2
Committee Findings and Recommendations
CHARGE TO THE COMMITTEE
As noted in the report (IOM, 2010), the Center for Food Safety and Applied Nutrition (CFSAN), in conjunction with the Food and Drug Administration’s (FDA’s) Center for Drug Evaluation and Research (CDER), approached the Institute of Medicine (IOM) in 2008 for advice on the topic of biomarker and surrogate endpoint evaluation in chronic disease. These FDA centers expressed concern regarding the limited number of surrogate endpoints available, the high cost of evaluating possible surrogate endpoints, and the absence of an agreed-upon, systematic, and transparent process for biomarker evaluation. They also wished to learn whether principles of biomarker qualification or evaluation learned in the drug development setting could be applied in other FDA-regulated product categories, such as foods. CFSAN thus requested that the IOM charge an expert committee with the following tasks:
An Institute of Medicine (IOM) committee will be convened to generate recommendations on the qualification process for biomarkers, with a focus on risk biomarkers and surrogate endpoints in chronic disease. These recommendations will consider existing prototypes for qualification of biomarkers used in drug development. The committee will recommend a framework for qualification and test it using case studies of risk biomarkers and surrogate endpoints for coronary heart disease (CHD)1 such as
LDL and high-density lipoprotein (HDL) cholesterol levels. In particular, the committee will:
-
Conduct a review of current approaches to qualifying biomarkers.
-
Recommend a framework that can be used to rank biomarkers according to the types and quality of evidence, considering context of use for a range of product types.
-
Demonstrate applications through case studies.
-
Make ancillary recommendations for the application, enhanced development, and use of risk biomarkers and surrogate endpoints in chronic disease.2
On the basis of this statement of task, the committee undertook its work to address these charges as ensured by the IOM external review process, said committee chair John R. Ball, senior advisor at the American Society for Clinical Pathology.
Committee Process and External Review Process
The IOM convened the committee, which comprised experts from a variety of related fields and was supported by a highly capable staff, said Dr. Ball. The committee met in person four times and had several teleconferences over the course of a year in order to fully develop their charge, set a plan of work, gather relevant evidence, and develop its findings and recommendations. The committee benefited from presentations by outside experts in a workshop format and from comments offered by interested parties. As part of its charge, the committee reviewed alternate biomarker evaluation models; this review can be found in Chapter 2 of the committee’s report (IOM, 2010).
The committee’s report underwent a rigorous external review, which helped focus and clarify their findings and recommendations. Fourteen reviewers participated in this process, and two individuals appointed by the National Research Council and the IOM oversaw the review, according to Dr. Ball.
Early in committee deliberations, Dr. Ball recalled that the committee recognized that “biomarkers are really useful when used carefully.” Biomarkers have served a variety of diverse uses, he noted, which include
-
Discovery and development of medical therapies and products,
-
Comparative effectiveness research,
-
Formation of clinical practice guidelines,
-
Basic biomedical research,
-
Clinical practice,
-
Public health practice, and
-
Understanding healthy nutrition and lifestyle choices.
However, Dr. Ball continued, the committee also quickly recognized that a biomarker’s usefulness is strongly dependent upon context. “No single biomarker is good for everything,” he said, which became a defining principle that informed the committee’s recommended framework for biomarker evaluation, discussed below. This framework and additional supporting recommendations were introduced in the workshop’s first session, following the definition of a series of terms relevant to biomarker evaluation and application. This session also reviewed examples of biomarker case studies to which the committee applied their evaluation framework.
DEFINITIONS
As noted in their report (IOM, 2010), “The committee observed a great deal of inconsistent and imprecise definition and use of terms relevant to biomarkers and biomarker evaluation.” Having determined that consistent, precise definition and use of terms is critical for biomarker evaluation, the committee strove to be both consistent with the spirit of previous efforts in this vein and also to clarify several potentially confusing definitions. The results of this process, which provided a foundation for the committee’s task, were presented at the workshop’s outset by committee member John A. Wagner, vice president of clinical pharmacology at Merck & Co., Inc.
Biomarker
Dr. Wagner noted that the Biomarkers Definitions Working Group (BDWG), convened by the National Institutes of Health (NIH), produced the definition of a biomarker that is widely used today (Biomarkers Definitions Working Group, 2001). The IOM committee’s version of this definition is: “a characteristic [for example, cholesterol level] that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a[n] … intervention.” The committee used this definition in its report, and further defined “objectively” to mean “reliably and accurately” in this context.
Risk Biomarker and Risk Factor
A related term, risk biomarker, was used by the FDA’s CFSAN in their request to the IOM to charge an expert committee to “generate recommendations on the qualification process for biomarkers, with a focus on risk biomarkers and surrogate endpoints in chronic disease” (IOM, 2010). The committee defined a risk biomarker to be “a biomarker that indicates a risk factor for a disease,” which includes genetic biomarkers, Dr. Wagner said. He noted that this definition contrasts with that used previously by CFSAN, which characterizes risk biomarkers as “biological indicators that signal a changed physiological state that is associated with the risk of a disease” (CFSAN, 2009), and therefore does not include genetic biomarkers.
Dr. Wagner said that the committee resolved to use the term biomarker instead of risk biomarker in order to clearly delineate between biomarkers and risk factors, which are defined in the report as “variables that predict outcomes and are composed of biomarkers and social and environmental factors” (IOM, 2010). As noted in the report, the value of a risk factor depends on its ability to predict an event.
Surrogate Endpoint and Clinical Endpoint
The widely accepted definition of surrogate endpoint was proposed by the BDWG in 2001, Dr. Wagner said. According to this definition, a surrogate endpoint is “a biomarker that is intended to substitute for a clinical endpoint. A surrogate endpoint is expected to predict clinical benefit (or harm or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence” (Biomarkers Definitions Working Group, 2001). For example, blood pressure has served as a surrogate endpoint for morbidity and mortality due to cardiovascular disease (CVD) in trials of several classes of antihypertensive drugs, Dr. Wagner said.
A surrogate endpoint represents a special use of a biomarker, in which the biomarker substitutes for a clinical endpoint. Closely following the BDWG definition, the committee defined clinical endpoint as “a characteristic or variable that reflects how a patient [or consumer] feels, functions, or survives” (Biomarkers Definitions Working Group, 2001). Death is one example of a clinical endpoint.
Dr. Ball and Dr. Wagner noted important examples of successes and failures of biomarkers that have been used as surrogate endpoints. Two key successes are blood pressure as a surrogate endpoint for CVD clinical endpoints, and HIV-1 RNA as an indicator of complete viral suppression for HIV interventions. By contrast, arrhythmia suppression proved a fail-
ure as a surrogate endpoint for interventions meant to reduce cardiac sudden death; similarly, low-density lipoprotein cholesterol (LDL-C) reduction through hormone replacement therapy failed to provide a surrogate endpoint for CVD clinical endpoints.
RECOMMENDATIONS
The committee concluded that focusing solely on biomarker qualification—the process of determining whether a biomarker of interest is associated with a specific clinical endpoint—would not sufficiently address the committee’s charge, said Dr. Wagner. The committee saw their primary task as identifying a process for biomarker evaluation, which they fulfilled in large part by recommending a three-part framework comprising
-
Analytical validation, which asks the question, is the biomarker able to be accurately measured?
-
Qualification, which asks the question, is the biomarker associated with the clinical endpoint of concern? and
-
Utilization, which asks the question, what is the specific context of the proposed use?
Dr. Wagner said that the first two pieces of the framework, analytical validation and qualification, are already commonly accepted; on the other hand, he noted, “the addition of utilization is a little bit more controversial, but nonetheless extremely useful.” He added that the committee envisioned all three elements of biomarker evaluation as interdependent components of an iterative cycle, as illustrated in Figure 2-1.
The committee’s recommendations are shown in Box 2-1. In accordance with their charge, the committee applied the biomarker evaluation framework to a series of case studies and made additional recommendations for implementing the framework, for supporting evidence-based decision making, and for promoting public health. Presentations by three committee members reviewed the background for and rationale behind each recommendation, and also presented in detail the committee’s case study of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) as biomarkers for cardiovascular risk, one of several such studies that appear in their report. These presentations were followed by a discussion session open to all workshop participants.
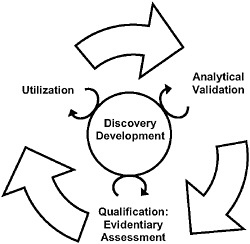
FIGURE 2-1 The steps of the evaluation framework are interdependent. While a validated test is required before qualification and utilization can be completed, biomarker uses inform test development, and the evidence suggests possible biomarker uses. In addition, the circle in the center signifies ongoing processes that should continually inform each step in the biomarker evaluation process.
SOURCE: IOM, 2010.
Recommendations 1 and 2: Biomarker Evaluation
Dr. Wagner discussed each component of the biomarker evaluation framework described in Recommendation 1 (see Box 2-1): analytical validation, qualification, and utilization. Analytical validation comprises analyses of the available evidence regarding the analytic performance of a particular assay or biomarker; this, he said, is a necessary first step to determining how a biomarker is performing. He defined qualification, a term which has in some cases been confused with validation, as the assessment of the available evidence that links a biomarker with a biological process, such as a disease state, a clinical outcome, or an intervention. Utilization, a concept subsumed under qualification in some previous biomarker evaluation frameworks, is a distinct process in the committee’s evaluation framework because it represents a subjective and contextual analysis—as contrasted with the objective analyses in the analytical validation and qualification steps—specific to the use of a given biomarker or surrogate endpoint, Dr. Wagner said. He added that the committee conceived of the three steps of biomarker evaluation as components of an interactive and iterative cycle, as illustrated in Figure 2-1.
An analysis of the many sources of variability in biomarker measurements—which include biological, sample collection, and analytical factors—led the committee to conclude that “biomarker tests need to
be reliable, reproducible across multiple laboratories and clinical settings, and maintain adequate sensitivity and specificity before data based on them can be used in subsequent evaluation steps,” Dr. Wagner said. This finding provided a particular focus and a foundation for the committee’s work.
Tumor Size and Analytical Validation
Per their charge, the committee used examples whenever possible to illustrate their findings. Dr. Wagner used the case study of tumor size—described in detail in the committee’s report on pages 135–142 (IOM, 2010)—to highlight the role of analytic validity in biomarker use. Measurements of tumor size are crucial in determining the efficacy of cancer therapeutics and in daily clinical practice, he said. However, tumor size can be defined by many different technologies (and different techniques within those technologies). Tumor size can be gauged by measuring tumor diameter, volume, or mass; these in turn are measured by a variety of platforms and techniques that include magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) imaging, Dr. Wagner said. Further, different contrast agents and different protocols may be used, all of which affect measurement precision. Thus, he concluded, “analytical validation of tumor size is complicated by multiple imaging platforms and other assay performance issues.”
CRP and Qualification
Biomarker qualification requires analysis of the nature and the strength of evidence for the relationship between a given biomarker and a disease-associated biologic pathway, and of evidence that interventions targeting the biomarker affect the clinical endpoints of interest, Dr. Wagner said. To illustrate this process, the committee examined C-reactive protein (CRP), a biomarker that has been shown in observational studies to serve as an independent predictor of future vascular events, including myocardial infarction (MI), ischemic stroke, peripheral vascular disease, and vascular death. The committee’s application of the biomarker evaluation framework to CRP found evidence for its prognostic value, but insufficient support for its use as a surrogate endpoint. As Dr. Wagner noted, CRP is a useful biomarker of CVD, but its utility as a surrogate endpoint has not been established (see also pages 142–153 of IOM, 2010).
Troponin and Utilization
The utilization component of the biomarker evaluation framework is used to determine whether the analytical validation and qualification conducted on a given biomarker provide sufficient support for a specific proposed use, Dr. Wagner explained. He emphasized that strong evidence and a compelling context are needed for the use of a biomarker as a surrogate endpoint—that is, as a substitute for the clinical endpoint.
This requirement is illustrated by the case of troponin, a biomarker used ubiquitously in acute settings to diagnose MI. Troponin can be elevated due to a variety of chronic heart conditions, inflammatory conditions, side effects from drugs, or organ failures, Dr. Wagner said. While there is evidence that prevention of MI reduces death rates, none supports the proposition that using an intervention specifically to decrease troponin levels improves mortality risk (IOM, 2010). Thus, he concluded, evidence is lacking to support the use of troponin as a surrogate endpoint for interventions in these situations (see also pages 153–159 in IOM, 2010).
Dr. Wagner acknowledged that decisions made regarding biomarker utilization are necessarily subjective, in contrast to qualification, which he characterized as a “data-gathering and evidentiary step.” This difference was a key rationale for separating qualification and utilization, he said (see also Recommendation 2b. in Box 2-1). However, he added, it is also the case that the three distinct elements of the biomarker framework are interrelated and may be pursued concurrently; moreover, the results of one evaluation step (for example, utilization) may reveal the need for revisions or additional work in one or both of the other steps (for example, analytical validation and/or qualification).
Beta-Carotene and the Biomarker Evaluation Process
The committee’s second recommendation (see Box 2-1) focuses on the evaluation by the FDA of biomarkers and biomarker tests with regulatory impact. The three parts of this recommendation specify that (1) such evaluations should be conducted by expert panels convened by the FDA; (2) analytical validation and qualification of biomarkers should be evaluated separately from utilization; and (3) biomarkers should be continually evaluated on a case-by-case basis.
Dr. Wagner offered the example of blood levels of beta-carotene, proposed as a biomarker for risk of CVD and cancer, to illustrate the need for an expert panel to periodically evaluate evidence associated with a particular biomarker. Years of epidemiological studies showed that diets rich in fruits and vegetables were associated with lower incidence of CVD and cancer, leading many to believe that beta-carotene was responsible
for the lower risk, he said. “However, definitive clinical trials showed that this hypothesis was incorrect and that supplementation with betacarotene did not lower risk for cancer or cardiovascular disease,” and in some cases, it increased risk (see also pages 168–175 of IOM, 2010). These circumstances suggest the need for periodic evaluation of the evidence for the analytical validation, qualification, and utilization of biomarkers with regulatory impact, he concluded.
LDL and HDL Case Study
Committee member Ronald Krauss, director of atherosclerosis research and senior scientist at the Children’s Hospital Oakland Research Institute, presented an in-depth analysis of the committee’s fifth case study, LDL-and HDL-cholesterol as biomarkers for cardiovascular risk, to illustrate the application of the biomarker evaluation framework (see also pages 159–168 in IOM, 2010).
The context for the LDL- and HDL-cholesterol case study, illustrated in Figure 2-2, is the relationship between both LDL-C and HDL-C and CVD risk, as established by the Framingham Heart Study (Castelli, 1988). LDL-C and HDL-C are, respectively, components of LDL and HDL particles. As Dr. Krauss noted, LDL comprises multiple subclasses of particles with differing composition. Research in many populations has affirmed that LDL-C is directly related to cardiovascular risk, independent of HDL-C, and that HDL-C is inversely related to cardiovascular risk. These findings led to the widespread use of LDL-C and HDL-C as biomarkers for evaluating the efficacy of pharmacologic and nutritional interventions for CVD. The committee’s case study evaluated each of these biomarkers in various contexts of use.
“Clearly, LDL stands as one of the major FDA-qualified surrogate endpoints for cardiovascular disease,” Dr. Krauss stated. LDL-C is often viewed as a benchmark biomarker, particularly for nutritional claims, he said, despite the fact that LDL-C is but one component of LDL particles, which vary in composition. “The evidence supporting LDL as a biomarker rests almost entirely on the measurement of LDL-C,” he concluded. He also noted that in some populations, LDL particle measurements provide better estimates of CVD risk than LDL-C.
Along with these issues, the committee considered the disease context for evaluating LDL-C or LDL particles as biomarkers, Dr. Krauss said. CVD, he noted, is a very complex disease that is increasingly recognized as a spectrum of pathologic and pathophysiologic effects, only one of which is primarily related to progression of the cholesterol content of plaques as a function of LDL-C in the blood. Atherosclerosis, he added, is “often indolent, progressive over time, and then is complicated by a
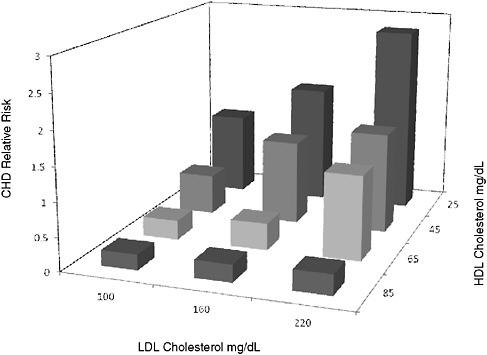
FIGURE 2-2 Relative risk of coronary heart disease after 4 years compared to several LDL-C to HDL-C ratios. Men aged 50–70 years in the Framingham Heart Study.
NOTE: HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; mg/dL = milligrams per deciliter; RR = relative risk; Y = years.
SOURCE: Castelli, 1988. Adapted, with permission, from the Pulsus Group, 2010. Adapted from the Canadian Journal of Cardiology 1988;4(SA):5–10.
number of additional factors that can convert a cholesterol-rich plaque to a more malignant form that destabilizes and is involved with both inflammation and thrombosis; immune changes can occur that could be critical.” Thus, he concluded, “it is rather simplistic to consider either LDL or HDL, or even the two of them together, as sufficient to explain these complex mechanisms.”
The predictive value of LDL-C for CVD events varies considerably as a function of health status, Dr. Krauss said. As shown in Figure 2-3, risk for cardiovascular events associated with high LDL-C in patients without diabetes and CVD was found to be significantly lower than LDL-C-associated event risk in patients with both conditions (Robinson and Stone, 2006). Likewise, he noted, when the effects of LDL-lowering interventions (for example, statins) were compared in a meta-analysis
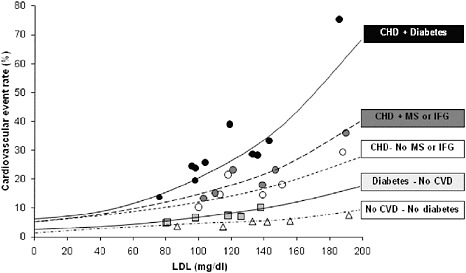
FIGURE 2-3 CVD risk varies over wide range of LDL-C.
NOTE: CHD = coronary heart disease; CVD = cardiovascular disease; IFG = impaired fasting glucose; MS = metabolic syndrome.
SOURCE: Robinson and Stone, 2006. Reprinted, with permission from Elsevier, 2010. Copyright 2006 by Elsevier.
(Baigent et al., 2005), some studies were found to deviate (but not significantly) from the generally linear relationship between reduction in LDL and major coronary events. “This was not unexpected, given the multiplicity of mechanisms involved, both in the disease process and in the mechanism of drug action,” Dr. Krauss observed.
“One of the classic examples of failure of LDL as a surrogate endpoint—and it actually applies to HDL as well—[is] the lesson from hormone replacement therapy,” he said. Postmenopausal hormone replacement therapy (HRT) was first thought to protect women from CVD based on both observational epidemiological data and the apparent beneficial effects of estrogen on both LDL and HDL and other biomarkers for CVD, he explained. “Fortunately,” he continued, “a number of people in the field held out for clinical trials, and some major clinical trials demonstrated that HRT had no benefit on CVD incidence in healthy women, and actually increased mortality in the first year of treatment in women with preexisting cardiovascular disease and increased the risk for thromboembolic events.”
Dr. Krauss summarized the conclusions of the committee’s case study of LDL-C as a biomarker of CVD risk as follows:
-
The strength of LDL-C as a surrogate endpoint is not absolute due to the heterogeneity of CVD processes, the heterogeneity of LDL-lowering drug effects, and the heterogeneity of LDL particles themselves.
-
Age, gender, and genetic factors have been shown to complicate the already complex dynamics of the LDL–CVD relationship; as a result, lowering LDL-C can never be considered a “perfect” indicator across all population groups.
-
Nonetheless, there is high probability that lowering LDL-C by certain interventions (for example statins) decreases risk of CVD, and LDL-C, although not perfect, is one of the best biomarkers for CVD.
Dr. Krauss then turned briefly to HDL-C and its potential as a biomarker for CVD risk based on the inverse relationship demonstrated in the Framingham Heart Study (Castelli, 1988; Gordon et al., 1977). “There is strong epidemiologic evidence for a relationship of HDL to cardiovascular risk, and there’s also quite a bit of pathophysiologic evidence indicating that certain therapeutic maneuvers, most of them based on genetic manipulations in animal models, can raise HDL to reduce atherosclerosis progression or disease risk in these models,” he stated. In humans, however, he noted that HDL “is even more heterogeneous than LDL and includes multiple subpopulations of particles with differing functional properties and disparate effects on atherogenic mechanisms.”
“There are multiple ways of raising HDL cholesterol and HDL particle concentrations, lifestyle being one of them,” Dr. Krauss continued. “But a number of drugs can raise HDL through different mechanisms, and understanding those drug effects simply by using HDL as an index of benefit does not address a fundamental question: is the HDL being raised by a process that would confer the expected benefit [reducing CVD risk]?” Thus, he concluded, “there is as yet no conclusive evidence in humans for an independent benefit of HDL increase on CVD outcomes, in large measure because trials aimed at raising HDL almost always have concordant effects on other cardiovascular risk markers that cannot easily be teased apart.”
As an example of these findings, Dr. Krauss presented the results of a large trial of the first HDL-C raising drug, a cholesteryl ester transfer protein inhibitor called torcetrapib (Barter et al., 2007). This trial demonstrated that torcetrapib had the capacity to raise HDL-C by over 70 percent from baseline over the course of the first year of treatment, and also to reduce LDL-C levels, he said. Nevertheless, he continued, despite the favorable apparent effect on these two CVD biomarkers, “the overall result of the trial showed not just the absence of a benefit, but actually a higher overall
mortality in individuals treated with this drug in combination with statin compared with statin alone.” This result “clearly represents a complex outcome that is not just a failure of the surrogate endpoint,” Dr. Krauss said. “We now know that there are off-target effects of this drug on cardiovascular disease itself, not manifested by any biomarker yet that we clinically use, that could have accounted for the adverse effect.”
Dr. Krauss then discussed the committee’s case study of LDL and HDL as CVD biomarkers in the context of a well-established paradigm for evaluating relationships between disease and intervention, surrogate endpoints, and clinical outcome, devised by committee member David DeMets, professor of biostatistics and medical informatics at the University of Wisconsin, and Thomas Fleming, who gave an overview presentation later in the discussion forum (see Chapter 7 in this volume) (Fleming and DeMets, 1996). As noted by Fleming and DeMets (1996), “even in the best of circumstances, it is possible for surrogate endpoints to be misleading by either overestimating or underestimating an intervention’s effect on clinical outcomes.”
This can happen, for example, if the surrogate endpoint is not in the causal pathway of disease. “We don’t have a real example of that here,” Dr. Krauss said, “because for the most part, the LDL and HDL measurements are in the causal pathway [for CVD] to some degree or another.” However, he added, one could argue that depending on the way by which LDL and HDL are measured (for example, as LDL-C and HDL-C), these biomarkers may not in fact reflect clinical outcomes.
Another scenario for biomarker failure under the paradigm occurs when an intervention (for example, statin) affects a biomarker (for example, LDL-C) favorably, contributing to improved clinical outcome, Dr. Krauss said. However, he observed, in this case the result “is not a perfect biomarker for the outcome of a trial, because the disease process itself has an extraordinary effect on the clinical outcome that’s independent of the intervention.”
In a third case of biomarker failure, the intervention affects a clinical outcome independently of a surrogate endpoint, according to Dr. Krauss. He offered the example of a diet intervention such as the Mediterranean diet in the Lyon Diet Heart Study, in which interventions improved clinical outcome without having any obvious benefit on LDL, HDL, or other surrogate endpoint (de Lorgeril et al., 1999).
Finally, a biomarker can fail when an intervention has multiple effects on the outcome itself, on the surrogate endpoint, and on the relationship of the disease to the outcome, Dr. Krauss stated; this is the case in the two previous examples involving the effects of torcetrapib and HRT on CVD. In these cases, “there may have been a benefit through the pathway of lowering LDL and raising HDL, but it was obscured by other effects of the drug and … of the disease process,” he said.
“In the end, biological complexity above and beyond simple measurements that we’re considering here lead to many opportunities for error,” Dr. Krauss concluded. As shown in Figure 2-4, interventions may influence disease outcomes through multiple pathways; as he noted, “this is certainly the case for LDL and HDL … which comprise particles arising through different pathways that may have different pathological effects, leading to different outcomes.” Thus, he concluded, the main lesson the committee learned from the LDL and HDL case study is that “interventions to address a multifactorial disease introduce potentially unforeseen effects, particularly when the causal disease pathways, the mechanisms of action of the intervention, and the characteristics of the biomarker itself are not fully understood.”
Dr. Ball added that this and the other case studies, among other evidence considered by the committee, led them to conclude that biomarkers “are good in many circumstances, but that it depends largely on the context of use.” He followed this with an anecdote from his own experience, which appears in the preface to the report. “Several years ago,” he said, “I had three episodes of atrial fibrillation, and after the third one, I realized that all three of those episodes had been associated with the drinking of two glasses of red wine.” Upon recognizing this correlation, he stopped drinking red wine, and thereafter has not had another episode of atrial fibrillation.
However, when he told his mother about his conclusions, she replied, “but I thought red wine was good for your heart.” As Dr. Ball learned, however, “it depends.” Red wine, he said, may be “good for the plumbing, the coronary arteries, apparently, but it was not so good for my pacemaker, the electrical system.” Similarly, he observed, sometimes an intervention affects biomarkers, sometimes it affects the disease itself, and sometimes it doesn’t have an effect—or it has a negative effect—on clinical outcomes. These circumstances, he said, led the committee to devise the three-part biomarker evaluation framework comprising analytic validation, qualification, and utilization, depending on context of use.
Discussion and Clarification of the Biomarker Evaluation Framework
In the discussion period that followed the committee presentations, several committee members sought to affirm that the focus of their work, per their charge, was to develop an evaluation framework for biomarkers, and not for FDA-regulated products or interventions (for example, drugs, biologics, medical devices, foods, or nutritional supplements) that might be identified or characterized through the use of biomarkers. Presenter Andrew Shao, senior vice president of scientific and regulatory affairs at the Council for Responsible Nutrition, raised this issue when he asked whether the committee had considered the case of biomarkers
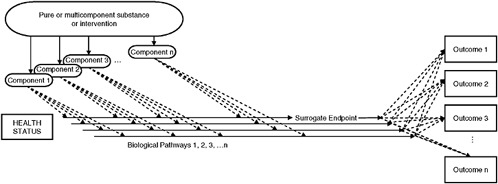
FIGURE 2-4 Multiple ingredients, multiple biological pathways, and multiple outcomes illustrate some of the complexities of the use of biomarkers and surrogate endpoints in chronic disease. Note that while the solid horizontal arrows indicate biological pathways, they do not necessarily indicate pathways of the particular disease or condition that a substance or intervention is meant to address. In other words, a surrogate endpoint may not be on the causal pathway of the disease process and a substance or intervention may have mechanisms of action independent of the disease process. Dotted lines indicate possible pathways.
SOURCE: IOM, 2010.
for nutrient exposure, which inform policies such as dietary guidelines, and if so, whether Recommendation 3 should be interpreted to mean that nutritional guidance should be developed according to the paradigm currently used for drugs.
“This committee was charged with evaluating the criteria for qualifying biomarkers themselves,” Dr. Krauss responded. “That’s a different question, I think, from the one you asked, because that information [biomarker qualification] can then be used by policy makers, dietary guidelines formulators, et cetera, in the context of the strength of the data, the strength of the basis for that qualification,” he explained. The goal of biomarker evaluation is to provide firm evidence on which such policy decisions can be made, he added.
The committee’s task was to identify criteria to be used for judging whether a given surrogate endpoint represents an appropriate way to monitor a specific disease or biological process, according to committee member Jennifer Van Eyk, professor in the departments of medicine and biological chemistry at Johns Hopkins University. The committee was not focused on the various entities that a biomarker or surrogate endpoint may be used to test, she said; instead, they considered the criteria by which surrogate endpoints should be judged and the challenges of developing surrogate endpoints that can be used throughout the regulatory process.
Some workshop participants sought further clarification of the distinction between utilization and qualification, as pertains to the recommended evaluation framework, and also between their use of the terms biomarker and surrogate endpoint. Presenter Marc Walton, medical officer at CDER, asked whether a biomarker (for example, HDL-C) determined to be inappropriate for use as a surrogate endpoint for a particular clinical endpoint (for example, CVD), can be said to have failed the qualification step as well as the utilization step.
Dr. Ball explained that the qualification step answers two questions: whether there is a relationship between the biomarker and the clinical endpoint, and whether the intervention of interest affects both the biomarker and the clinical endpoint in the same way. If that’s the case, utilization answers whether a particular context of use for the biomarker—which may or may not be as a surrogate endpoint—makes sense. “It probably is not possible to qualify HDL as a biomarker for everything,” Dr. Ball concluded. “The context of use matters.”
Indeed, Dr. Krauss pointed out, HDL failed on the second part of the qualification step because in certain situations—some of which he described in his presentation—an improvement in HDL-C levels did not improve disease outcomes (for example, when the intervention of interest is HRT). Clearly, HDL-C is a biomarker for CVD in a general sense,
he said, but it’s an imperfect biomarker in the sense that it doesn’t represent a component of HDL most responsible for its relationship to CVD. “One of the holy grails in our field is to identify a functional test for HDL that could actually correlate fairly consistently with efficacy in drugs or diets in reducing risk [for CVD] through measurement of that functional property of HDL,” he observed; such a test would replace HDL-C and, if it demonstrated a more direct connection with disease outcome, conceivably could achieve qualification and utilization as a biomarker.
“The analytical validation and qualification pieces are fit for the intended purposes,” Dr. Wagner added. The analytical validation and qualification requirements for a particular biomarker used in research would not be the same if that biomarker was to be used for a regulatory purpose; for example, although there are qualification issues with tumor size as a biomarker in oncology, it would be appropriate for use as a biomarker for evaluating a candidate anticancer agent under certain circumstances, especially given the dearth of cancer biomarkers. The committee recognized that the decision to use a biomarker for a regulatory purpose is a subjective one, he reported, so they separated utilization from qualification.
Utilization is the most challenging component of the biomarker evaluation framework from a policy-making perspective, noted committee member Roberta Ness, dean of the University of Texas School of Public Health. How to analyze and characterize a biomarker through testing are well understood, she observed, but the committee also recognized that many biomarkers work perfectly well in particular contexts, while failing in other contexts. “The parameters around the ‘it depends’ are complicated … and some of them are perhaps even counterintuitive,” she said. For instance, when assessing a biomarker in the context of a homogenous ill population for whom few medical interventions exist, Dr. Ness suggested that one might be much more willing to accept an imperfect biomarker as a surrogate endpoint, as a means to identifying better interventions, than in the context of a food consumed by the general population.
“My understanding of what the committee is saying is if one has a wealth of data … from clinical studies that [for example] establish that treatment effect on LDL is predicting treatment effect on death from MI, to what extent can we actually then implement that in the real world?” Dr. Fleming stated. “I would think the answer to that [question] would come in utilization,” he continued, “where you would say, if you had other classes of agents that were similar to those that were the source of the data, that extrapolation would be reasonable. But if you had a new, novel intervention such as … torcetrapib, that could readily be influencing off-target effects, one would be much more reserved about that degree of generalization.”
The same notion is true of blood pressure, Dr. Fleming added. As he noted in his presentation, considerable data show that effects on blood pressure predict effects on stroke for wide classes of agents, including low-dose diuretics, angiotensin-converting enzyme (ACE) inhibitors, beta blockers, calcium channel blockers, and angiotensin II receptor blockers (ARBs). In the case of a drug with a novel mechanism of action, however, “one would have more reservation about simply labeling [it] for stroke based on [its] effect on blood pressure.” Thus, he concluded, in the utilization step, one takes “the totality of the data from the analytical validation and the critically important qualification steps into a real-world setting to [ask], to what extent can we generalize those known relationships to determine whether the biomarker could be used in the specific instance as a basis for judging clinical benefit?”
On a more practical level, presenter Guy Johnson, principal of Johnson Nutrition Solutions, LLC, asked how biomarker utilization would be executed by the FDA. Given the agency’s long history of using advisory committees, Dr. Ball noted, it would be appropriate for the FDA to assemble groups of individuals with relevant expertise—including stakeholders with a range of perspectives—to address specific cases. These advisory committees would first assess the data on validation and qualification as they pertain to the biomarker under review, and then examine the particular use for which the biomarker is proposed.
Recommendations 3 and 4: Scientific Process Harmonization
Dr. Ness introduced the remaining recommendations in her presentation. While these recommendations, like the evaluation framework, apply to all products regulated by the FDA (i.e., drugs, medical devices, biologics, foods, and dietary supplements), Dr. Ness said she would focus her remarks on foods because, as she explained, this regulatory area was central to the committee’s charge.
Dr. Ness summarized Recommendation 3 (see Box 2-1) with the statement, “all regulatory areas should be considered equal.” She explained that the rationale for this recommendation came from the committee’s recognition that a very large number of people in the population may be exposed to any given food, and thereby, any toxic effects that might result from its consumption. “I think we lull ourselves into the idea that foods cannot be toxic, but clearly there are good examples where that’s in fact not the case,” she said, invoking Dr. Ball’s anecdotal experience with red wine, as well as medical allergies to foods, which have both broad and severe impact. In addition, she noted, currently, “there is no learned intermediary between a person and their consumption of food.”
Recommendation 4 (see Box 2-1) sends the message that “we should
not be considering biomarkers as a single element within a complex foodstuff and, in fact, within a complex diet,” Dr. Ness stated. Rather, she continued, “we need to really consider the whole food when talking about a particular relationship between a biomarker and an outcome.” Returning to the scenario depicted in Figure 2-4, Dr. Ness pointed out that a food is an example of a “pure or multicomponent substance or intervention,” illustrating the complex relationship that can exist between a food or individual components in food, biomarker measurements, and clinical outcomes. Therefore, she said, the committee concluded that to “minimize the uncertainty inherent when biomarkers are used to predict beneficial effects of a food substance, the substance’s effect should be evaluated in its context of use—the whole food product and dietary patterns associated with use.”
In the discussion period that followed this session, audience member Elaine Krul, molecular nutrition lead of Solae, LLC, observed that drug efficacy is often considered in the context of diet; for example, she said, “if you’re taking a statin, be careful when you take your grapefruit juice; when you’re taking warfarin, be very careful with your background diet and … vitamin K intake; if you’re taking synthroid, you have to be careful not to take other foods that inhibit the absorption of the drug.” However, she added, to extend such a scientifically rigorous analysis to foods would be difficult. Dr. Krauss replied that while it is important to examine the health effects of foods within the context that they are consumed, “the science is never going to be perfect.” He acknowledged a fundamental lack of certainty based on the science at hand, but asserted that probabilities of such effects should be determined, considered, and communicated to both medical professionals and consumers in ways that clearly convey their inherent uncertainty.
Referring to a publication considered to be a classic in the biomarker literature, Dr. Ness noted that Prentice (1989) not only insisted that biomarkers fully reflect clinical outcomes, but also that they must capture the entirety of the effect of an intervention on an outcome (for more detail about the Prentice criteria see pages 27 and 56 of IOM, 2010). “Clearly, if one takes just one component out of a complex foodstuff, one could not capture with a biomarker that whole complexity with respect to outcome,” she added. This viewpoint prompted considerable discussion. Presenter Stephen Williams, chief medical officer of SomaLogic, Inc., noted that analytical validation and qualification of biomarkers can always be improved, and the consequences of error are uncertain, and asked, “how good is good enough?” Dr. Wagner replied that the committee recommended the continuous and iterative evaluation of biomarkers.
Dr. DeMets also commented that the Prentice biomarker criteria are applied with consideration to a biomarker’s context of use, and that a
biomarker used as a surrogate endpoint must capture the effect of the intervention of interest, whether it is a medicine, a device, a food, or a supplement. “If you really are serious about evaluating the effect of any new intervention, from devices and drugs to foods, it really is an essential step,” he said. The failure of biomarkers such as LDL and beta-carotene to predict clinical endpoints resulted from incomplete knowledge of the biology of complex diseases such as cancer and CVD, he said. “The committee struggled with the issue of biomarkers versus clinical endpoints and the fact that they are all measurements, and they are all subject to measurement issues,” Dr. Wagner added.
Dr. Ness noted that the FDA has, in the case of several foods and supplements shown in Table 2-1, accepted health claims that do not reflect the entirety of a foodstuff. On the other hand, she continued, “just recently the FDA has decided that there are claims that are not acceptable to them.” For example, she said, the agency recently informed General Mills that their claim that eating Cheerios can lower cholesterol by 4 percent in 6 weeks exceeded the regulatory framework for food and would trigger a recategorization of this product as a drug. Dr. Ness reported that in March 2010, the FDA notified 17 food manufacturers regarding food labels that violated the Food, Drug, and Cosmetics Act by making unauthorized health claims, nutrient claims, and through their use of the word healthy.
Presenter Douglas Balentine, director of nutrition sciences at Unilever, Foods, noted that at present, very few foods make FDA-approved, ingredient-based health claims, and that most foods containing ingredients listed in Table 2-1 are marketed on the basis of general dietary guidance (for example, “Diets rich in fruits and vegetables may reduce the risk of some types of cancer”).
Ancillary Recommendations 5 and 6: Improving Evidence-Based Regulation
Dr. Ness introduced the committee’s ancillary recommendations intended to support the implementation of the biomarker evaluation framework across all areas regulated by the FDA (see Recommendations 5 and 6 in Box 2-1). According to Dr. Ness, the first ancillary recommendation strengthens recommendations made by previous panels, including the IOM committee that authored the report, The Future of Drug Safety (IOM, 2007b). These groups advised that the FDA be granted additional authority related to postmarket surveillance in areas beyond its existing focus for such studies: drugs and biologics.
TABLE 2-1 Health Claims Based on Surrogate Endpoints
|
Nutrient |
Disease |
Surrogate Endpoint |
Type of Claim |
|
Phytosterols, soy protein, corn oil, canola oil, and olive oil |
Coronary heart disease |
LDL and total cholesterol |
Phytosterols: Authorized Soy protein: Authorized Corn oil: Qualified Canola oil: Qualified Olive oil: Qualified |
|
Chromium picolinate |
Type 2 diabetes |
Insulin resistance |
Qualified |
|
Calcium and sodium |
Hypertension |
Systolic and diastolic blood pressure |
Calcium: Qualified Sodium: Authorized |
|
Calcium and vitamin D |
Osteoporosis |
Bone mineral density |
Authorized |
|
Calcium |
Colorectal cancer |
Colorectal polyps |
Qualified |
|
NOTE: LDL = low-density lipoprotein. SOURCE: Trumbo and Ellwood, 2009. |
|||
Postmarket Studies of Products Approved on the Basis of Surrogate Endpoints
“The current situation is that postmarket studies can be required [by the FDA] for drugs and biologics under certain circumstances, and in particular when there [is] an accelerated approval based upon a surrogate endpoint,” Dr. Ness stated. For example, for a drug approved for adults and subsequently used in pediatric population, or for a drug approved on the basis of animal studies because clinical trials in humans were unethical. For devices, she said, “postmarket surveillance is challenging due to a lack of reliable monitoring of adverse events and lack of publicly accessible information.” Surveillance of the effects of claims on foods is limited, and the process for removing harmful claims can be very slow, she added.
Dr. Ness observed that the FDA has recently strengthened its authority with respect to postmarket surveillance for drugs, and expressed hope that such surveillance could be extended to other regulatory areas, particularly medical devices and foods. The committee determined that surveillance of biomarker-based food health claims is needed, she said, because it is important to understand the impact of the actual foodstuffs on consumers. “When surrogate endpoints are used in product or claim approvals, data collection is needed to link the product or claim to health outcomes experienced by patients and consumers, whether for drugs, biologics, devices, foods, or supplements,” she said. The committee’s Rec-
ommendation 5a acknowledges that in order to achieve this goal, it will be necessary to adapt the FDA’s existing regulatory frameworks, which currently differ among product types, Dr. Ness explained.
In subsequent discussion, Dr. Williams expressed confusion as to the current state of the FDA’s authority to demand postmarket surveillance of the products it regulates. Noting that the committee’s report states that the enactment of the Food and Drug Administration Amendments Act (FDAAA) in 2007 granted the agency “new authorities to require post-market studies” that formerly would have been voluntary (IOM, 2007a, p. 207), he asked what gap in current legislation Recommendation 5a was intended to fill. Responding to Dr. Williams’ question, study director Christine M. Micheel, of the IOM, said that with little evidence to date as to whether the FDAAA effectively addressed the committee’s concerns, they wanted to emphasize the need for legislation—whether existing or strengthened—to require postmarket studies of products approved on the basis of surrogate endpoints. Dr. Ball added that the committee considered evidence from the FDA and others that some postmarket studies requested by that agency had not been undertaken by industry; whether these failures resulted from the FDA’s lack of authority or resources remained unclear, he said.
Responding to Dr. Balentine’s request for a description of an acceptable process for fully qualifying a food health claim, Dr. Ball observed that while the committee felt that the same scientific rigor ought to be brought to bear on such claims as on drugs or biologics approved on the basis of surrogate endpoints, they also recognized the need for practicality. “When pharmaceuticals go on the market, there’s a patent, and there’s a huge financial incentive for information to be developed and brought before the FDA,” he said. Because foods lack patents and the incentives they provide, there are practical difficulties in funding the gathering of information on foods. This gap, he said, is addressed in Recommendation 6 (discussed below), which states that the Department of Health and Human Services (HHS) should develop mechanisms for collecting and sharing data on biomarkers through public–private collaborations. “I think we do recognize a difference in the capabilities of the food industry and the capabilities of the pharmaceutical industry [to collect data],” Dr. Ball concluded, “but at the same time … when a claim is made on the basis of surrogate endpoints, there needs to be follow-up, because there can be unintended consequences of the claim.”
Given the committee’s position regarding the need for studies of food health claims based on surrogate endpoints, audience member Chor San Khoo, vice president of global nutrition and health at Campbell Soup Company, Inc., wondered whether the committee would recommend similar evaluation of diets. For example, “The DASH [Dietary Approaches to
Stop Hypertension] diet clearly is a very effective diet in reducing blood pressure … [and] it’s a composition of many foods … [so is gathering data related to use of that diet] also necessary?” Dr. Ness and Dr. Krauss responded that such a recommendation would exceed the committee’s charge, which pertained to products regulated by the FDA on the basis of biomarkers. The real question, Dr. Krauss added, is whether existing biomarkers are sufficient to assess the health effects of complex diets. “For any individual [biomarker], such as blood pressure, I think that the intervention can be a complex diet, with the understanding that there’s going to be other effects of that diet,” he said, “but blood pressure as a biomarker for one of those effects is legitimate.”
Consumer Understanding of Claims on Foods and Supplements
The second part of Recommendation 5 (see Box 2-1) derives from the committee’s recognition of a general lack of consumer understanding of biomarker-based claims on foods and supplements, according to Dr. Ness. For these products, “consumers must evaluate information without the advice of a learned intermediary in most circumstances, [so] consumer understanding of health information based on biomarkers is critically important,” she said. Dr. Ball added, “if a statement is made with regard to a biomarker, but the public has difficulty in understanding that, that’s an issue that we believe that FDA should examine and that industry should examine, as well.” The committee thus concluded that the FDA “needs the authority to request or require studies of consumer understanding, to continue conducting its own research in this area, and the authority to remove or require changes to claims or other information that are not understood by consumers,” Dr. Ness said.
The committee spent considerable time talking about the complex issue of consumer understanding of health claims, Dr. Ness said. Numeracy is a serious concern, as is the tendency of consumers to misunderstand food labels—particularly those that appear on the front of a package, which may cause them to ignore the required nutritional information provided on the side or back. This tendency has been reflected in research that further suggests that consumers have difficulty distinguishing among the various evidentiary levels of health claims on food labels and understanding the qualifying language that these claims frequently contain. The committee therefore concluded that the FDA needs the authority to request and require studies of consumer understanding and to make changes or remove claims that are misunderstood by consumers.
Recommendation 6 (see Box 2-1) supports the committee’s previous recommendations by encouraging the collection and sharing of biomarker-related data across the HHS, and in particular, the creation of
coordinated data infrastructure and surveillance systems, Dr. Ness said. To this end, she noted that the FDA has been building a program known as the Sentinel Initiative,3 a national electronic system for the postmarket safety tracking of drugs, biologics, medical devices, and eventually, all FDA-regulated products. She observed that ClinicalTrials.gov, which provides publicly accessible information about clinical trials, could provide “important guidance for biomarker data collection efforts” as well. Dr. Ness also recognized the following current and ongoing initiatives that may be brought to bear on Recommendation 6:
-
Biomarkers Consortium4—a public–private partnership to identify, develop, and qualify biomarkers;
-
Critical Path Institute5—an independent, publicly funded organization that supports the FDA’s Critical Path Initiative by working with industry, regulatory agencies, and academia on a range of projects, including the development of biomarkers;
-
CEO Roundtable on Cancer Life Science Consortium6—a precompetitive collaborative effort that is working with the National Cancer Institute (NCI) to select promising cancer biomarkers for development; and
-
Oncology Biomarker Qualification Initiative7—a collaboration between the NCI, FDA, and the Centers for Medicare & Medicaid Services (CMS).
While noting that each of these initiatives occupies a specific niche, she said that all of them could be more cohesively and uniformly leveraged, “bringing together data sources and partners to provide the best research infrastructure to further evaluate the relationship between interventions, biomarkers, and outcomes across regulatory areas.”


























