4
Risks Associated with Nanotechnology
Several participants noted that nanoparticles are commonly observed; these particles have both natural and human origins. “There are lots of nanoparticulates that we are exposed to every day. I am always amazed, when we think about these engineered nanoparticles as being such unusual beasts, because they are really not all that unusual,” Dr. Barker said.
Nonetheless, speakers noted that nanomaterials do pose several types of potential health risks, including short-term and long-term risks to the health of those taking nanomedicines, risks to the workers making nanomedicines, and contamination risks to the environment at large. “If you are looking at the challenges to nanotechnology, I think they are going to be about safety, and the agencies of the government need to get together and work this out,” Dr. Barker said.
DATA COLLECTION: BIODISTRIBUTION AND TOXICOLOGY
Dr. Ferrari and others listed several biological barriers that nanomedicines might have to surmount in order to reach their targets. These barriers include the reticuloendothelial system (RES) of the immune system, the kidneys, the liver, blood vessel walls, the tumor cell membrane, the cytosol or the nuclear membrane of a tumor cell, ionic and molecular pumps within tumor cells, and enzymatic degradation. In addition, nanomedicines might have to overcome the additional barrier posed by pressure that builds in tumors because of their leaky blood vessels, which
large molecules can penetrate. These molecules accumulate and draw in fluid, building pressure in tumor cells that impedes the entry of even small molecules, Dr. Li pointed out (see Figure 7).
The properties of nanomaterials make it difficult to predict how they will penetrate these various biological barriers or be metabolized, which in turn makes it difficult to assess their biodistribution and toxicity, several speakers noted. In most cases, one cannot predict in vivo biodistribution based on nanostructure physical and chemical properties, such as size and charge, Dr. Li noted. He added that nanostructures can distribute to various organs as intact nanoparticles or they can be metabolized or split up into different pieces, which can enter the cells of various organs and reside in them for an unknown amount of time before moving to other organs or being excreted.
“One of the most difficult parts is tracking the multiple components in vivo over time. Some may stay for a long time, some may stay for a short time. You don’t even know whether they stay as one whole piece the whole time. If they stay in the liver, how long are they going to stay, and what problems are they going to cause in the future?” said Dr. Li.
Dr. McNeil added that “a huge issue that we’ve uncovered is stability of the particles. If a nanomaterial is unstable, obviously it will come apart, and in some cases we’ve seen that within a minute of introducing
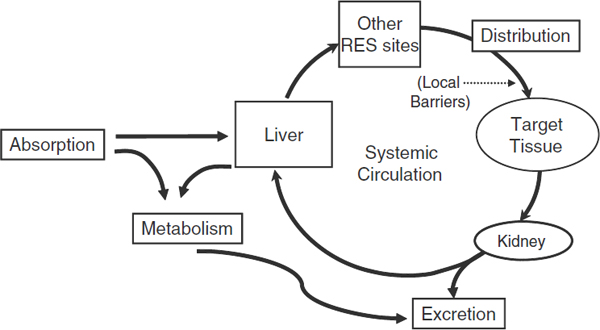
FIGURE 7 Pharmacokinetics; ADME diagram. ADME stands for absorption, distribution, metabolism, and excretion: the four biological processes that are assessed when a therapeutic or other systemic or topical drug, device, or biologic is evaluated for toxicity.
SOURCE: Li presentation (July 12, 2010).
it intravenously. Other particles are covalently bound and do not cleave, so the drug, even if it makes it to the tumor, will not come apart. It is not effective because the drug is not released and cannot interact with its target enzyme.”
Dr. Ruth Duncan, professor emerita of Cardiff University and visiting professor at the University of Greenwich, stressed that the pharmacokinetics (PK) are different for nanoparticles, given that they can enter cells, and that one needs to show microscopic distribution as well as macroscopic distribution. “It’s a different kind of paradigm from just using the old-fashioned cells that we were using for small molecules. The pharmacokinetics is totally different,” she said. “You need quantitative PK studies on the whole body as well as at the cellular level.” Dr. Li added, “macroscopic distribution doesn’t imply microscopic distribution. So even if you have macroscopic imaging, it doesn’t tell us enough of what is happening in vivo.” Dr. Gaspar stressed assessing both pharmacokinetics and pharmacodynamics when evaluating the biological effects of nanomedicines, and having translational models adapted to the specific questions that nanomaterials raise.
In addition, Dr. DeSimone cautioned that deformability is a characteristic that needs to be measured for nanomaterials. He described that deformability is commonly measured in biology: the age of a red blood cell can be estimated from its deformability and researchers have demonstrated that metastatic cancer cells are sometimes much more deformable than their non-cancerous counterparts (Suresh, 2007). In contrast to biological materials such as red blood cells or cancer cells, deformability has not been thoroughly explored as a characteristic impacting biodistribution and toxicity of nanomaterials. He described experiments in which his lab has begun to look into nanoparticle deformability; intravital microscopy—microscopic imaging done on live subjects in vivo—has resulted in a wealth of data. Results show that deformability can reduce both formation of aggregates in the lungs and uptake in the liver. In addition, Dr. DeSimone described how tuning nanoparticle deformability could help improve intracellular uptake of nanotherapeutics.
Reflecting Dr. Li’s statement that it is difficult to predict nanoparticle biodistribution and toxicity, Dr. DeSimone pointed out their experiences when testing the biological effects of PEG-based nanoparticles decorated with transferrin or antibodies to transferrin receptors (both proteins that bind transferring receptors); tranferrin receptors are overexpressed in some types of cancer. DeSimone and colleagues hypothesized that these nanoparticles could be loaded with anti-tumor drugs, the antibodies would target cells of interest, thus effecting preferential delivery of drug to tumor. However, when researchers tested the toxicity of the nanoparticles in the absence of any drug, it was found that the nanoparticles
themselves possessed the ability to induce cell death in certain types of cells (Wang et al., 2010).
Dr. Li pointed out that the route of exposure of nanomaterials will dictate, to some degree, the specific fate of them in the body. Nanomaterials applied via inhalation will have different biodistributions than those applied to the skin, taken orally, or taken intravenously. “If you inhale nanotubes versus inject them, you’ll have totally different biodistribution, toxicity profiles, and so on. Those considerations do not vary as much with small molecular agents,” Dr. Li said.
Further complicating biodistribution assessments is that the binding kinetics between nanomaterials and proteins are not well known, nor is it fully known how different components of nanostructures are metabolically processed and excreted. “All these special ADME [Absorption, Distribution, Metabolism, Excretion] considerations for nanomaterials that are quite distinct from those for small molecular drugs may hinder the development of nanomedicine, as this is just a partial list of the potential concerns that we have on different classes of different materials that we need to define before we get them into the clinic,” Dr. Li concluded, referring to the concerns shown in Figure 8.
Some effort to fill in these knowledge gaps have been made, Dr. Zhao noted, especially in regards to toxicity assessments. Studies have documented to a limited degree such factors as the relationship of response to nanomaterial dose, degree of aggregation, size, or structure, and methods have been developed to quantify nanoparticles in vivo, he said. Dr. Zhao and his colleagues at the Chinese Academy of Sciences have published about 60 papers in nanotoxicology, as well as completed a 10-volume set of nanosafety books that was published in Chinese by a scientific press in Beijing. He noted that there also is a book on nanotoxicology that was published in English in the United States in 2007 (Zhao and Singh Nalwa, 2006).
Dr. McNeil added that characterizations of more than 200 nanomaterials at the Nanotechnology Characterization Laboratory, including 50 animal studies have revealed a few basic principles about nanomaterials and their effects in the body. These studies indicate that nanoparticles with high surface charge are cytotoxic regardless of particle type, and that uncoated nanoparticles will accumulate in the liver and spleen, and they are more likely to be digested by phagocytes, unlike those that are PEGylated.
“We found that some of our in vitro results, at least for optimization, do in fact mimic what we’re seeing in vivo,” Dr. McNeil said. “We can begin to predict, for example, what PEG length is best for a particular protein that’s used for a targeting agent, but I can’t look at a nanoparticle and tell you X amount will go to the liver and X amount to the spleen.
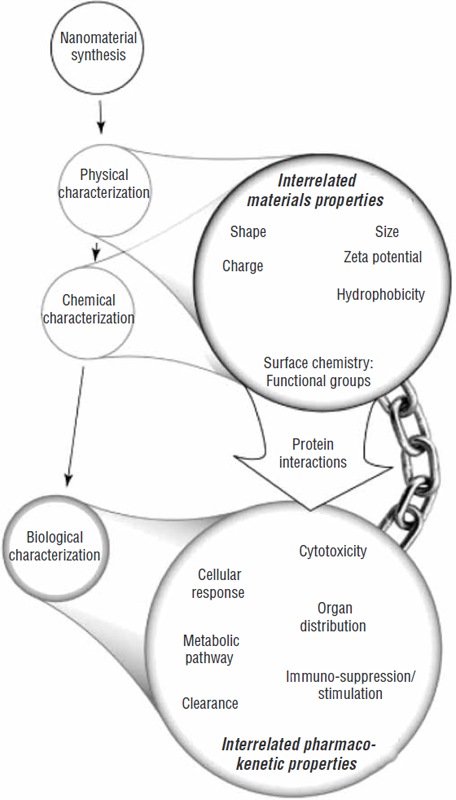
FIGURE 8 Special ADME considerations for nanomedicine.
NOTE: ADME = absorption, distribution, metabolism, excretion.
SOURCES: Li presentation (July 12, 2010) and Fischer and Chan (2007). Reprinted from Current Opinion in Biotechnology 18(6), H. C. Fischer and W. C. Chan, Nanotoxicity: The growing need for in vivo study, pp. 565–571, Copyright 2007, with permission from Elsevier.
We can’t provide that level of detail. All we can do is just point out trends at this point.”
Data at the National Characterization Laboratory show that surface charge, size, and hydrophobicity influence biocompatibility, he added. “We know that in our hands, every nanoparticle is unique. Just simply changing that particle—it’s surface charge or the length of the PEG—makes it almost a completely different entity, even if it’s still within the same class,” Dr. McNeil said.
Dr. Kulinowski noted that there are more than 4,000 papers in the International Council on Nanotechnology database relevant to nanosafety and nanotoxicology of medical or environmental nanomaterials. But little of this data is what she termed “regulator-ready” data. “The majority of the papers are hazard-related rather than exposure-related, and by far the majority of those are cell culture studies. So the relevance of those papers that say ‘nanoX kills 50 percent of the cells at this dose’ to a person taking a drug or using a consumer product is very low. While we might be able to appreciate that there’s a lot of work being done in this area, we’re not getting to that next stage yet where we can say what it means for decision making,” Dr. Kulinowski said.
The International Council on Nanotechnology conducted a workshop aimed at answering the question how long would it take to develop a model that would be able to predict nanomaterial behavior in biological systems and the environment. The outcome of that workshop was that it would take ten years to understand the dynamic nature of nanomaterials, Dr. Kulinowski reported. “We need to understand surface interactions much more than we do now, as well as a variety of other aspects in order to get to that goal of being able to look at the physical and chemical properties of a nanomaterial and be able to say, ‘well here’s how it’s going to interact in a cell, in a biological fluid, in a sand bed, river, etc.’”
So despite the emerging body of knowledge on nanotxoicity, often multiple studies are needed to characterize complex nanoparticles and show where they are likely to be distributed in the body when conducting clinical trials. Some of these studies are rather esoteric, Dr. Desai pointed out. For example, one might have to do x-ray diffraction to show the amorphous or crystalline characteristics of the nanoparticle, or electron microscopy, as well as other tests specific to the construct. “These can be complex constructs, where you have not just the drug, but you maybe have polymers, different targeting agents, and many other different components. It is very important to understand how all these interact,” Dr. Desai said.
Dr. Gaspar questioned the relevance of in vitro models and certain animal models when making biodistribution and toxicity assessments of nanomaterials, and stressed the need for in vivo studies.
OCCUPATIONAL SAFETY
“Occupational safety is a critical issue,” said Dr. Kulinowski. “No matter what we’re doing in nanotechnology, that has to be a consideration. Workers, whether they be researchers in the laboratory or production workers, are likely to be exposed to nanomaterials in higher quantities and for longer periods of time than consumers or even patients.”
Dr. Kulinowski noted that although there are numerous journal articles that touch on nanotechnology occupational safety issues, few address such practical questions as safe exposure levels for nanotechnology workers. “As a result, we don’t have any occupational exposure limit for nanoparticles,” Dr. Kulinowski said. She suggested translating the information the pharmaceutical industry has acquired on how to safely handle fine powders with high bioreactivity to workers handling nanomaterials. She also pointed out that the International Council on Nanotechnology recently established an open-source website for sharing information about occupational practices for the safe handling of nanomaterials that they call the “GoodNanoGuide.” Multiple stakeholders contribute, share, and discuss information on this site, which is modern, interactive, and up-to-date.1 “We’re looking at tasks that might be performed in a manufacturing or research environment and saying ‘here are the potential human exposures, and here are the potential controls that you might want to use,’” Dr. Kulinowski explained.
She added that there have been discussions about establishing medical registries and medical surveillance programs to document health risks in those who work with nanomaterials. The National Institute of Occupational Safety and Health’s most recent statement about this is that it is premature to set up a medical surveillance program or registry of workers, according to Dr. Kulinowski, but she added that the agency continues to explore this possibility. She noted that it is difficult to identify the demographics of the nanomaterials worker because nanotechnology is used in such a wide range of fields, including the chemical industry and the pharmaceutical industry. “Getting a handle on who they are and what the tasks are is very difficult,” she said, let alone what types of measurements and medical tests would be made on these workers.
In his talk, Dr. Zhao stressed the need to distinguish nano-specific risks from other manufacturing risks. He gave an example of a paper which linked exposure to nanomaterials of workers to serious lung disease (Song et al., 2009). This article created a media sensation, with Nature publishing a news article with the headline “Nanoparticle safety in doubt,” and most Chinese newspapers reporting that the nanoparticles had killed workers.
______________
But the situation was more complex than how it was initially reported. The patients with lung disease were working in a workshop used to heat plaster, and the plaster contained some titanium oxide nanoparticles that were released in the plaster fumes and found in the patients’ lungs. The nanoparticles were contained within polyacrylate esters. A study in animals by Dr. Zhao and his colleagues suggested that the lung toxicity was not due to the nanoparticles, but rather due to the fumes produced by the heating of the polyacrylate esters. He called for more assessment technologies and procedures to investigate potential nanotoxicities.
NANOMEDICINE SAFETY
Dr. Curley noted that the long-term toxicities linked to nanoparticles used in medicine are not known, giving the example of carbon nanotubes. “Single-walled carbon nanotubes are fascinating from a physical– chemical point of view, but they are also incredibly rigid and stable structures, so are those going to be safe to deliver to a patient over the long term?” Dr. Curley asked. He said one study found that aerosolized carbon nanotubes were toxic when delivered to the lungs of rats—they developed something akin to the black lung disease seen in coal miners. When asked by Dr. Bahadrasain how to reassure the public that the safety of nanomedicines is not a problem, Dr. Curley responded, “We need to do the preclinical and clinical toxicology and toxicity studies that will demonstrate that to the best of our ability, there are no long term effects with the nanomaterials we are using.
Dr. Duncan noted that the safety issues linked to nanomaterials depend not only on the material, but how it is used. She pointed out that using nanomaterials in MRI imaging, in which patients are given a very low dose of the materials only once or twice, poses different risks than treating them with a nanomedicine for months or longer. “It’s really important, when people ask the safety question, that we relate it to a particular material and a particular use, route of administration, and dose,” Dr. Duncan stressed. Dr. Curley agreed, noting that “you may be able to use things like quantum dots in an in vitro diagnostic system that you would never give to a patient.” Dr. Sackner-Bernstein added, “It doesn’t mean that carbon nanotubes are not a potential application as medical devices. It just means you’ve got to make sure that the occupational health issues are taken care of, and that you’re not using them as an inhaled device or drug.”
Dr. Libutti pointed out that “there is a lot of fear in the unknown. One of the biggest challenges for us is to turn the unknown to the known so we don’t have a lot of unrealistic fears.” Both he and Dr. Barker noted that this fear of the unknown slowed down the application of recombi-
nant DNA technology because of the numerous restrictions on how the technology could be used initially, but eventually those restrictions were relaxed once its safety was shown. “Because of the natural fears that folks have and the predilection for watching sci-fi movies, we are going to need to go through that same evolution with nanotechnology,” he said.
Based on his experience with Abraxane and five other nanomedicines, Dr. Desai said the standard battery of toxicology studies are sufficient to establish safety. “Whether you are testing a small molecule or a biologic or a nano-type construct, the tests are adequate to define the toxicology. Through the formal toxicology studies which any of you do in the standard development of drugs, those studies are pretty thorough. You look histologically at every possible organ, do all the blood chemistries, so if there is any particular toxicity, whether it be nanoparticle-related or not, you should be able to find it. I know it has been talked about that nanoproducts may have a different toxicology profile, but I think that the published papers, and maybe the little bit of hype in the lay press, has probably been more as a result of occupational exposure in the heavy industry settings … as opposed to the pharmaceutical applications,” he said. But he stressed designing and conducting studies to understand the disposition of the nanomaterial in vivo. “You have to understand the biodistribution, the metabolism, the excretion, and how these components degrade over time. These are all very important for the long-term understanding of the toxicology,” Dr. Desai said.
But Dr. Curley pointed out that Dr. Desai’s experience is with nanomedicines that have pharmacologic or biologic agents, and may not be applicable to metallic or semiconducting nanoparticles that may be used in vivo. Dr. Desai responded, “It is not so much to do with the fact that the particles we make are albumin and conventional drug molecule versus magnetic nanoparticles or whatever, but that the way to look at toxicology typically has been to take a detailed look at all the possible tissues and other biofluids. What else could anybody suggest that you look at that may give you a better idea of some other toxicology profile that isn’t caught by these kinds of studies?”
Dr. Li then pointed out that the major problem in assessing long-term toxicity of nanoparticles is that many are not metabolized and excreted, unlike most other examples of nanomedicines that have been used clinically. He noted that many inhaled particles, such as carbon nanotubes, might lodge in the lung for the long term, but that potential hazard would not be discerned in a short-term toxicity study. He said, for example, that acute toxicity assessments of asbestos would not indicate that it would cause any problems, but it does cause long-term toxicity. “I don’t think the acute ADME toxicology studies that we directly deal with using small molecular drugs would screen for those long term side effects,” Dr. Li
said. Dr. Zhao pointed out that his study of the metabolism of nanomaterials has revealed that many bind to proteins in the body, which impedes their excretion and metabolism. “They can stay there in the body for a long time—for nine months or longer,” he said. Dr. Curley added “from an evolutionary point of view, we have not evolved mechanisms to metabolize, excrete, or otherwise modify fullerenes or solid gold nanoparticles, etc.”
Dr. Desai agreed that one needs to discern if the particles do not degrade, and if they do accumulate in a particular organ, it raises different questions that require different studies, “but those aren’t outside of the realm of what the FDA will ask you for anyway,” he said. Dr. Josephson added that “The key thing is to make sure that the nanoparticle is gone at the end of your toxicity study. If it is still there, the interpretation is that there was no toxicity seen, but the animal didn’t live long enough.” Taking a lesson from history, he pointed out that gadolinium chelate contrast agents were shown to be rapidly eliminated by the kidney, and thus were touted as safe as saline by their manufacturer. But those studies neglected to look at people whose kidneys did not completely eliminate the compounds. This caused a buildup of gadolinium in their kidneys which was linked to their developing nephrogenic systemic fibrosis. Avoiding this syndrome is possible by knowing the renal status of patients prior to injecting them with the contrast. “But it has heightened the issue of elimination in nanotechnology—where do things go, how long to they stay, and can they cause toxicity years and months after they have been given.”
Dr. Barker noted that the safety issues raised by nanomedicines are not any different than what has been raised by biologics, and the biggest toxicity issues have not been related to long residence times of the agent in the body, but rather how these biologics alter factors that cannot be measured. For example, leukokines have prolonged toxicity that occurs long after they are administered, she said, for complex reasons that are currently unknown.
Dr. Duncan stressed “It is up to us as innovators and members of the public to continue with the regulatory agencies to evolve the process of safety assessment of nanomedicines, depending on what we are making.” But Dr. Desai and others cautioned against being overly cautious about nanomedicines. “It’s important that we don’t create hurdles for ourselves that make it more difficult in the long run to bring innovative technologies to the patients,” he said. Dr. Libutti added “We shouldn’t set the bar so high that it is difficult to cross, especially with respect to cancer therapies, as we should be so lucky if the patients live long enough to see long-term toxicities from the therapies. We shouldn’t regulate ourselves out of coming up with innovative therapies, worrying about fantastic toxicities that may never come to be. Certainly for the development of nanotherapies
for benign conditions, that may be more of an issue.” He pointed out that if high toxicity standards were adhered to 50 years ago, there would not be a single standard chemotherapeutic on the market now.
But Dr. Libutti added that the metrics for toxicity in preclinical trials may not measure toxicities in patients who are going to live long enough to manifest them. “It is reassuring and makes you feel comfortable if you check those boxes off for your toxicity runs, because you are more likely to get your IND through. But they don’t pretend to encompass as yet unrealized toxicities that new agents may develop,” he said.
Dr. Heath pointed out that “every application that I know of in nanotherapeutics that has gone into the people, the net result has been to decrease toxicity. The headline should be that we have been able to engineer away toxicity to a great extent. That is something that should be celebrated in this field. We are lowering toxicity of drugs.” Illustrating the importance of lowering the toxicity of current cancer medicines, Dr. Curley gave an example of one of his patients, who was a violinist when he was diagnosed with colorectal cancer metastatic to the liver. Although he has survived eight years post treatment, he experienced such severe neurotoxicity from his chemotherapy that he is no longer able to play his instrument. “We need to look not only at the survival of our patients, but what is the quality of that survival and what are the long-term effects,” Dr. Curley said. Dr. Hawk added that lowering the toxicity of cancer prevention agents is the main goal for applying nanotechnology to the cancer prevention field. “Our biggest challenge is making compounds safer, so this should be a very exciting future.”
RISK–BENEFIT ASSESSMENTS
Dr. Gaspar suggested that when it comes to nanomedicines, risk– benefit management is the approach that needs to be taken rather than risk assessment. “Every medicinal product has a risk. If we start to make decisions based only on risk assessment, we’ll end up withdrawing the pipeline of medicinal products as a whole, and not only the nanomedicines in particular,” he said. Dr. Kulinowski added that there is some social science research that indicates that consumers are willing to take greater risks for greater benefits. “It’s not just about risk, it’s about risk– benefit. When the benefit is low, there’s a lower tolerance for risk,” she said. Dr. Hawk added that risk–benefit assessments will especially underlie the usefulness of cancer preventives in a healthy population.
Dr. Sackner-Bernstein noted that FDA takes a risk-based approach when assessing the safety of medicines and devices, with more scrutiny given to those products likely to pose the most risk, but that the agency also considers risk–benefit assessments of those products, including the
potential impact on the public and whether there are alternatives that exist already to the product being considered. “We try to make sure that when there’s a product that actually has impact, the barriers that it faces are commensurate with the potential impact,” he said.
Dr. Duncan stressed engaging the public in risk–benefit assessments of nanotechnologies. “The public decides whether the risk–benefit is acceptable. As scientists and regulators, we have a duty to our patients to tell them accurately what the risks and benefits of the technology are,” she said. Dr. Li agreed that it is important to engage the public in these assessments, but he expressed concern about the public’s ability to make the scientific distinctions needed to adequately assess the risks and benefits of nanomedicines. He suggested educating the public about what nanotoxicology means in the environment or in their food versus what it means in medicine. He said that public understanding of risk–benefit is important in order for regulatory agencies to effectively communicate their work.












