9
Possible Opportunities in Waste Form Science and Technology
The previous chapters of this report have focused on the current state of development of waste form science and technology. The focus of this chapter is on the future: It describes some exciting trends and recent developments in materials science, processing technologies, and computational simulation and their potential applications to DOE programs, especially the Department of Energy-Office of Environmental Management’s (DOE-EM’s) cleanup program. This chapter is intended to address the last two charges of the committee’s statement of task (see Box 2.1. in Chapter 2) by providing examples of how scientific and technological advances may improve the DOE-EM cleanup program.
Advances in waste form science and technology could have important applications in other DOE programs as well. For example, the development of advanced nuclear fuel cycles by DOE’s Office of Nuclear Energy (DOE-NE) and others will require the design of new materials for recycling or immobilizing radionuclide streams that are unlike DOE’s legacy wastes, and also the development of new approaches for modeling nuclear fuels (e.g., Devanathan et al., 2010). Inert matrix fuels or new target materials, which are contemplated for use in reactors designed to “burn” transuranium elements, could be designed not only for their performance in those reactors, but also for ease of recycling and disposal (Peters and Ewing, 2007). Some of the examples provided in this chapter are potentially useful for these applications, especially for managing actinides.
Several recent workshops and studies have identified exciting new research opportunities in materials science, including the development of
improved waste forms, processing technologies, and computational capabilities. The reports include the following:
- Summary Report of the Nuclear Energy Research Initiative Workshop, April 23-25, 1998 (see the report of working group #4). Available at http://www.ne.doe.gov/pdfFiles/nerachWorkshop.pdf.
- Basic Research Needs for Advanced Nuclear Energy Systems, July 31-August 3, 2006 (see the panel #5 report on advanced waste forms). Available at http://www.er.doe.gov/bes/reports/files/ANES_rpt.pdf.
- Basic Research Needs for Geosciences: Facilitating 21st Century Energy Systems, February 21-23, 2007 (see sections related to subsurface geologic storage and modeling/simulation of geologic systems). Available at http://www.er.doe.gov/bes/reports/files/GEO_rpt.pdf.
- Basic Research Needs for Materials under Extreme Environments, June 11-13, 2007 (see section on nuclear energy). Available at http://www.er.doe.gov/bes/reports/files/MUEE_rpt.pdf.
- Global Nuclear Energy Partnership Integrated Waste Management Strategy Waste Treatment Baseline Study. GNEP-WAST-AI-RT-2007-00034. 2007 (see vol. 1 sections on processing and stabilization with different types of waste forms). Available at http://www.engconfintl.org/9arIWMS.pdf.
- Directing Matter and Energy: Five Challenges for Science and the Imagination, A Report from the Basic Energy Sciences Advisory Committee, 2007 (see chapter 7 on designing new materials). Available at http://www.er.doe.gov/bes/reports/files/GC_rpt.pdf.
- Alternative Waste Forms: Aqueous Processing (Ryan et al., 2009).
- Alternative Waste Forms for Electro-Chemical Salt Waste (Crum and Vienna, 2009).
Additionally, the recent National Research Council report Frontiers in Crystalline Matter from Discovery to Technology (NRC, 2009), which was sponsored in part by DOE, outlines an exciting agenda for the development of new materials for special applications. Although most of the examples in this report are for high-technology applications (e.g., microelectronics, superconductivity, and heterostructures), opportunities also exist for the development of new waste form materials.
The committee sees possible innovations developing from at least three directions:
- New waste form materials designed for specific performance functions (e.g., high durability in specific disposal environments; com-
-
patibility with specific waste streams) or designed to remain stable over different ranges of time, depending on the half-life of the radionuclide.
- New processing technologies that can handle complex, highly radioactive waste streams and produce more consistent waste form products.
- Advanced techniques for understanding and modeling waste form–near-field interactions.
In the sections that follow, the committee provides some examples of potential innovations in each of the three categories (i.e., materials, processing technologies, and models) mentioned above. These examples are presented to illustrate the wide variety of possibilities; they should not be viewed as inclusive or as recommendations for specific investigations. The examples are provided primarily to illustrate what might be developed by DOE-EM even with modest investments. Some of these examples are incremental in that they build on research programs that have obvious relations to the ongoing DOE-EM cleanup mission. Others are simply “outside-the-box” ideas that may warrant attention by DOE-EM.
9.1 NEW DEVELOPMENTS IN MATERIALS SCIENCE
The following examples illustrate how advances in materials science can be used to develop new and improved waste form materials for specific applications, for example, for immobilizing specific waste streams or for disposal in specific geological environments. These materials may benefit from further development or application; some have not been fully explored by the waste management community. These examples are intended to be illustrative, not comprehensive. The committee has made no effort to determine whether these materials are suitable for particular DOE-EM waste streams.
9.1.1 Amorphous Materials Designed with Short-Range and Intermediate-Range Order
There continues to be substantial progress in the characterization and understanding of the structure of glass and the interplay between glass composition, structure, and properties. This progress is the direct result of advances in materials characterization techniques, mainly spectroscopic techniques (Hawthorne, 1992; Pierce et al., 2010), including the use of synchrotron sources for X-ray scattering and X-ray absorption spectroscopy (Brown et al., 1995). A recent workshop has reviewed the possible applications of synchrotron radiation techniques to materials that contain radionuclides (ANL, 2010).
The application of advanced spectroscopy techniques has provided a greatly improved understanding of the structural properties of glasses, particularly short-range (nearest-neighbor atomic spacing) and intermediate-range (the connectivity extending across several metal-metal distances) order (Calas et al., 2010) (Figure 9.1). As a result, it now appears feasible to use amorphous network engineering to tailor glass compositions with specific atomic sites for incorporating radionuclides (e.g., Martin et al., 2002). Great progress has also been made in simulating glass structures and calculating the energetics that control glass durability (Garofalini, 2001; Poole et al., 1995). The improved knowledge of glass structure and durability should provide increased confidence in understanding glass behavior in disposal environments.
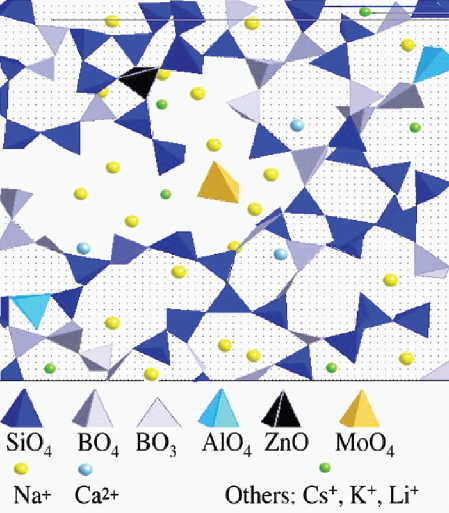
FIGURE 9.1 Schematic diagram showing the complexity of the structure of glass with both short-range (the individual coordination polyhedra) and intermediate range (extending across the different rings of polyhedra). With the increased understanding of the structure of glass, one can use the intermediate-range order for the atomic-scale incorporation of specific radionuclides.
SOURCE: Calas et al. (2010), Figure 2.
9.1.2 Glass-Ceramic Materials
Although glass may accommodate waste loadings of up to 38 weight percent, there are certain constituents (e.g., chromium, sulfate [SO4=], titanium, zirconium, phosphorus, and actinides) that have limited solubilities in certain glass compositions. Similarly, crystalline ceramics often have thin selvages of amorphous material along their grain boundaries. As noted in Chapter 3, there is a continuum of glass and crystalline phases within many materials. In fact, such glass-ceramic materials (GCMs) are probably more common than is generally appreciated because processing technologies are generally limited in their ability to provide phase-pure materials.
The multi-phase nature of GCMs makes them useful for immobilizing radioactive waste. As noted in Chapter 3, GCMs could be designed to incorporate long-lived radionuclides (e.g., actinides) into crystalline phases of greater durability and shorter-lived radionuclides (e.g., some fission products) into less durable glass phases. This approach was proposed more than 20 years ago (e.g., Hayward, 1988). However, recent advances in materials processing technologies may make it feasible to actually produce these materials at reasonable scales and costs.
There still remain a number of challenges for designing GCMs for radionuclide immobilization. The physical properties and leaching behavior of GCMs may differ from either pure glass or an assemblage of fully crystalline phases because of the coupling of processes between the phases. For example, crystalline phases that contain actinides may expand as radiation damage accumulates; this expansion may cause microcracking in the surrounding glass (Figure 9.2). The interface between the glass and crystal may be the point of maximum leach rate and radionuclide release on initial contact with water, resulting in a high instantaneous release followed by slower matrix dissolution.
Nevertheless, GCMs have already demonstrated utility as waste forms for iodine (Garino et al., in press). They can accommodate a greater range of radionuclides and achieve higher waste loadings (see Figure 3.1 in Chapter 3) than many materials now in use. They can also be produced at lower temperatures.
9.1.3 AOx Isometric Structures
Incremental changes in the composition and structure of a material can result in substantial improvements in its performance as a waste form. For example, uranium dioxide (UO2), which comprises the matrix of commercial nuclear fuels, has a simple, isometric fluorite (CaF2) structure, the same structure as many actinide oxides. During the past decade, derivative structures such as A2B2O7 pyrochlore, which contains two cation sites
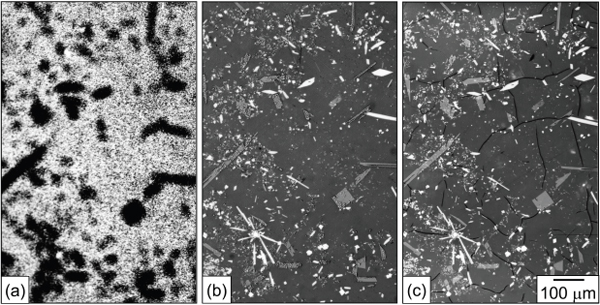
FIGURE 9.2 Effect of alpha-decay in crystalline phases on microstructure in a partially devitrified glass: (a) alpha-autoradiograph showing curium-244 concentrated in crystalline phases; (b) optical micrograph of region in (a) indicating no micro-cracks after 6 × 1015 alpha-decays/g; and (c) microcracking in same region from amorphization of crystalline phases after 2.4 × 1017 alpha decays/g.
SOURCE: (a) Courtesy of William J. Weber, University of Tennessee; (b) and (c) Weber et al. (1998), Figure 6.
(A, B) and one missing oxygen, have been examined for possible use for incorporating actinides, either as part of an inert matrix fuel or for direct disposal in a repository. A typical composition that has been investigated for this purpose is titanate pyrochlore (Gd,Pu)2(Ti,Hf)2O7. This material is very susceptible to radiation damage because of alpha-decay from the incorporated actinides (in this case plutonium), which causes the material to be transformed to an amorphous state. However, if the composition is changed by substituting zirconium for titanium to produce (Gd,Pu)2(Zr,Hf)2O7, the material has an entirely different response to radiation damage and does not become amorphous even at very high radiation doses.
This difference in behavior is illustrated schematically in Figure 9.3. The curves in the figure show the doses required to amorphize three pyrochlore materials as a function of temperature. The temperature at which thermal annealing dominates over damage accumulation—that is, the point at which the curves become vertical and a material can no longer be amorphized—is different for these three materials. By selecting a composition for which the thermal annealing occurs at low temperatures (e.g., Gd2ZrTiO7 in Figure 9.3), one can ensure that the waste form never becomes amorphous because of radiation damage.
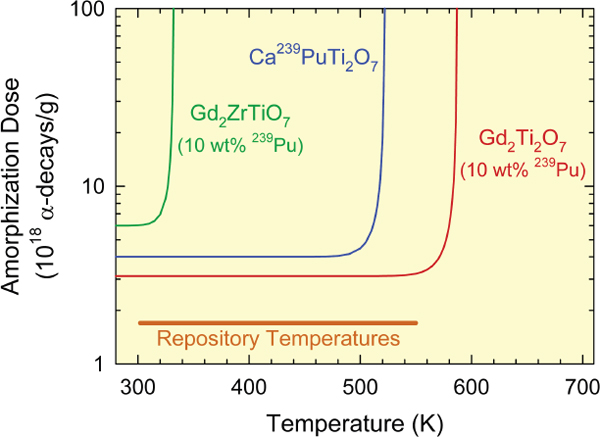
FIGURE 9.3 Predicted temperature dependence of amorphization in pyrochlore phases containing plutonium-239. The curves bend upward at elevated temperatures because of thermal annealing. Where the curves become vertical the material remains crystalline at high alpha-decay doses. The range of potential repository temperatures is indicated by the horizontal line. SOURCE: Ewing et al. (2004).
This annealing behavior could be used to advantage for disposing of minor actinides immobilized in pyrochlore waste forms. Such waste forms could be disposed of in boreholes drilled several kilometers into Earth’s crust, where temperatures are sufficiently elevated (because of the geothermal gradient) to prevent waste form amorphization (Figure 9.3).
Pyrochlore materials are potentially useful for immobilizing other radionuclides besides actinides. For example, Weck et al. (2010) recently synthesized a series of rare-earth technetate pyrochlores that can potentially be used to immobilize long-lived fission products such as technetium-99. Russian researchers are investigating new approaches for technetium immobilization (e.g., Laverov et al., 2010).
9.1.4 Complex Structure-Types
There are a number of more structurally complicated materials—for example, complex oxides, silicates, phosphates, and vanadates—that have
not been fully considered or developed as waste forms. Some of these materials were described in Chapter 3.
For example, murataite and garnet have recently received attention as potential host phases for actinides (Laverov et al., 2006, 2009a,b, 2010). Murataite-based ceramics (A6B12C4TX40-x)1 have atomic periodicities that are multiples of the basic AX2 structure of fluorite (CaF2); however, they have more complicated compositions and multiple cation sites. These sites can be used to immobilize waste streams that have complex compositions, thus eliminating the need for further chemical separation (Laverov et al., 2006, 2010; Lukinykh et al., 2008). These materials accommodate high actinide waste loadings and can be designed to remain crystalline over long periods of disposal, as was shown previously for pyrochlore (Yudintsev et al., 2007). However, phase-pure murataite is difficult to make. Usually, it is one phase in a polyphase assemblage containing (mostly) other titanates, which can help to encapsulate the actinide-bearing murataite.
Garnet (A3B2[TO4]3), which has an isometric structure with three cation sites that can accommodate actinides and lanthanides, may also be a useful waste form material. Recent work (Laverov et al., 2010) has shown that garnet leach rates and radiation response can be changed substantially by changing its composition. Crystalline phosphates of the NaZr2(PO4)3 (NZP2) family continue to be of interest mainly in the context of high-level radioactive waste (HLW) immobilization because the unique NZP structure can incorporate a variety of cations, including plutonium (Hawkins et al., 1997; Zyryanov and Vance, 1997). The NZP structure is a three-dimensional network of corner-sharing ZrO6 octahedra and PO4 tetrahedra in which plutonium can substitute for Zr, as in Na(Zr,Pu)2(PO4)3 (Orlova et al., 1994).
Apatite has a complicated, low-symmetry crystal structure: A10(BO4)6(OH, F, Cl)2, where A = calcium (Ca), sodium (Na), rare earths, fission products, and actinides; and B = silicon (S), phosphorus (P), vanadium (V), or chromium (Cr). It has been studied extensively as a host for toxic metals, and it also has great potential as an advanced waste form for complex radioactive waste streams because of its complex crystal chemistry, structural flexibility, and good chemical durability. A wide range of waste components (e.g., tri- and tetra-valent actinides, strontium, cesium) can be incorporated into the apatite structure by coupled substitutions at the cation and anion sites (Carpéna et al., 1998, 2001; Langmuir and Apted, 1992; Maddrell and Abratitis, 2004). Iodine, for example, can be incorporated in the structural channel in a lead vanadate apatite structure
________________________
1 Note: A = cations in 8-fold coordination; B = cations in 6-fold coordination; C = cations in a trigonal bipyramid (6-fold); T = cations in 4-fold coordination.
2 NZP denotes the sodium zirconium phosphate structural family.
(Pb10(VO4)6-x(PO4)xI2). The interest in this material has also resulted in the examination of new technologies, such as spark plasma sintering, for its synthesis (Campayo et al., 2009).
9.1.5 Metal-Organic Frameworks
Metal-organic frameworks (MOFs) are a relatively new class of porous materials that consist of metal atoms (ions) linked together by multifunctional organic ligands (Figure 9.4). An incredibly diverse group of MOFs have been synthesized because of the wide variations in possible linkages between the organic and inorganic components of each framework. With the diversity in framework topology and surface moities, MOFs can be constructed with extremely large surface areas and with surface adsorption molecules.
The field of “reticular” chemistry (Batten et al., 1995; Hoskins and Robson, 1990; Yaghi and Li, 1995; Yaghi et al., 2003) and the development of MOFs are less than a decade old (O’Keeffe et al., 2000). Nevertheless, MOFs have already been considered for many applications in the energy field, for example, hydrogen storage, carbon dioxide sequestration, and methane sequestration. The most recent development (Furukawa et al., 2010) has been the synthesis of three-dimensional crystal structures that have exceedingly high internal surface areas. These high surface areas could be useful for radionuclide separations. Further, pore spaces in MOFs can be engineered to specific sizes, which could also be useful for separations.
It is easy to imagine the design and use of MOFs for immobilizing iodine and technetium. In fact, there is ongoing research funded by DOE-NE on the use of MOFs as separation materials for radio-iodine (Sava et al., 2011). At Sandia National Laboratories, research is focused on existing and novel MOFs for high loadings of I2 separated from both liquid and gas streams. Published results have shown that various MOF/I2 phases (including ZIF-8 and HKUST-1 MOFs) can be successfully incorporated into low-temperature glasses to form GCMs.
9.1.6 Self-Assembled Mesoporous Materials
Mesoporous3 materials have attracted considerable attention since their discovery in the early 1990s (Beck et al., 1992; Kresgie et al., 1992). An important innovation has been to functionalize the surfaces of these materials (Anthony et al., 1993; Sayari, 1996) with self-assembled organic
________________________
3 Mesoporous materials have regularly arranged pores ranging from 2-50 nanometers in diameter. They have high surface areas (up to 1,500 square meters per gram) and uniform pore sizes and shapes.
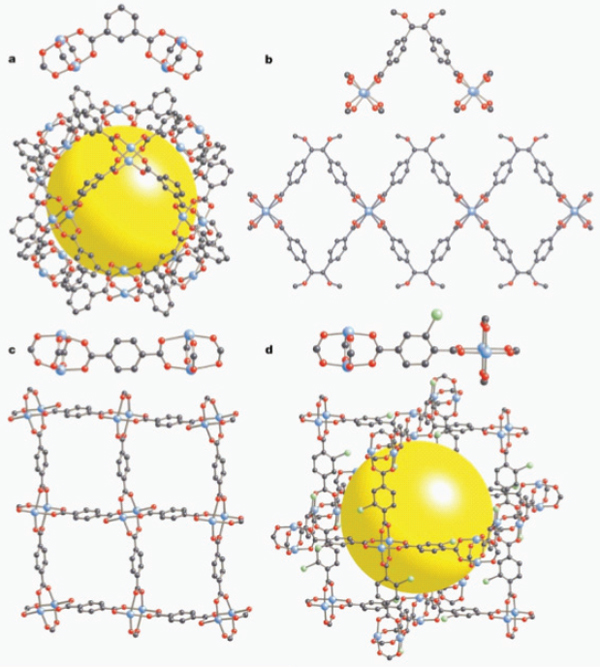
FIGURE 9.4 Framework structures are built of a linked framework of “paddle-wheel” units. The units can assume many different geometries and can accommodate a wide variety of elemental and molecular species. The small spheres are carbon, oxygen, bromine, and metal atoms. The large spheres illustrate the size of the cavities in some of the geometric arrangements.
SOURCE: Yaghi et al. (2003).
monolayers that provide a substrate with high chemical selectivity, allowing these materials to be used as chemical sensors (Kumar et al., 1994) and in chemical separation processes (Wirth et al., 1997).
Investigators at Pacific Northwest National Laboratory have extended this technology with the development of self-assembled mercaptan on meso-
porous silica (SAMMS) (Feng et al., 1997a,b) for the removal of mercury from waste water and organic wastes. A further extension of this technology would be the development of mesoporous materials that are functionalized for the separation of specific radionuclides and suitable for the synthesis of waste forms. Advantages include high radionuclide loadings, high selectivity, and the possibility of a chemically durable final product (Feng et al., 1997b).
There are many related structures that share the features of MOFs and mesoporous materials. Recently, a sulfide structure has been synthesized that is selective for cesium by incorporation into its open framework (Ding and Kanatzidis, 2010). Thus, even framework chalcogenide4 structures hold the potential for the sequestration of radionuclides.
9.1.7 Actinide Clusters and Frameworks
Nanoscale control of actinide materials is a new research field with potential applications in nuclear waste form development. Cage molecules containing from 20 to 60 uranium atoms as well as peroxide, hydroxyl, and oxygen have been reported over the past five years (Burns et al., 2005; Forbes et al., 2008; Sigmon et al., 2009a,b,c; Unruh et al., 2010). These clusters have diameters up to 3 nanometers. They are built of uranyl peroxide hydroxide hexagonal bipyramids that are linked through shared equatorial edges. This linkage results in topological squares, pentagons, hexagons, and a wide variety of cluster types. Several of these have fullerene topologies, including a cluster with 60 uranium atoms that is topologically identical to C60 buckminsterfullerene (Figure 9.5). It may be possible to tune the compositions and topologies of these structures for use as precursors in the creation of novel waste forms or nuclear fuels. Such clusters present the possibility of nanoscale control of chemical composition and properties of materials.
A new complex supertetrahedral framework has been recently discovered which consists of borate and thorium polyhedral (NDTB-1) (Wang et al., 2010). This compound has a cationic framework that contains anions within channels and cages that balance the framework charge. Borate polyhedra occur both in ordered positions within the framework and as disordered constituents of the channels. This compound has been shown to rapidly ion exchange these disordered borate groups with a variety of anionic groups, including TcO4–. This or other custom-designed materials with similar properties may be used to separate and/or sequester technetium to reduce its concentration in waste streams.
________________________
4 Materials containing sulfur, selenium, and tellurium, usually as sulfides, selenides, and tellurides.
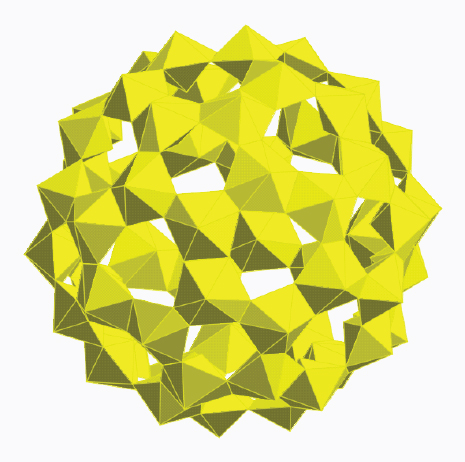
FIGURE 9.5 A cluster consisting of 60 uranium polyhedra (yellow) joined across shared edges of the polyhedra. This U60 cluster has the same topology as a C60 cluster, known as a “buckey ball.”
SOURCE: Courtesy of Peter Burns, University of Notre Dame.
9.1.8 Multi-Scale Computational Simulation of the Properties of Materials
One of the most rapidly developing research areas in materials science is the use of computational simulation to determine fundamental physical and chemical properties of materials. In the 1980s, the use of pseudopotentials to capture the behavior of chemically active electrons combined with density functional theory5 allowed the study of systems consisting of hundreds to thousands of atoms. At the same time, the rapid development of computer technology (faster processors and more efficient algorithms) led to the development of new tools for modeling the structure and properties of materials—and indeed, the new field of computational chemistry (Cygan, 2001).
There are now a wide variety of standard computational packages that are routinely used in studies of solid-state materials, including studies of
________________________
5 This theory is used in chemistry and physics to investigate the electronic structures of materials.
nuclear materials (Stan, 2009; Stan et al., 2007) and even potential waste form materials (Ferriss et al., 2010) at a number of scales (Figure 9.6). These techniques are particularly useful for the study of highly radioactive materials for which actual experiments are time consuming and expensive. Computational simulations can be used to investigate a wide range of compositions or structure types and to focus experimental efforts on the most critical, bench-marking data requirements. Computational simulations can be extended to study surface reactions and corrosion mechanisms (Rosso, 2001) and radiation effects in materials (Ewing and Weber, 2010).
9.1.9 Design of Materials for Specific Performance Requirements
With the dramatic advances being made in computational chemistry it is now becoming feasible to use computational techniques for materials discovery and design (NRC, 2009; see Figure 9.7). This innovation has
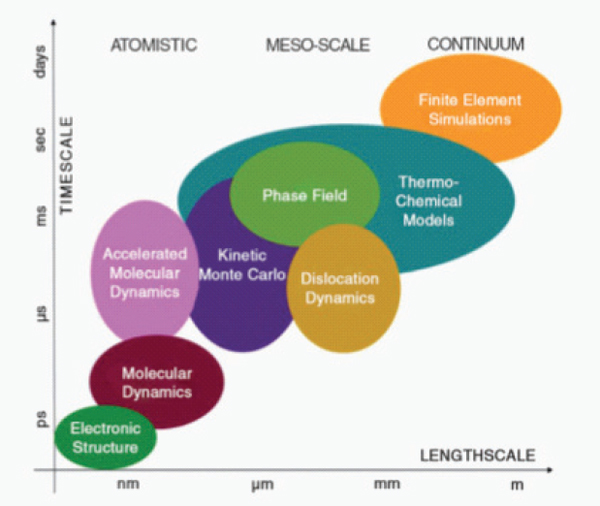
FIGURE 9.6 Multi-scale theoretical and computational methods used for materials model development and computer simulations.
SOURCE: Stan (2009).
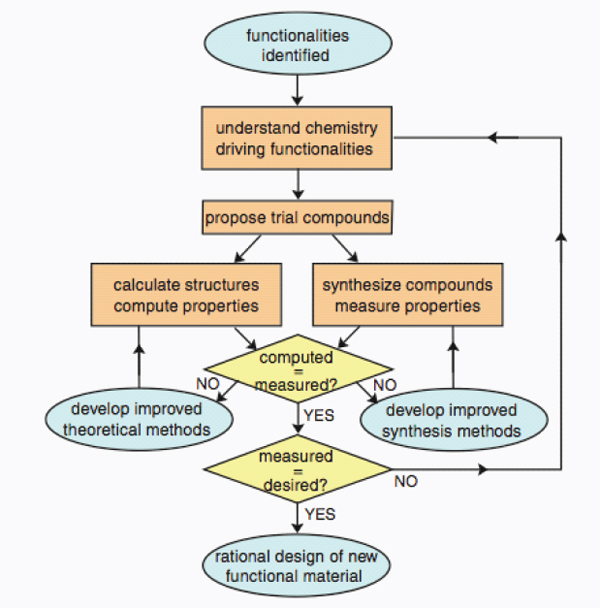
FIGURE 9.7 Schematic illustration of a methodology for the discovery and design of new materials.
SOURCE: NRC (2009).
potentially important applications to radioactive waste form development: Once a waste form’s performance requirements are established for a particular disposal environment, one can design materials to meet those requirements. As examples, glass waste form compositions might be changed to enhance chemical durability or crystalline waste form composition might be adjusted to enhance thermal annealing of radiation damage (as discussed in Section 9.1.2). Such applications are, in fact, in their infancy, but their potential is great.
Computational simulations can also be combined with experimental techniques now in routine use in chemistry and pharmacology. For exam-
ple, combinatorial chemistry methods reduce the time required to invent new catalysts and drugs. Xiang et al. (1995) has applied combinatorial chemistry techniques to the discovery of new superconducting materials. These techniques can be used in nuclear waste processing to invent new waste form formulations, increase theoretical understanding of material properties, evaluate the waste form performance, and shorten the time it takes to develop new waste processing technologies. Undoubtedly, adapting these techniques to waste form development will require additional development work.
9.2 NEW DEVELOPMENTS IN WASTE FORM PROCESSING TECHNOLOGIES
New waste form materials will be of use in the DOE-EM cleanup program only if they can be synthesized and produced at industrial scales. Fortunately, there have been many incremental improvements as well as important innovations in processing technologies that are potentially applicable to production of waste forms. Some examples are described in this section.
9.2.1 Computational Simulation of Material Processing
Recent advances in chemical engineering, materials science, and metallurgy provide the basis for development of new technologies for nuclear waste processing. Advances in computational science and recently emerging tools in Computational Fluid Dynamics (CFD) (e.g., the Ansys® suite of tools, MFIX by National Energy Technology Laboratory, and Barracuda® by CPFD Software) have led to significant incremental improvements in processing equipment and enhanced processing capabilities.
For example, such tools are currently in use to study flow patterns in Joule-heated melters equipped with gas bubblers (Matlack et al., 2008) and also for hot gas filter cleanup design (VanOsdol et al., 1996). Barracuda® and MFIX are being used to model hydrodynamic phenomena and chemical reactions in fluidized beds. This software enables engineers to develop trouble-free efficient process designs from the start; reduce the risk of scale-up from pilot to commercial plants; and avoid operational problems and downtime. These simulation tools are being used to scale-up fluidized bed gasifiers, polymerization reactors, and combustors.
These state-of-the-art computational capabilities can pave the way for the development of novel waste form processing techniques. For example, fluidized bed steam reforming is being developed for the processing of sodium-bearing waste at the Idaho National Laboratory (see Chapter 4). The operational challenges for this technology include generation of fines,
product agglomeration, and particle size control. CFD simulation tools can help to address such operational challenges, specifically to:
- Reduce attrition of bed material through the proper design and placement of internal components (e.g., reduce jets by the proper design of grids; design the internal cyclones and diplegs) and minimize carry-over.
- Optimize operating conditions through the proper choice of the bed support, which enables smoother fluidization.
- Reduce scale-up risks through the appropriate inclusion of the kinetics of particle growth combined with the bed hydrodynamics.
Once a simulation tool is built for these purposes it can also be used for troubleshooting during actual operations.
In recent years there have also been developments in tools to simulate the steady state and dynamic behavior of chemical processes. For example, the Aspen® suite of products (which were developed by Aspentech) is used widely in the chemical industry for such purposes. CFD models, when combined with such simulation software, can be a powerful way to monitor and control waste processing equipment such as fluidized beds and melters together with the entire plant associated with these processes.
9.2.2 Innovative Uses of Existing Technologies
Some existing processing technologies in use in other industries are also finding new applications for waste form production. These technologies are described in Chapter 4 and in the committee’s interim report (Appendix C). Fluidization technology, namely steam reforming, is being considered as a potential technology for the immobilization of a wide variety of high-sodium, low-activity wastes such as those existing at the Hanford Site, Idaho National Laboratory, and Savannah River Site. Cold crucible induction melting (CCIM), which is a well-established process in other industries, is being considered for immobilization of HLW and possible low activity waste in glass. As noted in Chapter 4, this technology can be used to immobilize waste streams containing chromium, aluminum, zirconium, sulfate, or phosphate, which can cause problems in Joule-heated melters. CCIM is also being integrated with an oxygen plasma to destroy organics and reduce post gas processing loads. Hot isostatic pressing (HIP), another well-established technology in other industries, is currently being considered for the production of waste forms containing calcine HLW at the Idaho National Laboratory.
9.3 NEW DEVELOPMENTS IN MODELING WASTE FORM–NEAR-FIELD INTERACTIONS
The ultimate objective of the DOE-EM cleanup program is the safe disposal of legacy waste. The evaluation of safety requires the ability to computationally simulate the behavior of radionuclides in the waste form and other engineered and geologic barriers in the near-field environment of a disposal facility. Recent advances in computational capacity and new conceptual approaches to reactive transport modeling (Steefel et al., 2005) offer new opportunities for understanding and simulating the release and mobility of radionuclides in disposal environments.
As discussed in some detail in Chapter 7, the development of high-fidelity models (Steefel et al., 2005) that realistically simulate radionuclide transport both at local and repository scales represents a conceptual advancement in simulation capabilities. Such models can be used to evaluate performance of a disposal facility not only with respect to radiological risk, but also for other performance metrics such as repository size.
9.4 DISCUSSION
As was noted in Chapter 2, the DOE-EM cleanup program is not expected to be completed for at least another four decades. Consequently, DOE-EM will have ample opportunities in the coming decades to incorporate advances in science and technology on waste forms, waste form processing technologies, and waste form–near-field modeling into its baseline approaches to increase program efficiencies, reduce lifecycle costs and risks, and advance scientific understanding and stakeholder confidence. The past 30 years have seen a steady increase in scientific and technological advances, perhaps best exemplified by the successful application of vitrification technologies to immobilize HLW. Still, these past successes do not preclude the exciting possibilities of new and innovative strategies for improving waste management systems.
The committee realizes that DOE-EM is already successfully immobilizing radioactive waste and making huge financial investments in the construction of facilities, such as the Waste Treatment Plant at the Hanford Site, which will be used to vitrify HLW. However, not every waste stream is a good match for vitrification, nor can all waste streams be accommodated in presently planned facilities. Consequently, prudence dictates that some fraction of the DOE-EM program should be devoted to capturing and using innovative science and technology. In fact, the “strategic initiatives” of the EM Engineering & Technology Roadmap (DOE, 2008) are entirely consistent with the development and use of new waste form materials,
new processing technologies, and improved modeling and computational capabilities.
- Advances in the science of materials design provide new methods for efficiently exploring innovative waste form materials. Computational simulation may provide an efficient and rapid means of surveying the properties of materials for immobilizing highly radioactive wastes without having to complete the full range of costly and time-consuming experiments. Once these computational surveys are completed, key experiments can be performed to confirm (or not) the results.
- Advances in materials processing can lead to improvements in waste form production rates and product quality at reduced production costs.
- Advances in computational capabilities, combined with new conceptual models for materials performance in disposal environments, can provide new insights into long-term materials performance in specific disposal environments.
In the development of new materials, technologies, and computational capabilities, the next 50 years is an eternity, and the prospects for innovative improvements are huge. The incorporation of new science and technology into the DOE-EM cleanup program need not interrupt the significant progress that is being made at present but can, if done well, enhance its prospects for future successes.
It can take decades to develop and introduce new technologies for processing radioactive waste. For example, it took about two decades from the decision to use borosilicate glass for HLW immobilization at Savannah River to the first “hot” (radioactive) operations at the Defense Waste Processing Facility. Some waste forms materials (e.g., ceramics) have been studied for almost 30 years but have not yet found widespread implementation for radioactive waste immobilization. It is imperative for DOE-EM to get this development of new processing technologies started in earnest to reap its benefits for the cleanup program in a timely fashion.
REFERENCES
ANL [Argonne National Laboratory]. 2010. Report of the Workshop on the Role of Synchrotron Radiation in Solving Scientific Challenges in Advanced Nuclear Energy Systems, Advanced Photon Source, Argonne, Ill. (April).
Anthony, R. G., C. V. Phillip, and R. G. Dosch. 1993. “Selective Adsorption and Ion Exchange of Metal Cations and Anions with Silico-titanates and Layered Titanates,” Waste Manage. 13, 503-512.
Batten, S. R., B. F. Hoskins, and R. Robson. 1995. “2 Interpenetrating 3D Networks Which Generate Spacious Sealed-Off Compartments Enclosing of the Order of 20 Solvent Molecules in the Structures of Zn(CN)(NO3)(Tpt)(2/3)Center-Dot-Solv (Tpt=2,4,6-Tri(4-Pyridyl)-1,3,5-Triazine, Solv=similar-to-3/4C(2)H(2)Cl(4)Center-Dot-3/4Ch(3)Oh or Similar-to-3/2Chcl(3)Center-Dot-1/3Ch(3)Oh),” Am. Chem. Soc. 117(19), 5385-5386.
Beck J. S., J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T-W Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins, and J. L. Schlenker. 1992. “A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates,” J. Amer. Chem. Soc. 114(27), 10834-10843.
Brown, G. E., F. Farges, Jr., and G. Calas. 1995. “X-ray Scattering and X-ray Spectroscopy Studies of Silicate Melts,” In Structure, Dynamics and Properties of Silicate Melts, J. F. Stebbins, P. F. McMillan and D. B. Dingwell (Eds.), Rev. Mineral. 32, 317-410.
Burns, P. C., K.-A. H. Kubatko, G. Sigmon, B. J. Fryer, J. Gagnon, M. R. Antonio, and L. Soderholm. 2005. “Actinyl Peroxide Nanospheres,” Angew. Chem. Int. Ed. 44(14), 2135-2139.
Calas, G., L. Galoisy, L. Cormier, J. M. Delaye, P. Jollivet, S. Peuget. 2010. “Structural Evolution of Nuclear Glasses Under Forcing Conditions (Irradiation, Alteration),” Mater. Res. Soc. Symp. Proc. 1265, 77-88.
Campayo, S., L. Gallet, Y. Grin, E. Courtois, F. Bernard, and F. Bart. 2009. “Spark Plasma Sintering of Lead Phosphovanadate P3(VO4)1.6(PO4)0.4,” J. European Ceram. Soc. 29, 1477-1484.
Carpéna, J., F. Audubert, D. Bernache, L. Boyer, B. Donazzon, J. LaCout, and N. Senamaud. 1998. “Apatitic Waste Forms: Process Review,” Mater. Res. Soc. Symp. Proc. 506, 543-549.
Carpéna, J., B. Donazzon, E. Céraulo, S. Prené. 2001. “Elaboration de Ciments Apatitiques Composites pour la Rétention du Césium et de l’iode,” Comptes Rendus de l Academie des Sciences-Series IIC-Chemistry 4, 301-308.
Crum, J. V. and J. D. Vienna. 2009. “Glasses for Immobilizing Lanthanide, Alkali, and Alkali-Earth Fission Products,” In Environmental Issues and Waste Management Technologies in the Materials and Nuclear Industries XII, A. Cozzi and T. Ohji (Eds.), Ceram. Trans. 207, 3-8.
Cygan, R. T. 2001. “Molecular Modeling in Mineralogy and Geochemistry,” In Reviews in Mineralogy and Geochemistry, R. T. Cygan and J. D. Kubicki (Eds.), 42, 1-36.
Devanathan, R., L. V. Brutzel, A. Chartier, C. Gueneau, A. E. Mattsson, V. Tikare, T. Bartel, T. Besmann, M. Stane, and P. Van Uffelenf. 2010. “Modeling and Simulation of Nuclear Fuel Materials,” Energy Environ. Sci. 3, 1406-1426.
Ding, N. and M. G. Kanatzidis. 2010. “Selective Incarceration of Caesium Ions by Venus Flytrap Action of a Flexible Framework Sulfide,” Nature Chemistry 2, 187-191.
DOE [U.S. Department of Energy]. 2008. Engineering and Technology Roadmap: Reducing Technical Risk and Uncertainty in the EM Program, Office of Environmental Management, Washington, D.C., Available at http://www.em.doe.gov/pdfs/FINAL%20ET%20Roadmap%20_3-5-08_.pdf.
Ewing, R. C., W. J. Weber, and J. Lian. 2004. “Pyrochlore (A2B2O7): A Nuclear Waste Form for the Immobilization of Plutonium and “Minor” Actinides” (Invited Focus Review), J. Appl. Phys. 95, 5949-5971.
Ewing, R. C. and W. J. Weber. 2010. “Chapter 35: Actinide Waste Forms and Radiation Effects,” In The Chemistry of the Actinides and Transactinide Elements 6, L. R. Morss, N. M. Edelstein and J. Fuger (Eds.), Springer, New York, 3813-3888.
Feng, X., G. E. Fryxell, L. Q. Wang, A.Y. Kim, J. Liu, and K. M. Kemner. 1997a. “Functionalized Monolayers on Ordered Mesoporous Supports,” Science 276, 923-926.
Feng, X., J. Liu, G. E. Fryxell, M. Gong, L-Q. Wang, X. Chen, D. E. Kurath, C. S. Ghormley, K. T. Klasson, and K. M. Kemner. 1997b. Self-Assembled Mercaptan on Mesoporous Silica (SAMMS) Technology for Mercury Removal and Stabilization, PNNL-11691, UC-2030, Pacific Northwest National Laboratory, Richland, Wash.
Ferriss, E. D. A., R. C. Ewing, and U. Becker. 2010. “Simulation of Thermodynamic Mixing Properties of Actinide-Containing Zircon Solid Solutions,” Amer. Mineral. 95, 229-241.
Forbes, T. Z., J. G. McAlpin, R. Murphy, and P.C. Burns. 2008. “Metal-Oxygen Isopolyhedra Assembled into Fullerene Topologies,” Angew. Chem.-Int. Ed. 47(15), 2824-2827.
Furukawa, H., N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O’Keeffe, J. Kim, and O. M. Yaghi. 2010. “Ultrahigh Porosity in Metal-Organic Frameworks,” Science 329, 424-428.
Garino, T. J., T. M. Nenoff, J. L. Krumhansl, D. Rademacher. In Press. “Recent Developments of Waste Forms for Radioactive Iodine,” Ceram. Trans.
Garofalini, S. H. 2001. “Molecular Dynamics Simulations of Silicate Glasses and Glass Surfaces,” In Molecular Modeling Theory: Applications in the Geosciences, R. T. Cygan and J. D. Kubicki (Eds.), Rev. Mineral. Geochem. 42, 131-168.
Hawkins, H. T., B. E. Scheetz, and G. D. Guthrie Jr. 1997. “Preparation of Monophasic (NZP) Radiophases: Potential Host Matrices for the Immobilization of Reprocessed Commercial High-Level Wastes,” Scientific Basis for Nuclear Waste Management XX, W. J. Gray and I. R. Triay (Eds.), 387-394.
Hawthorne, F. C. 1992. “Structure of Glasses of Geological Interest: Applying Spectroscopic Techniques,” Mater. Res. Soc. Bull. 17(5), 53-59.
Hayward, P. J. 1988. “Glass-Ceramics,” In Radioactive Waste Forms for the Future, W. Lutze and R. C. Ewing (Eds.), North Holland, Amsterdam.
Hoskins, B. F. and R. Robson. 1990. “Design and Construction of a New Class of Scaffolding-like Materials Comprising Infinite Polymeric Frameworks of 3D-linked Molecular Rods. A Reappraisal of the Zinc Cyanide and Cadmium Cyanide Structures and the Synthesis and Structure of the Diamond-related Frameworks [N(CH3)4] [CuIZnII(CN)4] and CuI[4,4’,4’’,4’’’-tetracyanotetraphenylmethane]BF4.xC6H5NO2,” Am. Chem. Soc. 112, 1546-1554.
Kresgie, C. T., M. E. Leonowicz, W. J. Roth, J. C. Vartuli, and J. S. Beck. 1992. “Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-crystal Template Mechanism,” Nature 359, 710-712.
Kumar, A., H. A. Biebuyck, and G. M. Whitesides. 1994. “Patterning Self -Assembled Monolayers: Applications in Materials Science,” Langmuir, 10, 1498-1511.
Langmuir, D. and M. Apted. 1992. “Backfill Modification Using Geochemical Principles to Optimize High-Level Nuclear Waste Isolation in a Geological Repository,” Mater. Res. Soc. Symp. Proc. 257, 13-24.
Laverov, N. P., S. V. Yudintsev, S. V. Stefanovsky, B. I. Omel’yanenko, and B. S. Nikonov. 2006. “Murataite as a Universal Matrix for Immobilization of Actinides,” Geol. Ore Depos. 48, 335-356.
Laverov, N. P., S. V. Yudintsev, and B. I. Omel’yanenko. 2009a. “Isolation of Long-lived Technetium-99 in Confinement Matrices,” Geol. Ore Depos. 51, 259-274.
Laverov, N. P., S. V. Yudintsev, E. E. Konovalov, T. O. Mishevets, B. S. Nikonov, and B. I. Omel’yanenko. 2009b. “Matrix for Immobilization of Radioactive Technetium,” Doklady Chem. 431(1), 71-75.
Laverov, N. P., S. V. Yudintsev, T. S. Livshits, S. V. Stefanovsky, A. N. Lukinykh, and R. C. Ewing. 2010. “Synthetic Minerals with the Pyrochlore and Garnet Structures for Immobilization of Actinide-Containing Wastes,” Geochem. Int. 48(1), 1-14.
Lukinykh, A. N., S. V. Tomilin, A. A. Lizin, S. V. Yudintsev, and S. V. Stefanovskii. 2008. “Radiation and Chemical Stability of a Polyphase Crystalline Matrix Based on Synthetic Murataite for Incoporation of Actinide Wastes,” Radiochem. 50, 541-546.
Maddrell, E. R. and P. K. Abraitis. 2004. “A Comparison of Wasteforms and Processes for the Immobilisation of Iodine-129,” In Scientific Basis for Nuclear Waste Management XXVII, Mat. Res. Soc. Symp. Proc. 807, 261-266.
Martin, J. D., S. J. Goettler, N. Fossé, and L. Ilton. 2002. “Designing Intermediate-Range Order in Amorphous Materials,” Nature 419, 381-384.
Matlack, K. S., H. Gan, M. Chaudhuri, W. Kot, W. Gong, T. Bardakci, I. L. Pegg and I. Joseph. 2008. Melt Rate Enhancement for High Aluminum HLW Glass Formulation: Final Report 08R1360-1, ORP-44236, Rev. 0, U.S. Department of Energy, Office of River Protection, Richland, Wash.
NRC [National Research Council]. 2009. Frontiers in Crystalline Material from Discovery to Technology, National Academies Press, Washington, D.C.
O’Keeffe, M., M. Eddaoudi, H. Li, T. Reineke, and O. M. Yaghi. 2000. “Frameworks for Extended Solids: Geometrical Design Principles,” J. Solid State Chem. 152, 3-20.
Orlova, A. I., Y. F. Volkov, R. F. Melkaya, L. Y. Masterova, I. A. Kulikov, and V. A. Alferov. 1994. “Synthesis and Radiation Stability of NZP Phosphates Containing F-elements,” Radiochem. 36(4), 322-325.
Peters, M. T. and R. C. Ewing. 2007. “A Science-based Approach to Understanding Waste Form Durability in Open and Closed Nuclear Fuel Cycles,” J. Nucl. Mat. 362, 395-401.
Pierce, E. M., L. R. Reed, W. J. Shaw, B. P. McGrail, J. P. Icenhower, C. F. Windisch, E. A. Cordova, and J. Broady. 2010. “Experimental Determination of the Effect of the Ratio of B/Al on Glass Dissolution along the Nepheline (NaAlSiO4)–Malinkoite (NaBSiO4) Join.” Geochim. Cosmochim. Acta 74, 2634-2654.
Poole, P. H., P. F. McMillan, and G. H. Wolf. 1995. “Computer Simulations of Silicate Melts,” In Structure, Dynamics and Properties of Silicate Melts, J. F. Stebbins, P. F. McMillan, and D. B. Dingwell (Eds.), Rev. Mineral. 32, 563-616.
Rosso, K. M. 2001. “Structure and Reactivity of Semiconducting Mineral Surfaces: Convergence of Molecular Modeling and Experiment,” In Reviews in Mineralogy and Geochemistry 42, R. T. Cygan and J. D. Kubicki (Eds.), 199-272.
Ryan, J. V., E. C. Buck, J. Chun, J. V. Crum, B. J. Riley, D. M. Strachan, S. K. Sundaram, L. A. Turo, and J. D. Vienna. 2009. Alternate Waste Forms: Aqueous Processing. AFCIWAST-PMO-MI-DV-2009-000360, Pacific Northwest National Laboratory, Richland, Wash. (September).
Sava, D., T. J. Garino, and T. M. Nenoff. 2011. “Iodine Confinement into Metal-Organic Frameworks (MOFs): Low Temperature Sintering Glasses to Form Novel Glass Composite Material (GCM) Alternative Waste Forms,” Ind. Eng. Chem. Res. DOI: 10.1021/ie200248g.
Sayari, A. 1996. “Catalysis by Crystalline Mesoporous Molecular Sieves,” Chem. Mater. 8(8), 1840-1852.
Sigmon, G. E., J. Ling, D. K. Unruh, L. Moore-Shay, M. Ward, M., B. Weaver, and P. C. Burns. 2009a. “Uranyl-Peroxide Interactions Favor Nano-cluster Self-assembly,” J. Amer. Chem. Soc. 131, 16648-16649.
Sigmon, G. E., D. K. Unruh, J. Ling, B. Weaver, M. Ward, L. Pressprich, A. Simonetti, and P. C. Burns. 2009b. “Symmetry Versus Minimal Pentagonal Adjacencies in Uranium-Based Polyoxometalate Fullerene Topologies,” Angew. Chem.-Int. Ed. 48(15), 2737-2740.
Sigmon, G. E., B. Weaver, K. A. Kubatko, and P. C. Burns. 2009c. “Crown and Bowl-shaped Clusters of Uranyl Polyhedra, Inorg. Chem. 48, 10907-10909.
Stan, M. 2009. “Discovery and Design of Nuclear Fuels,” Materials Today 12, 20-28.
Stan, M., J. C. Ramirez, P. Cristea, S. Y. Hu, C. Deo, B. P. Uberuaga, S. Srivilliputhur, S. P. Rudin, and J. M. Wills. 2007. “Models and Simulations of Nuclear Fuel Materials Properties,” J. Alloys and Comp. 444-445, 415-423.
Steefel, C. I., D. J. DePaolo, P. C. Lichtner. 2005. “Reactive Transport Modeling: An Essential Tool and a New Research Approach for the Earth Sciences,” Earth Planet. Sci. Lett. 240, 539-558.
Unruh, D.K., A. Burtner, L. Pressprich, G. E. Sigmon, and P. C. Burns. 2010. “Uranyl Peroxide Closed Clusters Containing Topological Squares,” Dalton Trans. 39, 5807-5814.
VanOsdol, J. G., R. A. Dennis, and F. D. Shaffer. 1996. A New Hot Gas Cleanup Filter Design Methodology, DOE/METC/C-97/7269, Advanced Coal-Fired Power Systems ‘96 Review Meeting (July), Available at http://www.osti.gov/bridge/servlets/purl/431655-PFJBMH/webviewable/431655.pdf.
Wang S. A., E. V. Alekseev, D. W. Juan et al., 2010, “A Supertetrahedral Cationic Framework That Removes TcO4- from Solution,” Angew. Chem. Int. Ed. 49(6), 1057-1060.
Weber, W. J., R. C. Ewing, C. R. A. Catlow, T. Diaz de la Rubia, L. W. Hobbs, C. Kinoshita, Hj. Matzke, A. T. Motta, M. Nastasi, E. K. H. Salje, E. R. Vance, and S. J. Zinkle. 1998. “Radiation Effects in Crystalline Ceramics for the Immobilization of High-Level Nuclear Waste and Plutonium,” J. Mat. Res. 13(6), 1434-1484.
Weber, W. J. and F. P. Roberts. 1983. “A Review of Radiation Effects in Solid Nuclear Waste Forms,” Nucl. Tech. 60(2), 178-198.
Weck, P. F., E. Kim, F. Poineau, E. E. Rodriguez, A. P. Sattelberger, and K. R. Czerwinski. 2010. “Structural and Electronic Trends in Rare-earth Technetate Pyrochlores,” Dalton Trans. 39, 7207-7210.
Wirth, M. J., R. W. Peter Fairbank, and H. O. Fatunmbi. 1997. “Mixed Self-assembled Monolayers in Chemical Separations,” Science 275, 44-47.
Xiang, X. D., X. Sun, G. Briceño, Y. Lou, K. A. Wang, H. Chang, W. G. Wallace-Freedman, S. W. Chen and P. G. Schultz. 1995. “A Combinatorial Approach to Materials Discovery,” Science 23(268), 1738-1740.
Yaghi, O. M. and H. Li. 1995. “Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels” Amer. Chem. Soc. 117, 10401-10402.
Yaghi, O. M., M. O’Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi, and J. Kim. 2003. “Reticular Synthesis and the Design of New Materials,” Nature 423, 705-714.
Yudintsev, S. V., S. V. Stefanovsky, and R. C. Ewing. 2007. “Actinide Host Phases as Radioactive Waste Forms,” In Structural Chemistry of Inorganic Actinide Compounds, S. V. Krivovichev, P. C. Burns, and I. G. Tananaev (Eds.), Elsevier, 457-490.
Zyryanov, V. N. and E. R. Vance. 1997. “Comparison of Sodium Zirconium Phosphate-Structured HLW forms and Synroc for High-Level Nuclear Waste Immobilization,” In Scientific Basis for Nuclear Waste Management XX, W. J. Gray and I. R. Triay (Eds.), Materials Research Society, Pittsburgh, Penn., 409-416.






















