9
Information Gaps and Research Needs
The purpose of this report is to review available data and establish science-based reference values for calcium and vitamin D, known as Dietary Reference Intakes (DRIs). The approach used has been that of risk assessment, as described in Chapter 1. This final chapter outlines information gaps and research needs identified by the committee in carrying out its charge. These gaps and research needs are also organized according to the risk assessment framework. The listings are not comprehensive, but offer the committee’s perspective on the major topic areas in need of attention. These needs are targeted to academic and medical researchers, national policy makers, the public health community, industry groups, and other relevant stakeholders and funding institutions. They provide a basis for organizing and prioritizing research efforts.
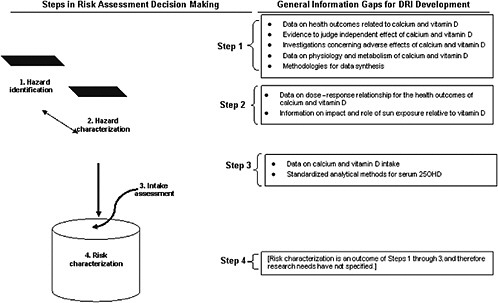
The general nature of the information gaps relevant to DRI development for calcium and vitamin D are outlined in Figure 9-1.
Although the uncertainties surrounding the DRIs have been described in this report and the scientific judgments made are documented, evidence from future research designed to overcome the limitations encountered by this committee can improve the ability to determine reference values in the future. Although the committee’s discussions form the basis for the identification of these research needs, other sources of research needs were noted, for example the National Institutes of Health Roundtable on Vitamin D Research Needs (Brannon et al., 2008) and the report from the Tufts Medical Center Evidence-based Practice Center (Chung et al., 2010).
Table 9-1 presents the identified research needs, which are then outlined.
TABLE 9-1 Vitamin D and Calcium Research Needs Organized by Risk Assessment Steps
|
Research Topic |
Research Questions and Identified Needs |
|
Step 1: “Hazard Identification” or Indicator Review and Selection |
|
|
Health Outcomes and Related Conditions |
|
|
Adverse Effects, Toxicity, and Safety |
|
|
Basic Physiology and Molecular Pathways |
|
|
Synthesizing Evidence and Research Methodology |
|
|
Step 2: “Hazard Characterization” or Intake-Response Assessment and Specification of DRIs |
|
|
Dose–Response Relationship |
|
|
Research Topic |
Research Questions and Identified Needs |
|
Sun Exposure |
|
|
Step 3: Intake Assessment |
|
|
Intake Assessment |
|
STEP 1:
“HAZARD IDENTIFICATION” OR INDICATOR REVIEW AND SELECTION
The committee found an overall lack of causal evidence from intervention studies for the task of identifying health outcome indicators. This was especially true for non-skeletal outcomes for vitamin D, but this was also true for skeletal outcomes, particularly in certain life stage groups. Data related to calcium were sparse for children and younger adults. Most vitamin D studies were conducted using older persons or postmenopausal women. Some available data suggested the possibility of ethnic differences in bone health, but this suggestion could not be further clarified. Very few studies explored the independent effects of calcium and vitamin D. Only limited data were available on adverse health effects. These information gaps, coupled with challenges in synthesizing disparate evidence for either calcium or vitamin D or their combination, presented challenges to DRI development. Further, lack of clarity concerning the physiology and metabolism of vitamin D was problematic as was the ability to judge the effects of vitamin D as a nutrient given its role as a prohormone.
Research Needs Related to Health Outcomes and Related Conditions
-
Clarify threshold effects of calcium and vitamin D on skeletal health outcomes by life stage and for different racial/ethnic
-
groups. Although there is a solid body of evidence related to bone health and the role of calcium and vitamin D, many data gaps remain for younger age groups and for the effect under menopausal conditions. The issue of “calcium economy” among certain groups and ethnic differences in vitamin D utilization require attention.
-
Elucidate inter-relationship between calcium and vitamin D, and specify independent effect(s) of each. There is a need for research protocols that examine the effects of vitamin D and calcium separately rather than as a combined administration, and which better clarify the nature of the inter-relationship. Without such data, the ability to identify requirements for calcium and for vitamin D is challenging.
-
Explore causal role for vitamin D in non-skeletal health outcomes. Investigation of causal relationships between vitamin D nutriture and potential non-skeletal health outcomes should undergo further research. These may include but are not limited to (no particular order): immune function and anti-inflammatory effects (especially related to obesity); total and site-specific cancers; cardiovascular disease; and diabetes. More data on the role of calcium and vitamin D, and their metabolism, during pregnancy and lactation is needed.
-
Determine appropriateness of serum 25-hydroxyvitamin D (25OHD) as a biomarker of effect. The ability to use the relatively accessible measure of serum 25OHD as a biomarker or surrogate is limited by a number of factors including not only its role as a prohormone, but also its variability, which is due to a number of non-nutritional factors. A better understanding of its relationship to specific health outcome would be beneficial, enhancing both the quality and quantity of research available. The measure should be studied for this purpose and also should be subject to a formal validation process.
-
Elucidate the effect of genetic variation, including that among racial/ethnic groups, and epigenetic regulation of vitamin D on developmental outcomes. This is an emerging field of study, which will likely prove relevant to DRI development. Studies in this area may contribute notably to an understanding of population differences related to chronic disease risk.
Research Needs Related to Adverse Effects, Toxicity, and Safety
-
Develop innovative methodologies to provide for identification and assessment of adverse effects of excess calcium and vitamin D. The ability to study adverse effects of calcium and vitamin D is
-
often limited due to ethical concerns. Creative approaches using an array of methodologies developed in other fields need to be adapted for nutritional use and incorporated into the approach for studying adverse effects of nutrients in vitro and in vivo using relevant animal models.
-
Elucidate adverse effects of long-term, high-dose calcium and vitamin D. The question of nutrient safety should not be a secondary aspect of study design nor can the failure to detect adverse effects as part of a study not designed for that purpose be considered an adequate assessment of safety. Dedicated studies are needed to assess adverse health effects related to long-term, high dose (although not necessarily “toxic”) levels of calcium and vitamin D.
-
Further explore the nature of vitamin D toxicity. Although toxicity is not the most appropriate goal for setting ULs, a better understanding of the timing, doses, and mechanisms associated with vitamin D toxicity (hypervitaminosis D) would be beneficial to understanding the impact of vitamin D on the human body. Of particular import is information about the metabolic fate and dynamics of high doses of vitamin D. The identification and use of animal models (particularly large animal models) would be especially helpful. Also needed is an understanding of how weight loss in obese individuals might affect vitamin D status and adverse outcomes (e.g., bariatric surgery patients).
Research Needs Related to Basic Physiology and Molecular Pathways
-
Examine the influence of calcium and phosphate on the regulation of vitamin D activation and catabolism through parathyroid hormone and fibroblast-like growth factor 23 (FGF23). Identify pathways that regulate vitamin D activation and catabolism through parathyroid hormone and FGF23 in order to understand the influence of calcium and phosphate intake on vitamin D regulation.
-
Clarify 25OHD distribution in body pools including storage and mobilization from adipose tissue. Understanding the distribution, storage, and mobilization of 25OHD in body pools would enhance the understanding regarding relationships among exposure to vitamin D from intake or endogenous synthesis, circulation serum levels of 25OHD, and health outcomes. The role of storage compartments and factors important to the mobilization of vitamin D is noticeably lacking.
-
Evaluate the nature and significance of extra-renal production of calcitriol for health outcomes. Determining the significance of extra-renal production of calcitriol for health outcomes is essential
-
to understand whether local production of calcitriol has an impact on health outcomes. In turn, the relevance of vitamin D nutriture and serum 25OHD for such an effect should be established.
-
Clarify the extent to which differences exist between vitamin D2and vitamin D3. Physiological responses as well as potential for differences in safety risks for the two forms of the nutrient should be further explored.
Synthesizing Evidence and Research Methodology
-
Explore enhanced methodologies for data synthesis. Alternative methods for synthesizing evidence from different study types and multiple parameters that consider uncertainties (including measurement error) include teleoanalysis, confidence profile predictive meta-analysis, and generalized multi-parameter evidence synthesis. In the case of calcium and vitamin D, such approaches should facilitate quantitative estimates of effect size and dose–response relationships as needed for DRI development.
-
Identify approaches to weight better potential health outcomes. In order to ensure the most objective and comprehensive systematic evidence reviews in the future, approaches to better weight potential health outcomes are needed.
STEP 2:
“HAZARD CHARACTERIZATION” OR INTAKE-RESPONSE ASSESSMENT AND SPECIFICATION OF DIETARY REFERENCE INTAKES
The committee encountered major challenges in determining the dose–response relationships for calcium and vitamin D. Sun exposure introduced further uncertainties regarding vitamin D.
Research Related to Dose–Response Relationships
-
Conduct studies to identify specific health outcomes in relation to graded and fully measured intakes of calcium and vitamin D. Too few studies are specifically designed to study the effects of graded doses of calcium or vitamin D on health outcomes, both overall and as part of the same study using the same subjects and outcome measures. Further, many studies in the calcium and vitamin D area are confounded by the failure to specify or measure and thereby take into account “background” intakes of the nutrient being studied when dose–response is being explored.
-
Clarify the influence of age, body weight, and body composition on serum 25OHD levels in response to intake/exposure. Information about how factors such as age, body weight, and body composition affect the variability in serum 25OHD response to intake or exposure would assist in the process of establishing requirements for vitamin D. Such information is also important to ascertaining the measure’s utility as a biomarker of effect and in making judgments about excess intake of the vitamin.
Research Needs Related to Sun Exposure
-
Investigate whether a minimal-risk ultraviolet B (UVB) radiation exposure relative to skin cancer exists that also enables vitamin D production. Whether a minimal or threshold UVB exposure level is possible to both enable subcutaneous vitamin D synthesis and avoid risk of skin cancer needs to be examined. Research should include assessment of the risk for skin cancer compared with the benefit of endogenous synthesis of vitamin D, particularly for at-risk populations.
-
Clarify how physiological factors such as skin pigmentation, genetics, age, body weight, and body composition influence vitamin D synthesis. Understanding how subcutaneous synthesis of vitamin D is affected by physiological factors and the impact of these factors on maintenance of serum 25OHD levels within normal physiologic ranges is important to integrating information about dietary intake and interpretation of serum 25OHD levels.
-
Clarify how environmental factors such as sunscreen use affect vitamin D synthesis. The impact of factors that affect endogenous vitamin D production, and notably the appropriate use of sunscreen for reducing cancer risk, needs to be determined to ascertain an appropriate risk-benefit profile for protected sun exposure as well as better elucidation of the role of sun exposure in determining vitamin D nutriture.
STEP 3:
INTAKE ASSESSMENT
Although great strides have been made recently in providing intake data on calcium and notably on vitamin D, more data as well as a consistent approach to data reporting would be helpful. The committee encountered challenges in identifying standardized and consistent data on vitamin D intakes across general populations in the United States and Canada, particularly for population subgroups who may be at risk for inadequate or
excessive intake. In addition, reliable data on the practice and impact of discretionary fortification on the part of food manufacturers is lacking.
-
Enhance dietary assessment methods and comparability for calcium and vitamin D intake, and methods for the measurement of calcium and vitamin D in foods and supplements. Methods related to dietary assessment have come far in recent years, and research in this area should continue. DRI development as it pertains to the North American population would benefit from targeted efforts to strive for comparability between the U.S. and Canadian surveys.
-
Investigate food and supplement sources of calcium and vitamin D for bioequivalence, bioavailability, and safety. The ability to assess whether different fortification delivery systems and food production methods affect the factors such as bioavailability or safety for both calcium and vitamin D is an important component of dietary intake assessment. Information on the practice of discretionary fortification by food manufacturers is needed.
-
Improve the standardization of the assay for serum 25OHD. Currently, different assays for the determination of serum 25OHD levels are in use, and they provide disparate results. In turn, reported measures are confounded by the need to understand the assay used and research reports contain results that are not readily compared. The role of standard reference materials and inter-laboratory collaboration is an important aspect of overcoming the challenges that the assay methodologies present.
RELATED RESEARCH NEED
Clinical practice was outside the scope of this committee convened to develop DRIs, which was tasked primarily with describing a distribution of requirements and upper levels of intake. However, as noted in Chapter 8, the cut-point levels of serum 25OHD intended to specify deficiency and sufficiency for the purposes of interpreting laboratory analyses and for use in clinical practice have been subject to a wide variation in specification without a systematic, evidence-based consensus development process. The importance of this specification to both the well-being of the North American population and to ensuring that the population is confident in their health and nutriture results in the committee calling attention to this research need. Its broad impact requires that it be addressed by a coalition of stakeholders under the auspices of a science-based organization such as the National Institutes of Health in conjunction with equivalent science-based organizations in Canada.
CONCLUDING REMARKS
The committee found that the greatest information gaps, and thus the most critical research needs, are related to the so-called hazard identification and hazard characterization steps in which the relationship between the nutrient and health outcomes are established. These needs for calcium and vitamin D DRI development relate to further exploring and describing both skeletal as well as non-skeletal health outcomes, long-term adverse effects of high levels of intake, and data to clarify the dose–response to intake. In the case of vitamin D, understanding the impact of sun exposure presents many challenges. Specific to the selected indicator (i.e., bone health), there is a need for more and better data related to the relatively unstudied life stage groups of children and young adults and the differences among racial/ethnic groups. Furthermore, the committee found a pressing public health need for development of consensus, science-based guidelines to establish cut-point levels for vitamin D deficiency and insufficiency.
REFERENCES
Brannon, P. M., E. A. Yetley, R. L. Bailey and M. F. Picciano. 2008. Summary of roundtable discussion on vitamin D research needs. American Journal of Clinical Nutrition 88(2): 587S-92S.
Chung, M., E. M. Balk, S. Ip, J. Lee, T. Terasawa, G. Raman, T. Trikalinos, A. H. Lichtenstein and J. Lau. 2010. Systematic review to support the development of nutrient reference intake values: challenges and solutions. American Journal of Clinical Nutrition 92(2): 273-6.