Workshop Overview
THE CAUSES AND IMPACTS OF NEGLECTED TROPICAL AND ZOONOTIC DISEASES: OPPORTUNITIES FOR INTEGRATED INTERVENTION STRATEGIES
Neglected tropical diseases1 (NTDs) have afflicted humanity since time immemorial and in their long histories have acquired notoriety as chronically disabling and deforming diseases. There is no single consensus definition for this group of diseases. Different organizations such as the World Health Organization, the U.S. Centers for Disease Control and Prevention, the U.S. Agency for International Development, and the Bill & Melinda Gates Foundation to name a few define the suite of these diseases differently. Be that as it may, these diseases, however they are defined, afflict more than 1.4 billion people, many of whom live on less than $1.25 per day. In the past, their serious impact on health and productivity led to considerable knowledge about the diseases, and effective control tools were developed for many. As living conditions improved in many parts of the world, opportunities for transmission were drastically reduced. As a result, these diseases are now rarely seen in populations that enjoy good access to health services and a reasonable standard of living.
NTDs today are a symptom of poverty and disadvantage. Those most affected are the poorest populations often living in remote, rural areas, in urban slums, or in conflict zones. With little political voice, NTDs have a low profile and status in public health priorities. Lack of reliable statistics coupled with the often unpronounceable names of these diseases have all held back efforts to bring them out of the shadows. Although medically diverse, NTDs share features that allow them to persist in conditions of poverty, where they cluster and frequently overlap. Approximately 1.4 billion people—one-sixth of the world’s population2—suffer from one or more NTDs. Conflict situations or natural disasters aggravate conditions that are conducive to the spread of these diseases. Around half of the world’s population is at risk of contracting these infections. The human NTDs are diseases of poverty, trapping the world’s poorest in a cycle of poverty. The global burden of the NTDs is equivalent to at least half of the combined global burden of HIV/AIDS, tuberculosis (TB), and malaria.
Several NTDs are zoonoses—infections that can be transmitted between animal and human hosts. Such infections can be transmitted directly; others are transmitted indirectly either through food and water or by means of a vector. One of the parasites that causes African trypanosomiasis, or sleeping sickness, can infect livestock and wild animals as well as humans and is transmitted by the bite of a tsetse fly. Additional neglected zoonotic diseases (NZDs) such as brucellosis, bovine tuberculosis, and rabies, which are not typically included among the NTDs, profoundly affect impoverished people not only through their direct effects on human health but also by sickening and killing the livestock upon which their livelihoods depend (WHO, 2006).
NTDs and NZDs not only share features that allow them to persist in conditions of poverty, where they cluster and frequently overlap, but they also present common opportunities for effective, integrated, intervention and control strategies. Significant (though imperfect) control measures—including drugs and vaccines, improvements in water and sanitation, and vector control measures, employed singly or in combination—have been developed for most NTDs and NZDs (Hotez and Pecoul, 2010; Spiegel et al., 2010). Policy makers and funding agencies have begun to acknowledge the public health and economic importance of the NTDs and NZDs, leading to increased support for the use of existing tools (such as the mass administration of drugs to combat several NTDs simultaneously) and the development of more effective integrated programs to control, and in some cases eradicate, these neglected diseases of poverty.
The Institute of Medicine’s (IOM’s) Forum on Microbial Threats hosted a two-day public workshop on September 21 and 22, 2010, in Washington, DC, to explore the scientific and policy dimensions of NTDs and NZDs. Through presentations and discussions, workshop participants discussed the origins and impacts of these diseases, both individually and as a collective phenomenon.
They reviewed the influence of NTDs and NZDs on human and animal health and on economic productivity, discussed prospects for disease control and mitigation, and considered opportunities for medical diplomacy and global engagement to reduce the profound, yet long-hidden, consequences of neglected diseases.
Organization of the Workshop Summary
This workshop summary was prepared for the Forum membership by the rapporteurs and includes a collection of individually authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on the presentations and discussions at the workshop.
The workshop summary is organized into sections as a topic-by-topic synthesis of the presentations and discussions that took place at the workshop. Its purpose is to present lessons from relevant experience, to delineate a range of pivotal issues and their respective problems, and to offer potential responses as discussed and described by the workshop participants. Manuscripts and reprinted articles, submitted by some but not all of the workshop’s participants, may be found in alphabetical order in Appendix A.
Although this workshop summary provides an account of the individual presentations, it also reflects an important facet of the Forum’s philosophy. The workshop functions as a dialogue among representatives from different sectors and allows them to present their beliefs and viewpoints about which areas may merit further attention. This report only summarizes the statements of participants at the workshop. It is not intended to be an exhaustive exploration of the subject matter nor does it represent the findings, conclusions, or recommendations of a consensus committee process.
Defining the NTDs
Workshop presentations and discussions reflected the dual nature of NTDs3 as both a public health phenomenon and a medically diverse group of diseases. This section examines common attributes of NTDs and, in particular, their inextricable association with poverty and conflict. The next section profiles individual diseases, their origins and effects on individuals and populations, and prospects for prevention and treatment. Brief descriptions of NTDs and NZDs described in this overview are presented in Box WO-6 (which appears on pages 86–107).
Burden of the “Bottom Billion”
NTDs4 comprise some of the most common infections of poverty and some of the leading causes of chronic disability in low- and middle-income countries (Hotez and Pecoul, 2010). As illustrated in Figure WO-1 and discussed further by Hotez in this volume, NTDs represent a group of more than a dozen major chronic, mostly parasitic, infectious diseases, with high endemicity in the developing countries of Africa, Asia, and the Americas (Hotez, 2010a; Musgrove and Hotez, 2009). It has been estimated that every person among the world’s poorest—the destitute “bottom billion”—suffers from co-infections by one or more NTD (Hotez et al., 2009b). The greatest health and economic burden of NTDs is borne by people whose existence is often overlooked: subsistence farmers and their families living in remote rural areas, and the teeming poor of urban slums and shantytowns (WHO, 2010b).
NTDs are not exclusively restricted to impoverished tropical regions of the world. Several of these diseases were once endemic in the United States and remain highly prevalent among the nation’s poorest residents (Hotez, 2008b, 2009a; Hotez and Wilkins, 2009). In the relatively poor south of Europe and in Turkey, ascariasis, trichuriasis, and a host of zoonotic helminth infections are associated with intestinal, neurological, and respiratory problems. Dogs in that region also serve as a reservoir for visceral leishmaniasis (Hotez, 2009a). Even impoverished Arctic natives contract helminth and protozoan infections through the consumption of undercooked wild animal meat and from contact with infected livestock such as reindeer and elk (Hotez, 2010b).
NTDs have plagued life on Earth for millennia. As noted in Box WO-1, accurate descriptions of these often painful and disfiguring parasitic infections appear in ancient texts including the Bible, Talmud, and Vedas; in the works of Hippocrates; and in Egyptian papyri (Cox, 2002; Hotez, 2010a).
In a presentation discussing NTDs in the United States, Peter Hotez, of the George Washington University and the Sabin Vaccine Institute, noted that most NTDs in the Americas—with the possible exceptions of Chagas disease and trachoma—were the living legacies of slavery and the slave trade. (Dr. Hotez’s contribution to the workshop summary report can be found in Appendix A, pages 221–293.) As illustrated in Figure WO-2, these parasites traveled to the Americas in the bodies of West African slaves, who worked in the sugar plantations of Latin America and the Carribbean and, to a lesser extent, in the cotton and sugar plantations of the American South (Hotez, 2009a). NTDs introduced into the United States by slavery, such as hookworm, schistosomiasis, and lymphatic filariasis (LF), became endemic in the New World (Hotez, 2009a).
The burden of hookworm disease in the American South inspired one of the
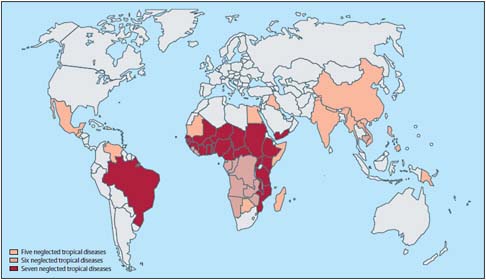
FIGURE WO-1 Geographical overlap and distribution of the seven most common neglected tropical diseases: ascariasis, hookworm infection, trichuriasis, schistosomiasis, lymphatic filariasis, onchocerciasis, and trachoma.
SOURCE: Hotez et al. (2009b). Reprinted from The Lancet, Vol. 373, Hotez PJ, Fenwick A, Savioli L, and Molyneux DH, Rescuing the bottom billion through control of neglected tropical diseases, pp. 1570–1575, Copyright (2009), with permission from Elsevier.
first large-scale, integrated attempts to combat the diseases now known as NTDs, which was launched by the philanthropist John D. Rockefeller in the first decade of the 20th century (CDC, 1993; Ettling, 2000; Humphreys, 2009). Viewing hookworm as a disease that stunted economic development as well as the lives of individuals and communities in the American South, the Rockefeller Sanitary Commission for the Eradication of Hookworm set out in 1909 to achieve the goal of hookworm eradication through the mass treatment of affected populations with anti-helminthic therapy. Although this effort reduced the severity of disease in infected individuals, it failed to eliminate the source of infection and re-infection occurred following the termination of therapy.
Hookworm remained endemic to the American South until profound economic development and urbanization occurred in the years prior to the beginning of World War II (Humphreys, 2009). The same can be said for three additional diseases of poverty that plagued the American South during the 19th and early 20th centuries: yellow fever, malaria, and pellagra. Humphreys (2009) observed that “the southern liberation from disease paralleled the end of sharecropping and the rise of prosperity in the South. It also occurred in decades that saw a vast migration from the countryside to the city and from the South to the North.” Similar transformations are now taking place in China, Hotez added. Over the
|
BOX WO-1 Ancient Scourges, New Names The illnesses we now call neglected tropical diseases have been plaguing humanity since the beginning of recorded history. Indeed, public health experts sometimes refer to NTDs as the “biblical diseases” because of their long history of causing human suffering (Hotez, 2006). Descriptions of parasitic worm and skin infections can be found in Egyptian papyri and the Vedas dating back to 1500 BC. These conditions are also described numerous times in the Bible, in the Talmud, and in the writings of ancient scholars such as Hippocrates (Cox, 2002; Hotez, 2010a). Early Descriptions of Parasitic Worm Infections Ancient Egyptian papyri represent some of the earliest written descriptions of parasitic worms infecting and causing disease in humans. For instance, the Ebers Papyrus, which dates back to 1500 BC, includes instructions for how to use pomegranate root to treat roundworm infections (Grove, 1990). Detailed descriptions of worm infections and ancient treatments for them can also be found in the writings of Hippocrates. In his Treatise on Diseases IV, Hippocrates outlines his theories on the origins of both flat and round worms, describing the courses of infection for each. For example, he writes, “When it [the worm] matches the gut in size, it keeps growing; and the parts which exceed the length of the gut separate off at the anus along with the feces, and what is expelled is like a cucumber seed, or often even larger” (Lonie,1981). The Bible also contains passages that scholars believe refer to parasitic worm infections. In Numbers, there is a passage that describes how God sent “fiery serpents” to attack the Israelites who had spoken out against him. Once the Israelites repent for their sins, God instructs Moses to “Make a fiery serpent and set it on a pole, and everyone who is bitten, when he sees it, shall live” (Numbers 21:8). Many historians believe that this passage is a reference to Guinea worm (Kristoff, 2010). Indeed, wrapping the worm around a stick and slowly pulling it from the body remains the standard method for removing the worms today. Early Descriptions of Skin Infections The Bible, the Talmud, and the Vedas all contain numerous references to skin diseases, which are often described as punishments for those who do not follow religious laws. For example, the Bible mentions “leprosy” (which religious scholars now believe refers to a number of different skin diseases including what we now consider leprosy) in Leviticus, Numbers, Samuel, Kings, Chronicles, Matthew, Mark, and Luke. Leviticus contains a detailed passage describing what to do when a person has a case of “leprosy.” In the passage, God explains to Moses and Aaron, “When a man is afflicted with a leprous disease, he shall be brought to the priest, and the priest shall look. And if there is a white swelling in the skin that has turned the hair white, and there is raw flesh in the swelling, it is a chronic leprous disease in the skin of his body, and the priest shall pronounce him unclean” (Leviticus 13:9–11). Similarly, Treatise XV of the Talmud discusses the signs and proper ways to handle skin diseases at length and includes detailed instructions on how they should be identified, inspected, and properly treated according to religious law (Barclay, 1878). |
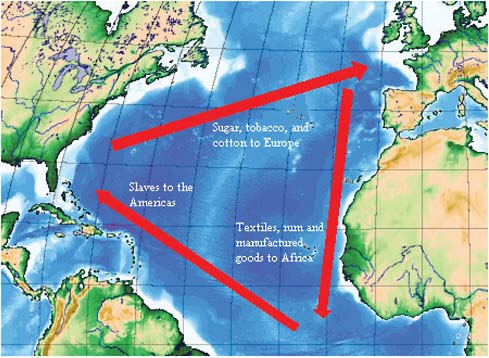
FIGURE WO-2 Depiction of the classical model of the Triangular trade. The use of African slaves was fundamental to growing colonial cash crops, which were then exported to Europe. European goods, in turn, were used to purchase African slaves, which were then brought on the sea lane west from Africa to the Americas, the so-called middle passage.
SOURCE: Triangular trade. http://en.wikipedia.org/wiki/Triangular_Trade (accessed November 12, 2010).
past decade, as the number of its people who live on less than a dollar a day has declined from 60 percent to 16 percent, there have been equally dramatic decreases in the prevalence of neglected infections of poverty, he stated.
Nevertheless, NTDs remain endemic to many less fortunate places left behind by socioeconomic progress. Collectively, these diseases cause an estimated 530,000 deaths per year and annual disability equivalent to 57 million life-years (Hotez et al., 2006, 2007b; Sachs and Hotez, 2006) (see Table WO-1). The heavy burden of disability associated with NTDs—which exceeds that of malaria and TB (Hotez at al., 2006)—reflects the chronic nature of these infections, coupled with the lack of health care delivery available to the vast majority of people who suffer from them.
As several speakers observed throughout the meeting, NTDs frequently present as co-infections with each other, as well as with malaria, TB, or HIV/AIDS (Brooker et al., 2006, 2007). Hotez noted that the geographic ranges of
TABLE WO-1 High-Prevalence and Other Vector-Borne Neglected Tropical Diseases
hookworm and schistosomiasis frequently overlap, as do schistosomiasis and HIV/AIDS, especially in rural areas. The additive effects of such multiple infections include profound anemia, complications of pregnancy, and physical and mental stunting in children, he said. Subsequent descriptions of the disability and disfigurement caused by individual diseases (collected in Box WO-6 and discussed in the next section of this overview), and of their magnified effects on women, children, and people living in conflict (below), further illustrate the broad and insidious public health impact of NTDs.
NTDs and Poverty
Poverty is by far the greatest risk factor for NTD infections, Hotez observed; and, he asserted, it is equally true that NTDs promote poverty and interfere with economic development. He and several other speakers featured poverty among their lists of common attributes of countries or regions afflicted with NTDs; workshop participants frequently connected the control of NTDs with the Millennium Development Goals (MDGs) (UNDP, 2010), a set of important benchmarks for poverty reduction, global health, and political equity.5 A major report on NTDs from WHO, released in the weeks following the workshop (WHO, 2010b), stated that “working to overcome the impact of NTDs represents a largely untapped development opportunity to alleviate the poverty of many populations and thereby make a direct impact on the achievement of the Millennium Development Goals (MDGs).”
NTDs and poverty are linked in numerous ways. Lack of access to clean water, sanitation, hygiene, housing, and health care leaves the poor vulnerable to a host of infections, including NTDs (Spiegel et al., 2010). Previous Forum workshops have examined how water, sanitation, and hygiene coupled with additional environmental factors—including climate change, extreme weather events, land use patterns, and ecosystem disruption—contribute to the persistence and geographic expansion of the NTDs, within the broader context of infectious disease emergence (IOM, 2008a,b, 2009). Water is an important common denominator in many of the NTDs. Water may serve as a medium through which parasites such as helminths are transmitted; as a breeding ground for vector species such as mosquitoes and tsetse flies; or as the habitat for intermediate hosts, such as snails that transmit schistosomiasis (see Table WO-2) and, therefore, represents an important route to NTD control.
Each of the three workshop presentations summarized below discussed additional important links between NTDs and poverty: the disproportionate burden of NTDs borne by women and children, the persistent effects of NTDs on cognitive
TABLE WO-2 Environmental Classification of Water- and Excreta-Related Infections
|
Category |
Example |
Control Strategies |
|
A. Fecal-oral (Potentially water-borne or water-washed) |
Viral |
Improve water quality (to prevent water-borne transmission), improve water availability, hygiene promotion (to prevent waterwashed transmission) |
|
Hepatitis A, E, and F Poliomyelitis Viral diarrhoeas |
||
|
Bacterial |
||
|
Campylobacteriosis Cholera Pathogenic E. coli Salmonellosis Typhoid, paratyphoid |
||
|
Protozoal |
||
|
Amoebiasis Cryptosporidiosis Giardiasis |
||
|
B. Purely water-washed |
Skin and eye infections |
Improve water availability, hygiene promotion |
|
Scabies Conjunctivitis Trachoma |
||
|
Louse-borne infections |
||
|
Relapsing fever |
||
|
C. Soil-transmitted helminths |
Ascariasis Trichuriasis Hookworm infection Strongyloidiasis |
Sanitation, hygiene promotion, treatment of excreta before re-use |
|
D. Food-borne diseases |
Bacterial |
As C above, plus meat inspection and cooking |
|
Cholera Campylobacter Salmonellosis and Shigellosis |
||
|
Viral infections |
||
|
Hepatitis A and E Norovirus Helminth infections Trichinellosis |
||
|
Food-borne trematode infections |
||
|
Clonorchiasis Opisthorchiasis Paragonimiasisis Tapeworms Diphyllobothrium infection Taenia solium infection Taenia saginata infection |
|
E. Water-based diseases |
Bacterial |
Reduce contact with/consumption of infected water, sanitation, treatment of excreta before re-use |
|
Cholera Legionellosis Leptospirosis |
||
|
Helminthic |
||
|
Schistosomiasis Clonorchiasis, and, Dracunculiasis |
||
|
F. Insect vector diseases |
Water-related |
Reduce number of potential breeding sites and need to pass near them, improve surface water drainage, use repellent/insecticide where appropriate |
|
Dengue Yellow fever Malaria West African trypanosomiasis |
||
|
Excreta-related |
||
|
Bancroftian filariasis Trachoma Fly- and cockroach-borne excreted infectionsa |
||
|
G. Rodent-borne diseases |
Rodent-borne excreted infections |
Rodent control, hygiene promotion, reduce contact with infected water |
|
Leptospirosis Tularaemia |
||
|
aExcreted infections comprise all those in categories A, C, and D plus helminthic diseases in category E. SOURCE: Adapted from Bartram and Cairncross (2010). |
||
development, and the role of conflict in making people more vulnerable to NTD infection and its consequences.
Impact on women’s and children’s health Speaker Marian McDonald, of the Centers for Disease Control and Prevention (CDC), examined the NTDs in terms of the MDGs and also of the “convergence model,” a framework for examining risk factors for disease emergence proposed in a seminal IOM report (IOM, 2003) that has informed much of the Forum’s work. (Dr. McDonald’s contribution to the workshop summary report can be found in Appendix A, pages 357–388.) As seen in Figure WO-3, the model consists of four sets of intersecting factors.
In the case of NTDs, these factors influence the burden of disease for women and children, as follows:
-
Genetic and biological factors. Women are biologically “at risk” for acquiring NTDs during pregnancy and birth, McDonald said. These vulnerabilities in turn affect children’s development. For example, she noted, soil-transmitted helminths (STHs) contribute to anemia in pregnant women, jeopardizing the health of both mother and fetus. Hotez noted
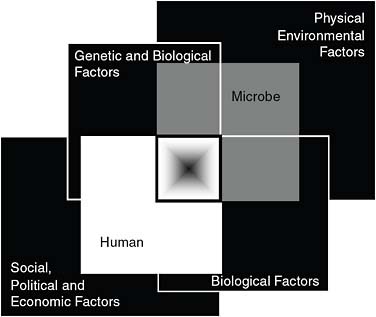
FIGURE WO-3 The convergence model. At the center of the model is a box representing the convergence of factors leading to the emergence of an infectious disease. The interior of the box is a gradient flowing from white to black; the white outer edges represent what is known about the factors in emergence, and the black center represents the unknown (similar to the theoretical construct of the “black box” with its unknown constituents and means of operation). Interlocking with the center box are the two focal players in a microbial threat to health—the human and the microbe. The microbe–host interaction is influenced by the interlocking domains of the determinants of the emergence of infection: genetic and biological factors; physical environmental factors; ecological factors; and social, political, and economic factors.
SOURCE: IOM (2003).
-
that up to one-third of pregnant women in sub-Saharan Africa are infected with hookworms.
-
Physical and environmental factors. While not unique to women and children, environmental and infrastructural conditions—such as substandard housing and inadequate sanitation—strongly affect NTDs, as described above.
-
Ecological factors. While not unique to women and children, ecological factors that influence NTDs—such as access to freshwater, and poultry and small livestock caretaking responsibilities—may have different consequences that result from women’s societal roles, McDonald observed. For example, she said, women account for 64 percent of all water collec-
-
tion, and men for 25 percent (the remainder is done by children, with girls twice as likely to carry water as boys); this task exposes the collector to parasites, infectious disease vectors, and, in regions where conflict is rife, violence as well, all of which raise the risk for NTDs.
-
Social, political, and economic factors. These have the greatest influence on NTD impact for women and children, according to McDonald. They include disproportionate rates of poverty and illiteracy, lack of education and land ownership, lack of political power, gender inequality, conflict, and war.
NTDs such as female genital schistosomiasis (FGS) and trichomoniasis shape women’s reproductive, sexual, social, and economic health, McDonald stated; they cause sexual dysfunction, increase risk for sexually transmitted infections, decrease fertility, and threaten pregnancy outcome. Stigma and exclusion experienced by people with disfiguring NTDs such as lymphatic filariasis (LF) are especially acute for women and can have severe economic consequences by preventing marriage or childbearing, she added. Caring for young children can also increase a woman’s risk of infection with an NTD; for example, she said, women frequently contract trachoma while caring for an infected child, and they are three times more likely than men to be permanently blinded by the disease. She also noted that FGS is associated with increased risk of acquiring HIV in women, as well as with ectopic pregnancy, which can be fatal.
Unfortunately, McDonald observed, one of the most effective strategies for prevention and control of NTDs, mass drug administration (MDA), can be problematic during pregnancy. STHs can be treated safely, and treatment of pregnant women with schistosomiasis was recommended by WHO in 2002. The lack of pregnancy safety trials for the drug praziquantel has restricted its use for that purpose, she said. LF cannot be safely treated in pregnant women, leaving them as a potential disease reservoir and at risk for infection, she added.
McDonald stated that NTDs are a scourge on children’s health globally, damaging children’s health and development in a number of ways. They also take children’s lives. NTDs are often considered diseases that make people sick but don’t kill them. That is not the case with children, as NTDs contribute to global child mortality (Black et al., 2010; Global Network, 2010; WHO, 2008f). She noted that many NTDs impair children’s growth and cognitive development, which—along with another frequent symptom, anemia—stunts their educational and economic prospects as well. The effects of stigma and social isolation associated with a disfiguring NTD are similarly devastating for children. In addition, she said that NTDs may increase the risk that a child will acquire HIV or malaria. Thus, the profound effects of NTDs on the lives of impoverished children not only increase their vulnerability to a range of chronic diseases and disabilities but also contribute to their being trapped in a cycle of poverty.
Parasite prevalence and the worldwide distribution of cognitive ability As McDonald noted, chronic NTD infections have been shown to not only inhibit children’s growth and development but also impair cognition and memory (Hotez et al., 2009b). It is therefore not surprising that these parasitic infections are associated with reduced educational performance and school attendance. Moreover, as speaker Christopher Eppig of the University of New Mexico suggested, this effect may influence average national intelligence, as measured by intelligence quotient (IQ) (Eppig et al., 2010). (Dr. Eppig’s contribution to the workshop summary report can be found in Appendix A, pages 155–172.)
“The topic of the worldwide variation of intelligence has been of interest to scientists for quite some time,” Eppig observed, “but it wasn’t until recently when Richard Lynn and Tatu Vanhanen published empirical data on average IQ by nation that formal studies were possible” (Lynn and Vanhanen, 2001, 2002, 2006). Eppig described a series of studies that followed this work, attempting to explain global patterns of intelligence distribution (as determined by Lynn and Vanhanen6) by such factors as education and employment prospects (Barber, 2005); climate (Templer and Arikawa, 2006); evolutionary novelty of environment, approximated as distance from Central Africa, where the human species is presumed to have originated (Kanazawa, 2008); and inbreeding depression studies (Saadat, 2008; Woodley, 2009). The study Eppig and coworkers conducted found the most robust association of them all: a strong inverse correlation between infectious disease burden (measured in disability-adjusted life-years, or DALYs) and IQ at the national level (Eppig et al., 2010).
The high metabolic demands of brain development and early childhood provide a plausible explanation for this effect, according to Eppig and coauthors (2010), who speculated that “a developing human will have difficulty building a brain and fighting off infectious diseases at the same time, as both are very metabolically costly tasks.”7 Moreover, Eppig said, “if our hypothesis is correct, that infectious disease is the primary driver of the worldwide distribution of human intelligence, then [this correlation should hold] … on other geographical scales, such as across the U.S. states.” To test this theory, they compared a measure of average state IQ, determined from scores on a commonly used standardized test, with a measure of infectious diseases–related stress based on statewide CDC data.
In the comparator group, they used three measures of wealth (median household income, income per capita, and gross state product) and two measures of education (expenditure per student and percentage of teachers ranked as “highly qualified” by the U.S. Department of Education). In this comparison too, infectious disease proved a far more important variable than education or wealth in predicting statewide IQ, Eppig stated, although education and wealth were found to be more significant to IQ than in their cross-national analysis.
Eppig maintained that a key interpretation of this finding is that efforts to increase the average IQ of an area should be focused on reducing infectious disease, especially those infectious diseases like the NTDs that affect brain development during early childhood. He also noted that considerable work still remained to test the relationship between IQ and infectious disease, including longitudinal studies tracking both variables among a cohort of individuals and continued exploration of possible mechanisms to explain their findings.
Eppig’s work points to additional types of studies that could be conducted with regard to NTDs that occur in the United States, Hotez observed (see discussion of these NTDs and their impact in next section). For example, he said, one could look at Toxocara infections as a risk factor in existing large asthma cohort studies, or examine the impact of cytomegalovirus infection on intellectual delays in African-American communities. “There are a number of important studies now that you could include in some large analyses that might already be being supported by the NIH and other agencies,” he advised.
Forum member Michael Osterholm, of the University of Minnesota, cautioned that the data Eppig and colleagues used to make their analyses were unreliable. “Even the data for across the states has [sic] to be very carefully looked at,” he said. “We have had many a Forum meeting here looking at disease surveillance, understanding how absolutely soft, erratic, and, in some cases, absolutely impossible to predict [sic] surveillance data are.”
“We fully admit that there are going to be sampling-error issues,” Eppig replied, adding that their hypothesis was far from proven. “Only by continuing to get finer and finer with the scale of our analyses we can say this is solved,” he added, but he also contended that the association between IQ and infectious disease burden had been supported on two very different geographical scales.
NTDs and conflict In a presentation titled, “Neglected Diseases, Civil Conflicts, and the Right to Health,” Chris Beyrer of the Johns Hopkins University discussed multiple mechanisms through which conflicts specifically increase vulnerability to NTDs, and how conflict situations contribute to an overall increase in neglect. (Dr. Beyrer’s contribution to the workshop summary report can be found in Appendix A, pages 132–155.) He also demonstrated that although interventions to address NTDs in conflict settings are challenging they can, nevertheless, have measurable impacts.
“NTDs are diseases of neglected peoples; conflicts fuel neglect,” Beyrer
stated. Conflicts cause displacement and overcrowding in camps and settlements and prevent access to adequate water, hygiene, food, and health care, he observed. These factors, in turn, increase exposures to and transmission of NTDs, and result in treatment gaps or delays. Conflicts and terror regimes further contribute to the neglect by obstructing or preventing disease surveillance and research, disabling health care systems, and, most directly, through the removal of health care providers, who either flee or are caught up in—and potentially harmed by—conflicts, he said. “The worst of these associations [occur during] … civil conflicts,” he added. “It’s really within country settings, and less about militaries fighting than it is about militaries contending in civilian spaces.”8
Several of these factors were in evidence during a civil conflict that erupted in Côte d’Ivoire in 2002, Beyrer recalled. While as much as one-half of the adult population became displaced from some areas during this period, 98 percent of the doctors and 86 percent of nurses fled some places (Betsi et al., 2006). The effects of conflict on health research are apparent in the numbers of HIV/AIDS and malaria studies conducted in the Democratic Republic of Congo (DRC). Beyrer observed that during the Mobutu regime, as political terror increased, those numbers rapidly declined to zero and have only recently begun to increase (Beyrer, 2007).
Similarly, he reported that cases of human African trypanosomiasis (HAT; sleeping sickness) in the DRC—which peaked in the 1930s, then declined as a result of control measures to about 1,000 cases per year and remained steady until the country gained independence from Belgium in 1960—rose as the Mobutu government began its violent regime. Between 1991 and 1994, cases of HAT reached their highest levels in the 20th century, more than 34,000 per year, coincident with the height of social and political chaos (Ekwanzala et al., 1996). The numbers of HAT infections, transmission rates, and the cost and toxicity of treatment in the DRC increased during this period, Beyrer observed. A contemporaneous “big-picture” analysis of HAT outbreaks in Central Africa found that nearly every outbreak has occurred in an area of conflict and that outbreak severity and conflict severity were strongly correlated across space and time (Berrang-Ford et al., 2010).
Since 1993, Beyrer has been working to address NTD infections and other critical health problems among people living inside conflict zones in eastern Burma (Myanmar). He described a successful intervention deployed across the Thai–Burma border called the Mobile Obstetric Medics program (Mullany et al., 2010). This program trains Burmese community health workers in a safe environment in Thailand. Upon completion of their training, these health care workers then return to Burma to provide basic health services, including mass de-
worming, to people living in conflict zones in eastern Burma. “These internally displaced populations are not reachable from the government sector and they are certainly not reachable from international organizations working in Burma,” Beyrer observed. In his formal remarks, Beyrer provided a range of evidence suggesting that the community health workers were able to deliver a basic set of services to significant numbers of displaced people.
That this program trained and empowered local health care workers was crucial to its success, Beyrer emphasized. The trainees, who represented each of four major ethnic groups in eastern Burma, were “the only people who can deliver those kinds of services in these conflict settings—people who can get into areas that none of us can and that international organizations really can’t,” he said. “Hopefully, we have been able, with this work and with the evaluation of it, to demonstrate, in an exceptionally challenging environment, interventions that might be useful and scalable in less challenging environments,” he concluded.
Why Are NTDs Neglected?
Despite their significant contribution to the global burden of disease, several workshop participants offered their opinions as to why the NTDs as a group are neglected, as well as the opportunity they present for poverty reduction and global development. Clearly, NTDs contribute to a cycle of poverty and disease in which each condition exacerbates and perpetuates the other. Many speakers emphasized that the lack of political voice among those most afflicted by NTDs is a primary reason for their neglect, as Beyrer expressed when he called them “neglected diseases of neglected people.”
The epidemiological characteristics of NTDs may also contribute to their neglect, several participants observed. Keynote speaker Ezekiel Emanuel of the White House Office of Management and Budget, among others, remarked that because these diseases cause high morbidity—which is often difficult to measure—and fewer deaths than HIV/AIDS and malaria, the health effects of NTDs are less apparent. Forum member Lonnie King, of the Ohio State University, noted that NTDs rarely cause explosive outbreaks, which tend to draw attention to infectious diseases. (Dr. Lonnie King’s contribution to the workshop summary report can be found in Appendix A, pages 342–346.) Speaker Lorenzo Savioli of WHO observed that, although NTDs are communicable, they are not easily or often exported to developed countries and are therefore not perceived as threats. (Dr. Savioli’s contribution to the workshop summary report can be found in Appendix A, pages 481–489.)
As both Emanuel and Savioli observed, another strike against the NTDs is their weakness as a “brand” if taken individually. Most diseases that comprise the NTDs have complex, hard-to-pronounce names, Emanuel observed. Exactly which diseases are NTDs is another unresolved question—a range of lists were presented at the workshop, from the “7 most common and/or most treatable

FIGURE WO-4 WHO list of neglected tropical diseases.
SOURCES: Hopkins (2010); Carter Center.
diseases” to a list of 30 “neglected diseases of poverty.” There was considerable controversy as to whether or not dengue is an NTD. Some argued that dengue is an acute emerging febrile illness, rather than a chronic, debilitating disease, and therefore does not fit the NTD model; others contended that dengue and other intermittent, acute diseases—which also include chikungunya and Japanese encephalitis—result in lifetime disability that is indeed neglected.
Keynote speaker Donald Hopkins, of the Carter Center, suggested that the field would benefit if a single list of diseases, such as WHO’s list of 13 NTDs,9 presented in Figure WO-4, served as a standard and guide for prioritizing research. (Dr. Hopkins’ contribution to the workshop summary report can be found in Appendix A, pages 208–221.) Pursue the diseases on this list as a cohort for a set period, he advised, and focus on eliminating or controlling this limited number of disease targets for the foreseeable future. “Adding and subtracting diseases in these different lists is unnecessarily confusing and defeats our objective,” he concluded.
Profiles of Neglected Diseases
Although NTDs have much in common, each disease has its own history, and each presents certain unique challenges and intervention opportunities. As speaker Eric Ottesen, of the Task Force for Global Health, observed, “we know that we are going to deal with these diseases in an integrated fashion … but it’s also important that we focus on the progress within each of the diseases. (Dr. Ottesen’s contribution to the workshop summary report can be found in Appendix A, pages 414–480.) Certainly we can integrate the implementation [of interventions], but we have to make sure that each of the diseases is being
addressed successfully.” The workshop presentations summarized in this section focused on individual NTDs and their specific health and socioeconomic effects, as well as prospects for reducing these burdens.
Diseases Targeted for Eradication and Elimination
Hopkins began his workshop presentation by defining the terms “elimination,” “eradication,” and “control,” as they apply to infectious diseases (see Box WO-2). “Eradication means reducing the incidence of a disease to zero worldwide, such that further control measures are unnecessary,” he said. “It means total interruption of transmission.” After a specified period when no cases of the disease are reported, WHO can certify that it is eradicated. “Eradication will always be a rare phenomenon,” he added.
Hopkins defined elimination as the cessation of transmission of a disease in a limited geographic area, where control measures may continue in order to combat or prevent reintroduction. He also noted that this term is frequently misused to describe control of a disease that persists in lesser amounts and that, therefore, poses an ongoing threat of transmission. “I believe such imprecise use of the word ‘elimination’ devalues the term, risks credibility, and confuses the public,” he observed (Hopkins, 2009). WHO is the only international organization that can legally declare a disease eradicated or eliminated, Hopkins stated; its governing body, the World Health Assembly, or one of its regional committees, can officially sanction targeting of any disease for eradication or elimination. Of the seven NTDs that have been targeted in this way (see Box WO-3), Hopkins focused his remarks on four diseases—dracunculiasis, onchocerciasis, trachoma, and LF—and the progress being made against them. LF was also the topic of a workshop presentation by Eric Ottesen, director of the Lymphatic Filariasis Support Center at the Task Force for Global Health, discussed below.
|
BOX WO-2 Definitions of Elimination, Eradication, and Control Elimination: cessation of transmission in a country, continent, or other limited geographic area; complete prevention of a clinical manifestation. Eradication: deliberate reduction of global incidence to zero, no further control measures necessary. Control: reduced incidence or prevalence, control measures still necessary. SOURCE: Hopkins (2010). |
|
BOX WO-3 NTDs Targeted by WHO for Elimination or Eradication Dracunculiasis: global eradication (WHA57.9) 2004 Onchocerciasis: regional elimination (interrupting transmission of the parasite) (PAHO CD48.R12) 2008 Lymphatic filariasis: global elimination as a public health problem (WHA50.29) 1997 Trachoma: global elimination of blinding trachoma (WHA51.11) 1998 Leprosy: global elimination (reduction of cases to less than 1 per 10,000 population) (WHA44.9) 1991; elimination as a public health problem (WHA51.15) 1998 Chagas disease: regional elimination of transmission “as technically feasible” (WHA51.14) 1998; “control and elimination” (WHA63.20) 2010 Visceral leishmaniasis: regional elimination (WHA60.13) 2007 SOURCE: Hopkins (2010). |
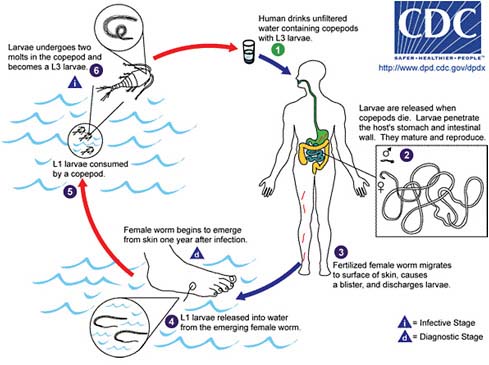
FIGURE WO-5 Life cycle for dracunculiasis.
SOURCE: Centers for Disease Control and Prevention.
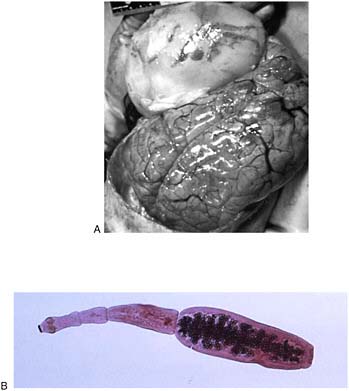
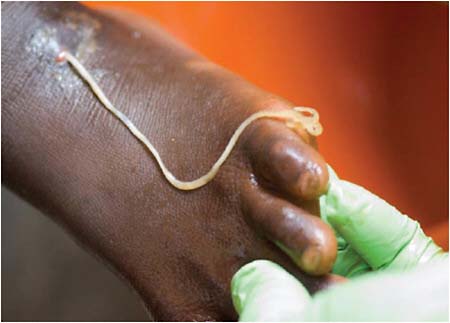
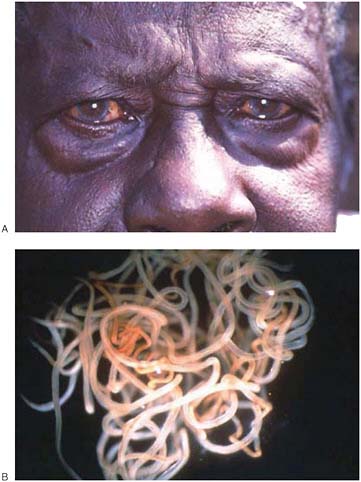
Dracunculiasis Also known as Guinea worm disease, dracunculiasis was the first (and to date, the only) NTD targeted by WHO for eradication, Hopkins said. It is a painful infection caused by the roundworm Dracunculus medinensis, which is transmitted through contaminated drinking water (see Box WO-6). Two- to 3-foot-long worms, each of which is capable of depositing hundreds of thousands of larvae, emerge directly through the skin approximately one year after infection, he explained. People with dracunculiasis endure weeks of pain and disability, preventing them from doing physical labor or from attending school. Figure WO-5 provides a schematic of the life cycle of dracunculiasis.
There is no pharmacological cure or vaccine for dracunculiasis, Hopkins observed, nor are recovered patients immune to future infections. Today, the only therapy for dracunculiasis is to physically extract the worm from the lower extremities or elsewhere. Humans, however, are the sole host for the parasite. Therefore, he said, dracunculiasis can be prevented through several relatively simple routes:
-
filtering drinking water;
-
keeping infected people from bathing or entering into drinking water sources;
-
applying larvicide to infected water sources; and
-
providing clean, safe water from wells.
Since 1986, the Carter Center has led efforts to eradicate dracunculiasis, in collaboration with the endemic countries, CDC, the United Nations Children’s Fund (UNICEF), and WHO, Hopkins said. Donors to this initiative have included manufacturers of water filtration fabric and larvicide, charitable foundations, the U.S. Agency for International Development (USAID), among many donors, as well as service volunteers from many countries. “Tens of thousands of grassroots village volunteers are the bedrock of the Guinea worm eradication program,” he observed. “They were the first to show the utility of village volunteers in Africa as the basis for a surveillance network that provides reliable village-based reports of a disease,” he continued, and he noted that village volunteers also educate their neighbors about disease prevention and distribute water filters. The dracunculiasis eradication program has also benefited from the strong political participation by several African heads of state and the leadership and engagement of former President Jimmy Carter.
In 2009, there were fewer than 3,200 cases of dracunculiasis reported in 645 endemic villages, compared with an estimated 3.5 million cases in more than 23,000 villages in 1986, Dr. Hopkins stated. Once prevalent in 20 nations in Africa and Asia, by 2009, only 4 countries in Africa reported cases. As of August 2010, 97 percent of all cases were reported from Sudan—especially from war-torn southern Sudan. According to Hopkins, the remaining cases are being reported from Ethiopia, Mali, and Ghana. Since 2005, when a peace agreement
was signed ending the most recent phase of the Sudanese civil war, significant progress has been made against dracunculiasis in southern Sudan, Hopkins observed. This occurred “despite pitiful infrastructure, low literacy, and sporadic insecurity that disrupted program operations 32 times in different places in 2009,” he added. “We are now aiming to stop transmission in southern Sudan by the end of 2012, political conditions permitting,” he stated.
Hopkins described five key benchmarks that have charted the dracunculiasis eradication program over the past 30 years:
-
a clear, quantitative goal, defined from the program’s outset;
-
identification of all endemic countries;
-
establishment of effective village-based active surveillance;
-
extension of interventions throughout endemic areas; and
-
monitoring of cases and status of interventions, followed by response, on a monthly basis.
“When the Guinea worm eradication program succeeds, it will set a precedent as the first parasitic disease of humans to be eradicated and as the first disease to be eradicated without a vaccine or curative treatment,” Hopkins observed. “Its legacy in endemic areas will include improved health, more productive agriculture, and better school attendance, as well as experienced health workers and village volunteers, and changed attitudes.”
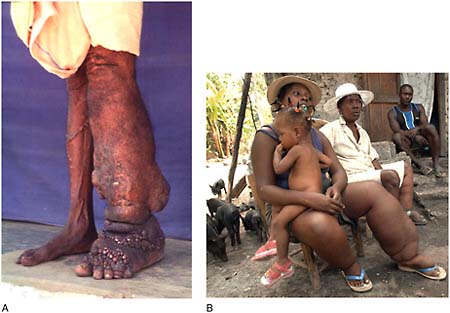
Onchocerciasis Also known as river blindness, onchocerciasis is a potentially blinding parasitic infection that is spread by the bite of black flies that breed in fast-flowing rivers, rapids, or dams. In Africa, suitable habitat for these flies has expanded with increased dam construction, Hopkins noted. Of an estimated 123 million people at risk for onchocerciasis in 37 mostly African countries, about 37 million are actually infected, he reported. Approximately half a million people are at risk in six countries in the Americas, and a few thousand in Yemen.
The infection can be treated or prevented by annual doses of ivermectin, a product of Merck & Co., Inc., but because the drug only affects the immature microfilariae, not the adult worms, annual treatments must continue for at least a decade, until the adult worms die out, he explained. In 1987, Merck made the precedent-setting announcement that it would provide unlimited amounts of its then-novel drug to treat people with onchocerciasis in poor countries free of charge, and for as long as necessary.
The Pan-American Health Organization (PAHO), WHO’s regional body in the Americas, targeted this disease in 1991 for interruption of transmission by 2012, Hopkins said. In the Americas, efforts to combat onchocerciasis among people living in 13 disease foci in the six affected countries (see Figure WO-6) have involved mass treatment with ivermectin at least twice per year, Hopkins stated, with a minimum coverage of 85 percent of the eligible population.
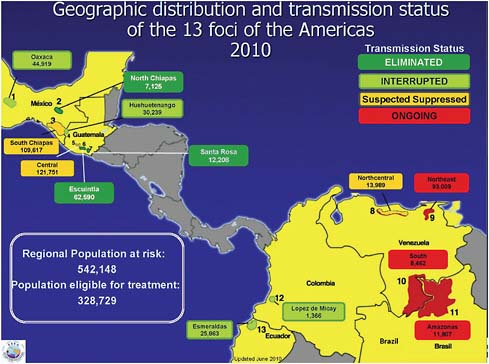
FIGURE WO-6 Geographic distribution and transmission status of the 13 onchocerciasis foci of the Americas (2010).
SOURCE: Hopkins (2010).
Some of the highly endemic communities in Mexico have been treated four times per year. At present, Hopkins stated, 10 of those foci have either been eliminated (that is, they have stopped semiannual treatments for at least three years, maintained surveillance, and have had no resurgence of disease), are believed to have suppressed transmission, or they are in post-treatment surveillance, having interrupted transmission and stopped semiannual treatments, but have not yet gone for three years without new cases. The remaining active foci are located in Venezuela and Brazil.
Most black fly species that carry onchocerciasis in the Americas do not transmit the disease as efficiently as do their African counterparts, Hopkins observed. That, in part, explains why onchocerciasis elimination is more feasible in the Americas than in Africa. The first African onchocerciasis control program—sponsored by WHO, the World Bank, and others—involved 11 West African countries from 1994 to 2002. By combining vector control—spraying rivers with insecticides to prevent breeding of black flies—with annual mass administration of ivermectin, transmission of onchocerciasis was completely eliminated across a vast area of Africa. He described that approach as effective but expensive and said
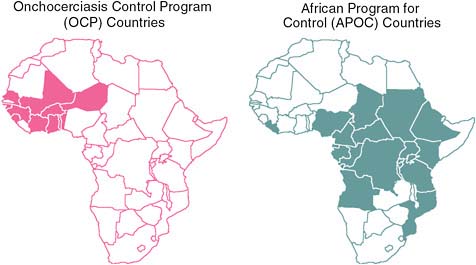
FIGURE WO-7 Onchocerciasis control programs in Africa.
SOURCES: Hopkins (2010); WHO/APOC.
that it would not be feasible in the other forested endemic areas of Africa. Fortunately, he added, ivermectin has been widely used in such areas under the African Program for Onchocerciasis Control (APOC). As illustrated in Figure WO-7, APOC reaches endemic regions of 19 African countries, and pioneered the use of volunteers selected by their own communities to distribute the drug.
Under APOC’s leadership, onchocerciasis prevalence was reduced from 47 percent in 1995 to 29 percent in 2008, Hopkins reported. However, studies conducted by the Carter Center in Cameroon and Nigeria have shown that transmission has persisted after 11 years of MDA in some areas, he continued, as well as in some untreated hypoendemic areas that were ineligible for APOC intervention. Hopkins went on to observe that these results suggested that, without continued external support, some endemic countries would be unable to sustain annual MDA with ivermectin. Indeed, in 2002, at a conference on the eradicability of onchocerciasis co-hosted by WHO and the Carter Center, participants concluded that the disease could not be eliminated throughout Africa without the development of a macrofilaricide to kill the adult worms. On the other hand, participants at this same meeting concluded that the disease could be eliminated with current tools in the Americas and in certain vulnerable foci in Africa and Yemen. Hopkins noted considerable progress toward these goals, including:
-
the elimination of onchocerciasis transmission in some hyperendemic foci in Mali and Senegal;
-
an effort to eliminate onchocerciasis in an isolated focus of the disease on the Sudanese island of Abu Hamad, in the Nile;
-
the launching of a nationwide onchocerciasis elimination program in Uganda; and
-
a 90 percent coverage rate of endemic areas in the Americas with ivermectin administration.
Hopkins noted that the presence of the Loa loa parasite presents a barrier to ivermectin use in 10 of the 30 endemic African countries, because people infected with Loa loa can develop potentially fatal neurological complications when treated with ivermectin, or with albendazole, a drug used to treat LF (see below). Rather than risk these consequences of MDA in Loa loa–endemic areas, he said, ivermectin must be administered only in non-endemic communities, and perhaps even on a patient-by-patient basis; however, he observed, more creative solutions will be needed if the parasite is to be eliminated in Africa. “There really is a tangible risk of inadvertently killing people,” he observed, and the reaction to such a blunder could cause ivermectin to be withheld from people who could benefit from it.
Trachoma Trachoma is a blinding bacterial infection spread by contaminated hands, cloths, and flies. An estimated 120 million people are at risk for the disease, Hopkins reported; 84 million people in 57 countries are infected.
The World Health Assembly established a target to eliminate blinding trachoma (but not all infections) by 2020, using a strategy known by the acronym SAFE:
-
Surgery to prevent progression to blindness;
-
Antibiotic administration to treat active infections and prevent spread;
-
Facial cleanliness; and
-
Environmental improvements (e.g., building latrines to suppress breeding of vector flies, which favor human feces deposited on the ground).
He said that it has yet to be determined how best to measure the quantifiable goals of this effort: reducing scarring trachoma to below 1 case per 1,000 population and reducing active trachoma below 5 percent in children ages 1 to 9.
Nevertheless, significant progress is being made in providing interventions such as latrine construction that offer broad public health benefits in addition to onchocerciasis control, Hopkins observed. For example, following an “explosion of latrine building” under way in the Amhara region of Ethiopia that has produced 1.8 million latrines since 2002, public attitudes toward hygiene have changed dramatically, he said, and similarly rapid and crucial behavioral changes occurred in response to the Guinea worm eradication program.
On the other hand, audience member Sheila West of the Johns Hopkins
University noted that some recent studies suggest that latrines are not as effective in controlling trachoma as once believed. West asked Hopkins whether other environmental measures to combat trachoma had been considered. Whatever their effectiveness against trachoma, latrines provide important public health benefits, he replied, “so I’m still a partisan of [latrine building], even if … the impact on trachoma is 20 percent rather than 60 or more percent.” Moreover, he added, latrines promote public understanding of the impact of hygiene on water-borne and water-washed diseases. “They are understanding that by these kinds of behavioral changes, they can improve their lives and the lives of their families,” he observed.
Hopkins noted that the antibiotic used to treat trachoma, azithromycin (Zithromax®), has been donated by its manufacturer Pfizer for use in MDA campaigns. These campaigns currently consume about 75 million doses per year and, as of 2008, reached approximately 40 million people, or 33 percent of the at-risk population, he reported. According to current estimates, 75 percent of the trachoma burden falls on 10 countries. In Ethiopia, perhaps the most severely affected country, an aggressive campaign has begun in the Amhara region, where the disease is most widespread and entrenched. This effort follows an important principle of elimination and eradication programs, which is, “if you can’t start in both highly endemic and low endemic areas simultaneously, start in the most highly endemic areas first, because it’s going to take the longest amount of time there,” Hopkins observed.
“Much more remains to be done,” Hopkins concluded. “Halfway to 2020, the global program to eliminate blinding trachoma is still uncertain about how many countries are still endemic, which areas require interventions, and how best to monitor progress.” On the other hand, West noted, a recent global scientific meeting hosted by WHO defined both targets and areas for trachoma elimination, and there has been a rapid decline in the number of cases worldwide.
Lymphatic filariasis (LF) A mosquito-borne infection is caused by the parasites Wuchereria bancrofti and Brugia malayi. It causes extreme, disfiguring swelling of the limbs and genitalia in about one-third of those infected, which can cause disability and stigma; some people suffer hidden damage from LF (Hopkins, 2010; Ottesen, 2010). Generally, LF is acquired in childhood, but its outward signs do not become apparent until adulthood. Of the 1.3 billion people at risk for LF in more than 80 countries in Africa, Asia, and the Americas, an estimated 120 million are infected.
LF can be prevented by annual MDA with diethylcarbamazine and albendazole over a period of at least five years, until the adult worms die (Hopkins, 2010; Ottesen, 2010). In Africa, except where Loa loa is endemic, LF is treated with ivermectin and albendazole. Merck, which produces ivermectin, and GlaxoSmithKline (GSK), which produces albendazole, donate these drugs for MDA. Albendazole and ivermectin have important secondary effects on intestinal helminths, including Ascaris and Trichuris, as well as on lice and scabies. Additional
interventions for LF include surgery to reduce disabling hydroceles in men with LF, and palliative care to mitigate secondary infections and swelling of some limbs; both tend to improve patients’ emotional and social health.
The Global Alliance to Eliminate Lymphatic Filariasis (GAELF),10 established in 2000, is a public–private partnership committed to helping LF-endemic countries achieve a minimum of 80 percent coverage for annual MDA with the appropriate drugs (Hopkins, 2010; Ottesen, 2010). Annual treatments to prevent LF rose from 10 million in 2000 to 496 million in 2008, representing 37 percent of the population at risk, he reported; however, LF-endemic areas in 15 countries remained to be mapped at that time. The group aims to conclude a successful global elimination program by 2020.
While acknowledging that “process indicators”—such as numbers of people treated—provide an incomplete (and sometimes deceptive) measure of progress against a disease, Ottesen nonetheless noted that the LF global elimination program11 provided more than 2.8 billion treatments during its first decade. Five countries have completed their MDA courses and are under active surveillance, as are several countries that did not require MDA due to a relatively low prevalence of LF, he said. The LF-related benefits of this program have been twofold, he explained: newborns have been protected from infection with the parasite, and people with asymptomatic infections have been prevented from progressing to overt disease (Ottesen et al., 2008).
The economic value of these health benefits for individuals treated during the first eight years of the program’s operation, and projected over the course of their lifetimes, is an estimated US$22 billion, and for health systems, US$2 billion (Chu et al., 2010). “This is an excellent investment in global health, with impressive economic rates of return,” Ottesen observed. Documenting both the health and the economic impacts of the global LF elimination program are crucial to ensuring its future and, indeed, that of any program targeting NTDs, he concluded.
Current status Table WO-3 summarizes the current status of elimination efforts against dracunculiasis, onchocerciasis, blinding trachoma, and LF. “These times of exceptional opportunities and inspiring progress are as exciting for us professionals as they are important to improving the human condition for this current
|
10 |
GAELF is a public–private partnership created in 2000 to assist in advocacy, resource mobilization, and program implementation. GlaxoSmithKline and Merck & Co. Inc., have pledged all the albendazole and Mectizan® (in Africa where onchocerciasis is prevalent) necessary to achieve elimination—the largest drug donations in history, valued at more than $1 billion. http://www.filariasis.org/who_we_are/index.htm (accessed November 15, 2010). |
|
11 |
In 1997, the Global Programme to Eliminate Lymphatic Filariasis was created in response to a specific resolution by the World Health Assembly. At that time WHO, having recently devised a strategy aimed at achieving LF elimination through MDA, received extraordinary pledges from two pharmaceutical companies (GlaxoSmithKline and Merck & Co., Inc.) for long-term drug donations of unprecedented size to jumpstart this nascent program (Ottesen et al., 2008; http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000317, accessed November 15, 2010). |
TABLE WO-3 Four NTDs Slated for Eradication or “Elimination”
|
Disease Program |
Dracunculiasis |
Onchocerciasis |
Lymphatic Filariasis |
Trachoma |
|
|
OEPA |
APOC |
||||
|
Goal |
Eradication 2009 |
Elimination (interrupt transmission) 2012 |
Control (public health problem) 2015 |
Elimination (public health problem) 2020 |
Elimination (blinding trachoma) 2020 (TF <5% in 1–9 y/o) |
|
Endemic Countries Known? |
Yes |
Yes |
Yes |
Mostly |
Uncertain |
|
Status of Surveillance |
Very good |
Excellent |
Good |
Incomplete |
Incomplete |
|
Coverage Target for Intervention |
100% |
>85% × 2 |
65% |
80% |
80% |
|
Extent of Intervention |
98% filters (2009) |
93% (2009) (0.626 m/0.672 m) |
63% (2008) (57 m/90 m) |
29% (2009) (385 m/1.333 b) |
33% (2009) (40 m/120 m) |
|
Monitor Disease/Intervention |
Monthly |
Monthly |
Annually |
Annually |
No |
|
SOURCES: Hopkins (2010); Carter Center. |
|||||
generation,” Hopkins observed. “Most NTDs cannot be eradicated or eliminated, but all can and should be much better controlled. The few NTDs that may be vulnerable to elimination or eradication should be pursued ruthlessly.”
Human African Trypanosomiasis (HAT) (Sleeping Sickness)
Caused by single-celled protozoan parasites belonging to genus Trypanosoma, HAT is transmitted to humans through the bite of the tsetse fly (genus Glossina) (Simarro et al., 2008). HAT is fatal if left untreated. Tsetse flies inhabit remote sub-Saharan rural areas where health systems are weak or non-existent (Simarro et al., 2008). People who live in these areas and who depend on agriculture, fishing, animal husbandry, or hunting are frequently exposed to the bite of the tsetse fly and therefore to HAT. Thirty-six sub-Saharan countries are considered endemic for HAT, although some of them have reported no cases in the last decade.
Together, HAT and the animal form of the disease, called nagana, have sig-
nificantly hindered development, agricultural productivity, and food security in Africa. Increased HAT transmission is associated with human migration, war, and poverty (Berrang-Ford et al., 2010; Simarro et al., 2008). As part of his presentation on the NZDs (see below), Forum member Lonnie King of the Ohio State University remarked that African trypanosomiasis has rendered cattle production unsustainable in parts of Africa that are heavily infested with tsetse flies. As a result, he said, human and animal populations have migrated from these areas in order to survive.
The vast majority of HAT infections involve the parasite Trypanosoma brucei gambiense, which causes a chronic infection that may remain asymptomatic for months to years (Simarro et al., 2008). Symptoms—such as severe headaches, sustained fever, sleep disturbances, and neurological disorders—may not emerge until the disease reaches an advanced stage and begins to affect the central nervous system. Trypanosoma brucei rhodesiense, which causes fewer than 10 percent of HAT cases, produces an acute infection (Simarro et al., 2008). Symptoms of its first stage occur within weeks to months and include chancre, occasional headaches, irregular fevers, pruritus, and the development of adenopathies. As with gambiense infections, the parasites eventually cross the blood–brain barrier and invade the central nervous system, producing severe neurological disease that is fatal if left untreated.
In the early part of the 20th century, HAT decimated populations in many parts of sub-Saharan Africa (Simarro et al., 2008). In his workshop presentation, Jean Jannin of WHO explained that, in the 1930s, colonial administrations established disease control programs that enabled the systematic screening, treatment, and follow-up of millions of people across the African continent; as a result, by the mid-1960s, HAT transmission was nearly stopped. (Dr. Jannin’s contribution to the workshop summary report can be found in Appendix A, pages 310–323.) Soon thereafter, however, many HAT control efforts were abandoned as many African countries achieved colonial independence and took on more immediate challenges. The disease slowly returned to past endemic areas, leading to outbreaks that began occurring in the 1980s. Social upheaval, war, and migration, combined with a lack of awareness and poverty, hindered the public health response to HAT and permitted its further spread.
Following a 1997 World Health Assembly resolution strongly advocating access to diagnosis and treatment for HAT and the reinforcement of surveillance and control activities, WHO established a network to strengthen coordination among all stakeholders, which led to stronger public- and private-sector support for HAT surveillance, control, and research (Simarro et al., 2008). Aided in part by the abatement of social upheaval and civil war in many countries where HAT was endemic, control of the disease has greatly improved in recent years. Between 1995 and 2006, the total number of new cases reported declined by 68 percent. Today, with fewer than 10,000 cases reported per year for the first time in 60 years, Africa is poised to eliminate HAT, Jannin observed.
Although a field diagnostic for HAT exists and is widely used for population screening, it is neither applicable to rhodesiense disease nor sufficiently sensitive to establish a definitive diagnosis (Simarro et al., 2008). A confirmatory diagnosis requires the microscopic evaluation of blood and lymph for parasites and, when positive results are found, assessment of the stage of infection, which requires a lumbar puncture and cerebrospinal fluid examination. Four parenteral drugs are used to treat HAT: suramin (developed in 1921) for first-stage rhodesiense disease; pentamidine (1940) for first-stage gambiense disease; melarsoprol (1949) for the second stage of both forms of HAT; and eflornithine (1990), which is only effective in the second stage of the gambiense form. Use of any of these drugs is cumbersome and risky, necessitating the support services of a well-trained staff. Over the past decade, WHO has distributed more than 175,000 HAT treatments, according to Jannin. All the drugs are donated to WHO by their manufacturers, sanofi-aventis (eflornithine, melarsoprol and pentamidine) and Bayer AB (suramine).
Jannin emphasized, however, that “we are killing—there is no other word—eight percent of our patients due to the toxicity of melarsoprol, and we are sure to face an increased number of resistant cases.” Halting the use of melarsoprol entirely would end treatment for second-stage rhodesiense infections and switch treatment of gambiense infections to eflornithine, which requires 56 infusions over a two-week period, he said. This would be “quite impossible for the healthcare facilities to do.” To address the latter dilemma, WHO and sanofi-aventis developed eflornithine kits to provide these facilities with everything they needed to administer the drug. In 2006, he said, such a kit weighed 20 kilograms and cost US$700. This load was lightened somewhat by the recent development of the drug combination nifurtimox12-eflornithine for HAT, which reduced the treatment kit’s weight to 9 kilograms and the cost to US$360. This combination also facilitates the administration of the drugs. Despite the considerable challenges associated with distributing these kits to remote locations, “everybody now, even in South Sudan or Democratic Republic of Congo can access these drugs,” Jannin said. “But it’s not enough to provide the drugs,” he continued. Patient management is a challenge, because it requires delivering the drugs by catheter over the course of days. To meet this challenge, WHO organized regional trainings for individuals who are now instructing health care providers at the country level, he said.
Currently, 19 African countries are reporting no cases of HAT, but only 7 of them are continuing regular surveillance, according to Jannin. To confirm these findings, WHO is developing regular surveys of historical foci. Eleven countries have fewer than 100 HAT cases per year, and 3 are reporting between 100 and 500, he observed. Only 2 countries—the Central African Republic and the DRC—are reporting more than 1,000 cases per year. Chad is reporting between
500 and 1,000 cases per year. At this rate (given that current prevalence and incidence estimates for HAT are considered accurate), elimination of HAT now appears feasible, Jannin said.
Among the steps currently being taken to pursue that goal, Jannin described the nearly complete “Atlas of Sleeping Sickness,” which maps 122,000 cases reported in the past decade. Efforts are also under way to develop new diagnostics for HAT that can be used in rural African treatment centers—ready to use, stable at room temperature, operable by workers with minimal training, and affordable by national health systems—and which provide a clear diagnosis of both forms of HAT (Simarro et al., 2008). To support the development of such diagnostics, WHO established a sample bank, currently housed at the Institut Pasteur in France, containing samples of relevant bodily fluids from hundreds of HAT patients and controls, Jannin reported. Attempts are also under way to develop a safe, affordable, orally administered drug effective against both types of HAT at both disease stages (Simarro et al., 2008). Although progress has been made in controlling the tsetse fly by various methods, none is ideal or universal; thus, vector control is also a subject of research and development to address HAT.
The quest to control HAT is now at a turning point, Jannin observed: should the world be content to maintain HAT at low levels, or should attempt to further reduce the number of cases? Moving forward means addressing the considerable limitations of HAT diagnostics, drugs, and their distribution. He added, though, that holding treatment back means risking the re-emergence of the disease, much as occurred in the last decades of the 20th century. This is a particular concern, he added, because of a looming drain on human resources to fight HAT. “The average age of our technicians is around 55 or more,” he said. “We have a system which is now working well. We have fantastic achievements. But it’s very, very fragile.” Despite the graying of the workforce and the fragility of the system, he concluded, the sustainable elimination of HAT can be achieved, and he anticipated that such an announcement would be made by WHO’s Director General in the coming months.
“To look at what has been accomplished against a terrible disease, but an almost even worse treatment, is incredible,” Hopkins observed in discussion following Jannin’s presentation. “It points to the other intangibles, besides the tools,” Hopkins continued. For example, he said, “look at what it takes to treat human African trypanosomiasis and compare it to what it takes to treat yaws (see Box WO-6): a single injection of long-acting penicillin, full stop. It doesn’t even require refrigeration. Yet there is still a lot of yaws in the world.”
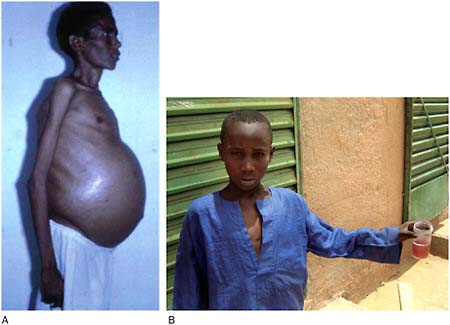
Schistosomiasis
Caused by several species of tremotodes (“flukes”), the parasitic worms of the genus Schistosoma, schistosomiasis (also known as bilharzia) may infect as many as 600 million people in Africa, the Middle East, South America, South-
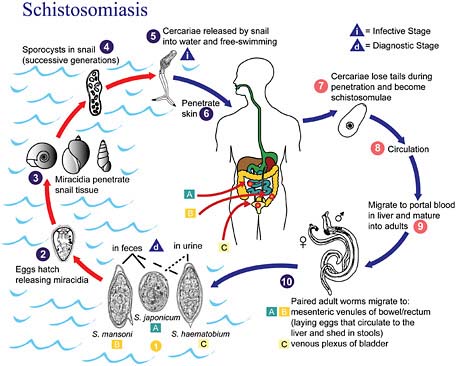
FIGURE WO-8 Life cycle of schistosomiasis.
SOURCE: Centers for Disease Control and Prevention.
east Asia, and the Philippines (King, 2007, 2010). Humans acquire this infection through contact with water infested with the parasite’s free-swimming larval stage. Eggs released into freshwater infect aquatic snails and develop into larvae. After leaving the snails, the larvae can live for about 48 hours before penetrating the skin of a mammalian host, such as a person who is bathing or swimming (CDC, 2008). Within several weeks, worms grow inside the blood vessels of the body and produce eggs. Some of these eggs travel to the bladder or intestines and are passed into the urine or stool, completing the cycle. The life cycle of schistosomiasis is illustrated in Figure WO-8.
Schistosomiasis is closely tied to the unsanitary disposal of human waste, and its spread has been closely linked to the construction of dams and irrigation systems. Following the construction of the Aswan High Dam in Egypt in the 1960s, which was intended to control alluvial flooding and to generate hydroelectric power from the Nile River, environmental changes allowed aquatic snails to proliferate (Malek, 1975). Soon after the dam’s completion, schistosomiasis, which previously had been rare in the region, spread widely and became endemic to the area. More recently, dam construction in Nigeria has dramatically altered
the freshwater environment, allowing freshwater snails to proliferate, which has resulted in an increase in the prevalence of schistosomiasis in the region (Oladejo and Ofoezie, 2006).
Speaker Charles King, of Case Western Reserve University, described recent progress in understanding this challenging disease. (Dr. Charles King’s contribution to the workshop summary report can be found in Appendix A, pages 323–342.) “We are rethinking what schistosomiasis is all about, which in turn helps to drive some of the decision making about who needs treatment, when they need treatment, and for how long,” he said. In the 1970s and 1980s, efforts to address schistosomiasis assumed that “the more infection you had, the more disease you had,” he observed. Therefore, rather than treating entire communities for schistosomiasis, control programs overlooked large numbers of people with subclinical infections and focused on the few with advanced disease, who were identifiable because they were passing high numbers of eggs. Indeed, he noted, some researchers concluded that the vast majority of people infected with schistosomiasis were not ill (Gryseels, 1989) and, consequently, that the disease did not represent a significant public health problem.
“This approach was wrong,” C. King concluded, because it ignored the physiological importance of subclinical disease and, in particular, its effects on development. Most people acquire schistosomiasis before the age of 5, he said. As a result, he said, these individuals suffer a host of systemic and organ-specific pathologies associated with the parasite’s presence, including anemia, stunting, wasting, lack of fitness, cognitive impairment, infertility, and genital lesions. Infection brings about a lifetime of disease, he observed, “starting when you are born, with poor vaccine response; as you start to become affected after the age of four, getting into problems with anemia, growth stunting, lack of fitness. This can persist as long as you are infected. You begin to lose schooling due to persistent symptoms. There is intermittent pain, then chronic organ dysfunction. As you get to reproductive years, there is genital schistosomiasis, both male and female, and infertility in women, with both primary and secondary infertility, probably due to mechanical obstruction.” As previously noted by McDonald, schistosomiasis takes a particularly heavy toll on women, for whom it is associated with increased risk of HIV infection, ectopic pregnancy, and painful intercourse. In Zimbabwe, Hotez reported on studies conducted by scientists at the University of Oslo who showed that female genital schistosomiasis has increased horizontal transmission of HIV/AIDS by at least threefold in Zimbabwe (Hotez et al., 2009a; Kjetland et al., 2006). These problems typically occur before the onset of advanced disease, which tends to occur after age 30, C. King observed. Therefore, “if we focus only on advanced disease,” he said, “that’s a very small subset of the population. It’s also a very small subset of the disease.”
These insights prompted C. King to redefine schistosomiasis as “a preventable, chronic, inflammatory condition caused by present or previous infection with metazoan parasitic blood flukes of Schistosoma species.” He estimated that
more than 440 million people fit this description and that many of them suffer complications from past infection. “We are beginning to refocus on the lifetime impact and the ecology of infection and disease, because, as it turns out, these are actually the more important outcomes that we need to be using as markers in our monitoring and evaluation,” he observed.
C. King then went on to discuss how a broader understanding of schistosomiasis was being translated into efforts to control the disease and mitigate its effects. Mathematical simulations suggest that children treated for schistosomiasis every one to two years through adolescence will attain full growth and experience reduced symptoms, he said. Because schistosomiasis is embedded in ecosystems, he added, “we are beginning to talk about issues of transmission control, not elimination or eradication. We need to do more than just drug therapy to really protect the people in these communities.” An integrated approach, combining education, behavior modification, sanitation, a protected water supply, snail habitat modification, and molluscicide treatment, could significantly reduce transmission of schistosomiasis in endemic communities, he stated. According to their model, reducing child-to-child transmission by 75 percent would effectively eliminate the disease. Ultimately, he said, schistosomiasis demands the same suite of interventions that liberated the American South from hookworm, malaria, and pellagra: disease control, micronutrient supplementation, agricultural extension programs, education, and rural electrification (in part to ensure clean water).
Chagas Disease
Sometimes called “American trypanosomiasis,” Chagas disease is also caused by a trypanosome parasite, T. cruzi, and spread by an insect vector—not by a fly, but by members of the family Reduviidae commonly known as “kissing bugs” or “assassin bugs.” Speaker Rick Tarleton, of the University of Georgia and the Chagas Disease Foundation, explained that reduviid bugs, which feed on warm-blooded animals, infest the walls and roofs of houses in areas where Chagas disease is endemic. (Dr. Tarleton’s contribution to the workshop summary report can be found in Appendix A, pages 505–522.) Parasites present in the bug’s feces can be transmitted to mammals as the insect feeds on them, or through ingestion of contaminated food. In its mammalian host, the parasite cycles between a brief extracellular stage that circulates in the bloodstream and the disease-producing intracellular stage that penetrates muscle, fat, and sometimes nerve cells, causing cardiac disease—the most common consequence of infection—as well as gastrointestinal disorders. These symptoms, which result from years of accumulated tissue damage due to the body’s immune response to low levels of parasites (which it can suppress, but not easily clear), typically arise decades later and tend not to be treated, he said.
Surveillance for Chagas disease is poor, according to Tarleton, who estimated that 10 to 20 million people, most of whom live in Latin America, are infected
with the parasite. The southern United States is endemic for Chagas disease, and the parasite is present in many animal species in that region. He noted, however, that good housing conditions in the region often limit transmission to humans. However, physician awareness of Chagas disease is low and screening and treatment capabilities are limited in the United States and in other wealthy countries (e.g., Spain, Canada, Australia, and Japan) where the disease infects relatively few people, most of whom are immigrants. Transmission of Chagas disease within these countries occurs mainly congenitally or between blood and organ donors and recipients, he said.
Nevertheless, Hotez observed, Chagas disease transmission does appear to be occurring at low levels in areas of the United States inhabited by reduviid bugs. He noted that zoonotic transmission from dogs is also a concern in the United States, as is the possibility that climate change will expand the geographic range of reduviid bugs, thereby increasing the potential distribution of Chagas disease.
Owing to the low parasite load typical of T. cruzi infection, diagnosis is difficult, Tarleton observed. The standard for detection is a blood smear, which is often inconclusive (CDC, 2010c).13 Positive results on at least two of three different serological tests is often accepted as a definitive diagnosis, he explained. Different groups use different sets of tests to determine seropositivity or negativity for Chagas disease, he said. Even using best-two-out-of-three, it may not be possible to detect every infected individual. The most sensitive method for T. cruzi diagnosis, known as xenodiagnosis, involves allowing lab-reared (sterile) reduviid bugs to feed on patients, according to Tarleton; if the bugs become infected, the parasite will multiply to detectable levels within four to six weeks. This technique is still used frequently, especially on young children, he noted—and, like all other parasite detection methods for Chagas disease, it is not sufficiently sensitive to monitor treatment efficacy.
As Tarleton pointed out, the upside of the diagnosis dilemma for Chagas disease is the lack of need for rapid or point-of-care tests; most patients with symptoms are already chronically infected, and the treatment course lasts 30 to 60 days. There is no urgency to treat them at the point of detection, he explained; testing and treatment can be performed at centralized locations. He added, however, that disease diagnosis needs to involve a single, uniform, and rigorously certified test that is quantitative so that it can also be used to monitor changes during and following treatment. Rather than attempt to detect small numbers of parasites, it might be preferable to identify robust protein or gene-expression markers and incorporate them into multiplex serodiagnostic assays, he added.
|
13 |
The diagnosis of Chagas disease can be made by observation of the parasite in a blood smear by microscopic examination. Thick and thin blood smears are made and stained for visualization of parasites. However, a blood smear works well only in the acute phase of infection, when parasites are seen circulating in blood. CDC Chagas Disease—Diagnosis. http://www.cdc.gov/chagas/diagnosis.html (accessed November 15, 2010). |
“Development of a test that absolutely certifies parasitological cure is also going to be very difficult,” he concluded, “but I don’t think this is unique to T. cruzi or Chagas disease.”
Two drugs are widely used to treat Chagas disease, Tarelton stated: benznidazole, the mainstay of current treatment, and nifurtimox, which was used in the past and has recently returned to use. Both produce considerable but generally manageable side effects, he added, and they require a 30- to 60-day course of treatment. Their biggest drawback stems from the lack of diagnostic methods to assess their efficacy. “It’s easy to tell if somebody is acutely infected and has the circulating parasites that a drug works,” he observed. “It’s not so easy when you can’t monitor what happens in somebody who is chronically infected … [and for whom] knocking down the parasites below a detectable level is not a cure.” There is, however, evidence from a long-term study (that was neither randomized nor placebo-controlled) that treatment with benznidazole reduces the cardiac sequelae of Chagas disease, Tarleton noted. In this study, patients were followed for up to 15 years to compare the progression of clinical disease after treatment with the drug, or in the absence of treatment (Viotti et al., 2006). The researchers found that progression toward cardiac disease was reduced by approximately 75 percent in patients who received benznidazole. However, he added, “you certainly can’t test a drug this way, so one thing that is really needed is accessible tests of cure, in order to evaluate new therapeutics [for Chagas disease].”
There are much improved high- and medium-throughput screening assays available to find leads for Chagas disease drugs, Tarleton continued, as well as good in vivo screens with which to follow up promising candidates. He noted that several companies, public–private partnerships, and nonprofits have begun to pursue this route. However, he added, inability to determine parasite load represents a major stumbling block to drug development for Chagas disease.
Treating houses with insecticide is the most effective means available for controlling T. cruzi infection, Tarleton said. This was demonstrated in initiatives coordinated by WHO and PAHO in the Southern Cone of South America in the 1980s and 1990s. However, he continued, reduviid bugs have developed insecticide resistance in some locations—a situation that is likely to worsen, particularly because the same insecticides are used in agriculture. Moreover, spraying houses is time-consuming, labor-intensive, and effective only for a few months. Integrated and sustainable vector control will be necessary to address Chagas over the long term, and it must be based on data, rather than anecdote, he concluded.
We are “just at the tip of the iceberg” in understanding Chagas disease, Hotez observed, and particularly its associated burden of disability. Speaker Mauricio Barreto, of Federal University of Bahia in Salvador, Brazil, recalled that in the 1970s, when his country experienced intense transmission of Chagas disease, it was estimated that more than 5 million people were infected with the parasite. An intensive vector control program eliminated its main vector, which (along with
monitoring of the blood supply) reduced transmission to very low levels, he said. Nevertheless, it is important to recognize the chronic nature of Chagas disease and to develop treatments for its sequelae in addition to preventive measures, he concluded.
NTDs in the United States
In a workshop presentation titled “Left Behind in America: Our Nation’s Neglected Infections of Poverty,” Hotez described how he came to the conclusion that several NTDs and related neglected infections of poverty remain highly prevalent among the poorest residents of the United States (Hotez, 2008b, 2009a). Following a database search through PubMed over 25 years using terms such as “neglected diseases” and “poverty,” as well as specific geographic regions, racial, ethnic, and socioeconomic groups, and the names of the 30 NTDs listed on the website of PLoS Neglected Tropical Diseases, he estimated disease prevalence rates among selected communities in which studies had been done. He then multiplied these local rates by published estimates of at-risk populations in socioeconomically disadvantaged groups to produce the estimates shown in Table WO-1.
“The diseases that really stuck out from this analysis were what we sometimes now call the three C’s and the three T’s,” Hotez said. Two of the Ts, toxocariasis and trichomoniasis, are prevalent among impoverished African American populations, as is congenital cytomegalovirus, particularly among women and children; among impoverished Hispanic Americans, he found relatively high rates of cysticercosis, Chagas disease, and toxoplasmosis. Hotez provided the following brief descriptions of these diseases as manifested in U.S. populations (see above for a discussion of Chagas disease in the United States).
Toxocariasis Although “not a disease most people think about,” Hotez said he believes toxocariasis to be the most common helminth infection in the United States (Hotez and Wilkins, 2009). The parasite is transmitted from infected dogs and cats—in the United States, dogs in the inner city and stray dogs in the South—which shed eggs that people ingest; the larval stages then migrate either through internal organs or the central nervous system (visceral larva migrans) or they migrate to the eyes or optic nerve (ocular larva migrans). Several European studies (Buijs et al., 1994, 1997; Taylor et al., 1988) link toxocariasis to asthma and developmental delays, he reported. A recent CDC survey found an age-adjusted seroprevalence for the disease of 21 percent among non-Hispanic blacks versus 12 percent among whites, with the highest rates in the American South (Won et al., 2008); Hotez noted that the risk factors associated with infection included low levels of education, poverty, and elevated lead levels. His own estimates suggest that about 2.8 million African Americans in the United States have been exposed to or infected by toxocariasis.
Trichomoniasis African American women have a 10-fold higher prevalence of this parasitic sexually transmitted infection compared to whites, according to Hotez, with some of the highest rates occurring in Louisiana. Apparent risk factors for trichomoniasis infection in the United States include low educational levels and poverty. Hotez called his original estimate of approximately 900,000 cases in African American women “very conservative.” Trichomoniasis is associated with preterm delivery and low birth weight, he reported, and the disease has also been identified as an important risk factor in the HIV/AIDS epidemic in the American South, because of increased viral shedding associated with co-infection (Kissinger et al., 2008; Sutton et al., 2007).
Congenital cytomegalovirus (CMV) infection A leading cause of hearing loss, vision loss, and mental retardation, CMV occurs at strikingly high rates among African American women, Hotez said. “There are an estimated 27,000 new CMV infections among seronegative pregnant women every year who acquire the virus during pregnancy,” he noted, reflecting a 50-fold increase in risk among pregnant African American teenagers and a fourfold increase in risk overall (Colugnati et al., 2007). CMV is therefore “extraordinarily common and a major reason why kids are institutionalized here in the United States,” he concluded.
Cysticercosis This parasitic infection of the brain currently represents the leading cause of epilepsy among Hispanic Americans, Hotez reported, adding that approximately 10 percent of seizures now presenting to emergency departments in Los Angeles result from cysticercosis. According to Hotez’s estimates, between 40,000 and 169,000 Americans are infected by the tapeworms Taenia solium (the pork tapeworm) and Taenia saginata (the beef tapeworm).
Congenital toxoplasmosis Hotez estimates that about 4,000 cases of this disease, caused by the protozoan parasite Toxoplasma gondii, occur in the United States each year. According to L. King, toxoplamosis is the nation’s third leading cause of death attributed to food-borne illness. A fetus can become infected with toxoplasmosis if the mother becomes infected during pregnancy, which may occur through improper handling of cat litter, or by touching or ingesting contaminated meat. Domestic cats contract the parasite from eating infected rodents or birds. Up to half of the fetuses infected with T. gondii are born preterm, and it can damage their eyes, nervous system, skin, and ears (National Library of Medicine, 2009).
Hotez noted that newborns with toxoplasmosis are often asymptomatic at birth; it can take up to a year for clinical manifestations, such as retinitis or intellectual deficits, to become apparent. “You actually have a year of opportunity to intervene with specific antiprotozoal chemotherapy,” he observed, yet in the United States, only two states—Massachusetts and New Hampshire—routinely
screen newborns for toxoplasmosis.14 “The cost-effectiveness studies are there to show that we should be doing screening throughout the United States,” he asserted. Moreover, rates of disease—and consequences for children—are probably far higher in other countries. “This is probably an extremely important infection that we are just beginning to understand the full extent of,” Hotez said, adding that congenital toxoplasmosis is not included in current global burden of disease assessments.
Hotez reported on new legislation, titled “The Neglected Infections of Impoverished Americans Act of 2010,” which was introduced by Rep. Hank Johnson of Georgia and just passed in the U.S. House of Representatives. The legislation requests that the Secretary of the Department of Health and Human Services report to the U.S. Congress on the state of the neglected infections of poverty, especially the three Cs and Ts, as a beginning for understanding the true extent of these conditions, their modes of transmission, and research needs.
Dengue
A vector-borne viral disease spread by mosquitoes, dengue currently threatens 40 percent of the global population and causes up to 100 million infections and 25,000 deaths each year (WHO, 2010a). Dengue differs from other NTDs in that it causes acute illness rather than chronic disease (as noted in previous discussion regarding the inclusion of dengue among the NTDs); these include the complications dengue hemorrhagic fever (DHF) and the frequently fatal and more severe manifestation of the disease as dengue shock syndrome (DSS) (Morens and Fauci, 2008). Dengue incidence has increased rapidly over recent decades, and explosive outbreaks have occurred. As several workshop participants observed, dengue is underreported throughout the world (see below).
Brazil has been hard hit by dengue, particularly in its urban centers (Teixeira et al., 2009). The most recent epidemic in Rio de Janeiro, which occurred in 2008, claimed more than 200 lives, Mauricio L. Barreto of the Instituto de Saúde Coletiva, Federal University of Bahia, Salvador, Brazil, said. Dengue re-emerged in Brazil in the early 1980s when the DENV-1 serotype was introduced into the country, preceded a few years earlier by the re-introduction of Aedes aegypti; it has since spread throughout the country, transmitting three of the four dengue viruses (DENV-1 through DENV-3). Barreto reported that the presence of DENV-4 was officially confirmed as of July 2010. Although dengue is a very important disease intervention priority, Barreto observed that its control in Brazil presents major intervention challenges: its range has expanded rapidly, its vector is ubiquitous, and the factors that influence why some cases develop into a deadly hemor-
rhagic fever have yet to be determined.15 He expressed concern that a “massive epidemic in large cities in Brazil” could occur as early as 2011.
In his presentation to the workshop, the chief of the CDC’s dengue branch, Harold Margolis, discussed the possible re-emergence of dengue in the continental United States. In his formal remarks at the workshop, he noted that more than 4 million Americans live in the dengue-endemic areas of Puerto Rico, the Virgin Islands, and the Pacific islands. In Puerto Rico, dengue is prototypically seasonal (with low rates of ongoing transmission during cooler, drier periods), prone to epidemics, and under-recognized, he said. The epidemiology of dengue in Puerto Rico (as well as Brazil) differs from that of other dengue-endemic locations in the region—nearly half of all cases occur in older children, young adults, and adults, he observed. In Asian countries such as Thailand and Vietnam, as well as in Central America, nearly all dengue infections and disease occur in very young children. In Puerto Rico, dengue “jumped the epidemic threshold” in July 2010, following heavy El Niño rains, Margolis said. At the time of his presentation, in mid-September 2010, more than 14,000 cases had been reported.
Between 1946 and 1980, there were no reported cases of dengue acquired in the continental United States and, until 2009, no locally acquired cases beyond the Texas–Mexico border (CDC, 2010a). In 2009, a locally acquired case of dengue was detected in Key West, Florida, heralding a local outbreak involving 27 residents; locally acquired cases have continued to be reported there. Margolis described the response to this outbreak, which included a serologic survey of 240 residents in which 13 were found to be seropositive for DENV-1. At the time of the workshop, Margolis stated that despite intensive vector control efforts a total of 44 locally acquired cases had been reported in 2010.
Based on the serologic survey, CDC estimated that 5 percent of Key West residents were seropositive for DENV-1 (CDC, 2010b). Hotez noted that DENV-2 has been prevalent along the Texas–Mexico border for several decades, particularly in low-income communities lacking window screening and garbage pickup (let alone air conditioning). These circumstances, Hotez warned, could precipitate urban dengue outbreaks throughout the Gulf Coast region that could become especially severe if the two viruses—DENV-1 and DENV-2—overlap, increasing the risk for DHF.
The potential for dengue to spread further into the United States depends to a large extent on the ranges of its vector species, Aedes aegypti and Aedes albopictus, Margolis said. Aedes aegypti is the fittest vector for dengue transmission, because it feeds exclusively on human blood. Aedes albopictus—also known as the Asian tiger mosquito—has a much wider range; it feeds on other warmblooded animals and undergoes diapause during the winter, he explained. Where the two species overlap, or where only A. albopictus lives, dengue transmission is
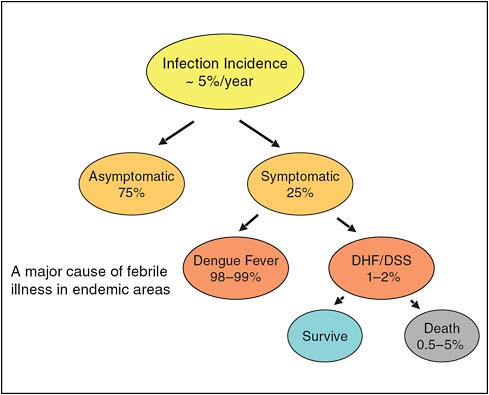
FIGURE WO-9 Dengue virus infection.
SOURCE: Margolis (2010); adapted from Vaccine 2002; 3043–3046.
not sustained as it is in areas inhabited solely by A. aegypti, such as Puerto Rico and Key West, Margolis stated.
Turning to the global re-emergence of dengue, Margolis discussed its under-recognition, which represents a significant barrier to addressing the disease. Figure WO-9 depicts the symptomatic cascade that results from dengue infection in endemic areas.
Only about one in four infections is symptomatic, he said; even so, dengue is a major cause of febrile illness in many tropical areas, sometimes co-circulating with influenza. Comparisons of laboratory-confirmed dengue in study sites with national incidence figures from several endemic locations, shown in Table WO-4, reveal major discrepancies suggestive of widespread under-diagnosis. Similarly, case reviews of suspected dengue deaths in Puerto Rico indicate that dengue-associated mortality is under-reported, much to Margolis’ dismay. “In a country where you ought to be recognizing a severe disease like this, we are not recognizing it,” he acknowledged.
TABLE WO-4 Laboratory-Confirmed Dengue Fever in Study Sites Compared to Reported National Incidence
|
Study Site |
Design |
Age Group |
Incidence |
Incidence (National Surveillance) |
|
Patillas, Puerto Rico |
Enhanced surveillance |
All |
0.8% |
0.07% (10) |
|
Managua, Nicaragua |
Cohort |
4–16 years |
0.9% |
0.026% (34) |
|
Kolkata, India |
Cohort |
All |
0.5% |
0.002% (250) |
|
Ratchaburi, Thailand |
Cohort |
3–13 years |
1.7% |
0.075% (23) |
|
Kampong Cham, Cambodia |
0–15 years |
Active surveillance |
1.0% |
0.028% (33) |
|
SOURCE: Margolis (2010); Pediatric Dengue Vaccine Initiative Field Site Consortium data. |
||||
On a more positive note, Margolis described some recent improvements in the “dengue toolbox,” comprised of integrated vector control, case management, diagnostics, vaccines, and antivirals. Although integrated vector control has been the mainstay of primary prevention for dengue, “it has really not prevented nor has it ever stopped an epidemic that is in progress,” he said. Integrated vector control might be possible through the release of genetically modified, sterile mosquito vectors, which are currently being developed. He also held out hope that reclassification guidelines from WHO, introduced in 2009, might improve identification of dengue cases and, therefore, their outcomes, because good case management has been demonstrated to reduce dengue mortality from 5 percent to less than 0.3 percent. Margolis further noted that primary care physicians in Puerto Rico, in order to maintain their medical licenses, must now take a course in dengue case management by 2013.
Margolis also cited recent improvements in dengue diagnostics, which he characterized as having been complicated, difficult to interpret, too slow for clinical diagnosis, and expensive. “Dengue is tough,” he said. “You get viremia that actually goes on for a fairly long period of time at high titers. There is a soluble antigen, a nonstructural antigen … [but] IgM [immunoglobulin M] doesn’t become detectable until about day 4. We don’t know if it’s not produced or if our tests aren’t that good.” As a result, dengue diagnosis previously required two specimens: one from the acute stage, and one from the convalescent stage—fine for surveillance, he observed, but no help to clinicians. Moreover, he added, in subsequent infections, IgM titer tends to be lower. However, a combined virus
detection strategy, using a molecular or NS116 test with the IgM anti-DENV,17 circumvents the need for paired samples (Lima et al., 2010). Margolis added, however, that this detection method has not yet been commercialized or sold in Food and Drug Administration (FDA)-approved kit form. Its availability, therefore, is quite limited, especially in low-income countries.
Margolis reported that several companies are developing tetravalent dengue vaccines, although most are in early (Phase I) stages of evaluation. Dengue vaccines must overcome some significant challenges including
-
interference, as multi-valent formulations of live, attenuated vaccines result in decreased immunogenicity and increased number of vaccine doses;
-
the need to protect against multiple DENV types; and
-
the distribution and delivery of vaccines to low-resource settings.
There is also a theoretical potential for vaccination to cause immune-enhanced disease, which Margolis said could be studied in animal models. In the course of these vaccine trials, about 800 children have been immunized with monovalent vaccines and challenged with another dengue infection. None of these children, now adults, has had severe dengue upon reinfection. These data suggest (although with small numbers and large confidence intervals) that immune-enhanced disease will be a rare event, he concluded—although active surveillance is being conducted to confirm this suggestive evidence.
In response to Margolis’ presentation and the ongoing discussion about whether dengue should be included among the NTDs, Dr. Lorenzo Savioli, Director of WHO’s Department of Control of the Neglected Tropical Diseases, related discussions at WHO’s NTD Strategic and Technical Advisory Group in 2010 that dengue should be distinguished from the NTDs with a special program all its own because of its present expansion. Dengue outbreaks can be effectively contained—at least in some endemic areas—with appropriate surveillance, integrated vector management, and proper tools, Savioli observed. WHO has taken up this challenge, he said, “because indeed it’s something that is getting out of control.”
|
16 |
The flavivirus non-structural protein NS1, a highly conserved and secreted glycoprotein, is a candidate protein for rapid diagnosis of dengue in endemic countries. The NS1 antigen test (the Platelia Dengue NS1 Ag assay) is a test for dengue made by Bio-Rad Laboratories and Pasteur Institute, introduced in 2006. It allows rapid detection on the first day of fever, before antibodies appear some five or more days later. For further information, see NS1 Antigen Test, http://en.wikipedia.org/wiki/NS1_antigen_test (accessed November 16, 2010). |
|
17 |
The most used techniques for dengue serodiagnosis are based on the anti-DENV IgM and immunoglobulin G (IgG) detection by using MAC-ELISA and IgG-ELISA. SOURCE: Lima et al. (2010). |
Neglected Zoonotic Diseases (NZDs)
Approximately 60 percent of all human pathogens are zoonoses: microbes that are naturally transmitted between animals and humans. Zoonotic diseases such as anthrax, bovine tuberculosis, brucellosis, cysticercosis, echinococcosis (hydatid disease), and rabies are endemic in many developing countries of Africa, Asia, and South and Central America. Neglect of their control persists because of a lack of information and awareness about their distribution, a lack of suitable tools and managerial capacity for their diagnosis, and a lack of appropriate and sustainable strategies for their prevention and control. Furthermore, many of the most affected countries have poor or non-existent veterinary public health infrastructures. This situation has marginalized control of zoonoses to the gap between veterinary responsibilities and medical needs, generating a false perception that their burden and impact on society are low. As a result, neither the human and animal health resources nor the research needed for their control are available, spawning a category of NZDs.
Introducing a workshop presentation that described individual NZDs and issues in their control as a group, Forum member Lonnie King of the Ohio State University characterized these diseases as a subset of NTDs. In particular, he noted, NZDs disproportionately burden the poor, who are more at risk of acquiring these illnesses because of their frequent contact with infected animals, and because poverty reduces opportunities for effective treatment. A general lack of awareness of the importance of NZDs has resulted in a concomitant dearth of suitable diagnostic tests and surveillance for these diseases, and of appropriate and sustainable prevention and control strategies to address them, he stated.
NZDs are doubly burdensome, as they sicken and kill valuable livestock as well as humans. This impact falls largely on the rural poor in developing countries, many of whom depend on animals for food, transportation, and farm work. NZDs, moreover, also affect the urban poor through their consumption of contaminated meat and milk products (WHO, 2006, 2010d,e). Agricultural development programs, a potential source of support for animal health in developing countries, have tended to focus on crops rather than livestock.
Because NZDs largely affect impoverished livestock caretakers and their families, prevention and control of these diseases can save lives, secure livelihoods, and help alleviate poverty, L. King observed. He estimated that livestock contributes to the livelihoods of 70 percent of the world’s poor people, and that approximately 800 million poor livestock keepers are dependent upon their animals and animal agriculture. The animals, in turn, support farmers, consumers, laborers, and trade in developing countries in major ways. According to the United Nations Food and Agriculture Organization (FAO), livestock numbers will need to increase by 50 percent in the next decade to meet increased global demand for proteins from animal sources (FAO, 2002), increasing the opportunity for transmission of a broad range of zoonotic diseases, he added. To meet these challenges, the unique position of NZDs at the interface between human and
animal health must be recognized,18 and the two constituencies must engage in effective collaboration, he concluded.
L. King provided the following descriptions of representative NZDs (see also discussion of African trypanosomiasis, above, and disease descriptions in Box WO-6), and he noted that all zoonotic diseases in animals are neglected in developing countries.
Anthrax Caused by the spore-forming Bacillus anthracis, anthrax is almost always fatal in animals. In humans, the disease occurs in three forms: inhalation anthrax, an occupational disease that affects humans only in industrialized countries; gastrointestinal anthrax, which results from eating meat from an infected animal; and cutaneous anthrax, acquired through contact with skin lesions of infected animals, which comprises 95 percent of cases in developing countries.
Bovine tuberculosis Caused by the bacterium Mycobacterium bovis, this form of TB produces extra-pulmonary infection that seldom causes death in cattle but can cause significant morbidity in cattle in developing countries. In humans, bovine TB may be clinically indistinguishable from M. tuberculosis infection or it may be manifested as an extra-pulmonary infection. Patients often do not respond to common TB drugs. As a result, improper diagnosis and treatment may prove fatal. Effective treatment of bovine TB in humans requires specific and expensive drugs. Both forms of human TB infections appear to be increasing worldwide, and at similar rates, but both diseases are known to be under-reported. Prior to the establishment of milk pasteurization in the United States, an estimated 20 to 30 percent of domestic TB cases resulted from infection with M. bovis.
Brucellosis Caused by bacteria of the genus Brucella spp., this disease infects cattle, sheep, pigs, and goats, among other species. Livestock infection contributes to abortion, permanently reduced fertility, and chronically reduced milk yields. Brucellosis is transmitted from infected animals to humans by direct contact, or by drinking their milk. In humans, the resulting chronic debilitating disease in tropical areas is often mistaken for drug-resistant malaria. In disease-endemic areas, brucellosis results in large losses to livestock producers.
Cysticercosis Caused by the pork tapeworm Taenia solium, cyticercosis infects humans who consume food contaminated with T. solium eggs. After the larvae hatch in the digestive system, they migrate to the muscles, brain, or eyes, where
they form cysts. Cyst formation in the brain can induce seizures. Cysticercosis represents a major cause of preventable epilepsy, especially in developing countries. An estimated 50 million people are infected with T. solium, most of whom live in areas distinguished by inadequate sanitation and poor pig husbandry practices and that lack effective systems for meat inspection and infection control. Because the disease travels with both humans and pigs, it spreads easily. In the United States, livestock in some border areas have relatively high rates of cysticercosis because of their increased exposures to infected people.
Echinococcosis Also known as hydatid disease, echinococcosis is caused by the tapeworm Echinococcus granulosus,19 which moves from sheep, to dogs, to people who ingest tapeworm eggs through contaminated water and food. The resulting cysts, typically located in the abdomen, grow slowly but ultimately must be surgically removed. The disease can be controlled by deworming dogs and keeping them from eating sheep offal. Echinococcosis is distributed globally and is associated with a significant burden of disease, especially in northern Africa and the Middle East.
Rabies Although well-controlled in developed countries, rabies causes an estimated 55,000 to 60,000 deaths per year in the developing world. Domestic dog bites are responsible for more than 95 percent of human infections, although bats are a major reservoir for the virus. Post-exposure prophylaxis for rabies is administered to approximately 10 million people each year. In developing countries, veterinary care tends to be poorly supported and there is little collaboration between animal and human health sectors. Not surprisingly, rabies is endemic in settings where large groups of unvaccinated and unconfined domestic dogs are present, coupled with few sustainable control measures, a lack of rabies education among traditional healers, and political or economic instability.
L. King also briefly discussed several diseases he termed animal-associated NZDs. These include dengue, toxoplasmosis, Chagas disease, and toxocariasis.20 Highlights of his remarks on some of these diseases, and disease agents not discussed elsewhere in this overview, are noted below and in Box WO-6.
Bartonella and other rickettsial diseases These infections—among them, cat scratch disease, spotted fevers, typhus, and scrub typhus—are caused by related organisms, which include members of the genera Bartonella, Anaplasma, and Ehrlichia. A wide range of vectors including sand flies, lice, fleas, biting flies, and ticks transmit the organisms to humans. As more is learned about Bartonella, L. King observed, the diseases associated with these bacteria appear to be “more
widespread in the world than anybody ever thought especially in immunocompromised populations.”
Q fever Coxiella burnetii bacteria, which are shed in the milk, urine, feces, amniotic fluids, and placental materials of animals, can infect humans, causing the flu-like symptoms of Q fever. About 1 percent of infected individuals develop chronic infection. The bacterium is distributed worldwide and has recently caused major outbreaks of disease in the Netherlands, where more than 50,000 pregnant sheep were slaughtered in 2010 in an attempt to curb its spread; 8 percent of sheep in the Netherlands are estimated to be seropositive for Coxiella. A recent U.S. study determined that more than 3 percent of the general U.S. population (~9.5 million people), and about 22 percent of U.S. veterinarians, were seropositive for the bacterium.
Leishmaniasis Recognized by Hotez as “one of the most important protozoan infections of humans after malaria,” leishmaniasis (also known as kala-azar) is actually a complex of diseases involving more than 20 parasite species, more than 30 vector species of sandflies, and a broad range of animal hosts including wild rodents, marsupials, hyraxes, edentates, and dogs, as well as humans. A disfiguring and sometimes fatal disease, leishmaniasis mainly affects residents of low- and middle-income countries who reside in areas where housing and sanitation are inadequate. The disease tends to disproportionately affect the very young and very old.
Leptospirosis Perhaps the most under-reported NZD in the world, leptospirosis is a bacterial infection that is usually transmitted to humans through contact between skin or mucous membranes and water contaminated with the urine of an infected animal. Because leptospirosis is a febrile illness without any “unique” early signs or symptoms of infection, it is difficult to diagnose. Moreover, surveillance for leptospirosis is poor, and its prevalence remains unknown. It is, however, perceived to be a growing threat in peri-urban settings with large rodent populations.
Food-borne trematodes More than 70 species of trematodes (flukes) are known to be associated with a variety of diseases in people. Transmission occurs through a complex life cycle that involves snails as carriers, and, secondarily, animals that transport infected snails along with mud in their hooves. Trematodes currently infect an estimated 40 million people; 10 percent of the world’s population is at risk of contracting such infections, with opisthorchiasis and clonorchiasis linked to high rates of cholangiocarcinoma (bile duct cancer) in China, Southeast Asia, and elsewhere.
Hepatitis E21 While L. King noted only that the number of cases of this disease appears to be increasing rapidly in both developed and developing countries, Forum member Rima Khabbaz, of CDC, observed that hepatitis E is a potentially vaccine-preventable disease and that several promising candidate vaccines have been developed. The lack of a well-defined burden of disease associated with hepatitis E, or a clear picture of its geographic distribution, serves as a disincentive for vaccine development. Manufacturers have been reluctant to continue studies necessary for vaccine licensure if the market for hepatitis E vaccine is limited to developing countries alone, she noted.
Several of L. King’s descriptions of NZDs demonstrate that, while many zoonoses pose a particularly serious risk to humans, the most effective way to control these diseases in humans is to address the disease burden of livestock and wild animals. Interventions designed to prevent and control NZDs require concerted action between veterinary, livestock, and human health sectors through a comprehensive, integrated, and interdisciplinary approach, he contended. Working across disciplines and professions—a skill he characterized as “meta-leadership”—will be essential.
L. King identified the following “critical actions and approaches” for addressing NZDs:
-
Raise global awareness;
-
Improve impact measures to better determine the burden of disease associated with NZDs and to inform cost-benefit analyses for interventions;
-
Implement surveillance to systematically collect incidence data and identify populations at risk for NZDs;
-
Invest in developing more accurate, affordable, and easy-to-use diagnostics; and
-
Build effective animal health infrastructure.
Reflecting on the need for better surveillance of NZDs, Hotez observed that the determination of prevalence and disease-burden estimates for the largely non-zoonotic NTDs such as schistosomiasis, soil-transmitted helminthiases, and LF raised awareness of these diseases and increased support for addressing them. “I don’t see that with the zoonotic diseases,” he said. “I think that’s what is holding back the community in a big way.” He suggested that the zoonotic diseases make a “hit-list” of the top five zoonotic infections to be targeted for control in order to focus awareness and galvanize advocacy for this group of truly neglected diseases.
L. King agreed with this notion, but he said that the dearth of funding for
animal health would prove a barrier to such efforts. He added, however, that overcoming the “downward spiral of lack of appreciation, and consequently … lack of advocacy” might be possible if agencies such as FAO and WHO joined forces with the World Organization for Animal Health (OIE). He also noted that integrated control programs (described in the next section of this overview) could address NZDs along with other more “visible” NTDs. The “toolkit” of approaches to controlling NZDs is growing, L. King said, but its rate of growth reflects accessibility to markets and the marketability of animal health products.
Animal health in general has received comparatively little philanthropic encouragement compared with human health, King observed. Control of zoonotic diseases requires coordination between the veterinary and human medical and public health sectors, which is particularly difficult to achieve under conditions of limited resources and inadequate governance structures. The lack of animal health infrastructure in many developing countries fosters the perception that zoonotic diseases do not impose a significant health burden. On the contrary, evidence-based studies on some NZDs have determined that their true incidence can be 100-fold higher than reported, as was the case for rabies in Africa (WHO, 2006).
NZDs are doubly burdensome, as they sicken and kill valuable livestock as well as humans. This impact falls largely on the rural poor in developing countries, many of whom depend on animals for food, transportation, and farm work. NZDs, moreover, also affect the urban poor through their consumption of contaminated meat and milk products (WHO, 2006, 2010d,e). Agricultural development programs, a potential source of support for animal health in developing countries, have tended to focus on crops rather than livestock.
The under-diagnosis (and therefore, under-treatment) of NZDs in humans, another aspect of poverty, adds to the cumulative burdens they impose. The magnitude of that burden remains unknown since NZDs are also under-reported. The following statistics from WHO (Box WO-4) illustrate a variety of adverse consequences of some NZDs (WHO, 2010f).
With the projected rapid growth of animal populations to supply the protein demands of the expanding human populations in the developing world, the return on investment for supporting animal health becomes increasingly clear. Controlling zoonotic diseases will not only reduce livestock and human illness but will also improve the livelihood of millions of impoverished livestock keepers and their families. This, in turn, could lead to broad-based improvements in public health. In addition, L. King said, because zoonoses can travel as widely as their hosts, controlling NZDs where they normally occur can reduce the threat of global disease proliferation.
Approaches to Integrated Control of NTDs and NZDs
The experiences of the Rockefeller Sanitary Commission in its attempt to eradicate hookworm from the American South in the early 20th century (dis-
|
BOX WO-4 Cumulative Burdens of the NZDs
SOURCE: WHO (2010f). |
cussed earlier) highlight key aspects of current attempts to control neglected diseases of poverty and to mitigate their health and economic consequences. These efforts employ two basic approaches, either singly or in combination: mass drug administration and environmental interventions, encompassing both human-built and natural environments (Spiegel et al., 2010). Over the past two decades, mass administration of drugs has been the mainstay of NTD initiatives that have achieved, for example, the successful control and elimination of onchocerciasis and significant reduction of LF in parts of Africa. Some of the unmet needs for the control of NTDs in sub-Saharan Africa are illustrated in the map in Figure WO-10 (from Fenwick et al., 2009). Meanwhile, dracunculiasis—for which no vaccine or therapy exists—stands at the brink of global eradication following the widespread adoption of a suite of measures to provide safe drinking
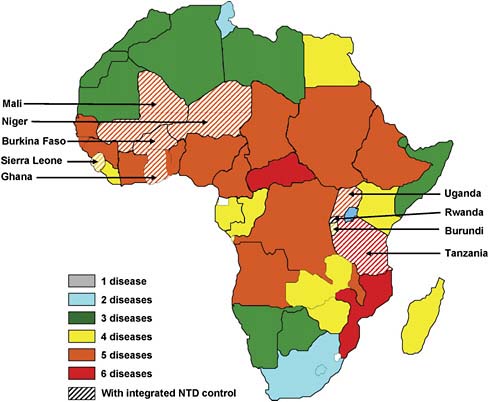
FIGURE WO-10 Distribution of NTDs in Africa (modified according to Figure 1 in Molyneux et al., 2005) and countries with integrated NTD control programs in sub-Saharan Africa. Those currently covered by the integrated NTD control programs assisted by the Schistosomiasis Control Initiative (SCI) and other organizations are indicated. Zambia was included in the SCI schistosomiasis and STH control program, but because of funding the program is currently interrupted. The NTD control program in southern Sudan is not indicated on the map.
SOURCE: Fenwick et al. (2009). Reprinted from International Health, 1/1, Fenwick A, Zhang Y, and Stoever K, Control of the Neglected Tropical Diseases in sub-Saharan Africa: the unmet needs, pp. 61–70, Copyright (2009), with permission from Elsevier.
water for at-risk populations (WHO, 2009). Vector control and other preventive environmental measures remain the only options for controlling several NTDs, including dengue and trachoma.
Every NTD and NZD presents unique challenges and is represented by a specific public health constituency. Because these diseases are often found in overlapping geographic areas and host populations, and frequently polyparasitize the same individual or animal host(s), groups of these diseases sharing similar
|
BOX WO-5 Common Features of Integrated Control Programs for Overlapping NTDs and NZDs, Malaria, and Other Infectious Diseases of Poverty Principles for Integrating Actions:
Common Interventions:
Common Delivery Platforms:
SOURCE: Adapted from Ault (2010). |
“lifestyles” may be most effectively and efficiently addressed through a coordinated intervention strategy composed of a “rapid impact” package of drugs and other interventions (Hotez, 2009b; Hotez and Pecoul, 2010; Hotez et al., 2007a; Spiegel et al., 2010). A central tenet of global strategies to reduce the burden of NTDs and NZDs—integrated control—reflects common principles, interventions, and platforms, as described by speaker Steven Ault of PAHO (see Box WO-5). (Dr. Ault’s contribution to the workshop summary report can be found in Appendix A, pages 115–131.)
Workshop presentations and discussions considered integrated control from a variety of perspectives. These ranged from discussions of MDA to control multiple NTDs, which represent the narrowest interpretation of “integration,” to broad-based strategies combining biomedical, vector control, and behavioral interventions to address neglected diseases of poverty as a social phenomenon. Many reflected a point of view expressed by L. King, who endorsed a coordinated response to NTDs and NZDs rather than dealing with “one outbreak at a time.” As a model for such a strategy, he invoked the One Health Initiative, which emphasizes connections between human, animal, and environmental health (One Health Initiative, 2010). One Health, he explained, is “the collaborative effort of multiple disciplines, especially on the veterinary and human health side, working collaboratively, nationally, locally, globally, to attain optimal health of people, animals and our environment.”
Addressing “Tool-Ready” Diseases
Among the speakers who presented various classification schemes of NTDs, Savioli offered a practical distinction: “tool-ready” versus “tool-deficient” diseases. Tool-ready diseases can be addressed without disease-specific services or even individual diagnosis, he explained. These include LF, leprosy, onchocerciasis, schistosomiasis, soil-transmitted helminthiasis, and trachoma, along with yaws. These diseases can be controlled through MDA, which Savioli characterized as treatment that can be given “by non-specialized teams and integrated through other systems, using either community volunteers, schools, or other means to ensure treatment and expansion of intervention.” Other diseases—including leishmaniasis, HAT, Chagas disease, and Buruli ulcer—are “tool-deficient,” according to Savioli, because they are difficult to diagnose and/or complicated and costly to treat. Tool-deficient diseases are not amenable to preventive chemotherapy, he explained. Controlling these diseases requires more innovative methods, such as the interventions developed to address dracunculiasis—a disease for which no vaccine, treatment, or diagnostic tools exist. Hotez pointed out, however, that in reality all NTDs are tool-ready, meaning that there are interventions available for each of the major human NTDs, including the major kinetoplastid infections such as HAT and Chagas disease, while (ironically) almost all of the NTDs are also tool-deficient, including hookworm and schistosomiasis, referring to the fact that high rates of drug failure and emerging resistance occur for most of the NTDs that have drugs available for purposes of MDA (Hotez and Pecoul, 2010).
In her keynote address to the workshop, Christy Hanson, of USAID, described her agency’s efforts to target seven of the high-prevalence NTDs—three soil-transmitted helminthiases (ascariasis, trichuriasis, and hookworm), LF, onchocerciasis, schistosomiasis, and trachoma—for MDA. (Dr. Hanson’s contribution to the workshop summary report can be found in Appendix A, pages 183–208.) Describing the growth of USAID’s NTD program from an initial
federal investment of $15 million in 2006 to the expected influx of approximately $100 million from the Global Health Initiative in 2011, Hanson noted that the program initially focused on “five countries where there was an overlapping burden of at least three of the seven NTDs that we wanted to deal with, where the governments were committed to an integrated approach, where at least one of the pillar programs [addressing individual NTDs] was functioning well … [and was] something that we could build upon, and where there were technical partners already on the ground, with good working relationships with the ministries.” As a result, she continued, “we felt we could get technical assistance, partner with the governments, and really move quickly.”
After three years at that funding level, followed by a near-doubling of funding in 2009, the program is now active in 14 countries and has delivered more than 255 million treatments for NTDs, according to Hanson. “We found tremendous efficiency gains both by integrating the different NTD programs and, more importantly, we look in every country at the existing infrastructure and the existing health platforms and education platforms,” she said. “We work through the schools. We work through child health days. We work through existing programs, existing partners, existing infrastructure…. [W]herever we can, we piggyback on what is already working at the country levels. This adds to sustainability, but it also has allowed us to expand rapidly at very low cost.”
The mapping of disease-endemic areas, as illustrated in Figure WO-11, has been crucial to these efforts, Hanson explained. Such maps ensure that governments satisfy the requirements of pharmaceutical companies that donate drugs, and they support ongoing NTD control efforts by the governments of disease-affected countries. “Even if we were to walk out the door tomorrow, they are able to access those drug donations,” she said. USAID continues to track host-country contributions toward these NTD programs to ensure ongoing commitment to disease control. “We do not want to see the government commitment to NTDs go down,” she observed, “we want to see it shift [to other NTD targets].” Typically, ministries of health in countries supported by USAID contribute one-third of the cost of NTD control, Hanson stated.
Mobilizing individual communities to undertake MDA requires time and commitment, Hanson observed, but once the intervention is accepted, it is a relatively efficient and inexpensive process, because most of the drugs used are donated.22 This has enabled USAID to build considerable capacity at the community level, she said. “It’s not just about passing out the pill. It is the health education messages, the safe strategies, the hygiene practices … along with how
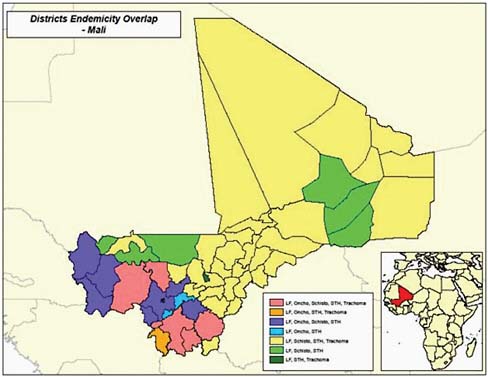
FIGURE WO-11 Endemic zoonotic diseases by district in Mali.
SOURCES: Hanson (2010); Ministry of Health, Mali (2010).
you deliver the drug,” she noted. At the ministerial level, USAID is developing an international training course to educate program managers in the financial and resource planning skills required for coordinating an integrated disease control program.
In partnership with WHO, Hanson and her colleagues are also developing a “rollout package”—consisting of guidelines for action, budgeting, obtaining drug donations, logistics, and reporting—that is designed to streamline the implementation of national MDA programs. Savioli of WHO also emphasized the importance of capacity building to ensure that there is an adequate workforce in place to sustain these programs. Noting that many experts in this field—himself included—are nearing retirement, he said, “We need a new generation of people that will take over from us.”
Summing up the impact of USAID’s NTD control efforts, Hanson noted that, while they are proud of the number of treatments they have been able to provide, their goal is to reduce NTD prevalence to low enough levels that MDA is no longer necessary. In addition, the agency is focused on increasing the efficiency of the program in ways that can be documented and reproduced, and encouraging
sustainable support by the governments of the affected countries. Ultimately, she said, USAID wants to be able to demonstrate that gains against NTDs advance the broader aims of development, poverty reduction, and education, as expressed in the MDGs.
Regional Control Efforts
The principles of integrated control have been widely adopted to address NTDs, but the targets of such efforts vary on a regional basis, Savioli observed. Diseases targeted for elimination by PAHO differ from likely candidates in Africa, he noted. Moreover, outside of the Caribbean region, schistosomiasis elimination is currently possible in the Middle East, Asia, and South America but not yet in Africa south of Sahara, only in the eastern Mediterranean region. The leishmaniasis elimination activities currently under way in WHO’s Southeast Asia region (SEARO) will not yet be possible in either Africa or the Americas, he observed. “We have to recognize that regions have their own targets,” he concluded, while acknowledging that such specificity complicates global efforts to reduce the burden of NTDs. Quoting the advice of Ferrari formula one driver Niki Lauda—“If you think you have everything under control, you are not going fast enough”—Savioli added, “that’s the way we need to deal with the issue sometimes.”
Africa’s NTD burden has received disproportionate attention, Savioli contended, even though—based on current burden of disease estimates—Southeast Asia’s NTD burden is comparable. The attention focused on Africa is appropriate, he continued, because many different NTDs are endemic to the continent, but this should not be at the expense of other parts of the world that need to control “their” NTDs in order for their economies to grow. He therefore applauded the expansion of USAID’s NTD program to include Bangladesh and Nepal, as well as Haiti (USAID, 2010).
Latin America and the Carribbean (LAC), another key region for NTD control, was the focus of Ault’s workshop presentation. Soil-transmitted helminth infections, Chagas disease, and schistosomiasis (which frequently occur as a coinfection with hookworm) are the most prevalent and burdensome NTDs in this region, afflicting many of its poorest people (Hotez et al., 2008b). Onchocerciasis and LF, as well as schistosomiasis, persist in LAC as legacies of the transatlantic slave trade, Ault noted.
Ault predicted that recent successes in controlling such infectious diseases as smallpox, polio, and measles in LAC would bode well for targeted control efforts to address NTDs. To date, onchocerciasis transmission has been interrupted in 7 of 13 LAC foci; transmission of Chagas disease by both vector and blood transfusion has been significantly reduced, as have dog-transmitted rabies cases, he reported.
PAHO has been engaged in an effort to map diseases it has designated

FIGURE WO-12 Classification of NIDs and other poverty-related infections.
SOURCE: Ault (2010).
“neglected diseases and other infections related to poverty,” or NIDs, to the first administrative level in 14 LAC countries (PAHO, 2009), Ault said. In addition to collecting key epidemiological data, it has also identified major operational strategies to control each disease. Based on these results, PAHO grouped the NIDs into three categories (see Figure WO-12).
Group 1—illustrated in Figure WO-13—is composed of diseases targeted for elimination of transmission or elimination as a public health problem23 in LAC.
-
Lymphatic filariasis
-
Onchocerciasis
-
Trachoma
-
Chagas disease (elimination of transmission by domestic vectors and blood transfusion)

FIGURE WO-13 Group I: elimination targets. *Elimination of transmission by domestic vectors and blood transfusion; **elimination in Hispaniola and Central America.
SOURCE: Ault (2010), taken from the draft report for the 144th Executive Committee, 2009, on Elimination of Neglected Diseases and Other 10 Infections Related to Poverty.
-
Malaria (elimination in Hispaniola and Central America)
-
Human rabies transmitted by dogs
-
Plague
-
Leprosy
-
Neonatal tetanus
-
Congenital syphilis
Group 2—diseases for which prevalence and burden of disease remain to be determined—includes schistomiasis caused by S. mansoni and soil-transmitted helminthiases caused by Ascaris, Trichiuris, and hookworm (see Figure WO-14).
“We are assuming for practical purposes that all countries in the region are at risk [for these diseases, and] that there is some burden,” Ault stated. “Whether it represents a public health problem or not is a question that has to be investigated.” Ault estimated that at least 13 million preschool-aged children and 33 million school-aged children in LAC are at risk for soil-transmitted helminthiases, based on their lack of access to basic sanitation. Mexico and Nicaragua provide preven-

FIGURE WO-14 Diseases targeted for drastic disease burden reductions.
SOURCE: Ault (2010), taken from the draft report for the 144th Executive Committee, 2009, on Elimination of Neglected Diseases and Other Infections Related to Poverty.
tive STH treatment for all schoolchildren, he added, but this must be extended to other countries, as well as to preschoolers. The diseases in Group 3—which Ault characterized as “diseases for which new strategies and tools [were] needed for sustainable control”—included leishmaniasis, cysticercosis, echinococcosis, and food-borne trematodiasis, and other parasitic zoonoses.
In addition to ongoing mapping of NTDs and analysis of progress toward their control, Ault reported, PAHO is conducting demonstration projects on integrated approaches to NTD control in Chiapas, Mexico, and Recife, Brazil. He went on to state that the goal of this regional collaboration involving PAHO, the Inter-American Development Bank, and the Global Network for Neglected Tropical Diseases is “to see how well this integration functions and what outcomes we can actually measure as a result of the integrated implementation.”
Recognizing and Addressing the Social Determinants of NTDs
Brazil stands out among the LAC countries for its heavy burden of NTDs, Hotez observed (Hotez, 2008a). “I realize Brazil is the biggest country in the Western Hemisphere, accounting for about 30 to 40 percent of the population in Latin America,” he said. “They still have more than 50 percent, in some cases up to 97 percent of the neglected tropical diseases in the Western Hemisphere … [including] almost all of the trachoma, most of the leprosy, hookworm, and schistosomiasis.” The social and environmental determinants of infectious disease occurrence in Brazil, which contribute to its high NTD burden, were a focus of Barreto’s workshop presentation. Like McDonald, speaker Jerry Spiegel, of the University of British Columbia, characterized social determinants of NTDs as profoundly influencing not only disease prevalence but also the ability to carry out effective and sustained interventions, and therefore as essential targets of integrated control efforts. (Dr. Spiegel’s contribution to the workshop summary report can be found in Appendix A, pages 490–505.)
Social and environmental factors in Brazil Barreto noted several key changes in Brazil’s population in recent decades: a shift to more than 80 percent of people living in cities, as compared with 31 percent in 1940; a rapid decline in fertility over the past 20 years; and an increase in income and life expectancy such that both now approximate the global mean. Although many Brazilians have access to a high-quality water source, many lack adequate sanitation, he reported. Nonetheless, mortality due to infectious disease accounts for only about 8 percent of the deaths in the country, and it continues to decrease.
Brazil, a federation of 26 states and one federal district, comprises five major regions, which differ markedly in terms of their socioeconomic status—and also in terms of their patterns of infectious disease occurrence, Barreto observed. The south and southeast regions, where gross domestic product (GDP) is highest, have the highest rates of HIV/AIDS-related mortality and the lowest number of deaths associated with NTDs such as schistosomiasis and leishmaniasis, as well as with diarrhea, malaria, and TB; these figures are reversed in the poorer north and northeast. Chagas disease deaths are concentrated in the central west. “In terms of infectious disease epidemiology, there are marked regional differences in this large country,” he concluded.
Examples of infectious diseases that have been successfully controlled in Brazil include vaccine-preventable diseases and Chagas disease, Barreto said. In each such case, he observed, a clearly defined and effective prevention strategy (e.g., vaccines, elimination of an intradomiciliary vector, and provision of water and sanitation) was effectively implemented through policies that either addressed key determinants of disease (e.g., water quality, basic sanitation, or vector elimination) or that provided access to preventive resources such as vaccines or that expanded access to primary health care. For example, he noted,
following implementation of a major sanitation project in the city of Salvador, diarrhea incidence and prevalence declined rapidly in the poorest parts of the city, where sewer coverage had been increased from 26 percent to 80 percent of all households (Barreto et al., 2007; Genser et al., 2008). Within 5 years of the project’s completion, the prevalence of several parasitic infections—including ascariasis and trichuriasis—were found to have declined more than 50 percent (Barreto et al., 2010; Mascarini-Serra et al., 2010). Moreover, Barreto reported, “in Salvador there is no more hookworm in small kids—practically zero.” These results suggest that investments in environmental interventions such as sanitation have a major impact on NTDs, as well as on other common infectious diseases, he concluded.
Barreto also identified NTDs that have been less successfully addressed in Brazil, including leprosy and schistosomiasis, which he characterized as partial successes, and dengue and leishmaniasis, which he described as failures. Leprosy and schistosomiasis are chronic infectious diseases with long infectious periods and, in the case of schistosomiasis, transmitted by vector species that have proven difficult to control, he said; dengue and leishmaniasis have eluded control in part through urbanization, and also because they lack safe and/or specific treatments.
Addressing social determinants of NTDs through integrated control strategies “To understand the challenges of NTDs in women and children, we must understand how health is transmitted across lifetimes and generations,” McDonald observed. “Adult health is shaped by the social and economic opportunities that adults face, along with living and working conditions experienced. Adult health, in turn, shapes family health and well-being. Family health and well-being, in turn, shapes childhood health, which forms the basis of health in the adult (RWJF, 2008). The global cycle of poverty and substandard living conditions is what we must break in order to address NTDs and make them neglected no more.”
Taking a closer look at these relationships, Spiegel examined NTDs from a social perspective and urged that integrated control strategies address factors—such as poverty, lack of infrastructure, and inadequate community-based primary care—that “predispose the ill health that we are trying to address.” Without attention to such social determinants of health, he argued, interventions cannot be effectively sustained (Spiegel et al., 2010). To illustrate this point, Spiegel recalled a Jewish folk tale about the mythical, backward village of Chelm, which stood at the edge of a dangerous precipice. When the wisest men of Chelm were asked how to prevent people from falling to their deaths, they replied, “build a hospital at the bottom of the precipice.”
To truly evaluate the impact and outcomes of integrated control strategies in promoting health, rather than simply measure activity in responding to illness (e.g., counting the number of treatments distributed), four basic questions must be answered, Spiegel said:
-
Do we have an efficacious treatment?
-
Is it safe?
-
Do we have the services and systems to bring that to bear?
-
Do we have a way of addressing the conditions that are producing the effect that’s calling for this attention?
A “yes” to all four ensures capability to scale effective interventions to sustainably control disease, not just to stifle transmission and treat victims, he explained. This is important, because populations most vulnerable to NTDs have been neglected not only because drugs and diagnostics have not been developed for these diseases, he observed, but also because their health systems and infrastructure have similarly not received adequate attention. “We are really dealing with more than an individual’s illness,” he added, “because all individuals are stamped by the insecurity of acquiring that illness.” Therefore, he argued, health represents “an excellent platform for developing a common security globally [sic].”
To comprehensively consider biomedical, environmental, and social determinants of health, Spiegel and his colleagues work with a framework called the “ecosystem approach to human health” (Lebel, 2003; Webb et al., 2010) that emphasizes transdisciplinarity, participation, and equity. Applying these principles in Havana, Cuba, they created an integrated surveillance system for dengue (Bonet et al., 2007), establishing a socially connected network and linking people in various capacities—from government epidemiologists, local physicians, and environmental officers to resident citizens. Information derived from this system was used to identify “hot spots” and monitor transmission trends and to develop a geographic information system for mapping the disease. The researchers then conducted a mosquito breeding site case-control study to help them identify social and environmental risk factors that could be reduced to improve community dengue control (Spiegel et al., 2007). In addition to such expected factors as lack of larvicide in water tanks, the researchers found that practitioners of the Afro-Caribbean religion Santeria placed themselves at higher risk for dengue through the use of ceremonial flower vases, in which mosquitoes could breed. Therefore, they concluded, efforts to reduce dengue in Havana should involve community religious leaders to help promote safe practices, and local actions to do this were conducted.
While cautioning against exclusive reliance on short-term medical solutions to neglected diseases of poverty, Spiegel acknowledged that, in a crisis, affected individuals must be treated as quickly and effectively as possible. However, he continued, it is important to recognize that taking such actions may exhaust possibilities for intervening at other levels. “One of the concerns of having an overemphasis on a technical cure-all, as effective as it may be, [is that] circumstances change,” he said, noting, for example, that dengue flourished after its mosquito vector developed resistance to DDT. In addition, providing technical “fixes” encourages communities to rely on a “paternalistic provision of things,” rather than
involve themselves in identifying and targeting systemic risk factors for disease, Spiegel said. “Can we really achieve the outputs that we want to achieve, without having some fundamental infrastructure in place?” he asked. “I don’t think so.” His argument is supported by the legacy of the Rockefeller Sanitary Commission for the Eradication of Hookworm, which failed to eradicate hookworm from the American South solely through MDA.
In order to research and fund interventions directed at the social determinants of NTDs, Spiegel and colleagues have proposed a mechanism they call “social offsets,” by which a proportion of funding for any NTD research program is set aside to address related socio-environmental and health system factors (Spiegel et al., 2010). For example, in order to gain permission to distribute NTD drugs in a country, pharmaceutical companies would need to contribute to funding its health systems or to addressing other social determinants of NTDs. However, he added, there is no advantage to addressing the social determinants of NTDs if it does not produce demonstrable benefits. He insisted that, like biomedical interventions, social and environmental approaches to NTDs must be held to account, evaluated, and adjusted to ensure that they lead to desired outcomes and complement other interventions.
In an informal luncheon address to workshop participants, Forum member Jesse Goodman, Chief Scientist at FDA, applauded the inclusion of efforts to address social determinants of health in the development of integrated disease control programs. “If you just go into a community and say, ‘I’m going to make you healthy,’ but it’s not going to be tied to other efforts to help in other ways in that community, efforts which actually may address even more important determinants of health, that’s a missed opportunity,” he observed. Moreover, he said, “You really want to build an integrated system that isn’t just for 15 infectious diseases, but will work as people transition from one set of health problems to another.”
Addressing Unmet Needs
Increasing recognition of the health and socioeconomic consequences of NTDs and NZDs is spurring efforts to identify and develop medical products, strategic plans, and workforce to reduce this significant burden of disease. The two previous sections of this overview (“Profiles of Neglected Diseases” and “Approaches to Integrated Control of NTDs and NZDs”) describe a range of disease- and setting-specific needs for preventive and therapeutic treatments and diagnostics, as well as opportunities to maximize the impact of such interventions through integrated control programs. In a workshop session devoted to “unmet needs,” presentations and discussions reviewed recent advances in the development of drugs, vaccines, and diagnostics to control and treat NTDs and NZDs. Participants also considered the essential role of partnerships—involving industry, governments, nongovernmental organizations (NGOs), academic
researchers, and funding agencies—in developing tools and strategies to address neglected diseases, and ways to improve and expand current models of cooperation and collaboration.
Drug Development Through Partnership
Pharma perspective Using examples from his company’s considerable experience in developing drugs and vaccines for neglected diseases, Forum member and speaker Mark Feinberg, of Merck & Co., Inc., examined lessons learned from these public–private partnerships, as well as current challenges and future opportunities in addressing the diseases of poverty. In addition to the significant hurdles that any drug candidate confronts along the development pathway, products targeted to treat NTDs face several additional obstacles, Feinberg observed. First, he noted, it is often difficult for regulatory authorities in the developed world to assess the relative risks and benefits of products targeting diseases that are largely or exclusively present in developing countries. “It’s not that [regulatory officials] aren’t working at it,” he added, “but there aren’t a lot of models where that [process] has been successfully navigated so far.” In addition, he said, evaluating treatments for the several NTDs that do not present as clinical disease until long after the onset of infection requires the identification of appropriate surrogate endpoints, of which very few have been approved by regulatory authorities. Finally, target profiles for NTD products have historically often failed to address factors that strongly influence implementation efforts, such as cost, scalability, route of administration, duration of treatment, or delivery logistics.
Despite these odds, Merck and other companies are engaged in a range of product-development partnerships (PDPs), spanning every stage of development, to produce drugs, vaccines, and diagnostics to treat diseases that are largely restricted to the developing world and, therefore, unlikely to be profitable, Feinberg observed. One of the best-known—and in many ways pioneering—examples of such a partnership began in 1987, when Merck first committed to supply the drug ivermectin (Mectizan) free of charge, and for as long as it is needed, to campaigns against onchocerciasis (also known as river blindness). In addition to its having an important impact on the control of onchocerciasis, Feinberg also noted that this partnership helped bring together partnerships and support the development of country-level health delivery infrastructure that has also provided a pathway to deliver ancillary interventions, including vitamin A supplementation and vaccination programs, to communities receiving ivermectin treatment.
To date Merck has donated more than 600 million ivermectin treatments (worth more than US$3 billion) for use in 34 countries, Feinberg reported. Treatment with ivermectin is estimated to prevent 40,000 cases of river blindness each year and allows farmers to reclaim significant areas of arable land previously rendered uninhabitable because of the presence of the black fly, he added. In 1998,
Merck launched an additional ivermectin donation program for the treatment of LF in combination with albendazole, which is donated by GSK.
Feinberg summarized lessons learned over the course of two decades of ivermectin donations by describing the factors that contributed to the success of this PDP (Thylefors et al., 2008):
-
A broad partnership with clearly recognized common agenda and identified roles for all parties;
-
Appropriate assessment tools to easily identify target populations for treatment;
-
An appropriate community base for treatment with motivation and local support;
-
Streamlining of all drug management aspects;
-
An ongoing operations research agenda to address present and upcoming challenges; and
-
Long-term commitments and long-term investments.
He also noted that this partnership has raised several important questions and implications:
-
Would the program have been so successful if it was not predicated on a drug-donation model?
-
Is drug donation, in and of itself, a viable model for addressing NTDs, or is it insufficiently sustainable?
-
If novel agents to address NTDs are developed through PDPs, who will be responsible for their production, procurement, and delivery?
-
Who will assess their impact and safety?
“I think none of those questions are available for answer now,” he concluded.
Feinberg then examined Merck’s participation in several initiatives to increase access to important vaccines in developing countries. These include an agreement, reached in the late 1980s, to provide the Chinese government with technology for producing recombinant hepatitis B vaccine. Merck did not profit or receive royalties from this partnership, which today provides about 65 percent of China’s supply of the vaccine, he stated. The vaccine produced in this venture has prevented an estimated 30 million chronic hepatitis infections and is estimated to have averted approximately 3 million deaths, he added.
Another partnership, involving a new rotavirus vaccine produced by Merck, was launched to show that a vaccine could be rapidly and effectively introduced into a developing country to demonstrably reduce disease burden, Feinberg said. Although almost all children worldwide will become infected with rotavirus before the age of 5 years, the greatest impact of the infection is seen in low-income countries where an estimated 600,000 children die of rotavirus gastroenteritis
each year. To help demonstrate the feasibility and impact of rotavirus vaccine in a low-income country, Merck partnered with the Nicaraguan government to provide rotavirus vaccine and administrative support so that every child born within a three-year period was immunized and the impact on rates of severe rotavirus disease were monitored thereafter, he explained; additional studies conducted by PAHO and CDC were also conducted to examine the vaccine’s impact (Patel et al., 2009). Importantly, rates of rotavirus disease have fallen substantially in Nicaragua since introduction of the vaccine, and immunization rates quickly reached and have been maintained at rates among the highest in the world (including in much wealthier countries such as the United States), he reported. Efficacy studies of the rotavirus vaccine have also been conducted through the Global Alliance for Vaccines and Immunization (GAVI) in Mali, Ghana, Kenya, Vietnam, and Bangladesh (Armah et al., 2010; Zaman et al., 2010); based on their outcomes, along with other data demonstrating the efficacy in GAVI-eligible countries in Africa of a different rotavirus vaccine produced by GSK, WHO now recommends immunization for every infant worldwide, he said. Nevertheless, he added, the rollout of rotavirus vaccines in developing countries has, to date, been disappointingly slow, largely because the current global financial crisis has severely curtailed GAVI’s financial resources to support new vaccine introduction.
Merck’s experiences introducing hepatitis B and rotavirus vaccines to developing countries revealed “tremendous opportunities and tremendous needs to develop new and better vaccines for low-income countries,” Feinberg said, as well as the potential to develop new and better models to meet these needs. This is the aim of its recent joint venture, the MSD Wellcome Trust Hilleman Laboratories. Located in India and funded by Merck and the Wellcome Trust,24 this facility is specifically dedicated to the development of new vaccines targeting diseases prevalent in low-income countries and of optimized versions of existing vaccines so that they may be more effectively implemented in resource-limited settings, he explained. In addition to the financial support provided by both Merck and the Wellcome Trust, Merck provides the laboratories with technical support, enabling technologies, and access to relevant intellectual property.
The Merck–Wellcome partnership is intended to bring together diverse partners from academic and government laboratories as well as other industrial partners (both multinational companies and developing-world manufacturers) and to serve as a model for collaborative engagement—to provide innovative products to address important unment medical needs in developing countries, Feinberg said. “The goal here is to really recapitulate the end-to-end view that exists in typical drug-development or vaccine-development programs in industry,” he observed—in particular, to support proof-of-concept determinations, which tend to be a major stumbling block in the drug development pathway.
“There are opportunities to broaden the definition of the partnership model
[to accommodate] a broader vision of integration and prioritization,” Feinberg observed. “Certainly there can be public–private partnerships. There can be partnerships between private-sector entities,” he added. “There can be partnerships that bring together multiple private-sector entities, with potentially private-sector partners, public-sector partners, when you have people who have the greatest potential to contribute.”
Organization perspective The Drugs for Neglected Disease initiative (DNDi) is a collaborative, non-profit drug research and development (R&D) organization that establishes partnerships with industry, governments, and private funders to develop new treatments for neglected diseases including malaria, visceral leishmaniasis (VL), HAT, and Chagas disease (DNDi, 2010).25 In his workshop presentation, Shing Chang, DNDi’s director of research and development, described their efforts to develop new drugs for three of the deadliest NTDs: HAT, leishmaniasis, and Chagas disease.
Chang began with an explanation of DNDi’s tripartite approach to drug development. In some cases, he said, it is necessary to adopt the long-term strategy of screening of compound libraries for leads that can be adapted to produce simplified, cost-effective, short-course treatments that also contribute to disease elimination, if possible. DNDi also pursues novel formulations and new indications of existing drugs, beginning with the pre-clinical phase of development, he said. For the short term, they identify drugs that are already approved in one geographic region that can be made available in another, in order to expand available treatment options.
For HAT—which, as Jannin previously noted, has lacked good treatment options—DNDi developed a less expensive, more easily administered alternative, nifurtimox-eflornithine combination therapy (NECT). This is still a stop-gap measure, according to Chang, because the drug must be infused, limiting its use to patients who have access to health care facilities and skilled medical personnel. Ideally, HAT will be treated orally with a combination of two drugs, he said, but there are few promising oral candidates in development.
For VL, the most severe and potentially fatal form of leishmaniasis, current treatments are difficult to administer and often poorly tolerated, Chang observed. DNDi is pursuing a range of region-specific strategies to improve the efficacy of existing VL drugs while attempting to identify promising novel compounds. In India, where resistance has arisen to one of the key drugs—pentavalent anti-
monial—DNDi is attempting to develop combinations of existing treatments for VL, he said, while in Africa, they are registering drugs available in India and using them to develop combination therapies. In Latin America, the organization is participating in the clinical investigation to determine the efficacy of the standard treatment and compare it with a combination. In Asia, DNDi is testing combinations of three VL drugs. However, collaboration with another organization that focuses on developing drugs for tuberculosis (TB Alliance) has yielded two promising compounds that are orally active against VL, Chang reported, “so we’re looking forward to potentially having a VL oral drug.”
The challenge of Chagas disease Several partnerships are pursuing much sought-after treatments for Chagas disease (see previous discussion in “Profiles of Neglected Diseases”), for which only two drugs are currently available: nifurtimox and benznidazole. Both require a long treatment period, are toxic, and are unavailable in pediatric formulations, Chang noted. DNDi has developed pediatric-strength benznidazole, which should be available by early 2011. Mean-while, DNDi, Merck, and others are also investigating a group of antifungal drugs, the azoles, which have promising activity against the Chagas trypanosome in animal models.
Merck owns one of these compounds, posconazole, which has the double advantage of a long half-life and extensive distribution throughout the body, according to Feinberg. Posconazole is licensed in the United States, the European Union, and in other countries for the prevention of invasive fungal infections and for the treatment of oropharyngeal candidiasis, he said. A Phase II proof-of-concept study for the treatment of Chagas disease is slated to begin in late 2010, with results anticipated by 2012.
Although these developments are promising, Feinberg observed, they raise a new set of challenges—how does one assess the efficacy of a Chagas drug in the absence of a robust, validated, and accepted surrogate laboratory measure to assess drug efficacy in an infection for which clinical disease can take years to develop after initial infection? In the planned proof-of-concept study to assess the efficacy of posaconazole in the treatment of chronic Chagas disease, Merck will monitor parasite levels using polymerase chain reaction (PCR), he noted, but he wondered if a proof of concept based on such a surrogate endpoint would be sufficient to support licensure of the drug in the many countries—including the United States—where Chagas disease is also present.
Antipoverty Vaccines
In a second presentation to the workshop, Hotez described progress toward the development of vaccines to control the helminth infections hookworm and schistosomiasis, which, measured together in terms of DALYs, rank closely behind malaria as the world’s most prevalent parasitic diseases (Hotez et al., 2008a).
Currently, two drugs, albendazole or mebendazole, are the only treatment options available to the more than 1 billion people infected with hookworm, he said, while praziquantel is the sole option for the nearly 800 million people chronically infected with schistomiasis.
Although resistance to these drugs has not yet occurred on a large scale, it does present a significant threat to the “massive scale-up” required to treat vast numbers of people infected with these diseases, Hotez said. In particular, he noted, albendazole and mebendazole are members of a class of drugs known as antihelminthic benzimidazoles, which have been used for decades to deworm cattle and sheep throughout the Southern Hemisphere and to which “widespread resistance” has already arisen. Even so, he observed, “What are we doing to monitor for resistance in humans? Essentially nothing! There are a couple of research groups around the world, but there is, for all practical purposes, no resistance monitoring in place [for anthelminthic benzimidazoles].” Moreover, Hotez continued, several analyses show that mebendazole, administered in a single dose (a common practice of annual MDA programs in developing countries), failed to cure or even significantly reduce worm burden in the vast majority of many cases (Keiser and Utzinger, 2008).
Responding to the significant limitations of antihelminthic drugs, the Sabin Vaccine Institute, which Hotez directs, is seeking to develop vaccines that target hookworm and schistosomiasis, as well as a vaccine for malaria. “There [are] now good evidence and a lot of data showing how neglected tropical diseases impair intellectual and physical development in children,” he observed; for example, chronic hookworm infection in childhood has been shown to reduce future wage earning by 40 percent and also to contribute to adverse pregnancy outcomes (Hotez et al., 2009b). Thus, Hotez and his colleagues refer to the products they pursue as “antipoverty vaccines.”
To produce these vaccines, the institute established a PDP known as Sabin Vaccine Development, Hotez explained. With the strong support of the Brazilian and Dutch governments, the National Institutes of Health (NIH), and the Bill & Melinda Gates Foundation, as well as individuals and family foundations led by Mort Hyman and Len Blavatnik, the PDP engages in all phases of vaccine discovery, from antigen discovery through genomics and proteomics, and to Phase I and II trials and beyond, he said. Other groups pursuing “antipoverty” vaccines include the Hilleman Laboratories, as described earlier by Feinberg, Novartis Global Health Vaccines, the Infectious Disease Research Institute, the International Vaccine Institute of Korea, and the Program for Appropriate Technology in Health, together with its subsidiary, the Malaria Vaccine Initiative. Hotez noted that a key advantage of such partnerships is their ability to propel vaccine development through the notoriously difficult transition from R&D to production. In Brazil, this transition is simplified because two public-sector, not-for-profit vaccine manufacturers produce two-thirds of the country’s vaccines. Brazil—
despite its high rates of poverty and NTDs—stands out as a so-called innovative developing country (IDC).
Focusing on the human hookworm vaccine, Hotez observed that making a vaccine against a parasite, rather than an antigen, requires a novel approach—in this case, attempting to interfere with its ability to feed on human blood. Using this strategy, the PDP has identified two effective vaccine antigens (Asojo et al., 2007; Hotez et al., 2010; Loukas et al., 2005), has completed manufacture of one of the antigens in preparation for Phase I trials in Brazil, and anticipates completion of a large Phase II study by 2017, he reported.
“Wherever you find hookworm, you find schistosomiasis,” Hotez observed. “If you have hookworm, you are more likely to have schistosomiasis, especially intestinal schistosomiasis caused by Schistosoma mansoni.” Therefore, he reasoned, “if we are going into these endemic areas to vaccinate people against hookworm, why not try to do this for schistosomiasis as well?” Taking advantage of the complete genome sequence of S. mansoni, Alex Loukas, Jeff Bethony, and their colleagues working with Sabin Vaccine Development identified a promising target in the form of a family of proteins that span cell membranes, called tetraspanin surface antigens. They also determined that, by disabling these proteins using RNA interference, they could disrupt the integrity of the surface membrane and thereby halt worm development (Tran et al., 2010). Additional research demonstrating that some people who are apparently resistant to schistosomiasis possess antibodies to tetraspanin surface antigens (Tran et al., 2006) supports the viability of this target.
An anti-schistomiasis vaccine could also provide a “back door” route to AIDS prevention, Hotez speculated, because genital schistomiasis appears to increase risk for HIV infection in women. “If you actually look at a map of schistosomiasis with HIV/AIDS, it’s amazing how the two, especially in rural areas, overlap so tightly,” he said. Similarly, he continued, a hookworm vaccine might reduce the impact of malaria, because many people suffer co-infections of those parasites.
“The dream, moving forward, is to ultimately create a multivalent, antihelminthic antipoverty vaccine,” Hotez concluded. He anticipated that such a vaccine could save 26 million DALYs, or about half the DALYs attributed to malaria or HIV/AIDS, and lift a significant portion of Brazil’s overall burden of disease. If an antihelminthic vaccine were added to “rapid impact” packages, which currently consist of drugs targeting the seven most common NTDs, the resulting “vaccine-linked chemotherapy” package could be administered through school-based health systems or on designated Child Health Days, Hotez said.
Diagnostics: Essential to Progress
Speaker Patrick Lammie, of CDC, observed that precise, robust, field-friendly, affordable diagnostics are needed across the NTD portfolio to direct
treatment and also to support surveillance. (Dr. Lammie’s contribution to the workshop summary report can be found in Appendix A, pages 346–356.) Diagnostics appropriate for individual case management differ from those used to guide and monitor MDA programs in which entire communities are treated, he said; both types of diagnostic tools may be employed at different stages of a disease control program, supported by appropriate sampling methodologies. In the initial disease-mapping phase of control programs, convenience sampling provides enough information to make appropriate decisions, he said. However, determining when and how to alter MDA frequency requires more precision (i.e., measuring a diagnostic marker indicative of infection), whereas post-MDA and surveillance diagnostics must capture transmission, he explained. Thus, he said, as the control program progresses, sampling becomes more systematic, and decision-making demands more robust information.
Although “19th-century” microscopic diagnostic techniques currently used in the initial phases of NTD control are adequate for the task, recent advances in genomics, proteomics, and metabolomics could vastly improve monitoring efforts in the late stages of control programs, Lammie said. Antigen-detection assays can be developed to monitor residual levels of infection in communities where MDAs are conducted, to inform decisions about altering treatment frequency or stopping it altogether, he said. He noted that such tests exist for LF, and that another is in development for schistosomiasis. There is a critical need for a similar tool to monitor trachoma transmission, he noted; currently, parasite presence is gauged by inflammation, a symptom that may persist beyond the infective stage. L. King also observed that the lack of specific tests for zoonotic parasites means that cases of febrile illnesses tend to be diagnosed as malaria, based on clinical signs and symptoms, but may actually result from zoonotic infections such as leptospirosis and Q fever.
To achieve elimination of NTDs through MDA, infection prevalence must be driven below levels capable of sustaining transmission in a host population, Lammie explained. “We are not going to be measuring … the number of [transmission] cycles and the initial prevalence with enough precision that we are really going to be able to rely on a standard algorithm to make decisions about when it’s safe to stop [treatment],” he observed; rather, data must drive decision making. Surveillance at this stage of a control program is intended to detect any recrudescent transmission. Unfortunately, he said, researchers largely lack the tools to conduct that sort of surveillance in the field.
In an earlier discussion, audience member Joel Breman of the Fogarty International Center at NIH stated that “[i]t is essential that diagnostic tests be able to detect particularly low-level microbe infections when one gets down to the final mile, much less the final inch, in [NTD elimination or eradication] programs, and, in fact, discern where those microbes have come from.” He observed that PCR for malaria can detect infection better than microscopy or even rapid diagnostic tests,
and he concluded that similarly sensitive tests are needed to detect low levels of microfilariae (for onchocerciasis and LF).
Describing his own experiences working on diagnostics for LF, Lammie recounted the results of a multi-center tool validation trial of rapid diagnostic tests in several different developing-country settings. Much to the researchers’ dismay, test concurrence proved lower than anticipated, suggesting that technicians had been inadequately trained to perform the tests and that the commercially manufactured tests had not been well standardized. Lacking laboratory capacity to conduct serologic tests, the researchers concluded that point-of-care diagnostics would be a better choice for field settings—except that they are not available for onchocerciasis, schistosomiasis, or trachoma. NTD colleagues have now developed a statistically robust survey protocol, based on detection of antigen-positive (or antibody-positive) children that program managers can use routinely to determine whether any MDA program (regardless of its disease target) should be continued.
After stopping an MDA program, surveillance for incident infections must continue, Lammie observed; however, with current diagnostics, such events are difficult to detect in their early stages. In theory, antibody-based tests offer an early, sensitive indicator of exposure to pathogens and could be multiplexed to test for several diseases, but existing antibody tests for NTD pathogens are neither sufficiently robust nor specific for this purpose, he said.
In addition to improving patient care and programmatic decision making, better diagnostic tools will advance understanding of NTD transmission dynamics, Lammie observed. For example, he said, when transmission is reduced to “islands” within a geographic area where a disease is otherwise eliminated, it remains to be determined whether and how long transmission persists within those foci, or if they represent potential sources for resurgence of transmission within the surrounding population.
The Role of Regulatory Science
Goodman discussed FDA’s current and potential role in addressing NTDs, working with innovators as they develop novel products and devising tools to evaluate products. He also drew scientific and policy parallels between the development of medical countermeasures against biological, chemical, radiological, and nuclear threats and emerging infectious diseases (Hamburg, 2010)—a major initiative undertaken by FDA—and efforts to develop drugs, vaccines, and diagnostics for NTDs.
Both pursuits demand and benefit from collaboration within government agencies and between governments and non-governmental entities to overcome significant barriers to development, Goodman observed. “Showing safety and effectiveness of [both NTD drugs and medical countermeasures] … is something that we often don’t have the tools and models and methods to do,” he said.
“Doing clinical trials in less developed countries is something that is a challenge that we frequently don’t have the regulatory structures or methods to address.” Manufacturing capacity, quality, and oversight present additional challenges to product development, as do the environments in which the products must be effective as delivered and used.
The medical countermeasures initiative seeks to address these challenges through a series of measures that are directly relevant to neglected diseases, Goodman stated. The FDA Medical Countermeasures Initiative (MCM) provides resources to support interactive science-based review and regulatory science to aid in translation from basic science into practical products, since there are often little or uncertain economic incentives to develop such products. Also, basic scientists may not recognize potentially powerful applications for their discoveries that could help enable MCM or NTD product development. In addition, FDA is providing interactive regulatory and technical support for planned DHHS-funded and other efforts to develop advanced development and manufacturing facilities and approaches for medical countermeasures using novel platform technologies such as flexible, modular, scalable, and disposable methods that may also prove appropriate for manufacturing products needed for both emerging threats and NTDs, including in other areas of the world, he observed.
FDA will work with the sponsors of each countermeasure in development, and with colleagues in the Department of Health and Human Services (HHS) and the Department of Defense (DOD), to develop a regulatory science plan that identifies gaps in applied science and determines how to fill them, Goodman said. This effort is being substantially resourced for medical countermeasures and for emerging infectious diseases, he continued. Ideally, it would extend to all diseases of global health importance. In the meantime, the medical countermeasures initiative should benefit the cause of neglected diseases by raising awareness that the health of each country affects the health of the world, and by advancing regulatory science and driving multi-use technological innovations and supporting companies that engage in it.
Goodman encouraged the NTD community to view FDA as a resource to help guide improvements in the quality of treatments and in the manufacturing processes that produce them. “We are putting a tremendous amount of energy into issues like information sharing with our regulatory partners, developing approaches to jointly assess new products so that we work together with the rest of the world in using science-based approaches to assessing product safety and efficacy,” he said. Indeed, he emphasized, FDA accepts the results of clinical trials conducted overseas, as long as they include the appropriate protections and methods. The agency also evaluates and approves products for the United States even when they may be used primarily outside the United States. “Our regulatory decisions are risk/benefit decisions, not risk decisions,” Goodman insisted. “We look at a decision, if it’s a drug for onchocerciasis or a vaccine for malaria, in
terms of whether there is a favorable risk/benefit situation. We don’t apply some U.S. standard that says things have to have no risk and 100 percent benefit.”
Goodman also noted that FDA works with WHO on a number of issues with relevance to NTDs, including vaccine pre-qualification, establishing standards (e.g., for regulating blood testing and screening for Chagas disease), prequalifying drug and vaccine manufacturing facilities, and efforts to reduce drug counterfeiting. Ultimately, he said, FDA hopes to “make progress in educating both our country and the world that the health problems of one country are the health problems of every country.” And to work together to prevent and attack those problems.
Investing to Address Neglected Diseases
In addition to the significant contributions of pharmaceutical companies to reduce the burden of NTDs, these efforts also receive support from countries, NGOs, and philanthropies, all of which were represented among workshop speakers. They described their current commitments and goals regarding research, development, delivery, and implementation of measures to control NTDs, and they considered how to coordinate investment in NTDs in order to optimize its impact. In the course of these presentations and subsequent discussions, participants examined ways to sustain and expand recent gains made against NTDs, so as to address significant unmet needs for effective measures to prevent and treat neglected diseases, and to mitigate the health and socioeconomic consequences of neglected diseases.
Current Investment in NTDs
The U.S. Global Health Initiative (GHI) In a keynote address that opened the workshop, Emanuel described the overall goals of the GHI and its areas of specific focus, which include the NTDs. Announced by President Obama in May 2009, the GHI will provide $63 billion over six years to address the following issues in developing countries:
-
Increasing coverage and integration of pneumococcus and rotavirus vaccines in immunzation programs;
-
Improving maternal and infant outcomes for childbirth;
-
Preventing and treating malaria;
-
Preventing HIV/AIDS; and
-
Preventing and treating NTDs.
As Hanson had noted, this commitment dwarfs previous U.S. contributions to NTDs, which, beginning in 2003, amounted to $45 million over the course of the next five years. During that time, Emanuel said, more money was spent research-
ing NTDs at NIH than in distributing effective treatments to people in need. These meager efforts were, however, supported by donations of NTD drugs from Merck, Pfizer, GSK, and other pharmaceutical companies, and also by contributions from the British government (discussed below).
Emanuel presented the following list of NTD targets for the GHI:
-
Reducing the prevalence of the seven high-prevalence NTDs,26 by 50 percent, among 70 percent of the affected populations;
-
Eliminating onchocerciasis in Latin America by 2016; and
-
Eliminating LF and leprosy globally by 2017.
Achieving these goals will require the establishment of enduring and accountable public–private partnerships, Emanuel said. In an immediate step to increase its potential impact, the GHI will fund the creation of publicly accessible, global maps of NTDs overlaid with the locations of schools and health clinics where medications can be distributed.
The U.S. commitment to controlling and eliminating NTDs recognizes their significant health and economic impact, as well as the promise that this burden can be substantially reduced, Emanuel said. “We have effective treatments and treatment strategies [for NTDs],” he observed. “They are a great example of public–private partnerships, where we get much more leverage for what we spend.”
The U.K. commitment to NTD control Contributions from the United Kingdom to address NTDs derive from diverse donors, including the British government, its Medical Research Council, and organizations focused on implementation of control measures, according to speaker Alan Fenwick of London’s Imperial College. (Dr. Fenwick’s contribution to the workshop summary report can be found in Appendix A, pages 172–183.) Since 2000, he said, the British government has been a steady source of support for the Centre for Neglected Tropical Diseases (CNTD) at Liverpool University, the Schistomiasis Control Initiative (SCI) at Imperial College (which Fenwick directs), and APOC, among others. In September 2008, Prime Minister Gordon Brown pledged an additional 50 million pounds over five years for NTDs,27 a commitment that his successor, David Cameron, has agreed to honor, Fenwick said. The Wellcome Trust and Medical Research Council exclusively support research and development related to NTDs; neither
organization funds implementation programs. GSK, which donates its drug, albendazole, to LF control programs, also has a significant presence in the United Kingdom. Fenwick also mentioned a range of potential private donors—including individual citizens—whom he hopes can be persuaded to recognize and support the cause of NTDs in the near future.
In addition to the university-based initiatives SCI and CNTD, which specifically support the implementation of control measures against NTDs, other groups—such as Imperial College’s Programme for Child Development, and two NGOs specializing in eye diseases—contribute to such efforts through integrated programs, Fenwick said. He also noted that SCI and CNTD have joined forces to direct their respective government funds toward the same recipient countries, in order to support the implementation at scale of both MDA and (in some cases) targeted chemotherapy against schistosomiasis, STHs, and LF in an integrated fashion.
As increasing numbers of organizations become involved in NTD control programs—both within and outside Britain—Fenwick urged greater coordination and collaboration, and cautioned against duplication of effort. He encouraged an expanded approach to integrated control to incorporate the provision of bed nets and nutritional supplementation for preschool-age children. In addition, he advised greater attention to the behavioral aspects of MDA, to increase compliance and ensure “that the people who are swallowing the drugs understand exactly why,” he said. For example, most children treated for schistosomiasis have yet to experience symptoms, so they are being asked “to take a leap of faith and to take drugs that we are offering to them, when they can’t necessarily see that they particularly need them,” he said. He also echoed several other speakers and participants in emphasizing the importance of local ownership of MDA programs.
The Bill & Melinda Gates Foundation support for NTD control Speaker Julie Jacobson, of the Bill & Melinda Gates Foundation, observed that supporting efforts to combat NTDs fits the Foundation’s basic premise: “Every person deserves the chance to live a healthy and productive life.” (Dr. Jacobson’s contribution to the workshop summary report can be found in Appendix A, pages 293–309.) Within its Global Health Division, the Bill & Melinda Gates Foundation currently awards grants for initiatives directed at a list of neglected diseases that includes WHO’s “standard 13” as well as dengue and Japanese encephalitis; since 1998, the Foundation has invested US$544 million to address NTDs, she reported. Because DALYs drive the Foundation’s giving strategy, she said, and as a result of the lack of firm DALY estimates for many NTDs, the Foundation is sponsoring efforts to improve those measurements. Grantees submit annual reports on their progress and receive payment from the Foundation linked to the achievement of project milestones.
Rather than set eradication or elimination goals for particular NTDs, the Bill & Melinda Gates Foundation invests strategically in drugs and treatments,
vaccines, diagnostics, biomarkers, vector controls, and implementation strategies, Jacobson explained. “We don’t implement programs, but we do things to make sure that programs that are implemented can be done well and achieve more,” she added. Current investments include the development of a macrofilaricide capable of killing adult worms in patients with onchocerciasis and LF, thereby dramatically reducing treatment duration (which currently can last as long as 17 years). Other grants under consideration include efforts to develop traps for black flies to permit analysis of parasite transmission in vector populations, a more sensitive diagnostic for onchocerciasis for monitoring post-MDA transmission, and an examination of the ancillary benefits gained through MDA. An ongoing project to develop a vaccine and drug to prevent and treat cysticercosis in pigs and humans has enabled the local elimination of that disease in a site in northern Peru, so it is working to expand and refine the model strategy and encourage uptake, she said.
The Bill & Melinda Gates Foundation’s approach to NTDs has largely focused on research and development of new tools and strategies for integrated program development, Jacobson said, but they are increasingly cognizant of the need to reach more communities and optimize the impact of these innovations. To meet this challenge, the Foundation has developed communications and advocacy plans “to get the word out” about promising developments, she said. “If we work with a grantee to make the perfect tool, it means nothing if it doesn’t get out there,” she concluded.
Overview of investment in R&D Speaker Mary Moran, of Policy Cures—an independent group that provides research, information, decision-making tools, and strategic analysis for those involved in the creation of new pharmaceuticals for neglected diseases (Policy Cures, 2010)—reviewed the current state of R&D investment in NTDs, as described in a recent report from her organization (Moran et al., 2009). (Dr. Moran’s contribution to the workshop summary report can be found in Appendix A, pages 388–414.) Known by its abbreviated title, G-FINDER (for Global Funding of Innovation for Neglected Diseases), the report summarizes the results of an annual survey of 208 organizations that invest specifically in the R&D of new products aimed at a group of 31 neglected diseases, of which approximately half are NTDs.
From these highly detailed data, Moran extracted several “big picture” characteristics, beginning with the bottom line: in 2008, she reported, the world spent about US$3 billion to develop new products for neglected diseases, of which nearly three-quarters was spent on HIV/AIDS, TB, and malaria. The NTDs, among which Moran included WHO’s list of 15 diseases, collectively receive approximately 12 percent of this total, or just under US$350 million; Figure WO-15 shows how that amount was divided among classes of NTDs, with kinetoplastid diseases (Chagas disease, HAT, and leishmaniasis) and dengue receiving the majority of funding. “Funder investment in this area is very clearly stratified,” she observed.
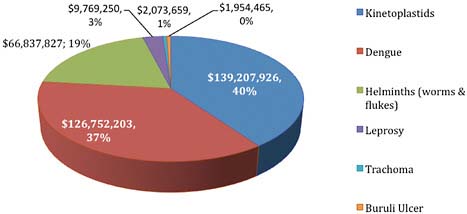
FIGURE WO-15 Global funding for NTDs by disease.
SOURCE: Adapted from Moran (2010).
The top 12 NTD R&D funding organizations are shown in Figure WO-16. NIH is the biggest funder—followed by the Bill & Melinda Gates Foundation—for nearly every disease, Moran said. She also noted that the vast majority of pharmaceutical industry funding is directed at dengue, while nearly all R&D funding for kinetoplastid and helminth diseases and trachoma derives from public and non-profit sources. “The big funders [NIH and Gates] provide about 60 percent of funding, just those two groups,” she observed. “After that, you drop to very small figures. If you want to be one of the top 10 global funders [of NTD R&D], you only need about $15 million, $20 million, and, based on these figures, you will be providing more than Japan, more than Germany.” On the other hand, she said, IDCs like Brazil and India now provide 10 percent of global investment in NTD R&D.
A closer examination of the breakdown of funding by disease and disease class reveals the following details, of which Moran made note:
-
Whereas R&D funding for most NTDs is spent on basic research, this is not the case for kinetoplastid diseases, largely because of DNDi’s efforts to develop drugs for HAT and leishmaniasis. This statistic illustrates a general trend that the existence of PDPs—which are not necessarily reflective of need—drive investment in R&D, Moran observed.
-
Dengue is exceptional in receiving significant funding from the pharmaceutical industry—nearly 85 percent of its US$52 million total contribution to NTDs; this is because dengue affects wealthier countries and thus has a bigger market than most NTDs, Moran said. Dengue R&D is also relatively well-funded by Brazilian groups because of the impact of the
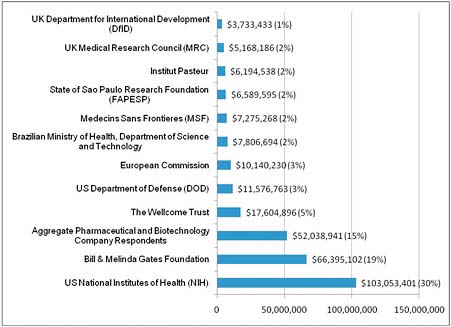
FIGURE WO-16 Top 12 funders of NTD research, 2008 (US$).
SOURCE: Adapted from Moran (2010).
-
disease in that country and their capacity to do research (as previously noted by Hotez), she added. Moran characterized such demand-driven funding as “much more sustainable” than funding driven by charitable grants or donations.
-
Most of the relatively meager funding for helminth diseases is spent on basic research, reflecting the absence of a group devoted to applied work for most of these diseases. Here again, she observed, “the funding for basic versus applied research doesn’t appear to reflect actual need. It’s driven by other things.”
From these findings, Moran concluded, there is a “lack of correlation between investment patterns and R&D needs and possibilities,” or more simply, a “disconnect between policy and innovation.” For years, out of necessity, public health policy has been focused on making do with little, she said. “Now innovations come along, and I think we are all at a bit of a loss. Policy hasn’t caught up to provide the tools to stimulate investment, to guide investment, and to make sure innovations get out there quickly.”
Several crucial pieces of information are needed to fill this funding gap, Moran observed. Funders need accurate assessments of health return on investment—a difficult calculation to make, she added, because the investment needed
to drive a product reflects a host of factors, including the impact of the disease (e.g., in DALYs), the severity of underfunding, and the size of the treatment gap. “If you are putting money into an area where there are already products and you are going to make a “top-up” product, your impact is much less than if you are making a product where there is nothing,” she observed. Funders also need to know the state of science in the field, she said. For example, are (relatively cheap) diagnostics needed, or (relatively expensive) vaccines? “When funders think about where to put their money to get the biggest bang for the buck, there are a whole bunch of issues that they need to know about, and I don’t think they clearly get this information,” she concluded; moreover, funders lack the analytical tools they need to determine their health return on investment.
Those who receive funding also lack important information about how to get their products into use, Moran observed. “There is often a gap between a product being available and when it gets out there,” she said, adding that a backlog of more than 100 diagnostic products currently await prequalification by WHO. However, even pre-qualified products often fail to provide potential end users with information they need to help them decide if the product suits their needs, she added. Moran described a promising new multi-drug resistant MDR-TB diagnostic that detects the disease in about 97 percent of patients and which a technician with very little training can perform in two hours—something public health has wanted for decades. Unfortunately, she continued, “we have the machine, but we don’t have the advice. What if you are sitting in Brazil? What’s the entry point for this test? Is it district hospital? Is it urban? When does it displace my existing system of testing? Who’s going to fund it? Should I use it for HIV? The test is very sensitive at picking up smear-negative patients, about 90 percent. When do I use it for HIV, or should I just use it for MDR-TB? How do I scale it up? How do I implement it? Where’s the entry point? How do I roll it out?”
Ministries of health need information on cost-effectiveness and implementation (e.g., if existing laboratories and/or workforce are sufficient) in order to determine whether to purchase a product, Moran explained. If this information were provided in “policy packages” accompanying each product, end-users could make better decisions as to whether or not to adopt a novel intervention, she said.
Responding to the suggestion that focusing on product development overlooks health-system approaches to NTDs, and therefore results in opportunity costs, Moran said that both approaches were needed, noting that past experience (e.g., in determining whether to emphasize treatment or prevention of HIV/AIDS) suggests that treating people leads to community mobilization to address broader health issues. “Our job as policymakers is to make sure that the innovations are brought to patients as soon as can be,” Moran concluded. “For every delay in that, I actually think we’re starting to be responsible for some of this mortality. We have always pointed at poor infrastructure, or lack of industry investment in R&D for neglected diseases. But now that innovations are coming along, delays in rolling them out really lay at our door.”
Gauging health return on investment The concept of “health return on investment,” raised by Moran, sparked considerable discussion. Forum member Carole Heilman, of NIH, noted that health return can be variously defined, but that death is the easiest way to measure it. “Preventing death is really something you can grab onto,” she said. It is far easier to motivate funders to focus resources on “killer” diseases such as HIV, TB, and malaria than it is for chronic, debilitating diseases. Curing poverty is an attractive alternative, she continued, but not unless an intervention is measured using solid indicators of poverty (e.g., changes in gross domestic product [GDP] or in measures of intelligence).
“There [are] genuine data showing impressive economic rates of return when you can keep people free of these neglected tropical diseases,” Hotez replied. “They truly are antipoverty measures.”
But, Heilman asked, can you really demonstrate that a particular implementation measure produced those changes? Were there other confounding factors that you could control for or deal with?
That’s a legitimate question, Lammie observed, but the reality is that programs are already in place under the assumption that they will alleviate poverty—so it is important to collect impact data to see if that is the case. “Do I have confidence that there are relationships between the presence of these diseases and poverty? Yes. Are they linear? Are they direct? Probably not,” he concluded. “But I think, as we have seen the programs go to scale … the reality is that our collection of data, our gathering of data related to impact has really lagged behind. We have a long way to go to get to the point where I think we are going to be able to satisfy you with an answer.”
With regard to specific interventions, Feinberg noted that, in the cases of pneumococcal and rotavirus vaccines, not only did projections derived from clinical studies anticipate economic benefits, but these were borne out in post-implementation studies by CDC. He added that he is not aware of similar efforts focused on NTDs.
Forum chair David Relman raised the possibility of conducting public health intervention experiments, controlling for as many potential confounds as possible, to explore the poverty–NTD connection. It would be unethical to perform case-control evaluations and withhold treatments from a community, Lammie replied, but it is possible to capture disease-specific impacts of integrated interventions in terms of such factors as children’s growth rate or anemia prevalence. Such studies could potentially be conducted at “demographic and surveillance sites where there have been huge investments made over the last decade to look at public health interventions in the context of longitudinally monitored populations,” he said.
On this point, Hopkins noted that one of the Carter Center’s most powerful advocacy tools for funding dracunculiasis eradication was a UNICEF study in southwest Nigeria that was funded for US$5,000. The disease was highly prevalent in this area, which was also a very fertile rice-growing region, and the study demonstrated that rice farmers alone (among a total population of 1.6 million) were
losing $20 million per year due to dracunculiasis. When extrapolated to the entire agricultural sector of Nigeria, that figure was huge, he said—and persuasive.
“Is it indeed possible to come up with some kind of metric for health return on investment?” Moran asked. Companies gauge return on investment with one set of metrics, and cost-effectiveness with another, she observed; most governments compare the health benefit of a product with its cost. “I’m trying to look at where there is the opportunity to improve information—perfect information, no; a single number, no; a list of priorities, no—just a tool to help—because, honestly, we look at the R&D funding stats all the time, and it’s just random,” she said. Rather than attempt to discern precise measures of health impact, she said, she hopes to identify “simple rubrics” and “distinguishing factors” to guide investment decisions, which often come down to choosing to develop or to distribute a drug for one NTD, versus one for another.
Guiding Principles and Next Steps
In his presentation on the GHI, Emanuel described the following seven principles upon which the initiative is based. All seven were raised and elaborated upon in subsequent workshop presentations and discussion and thus may be viewed as representative of the thinking behind investment in neglected diseases, a critical component of global health strategy.
Focus on women’s and children’s health As both Emanuel and McDonald noted, women are primarily responsible for childrearing and for providing and seeking health care for their families; they also manage water and nutrition. It is often said that the death of a mother increases tenfold the chance that her child will die, Emanuel noted—so, if you think like an economist, you can double your return on investment by improving women’s health. Since women and children suffer disproportionately from NTDs and their consequences, as McDonald observed (see previous discussion in “Defining the NTDs”), such an investment should have even greater impact. Hotez echoed the importance of NTDs as problems in maternal and child health (Hotez, 2009c).
Through support for long-term systematic change related to women and children through the monitoring and evaluation of women’s health, the GHI aims to enhance productivity and social and economic participation, Emanuel said; similar gains should be realized by treating children to prevent physical and cognitive effects of NTDs that drive the poverty cycle. Ault agreed, noting that one way such gains could be realized quickly was by extending programs such as school-based de-worming to preschool-age children.
Coordination and integration The specific directives of the GHI reflect consensus on the value of coordinating efforts to reduce NTD burden and on the need to integrate approaches to simultaneously and efficiently address several diseases
(including malaria and HIV/AIDS). Under the GHI, all agencies of the U.S. government that deal with international health—the State Department, USAID, HHS—will be encouraged to work together and to coordinate their activities with host-country staff, Emanuel stated. Points of care and supply chains will be integrated, to reflect the fact that patients rarely are infected with only one parasite, nor do they have the flexibility, time, or resources to attend multiple, disease-specific clinics, he added.
Partnership The health needs of people in developing countries are too vast, and the challenges too great, for any one country or organization to manage alone, Emanuel observed; this effort must involve other countries, NGOs, private philanthropies, and the private sector. Regarding NTDs, his commitment to “coordinate activities among various donors so we don’t duplicate what we are doing and we are as efficient as possible” was affirmed by several others, including Feinberg and Moran. Savioli described global efforts to address NTDs as a “roundtable of partners that are really working together as a single group,” in which WHO participates by defining problems and seeking their solutions (e.g., determining whether drugs such as ivermectin can be safely administered during pregnancy and infancy).
Country ownership Many participants extolled the importance of national or local management of and participation in NTD control programs. The ultimate responsibility for health interventions rests with the countries in which they are delivered, Emanuel observed, so the United States will work closely with governments and in-country health organizations to ensure that investments are aligned with national priorities; this may involve helping countries to develop health plans and the capacity to carry them out (e.g., computers, information, budgeting strategies, and training).
Sustainability Several participants emphasized that infrastructural improvements will be necessary to sustain interventions against NTDs over the long term. “The aim isn’t to manage needs, but to address them in a way that permanently reduces and eliminates these health problems,” Emanuel said. “Health systems, therefore, have to be improved both institutionally and structurally.” There is an especially critical need to develop adequate workforce to address health needs in developing countries, he added.
When asked how to resolve tension between disease-specific approaches to NTDs and the need to develop overall public health infrastructure, Fenwick observed that political will within each recipient country is important to striking this balance, which can only occur if there is support for infrastructural development. “There are often trained people in [developing] countries for addressing NTDs, but they don’t have resources they need,” he observed. “If you provide those resources, they can do great things.”
Monitoring and evaluation “We need to base our policy decisions on hard, good data,” Emanuel insisted. “In the end, it’s not the budget numbers, it’s not the number of bed nets used or the number of pills distributed that really matter to us, but it’s the lives saved, the health improved, and the health system functionality that we really ultimately care about.” Even more important than doing what works is figuring out what doesn’t work, and stopping it quickly, he observed; on the other hand, he added, “we need to identify, take to scale, and refine proven approaches. When something works, we need to quickly disseminate it.”
Exactly how to know if something works remains a challenge, Emanuel acknowledged; thus, robust metrics must be developed to measure the impact of interventions. As economist and Nobel Prize winner Joseph Stiglitz has argued, “What you measure affects what you do—if you don’t measure the right thing, you don’t do the right thing” (Goodman, 2009).
Research and innovation Anticipating a key point Moran made in her presentation, Emanuel noted several disappointing lapses in the adoption of proven measures to improve global health, such as circumcision to prevent mother-to-child HIV transmission. “We have not scaled up,” he admonished. “We need to implement findings as part of our research.” Under the GHI, he said, “we are going to support innovations that promote results-oriented rather that input-oriented expenditures.” He also envisioned testing a variety of strategies for health services delivery involving such things as incentives to encourage people to attend clinics or to adhere to treatment.
Expanding Medical Diplomacy
In recent years, several workshop participants observed, NTDs have become increasingly less neglected. A major advance came with the recognition that NTDs represented an important opportunity for medical diplomacy28: the provision of needed health services as a means to developing positive international relations. Hotez took up this topic at the conclusion of his presentation on antipoverty vaccines, while displaying the quote from John Gardner in Figure WO-17.
Figure WO-17 also displayed the results of an analysis Hotez and colleagues performed, revealing that 40 percent of the world’s seven most common NTDs occur in the world’s Islamic countries: Indonesia, Bangladesh, Pakistan, Somalia, Sudan, West Africa, Chad, and Niger (Hotez, 2009d). Another 20 to 30 percent of the NTDs occur in large “upper middle” and “lower middle” income countries—India, China, Iran, North Korea, and Syria—that possess nuclear weapons (Hotez,
FIGURE WO-17 Opportunities for “vaccine diplomacy.”
SOURCE: Hotez (2010c).
2010d). Given the geopolitical importance of these countries, health diplomacy addressing NTDs is more critical than ever, Hotez suggested; so important, in fact, that its purview should be expanded beyond MDA to include joint cooperative scientific ventures. Recalling Albert Sabin’s joint effort with Soviet scientists that produced the oral polio vaccine—which occurred at the height of the Cold War—as an example of how two countries can put aside their ideologies for purposes of biomedical research, he posed the following question: “Can vaccinations help to resolve conflicts and nurture diplomacy?” (Hotez, 2010c).
Forum member Terence Taylor, of the International Council for the Life Sciences, affirmed Hotez’s idealism. He offered the contemporary example of consortia on health decisions between Israel and its Arab neighbors, which have enabled them jointly to pursue leishmaniasis control and to share outbreak data on a day-to-day basis. In Southeast Asia, in southern Africa, and even in North Korea, health consortia are currently working across political divides, he added. Such groups could profitably turn their attentions to NTDs and, in particular, to the establishment of public health priorities in addressing these diseases, he said. “Health issues,” Taylor concluded, “can cross the most difficult boundaries, and it is happening now.”
In the end it is not the budget numbers, the number of bednets used, or the pills distributed that matter, but the lives saved, the health improved, and the health systems functionality.
—Emanuel keynote remarks
 FIGURE WO-6-4 Brucellosis. (A) Hygromasa on the knee joints. (B) Brucella melitensis/Brucella suis. SOURCES: Food and Agriculture Organisation of the United Nations (FAO); Defense Threat Reduction Agency (DTRA). Infectious agent: Various bacteria of genus Brucella Routes of transmission to humans: Contact with infected animals, drinking unpasteurized milk from infected animals Health effects: Recurrent fever (often misdiagnosed as drug-resistant malaria); joint pain, fatigue, depression Incidence, prevalence, and mortality (estimated): More than 500,000 new cases per year Prevention: Pasteurization of milk, vaccination of cattle in high-prevalence areas, testing and culling of cattle in low-prevalence areas Treatment: Antibiotic therapy |
 FIGURE WO-6-6 Chagas disease. (A) Acute Chagas disease in a young child. The eye sign of Romana is present. This is frequently seen in acute cases and is presumed to mark the point of entry of the parasite. (B) Trypanosome trypomastigote (Trypanosoma sp.) A hemoflagellated protozoan parasite that causes trypanosomiasis (Chagas disease, African sleeping sickness). SEM 800x magnification. SOURCES: Special Programme for Research and Training in Tropical Diseases (TDR) Research Image Library (WHO/TDR); http://www.denniskunkel.com/product_info.php?products_id=13508 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Trypanosoma cruzi, a protozoan parasite Routes of transmission to humans: Bites from insects known as “assassin bugs” or “kissing bugs”; blood transfusions; mother-to-fetus Health effects: Cardiac or digestive complications; risk of death from heart problems Incidence, prevalence, and mortality (estimated): 8 to 9 million people infected; up to 2.8 million with severe disease; 14,000 deaths per year Prevention: Vector control; improved housing and food hygiene; screening of blood and organ donors Treatment: Anti-parasitic drugs; most effective if given at onset of infection |
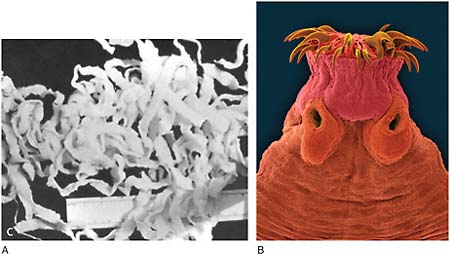 FIGURE WO-6-8 Cysticercosis. (A) Adult Taenia solium worm. (B) Mammal intestine tapeworm (Taenia spp.). The adult has a head (scolex) with suckers and/or hooks that are used to attach to the host (SEM 16x magnification). SOURCES: Palmer and Reeder/USUHS, Courtesy of Dr. Herman Zaiman; http://www.denniskunkel.com/product_info.php?products_id=1173 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Tapeworms that infect pigs (Taenia solium) and cattle (Taenia saginata) Routes of transmission to humans: Consumption of raw or undercooked pork (cysticercosis) or beef (taeniasis) containing larvae; contact with tapeworm carriers (who shed eggs in feces) or with egg-contaminated water or food Health effects: Depending on the location of the larval cysts, infections can cause lumps under the skin, impaired vision, or—when cysts form in the brain or spinal cord (NCC)—neurological symptoms including seizures, headaches, cognitive impairment, balance difficulties, and swelling of the brain Incidence, prevalence, and mortality (estimated): 50 million cases worldwide; 50,000 deaths per year Prevention: Cooking pork and beef, hygiene and sanitation Treatment: Cystercercosis treatment involves both antibiotics and steroids and is not always successful; antibiotic treatment of taeniasis is effective |
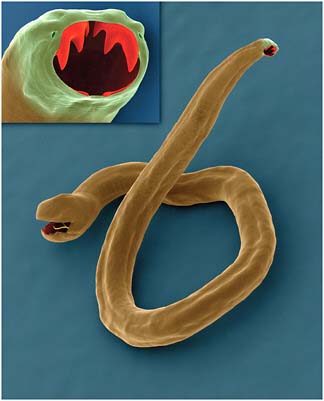 FIGURE WO-6-11 Hookworm (Nematode Ancylostoma caninum). The adult parasites are small cylindrical worms 0.5–1.5 mm long. The genus Ancylostoma have pairs of teeth on the ventral margin of the buccal capsule (SEM 8x magnification). SOURCE: http://www.denniskunkel.com/product_info.php?products_id=1093 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Necator americanus (85 percent of the cases) and Ancylstostoma duodenale, parasitic nematodes (roundworms) Routes of transmission to humans: Larvae in contaminated soil enter through skin Health effects: Intestinal blood loss, anemia, protein malnutrition; cognitive impairment, delayed mental and physical development; maternal morbidity and mortality during pregnancy Incidence, prevalence, and mortality (estimated): 576 million people infected, of which 60 million are “highly infected,” with the largest number of cases in sub-Saharan Africa, South and East Asia, and in tropical regions of the Americas Prevention: Improved hygiene and sanitation; periodic drug treatment of at-risk populations Treatment: Antihelminthic therapy |
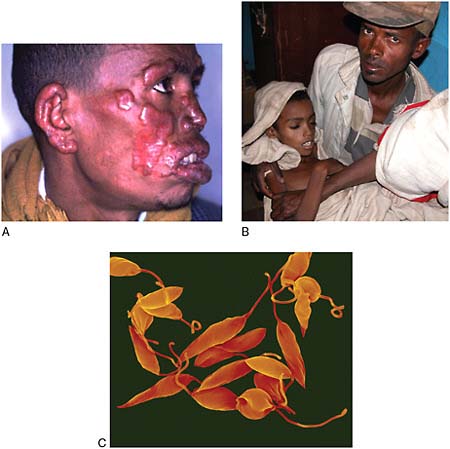 FIGURE WO-6-12 Leishmaniasis (Leishmania). (A) Patient with diffuse cutaneous leishmaniasis. (B) Patient awaiting treatment during a 2005 leishmaniasis outbreak in Libo Kemkem, Highlands, Ethiopia. (C) Parasitic promastigotes that cause leishmaniasis in humans (Leishmania donovani). SOURCES: WHO/TDR/Crump; WHO; http://www.denniskunkel.com/product_info.php?products_id=12507 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Protozoan parasites of the genus Leishmania Routes of transmission to humans: Insect bites, especially those of sandflies Health effects: Fever, weight loss, skin sores, disfigurement; visceral leishmaniasis can be fatal Incidence, prevalence, and mortality (estimated): 12 million people infected; up to 50,000 deaths per year Prevention: Vector control Treatment: Several effective drugs, all with serious drawbacks |
 FIGURE WO-6-13 Leprosy (Mycobacterium leprae). (A) The face of a patient with active, neglected nodulous lepromatous leprosy. (B) Mycobacterium leprae—Gram-positive rod prokaryote, cause of leprosy. SOURCE: WHO/TDR/McDougall; http://www.denniskunkel.com/product_info.php?products_id=8519 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Mycobacterium leprae, a bacterium Routes of transmission to humans: Human-to-human contact via respiratory droplets; may be additional modes Health effects: Loss of sensation leading to damage to extremities and disability; disfigurement Incidence, prevalence, and mortality (estimated): 900,000 people infected Prevention: None (not highly infectious) Treatment: Antibiotic therapy (multi-drug) |
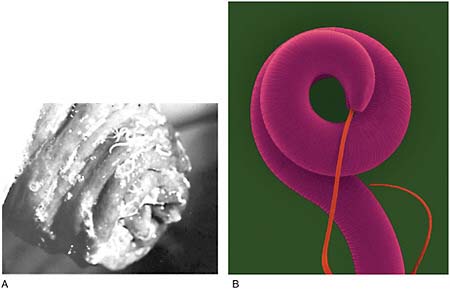 FIGURE WO-6-20 Trichuriasis (Whipworm). (A) Close-up of the prolapsed rectum of a child with numerous Trichuris trichiura clinging to the rectal mucosa. (B) Nematode (Trichuris spp.) helminth (whipworm), intestinal parasite. SOURCES: Palmer and Reeder/USUHS, courtesy of Dr. Herman Zaiman; http://www.denniskunkel.com/product_info.php?products_id=668 (accessed January 19, 2011). Copyright Dennis Kunkel Microscopy, Inc. Infectious agent: Trichuris trichiura, a parasitic roundworm Routes of transmission to humans: Ingestion of eggs from contaminated soil; worm embeds in wall of large intestine Health effects: Cognitive impairment, colitis and inflammatory bowel disease, delayed physical and mental development Incidence, prevalence, and mortality (estimated): 604 million people infected of whom 27 million are “highly infected” Prevention: Improved hygiene and sanitation; periodic drug treatment of at-risk populations Treatment: Antihelminthic therapy |
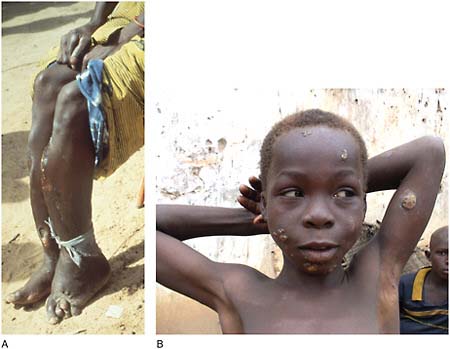 FIGURE WO-6-22 Yaws infection (Treponema pertenue). (A) Tibial “saber” deformity as a result of yaws. (B) Child presenting lesions caused by yaws, Côte d’Ivoire, 2009. SOURCES: Rinaldi (2008), previously published in PLoS Neglected Tropical Diseases; WHO. Infectious agent: Treponema pertenue, a bacterium Routes of transmission to humans: Person-to-person via skin contact (infects only humans) Health effects: Chronic disfigurement due to skin and cartilage lesions; painful bone lesions; rarely fatal Incidence, prevalence, and mortality (estimated): No recent estimates; in the 1990s, WHO determined that 2.5 million people were infected, of which 460,000 represented new cases Prevention: A candidate for eradication via antibiotic treatment to interrupt transmission Treatment: Antibiotic therapy SOURCE: Adapted from Health Affairs (2009) with additional information from Ayele et al. (2004), CDC (2009, 2010a), Cosivi et al. (1998), Hotez (2010a), Hotez and Wilkins (2009), Pappas et al. (2006), and WHO (2008a–g, 2010c–g); The Merck Manual (http://www.merckmanuals.com/professional/sec14/ch183/ch183h.html (accessed on January 21, 2011). |
WORKSHOP OVERVIEW REFERENCES
Armah, G. E., S. O. Sow, R. F. Breiman, M. J. Dallas, M. D. Tapia, D. R. Feikin, F. N. Binka, A. D. Steele, K. F. Laserson, N. A. Ansah, M. M. Levine, K. Lewis, M. L. Coia, M. Attah-Poku, J. Ojwando, S. B. Rivers, J. C. Victor, G. Nyambane, A. Hodgson, F. Schodel, M. Ciarlet, and K. M. Neuzil. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastro-enteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 376(9741):606–614.
Asojo, O. A., K. Homma, M. Sedlacek, M. Ngamelue, G. N. Goud, B. Zhan, V. Deumic, O. Asojo, and P. J. Hotez. 2007. X-ray structures of Na-GST-1 and Na-GST-2 two glutathione S-transferase from the human hookworm Necator americanus. BMC Structural Biology 7:42.
Ault, S. 2010. Regional approaches to NTD control in the Americas. Presentation given at the September 21–22, 2010, public workshop, “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Ayele, W. Y., S. D. Neill, J. Zinsstag, M. G. Weiss, and I. Pavlik, 2004. Bovine tuberculosis: An old disease but a new threat to Africa. International Journal of Tuberculosis and Lung Disease 8 (8):924–937.
Barber, N. 2005. Educational and ecological correlates of IQ: A cross-national investigation. Intelligence 33:273–284.
Barclay, J. 1878. The Talmud. London: John Murray.
Barreto, M. L., B. Genser, A. Strina, M. G. Teixeira, A. M. Assis, R. F. Rego, C. A. Teles, M. S. Prado, S. M. Matos, D. N. Santos, L. A. dos Santos, and S. Cairncross. 2007. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: Assessment by two cohort studies. Lancet 370(9599):1622–1628.
Barreto, M. L., B. Genser, A. Strina, M. G. Teixeira, A. M. Assis, R. F. Rego, C. A. Teles, M. S. Prado, S. M. Matos, N. M. Alcantara-Neves, and S. Cairncross. 2010. Impact of a city-wide sanitation programme in northeast Brazil on intestinal parasites infection in young children. Environmental Health Perspectives. 118(1):1637–1642.
Bartram, J., and S. Cairncross. 2010. Hygiene, sanitation, and water: Forgotten foundations of health. PLoS Medicine 7(11):1–9.
Berrang-Ford, L., J. Lundine, and S. Breau. 2010. Conflict and human African trypanosomiasis. Social Science & Medicine 72(3):398–407.
Betsi, N. A., B. G. Koudou, G. Cisse, A. B. Tschannen, A. M. Pignol, Y. Ouattara, Z. Madougou, M. Tanner, and J. Utzinger. 2006. Effect of an armed conflict on human resources and health systems in Côte d’Ivoire: Prevention of and care for people with HIV/AIDS. AIDS Care 18(4):356–365.
Beyrer, C. 2007. Civil conflict and health information: The case of DR Congo. In Public health and human rights: Evidence based approaches. Baltimore, MD: The Johns Hopkins University Press.
Black, R. E., S. Cousens, H. L. Johnson, J. E. Lawn, I. Rudan, D. G. Bassani, P. Jha, H. Campbell, C. F. Walker, R. Cibulskis, T. Eisele, L. Liu, and C. Mathers. 2010. Global, regional, and national causes of child mortality in 2008: A systematic analysis. The Lancet 375(9730):1969–1987. http://www.ncbi.nlm.nih.gov/pubmed/20466419 (accessed October 20, 2010).
Bonet, M., J. M. Spiegel, A. M. Ibarra, G. Kouri, A. Pintre, and A. Yassi. 2007. An integrated ecosystem approach for sustainable prevention and control of dengue in central Havana. International Journal of Occupational and Environmental Health 13(2):188–194.
Brooker, S., A. C. Clements, P. J. Hotez, S. I. Hay, A. J. Tatem, D. A. Bundy, and R. W. Snow. 2006. The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malaria Journal 5:99.
Brooker, S., W. Akhwale, R. Pullan, B. Estambale, S. E. Clarke, R. W. Snow, and P. J. Hotez. 2007. Epidemiology of plasmodium-helminth co-infection in Africa: Populations at risk, potential impact on anemia, and prospects for combining control. American Journal of Tropical Medicine and Hygiene 77(6 Suppl):88–98.
Buijs, J., G. Borsboom, J. J. van Gemund, A. Hazebroek, P. A. van Dongen, F. van Knapen, and H. J. Neijens. 1994. Toxocara seroprevalence in 5-year-old elementary schoolchildren: Relation with allergic asthma. American Journal of Epidemiology 140:839–847.
Buijs, J., G. Borsboom, M. Renting, W. J. A. Hilgersom, J. C. van Wieringen, G. Jansen, and J. Neijens. 1997. Relationship between allergic manifestations and Toxocara seropositivity: A cross sectional study among elementary school children. European Respiratory Journal 10:467–475.
Burns, M. 2010. Lifesaving drug praziquantel too expensive for Africa. http://www.miller-mccune.com/health/lifesaving-drug-praziquantel-too-expensive-for-africa-23538/ (accessed October 29, 2010).
CDC (Centers for Disease Control and Prevention). 1993. Recommendations of the International Task Force for Disease Eradication. MMWR Recommendations and Reports 42(RR-16):1–38.
———. 2008. Schistosomiasis fact sheet. http://www.cdc.gov/ncidod/dpd/parasites/schistosomiasis/factsht_schistosomiasis.htm (accessed October 22, 2010).
———. 2009. Rabies. http://www.cdc.gov/rabies/ (accessed January 6, 2011).
———. 2010a. Locally acquired dengue—Key West, Florida, 2009–2010. Morbidity and Mortality Weekly Report 59(19):577–581.
———. 2010b. Report suggests nearly 5 percent exposed to dengue virus in Key West. http://www. cdc.gov/media/pressrel/2010/r100713.htm (accessed October 24, 2010).
———. 2010c. Chagas disease—diagnosis. http://www.cdc.gov/chagas/diagnosis.html (accessed January 6, 2011).
Chu, B. K., P. J. Hooper, M. H. Bradley, D. A. McFarland, and E. A. Ottesen. 2010. The economic benefits resulting from the first 8 years of the Global Programme to Eliminate Lymphatic Filariasis (2000–2007). PLoS Neglected Tropical Diseases 4(6):e708.
Chukwuekezie, O., E. Ampadu, G. Sopoh, A. Dossou, A. Tiendrebeogo, L. Sadiq, F. Portaels, and K. Asiedu. 2007. Buruli ulcer, Nigeria. Emerging Infcctious Diseases 13(5):782–783.
Colugnati, F. A., S. A. Staras, S. C. Dollard, and M. J. Cannon. 2007. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infectious Diseases 7:71.
Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerging Infectious Diseases 4 (1):59–70.
Cox, F. E. 2002. History of human parasitology. Clinical Microbiology Reviews 15(4):595–612.
DNDi (Drugs for Neglected Diseases initiative). 2010. Drugs for Neglected Diseases initiative— overview. http://www.dndi.org/index.php/overview-dndi.html?ids=1 (accessed November 4, 2010).
Ekwanzala, M., J. Pepin, N. Khonde, S. Molisho, H. Bruneel, and P. De Wals. 1996. In the heart of darkness: Sleeping sickness in Zaire. Lancet 348(9039):1427–1430.
Eppig, C., C. L. Fincher, and R. Thornhill. 2010. Parasite prevalence and the worldwide distribution of cognitive ability. Proceedings. Biological Sciences. The Royal Society 277(1701):3801–3808.
Ettling, J. 2000. The germ of laziness: Rockefeller philanthropy and public health in the New South. Cambridge, MA: Harvard University Press.
FAO (Food and Agriculture Organization). 2002. Global and Regional Food Consumption Patterns and Trends, FAO, Rome. http://www.fao.org/docrep/005/ac911e/ac911e05.htm (accessed January 6, 2011).
Feinsilver, J. M. 2006. Cuban medical diplomacy: When the left has got it right. http://www.coha.org/cuban-medical-diplomacy-when-the-left-has-got-it-right/ (accessed August 1, 2010).
Fenwick, A., Y. Zhang, and K. Stoever. 2009. Control of the neglected tropical diseases in sub-Saharan Africa: The unmet needs. International Health 1:61–70.
Genser, B., A. Strina, L. A. dos Santos, C. A. Teles, M. S. Prado, S. Cairncross, and M. L. Barreto. 2008. Impact of a city-wide sanitation intervention in a large urban centre on social, environmental and behavioural determinants of childhood diarrhoea: Analysis of two cohort studies. International Journal of Epidemiology 37(4):831–840.
Global Network (Global Network for Neglected Tropical Diseases). 2010. “About NTDs.” Sabin Vaccine Institute. Washington, DC. http://globalnetwork.org/about-ntds (accessed November 16, 2010).
Goodman, P. 2009. Emphasis on growth is called misguided. New York Times, September 23.
Grove, D. I. 1990. A history of human helminthology. Wallingford, UK: CAB International.
Gryseels, B. 1989. The relevance of schistosomiasis for public health. Tropical Medicine and Parasitology 40(2):134–142.
Hamburg, M. A. 2010. Margaret A. Hamburg, M.D., Commissioner of Food and Drugs—remarks at the medical countermeasures review roll out. http://www.fda.gov/NewsEvents/Speeches/ucm226351.htm (accessed November 3, 2010).
Hanson, 2010. Keynote Remarks. Presentation given at the September 21–22, 2010, public workshop, “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Health Affairs. 2009. Tropical diseases: The price of neglect (editorial). Health Affairs (Millwood) 28(6):1688–1690.
Hopkins, D. 2009. The allure of eradication. Global Health Magazine Summer 2009(03):14–17.
———. 2010. NTDs slated for elimination and eradication. Paper read at “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” September 21, 2010, Washington, DC.
Hotez, P. J. 2006. The “biblical diseases” and U.S. vaccine diplomacy. Brown Journal of World Affairs 12:247–258.
———. 2008a. The giant anteater in the room: Brazil’s neglected tropical diseases problem. PLoS Neglected Tropical Diseases 2(1):e177.
———. 2008b. Neglected infections of poverty in the United States of America. PLoS Neglected Tropical Diseases 2(6):e256.
———. 2009a. Neglected diseases amid wealth in the United States and Europe. Health Affairs (Millwood) 28(6):1720–1725.
———. 2009b. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. Clinical Pharmacology and Therapeutics 85(6):659–664.
———. 2009c. The neglected tropical diseases and their devastating health and economic impact on the member nations of the Organisation of the Islamic Conference. PLoS Neglected Tropical Diseases 3(10):e539.
———. 2010a. A plan to defeat neglected tropical diseases. Scientific American 302(1):90–94, 96.
———. 2010b. Neglected infections of poverty among the indigenous peoples of the Arctic. PLoS Neglected Tropical Diseases 4(1):e606.
———. 2010c. Peace through vaccine diplomacy. Science 327(5971):1301.
———. 2010d. Nuclear weapons and neglected diseases: The “ten-thousand-to-one gap.” PLoS Neglected Tropical Diseases 4(4):e680.
Hotez, P. J., and B. Pecoul. 2010. “Manifesto” for advancing the control and elimination of neglected tropical diseases. PLoS Neglected Tropical Diseases 4(5):e718.
Hotez, P. J., and P. P. Wilkins. 2009. Toxocariasis: America’s most common neglected infection of poverty and a helminthiasis of global importance? PLoS Neglected Tropical Diseases 3(3):e400.
Hotez, P. J., D. H. Molyneux, A. Fenwick, E. Ottesen, S. Ehrlich Sachs, and J. D. Sachs. 2006. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Medicine 3(5):e102.
Hotez, P., S. Raff, A. Fenwick, F. Richards, Jr., and D. H. Molyneux. 2007a. Recent progress in integrated neglected tropical disease control. Trends in Parasitology 23(11):511–514.
Hotez, P. J., D. H. Molyneux, A. Fenwick, J. Kumaresan, S. E. Sachs, J. D. Sachs, and L. Savioli. 2007b. Control of neglected tropical diseases. New England Journal of Medicine 357(10):1018–1027.
Hotez, P. J., J. M. Bethony, S. C. Oliveira, P. J. Brindley, and A. Loukas. 2008a. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Review of Vaccines 7(6):745–752.
Hotez, P. J., M. E. Bottazzi, C. Franco-Paredes, S. K. Ault, and M. R. Periago. 2008b. The neglected tropical diseases of Latin America and the Caribbean: A review of disease burden and distribution and a roadmap for control and elimination. PLoS Neglected Tropical Diseases 2(9):e300.
Hotez, P. J., A. Fenwick, and E. F. Kjetland. 2009a. Africa’s 32 cents solution for HIV/AIDS. PLoS Neglected Tropical Diseases 3(5):e430.
Hotez, P. J., A. Fenwick, L. Savioli, and D. H. Molyneux. 2009b. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373(9674):1570–1575.
Hotez, P. J., J. M. Bethony, D. J. Diemert, M. Pearson, and A. Loukas. 2010. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nature Reviews Micriobiology 8(11):814–826.
Humphreys, M. 2009. How four once common diseases were eliminated from the American South. Health Affairs (Millwood) 28(6):1734–1744.
Iglehart, J. K. 2004. Advocating for medical diplomacy: A conversation with Tommy G. Thompson. http://content.healthaffairs.org/cgi/content/full/hlthaff.w4.262v1/DC1 (accessed August 1, 2010).
IOM (Institute of Medicine). 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
———. 2008a. Global climate change and extreme weather events: Understanding the contributions to infectious disease emergence. Washington, DC: The National Academies Press.
———. 2008b. Vector-borne diseases: Understanding the environment, human health, and ecological connections. Washington, DC: The National Academies Press.
———. 2009. Global issues in water, sanitation, and health. Washington, DC: The National Academies Press.
Kanazawa, S. 2008. Temperature and evolutionary novelty as forces behind the evolution of general intelligence. Intelligence 36.
Keiser, J., and J. Utzinger. 2008. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. Journal of the American Medical Association 299(16):1937–1948.
King, C. H. 2007. Lifting the burden of schistosomiasis—defining elements of infection-associated disease and the benefits of antiparasite treatment. Journal of Infectious Diseases 196(5):653–655.
———. 2010. Parasites and poverty: The case of schistosomiasis. Acta Tropica 113(2):95–104.
Kissinger, P., W. E. Secor, J. S. Leichliter, R. A. Clark, N. Schmidt, E. Curtin, and D. H. Martin. 2008. Early repeated infections with Trichomonas vaginalis among HIV-positive and HIV-negative women. Clinical Infectious Diseases 46(7):994–999.
Kjetland, E. F., P. D. Ndhlovu, E. Gomo, T. Mduluza, N. Midzi, L. Gwanzura, P. R. Mason, L. Sandvik, H. Friis, and S. G. Gundersen. 2006. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20(4):593–600.
Kristoff, N. 2010. Winning the worm war. New York Times, April 28. http://www.nytimes. com/2010/04/29/opinion/29kristof.html (accessed August 20, 2010).
Lebel, J. 2003. In focus: Health—an ecosystem approach. Ottawa: International Development Research Centre.
Lima, M. D. R. Q., R. M. R. Nogueira, H. G. Schatzmayr, and F. B. D. Santos. 2010. Comparison of three commercially available dengue NS1 antigen capture assays for acute diagnosis of dengue in Brazil. PLoS Neglected Tropical Diseases 4(7):e738.
Lonie, I. M. 1981. The Hippocratic treatises, “On generation,” ”On the nature of the child,” “On diseases IV.” Berlin: De Gruyter.
Loukas, A., J. M. Bethony, S. Mendez, R. T. Fujiwara, G. N. Goud, N. Ranjit, B. Zhan, K. Jones, M. E. Bottazzi, and P. J. Hotez. 2005. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Medicine 2(10):e295.
Lynn, R., and T. Vanhanen. 2001. National IQ and economic development: A study of eighty-one nations. Mankind Quarterly 41(4):415–421.
———. 2002. IQ and the wealth of nations. Westport, CT: Praeger.
———. 2006. IQ and global inequality. Athens: Washington Summit.
Malek, E. A. 1975. Effect of the Aswan high dam on prevalence of schistosomiasis in Egypt. Tropical and Geographical Medicine 27(4):359–364.
Margolis, H. 2010. Reemergence of dengue in the United States? Presentation given at the September 21–22, 2010, public workshop, “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Mascarini-Serra, L. M., C. A. Telles, M. S. Prado, S. A. Mattos, A. Strina, N. M. Alcantara-Neves, and M. L. Barreto. 2010. Reductions in the prevalence and incidence of geohelminth infections following a city-wide sanitation program in a Brazilian urban centre. PLoS Neglected Tropical Diseases 4(2):e588.
Molyneux D. H., P. J. Hotez, and A. Fenwick. 2005. “Rapid-Impact Interventions”: How a policy of integrated xontrol for Africa’s neglected tropical diseases could benefit the poor. PLoS Medicine 2(11): e336.
Moran, 2010. Neglected tropical and zoonotic diseases and their impact on women and children’s health. Presentation given at the September 21–22, 2010, public workshop, “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Moran, M., J. Guzman, A. L. Ropars, A. McDonald, N. Jameson, B. Omune, S. Ryan, and L. Wu. 2009. Neglected disease research and development: How much are we really spending? PLoS Medicine 6(2):e30.
Morens, D. M., and A. S. Fauci. 2008. Dengue and hemorrhagic fever: A potential threat to public health in the United States. Journal of the American Medical Association 299(2):214–216.
Morse, S. 2006. Making development simple. The genetic deterministic hypothesis for economic development. Ecological Economics 56(1):79–88.
Mullany, L. C., T. J. Lee, L. Yone, C. I. Lee, K. C. Teela, P. Paw, E. K. Shwe Oo, C. Maung, H. Kuiper, N. F. Masenior, and C. Beyrer. 2010. Impact of community-based maternal health workers on coverage of essential maternal health interventions among internally displaced communities in eastern Burma: The MOM project. PLoS Medicine 7(8):e1000317.
Musgrove, P., and P. J. Hotez. 2009. Turning neglected tropical diseases into forgotten maladies. Health Affairs (Millwood) 28(6):1691–1706.
National Library of Medicine. 2009. Congential toxoplasmosis. http://www.nlm.nih.gov/medlineplus/ency/article/001360.htm (accessed October 23, 2010).
Oladejo, S. O., and I. E. Ofoezie. 2006. Unabated schistosomiasis transmission in Erinle River Dam, Osun State, Nigeria: Evidence of neglect of environmental effects of development projects. Tropical Medicine & International Health 11(6):843–850.
One Health Initiative. 2010. One Health Initiative will unite human and veterinary medicine. http://www.onehealthinitiative.com/index.php (accessed October 29, 2010).
Ottesen, E. 2010. Progress in control and elimination of lymphatic filariasis. Paper read at “The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Implications for Global Health and Opportunities for Novel Intervention Strategies,” September 21, 2010, Washington, DC.
Ottesen, E. A., P. J. Hooper, M. Bradley, and G. Biswas. 2008. The Global Programme to Eliminate Lymphatic Filariasis: Health impact after 8 years. PLoS Neglected Tropical Diseases 2(10):e317.
PAHO (Pan-American Health Organization). 2009. Epidemiological profiles of neglected diseases and other infections related to poverty in LAC. Washington, DC: PAHO. http://new.paho.org/hq/index.php?option=com_content&task=view&id=1247&Itemid=259&lang=en (accessed November 18, 2010).
Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infectious Diseases 6 (2):91–99.
Patel, M., C. Pedreira, L. H. De Oliveira, J. Tate, M. Orozco, J. Mercado, A. Gonzalez, O. Malespin, J. J. Amador, J. Umaña, A. Balmaseda, M. C. Perez, J. Gentsch, T. Kerin, J. Hull, S. Mijatovic, J. Andrus, and U. Parashar. 2009. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. Journal of the American Medical Association 301:2243–2251.
Policy Cures. 2010. http://www.policycures.org/ (accessed November 4, 2010).
Rinaldi, A. 2008. Yaws: A Second (and Maybe Last?) Chance for Eradication. PLoS Neglected Tropical Diseases 2(8): e275.
RWJF (Robert Wood Johnson Foundation). 2008. Commission to Build a Healthier America. Social advantage and health across lifetimes and generations [chart]. Princeton, NJ: RWJF. http://www.commissiononhealth.org/PDF/hlth_gens.pdf (accessed October 20, 2010).
Saadat, M. 2008. Consanguinity and national IQ scores. Journal of Epidemiology and Community Health 62(6):566–567.
Sachs, J. D., and P. J. Hotez. 2006. Fighting tropical diseases. Science 311(5767):1521.
Simarro, P. P., J. Jannin, and P. Cattand. 2008. Eliminating human African trypanosomiasis: Where do we stand and what comes next? PLoS Medicine 5(2):e55.
Spiegel, J. M., M. Bonet, A. M. Ibarra, N. Pagliccia, V. Ouellette, and A. Yassi. 2007. Social and environmental determinants of Aedes aegypti infestation in central Havana: Results of a case-control study nested in an integrated dengue surveillance programme in Cuba. Tropical Medicine & International Health 12(4):503–510.
Spiegel, J. M., S. Dharamsi, K. M. Wasan, A. Yassi, B. Singer, P. J. Hotez, C. Hanson, and D. A. Bundy. 2010. Which new approaches to tackling neglected tropical diseases show promise? PLoS Medicine 7(5):e1000255.
Sutton, M., M. Sternberg, E. H. Koumans, G. McQuillan, S. Berman, and L. Markowitz. 2007. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clinical Infectious Disease 45(10):1319–1326.
Taylor, M. R. H., C. T. Keane, P. O’Connor, E. Mulvihill, and C. Holland. 1988. The expanded spectrum of toxocaral disease. Lancet 1:692–694.
Teixeira, M. G., C. Costa Mda, F. Barreto, and M. L. Barreto. 2009. Dengue: Twenty-five years since reemergence in Brazil. Cadernos Saude Publica 25(Suppl 1):S7–S18.
Templer, D. I., and H. Arikawa. 2006. Temperature, skin color, per capita income, and IQ: An international perspective. Intelligence 34:121–139.
Thylefors, B., M. M. Alleman, and N. A. Twum-Danso. 2008. Operational lessons from 20 years of the Mectizan donation program for the control of onchocerciasis. Tropical Medicine & International Health 13(5):689–696.
Tran, M. H., M. S. Pearson, J. M. Bethony, D. J. Smyth, M. K. Jones, M. Duke, T. A. Don, D. P. McManus, R. Correa-Oliveira, and A. Loukas. 2006. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nature Medicine 12(7):835–840.
Tran, M. H., T. C. Freitas, L. Cooper, S. Gaze, M. L. Gatton, M. K. Jones, E. Lovas, E. J. Pearce, and A. Loukas. 2010. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathogens 6(4):e1000840.
UNDP (United Nations Development Programme). 2010. What are the Millennium Development Goals? http://www.undp.org/mdg/basics.shtml (accessed November 9, 2010).
USAID (U.S. Agency for International Development). 2010. Neglected tropical disease program— where we work. http://ntd.rti.org/index.cfm?fuseaction=wherewework (accessed October 30, 2010).
Viotti, R., C. Vigliano, B. Lococo, G. Bertocchi, M. Petti, M. G. Alvarez, M. Postan, and A. Armenti. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: A nonrandomized trial. Annals of Internal Medicine 144(10):724–734.
Webb, J. C., D. Mergler, M. W. Parkes, J. Saint-Charles, J. Spiegel, D. Waltner-Toews, A. Yassi, and R. F. Woollard. 2010 (accepted for publication). Tools for thoughtful action: The role of ecosystem approaches to health in enhancing public health. Canadian Journal of Public Health 101(6):439–441.
WHO (World Health Organization). 2006. The control of neglected zoonotic diseases. Geneva: WHO.
———. 2008a. Anthrax in humans and animals, 4th Edition. Geneva: World Health Organization.
———. 2008b. Dengue and dengue hemmorhagic fever fact sheet. http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed 10 August 2008).
———. 2008c. Intestinal worms: epidemiology. http://www.who.int/intestinal_worms/epidemiology/en/ (accessed 10 August 2008).
———. 2008d. Onchocerciasis disease information. http://www.who.int/tdr/diseases/oncho/diseaseinfo.htm (accessed 10 August, 2008).
———. 2008e. Soil-transmitted helminths. http://www.who.int/intestinal_worms/en/ (accessed 10 August, 2008).
———. 2008f. Water-related diseases: guinea-worm disease (dracunculiasis). http://www.who.int/water_sanitation_health/diseases/guinea/en/ (accessed 10 August, 2008).
———. 2008g. Measuring child mortality. http://www.who.int/child_adolescent_health/data/child/en/index.html. (accessed May 4, 2010).
———. 2010a. Dengue and dengue haemorrhagic fever. http://www.who.int/mediacentre/factsheets/fs117/en/index.html (accessed July 29, 2010).
———. 2010b. Working to overcome the global impact of neglected tropical diseases. Geneva.
———. 2010c. Fact sheets. http://www.who.int/mediacentre/factsheets/en/ (accessed July 31, 2010).
———. 2010d. Neglected zoonotic diseases. http://www.who.int/neglected_diseases/diseases/zoonoses/en/index.html (accessed July 31, 2010).
———. 2010e. Neglected zoonotic diseases (NZD). http://www.who.int/neglected_diseases/zoonoses/en/ (accessed July 31, 2010).
———. 2010f. Some figures on the impact of neglected zoonotic diseases. http://www.who.int/ne-glected_diseases/diseases/zoonoses_figures/en/index.html (accessed July 31, 2010).
———. 2010g. Dracunculiasis eradication—global surveillance summary, 2009. Weekly Epidemiological Record 85(19):166–176.
Won, K. Y., D. Kruszon-Moran, P. M. Schantz, and J. L. Jones. 2008. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. American Journal of Tropical Medicine and Hygiene 79(4):552–557.
Woodley, M. A. 2009. Inbreeding depression and IQ in a study of 72 countries. Intelligence 37:268–276.
Zaman, K., D. A. Dang, J. C. Victor, S. Shin, M. Yunus, M. J. Dallas, G. Podder, D. T. Vu, T. P. Le, S. P. Luby, H. T. Le, M. L. Coia, K. Lewis, S. B. Rivers, D. A. Sack, F. Schodel, A. D. Steele, K. M. Neuzil, and M. Ciarlet. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet 376(9741):615–623.