E
Materials Development Case Studies
The U.S. propulsion community has a long and successful history of bringing advanced materials to fruition as measured in terms of more capable military systems and commercial systems. It is instructive to examine several of the advanced materials through the development process to identify how the successful materials and manufacturing processes produced propulsion advances or in some cases did not realize the expected potential of the material.
E.1
SUCCESS STORY IN MATERIALS, GAMMA TITANIUM ALUMINIDES (GEnxTM)
The history of GE’s efforts to utilize gamma titanium aluminides (TiAl) is an excellent case study of how the development process evolves.1 In this case, the advanced material went through the traditional materials development process, but found application in a commercial engine rather than in the originally targeted military systems.
Realizing the benefits of gamma TiAl has been the goal of the aerospace industry for more than three decades. The promise of a materials system with half the density of nickel alloys and acceptable strength above 800ºC is considerable, in particular for rotating airfoils. However, gamma TiAl alloys are a major departure from conventional Ti, Al, or Ni alloys and consequently required a significant level of
learning, process and facility development, and engineering application precursor work before they could be introduced into products. A clear lesson learned in this regard is the need to transition materials development from “technology push” to “technology pull” as quickly as possible so that all the requirements of the end application are identified early.
The amount of effort required for a new material introduction, especially for a new class of materials like TiAl, is beyond the level that any industrial company alone can support over the lengthy development process. Collaborative industry-government efforts were key to sustaining the needed “constancy of purpose” required to introduce new materials and processes into applications.
E.1.1
Timeline Outlining the History of Gamma TiAl Introduction at GE Aviation
1970s Through Early 1980s
In the period of the 1970s through the early 1980s, little direct GE work was done; there was considerable Air Force funding of work at government laboratories, universities, and Pratt and Whitney, including work to produce and rig test low-pressure turbine (LPT) blades and other engine components.
Mid- to Late 1980s
In the mid- to late 1980s, gamma TiAl research and development (R&D) continued work at Pratt and Whitney, government laboratories, and universities. Alloy development work was funded both internally and by the Air Force at GE’s Global Research Center (GE GRC). GE alloy 48Al-2Cr-2Nb resulted from a chemistry matrix that was part of the internally funded work.
With respect to the criticality of the government-funded work, it is unlikely that GE would have pursued gamma TiAl without the precursor efforts or the parallel funding for alloy development work at GE GRC.
The end result of the 1980s’ alloy work was the establishment of GE 48-2-2, a material that has a nominal room temperature elongation of approximately 2 percent and environmental resistance so that it does not require coatings for applications up to approximately 800ºC. These are significant characteristics, as they make GE 48-2-2 attractive from an engineering and manufacturing standpoint. For comparison purposes, competing gamma TiAl alloys typically had nominal ductilities that were less than 1 percent and required environmental barrier coatings for extended use in the operational environment.
Approximately 1988
In about 1988, GE recognized the potential of GE 48-2-2 as a viable engineering material and began significant work on application-specific development, including casting, forging, and powder processing of GE 48-2-2. The funding of this work was entirely GE Aviation industry research and development (IR&D). Cast gamma TiAl work on GE 48-2-2 began in 1989. Fundamental producibility and cost assessments of the alternate approaches convinced GE that casting was the preferred method of manufacture for gamma TiAl engine parts.
1990-1994
During the 1990-1994 period, baseline GE90 weight requirements inspired a detailed assessment of the potential of TiAl applications. A review of GE-generated and historical government contract (Pratt and Whitney) reports convinced GE to pursue LPT blades. GE generated the first draft of the LPT blade design practice for a TiAl blade, including attachment considerations and key materials property requirements. An initial draft of a materials specification was also done.
To facilitate progress toward implementation of LPT blades, GE committed to designing, producing, and engine testing CF6-80C stage 5 LPT blades from GE 48-2-2. Howmet (Whitehall) cast the blades overstock. The blades were subsequently electrochemically milled to final shape. Since GE was not able to optimize the design for the TiAl material (the parts had to fit into the existing stage 5 space), damper pins were employed to avoid resonant frequencies during engine testing.
The ability (or lack of ability) by melters to control aluminum content within the range of the narrow specification (32 to 33.5 weight percent) was brought to light in the CF6 LPT blade program. Howmet vacuum arc re-melting (VAR) was unsuccessful in hitting the required range, as was Timet. At the time, the state of the art was ±3 wt% Al within the ingot. In order to mitigate this, GE developed a heat treatment that yielded relatively uniform mechanical properties in cast blades in spite of the wide variation in aluminum content from blade to blade. This allowed GE Aviation to make full sets of parts for the CF6 program. Subsequent work at Oremet-Wah Chang (now Allvac), using an internally developed VAR method, was shown to be capable of meeting GE’s chemistry specification, and has been the method of choice for current production.
CF6 hardware was successfully produced, and GE ran 1,027 cycles in 286 hours of engine testing in the summer of 1993. The LPT module was disassembled, parts were inspected, and a decision was made to reinstall the gamma blades and run another block of engine testing consisting of another 502 cycles, which were successfully completed in 1994. This engine testing demonstrated that it was possible to manufacture, assemble, disassemble, inspect, reassemble, and run gamma TiAl LPT blades.
Subsequently, F414 seal support rings (1995-1996) and ESPR (Engineering Research Association for Supersonic Transport Propulsion System; Japanese government funding) shroud support rings (2002) were successfully produced and engine-tested. Production and engine testing of these three significantly different components significantly grew the organizational confidence needed to introduce gamma TiAl.
Government funding, in particular NASA and Navy contracts, greatly advanced GE’s understanding of 48-2-2 and the practical capability of alternate alloy systems. Invention of NCG 359 (Navy cast gamma 359—the 359 is half of 718) helped in the understanding of the functional limitation of gamma alloys—especially crack growth rate, which was found to be difficult to enhance beyond 48-2-2. GE subsequently minimized the pursuit of other TiAl chemistries to focus on understanding Ti 48-2-2 behavior and application requirements.
1995-1996
NASA Enterprise Project Management contracts that produced large Ti 48-2-2 castings at Precision Castparts Corporation (PCC) generated welding procedures (resulting in the construction of the welding facility currently being used in production of the GEnx blades at PCC). NASA work on impact resistance and subsequent fatigue behavior was instrumental in defining blade leading-edge geometry and solidifying the GE design practice for LPT blades. Additionally, GE Aviation received a contract to develop a defect-tolerant design approach for Ti 48-2-2, called Damage Tolerant Design of Gamma (DTDG). All of this work was performed in the 1990s, contributed to the understanding of Ti 48-2-2, and was instrumental in defining blade leading-edge geometry and solidifying GE’s design practice for LPT blades.
1997
In 1997, the GE90-115 reduced all of the above information into a complete design practice. Based on its Enterprise Project Management work, PCC was selected to work conventional casting processes. Cost targets were met on a projected basis, but due to the unknown risk of achieving the cost-versus-weight benefit, plus immaturity of the supply chain with respect to meeting aggressive engine development program commitments, implementation was not pursued.
1997-2004
During the 1997-2004 time frame, only Japan’s ESPR funding kept gamma TiAl research alive at GE. Without this program, GE would not have been able to
start the GEnx program in time for the first engine to test (FETT). PCC had an inventory of 8,000 pounds of GE Ti 48-2-2 that was not used for ESPR, and this material became the original stock used to rapidly start the GEnx program.
2004 to Today
After 2004, GE 48-2-2 was selected for the GEnx1B stage 6 and 7 LPT blades, launching the development of an overstock casting process at PCC, a full design database, machining studies, and implementation of a gamma LPT design practice at GE.
During the late 1990s, Air Force funding (Efficient Processing of Near-Gamma Titanium Aluminide) evaluated the cost-effectiveness of conventional forging, powder metallurgy, and casting for the producibility of several components and determined that casting was the most cost-effective route. Advances in forging and alternate casting processes have been made in the past 7 years, particularly in the European Union (EU), where significant government funding has been put into TiAl processing for both the gas turbine and automobile (turbochargers and valve) businesses. GE evaluated the current forging and powder processes and assessed casting as the best long-term cost approach for producing GEnx LPT blades. Future advances in manufacturing processes, including the industrialization of meltless TiAl, are needed to facilitate practical, cost-effective alternatives to casting. In the meantime, casting processes will be improved as well.
The lack of an established industrial base for the production of gamma TiAl has been the most significant impediment to implementation. The costs of the 20 years of GE R&D to understand the material, to develop the design practices, to conduct engine tests, and to certify TiAl are on the order of $40 million. The additional cost that GE has incurred to industrialize gamma TiAl LPT blades is in excess of $85 million.
E.1.2
Development of Gamma TiAl at Allison Gas Turbine
A range of Air Force and Navy contracts supported titanium aluminides technology development at Allison Gas Turbine (now Rolls-Royce North America, Inc.) in the 1980s through the early 2000s, including the following: Hot Rolling of TiAl Sheet; Turbine AF applications; Composite Disk Validation; Gamma Ti Aluminides Development; High Temperature Coatings for Ti Aluminides; Joining of Aluminides and Metal Matrix Composites; Damage Tolerant Design with Gamma; and Integrated High Performance Turbine Engine Technology (IHPTET) and Core and Engine Structural Assessment Research (CAESAR) demonstrators in the late 1990s and 2000s. Developments for NASA were cast gamma airfoils for regional engines in 1995.
In addition to these contracts, many development activities were funded by IR&D that led to the development of Alloy 7, which is one of the highest-strength and best-performing wrought TiAl alloys. The current generations of high Nb alloys at Rolls Royce are all based on the baseline Alloy 7 compositions. Most of the development work and understanding of gamma TiAl led to the development of compressor blades and vanes, and these were successfully tested in both Advanced Turbine Engine Gas Generator/Joint Technology Demonstrator (ATEGG/JTDE) and CAESAR test engines, but have not yet found production applications.
E.2
ALPHA-2 Ti3Al DEVELOPMENT—LESS THAN EXPECTED POTENTIAL
As noted above, all successful materials possess one to several of the following criteria:
-
Satisfies property and processing requirements,
-
Technology pull matched technology push,
-
Insertion timeliness meshed with application need,
-
Strong systems pull,
-
Amenable to modification to improve properties,
-
Stable industrial base,
-
Maturity—no major surprises,
-
Key structure-property relationships understood,
-
Industry willing to take risks,
-
High confidence,
-
No other material will work, and
-
Adaptable/flexible properties and processing.
Meeting property requirements may seem to be an obvious minimum criterion. However, there are materials that are deficient in key properties but that are in routine use, in some cases in very demanding applications. This is possible if the property deficiencies can be overcome by establishing limits on the use of a material or by changing a design. For example, a material that does not have the required “time at temperature” capability may be used if a routine replacement of a component after a specified time interval is feasible. In other cases, design changes can compensate for material deficiencies and allow higher use temperatures or increased times at temperature. Unfortunately, there are penalties associated with compensating for the material. Limits on lifetimes can have major cost implications, and design changes can add weight and producibility issues.
Some materials, however, never transition into routine applications. Such materials may be so deficient that maintenance or design “work-arounds” are not feasible because the associated penalties are too severe. It is also possible that these
materials’ deficiencies are overcome by materials substitution; that is, an alternate material that meets the requirements becomes available. An example of a material that has not had a successful history is alpha-2 Ti3Al.
When the National Aerospace Plane (NASP) project (Figure E.1) was initiated in the early to mid-1980s, speed and reusability were major drivers. The speed requirement would result in airframe temperatures reaching 1000ºC over a large portion of the plane’s surface, with leading edges reaching temperatures as high as 1650ºC. The reusability requirement meant that time at temperature was also critical to the plane’s mission. Consequently, both alpha-2 and gamma titanium aluminides were in the mix early as candidate airframe materials. Alpha-2 was considered the more promising candidate, offering higher ductility, higher tensile strengths across the entire temperature range, and processibility.
As the development cycle for the application of this material to NASP progressed, testing of the material in key environmental conditions was carried out. A key environmental requirement associated with reusability is properties at elevated temperatures in air over extended periods of time. The results of these tests revealed that alpha-2’s oxidation resistance over time was unsatisfactory and was actually no better than high-temperature titanium alloys such as Ti-6242 or Ti-834.

FIGURE E.1 Artist’s concept of National Aerospace Plane (NASP) vehicle. SOURCE: NASA.
Approaches to improving this key deficiency involved the addition of large amounts of expensive, relatively dense alloying elements, causing the material to become more expensive and heavier. In the final analysis, these changes did not yield a significant improvement in oxidation resistance. Consequently, two of its initially attractive features, weight savings and high-temperature properties, were not realized.
While the work on alpha-2 was yielding discouraging results, the gamma alloy continued to improve, especially with castings, to the point at which they had sufficient ductility to be considered for structural alloys. In addition, this material had a naturally higher specific stiffness and high-temperature oxidation resistance as compared with alpha-2.
As applications to high-temperature airframes were disappearing, applications to turbine engines would seem to have potential. Engine companies were requiring higher and higher temperature capability. Unfortunately, alpha-2’s oxidation resistance and higher density made it unattractive for turbine engine applications. The risk-averse nature of the engine community was especially incompatible with this material’s history.
In summary, after the investment of substantial resources, the initial potential of this material was never realized. It failed to meet several of the key criteria associated with the applications at the time, and an alternate material was improved to the point that it became the prime candidate. Eventually, the major airframe systems pull disappeared altogether owing to the cancellation of the NASP Program. Key deficiencies and competition from other materials also took it out of consideration for turbine engines.
E.3
NICKEL-BASED SUPERALLOY SUCCESS STORY
The characteristics of materials that successfully transition from development to application are not “universal.” There is no set template that “fits all” materials. However, all successful materials do possess one to several of the criteria listed above (see Section E.2).
Superalloys are based on Group VIIIB elements, located within the transition metals section of the Periodic Table. The base metals are typically nickel, iron, and cobalt, with alloying additions of cobalt, chromium, nickel, iron, tungsten, aluminum, titanium, niobium, and tantalum. Superalloys based on nickel are the predominant materials in terms of commercial applications. They have been providing exceptional high-temperature properties and processing for more than 60 years. As early as World War II they were employed in critical high-temperature military applications. Over the ensuing years the number of applications greatly expanded, as did the amount of the alloys in existing applications. As discussed above, major advances in gas turbine engine performance were the direct result of
increased temperatures, which in turn were made possible by improved materials. The hottest and most demanding applications in today’s high-performance engines, the combustor and high-pressure turbine (Figure E.2), are dominated by nickel-based alloys. One of the major attributes of these materials is that their temperature capability has progressed steadily over the years, as shown in Figure E.2. This evolution in temperature capability has resulted from improvements in alloy composition and processing, from wrought, to conventionally cast, to directionally solidified, to single crystal. This progression in temperature capability has allowed the material to keep pace with the design. A key indicator of their success is that they now comprise over 50 percent of the weight of a high-performance turbine engine.
This class of alloys has been very successful because it possesses many of the characteristics of successful materials cited above: outstanding properties for many demanding applications (e.g., high specific properties, high-temperature mechanical and thermal stability, low-temperature ductility, and processability); no other materials will work (it is the only material that will provide the required properties for the combustor and turbine—the hottest and most demanding sections of a gas turbine engine); excellent adaptability/flexibility (an unparalleled ability to
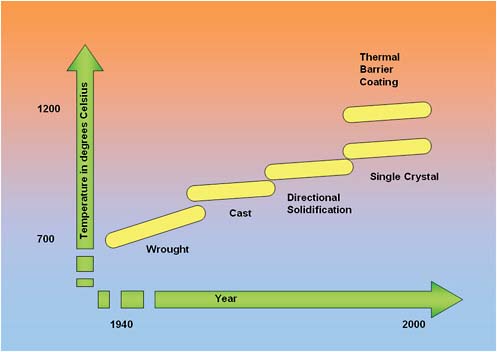
FIGURE E.2 Improvements in alloy temperature capability.
respond to the designer’s desire for higher temperature, as shown in Figure E.2); a constant evolution of understanding how to provide the user with a high degree of confidence in the material; and no show-stoppers or major surprises.
To understand why these materials have been successful, one needs to understand the material’s chemistry and chemical behavior. Nickel has no phase transformation between room temperature and its melting point. Its face-centered cubic structure is amenable to facile processing and alloying. For intermediate temperatures, it can be either solid-solution-strengthened or precipitation-strengthened-solid solution, and it can be precipitation-strengthened for high temperatures. There are a variety of compatible alloying element chemistries, including aluminum, chromium, cobalt, iron, and tungsten. In the case of nickel superalloys, there has been a tremendous synergy between scientific understanding and applications—scientific understanding of the relationships between microstructure and high-temperature properties, and applications that have benefited from this understanding in the form of improved materials and processes. This synergy has been a “perfect storm” for a successful story.










