THE ROLE OF PLANT AND MICROBIAL RESEARCH IN
EXPLORING THE EFFECTS OF MICROGRAVITY
Earth’s gravity has a profound effect on biological systems. The most obvious effect is to define the weight of organisms, shaping the evolution of features such as bone and muscle mass in animals and the development of supportive tissues in plants. However, subtler gravitational effects are also integral to biological processes on Earth. For example, gravity-driven buoyancy is responsible for convection and so contributes to processes as diverse as the cooling of the surface of leaves or the distribution of signaling molecules between bacteria. Such gravity-related processes are connected to the functioning of terrestrial biology at scales from the whole organism to individual cells. Life has evolved with this constant background of Earth-normal 1 g and so has never had to develop the capacity to adapt to any other gravitational environment. The reduced gravity of spaceflight is therefore outside the limits that have shaped terrestrial biology. This fact provides the overarching principle for the need to understand the biological impacts of spaceflight environments. Understanding how terrestrial biology responds to microgravity and partial gravity will reduce exploration risks to crews by allowing us to understand responses and so design countermeasures to potential problems. However, spaceflight also offers the unique environment of reduced gravity in which to probe and dissect biological mechanisms. Therefore, research into how terrestrial biology responds to spaceflight will both refine our understanding of what it takes to explore space and help define how conditions on Earth have shaped biology here.
Novel environments, such as spaceflight, provide science with a tool to help define the range of adaptive processes present in biological systems and set the limits on where terrestrial biology can thrive. For the National Aeronautics and Space Administration (NASA), there is also a practical and tactical reason for this research: to best enable the entire exploration process. A deeper understanding of biological processes—all biological processes—within the spaceflight environment produces a more resilient, safer, and better informed exploration architecture. Yet there is also a fundamental question at stake: can life as we know it survive and thrive off the face of Earth, and by extension, what are the limits of our kind of life, terrestrial life, in the universe? There was a time when “life” in this context would have been limited to the human organism, and the question might have been limited to the human dimension. However, given the results of recent genome sequencing projects, we now more clearly understand the relatedness and interrelatedness of all life on Earth down to its molecular underpinnings. Over the past decade, the unraveling of genomes within the tree of life has highlighted structural and functional similarities among terrestrial life forms, while exposing the key differences that allow species and individuals to be distinct.
Therefore, there is a realization that insights into plants and microbes inform us not only about their responses but also about ourselves and our relationships within the entire tree of life as it has developed on the planet Earth.
Plants and microbes are also projected to be key elements in long-term life support efforts in extraterrestrial habitats through providing fresh food and recycling air, water, and waste products. These organisms will likely have other positive, as well as potentially negative, impacts on the success of long-duration spaceflight. For example, in numerous anecdotal reports, Russian cosmonauts and U.S. astronauts mention that cultivating plants in space is different from the usual mission operations in that it provides a link with Earth. Indeed, plants have been shown to be useful as countermeasures for the mental health-related difficulties experienced by humans living in a variety of isolated, stressful, or extreme environments.1,2,3 Similarly, maintaining proper internal microbial ecosystems is essential to human health and survival, even though microbes can present engineering challenges due to their fouling of equipment. Indeed, the effects of spaceflight on microbial populations represent a poorly defined risk to astronauts on long-term missions. Understanding the impacts of spaceflight and reduced-gravity environments on plants and microbes thus becomes an important goal to support safe, long-term human habitation in extraterrestrial environments.
This chapter focuses on the value of fundamental biological research on plants and microbes related to the exploration process. Model systems, underlying molecular mechanisms, and the need for rigorous experimental design are highlighted as the means to best develop a mechanistic understanding of the responses of microbes and plants to the spaceflight environment. That knowledge can be applied to solve exploration needs and predict solutions to anticipated challenges. In addition, and as an added benefit, a better understanding of the underlying molecular mechanisms of gravity sensing and adaptation to microgravity environments may also lead to unanticipated advances that help human life and health on Earth. For example, an improved understanding of how to optimize plant growth in the extreme environment of spaceflight may lead to strategies to increase the efficiency of terrestrial crop production, and insights into how microbial populations change in response to the stresses of spaceflight could provide clues to how microbial populations might be managed in terrestrial settings.
Overview: The Need for Modern Analyses Applied to Model Systems
The past decade has redefined our understanding of biology in terrestrial settings at the molecular, developmental, and cellular levels. The study of spaceflight biology is poised to take advantage of this new knowledge. It is clear that the recent massive strides in genome sequencing, for example, could revolutionize the design of experiments that can be conducted in space and allow scientists to answer fundamental questions about the role of gravity in biological systems. Such knowledge can also be used to optimize plant and microbial systems for the spaceflight environment. It is also clear that our collective understanding of environmental sensing and response is beginning to illuminate underlying mechanisms at the cellular and molecular levels, largely due to the rapid progress enabled by well-characterized model systems. Therefore use of such systems and of molecular analyses that allow us to directly probe these mechanisms will be key to expanding our understanding of biological processes in space. Importantly, insights from these model systems have far-reaching, cross-kingdom applications. Presented below are key issues for space-related research relating to specific sensory and response systems such as those used to react to changes in gravity, mechanical forces, atmosphere, and radiation, and to the integration of these varied stimuli acting simultaneously into a response that is likely unique to terrestrial biology in the extraterrestrial environment.
Sensory Mechanisms I: Gravity Sensing and Response Mechanisms in Plants
All Earth organisms have evolved in an environment at a constant 1 g, and much of their development is entrained to this all-pervasive cue. Yet the mechanisms whereby plants sense and respond to the gravity vector remain in large part unknown. Plants have developed a sensitive system to monitor the gravity vector and to respond with directional, or gravitropic, growth.4,5,6 Such gravitropism allows the plant to maintain the correct orientation
of its organs and so helps define the architecture of both the root and shoot systems. In fact, diverse aspects of plant development can be altered by gravity; for example, the weight of a branch leads to stress within the plant and the production of additional supporting materials called reaction wood.7 The past decade has seen significant advances in our understanding of how gravity is sensed and translated into changes in plant growth and development; it has also highlighted critical gaps in our knowledge of how these events are induced.
Perception of gravity in plants is now generally accepted to involve specialized cells containing mobile starch-filled organelles, called amyloplasts, that likely act as the gravity-responsive masses triggering cellular responses.8 In the aerial parts of the plant, these sensors lie in the vasculature, a sheath of endodermal cells surrounding tissues specialized for water and nutrient transport, whereas in the root, they are localized to the extreme tip in the root cap. Evidence also points to an unidentified second root gravity sensory system outside of the root cap.9,10 A range of molecular components that are linked to gravity perception have been identified,11,12 but currently still unknown are the precise molecular identities of the receptors that translate the physical force of gravity to cellular signal(s). Similarly, the identity of the immediate signals generated by this sensory system and the associated response components that encode the directional information remain to be defined. As stated in a previous National Research Council (NRC) report on space biology13 our ignorance of the cellular gravity perception machinery remains a fundamental gap in our understanding of how gravity can affect plant growth and development. A NASA research thrust into these fundamental control mechanisms underlying plant growth and development would provide knowledge needed to design plant-based systems as an integral component of bioregenerative life support systems for extended human spaceflight, as well as provide a better understanding of plant growth control mechanisms on Earth.
The mechanisms underlying the control of subsequent plant growth responses have received intensive study, with directional transport of the plant hormone auxin emerging as a significant regulatory element. For example, proteins of the AUX/LAX, PIN, and ABCB families are now known to represent the major transporters that direct the flow of this growth-regulating hormone.14 However, the mechanisms linking gravity perception to the correct placement and relocalization of these transporters, and to the systems that regulate their activities, still remain to be defined.15,16 Other hormones and signals such as cytokinins, ethylene, and reactive oxygen species have also been proposed to be integral regulators of plant gravity-responsive growth,17 and there remains a significant open question as to the interrelationships between these regulatory systems. Advancing and integrating our knowledge of plant growth control are further critical components of research for NASA to pursue. Such analyses will contribute fundamental knowledge of the controls of plant form, with potentially widespread application on Earth, where features such as growth habit (which underpinned the green revolution18) and even responsiveness to gravity (crop recovery after lodging,19 where the weather has bent a crop flat to the ground) have important impacts on crop yields and harvesting. This insight into plant development and physiological responses would also be critical to our ability to design bioregenerative life support systems that incorporate plants to provide sustained replenishment of water and air and to provide food for extended crewed missions into space.
The past decade has also seen an increasing realization that the responses of plants to gravity are inextricably intermingled with those to other stimuli. Thus, perceptions of light, touch, and water gradients have all been shown to modulate the gravity response20 and vice versa. For example, touch stimulation of a plant causes decreased gravity response,21 whereas gravitropism may suppress the growth response of roots toward water sources.22,23 In the spaceflight environment, plants are exposed to many stimuli other than reduced gravity. Light levels and quality, atmospheric composition, nutrient levels, and water availability are all critical elements shaping plant growth in space. Profiling the alterations in gene expression seen upon changes in gravity has also exposed a complex response network that shares common elements with reactions to other stimuli such as touch and light.24,25 Illuminating the integration of multiple stimuli is key to understanding the effects of gravity and spaceflight. A program focused on such mechanistic understanding would present opportunities for collaboration with programs at the National Science Foundation (NSF), the U.S. Department of Agriculture, and the National Institutes of Health (NIH).
Considering the presence of a multiplicity of stimuli and the often extreme environments of spaceflight, these interactions of the gravity perception machinery with other signaling systems may have important and likely unexpected effects on plant growth in space. Robust transcriptional profiling, coupled to proteomic and metabolic
analyses of the changes elicited by extended growth in microgravity and partial-gravity environments, will be essential to characterize the responses of plants and microbes to the unique challenges of spaceflight. Release of such data to the scientific community for intensive study as rapidly as possible will maximize the science return from each experiment. Raw datasets, including unprocessed molecular data that can be subjected to multiple subsequent analyses, will broaden the community of researchers engaged in elucidating the mechanisms underlying plant responses to space environments. It is therefore imperative for NASA to develop both guidelines and tools for the rapid and efficient dissemination of such datasets, in addition to publishing research studies. This effort will require balancing the needs for assessment of the robustness of the data and the interests of the principal investigators collecting the data with the need for public dissemination.
Sensory Mechanisms II: Gravity and Mechanical Sensing in Microbes
The space environmental factors that shape plant growth (e.g., microgravity, light and gas levels and quality, and nutrient and water availability) represent key common parameters across kingdoms and have critical effects on other biological systems that will be intentionally and unintentionally taken into space. These include the microbiota present within the spaceflight environment (Figure 4.1), including the large microbial populations present within and upon the crew. Despite the strictest decontamination procedures, it will not be possible to remove microbial populations from spaceflight environments and, in fact, microbiota are essential to human health and survival. For future long-duration missions, it is thus imperative to more fully understand the responses of microorganisms to the unique environment of space at levels from the individual cell and its sensory machinery to populations and microbial communities. Our current lack of an understanding of how microbial populations change during extended spaceflight represents a significant limitation on our ability to ensure the safety of the crew on such missions. Research in this area should receive high priority. In addition, an understanding of the molecular mechanisms underlying how these systems respond to conditions in space may one day allow us to genetically engineer beneficial microbes (and plants)—for example, those involved in a bioregenerative life support system—so they can better handle the environments involved in space exploration.
There has been an ongoing interest in the responses of bacteria to the spaceflight environment,26 and these studies indicate that when grown in a liquid environment in microgravity, bacteria behave differently than when grown in the same environment under normal gravity. As described below, the limited data available indicate that spaceflight alters gene expression patterns, the transcriptome, of at least one bacterial species. There is some evidence to suggest that bacteria can acquire an increased resistance to stresses such as acid shock after experiencing a low-shear or microgravity environment.27 However, it is unclear if bacteria and other microbes directly sense gravity. An alternative possibility that has yet to be fully explored is that the microgravity environment generates secondary physical parameters that affect microbial activity. Regardless of whether microbes sense gravity directly, the study of mechanisms by which bacteria sense and respond to microgravity environments will still serve several purposes: It provides a simple and tractable model system for how an organism detects and adapts to a microgravity environment and/or mechanical forces, it may lead to protocols that help avoid infections during long-duration spaceflight, and it will provide fundamental research that may enhance the treatment of infections on Earth.
Although it has not been conclusively demonstrated that bacteria directly sense gravitational forces, it is undeniable that they do detect mechanical signals. One of the primary ways any organism detects such forces is by mechanosensitive channels. Bacteria harbor at least two classes of such channels: the MscL and MscS families.28 A well-defined and supported function of these channels is as biological “emergency release valves” that allow bacteria to survive acute decreases in external osmotic strength.29 Although further study is required to determine if any of them play a role in sensing microgravity environments, these bacterial mechanosensors have given researchers insight into the mechanisms by which bacteria detect osmotic forces.30 They have served, and will continue to serve, as a paradigm for how mechanosensors, including those in plants and animals, detect and respond to mechanical forces including gravity,31 indicating that a robust basic research program will have far-reaching impact on our understanding of how organisms in general may be affected by spaceflight. For example, although plants are exquisitely sensitive to mechanical forces, such as the touch stimulations and pervasive vibrations experienced in the spaceflight environment, a plant touch sensor has yet to be characterized at the molecular level. Similarly, the

FIGURE 4.1 Microbial contaminants growing on the interior wall of the International Space Station where crew placed their clothing after working out. The panel eventually had to be replaced after attempts at decontamination failed. SOURCE: NASA Image ISS010E11563, available at http://www.nasa.gov/mission_pages/station/science/experiments/Environmental_Monitoring.html#images.
molecular sensors that detect amyoplast settling related to plant gravity detection within roots and shoots have yet to be identified. Some of the strongest candidates for such plant mechanosenors are homologs of the MscS family of bacterial channels.32,33,34 Thus, a comparative analysis of sensors derived from model systems has enormous potential to discover fundamental principles underlying mechano- and gravity-sensing across kingdoms.
Recent work has revealed that bacteria also contain homologs of the cytoskeletal elements actin and tubulin found in eukaryotic cells. Previous work suggested that mammalian cells use tensegrity-based cytoskeletal architecture (triangular, geodesic shapes) to translate changes in mechanical forces at the membrane to corresponding molecular responses inside the cell, including changes in cell signaling.35,36 Consistent with these ideas, in plants the actin cytoskeleton appears to play an inhibitory role in gravity perception.37,38,39 Thus, deformation of the cytoskeleton may itself serve as a widespread component of mechano- or gravity-sensors. However, it is presently unclear if this mechanism is used by bacteria, and the concept warrants study. Here again the observations reinforce the need to develop a research portfolio aimed at searching across kingdoms for commonalities and differences in the themes of sensing and response.
Finally, bacteria have intercellular communication systems that work through molecules known as quorum sensing signals.40,41 We have no idea how these signals may function in spaceflight environments. It is possible that what appears to be microgravity sensing may actually be a modification of other sensing systems, perhaps because of the decrease in shear forces and convection currents associated with reduced gravity environments. Studies of the activity of these intercellular communication systems and the intracellular signaling events they elicit may help resolve effects of partial gravity on bacterial activities as being direct or indirect, and may ultimately provide a probe for effects secondary to microgravity.
The study of mechanosensitive channels and cytoskeletal elements therefore holds promise for understanding the molecular mechanisms underlying how microbes detect mechanical forces. Further study should be performed to determine how these systems, as well as quorum sensing, are affected by a microgravity environment. The study of bacterial cell signaling affords the possibility of improving our understanding of the effects of microgravity on bacterial metabolic activities and provides a model to understand how microgravity might affect cellular signaling systems in general. The broad study of all of these potential sensory systems and their potential activation of microbial virulence or other morphological factors is of mutual interest to many funding agencies including NIH, NSF, and the Department of Defense, thus providing opportunities for collaboration with these agencies. Evolutionary similarities between mechanosensitive channels and cytoskeletal elements across kingdoms indicate that microbial studies would have implications over a wide range of other organisms. Only through an integrated, cross-kingdom research portfolio will NASA be able to approach these critical questions of the degree to which biological systems share fundamental themes of sensory systems and signal processing responsive to the environmental milieu of spaceflight.
Similar questions about mechanisms of sensing and response in microbes arise when considering the cells of more complex organisms. Although it is clear that some single-celled organisms can sense and respond to gravity, such as the directional swimming response of Euglena in a gravitational field (so called gravitaxis), the answers to the most basic questions such as, How does a eukaryotic cell sense and respond to gravity? or, Do all cells sense and/or respond to gravity? remain unclear. Yet cell research is a vital tool to extend our presence in space. Single-celled organisms and cells in culture afford specific research advantages for spaceflight because, relative to more complex forms of life, they present fewer logistical demands, replicate more frequently, and are a microcosm for ways in which more complex systems respond to space environments. Understanding their response to spaceflight represents a first step toward establishing what levels of gravity are necessary to sustain normal cellular function. Alterations in gravity, space radiation, and the host of environmental factors in spacecraft are thought to impact cells in many ways, including affecting proliferation, inducing chromosomal aberrations, and affecting gene expression. To determine how and why cellular reactions in space occur, consequences of the physical environment of spaceflight and reduced gravity such as alterations in convection must be differentiated from direct biological effects through sensory systems directly monitoring and responding to gravitational force.
Because complex organisms consist of many different cells and cell systems, responses of one cell type are not necessarily predictive of other cell types, but developing models of simple cells and their methods of detecting and responding to gravity is a critical step in understanding the reaction of complex multicellular organisms and their capacity to thrive in space. Modern cell biology techniques, such as analyses of gene expression, protein expression, and structure that help identify signals and their responses arising in space environments, will enable elucidation of underlying mechanisms.
Radiation Effects on Plants and Microbes
The sessile, photosynthetic lifestyle of plants means that many have evolved to tolerate levels of solar radiation reaching the surface of Earth, such as ultraviolet wavelengths of light, that can be extremely deleterious to animal biology. Similarly, microbes represent some of the most radiation-resistant organisms known.42 However, the response of these organisms to the radiation experienced in space remains extremely poorly defined. As described more fully in Chapter 7 and a recent report from the NRC,43 the space radiation environment presents significant challenges to biology because it differs substantially from the radiation environment on Earth and includes high-energy protons and atomic nuclei of the heavier elements. The highly charged and energetic nuclei, referred to as HZE particles, are important components of galactic cosmic radiation. HZE particles are less abundant than protons but far more hazardous. Solar disturbances can also contribute to the space radiation environment when they include solar particle events, in which high-energy protons are often the principal form of radiation. An additional source of radiation can be found in low Earth orbit, where protons are trapped in radiation belts at certain altitudes. Protons and HZE particles interact with the material of space habitats, space suits, and biological organisms. Consequently, the intensity, energy spectral characteristics, and quality of radiation inside a spacecraft or habitat differ from that in free space. The radiation dose received by biological organisms therefore varies according to the architecture of the spacecraft/habitat and when and where that organism moves around within the spacecraft.
The implementation of radiation protection is based on current understanding of the biological effects of exposure to radiation. Techniques to mitigate radiation risks include operational and scheduling procedures and physical shielding to reduce exposure to a level “as low as reasonably achievable” (based on known risks to humans). NASA’s research strategy for implementing better radiation health protection centers on the premise that space radiation can be simulated in ground-based laboratories. Much NASA-funded radiation research uses ground-based accelerators at the Brookhaven National Laboratory, where dedicated beam lines and research support facilities constitute the NASA Space Radiation Laboratory (NSRL). Both HZE particles and protons can be produced at the NSRL to perform focused, mechanistic studies on the biological consequences of exposure to the components of space radiation environments. The basic knowledge obtained from these experiments enables the prediction of effects attributable to space radiation and will reduce the associated uncertainties, thus reducing the need for large safety margins and expensive shielding. At present there are indications that microbes can show high resistance to some kinds of radiation exposure,44 but lacking is the systematic analysis of the sensitivity and responses of plants and microbes to the expected radiation environment of extended spaceflight or possible planetary outposts. Thus, research needs in spaceflight radiation include determining the fundamental effects of radiation on plants and microbes. Understanding these effects and the underlying mechanisms will foster development and implementation of strategies for operational countermeasures.
Plant and Microbial Growth Under Altered Atmospheric Pressures
The pressure and composition of Earth’s atmosphere evolved over the eons to its current levels, approximately 101 kPa (1 atmosphere) with approximately 20 percent oxygen, which support current terrestrial biology. However, providing a constant 1 atmosphere of pressure throughout the entire habitable portion of a spacecraft presents significant engineering challenges. Reducing pressures where possible has been seen as an attractive option to reduce both structural mass requirements and the levels of stored gases needed to support the crew. The lower limit of pressure for human comfort during routine activities is about 34 kPa, but only if the partial pressure of oxygen is maintained at 15 to 20 kPa. While humans could safely breathe an atmosphere almost entirely of oxygen at even
lower pressures, fire hazard becomes a concern unless a quenching gas and water vapor are added such that the total atmospheric pressure is 30 to 50 kPa.45 The Mercury, Gemini, Apollo, and Skylab programs ran at 34 kPa. The space shuttle and the ISS usually run at 101 kPa, though the shuttle pressures are reduced to approximately 75 kPa during extravehicular activities (EVAs).46,47 The atmospheric pressures aboard future vehicles and habitats are likely to be similarly variable. The Orion Crew Exploration Vehicle, for example, is designed to run at total atmospheric pressures ranging from approximately 100 kPa during ISS docking phases to approximately 54 kPa while associated with Altair and within Altair and lunar surface systems.48,49 The choices of atmospheric pressure, which are driven by operational and engineering limitations that involve containment and transition to EVA, are likely to have profound effects on plant and microbial development.
To survive, the microbes and plants present in any spaceflight habitat or environment must be able to adapt to the environmental pressures imposed upon them. However, understanding basic physiology at lower atmospheric pressures has been largely unaddressed by NASA science. This is due in part to the fact that microbial and plant spaceflight experiments have occurred largely in the shuttle and ISS eras in which 101 kPa has been the operational norm. In addition to considerations relative to plants and microbes within human spaceflight environments, a primary and long-term goal of sustaining life in remote space locations such as the Moon or Mars is to minimize the overall cost by employing bioregenerative life support systems. Because plants tolerate pressures much lower than those required for humans, well below 25 kPa depending on the plant and its stage of growth,50,51 plants could be grown within low-pressure habitats, thereby saving on the resources needed to maintain high atmospheric pressures.
During the past 10 years, some fundamental strides have been made in understanding the effects of atmospheric pressure and gas composition on plants and microbes. Studies at universities and international partner institutions have revealed that plants undergo a dramatic shift in gene expression as they alter metabolism in low atmospheric pressures, but they do adapt successfully in terms of plant productivity.52,53,54 Microbial survival has been shown to depend on atmospheric pressure, especially at extremely low pressures.55 However, for both plant and microbial responses, these studies have been limited and leave much unexplored. Such experiments can be performed in ground-based facilities.
It is critical that fundamental biological responses of plants and microbes be examined at the pressures and gas compositions that have been chosen for future spaceflight and planetary outpost systems and EVA subsystems. Because biological life support systems could be managed at lower atmospheric pressures, biological responses over a wide range of operational pressures should be investigated. Given that some vehicles and habitats may be inactivated for extended periods, the ecology of closed systems at low pressures and inactive conditions (such as reduced temperature regulation or air flow) should also be explored.
Spaceflight Syndrome I: Response to the Integrated Spaceflight Environment
Understanding the role of spaceflight environments—as a collective set of environmental components—in metabolic and physiological processes in biology, including the biology of plants and microbes, is necessary to understanding both the fundamental impacts of spaceflight on biological systems and how those impacts will influence human life support options. Although the most obvious environmental component in space is the lack of a gravity vector, there are many additional factors influencing the spaceflight environment that must also be considered and overcome. Some of those factors are independently identifiable. Radiation, for example, certainly influences biological systems. However, the spaceflight environment is actually a complex and interrelated environmental collective that arises from a range of inputs that are either intrinsic and natural (e.g., radiation, gravity) or derived from the spacecraft habitat (atmospheric composition, pressure, volatile organic compounds, variations in light spectrum, vibrations, noise, etc.). The spacecraft environment is a highly engineered volume that brings multiple potential components into environmental interactions. The integrated response to these multiple signals is likely to yield unexpected effects, especially against the unique background of microgravity. While the past decade has seen successful plant and microbial growth experiments in space, a full understanding of the deeper impacts of spaceflight on plant and microbial growth and development remains an imperative yet to be achieved.
There remain, for example, unresolved questions of whether the microgravity environment itself is deleterious to basic physiological processes in plants.56,57 Plants grown in orbiting vehicles display reduced or altered gravitropic orientations and growth forms.58,59 Physical processes that depend on gravity are diverse and include
such important functions as particle sedimentation, isothermal settling, and buoyancy-driven convection. These functions in turn determine cellular processes such as sedimentation of organelles, chromosome movement, macromolecular assembly, convection and intracellular transport, diffusion of molecules between cells, and cytoskeletal organization.60 Thus, the absence of gravity may by itself have profound effects on plant morphology and cellular physiology.
That said, the past decade has seen many experiments that resulted in essentially normal plant growth and development, including demonstrations of generational seed-to-seed growth on orbit,61,62,63 which was called out as a priority in the 1998 NRC report.64 However, changes in plant growth relative to Earth-bound controls have been documented.65-69 Many of the recent successful plant growth experiments, for example, took place in advanced hardware that removed engineering-imposed environmental stresses and compensated for altered physical components such as diffusion and convection.70,71 These successes were based on fundamental studies that had preceded them. Other examples have shown less than optimal growth or altered states of physiology or metabolism.72,73 Some alterations appear to be caused by hypoxic root zone74,75,76 conditions due to the lack of convection and plant-atmosphere gas exchange.77,78 The data indicate that such secondary effects as root zone hypoxia remain influential in directing spaceflight changes in metabolism.79-82 These aeration-related problems, as well as chromosomal abnormalities, lack of gravitropism, and reduced lignification, have all contributed to difficulties in growing completely normal plants in spaceflight environments.83,84,85
Data from the past decade show that the practice of growing plants in space continues to improve, therefore allowing a direct approach to fundamental understanding of spaceflight-induced (rather than hardware-induced) changes in metabolism. Moderately comprehensive gene expression studies are revealing genome-wide changes in gene expression of plants grown in space, relative to the controls,86,87 but the number of replicated experiments is very low, limiting the depth of understanding. Similarly, detailed examinations of plant physiology during spaceflight have revealed both fundamental conservation of some metabolisms on orbit88 and differences in others.89,90 The data suggest that much remains to be learned about plant responses to the spectrum of spaceflight and exploration environments. A high-priority research program in this area will drive both practical insight into how plants (and microbes, see below) can be grown successfully in space and advances in understanding how plants respond to their environment. The improvements in hardware for plant growth in space suggest that future experiments will need to address fewer of the syndrome components due to intrinsic factors such as hardware limitations and can instead focus on fundamental extrinsic factors such as microgravity and partial gravity or radiation exposure during both spaceflight and excursions to planetary surfaces such as the Moon and Mars. Thus, continuing to apply insights gained from basic research to improving the hardware for plant-growing systems should be integral to NASA’s vision for the next decade and beyond.
Transitions between microgravity, partial gravity, hyper-gravity, and 1 g occur as integral components of launches such as those for flights to the ISS and will occur during excursions to planetary surfaces including the Moon or Mars. Current evidence suggests that plants and microbes undergo multiple molecular and cellular responses during such transitions, yet survive launch and microgravity without gross disruption of growth and development. However, complete understanding of those transitional responses is yet to be achieved. Moreover, the fundamental question of how organisms that have spent extended periods, or their entire life, in space respond to such gravitational transitions remains unanswered. Yet these transitions will be experienced both in research settings where samples are delivered to, or recovered from spaceflight and as part of exploration missions. Exposure to spaceflight may affect subsequent plant and microbial responses to gravity and help define the response systems these organisms have to monitoring and/or responding to gravitational signals. The ability to conduct research in transitional gravity environments, together with the ability to grow plants and microbes through multiple generations offered by facilities such as the ISS, should allow for these studies to be performed on organisms entrained at specific gravity levels and thereby elucidate the biological responses to gravity transitions.
Spaceflight Syndrome II: Microbial Ecosystems and Environments
There are more bacterial than human cells in and on the human body. This microbiota is beneficial for several physiological functions including food digestion. However, the influences of microgravity and partial-gravity environments on the human microbiota have yet to be fully examined. Disturbances in the spectrum of these microbes
are known to affect human health; for example, the overgrowth of certain bacteria, such as Clostridium difficile, in the large intestine can lead to potentially life-threatening illness. Studies need to be performed on scraped skin and fecal samples to determine the bacterial species that thrive under flight conditions, determine the effectiveness of hygiene within spacecraft, and establish whether the composition of the microbiota differs consistently from that of normal terrestrial microbiota. Some environmental conditions may accelerate changes in microbiota. For example, antibiotics can alter the normal intestinal flora. How repopulation by microbes after removal of antibiotic selection might occur in space is not known, yet a normal intestinal microbiota is critical for human health. Thus, if astronauts are treated with antibiotics, a study should be done of whether their intestinal flora returns and if so, how rapidly, whether it reflects a normal intestinal flora, and whether some oral treatment with viable microbial cultures would be beneficial in re-establishing a microbial population conducive to health.
Species that are uncommon, or that have significantly increased or decreased in number, can be studied in a “microbial observatory” on the ISS, in ground-based facilities, or both. If these studies suggest that permanent changes have occurred within the species, approaches such as microarray analysis and whole-genome sequencing can be used to determine what modifications or mutations may have occurred to shift the microbial population dynamics. The continuing decline in the cost and increase in speed of genomic analysis should facilitate the comprehensive study of any changes in these microbial populations in space. Wide dissemination of this rich collection of raw data within the scientific community will allow a variety of scientific investigations. Recent advances in defining the human microbiome make this decade an ideal time to probe the influence of spaceflight on human microbiota.
Spaceflight Syndrome III: Changes in the Virulence of Pathogens
Although several studies report reactivation of latent viruses during spaceflight, the data so far suggest that this reactivation is due to dysregulation or decreases in the host’s immune response. This area is covered in depth in Chapter 6 of this report, where recommendations for future study are proposed. One must also consider fungal and bacterial infections of immunocompromised humans as potential issues during spaceflight. Bacterial pathogens pose a danger to astronauts, particularly on long spaceflights during which medical evacuation is not an option. When challenged by certain stresses, bacteria can become more resistant to antimicrobial agents. Infections forming biofilms within the body or at a tissue/catheter interface can be extremely difficult to treat. Studies have shown that Pseudomonas aeruginosa and E. coli can form biofilms in low-shear modeled microgravity,91 suggesting that biofilms could be a problem in space as they are on Earth. Spaceflight experiments are needed to augment these limited preliminary studies to clarify whether biofilms are more common or functionally different in microgravity conditions than they are at 1 g.
Other studies suggest that virulence of bacteria may increase independent of biofilm formation. The best-studied case is Salmonella enterica serovar Typhimurium, which consistently becomes more resistant to an acid shock and modestly more virulent to mice at 1 g after bacterial growth under microgravity conditions.92 Experiments aimed at understanding the underlying mechanism for this phenomenon have not yielded a solid explanation.
The ISS provides a limited platform to understand the relationship of microgravity and other aspects of space and spaceflight environments to bacterial virulence. Currently, bacterial virulence is assayed post-flight in ground-based facilities. Ultimately, one would like to study bacterial-host interactions under microgravity conditions. However, given the difficulties of containment of the potentially virulent bacteria from astronauts in a crewed flight on the ISS and the current lack of an independent life support system for such studies on these platforms, such protocols are complicated. To pursue this question, NASA should consider the use of alternative platforms such as free-flyers, as well as simple animal model systems including invertebrates, which would not pose a risk to crew health.
Microbe-Microbe Interactions
It remains unclear how space environments influence different species of bacteria and other microbes. It is conceivable, if not highly probable, that the microgravity environment, as well as other variables such as increased
radiation, changes the dynamics between species competing for specific niches. Quorum sensing is a mechanism whereby bacteria influence each other’s behavior.93 Because of the changes in fluid dynamics and convection currents in microgravity and partial-gravity environments, sensing of quorum signals could be altered in the space environment. Thus, the study of quorum sensing signals in microgravity environments could yield important insight into how bacteria compete and cooperate during spaceflight.
The NASA program called Surface, Water, and Air Biocharacterization (SWAB) carries out continued classical identification and characterization of microbes, including bacteria and molds, on surfaces and in the atmosphere of the ISS.94 Data from SWAB, including the relative abundance of each organism, may provide clues to microbial species that are more resilient in the space environment and thus should be made available to the general scientific community as a ready-made “microbial observatory.” In addition to the collection of ambient samples, direct experiments that compare mixed cultures with ground-based controls should be carried out to determine how the spaceflight environment influences microbial competition. The subsequent analyses and pursuit of the molecular mechanisms underlying any selective advantages found would be of extraordinary scientific benefit, as they would yield clues about how beneficial species (e.g., microbes for a bio-recycling center or for nitrogen fixation for plants) could be genetically altered to better compete and survive the space environment.
With current capabilities and costs for whole-genome sequencing of bacteria, it is within reach to use model bacteria to follow genomic evolution of bacteria over time in space. This will provide information about selective forces and the targets of selection during spaceflight. As reflected in the recommendations at the end of this chapter, the panel favors conducting simple genome sequencing-based experiments during the next decade on the evolution of bacteria in space.
Microbe-Plant Interactions
Plants are subject to microbial and viral pathogens. As with microbe-human interactions, studies will be required to determine any changes that may occur for these pathogen-host interactions in a low-gravity environment or generally within spaceflight or surface habitats. Currently, essentially all of the programs studying the possibility of growing viable food crops in low-gravity environments have used relatively sterile conditions and hydroponics for nutrients, yet outbreaks of plant pathogens have been seen in space-flown materials.95,96,97 However, it is unclear what microbes are likely to infect the plants, fluids, and workings of the hydroponic system in a low-gravity environment. If such facilities are to be established, for example on a lunar station, programs should be established to determine the microbiota likely to be established within these systems. As mentioned above, a long-term goal of these studies should be to determine if beneficial microbes can be selected or genetically modified to better compete with other organisms and survive the low-gravity environment.
It will be critical to determine whether changes in microbial populations and plant pathogenicity are a response to the unique elements of the spaceflight environment, such as direct effects of microgravity on microbial or plant physiology, or are effects that relate to suboptimal growth conditions such as poor humidity control or nutrient delivery in plant growth chambers designed for use in space. Such studies may not only have long-term benefits for life support systems in low-gravity environments; an increase in our understanding of how to optimize plant growth conditions/responses in the controlled environments of spaceflight may also benefit crop production in controlled environments on Earth.
Role of Plants and Microbes in Long-Term Life Support Systems
Eventually space travel will require the ability for self-sufficiency. Once mission profiles extend beyond short trips to the lunar surface, the duration of each mission will mean it will no longer remain cost-efficient or indeed feasible to dispose of all waste and resupply oxygen, water, and food to crew members from Earth. NASA has acknowledged this reality for more than two decades with programs exploring the development of both physico-chemical and bioregenerative life support systems (Figure 4.2). The program on bioregenerative capabilities arose from observations that the only truly long-term, self-sustaining life support system that has a demonstrated stability and efficacy relies upon biological systems for its function; that system is the life support afforded by Earth.
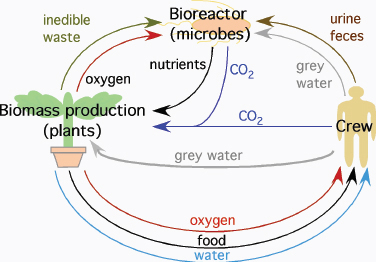
FIGURE 4.2 Bioregenerative life support systems may utilize plants and microbes to recycle waste and provide food, oxygen, and water for crews on long-duration missions. SOURCE: After D.A. Koerner, Planting Spaces in Outer Spaces, NASA Kennedy Space Center, Plantation, Fla., 2004; available at http://www.plantation.org/docs/landscape/planting-spaces.pdf; and P.L. Barry, Leafy Green Scientists, NASA Marshall Space Flight Center, Huntsville, Ala., 2001; available at http://science.nasa.gov/science-news/science-at-nasa/2001/ast09apr_1/.
NASA’s Closed Ecological (or Controlled Environment) Life Support System (CELSS) Program of the 1980s focused primarily on food crops as key for sustained production of essential life support consumables (i.e., food, oxygen, water) to make space habitats independent of periodic resupply from Earth.98,99,100 Initial research and development efforts maximized crop productivity and yield in controlled environments by elevating light levels, enriching atmospheric CO2 concentrations, optimizing growth temperatures, and using well-managed hydroponic culture to prevent water and mineral nutrients from limiting crop yield. Early studies focused on wheat, potato, soybean, and lettuce as model CELSS candidate crop species.101-104 The common outcome of these studies was yield rates exceeding world records for those crops in the field.105,106 Calculations indicated that caloric and nutritional requirements for a space-crew diet could be met by ≤50 m2 of crop-growth space per person, with air revitalization occurring by default.107,108
The subsequent Advanced Life-Support Program supported research on resource recovery, systems analysis, food technology, and some plant research during the 1990s.109-114 Several multi-institutional NASA Specialized Centers of Research and Training (NSCORTs) were established in the 1990s and early 2000s to address systems integration issues for closing loops between edible-biomass production, food processing, human activities, and resource recovery in a complex life support system that would be closed with respect to mass but open with respect to energy.115,116 Hypobaric pressure was also evaluated to reduce the structural mass required for growing plants in space.117 Physico-chemical resource recovery was added to bioregenerative approaches. A major conclusion from this work and associated systems analysis was that growing plants for maximum productivity would cost large amounts of energy, and those requirements were well beyond the likely capabilities of an early outpost.118 Since these energy needs have been identified as a key potential limitation to the large-scale cultivation of plants in space, it is expected that research will address the development of solar collection/photovoltaic-electrical storage to enable fiber-optic plant-growth lighting and energize light-emitting diodes (LEDs) to sustain crop production.
Investigating planetary in situ resource utilization may reduce the need for complete self-sufficiency of life support systems for habitation scenarios that are energy and/or resource limited.119 It should therefore be an integral component of research aimed at lunar or Mars outposts. Food plants and microbes should be selected, bred, and/or engineered for functional efficacy as well as for adaptability to particular space environments. Such a selection strategy will be viable only if supported by a vigorous program in basic plant and microbial research to define how these organisms sense and respond to the varied environments presented in space.
NASA therefore should commit to a program aimed at defining the degree to which a bioregenerative life support system could enable human habitation of space that eventually becomes independent of resupply from Earth. Implementation will need to occur in stages that are affordable, incremental, and have long-term commitment of resources. Food plants will need to be a cornerstone of this effort because they alone can synthesize nutritious, edible biomass from CO2, inorganic nutrients, and water while revitalizing the atmosphere using the energy of light. However, microbial reactors will likewise need to receive attention to provide reliable and efficient processing of the solid, liquid, and gaseous wastes of habitation. Bioregenerative systems must compete effectively with the physico-chemical/resupply strategy, so equivalent system mass needs to be reduced for crop production, for food preparation/storage, and for resource recovery. This research area may also inform the critical emerging field of sustainability science.
Because international collaborations will be essential to make rapid progress with these aims, NASA should support collaborations, where appropriate, with partners that are already pursuing these goals, such as European scientists developing component parts to the Micro-Ecological Life Support System Alternative (MELiSSA).120
AVAILABLE AND NEEDED PLATFORMS
To elucidate the effects of space environments on life, provide an understanding of life’s foundations on Earth, and facilitate exploration, access to a variety of research platforms will be required. A portfolio approach that relies on communities of investigators using model organisms, robust technology development, and all available ground and flight platforms will greatly facilitate this endeavor. It will allow repeat investigations for critical new discoveries, decrease time from selection to flight, shorten the discovery confirmation process, and enhance the mission-driven nature of life sciences research.
Ground-based research provides the basis for the design of flight research and broadens our fundamental knowledge. Space radiation in particular can be well simulated in ground-based laboratories. Accelerators at the NSRL produce both high-energy protons and the energetic nuclei of heavier elements that are important components of galactic cosmic radiation. Access to this facility will allow focused, mechanistic studies on the biological consequences of exposure of plants and microbes to the components of space radiation environments, although analysis of the effects of long-term radiation exposure for months to years simulating extended exploration missions will likely still need to be made in space.
Ground-based analysis of shared specimens and data from space experiments using new technological approaches such as transcript profiling would increase the value of research and build a broad community of researchers engaged in solving the central issues of space biology. In addition, ground-based facilities that would allow systems integration and validation for a bioregenerative life support system will need to be implemented, with the goal of testing such integration toward the end of the next 10 years, once the applicability of individual components has been rigorously verified.
Only in flight can key questions about biological responses to microgravity be addressed, providing the unique opportunity of examining terrestrial organisms without the otherwise ever-present stimulus of gravity. The ISS will provide a platform for experiments to be performed in low Earth orbit to both understand the effects of micrograv-
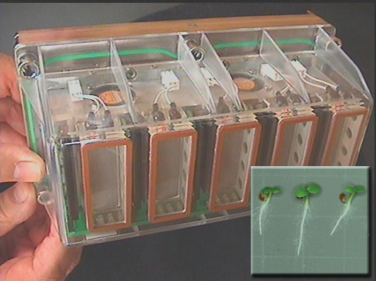
FIGURE 4.3 Current growth facilities on the ISS, such as the plant culture chambers of the European Modular Cultivation System pictured above, already provide limited access to experimental platforms to understand how the spaceflight environment affects plant growth and development. SOURCE: NASA Image 183375, available at http://www.nasa.gov/images/content/183375main_EMCS2.jpg.
ity and serve as a preparatory step to interplanetary journeys. The upcoming completion of the ISS is leading to a new era in which operations can focus on research and the development and validation of technologies to enable space exploration. In this new era, a combination of experimental design and appropriate equipment can begin to exclude the extraneous variables of the spacecraft environment to focus on critical features inherent to spaceflight such as reduced gravity and altered radiation exposures (Figure 4.3). Free-flyers would complement the research possible on the ISS. They would be especially well suited for experiments involving virulent organisms or toxic, radioactive, or otherwise dangerous materials that pose a risk to humans. Short-duration microgravity programs address the critical transition between gravity at 1 g and microgravity, over time frames in which many biological responses involved in adaptation are likely to occur. Suborbital platforms and parabolic flights are key platforms in the delivery of short-duration microgravity for biological studies.
The Lunar Surface as a Platform
While the currently existing and planned hardware on the ISS will allow microgravity experiments, manipulating gravitational stimulus via centrifuges to provide fractional gravity will only be possible for cells and very small organisms. We currently know that biological processes that operate properly at 1 g do not in microgravity, but the threshold for restoring proper function is unknown. To reduce risk and uncertainty in planning for human exploration of Mars, we need to know which biological functions will be normal in 1/3 g. The capability to carry out biological experimentation (especially if centrifuges are available) and test bioregenerative life support systems at lunar bases or potentially on robotic lunar landers will allow those questions to be answered. If biological functions are normal in the 1/6 g environment of the Moon, then they should certainly be fine on Mars. If they are still altered on the Moon, then centrifugation studies can be used to determine the impact of 1/3 g.
Progress in understanding the research issues outlined in this chapter will require access to space-adapted versions of the advanced tools and techniques that now support research in Earth-based laboratories, as well as to new tools developed to take advantage of the space environment. The following are some of the more important of these enabling technologies:
• Microanalytical technologies—molecular tags, liquid protein and gene arrays, reporter-based polymerase chain reaction, high-throughput sequencing;
• Miniaturized, autonomous processing and control systems for space biological research;
• In situ imaging systems to visualize changes in cell shape, configuration, and molecular tags;
• Advanced in-flight systems and modules for growth and nurturing of cells, microbes, and plants;
• In situ analysis and automated sample management/handling systems to permit remote measurements and data return;
• Advanced fixation and cryopreservation systems;
• Flight and lunar surface centrifuges;
• Noninvasive technologies to quantify radiation-induced damage to cells and tissue; and
• Ionizing radiation sources for synergistic studies on space-based platforms.
Some critical analytical approaches that can be performed on Earth in support of in-space experimentation include:
• Bioinformatics for discovery of key cellular and molecular systems necessary for biological organisms to thrive in space environments; and
• Computational models of molecular systems.
PRIORITIZED RESEARCH RECOMMENDATIONS
The research recommendations set forth below provide a delineated set of goals and approaches that encompass the science needed in support of the exploration mission and the science that is uniquely enabled by that exploration. These two closely connected concepts—the science that enables exploration and the science enabled by exploration—speak strongly to the powerful role of science within the human spaceflight endeavor. These research recommendations also depend on and define the resources needed to accomplish the delineated goals. Those resources include hardware and flight opportunities together with robust ground-based programs that place highly evolved experiments in the best position for spaceflight access.
1. NASA should establish a microbial observatory program on the ISS to conduct long-term, multigenerational studies of microbial population dynamics. The effects of the spaceflight environment on microbial population dynamics are largely unknown and represent both a significant gap in our knowledge and an important opportunity to study the evolution of microbial populations and predict health and engineering risks during long-term space exploration. As part of this effort, NASA should:
a. Capitalize on the technological maturity, low cost, and speed of genomic analyses and the rapid generation time of microbes to monitor the evolution of microbial genomic changes in response to the selective pressures present in the spaceflight environment;
b. Study changes in microbial populations from the skin and feces of the astronauts, plant and plant growth media, and environmental samples taken from surfaces and the atmosphere of the ISS; and
c. Establish an experimental program targeted at understanding the influence of the spaceflight environment on defined microbial populations.
Once fully implemented, within 10 years this microbial observatory program could provide significant insight into spaceflight-induced changes in the populations of beneficial and potentially harmful microbes. The program would also provide both mechanistic understanding of these changes, for example cataloging population changes and mapping/linking these to environmental niche and genomic changes, as well as insight into practical countermeasures for mitigating risks to humans and hardware. (P1)
2. NASA should establish a robust spaceflight program of research analyzing plant and microbial growth in spaceflight environments and physiological responses to the multiple stimuli encountered in those environments. The effects of the complex environment of spaceflight on plant and microbial growth, physiology, and development, such as effects of altered gravity or radiation, remain poorly characterized. Understanding these responses will be invaluable for defining how biological systems respond to spaceflight. It will provide critical information required for the successful incorporation of plants and microbes into a bioregenerative life support system and have critical impact on understanding effects of importance to human exploration of space, such as possible changes in virulence of plant and animal pathogens in space. A successful research program addressing these questions would:
a. Establish a robust spaceflight program of research analyzing plant and microbial growth and physiological responses to the multiple stimuli encountered in spaceflight environments;
b. Encourage research studying the responses to individual components of spaceflight environments, such as altered gravity, radiation, and atmospheric composition, and to the integrated effects of these multiple factors; and
c. Establish as goals for this research program both characterizing the changes elicited by the components of the spaceflight environments and conducting fundamental research to understand the basic mechanisms of plant and microbial sensing and response to these stimuli.
This program should take advantage of the many recently emerged, systems-level analytical technologies such as genomics, transcriptomics, proteomics, and metabolomics. It should also apply modern cellular and molecular approaches and integrate a vigorous flight-based and ground-based research program.
By the end of 2020, a well-targeted program using model systems and looking at the spaceflight environmental factors that are currently believed to have the largest effects (such as radiation, partial gravity, and atmospheric composition) should be able to assess the individual contributions of these stimuli to the changes that have been observed in plants and microbes growing in space. This time frame also allows for significant progress toward providing a mechanistic basis for these observed changes and the development of countermeasures to mitigate changes having significant detrimental effects on the growth of plants and beneficial microbes in space (P2).
3. NASA should develop a research program aimed at demonstrating the roles of microbial-plant systems in long-term life support systems. Incorporation of plants and microbes into a bioregenerative life support system represents one highly attractive avenue to sustain the crew on long-duration missions without resupply. However, the state of the technology is far from testing the feasibility of developing a robust, sustainable life support system incorporating biological systems as a major component. Such a program should:
a. Ensure careful development of each component of such a life support system in a rigorous ground-based research program with eventual validation in space; and
b. Establish the long-term goal of the program to be integration of each validated component into a life support system that can contribute to water and air purification, waste processing, and crew nutrition either alone or in conjunction with physico-chemical approaches.
A realistic time frame for this work would be construction of a ground-based test bed for systems integration toward the end of the next 10 years, after component systems have matured. Efficient systems integration could be expected in the 10-20 year time frame, but the technology may not be ready for deployment as an integrated,
operational life support system on the Moon and beyond for at least 20 years. Planning should begin for ground-based life support test beds including crops, food and waste processing, resource recovery, humans or their surrogates, and systems control, but such integration efforts should not dominate the space life support agenda or budget until critical components of the system have been identified, developed, and rigorously tested for appropriate functionality. Table 4.1 gives an overview of the research timeline. (P3)
Table 4.1 breaks down these broad research recommendations and technology/platform requirements along a timeline based on the current status of knowledge, goals for the next decade, and long-term aims for 2020 and beyond. It also defines advances in knowledge and capabilities that can be expected if these research emphases are pursued.
PROGRAMMATIC ISSUES AND RECOMMENDATIONS
As emphasized in the 1998 NRC strategy report on space biology,121 spaceflight opportunities should unconditionally give maximal access to peer-reviewed experiments that have a strong basis in ground tests and spaceflight performance verifications. Given the intense limitations of actual spaceflight access, no spaceflight opportunities should carry science that is not vetted by peer review within NASA or formal NASA science partnerships. NASA should coordinate agency assets, commercial payload developers, and flight systems developers in a manner that serves the best science.
Spaceflight science should also maximize repeated, multiple-sample experiment designs. All experiment profiles should include a statistical treatment that allows strong conclusions from the data. Experiments that are single runs without replication of samples and that are devoid of statistical treatment should be eliminated.
A long-term, well-supported funding base in fundamental and applied biology in space will develop a scientific community to carry out the research required to meet the prioritized science objectives. However, recent funding activities and policies have left the space biology community fragmented and less than fully committed to NASA activities. Given the time frame required for completion of the types and scales of experiments indicated in this report, typical grant funding durations should cover multiple years, with contingencies for delays in flight experiments. Stable funding of multiyear durations is essential for implementing projects that will enable a scientific community that is not only immediately responsive to short-term issues but also capable of educating the next generations of space biology scientists.
The space biology research programs will advance rapidly when supported by a robust ground research program. The ground research program will produce and refine the questions to be addressed in space. Ground research will refine the technologies to be employed in space. Ground programs also produce the range of mutant strains and other biological resources that allow spaceflight experiments to embrace and engage the most modern innovations in biological science. Ground research is critical to the longer-term biological sciences that will be engaged for life support systems. With limited orbital capacity for plant growth production, life support principles that address the scalability and applicability of plant growth in life support functions must be conducted in ground-based facilities.
Modern analytical techniques such as those employed in genomics, transcriptomics, proteomics, and metabolomics offer an immense opportunity to understand the effects of spaceflight on biological systems. Such techniques generate considerable amounts of data that can be mined and analyzed for information by multiple researchers. The creation of formalized program to promote the sharing and analysis of such data would greatly enhance the science derived from flight opportunities. Elements of such a program would include guidelines on data sharing and community access, with a focus on rapid release of these datasets while respecting the rights of the investigators conducting the experiments. A program of analysis grants, dedicated to the analysis of spaceflight-derived datasets, would provide value-added interpretation while ensuring that all data are maximally mined for information. Larger-scale multiple investigator experiments, with related science objectives, methods, and data products, would result in the production of large datasets and would emphasize analysis over implementation. Key aspects of such large-scale experiments would be replicates and statistical strength.
Biological experiments in space will benefit from a considered intermingling of automated and scientist-in-the-loop implementation. Increased automation will be required for sophisticated experiments on the ISS, free-flyers, and other platforms. Telemetric science without sample return will greatly facilitate increased sophistication in the
TABLE 4.1 Overview of Research Timeline
| Research Targets | Current Status | 2010-2020 | 2020 and Beyond | Outcomes |
| Understand the effects of space environments on microbial populations |
SWAB program samples ISS populations, little experimentation |
• Establish Microbial Observatory on the ISS to study population dynamics and genomic alterations • Generational experiments and metagenomics to investigate microbial evolution in space |
Increased understanding of the fundamental mechanisms and designs of life on Earth Ability to predict the adaptation processes of cell, microorganisms, plants, and ecosystems in response to space environments |
|
| Determine how space environments affect organisms at critical stages of growth and development |
Impacts documented, but mechanisms poorly characterized and understood Components of spaceflight syndrome not clearly separated |
• Carry out controlled comprehensive analysis with model systems • Focus rigorous, highly replicated “omic”a analyses on mechanistic questions using a single stimulus |
• Apply systems-level analyses to responses to multiple stimuli |
|
| Understand gravity sensing and response systems |
No consensus on gravity sensing in microbes Components identified in plants, but little systems understanding |
• Resolve basis of cell and microbial responses to microgravity • Define and test molecular basis using spaceflight experiments in combination with centrifugation |
• Test interactions with other stimuli • Systems analysis establishing interactions of gravity responses with other stimuli |
|
| Mitigate and manage human infectious disease risks |
Indications of altered virulence of single species of microorganisms in spaceflight conditions |
• Characterize and assess critical risks by assessing effects of space environments on pathogenic and cooperative interactions among species • Advance understanding of mechanisms |
• Develop and evaluate candidate countermeasures with ground analogs and spaceflight |
Risks to the exploration process decreased by a mechanistic appreciation of the effects of radiation, gravity, and closed environments on plant and microbe systems, and on human health and performance |
| Reduce exploration costs and risks through robust, sustainable, bioregenerative life support components |
ISS baseline is 90-day resupply Low-level effort in United States after five decades of research International efforts strong |
• Identify critical components for rigorous ground-based programs • Emphasis on fresh food first • Trade studies for low Earth orbit, the Moon, Mars |
• Integrated testing of lower equivalent system mass life support technologies and subsystems in relevant environments • Select space-optimized plants and microbes • On-orbit/lunar surface validation |
|
| Reduce uncertainties about the risks of space radiation environments to microbes and plants |
Little information about the impacts of space radiation on plants and microbes |
• Expand knowledge of risk using comprehensive analyses of model systems in ground facilities, especially the NASA Space Radiation Laboratory • Validation in the combined space environment using free-flyers and external platforms on the ISS |
• Establish acceptable levels of risk • Develop and test countermeasures including genetic resistance |
|
| Research Targets | Current Status | 2010-2020 | 2020 and Beyond | Outcomes |
| Enabling technologies |
Very limited on-orbit technology for molecular or cellular analysis; no technology development program |
• Robust development program for on-orbit/lunar-surface technologies, esp. molecular and cellular • Coordinated, comprehensive large-scale flight experiments • Programs to facilitate community analysis of datasets |
• Microanalytical technologies • Miniaturized growth, processing, and control systems for space biological research • In situ imaging systems • Centrifuges |
Automated analytical capacity off-planet (ISS, free-flyers, lunar surface) and data-sharing program to maximize research return |
| Research platform |
Ground labs including analogs; little access to shuttle, ISS |
Ground laboratories including analogs; robust programs on the ISS and free-flyers |
Ground laboratories including analogs; shuttle, the ISS, free-flyers, and lunar science stations | |
a“Omic” refers to genomics, proteomics, transcriptomics, and metabolomics.
design of space biology experimentation. However, there should be a continued emphasis on keeping scientists engaged during the conduct of the experiment, to allow the experiment to be facile and responsive to the flight profile or experiment progress. That emphasis could be accomplished by designing autonomous hardware to be communicative and responsive to remote input. Future science will be enhanced by a robust technology development program that advances these principles. That emphasis can also be accomplished by keeping scientists actively involved in the conduct of the scientist-tended experiments wherever possible, such as on parabolic, suborbital, and when possible, orbital platforms.
Space biology represents a potential opportunity for coalescing disparate programmatic elements within NASA and its international partners. Biological studies discussed in this report currently have representation in multiple parts of NASA, including astrobiology, planetary protection, fundamental space biology, and exploration life sciences. A cohesive and visible voice at NASA headquarters would leverage the biological representation among programs such as planetary protection, astrobiology, and bioastronautics. Coordination with international partners, the ISS National Laboratory partners, and commercial partners would help complete the vision of space biology.
1. Kanas, N., and Manzey, D. 2003. Space Psychology and Psychiatry. Kluwer Academic Publishers, Dordecht, The Netherlands.
2. Ulrich, R.S. 1984. View through a window may influence recovery from surgery. Science 224(4647):420-421.
3. Ulrich, R.S., Simons, R.F., Losito, B.D., Fiorito, E., Miles, M.A., and Zelson, M. 1991. Stress recovery during exposure to natural and urban environments. Journal of Environmental Psychology 11:201-230.
4. Hoson, T., and Soga, K. 2003. New aspects of gravity responses in plant cells. International Review of Cytology 229:209-244.
5. Morita, M.T., and Tasaka, M. 2004. Gravity sensing and signaling. Current Opinion in Plant Biology 7(6):712-718.
6. Perrin, R.M., Young, L.S., Murthy, U.M.N., Harrison, B.R., Wang, Y., Will, J.L., and Masson, P.H. 2005. Gravity signal transduction in primary roots. Annals of Botany (London) 96(5):737-743.
7. Hoson, T., and Soga, K. 2003. New aspects of gravity responses in plant cells. International Review of Cytology 229:209-244.
8. Perrin, R.M., Young, L.S., Murthy, U.M.N., Harrison, B.R., Wang, Y., Will, J.L., and Masson, P.H. 2005. Gravity signal transduction in primary roots. Annals of Botany (London) 96(5):737-743.
9. LaMotte, C.E., and Pickard, B.G. 2004. Control of gravitropic orientation. II. Dual receptor model for gravitropism. Functional Plant Biology 31(2):109-120.
10. Wolverton, C., Mullen, J.L., Ishikawa, H., and Evans, M.L. 2002. Root gravitropism in response to a signal originating outside of the cap. Planta 215(1):153-157.
11. Morita, M.T., and Tasaka, M. 2004. Gravity sensing and signaling. Current Opinion in Plant Biology 7(6):712-718.
12. Blancaflor, E.B., and Masson, P.H. 2003. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiology 133(4):1677-1690.
13. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
14. Titapiwatanakun, B., and Murphy, A.S. 2009. Post-transcriptional regulation of auxin transport proteins: Cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. Journal of Experimental Botany 60(4):1093-1107.
15. Titapiwatanakun, B., and Murphy, A.S. 2009. Post-transcriptional regulation of auxin transport proteins: Cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. Journal of Experimental Botany 60(4):1093-1107.
16. Feraru, E., and Friml, J. 2008. PIN polar targeting. Plant Physiology 147(4):1553-1559.
17. Morita, M.T., and Tasaka, M. 2004. Gravity sensing and signaling. Current Opinion in Plant Biology 7(6):712-718.
18. Khush, G.S. 1999. Green revolution: Preparing for the 21st century. Genome 42(4):646-655.
19. Pinthus, M.J. 1973. Lodging in wheat, barley and oats; the phenomenon—Its causes and preventative measures. Advances in Agronomy 25:209-263.
20. Gilroy, S., and Masson, P.H. 2008. Plant Troipisms. Blackwell, Oxford, U.K.
21. Massa, G.D., and Gilroy, S. 2003. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant Journal 33(3):435-445.
22. Kobayashi, A., Takahashi, A., Kakimoto, Y., Miyazawa, Y., Fujii, N., Higashitani, A., and Takahashi, H. 2007. A gene essential for hydrotropism in roots. Proceedings of the National Academy of Sciences U.S.A. 104(11):4724-4729.
23. Takahashi, H., Miyazawa, Y., and Fujii, N. 2009. Hormonal interactions during root tropic growth: Hydrotropism versus gravitropism. Plant Molecular Biology 69(4):489-502.
24. Kimbrough, J.M., Salinas-Mondragon, R., Boss, W.F., Brown, C.S., and Sederoff, H.W. 2004. The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiology 136(1):2790-2805.
25. Salinas-Mondragon, R., Brogan, A., Ward, N., Perera, I., Boss, W., Brown, C.S., and Sederoff, H.W. 2005. Gravity and light: Integrating transcriptional regulation in roots. Gravitational and Space Biology Bulletin 18(2):121-122.
26. Horneck, G., Mancinelli, R., and Klaus, D. 2010. Space microbiology. Microbiology and Molecular Biology Reviews 74(1):121-156.
27. Wilson, J.W., Ott, C.M., Höner zu Bentrup, K., Ramamurthy, R., Quick, L., Porwollik, S., Cheng, P., McClelland, M., Tsaprailis, G., Radabaugh, T., Hunt, A., et al. 2007. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proceedings of the National Academy of Sciences U.S.A. 104(41):16299-16304.
28. Pivetti, C.D., Yen, M.R., Miller, S., Busch, W., Tseng, Y.H., Booth, I.R., and Saier, M.H., Jr. 2003. Two families of mechanosensitive channel proteins. Microbiology and Molecular Biology Reviews 67(1):66-85.
29. Levina, N., Tötemeyer, S., Stokes, N.R., Louis, P., Jones, M.A., and Booth, I.R. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: Identification of genes required for MscS activity. The EMBO Journal 18(7):1730-1737.
30. Blount, P., Iscla, I., and Li, Y. 2008. Mechanosensitive channels and sensing osmotic stimuli in bacteria. Pp. 25-47 in Sensing with Ion Channels (B. Martinac, ed.). Springer-Verlag Press, Berlin, Germany.
31. Blount, P., Li, Y., Moe, P.C., and Iscla, I. 2008. Mechanosensitive channels gated by membrane tension: Bacteria and beyond. Pp. 71-101 in Mechanosensitive Ion Channels (A. Kamkin and I. Kiseleva, eds.). Mechanosensitivity in Cells and Tissues, Volume 1. Springer Press, New York.
32. Haswell, E.S., and Meyerowitz, E.M. 2006. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Current Biology 16(1):1-11.
33. Haswell, E.S., Peyronnet, R., Barbier-Brygoo, H., Meyerowitz, E.M., and Frachisse, J.M. 2008. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Current Biology 18(10):730-734.
34. Peyronnet, R., Haswell, E.S., Barbier-Brygoo, H., and Frachisse, J.M. 2008. AtMSL9 and AtMSL10: Sensors of plasma membrane tension in Arabidopsis roots. Plant Signaling and Behavior 3(9):726-729.
35. Ingber, D.E. 1997. Integrins, tensegrity, and mechanotransduction. Gravitational and Space Biology Bulletin 10(2):49-55.
36. Ingber, D.E. 2003. Tensegrity II. How structural networks influence cellular information processing networks. Journal of Cell Science 116(Pt 8):1397-1408.
37. Hou, G., Kramer, V.L., Wang, Y.S., Chen, R., Perbal, G., Gilroy, S., and Blancaflor, E.B. 2004. The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant Journal 39(1):113-125.
38. Hou, G., Mohamalawari, D.R., and Blancaflor, E.B. 2003. Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiology 131(3):1360-1373.
39. Yamamoto, K., and Kiss, J.Z. 2002. Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiology 128(2):669-681.
40. Ng, W.L., and Bassler, B.L. 2009. Bacterial quorum-sensing network architectures. Annual Review of Genetics 43:197-222.
41. Fuqua, C., and Greenberg, E.P. 2002. Listening in on bacteria: Acyl-homoserine lactone signalling. Nature Reviews Molecular Cell Biology 3(9):685-695.
42. DeVeaux, L.C., Müller, J.A., Smith, J., Petrisko, J., Wells, D.P., and DasSarma, S. 2007. Extremely radiation-resistant mutants of a halophilic archaeon with increased single-stranded DNA-binding protein (RPA) gene expression. Radiation Research 168:507-514.
43. National Research Council. 2008. Managing Space Radiation Risk in the New Era of Space Exploration. The National Academies Press, Washington D.C.
44. DeVeaux, L.C., Müller, J.A., Smith, J., Petrisko, J., Wells, D.P., and DasSarma, S. 2007. Extremely radiation-resistant mutants of a halophilic archaeon with increased single-stranded DNA-binding protein (RPA) gene expression. Radiation Research 168:507-514.
45. Paul, A.L., and Ferl, R. 2006.The biology of low atmospheric pressure—Implications for exploration mission design and advanced life support. Gravitational and Space Biology 19:3-17.
46. Paul, A.L., and Ferl, R. 2006. The biology of low atmospheric pressure—Implications for exploration mission design and advanced life support. Gravitational and Space Biology 19:3-17.
47. NASA Exploration Atmospheres Working Group. 2006. Recommendations for Exploration Spacecraft Internal Atmospheres. NASA Johnson Space Center, Houston, Tex.
48. NASA Exploration Atmospheres Working Group. 2006. Recommendations for Exploration Spacecraft Internal Atmospheres. NASA Johnson Space Center, Houston, Tex.
49. Anderson, M., Curley, S., Stambaugh, I., and Rotter, H. 2009. Altair lander life support: Design analysis cycles 1, 2, and 3. SAE 39th International Conference on Environmental Systems. Savannah, Ga., July 12-16, 2009. SAE Technical Paper 2009-01-2477. SAE International, Warrendale, Pa.
50. Andre, M., and Richaux, C. 1986. Can plants grow in quasi-vacuum? Pp. 395-404 in CELSS 1985 Workshop. NASA Publication TM 88215. NASA Ames Research Center, Moffett Field, Calif.
51. McKay, C.P., and Toon, O.B. 1991. Making Mars habitable. Nature 352:489-496.
52. Paul, A.L., Schuerger, A.C., Popp, M.P., Richards, J.T., Manak, M.S., and Ferl, R.J. 2004. Hypobaric biology: Arabidopsis gene expression at low atmospheric pressure. Plant Physiology 134(1):215-223.
53. Richards, J.T., Corey, K.A., Paul, A.L., Ferl, R.J., Wheeler, R.M., and Schuerger, A.C. 2006. Exposure of Arabidopsis thaliana to hypobaric environments: implications for low-pressure bioregenerative life support systems for human exploration missions and terraforming on Mars. Astrobiology 6(6):851-866.
54. Corey, K.A., Barta, D.J., and Henninegr, D.L. 1999. Photosynthesis and respiration of a wheat stand at reduced atmospheric pressure and reduced oxygen. Advances in Space Research 20:1869-1877.
55. Schuerger, A.C., and Nicholson, W.L. 2006. Interactive effects of low pressure, low temperature, and CO2 atmospheres inhibit the growth of Bacillus spp. under simulated martian conditions. Icarus 181:52-62.
56. Halstead, T.W., and Dutcher, F.R. 1984. Status and prospects. Annals of Botany 54(Suppl. 3):3-18.
57. Dutcher, F.R., Hess, E.L., and Halstead, T.W. 1994. Progress in plant research in space. Advances in Space Research 14(8):159-171.
58. Volkmann, D., Behrens, H.M., and Sievers, A. 1986. Development and gravity sensing of Cress roots under microgravity. Naturwiss 73:438-441.
59. Kern, V.D., Schwuchow, J.M., Reed, D.W., Nadeau, J.A., Lucas, J., Skripnikov, A., and Sack, F.D. 2005. Gravitropic moss cells default to spiral growth on the clinostat and in microgravity during spaceflight. Planta 221(1):149-157.
60. Todd, P. 1989. Gravity-dependent phenomena at the scale of the single cell. American Society for Gravitational and Space Biology Bulletin 2:95-113.
61. Musgrave, M.E., and Kuang, A. 2003. Plant reproductive development during spaceflight. Advances in Space Biology and Medicine 9:1-23.
62. Musgrave, M.E., and Kuang, A. 2001. Reproduction during spaceflight by plants in the family Brassicaceae. Journal of Gravitational Physiology 8(1):P29-P32.
63. Musgrave, M.E., Kuang, A., Xiao, Y., Stout, S.C., Bingham, G.E., Briarty, L.G., Levenskikh, M.A., Sychev, V.N., and Podolski, I.G. 2000. Gravity independence of seed-to-seed cycling in Brassica rapa. Planta 210(3):400-406.
64. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
65. Musgrave, M.E., and Kuang, A. 2003. Plant reproductive development during spaceflight. Advances in Space Biology and Medicine 9:1-23.
66. Musgrave, M.E., and Kuang, A. 2001. Reproduction during spaceflight by plants in the family Brassicaceae. Journal of Gravitational Physiology 8(1):P29-P32.
67. Paul, A.L., Daugherty, C.J., Bihn, E.A., Chapman, D.K., Norwood, K.L., and Ferl, R.J. 2001. Transgene expression patterns indicate that spaceflight affects stress signal perception and transduction in arabidopsis. Plant Physiology 126(2):613-621.
68. Stutte, G.W., Monje, O., Hatfield, R.D., Paul, A.L., Ferl, R.J., and Simone, C.G. 2006. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 224(5):1038-1049.
69. Stout, S.C., Porterfield, D.M., Briarty, L.G., Kuang, A., and Musgrave, M.E. 2001. Evidence of root zone hypoxia in Brassica rapa L. grown in microgravity. International Journal of Plant Sciences 162(2):249-255.
70. Paul, A.L., Daugherty, C.J., Bihn, E.A., Chapman, D.K., Norwood, K.L., and Ferl, R.J. 2001. Transgene expression patterns indicate that spaceflight affects stress signal perception and transduction in arabidopsis. Plant Physiology 126(2):613-621.
71. Stutte, G.W., Monje, O., Hatfield, R.D., Paul, A.L., Ferl, R.J., and Simone, C.G. 2006. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 224(5):1038-1049.
72. Halstead, T.W., and Dutcher, F.R. 1984. Status and prospects. Annals of Botany 54(Suppl. 3):3-18.
73. Dutcher, F.R., Hess, E.L., and Halstead, T.W. 1994. Progress in plant research in space. Advances in Space Research 14(8):159-171.
74. Cowles, J.R., Scheld, H.W., Lemay, R., and Peterson, C. 1984. Growth and lignification in seedlings exposed to eight days of microgravity. Annals of Botany 54(Suppl. 3):3-18.
75. Krikorian, A.D., and O’Conner, S.A. 1984. Karyological observations. Annals of Botany 54(Suppl. 3):49-63.
76. Slocum, R.D., Gaynor, J.J., and Galston, A.W. 1984. Cytological and ultrastructural studies on root tissues. Annals of Botany 54(Suppl. 3):65-76.
77. Musgrave, M.E., Kuang, A., and Matthews, S.W. 1997. Plant reproduction during spaceflight: Importance of the gaseous environment. Planta 203(Suppl.):S177-S184.
78. Michael, G., Musgrave, M., Bard, V., Thwaites, S., Beney, J., Sumners, S., Krasner, D., and Wyndham, M. 1988. Self referral to consultants [letter]. British Medical Journal (Clinical Research Ed.) 296(6622):640.
79. Slocum, R.D., Gaynor, J.J., and Galston, A.W. 1984. Cytological and ultrastructural studies on root tissues. Annals of Botany 54(Suppl. 3):65-76.
80. Hullinger, R.L. 1993. The avian embryo responding to microgravity of space flight. Physiologist 36(1 Suppl):S42-S45.
81. Vartapetian, B.B. 1991. Flood sensitive plants under primary and secondary anoxia: Ultrastructure and metabolic responses. Pp. 201-216 in Plant Life Under Oxygen Deprivation (M.B. Jackson, D.D. Davies, and H. Lambers, eds.). SPB Academic Publishing, The Hague, Netherlands.
82. Davies, W.J., Metcalfe, J.C., Schurr, U., Taylor, G., and Zhang, J. 1987. Hormones as chemical signals involved in root to shoot communication of effects of changes in the soil environment. Pp. 206-216 in Hormone Action in Plant Development, A Critical Appraisal (G.V. Hoad, M.B. Jackson, J.R. Lenton, and R. Atkin, eds.). Butterworths, London, U.K.
83. Cowles, J.R., Scheld, H.W., Lemay, R., and Peterson, C. 1984. Growth and lignification in seedlings exposed to eight days of microgravity. Annals of Botany 54(Suppl. 3):3-18.
84. Krikorian, A.D., and O’Conner, S.A. 1984. Karyological observations. Annals of Botany 54(Suppl. 3):49-63.
85. Slocum, R.D., Gaynor, J.J., and Galston, A.W. 1984. Cytological and ultrastructural studies on root tissues. Annals of Botany 54(Suppl. 3):65-76.
86. Paul, A.L., Poppb, M.P., Gurleyc, W.B., Guyd, C., Norwoode, K.L., and Ferla, R.J. 2005. Arabidopsis gene expression patterns are altered during spaceflight. Advances in Space Research 36(7):1175-1181.
87. Solheim, B.G., Johnsson, A., and Iversen, T.H. 2009. Ultradian rhythms in Arabidopsis thaliana leaves in microgravity. The New Phytologist 183(4):1043-1052.
88. Monje, O., Stutte, G., and Chapman, D. 2005. Microgravity does not alter plant stand gas exchange of wheat at moderate light levels and saturating CO2 concentration. Planta 222(2):336-345.
89. Stutte, G.W., Monje, O., Goins, G.D., and Tripathy, B.C. 2005. Microgravity effects on thylakoid, single leaf, and whole canopy photosynthesis of dwarf wheat. Planta 223(1):46-56.
90. Tripathy, B.C., Brown, C.S., Levine, H.G., and Krikorian, A.D. 1996. Growth and photosynthetic responses of wheat plants grown in space. Plant Physiology 110(3):801-806.
91. McLean, R.J., Cassanto, J.M., Barnes, M.B., and Koo, J.H. 2001. Bacterial biofilm formation under microgravity conditions. FEMS Microbiology Letters 195(2):115-119.
92. Wilson, J.W., Ott, C.M., Höner zu Bentrup, K., Ramamurthy, R., Quick, L., Porwollik, S., Cheng, P., McClelland, M., Tsaprailis, G., Radabaugh, T., Hunt, A., et al. 2007. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proceedings of the National Academy of Sciences U.S.A. 104(41):16299-16304.
93. Ng, W.L., and Bassler, B.L. 2009. Bacterial quorum-sensing network architectures. Annual Review of Genetics 43:197-222.
94. Evans, C.A., Robinson, J.A., Tate-Brown, J., Thumm, T., Crespo-Richey, J., Baumann, D., and Rhatigan, J. 2008. International Space Station Science Research Accomplishments During the Assembly Years: An Analysis of Results from 2000-2008. NASA/TP-2009-213146-REVISION A. NASA Center for AeroSpace Information, Hanover, Md.
95. Bishop, D.L., Levine, H.G., Kropp, B.R., and Anderson, A.J. 1997. Seedborne fungal contamination: Consequences in space-grown wheat. Phytopathology 87(11):1125-1133.
96. Leach, J.E., Ryba-White, M., Sun, Q., Wu, C.J., Hilaire, E., Gartner, C., Nedukha, O., Kordyum, E., Keck, M., Leung, H., and Guikema, J.A. 2001. Plants, plant pathogens, and microgravity—A deadly trio. Gravitational and Space Biology Bulletin 14(2):15-23.
97. Ryba-White, M., Nedukha, O., Hilaire, E., Guikema, J.A., Kordyum, E., and Leach, J.E. 2001. Growth in microgravity increases susceptibility of soybean to a fungal pathogen. Plant Cell Physiology 42(6):657-664.
98. Galston, A.W. 1992. Photosynthesis as a basis for life-support on Earth and in space. Bioscience 42(7):490-493.
99. MacElroy, R.D., and Bredt, J. 1985. Controlled Ecological Life Support System: Life Support Systems in Space Travel. NASA Conference Publication 2378. NASA Ames Research Center, Moffett Field, Calif.
100. NASA Office of Space Science and Applications, Life Sciences Division. 1985. Controlled Ecological Life Support Systems (CELSS) Program Plan. NASA, Washington, D.C.
101. Bugbee, B.G., and Salisbury, F.B. 1988. Exploring the limits of crop productivity. 1. Photosynthetic efficiency of wheat in high irradiance environments. Plant Physiology 88(3):869-878.
102. Knight, S.L., and Mitchell, C.A. 1983. Enhancement of lettuce yield by manipulation of light and nitrogen nutrition. Journal of the American Society for Horticultural Science 108(5):750-754.
103. Raper, C.D., Vessey, J.K., and Henry, L.T. 1991. Increase in nitrate uptake by soybean plants during interruption of the dark period with low intensity light. Physiologia Plantarum 81(2):183-189.
104. Wheeler, R.M., and Tibbitts, T.W. 1987. Utilization of potatoes for life-support-systems in space. 3. Productivity at successive harvest dates under 12-H and 24-H photoperiods. American Potato Journal 64(6):311-320.
105. Bugbee, B., and Monje, O. 1992. The limits of crop productivity. Bioscience 42(7):494-502.
106. Knight, S., and Mitchell, C.A. 1987. Stimulating productivity of hydroponic lettuce by foliar application of triacontanol. Horticultural Science 22:1307-1309.
107. Salisbury, F.B., and Bugbee, B. 1984. Wheat farming in a lunar base. Pp. 635-645 in Lunar Bases and Space Activities of the 21st Century (W.W. Mendel, ed.). Lunar and Planetary Institute, Houston, Tex.
108. Wheeler, R.M. 1996. Gas balances in a plant-based CELSS. Pp. 207-216 in Plants in Space Biology (H. Suge, ed.). Tohoku University, Sendai, Japan.
109. Averner, M. 1993. NASA Advanced Life Support Program Plan. Office of Life and Microgravity Sciences and Applications Division, NASA, Washington, D.C.
110. Hill, W.A., Loretan, P.A., Bonsi, C.K., Morris, C.E., Lu, J.Y., and Ogbuehi, C. 1989. Utilization of sweet potatoes in controlled ecological life support systems (CELSS). Advances in Space Research 9:1631-1635.
111. Hoff, J.E., Howe, J.M., and Mitchell, C.A. 1982. Nutritional and Cultural Aspects of Plant Species Selection for a Controlled Ecological Life Support System. NASA Contractor Report 166324. NASA Ames Research Center, Moffett Field, Calif.
112. Ohler, T.O., and Mitchell, C.A. 1995. Effects of carbon dioxide level and plant density on cowpea canopy productivity for a bioregenerative life support system. Life Support and Biosphere Science 2:3-9.
113. Tibbits, T.W., and Alford, D.K. 1982. Controlled Ecological Life Support System Use of Higher Plants. NASA Conf. Publ. 2231. NASA Ames Research Center, Moffett Field, Calif.
114. Volk, G.M., and Mitchell, C.A. 1995. Photoperiod shift effects on yield characteristics of rice. Crop Science 35(6):1631-1635.
115. Stutte, G.W., Monje, O., Goins, G.D., and Tripathy, B.C. 2005. Microgravity effects on thylakoid, single leaf, and whole canopy photosynthesis of dwarf wheat. Planta 223(1):46-56.
116. Tripathy, B.C., Brown, C.S., Levine, H.G., and Krikorian, A.D. 1996. Growth and photosynthetic responses of wheat plants grown in space. Plant Physiology 110(3):801-806.
117. Corey, K.A., Barta, D.J., and Henninegr, D.L. 1999. Photosynthesis and respiration of a wheat stand at reduced atmospheric pressure and reduced oxygen. Advances in Space Research 20:1869-1877.
118. Mitchell, C.A., Dougher, T.A.O., Nielsen, S.S., Belury, M.A., and Wheeler, R.M. 1996. Costs of providing edible biomass for a balanced vegetarian diet in a controlled ecological life-support system. Pp. 245-254 in Plants in Space Biology (H. Suge, ed.). Tohoku University, Sendai, Japan.
119. Monje, O., Stutte, G., and Chapman, D. 2005. Microgravity does not alter plant stand gas exchange of wheat at moderate light levels and saturating CO2 concentration. Planta 222(2):336-345.
120. de Micco, V., Aronne, G., Joseleau, J.P., and Ruel, K. 2008. Xylem development and cell wall changes of soybean seedlings grown in space. Annals of Botany (London) 101(5):661-669.
121. National Research Council. 1998. A Strategy for Research in Space Biology and Medicine in the New Century. National Academy Press, Washington, D.C.
























