3
The U.S. Medical Countermeasures Enterprise and Recent Reviews and Current Operation of the Special Immunizations Program
The Special Immunizations Program (SIP) remains a distinct but small component, but it is part of the overall U.S. military and civilian medical countermeasures (MCM) enterprise, so its effectiveness must be considered in this broader framework
3.1
THE U.S. MEDICAL COUNTERMEASURES ENTERPRISE
Overarching U.S. security goals and objectives relevant to MCM against biological threats and infectious diseases are derived from strategic documents, such as the U.S. National Security Strategy (White House 2010), the National Strategy for Countering Biological Threats (NSC 2009), the National Health Security Strategy (HHS 2009b), Homeland Security Presidential Directive 18 (White House 2007), and Quadrennial Defense Reviews (DOD 2010). Assessing which pathogens and toxins pose the most important risks to U.S. national security and establishing initiatives to develop and acquire MCM involve coordination among multiple agencies and offices of the federal government, as depicted in Figure 3.1. Assessments of risk and priority-setting guide the establishment of acquisition requirements, with the Department of Defense (DOD) assuming primary responsibility for the development and acquisition of MCM against military threats and the Department of Health and Human Services (HHS) focusing on threats to the civilian population. Those agencies also interact with extramural researchers and industry to develop the products needed for the MCM pipeline.
Within HHS, the Office of the Assistant Secretary for Preparedness and Response (ASPR) assumes a primary role for the oversight of programs to develop and acquire MCM for use in the civilian population. The office is re-
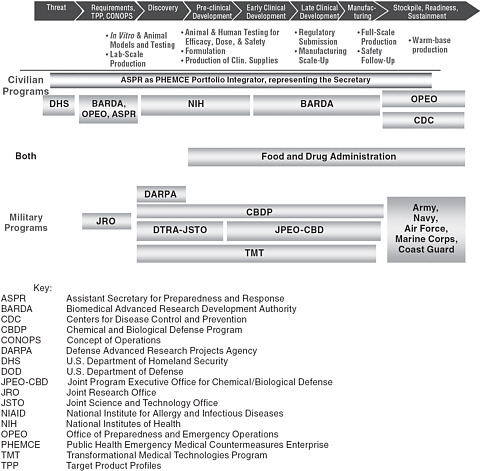
FIGURE 3.1 Government agencies involved in the civilian and military MCM pathway. SOURCE: Adapted from NBSB 2010b.
sponsible for leading the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) and houses the Biomedical Advanced Research and Development Authority (BARDA), which provides an integrated, systematic approach to the development and purchase of vaccines, drugs, other therapies, nonpharmaceutical countermeasures, and diagnostic tools for public health medical emergencies, manages Project Bioshield, and participates in such initiatives as the Integrated Portfolio for CBRN Medical Countermeasures.1 In addition to BARDA, created under the Pandemic and All-Hazard Preparedness Act of 2006, HHS supports MCM development and research through the
|
1 |
Further information is available at the HHS Web site MedicalCountermeasures.gov at https://www.medicalcountermeasures.gov/default.aspx. |
National Institutes of Health (NIH), particularly through the National Institute of Allergy and Infectious Diseases (NIAID 2007; HHS 2007, 2009b, 2010a). NIAID’s research agenda includes construction and renovation of biosafety level 3 (BSL-3) and BSL-4 laboratories around the country, including an NIAID Integrated Research Facility at Fort Detrick, MD, an Integrated Research Facility at NIAID’s Rocky Mountain Laboratories in Hamilton, MT, National Biocontainment Laboratories at Boston University and at the University of Texas Medical Branch at Galveston; and construction or renovation of numerous BSL-3 and BSL-2 biocontainment laboratory suites. The Centers for Disease Control and Prevention (CDC) also plays a role in the national enterprise by being the lead agency in the diagnostic and immediate public health response to emerging infections and maintaining the Strategic National Stockpile (SNS), which contains vaccines, therapeutics, and medical supplies that can be rapidly deployed in the event of a public health emergency.
The military MCM pipeline is complex, with important research and development roles played by the DOD Chemical and Biological Defense Program (CBDP), the Defense Advanced Research Projects Agency, the Joint Science and Technology Office for Chemical and Biological Defense (JSTO) which is part of the Defense Threat Reduction Agency (DTRA), and the DOD Transformational Medical Technologies (TMT, formerly TMTI) program. The military also maintains the Joint Program Office for Chemical and Biological Defense (JPEO-CBD), which includes the Joint Vaccine Acquisition Program (JVAP) as one of its activities. The JVAP plays an important role in the advanced development2 of vaccines that have been identified as military needs and includes as its mission to “develop, produce, and stockpile U.S. Food and Drug Administration (FDA)–licensed vaccine systems to protect the Warfighter against biological warfare agents” (JPEO-CBD 2011). As a result, JVAP has a role that complements but is distinct from that of the SIP, which also serves researchers who are working at earlier stages in the scientific R&D pipeline and which offers Investigational New Drug (IND) vaccines that may not yet have been identified as direct warfighter needs or been transitioned into the advanced development pipeline. Although the individual armed services are depicted to the right of Figure 3.1 under the acquisition, stockpiling, and readiness of licensed products, research programs within the services, such as those at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), also make important contributions to R&D efforts. As discussed in Chapter 2, the U.S. Army and USAMRIID in particular have a long history of research on hazardous pathogens, and USAMRIID operates the only BSL-4 facility in
DOD. As provided by DOD Directive 5160.05E (DOD 2008) and Chairman of the Joint Chiefs of Staff Instruction CJCSI 3112.01A (Joint Chiefs 2010), the Army also serves as the DOD executive agent for the CBDP, further highlighting its central role in U.S. biodefense efforts.
3.2
NATIONAL BIODEFENSE AND MEDICAL COUNTERMEASURES PRIORITIES
Through their assessment and requirements-setting processes, DOD, the Department of Homeland Security (DHS), and HHS establish lists of priority agents against which MCM are desired. They include historical pathogens of concern and, increasingly, more flexible strategies, such as broad-spectrum countermeasures, platform technologies, and products targeting new or emerging infectious agents.
Although the military (DOD-led) and civilian (HHS-led) MCM efforts have somewhat different missions and establish their own priorities, the agencies have also recognized their common interest in advancing the MCM pipeline and have attempted to coordinate and integrate their needs better. An understanding of pathogen priorities identified by the agencies can be discerned in recent program reports and reviews. For instance, the February 2010 report from the National Biodefense Science Board (NBSB), Optimizing Industrial Involvement with Medical Countermeasure Development, contains “Table 1: Top Priority Medical Countermeasures (MCMs) against Chemical, Biological, Radiological, and Nuclear Threats, Annotated by License and Stockpile Status, Reflecting HHS and DOD Programs, February 2010” (NBSB 2010a: 13), which includes the pathogens Bacillus anthracis (anthrax), Clostridium botulinum (botulism), filoviruses (Ebola and Marburg), Junin virus, variola major virus (smallpox), Burkholderia mallei (glanders) and Burkholderia pseudomallei (melioidosis), Yersinia pestis (plague), Francisella tularensis (tularemia), and Rickettsia prowazekii (typhus). DOD and HHS have also established the Integrated Portfolio for CBRN Medical Countermeasures Portfolio (also commonly called “One Portfolio”) (Newmark 2009). As presented in 2009 by the JPEO-CBD, priority biological countermeasures can be classified in three categories: DOD-unique, HHS-unique, and common (Newmark 2009):
-
DOD-unique:
-
Brucella spp. (brucellosis) (prophylactic use).
-
Venezuelan equine encephalitis (VEE) virus, eastern equine encephalitis (EEE) virus, and western equine encephalitis (WEE) virus (prophylactic and therapeutic uses).
-
Yersinia pestis (plague) (prophylactic use).
-
Clostridium botulinum (botulism) (prophylactic use).
-
Staphylococcal enterotoxin B (prophylactic and therapeutic uses).
-
-
-
Francisella tularensis (tularemia) (prophylactic use).
-
Ricin (prophylactic and therapeutic uses).
-
Other, unfunded.
-
-
HHS-unique
-
Burkholderia spp. (therapeutic use).
-
Junin virus (therapeutic use).
-
Yersinia pestis (plague) (therapeutic use).
-
-
Common
-
Bacillus anthracis (anthrax) (prophylactic and therapeutic uses).
-
Variola virus (smallpox) (prophylactic and therapeutic uses).
-
Ebola and Marburg viruses (prophylactic and therapeutic uses).
-
Francisella tularensis (tularemia) (therapeutic use).
-
Clostridium botulinum (botulism) (therapeutic use).
-
A recent presentation by DOD’s TMT program, which seeks to exploit novel technologies for the development of next-generation countermeasures, similarly illustrates the universe of priority pathogen threats (Hough 2010), including additional infectious pathogens and toxins of interest to DOD that are not presented in the NBSB and One Portfolio documents and priorities for the development of countermeasures against broad-spectrum targets and emerging threats and is presented as Figure 3.2.
The creation of stockpiles of vaccines and other MCM for civilian use is supported through Project BioShield and added to the SNS as they are acquired. The acquisitions have focused largely on vaccines and therapeutics
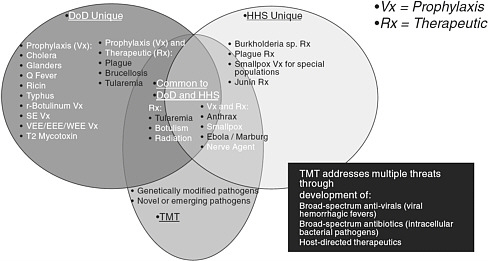
FIGURE 3.2 U.S. MCM needs. SOURCE: Hough 2010.
against a narrow selection of pathogens—notably Bacillus anthracis (anthrax), the variola major virus (smallpox), and Clostridium botulinum (botulism)—with countermeasures against radiation exposure.
3.3
REVIEW OF PREVIOUS REPORTS RELEVANT TO BIODEFENSE MEDICAL COUNTERMEASURES
Numerous reports and reviews since 2001 have discussed the military and civilian U.S. MCM and biodefense vaccines pipeline.
3.3.1
The Military Medical Countermeasures Enterprise
Since the report to the deputy secretary of defense by an independent panel of experts (referred to as the Top Report) in 2001, there have been several external analyses of DOD’s biodefense programs and strategies for MCM (Top et al. 2000 in DOD 2001; IOM 2002, 2004). The panel of experts that produced the Top Report noted the large number of DOD units involved in the development and acquisition of biodefense and infectious disease products at that time and the “fragmented” nature of the program, observed that DOD was not following best practices found in industry, and recommended creation of a dedicated DOD government-owned, contractor-operated (GOCO) vaccine production facility.3 A 2002 Institute of Medicine (IOM) report likewise criticized DOD’s administrative separation of the acquisition processes for vaccines intended to protect against naturally occurring infectious diseases and those for defense against biowarfare as scientifically and organizationally “unsound.” The report recommended manufacturing arrangements to ensure “consistent vaccine availability” and suggested that DOD seek a “new paradigm” with FDA for regulation of special-use vaccines likely to remain in IND status.
The 2004 IOM report Giving Full Measure to Countermeasures recommended that funding for DOD countermeasures double in 5 years to $300–600 million and noted that new countermeasures require “substantial and sustained” effort, including having a strong scientifically knowledgeable leadership and adequate funding. It also recommended that the MCM program be given “genuine priority.” To accomplish that, the report suggested that Congress authorize the creation of a new agency within DOD to provide the necessary infrastructure and creation of an external review committee of vaccinologists and other scientists to review and evaluate the DOD research program. Finally, the report called for more effective collaboration between academe, industry, and government and noted a need to address MCM regulatory challenges.
Those external reviews generally noted a complex and fragmented system
of offices responsible for the DOD MCM development and acquisition pipeline and consistently recommended new approaches to achieving military vaccine development and acquisition goals, increased collaboration with the private sector, and development of a dedicated vaccine production facility. Although a new agency was not created within DOD to oversee all aspects of MCM, DOD restructured aspects of its CBDP in 2003. The current system of CBD responsibilities outlined in the CBDP annual report to Congress (CBDP 2010) includes overall coordination responsibilities through the Office of the Assistant to the Secretary of Defense for Nuclear and Chemical and Biological Defense Programs; the Army as executive agent for chemical, biological, radiological, and nuclear (CBRN) programs; and DTRA assuming substantial responsibilities for science and technology. The 2003 implementation plan was intended to streamline operations and resulted in the creation of the JPEO-CBD executed by the Army and JSTO-CBD responsibilities in DTRA.
3.3.2
The Civilian Medical Countermeasures Enterprise
Recent reports have also reviewed issues in the civilian MCM pipeline. The NBSB, which like BARDA was created under the authority of the 2006 Pandemic and All-Hazards Preparedness Act, provides the secretary of HHS expert advice on public health emergency preparedness, including biological threats and natural infectious diseases. In addition to voting members, the NBSB includes ex officio members who represent a variety of federal agencies. In 2010, the NBSB released two reports on medical countermeasures: Optimizing Industrial Involvement with Medical Countermeasure Development (Optimizing Report) and Where Are the Countermeasures? Protecting America’s Health from CBRN Events (Countermeasures Report) (NBSB 2010a,b).
The Optimizing Report calls for “concerted action” among the various departments, agencies, and other entities of the U.S. government; expansion of MCM markets to include international partners, first responders, state and local governments, and laboratories; and innovative partnerships with industry, including long-term commitments and consistent funding. The report also identifies several barriers to government–industry collaborations to develop MCM, including difficulty in contracting, the need for clarity about MCM requirements, lack of coordination among federal agencies that have MCM development activities, inadequate understanding of the commercial biopharmaceutical enterprise in the federal government, an immature commercial market for MCM to create incentives for industry; and inadequate mechanisms to maintain industry involvement in MCM development by preserving manufacturing capacity after initial lots of MCM have been produced.
The Countermeasures Report points to a continued need to foster a shared vision and strong coordination among HHS-led efforts and across DOD and HHS countermeasures missions. The report emphasizes the need for a com-
prehensive national strategy and common purpose and the need to address regulatory concerns in MCM development, such as the application of FDA’s “Animal Rule” (discussed in Section 4.2.3). The report highlights the progress made by the Integrated Portfolio approach being pursued by HHS and DOD and notes (NBSB 2010b: 9) that
it is in the national interest to have distinct DOD and HHS programs in MCM development, and the Integrated Portfolio approach jointly adopted by these two Departments offers an impressive example of coordination and collaboration that other agencies could well use as a model. Collaboration between DOD and HHS, however, needs to continue to mature and broaden.
The report’s executive summary (NBSB 2010b: 5) notes further that
the federal MCM program to date can be characterized as a good effort conducted by talented people, but lacking in centralized leadership and with poor synchronization of the agencies within the Department of Health and Human Services (HHS). The effort has not fully tapped the talent of the Department of Defense (DOD) and the Department of Homeland Security (DHS). The combined effort is under-resourced and has largely failed to mobilize the productive skills and efforts of industry. There is no unified national strategy that prioritizes the array of threats and encompasses all aspects of responsiveness, from creating to stockpiling to distributing MCMs. Instead, development of MCMs has been too much a matter of selecting projects to fit within available budgets, instead of allocating the necessary funds to tackle a prioritized list of threats.
Recognizing those challenges, in December 2009 the secretary of HHS requested a review of the civilian countermeasures efforts. The IOM held a workshop on this topic in February 2010 and NBSB also published the two reports cited above. The review requested by the secretary and led by ASPR, The Public Health Emergency Medical Countermeasure Enterprise Review: Transforming the Enterprise to Meet Long Range National Needs was released by ASPR in August 2010 (HHS 2010b; IOM 2010).
The recommendations made in those military and civilian countermeasures reports highlight special challenges inherent in the development of vaccines and other countermeasures against biothreat agents and emerging infectious diseases, including limited commercial markets, the difficulty of conducting clinical trials and following a traditional path to FDA licensure, and a need to continue anticipating and addressing new and emerging threats. The reports conclude that U.S. countermeasures efforts are of value but that improvement can and should be made to enhance their effectiveness. Table 3.1 presents selected findings and recommendations from the studies, which broadly are in several categories:
TABLE 3.1 Selected Findings and Recommendations from Extramural Reviews of Biodefense Vaccines and MCM
|
Theme |
Selected Findings and Recommendations |
|
Leadership and priority-setting |
“Establish a unified process for identifying and prioritizing threats and requirements” (Top et al. 2000 in DOD 2001). “DOD needs to consolidate and integrate its vaccine research, development, and acquisition programs for BW defense and endemic disease protection” (Top et al. 2000 in DOD 2001). “DOD must adopt industry practices, capture industry interest, and invest its own corporate resources in the management and execution of the AVP [Acquisition of Vaccine Production] program if it has any hope of solving its vaccine requirements” (Top et al. 2000 in DOD 2001). “Combine all DOD vaccine acquisition responsibilities under a single DOD authority that includes the entire spectrum of responsibility—from potential threat definition through research and development, advanced product development, clinical trials, licensure, manufacture, procurement, and continued maintenance of manufacturing practice standards and regulatory compliance” (IOM 2002). “Consolidate infrastructure, funding, and personnel for DOD acquisition programs for biodefense and naturally occurring infectious disease vaccines” (IOM 2002). “Actively encourage the development, distribution, and use of a well-defined and validated research priority-setting mechanism, which could involve prioritized, weighted lists of infectious disease threats and formal scenario-planning exercises” (IOM 2002). “The Secretary of Defense and Congress must make the DOD program for medical biodefense countermeasures a high priority” (IOM 2004). “Congress should authorize the creation of the Medical Biodefense Agency, a new DOD agency responsible for the research and development program for medical countermeasures against biological warfare agents (IOM 2004)” “Congress should establish an external review committee of experts in the development of vaccines and drugs to review and evaluate the program and performance of the DOD research and development program for medical biodefense countermeasures each year” (IOM 2004). “The Secretary of HHS promptly tasks senior HHS leaders to develop a common set of prioritized research goals, prioritized product requirements, and prioritized dispensing goals for civilian populations; and coordinates these priorities with DOD” (NBSB 2010b). “For FY2012 and beyond, the Secretary of HHS develops a coordinated budget request relevant to CBRN MCM budget lines within NIH, NIAID, BARDA, CDC, FDA, and ASPR (and in conjunction with DOD)” (NBSB 2010b). “The Secretary of HHS develops a legislative plan to seek multi-year funding authority for CBRN MCM efforts” (NBSB 2010b). Recommendations under Enhancements to the MCM Enterprise: 1. Strategic Leadership, Program, and Administrative Changes; 2. Updating the Requirements for Current and Future Products; 3. Multiyear Planning Process (HHS, 2010b) |
|
Partnerships |
“Use an integrated strategy that includes: GOCO…, PSC, DOD biomedical laboratories, and DOD partnerships with commercial companies (including appropriate incentives), National Institutes of Health, Public Health Service, and academia” (Top et al. 2000 in DOD 2001). “Leverage DOD research efforts by building greater interactions and an effective formalized coordinating structure that links DOD research to vaccine development activities carried out by the Department of Health and Human Services and other public and private groups” (IOM 2002). “Ensure that there is an effective, ongoing senior advisory group—one providing perspectives from both within and outside of DOD—to assess program priorities and accomplishments, to act as a proponent for vaccines and other infectious disease countermeasures, and to maintain active relationships with current science and technology leaders in academic, government, and corporate sectors” (IOM 2002). Several recommendations under “Establishing Effective Collaboration with Academia and the Private Sector” (IOM 2004). “Various departments, agencies, and entities of the U.S. Government must act in concert to ensure success“(NBSB 2010a). “The U.S. Government must create, sustain, and enhance innovative partnerships with private industry” (NBSB 2010a). |
|
Manufacturing and regulatory barriers |
“Work toward manufacturing arrangements that ensure consistent vaccine availability by addressing long-term commitment, predictable volumes and prices, indemnification, and intellectual property issues. These arrangements should include consideration of vaccine-specific, government partnerships with individual private manufacturers, a private manufacturer consortium, and government-owned, contractor-operated vaccine-production facilities” (IOM 2002). “Vigorously seek a new paradigm for the regulation of special-use vaccines that remain in Investigational New Drug status with the Food and Drug Administration without reasonable prospects of licensure under current rules, ensuring demonstration of the safety and efficacy of these products commensurate with their anticipated use” (IOM 2002). Meeting the Challenges of the Regulatory Process: several recommendations including, “the DOD agency should work with NIH and engage FDA to develop additional animal models that will be useful for specific agents or products of particular concern to DOD…. FDA should work with the scientific community to enrich the science base that the agency will have to draw on in order to apply the Animal Efficacy Rule….” (IOM 2004). “[P]articipate in interdepartmental efforts to make a formal assessment of the need for facilities for animal testing and holding and for GMP-compliant manufacturing of material for clinical testing that will arise from research efforts to develop medical countermeasures to biowarfare or bioterrorism agents that are under way, planned, or likely” (IOM 2004). “The ASPR promptly provides a plan to the Secretary of HHS to provide for centralized advanced development and manufacturing of selected biological MCMs, based on one or more public-private partnerships (PPPs) or federally funded research-and-development centers (FFRDCs)” (NBSB 2010b). Several recommendations under New Infrastructure Initiatives, including: 1. 21st-Century Regulatory Science; 2. Flexible Manufacturing and Advanced Development Core Services Partnerships; 3. Expanding the Product Pipeline and Addressing Multiuse Potential (HHS 2010b). |
|
Workforce and other needs |
“The Medical Biodefense Agency should define the capabilities needed for its medical countermeasures workforce….” (IOM 2004). “The U.S. Government should expand MCM markets to include international partners, State, local, and tribal governments, laboratorians, and first-responders in each of these sectors. These markets are relatively small, but including them would send industry an important message that the U.S. Government is not the only market. Adding MCMs to Standardized Equipment Lists (SELs) and Authorized Equipment Lists (AELs) would allow State and local first-responders to use federal grant funds to protect these personnel against occupational hazards” (NBSB 2010a). |
-
Strengthen leadership and priority-setting. The reports suggest a need for additional mechanisms to coordinate and centralize authority throughout the life cycle of MCM development and acquisition, a need to establish and communicate MCM priorities with the development of a process of regular review and adjustment, and a need for consistent and multiyear funding.
-
Build partnerships. The reports suggest a need to promote communication between military and civilian countermeasures programs to maximize the effectiveness of the enterprise and a need to strengthen partnerships with private industry.
-
Address manufacturing and regulatory barriers. The reports suggest a variety of strategies to ensure MCM manufacturing capacity, such as creation of a GOCO vaccine facility, changes in the development and acquisition process to strengthen industry involvement in development and manufacturing, and new regulatory science in partnership with FDA.
-
Other needs. The reports include calls to support a qualified and effective workforce and a suggestion to expand MCM market planning to consider international uses and uses in occupational health settings.
3.4
RECENT DEVELOPMENTS REGARDING THE SPECIAL IMMUNIZATIONS PROGRAM (2000–2010)
The committee examined both the recent history of the SIP (2000–2010) and the structure and operations of the current program in the context of efforts to review the overall MCM enterprise.
As noted in Chapter 2, immunizations provided through the SIP consist largely of legacy investigational vaccines produced by the Salk Institute Government Services Division (TSI GSD) facility, which is now closed. Using those supplies, the SIP continues to operate to provide IND and licensed vaccines to
at-risk military and civilian personnel, ensure the safety and occupational health of participants through continuous medical evaluation, provide evaluation of and treatment for occupational exposures, and collect safety and immunogenicity data (including long-term follow-up data) to further medical research.
The expansion of research in both emerging infectious diseases and biothreat agents created a larger scientific community potentially at risk, with the potential to require a corresponding increase in the immunization services provided by the SIP. From 2000 to 2002, the number of volunteers enrolled in the SIP grew from 600 to about 800 patients. The substantial cost of operating the program was borne solely by USAMRIID and its parent command, the U.S. Army Medical Research and Materiel Command (USAMRMC), in an unsustainable situation.
A report by an external biosafety assessment team issued in 2002 (Findings and Recommendations on Alternative Strategies for Worker Protection) noted that select agent vaccine support for “added” protection of laboratory workers provided by the SIP was essential not only for USAMRIID but for institutions throughout the United States in which work on special pathogens was being conducted. The report suggested that CDC’s Advisory Committee on Immunization Practices (ACIP), not USAMRIID, should be responsible for deciding immunization practice with these experimental vaccines at the national level (Boudreau 2010).
In November 2002, at the American Society of Tropical Medicine and Hygiene meeting, a draft decision memo was developed by USAMRMC, NIAID, and CDC to explore possible solutions to the mounting costs and expertise required to ensure proper IND vaccine testing and volunteer safety and compliance with FDA requirements. In January 2003, an SIP interagency working group was formed to explore solutions to those problems, and an SIP subgroup of the Biodefense Vaccine and Immunologics Committee was convened on March 14, 2003. The subgroup comprised representatives of DOD, the U.S. Department of Agriculture (USDA), DHS, and HHS (CDC, NIAID, FDA, and NIH) and was chaired by a representative of HHS.
The SIP subgroup held five meetings to develop options, refine program costs, and validate enrollment projections; held executive briefings on its interim report for the commander of USAMRMC, the director of CDC, the director of NIAID, the commander of the U.S. Army Medical Command and the Army surgeon general, the deputy assistant secretary for defense for chemical and biological defense, the assistant secretary of defense for health affairs, DHS, the Office of Science and Technology Policy, and the associate administrator of the USDA Agricultural Research Service; held five interim meetings with the U.S. government interagency working group co-chair, Philip Russell; and gave an initial briefing to the full Weapons of Mass Destruction Medical Countermeasures (WMDMC) Subcommittee on June 20, 2003. The guidance
received at those executive briefings included the following: USAMRMC would be willing to accept the SIP mission with consistent supplemental funding from non-DOD users of the program; USAMRMC would maintain its own program to protect Army and DOD users. DHS would not designate funds to the program, nor could Project BioShield be used to support the program (Eitzen 2010). In 2003, the commander of USAMRIID also sent a memo to all agencies stating that a charge of $6,457 per person enrolled in the SIP (paid in advance) would be assessed to cover the continuing costs of the program. All enrolled participants would also be required to undergo annual medical evaluation (Henchal 2003).
At a briefing on May 27, 2004, Office of Management and Budget (OMB) representatives supported the developing SIP subgroup recommendations. The final report was given to the WMDMCS Senior Group (July 9, 2004), and on December 16, 2004, the chair of the SIP subgroup, Edward Eitzen, briefed the U.S. Homeland Security Council Policy Coordinating Committee (HSC PCC) on the proposed SIP recommendations (Eitzen 2004, 2010). The subgroup recommended that vaccines against the following be included in the SIP: pentavalent botulinum toxoid, tularemia, Rift Valley fever (RVF), VEE TC 83, VEE C 84, EEE, WEE, and Q fever. It also presented three options for the program structure:
-
An Army-sponsored SIP whereby the Army (USAMRMC) executes the SIP with funding provided by all participating federal agencies in a fully burdened cost-sharing arrangement according to use of the program (an option that also noted possible involvement of regional SIP sites).
-
An HHS- or DHS-sponsored SIP with regional immunization sites.
-
Two separate programs—an Army-sponsored SIP for military and DOD personnel and a civilian agency-sponsored SIP for civilian participants.
The subgroup concluded that a cost-sharing arrangement among agencies, according to program use, with one agency as sponsor of the program was the “only acceptable arrangement” (Eitzen 2004, 2010) and recommended option 1 because
-
One program would maximize program management and avoid duplication of costs that would occur with two programs.
-
The Army was willing to accept management of an expanded program with equitable cost-sharing agreements for the distribution of the fully burdened program costs among all participants.
-
The approach would ensure that DOD contingency protocols are supported.
-
The approach would provide experienced and trained staff (although this would require augmentation and facility site expansion to accommodate program growth and coverage of other agencies).
-
A new program start with a separate IND holder would not be required (so there would be much less lag time to availability of vaccines to civilian researchers).
-
Existing investments in an automated clinical database-management system would not require duplication.
-
Proven efficiency in vaccine storage and shipment would be preserved.
-
Having one sponsoring agency would conserve product availability.
Projected enrollment in the expanded SIP anticipated by the subgroup in its deliberations included 660 DOD users, about 800 HHS users (CDC, 550; NIH, 200; and FDA, 45), and additional users in USDA (60), DHS (100), other federal agencies (60), and private organizations (260). Cost estimates also were provided for the SIP at its current size (about 600 enrollees) and for an expanded program (of about 2,000 enrollees with the option of expansion to regional immunization sites—discussed in more detail in Section 3.5). The characteristics of the expanded SIP would include the following (Eitzen 2004, 2010):
-
Overall cost sharing as a percentage of the total cost. Each agency’s percentage would be calculated on the basis of estimates of the number of vaccines requested by it.
-
Variable costs for program expenses related to different vaccines, which reflect the number of doses required for the primary series, the number of protocol-mandated follow-up visits, and the number of booster vaccinations anticipated per year (no charge is assessed for the investigational vaccines themselves).
-
No individual invoicing by volunteer and no end-of-year refunds if total vaccines initially requested were not required.
-
Overall program costs calculated on the basis of salaries of clinical and regulatory staff, equipment expense, inventory and storage of vaccines, vaccine-stability testing, medical supplies, diagnostic testing, overhead, training, travel, office supplies, and information technology support for clinical and electronic document databases and product testing and storage.
The total costs were to be divided by the total number of injections requested annually, and the appropriate share of program costs would be invoiced to individual agencies according to their stated requirements.
Those recommendations were in accord with the earlier (May 2003) memo by the deputy assistant secretary of the Army, which stated that the SIP was to be a fully reimbursable program that used both FDA-licensed and unlicensed vaccines, the latter administered under IND protocols, and that the U.S. Army Medical Materiel Development Activity (USAMMDA) was to be the proponent for the SIP. Although receipt of investigational vaccines was to be completely voluntary, mandatory risk assessments were required before receipt of investigational vaccines. Furthermore, there was to be an evaluation of all persons then enrolled in the SIP to ensure they met the newly issued requirements, and re-enrollment in the SIP was to be on a cost-reimbursable basis with a fee schedule established by USAMMDA (Fatz 2003).
The HSC PCC approved the expanded SIP program and ordered that it be implemented with fully burdened funding contributed by the participating departments and agencies according to their percentage of SIP use.4 Under that arrangement, USAMRMC would continue to manage the program at Fort Detrick with a proposed expansion to three satellite locations. At this meeting, all participating agencies were in agreement that a cost-sharing program administered by DOD would be the model for a nationwide SIP, and the program was to be implemented in FY 2006 for a 5-year period. In its consideration of options and budget projections, the SIP subgroup included one-time costs of starting up regional sites and costs of testing and vialing new lots from bulk stocks of the existing SIP vaccines to meet the projected user base of 2,000 enrollees (Eitzen 2010). In his 2004 briefing paper, the subgroup chair noted that four additional IND vaccines might be of potential interest to the SIP (for Chikungunya virus, Hantavirus, Junin virus, and tickborne encephalitis virus) (Eitzen 2004). The subgroup did not, however, address the issue of existing vaccine supply for the SIP beyond considering the potential costs of vialing of new lots of existing Salk GSD vaccines from bulk stocks. The subgroup also did not consider in detail the addition of other existing vaccines or the development of new vaccines for inclusion in the SIP.
In February 2005, the Division of Medicine of USAMRIID and the Division of Regulatory Affairs of USAMMDA at Fort Detrick were asked to compile the number of vaccines requested by each government agency and to update budgetary projections for OMB. An initial estimate presented to OMB in January 2005 was $16.8 million, and the budget estimate for 2006 was $13.8 million. Those estimates allowed initiation of two new regional sites for vaccine administration in the southeastern and southwestern United States. Those regional sites were not established, however, because of budget constraints.
In a progress report written in May 2007, it was noted that in 3½ years since the HSC PCC ordered implementation of the expanded SIP, agencies had not set aside funding for an expanded SIP accessible to non-DOD as well
as DOD researchers. Therefore, some potential users may have been working without access to potentially protective IND vaccines available in the SIP. The report suggested that an interagency implementation and oversight body be established to drive implementation of the HSC PCC order (Eitzen 2010). Table 3.2 summarizes some of these significant developments regarding the SIP.
In summary, both the 2002 and 2004 reviews of the SIP recommended that an expanded SIP be implemented to include workers in government agencies beyond the Army, both federal and state, and academe and industry.
3.5
THE CURRENT SPECIAL IMMUNIZATIONS PROGRAM
3.5.1
Scope and General Structure of the Program
The committee noted that the SIP is the only program in the United States that provides investigational vaccines for laboratory workers exposed to hazardous pathogens and toxins.5 Although the SIP was conceived in support of laboratory personnel working at what is now USAMRIID, program reviews and agreements have expanded the array of participating organizations. As a result, the SIP currently offers selected investigational and licensed products to both military and civilian personnel working in a biohazardous environment who are at risk for pathogen and toxin exposure. The program also conducts medical monitoring of participants.
The SIP is housed in DOD under USAMRMC. It continues to operate within the Medical Division of USAMRIID, although a variety of offices in and outside USAMRIID support aspects of the overall program. The SIP facilities include the SIP clinic for administration of vaccines and medical follow-up and the SIP clinical laboratory. USAMRIID physicians from the Division of Medicine serve as principal investigators and subinvestigators for the protocols that govern the administration of investigational SIP vaccines.6 Review of the IND protocols, enrollment of personnel receiving vaccines, and other regulatory compliance issues are also subject to oversight by the USAMRIID Institutional Review Board, the USAMRMC Office of Human Research Protection, and the Quality Assurance and Regulatory Compliance Office. In addition, the USAMRIID Medical Division houses the necessary medical monitoring services for SIP IND protocols and encompasses the medical surveillance function of the SIP. USAMRMC’s USAMMDA serves as the IND sponsor representative on
TABLE 3.2 Important Events and Recent Reviews of the SIP (2000–2010)
|
Decade |
Important Events |
|
2000s |
Increase in U.S. biodefense research Army submits reports to FDA analyzing previous SIP immunization data and writes new investigational vaccination protocols Report of external biosafety assessment team (2002) Formation of SIP subgroup (2003) HSC PCC SIP agreement on “expanded” SIP to be used by multiple agencies with cost sharing (2004) Memo on status of implementation of 2004 agreement (2007) |
|
Panel |
Conclusions and Recommendations |
|
Findings and Recommendations on Alternative Strategies for Worker Protection (2002) |
Vaccines provide an essential extra measure of safety for laboratory workers. SIP is essential for internal and external customers conducting special pathogens work. CDC, via ACIP, not USAMRIID, should decide on use of IND vaccines at the national level. |
|
WMDMC Subcommittee, SIP subgroup (2004) |
Recommended SIP expansion with Army managing program on a pay-as-you-go, cost-sharing basis. |
behalf of the U.S. Army Medical Department Office of the Surgeon General, and USAMMDA also supports the SIP through product management and FDA regulatory support. That support includes oversight of regulatory compliance in such fields as vaccine-stability testing and product accountability. Finally, the stockpiles of IND vaccines used in the SIP are managed through the Joint Vaccine Acquisition Program (JVAP) of the Chemical Biological Medical Systems Joint Project Management Office (CBMS-JPMO), part of DOD’s Joint Program Executive Office for Chemical and Biological Defense (JPEO-CBD). Those activities include vaccine storage and vial distribution. Figure 3.3 is a simplified representation of offices relevant to the SIP in DOD.
Personnel in USAMRIID are able to enroll directly in the program. Civilian personnel in federal agencies enroll under the establishment of an appropriate memorandum of agreement (MOA) between the relevant agency and USAMRMC, and civilian personnel in nonfederal institutions enroll after establishment of a cooperative research and development agreement (CRDA) with the relevant institution. An agreement takes about 3 months for approval by USAMRMC, USAMRIID, and USAMMDA.
Before participation in the SIP, an individual risk assessment is performed by a supervisor and safety officer at the referring agency or institution to establish the potential for exposure to pathogens and toxins, based on activities or tasks performed in the laboratory and by the SIP Physician for medical eligibility to enroll in the SIP. The SIP has established detailed protocols and standard
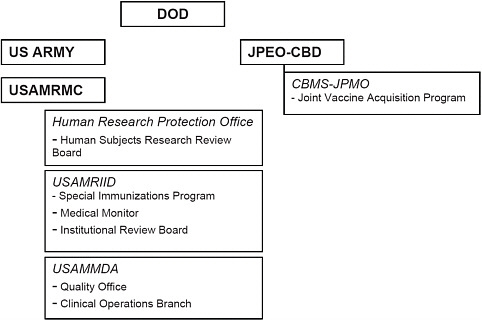
FIGURE 3.3 DOD offices relevant to the SIP.
operating procedures for enrollment in the program based on relevant medical criteria along with informed-consent procedures (USAMRIID 2004; DOD 2009). The committee noted the importance of the risk assessment process and emphasized that SIP vaccines should be given only to at-risk persons.
3.5.2
Special Immunizations Program Customers
At the time of the 2004 agreement on the proposed SIP expansion and agency cost-sharing arrangements, about 600–800 participants were enrolled in the SIP. HHS, however, had substantially increased funding for research related to biodefense, largely through NIAID. HHS projected increases in SIP participants on the basis of potential request for proposal responses to new funding opportunities offered through NIH and BARDA. During the 2004 SIP review and discussions, NIAID alone anticipated that its needs would be about 1,000 vaccinees in 2005 and increase to over 1,800 in 2010. That would have represented 42% of SIP use by HHS. It also estimated that about 1,000–5,000 workers in BSL-3 suites would have sufficient potential exposure to warrant consideration of immunization (Crumrine 2010). As a result of agency estimates used by the 2004 SIP subgroup, use of the SIP program was projected
to increase from about 600 participants per year to 2,000 participants per year (Eitzen 2010).
The expected increases in SIP use did not occur. Although the SIP experienced an initial increase to over 900 participants (including both DOD and non-DOD users), participation has since declined to a steady number of about 600. In 2010, the number of participants in the SIP was 623—395 in USAMRIID and 228 in other government agencies and external organizations (Boudreau 2010). It is unclear whether the decline reflects a decrease in laboratory workers who require immunization or is due to the high cost and inconvenience of the SIP program, which discourages participation. The organizations that had SIP enrollees in 2010 now encompass multiple DOD customers, non-DOD federal agencies, state health departments, academic institutions, and industry. As discussed below, each participating organization pays a share of the SIP operating costs and covers regulatory costs associated with the specific vaccines received by the enrollees that it supports. These groups include (Boudreau 2010):
-
DOD
-
Soldier Biological and Chemical Command
-
U.S. Army, Dugway Proving Ground
-
USAMRIID
-
U.S. Navy, Naval Medical Research Center
-
Defense Intelligence Agency
-
-
Non-DOD Government
-
U.S. Department of State
-
CDC, Fort Collins
-
USDA, Ames, Iowa; University of Wyoming; Plum Island Animal Disease Center
-
DHS, National Bioforensic Analysis Center at the National Biodefense Analysis and Countermeasures Center
-
U.S. Department of Energy, Lovelace Respiratory Research Institute
-
HHS, NIAID
-
-
Nongovernment
-
AlphaVax
-
Colorado State University
-
University of North Carolina
-
Johns Hopkins Applied Physics Laboratory
-
Boehringer Ingelheim
-
Southern Research Institute
-
New York State Department of Health
-
University of South Florida
-
University of Pittsburgh
-
University of Texas Medical Branch, Galveston
-
The current SIP customer base of about 20 organizations clearly fails to reflect program use by many of the researchers handling Select Agents. As of 2009, 388 “entities” were authorized to work with select agents: federal laboratories (65 entities), nonfederal government laboratories (121 entities), academic organizations (120 entities), and commercial and private entities (82 entities) (NRC 2009: 53). Overall, more than 13,000 individuals were cleared for involvement in Select Agent work in some fashion (NRC 2009: 8); this number far exceeds the number enrolled in the SIP.7
In the view of the SIP leadership, several factors may have influenced the observed magnitude of use. In some instances, participants enrolled in the SIP, received a single vaccine, and later left the program (Boudreau 2010); in these cases, SIP use was a discrete, one-time event. In other cases, laboratory personnel working with pathogens may not have been evaluated to be truly at risk and to benefit from SIP enrollment, because of the nature of the particular pathogens they were working with, the procedures they were conducting, or the type of biosafety environment in which they were working. The committee observed that use may have been lower than projected as agencies and organizations involved in medical countermeasures research and development focused on pathogens for which vaccines are not available from the SIP. In HHS, for example, an important component of BARDA’s mission is preparedness for pandemic influenza, vaccines for which are not in the current SIP but may be available by other mechanisms. As noted in Chapter 1, BARDA’s other significant mission focuses on the advanced development, and acquisition under Project Bioshield, of vaccines and therapeutics for civilian use against CBRN threats, particularly anthrax, smallpox, and botulinum toxin.8 Although vaccines against those diseases are included in the current SIP, licensed products for anthrax and smallpox exist, and the IND for at least one product against botulinum toxin is held by CDC. As a result, those vaccines can be obtained for occupational immunization from sources other than the SIP. While NIAID researchers remain potential SIP customers, other potentially relevant agencies, such as contractors performing work sponsored by BARDA, appear to have been able to meet most of their occupational immunization needs through mechanisms outside of the SIP.
3.5.3
Vaccines Offered in the Special Immunizations Program
The SIP provides access to 8 licensed vaccines against six diseases and to 10 investigational products (nine vaccines and the Q fever skin test) maintained under IND status. Tables 3.3 and 3.4 list current investigational and licensed
TABLE 3.3 Current SIP Vaccines (IND)
|
IND Vaccines |
Year of Manufacture (FDA Submission) |
Years of Supply Lefta |
|
Botulinum toxoidb (only nonlyophilized) |
1994 |
83 |
|
Eastern equine encephalitis (TSI-GSD 104 Lot 2-1-89) (inactivated, dried) |
1989 (1967) |
73 |
|
Rift Valley fever (TSI GSD 200 lot 7-2) (inactivated, dried) |
1978 (1969) |
11 |
|
Rift Valley fever—MP12c (TSI-GSD 223 lot 7-2-88) (live, attenuated, lyophilized) |
1988 (1991) |
10 |
|
Venezuelan equine encephalitis TC83 (NDBR 102 lot 4-3) (live, attenuated, lyophilized) |
1971 (1965) |
73 |
|
Venezuelan equine encephalitis C-84 (TSI-GSD 205, lot 7-1) (inactivated, dried) |
1981 (1975) |
12 |
|
Western equine encephalitis (TSI-GSD 210 lot 3-1-92) (inactivated, dried) |
1992 (1984) |
46 |
|
Q feverd (NDBR 105 lot 4) (inactivated, dried) |
1970 (1972) |
15 |
|
Tularemia (LVS) (NDBR 101 lot 4) (live, attenuated) |
1962 (1965) |
18 |
|
SOURCE: Boudreau 2010. aExact number of doses is confidential. Years of supply assumes use by the SIP on the basis of about 4 times the current use rates. bCDC holds the IND. cThe inclusion of Rift Valley fever MP-12 in the SIP has been discussed and a draft clinical protocol for its use has been developed. However, the vaccine needs to be revialed into single-dose vials for use in the program (Ellen Boudreau and Judy Pace-Templeton, USAMRIID, personal communication). dQ fever vaccine use is currently limited by skin test availability. There have been Q fever skin test potency issues: Q fever NDBR 105 inactivated vaccine requires prevaccination with the IND Q fever skin test. FDA placed a hold on the skin test because of potency issues. The skin test was remanufactured, and data were submitted to FDA in January 2010, but further documentation was requested from the production facility. One solution is the Australian Q fever vaccine Q-Vax, whose maker has been encouraged to submit a Biologics License Application in the United States. Administration of this vaccine in Australia also makes use of a prevaccination skin test, in this case intradermal administration of diluted vaccine (Gidding et al. 2009). |
||
TABLE 3.4 Current SIP Vaccines (Licensed)
|
Vaccine Against |
Product |
Manufacturer |
|
Anthrax |
Biothrax® (anthrax vaccine adsorbed) |
Emergent BioDefense Operations Lansing Inc. |
|
Hepatitis B |
Engerix-B® (recombinant) |
GlaxoSmithKline |
|
Recombivax® (recombinant) |
Merck |
|
|
Japanese encephalitis |
IXIARO® (inactivated virus) |
Intercell AG |
|
Rabies |
Imovax® (inactivated virus) |
sanofi pasteur |
|
RabAvert® (inactivated virus) |
Novartis Vaccines and Diagnostics |
|
|
Smallpox |
ACAM2000® (live virus) |
sanofi pasteur |
|
Yellow fever |
YF-VAX® (live virus) |
sanofi pasteur |
SIP vaccines, respectively, and Table 3.5 provides information on vaccine characteristics. All but one of the IND vaccines in the SIP are lyophilized preparations stored at −20°C ± 10°C. Typically, the time to use of a reconstituted vaccine is within 1–8 h.
The IND vaccines offered through the SIP would be unavailable to researchers and other at-risk personnel outside enrollment in the SIP. The SIP also offers licensed products against hazardous pathogens to provide continuity of care to personnel enrolled in the SIP (Boudreau 2010). Although immunization with licensed vaccines may be available to researchers from sources other than the SIP, access to some of them remains challenging (Pouch Downes 2010). The SIP provides an integrated mechanism for offering immunizations to at-risk researchers in support of occupational health and biosafety. The extensive medical monitoring and follow-up that are part of the SIP may also be an important source of data and allow the SIP to serve as a clinical test bed.
3.5.4
Logistics of Administration
The IND vaccines used in the SIP remain in extended Phase II testing, and this poses substantial cost, regulatory, and logistical burdens on SIP staff.
In 1998, 117 extramural sites were administering investigational vaccines under the USAMRMC-held INDs; USAMRIID provided the principal investigators while the extramural sites provided associate principal investigators. In 1999, USAMRMC closed all the extramural sites when they could no longer meet the rigorous current Good Clinical Practice (cGCP) regulatory requirements necessary for monitoring investigational vaccine use. Currently, all SIP immunizations are administered by USAMRIID, and subjects must go to Fort Detrick for this service. Up to 900 people have traveled to USAMRIID, and
TABLE 3.5 Characteristics of SIP IND Vaccines
|
Vaccine Against |
Schedule |
Immunogenicity |
Boost |
Systemic Adverse Effects |
Injection-Site Adverse Effects |
|
Botulinum toxoid |
Days 0, 14, 84, 180, 365 |
Acceptable for serotypes A/B |
annual |
10–15% (fever, headache, myalgia) |
20–40%, increasing after boosters |
|
EEE |
Days 0, 28 |
65–70% primary; 85–90% booster |
Mandatory 6 mo, then as needed according to titer (1:40) |
10–15% |
10% |
|
Rift Valley fever |
Days 0, 7, 28, 180 |
79% primary series; 96% booster response |
As needed according to titer (1:40) |
8–10% |
3–5% |
|
Rift Valley fever MP12 |
Day 0 |
93–95% |
As needed according to titer |
30% (headache), 10% (myalgia and fatigue) |
40% |
|
VEE TC83 |
Day 0 |
75%, lasts 8 yr |
VEE C-84 according to titer (1:20) |
50–60% |
Rare |
|
VEE C-84 |
Day 0 VEE TC83 responders; or days 0, 30, 60 for nonresponders |
86–95% |
As needed according to titer (1:20) |
20–30% |
10% |
|
WEE |
Days 0, 7, 28 |
Titer > 40 100% primary series (preliminary data) |
Mandatory 6 mo |
Not available yet |
Not available yet |
|
Q fever |
Day 0 |
Assumed to be lifelong |
None |
30–35% |
Expected response |
|
Tularemia |
Day 0 |
99% “take” + 98% + microagglutination titer |
If initial take negative, every 10 yr |
30–35% |
Expected response |
|
SOURCE: Boudreau 2010. |
|||||
USAMRMC initially bore the immunization costs of this program. Since 2003, other agencies enrolling employees in the SIP have been required to pay at least a share of the costs, and a fully burdened cost-sharing agreement has been in place since 2004.
For entry into the SIP, a Cooperative Research and Development Agreement (CRDA) is required between USAMRIID, USAMMDA, and nonfederal institutions, and a Memorandum of Agreement (MOA) is required for federal agencies. Those agreements take about 3 months for approval by USAMRMC, USAMRIID, and USAMMDA. A risk assessment is performed by a supervisor and biosafety officer at the referring institutions to establish the potential for exposure to pathogens and toxins and by an SIP physician for medical eligibility. Travel and return visits by extramural participants are required. Three SIP vaccines require only a single dose or a single dose and a booster dose, but five vaccines are administered in multiple doses. As a result, the extent of travel and associated costs depend on the number and type of SIP vaccines needed by an enrollee. Vaccine recipients who live out-of-state must have an occupational health-care provider available to contact to assess any medical problems or adverse events. Such information is communicated to the SIP personnel by telephone or e-mail. For all SIP participants, an annual medical review at USAMRIID, physical examination, and laboratory tests are required, as is the recording of continuing medications, intercurrent illnesses and accidents, and surgical procedures. Compliance is required for shipping of serum specimens to USAMRIID for antibody titers, baseline electrocardiography (ECG), and chest x rays. Recipients of the tularemia live vaccine strain (LVS) vaccine must remain at Fort Detrick for up to 3 days to assess the “take” after scarification. A similar stay is required for a Q fever vaccine9 and smallpox vaccine.
Although it increases the travel burden on non-USAMRIID participants in the SIP, having one site for the SIP has advantages, including the following:
-
It facilitates annual medical review.
-
It centralizes physical examinations and laboratory evaluation, baseline ECG, and the taking of chest x rays.
-
It facilitates recording of medications, illness, accidents, and surgical operations.
-
It allows serum samples to be assayed in the SIP clinical laboratories and reduces the need to ship samples for titers.
3.5.5
SIP Vaccine Supply and Stockpile Management
The bulk of the SIP vaccine supply is made up of legacy vaccines manufactured at the Salk Institute vaccine-production plant in Swiftwater, PA, in the
1960s–1980s. Since the facilities’ acquisition by Institut Merieux in 1989 (later Pasteur Merieux Connaught and now sanofi pasteur) and closure of the Salk GSD in 1998, no new lots of those vaccines have been produced. The existing stockpiles of these legacy investigational vaccines are maintained by the DOD Chemical Biological Medical Systems (CBMS). CDC manages the government stockpile of licensed vaccines available through the CDC Drug Service.
Numerous lots are available for most of the SIP investigational vaccines, and conservative estimates of IND vaccine supply range from 10 or 11 years for Rift Valley fever lots to 73 years for some VEE and EEE lots (see Table 3.4). The use of additional lots with confirmed potency by virtue of stability assessments would expand the supply by a factor of 2–10. As vaccines against hazardous pathogens of interest to the SIP have become licensed (for example, vaccines against Japanese encephalitis, hepatitis B, rabies, anthrax, smallpox, and yellow fever), the SIP has continued to purchase these vaccines and administer them to eligible personnel. However, no specific budgetary line provides for this purchase.
No new IND vaccines currently administered in the SIP have been added since 1992 (WEE); most were placed in the program in 1964–1981 (see Table 3.4). Some vaccines are now over 40 years old, but no vaccine has yet been withdrawn because of low potency or failure to meet other stability assessments.10 With regular testing of the vaccine lots, there has also been some minimal loss of vaccine stock due to loss of vacuum or sterility. To preserve the existing supply of vaccine, revialing of new lots from bulk stocks may be necessary, but this would be an expensive undertaking with no visible source of funding, no clear manufacturer to assume the effort, and uncertain ability to release such materials based on quality assessments.
Requirements for maintaining SIP vaccines under IND status include
-
Regulatory reporting.
-
Potency testing every 2 years.
-
Sterility testing every 3 years.
-
Compliance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines for study conduct.
-
Intensive documentation and training of personnel.
-
Protocol compliance by volunteers and investigators.
-
Quality-assurance and quality-control monitoring.
From 2000 to 2003, lot release tests were repeated on all SIP vaccines for potency, bacteriostasis and fungistasis, mycoplasma, endotoxin, general safety, residual moisture and product enhanced reverse transcriptase testing, vial integrity (if vacuum-packed), and spark testing or sterility testing (if nitrogen-packed). Potency testing is conducted every 2–3 years and the other lot release tests could be performed if they were requested by the sponsor or FDA. There is no current budget for new lot release testing (Boudreau 2010).
IND vaccines in the SIP remain in prolonged IND status and are unlikely to transition to licensure for several reasons (Boudreau 2010):
-
There are no current manufacturers or pharmaceutical companies that can expect a profit in producing the vaccines.
-
Vaccine effectiveness and safety studies have been conducted in animal models. Vaccine effectiveness in preventing disease in humans is difficult to test because of ethical or safety concerns in conducting traditional clinical trials for efficacy; the population of at-risk individuals is also small. To date, no vaccine has been approved for licensure under the “animal rule” instituted by FDA in June 2002.
-
FDA permits their use under IND protocol, which may also reduce incentives to proceed to further regulatory milestones.
There are disadvantages of maintaining prolonged IND status for these vaccines, including the fact that no or few controlled clinical efficacy trials have been performed in humans, and questions about whether vaccine potency and sterility will continue to be maintained with aging vaccine product (Boudreau 2010). The SIP IND vaccines were also developed using cell culture production practices that might not meet current standards, and as seen in Table 3.5, the levels of reactogenicity and of local and systemic adverse events for some of them may be higher than desirable. Because the vaccines are administered under clinical trials, there is also a need to monitor titers, perform medical assessments, and analyze and submit clinical data to FDA.
In addition, individuals receiving the IND vaccines may experience adverse events, there may be populations of workers ineligible to receive the vaccines, the benefits of the vaccine may be less in cases of high-dose aerosol exposure, and it is possible that receiving the immunization may lead to an unwarranted sense of safety and security that results in lax laboratory practices (Boudreau 2010). Although some of these considerations might apply to licensed vaccines and to vaccines developed using today’s technology, these may be greater concerns given that the SIP IND vaccines were largely developed decades ago prior to the establishment of modern cGMP standards.
Despite the cost of their maintenance, the committee noted that IND vaccines can potentially offer additional protection to personnel in the event of inevitable accidents and percutaneous injury. In such situations, the severity of
diseases being covered and the lack of licensed vaccine support consideration of the use of IND vaccines if they are available and considered to be safe. The immune response may be primed before pathogen or toxin exposure (prophylactic use), or the vaccine may in some cases be able to be administered after a potential exposure in an effort to minimize adverse consequences (therapeutic use), as is the case for smallpox vaccine. Alternatively, the presence of a population of immunized people may allow serum isolated from vaccinated people to be used therapeutically if unimmunized people are exposed. In addition, the data on safety and immunogenicity accumulated through the SIP IND clinical trials could be used to enable FDA to make a determination for an Emergency Use Authorization if this is required.
3.5.6
Costs of the Special Immunizations Program
According to SIP leadership, entry into the SIP program currently costs about $10,000–15,000 per volunteer per year (depending on the number and type of SIP vaccines needed).11 That is based on audited costs of operating the program, which include (Boudreau 2010)
-
Periodic potency and lot release testing of the product.
-
Storage and maintenance of the stockpile.
-
Regulatory submissions, including continuing review reports, annual reports, summary reports every 5 years, investigator brochures, periodic safety and serious adverse event reporting, informed-consent form updates, responses to periodic audits, and clinical monitoring visits.
-
Intensive physician and nurse efforts because volunteers are enrolled in multiple clinical studies.
-
Clinical database and document control compliant with the record-keeping requirements of 21 CFR Part 11.
-
Maintenance of the SIP clinical-record archive, which contains information on the last 40 years of the SIP.
Invoices are sent to participating institutions on the basis of those audited costs of operating the program. Payment is required in advance for SIP enrollees.
In addition to the above-mentioned costs, operation of the SIP entails considerable overhead costs, for example, for staffing, facilities, equipment, and product maintenance. Estimates of costs for the SIP provided during the
2004 subgroup review for the single-location SIP serving about 600 enrollees included 79 staff assignments ($8.9 million per year), with enlisted personnel supplementation from outside of the USAMRIID Division of Medicine as needed. Additional estimates of costs provided during the 2004 review for a single-location SIP serving 600 enrollees included $0.8 million for facilities, $0.2 million for equipment, and $6.1 million for product storage, testing, and documentation.12 Those data indicated a total overhead cost of about $16.0 million per year (Eitzen 2010). It was projected that SIP expenses would increase by about $0.9 million per year over the following 5-year period (Eitzen 2010). Current staffing and related costs appear to be different than those used during the 2004 review, and are discussed in more detail below.
At the time of the 2004 HSC PCC agreement estimating that SIP enrollment would expand to 2,000 subjects, it was projected that such an expanded program would require additional staff costs and facilities costs for the projected regional sites, which would result in an estimated annual cost of $35.6 million. On the basis of projected use by the primary federal government stakeholders, the estimated annual expenses would be $11 million for DOD and $15 million for HHS, with USDA, DHS, other federal agencies, and private users making up the balance. It was also estimated that considerable filling and finishing of existing bulk vaccines would be required for the expanded program at an estimated one-time cost of $28 million for 5 years (Eitzen 2010).
According to SIP leadership, the total number of staff currently in the SIP is about 40–45 at USAMRIID and approximately 8 part-time staff at USAMMDA. They include 10 physicians (5 for the SIP, 4 for after-hours calls for USAMRIID personnel, and 1 medical monitor), 6 registered nurses and 6 licensed practical nurses, 3 administrators, and several vaccine verifiers (enlisted personnel) and vital-signs takers. Management of the vaccination protocols also requires data-entry personnel (4) and data managers (2). In addition, USAMMDA provides 10–15 regulatory personnel, including quality-assurance managers, study monitors, product managers, and managers for product testing (USAMRIID 2009). The cost trend for the present scope of the SIP—including immunizations and medical management of participants at USAMRIID, and regulatory management though USAMMDA Regulatory Affairs—is estimated to be about $9 million per year (Boudreau 2010). CBMS/JVAP contracts with third parties for product storage and potency testing.
The SIP operates on a pay-as-you-go basis, and funding to support the
extensive infrastructure and staffing of the program appears insecure. There is no line item in the USAMRMC budget for the SIP, and each participating stakeholder, whether DOD or non-DOD, pays for SIP immunizations out of discretionary money in its budget. The vaccines are stored at two off-site facilities, and costs of CBMS stockpile management have been estimated at $1.5 million per year (Boudreau 2010). However, there is no line item in the budget for further vaccine storage and testing. CBMS currently manages these activities on a year-to-year basis; no other office has agreed to undertake this management and no long-term mechanism has yet been identified (Boudreau 2010).
3.5.7
Governance of and Priority-Setting in the Special Immunizations Program
As discussed in Section 3.2, national priorities for military and civilian MCM are currently set separately from the process of governance that determines the current and future capabilities of the SIP. These national MCM priorities include agents of specific interest to the military, agents of interest to civilian authorities such as HHS, and agents of interest to both. In addition to products directed against traditional pathogens and toxins, strategies are being pursued to develop broad-spectrum antivirals and antibiotics and potential countermeasures against genetically modified or novel agents.
Against the backdrop of the national MCM enterprise, there is a need to consider whether the current portfolio of products available in the SIP aligns with national research agendas and whether there is a decision-making process by which a vaccine becomes available to or is removed from the SIP.
Table 3.6 compares the current list of investigational and licensed products in the SIP with several current national priority lists. As can be seen in the table, the SIP continues to contain a subset of investigational vaccines that largely reflect historical R&D efforts in USAMRIID and traditional military biodefense priorities. It does not, for example, include investigational vaccines against the Ebola and Marburg filoviruses, which are of interest to both DOD and HHS, or against pathogens of particular HHS priority, such as Junin and Burkholderia species. Initial development and production of seed vaccine stocks for two products against pathogens that may be of interest to civilian researchers, Junin virus and Chikungunya virus, were developed at USAMRIID but have since been transferred to institutions outside the United States and are no longer available in the SIP. The use of those vaccine stocks in other countries is discussed in Chapter 5. The committee believes that this illustrates a general limitation of the current SIP for pathogens having primarily civilian but not military interest.
As discussed in Chapter 5, other relevant countermeasures development efforts may be under way (for example, against Ebola and Marburg viruses) and could be considered for inclusion in the SIP once efforts have reached a suit-
TABLE 3.6 Comparison of SIP Provision of Vaccines with DOD and HHS Priorities
|
SIP |
PHEMCE Implementation Plan |
JPEO-CBD |
Transformational Medical Technologies |
|
B. anthracis (anthrax) C. botulinum (botulism) Variola major (smallpox) F. tularensis (tularemia) EEE, VEE, WEE viruses Rift Valley fever virus C. burnetii (Q fever) Hepatitis B (licensed) Japanese encephalitis virus (licensed) Rabies (licensed) Yellow fever (licensed) |
Anthrax Botulism Smallpox Tularemia |
Anthrax Botulism Smallpox Tularemia EEE, VEE, WEE |
Anthrax Botulism Smallpox Tularemia EEE, VEE, WEE |
|
|
|
Q fever |
|
|
|
Ebola/Marburg Burkholderia spp. Junin virus Yersinia pestis (plague) Rickettsia prowazekii (typhus) |
Ebola/Marburg Burkholderia spp. Junin virus Yersinia pestis |
Ebola/Marburg Burkholderia spp. Junin virus Yersinia pestis Typhus |
|
|
|
Brucella spp. Staphylococcal enterotoxin B Ricin |
Brucella spp. Staphylococcal enterotoxin B Ricin Vibrio cholerae T2 mycotoxin |
|
SOURCES: HHS 2007; Newmark 2009; Hough 2010; NBSB 2010a. |
|||
able stage of development. HHS has also made development and acquisition of vaccines against pandemic influenza strains such as H5N1 a priority. It has solicited requests for proposals for influenza manufacturing capacity (see Section 5.2.4) and a licensed H5N1 vaccine has been produced (sanofi pasteur, licensed in 2007). The vaccine is for pre-pandemic use; it is not included in the SIP.
The process by which DOD sets its priorities for biodefense and infectious disease vaccines and undertakes the R&D and acquisition activities that it supports is complex.13 As the committee understands the current process of
governance of the SIP, for an IND vaccine to be placed into the SIP by DOD there must be a formal document establishing the military need for it. If the need is established, an effort is initiated, and the vaccine must be placed into the category of advanced development. There is no funding for any vaccine that has not achieved those milestones. The DOD vaccine enterprise is focused largely on developing and acquiring licensed products for potential larger-scale use in force protection, not on the more limited role of vaccines (including investigational products that may never progress to full licensure and larger-scale manufacture) for protecting personnel who work to develop next-generation countermeasures.
Within USAMRIID, a Special Immunizations Committee chaired by the chief of the Medical Division is charged with oversight of the SIP program and is able to recommend DOD vaccines for incorporation into the SIP. Its mission is to “outline and evaluate policy and procedure for administration of special immunizations for personnel requiring entry into USAMRIID Biosafety Level 3 and 4 laboratories, and to make immunization policy recommendations to the Commander” (USAMRIID 1995). The current system of SIP oversight and the requirement for a documented military need for a vaccine to be added to the SIP suggest that there is no procedure for placing an investigational vaccine that is deemed critical to the protection of non-DOD laboratory workers into the SIP. Further, the committee could not find evidence of an interagency process for undertaking regular reviews and making broader systematic decisions on vaccines to be included in the SIP. The gap appears particularly noteworthy in the context of the “expanded” SIP agreements implemented since 2004 that include enrollment of at-risk laboratory workers in non-DOD agencies, academe, and industry.
3.6
FINDINGS AND CONCLUSIONS ON THE MEDICAL COUNTERMEASURES ENTERPRISE AND THE CURRENT SPECIAL IMMUNIZATIONS PROGRAM
During the last decade, numerous outside reviews of military and civilian biodefense vaccine programs have recommended a substantial increase in the funding of and the priority given to these programs, including the establishment of production facilities and agencies that have oversight functions to direct the efforts. More recently, recommendations have been offered to create incentives for industry and the private sector to establish better collaboration with government and academe and to overcome regulatory obstacles. Previous reviews of the SIP have consistently recommended that the program be open to at-risk researchers beyond USAMRIID and cost-sharing mechanisms to support the additional use.
In this context, the committee concluded that several findings about the SIP are evident:
-
Finding 3: The SIP remains the only formal program that exists to provide multiple vaccines (both licensed and IND) to at-risk laboratory workers and other occupationally exposed personnel who work with hazardous pathogens.
-
Finding 4: USAMRMC has the history, personnel, clinic facilities, protocols, standard operating procedures, and regulatory infrastructure to administer, monitor, and document immunizations provided through the SIP.
-
Finding 5: The SIP generally functions well for USAMRIID customers but has not met the expected need of customers outside of USAMRIID, particularly personnel involved in civilian biodefense countermeasures, public health research, and the veterinary communities.
SIP enrollment remains at about 600–700 per year, rather than the roughly 2,000 projected in 2004, and this suggests a need to reevaluate the potential stakeholders and related customers whose access to the SIP would enhance their biosafety-practices program. Some workers at risk for exposure to dangerous pathogens against which a vaccine exists are able to access the program successfully, but it appears that others are conducting research without the potential protection that a vaccine may provide. Given the current cost structure, potentially relevant users may be unable to afford the price of participation in the program. In the current economic climate, that problem is likely only to worsen. The supply of investigational vaccines in the SIP is sufficient for the immediate term but will be more rapidly depleted if additional participants enter the SIP and require immunizations. The current SIP clinical lots may be unable to accommodate the additional demand from all laboratory workers at risk for exposure. To meet the additional demand, further vialing from bulk stocks may be required, and this would add substantially to the costs of maintaining the SIP in the absence of a clear understanding regarding how these costs would be paid. It is often difficult for stakeholders to obtain the vaccines outside the SIP.
-
Finding 6: Other than the USAMRIID SIP committee, which is limited to military needs, the SIP appears to lack a governance structure that enables regular strategic review of the investigational and licensed vaccines included in it and to lack mechanisms to address identified gaps.
Further expansion of the SIP to accommodate civilian countermeasure priorities will require a better mechanism for deciding which IND vaccines should be included. Currently, for an IND vaccine to be in the SIP, the DOD process requires the establishment of a military need for a specific vaccine to be developed, and it must reach the end of Phase II testing with an adequate safety profile. IND vaccines developed outside DOD and not required by the military
might well be useful to other stakeholders. A mechanism by which requests to include such vaccines are considered should be implemented.
-
Finding 7: The SIP lacks consistent funding, including a lack of line items in USAMRIID and other agency budgets to support continued maintenance of the existing stockpiles, vialing additional bulk vaccine from existing stocks, and costs associated with acquiring new vaccines for inclusion in the SIP.


































