8
Prevention
The societal burden of tick-borne diseases (TBDs) is substantial. In the United States alone, every year this group of diseases produces tens of thousands of illnesses, many of which are severe and result in hospitalization, long-term sequelae, including disabilities or deaths. Research efforts have been focused on ameliorating the symptoms and consequences of disease through treatment. However, the development, deployment, and evaluation of strategies to prevent the occurrence of TBDs should also be a major area of scientific inquiry. This is one aspect of the debate about tick-borne diseases where there is no controversy. Prevention of disease is far preferable to treating the short- and long-term consequences once they occur.
The incidence rate of all of the diseases discussed in this workshop has been on the increase. They have also been expanding in geographic range, and new human tick-borne pathogens continue to be recognized. These trends result in an ever-larger number of persons requiring treatment, placing a greater financial impact on the healthcare system and individual patients, and, ultimately, a greater burden on society. The escalating burden of TBDs is a clear demonstration that the available prevention measures have been ineffective. Whether this is because they simply do not work or because they have been underused is far less clear. But a wider array of simple and effective prevention modalities would be very beneficial and would, it is hoped, change the current trajectory of TBD incidence.
Prevention measures can be divided into two categories: Pharmacologic preventive measures such as antibiotic prophylaxis or vaccines, and non-pharmacologic interventions such as behavior change or tick-targeted strategies (e.g., tick checks or tick reduction). In this workshop, information
was presented for both categories, but there was not enough time to cover the entire range of available or potential approaches. Two presentations addressed current and future opportunities for vaccine development. One presentation addressed the role and effectiveness of behavior change and another addressed vector-control strategies.
CURRENT VACCINES FOR TICK-BORNE DISEASES AND VACCINE DEVELOPMENT
Jere W. McBride, Ph.D., M.S., Center for Biodefense and Emerging Infectious Diseases, University of Texas Medical Branch at Galveston
Currently, no tick-borne disease vaccines for humans are licensed in the United States. The U.S. Food and Drug Administration licensed a vaccine for Lyme disease in 1998 and it was withdrawn from the market in 2002. While the vaccine—based on outer surface protein A (OspA) emulsified in aluminum hydroxide adjuvant—prevented transmission of Borrelia burgdorferi from ticks to humans by killing spirochetes in ticks, three doses were needed to provide 80 percent protection against infection. A number of problems contributed to the withdrawal of the vaccine. The antibody titers did not persist for long time periods, which required individuals to receive multiple boosters to maintain protective immunity. Furthermore, a number of autoimmune-related side effects, including arthritis and neuropathology, were reported to possibly be associated with the vaccine (Schuijt et al., 2011). A short stretch of amino acids in OspA with the potential for molecular mimicry with human LFA-1 (lymphocyte function associated antigen) was identified as a possible cause for the autoimmune-related responses, but this finding remains controversial (Steere et al., 2001; Ball et al., 2009). In Europe, current efforts are focused on developing vaccines with a modified OspA that does not contain the sequence linked to autoimmune responses. Other vaccines for Borrelia are in varying stages of development as either single- or multiple-antigen vaccines that include OspB, OspC, or DNA-binding protein HU-alpha.
Experimental veterinary vaccines for bovine anaplasmosis and infection and treatment strategies for heartwater—an ehrlichiosis of ruminants—demonstrate that vaccines for human rickettsial diseases are feasible. To develop such vaccines, however, some basic research on these emerging infectious diseases needs to be done, including:
-
Defining immunoprotective pathogen proteins;
-
Understanding and defining pathogen antigenic variation;
-
Understanding variations in pathogenicity;
-
Understanding protective immune mechanisms;
-
Developing appropriate animal models;
-
Understanding host influence on pathogen phenotype and transmission;
-
Defining pathogen and host gene expression during infection to identify vaccine candidates;
-
Understanding molecular host-pathogen interactions that can be blocked by the host immune response; and
-
Identification and development of vector vaccine components.
A number of studies have elucidated a host’s protective immune mechanisms against Ehrlichia and Anaplasma. For example, humoral and cellular immune mechanisms have been shown to be important in controlling acute bacteremia in Ehrlichia (Winslow et al., 2000; Feng and Walker, 2004); passive transfer of antibodies against the pathogen’s outer membrane proteins can protect a host (Winslow et al., 2000); and interferon gamma (IFN-γ) is important in activating a host’s monocytes and clearing infection (Feng and Walker, 2004). Furthermore, in mice, lymphocytes (CD4 and CD8) protect against fatal infection (Feng and Walker, 2004), and the major histocompatibility complex (MHC) class II is important for pathogen clearance (Ganta et al., 2002). For Anaplasma, while protective adaptive immune responses include cellular and humoral immune mechanisms, the innate immune mechanisms appear to be dispensable for control of the infection (von Lowenich et al., 2004). Elimination of A. phagocytophilum is mediated by antibodies and IFN-γ (Sun et al., 1997; Akkoyunlu and Fikrig, 2000; Wang et al., 2004). High-antibody titers are associated with immunity, although antigenic variation provides an immune evasion mechanism for the pathogen (Barbet et al., 2003; Granquist et al., 2010).
For Ehrlichia, some proteins are promising for vaccine development. Initial efforts have focused on immunoreactive proteins, surface-exposed proteins, and effector proteins that either play a role in arthropod infection and transmission, induce both cell-mediated and humoral immunity, or are recognized by the acute-stage immune response. Initial experiments to molecularly characterize potential effector proteins have shown they contain tandem repeats (TRPs). Thus far, 12 TRPs have been identified as being encoded by the Ehrlichia chaffeensis genome. Of these 12, 8 are predicted to be secreted. Two of those TRPs—TRP120 and TRP47—have mucin-like properties: that is, they have high serine/threonine content, and are strongly acidic. These tandem repeat proteins are of high interest because TRPs in other microorganisms have been associated with immune resistance, antigenic diversity, adhesion, and protein–protein interactions.
To determine the major B cell epitopes—that is, the regions that a host’s immune system recognizes—TRP120 and TRP47 were expressed as recombinant fragments, including the N-terminal portion, the tandem
repeat region, and the C-terminal portion. These fragments were probed with serum from a dog or a patient infected with E. chaffeensis. Antibodies in the host serum strongly recognized the tandem repeat region, but not the other components, revealing that the major immunogenic epitope are in the tandem repeat region. Similarly, epitopes have been mapped in the tandem repeat region of other TRPs.
By mapping this epitope to the tandem repeat region and using overlapping peptides, a 22-amino-acid peptide in TRP120 that contained the dominant immunogenic epitope was identified. In experiments where antibodies against this peptide were passively transferred into severe combined immunodeficiency (SCID) mice infected with E. chaffeensis, a significant reduction in the bacterial load was reported. The spleen weights of the mice treated with antibodies were much less than those of controls, consistent with the findings related to increased bacterial loads in controls. Similar protection was observed after passive transfer of antibodies to TRP32 and TRP47 into SCID mice infected with E. chaffeensis. These results suggest that antibodies to these three TRPs induce a measure of protection in the mammalian host.
Microarrays have been used to understand the protein expression levels of TRPs in both mammalian and arthropod hosts. Such studies are important in revealing how a pathogen cycles through the arthropod host and into the mammalian host, for example, and which proteins are actually expressed—and therefore would be good targets for a vaccine. Many TRPs appear to help the pathogen transition into the mammalian host and survive in the macrophage. These proteins are highly upregulated after the pathogen enters the mammalian host. For example, TRP47 is the most highly expressed gene in the Ehrlichia genome in the macrophage. Further, one tandem repeat protein—TRP120—is expressed in both the tick and the mammalian host. Two outer membrane proteins are also expressed—one that is expressed in the mammalian and tick hosts—and one, outer membrane protein 1B expressed only in the tick host, which could be considered for a tick-specific vaccine for E. chaffeensis. Antibodies directed at outer membrane proteins as well as TRPs have been shown to be protective in mammalian hosts. Some of the earliest antibody responses are directed at these TRPs, which are involved in molecular host–pathogen interactions that may be blocked by antibody. OMP-1 expression is also restricted in the tick, suggesting that vaccines targeting the appropriate outer membrane protein could have bactericidal activity in the tick. Similarly, TRP120 may be a candidate as a vaccine to prevent vector feeding or transmission, or inhibiting infection in the mammalian host. For A. phagocytophilum, seven major immunoreactive antigens have been identified, including 44-, 55-, 72-, 100-, and 160 kDa proteins. P44 is an outer membrane protein that recombines and is involved in antigenic variation. Antibodies directed at
conserved regions of P44 partially protect a host from a pathogen challenge. Antibodies directed at two cotranscribed surface proteins, Anaplasma surface proteins 62 and 55, also partially neutralize A. phagocytophilum infection.
Rickettsia pathogens differ from Ehrlichia and Anaplasma in that they lyse the membrane of host cells, and are therefore free in the cytosol. Three mechanisms help define the virulence of Rickettsia in a mammalian host. Rickettsia enters a cell when adhesins such as OmpA and OmpB and stem cell antigen-1 (Sca-1) and stem cell antigen-2 (Sca-2) interact with Ku70, a surface protein on host cells, and induce them to phagocytose the Rickettsia. Second, internalized Rickettsiae are initially bound within a phagosome. However, the organism uses four enzymes to quickly lyse the vacuole membrane to escape it. Last, three spotted-fever group Rickettsiae use actin-based motility to move inside the cytoplasm and between cells. The actin tail is formed by polymerization of the host actin at one end of the bacterium with the rickettsial protein RickA or Sca-2. Some of the protective immune mechanisms used by the host against Rickettsia have been defined. For example, pro-inflammatory cytokines, IFN-γ and TNF-α, activate endothelial cells to kill intracellular organisms via nitric oxide synthase-dependent mechanisms. Cytotoxic CD8 T cells lyse infected target cells via pathways involving perforin and granzymes and are more important than CD4 T cells in clearing infection against Rickettsia. Humoral immunity may be more important in preventing reinfection than in clearing primary infection.
There have been some preliminary experimental vaccines for the rickettsioses. For example, formalin-inactivated vaccines against Rickettsia rickettsii have been developed from infected tissue, yolk sacs, and cell culture. These have not been proven to be effective, and subunit vaccines based on outer membrane proteins A and B appear to provide some protection in animals, but their efficacy in humans has not been studied. A newer avenue of vaccine development involves Rickettsia gene knockouts of virulence determinants. Two examples, which appear to be avirulent in guinea pigs, are Rickettsia prowazekii with the phospholipase D gene removed, and R. rickettsii with the sca-2 gene removed. The sca-2 gene mutant displays a small-plaque phenotype, but replicates at wild-type levels within Vero cells (Kleba et al., 2010). Furthermore, the Rickettsia with the sca-2 knockout also lacks actin-based motility. A live attenuated vaccine based on that knockout that mimics natural infection would seem promising.
Other efforts have focused on vector proteins that impair tick feeding while also modulating immune responses and coagulation in a mammalian host. Several tick proteins have shown some protective ability, including those involved in iron transport and inflammation regulation, those with antihemostatic and anti-inflammation properties, antioxidants and immune
shields, and those involved in IgG binding and secretion. For example, subolesin—a well-conserved protein among tick species—provides protection in mammals against both Ixodes and Amblyomma tick feeding.
Knowledge Gaps and Research Opportunities
McBride noted there are a number of avenues for future work on vaccines against tick-borne diseases:
-
For Rickettsia, live attenuated vaccine mimics the natural infection; thus Rickettsia that are genetically attenuated by gene knockouts are promising for future development of vaccines for rickettsial diseases.
-
Vector proteins are attractive vaccine candidates. Targeting vector proteins that impair tick feeding, host immune modulating and coagulation activities, and tick proteins that interact with pathogen are under investigation.
-
Vaccines with effective combinations (elements directed against both vector and pathogen) of protective mechanisms are likely to be attractive future approaches to vaccine development.
DEVELOPING OPPORTUNITIES FOR FUTURE VACCINES
Wendy Brown, Ph.D., M.P.H., Department of Veterinary Microbiology and Pathology, Washington State University
Many lessons can be drawn from the study of related organisms in veterinary species, such as cattle, that can inform our understanding of human immunology, pathology, and vaccine development for tick-borne pathogens. From this research, it is known that not all tick-borne pathogens are equal. They consist of a variety of different agents, including viruses, bacteria, and protozoa. Their complexity increases accordingly with their genome size. For example, most viral pathogens have very small genomes, whereas protozoa such as Babesia bovis, Theileria parva, or malaria have genomes 1,000-fold larger and considerably more complicated.
Currently, the only vaccines for tick-borne diseases in humans are for viral infections, such as flaviviruses. One of the reasons that existing viral vaccines are effective is that viremia can be controlled by the immune system by increasing titers of IgM and IgG antibodies. Thus, immunizing with an inactivated, live viral vaccine or a killed vaccine can induce a rapid immune response, which can result in the virus being quickly controlled when the animal or human comes in contact with it. The hallmark of these protective vaccines is that they induce a neutralizing antibody response
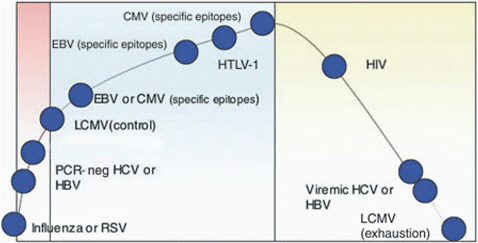
FIGURE 8-1 Pathogens with high antigen load cause immune dysfunction.
SOURCE: Klenerman and Hill, National Immunology, 2005.
involving long-lived B lymphocytes and plasma cells, and a long-lived memory B cell and T cell response. This type of protective immune response is the goal of most work on human vaccines against tick-borne pathogens.
Bacterial and protozoal tick-borne pathogens are more complicated than viruses because they involve multiple stages in the host cell, and because they have evolved different strategies to evade the host’s immune response. For example, a number of tick-borne pathogens, including Borrelia burgdorferi, B. hermsii, Anaplasma. phagocytophilum, A. marginale, and Babesia bovis employ antigenic variation to evade a host immune’s response. Furthermore, vector-borne pathogens such as A. marginale, B. bovis, and malarial parasites that cause high levels of pathogen load throughout infection persist in the host and induce a dysfunctional CD4 T lymphocyte response that includes overexpression of inflammatory cytokines and deletion of antigen-specific T cells. Many viruses that induce high viremia also produce severe immune dysfunction that correlates with the load of pathogen in the host (Figure 8-1). Common examples are HIV, hepatitis C virus, and hepatitis B virus, which induce T cell dysfunction leading to T cell exhaustion or deletion.
As research leads to understanding of the molecular characteristics and the cellular pathways involved in pathogenesis, a number of targets have been identified for developing potential vaccines. These include
-
Outer membrane or surface proteins produced during the host stage to prevent infection.
-
Proteins on the surface of infected cells. For example, one target would be CD8 cytotoxic T cells, which recognize parasite antigens on the surface of infected lymphocytes.
-
Tick-stage pathogen antigens (e.g., OspA) that would prevent transmission.
-
Antigens produced within the tick, such as those active in its gut to reduce tick feeding and to reduce or prevent transmission of the pathogen.
Vaccines based on outer membrane proteins are of particular interest as they serve as the interface between a pathogen and a host, and are a primary target of the host’s immune response. A pathogen uses the outer membrane to adhere to and invade host cells, and often for intracellular survival and growth. The outer membrane of bacterial pathogens expresses complexes of proteins, such as the type III and type IV secretion systems, which serve as virulence factors and send effector molecules into host cells, disrupting their function. Protective vaccines based on purified outer membranes or outer membrane vesicles include Treponema, Neisseria, Hemophilus influenza, Chlamydia, Francisella, and Anaplasma. Immunizing cattle with outer membranes purified from A. marginale, for example, results in complete protection against infection in approximately one-third of the animals. However, vaccines based on individual outer membrane proteins instead of whole membranes induce poor protective immunity. Early efforts on individual outer membrane proteins focused on serologically reactive proteins, which are immunodominant proteins and recognized by serum antibody from an infected individual or an immunized animal. For the five or six immunodominant outer membrane proteins of A. marginale, these proteins did not induce protection equivalent to that of whole outer membranes. There are a number of hypotheses to explain why vaccines based on whole outer membranes are protective, while those based on individual outer membrane proteins are not. For example, it is possible that the antigens tested thus far are not the ones that can induce protection, that multiple antigens need to be included in a vaccine, or that the association of proteins within the outer membrane confers ability to induce protective immunity through linked recognition of T cell and B cell epitopes.
Individual outer membrane proteins that stimulate antibodies after infection or immunization have been typically targeted for vaccine development because it is easier to perform than screening antigens to find those that increase production of CD4 T cells. However, the latter is important because those antigens are essential for enabling B cells to switch isotypes to make IgG, the antibody that is important in opsonization. Antigens that effect both IgG antibody and CD4 T cell responses in various hosts are likely to advance our knowledge.
Immunodominant surface proteins have also been typically targeted for vaccine development. For example, B. bovis has two major surface antigens, MSA-1 and RAP-1. MSA-1 is abundant and undergoes antigenic variation between strains, and along with RAP-1, it has been shown to provide incomplete or no protection (Brown et al., 2006). Similarly, in A. marginale, the two major surface proteins, Msp2 and Msp3, undergo antigenic variation, resulting in numerous antigenic variants in an individual and a population at any given time. These immunodominant proteins conveyed incomplete or no immunoprotection (Palmer et al., 1999). The Waksman Postulate suggests a working hypothesis for why these proteins might be abundant, but not essential. The Postulate noted that “any antigen against which parasites allowed the host to mount an immune response is by definition unimportant for the survival of the organism” (Sher, 1988). It was also noted that “antigens that induce poor or immunosuppressed responses during natural infection should not be ignored since they may be molecules essential for parasite survival” (Sher, 1988). Based on these results, the subdominant, but conserved antigens may be better candidates for vaccines. To begin to understand their role, there is a need to understand how subdominant, conserved antigens associate with other proteins in the outer membrane and how this interaction might affect the generation of immune responses through the linked recognition of T cell and B cell epitopes.
In preliminary studies, proteins were selected based on immunoblots from A. marginale that were not immunodominant. Sixty proteins that were recognized by immune sera from three different animals immunized with outer membrane from the pathogen were selected by mass spectrometry. Twenty-one proteins, including three structural proteins of the type IV secretion system, were identified that were mildly reactive with the immune sera. Of these new proteins, 70 percent were recognized by immune serum from all three vaccinated animals with different major MHC class II haplotypes that represent the majority of MHC class II molecules in Holstein cattle. Using a high-throughput in vitro transcription and translation system to express those proteins, tags were added to the proteins. The proteins were bound to beads, and then fed to antigen-presenting cells that present antigen to immune T cells. Most of the antigens, including the type IV secretion system proteins, stimulated very strong CD4 T cell responses (Lopez et al., 2008).
Linked recognition of T and B cell epitopes on proteins associated through covalent or non-covalent bonding may be important for these antigens to stimulate antibody to undergo isotype switching. For example, B cells recognize antigen 1, which may or may not be associated with another protein, through their B cell immunoglobulin receptor. If it is associated with antigen 2, then that complex of antigens is taken into the B cell, and those proteins are processed and presented on the cell surface, where
they are presented to CD4 T cells that recognize antigen. If the T cells have a receptor for antigen 1 or antigen 2, they can provide help to B cells to make antibody to both antigens 1 and 2. Using MSP1, a heteromer of MSP1-A and MSP1-B surface proteins, to investigate the process in A. marginale, some animals have T cell responses to only MSP1-A, but have good antibody responses to both proteins. Thus, MSP1A-specific T cells provide help to B cells to make antibody to both MSP1-A and MSP1-B. There is additional evidence for linked recognition of another outer membrane protein. Several cattle immunized with outer membranes had antibody responses to the type IV secretion system protein VirB9-1, but no T cell response to this outer membrane protein. However, there was a strong T-cell response to VirB9-2 and VirB10. These three proteins are components of a type IV secretion system complex, where the two VirB9 and VirB10 proteins are associated. We hypothesize that in these individuals, VirB10 stimulates T cells that provide help for B cells to make antibody to VirB9-1. The next step is to immunize cattle with these combinations of proteins, either linked or unlinked, to see if they produce protective immunity.
These findings underscore the importance of studying immune responses to tick-borne pathogens in natural hosts. Mouse models have a number of limitations, including the fact that mice cannot be infected with A. marginale, for a number of possible reasons. Mice diverged from humans 65 million year ago. Research mice are inbred and have numerous recessive defects that skew immune responses. Finally, there is limited representation of the MHC haplotypes, which present antigens differently among mammalian species. MHC molecules are also highly polygenic and polymorphic within a population. As a result, the T cell epitopes are going to differ between individuals and certainly between species.
Knowledge Gaps and Research Opportunities
Brown noted that researchers should conduct the following work on vaccines against tick-borne diseases:
-
Examine surface-exposed, conserved proteins are priority targets.
-
Target the subdominant antigens for persistent pathogens.
-
Use naturally associated proteins to increase T cell responses.
-
Focus on T cell epitopes recognized by the majority of individuals within a population, as well as by antibodies.
-
Study a pathogen in its human host, or an outbred large animal model.
-
Develop metrics to measure protective immune responses in these species.
DISCUSSION
Developing methods to protect humans against tick-borne diseases remains controversial, but many new avenues are being explored. Schutze suggested that researchers put more emphasis on a general vaccine aimed to reduce prolonged tick attachment regardless of the tick genus or species. Another participant questioned whether it was possible to design a vaccine based on salivary proteins from ticks that are conserved among tick populations to prevent prolonged attachment of the tick to humans. McBride noted that vaccines against evolutionary conserved proteins among tick populations such as subolesin, a salivary protein, show promise. Within the vaccine field, there is interest in research on tick salivary proteins and other proteins produced by the tick and pathogens as vaccine candidates. Brown further noted that a few tick genomes have been sequenced so that proteomics are now being applied to identify specific salivary proteins.
Another participant questioned how highly specific bands to Borrelia, such as 31-KD OspA and 34-KD OspB, could be used for vaccine development, but not for disease detection. McBride noted that the highly specific bands to Borrelia confound diagnosis because despite a demonstrated immune response to those bands, it becomes difficult to differentiate between an infection and a vaccinated individual.
EDUCATION, BEHAVIOR CHANGE, AND OTHER NON-PHARMACEUTICAL MEASURES AGAINST LYME AND OTHER TICK-BORNE DISEASES
Paul Mead, M.D., M.P.H., Centers for Disease Control and Prevention
Although the topic of prevention is often relegated to the end of a meeting or report, this placement does not diminish its importance. For tick-borne diseases, prevention is clearly preferable to treatment, and it should be a foremost concern. Unfortunately, current methods and opportunities for prevention are limited, and there is urgent need for new methods and new approaches.
Personal protective measures—as distinct from community-level measures—represent the final point of intervention for preventing human infection. Familiar personal protective measures include frequent tick checks to remove crawling or attached ticks; use of protective clothing such as long-sleeved shirts, long pants, and light-colored clothes; tucking pants into socks; using repellent; and avoiding tick habitat. These strategies have yielded mixed results in analytic studies.
Some personal protection methods, including some recommended by the Centers for Disease Control and Prevention (CDC), have not been
shown to prevent tick-borne diseases. For example, the method of tick removal, tucking pants into one’s sock, or wearing light-colored clothing have not been shown to be protective (Smith et al., 1988; Schwartz and Goldstein, 1990; Ley et al., 1995; Orloski et al., 1998; Connally et al., 2009). There is at least one study (Stjernberg and Berglund, 2005) suggesting that individuals are actually more likely to acquire ticks from the environment when wearing light-colored clothing than when wearing dark-colored clothing. To be clear, the CDC does still recommend use of fine-tipped tweezers as a quick and definitive way to remove ticks.
Insect repellent use has been the subject of a number of case-control or cross-sectional observational studies over the past 20 years. The results have been mixed, with some studies showing a protective effect, while others did not (Smith et al., 1988, 2001; Schwartz and Goldstein, 1990; Lane et al., 1992; Ley et al., 1995; Klein et al., 1996; Orloski et al., 1998; Phillips et al., 2001; Vazquez et al., 2008; Connally et al., 2009). The studies have varied as to whether the repellent was applied to the skin or clothing. Other studies focused on whether the repellent was used in the yard, away from the house, at work, or at leisure. Because of this variation in design and the fact that accurate dosage of repellent was not collected, it is difficult to make direct comparisons among these studies.
Tick checks are a potentially important strategy for reducing tick-borne diseases because recognition and removal of ticks before attachment—or even shortly after—can effectively block pathogen transmission. Nevertheless, most studies have failed to demonstrate a protective effect of tick checks. This may be because ticks, especially nymphal ticks, are very small, and there may be practical limitations on people’s ability to detect them.
Detailed examination of two studies further illustrates the complexity of evaluating the effectiveness of personal protective measures. During 2000–2003, Vazquez and colleagues conducted a case-control study to evaluate the use of protective clothing, repellents, and tick checks among 700 Connecticut patients with clinically diagnosed Lyme disease and approximately 1,100 matched controls (Vazquez et al., 2008). Participants were divided into those with definite, possible, or unlikely Lyme disease, based on clinical and laboratory evidence. Participants with definite Lyme disease were significantly less likely to report using protective clothing—long-sleeved shirts, long pants, and light-colored clothing—than were controls (46 versus 60 percent). Patients with Lyme disease were also significantly less likely to report using repellent than matched controls. However, about 77 percent of both patients and controls reported checking for ticks.
A second study conducted in Connecticut a few years later examined a wide variety of personal protective behaviors and home landscaping practices among approximately 360 patients with Lyme disease and a similar number of matched controls. Unlike the Vazquez study, these researchers
found that protective clothing did not reduce participants’ risk of Lyme disease. They did find, however, that performing tick checks within 36 hours of time spent in the yard was protective (with an adjusted odds ratio of 0.55). Furthermore, bathing within 2 hours of spending time in the yard was also protective (with an adjusted odds ratio of 0.4). Wearing repellent trended toward being protective, but this association was not statistically significant.
Bathing within 2 hours of potential tick exposure may be a behavior worthy of further promotion. Bathing allows people a good opportunity to search for attached or engorged ticks, while soap and water may wash off those they miss that are not yet attached. Furthermore, as people typically do not put the same clothes back on after bathing, this may reduce their risk to exposure from tick remaining on their clothing. Finally, bathing is an activity that people perform often. Prevention behavior researchers note that an ideal prevention behavior is one that can be easily incorporated into everyday activities. Making people aware of the protective value of bathing might spur them to time that activity for its greatest effect in preventing tick-borne disease.
A number of caveats are important when considering these observational studies. First, comparisons are difficult to make across studies due to differences in outcome measures. For example, investigators have relied on case-control and cross-sectional studies to evaluate the efficacy of using insect repellent. However, most of these researchers did not collect or did not provide information on the type or dose of repellent that participants actually used. Second, the lack of statistical significance does not mean that an intervention is useless, only that the magnitude of the effect is limited. Third, multiple protective factors may mask or dilute the significance of any one protective behavior. Despite these complexities, personal protective measures are relatively benign even if not always very effective, and there is little downside to encouraging people to use them.
Assuming personal protective measures are effective, questions remain as to whether a sufficient number of individuals can be encouraged to practice these behaviors regularly. General knowledge of disease risk is usually necessary but not sufficient to motivate adoption of new behaviors. Individuals need to have the confidence that they can perform the behavior and need to believe that it is worthwhile (i.e., believing that the disease is a real problem, believing that the behavior is effective in preventing the disease, and believing that the benefits of performing the behavior outweighs the inconvenience of the behavior).
Two studies highlight the range of outcomes for prospective interventional studies. One study randomly assigned passengers on the ferry from Hyannis to Nantucket to watch an entertaining educational demonstration on either bike safety or tick-bite prevention behavior and tick removal
(Daltroy and Phillips, 2007). Two months later, researchers asked participants about recent symptoms of tick-borne disease, and reviewed their medical records when possible. The study enrolled 30,000 participants over a 3-year period.
In the main-effects model, patients who received the educational intervention on tick bites had a lower risk of reporting tick-borne illness, although the finding was not statistically significant. In the more complex intervention-effects model, which accounted for interactions among the variables, there was some evidence that educational intervention was beneficial depending on the visitors’ length of stay on the island. The researchers found no difference in risk between intervention and control groups among passengers who stayed on Nantucket for less than 2 weeks. They did find a significantly lower risk, however, among passengers who received the intervention and stayed longer than 2 weeks. Nantucket residents who received the invention also had a slightly lower risk of tick-borne disease, although that finding was not statistically significant.
The interpretation of these results is complicated. The reduced risk of tick-borne disease among passengers staying more than 2 weeks is encouraging. Nevertheless, disease incidence was still very high among passengers staying less than 2 weeks (3 cases per 1,000), and these passengers made up approximately 85 percent of study participants. The lack of a demonstrable effect among this group is surprising and disappointing.
In a second study, intensive, community-wide, multiyear educational programs were evaluated in three health districts in Connecticut (Gould et al., 2008). These programs were developed with input from the communities and provided environmental management and personal protective behavior education to a wide variety of audiences, including schoolchildren, landscapers and gardeners, hikers, and so forth. Approximately 2,800 participating households completed before and after surveys.
During the baseline period, more than 80 percent of respondents said they knew a lot or some about Lyme disease, and more than 70 percent thought Lyme disease was a very serious or somewhat serious problem in their town. More than 50 percent of respondents also thought it was very likely or somewhat likely that someone in their household would contract Lyme disease in the next year. Approximately 5 percent of participants reported a previous diagnosis of Lyme disease.
Given their comprehension of the burden of disease and their personal experience, the study investigated the frequency of performing preventive behaviors. Approximately 99 percent of participants reported that they used some protective behavior some of the time. After the intervention, the proportion of people who reported always checking for ticks rose from around 50 to 57 percent, the proportion who reported using repellent rose from 21 to 28 percent, and the proportion who reported tucking pants into their socks
slightly decreased. These findings suggest that—even in a highly motivated population—voluntary behavior change is hard work, and that a portion of the population will not respond to efforts to encourage such change.
In conclusion, there are a few points for state-of-the-science prevention research. First, researchers have repeatedly studied the efficacy of personal protective measures against tick-borne illness. This is not a research gap. Second, these studies show that some behaviors provide some benefit, while others do not. Third, educational campaigns should target behaviors that have some evidence of benefit, such as bathing, and not those that do not, such as tucking pants into socks. Finally, it is important to recognize that the personal protective measures are unlikely to have a major public health benefit for tick-borne diseases. Public health officials have been educating people about Lyme disease for 20 years, for example, but cases have continued to climb, and the disease is spreading to new areas.
Mead noted that these conclusions suggest the need to think more broadly about public health interventions, which can occur at several levels. At one level are interventions targeted toward individuals—behaviors that people must decide to do, and then perform correctly and routinely. At the next level are interventions that involve the individual, but that are enforced legally or through public health policy, such as seat belt laws and vaccination programs. At the third level are interventions implemented at the community level that do not involve the individual, such as municipal water and sewer systems and the pasteurization of milk. The history of public health impact and disease control favors interventions in the third category. In identifying research gaps, future research on preventing Lyme and other tick-borne diseases should focus on interventions implemented at the community level, such as controlling deer populations. Regardless of the method, prevention measures should be judged by their ability to prevent human illness in endemic communities, and not just kill ticks.
VECTOR- AND HOST-TARGETED STRATEGIES FOR PREVENTION OF TICK-BORNE DISEASES
José M.C. Ribeiro, M.D., Ph.D., National Institute of Allergy and Infectious Diseases
As mentioned previously, there are a number of options to develop prevention strategies for tick-borne diseases. Common approaches focus on the vector (tick) directly, their mammalian hosts, or human behavior. This presentation will cover the use of pesticides to control for ticks, targeting the mammalian hosts, and targeting the tick’s saliva.
A common approach for controlling vectors is to spray areas where ticks are endemic with acaracides. Given the fact that disease transmission
primarily occurs in the forest edge (the first 10 meters), targeted application can be more effective then widespread pesticide application. Pesticide resistance should not be a problem as its development requires that at least 70 percent of the tick population be exposed to a high dose of acaricides. With targeted application, there is the intermixing of the wild-type, insecticide-susceptible ticks that will breed with ticks in the forest edge, thus diluting insecticide resistance genes that may appear.
A second approach to target the vector is to spray an individual’s clothing with permanone, an acaricide that kills ticks, or to bathe with soaps containing permethrin soon after potential tick exposure to kill any attached ticks before they can deliver the pathogens, which takes from 12 to 24 hours to occur. However, human toxicity of acaricides is a concern, and some researchers are working on organic or green formulas that could be less toxic.
Vertebrate hosts are needed to sustain the tick population, so targeting mammalian hosts is another prevention strategy. Tick larvae feed mainly on Peromyscus leucopus, the white-footed mouse, while adult ticks attach mostly to deer. Those two animals account for the largest mammalian biomass in New England. The biomass decreases by an order of magnitude when other potential hosts are considered. Culling the deer population is often discussed as a strategy for controlling the tick population. With few exceptions, this approach is only successful in the short term. Deer herds need to be significantly reduced or eliminated because if the deer population drops by only half, twice as many ticks will simply attach to each remaining animal (Rand et al., 2004; Jordan et al., 2007).
Another approach is to administer acaricides using a tick’s mammalian hosts, such as bait boxes. For the white tail deer, the ticks are concentrated on the deer in late fall and early spring. As mentioned previously, there are self-medicating applicators such as the four-poster to apply insecticides to the host. To be effective, however, researchers need to understand the behavior of the host animal and the interaction with the ticks. Larvae feed on the white foot mouse in August and drop off to molt to a nymphal tick over the winter. Mice are nocturnal animals and sleep during the day, and the tick feeds for a few days and drops off the host in the late afternoon. This means that the ticks are dropping off their host at a time of the day when the mouse is in the burrow. Furthermore, Permyscus leucopus makes its nests under the frost line, which means that the ticks are protected during the winter. As the weather becomes colder, it will gather nesting materials to take to the nest. One strategy that has been implemented is permethrin-impregnated cotton that the mice will scavenge to use in their nests. Although rodent-targeted techniques for distributing acaricides are effective in reducing the tick population, they are cumbersome. For example, the impregnated cotton must be distributed every 10 yards because the home range of the mice is 10 to 20 yards. Distribution must also cover a relatively
large area to be effective. A single administration on the edge of relatively small area does not work (Deblinger and Rimmer, 1991; Stafford, 1991).
The third approach for vector–host interactions is to target transmission once a tick has attached and begins feeding. During a blood meal, ticks have evolved to be highly adaptive to remain attached to a host’s skin for several days and to ingest blood during the entire time. When a tick first attaches to a host, the ticks are coming out of a dormant state. As they start to inject a cementing protein, their metabolism begins to increase. Transcription of various proteins increase, and this new metabolic state likely triggers the pathogen. The feeding phases can be characterized as a slow feeding phase when the metabolic system is increasing followed by a rapid feeding phase. This is the primary reason for targeting tick removal in the first 24 hours.
During the feeding process, a tick faces its host’s barrier of the hemostasis, a highly redundant process consisting of blood clotting, platelet aggregation, and vasoconstriction at the site of the attachment. To counter these obstacles, the tick produces a complex salivary potion that neutralizes these host defenses. The site of a tick bite is in an immunosuppressed state as tick saliva contains proteins that will block the activation of histamine, ATP, serotonin, bradykinin, and leukotriene B4, which trigger pain and itching in the hosts, thus the attached tick can go unnoticed unless that person happens to develop an allergy to one or more of its salivary proteins. In brief, tick saliva has antihemostatic, anti-inflammatory, and immunosuppressive properties. Furthermore, the attachment site is high in interleukin 4, for example, and has substances which inhibit the maturation of dendritic cells and the activation of lymphocytes. This environment protects pathogens during transmission.
One prevention strategy is the use of an anti-tick saliva vaccine to prevent disease acquisition. Vaccines traditionally target proteins on the surface of the pathogens, but anti-tick saliva vaccine targets the compounds that aid in the transmission of the pathogen to the new host. For example, rabbits are killed when they are bitten by a tick infected with Francisella tularensis. When rabbits were presensitized by exposure to a clean tick, they developed an allergy to the tick bite that triggered a protective response to an infected tick bite (Bell et al., 1979). Furthermore, individual antibodies developed to inhibit a tick protein such as Salp 15, which is known to inhibit the response of mammalian T cells, enabled mice to be protected against B. burgdorferi.
Other tick saliva proteins also may be targets for vaccine development. When guinea pigs are exposed to ticks, they develop a large quantity of antibodies that results in rejection of a tick during a second exposure. However, the guinea pigs do not make antibodies against cathepsin inhibitor, a protein that prevents the maturation of dendritic cells, because it is present in very low concentrations in tick saliva. In one experiment, immunity
against recombinant cathepsin inhibitor impaired the ability of Ixodes scapularis to feed. Thus some antibody responses may be more important than others. The myriad compounds create the redundancy and resiliency of the salivary system, but they also offer a large number of vaccine targets. Future research needs to identify which proteins provide a protective response for the transmission of tick-borne pathogens, including identification of early salivary antigens that might lead to an antibody that trigger a local reaction and alert the vaccinated person to detect and detach the tick soon after attachment. As spirochetes are transmitted 24 hours after exposure, an immediate or even a few hours of a delayed reaction to tick saliva could alert an individual to the need to remove a tick.
Genomics are a promising area of research to aid in identifying these proteins. Ticks have large genomes in comparison to both animals and humans. I. scapularis and I. pacificus have about two gigabases of genome, more than twice that of the chicken. Another tick, Boophilius microplus, has approximately seven gigabases of genome, which is on the same order of the human genome. It is a large genome with approximately 20 chromosomes, which is a result of genome duplication. With transcriptome analysis, more than 300 proteins in 26 families in tick’s salivary glands have been annotated (Ribeiro et al., 2006). For most of these proteins, however, their function is unknown. What is known is that tick proteins have been at a fast pace of evolution. Ixodes ticks have protein families that are only expressed in them, so each genus of ticks have evolved independent protein families.
In summary, Ribeiro noted that tick-borne diseases continue to be a problem in the United States. There is a need to use integrated pest management as a strategy to minimize the risk of disease transmission. At the same time, basic research is needed to understand the cellular processes that may aid in developing a vaccine to prevent transmission.
DISCUSSION
Many non-pharmaceutical methods for prevention exist and range from education to community-level interventions. Awareness is the key for generating community-level action, but action must start at the individual level including the healthcare provider. One participant noted that some cases of Lyme disease are not being diagnosed in a timely fashion. Mead agreed that physician education is important. The CDC has produced some educational materials for physicians, but more work needs to be done. Another participant noted that often the last diagnosis a doctor will consider is Lyme disease, when it should be one of the first.
Another participant noted that everyone needs to be a part of the education process because the entire community needs to consider tick-borne diseases as a serious health concern before protective measures will be effectively
employed. To make people more aware of ticks, one participant suggested that the CDC target neighborhoods, present a positive message regarding potential disease risks, and incorporate measures of community risk such as tick drags. Mead noted that awareness is the key, whether for the physicians, the patients, or the community. He noted that community awareness could lead to further adoption of community-level interventions, but there is a need to strategically plan the approach. Another participant noted that prevention is key for reducing the burden of disease and four-posters need to be a part of the strategy for deer. Mead noted that in some states, using four-posters is a problem because wildlife departments are concerned about transmission of chronic wasting disease or the effect of permethrin on hunters. The CDC is starting some research to look at the efficacy of four-posters in various areas. To reach the public, some participants suggested developing public service announcements because many communities are not aware of the importance of tick-borne diseases. Mead noted that the CDC recognizes that Lyme disease is a growing problem and is among the top seven reportable disease in the country. In Northeastern United States, it is among the top two or three. As part of the recognition of the importance of this disease, the CDC has received funding to strengthen their communications activities to more aggressively disseminate effective prevention messages.
A multitude of other preventative strategies beyond promoting awareness have shown promise but require tweaking before they can be implemented. Some participants asked when new vaccines may be developed. McBride noted that vaccines for humans are in the near future. While these vaccines still face numerous obstacles, progress has been made for Ehrlicia and Rickettsia in particular. A vaccine is currently being tested in Europe and if it is successful, it may be available in the United States in the future.
Finally, another participant questioned why it takes 24 hours for a tick to begin to transmit pathogens. Ribeiro noted that studies using fluorescent spirochetes did not see evidence of pathogen in the salivary glands until 48 hours after attachment (De Silva and Fikrig, 1995; Ribeiro et al., 2006). The pathogen resides in the tick gut, and once the tick attaches to feed, the Borrelia begins to multiply. They increase in size and begin to be seen in the hemocele by 24 hours, and then by 48 hours, they are present in the salivary gland.
CONCLUDING THOUGHTS ON PREVENTION
Stephen M. Ostroff, M.D., Bureau of Epidemiology, Pennsylvania Department of Health
Efforts to develop and implement preventive measures against tick-borne infections—both pharmacological and non-pharmacological—have so far proved disappointing. These measures produce short-term or
sustained reductions in the incidence of disease in only limited cases. In no cases have preventive measures halted the geographic spread of disease-carrying ticks or the pathogens they harbor. The incidence of tick-borne infections has therefore grown continuously over the past several decades. That sends an unfortunate—and to a certain degree self-fulfilling—message that these diseases are not preventable.
However, tick-borne diseases should be as amenable to preventive measures as other infections of public health significance. Prevention offers the best opportunity over the long-term to truly reduce the burden and impact of tick-borne infections. Investments in safe, effective, and simple preventive interventions are therefore essential. A combination of these approaches would likely have the greatest success in reducing the overall burden of tick-borne disease.
Among pharmacological interventions, vaccination appears to hold the most promise. Significant efforts are under way to develop vaccines for tick-borne infections, although no human vaccines are now available for any of the major pathogens, and none are close to licensing or even in clinical trials.
Efforts to develop vaccines against Rocky Mountain spotted fever date back to the 1930s, although none has offered significant protection. However, the development and licensing of an outer-surface protein vaccine against Lyme disease in the late 1990s shows that vaccination for tick-borne disease is a viable option. Clinical trials demonstrated that the OspA vaccine was clearly effective in preventing Lyme disease. However, the manufacturer withdrew the vaccine after only 2 years on the market for many other reasons. That setback shows that any future vaccines for tick-borne infections must not only be effective but also easy to administer, cost-effective, and free of concerns about potential short- and long-term side effects.
The two workshop speakers who covered vaccines focused on ehrlichiosis, anaplasmosis, and rickettsial infections. In targeting these infections in both humans and animals, investigators have explored both subunit vaccines and live attenuated vaccines. They are making progress, but must still overcome key challenges. These include better defining immunological responses to infection, understanding mechanisms of immune-based protection, developing the ability to measure protection, and creating animal models to study potential vaccine candidates.
A significant challenge for vaccine development is the sheer diversity of human tick-borne pathogens, along with their antigenic variation. Tick-borne pathogens include rickettsial agents, several bacteria species, and Babesia parasites. Each agent has different incidence, geography, epidemiologic features, risk groups, and clinical impact. It is hard to imagine a scenario where vaccines become available for all these agents. Even if they
were developed, questions regarding how each vaccine would be used—and whether there is a market large enough to justify their development and licensure—would remain.
An attractive alternative discussed during the workshop is to develop vaccines that generically target the host–tick interaction, rather than pathogen-specific vaccines. Such vaccines could be directed against critical proteins in tick saliva needed for attachment, or they could limit the ability of a tick to take a blood meal if it does attach. Such options are made even more attractive by the fact that the number of tick species responsible for transmitting tick-borne pathogens is quite small. Each species can transmit multiple agents. A single vaccine directed against attachment and feeding of Ixodes species, for example, would protect against multiple agents.
Mead explored multiple non-pharmaceutical interventions. The best studied are personal protective measures that involve behavioral change, such as the use of tick checks, long-sleeved clothing, and insecticides, but the results have been variable. Some studies have found a protective effect while others have not. The inconsistencies are likely related to study design or outcome measures, but they highlight the difficulty of implementing and sustaining complex behavioral change. Such interventions need to be simple, used consistently, and reinforced. Mead concluded that despite limited evidence that such interventions work, public officials should still recommend them.
Preliminary data presented by Weber earlier in the workshop showed that the use of permethrin-impregnated clothing by outdoor workers reduced tick bites by 93 percent, and may therefore be an effective strategy to prevent tick-borne infections among people in high-risk occupations. The U.S. military has used such clothing extensively. Although no one has systematically studied such an approach in the general population, it might offer significant protective benefits in high-incidence locations.
Environmental preventive measures, such as the use of acaracides against deer or rodent populations, have been found to be effective. The same applies to targeted deer removal. However, none of these approaches has been widely adopted because of cost, environmental concerns, or difficulties applying them on a large enough scale or over a sustained period of time to have a significant impact on disease occurrence. Ostfeld suggested that approaches to habitat management that promote species diversity would likely be highly effective in reducing the transmission of Lyme disease. Ribiero proposed another innovative approach: identifying endosymbiotic microorganisms that would inhibit or destroy pathogens within the tick vector, or interrupt the tick maturation cycle.
Finally, workshop participants repeatedly emphasized the importance of efforts to educate the public about tick-borne diseases and how to prevent them. Even high-incidence areas have often lacked intense or sustained
social marketing campaigns like those designed to combat other diseases. One innovative educational campaign conducted on Massachusetts ferries had a favorable impact on people’s knowledge of Lyme disease and efforts to reduce their exposure to ticks. Public health officials should consider similar campaigns—even in lower incidence locations, or targeted to school-children, people in high-risk occupations, and outdoor enthusiasts—as they may improve adherence to prevention measures and reduce the incidence of disease.






















