1
Introduction
If you want to summarize the promise of nanomedicine in one word, that word is “control.” For 3,000 years we have been giving drugs to patients, and the drugs go wherever they want and can cause toxicity. They may treat the right things, but we lose control. The promise of nanomedicine is to allow you to bring back that control. Imagine the day you can say, “drug, come here; drug, turn on; drug, turn off.” That will be the day that we have revolutionized medicine. To be able to engineer that in a small molecule is almost impossible. There are not enough elements in a small molecule to allow you to build in those controls. Whereas in nanomedicine, you theoretically can program that particular nanomaterial to do what you want it to do using local or remote signals. (Li, 2010)
Increasingly, the nature of cancer and how to prevent, diagnose, and treat it is being understood. Biological interactions important for cancer pathophysiology include genetic, intracellular, and intercellular processes—many of which take place on a scale between 1 nanometer and several microns. According to the National Nanotechnology Initiative, “nanotechnology is the understanding and control of matter at dimensions between approximately 1 and 100 nanometers (see Figure 1), where unique phenomena enable novel applications. Encompassing nanoscale science, engineering, and technology, nanotechnology involves imaging, measuring, modeling, and manipulating matter at this length scale” (NNI, 2010). Nanotechnology has the potential to translate recent discoveries in cancer biology into clinical advances in oncology.
Consequently, public investment in nanotechnology for cancer continues to increase, and medical products based on nanotechnology are
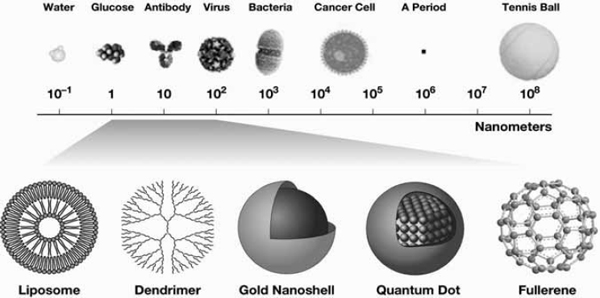
FIGURE 1 Comparison of size of objects relative to a nanometer. Images on the bottom are examples of objects that often have sizes in the 1–100 nm range.
SOURCES: Barker presentation (July 12, 2010) and NCI (2010b).
already on the market. Several of the first FDA-approved uses of nanotechnology in medicine were for new formulations of standard chemotherapeutic agents to enhance delivery of the drugs to cancer cells and reduce side effects suffered by patients. Researchers have also used nanotechnology to improve contrast materials for imaging tumors. Nanotechnology holds promise for diagnostic tools and multifunctional products such as theranostics, which combine diagnostic tests with therapeutic agents.
A great deal of research funding is currently being devoted to research in nanomedicine, providing ample opportunity for scientific advances and new products. Even so, there are substantial challenges to overcoming clinical research and translational science hurdles. These challenges include
- Bridging interdisciplinary gaps to gather basic knowledge in order to more effectively design, develop, test, and regulate nanomedicines
- Developing appropriate standards for testing, manufacturing, and regulation of nanotechnology, and closing current regulation gaps
- Discerning and balancing the risks and benefits of nanotechnology, as well as conveying these risks and benefits to both policymakers and the public.
To explore what nanomedicine is, what it can do, its potential risks and benefits, and the state of the art for standards and regulation, all with respect to nanomedicine in oncology, the National Cancer Policy Forum held a workshop in Washington, DC, on July 12–13, 2010, titled “Policy Issues in Nanotechnology and Oncology.” This document is a summary of the workshop. The workshop discussions fell under several main categories: uses of nanotechnology in oncology and cancer research, research and development of new cancer nanomedicines, risk management, and nanotechnology and the public. These topics form the chapters of this summary; policy issues, such as regulation of nanotechnology-based products and the challenges of facilitating interdisciplinary education, research, and development are discussed as they arose in the workshop proceedings. The views expressed in this summary are those of the speakers and discussants, as attributed to them, and are not the consensus views of workshop participants of members of the National Cancer Policy Forum. Further information on nanomedicine and nanotechnology can be found by starting with reviews in the scientific literature (Boisselier and Astruc, 2009; Debbage, 2009; Gao et al., 2009; Kostarelos et al., 2009; Moghimi et al., 2005; Nie et al., 2007; Peer et al., 2007; Riehemann et al., 2009). This summary organizes the themes of discussion by topic, and topics and quotations are not necessarily arranged in chronological order.
WHAT ARE NANOTECHNOLOGY AND NANOMEDICINE?
Dr. Mauro Ferrari, president, chief executive officer and director of the Methodist Hospital Research Institute, began the workshop by pointing out that there are multiple operational definitions of nanotechnology in different agencies and countries. But the key features these definitions tend to all share are that nanotechnology is engineered materials that make use of the unique physical properties possessed by the materials due to their size—properties that blend atomic or molecular properties with more commonly encountered bulk properties. The unique properties of nanomaterials enable novel applications, but, as several speakers pointed out, require the harnessing of multiple disciplines, such as physics, chemistry, engineering, materials science, and biology to further the field of nanotechnology.
Dr. Ferrari used the term “nanomedicine” to broadly define the application of nanotechnology to medicine, which would include imaging applications. But others, as he pointed out, use the term nanomedicine to refer to specific therapeutic agents made with nanotechnology. Dr. King Li, senior member of the Methodist Hospital Research Institute, chair of the department of radiology at the Methodist Hospital, director of the molecular imaging program and professor of radiology at the Weill
Medical College of Cornell University added that “cancer nanomedicine is more than just the materials. I think we can achieve more if you think about it as a combination of materials science, focused energy [delivery], targeted drug delivery, and molecular biology all intersected in the middle. That is the direction that we are moving,” he said. Others debated the definition of nanotechnology used for regulatory purposes. This debate will be covered in Chapter 5.
BOX 1
Nanomaterials Used in Medicine
Several different kinds of nanomaterials are used in diagnostics and therapeutics, including nanoparticles, nanoshells, quantum dots, nanowires, fullerenes, micelles, liposomes, and dendrimers. See below for descriptions of these nanomaterials.
Nanoparticles
In addition to many other uses, nanoparticles are nano-size particles used to target tumor and other cells of interest for imaging or treatment purposes. These particles are composed of a variety of materials and can be made to contain therapeutic molecules that they release when they bind to their target. The term nanoparticle generally refers to materials made from a wide variety of inorganic materials, such as metals, semiconductors, or oxides. Nanoparticles made from semiconductor materials, sometimes referred to as quantum dots, are also described in this box. Nanoparticles are also referred to as nanocrystals when the materials composing the particles are crystalline.
Nanoshells
These usually have a core of silica and a metallic outer layer and can be decorated with molecular probes for cancer-related compounds. Nanoshells can be used to image tumors and for theranostics. For the latter, energy is directed at a tumor site and selectively absorbed by the nanoshells that accumulate in tumor cells. The heat of the energized nanoshells kills the tumor cells. Nanoshells are also used to provide targeted delivery of drugs to tumor cells.
Quantum dots
The term “quantum dot” refers to nanoparticles made from semiconductor materials such as cadmium selenide surrounded by a shell of zinc sulfide. When linked to an antibody or other molecule capable of binding to a target of interest, quantum dots can be concentrated at target-rich areas in an organism or tissue sample. Because of the multitude of colors with which they can emit light, quantum dots can be combined to create assays capable of detecting multiple substances simultaneously.
PHYSICAL PROPERTIES OF NANOMATERIALS
Several speakers gave examples of nanomaterials (see Box 1) and the different physical properties that nanotechnology products have, including different kinetics, diffusion characteristics, aerosol dynamics, fluid dynamics, size, and surface to volume ratio. Dr. Anna Barker, former deputy director of the National Cancer Institute, noted that the increased
Nanowires
These are wires of metal, oxide, or semiconductor materials, and they possess diameters in the nanometer range. Lengths can be hundreds of nanometers to centimeters or longer. These are valued for both their structural and electronic properties.
Fullerenes
The best-known fullerene is C60, also called a buckyball. C60 is 60 atoms of carbon with icosahedral symmetry, similar to that of soccer balls. Single-walled and multi-walled carbon nanotubes are tubes formed from graphitic carbon. Carbon nanotubes can possess diameters of only a few nanometers and lengths from the tens to hundreds of nanometers up into the millimeter range. Carbon nanotubes may be bundled together or used singly. Fullerenes have exceptional strength and unique electrical and thermal properties.
Micelles and Liposomes
Biomolecules can also be used as building blocks for nanomaterials. Sometimes referred to as nanoparticles, micelles and liposomes are particles made from lipids. Lipid molecules are hydrophilic on one end and hydrophobic on the other end. Micelles consist of single lipid layers arranged into a sphere. In aqueous solutions, the hydrophobic ends point towards the interior of the sphere, allowing hydrophobic molecules to be transported in aqueous solutions. In hydrophobic solutions, the hydrophilic ends point towards the interior of the sphere, allowing water soluble cargo. Liposomes are small particles constructed from lipid bilayers;if the hydrophilic end of the outer layer is pointing outward, then the hydrophilic ends of the interior layer are pointing inward. Like micelles, liposomes can carry molecules in their interior cavity; in aqueous solutions, cargo is generally water soluble. Lipidic structures such as micelles or liposomes may surround chemotherapeutic agents and passively accumulate in tumors, where they release their drugs, or they may be decorated with antibodies that target tumor-specific proteins.
Dendrimers
These are ordered, branched polymers. Dendrimers enable multiple functions to be achieved with a single nanoparticle as each branch can be designed to have a different nanomedicine or diagnostic component.
surface to volume ratio of some nanomaterials enables researchers to attach more components per unit volume. In addition, different types of molecules with differing targets can be attached to single nanoparticles. She added that their small size enables nanomaterials to easily enter most cells, where they can readily interact with biomolecules on both the cell surface and within the cell.
Dr. Joseph DeSimone, Chancellor’s Eminent Professor of Chemistry at the University of North Carolina at Chapel Hill and William R. Kenan Jr. Distinguished Professor of Chemical Engineering at North Carolina State University, noted that size and shape influence the likelihood that particles will enter the cell via endocytosis. Nanoparticles, especially rodshaped particles, are more likely to enter a cell by this process, he said. This is advantageous because endocytosis protects the particle’s payload from being ejected by cellular pumps, which are known to confer drug resistance (Heath et al., 2009).
The size of nanomaterials can also be advantageous for targeting tumor cells. Nanomaterials are not so small that they are rapidly eliminated through the kidney, yet they are small enough that they are more likely to penetrate the leaky blood vessels that feed tumors and concentrate in tumor tissue, as opposed to normal tissue, many speakers pointed out. “The sweet spot for cancer applications is that just by the fact that particles are roughly a hundred nanometers in size; there’s passive accumulation in tumors,” said Dr. Scott McNeil, director of the Nanotechnology Characterization Laboratory.
Dr. DeSimone added that the inhalation properties of medicines are also strongly influenced by their size and shape. By engineering those properties on the nanoscale, one can acquire the aerodynamics needed to effectively deliver inhaled medicines deep into the lungs, his dog animal model suggests. There also can be sustained release and residence in certain tissues due to the unique features of various nanomaterials that other classic small molecules do not have, according to Dr. Barker.
Lastly, Dr. Scott Manalis, associate professor of biological and mechanical engineering at Massachusetts Institute of Technology, pointed out that the small size of nanomaterials such as cantilevers give them a greater sensitivity to mass. These microscopic, flexible beams can provide rapid and sensitive detection of altered weight and other traits of individual cells. In addition, cantilevers can be coated with genetic or other molecular probes for cancer-related molecules. When target molecules in solution bind to the cantilever-bound probes, bending of the cantilevers occurs, which triggers an electrical or visual signal that can be detected. How cells differ in weight may have diagnostic or prognostic significance in cancer, which alters cell growth rates, he said (see also Box 2).
The unique properties of nanomaterials will influence the tests used
BOX 2
Single-Cell Diagnostics
Drs. James Heath, Elizabeth W. Gilloon Professor and professor of chemistry at the California Institute of Technology, professor of molecular and medical pharmacology at the University of California, Los Angeles, and director of the NanoSystems Biology Cancer Center, and Scott Manalis, associate professor of biological and mechanical engineering at Massachusetts Institute of Technology, showed how it is possible to do single-cell diagnostics using nanotechnology. Dr. Heath uses his barcode nanotechnology, as described previously, to detect as many as 20 cancer-triggering P13K pathway proteins from single cells. “Out of this type of analysis, one can develop a network of the system,” he said.
Such single-cell analyses are revealing some unexpected findings that were not previously discovered using microarray technology on multiple cells, and may be able to counteract the misinterpretations that arise when multiple heterogeneous cancer cells are combined in a single analysis, generating average results that misrepresent the more diverse results found in the sample. For example, some cells may have high amounts of epidermal growth factor (EGF) and erlotinib, which targets EGF, while others have low amounts of the growth factor and this drug that targets it. By mixing these cells in a single analysis, one may miss a correlation between having high amounts of EGF and also having high amounts of erlotinib because the average of these cells does not indicate this correlation.
In his lab, Dr. Manalis has been pursuing single-cell assessments of physical properties such as mass, weight, and density. “It should be useful to measure these types of parameters, not only to understand cancer and its disregulation of the cell cycle and cell growth, but also how cells respond to drugs that can be ultimately used to predict which therapies are likely to work in patients,” he said.
He has used cantilevers with U-shaped channels for fluid flow to determine the mass of single cells. With this setup, he has dramatically increased the ability to measure mass over conventional methods. The smaller the cantilevers, the better the resolution. “Not only can we weigh a single cell, but we can weigh it with a precision that is about a thousand times less than the cell itself. So we can measure very, very small changes in the weight of the cell,” Dr. Manalis said.
Dr. Manalis has also developed similar nanodevices that weigh single cells in two different fluids to determine the density of individual cells. Using these measurements, he has been able to show differences between red blood cells from anemic versus normal individuals, and to discern actively dividing lymphoblasts from normal white blood cells.
He also has developed a way to pause cells in transit in his devices so their weight and density can be measured repeatedly over time. Using this method, he is currently assessing the effects of various treatments on the weight and density of individual cancer cells, which are measured before and after the treatment. He is also using fluorescent tags for stages of the cell cycle while simultaneously measuring mass or density. “We envision this as a way to study how density and mass [as indicators of cell growth] are perturbed by giving cells drugs at different parts of the cell cycle,” Dr. Manalis said.
to characterize them, both Drs. McNeil and Yuliang Zhao pointed out. “Very naively we thought five and six years ago that we could simply take an off-the-shelf kit and characterize nanomaterials, and that’s not the case. [The nanomaterials] will interfere with the assay—many nanoparticles will absorb at the same wavelength that the colorimetric assays do. Some particles are catalytic and will cleave a substrate so you get false positives,” said Dr. McNeil. Dr. Yuliang Zhao, director of the Chinese Academy of Sciences’ Key Lab for Nanosafety added that additional parameters are required when characterizing the toxicity of nanomaterials, besides mass concentration, reactivity, solubility and other standard parameters. These additional parameters include quantum effects, structure, shape, particle concentration, number, size, size distribution, surface chemistry, and tendency to aggregate or self assemble.








