiece respirators. Finally, inert particles have penetration performance similar to virus particles (Eninger et al., 2008a; Wander and Heimbuch, 2009).
Alternative filter materials In the event of a pandemic, there may not be enough respirators available to meet demand. Rengasamy and colleagues (2010a) examined the filtration performance of common cloth materials, such as sweatshirts, T-shirts, towels, scarves, and cloth masks, against nanoparticles using polydisperse and monodisperse aerosols (20 to 1,000 nm) at two face velocities. The cloth materials had penetration levels of 40 to 90 percent for polydispersed NaCl, well above that of N95 respirators. Penetrations of 9 to 98 percent were obtained for different monodisperse NaCl aerosol nanoparticles. These materials had penetration levels similar to some face masks that were tested previously. They concluded that only minimal protection would be provided by wearing masks made out of these cloth materials, especially when considering that face seal leakage will decrease protection further.
Fit
Face seal leakage is a critical factor in the amount of protection provided by a respirator. Although much research has been done on filtering media and improving filter efficiency, the fit side of the equation has not been explored in such depth. Several recent studies examined aspects of fit related to healthcare personnel.
Face masks Two recent studies examined the extent to which face masks fit the face. Duling and colleagues (2007) assessed six face masks. The simulated workplace protection factor fifth percentile value was 1.4 and the lower 90 percent confidence limit was 1.2, indicating that none of the
masks provided adequate protection. Oberg and Brosseau (2008) evaluated facial fit of 5 face masks using qualitative and quantitative fit tests with 20 human volunteers. When the subjects put on the face masks themselves, they all failed the qualitative fit test. When they were assisted with donning the face masks, 18 subjects failed the fit test. For unassisted donning, average quantitative fit factors were 2.5 to 6.9; for assisted donning, they ranged from 2.8 to 9.6. None of the masks tested attained an individual fit factor of 100, the minimum passing level required by the Occupational Safety and Health Administration (OSHA) for a half-mask filtering facepiece respirator.
Loose-fitting PAPRs Loose-fitting PAPRs may be worn by healthcare personnel who have beards or who cannot otherwise wear an N95 filtering-facepiece or elastomeric air-purifying respirator. The unfiltered, exhaled air from the PAPR may transmit virus from the wearer to others. An N95 respirator may be worn inside the PAPR to prevent this from happening. Roberge and colleagues (2008) used a manikin to assess the protection factor of a loose-fitting PAPR with and without an N95 respirator glued to the manikin. Flow rates were 25 L/min and 40 L/min. The N95 significantly increased the PAPR protection factor even when the PAPR blower was turned off. However, consideration should be given to the possible negative impact of the additional physiological burden of wearing an N95 respirator inside a PAPR (Roberge, 2008). Additionally, their results might not hold in the work setting because the N95 was glued to the face (Roberge et al., 2008). Some loose-fitting PAPRs do not fully encapsulate the head, making it possible for the wearer to overbreathe the blower and possibly be exposed to contaminants (Roberge et al., 2008). Johnson and colleagues (2008) found that the 1.1 L of air inside the loose-fitting PAPR they tested would act as a buffer against contaminated air that leaks into the respirator due to overbreathing the blower. That volume could also help if an N95 were worn under the PAPR and face seal leaks occurred.
Fit testing and inward leakage Several large-scale fit tests of healthcare personnel were completed recently (Lee et al., 2008b; McMahon et al., 2008; Oestenstad et al., 2007; Wilkinson et al., 2010; Winter et al., 2010). McMahon and colleagues (2008) found that 5 percent of men and 15 percent of women could not pass the fit test with the first respirator tried, while Lee and colleagues (2008b) had 26 percent of workers fail the fit test with the first respirator. Winter and colleagues (2010) found
that 28 percent of 50 staff members did not fit the 3 respirators tested. Wilkinson and colleagues (2010) found that 82.9 percent of 6,160 healthcare personnel were successfully fitted with the first respirator, 12.3 percent required testing with a second respirator, and 4.8 percent required testing with 3 or more respirators. Therefore, multiple respirators are likely to be needed to get passing fit tests for all staff. First-time pass rates may improve after NIOSH incorporates the new sizing panels (Zhuang et al., 2007, 2008) into its TIL certification requirement.
Gender and age in women may be significant factors in achieving a successful fit (McMahon et al., 2008), though Oestenstad and colleagues (2007) did not find a gender difference in the 41 subjects they tested. Gender, respirator brand, and test repetition did not have any significant effects on location or shape of leaks assessed on half-mask respirators using a fluorescent tracer during fit tests (Oestenstad and Bartolucci, 2010). There was a difference in fit test leak-site distribution for women, and the authors suggested that facial dimensions may be an important factor. In fact, their prior research showed that fit was significantly associated with face length and lip width and possibly face width (Oestenstad et al., 2007). Weight gain during pregnancy may impact fit due to changes in facial anthropometrics (Roberge, 2009). Wilkinson and colleagues (2010) found that personnel who reported their race as Asian had the highest failure rate and that race was correlated with facial shape. Training improved the fit test pass rate (Lee et al., 2008b; Winter et al., 2010). However, as time elapsed from the fit test, pass rates were similar to those prior to training, although frequent use after training led to increased pass rates (Lee et al., 2008b).
Experience of the fit testers was found to be a significant factor in achieving a successful fit test with the first respirator tried (Wilkinson et al., 2010). Their testers selected a respirator based on observations of the subject’s facial characteristics, the physical fit of the respirator, and the “real-time” option on the PortaCount® fit tester. Janssen and colleagues (2007) evaluated the workplace protection factor of an N95 filtering face-piece respirator during light, moderate, and heavy intensity tasks in a steel foundry and found a large variability in protection because of removing and re-donning the respirator. This may also be a problem in healthcare settings. They suggested that a time-weighted, average workplace protection factor be considered to estimate ongoing protection.
Participants at a NIOSH-sponsored workshop (Brosseau, 2009) expressed interest in developing a respirator that did not require initial and annual fit tests and provided suggestions for improving the fit capabili-
ties of respirators. Au and colleagues (2010) proposed using a customizable, reusable mask with high-efficiency air filters. They investigated the efficacy of this mask without fit testing versus a fit tested N95 respirator in 22 volunteers. The median filtration factor was significantly higher for the N95 respirators compared to the mask that is cut to size. Only 16 of the 22 volunteers had a fit factor greater than 100. This was lower than the pass rate for the N95 (19/22), but was not significantly different. The authors concluded that the customizable mask should be studied further, but that it should not be used without fit testing at this time.
Face seal leakage is an important factor in respirator protection, and it depends on several factors, including proper respirator selection, fit, and donning. Cho and colleagues (2010) found that most particle penetration occurs through face seal leakage even when the respirator fits well (workplace protection factor = 515), and that particle penetration of the face seal decreases with increases in breathing rate and particle size. Similarly, Grinshpun and colleagues (2009) found that the number of particles penetrating through the facepiece seal far exceeded penetration through the filter medium for both an N95 respirator and a face mask using challenge particles in the 0.03 to 1 μm range. Lee and colleagues (2008c) investigated the protection factor of four N95 respirators and three face masks with a challenge of particles representing bacterial and viral size ranges (aerodynamic size: 0.04 to 1.3 μm). Prior research (Coffey et al., 2004) had demonstrated high protection levels for Respirator A and medium protection for Respirator B. Respirators C and D were the same except D had an exhalation valve. Overall, 29 percent of N95 respirators and 100 percent of face masks had protection factors of < 10, the assigned protection factor for the N95 (Lee et al., 2008c). The percentages of N95 respirators with protection factors of > 10 for all particle sizes tested were 86, 36, 89, and 78 percent for Respirators A to D, respectively. There were no significant differences in the protection factor between the N95 and N95 with the exhalation valve. The protection factors of the N95 were an average of 8 to 12 times greater than those of face masks.
Particle size–dependent face seal leakage has not been fully investigated (Shaffer and Rengasamy, 2009). However, NIOSH has initiated studies to determine whether face seal leakage of nanoparticles is consistent with the leakages observed for gases/vapors and larger particles. Further research on leakage of nanoparticles is important to better understand the effectiveness of filtering facepiece respirators in workplaces where nanoparticles are present.
Preventing a Patient from Spreading Virus
One suggestion for protecting personnel from virus exposure is to place a face mask or N95 respirator on a patient with confirmed influenza. Huang and Huang (2007) demonstrated greater bacterial leakage at close range for a face mask (15.8 percent of no mask control) than for an N95 respirator (4.1 percent of control). Placing positively charged polypropylene edging on the mask and respirator decreased leakage to 4.5 and 1.8 percent of control, respectively. This indicates that masks and respirators could be modified easily to decrease virus transfer.
Tang and colleagues (2009) used a schlieren optical method to visualize cough flows of volunteers wearing either a face mask or N95 respirator. With the N95, more of the cough went through the front of the respirator compared to the face mask, and the N95 was better at preventing leakage of the cough. The face mask on the standing people who coughed blocked the forward motion of the cough jet and directed it upward, downward, and out of the sides of the mask. The leakage air from both the mask and respirator had little momentum. They concluded that both face masks and N95 respirators decelerated and redirected the expelled air, which then joined the general upward motion of the cougher’s thermal plume created by body heat. This prevented the cough from being projected forward as a rapid turbulent jet over distances sufficient to reach the breathing zones of other individuals.
Diaz and Smaldone (2010) recently developed a headform-based system to evaluate the effectiveness of N95 respirators and loose- or tight-fitting face masks in preventing transmission of infectious aerosols from a source to a receiver. Although an N95 on the source filtered significantly more particles than the face masks, the simulated workplace protection factor did not differ for the unmasked receiver. When the receiver wore each mask or respirator, < 1 percent of the particles were filtered and the simulated workplace protection factor was 1.4 to 2.2. Sealing an N95 to the source using Vaseline® yielded a simulated workplace protection factor of 4,082, and sealing it to the receiver resulted in a protection factor of 118. The authors concluded that the face masks worn at the source resulted in greater protection than a face mask or respirator worn on the receiver.
Both Huang and Huang (2007) and Tang and colleagues (2009) showed greater leakage with the face mask than with the N95 respirator. This seems to contrast with the results of Johnson and colleagues (2009), who showed that both fully blocked virus expulsion. However, Huang
and Huang as well as Tang and colleagues considered leakage from the sides of the face mask and respirator, while Johnson and colleagues had volunteers cough directly onto the collection plate. Although Tang and colleagues (2009) indicated that the cough flow was directed away from the receiver, they did not assess filtration, so virus particles that could not be observed with the schlieren optics could have reached the receiver. Diaz and Smaldone (2010) did not use heated manikins, so there were no thermal plumes that may have directed the particle flow upward and away from the receiver. More research is needed to understand the particle dynamics of exhaled viruses while wearing a face mask or respirator in order to make a recommendation regarding patient mask or respirator wear.
Summary: Respirator and Face Masks—Fit and Filtration
Several studies have shown that face masks do not have sufficient filtration or fit to provide adequate inhalation protection of small particles to wearers. NIOSH-certified N95 respirators have been shown by several studies to provide expected filtration levels of nanoparticles. However, high filtration efficiency alone does not ensure that the wearer will be protected. Mask D in Oberg and Brosseau’s study (2008) had the second highest filtration efficiency, but the lowest fit factor. Because face seal leakage far exceeds penetration through the filter (Cho et al., 2010; Grinshpun et al., 2009), future research should focus on improving respirator fit.
Although Lee and colleagues (2008c) showed that some N95 respirators may not achieve an assigned protection factor of 10 when nanoparticles are present, whether higher fit factors would have been achieved with a different model respirator is unknown. The rates of failure from their study were similar to the fit-factor failure rates of large-scale protection-factor tests performed on healthcare personnel (Lee et al., 2008b; McMahon et al., 2008; Winter et al., 2010). Multiple respirators should be available for healthcare personnel, as currently required by OSHA’s respirator standard, because it is unlikely that one model or size will fit all employees. Long-term storage of these additional respirators should have little impact on penetration levels (Viscusi et al., 2009a). Participants in a no-fit respirator workshop (Brosseau, 2009) identified the need for a user seal check that works, continuous fit checking rather than a no-fit test, and a respirator that can be put on easily multiple times.
These features would be beneficial because protection varies with repeated donning (Janssen et al., 2007).
Respirators: Decontamination
Reuse of filtering facepiece respirators has been suggested as a strategy to counteract anticipated supply shortages during a pandemic and to reduce costs. The potential for the respirator to serve as a fomite is a serious concern. Safe, effective decontamination methods that inactivate the virus without altering the respirator performance and physical characteristics are necessary for this to occur. Respirators impregnated with antiviral particles may decrease the risk of virus transmission because of improper handling of virus-exposed protective equipment. To study this issue, methods are first needed to effectively deposit virus onto respirators, and then their potential to act as fomites can be investigated. Finally, the effectiveness of treated filter media and decontamination methods can be assessed.
To assess the efficacy of decontamination methods, reliable and repeatable methods of applying contaminant aerosols to filter media are needed. NIOSH has developed two systems for applying virus aerosols to respirator filter media that pass the aerosol through the filter media rather than depositing it on the surface. The bioaerosol respirator test system (Fisher et al., 2009) loads filter media with virus-containing particles while the droplet-phase aerosol respirator test system (Vo et al., 2009) applies droplets onto media. Another system, the Dry Aerosol Deposition Device (Heimbuch et al., 2009), deposits biological aerosols onto the surface using impaction, a system that allows the aerosol to be delivered quickly and with reproducible loading.
Additionally, to assess the number of viable particles on PPE, recovery methods are needed that allow removal without killing the virus. Casanova and colleagues (2009) developed a new technique for recovering bacteriophage MS2, a non-enveloped virus, from contaminated gloves, gowns, respirators, and goggles. Recovered viruses were not inactivated, indicating that this technique can be used for viral survival studies.
Rate and length of virus survival are key facts needed to assess the potential for respirators to serve as fomites. Rengasamy and colleagues (2010b) applied MS2 as both an aerosol and as liquid drops onto N95 filter coupons. They demonstrated that > 10 percent of the challenge
MS2 bacteriophage survived for 20 hours at 22ºC and 30 percent relative humidity. Fisher and Shaffer (2010) subsequently assessed MS2 survivability at 1, 2, 3, 4, 5, and 10 days. The authors found that 10 percent of initial MS2 survived for 4 days for both deposition methods, and that all samples had detectable levels of MS2 on the tenth day. Because MS2 is a non-enveloped virus, enveloped influenza virus survivability cannot be estimated from the current study. MS2 survives for longer periods of time on surfaces than influenza does. Because fomites may be a source for indirect contact transmission, decontamination may be required before reuse.
Decontamination Methods
Studies of various types of decontamination methods have not yet been successful in identifying an effective means for decontamination that does not affect the structure and integrity of the respirator. Viscusi and coworkers (2007, 2009b) assessed the effect of decontamination methods on filter aerosol penetration, physical appearance, and airflow resistance. From their initial list of 10 potential decontamination methods (autoclave, isopropyl alcohol, bleach, hydrogen peroxide, microwave, soap and water, ultraviolet [UV] radiation, dry heat, vaporized hydrogen peroxide, and ethylene oxide) (2007), liquid hydrogen peroxide, vaporized hydrogen peroxide, and UV radiation caused the least change in filter performance. The authors (2009b) then further evaluated five decontamination methods (UV germicidal irradiation, ethylene oxide, vaporized hydrogen peroxide, microwave oven irradiation, and bleach) on three models each of N95, P100, and surgical N95 filtering facepiece respirators. Ethylene oxide and UV germicidal irradiation were the only methods that did not cause any observable physical changes to the respirators. The bleach method left a noticeable odor, even after overnight drying, and bleach off-gassed when the decontaminated respirators were rehydrated with deionized water. UV germicidal irradiation, ethylene oxide, and vaporized hydrogen peroxide were the most promising decontamination methods, although there are concerns regarding throughput for the latter two methods.
Fisher and colleagues (2009) investigated a process for applying viral droplets to respirators to evaluate decontamination methods. Two decontamination methods, one physical (steam) and one chemical (bleach), were examined as well as two concentrations of organic matter (protec-
tive factor) in the aerosol medium. The organic matter had a protein concentration similar to the organic challenge in ASTM E1053-97, Standard Test Method for Efficacy of Virucidal Agents Intended for Inanimate Environmental Surfaces. The organic matter may protect the virus by neutralizing the decontamination solution or by providing a physical barrier to the decontaminant. Detectable differences were noted in the efficacy of the bleach decontamination, depending on the concentration of bleach and protective factor. Steam showed an effect for treatment time, but not protective factor. Viruses recovered were more likely to be found on the outer layer than when low concentrations of organic matter were used. This would be important for a decontamination method such as UV light that only reaches the outer layer of filter material.
A study of bleach and UV radiation (Vo et al., 2009) found that bleach concentration and UV exposure time were factors in decontamination. UV radiation was recommended for further study, such as assessing the impact of pleats or folds in filtering facepiece respirators, because it was non-toxic and did not leave an odor. Low-dose UV radiation resulted in 3-log10 reductions in MS2, while higher doses resulted in no detectable MS2. The authors discussed that further research is needed because non-enveloped MS2 may not behave in the same manner as enveloped viruses, and because the composition and size of particles used might not exactly mimic respiratory secretions.
Finally, Salter and colleagues (2010) assessed the amount of residual chemicals on six models of filtering facepiece respirators after seven decontamination methods: ethylene oxide, vaporized hydrogen peroxide, UV light, and four liquids (hydrogen peroxide, bleach, mixed oxidants, and dimethyl dioxirane). Six of the seven methods did not deposit significant amounts of toxic residue. The ethylene oxide–treated respirators had detectable levels of 2-hydroxyethyl acetate, a hazardous byproduct that may have been formed when the ethylene oxide reacted with rubber parts of the respirator. As noted by other authors, bleach-treated respirators had a bleach odor after treatment. The bleach also corroded metal parts and discolored others. Dimethyl dioxirane and mixed oxidants also oxidized metal parts and had distinct odors.
Practical decontamination methods are needed as decontamination of filtering facepiece respirators is not currently an option. Although bleach is readily available and inexpensive, it has been shown to cause offensive odors and can cause corrosion. Ethylene oxide may react with rubber straps to create a hazardous byproduct. It also has problems with throughput, as does vaporized hydrogen peroxide. Other techniques such
as soap and water, isopropyl alcohol, and microwaving can cause changes to the physical characteristics of the respirator. UV radiation shows promise, although its effectiveness on inner filter layers and pleats is unknown, as is its affect on electrostatic properties of electret filters. Additional research with enveloped influenza viruses is needed.
Treated Filter Media
Several studies investigated the decontamination efficacy of treated filter media. Oxford and colleagues (2007) found that masks impregnated with QR-435, a green tea extract mix, trapped a significantly greater amount of virus compared to an untreated mask. Lee and colleagues (2008a) exposed iodine-treated filter media to aerosolized bacteria at an equivalent of 85 L/min flow. Viability of collected spores from control and treated media were assessed. Survival fraction was significantly lower for the treated filter versus untreated at room temperature and low relative humidity. However, there were no differences in viability at room temperature and high relative humidity or at high temperature and high relative humidity. Lee and colleagues (2009) then investigated the efficacy of iodine-treated filter media against MS2 aerosols. Treated media showed significantly higher viable removal efficiencies than untreated. Rengasamy and colleagues (2010b) applied MS2 virus droplet nuclei onto four coupons from antimicrobial respirators and controls. Antimicrobial agents included iodine, embedded silver-copper throughout the fibers of the outer layer of the mask, EnvizO3-Shield technology on the outer layer, and titanium dioxide–coated filter layers beneath the outer layer. MS2 is less sensitive to many antimicrobial agents because it is a non-enveloped virus, which is hardier and able to survive longer than enveloped viruses such as influenza. The iodinated fibers from Respirator C had a significant increase in the log10 reduction of MS2 at 37ºC and 80 percent relative humidity, but not at lower temperatures and relative humidity. MS2 droplet nuclei survived for more than 20 hours at room temperature and 30 percent relative humidity. For an antimicrobial agent to be effective, it needs to reduce viability faster. At 22ºC, 30 percent relative humidity, all four antimicrobial respirators had < 1 log10 reduction, which was not significantly different from the control. The iodinated respirator showed 3.7 log10 reduction of MS2 at 4 hours. This was significantly higher than the control, while the others were not significantly different from the control. Therefore, the decontamination efficacy
of the antimicrobial respirators depends on storage conditions and the antimicrobial agent. Iodine from the filter that enters the extraction medium may also inactivate virus.
Borkow and colleagues (2010) investigated the antiviral properties of a copper oxide–containing N95 respirator. Treated and control respirators were exposed to aerosolized human influenza A virus and avian influenza virus at a constant airflow rate for 1 minute. The number of infectious virus titers recovered from the masks was measured 30 minutes after exposure. The researchers found that the copper oxide particles did not impact filtration efficiency, but there was a statistically significant difference in retrieved infectious influenza between the control and copper oxide–impregnated masks. This could offer the possibility of reducing the risk of hand or environmental contamination, with potential subsequent infection due to improper handling of exposed respirators.
Summary: Decontamination
Various techniques have been developed for applying viruses to respirators. Recent research has shown that non-enveloped viruses can persist on respirators for up to 10 days in hospital-like environments (Fisher and Shaffer, 2010). Although enveloped viruses such as the influenza virus may not survive as long, it is reasonable to assume that the virus will survive long enough to render the respirator a potential fomite. Current decontamination research has not demonstrated an effective technique for killing viruses that does not also have a detrimental impact on respirator physical characteristics or function. More research in this area is needed. Iodine-treated and copper oxide–impregnated filter media show promise for inactivating virus in the respirator.
Respirators: Tolerance, Physiological Responses, and Communications
Respirators worn by healthcare personnel have the potential to impact comfort, physiological responses, task performance, and communications with each other and with patients. Protective equipment, such as respirators or face shields, may create pressure points that cause discomfort. The breathing resistance and dead volume of the respirators may alter respiration and lead to a build-up of carbon dioxide. Because respi-
rators may alter the wearer’s field of view, some tasks may be more difficult to perform and thus may take longer. The respirator covers the mouth, muffling speech and removing visual cues for the listener. Additionally, the noise created by a PAPR blower and environmental noise both hinder communications. Several researchers have investigated the impact of respirators on healthcare personnel.
Respirator Tolerance
Consistent use of PPE by healthcare personnel during exposure to hazards is essential to achieve the benefits afforded by the devices. One of the critical features in helping to achieve consistent compliance in the wearing of PPE may be the comfort and tolerability of the equipment itself.
Although most healthcare personnel appear to be assigned N95 respirators for use, elastomeric respirators represent an alternative type of respiratory protection that could be used. Roberge and colleagues (2010c) reported the comfort responses of healthcare personnel wearing a half-face elastomeric respirator who completed exercise regimes for a 1-hour period. The healthcare personnel’s mean comfort scores were low, indicating that the elastomeric respirators were generally comfortable, and the comfort scores were not significantly different from controls (no respirator). Complaints of subjective symptoms and design features of the respirator included facial heat, skin irritation, and weight of the respirator, among others.
Little is known about the tolerability of healthcare personnel to wearing respirators, particularly during an influenza pandemic where they likely would be required to be worn for long periods of time within an 8-hour work shift over a duration involving a number of weeks or more. Radonovich and colleagues (2009) determined the mean tolerance time that healthcare personnel would be willing to wear a variety of respirator ensembles (including N95s, PAPRs, half-face elastomerics, and face masks in various combinations) while performing their normal job duties. Healthcare personnel stopped wearing their respirator ensemble before the end of an 8-hour shift in 59 percent of the total work shifts evaluated by the study. Reasons given for discontinued wear included communication difficulties (visual, auditory, or vocal), heat, pressure or pain, and dizziness or difficulty concentrating, among others. Median tolerance times varied by the respirator ensemble worn, ranging from 4.1
to 7.7 hours. Wearers of disposable models often complained of facial heat and pressure, while users of reusable models often reported communication problems. A cup-shaped N95 with an exhalation valve had a greater median tolerance time (7.7 hours) compared to a similar model that was not equipped with a valve (5.8 hours). The ensemble combination of cup N95 plus a face mask had the shortest median tolerance time of 4.1 hours. The PAPR had a slightly lower median tolerance time (7.6 hours) than either the cup-shaped N95 with exhalation valve or face mask, which had the same median tolerance time (7.7 hours). Although the difference is not likely to be clinically significant, it is surprising that the tolerance time for the PAPR was lower than that for the N95 with exhalation valve. It is also interesting to note that the tolerance time for the face mask was similar to both the PAPR and the N95 with exhalation valve. However, the N95 with exhalation valve had a higher number of complaints (24) than the PAPR (17) or face mask (17).
Harber and colleagues (2009) evaluated a number of subjective tolerance measures to respirator use by subjects selected from the general population, including some with mild respiratory impairment, while engaging in exercise and work simulation. Respirators evaluated included half-face elastomerics and N95s. Under work simulation, the subjective tolerance measures (e.g., comfort, heat, speech) for all variables assessed, except heat, were more “adverse” for the elastomeric half-mask respirator than for the N95. The largest adverse subjective ratings were for comfort, face, breathing, heat, and heavy weight of the device. The largest differences between the two types of respirators were for subjective measures of nose impact and heavy weight, with the elastomeric model demonstrating the greater adverse impact among subjects on these two measures. Overall, however, the study concluded that while both respirator types were “relatively well tolerated,” N95 respirators may be preferable to elastomeric respirators.
Although not much is known about what workers would like to see incorporated into future respirator designs to assist in increasing compliance, one recent study surveyed healthcare personnel to assess their perspectives on this issue (Baig et al., 2010). Of 149 survey respondents, only 24 percent reported that their N95 respirator was comfortable most of the time or always, and only 6 percent indicated that they would be able to tolerate wearing an N95 respirator continuously for an 8-hour shift. Overall, 56 percent of respondents believe there is a need to develop a new N95 respirator for healthcare personnel, 44 percent preferred an N95 that does not require fit testing, and 60 percent preferred wearing a
disposable device. Problems identified by respondents that need to be addressed in future respirator design included discomfort, difficulty breathing, heat, and low tolerability for use over extended time periods.
Physiological Impact
The physiological impact of respirators on workers is an important factor in designing wearable PPE. Recent studies have investigated the physiological impact of respirators during physical tasks and while walking on a treadmill. Vojtko and colleagues (2008) found small but statistically significant increases in both inhalation and exhalation resistances of a face mask placed over an N95 filtering facepiece respirator for minute volumes of 25 and 40 L/min, but the total did not exceed NIOSH limits (although those are measured at 85 L/min). Although their study was performed with a simulator, the authors concluded that the slight increases in resistance should not impact the wearer’s respiratory effort.
Bansal and colleagues (2009) assessed the impact of one dual-cartridge elastomeric and one N95 half-face mask respirator on both normal and respiratory-impaired volunteers (respiratory impairments were listed as chronic rhinitis, mild chronic obstructive pulmonary disease, and mild asthma). No control condition was used. Sedentary (bolt sorting), low-intensity (walk across room and put papers in appropriate bins), and moderate-intensity (stock shelves with cereal boxes and juice jugs) tasks were included. The authors found small but statistically significant differences between the two respirators for inhalation time (longer for half-face models, 1.14 versus 1.10), exhalation time (shorter for half-face models, 1.39 versus 1.44), and increased duty cycle for half-face models (0.46 versus 0.45). However, the differences are not likely to be clinically significant. The authors stated that neither respirator should cause hypoventilation and that both types of respirators should be well tolerated for most individuals, including those with mild respiratory impairments.
Another study used thermal imaging to assess surface temperature of two N95 respirators and two N95 respirators with exhalation valves from the same manufacturer (Monaghan et al., 2009). Respirators were placed on a headform with an Automated Breathing Metabolic Simulator supplying air at 100 percent relative humidity and 33ºC at 10 L/min for an hour. The authors concluded that at the breathing rate used, exhalation valves provided no heat dissipation benefits over a mask without one.
However, they also noted that the exhalation valve was not activated at the low breathing rate.
Roberge and colleagues (2010a,b,c) completed a number of studies that assessed the physiological impact of various respirators during a 1-hour treadmill walk at 1.7 and 2.5 mph. There were no significant differences between N95 filtering facepiece respirators with and without exhalation valves and the control in physiological variables, exertion scores, or comfort scores. The comparison of the N95 with and without the exhalation valve found no differences in the partial pressure of carbon dioxide (CO2). Similar results were reported for face masks worn over the N95s either with or without the exhalation valve. The authors did note that face masks decreased oxygen levels in the filtering facepiece respirators at the low work rate and in the respirators with the exhalation valves at the higher work rate. An elastomeric air-purifying respirator resulted in decreased breathing rates and higher tidal volumes at both work rates, although the minute ventilation did not differ. While transcutaneous CO2 values did not statistically differ (elastomeric air-purifying respirator versus control), some subjects had elevated levels that the authors suggested should be investigated further.
The elastomeric air-purifying respirator imposed little additional physiological burden over an hour of wear at the work rates assessed. Exhalation valves in N95 respirators may decrease exhalation resistance and help dissipate heat and CO2 build-up; however, at low flow rates, these benefits may not be realized. Monaghan and coworkers (2009) noted that the exhalation valve was not activated at 10 L/min ventilation rate. This means wearers may not notice any difference in thermal sensation of the face during sedentary or low-intensity activities. Roberge and colleagues (2010b) found that at low work rates, excess CO2 was not a problem. Therefore, the benefit of the exhalation valve may be seen only at higher work rates.
Healthy workers and those with mild respiratory impairments should be able to physiologically tolerate respirators. Little physiological burden should be imposed by filtering facepiece respirators (with or without a face mask) or by elastomeric respirators. However, the impact of filtering facepiece respirators on pregnant women is not well understood (Roberge, 2009). Respirators designed to accommodate the respiratory limitations of pregnant healthcare personnel may improve comfort and tolerability for other wearers (Roberge, 2009). Although loose-fitting PAPRs were not investigated, they should not impose a respiratory bur-
den due to their positive air flow. However, the weight of the blower may increase the physiological workload.
Communications
Healthcare personnel must be able to communicate effectively and accurately with each other and with patients while wearing PPE. Communications while wearing PPE typical of healthcare personnel has been investigated by Mendel and colleagues (2008) and Radonovich and colleagues (2010). A third study involving air traffic controllers also provides useful information (Hah et al., 2009).
Mendel and coworkers (2008) assessed the impact of face masks on speech intelligibility using the Connected Speech Test with normal and hearing-impaired listeners in either a quiet room or with prerecorded noise from a dental drill played at 45 dBA. No impact was reported on speech intelligibility for either the normal or hearing-impaired listeners. A small but significant difference in scores for both mask and no-mask scores was noted due to background noise. Radonovich and coauthors (2010) used a Modified Rhyme Test with modified scoring to investigate the impact of face masks and respirators on speech communications. Reported scores were not corrected for guessing. The first set of trials was performed in an intensive care unit (ICU) room with simulated noise, with only the speaker wearing the device. The scores of the N95 cup-shaped respirator, duck bill N95, face mask, and control were statistically the same at both 3 and 7 feet in an ICU room with simulated noise. The elastomeric half-mask respirator with exhalation valve was the worst performer at 72 percent, scoring even lower than the loose-fitting PAPR (84 percent). However, the cup-shaped N95 with a face mask, cup-shaped N95 with an exhalation valve, PAPR, and cup-shaped N95 with exhalation valve and overlying face mask all had scores significantly lower than the control.
The second set of trials was performed with elastomeric respirators with and without exhalation valves in an audiometric test room with background noise. All six respirators had scores significantly different from the control, and the three respirators with speech augmentation performed better than those without augmentation. The final set of trials was performed in an audiometric test room with background noise with only the listener wearing a PAPR. The PAPR significantly impacted scores (79 percent) compared to the control (90 percent). Intelligibility was
higher at 3 feet than at 7 feet, but was not significantly different. Control scores were higher in the audiometric room than in the ICU, possibly due to reverberation and distractions in the ICU area. The authors suggested that respirators should be developed that improve communication among coworkers in noisy medical settings.
Hah and colleagues (2009) investigated loose-fitting PAPRs and N95s for air traffic controllers who would have to work during an influenza pandemic. Although they focused on communications using headsets, some of their observations are applicable to the healthcare environment. The blowers for the three loose-fitting PAPRs that they investigated created noise between 52 dBA and 81 dBA. As expected, the respirator with the highest blower noise had the lowest speech intelligibility scores. Although the authors used a modified rhyme test that was different than the test employed by Radonovich and colleagues (2010), these authors found error rates of 3 to 18 percent with the PAPRs for electronic communications and error rates of 32 to 55 percent with 3 subjects performing face-to-face communications. The quietest PAPR had the lowest error rates. Their N95 tests with two filtering facepiece respirators and one elastomeric respirator had error rates of 0 to 16 percent, though only 2 volunteers were used. The highest errors were with the elastomeric respirator, similar to the Radonovich findings. The elastomeric respirator was rated as obstructing maintenance tasks more than the filtering facepiece respirator.
These three studies showed that background noise, whether it is from a dental drill, ICU room, or PAPR, has a detrimental effect on speech communications. Efforts should be made to decrease ambient noise, select PAPRs with low-noise blowers, or develop and certify PAPRs with lower flow rates that could be used by healthcare personnel. Speech augmentation devices or voice projection units may also improve communications. The Department of Homeland Security has a current Small Business Innovation Research project (Topic Number H-SB09.2-006) (Department of Homeland Security, 2009) that is investigating improved speech intelligibility in noise for first responders. Some of the techniques being developed may be applicable to PAPRs and elastomerics, the respirators that the studies by Radonovich and Hah and colleagues identified as having the greatest decrement on speech recognition. Additionally, the impact of decreased communications on patient care and procedural outcome is unknown.
Current Research Directions
NIOSH recently supported a research effort (Brosseau, 2009) that examined ways to improve fit for half-mask respirators. The effort culminated in a workshop that included presentations on recent research efforts, innovation, design, and impediments to bring new products to market as well as breakout sessions during which participants discussed characteristics of good design, areas where research is needed, wearer characteristics that impact fit, and necessary design changes. Characteristics of good design identified during the breakout session included “low weight, able to fit many facial profiles, does not impair field of vision, uniform pressure on the face, good strap design, can be easily donned multiple times, has a limited number of parts, does not interfere with communication and is portable and easy to store” (Brosseau, 2009, p. 33). Participants identified the following as important research areas: decontaminating respirators, cross-contamination occurrence during repeat donning, efficacy of biocidal coatings, and whether respirator use reduces infection rates in emergency departments. Wearer characteristics that the group identified as contributing to fit were symmetry, chin characteristics, sweat, nose dimension, and head dimension. Participants also identified the need for a user seal check that works and said they would like continuous fit checking rather than a no-fit test. Overarching recommendations for future research were to further investigate the relationship between respirator design and fit; to clarify the impact of facepiece design, facepiece sizes, and aging on the relationship between facial measurements and respirator fit; to determine how user seal check impacts respirator fit; to explore new methods for checking facepiece seals; and to determine the impact of environmental conditions and other protective equipment on respirator fit.
The Department of Veterans Affairs is leading a collaborative effort with the National Personal Protective Technology Laboratory (NPPTL) to develop a respirator specifically designed for healthcare personnel entitled Project Better Respiratory Equipment Using Advanced Technologies for Healthcare Employees (B.R.E.A.T.H.E.) (Department of Veterans Affairs, 2010). The project has been divided into four stages: interagency working group information exchange, prototype development, prototype lab and human subject testing, and commercialization. Nine federal departments and agencies are participating in the working group. The group met in 2008 to discuss issues of safety and effectiveness, impact on occupational activities, comfort and tolerability, and
healthcare policies and practices. On December 14, 2009, a notice was placed in the Federal Register describing the project and seeking letters of interest from commercial organizations with the ability to design and manufacture a respirator that meets the needs of Project B.R.E.A.T.H.E. (Federal Register, 2009). It is anticipated that the report will be published in 2011.
Summary
Efforts are under way to develop more comfortable and easier to fit respirators that will be more conducive to interactions with patients, colleagues, and family members. Research to date has shown that the low inhalation and exhalation resistance rates of filtering facepiece respirators do not significantly impact respiration. Although hypoventilation occurs with full-facepiece air-purifying respirators, neither the Bansal and colleagues (2009) nor the Roberge and colleagues (2010a,b,c) studies showed any evidence of such a burden with N95 filtering facepiece respirators or elastomeric respirators. Respirators do impact communications. However, the impact of decreased speech intelligibility on task performance is unknown. Loose-fitting PAPRs may make many healthcare tasks more difficult because of their bulkiness, added weight of the blower, flexible visor, and blower noise.
Protective Clothing
Gowns and other forms of protective clothing are designed for healthcare personnel primarily to act as a barrier to prevent the penetration of liquids or solids from coming into contact with the wearer’s skin and clothing. As the barrier properties of the protective clothing increase, the breathability of the material generally decreases, which then has the potential to impact comfort and tolerability (i.e., cause an increase in heat levels experienced by the wearer). A recent study has demonstrated that medical personnel who wear chemical protective clothing while performing basic life-saving tasks (e.g., connecting an intravenous [IV] line) experience discomfort and heat stress along with needing more time to perform the task (Rissanen et al., 2008). Using phase-change materials for the construction of protective clothing offers the potential to help reduce heat stress and improve thermal comfort for healthcare personnel.
Phase-change materials provide cooling by absorbing heat when they change from a solid to a liquid state. A surgeon wearing a vest containing phase-change materials reported subjective improvements in thermal comfort over that compared to regular clothing (Reinertsen et al., 2008).
A 2008 review of protective clothing for healthcare personnel highlighted additional issues that are considered in designing protective clothing, including antibacterial finishing treatments, the impact of temperature and relative humidity in the work environment, the use of multiple layers, and changes that can result from laundering or cleaning that might impact the protective effects of the clothing (Laing, 2008). The issue of laundering was further reviewed by Wilson and colleagues (2007), who assessed the limited available literature and found that industrial laundering and home laundering both were effective in decontamination of healthcare clothing, including lab coats.
A large-scale study (Manian and Ponzillo, 2007) examined compliance of gown wear for hospital personnel (n = 1,150) and visitors (n = 392) to general wards and an ICU. Overall compliance by hospital personnel was 76 percent, while visitors complied 65 percent of the time. However, there were differences among healthcare personnel by occupation, with respiratory therapists having the highest compliance rate (96 percent) and physicians having the lowest (67 percent). Female healthcare personnel (79 percent) were more likely than males (66 percent) to wear gowns. Overall compliance was higher in the ICU (83 percent) than in the general wards (71 percent). The study authors did not note whether an individual healthcare professional was included in multiple observations. Additionally, knowing they were being watched may have influenced the decision to wear a gown. The reasons for non-compliance are unknown. However, the study showed that improvement is needed in gown compliance and that educational efforts should focus on male healthcare personnel as well as both healthcare personnel and visitors to general wards.
Gloves
Gloves also serve as barrier protection, although the role of gloves in preventing the transmission of influenza or other respiratory viruses is unknown. For bloodborne pathogens, gloves can prevent transmission through direct contact with non-intact skin. Gloves can provide a barrier between contaminated surfaces and the skin and can serve as a reminder
to avoid self-inoculation. However, gloved hands can be a means of self-inoculation if healthcare personnel inadvertently touch their mouth, nose, or eyes with contaminated hands (IOM, 2008). Changing gloves between patients and paying rigorous attention to hand hygiene protocols can reduce contamination between patients or self-inoculation. Glove donning and doffing procedures have been examined to reduce contamination (Jones et al., 2010; Newman et al., 2007).
As with gowns and protective clothing, one active area of research is the use of antiviral coatings or other virus-inhibiting mechanisms. Caillot and Voiglio (2008) examined the tolerance and ease of use of gloves that had a disinfecting agent between the two layers of the glove. They found that tactile feeling, grip quality, and other measures were comparable to double latex gloving. Other areas of study are indicators of perforation in gloves, double gloving, and tolerance of powder or other irritant reducers (e.g., aloe vera) (Fry et al., 2010; Hubner et al., 2010; Korniewicz and El Masri, 2007; Partecke et al., 2009).
Eye Protection and Face Shields
Transmission of influenza through the mucosa of the eyes, nose, and mouth is plausible, but not confirmed (Chan et al., 2010). Case reports of conjunctivitis have been noted with the H7N7 avian influenza virus (Fouchier et al., 2004). Protective eyewear and face shields can reduce self-inoculation and may provide protection against droplet spray. Little is known about how well these devices protect the wearer from direct contact, when this protection is needed regarding transmission from patients to healthcare personnel, and the extent to which extra precautions are needed during aerosol-generating procedures.
Face shields may be a useful form of protection in lieu of face masks for workers exposed to droplet spray, particularly regarding comfort and tolerability issues, reduced breathing resistance, and improved speech communication. Decontamination and reuse may be possible for face shields, but much remains to be learned. It is unknown whether healthcare personnel would find face shields to be an acceptable alternative to masks and whether face shields would provide similar or superior (by protecting the eyes) protection to masks.
The recent literature on eye protection and face shields appears to be limited and focused on blood-splash concerns. A study by Mansour and colleagues (2009) examined eye protection during orthopedic surgery
and made comparisons of several types of glasses, loupes, or face shields. Modern prescription glasses offered no benefit over the control condition, with both resulting in contamination rates of 83 percent. These rates were significantly lower for all other eye-protective devices (50 percent for standard surgical telescopic loupes, 30 percent for face-mask and eye-shield combinations, and 3 percent for disposable glasses). Studies have also examined eye protection for blood-splash concerns (Davies et al., 2007; Wines et al., 2008) and found that face masks, eye shields, and glasses worn by surgeons and scrub nurses had high incidence of blood and body-fluid splashes. Similar studies would be useful to evaluate the exposure of healthcare personnel to droplet spray during patient care and to assess which types and combinations of eye protection and face shields provide the greatest protection.
Cross-Cutting Issues: Task Performance and New Materials
Task Performance
The ability of medical staff to perform tasks and medical procedures while wearing PPE is a concern. Several recent studies assessed the impact of various levels of PPE on the ability of healthcare personnel to perform medical tasks. The ability to complete a simulated resuscitation was investigated for paramedics wearing an air-purifying respirator and PAPR (Schumacher et al., 2009) and for paramedics and anesthesia trainees wearing an air-purifying respirator with a binocular or panoramic lens (Brinker et al., 2007; Schumacher et al., 2008). No differences in task completion times were found in any of these three studies. Delays resulting from donning a protective gown were seen in the initiation of chest compressions and cardiopulmonary resuscitation in a study of fire-fighter defibrillator instructors who were videotaped performing cardiac arrest scenarios (Watson et al., 2008). Rissanen and colleagues (2008) found that completing two medical tasks while wearing chemical, biological, radiological, and nuclear (CBRN) PPE, including an impregnated charcoal suit, overalls, an air-purifying respirator, and cotton and rubber gloves, took longer—19 percent for ventilation and 18 percent for connecting an IV line. Udayasiri and colleagues (2007) assessed the ability of emergency department doctors and nurses to perform trauma resuscitation in level C PPE (air-purifying respirator, hooded suit, and inner and
outer gloves) versus gowns and gloves. Although volunteers believed the PPE impaired pulse assessment, IV cannulation, IV-line attachment, mini-jet use, bag and mask ventilation, and communication, only the time to control hemorrhage (38 to 47 sec, p = 0.02) was significantly impacted. Castle and colleagues (2009) assessed the ability of 64 clinicians to perform intubation, laryngeal mask airway placement, and insertion of an IV cannula and intraosseous needle twice while in CBRN PPE and once unsuited. Eight percent of intubation and 12 percent of IV cannulation attempts were unsuccessful in CBRN PPE.
Donning of PPE has also been noted to delay the onset of patient care. Watson and colleagues (2009) conducted a simulation of a patient on a pediatric ward who developed respiratory failure. Donning of full PPE delayed the first response to the simulated “code” situation by 2 minutes, and other care measures were also delayed.
These results show that many medical tasks can be performed in various levels of PPE, but that the tasks may take longer. No studies were performed that assessed the impact of filtering facepiece respirators, elastomeric respirators, or loose-fitting PAPRs on task performance. However, a filtering facepiece respirator or elastomeric respirator is unlikely to have a greater impact on task performance than a full-facepiece air-purifying respirator. The bulkiness and visor of a loose-fitting PAPR may have a different impact on performance than the more close-fitting air-purifying respirator and PAPR used in these studies. Castle and colleagues (2009) report that the reason some tasks could not be completed may be because clinicians were wearing full chemical, biological suits. The individual impact of respirators, suits, and rubber gloves cannot be separated. Additional studies that assess the impact on task performance of PPE likely to be worn during an influenza pandemic or outbreak of other viral respiratory diseases are warranted.
Using Multiple Types of PPE: Integration and Use Issues
Because healthcare personnel frequently need to use several PPE items for protection against bloodborne or other infectious agents, including a respirator, gloves, a gown, eye or face protection, and in some cases head and shoe coverings, issues arise about the integration and interface of these items and how best to don and doff the PPE to ensure that maximum protection is provided and contamination is avoided. Workers in fields such as law enforcement, firefighting, and health care
have critical needs to be able to perform specific tasks while wearing multiple types and models of PPE. The impact of PPE on occupation-specific tasks and the equipment used to perform those tasks should be considered in designing and selecting PPE.
Studies on the impact of PPE on medical tasks have focused on chemical and biological protective equipment (Brinker et al., 2007; Castle et al., 2009; Rissanen et al., 2008; Schumacher et al., 2008, 2009; Udayasiri et al., 2007). These studies did not address healthcare-specific PPE used to protect against influenza transmission. The impact of individual PPE items was not assessed, so it is not known whether the respirator, gloves, or suits or a combination of equipment caused the interference. Additionally, face shields were not used in these studies. Integration of common healthcare PPE with medical equipment should be assessed and their impact on task performance determined.
SUMMARY OF PROGRESS
Personal protective equipment is a critical component in the hierarchy of controls used to protect healthcare personnel from influenza and other viral respiratory diseases. Understanding the functional issues related to the design of PPE as well as the factors that impact use are critical to ensuring that healthcare personnel are adequately protected, comfortable, and able to perform their jobs. Important advances have been made in some areas since the 2008 IOM report, but other areas, particularly regarding improvements in gowns, gloves, face masks, and face shields, need to be more fully addressed. Much research has been done regarding filtration of respirator media, but ways to improve fit, including new technologies specifically for filtering facepiece respirators, need more research because face seal leakage greatly exceeds filter penetration in the overall TIL of respirators. The physiological impact of respirators has been studied in-depth, but research in this area is lacking for other types of PPE. Integration issues concerning PPE and medical equipment and the impact on operational performance have not been adequately studied. Effective decontamination methods that do not impact the physical characteristics of respirators have been studied for some types of respirators, but with inconclusive results. Finally, the characteristics of a respirator that would specifically address the needs of healthcare personnel (e.g., patient–provider interaction, comfort, reduced physiological burden)
have been identified. Addressing these issues is important for developing PPE for healthcare personnel that is safe, effective, and comfortable.
FINDINGS AND RESEARCH NEEDS
This chapter provides an overview of the range of ongoing work on designing and engineering effective PPE to prevent transmission of influenza and other viral respiratory diseases. At its June 2010 workshop and through its literature review, the committee realized that many research efforts have been completed recently and that ongoing research efforts in this area continue. The challenge will be to sustain these efforts and to broaden them into areas that will result in wearable and effective PPE for healthcare personnel. Box 3-1 highlights the committee’s findings in this area.
-
Wearability:
-
Respirators: Continue examining the features of N95s, PAPRs, and elastomeric respirators that impact comfort and tolerability among healthcare personnel. Identify alterations in respirator design and construction that show promise in improving problem features that adversely impact comfort and tolerability.
-
Other PPE: Initiate research to identify factors affecting the comfort and tolerability of protective eyewear and clothing, and identify changes having the potential to positively influence comfort and tolerability. Evaluate differences between short- and long-term use of PPE as it affects comfort and tolerability. Develop and field test new designs and features for PPE for healthcare personnel that offer potential for improving comfort and tolerability.
-
-
Decontamination and Feasibility of Reuse:
-
Decontamination methods: Continue to assess promising decontamination methods for all types of PPE, including research on the impact of decontamination methods on respirator protection and on the physical characteristics of
-
|
BOX 3-1 Findings
|
-
-
the respirator (inner, middle, and outer layers). Assess decontamination effectiveness using either influenza virus or a suitable surrogate.
-
Feasibility of reuse: Develop a protocol for donning and doffing PPE to minimize self-inoculation.
-
-
TIL and Protection:
-
TIL: Finish development of the TIL certification requirements for half-mask air-purifying respirators. Assess TIL of very small particles (< 100 nm) with respirators.
-
Face masks and face shields: Assess the TIL of face masks against droplet spray. Conduct research using manned and unmanned tests to determine if face shields can offer suitable alternative protection to goggles and/or face masks to protect healthcare personnel against droplet spray.
-
Fit: Evaluate the impact of facepiece materials and design on improving the fit of filtering facepiece respirators. Develop improved and simpler fit testing methods. Examine the effectiveness of performing a user seal check for an N95 respirator each time it is donned.
-
Workplace protection studies: Conduct workplace protection studies to assess protection during typical tasks and time
-
-
changes in protection. Determine how using typical instruments impacts protection, and identify integration issues.
-
Equipment and Technologies:
-
Integration: Conduct human factors (field of view, visual acuity, communication) and operational performance studies to assess the ability of healthcare personnel to perform medical procedures in typical healthcare-specific PPE ensembles.
-
New technologies: Continue development of an air-purifying respirator that specifically addresses the needs of healthcare personnel. New materials and technologies should be developed specifically for filtering facepiece respirators to improve fit, comfort, and tolerability. A new low-noise, lightweight, PAPR and a face shield that is reusable and easy to clean should be designed and developed. The efficacy and effectiveness of antiviral-coated PPE and impacts on maintenance and reuse of PPE should be assessed.
-
RECOMMENDATIONS
Recommendation: Continue and Expand Research on PPE for Healthcare Personnel
NPPTL and other agencies, private-sector companies, and other organizations should continue to advance research in designing and evaluating the effectiveness of respirator protection for healthcare personnel and expand its research efforts to improve and evaluate the effectiveness of gloves, gowns, eye protection, face shields, and face masks in preventing the transmission of influenza or other viral respiratory diseases. Areas of focused research needs include
-
effectiveness in preventing fomite, droplet spray, or aerosol transmission;
-
decontamination and feasibility of reuse;
-
comfort, fit, and usability;
-
impact on task performance; and
-
development of technologies specifically for healthcare personnel.
Recommendation: Improve Fit Test Methods and Evaluate User Seal Checks
NPPTL should develop novel, simpler fit test methods and evaluate the effectiveness of performing user seal checks on N95 respirators.
Recommendation: Develop and Certify PAPRs for Healthcare Personnel
NPPTL should develop certification requirements for a low-noise, loose-fitting PAPR for healthcare personnel.
Recommendation: Examine the Effectiveness of Face Masks and Face Shields as PPE
NPPTL should investigate the effectiveness of face masks and face shields in preventing transmission of viral respiratory diseases.
REFERENCES
Au, S. S., C. D. Gomersall, P. Leung, and P. T. Li. 2010. A randomised controlled pilot study to compare filtration factor of a novel non-fit-tested high-efficiency particulate air (HEPA) filtering facemask with a fit-tested N95 mask. Journal of Hospital Infection 76(1):23-25.
Baig, A. S., C. Knapp, A. E. Eagan, and L. J. Radonovich, Jr. 2010. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. American Journal of Infection Control 38(1):18-25.
Bansal, S., P. Harber, D. Yun, D. Liu, Y. Liu, S. Wu, D. Ng, and S. Santiago. 2009. Respirator physiological effects under simulated work conditions. Journal of Occupational and Environmental Hygiene 6(4):221-227.
Borkow, G., S. S. Zhou, T. Page, and J. Gabbay. 2010. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 5(6):e11295.
Brinker, A., S. A. Gray, and J. Schumacher. 2007. Influence of air-purifying respirators on the simulated first response emergency treatment of CBRN victims. Resuscitation 74(2):310-316.
Brosseau, L. M. 2009. Toward better fitting respirators: No fit test respirator workshop and research roadmap, RFQ 2008-Q-10205. http://www.sph.umn.edu/ce/presentations/docs/Final_NoFitRespiratorReport_July28_2009.pdf (accessed November 30, 2010).
Brosseau, L. M., and K. Harriman. 2007. Commentary on “in vivo protective performance of N95 respirator and surgical facemask.” American Journal of Industrial Medicine 50(12):1025-1026; author reply 1027-1029.
Caillot, J. L., and E. J. Voiglio. 2008. First clinical study of a new virus-inhibiting surgical glove. Swiss Medical Weekly 138(1-2):18-22.
Casanova, L., W. A. Rutala, D. J. Weber, and M. D. Sobsey. 2009. Methods for the recovery of a model virus from healthcare personal protective equipment. Journal of Applied Microbiology 106(4):1244-1251.
Castle, N., R. Owen, M. Hann, S. Clark, D. Reeves, and I. Gurney. 2009. Impact of chemical, biological, radiation, and nuclear personal protective equipment on the performance of low- and high-dexterity airway and vascular access skills. Resuscitation 80(11):1290-1295.
Chan, M. C., R. W. Chan, W. C. Yu, C. C. Ho, K. M. Yuen, J. H. Fong, L. L. Tang, W. W. Lai, A. C. Lo, W. H. Chui, A. D. Sihoe, D. L. Kwong, D. S. Wong, G. S. Tsao, L. L. Poon, Y. Guan, J. M. Nicholls, and J. S. Peiris. 2010. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. American Journal of Pathology 176(4):1828-1840.
Cho, K. J., T. Reponen, R. McKay, R. Shukla, H. Haruta, P. Sekar, and S. A. Grinshpun. 2010. Large particle penetration through N95 respirator filters and facepiece leaks with cyclic flow. Annals of Occupational Hygiene 54(1):68-77.
Coffey, C. C., R. B. Lawrence, D. L. Campbell, Z. Zhuang, C. A. Calvert, and P. A. Jensen. 2004. Fitting characteristics of eighteen N95 filtering-facepiece respirators. Journal of Occupational and Environmental Hygiene 1(4):262-271.
Davies, C. G., M. N. Khan, A. S. Ghauri, and C. J. Ranaboldo. 2007. Blood and body fluid splashes during surgery—the need for eye protection and masks. Annals of the Royal College of Surgeons of England 89(8):770-772.
Department of Homeland Security. 2009. Noise cancellation for voice operated switch (VOX) communications: Small Business Innovation Research project topic number H-SB09.2-006. https://www.sbir.dhs.gov/reference/DHS_SBIR-2009.2_Full_Solicitation.doc (accessed November 4, 2010).
Department of Veterans Affairs. 2010. National Center for Occupational Health and Infection Control: Key projects. http://www.publichealth.va.gov/employeehealth/epidemiccontrol/projects.asp#breathe (accessed November 4, 2010).
Diaz, K. T., and G. C. Smaldone. 2010. Quantifying exposure risk: Surgical masks and respirators. American Journal of Infection Control 38(7):501-508.
Duling, M. G., R. B. Lawrence, J. E. Slaven, and C. C. Coffey. 2007. Simulated workplace protection factors for half-facepiece respiratory protective devices. Journal of Occupational and Environmental Hygiene 4(6):420-431.
Eninger, R. M., T. Honda, A. Adhikari, H. Heinonen-Tanski, T. Reponen, and S. A. Grinshpun. 2008a. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Annals of Occupational Hygiene 52(5):385-396.
Eninger, R. M., T. Honda, T. Reponen, R. McKay, and S. A. Grinshpun. 2008b. What does respirator certification tell us about filtration of ultrafine particles? Journal of Occupational and Environmental Hygiene 5(5):286-295.
Eshbaugh, J. P., P. D. Gardner, A. W. Richardson, and K. C. Hofacre. 2009. N95 and P100 respirator filter efficiency under high constant and cyclic flow. Journal of Occupational and Environmental Hygiene 6(1):52-61.
Federal Register. 2009. Notice: Department of Veterans Affairs: Project Better Respiratory Equipment Using Advanced Technologies for Healthcare Employees (B.R.E.A.T.H.E.). http://frwebgate3.access.gpo.gov/cgi-bin/PDFgate.cgi?WAISdocID=ceFTAw/1/2/0&WAISaction=retrieve (accessed November 4, 2010).
Fisher, E., and R. Shaffer. 2010. Survival of bacteriophage MS2 on filtering facepiece respirator coupons. Applied Biosafety 15(2):71-76.
Fisher, E., S. Rengasamy, D. Viscusi, E. Vo, and R. Shaffer. 2009. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Applied and Environmental Microbiology 75(6):1500-1507.
Fouchier, R. A. M., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. G. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. J. van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. M. E. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proceedings of the National Academy of Sciences (U.S.A.) 101(5):1356-1361.
Fry, D. E., W. E. Harris, E. N. Kohnke, and C. L. Twomey. 2010. Influence of double-gloving on manual dexterity and tactile sensation of surgeons. Journal of the American College of Surgeons 210(3):325-330.
Grinshpun, S. A., H. Haruta, R. M. Eninger, T. Reponen, R. T. McKay, and S. A. Lee. 2009. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: Two pathways for particle penetration. Journal of Occupational and Environmental Hygiene 6(10):593-603.
Hah, S., T. Yuditsky, K. A. Schulz, H. Dorsey, A. R. Deshmukh, and J. Sharra. 2009. Evaluation of human performance while wearing respirators. http://hf.tc.faa.gov/technotes/dot-faa-tc-09-10.pdf (accessed November 3, 2010).
Harber, P., S. Bansal, S. Santiago, D. Liu, D. Yun, D. Ng, Y. Liu, and S. Wu. 2009. Multidomain subjective response to respirator use during simulated work. Journal of Occupational and Environmental Medicine 51(1):38-45.
Heimbuch, B. K., K. Kinney, B. Nichols, and J. D. Wander. 2009. The dry aerosol deposition device (DADD): An instrument for depositing microbial aerosols onto surfaces. Journal of Microbiological Methods 78(3):255-259.
Huang, J. T., and V. I. Huang. 2007. Evaluation of the efficiency of medical masks and the creation of new medical masks. Journal of International Medical Research 35(2):213-223.
Hubner, N. O., A. M. Goerdt, N. Stanislawski, O. Assadian, C. D. Heidecke, A. Kramer, and L. I. Partecke. 2010. Bacterial migration through punctured surgical gloves under real surgical conditions. BMC Infectious Diseases 10:192.
IOM (Institute of Medicine). 2008. Preparing for an influenza pandemic: Personal protective equipment for healthcare workers. Washington, DC: The National Academies Press.
Janssen, L. L., T. J. Nelson, and K. T. Cuta. 2007. Workplace protection factors for an N95 filtering facepiece respirator. Journal of Occupational and Environmental Hygiene 4(9):698-707.
Johnson, A. T., F. C. Koh, S. Jamshidi, and T. E. Rehak. 2008. Human subject testing of leakage in a loose-fitting papr. Journal of Occupational and Environmental Hygiene 5(5):325-329.
Johnson, D. F., J. D. Druce, C. Birch, and M. L. Grayson. 2009. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clinical Infectious Diseases 49(2):275-277.
Jones, C., B. Brooker, and M. Genon. 2010. Comparison of open and closed staff-assisted glove donning on the nature of surgical glove cuff contamination. Australian and New Zealand Journal of Surgery 80(3):174-177.
Korniewicz, D. M., and M. El Masri. 2007. Effect of aloe-vera impregnated gloves on hand hygiene attitudes of health care workers. MEDSURG Nursing 16(4):247-252.
Laing, R. M. 2008. Protection provided by clothing and textiles against potential hazards in the operating theatre. International Journal of Occupational and Safety Ergonomics 14(1):107-115.
Lee, J. H., C. Y. Wu, K. M. Wysocki, S. Farrah, and J. Wander. 2008a. Efficacy of iodine-treated biocidal filter media against bacterial spore aerosols. Journal of Applied Microbiology 105(5):1318-1326.
Lee, J. H., C. Y. Wu, C. N. Lee, D. Anwar, K. M. Wysocki, D. A. Lundgren, S. Farrah, J. Wander, and B. K. Heimbuch. 2009. Assessment of iodine-treated filter media for removal and inactivation of MS2 bacteriophage aerosols. Journal of Applied Microbiology 107(6):1912-1923.
Lee, M. C., S. Takaya, R. Long, and A. M. Joffe. 2008b. Respirator-fit testing: Does it ensure the protection of healthcare workers against respirable particles carrying pathogens? Infection Control and Hospital Epidemiology 29(12):1149-1156.
Lee, S. A., S. A. Grinshpun, and T. Reponen. 2008c. Respiratory performance offered by N95 respirators and surgical masks: Human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Annals of Occupational Hygiene 52(3):177-185.
Li, Y., T. Wong, J. Chung, Y. P. Guo, J. Y. Hu, Y. T. Guan, L. Yao, Q. W. Song, and E. Newton. 2006. In vivo protective performance of N95 respirator and surgical facemask. American Journal of Industrial Medicine 49(12):1056-1065.
Manian, F. A., and J. J. Ponzillo. 2007. Compliance with routine use of gowns by healthcare workers (HCWs) and non-HCW visitors on entry into the rooms of patients under contact precautions. Infection Control and Hospital Epidemiology 28(3):337-340.
Mansour, A. A., 3rd, J. L. Even, S. Phillips, and J. L. Halpern. 2009. Eye protection in orthopaedic surgery. An in vitro study of various forms of eye protection and their effectiveness. Journal of Bone and Joint Surgery 91(5):1050-1054.
McMahon, E., K. Wada, and A. Dufresne. 2008. Implementing fit testing for N95 filtering facepiece respirators: Practical information from a large cohort of hospital workers. American Journal of Infection Control 36(4):298-300.
Mendel, L. L., J. A. Gardino, and S. R. Atcherson. 2008. Speech understanding using surgical masks: A problem in health care? Journal of the American Academy of Audiology 19(9):686-695.
Monaghan, W. D., M. R. Roberge, M. Rengasamy, and R. J. Roberge. 2009. Thermal imaging comparison of maximum surface temperatures achieved on N95 filtering facepiece respirators with and without exhalation valves at sedentary breathing volumes. Journal of the International Society for Respiratory Protection 26:12-19.
Newman, J. B., M. Bullock, and R. Goyal. 2007. Comparison of glove donning techniques for the likelihood of gown contamination. An infection control study. Acta Orthopaedica Belgica 73(6):765-771.
Oberg, T., and L. M. Brosseau. 2008. Surgical mask filter and fit performance. American Journal of Infection Control 36(4):276-282.
Oestenstad, R. K., and A. A. Bartolucci. 2010. Factors affecting the location and shape of face seal leak sites on half-mask respirators. Journal of Occupational and Environmental Hygiene 7(6):332-341.
Oestenstad, R. K., L. J. Elliott, and T. M. Beasley. 2007. The effect of gender and respirator brand on the association of respirator fit with facial dimensions. Journal of Occupational and Environmental Hygiene 4(12):923-930.
Oxford, J. S., R. Lambkin, M. Guralnik, R. A. Rosenbloom, M. P. Petteruti, K. Digian, and C. Lefante. 2007. Preclinical in vitro activity of QR-435 against influenza A virus as a virucide and in paper masks for prevention of viral transmission. American Journal of Therapeutics 14(5):455-461.
Partecke, L. I., A. M. Goerdt, I. Langner, B. Jaeger, O. Assadian, C. D. Heidecke, A. Kramer, and N. O. Huebner. 2009. Incidence of microperforation for surgical gloves depends on duration of wear. Infection Control and Hospital Epidemiology 30(5):409-414.
Radonovich, L. J., Jr., J. Cheng, B. V. Shenal, M. Hodgson, and B. S. Bender. 2009. Respirator tolerance in health care workers. Journal of the American Medical Association 301(1):36-38.
Radonovich, L. J., Jr., R. Yanke, J. Cheng, and B. Bender. 2010. Diminished speech intelligibility associated with certain types of respirators worn by healthcare workers. Journal of Occupational and Environmental Hygiene 7(1):63-70.
Reinertsen, R. E., H. Faerevik, K. Holbo, R. Nesbakken, J. Reitan, A. Royset, and M. Suong Le Thi. 2008. Optimizing the performance of phase-change materials in personal protective clothing systems. International Journal of Occupational Safety and Ergonomics 14(1):43-53.
Rengasamy, S., W. P. King, B. C. Eimer, and R. E. Shaffer. 2008. Filtration performance of NIOSH-approved N95 and P100 filtering facepiece respirators against 4 to 30 nanometer-size nanoparticles. Journal of Occupational and Environmental Hygiene 5(9):556-564.
Rengasamy, S., B. C. Eimer, and R. E. Shaffer. 2009. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Annals of Occupational Hygiene 53(2):117-128.
Rengasamy, S., B. Eimer, and R. E. Shaffer. 2010a. Simple respiratory protection—evaluation of the filtration performance of cloth masks and common fabric materials against 20-1000 nm size particles. Annals of Occupational Hygiene 54(7):789-798.
Rengasamy, S., E. Fisher, and R. E. Shaffer. 2010b. Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. American Journal of Infection Control 38(1):9-17.
Rissanen, S., I. Jousela, J. R. Jeong, and H. Rintamaki. 2008. Heat stress and bulkiness of chemical protective clothing impair performance of medical personnel in basic lifesaving tasks. Ergonomics 51(7):1011-1022.
Roberge, M. R., M. R. Vojtko, R. J. Roberge, R. J. Vojtko, and D. P. Landsittel. 2008. Wearing an N95 respirator concurrently with a powered air-purifying respirator: Effect on protection factor. Respiratory Care 53(12):1685-1690.
Roberge, R. J. 2008. Evaluation of the rationale for concurrent use of N95 filtering facepiece respirators with loose-fitting powered air-purifying respirators during aerosol-generating medical procedures. American Journal of Infection Control 36(2):135-141.
———. 2009. Physiological burden associated with the use of filtering facepiece respirators (N95 masks) during pregnancy. Journal of Women’s Health 18(6):819-826.
Roberge, R. J., A. Coca, W. J. Williams, A. J. Palmiero, and J. B. Powell. 2010a. Surgical mask placement over N95 filtering facepiece respirators: Physiological effects on healthcare workers. Respirology 15(3):516-521.
Roberge, R. J., A. Coca, W. J. Williams, J. B. Powell, and A. J. Palmiero. 2010b. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respiratory Care 55(5):569-577.
———. 2010c. Reusable elastomeric air-purifying respirators: Physiologic impact on health care workers. American Journal of Infection Control 38(5):381-386.
Salter, W. B., K. Kinney, W. H. Wallace, A. E. Lumley, B. K. Heimbuch, and J. D. Wander. 2010. Analysis of residual chemicals on filtering facepiece respirators after decontamination. Journal of Occupational and Environmental Hygiene 7(8):437-445.
Schumacher, J., J. Runte, A. Brinker, K. Prior, M. Heringlake, and W. Eichler. 2008. Respiratory protection during high-fidelity simulated resuscitation of casualties contaminated with chemical warfare agents. Anaesthesia 63(6):593-598.
Schumacher, J., S. A. Gray, L. Weidelt, A. Brinker, K. Prior, and W. M. Stratling. 2009. Comparison of powered and conventional air-purifying respirators during simulated resuscitation of casualties contaminated with hazardous substances. Emergency Medicine Journal 26(7):501-505.
Shaffer, R., and S. Rengasamy. 2009. Respiratory protection against airborne nanoparticles: A review. Journal of Nanoparticle Research 11(7):1661-1672.
Tang, J. W., T. J. Liebner, B. A. Craven, and G. S. Settles. 2009. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. Journal of the Royal Society Interface 6(Suppl. 6):S727-S736.
Udayasiri, R., J. Knott, D. T. D. Mc, J. Papson, F. Leow, and F. A. Hassan. 2007. Emergency department staff can effectively resuscitate in level C personal protective equipment. Emergency Medicine in Australasia 19(2):113-121.
Viscusi, D., W. King, and R. Shaffer. 2007. Effect of decontamination on the filtration efficiency of two FFR models. Journal of the International Society for Respiratory Protection 24:93-107.
Viscusi, D. J., M. Bergman, E. Sinkule, and R. E. Shaffer. 2009a. Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. American Journal of Infection Control 37(5):381-386.
Viscusi, D. J., M. S. Bergman, B. C. Eimer, and R. E. Shaffer. 2009b. Evaluation of five decontamination methods for filtering facepiece respirators. Annals of Occupational Hygiene 53(8):815-827.
Vo, E., S. Rengasamy, and R. Shaffer. 2009. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Applied and Environmental Microbiology 75(23):7303-7309.
Vojtko, M. R., M. R. Roberge, R. J. Vojtko, R. J. Roberge, and D. P. Landsittel. 2008. Effect on breathing resistance of a surgical mask worn over a N95 filtering facepiece respirator. Journal of the International Society for Respiratory Protection 25:1-8.
Wander, J., and B. Heimbuch. 2009. Challenge of N95 and P100 filtering facepiece respirators with particle containing viable H1N1. Pittsburgh, PA: National Institute for Occupational Safety and Health.
Watson, C. M., M. C. McCrory, J. M. Duval-Arnould, and E. A. Hunt. 2009. Abstract P212: Simulated pediatric resuscitation during novel H1N1 influenza outbreak. Circulation 120:S1487-S1488.
Watson, L., W. Sault, R. Gwyn, and P. R. Verbeek. 2008. The “delay effect” of donning a gown during cardiopulmonary resuscitation in a simulation model. Canadian Journal of Emergency Medical Care 10(4):333-338.
Wilkinson, I. J., D. Pisaniello, J. Ahmad, and S. Edwards. 2010. Evaluation of a large-scale quantitative respirator-fit testing program for healthcare workers: Survey results. Infection Control and Hospital Epidemiology 31(9):918-925.
Wilson, J. A., H. P. Loveday, P. N. Hoffman, and R. J. Pratt. 2007. Uniform: An evidence review of the microbiological significance of uniforms and uniform policy in the prevention and control of healthcare-associated infections. Report to the Department of Health (England). Journal of Hospital Infection 66(4):301-307.
Wines, M. P., A. Lamb, A. N. Argyropoulos, A. Caviezel, C. Gannicliffe, and D. Tolley. 2008. Blood splash injury: An underestimated risk in endourology. Journal of Endourology 22(6):1183-1187.
Winter, S., J. H. Thomas, D. P. Stephens, and J. S. Davis. 2010. Particulate face masks for protection against airborne pathogens—one size does not fit all: An observational study. Critical Care and Resuscitation 12(1):24-27.
Zhuang, Z., B. Bradtmiller, and R. E. Shaffer. 2007. New respirator fit test panels representing the current U.S. civilian work force. Journal of Occupational and Environmental Hygiene 4(9):647-659.
Zhuang, Z., D. Groce, H. W. Ahlers, W. Iskander, D. Landsittel, S. Guffey, S. Benson, D. Viscusi, and R. E. Shaffer. 2008. Correlation between respirator fit and respirator fit test panel cells by respirator size. Journal of Occupational and Environmental Hygiene 5(10):617-628.










































