The final charge of the statement of task for this study (see Box 2.1 in Chapter 2) calls for the identification and description of “potential new waste forms that may offer enhanced performance or lead to more efficient production.” This chapter, which is written primarily for technical audiences, addresses this charge by providing a brief review of waste form materials and an assessment of their potential applicability to Department of Energy, Office of Environmental Management (DOE-EM) waste streams. Possible applications are also highlighted in Chapter 1.
A voluminous technical literature on waste form materials has developed over the past six decades. A comprehensive review of this literature is well beyond the scope of this study. However, the committee has included key historical and review article references in this chapter for interested readers.
3.1 WASTE FORM DEVELOPMENT
The concept of immobilizing radioactive waste in either vitreous or crystalline materials is more than 50 years old. In 1953, Hatch (1953) of Brookhaven National Laboratory introduced the concept of immobilizing radioactive elements in an assemblage of mineral phases. The first borosilicate glass formulations were developed in the United States between 1956 and 1957 by Goldman and others at the Massachusetts Institute of Technology (Eliassen and Goldman, 1959; Goldman et al., 1958; Mawson, 1965). These researchers examined calcium-aluminosilicate porcelain glazes
to which boron oxide (B2O3) had been added to achieve a pourable glass and minimize radionuclide volatilization. The most promising vitreous systems for future development were determined to be borosilicate based, e.g., CaO-Al2O3-B2O3-SiO2 and Na2O-CaO-Al2O3-B2O3-SiO2.
In 1970, the singular requirement for a waste form from the Office of Nuclear Waste Isolation1 (ONWI) was that it be a stable solid (DOE, 1981; Walton et al., 1983). By the mid-1970s, innovative proposals for producing stable solid waste forms were being offered—for example, supercalcine ceramics by Rustum Roy and colleagues at Pennsylvania State University (McCarthy, 1977; Roy, 1975, 1977, 1979); alumina-based tailored ceramics by Rockwell International Science Center (Jantzen et al., 1982b; Morgan et al., 1981); and titania-based SYNthetic ROCk (SYNROC) by Ted Ringwood and colleagues at the Australian National University and the Australian Nuclear Science and Technology Organisation (Reeve et al., 1984; Ringwood, 1978, 1985; Ringwood et al., 1978). The first systematic compilations of potential crystalline waste form phases were also made at this time (Haaker and Ewing, 1981).
There were extensive research and development (R&D) programs on nuclear waste forms during the late 1970s and early 1980s, resulting in the examination of a wide variety of single-phase and polyphase ceramics. By this time “low leachability” had become the main criterion for waste form comparisons (DOE, 1981; Walton et al., 1983), and such comparisons between crystalline ceramics and glass generated considerable controversy (Kerr, 1979a,b).
Beginning in 1978, there was intense study of alternative waste forms that culminated in a review (Garmon, 1981) that recommended borosilicate glass for immobilizing high-level radioactive waste (HLW) at the Savannah River Site (SRS) in South Carolina and West Valley in New York and also identified SYNROC/tailored ceramics as promising alternatives (Hench et al., 1981). Glass was considered to be a more proven technology, and there were questions about the maturity of production technologies for ceramic waste forms. Nevertheless, Hench et al. (1981) made a strong recommendation for continued research and development for ceramic waste forms, including SYNROC and titanate- and alumina-based ceramics. These alternative waste forms were later determined to be difficult to process, more costly to implement, and not as flexible for accommodating variations in waste composition as borosilicate glass (De et al., 1976; Dunson et al., 1982; Lutze et al., 1979; McCarthy, 1973; McCarthy and Davidson, 1975; Morgan et al., 1981; Ringwood et al., 1981; Schoebel, 1975), even though
________________________
1 The Office of Nuclear Waste Isolation was located at the Battelle Memorial Institute. It conducted research and published technical reports on technical aspects of nuclear waste isolation.
many were found to have superior product quality (Hench et al., 1981; Walton et al., 1983).
High-temperature processing of these alternative waste forms frequently resulted in the formation of an intergranular glass phase, especially when alkali-containing wastes were processed. This intergranular glass limited product stability and durability because radionuclides such as cesium-137 and strontium-90, which were frequently incorporated into the intergranular glass phases (Buykx et al., 1988; Clarke, 1981; Cooper et al., 1986; Zhang and Carter, 2010), were determined to leach at the same rates as those from glass waste forms (Jantzen et al., 1982a). Because little was understood at the time about the degradation mechanism of a single-phase glass versus glass-ceramic materials (i.e., materials that contain both glass and crystalline phases), borosilicate glasses were selected for continued development over the alternative waste forms (Walton et al., 1983).
Research activity on alternative waste forms was severely curtailed as a result of the 1981 decision in the United States to immobilize defense HLW in borosilicate glass and the subsequent construction of the Defense Waste Processing Facility (DWPF) at SRS and the West Valley Demonstration Project (WVDP) at West Valley. The R&D effort on nuclear waste forms during this period has been summarized by Lutze and Ewing (1988).
More recently, there has been a resurgence of interest in crystalline waste forms because of the need to develop durable materials for the stabilization and disposal of actinides such as plutonium from defense and civilian programs (Burakov et al., 2010; Ewing, 1999; Ewing et al., 1995b; Oversby et al., 1997). There has been additional R&D work on minerals and their analogues (e.g., apatite, monazite, zirconolite, zircon, and pyrochlore) (Ewing et al., 1995a) and SYNROC formulations (Ryerson and Ebbinghaus, 2000) as well as another down selection between glass and ceramic waste forms (Meyers et al., 1998).
Crystalline waste forms made from clay have been studied almost continuously since 1953 (Hatch, 1953; Lutze et al., 1979). Roy (1981) proposed low-temperature, hydrothermally processed, low-solubility phase assemblages consisting of mineral analogues of mica, apatite, pollucite, sodalite-cancrinite, and nepheline, many of which could be made from reactions between clays (kaolin, bentonite, and illite) and waste. Mineral analogue waste forms made from clays have been recently re-examined for the immobilization of high-sodium, salt supernate HLW at the Hanford Site in Washington; high-sodium recycle streams from tank cleaning at the Idaho National Laboratory (INL), and low-activity waste melter off-gas condensates at Hanford. These mineral analogue waste forms are made using a moderate-temperature (700°C-750°C) thermal pyrolysis treatment (Mason et al., 1999, 2003) (i.e., steam reforming; see Chapter 4) by adding clay to the waste to form feldspathoid mineral analogues (sodalite and
nepheline) or dehyroxylated mica (Jantzen et al., 2008), depending on clay composition.
Stabilization and solidification with cement-based binders has been used to immobilize radioactive wastes since the beginning of the nuclear age. The process has been used to encapsulate solid waste, solidify liquid waste (including tritiated water), stabilize contaminated soils, stabilize tank-heel residues after tanks are emptied, and as low-permeability barriers. Cements have also been used as binders and to encapsulate granular or cracked waste forms. A recent comprehensive review of cement systems for radioactive waste disposal can be found in Pabalan et al. (2009). Long-term cement durability comparisons have been made using ancient cements, geopolymers, and mortars (Jiang and Roy, 1994; Kovach and Murphy, 1995; Krupka and Serene, 1998; Miller et al., 1994; Roy and Langton, 1983, 1984, 1989; Steadman, 1986), some of which may also serve as natural analogues for geopolymer waste forms (Barsoum et al., 2006, but see also Jana, 2007).
Recent reviews of developments in waste form research are provided in the following papers: Caurant et al. (2009); Donald et al. (1997); Ewing (1999, 2001); Ewing et al. (2004); Lee et al. (2006); Lumpkin (2001, 2006); Lutze and Ewing (1988); Ojovan and Lee (2005, 2007); Stefanovsky et al. (2004); Weber et al. (2009); and Yudintsev et al. (2007). The most recent interest has been associated with the desire to create new waste forms as part of advanced nuclear fuel cycles involving recycling of irradiated fuel (Peters and Ewing, 2007). Recent reviews of radiation effects in waste forms can be found in a series of papers by Ewing and others (1995b); Ewing and Weber (2010); and Weber and others (1997, 1998). Reviews of natural analogues that provide long-term data on the durability of glass and crystalline ceramics have been provided in a number of papers, including Allen (1982); Ewing (1979, 1999); Haaker and Ewing (1981); Jantzen and Plodinec (1984); Malow et al. (1984); Morgenstein and Shettel (1993); and Verney-Carron et al. (2010).
3.2 ROLE OF WASTE FORM IN WASTE IMMOBILIZATION
As noted in Chapter 2, the primary role of a waste form is to immobilize radioactive and/or hazardous constituents (hereafter simply referred to as constituents) in stable, solid matrices for storage and eventual disposal. Immobilization can occur through chemical incorporation, encapsulation, or a combination of both processes. Table 3.1 provides a pictorial representation of the different combinations of chemical incorporation and encapsulation for the waste form materials described in Section 3.3.
Encapsulation is achieved by physically surrounding and isolating constituents in a matrix material, which traps waste ions on grain boundaries and in some cases sequesters constituents in hydrated products. Cements,
TABLE 3.1 Key Properties of the Waste Form Materials Described in this Chapter
| Waste Form Class (see text) | Retention Mechanism | Graphical Representation | Waste Form Properties | Examples |
| 1. Single-Phase Glasses Constituents are atomically bonded in the glass structure, usually to oxygen that is also bonded to other matrix elements (e.g., Si, Al, B, P) by short-range order (SRO) and medium-range order (MRO). |
Chemical incorporation |  |
a. Moderate waste loading b. Good overall durability c. Easy to model constituent release from a single phase | Borosilicate glasses, aluminosilicate glasses, phosphate glasses |
2a. Glass-Ceramic Material Constituents are present in the glass matrix, and benign crystals such as spinels (Cr, Ni, and Fe species) ( |
Chemical incorporation |
 |
a. Higher waste loadings for high Cr, Ni, and Fe wastes b. Good overall durability c. Easy to model constituent release from single-phase glass because there is minimal impact from grain boundary dissolution (has to be determined by experimentation) |
Higher waste-loaded borosilicate, alumino-silicate, or phosphate glasses |
| Waste Form Class (see text) | Retention Mechanism | Graphical Representation | Waste Form Properties | Examples |
| 2b. Glass-Ceramic Material Constituents are present in the glass matrix and in the crystalline phases. Example shows Cs in the glass and in a secondary phase ( |
Chemical incorporation and encapsulation |  |
a. Higher waste loadings if soluble constituent phases are not formed b. Overall durability may be greater or less than homogeneous glass c. More complex to model constituent release from multiple phases (glass and crystal) and grain boundaries (has to be determined by experimentation) | Glass-bonded sodalite |
3a. Crystalline Ceramics: Single Phase 4a. Metals: Single Phase Consists of only one main crystalline phase which contains the same radionuclide(s). May be granular or monolithic. |
Chemical incorporation |
 |
a. High waste loading for single constituents b. Good overall durability c. Easy to model constituent release from a single phase |
Pyrochlores for single actinide stabilization, zeolites for single radionuclide stabilization |
| 3b. Crystalline Ceramics: Multi-Phase 4b. Metals: Multi-Phase Individual phases contain one or multiple constituents (e.g., solid solution indicated between UO2 and ThO2). Some phases do not incorporate any constituents (gray shading). May be granular or monolithic. |
Chemical incorporation |  |
a. High waste loading b. Superior overall durability c. Difficult to model constituent release from multiple phases d. Need to tailor for and know/determine radionuclide partitioning amongst phases e. May require precalcining for waste form processing to work efficiently | SYNROC, tailored ceramics, Pu ceramics, supercalcines, minerals for actinide stabilization |
3a, 3b. Granular Crystalline Ceramic 4a, 4b. Metal Composites Granular waste forms must be monolithed for disposal if not containerized. The monolithing agent does not incorporate constituents (gray shading). Also known as composite waste forms. |
Chemical incorporation and encapsulation |
 |
a. High waste loadings only if binder (monolithing agent) is minimized b. Superior overall durability-double containment c. Difficult to model constituent release from multiple phases d. Need to tailor for and know/determine radionuclide partitioning amongst phases e. May require precalcining for waste for processing to work efficiently |
Fluidized bed seam reforming for Hanford low-activity waste or Waste Treatment Plant secondary wastes in geopolymer matrices |
| Waste Form Class (see text) | Retention Mechanism | Graphical Representation | Waste Form Properties | Examples |
| 5, 6, 7, 8. Cements, Geopolymers, Hydroceramics, Ceramicretes Hydrated phases weakly incorporate constituents or retain them by sorption. Encapsulation is by solidification or precipitation of constituents on grain boundaries where non-constituent phases hydrate or crystallize. Example shows Tc sequestered by C-S-H hydrates and sequestered by secondary fly-ash granules. |
Encapsulation |  |
a. Low waste loading b. Lower overall durability c. Difficult to model constituent release from multiple phases and hydrated secondary phases d. Easy to process—usually mix and set | Savannah River Site Saltstone |
Key:
Pu Tc U Cs
geopolymers, ceramicrete, and hydroceramics (see Section 3.3) can be used as waste forms or as binders for other waste form materials. Encapsulation is typically used to immobilize low-level or intermediate-level wastes.
Chemical incorporation involves the atomic-scale bonding of radioactive constituents into radiophases of the waste form material, which are durable structures with any combination of short-range order (SRO),2 medium-range order (MRO),3 or long- range order (LRO).4 Glasses incorporate constituents into their atomic structures by SRO and MRO. Recent experimentation has shown the existence of large, cation-rich clusters in glass. These more highly ordered regions of MRO often have atomic arrangements that approach those of crystals (Box 3.1). Crystalline ceramics incorporate constituents by a combination of SRO, MRO, and LRO. LRO defines the periodic structural units characteristic of crystalline ceramics.
There are two approaches for immobilizing radioactive waste in crystalline materials (Roy, 1975, 1977):
- Radionuclides can be incorporated into the atomic structure of the phase. Individual radionuclides occupy specific sites in the structure, generally according to atomic size and charge constraints. For complex waste streams, crystalline structures with multiple cation sites are required to accommodate different radionuclides.
- The radionuclide-bearing radiophases can be encapsulated in another non-radionuclide bearing material to form a composite waste form.5 Encapsulating materials, such as TiO2 or ZrO2, can have high durability.
3.3 WASTE FORM MATERIALS
A wide range of materials are potentially suitable for immobilizing radioactive waste. For simplicity of discussion, these waste form materials have been grouped into eight classes based on their phase properties:
- Single-phase (homogeneous) glasses
- Glass-ceramic materials
________________________
2 SRO: radius of influence ~1.6Å-3Å around a central atom, e.g., such as tetrahedral and octahedral structural units.
3 MRO: radius of influence ~3Å-6Å encompasses second- and third-neighbor environments around a central atom. The more highly ordered regions, referred to as clusters or quasicrystals, often have atomic arrangements that approach those of crystals.
4 LRO extends beyond third-neighbour environments and gives crystalline ceramic/mineral structures their crystallographic periodicity.
5 For example, waste constituents can be chemically incorporated into a crystalline ceramic phase and then encapsulated in another material that provides an additional barrier to release.
BOX 3.1
How Structural Characteristics of Borosilicate Waste
Glass Control Physical and Chemical Characteristics
The polymerization of the SRO and MRO in borosilicate glasses provides more flexibility for atomically bonding waste constituents than in crystalline materials. The glass-forming SRO structural groups are usually tetrahedral Si, B, Al, Fe, P surrounded by four oxygen atoms or trigonal B surrounded by three oxygen atoms. The tetrahedra link to each other via bridging oxygen bonds (BO). The remaining non-bridging oxygen atoms (NBO) carry a negative charge and, in turn, ionically bond to positively charged cations such Cs+, Sr+2, Ca+2, and other positively charged constituents. These linkages create MRO structural groups, e.g., (Cs,K,Na,Li)AlO2, (Cs,K,Na,Li)FeO2, (Cs,K,Na,Li)BO2, (Cs,K,Na,Li)SiO4 (Ellison and Navrotsky, 1990), or (Cs,K,Na)AlSiO4 (Li et al., 2000), which form sheet-like units, chain-like units, and monomers (White, 1988) that further bond waste constituents ionically.
The modified random network model (MRN Model) (Porai-Koshits, 1958; Warren, 1933; Zachariasen, 1932, 1933) for glass is able to account for the existence of large cation-rich clusters in glass (e.g., clusters of Ca in CaSiO3 glasses and Na in Na2MoO4). These more highly ordered regions of MRO, which are referred to as clusters (or quasicrystals in the older literature) can have atomic arrangements that approach those of crystals (Burnham, 1981). These clusters govern constituent solubility (Calas et al., 2003; Cauranta et al., in press; Hyatt et al., 2004; Nyholm and Werme, 1981) (see Box Table) and crystal formation during cooling. The process model in use at the DWPF uses a quasicrystal model to prevent unwanted crystallization in the Joule heated melter.
In the MRN model, tetrahedra define the network regions and the NBO-cation regions represent percolation channels (see Box Figures and Figure 9.1 in Chapter 9) that can act as ion-exchange paths for elements that are ionically bonded to the NBO. Such percolation channels are also found in rare-earth (lanthanide) alumino-borosilicate (LaBS) glasses (Caurant et al., 2009). The molecular structure of glass controls constituent release by establishing ion exchange sites, hydrolysis sites, and the access of water to those sites through the percolation channels. The mechanisms of constituent release are similar to those for natural analogues glasses (basalts) and minerals.
BOX TABLE Solubility of Elements in Silicate Glass
| Element | Solubility (mass %) |
| Al, B, Ca, Cs, K, Na, Pb, Rb, Si, U | >25 |
| Ba, Fe, La, Li, Mg, Nd, Sr, Zn | 15-25 |
| Be, Bi, Cu, F, Ga, Ge, Mn, P, Pr, Pu, Th, Ti, V, Zr | 5-15 |
| Am, As, C, Cd, Ce, Cl, Cm, Co, Cr, Cy, Eu, Hf, Mo, Ni, Np, Pm, Re, S, Sb, Se, Sm, Sn, Tc, Te, Tl, W, Y | 1-5 |
| Ag, Au, Br, Hg, I, N, Pd, Pt, Rh, Ru | <1 |
SOURCE: Ojovan and Lee (2007).
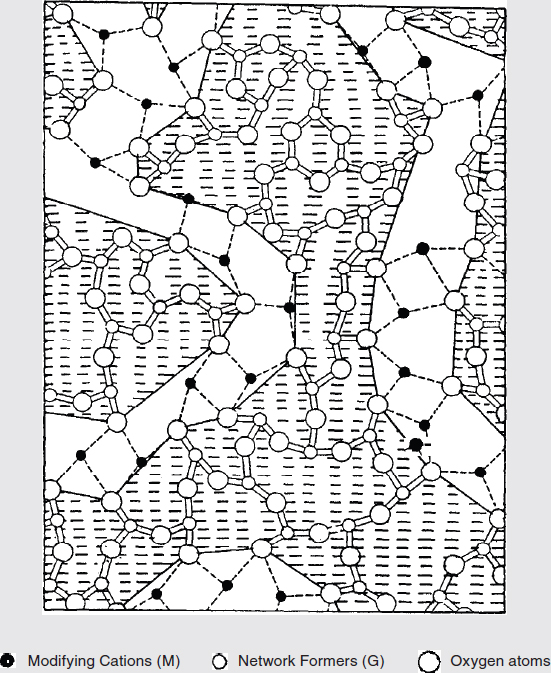
BOX FIGURE 1 A modified random network (MRN) for a glass of nominal composition M2O3(G2O3)2, where M represents the modifying cations and G represents the tetrahedral cations. Covalent bonds are shown by the solid lines and ionic bonds by the dotted lines. The dashed regions are defined by the boundary, which runs along the G–O (i.e., non-bridging) bonds. The undashed regions represent the percolation channels defined by the M–O bonds that run through the glass network. SOURCE: Greaves (1989).
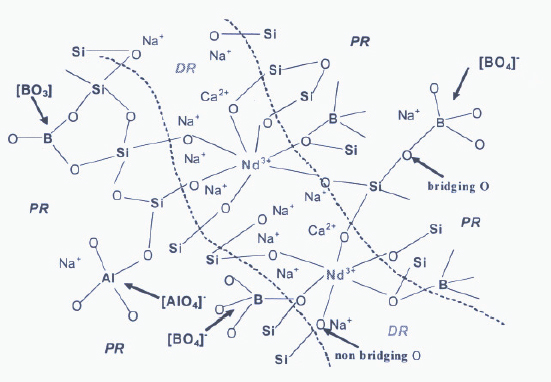
BOX FIGURE 2 Proposed polymerization of SRO and MRO in the atomic structure of a French HLW rare earth bearing aluminoborosilicate glass. Na+, Ca2+, and Nd3+ exist in the proposed percolation channels. SOURCE: Caurant et al., (2009).
- Crystalline ceramics
- Metals
- Cements
- Geopolymers
- Hydroceramics
- Ceramicretes
Brief descriptions of these materials are provided in the following sections. More detailed descriptions of these materials are provided in the accompanying tables.
3.3.1 Single-Phase (Homogeneous) Glasses
Glass is an amorphous solid material produced by cooling a material from a molten to a solid state without crystallization. Glass waste forms can have a wide range of compositions, but they are generally classified using
the dominant tetrahedral structural groups forming its structure, similar to the nomenclature for aluminosilicates, borates, and phosphates:
- Borosilicate glasses contain (SiO4)–4, (BO4)–5, (BO3)–3, and some (AlO4)–5 structural units.
- Aluminosilicate glasses contain (SiO4)–4 and (AlO4)–5 units.
- Aluminoborate glasses contain only (BO4)–5, (BO3)–3, and some (AlO4)–5 units.
- Aluminophosphate glasses contain (PO4)–3 and (AlO4)–5 units.
- Iron phosphate glasses contain (PO4)–3 and (FeO4)–5 units.
The major properties of glass waste form materials are summarized in Table 3.2.
Glass is being used worldwide to immobilize HLW from reprocessing of spent nuclear fuel and targets. The immobilization process, vitrification, is a continuous process capable of handling large-volume waste streams. This process is a well-demonstrated technology with more than 40 years of industrial experience (see Chapter 4).
Glass has several advantages for HLW immobilization: It can generally accommodate a wide range of waste stream compositions; has adequate-to-good durability in many disposal environments (see Chapters 5 and 8); and has good thermal and mechanical stability properties. In particular, borosilicate glasses melt at temperatures between 1,050°C-1,200°C, which limits the volatility of radionuclides such as technetium-99, cesium-137, and iodine-129. The melts are generally less corrosive than commercial glass melts, such as Pyrex, because of their lower temperatures.
The worldwide production of HLW glass is summarized in Table 3.3. To date, more than 5,000 metric tons of HLW borosilicate glass have been produced at SRS and 500 metric tons have been produced at West Valley, New York. More than 6,700 metric tons of HLW borosilicate glass have been produced in France. The compositions of these glasses, including the incorporated wastes, fall into a common region in the borosilicate glass forming system (Jantzen, 2011; Ramsey, 1989; Wicks et al., 1985). They contain ~60 weight percent or more of glass forming oxides (SiO2, B2O3, ZrO2, Al2O3, P2O5, and fission products), >15 weight percent glass modifier oxides (Na2O, K2O, Li2O, CaO, MgO, SrO, and ZnO), and 0-25 weight percent glass intermediate oxides (Cr2O3, Fe2O3, CuO, NiO, MnO, PbO, TiO2, and actinides).
3.3.2 Glass-Ceramic Materials
Glass-ceramic materials (GCMs) are materials that contain both crystalline and glass phases (see Table 3.1). Depending on the intended appli-
TABLE 3.2 Single-Phase (Homogeneous) Glass Waste Forms (Type 1 in Table 3.1)
| Glass Type | Major Structural Components | Comments | Selected References |
| Alkali borosilicate | (SiO4)–4, (BO4)–5, (BO3)–3, and some (AlO4)–5 structural (tetrahedral or trigonal units) to which alkali, alkaline earth, and waste species bond | Flexible easy processing; low melt temperatures 1,150°C -1,200°C to minimize volatility; cold cap production (if feasible) minimizes volatility; most waste cations highly soluble in glass; overall waste solubility 25-40 weight percent. | Caurant et al., 2009; Donald et al., 1997; Jantzen, 2011; Lee et al., 2006; Lutze and Ewing, 1988; Ojovan and Lee, 2005, 2007; Stefanovsky et al., 2004 |
| Lanthanide borosilicate (LaBS) | (SiO4)–4, (BO4)–5, (BO3)–3, and some (AlO4)–5 structural (tetrahedral or trigonal units) to which lanthanides, alkaline earth, and other waste species bond | Higher solubility for actinides than alkali borosilicate glass; many of the lanthanides used have large thermal neutron cross-sections and serve as neutron absorbers; 1,300°C-1,500°C melt temperatures cause increased volatilization of some radionuclides; corrosion behavior of LaBS glass is similar to HLW glasses in current use by DOE; existing repository models were deemed to be directly applicable to LaBS glass; waste solubility 16-59 weight percent lanthanides. | Bardez et al., 2006; Harrison and Scales, 2008a,b; Jantzen, 2011; Marra et al., 2006; Ramsey et al., 1995 |
| Aluminosilicate glasses and/or alkali aluminosilicate glasses | (SiO4)–4 and (AlO4)–5 | Higher melting temperature (~1,600°C) causes volatilization of radionuclides; waste loading dependent on rapid cooling, e.g., 20 weight percent UO2 if cooled rapidly but <10 weight percent if cooled slowly; improved durability over borosilicate glass. | Donald et al., 1997; Lutze and Ewing, 1988 |
| Aluminoborate glasses | (BO4)–5, (BO3)–3, and some (AlO4)–5 | Not used for waste vitrification. | |
| High-silicate glasses (sintered glasses) | (SiO4)–4 | High SiO2 glasses must be made by hot pressing and sintering at 650°C -800°C to retain volatile fission elements such as Cs, Ru, Mo, and Tc; waste solubility 5-35 weight percent reported. | Donald et al., 1997; Lutze and Ewing, 1988 |
| Alkali alumino-phosphate | (PO4)–3and (AlO4)–5 | Phosphate can be added remotely as H3PO4; phosphate precipitates formed in feed solution are hydrous and non-caking; phosphate systems form melts at lower temperatures than silicate or borosilicate systems; most cations are readily incorporated into homogeneous phosphate melts; phosphate systems can be devised to accommodate more than 10 weight percent sulfate; melts are corrosive to materials of construction; glass product has a tendency to devitrify; durability can be comparable to borosilicate glass if alumina content is sufficient; composition ~24-27 weight percent Na2O, 20-24 weight percent Al2O3+MemOn, 50-52 weight percent P2O5. | Caurant et al., 2009; Donald et al., 1997; Ewing and Wang, 2002; Gombert, 2007; Hatch et al., 1962; Lee et al., 2006; Ojovan and Lee, 2005, 2007; Schneider, 1969; Stefanovsky, 2009; Stefanovsky et al., 2004 |
| Lead iron phosphate | (PO4)–3and (FeO4)–5 | Composition ~PbO, 40 to 66 weight percent; P2O5, 30 to 55 weight percent; and Fe2O3, 0 to 10 weight percent depending on the amount of iron in the waste; low melting (850°C -1,050°C); abandoned because of RCRA issues with Pb component, low waste loading (~20 weight percent); poor solubility of certain species compared to borosilicate glass; tendency to devitrify. | Chick et al., 1986; Donald et al., 1997; Jantzen, 1986a,b; Kahl, 1986; LLNL, 1996; Lutze and Ewing, 1988; Marasinghe et al., 2000; Sales and Boatner, 1984 |
| Glass Type | Major Structural Components | Comments | Selected References |
| Iron phosphate | (PO4)–3and (FeO4)–5 | Good chemical durability; high solubility for many heavy metals (e.g., uranium, chromium, zirconium, cesium, molybdenum), noble metals, and rare earths; low melting temperatures (950°C- 1,100°C); rapid melt rates (viscosity typically below one poise); low corrosion of oxide refractories and Inconel alloys commonly used in glass melting furnaces; waste loadings 25-50 weight percent; tendency to devitrify. | Badyal et al., 1999; Chen and Day, 1999; Day et al., 1997, 1998, 2004; Karabulut et al., 1999; Kim et al., 2003; Marasinghe et al., 1997, 1998, 1999, 2000, 2001; Mesko and Day, 1999; Mesko et al., 1998, 2000; Mogus-Milankovic et al., 1998; Ray et al., 1999; Yu and Day, 1995 |
| Chalcogenide | TeO2-XCl-B2O3 TeO2-XCl-Li2O TeO2-XCl-Na2O | S, Se, and Te glasses for radionuclides that are otherwise difficult to immobilize (e.g., I-129) in conventional glass or borosilicate glass systems; gels such as Pt2Ge4S9.6 are used to immobilize actinides, noble gases, carbon dioxide, and mixed chlorides. | Crum et al., 2009; Ryan et al., 2009 |
| XCl = “mixed chlorides” simulant at ~19 weight percent | |||
TABLE 3.3 Global Production of HLW Glass at Industrial Scales
| Plant Name | Location | Vitrification Process | Waste Glass Quantities (metric tons) | Waste Loading Range (weight percent) | Canister Size (Meters) [Number of Canisters Produced] | Activity (TBq)a |
| Defense Waste Processing Facility (DWPF) | Aiken, South Carolina, USA | Joule-heated melter | 5,000b | 28-40c | 0.6 × 3 [2,845] | 7.7 × 105 |
| West Valley Demonstration Project (WVDP) | West Valley, New York, USA | Joule-heated melter | ~500d | ~20.4-23.5e | 0.6 × 3 [275] | 8.9 × 105 |
| Waste Vitrification Plant (WVP), BNFL | Sellafield, UK | Induction, hot crucible | ~1,800f | ~25g | 0.43 × 1.34 [4,319h] | 1.9 × 107 |
| Areva NC (R7/T7)i | La Hague, France | Induction, hot crucible | 5,573j | 12-18k | 0.43 × 1 [14,045] | 2.38 × 108 |
| AVM or Atelier de Vitrification de Marcoulei | Marcoule, France | Induction, hot crucible | 1,138m | 12-18k | 0.43 × 1 [3,159] | 1.69 × 106 |
| Pamela | Mol, Belgium | Joule-heated melter | 500l | 15-25k | 0.30 × 1.2 0.43 × 1.34 [2,200] | 4.5 × 105 |
| Tokai Vitrification Facilityo (TVF) | Tokaimura, Ibaraki Perfecture, Japan | Joule-heated melter | >100 | 20-30k | 0.43 × 1 [247n] | 1.5 × 104 |
| Plant Name | Location | Vitrification Process | Waste Glass Quantities (metric tons) | Waste Loading Range (weight percent) | Canister Size (Meters) [Number of Canisters Produced] | Activity (TBq)a |
| Mayak Vitrification Facilityp (EP-500) | Ural Region, Russia | Joule-heated melter | ~8,000 | 33k | 0.57 × 1 [17,600] | 3.33 × 107 |
a 1 Tera-Becquerel (TBq) = 1012 decays per second.
b 1996-2009.
c Jantzen et al. (2005).
d 1996-2002; mission complete.
e Perez et al. (2001).
f 1991-2007 at 150 L glass per canister and an assumed glass density of 2.75 g/cm3.
g Riley et al. (2009).
h Predicted mission completion is 6582 canisters.
i Caterine Veyer of AREVA, personnel communication (2010).
j P.P. Poluektor, personal communication (2010).
k Acidic waste loadings are comprised of fission products and minor actinides; corrosion products and alkali are not included as for neutralized wastes.
l 1978-2008
m 1985-1991
n 1995-2006
o Seiichiro Mitsui of Japan Atomic Energy Association (JAEA), personnel communication (2010).
p 1989-2008
cation, the major component may be a crystalline phase with a vitreous phase acting as a bonding agent. Alternatively, the vitreous phase may be the major component with particles of a crystalline phase dispersed in the vitreous matrix. GCMs can be formed by a number of processes, including melt crystallization (controlled or uncontrolled), multiple heat treatments, or by encapsulation of ceramic material in glass (Lee et al., 2006).
In practice, crystalline materials frequently contain glass remnants along grain boundaries, and unreacted radiophases may persist rather than be completely incorporated into the crystal structures. A virtual continuum exists between 100 percent glass and 100 percent crystalline materials with GCMs falling in between as shown in Figure 3.1.
GCMs offer a useful compromise between glasses and ceramics. They are easier and less expensive to prepare than conventional ceramics but offer higher durability than glasses, provided that the soluble species that can sequester radionuclides (e.g., Na2SO4, which can sequester cesium and strontium) (Plodinec and Wiley, 1979) are prevented from crystallizing (Figure 3.1 and Table 3.1).
GCMs offer several potential advantages over glass for use as waste form materials, including increased waste loadings, increased waste form density, and thus smaller disposal volumes. These waste forms can also be used to immobilize glass-immiscible components such as sulphates, chlorides, molybdates, and refractory materials that have very high melting temperatures. They can also be used to immobilize long-lived radionuclides (e.g., actinides) by incorporating them into the more durable crystalline phases; short-lived radionuclides (e.g., many fission products) can be accommodated in the less durable vitreous phase (Lee et al., 2006). Relatively low leach rates (see Chapter 5) have been observed for some glass ceramics, which may make them potential candidates for immobilization of HLW. For example, allowing the formation of crystalline phases that have little to no impact on durability (see Figure 3.1) would be an achievable incremental strategy for increasing HLW loading in glass (and throughput of waste) at SRS and Hanford.
A number of different GCMs have been proposed for the immobilization of HLW; summaries can be found in Donald et al. (1997), Lee et al. (2006), and Stefanovsky et al. (2004). A brief synopsis of GCMs is given in Table 3.4.
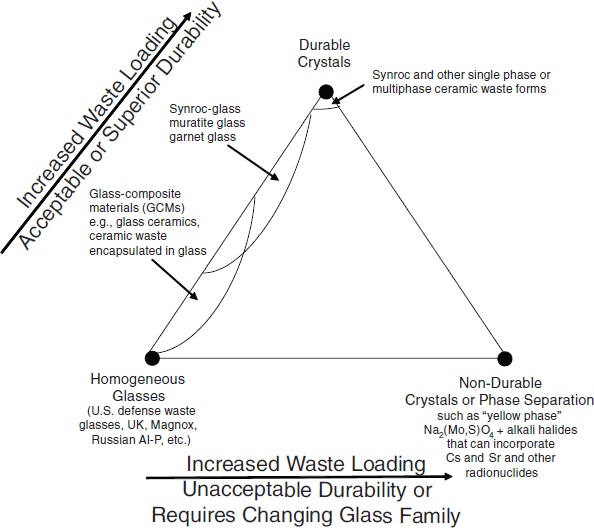
FIGURE 3.1 Schematic diagram illustrating the durability and waste loading of waste form materials relative to single-phase (homogeneous) glass. Single-phase glass formulations are shown in the lower left corner of the triangle. If crystals (e.g., spinels) are allowed to form in the glass, then glass-ceramic materials (GCMs) can be produced (left leg of triangle) that have superior waste loading and durability relative to single-phase glass. Fully crystalline materials (upper corner of triangle) are considered to be exceptionally durable waste forms with high waste loadings relative to single-phase glass. However, the incorporation of certain species (e.g., Mo, S, and P) into glass creates non-durable secondary phases (lower right corner or triangle) that may have unacceptable durabilities.
SOURCE: After Ojovan and Lee (2007).
3.3.3 Crystalline Ceramics
Crystalline ceramics are inorganic, non-metallic solids that contain one
TABLE 3.4 Glass Ceramic Materials (Types 2a and 2b in Table 3.1)
| Material | Glass Phase | Crystalline Phase(s) | Comments | Selected References |
| Borosilicate based | ||||
| Alkali borosilicates | Borosilicate | NiFe2O4 spinels ZrSiO4 Al2O3 | Glasses are allowed to partially crystallize in the melt pool or spontaneously crystallize during cooling; spinels do not sequester radionuclides and have little or no impact on glass durability; zircon and alumina are likewise benign; crystallization alters the glass viscosity, making it non-newtonian; soluble phases like Na2SO4 should be avoided because they sequester Cs and Sr. | Bickford and Jantzen, 1984; Cicero et al., 1993; Hrma et al., 1994; Jantzen et al., 1984; Plodinec and Wiley, 1979 |
| Glass-bonded sodalites | Borosilicate | I in NaI I in sodalite: Na8Al6Si6O24(I)2, Cl in sodalite: Na8Al6Si6O24(Cl)2 | Glass-bonded sodalites are formed by hot isostaic pressing or cold pressing/sintering of high alkali containing electrorefiner wastes at 1,000°C-1,200°C; release of radionuclides is controlled by the dissolution rate of the binder glass. | Ebert, 2005; Morss et al., 2000; Moschetti et al., 2000; Sinkler et al., 2000 |
| Synroc aluminoborosilicates | Aluminoborosilicate | Zirconolite: CaZrTi2O7 | Made by hot isostatic pressing; zirconolite is the major crystalline phase; for low-purity actinide wastes, Pu partitions into the zirconolite over the glass phase by a factor of 100:1 and Gd neutron absorbers partition into zirconolite; accommodates both actinides and any associated impurities: proliferation resistant because of the low purity and concentration of Pu in the waste form. | B. Begg and K. Smith, ANSTO, written communication |
| Material | Glass Phase | Crystalline Phase(s) | Comments | Selected References |
| Barium aluminosilicates Celsian analogues | Borosilicate (sodium aluminosilicate with 2-7 weight percent B2O3 and 3-4 weight percent TiO2) | Celsian: BaAl2Si2O8 Pyrochlore: RE2Ti2O7; RE = rare earth Scheelite: BaMoO4 Pollucite: CsAlSi2O6 Molybdenum-nosean: Na8Al6MoO4(SiO4)6 Perovskite: CaTiO3 Diopside: CaMgSi2O6 Eucryptite: LiAlSi2O6 Spodumene: LiAlSi2O6 Nepheline: NaAlSiO4 | Melt temperatures from 1,100°C-1,400°C; controlled crystallization temperatures between 530°C -720°C; pyrochlore phase is a host for actinides and Sr; pollucite is a host for Cs and Rb; noble metal fission products form small metallic droplets; leaching characteristics have been noted to be comparable to the borosilicate glasses; research on this material has been limited. | Donald et al., 1997; Lutze and Ewing, 1988; Lutze et al., 1979 |
| Diopside borosilicates | Borosilicate | Diopside: CaMgSi2O6 Powellite: CaMoO4 Perovskite: CaTiO3 | Waste loadings of ~30 weight percent could be achieved using these materials for European and Japanese commercial wastes, which normally accommodate ~16 weight percent; glasses were melted at 1,300°C and controlled crystallization was carried out at temperatures in the range 800°C-1,100°C; Cs was in the diopside phase; La, Ce, Nd, and Pr were in the perovskite phase; Sr and Sm were in the glass phase; noble metals were metallic. | Donald et al., 1997; Lutze and Ewing, 1988; Lutze et al., 1979; Ninomiya et al., 1981 |
| Titania based | ||||
| SYNROC and sphene | Sodium aluminosilicate with TiO2 and CaO | Sphene: CaTiSiO5 Pyrochlore: Ca(RE,U) Ti2O7 Zirconolite: CaZrTi2O7 Perovskite: (Ca,Re,U,Sr) TiO3 Anorthite: CaAl2Si2O8 | GCMs containing TiO2 are known as sphene glass ceramics; other formulations that produce the same phases as the SYNROC crystalline ceramic forms, mainly zirconolite, can also be produced. Forms between 1,300°C-1,500°C. Actinides and REEs, and some Sr are incorporated into zirconolite phase; Cs and the remaining Sr are incorporated into the vitreous phase. As a rule, leach rates for the actinides and REEs from zirconolite glass-ceramics are between 10–4 and 10–2g/m2day. | Advocat et al., 1998; Donald et al., 1997; Hayward, 1988; Lashtchenova and Stefanovsky, 1998a,b; Loiseau et al., 2001; Lutze and Ewing, 1988; Martin et al., 2002; McGlinn et al., 2001; O’Holleran et al., 1997; Stefanovsky et al., 2004; Vance et al., 1996a,b; Zyryanov and Vance, 1997 |
| Alkali titanium silicate | Sodium titanium silicates | Corundum: Al2O3 Cristobalite: SiO2 Albite: NaAlSi3O8 Zirconolite: CaZrTi2O7 Perovskite: (Ca,Re,U,Sr) TiO3 Zircon: ZrSiO4 | Formed by HIPing calcine (70 weight percent) with Si, Ti, Al metal, and alkali oxides; for high Zr containing ICPP wastes. | Donald et al., 1997; Lutze and Ewing, 1988; Vinjamuri, 1995 |
| Material | Glass Phase | Crystalline Phase(s) | Comments | Selected References |
| Barium titanium silicate | Barium silicates with TiO2 | Fresnoite: Ba2TiSi2O8 Ba priderite: BaFe2Ti6O16 Pyrochlore: RE2Ti2O7 Scheelite: BaMoO4 | Forms at 1,200°C. Fresnoite phase hosts Ba and Sr; priderite phase hosts Ba; pyrochlore phase hosts RE, actinides, and Sr. Cs remains in the glassy phase. Glass is 50 percent and crystalline phases are 50 percent. A reheat step is required and Ba is a RCRA metal. | Donald et al., 1997; Hayward, 1988; Lutze and Ewing, 1988; Stefanovsky et al., 2004 |
| Silicate based | ||||
| Basalt | Complex natural oxide based on Si, Ca, Mg, Fe, Al, and Ti | For Purex wastes: Augite: (Ca, Mg, Fe)2Si2O6 Powellite: (Ca, Sr) MoO4 Spinel: (NiFe2O4) | Different waste loadings from different waste sources are possible; glasses melt in the range 1,300°C-1,400°C. Crystallization is carried out at temperature ranges 670°C-700°C and 900°C-950°C. The chemical durabilities of these glass-ceramic materials are superior to borosilicate glass. | Donald et al., 1997; Hayward, 1988; Lutze and Ewing, 1988; Saidl and Ralkova, 1966; Stefanovsky et al., 2004; Tolstova et al., 2002 |
| Iron enriched basalt (IEB) | Aluminosilicate glass | Iron spinel Feldspars: NaAlSi3O8 to CaAl2Si2O8 Augite: (Ca, Mg, Fe)2Si2O6 Fluroapatite: Ca5(PO4)3F Zircon: ZrSiO4 Fluorite: CaF2 Cristobalite: SiO2 Hematite: Fe2O3 Mullite: Al6Si2O13 | Applications to commercial and defense wastes, including decontamination of Three Mile Island containment water together with core debris; Melts at 1,400°C-1,500°C and controlled cooling after casting the glass into containers. | Donald et al., 1997; Hayward, 1988; Lutze and Ewing, 1988; Stefanovsky et al., 2004 |
| IEB with TiO2 and ZrO2 | Aluminosilicate glass | Same as above plus: Zirconolite Pseudobrookite: Fe2TiO5 Chevkinite: Ce4Fe2Ti3Si4O22 | Cast glasses crystallized by holding at 1,200°C for 16 h; Ti phases retain actinides. | Donald et al., 1997; Hayward, 1988; Lutze and Ewing, 1988; Stefanovsky et al., 2004 |
| Magnesium aluminosilicate (MAS) | Magnesium aluminosilicate | Enstatite: MgSiO3 Indialite/Corderite: Mg2Al4Si5O18 | Used as an encapsulant for Zr alloy cladding wastes; made by press and sinter and accommodated 20 weight percent ZrO2. | Donald et al., 1997 |
| Phosphate-based | ||||
| Apatite/monazite glass ceramics | Calcium phosphate | Apatite: Ca5(PO4)3(F,Cl) Monazite: (Ce,U)PO4 | Apatite hosts Ca, P, F, Cl, S, Sr, Cs, As, Pb, Ba, Hg, Cd, Cr, U, and Ce; Melts at 1,400°C, crystallizes at 1,150°C and allowed to furnace cool; investigated primarily for phosphate-rich or fluoride-rich waste streams including ICPP CaF2 wastes. | Knecht and Berreth, 1981; Wronkiewicz et al., 1996; Zhao et al., 2001 |
| Low temperature sintering glasses | Bi-oxide based glasses | Mordenite zeolite | Low-temperature sintering allows for glass encapsulation of volatile gas-containing matrices (e.g., AgI-MOR) | Garino and Nenoff, 2011; Garino, et al., 2010; Sava et al, 2011 |
TABLE 3.5 Single-Phase Crystalline Ceramic Materials (Type 3a in Table 3.1)
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Simple Oxides | ||
| XO2 Oxides | ZrO2, UO2, ThO2, HfO2, and PuO2 have the simple fluorite CaF2 cubic structure. | Burghatz et al., 1998; Gong et al., 2000; Poinssot et al., 2005; Sickafus et al., 1999 |
| Complex Oxides | ||
| Pyrochlore | A derivative of the fluorite structure type, A2B2O7, where A-site contains large cations (Na, Ca, U, Th, Y, and lanthanides) and the B-site contains smaller, higher-valence cations (Nb, Ta, Ti, Zr, and Fe3+). | Chakoumakos, 1984; Chakoumakos and Ewing, 1985; Ewing et al., 2004; Laverov et al., 2010 |
| Murataite | Also a derivative of the isometric fluorite structure A6B12C5TX40-x with multiple units of the fluorite unit cell; hosts U, Pu, and rare earth elements. | Lian et al., 2002; Morgan and Ryerson, 1982; Sobolev et al., 1997a,b; Stefanovsky et al., 2004, 2007a,b,c; Urusov et al., 2005; Yudintsev et al., 2007 |
| Zirconolite | Monoclinic CaZrTi2O7 has a fluorite-derived structure closely related to pyrochlore, where plutonium may be accommodated on the Zr-site, as in the case of Ca(Zr,Pu)Ti2O7. | Boult et al., 1987; Clinard et al., 1982, 1984; Vance et al., 1994a,b; Zhang et al., 2009 |
| Perovskite | CaTiO3 has a wide range of compositions as stable solid-solutions; orthorhombic; consists of a 3-dimensional network of corner-sharing TiO6 octahedra, with Ca occupying the large void spaces between the octahedra (the corner-sharing octahedra are located on the eight corners of a slightly distorted cube); plutonium, other actinides, and rare-earth elements can occupy the Ca site in the structure, as in (Ca,Pu) TiO3. | Boult et al., 1987; Vance et al., 2004 |
| Ba-Hollandite | Ba1.2(Al,Ti)8O16 | Carter et al., 2002, 2004 |
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Ferrite garnet | [8]A3[6]B2[TiO4]3, e.g.,.[8](Ca,Gd, actinides)[6]Fe2[4]Fe3O12 | Laverov et al., 2010 |
| Crichtonite | (Sr,La,Ce,Y)(Ti,Fe3+,Mn)21O38 | Gong et al., 1995 |
| Freudenbergite | Na2(Ti,Fe)8O16 | Vance et al., 1994c |
| Simple Silicates | ||
| Zircon/Thorite | ZrSiO4/ThSiO4; zircon is an extremely durable mineral that is commonly used for U/Pb age-dating, because high uranium concentrations (up to 20,000 ppm) may be present. | Ewing et al., 1995a; Meldrum et al., 2000 |
| Titanite (sphene) | CaTiSiO5 | Hayward, 1988; Park et al., 2009 |
| Garnet | A3B2(XO4)3; distorted cubic structure; BO6 octahedra and XO4 tetrahedra establish a framework structure alternately sharing corners; A and B sites can host actinides and rare earth elements, and X =Si4+, Fe3+, Al3+, Ga3+, Ge4+, and V5+, making silicate, ferrite, aluminate, gallate, germinate, and vanadate garnets. | Utsonomiya et al., 2002; Yudintsev, 2001, 2003; Yudintsev et al., 2002 |
| Britholite (silicate apatite) Also known as oxy-apatites in the literature. |
(REE, Ca)5(SiO4,PO4)3(OH,F); i.e., Ca2Nd8(SiO4)6O2, Ca2La8(SiO4)6O2, where REE = rare earth elements; based on ionic radii of Nd3+, La3+, and Pu3+, an extensive range of solubility for Pu3+ substitution for the Nd or La, particularly on the 6h site, is expected. Because there is an extensive range in the Ca/REE ratio in these silicate apatites, a fair amount of Pu4+ substitution may be possible; La3+ through Lu3+ can substitute for Ca2+ and form oxyapatites, RE4.67□0.33[SiO4]3O (where □ = vacancy site) and can also accommodate Sr and Cs. | Fahey et al., 1985; Felsche, 1972; Jantzen and Glasser, 1979; McCarthy and Davidson, 1975; Utsunomiya et al., 2003; Weber, 1983, 1993 |
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Framework Silicates | ||
| Zeolitesa | Xx/n(AlO2)x (SiO2)y where X is the charge balancing counter-ion, n is the charge of the counter-ion, x is the number of charge-deficient alumina sites, and y is the number of charge-neutral silica sites; characterized by internal voids, channels, pores, and/or cavities of well-defined size in the nanometer range, ≈4Å-13Å; channels and/or cavities may be occupied by charge-compensating ions and water molecules; zeolites such as Ag-Mordenite selectively sorbs I2 (I-129); certain zeolites can be converted to condensed oxide ceramics by heating. This process is particularly attractive for waste form fabrication because capture and storage is preformed with minimal steps. | Breck, 1974; Chapman et al., 2010; Cronstedt, 1756; Higgins et al., 2002; Smith, 1963, 1976; Yudintsev et al., 2002 |
| Pollucite | (Ca, Na)2Al2Si4O12•2H2O; host for fission products such as Cs-137. | Gallagher and McCarthy, 1981; Kaminski et al., 2009; Komameni and White, 1981; Mimura et al., 1990; Strachan and Schulz, 1979; Yanagisawa et al., 1987 |
| Pollucite (Cs/Ti version) | CsTiSi2O6.5 | Anthony et al., 1993; Balmer and Bunker, 1995; Garino et al., 2009; McCready et al., 1997; Su et al., 1996, 1999; Xu et al., 2000, 2001 |
| Nepheline | NaAlSiO4 silica “stuffed derivative” ring type structure; some polymorphs have large nine-fold cation cage sites while others have 12-fold cage-like voids that can hold large cations (Cs, K, Ca); natural nepheline structure accommodates Fe, Ti and Mg. | American Mineralogist Crystal Structure Database, 2010; Berry and Mason, 1959; Deer et al., 1963; Jantzen, 2008; Kim et al., 2007; Klingenberg and Felsche, 1986; Sinkler et al., 2000 |
| Leucite | KAlSi2O6; K analogue of nepheline | |
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Sodalite group (Note: The name of the mineral changes with anions sequestered in cage structure). |
Sodalite Na8Cl2Al 6Si 6O24, also written as (Na,K)6[Al6Si6O24]•(2NaCl) to demonstrate that 2Cl and associated Na atoms are in a cage structure defined by the aluminosilicate tetrahedra of six adjoining NaAlSiO4; a naturally occurring feldspathoid mineral; incorporates the alkali, alkaline earths, rare earth elements, halide fission products, and trace quantities of U and Pu; sodalite is being investigated as a durable host for the waste generated from electrorefining operations deployed for the reprocessing of metal fuel; minor phases in HLW supercalcine waste formsb where they retained Cs, Sr, and Mo, e.g., Na6[Al6Si6O24](NaMoO4)2; sodalite structures are known to retain B, Ge, I, Br, and rare earth elements in cage-like structures. | Brookins, 1984; Buhl et al., 1989; Deer et al., 1963; Fleet, 1989; Jantzen, 2008; Kim et al., 2007; Mattigod et al., 2006; McFarlane et al., 1997; Olson et al., 2004; Nakazawa et al., 2001; Sinkler et al., 2000 |
| Nosean: (Na,K)6[Al6Si6O24](Na2SO4), silica “stuffed derivative” sodalite cage-type structure host mineral for sulfate or sulfide species. | ||
| Hauyne: Na6[Al6Si6O24]((Ca,Na) SO4)1-2 sodalite family; can accommodate either Na2SO4 or CaSO4. | ||
| Helvite: Mn4[Be3Si3O12]S; Be can be substituted in place of Al and S2 in the cage structure along with Fe, Mn, and Zn. | ||
| Danalite (Fe4[Be3Si3O12]S). | ||
| Genthelvite (Zn4[Be3Si3O12]S). | ||
| Lazurite (Ca,Na)6[Al6Si6O24]((Ca,Na) S, SO4,Cl)x; can accommodate either SO4 or S2, Ca or Na and Cl. |
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Cancrinite | (Na,Ca,K)6[Al6Si6O24] ((Na,Ca,K)2CO3)1.6•2.1H2O: Only found in hydroceramic waste forms. | International Zeolite Association (IZA) website |
| Crystalline silicotitanate (CST) | Na2(H2O)2Ti4O5(OH) (SiO4)2Na(H2O)1.7; similar in structure to natural sitinakite: Na2(H2O)2Ti4O5(OH) (SiO4)2K(H2O)1.7 and natural titanosilicate pharmocosiderite minerals; selectivity for monovalent cations (e.g., Cs); enhanced selectivity for Cs over entire pH range when doped with specific transition metals (e.g., Nb). | Andrews and Harbour, 1997; Anthony et al., 1993; Miller and Brown, 1997; Nyman et al., 2001; Sokolova et al., 1989; Yakovenchuk et al., 2008; Yu et al., 2002; Xu et al., 2000 |
| Phosphates | ||
| Monazite | CePO4 or LaPO4; very corrosion resistant and can incorporate a large range of radionuclides including actinides and toxic metals into its structure; has been proposed as a potential host phase for excess weapons plutonium and as a host phase for radionuclides and toxic metals in glass-ceramic waste forms for low-level and hazardous wastes. | Boatner and Sales, 1988; Ewing and Wang, 2002; Ewing et al., 1996; Glorieux et al., 2009; Montel et al., 2006; Wronkiewicz et al., 1996; Zhang and Vance, 2008 |
| Apatite | Ca4-xRE6+x(SiO4)6-y(PO4)y(O,F)2; actinide-host phases in HLW glass, glass-ceramic waste forms, ceramic waste forms, and cement; actinides can readily substitute for the rare earth elements in the crystal structure, as in Ca2(Nd,Cm,Pu)8(SiO4)6O2, and fission products are also readily incorporated; however, the solubility for tetravalent Pu may be limited without other charge-compensating substitutions; has been proposed as a potential host phase for Pu and high-level actinide wastes. | Audubert et al., 1997; Boyer et al., 1997; Bros et al., 1996; Carpena and Lacout, 2005; Carpena et al., 2001; De et al., 1976; Ewing and Wang, 2002; Ewing et al., 1996; Jantzen and Glasser, 1979; Kim et al., 2005; McCarthy, 1977; McCarthy and Davidson, 1975; Park et al., 2002; Weber, 1982, 1993; Weber et al., 1997a,b; Wronkiewicz et al., 1996 |
| Crystalline Ceramic Phase | Nominal Composition(s) | Selected References |
| Xenotime | YPO4 | Ewing and Wang, 2002 |
| Sodium zirconium phosphate (NZP) | NaZr2(PO4)3; structure can incorporate a complex variety of cations, including plutonium; three-dimensional network of corner-sharing ZrO6 octahedra and PO4 tetrahedra in which plutonium can substitute for Zr, as in Na(Zr,Pu)2(PO4)3; complete substitution of Pu4+ for Zr has been demonstrated in NZP. | Ewing and Wang, 2002; Orlova et al., 1994; Scheetz and Roy, 1988; Scheetz et al., 1994 |
| Thorium phosphate diphosphate | Th4(PO4)4P2O7; a unique compound for immobilization of Pu and U; partial substitution of Pu for Th has been demonstrated (up to 0.4 mole fraction), complete substitution is not possible. | Dacheux et al., 1998a,b; Ewing and Wang, 2002; Pichot et al., 2000 |
a In recent usage, zeolite represents the framework topology of the 3D porous crystalline inorganic frameworks (with or without water); it can be applied to the framework of related minerals, their synthetic analogues, and/or non-aluminosilicate porous crystalline 3D frameworks. Structural information from the International Zeolite Association (IZA) website, http://www.iza-structure.org/databases/; see Analcime analogues (ANA).
b Supercalcines were the high-temperature silicate-based “natural mineral” assemblages proposed for HLW waste stabilization in the United States (1973-1985).
or more crystalline phases.6 Single-phase crystalline ceramics (Table 3.5) can be used to immobilize separated radionuclides (e.g., plutonium-239) or more chemically complex waste streams (e.g., HLW). In the latter case, the atomic structure of the ceramic phase must have multiple cation and anion sites that can accommodate the variety of radionuclides present in the waste stream. These materials are potentially attractive for immobilizing long-lived alpha emitting actinides such as plutonium, neptunium, and americium (Burakov et al., 2010). However, some of these materials are susceptible to radiation damage effects associated with alpha decay from actinides; these effects have recently been summarized in Ewing and Weber (2010).
________________________
6 However, as noted previously, even materials that are predominantly a single phase will frequently have trace amounts of other phases segregated along grain boundaries or as inclusions of unreacted material.
TABLE 3.6 Multiphase Crystalline Ceramic Materials (Type 3b in Table 3.1)
| Multiphase Ceramic Waste Form | Description | Selected References |
| SYNROC | ||
| Titania Based | Dense, multiphase titanate-based waste form designed for the immobilization of HLW; interest in this multiphase crystalline ceramic stems from the robust nature of individual phases and because this phase assemblage is very tolerant to changes in the waste-to-precursor-titanate oxide ratio; in most cases the ratios of the phases vary as the waste loading changes but new phases are not introduced. The original SYNROC phase assemblage included: zirconolite (CaZrTi2O7), which incorporates rare earths and actinides; perovskite (CaTiO3), which incorporates rare earths, actinides, and Sr; and hollandite (BaAl2Ti6O16), which incorporates Cs, Rb and Ba, and rutile (TiO2). Though the original SYNROC-C was designed for the immobilization of HLW, new variants for actinides and Pu and U have been developed. | Ewing and Weber, 2010; Maddrell, 2001; Reeve et al., 1984; Ringwood, 1978,1985; Ringwood et al., 1978, 1979, 1988; Vance, 1994; Vance et al., 1996b |
| Supercalcine Ceramics | ||
| Silicate based | Supercalcines are silicate-, phosphate-, and oxide-based ceramics and can include some minor titanate phases. They are specifically tailored for HLW from different reprocessing schemes. They have very high loadings of fission products, typically 70 weight percent (simulated by stable isotopes in the experimental work), and the chemistry of the different phases is driven by the fission products as majority components. Typical phases are pollucite (CsAlSi2O6); powellite (CaMoO4); and rare earth apatites and phosphates (e.g., monazite, REPO4, where RE = trivalent rare earths) with monazite; fluorite ((U,Zr,Pu)O2), scheelite (SrMoO4); corundum, spinel, rutile, and sodalite phases found as well. | Felsche, 1972; Jantzen and Glasser, 1979; McCarthy, 1977; McCarthy et al., 1979a,b; Roy, 1977, 1979 |
| Multiphase Ceramic Waste Form | Description | Selected References |
| Tailored Ceramics | ||
| Alumina based | Tailored ceramics are primarily aluminate-based with compatible phases of alumina, spinel (nominally MgAI2O4), magnetoplumbite (nominally X(Al, Fe)12O19 where X = Sr, Ba, or charge substituted paris such as [Cs0.5 + La0.5), and a fluorite-related uraninite ((U,Th) O2). For wastes containing a high concentration of sodium, an additional crystalline phase, nepheline (NaAISiO4) is produced to accommodate the monovalent ion. The magnetoplum-bite phase acts as a host for the radionuclides Sr and Cs, and the phase assemblage is analogous to a naturally occurring placer deposit of alumina-spinel-hibonite (magnetoplumbite)-thoria, found in Fort Dauphin, Malagasy. | Clarke et al., 1982; Harker, 1988; Harker et al., 1981a,b, 1983; Jantzen et al., 1982a,b; Morgan et al., 1981 |
Multiphase crystalline ceramics (Table 3.6) consist of an assemblage of crystalline phases. Individual phases are selected for the incorporation of specific radionuclides, with the proportions of phases varying depending on the composition of the waste stream. An individual phase can host one or more radionuclides, including solid solutions of radionuclides. However, not all phases will host radionuclides.
The three best known examples of multiphase waste forms are SYNROC, which is based on titanium ceramic phase assemblages; supercalcine ceramics, which are silicate-based ceramic phase assemblages; and tailored ceramics, which are alumina-based ceramic phase assemblages.
3.3.4 Metals
Several different types of metallic materials have been studied as potential waste forms. Like crystalline ceramics, metal waste forms can consist of single or multiple phase assemblages, and the waste form itself can be granular or monolithic. Metal waste forms can be fabricated sintering or casting. Each of these techniques has drawbacks; in particular, it can be
difficult to find metal compositions and processes that effectively wet and encapsulate dispersed phases or fines.
Metal waste forms composed predominantly of lead and lead-based alloys are being used in Russia (and have been used in Belarus and Ukraine) to immobilize radioactive sources (Arustamov et al., 1999; Ojovan et al., 2004). Metal waste forms are being used by DOE-EM to immobilize metallic fuel waste, including fuel hulls, at INL. The waste is solidified by melting at 1,560°C into a uniform, homogeneous waste form. Typically, zirconium is added to the waste, which lowers its melting point and generates enough Fe2Zr intermetallic phase to accommodate the transuranic elements (Ebert, 2005). As a result, waste loading is typically above 90 percent (Marsden and Westphal, 2006).
Metallic composite waste forms produced by hot isostatic pressing (see Chapter 4) are under study by the Australian Nuclear Science and Technology Organisation (ANSTO) for applications in the United Kingdom (Begg and Smith, written communication). Metal encapsulation will be used to immobilize debris waste streams (e.g., cermets, silicon carbide, graphite, broken fuel pins, fuel hulls) that are not economically feasible to process by other means. The encapsulant material provides a second level of protection to the release of radionuclides.
3.3.5 Cements
Cements are inorganic materials that set and harden as a result of hydration reactions. Cements microencapsulate wastes (Table 3.1), although there is recent evidence that during hydration three binding mechanisms can also occur between the cement and metal ions in the waste (Cocke and Molla, 1993; Cougar et al., 1996; Glasser, 1997):
- Precipitation of metal ions into the alkaline matrix as an oxide, mixed oxide, or as another discrete solid phase.
- Adsorption or (co-)precipitation of metal ions onto the surface of cement minerals.
- Incorporation of metal ions into hydrated cement minerals as they crystallize.
These processes are not mutually exclusive.
The most common type of cement, Portland cement, consists of calcium silicates, other aluminum and iron containing phases, and additives such as gypsum to control set time. Portland cement, when mixed with water and aggregates, hydrates to form concrete. Grout, a mixture of Portland cement and various sand mixtures, is commonly used to encapsulate radioactive
wastes. DOE-EM is filling emptied HLW tanks at SRS and INL with grout to immobilize waste heels and provide structural support.
Cements are used to immobilize waste having relatively low levels of radioactivity (i.e., low- or intermediate-level radioactive wastes). Higher-activity wastes can result in radiolysis and production of hydrogen gas from the breakdown of water or hydroxyl groups in the cement (Bibler, 1980). To minimize radiolysis, the use of concrete formed under elevated temperature and pressure (FUETAP), which reduces the amount of entrapped water to about 2 percent, was considered for the immobilization of HLW (McDaniel and Delzer, 1988; Moore, 1981). This idea was eventually abandoned because of the complexity of processing. To the committee’s knowledge, no waste processing programs are pursuing this technology today.
Modeling has shown that cements can be “designed” to retain radioactive and hazardous constituents (McPhee and Glasser, 1993). In fact, much research has focused on improving the effectiveness of grout in adverse environments associated with the disposal of radioactive waste (Ghattas et al., 1998; Mattus, 1998; Merz and Khalil, 1992). As discussed in these references, a variety of cement-polymer composites have been investigated as a means of making grouts more compatible with the radioactive and chemical constituents in waste.
For example, the addition of blast furnace slag to Saltstone,7 which is being used to dispose of low-activity waste at SRS (see Chapter 2), provides a chemical reductant [iron(II)] and a precipitating agent (sulfide) that chemically binds contaminants such as chromium and technetium as insoluble species, thus reducing their tendency to leach from the waste form. Experimentation has shown that leaching of chromium and technetium was effectively reduced to levels that would allow all projected future salt solution compositions to be processed into Saltstone (MMES et al., 1992). These experiments showed that the addition of slag essentially stopped technetium-99 leaching, although it did not reduce nitrate leaching.
3.3.6 Geopolymers and Hydroceramics
Less mature technologies have been investigated for low-activity waste disposal since the mid-1990s. These include geopolymers and hydroceramics, which are described in the following subsections.
________________________
7 The Saltstone that is being used to immobilize low-activity waste at Savannah River contains 5 weight percent cement, 25 weight percent fly ash, 25 weight percent blast furnace slag, and 45 weight percent salt solution.
3.3.6.1 Geopolymers
Geopolymers are ceramic-like, inorganic polymers made from aluminosilicates cross-linked with alkali metal ions (Barbosa et al., 2000; Davidovits, 1994; Kriven et al., 2004). A low water content is used (H2O/M2O ~10-25 weight percent, where M denotes the alkali metals sodium, potassium, or cesium) so that an amorphous geopolymer forms instead of crystalline zeolites. A nominal composition of 4SiO2•Al2O3•M2O is often used to represent the geopolymer matrix, although Si:Al ratios can vary from 1 to 3 depending on the intended application. For cement- and concrete-like applications a ratio of 2:1 is nominally used (Sheppard, 2005). Geopolymers appear to be excellent low-temperature binders and are environmentally more acceptable8 than cement waste forms.
Geopolymers are typically made by mixing an aluminosilicate such as metakaolinite (Al2O3•2SiO2) or Class F fly ash with a highly caustic hydroxide solution and an alkali silicate solution of the type (Na,K,Cs)2Si2O5. Geopolymers can be formed under ambient conditions (Kriven et al., 2004) but often are autoclave cured between 80°C-120°C to produce an amorphous, cross-linked, three-dimensional structure. Curing of large-scale monoliths in a steam room at 40°C-45°C has been found to be adequate for stabilization of mining wastes (Davidovits, 1995; Hermann et al., 1999; van Jaarsveld et al., 1996).
Geopolymers and geopolymeric cements, including but not limited to fly ash-based geopolymeric concretes, are candidates for environmental applications, including permanent encapsulation of radioactive waste (Hanzlicek and Steinerova-Bopndrakova, 2006; Hanzlicek et al., 2006) and other hazardous waste (Perera et al., 2005), and also as sealants, caps, barriers, and other structures necessary for remediation of contaminated sites. Geopolymers have been used in pilot-scale demonstrations on both mining wastes and uranium mill tailings in Europe (Davidovits, 1995; Hermann et al., 1999; van Jaarsveld et al., 1996) and were also investigated in the mid to late 1990s for the disposal of radioactive wastes (Khalil and Merz, 1994; Zosin et al., 1998).
More recently, geopolymers have been investigated as a waste form for stabilization of Resource Conservation and Recovery Act metals; as binders for granular mineral wastes produced by fluidized bed steam reforming for low-activity waste at Hanford; and for the stabilization of cesium-137 and strontium-90 wastes.
Special geopolymer formulations, marketed under the name DuraLith,
________________________
8 Production of the raw materials used to make cement emits CO2. Production of geopolymer materials emits only water vapor; moreover, geopolymers can be made from fly ash, a byproduct and waste from coal-fired power plants.
have been patented (Gong et al., 2006) by the Vitreous State Laboratory (VSL) in Washington, D.C. Testing of DuraLith formulation by Pacific Northwest National Laboratory (Gong et al., 2006) and VSL (W. Lutze, VSL, written communication) showed great promise for stabilization of technetium.
3.3.6.2 Hydroceramics
Hydroceramics are concrete-type materials that are made by curing a mixture of inorganic waste, calcined clay (metakaolin, Al2O3•2SiO2), vermiculite, Na2S, and NaOH with water under hydrothermal conditions (60ºC-200ºC) to form a matrix containing crystalline tectosilicates (zeolites) embedded in a sodium aluminosilicate matrix (Bao et al., 2004). The solidification process occurs as a result of hydration reactions. The NaOH solution dissolves the metakaolin much the same as in geopolymers, but the presence of abundant water or hydroxide results in the production of crystalline silicates instead of an amorphous matrix. These silicates have sodalite and cancrinite structures, which can trap constituents and render them insoluble.
Hydroceramic waste forms have several potential applications to DOE-EM waste streams. They have been shown to be effective for immobilizing low-activity sodium-bearing waste at INL. Additionally, Scheetz and Olanrewaju (2001) have developed hydroceramic waste forms for HLW calcines at INL. However, the need for denitration and high-temperature curing will likely preclude the use of hydroceramics for immobilizing low-activity waste streams at SRS and INL.
3.3.7 Ceramicretes
Ceramicretes are phosphate-bonded ceramics, also known as chemically bonded phosphate ceramics. These materials are produced by reacting magnesium oxide, monopotassium phosphate, and the stoichiometrically required amount of water according to the reaction
MgO + KH2PO4 + 5H2O → MgKPO4•6H2O
This reaction is exothermic and occurs rapidly at room temperature. The reaction product, ceramicrete (MgKPO4•6H2O), is a hard, insoluble phosphate ceramic (Wagh et al., 2003) that contains a considerable amount of bound water. Waste constituents react with the material to form insoluble phosphates or are encapsulated in the matrix.
A patented technology for producing ceramicretes (Wagh and Singh,
1997) has been licensed to treat mixed and low-level wastes and is being used for macroencapsulation and containerization of uranium. Phosphate ceramics are also used for road and highway repairs, and the oil industry is testing these materials for drilling casing and capping. The medical/dental industry is also using several phosphate-ceramic formulations.
DOE has also examined the suitability of this waste form for both micro- and macro-encapsulation of radioactive and hazardous waste streams, e.g., low-activity waste streams typical of Hanford and other sites. Testing of ceramicrete made with INL’s sodium-bearing waste and Hanford Waste Treatment Plant Secondary Waste has been performed at Pacific Northwest National Laboratory.
3.4 DISCUSSION
As the discussion in this chapter illustrates, there are a wide variety of waste form materials that could potentially be used to immobilize radioactive and chemically hazardous wastes. Some waste form materials have been demonstrated in real-world applications on radioactive and hazardous wastes, whereas other have only been researched or demonstrated in the laboratory or at pilot scales.
The committee judges that there are many potential applications of new and improved waste form materials to DOE-EM waste streams. Table 3.7 illustrates the potential compatibility of the waste form materials described in this chapter to some of the DOE-EM waste streams that were described in Chapter 2. Note particularly:
- All eight classes of waste form materials that are described in this chapter are potentially compatible with one or more DOE-EM waste streams.
- Borosilicate glass is already being used by DOE-EM to immobilize HLW, and DOE-EM plans to use glass to immobilize low-activity waste at Hanford (see Chapter 2 and Appendix C). However, other waste form materials, in particular GCMs and crystalline ceramics, are potentially suitable for both of these applications.
- Glass, GCMs, and crystalline ceramic materials have the widest range of potential compatibilities with DOE-EM waste streams—in particular for HLW from tanks and high-sodium wastes, but also for cesium and strontium capsules (should DOE-EM decide not to dispose of those capsules directly) and excess plutonium.
- One or more of the encapsulant waste form materials (i.e., cements, geopolymer, ceramicrete, and hydroceramic waste forms) are potentially compatible with high-sodium wastes, cesium and strontium capsules, and wastes containing iodine-129 and technetium-99.
TABLE 3.7 Waste Form Compatibility with Selected DOE-EM Waste Streams
| Waste Characteristics | Waste Forms | |||||||||
| Waste Types | Radionuclides | Borosilicate Glass | Other Glasses | Glass Composite Materials | Mineral Ceramics (Synroc) | Simple Oxides | Metal Matrix | Zeolites/Hydroceramics | Cements/Cermicrete | Geopolymers |
| HLW Tank Waste | ||||||||||
| INL: Calcine | Cs, Sr, Tc, I | Pa | C? /P | P | ||||||
| SRS: Sludge (Al, Fe) | C | Pd | P | |||||||
| Hanford: Sludge (Al, Fe, Cr, Bi-P) | C | Pc | Pe | P | ||||||
| High-Sodium Waste | ||||||||||
| INL: Sodium-bearing waste | Fission Products (FP), low U, Pu | P | P | C | C | P | P | |||
| SRS: Low-activity | ||||||||||
| waste/saltcake/ supernate | P | P | P | P | C | P | ||||
| Hanford: Low- | ||||||||||
| activity waste/ saltcake/supernate | C | P | P | P | P | P | P | |||
| Other | ||||||||||
| Spent fuel and non-irradiated/ irradiated | Pu, FP, TRU, U | P | ||||||||
| Cesium-strontium capsules | Cs, Sr | P | P | P | P | P | ||||
| Tank heels | FP, U | C | ||||||||
| Excess plutonium oxides | Pu | Pb | P | P | P | P | ||||
| Waste Characteristics | Waste Forms | |||||||||
| Waste Types | Radionuclides | Borosilicate Glass | Other Glasses | Glass Composite Materials | Mineral Ceramics (Synroc) | Simple Oxides | Metal Matrix | Zeolites/Hydroceramics | Cements/Cermicrete | Geopolymers |
| EBR-2 (Experimental Breeder Reactor) | I, Cl | C | P | P | P | |||||
| Melter recycle (Hanford) | I, Tc | P | ||||||||
| I and Tc99 | I, Tc | P | P | P | ||||||
| Orphan wastes | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Depleted Uranium | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
NOTES:
C = current application; P = possible application
a With blending, dissolving, low waste loading.
b Pu solubility low.
c With Bi-P or high SO4.
d High waste loading.
e With high PO4, high waste or high Cr.
Of course, compatibility is just one of several considerations in selecting a waste form material to immobilize a specific waste stream. Other considerations include the ease of processing, cost, and risk. Processes for waste form production are described in the next chapter. The committee’s finding on the fourth charge of its task statement (Box 2.1. in Chapter 2) is given in Chapter 1.
REFERENCES
Advocat, T., C. Fillet, J. Marillet, G. Leturcq, J. M. Boubais, and A. Bonnetier. 1998. “Nd-doped Zirconolite Ceramic and Glass-ceramic Synthesized by Melting and Controlled Cooling,” Mat. Res. Soc. Symp. Proc. 506, 55-62.
Allen, C. C. 1982. “Stability and Alteration of Naturally Occurring Low-Silica Glasses: Implications for the Long Term Stability of Waste Form Glasses,” Scientific Basis for Nuclear Waste Management V, W. Lutze (Ed.), Elsevier Science Publ., New York, 37-44.
American Mineralogist Crystal Structure Database. 2010. http://rruff.geo.arizona.edu/AMS/result.php?mineral=Nepheline.
Andrews, M. K. and J. Harbour. 1997. “Glass Formulation Requirements for Hanford Coupled Operations Using Crystalline Silicotitanates (CST),” WSRC-RP-97-0265. Westinghouse Savannah River Company, Aiken, S.C.
Anthony, R. G., C. V. Phillip, and R. G. Dosch. 1993. “Selective Adsorption and Ion Exchange of Metal Cations and Anions with Silico-Titanates and Layered Titanates,” Waste Manage. 13, 503-512.
Arustamov, A. E., M. I. Ojovan, and M. B. Kachalov. 1999. “Lead and Lead Based Alloys as Waste Matrix Materials,” Mat. Res. Soc. Symp. Proc. 556, 961-966.
Audubert, F., J. Carpena, J. L. Lacout, and F. Tetard. 1997. “Elaboration of an Iodine-Bearing Apatite Iodine Diffusion into a Pb3 (VO4)2 Matrix,” Solid State Ionics 95(1-2), 113-119.
Badyal, Y., M. Karabulut, G. K. Marasinghe, M. L. Saboungi, D. Haeffner, S. Shastri, D. E. Day, and C. S. Ray. 1999. “The Effects of Uranium Oxide High-Level Waste on the Structure of Iron Phosphate Glasses,” In Scientific Basis for Nuclear Waste Management XXII (v. 556), Materials Research Society, Pittsburgh, Penn., 297-303.
Balmer, M. L. and B. C. Bunker. 1995. “Inorganic Ion Exchange Evaluation and Design-Silicotitanate Ion Exchange Waste Conversion,” PNL-10460, Pacific Northwest Laboratory, Richland, Wash.
Bao, Y., S. Kwan, D. D. Siemer, and M. W. Grutzeck. 2004. “Binders for Radioactive Waste Forms Made from Pretreated Calcined Sodium Bearing Waste,” J. Mat. Sci. 39, 481-488.
Barbosa, V. F. F., K. J. D. MacKenzie, and C. Thaumaturgo. 2000. “Synthesis and Characterisation of Materials Based on Inorganic Polymers of Alumina and Silica: Sodium Polysialate Polymers,” Int. J. Inorg. Mat. 2, 309-317.
Bardez, I., D. Caurant, J. L. Dussossoy, P. Loiseau, C. Gervais, F. Ribot, D. R. Neuville, N. Baffier, and C. Fillet. 2006. “Matrices Envisaged for the Immobilization of Concentrated Nuclear Waste Solutions,” Nucl. Sci. and Eng. 153, 272-284.
Barsoum, M. W., A. Ganguley, and G. Hug. 2006. “Microstructural Evidence of Reconstituted Limestone Blocks in the Great Pyramids of Egypt,” J. Amer. Ceram. Soc. 89(12), 3788-3796.
Berry, L. G. and B. Mason. 1959. “Mineralogy Concepts, Descriptions, Determinations,” W.H. Freeman & Co., San Francisco, Calif.
Bibler, N. E. 1980. “Radiolytic Gas Generation in Concrete Made With Incinerator Ash Containing Transuranium Nuclides,” Scientific Basis for Nuclear Waste Management II, 585-592.
Bickford, D. F. and C. M. Jantzen. 1984. “Devitrification of SRL Defense Waste Glass,” Scientific Basis for Nuclear Waste Management VII, G. L. McVay (Ed.), Elsevier Publishing, New York, 557-565.
Boatner, L. A. and B. C. Sales. 1988. “Monazite,” In Radiation Waste Forms for the Future, W. Lutze and R. C. Ewing (Eds.), Amsterdam, Holland, North-Holland Press, 495-564.
Boult, K. A., J. T. Dalton, J. P. Evans, A. R. Hall, A. J. Inn, J. A. C. Marples, and E. L. Paige. 1987. “The Preparation of Fully-active Synroc and its Radiation Stability,” Report No. AERE-R 13 318.
Breck, D. W. 1974. “Zeolite Molecular Sieves: Structure, Chemistry and Use,” John Wiley & Sons, New York.
Brookins, D. G. 1984. “Geochemical Aspects of Radioactive Waste Disposal,” Springer-Verlag, New York.
Buhl, J. Ch., G. Englehardt, and J. Felsche. 1989. “Synthesis, X-ray Diffraction, and MAS n.m.r. Characteristics of Tetrahydroxoborate Sodalite, Na8[AlSiO4]6[B(OH)4]2,” Zeolites 9, 40-44.
Burakov, B. E., M. J. Ojovan, and W. E. Lee. 2010. Materials for Engineering—Vol. 1, Crystalline Materials for Actinide Immobilisation. Imperial College Press, London.
Burghatz, M., H. Matzke, C. Leger, G. Vambenepe, and M. Rome. 1998. “Inert Matrices for the Transmuation of Actinides; Fabrication, Thermal Properties and Radiation Stability of Ceramic Materials,” J. Nucl. Mat. 271, 544-548.
Burnham, C. W. 1981. “The Nature of Multicomponent Aluminosilicate Melts,” Phys. Chem. of the Earth 13-14, 191-227.
Buykx, W. J., K. Hawkins, D. M. Levins, H. Mitamura, R. St. C. Smart, G. T. Stevens, K. G. Watson, D. Weedon, and T. J. White. 1988. “Titanate Ceramics for the Immobilization of Sodium-Bearing High-Level Nuclear Waste.” J. Am. Ceram. Soc. 71(8), 678-688.
Calas, G., M. Le Grand, L. Galoisy, D. Ghale. 2003. “Structural Role of Molybdenum in Nuclear Glasses: an EXAFS Study,” J. Nucl. Mat. 322, 15-20.
Carpena, J., B. Donazzon, E. Ceraulo, and S. Prene. 2001. “Composite Apatitic Cement as a Material to Retain Cesium and Iodine,” Comptes Rendus de L Academie Des Sciences Serie II Fascicule C-Chimie 4, 301-308.
Carpena, J. and J. L. Lacout. 2005. “Calcium Phosphate Nuclear Materials: Apatitic Ceramics for Separated Wastes,” Actualite Chimique, 66-71.
Carter, M. L., E. R. Vance, D. R. G. Mitchell, J. V. Hanna, Z. Zhang, and E. Loi. 2002. “Fabrication, Characterization, and Leach Testing of Hollandite, (Ba,Cs)(Al,Ti)2Ti6O16,” J. Mat. Res. 17, 2578-2589.
Carter, M. L., E. R. Vance, and H. Li. 2004. “Hollandite-rich Ceramic Melts for the Immobilization of Cs.” Mat. Res. Soc. Proc. 807, 249-254.
Caurant, D., P. Loiseau, O. Majerus, V. Aubin-Chevaldonnet, I. Bardez, and A. Quintas. 2009. “Glasses, Glass-Ceramics and Ceramics for Immobilization of Highly Radioactive Nuclear Waste,” Nova Science Publishers, Inc., New York.
Cauranta, D., O. Majérusa, E. Fadela, A. Quintasa, C. Gervaisb, T. Charpentierc. In press. “Structural Investigations of Borosilicate Glasses Containing MoO3 by MAS NMR and Raman Spectroscopies,” J. Nucl. Mat.
Chakoumakos, B. C. 1984. “Systematics of the Pyrochlore Structure Type, Ideal A2B2X6Y,” J. Solid State Chem. 53 120-129.
Chakoumakos, B. C. and R. C. Ewing. 1985. “Crystal Chemical Constraints on the Formation of Actinide Pyrochlores,” Mat. Res. Soc. Symp. Proc. 44, 641-646.
Chapman, K., P. Chupas, and T. M. Nenoff. 2010. “Radioactive Iodine Capture in Silver-Loaded Zeolites Through Nanoscale Silver Iodide Formation,” J. Amer. Chem. Soc. 132(26), 8897-8899.
Chen, F. and D. E. Day. 1999. “Corrosion of Selected Refractories by Iron Phosphate Melts,” Ceram. Trans. 93, 213-220.
Chick, L. A., L. R. Bunnell, D. M. Strachan, H. E. Kissinger, and F. N. Hodges. 1986. “Evaluation of Lead-Iron-Phosphate Glass as a High Level Waste Form,” Advan. in Ceram. 20, 149-156.
Cicero, C. A., S. L. Marra, and M. K. Andrews. 1993. “Phase Stability Determinations of DWPF Waste Glasses,” WSRC-TR-93-227, Rev. 0, Westinghouse Savannah River Company, Aiken, S.C. (May).
Clarke, D. R. 1981. “Preferential Dissolution of an Intergranular Amorphous Phase in a Nuclear Waste Ceramic.” J. Am. Ceram. Soc. 64(6), C-89–C-90.
Clarke, D. R., C. M. Jantzen, and A. B. Harker. 1982. “Dissolution of Tailored Ceramic Nuclear Waste Forms,” Nucl. and Chem. Waste Man. 3, 59-66.
Clinard Jr., F. W., L. W. Hobbs, C. C. Land, D. E. Peterson, D. L. Rohr, R. B. Roof. 1982. “Alpha Decay Self-Irradiation Damage in 238Pu-substituted zirconolite,” J. Nucl. Mat. 105, 248-256.
Clinard Jr., F. W., D. E. Peterson, D. L. Rohr, and L. W. Hobbs. 1984. “Self-Irradiation Effects in 238Pu-Substituted Zirconolite: I. Temperature Dependence of Damage,” J. Nucl. Mat. 126, 245-254.
Cocke, D. L. and M. Y. A. Molla. 1993. “The Chemistry and Leaching Mechanisms of Hazardous Substances in Cementitious Solidification/Stabilization Systems,” In Chemistry and Microstructure of Solidified Waste Forms, R. D. Spence (Ed.), Lewis Publishers, Boca Raton, Fla., 187-242.
Cooper, J. A., D. R. Cousens, J. A. Hanna, R. A. Lewis, S. Myhra, R. L. Segall, R. St. C. Smart, P. S. Turner, and T. J. White. 1986. “Intergranular Films and Pore Surfaces in Synroc C: Structure, Composition, and Dissolution Characteristics.” J. Amer. Ceram. Soc. 69(4), 347-352.
Cougar, M. D. L., B. E. Scheetz, and D. M. Roy. 1996. “Ettringite and C-S-H Portland Cement Phases for Waste Ion Immobilization, A Review.” Waste Manage. 16, 295-303.
Cronstedt, A. F. 1756. “Observation and Description of an Unknown Kind of Rock to be Named Zeolites,” Kongl. Vetenskaps Akad. Handl. Stockholm 17, 120-123 (in Swedish).
Crum, J. V., B. J. Riley, S. K. Sundaram, S. A. Arreguin, J. Matyas, M. J. Schweiger, B. T. Rieck, and J. D. Vienna. 2009. “Alternative Waste Forms for Electro-Chemical Salt Waste,” AFCI-WAST-PMO-MI-DV-2009-000293, Pacific Northwest National Laboratory, Richland, Wash. (October).
Dacheux, N., R. Podor, B. Chassigneux, V. Brandel, and M. Genet. 1998a. “Actinides Immobilization in New Matrices Based on Solid Solutions: Th4-xMxIV(PO4)(4)P2O7, (M-IV = U-238, Pu-239),” J. Alloys and Comp. 271, 236-239.
Dacheux, N., R. Podor, V. Brandel, and M. Genet. 1998b. “Investigations of Systems ThO2MO2-P2O5 (M=U, Ce, Zr, Pu). Solid Solutions of Thorium-Uranium(IV) and Thorium-Plutonium(IV) Phosphate-Diphosphates,” J. Nucl. Mat. 252(3), 179-186.
Davidovits, J. 1994. “Geopolymers: Man-Made Rock Geosynthesis and the Resulting Development of Very Early High Strength Cement,” J. Mat. Ed. 16(2-3), 91-137.
Davidovits, J. 1995. “Recent Progresses in Concretes for Nuclear Waste and Uranium Waste Containment,” Concrete Int. 16, 53-58.
Day, D. E., X. Yu, G. J. Long, and R. K. Brow. 1997. “Properties and Structure of Sodium-Iron Phosphate Glasses,” J. Non-Cryst. Solids 215(1), 21-31.
Day, D. E., Z. Wu, C. S. Ray, and P. R. Hrma. 1998. “Chemically Durable Iron Phosphate Glass Wasteforms,” J. Non-Cryst. Solids 241(1), 1-12.
Day, D. E., C. S. Ray, and C. W. Kim. 2004. “Iron Phosphate Glasses: An Alternative for Vitrifying Certain Nuclear Wastes,” Final Report for the U.S. Department of Energy’s Environmental Management Science Program. Available at http://www.osti.gov/em52/2001projsum/73976.pdf.
De, A. K., B. Luckscheiter, W. Lutze, G. Malow, and E. Schiewer. 1976. “Development of Glass Ceramics for the Incorporation of Fission Products.” Ceram. Bull. 55, 500-503.
Deer, W. A., R. A. Howie, and J. Zussman. 1963. “Rock-Forming Minerals, Vol. IV,” John Wiley & Sons, Inc., New York.
DOE [U.S. Department of Energy]. 1981. Interim Performance Specifications for Waste Forms for Geologic Isolation. NWTS-19 Draft. Office of Nuclear Waste Isolation, Columbus, Ohio (October).
Donald, I. W., B. L. Metcalfe, and R. N. J. Taylor. 1997. “Review: The Immobilization of High Level Radioactive Wastes Using Ceramics and Glasses,” J. Mat. Sci. 32, 5851-5887.
Dunson Jr., J. B., A. M. Eisenberg, R. L. Schuyler III, H. G. Haight Jr., V. E. Mello, T. H. Gould Jr., J. L. Butler, and J. B. Pickett. 1982. Assessment of Processes, Facilities, and Costs for Alternative Solid Forms for Immobilization of SRP Defense Wastes. DP-1625, E.I. DuPont deNemours & Co., Savannah River Laboratory. Aiken, S.C. (March).
Ebert, W. L. 2005. “Testing to Evaluate the Suitability of Waste Forms Developed for Electrometallurgically Treated Spent Sodium-Bonded Nuclear Fuel for Disposal in the Yucca Mountain Repository,” ANL Report 05/43, Argonne National Laboratory, Argonne, Ill. (September).
Eliassen, R. and M. I. Goldman. 1959. Disposal of High-Level Wastes by Fixation in Fused Ceramics. Hearings on Industrial Radioactive Waste Disposal, Vol. 3, R. L. Doan (Ed.), U.S. Government Printing Office, Washington, D.C.
Ellison, A. J. G. and A. Navrotsky. 1990. “Thermochemistry and Structure of Model Waste Glass Compositions,” Scientific Basis for Nuclear Waste Management XIII, V.M. Oversby and P. W. Brown (Eds.), Materials Research Society, Pittsburgh, Penn., 193-207.
Ewing, R. C. 1979. “Natural Glasses: Analogues for Radioactive Waste Forms,” Scientific Basis for Nuclear Waste Management I, G. J. McCarthy (Ed.), Plenum Press, New York, 57-68.
Ewing, R. C. 1999. “Nuclear Waste Forms For Actinides,” Proc. Natl. Acad. Sci. 96, 3432-3439.
Ewing, R. C. 2001. “The Design and Evaluation of Nuclear-waste Forms: Clues from Mineralogy,” Can. Mineral. 39, 697-715.
Ewing, R. C., W. Lutze, and W. J. Weber. 1995a. “Zircon: A Host-phase for the Disposal of Weapons Plutonium,” J. Mat. Res. 10(2), 243-246.
Ewing, R. C., W. J. Weber, and W. J. Clinard. 1995b. “Radiation Effects in Nuclear Waste Forms,” Progress in Nuclear Energy 29(2), 63-127.
Ewing, R. C., W. J. Weber, and W. Lutze. 1996. “Crystalline Ceramics: Waste Forms for the Disposal of Weapons Plutonium.” In Disposal of Ex-Weapons Plutonium as Waste, E. R. Merz and C. E. Walter (Eds.), NATO ASI Series, Kluwer Academic Publishers, Dordrecht, The Netherlands, 65-83.
Ewing, R. C. and L. M. Wang. 2002. “Phosphates as Nuclear Waste Forms” In Reviews in Mineralogy & Geochemistry 48, M. J. Kohn, J. Rakovan, and J. M. Hughes (Eds.), 673-699.
Ewing, R. C., W. J. Weber, and J. Lian. 2004. “Pyrochlore (A2B2O7): A Nuclear Waste Form for the Immobilization of Plutonium and “Minor” Actinides,” J. Appl. Phys. 95, 5949-5971.
Ewing, R. C. and W. J. Weber. 2010. “Chapter 35: Actinide Waste Forms and Radiation Effects,” In The Chemistry of the Actinides and Transactinide Elements 6, L. R. Morss, N. M. Edelstein, and J. Fuger (Eds.), Springer, New York, 3813-3888.
Fahey, J. A., W. J. Weber, and F. J. Rotella. 1985. “An X-ray and Neutron Powder Diffraction Study of the Ca2+xNd8-x(SiO4)6O2-0.5x System,” J. Solid State Chem. 60, 145-158.
Felsche, J. 1972. “Rare Earth Silicates with the Apatite Structure,” J. Solid State Chem. 5(2), 266-275.
Fleet, M. E. 1989. “Structures of Sodium Alumino-Germanate Sodalites,” Acta Cryst. C45, 843-847.
Gallagher, S. A. and G. J. McCarthy 1981. “Preparation and X-ray Characterization of Pollucite (CsAlSi2O6),” Inorg. Nucl. Chem. 43, 1773-1777.
Garino, T. J., T. M. Nenoff, T. J. Park, and A. Navrotsky. 2009. “The Crystallization of Ba-Substituted CsTiSi2O6.5 Pollucite using CsTiSi2O6.5 Seed Crystals,” J. Amer. Ceram. Soc. 92(9), 2144-2146.
Garino, T. J., T. M. Nenoff, J. L. Krumhansl, and D. X. Rademacher, 2010. “Development of Iodine Waste Forms using Low-Temperature Sintering Glass,” In Materials Challenges in Alternative and Renewable Energy, G. Wicks, J. Simon, R. Zidan, E. Lara-Curzio, T. Adams, J. Zayas, A. Karkamkar, R. Sindelar, and B. Garcia-Diaz (Eds.), Ceram. Trans. 224, John Wiley & Sons, Inc., Hoboken, N.J., 305-314.
Garino, T .J. and T. M. Nenoff. 2011. “Low-Temperature Sintering Bi-Si-Zn Oxide Glasses for Use in Either Glass Composite Materials or Core/Shell 129I Waste Forms,” J. Amer. Ceram. Soc. Available at http://onlinelibrary.wiley.com/doi/10.1111/j.1551-2916.2011.04542.x/pdf.
Garmon, L. 1981. “The Box Within a Box Within a Box,” Sci. News 120, 396-399.
Ghattas, N. K., S. B. Eskander, H. A. Shatta, H. Ghorab, and N. E. Ikladious. 1998. “Some Applications of Polymer Modified Cement for Nuclear Wastes,” Spectrum 1998, Denver, Colo., 645-651.
Glasser, F. P. 1997. “Fundamental Aspects of Cement Solidification and Stabilization,” J. Haz. Mat. 52(2-3), 151-170.
Glorieux, B., J. M. Montel, and M. Matecki. 2009. “Synthesis and Sintering of a Monazite-Brabantite Solid Solution Ceramics Using Metaphosphate,” J. Eur. Ceram. Soc. 29, 1679-1686.
Goldman, M. I., J. A. Servizi, R. S. Daniels, T. H. Y. Tebbutt, R. T. Burns, and R. A. Lauderdale. 1958. “Retention of Fission Products in Ceramic-Glaze-Type Fusions,” Proc. 2nd UN International Conference on Peaceful Uses of Atomic Energy, Geneva, United Nations, New York.
Gombert, D. 2007. “Global Nuclear Energy Partnership Integrated Waste Management Strategy Waste Treatment Baseline Study,” GNEP-WAST-WAST-AI-RT-2007-000324, Idaho National Laboratory, Idaho Falls, Idaho.
Gong, W. L., R. C. Ewing, L. M. Wang, and H. S. Xie. 1995. “Crichtonite Structure Type (AM21O38 and A2M19O36) as a Host Phase in Crystalline Waste Form Ceramics,” Scientific Basis for Nuclear Waste Management XVIII, T. Murakami and R. C. Ewing (Eds.), Proc. Mat. Res. Soc. 353, 807-815.
Gong, W. L., W. Lutze, and R. C. Ewing. 2000. “Zirconia Ceramics for Excess Weapons Plutonium Waste,” J. Nucl. Mat. 277(2-3), 239-249.
Gong, W. L., W. Lutze, and I. L. Pegg. 2006. “Low-Temperature Solidification of Radioactive and Hazardous Wastes,” U.S. Patent 2006/0211908A1.
Greaves, G. N. 1989. “EXAFS, Glass Structure and Diffusion,” Phil. Mag. B 60(6), 793-800.
Haaker, R. F. and R. C. Ewing. 1981. Naturally Occurring Crystalline Phases: Analogues for Radioactive Waste Forms. PNL-3505, Pacific Northwest Laboratory, Richland, Wash..
Hanzlicek, T. and M. Steinerova-Bopndrakova. 2006. “Immobilization of Toxic Metals in Solidified Systems of Siloxo-Sial Networks,” J. Amer. Ceram. Soc. 89(3), 968-970.
Hanzlicek, T., M. Steinerova, and P. Straka. 2006. “Radioactive Metal Isotopes Stabilized in Geopolymer Matrix: Determination of a Leaching Extract by a Radiotracer Method,” J. Amer. Ceram. Soc. 89(11), 3541-3543.
Harker, A. B., C. M. Jantzen, D. R. Clarke, and P. E. D. Morgan. 1981a. “Tailored Ceramic Nuclear Waste Forms: Preparation and Characterization,” Scientific Basis for Nuclear Waste Management III, J. G. Moore (Ed.), Plenum Press, New York, 139-146.
Harker, A. B., D. R. Clarke, C. M. Jantzen, and P. E. D. Morgan. 1981b. “Surface and Inter-facial Characterization of Tailored Ceramic Nuclear Waste Forms,” In Surfaces and Interfaces in Ceramic and Ceramic-Metal Systems, J. Pask and A. Evans (Eds.), Materials Sci. Res. Symp., 14, Plenum Press, New York, 207-216.
Harker, A. B., P. E. D. Morgan, D. R. Clarke, J. J. Flintoff, and C. M. Jantzen. 1983. “Formulation and Processing of Alumina Based Ceramic Nuclear Waste Forms,” DOE/ET/41900-18, Rockwell International Corp., Thousand Oaks, Calif., 47-107.
Harker, A. B. 1988. “Tailored Ceramics,” In Radioactive Waste Forms for the Future, W. Lutze and R. C. Ewing (Eds.). Amsterdam, North-Holland, 335-392.
Harrison, M. T. and C. R. Scales. 2008a. “Development of Borosilicate Glass Compositions for the Immobilizaion of the UK’s Separated Plutonium Stocks,” Mat. Res. Soc. Symp. Proc. 1107, 405-412.
Harrison, M. T. and C. R. Scales. 2008b. “Durability of Borosilicate Glass Compositions for the Immobilisation of the UK’s Separated Plutonium Stocks,” Mat. Res. Soc. Symp. Proc. 1107, 429-436.
Hatch, L. P. 1953. “Ultimate Disposal of Radioactive Wastes,” Am. Sci. 41, 410-421.
Hatch, L. P., G. G. Neth, and E. J. Tuthill. 1962. “Ultimate Disposal of High Level Radioactive Wastes—Fixation in Phosphate Glass with Emphasis on the Continuous Mode of Plant Operation,” BNL-6321, Brookhaven National Laboratory, Upton, New York (October).
Hayward, P. J. 1988. “Glass-Ceramics,” In W. Lutze and R. C. Ewing (Eds.), Radioactive Waste Forms for the Future, North Holland, Amsterdam, 427-494.
Hench, L. L., R. J. Charles, A. R. Cooper, R. C. Ewing, J. R. Hutchins, D. W. Readey, F. L. VerSnyder, and S. M. Widerhorn. 1981. The Evaluation and Selection of Candidate High Level Waste Forms, Report No. 3, USDOE/TIC-11472, Alternative Waste Forms Peer Review Panel, National Technical Information Service, Springfield, Va.
Hermann, E., C. Kunze, R. Gatzweiler, G. Kiebig, and J. Davidovits. 1999. “Solidification of Various Radioactive Residues by Geopolymere with Special Emphasis on Long-Term Stability,” Geopolymere 99 Proceedings.
Higgins, F. M., N. H. de Leeuw, and S. C. Parker. 2002. “Modeling the Effect of Water on Cation Exchange in Zeolite A,” J. Mat. Chem. 12, 124.
Hrma, P. R., G. F. Peipel, M. J. Schweiger, D. E. Smith, D. S. Kim, P. E. Redgate, J. D. Vienna, C. A. LoPresti, D. B. Simpson, D. K. Peeler, and M. H. Langowski. 1994. “Property/Composition Relationships for Hanford High-Level Waste Glasses Melting at 1150°C, Vols. 1 and 2,” U.S. DOE Report PNL-10359, Pacific Northwest Laboratory, Richland, Wash. (December).
Hyatt, N. C., R. J. Short, R. J. Hand, W. E. Lee, F. Livens, J. M. Charnock, and R. L. Bilsborrow. 2004. “The Structural Chemistry of Molybdenum in Model High Level Nuclear Waste Glasses, Investigated by Mo K-Edge X-Ray Absorption Spectroscopy,” Ceram. Trans. 168, 179-187.
Jantzen, C. M. 1986a. “Investigation of Lead-Iron-Phosphate Glass for SRP Waste,” DP-1729, E.I. du Pont de Nemours & Co., Savannah River Laboratory, Aiken, S.C. (October).
Jantzen, C. M. 1986b. “Investigation of Lead-Iron-Phosphate Glass for SRP Waste,” Advances in Ceramics 20, D. E. Clark, W. B. White and A. J. Machiels (Eds.), American Ceramic Society, Westerville, Ohio, 157-165.
Jantzen, C. M. 2008. “Mineralization of Radioactive Wastes by Fluidized Bed Steam Reforming (FBSR): Comparison to Vitreous Waste Forms, and Pertinent Durability Testing,” WSRC-STI-2008-00268, Savannah River National Laboratory, Aiken, S.C.
Jantzen, C. M. 2011. “Development of Glass Matrices for High Level Radioactive Wastes,” In Handbook of Advanced Radioactive Waste Conditioning Technologies, M. I. Ojovan (Ed.), Woodhead Publishing, U.K., 230-292.
Jantzen, C. M. and F. P. Glasser. 1979. “Stabilization of Nuclear Waste Constituents in Portland Cement,” Amer. Ceram. Soc. Bull. 58(4), 459-466.
Jantzen, C. M., D. R. Clarke, P. E. D. Morgan, and A. B. Harker. 1982a. “Leaching of Polyphase Nuclear Waste Ceramics: Microstructural and Phase Characterization.” J. Amer. Ceram. Soc. 65(6), 292-300.
Jantzen, C. M., J. Flintoff, P. E. D. Morgan, A. B. Harker, and D. R. Clarke. 1982b. “Ceramic Nuclear Waste Forms,” In Proceedings of International Seminar on Chemistry and Process Engineering for High Level Liquid Waste Solidification, Julich, FRG, Juel-Conf 42(2), 693-706.
Jantzen, C. M. and M. J. Plodinec. 1984. “Thermodynamic Model of Natural, Medieval, and Nuclear Waste Glass Durability,” J. Non-Cryst. Solids 67, 207-233.
Jantzen, C. M., D. F. Bickford, D. G. Karraker, and G. G. Wicks. 1984. “Time-Temperature-Transformation Kinetics in SRL Waste Glass,” Advan. in Ceram. 8, 30-38.
Jantzen, C. M., A. D. Cozzi, and N. E. Bibler. 2005. “High Level Waste Processing Experience with Increased Waste Loadings,” In Environmental Issues and Waste Management Technologies X, J. D. Vienna, C. C. Herman, and S. L. Marra (Eds), Ceram. Trans. 168, 31-49.
Jantzen, C. M. and M. R. Williams, 2008, “Fluidized Bed Steam Reforming (FBSR) Mineralization for High Organic and Nitrate Waste Streams for the Global Nuclear Energy Partnership (GNEP),” WM2008, Conference, Phoenix, Ariz., Available at http://www.wmsym.org/archives/2008/pdfs/8314.pdf.
Jantzen, C. M., M. R. Williams, N. E. Bibler, C. L. Crawford, and A. R. Jurgensen. 2008. “Fluidized Bed Steam Reformed (FBSR) Mineral Waste Forms: Application to Cs-137/Sr-90 Wastes for the Global Nuclear Energy Partnership (GNEP),” WSRC-MS-2008-00013, Westinghouse Savannah River Company, Aiken, S.C.
Jiang, W. and D. M. Roy. 1994. “Ancient Analogues Concerning Stability and Durability of Cementitious Wasteform,” In Scientifc Basis for Nuclear Waste Management XVII, A. Barkatt and R. A. Van Konynenburg (Eds.), Mat. Res. Soc. Symp. Proc. 33, 335-340.
Kahl, L. 1986. “Hydrolytic Durability of Lead-Iron-Phosphate Glasses,” Advances in Ceramics, 20, D. E. Clark, W. B. White and A. J. Machiels (Eds.), American Ceramic Society, Westerville, Ohio, 141-148.
Kaminski, M. D., C. J. Mertz, M. Ferrandon, N. L. Dietz, and G. Sandi. 2009. “Physical Properties of an Alumino-silicate Waste Form for Cesium and Strontium,” J. Nucl. Mat. 392, 510-518.
Karabulut, M., G. K. Marasinghe, C. S. Ray, D. E. Day, O. Ozturk, and G. D. Waddill. 1999. “X-ray Photoelectron and Mossbauer Spectroscopic Studies of Iron Phosphate Glasses Containing U, Cs, and Bi,” J. Non-Cryst. Solids 249(2-3), 106-116.
Kerr, R. A. 1979a. “Nuclear Waste Disposal: Alternatives to Solidification in Glass Proposed,” Science 204, 289-291.
Kerr, R. A. 1979b. “Academy Squabbles over Radwaste Report,” Science 205, 287-289.
Khalil, M. Y. and E. Merz. 1994. “Immobilization of Intermediate-Level Wastes in Geopolymers,” J. Nucl. Mat. 211, 141-148.
Kim, D. S., W. C. Buchmiller, M. J. Schweiger, J. D. Vienna, D. E. Day, C. W. Kim, D. Zhu, T. E. Day, T. Neidt, D. K. Peeler, T. B. Edwards, I. A. Reamer, and R. J. Workman. 2003. “Iron Phosphate Glass as an Alternative Waste-Form for Hanford LAW,” PNNL-14251, Pacific Northwest National Laboratory, Richland, Wash. (February).
Kim, J. G., J. H. Lee, I. T. Kim, and E. H. Kim. 2007. “Fabrication of a Glass-Bonded Zeolite Waste Form for Waste LiCl Salt,” J. Ind. Eng. Chem. 13(2), 292-298.
Kim, J. Y., Z. L. Dong, and T. J. White. 2005. “Model Apatite Systems for the Stabilization of Toxic Metals: II, Cation and Metalloid Substitutions in Chlorapatites,” J. Amer. Ceram. Soc. 88, 1253-1260.
Klingenberg, R. and J. Felsche. 1986. “Interstitial Cristobalite-type Compounds (Na2O)0.33Na[AlSiO4]),” J. Solid State Chem. 61, 40-46.
Knecht, D. A. and J. R. Berreth. 1981. “Immobilization of Idaho Chemical Processing Plant High Level Wastes,” In Proceedings of the International Seminar on Chemistry and Process Engineering for High-Level Liquid Waste Solidification, R. Odoj and E. Merz (Eds.), Julich, FRG, Juel-Conf 42(1), 252-276.
Komameni, S. and W. B. White. 1981. “Stability of Pollucite in Hydrothermal Fluids,” Scientific Basis Nuclear Waste Management III, J. G. Moore (Ed.), New York, Plenum Press, 387-396.
Kovach, L. A., and W. M. Murphy (Eds.) 1995. Proceedings of the Workshop on the Role of Natural Analogs in Geologic Disposal of High-Level Nuclear Waste, NUREG/CP-0147 (CNWRA 93-020), Center for Nuclear Waste Regulatory Analyses, San Antonio, Texas.
Kriven, W. M., M. Gordon, and J. Bell. 2004. “Geopolymers: Nanoparticulate, Nanoporous Ceramics Made Under Ambient Conditions,” Microscopy and Microanalysis ‘04 (Proc. 62nd Annual Meeting of Microscopy Society of America) 10, 404-405.
Krupka, K. M. and R. J. Serene. 1998. Effects on Radionuclide Concentrations by Interactions in Support of Performance Assessment of Low-Level Radioactive Waste Disposal Facilities, NUREG/CR-6377 (PNNL-11408), Pacific Northwest National Laboratory, Richland, Wash. (May).
Lashtchenova, T. N. and S. V. Stefanovsky. 1998a. “Immobilization of Incinerator Ash in Synroc-Glass Material,” In Proceedings of the IT3 International Conference on Incineration & Thermal Treatment Technologies, Salt Lake City, Utah, 603-607.
Lashtchenova, T. N. and S. V. Stefanovsky. 1998b. “Titanium-Silicate Based Glass-Crystalline Wasteforms,” In M. K. Choudhary, N. Y. Huff, and C. H. Drummond III (Eds.), Proceedings of the XVIII International Congress on Glass, San-Francisco, Calif.
Laverov, N. P., S. V. Yudintsev, T. S. Livshits, S. V. Stefanovsky, A. N. Lukinykh, and R. C. Ewing. 2010. “Synthetic Minerals with the Pyrochlore and Garnet Structures for Immobilization of Actinide-Containing Wastes,” Geochem. Int. 48, 1-14.
Lee, W. E., M. I. Ojovan, M. C. Stennett, and N. C. Hyatt. 2006. “Immobilisation of Radioactive Waste in Glasses, Glass Composite Materials and Ceramics,” Adv. in Appl. Ceram. 105(1), 3-12.
Li, H., Y. Su, J. D. Vienna, and P. Hrma. 2000. “Raman Spectroscopic Study—Effects of B2O3, Na2O, and SiO2 on Nepheline (NaAlSiO4) Crystallization in Simulated High Level Waste Glasses,” Ceram. Trans. 107, 469-477.
Lian, I., S. V. Yudintsev, S. V. Stefanovsky, O. I. Kirjanova, and R. C. Ewing. 2002. “Ion Induced Amorphization of Murataite,” Mat. Res. Soc. Symp. Proc. 713, 455-460.
LLNL (Lawrence Livermore National Laboratory). 1996. Fissile Material Disposition Program–Screening of Alternate Immobilization Candidates for Disposition of Surplus Fissile Materials, UCRL-ID-118819, L-20790-1, LLNL, Livermore, California.
Loiseau, P., D. Caurant, N. Baffler, L. Mazerolles, and C. Fillet. 2001. “Development of Zirconolite-Based Glass-Ceramics for the Conditioning of Actinides,” Mat. Res. Soc. Symp. Proc. 663, 179-187.
Lumpkin, G. R. 2001. “Alpha-decay Damage and Aqueous Durability of Actinide Host Phases in Natural Systems,” J. Nucl. Mat. 289, 136-166.
Lumpkin, G. R. 2006. “Ceramic Waste Forms for Actinides,” Elements 2, 365-372.
Lutze, W., J. Borchardt, and A. K. De. 1979. “Characterization of Glass and Glass Ceramic Nuclear Waste Forms,” in Scientific Basis for Nuclear Waste Management I, G. J. McCarthy (Ed.), Plenum Press, New York, 69-81.
Lutze, W. and R. C. Ewing. 1988. Radioactive Waste Forms for the Future, North Holland, Amsterdam.
Maddrell, E. R. 2001. “Generalized Titanate Ceramic Waste Form for Advanced Purex Reprocessing,” J. Amer. Ceram. Soc. 84, 1187-1189.
Malow, G., W. Lutze, and R. C. Ewing. 1984. “Alteration Effects and Leach Rates of Basaltic Glasses: Implications for the Long-Term Stability of Nuclear Waste Form Borosilicate Glasses,” J. Non-Cryst. Solids 67, 305-321.
Marasinghe, G. K., M. Karabulut, C. S. Ray, D. E. Day, M. G. Shumsky, W. B. Yelon, C. H. Booth, P. G. Allen, and D. K. Shuh. 1997. “Structural Features of Iron Phosphate Glasses,” J. Non-Cryst. Solids 222, 144-152.
Marasinghe, G. K., M. Karabulut, C. S. Ray, D. E. Day, C. H. Booth, P. G. Allen, and D. K. Shuh. 1998. “Redox Characteristics and Structural Properties of Iron Phosphate Glasses: A Potential Host Matrix for Vitrifying High Level Nuclear Waste,” Ceram. Trans. 87, 261-270.
Marasinghe, G. K., M. Karabulut, C. S. Ray, D. E. Day, P. G. Allen, J. J. Bucher, D. K. Shuh, Y. Bagyal, M. L. Saboungi, M. Grimsditch, S. Shastri, and D. Heaffner. 1999. “Effects of Nuclear Waste Composition on Redox Equilibria, Structural Features, and Crystallization Characteristics of Iron Phosphate Glasses,” Ceram. Trans. 93, 195-202.
Marasinghe, G. K., M. Karabulut, X. Fang, C. S. Ray, and D. E. Day. 2000. “Vitrified Iron Phosphate Nuclear Waste Forms Containing Multiple Waste Components,” Ceram. Trans. 107, 115-122.
Marasinghe, G. K., M. Karabulut, X. Fang, C. S. Ray, and D. E. Day. 2001. “Iron Phosphate Glasses: An Alternative to Borosilicate Glasses for Immobilizing Certain Nuclear Wastes,” Environmental Issues and Waste Management Technologies VI, Ceram. Trans. 119, 361-368.
Marra, J. C., D. K. Peeler, and C. M. Jantzen. 2006. “Development of an Alternate Glass Formulation for Vitrification of Excess Plutonium,” WSRC-TR-2006-00031, Westinghouse Savannah River Laboratory, Aiken, S.C. (January).
Marsden, K. C. and B. Westphal. 2006. “Production-Scale Metal Waste Process Qualification,” Proc. 2006 Int. Pyroprocessing Conf., Idaho Falls, Idaho, August 8-10.
Martin, C., I. Bibet, and T. Advocat. 2002. “Alteration of a Zirconolite Glass-ceramic Matrix under Hydrothermal Conditions,” Mat. Res. Soc. Symp. Proc. 713, 405-411.
Mason, J. B., T. W. Oliver, M. P. Carson, and G. M. Hill. 1999. “Studsvik Processing Facility Pyrolysis/Steam Reforming Technology for Volume and Weight Reduction and Stabilization of LLRW and Mixed Wastes,” WM’99 Conference, Tucson, Ariz., Available at http://www.wmsym.org/archives/1999/60/60-3.pdf.
Mason, J. B., J. McKibben, K. Ryan, and D. Schmoker. 2003. “Steam Reforming Technology for Denitration and Immobilization of DOE Tank Wastes,” WM ‘03 Conference, Tucson, Ariz., Available at http://www.wmsym.org/archives/2003/pdfs/302.pdf.
Mattigod, S. V., B. P. McGrail, D. E. McCready, L. Wang, K. E. Parker, and J. S. Young. 2006. “Synthesis and Structure of Perrhenate Sodalite,” J. Microporous & Mesopourous Mat. 91(1-3), 139-144.
Mattus, C. H. 1998. “Sulfur Polymer Cement for Macro Encapsulation of Mixed Waste Debris,” Spectrum ’98, Denver, CO, 652.
Mawson, C. A. 1965. Management of Radioactive Wastes. D. VanNostrand Co., Inc., Princeton, New Jersey.
McCarthy, G. J. 1973. “Quartz-Matrix Isolation of Radioactive Wastes.” J. Mat. Sci. 8, 1358-1359.
McCarthy, G. J. 1977. “High Level Waste Ceramics: Materials Considerations, Process Simulation and Product Characterization.” Nucl. Tech. 32, 92-105.
McCarthy, G. J. and M. T. Davidson. 1975. “Ceramic Nuclear Waste Forms” Am. Ceram. Soc. Bull. 54(9), 782-786.
McCarthy, G. J., J. G. Pepin, and D. D. Davis. 1979a. “Crystal Chemistry and Phase Relations in the Synthetic Minerals of Ceramic Waste Forms: I. Fluorite and Monazite Structure Phases,” Scientific Basis for Nuclear Waste Management, Plenum Press, New York, 297-306.
McCarthy, G. J., J. G. Pepin, D. E. Pfoertsch, and D. R. Clarke. 1979b. “Crystal Chemistry of the Synthetic Minerals in Current Supercalcine-Ceramics,” Ceramics in Nuclear Waste Management, CONF-790420, National Technical Information Service, Springfield, Virginia, 315-320.
McCready, D. E., M. L. Balmer, and K. D. Keefer. 1997. “Experimental and Calculated X-ray Diffraction Data for Cesium Titanium Silicate, CsTiSi2O6,5: A New Zeolite,” Powder Diffr. 12(1), 40-46.
McDaniel, E. W. and D. B. Delzer. 1988. “FUETAP Concrete,” In Radioactive Waste Forms for the Future, W. Lutze and R. C. Ewing (Eds.), North-Holland, Amsterdam, 565-588.
McFarlane, H. F., K. M. Goff, F. S. Felicione, C. C. Dwight, and D. B. Barber. 1997. “Hot Demonstrations of Nuclear-Waste Processing Technologies,” JOM 49(7), 14-21.
McGlinn, P. J., T. Advocat, E. LoI, G. Leturcq, and J. P. Mestre. 2001. “Nd- and Ce-doped Ceramic Glass Composites: Chemical Durability under Aqueous Conditions and Surface Alteration in a Moist Clay Medium at 90°C,” Mat. Res. Soc. Symp. Proc. 663, 249-258.
McPhee, D. E. and F. P. Glasser. 1993. “Immobilization Science of Cement Systems,” Mat. Res. Soc. Bull. 18, 66-71.
Meldrum, A., L. A. Boatner, and R. C. Ewing. 2000. “A Comparison of Radiation Effects in Crystalline ABO4-type Phosphates and Silicates,” Mineral. Mag. 64, 185-194.
Merz, R. and M. Y. Khalil. 1992. “Geopolymer and Cement Glass: Promising Fixation and Solidification Matrices for Nuclear and Hazardous Wastes,” Spectrum ’92, International Topical Meeting, Nuclear and Hazardous Waste Management, Vol. 2, Boise, Idaho, 914.
Mesko, M. and D. E. Day. 1999. “Immobilization of Spent Nuclear Fuel in Iron Phosphate Glass,” J. Nucl. Mat. 273(1), 27-36.
Mesko, M., D. E. Day, and B. C. Bunker. 1998. “Immobilization of High-Level Radioactive Sludges in Iron Phosphates Glasses,” In Science and Technology for Disposal of Radioactive Tank Waste, W. W. Schulz and N. J. Lombardo (Eds.), Plenum Publishing Corp., New York, 379-390.
Mesko, M., D. E. Day, and B. C. Bunker. 2000. “Immobilization of CsCl and SrF2 in Iron Phosphate Glass,” Waste Manage. 20(4), 271-278.
Meyers, B., G. A. Armantrout, C. M. Jantzen, A. Jostsons, J. M. McKibben, H. F. Shaw, D. M. Strachan, and J. D. Vienna. 1998. “Technical Evaluation Panel Summary Report: Ceramic and Glass Immobilization Options,” UCRL-ID-129315, Lawrence Livermore National Laboratory, Livermore, Calif. (January).
Miller, J. E. and N. E. Brown. 1997. “Development and Properties of Crystalline Silicotitanate (CST) Ion Exchangers for Radioactive Waste Applications,” SAND97-0771, Sandia National Laboratories, Albuquerque, N.M.
Miller, W., R. Alexandeq, N. Chapman, I. McKinley, and J. Smellie. 1994. “Natural Analogue Studies in the Geological Disposal of Radioactive Wastes,” Studies in Environmental Science 57, Elsevier, New York.
Mimura, H., M. Shibata, and K. Akiba. 1990. “Surface Alteration of Pollucite Under Hydrothermal Conditions,” J. Nucl. Sci. Tech. 27, 835-843.
MMES (Martin Marietta Energy Systems, Inc.), EG&G Idaho, Inc., Westinghouse Company, and Westinghouse Savannah River Company. 1992. Radiological Performance Assessment for the Z-Area Saltstone Disposal Facility, WSRC-RP-92-1360, Westinghouse Savannah River Company, Aiken, S.C.
Mogus-Milankovic, A., M. Fajiic, A. Drasner, R. Tojiko, and D. E. Day. 1998. “Crystallization of Iron Phosphate Glasses,” Phys. and Chem. of Glasses 39(2), 70-75.
Montel, J. M., B. Glorieux, A. M. Seydoux-Guilaume, and R. Wirth. 2006. “Synthesis and Sintering of a Monazite-brabantite Solid Solution Ceramic for Nuclear Waste Storage,” J. Physics and Chemistry of Solids 67, 2489-2500.
Moore, J. G. 1981. “A Survey of Concrete Waste Forms,” In Proceedings of a Conference on Alternative Nuclear Waste Forms and Interactions in Geologic Media, J. G. Moore, L. A. Boatner, and G. C. Battle (Eds.), NTIS 194-216, National Technical Information Service, Springfield, Va., 194-216.
Morgan, P. E. D., D. R. Clarke, C. M. Jantzen, and A. B. Harker. 1981. “High Alumina Tailored Nuclear Waste Ceramics,” J. Am. Ceram. Soc. 64(5), 249-258.
Morgan, P. E. D. and F. J. Ryerson. 1982. “A New “Cubic” Crystal Compound,” J. Mat. Sci. Lett. 1, 351-352.
Morgenstein, M. E. and D. L. Shettel Jr. 1993. “Evaluation of Borosilicate Glass as a High-Level Radioactive Waste Form,” In High Level Radioactive Waste Management, Proc. Fourth Annual International Conf., Vol. 2, Am. Nuclear Society, La Grange Park, Ill., 1728-1734.
Morss, L. R., M. L. Stanley, C. D. Tatko, and W. L. Ebert. 2000. “Corrosion of Glass Bonded Sodalite as a Function of pH and Temperature,” Scientific Basis for Nuclear Waste Management XXIII, R. W. Smith and D. W. Shoesmith (Eds.), Materials Research Society, Pittsburgh, Penn., 733-738.
Moschetti, T., W. Sinkler, T. Disanto, M. H. Hois, A. R. Warren, D. Cummings, S. G. Johnson, K. M. Goff, K. J. Bateman, and S. M. Frank. 2000. “Characterization of a Ceramic Waste Form Encapsulating Radioactive Electrorefiner Salt,” Scientific Basis for Nuclear Waste Management XXIII, R. W. Smith and D. W. Shoesmith (Eds.), Materials Research Society, Pittsburgh, Penn., 577-582.
Nakazawa, T., H. Kato, K. Okada, S. Ueta, and M. Mihara. 2001. “Iodine Immobilization by Sodalite Waste Form,” Mat. Res. Soc. Symp. Proc. 663, 51-57.
Ninomiya, M., T. Yamanaka, T. Sakane, M. Hora, S. Nakamura, and S. Kawamura. 1981. “Diopside Glass-Ceramic Material for the Immobilization of Radioactive Wastes,” Proceedings of the International Seminar on Chemistry and Process Engineering for High-Level Liquid Waste Solidification, R. Odoj and E. Merz (Eds.), Julich, FRG, Juel-Conf 42(1), 675-692.
Nyholm, R. and L.O. Werme. 1981. “An ESCA Investigation of Molybdenum Containing Silicate and Phosphate Glasses,” Scientific Basis for Nuclear Waste Management III, Plenum Press, New York.
Nyman, M. D., T. M. Nenoff, and T. J. Headley. 2001. “Characterization of UOP IONSIV IE-911.” SAND2001-0999, Sandia National Laboratories, Albuquerque, N.M.
O’Holleran, T. P., S. G. Johnson, S. M. Frank, M. K. Meyer, M. Noy, E. L. Wood, D. A. Knecht, K. Vinjamuri, and B. A. Staples. 1997. “Glass-Ceramic Waste Forms for Immobilizing Plutonium,” Mat. Res. Soc. Symp. Proc. 465, 1251-1258.
Ojovan, M. I., W. E. Lee, I. A. Sobolev, O. K. Karlina, and A. E. Arustamov. 2004. “Metal Matrix Immobilisation of Sealed Radioactive Sources for Safe Storage, Transportation and Disposal,” Proc. WM’04 Conference, Tucson, Ariz., Available at http://isl.group.sheffield.ac.uk/papers/MIOWM04Metalpaper.pdf.
Ojovan, M. I. and W. E. Lee. 2005. An Introduction to Nuclear Waste Immobilisation, Elsevier, Amsterdam.
Ojovan, M. I. and W. E. Lee. 2007. New Developments in Glassy Nuclear Wasteforms, Nova Science Publishers, New York.
Olson, A. L., N. R. Soelberg, D. W. Marshal, and G. L. Anderson. 2004. “Fluidized Bed Steam Reforming of INEEL SBW Using THOR Mineralizing Technology,” INEEL/EXT-04-02564, Idaho National Laboratory, Idaho Falls.
Orlova, A. I., Y. F. Volkov, R. F. Melkaya, L. Y. Masterova, I. A. Kulikov, and V. A. Alferov. 1994. “Synthesis and Radiation Stability of NZP Phosphates Containing F-elements,” Radiochem. 36(4), 322-325.
Oversby, V. M., C. C. McPheeters, C. Degueldre, and J. M. Paratte. 1997. “Control of Civilian Plutonium Inventories Using Burning in a Non-fertile Fuel,” J. Nucl. Mat. 245, 17-26.
Pabalan, R. T., F. P. Glasser, D. A. Pickett, G. R. Walter, S. Biswas, M. R. Juckett, L. M. Sabido, and J. L. Myers. 2009. “Review of Literature and Assessment of Factors Relevant to Performance of Grouted Systems for Radioactive Waste Disposal,” CNWRA 2009-001, Center for Nuclear Waste Regulatory Analysis, San Antonio, Texas.
Park, H. S., I. T. Kim, H. Y. Kim, K. S. Lee, S. K. Ryu, and J. H. Kim. 2002. “Application of Apatite Waste Form for the Treatment of Water-soluble Wastes Containing Radioactive Elements. Part 1: Investigation on the Possibility,” J. Indus. and Eng. Chem. 8, 318-327.
Park, T. J., S. Li, and A. Navrotsky. 2009. “Thermochemistry of Glass Forming Y-Substituted Sr-Analogues of Titanite (SrTiSiO5),” J. Mat. Res. 24, 3380-3386.
Perera, D. S., A. Zaynab, E. R. Vance, and M. Mizumo. 2005. “Immobilization of Pb in a Geopolymer Matrix,” J. Am. Ceram. Soc., 88(9), 2586-2588.
Perez, J. M., Jr., D. F. Bickford, D. E. Day, D. S. Kim, S. L. Lambert, S. L. Marra, D. K. Peeler, D. M. Strachan, M. B. Triplett, J. D. Vienna, and R. S. Wittman. 2001. High-Level Waste Melter Study Report, PNNL-13582, Pacific Northwest National Laboratory, Richland, Wash.
Peters, M. T. and R. C. Ewing. 2007. “A Science-Based Approach to Understanding Waste Form Durability in Open and Closed Nuclear Fuel Cycles,” J. Nucl. Mat. 362, 395-401.
Pichot, E., N. Dacheux, V. Brandel, and M. Genet. 2000. “Investigation of Cs-137(+), Sr-85(2+) and Am-241(3+) Ion Exchange on Thorium Phosphate Hydrogenphosphate and Their Immobilization in the Thorium Phosphate Diphosphate,” New J. Chem. 24, 1017-1023.
Plodinec, M. J. and J. R. Wiley. 1979. “Evaluation of Glass as a Matrix for Solidifying Savannah River Plant Waste: Properties of Glasses Containing Li2O,” U.S. DOE Report DP-1498, E.I. duPont deNemours & Co., Savannah River Laboratory, Aiken, S.C. (February).
Poinssot, C., C. Ferry, M. Kelm, B. Grambow, A. Martinez-Esparza, L. Johnson, Z. Andriambololona, J. Bruno, C. Cachoir, J. M. Cavedon, H. Christensen, C. Corbel, C. Jegou, K. Lemmens, A. Loida, P. Lovera, F. Miserque, J. de Pablo, A. Poulesquen, J. Quiñones, V. Rondinella, K. Spahiu, and D. Wegen. 2005. “Spent Fuel Stability under Repository Conditions—Final Report of the European Project, European Commission,” Available at ftp://ftp.cordis.europa.eu/pub/fp5-euratom/docs/fp5-euratom_sfs_projrep_en.pdf.
Porai-Koshits, E. A. 1958. The Structure of Glass, E. B. Uvarov (Trans.), Consultants Bureau, NY.
Ramsey, W. G. 1989. Durability Study of Simulated Radioactive Waste Glass in Brine Environment, Unpublished M.S. Thesis, Clemson University, Clemson, S.C.
Ramsey, W. G., N. E. Bibler, and T. F. Meaker. 1995. “Compositions and Durabilities of Glasses for Immobilization of Plutonium and Uranium,” Waste Management ’95, Tucson, Ariz.
Ray, C. R., X. Fang, M. Karabulut, G. K. Marasinghe, and D. E. Day. 1999. “Iron Redox and Crystallization of Iron Phosphate Glass,” Ceram. Trans. 43, 187-194.
Reeve, D. D., D. M. Levins, J. L. Woolfrey, and E. J. Ramm. 1984. “Immobiliation of high-level Radioactive Waste in SYNROC,” Advan. in Ceram. 8, 200-208.
Riley, A., S. Walker, and N. R. Gribble. 2009. “Composition Changes and Future Challenges for the Sellafield Waste Vitrification Plant,” Scientific Basis for Nuclear Waste Management XXXIII, 267-273.
Ringwood, A. E. 1978. Safe Disposal of High-Level Nuclear Reactor Wastes: A New Strategy, Australian National University Press, Canberra.
Ringwood, A. E. 1985. “Disposal of High-Level Nuclear Wastes: A Geological Perspective,” Mineral. Mag. 49, 159-176.
Ringwood, A. E., S. E. Kesson, N. G. Ware, W. Hibberson, and A. Major. 1978. “Immobilisation of High Level Nuclear Reactor Wastes in SYNROC,” Nature 278, 219-223.
Ringwood, A. E., S. E. Kesson, N. G. Ware, W. O. Hibberson, and A. Major. 1979. “SYNROC Processes—Geochemical Approach to Nuclear Waste Immobilization,” Geochem. J. 13(4), 141-165.
Ringwood, A. E., V. M. Oversby, and S. E. Kesson. 1981. SYNROC: Leaching Performance and Process Technology, Proc. Seminar on Chemistry and Process Engineering for High-Level Liquid Waste Solidification, R.Odoj and E. Merz (Eds), Julich, FRG, Juel-Conf 42(1), 495-506.
Ringwood, A. E., S. E. Kesson, K. D. Reeve, D. M. Levins, and E. J. Ramm. 1988. “SYNROC,” In Radioactive Waste Forms for the Future, W. Lutze and R. C. Ewing (Eds.), North-Holland, Amsterdam, 233-334.
Roy, D. M. and C. A. Langton. 1983. “Characterization of Cement-Based Ancient Building Materials in Support of Repository Seal Materials Studies,” BMI/ONWI-523, The Pennsylvania State University, State College, Penn.
Roy, D. M. and C. A. Langton. 1984. “Longevity of Borehole and Shaft Sealing Materials: Characterization of Ancient Cement Based Building Materials,” In Scientific Basis for Nuclear Waste Management VII. G. L. McVay (Ed.), Materials Research Society Symposium Proceedings, North-Holland, New York, 543-549.
Roy, D. M. and C. A. Langton. 1989. “Studies of Ancient Concrete as Analogs of Cementitious Sealing Materials for a Repository in Tuff,” LA-11527-MS, Los Alamos National Laboratory, Los Alamos, N.M.
Roy, R. 1975. “Ceramic Science of Nuclear Waste Fixation,” Abstract, Am. Ceram. Soc. Bull. 54, 459.
Roy, R. 1977. “Rational Molecular Engineering of Ceramic Materials,” J. Amer. Ceram. Soc. 60, 358-359.
Roy, R. 1979. “Science Underlying Radioactive Waste Management: Status and Needs,” Scientific Basis for Nuclear Waste Management I, G.J. McCarthy (Ed.), Plenum Press, New York, 1-20.
Roy, R. 1981. “Hydroxylated Ceramic Waste Forms and the Absurdity of Leach Tests,” Proc. Seminar on Chemistry and Process Engineering for High-Level Liquid Waste Solidification, R. Odoj and E. Merz (Eds.), Julich, FRG, Juel-Conf 42(1), 576-602.
Ryan, J. V., E. C. Buck, J. Chun, J. V. Crum, B. J. Riley, D. M. Strachan, S. K. Sundaram, L. A. Turo, and J. D. Vienna. 2009. Alternate Waste Forms: Aqueous Processing, AFCIWAST-PMO-MI-DV-2009-000360, Pacific Northwest National Laboratory, Richland, Wash. (September).
Ryerson, F. J. and B. Ebbinghaus. 2000. Pyrochlore-rich Titanate Ceramics for the Immobilization of Plutonium: Redox Effects on Phase Equilibria in Cerium- and Thorium-Substituted Analogs, UCRL-ID-139092, Lawrence Livermore National Laboratory, Livermore, Calif. (May).
Saidl, J. and J. Ralkova. 1966. “Radioactive Waste Solidification by Means of Melting with Basalt,” Atomic Energy 10, 285-289 (in Russian).
Sales, B. C. and L. A. Boatner. 1984. “Lead-Iron Phosphate Glass: A Stable Storage Medium for High-Level Nuclear Waste,” Science 226, 45-48.
Sava, D., T. J. Garino, and T. M. Nenoff. 2011. “Iodine Confinement into Metal-Organic Frameworks (MOFs): Low Temperature Sintering Glasses to Form Novel Glass Composite Material (GCM) Alternative Waste Forms,” Ind. Eng. Chem. Res. DOI:10.1021/ie200248g.
Scheetz, B. E. and R. Roy. 1988. ”Novel Waste Forms,” In Radioactive Waste Forms for the Future. W. Lutze and R. C. Ewing (Eds.), North-Holland, Amsterdam, 596-599.
Scheetz, B. E., D. K. Agrawal, E. Breval, and R. Roy. 1994. “Sodium Zirconium-Phosphate (NZP) as a Host Structure for Nuclear Waste Immobilization–A Review,” Waste Manage. 14, 489-505.
Scheetz, B. E. and J. Olanrewaju. 2001. “Final Report Determination of the Rate of Formation of Hydroceramic Waste Forms made with INEEL Calcined Wastes,” Available at http://www.osti.gov/bridge/servlets/purl/801196-G2Zift/webviewable/801196.pdf.
Schneider, K. J. (Ed.) 1969. Waste Solidification Program, Vol. 1, Process Technology–Pot Spray, and Phosphate Glass Solidification Processes, BNWL-1073, Pacific Northwest Laboratories, Richland, Wash. (August).
Schoebel, R. O. 1975. “Stabilization of High Level Waste in Ceramic Form,” Bull. Amer. Ceram. Soc. 54(4), 459.
Sheppard, L. M. 2005. “Geopolymer Composites: A Ceramics Alternative to Polymer Matrices,” Available at http://composite.about.com/library/weekly/aa030529.htm (April).
Sickafus, K. E., R. J. Hanrahan, K. J. McClellan, J. N. Mitchell, C. J. Wetteland, D. P. Butt, P. Chodak, K. B. Ramsey, T. H, Blair, K. Chidester, H. Matzke, K. Yasuda, R. A Verrall, and N. Yu. 1999. “Burn and Bury Option for Plutonium,” Amer. Ceram. Soc. Bull. 78(1), 69-74.
Sinkler, W., T. P. O’Holleran, S. M. Frank, M. K. Richmann, and S. G. Johnson. 2000. “Characterization of a Glass-Bonded Ceramic Waste Form Loaded with U and Pu,” Scientific Basis for Nuclear Waste Management XXIII, R. W. Smith and D. W. Shoesmith (Eds.), Materials Research Society, Pittsburgh, Penn., 423-429.
Smith, J. V. 1963. “Structural Classification of Zeolites.” Mineral. Soc. Amer. Spec. Pap. 1, 281.
Smith, J. V. 1976. “Origin and Structure of Zeolites,” In Zeolite Chemistry and Catalysis 171, J. A. Rabo (Ed.), American Chemical Society.
Sobolev, I. A., S. V. Stefanovsky, B. I. Omelianenko, S. V. loudintsev, E. R. Vance, and A. Jostsons. 1997a. “Comparative Study of Synroc-C Ceramics Produced by Hotpressing and Inductive Melting,” Mat. Res. Soc. Symp. Proc. 465, 371-378.
Sobolev, I. A., S. V. Stefanovsky, S. V. Loudintsev, B. S. Nikonov, B. I. Omelianenko, and A. V. Mokhov. 1997b. “Study of Melted Synroc Doped with Simulated High-level Waste,” Mat. Res. Soc. Symp. Proc. 465, 363-370.
Sokolova, E. V., R. K. Ratsvetaeva, V. I. Andrianov, Y. K. Egorov-Tismenko, and Y. P. Men’shikov. 1989. “Crystal-Structure of a New Natural Sodium Titanosilicate,” Dokl. Akad. Nauk. Sssr 307, 114-117.
Steadman, J. A. 1986. “Archaeological Concretes as Analogues,” In Commission of the European Communities. Natural Analogue Working Group, Second Meeting, Interlaken, Switzerland, June 17-19, 1986, B. Cbme and N. A. Chapman (Eds.), CEC Report No. EUR 10671, Commission of the European Communities, Luxembourg, 165-171.
Stefanovsky, S. V. 2009. “Ceramic and Phosphate Glass Waste Forms and Cold Crucible Melting Technology,” presented at the Committee on Waste Forms Technology and Performance, Meeting #3, November 4, 2009, Washington, D.C.
Stefanovsky, S. V., S. V. Yudintsev, R. Giere, and G. R. Lumpkin. 2004. “Nuclear Waste Forms,” Geological Society of London Special Publications 236, 37-63.
Stefanovsky, S. V., A. G. Ptashkin, O. A. Knyazev, S. A. Dimitriev, S. V. Yudintsev, and B. S. Nikonov. 2007a. “Inductive Cold Crucible Melting of Actinide-bearing Murataite-based Ceramics,” J. Alloys and Compounds 444, 438-442.
Stefanovsky, S. V., S. V. Yudintsev, B. S. Nikonov, O. I. Stefanovsky. 2007b. “Rare Earth-Bearing Murataite Ceramics,” Mat. Res. Soc. Symp. Proc. 985, 175-180.
Stefanovsky, S. V., S. V. Yudintsev, S. A. Perevalov, I. V. Startseva, G. A. Varlakova. 2007c. “Leach Resistance of Murataite-based Ceramics Containing Actinides,” J. Alloys and Compounds 444, 618-620.
Strachan, D. M. and W. W. Schulz. 1979. “Characterization of Pollucite as a Material for Long-Term Storage of Cesium-137,” Amer. Ceram. Soc. Bull. 58(9), 865-871.
Su, Y., M. L. Balmer, and B. C. Bunker. 1996. “Evaluation of Cesium Silicotitanates as an Alternate Waste Form,” Mat. Res. Soc. Symp. Proc. 465, 457-464.
Su, Y., M. L. Balmer, L. Wang, B. C. Bunker, M. Nyman, T. Nenoff, and A. Navrotsky. 1999. “Evaluation of Thermally Converted Silicotitanate Waste Forms,” Mat. Res. Soc. Symp. Proc. 556, 77-84.
Tolstova, O. V., T. N. Lashtchenova, and S. V. Stefanovsky. 2002. “Glassy Materials from Basalt for Intermediate-Level Waste Immobilization,” Glass and Ceramic 6, 28-31 (in Russian).
Urusov, V. S., N. I. Organova, O. V. Karimova, S. V. Yudintsev, and S. V. Stefanovsky. 2005. “Synthetic “Murataits” as Modular Members of Pyrochlore-Murataite Polysomatic Series,” Doklady Earth Sci. 401(2), 319-325.
Utsonomiya, A. S., L. M. Wang, S. V. Yudintsev, and R. C. Ewing. 2002. “Ion Irradiation Effects in Synthetic Garnets Incorporating Actinides,” Mat. Res. Soc. Symp. Proc. 713, 495-500.
Utsunomiya, S., S. Yudintsev, L. M. Wang, and R. C. Ewing. 2003. “Ion-beam and Electron-beam Irradiation of Synthetic Britholite,” J. Nucl. Mat. 322, 180-188.
van Jaarsveld, J. G. S., J. S. J. van Deventer, and L. Lorenzen. 1996. “The Potential Use of Geopolymeric Materials to Immobilise Toxic Metals, Part I. Theory and Applications,” Miner. Eng. 10(7), 659-669.
Vance, E. R. 1994. “SYNROC—A suitable waste form for actinides,” MRS Bull 19(12), 28-32.
Vance, E. R., C. J. Ball, R. A. Day, K. L. Smith, M. G. Blackford, B. D. Begg, and P. J. Angel. 1994a. “Actinide and Rare Earth Incorporation in Zirconolite,” J. Alloys and Comp. 213/214, 406-409.
Vance, E. R., P. J. Angel, B. D. Begg, and R. A. Day. 1994b. “Zirconolite-Rich Titanate Ceramics for High-Level Actinide Wastes,” In Scientific Basis for Nuclear Waste Management XVII, A. A. Barkatt and R. Van Konynenburg (Eds.), Mat. Res. Soc. 333, 293-298.
Vance, E. R., P. J. Angel, D. J. Cassidy, M. W. A. Stewart, M. G. Blackford, and P. A. McGlinn. 1994c. “Freudenbergite: A Possible Synroc Phase for Sodium-Bearing High-Level Waste,” J. Amer. Ceram. Soc. 77, 1576-1580.
Vance, E. R., R. A. Day, M. L. Carter, and A. Jostons. 1996a. “A Melting Route to Synroc for Hanford HLW Immobilization,” Mat. Res. Soc. Symp. Proc. 412, 289-295.
Vance, E. R., R. A. Day, Z. Zhang, B. D. Begg, C. J. Ball, and M. G. Blackford. 1996b. “Charge Compensation in Gd-doped CaTiO3,” J. Solid State Chem. 124, 77-82.
Vance, E. R., M. L. Carter, Z. Zhang, K. S. Finnie, S. J. Thomson, and B. D. Begg. 2004. “Uranium Valences in Perovskite, CaTiO3,” Environmental Issues and Waste Management Technologies in the Ceramic & Nuclear Industries IX, Ceram. Trans. 155, 3-10.
Verney-Carron, A., S. Gin, P. Frugier, and G. Libourel. 2010. “Long-Term Modeling of Alteration-Transport Coupling: Application to a Fractured Roman Glass,” Geochim. Cosmochim. Acta 74, 2291-2315.
Vinjamuri, K. 1995. “Candidate Glass-Ceramic Waste Forms for Immobilization of the Calcines Stored at the Idaho Chemical Processing Plant,” Ceram. Trans. 61, 439-446.
Wagh, A. S. and D. Singh. 1997. “Method for Stabilizing Low-Level Mixed Wastes at Room Temperature,” U.S. Patent 5,645,518.
Wagh, A. S., M. D. Maloney, G. H. Thomson, and A. Antink. 2003. “Investigations in Ceramicrete Stabilization of Hanford Tank Wastes,” WM’03, Phoenix, Ariz., Available at http://www.wmsym.org/archives/2003/pdfs/257.pdf.
Walton, Jr., R. D., W. B. Wilson, and D. E. Gordon. 1983. “Department of Energy’s Selection of High Level Waste Forms,” In The Treatment and Handling of Radioactive Wastes, A. G. Blasewitz, J. M. Davis, and M. R. Smith (Eds), Battelle Press, Columbus Ohio, 307-311.
Warren, B. E. 1933. “X-ray Diffraction of Vitreous Silica,” Zeit. Krist. 86, 349-358.
Weber, W.J. 1982. “Radiation-Damage in a Rare-Earth Silicate with the Apatite Structure,” J. Amer. Ceram. Soc. 65, 544-548.
Weber, W. J. 1983. “Radiation-Induced Swelling and Amorphization in Ca2Nd8(SiO4)6O2,” Rad. Effects and Defects in Solids 77, 295-308.
Weber, W. J. 1993. “Alpha-Decay-Induced Amorphization in Complex Silicate Structures,” J. Amer. Ceram. Soc. 76(7), 1729-1738.
Weber, W. J., R. C. Ewing, and A. Meldrum. 1997a. “The Kinetics of Alpha-Decay-Induced Amorphization in Zircon and Apatite Containing Weapons-grade Plutonium or Other Actinides,” J. Nucl. Mat. 250(2-3), 147-155.
Weber, W. J., R. C. Ewing, C. A. Angell, G. W. Arnold, A. N. Cormack, J. M. Delaye, D. L. Griscom, L. W. Hobbs, A. Navrotsky, D. L. Price, A. M. Stoneham, and M. C. Weinberg. 1997b. “Radiation Effects in Glasses Used for Immobilization of High-Level Waste and Plutonium Disposition,” J. Mat. Res. 12(8), 1946-1978.
Weber, W. J., R. C. Ewing, C. R. A. Catlow, T. Diaz de la Rubia, L. W. Hobbs, C. Kinoshita, Hj. Matzke, A. T. Motta, M. Nastasi, E. H. K. Salje, E. R. Vance, and S. J. Zinkle. 1998. “Radiation Defects in Crystalline Ceramics for the Immobilization of High-level Nuclear Waste and Plutonium,” J. Mat. Res. 13(6), 1434-1484.
Weber, W. J., A. Navrotsky, S. Stefanovsky, E. R. Vance, and E. Vernaz. 2009. “Materials Science of High-Level Nuclear Waste Immobilization,” MRS Bull. 34, 46-53.
White, W. B. 1988. “Glass Structure and Glass Durability,” In Materials Stability and Environmental Degradation, A. Barkatt, E. D. Vernik, and L. R. Smith (Eds.), MRS Symp. Proc. 125, 109-114.
Wicks, G. G., W. D. Rankin, and S. L. Gore. 1985. “International Waste Glass Study—Composition and Leachability Correlations,” Scientific Basis for Nuclear Waste Management VIII, C. M. Jantzen, J. A. Stone, and R. C. Ewing (Eds.), Materials Research Society, Pittsburgh, Penn., 171-177.
Wronkiewicz, D. L., S. F. Wolf, T. S. Disanto. 1996. “Apatite- and Monazite-bearing Glass-Crystal Composites for the Immobilization of Low-level Nuclear and Hazardous Wastes,” Mat. Res. Soc. Symp. Proc. 412, 345-352.
Xu, H., A. Navrotsky, M. D. Nyman, and T. M. Nenoff. 2000. “Thermochemistry of Microporous Silicotitanate Phases in the Na2O-Cs2O-SiO2-TiO2-H2O System,” J. Mat. Res. 15, 815-823.
Xu, H., A. Navrotsky, M. L. Balmer, Y. Su, and E. R. Bitten. 2001. “Energetics of Substituted Pollucites Along the CsAlSi2O6-CsTiSi2O6,5 Join: A High-Temperature Calorimetric Strudy,” J. Am. Ceram. Soc. 84(3), 555-560.
Yakovenchuk, V. N., E. A. Selivanova, G. Yu. Ivanyuk, Y. A. Pakhomovsky, D. V. Spiridonova, and S. V. Krivovichev. 2008. “First Natural Pharmacosiderite-Related Titanosilicates and Their Ion-Exchange Properties,” Miner. Advan. Mat. I, 27-35.
Yanagisawa, K., M. Nishioka, and N. Yamasaki. 1987. “Immobilization of Cesium into Pollucite Structure by Hydrothermal Hot-Pressing,” J. Nucl. Sci. Tech. 24(1), 51-60.
Yu, B., J. Chen, and C. Song. 2002. “Crystalline Silicotitanate: A New Type of Ion Exchanger for Cs Removal from Liquid Waste,” J. Mater. Sci. Tech. 18(3), 206-210.
Yu, X. and D. E. Day. 1995. “Effect of Raw Materials on the Redox State of Iron and Properties of Iron Phosphate Glasses,” In Proceedings of the 17th International Congress on Glass 2, International Academic Publishers, Beijing, The Peoples Republic of China, 45-51.
Yudintsev, S. V. 2001. “Incorporation of U, Th, Zr, and Gd into the Garnet-structured Host,” In Proceedings of the 8th International Conference on Radioactive Waste Management and Environmental Remediation, The American Society of Mechanical Engineers, New York.
Yudintsev, S. V. 2003. “A Structural-Chemical Approach to Selecting Crystalline Matrices for Actinide Immobilization,” Geol. Ore Depos. 45, 151-165.
Yudintsev, S. V., M. I. Lapina, A. G. Ptashkin, T. S. Ioudintseva, S. Utsonomiya, M. L. Wang, and R. C. Ewing. 2002. “Accommodation of Uranium into the Garnet Structure,” Mat. Res. Soc. Symp. Proc. 713, 477-480.
Yudintsev, S. V., S. V. Stefanovsky, and R. C. Ewing. 2007. “Actinide Host Phases as Radioactive Waste Forms,” In S. V. Krivovichev, P. C. Burns and I. Tananaev (Eds.), Structural Chemistry of Inorganic Actinide Compounds, Elsevier, Amsterdam, 457-490.
Zachariasen, W. H. 1932. “The Atomic Arrangement in Glass,” J. Am. Chem. Soc 54, 3841-3851.
Zachariasen, W. H. 1933. “The Vitreous State,” J. Chem. Phys. 3, 162-163.
Zeolite structural information from the International Zeolite Association (IZA), website, http://www.iza-structure.org/databases/see: Cancrinite (CAN).
Zeolite structural information from the International Zeolite Association (IZA), website, http://www.iza-structure.org/databases/see: sodalite (SOD).
Zhang, Y. J. and E. R. Vance. 2008. “Plutonium in Monazite and Brabanite: Diffuse Reflectance Spectroscopy Study,” J. Nucl. Mat. 375, 311-314.
Zhang, Y., M. W. A. Stewart, H. Li, M. L. Carter, E. R Vance, S. Moricca. 2009. “Zirconolite-rich Titanate Ceramics for Immobilization of Actinides—Waste form/HIP Can Interactions and Chemical Durability,” J. Nucl. Mat. 395, 69-74.
Zhang, Z. and M. L. Carter. 2010. “An X-Ray Photoelectron Spectroscopy Investigation of Highly Soluble Grain-Boundary Impurity Films in Hollandite,” J. Am. Ceram. Soc. 93(3), 894-899.
Zhao, D., L. Li, L. L. Davis, W. J. Weber, and R. C. Ewing. 2001. “Gadolinium Borosilicate Glass-bonded Gd-Silicate Apatite: A Glass-Ceramic Nuclear Waste Form for Actinides,” Mat. Res. Soc. Symp. Proc. 663, 199-206.
Zosin, A. P., T. I. Priimak, and K. B. Avsaragov. 1998. “Geopolymer Materials Based on Magnesia-Iron Slags for Normalization and Storage of Radioactive Wastes,” Atomic Energy 85, 510-514.
Zyryanov, V. N. and E. R. Vance. 1997. “Comparison of Sodium Zirconium Phosphate-Structured HLW forms and Synroc for High-Level Nuclear Waste Immobilization,” In Scientific Basis for Nuclear Waste Management XX, W. J. Gray and I. R. Triay (Eds.), Materials Research Society, Pittsburgh, Penn., 409-416.


























































