6
Treatment of Drug-Resistant TB
Key Messages
- Aggressive drug therapy can increase the range of options for MDR TB patients.
- HIV infection is a major driving force behind the TB epidemic in adult populations.
- Adherence is the key to treatment success for both TB and HIV.
- The combination of aggressive drug treatment and surgery, which is widely used in Russia, can improve the outcomes of patients with MDR TB.
- An important component of an effective public health treatment program is the promotion of scientific research into new technologies and methods of diagnosing and treating patients and the rapid incorporation of scientific innovations into the program.
Clinical care is an important part of any meaningful response to drug-resistant TB. Even with XDR TB, Farmer noted, there is no escaping the clinical imperative of treating patients.
Farmer stated that XDR TB is not untreatable but is very difficult to treat, and failure rates are high even with the highest standard of care, which includes aggressive regimens that often must be tailored to patients on the basis of laboratory data. Yet the laboratory data needed to tailor
treatments often are difficult to obtain in those settings where drug-resistant TB takes its greatest toll. Other data also affect treatment regimens. For example, the degree of parenchymal damage should determine the duration and form of therapy; Russian physicians have demonstrated the importance in some cases of adjuvant surgery (see Chapter 3 and the further discussion of this approach later in this chapter). XDR TB is not a new genus or species, said Farmer. The disease is ultimately TB, and the issues that have been discussed for many years with regard to MDR TB apply also to XDR TB.
High-quality clinical care must keep pace not just with genetic mutations but also with social mutations, said Farmer. Social mutations in the Soviet Union, the United States, and other countries have been partially responsible for TB outbreaks in the past, which have consumed major resources before being brought under control. Yet the system of clinical care for drug-resistant TB has seen little innovation over the last 10 years. Most new drugs are really variants of old ones; no new class of drugs has made it through clinical trials and regulatory processes during this period. A few novel agents are in the drug development pipeline, but access to them for treatment is not yet close. Thus, as many workshop participants noted, MDR TB treatment must be provided with what is available today, tailored to the drug susceptibility patterns of patients.
Speakers addressed several topics related to treatment of drug-resistant TB: the need for tailored treatment regimens based on drug susceptibility testing, treatment of drug-resistant TB in the Russian Federation, treatment of patients coinfected with TB and HIV, and innovative research in MDR TB treatment.
PETTS: MAKING THE CASE FOR DRUG RESISTANCE TESTING AND TAILORED TREATMENT REGIMENS1
M.tb. has developed resistance within a few years to every anti-TB drug introduced in the past, from streptomycin and isoniazid in the 1940s and 1950s, to rifampin in the 1960s and 1970s, to the quinolones in the 1990s. As a result, said Cegielski, the GLC was established with three goals: (1) increase access to effective treatment of MDR TB with quality-assured second-line drugs, (2) prevent increasing resistance to those same drugs, and (3) contribute to the evidence base for policy guidelines.
Evaluating the GLC’s impact on preventing drug resistance was the stimulus for the Preserving Effective TB Treatment Study (PETTS), which has the objective of determining the frequency of and risk factors for acquired resistance to second-line drugs in a diverse group of MDR TB programs. The study is examining the characteristics of programs (includ-
![]()
1 This section is based on the presentation of Dr. Cegielski.
ing whether they were approved by the GLC), of patients, of the bacteria, and of the treatments received by patients. It also is seeking to determine the consequences for patients of acquired resistance to second-line drugs.
PETTS is a prospective follow-up study of MDR TB patients in nine countries: Estonia, Latvia, Peru, the Philippines, Russia, South Africa, South Korea, Taiwan, and Thailand. Consecutive consenting adults with pulmonary MDR TB, confirmed locally, are being enrolled at the start of treatment with second-line drugs. In other words, all eligible patients are informed about the study and invited to participate at the same time that they are being evaluated and started on treatment. The goal is to have the patients included in the study be representative of the patient population in general. Study participants have a baseline sputum culture taken within 30 days of the start of treatment, and follow-up sputum cultures are then taken monthly for 2 years or until treatment is complete. For consistency, all cultures are shipped to the CDC for centralized analysis.
The strategy is to compare the drug susceptibility test results for the first and last positive cultures from each patient. These isolates are tested for susceptibility to 12 drugs. If the drug susceptibilities have changed, those isolates are genotyped. If the genotypes are the same, acquired resistance is assumed; if the genotypes are different, resistance is most likely related to the strain differences.
PETTS sites are in the nine countries, with the global coordinating center being located in Atlanta at CDC. Enrollment for the study ended in December 2008, with viable and uncontaminated baseline cultures having been received from 1,398 patients. The patients differed considerably by site, but overall, 19 percent were HIV-infected (with 34 percent having uncertain HIV status), 13 percent had diabetes, 53 percent were hospitalized at the start of treatment, 83 percent were smear positive, 61 percent had cavitary lung disease, and 16 percent were cases never previously treated. Most of the patients, 71 percent, had been treated before with first-line drugs; a smaller percentage, 13 percent, had been treated before with second-line drugs.
Of the total group, 1,278 patients had confirmed MDR TB, with corresponding clinical data. Confirmation of results of local drug susceptibility testing as MDR TB at CDC’s laboratories was 96−100 percent for five countries and 84–90 percent for three countries.
A large number of these patients already had showed resistance to second-line drugs at the start of treatment. Resistance was 11 percent for the quinolones, 11−18 percent for the injectable agents, close to 20 percent for ethionamide, and almost 10 percent for para-aminosalycylic acid. Resistance to second-line drugs varied greatly from country to country—for example, from 4 to 31 percent for ethionamide. The overall high level and
diversity of drug resistance found at baseline suggests that standardized approaches to treating MDR TB are not advisable, said Cegielski.
Patients also were resistant to drug combinations. For example, half of the patients showed resistance to all four first-line drugs. More than 40 percent showed resistance to at least one second-line drug, with a range of 33 to 62 percent. Twenty percent showed resistance to at least one of the injectables (kanamycin, amikacin, and capreomycin), 10 percent to all three of the injectables, and 11 percent to the quinolones. Six percent of patients had XDR TB at the start of treatment. Again, the range of resistance across sites was broad, indicating that the treatment of MDR TB should be tailored to the unique epidemiology of specific populations and ideally individualized to patients’ susceptibility patterns.
Because these were all MDR TB patients, isoniazid and rifampicin could not be used for treatment. More than 60 percent of the patients already showed resistance to ethambutol and streptomycin, and 10−20 percent already showed resistance to at least one of the main drugs used in treating MDR TB. Ignoring this preexisting resistance would contribute to the development of XDR and TDR TB, said Cegielski.
Almost half of the patients were susceptible to a higher concentration of isoniazid, reflecting recent results on the efficacy of high-dose isoniazid, and 30 percent showed susceptibility to rifabutin. Microbiologists argue about whether these results are an artifact of testing or have in vivo significance, but Cegielski believes that these strategies should be tested in controlled clinical trials. He contends that we do not need to wait for new drugs to begin developing better treatments for MDR TB; rather, we can use drugs that are already approved and on the market more effectively. Similarly, of the patients whose TB was resistant to kanamycin, many were infected with strains susceptible to amikacin and capreomycin. Cegielski emphasized that these are in vitro results that need to be studied in controlled clinical trials.
When the number of drugs to which patients showed resistance is subtracted from the 12 that were tested, 4 potentially effective drugs remained for 70 percent of patients, which the WHO deems the minimum adequate treatment for MDR TB. If more aggressive and novel approaches are considered, the proportion of patients with the potential to respond to four effective drugs would hypothetically increase to well over 90 percent. Partners In Health has shown in projects around the world that treating patients more aggressively with more drugs yields higher cure rates. Especially given the toxicities of second-line drugs, being able to choose from a number of drugs is important.
Cegielski briefly discussed follow-up data on 477 isolates. The isolates have not yet been genotyped, so it is not known whether resistance was acquired, but the resistance patterns are available. From 10 to 18 percent of patients showed resistance to a second-line agent on their last posi-
tive culture that was not present at the beginning of treatment. Among patients who showed no fluoroquinolone resistance at the start of treatment, 11 percent exhibited such resistance on their last culture. Among those who initially showed no resistance to the injectables, 18 percent did so at the end of treatment. Among those who initially showed no second-line drug resistance at all, 17 percent showed resistance to at least one of the second-line drugs at the end of treatment. Also, 10 percent of patients who did not have XDR TB at the start of treatment had it at the end of the study period.
TREATMENT OF DRUG-RESISTANT TB IN THE RUSSIAN FEDERATION2
Vasilyeva reported on the treatment of MDR and XDR TB patients at the CTRI, Russian Academy of Medical Sciences, in Moscow. Treatment efficacy in newly detected TB patients in the Russian Federation was 57.8 percent in 2008. CTRI has 80 beds in its MDR TB department and another 50 beds in its TB department. MDR and XDR TB accounted for 40.6 and 2.2 percent, respectively, of cases at CTRI in 2008. Among previously treated patients at CTRI, 61.6 percent had MDR TB and 16.5 percent XDR TB.
As noted earlier, Russian TB patients are often treated with surgery aimed at healing lung cavities. According to Vasilyeva, when surgery (i.e., collapse therapy, artificial pneumothorax and/or pneumoperitoneum) is combined with chemotherapy based on drug susceptibility testing, better results can be obtained than with chemotherapy alone. Vasilyeva reported that treatment with chemotherapy and surgery at her institution was found to be 83 percent effective (sputum conversion) after 8 months, compared with 44 percent effectiveness for chemotherapy alone.
To illustrate, Vasilyeva presented a case study of a 27-year-old male patient who had been ill for 3 years and for whom previous therapy had produced no effect. When treated surgically and with aggressive chemotherapy based on the results of drug susceptibility testing, the patient had a negative culture and began putting on weight. In a second case study, a 21-year-old woman had been ill for 3 years with no effect of treatment, and an x-ray showed that her right lung had been destroyed. After a year of being prepared for surgery, she underwent the surgery and was treated with chemotherapy for 15 more months. At her 18-month follow-up, she was stable and culture negative.
According to Vasilyeva, treatment success depended on the length
![]()
2 This section is based on the presentation of Irina Vasilyeva, Central TB Research Institute, Russian Academy of Medical Sciences.
of time until diagnosis of drug-resistant TB, as well as the quality of the diagnostic tool. As CTRI implemented rapid biochip methods of MDR/ XDR TB diagnosis within 24 hours after sputum collection, the effectiveness of MDR TB treatment increased significantly as a result of the timely administration of second-line drugs. Vasilyeva concluded that the success of MDR TB treatment depends on the point at which MDR or XDR TB is detected, adequate long-term treatment regimens, the quality of second-line drugs, the use of surgical methods, and the early management of adverse drug effects.
DRUG-RESISTANT TB AND COINFECTION WITH HIV3
HIV is a driving force of today’s TB epidemic, said Shin. It increases the risk of contracting TB, the risk of having disease that is drug resistant, and the risk of excess morbidity and mortality. Shin suggested that HIV infection is a major factor in the failure to control TB. Another driving force of the epidemic in many locations is the underlying vulnerability of particular populations. For example, TB and HIV infection can be intertwined with physical and mental problems, including those caused by substance abuse (see Chapter 7).
Shin discussed her experiences in Peru, where a cohort of patients treated from 1996 through the end of 2005 consisted of about 100 patients infected with HIV who were receiving treatment for MDR TB. This cohort served as something of a natural experiment, said Shin, because a supply of antiretroviral drugs for this group became available only in 2004. Most of the patients had been previously treated for TB, and the majority had AIDS, with a mean CD4 cell count of 181.
A minority of the patients were cured, with death occurring in about half of the cohort. Survival was better among those who received antiretrovirals. Shin emphasized that immune reconstitution is essential if MDR TB is to be brought under control, even though adding antiretrovirals to complex anti-TB regimens is difficult. Indeed, the literature now advocates that treatment with antiretrovirals begin as quickly and aggressively as possible.
Adherence is the key to treatment success for both TB and HIV. According to Shin, adherence must be greater than 95 percent to avoid excessive virologic failure with HIV infection, although the actual percentage varies with the types of antiretrovirals used. One potential advantage of treating a coinfected population is that an aggressive approach to achieving adherence to TB therapy can help ensure that the patient also maintains adherence
![]()
3 This section is based on the presentations of Sonya Shin, Harvard Medical School and Brigham and Women’s Hospital, and Olga Frolova, Federal TB Healthcare Delivery Center for HIV-Infected Patients, Russian Federation.
to antiretroviral therapy (ART). In Peru, for instance, community-based directly observed therapy programs reduced mortality more for people on both therapies than for those on TB therapy alone. Directly observed therapy for coinfected patients also reduced hospital admissions. Patients who received this kind of community support required only five hospital days per person-year versus 15 for other patients, thus saving costs as well.
In Russia, said Frolova, the monitoring of TB and HIV infection is combined, with the resulting data being delivered to the TB specialist responsible for HIV coinfection in the area. In a general hospital, data on such cases are transferred to this specialist, as are reports on such cases from autopsies. Confidentiality is ensured by having just one person in charge of the data, and the data are encoded. Through this system, information on patients coinfected with TB and HIV is available for all of Russia.
In a review of drug susceptibility data for about 4,500 HIV-positive patients, resistance to one drug was found in 11 percent and resistance to two or more drugs in 55 percent (about half of whom also showed resistance to rifampicin and isoniazid), and 34 percent remained drug-susceptible. Among coinfected patients, three-quarters of men and just more than half women had become infected by HIV through injecting drug use. This is an important group, said Frolova, because they can leave institutions, move to metropolitan areas, and infect other people in the community.
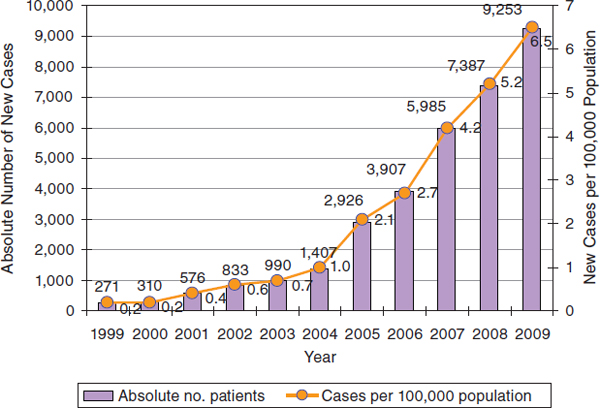
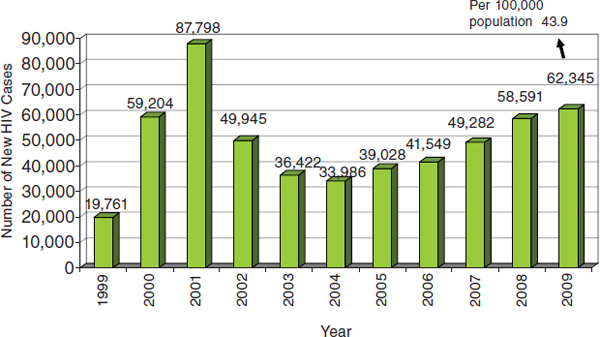
Frolova stated that the number of new cases of TB-HIV coinfection has been increasing over the past decade, to more than 6.5 per 100,000 population in 2009 (Figure 6-1). Similarly, the number of new cases of HIV infection in the Russian Federation has been increasing in recent years following a decline early in the 2000s (Figure 6-2). The direct cause of death for more than half of HIV-infected patients is TB.
According to Frolova, most HIV-infected patients in the Russian Federation are examined for TB. In 2009, more than 15,000 people with HIV received TB treatment, including more than 7,000 who received ART. Among those receiving ART, 12 percent were simultaneously undergoing anti-TB therapy.
Frolova cited several challenges related to treatment of coinfected patients. For example, international guidelines call for ART to be given to patients with extrapulmonary TB, but there is no universal definition of extrapulmonary TB. At the same time, patients are supposed to be given numerous antiretroviral and anti-TB drugs, but Frolova expressed concern that many suffer from severe liver disorders that interfere with drug treatment. In general, she said, there is little experience in Russia to call on in the use of antiretroviral drugs in patients coinfected with TB and HIV.
To stop the spread of TB-HIV coinfection, said Frolova, the Russian Federation must:
- develop and implement a clear and strict TB control plan;
- improve legislation allowing TB control measures to be implemented;
- open inpatient facilities and hostels for patients with TB and HIV coinfection; and
- devise a system for delivering TB care to HIV patients and drug addicts along with drug services.
INNOVATIVE RESEARCH IN MDR TB TREATMENT
Speakers described two areas of innovative research in MDR TB treatment: immunological and stem cell therapy and the use of bone marrow cells.
Immunological and Stem Cell Therapy in MDR TB Patients4
The advent of TDR TB demands new approaches to the treatment of TB, said Maeurer. He described several recent research advances that have brought the field closer to such treatments.
In a recent experiment using nonhuman primates, three groups of monkeys received different vaccines and then were challenged with virulent M.tb. The first group received saline solution and acted as a control. The second received a genetically altered Bacille Calmette Guérin (BCG) vaccine, and the third received normal BCG vaccine. The latter two groups survived well compared with the control group. To identify a correlate of success, Maeurer and colleagues surveyed the immunological reactions of the monkeys. The animals that survived had a very strong gamma-interferon response. But Maeurer observed that 4,500 other antigens also could be monitored as a measure of immunological success. The questions to be answered were which antigens should be chosen, which confer protection, and whether there are any other immune correlates in the T cell compartments.
To provide at least partial answers to these questions, Maeurer and colleagues examined the cytokines released by polyfunctional T cells, which are able to make three or four different cytokines simultaneously. Monkeys with T cells capable of producing gamma-interferon, tumor necrosis factor, and antileukin-2 at the same time were well protected. Again, Maeurer asked which other proteins could provide a good measure of resistance to TB.
As part of this work, Maeurer’s team used a method known as tetramer-
![]()
4 This section is based on the presentation of Markus Maeurer, Karolinska Institute, Stockholm, Sweden.
gated detection to examine a patient infected with TB and a second patient infected with another mycobacterium. The patient infected with TB had a very specific T cell response compared with the other patient. “This opens an entirely new avenue of immune diagnostic which is fast and robust and independent of cytokines,” said Maeurer.
To test a larger number of proteins, Maeurer’s group extracted proteins from serum, fragmented them, and screened them using a microarray containing tens of thousands of peptides. Using just 3 microliters of serum, they were able to identify correlates of TB infection and response to treatments. For example, specific peptides were detected in 34 of 34 people with TB but not in any healthy individuals. Individuals who are infected with TB, who are not infected, and who have varying reactions to TB treatments can all be identified quickly, robustly, and with a minimum amount of serum. “This powerful technology allows us … to decipher who is sick, who is healthy, and what happens if we treat a patient with novel therapies or if a patient receives a tuberculosis vaccine,” said Maeurer. It also is a way to identify drug treatment targets in patients who have XDR TB versus normal TB, which aids in clinical treatment and development and the measurement of success and failure.
Maeurer and his colleagues have also used IL-7 to treat patients with XDR TB. IL-7 is the most potent T cell survival factor and the most potent suppressor of TGF-beta. TGF-beta creates connective tissue, which interferes with breathing. The human immune system is “bathed” in IL-7, which is produced in the thymus, the kidney, and the intestines. When mice lack the ability to produce IL-7, they have combined severe immunodeficiency syndrome, even though the loss of other cytokines does not have such severe effects. IL-7 activates the RAT1 and RAT2 genes, it programs T cell development in the thymus, it helps rearrange the T cell receptor, and it is important for T cell differentiation.
More than a decade ago, Maeurer performed an experiment that involved infecting mice with M.tb. and then treating them with various cytokines, including IL-7. The experiment showed that mice injected with IL-7 lived much longer than those injected with other cytokines. However, the protective effect was much stronger for IL-7 cells extracted from mice already challenged with M.tb. than for IL-7 alone.
Injection of monkeys with IL-7 produced much higher activity in bone marrow and thymic tissue. IL-7 appears to broaden the immune repertoire, said Maeurer. “Since the thymus is activated, we have more barriers; we have more T cells available, which are able to recognize tuberculosis-infected cells. This may be a very, very important point, because the immune system may be exhausted after chronic, long-term tuberculosis infection.”
The Use of Bone Marrow Cells in Treating TB5
Bone marrow cells offer an intriguing approach to the treatment of TB, said Gergert. To determine the feasibility of this approach, he and his colleagues have been studying the effects of bone marrow cell transplants on the growth of M.tb. in infected mice. For this study, they used an inbred, TB-susceptible strain of mice and bone marrow cells taken from TB-resistant mice. They then introduced a strain of M.tb. into the mice and measured their survival rate after infection under various experimental treatment protocols.
One group of mice received no treatment and served as a control. Three other groups received isoniazid therapy, isoniazid plus bone marrow cells, or just bone marrow cells. The isoniazid was administrated to the mice intragastrically 3 days after infection and then daily for 2 months. The bone marrow cells were introduced intravenously, also 3 days after infection and then once a week for 2 months.
Among the control group that received no treatment, all were dead within 25 days. The mice in all three treated groups survived throughout the experimental period. Thus, said Gergert, the transplant of bone marrow cells into infected mice significantly improved their survival rate with no other intervention. The life spans of these mice were comparable with those of the groups of animals that received chemotherapy or chemotherapy plus bone marrow cell transplants.
The introduction of bone marrow cells also significantly inhibited the growth of M.tb. in the organs of infected mice, although not quite as much as in the groups treated with isoniazid. In addition, the mice treated with bone marrow cells had higher levels of specific cell immunity, as measured by the delayed hypersensitivity reaction and interferon-gamma levels. Thus, the transplantation of bone marrow cells strengthened the anti-TB immune response, said Gergert.
![]()
5 This section is based on the presentation of Vladislav Gergert, Central Scientific Research Institute of TB, Russian Academy of Medical Sciences.
This page intentionally left blank.