Components of US Medical-Device Regulation
From the inception of device regulation under the 1976 Medical Device Amendments, it was understood that devices would be subject to various degrees of premarket review, depending on their risk classification (see Chapter 2). In addition, all devices, once they entered the marketplace (by whatever review mechanism) would be subject to a wide array of regulatory requirements. Premarket review and regulatory control after market entry were intended to operate together to provide a structure that would protect the public’s health while not inhibiting innovation.
The committee notes several times throughout this report that the 510(k) process does not operate in isolation. It is part of a larger framework of regulatory tools. This chapter provides an overview of the components of medical-device regulation that come into play after a product is marketed and then an introduction to the available paths for premarket review of devices, including the 510(k) clearance process.
TOOLS AND AUTHORITIES FOR REGULATING MARKETED DEVICES
In general, medical-device regulation is described by following the typical life cycle of a device, starting with research and development, progressing to premarket review by the Food and Drug Administration (FDA), following with regulatory requirements while the device is marketed, continuing with evolution of the device into several (often many) iterations and newer variants, and ending with product obsolescence (IOM, 2010; Kahan, 2009). To focus attention on the incremental aspects of premarket review, however,
this chapter will begin with the FDA’s “general controls”—the regulatory system applicable to devices once they enter the marketplace. Once the “postmarket” system is understood, the supplemental controls provided by premarket review can be better appreciated.
In its “Preliminary Report and Recommendations,” the FDA Center for Devices and Radiological Health (CDRH) 510(k) Working Group states (FDA, 2010a) that
the aim of the 510(k) program is two-fold: (1) to assure, through a quality review process, that marketed devices, subject to general and applicable special controls, provide a reasonable assurance of safety and effectiveness; and (2) to foster innovation. Robust premarket review is an essential component of CDRH’s medical device oversight. CDRH’s postmarket tools, while valuable, have important limitations and are not sufficient to serve as a substitute for high-quality premarket review [emphasis added].
This chapter explores those potential or perceived limitations and the procedures by which the controls are imposed and enforced.
Controls Affecting Marketed Devices
The statute establishes a combination of prohibitions and mandates with which a device and its manufacturer must comply. Some of the mandates are imposed on specific devices by order of the FDA at its discretion; others are adopted through regulations issued by notice-and-comment rule-making. This discussion covers only the legal authority of the FDA, not how that authority may have been used through individual enforcement actions or the exercise of discretion not to act.
Many of the controls apply to all devices, regardless of risk presented or classification; others apply to a subset of devices based on risk but not classification; and a few apply only to devices classified in Class II or Class III. Compliance with the controls is monitored and enforced by the FDA through its own resources and with the assistance of the US Department of Justice (DOJ) and federal courts.
The following text will review prohibitions and mandates related to all devices, prohibitions and mandates related to higher-risk devices, FDA monitoring systems, FDA enforcement powers, and procedural requirements that may affect the FDA’s ability to use the powers granted by law.
Controls Applicable to All Devices
Prohibitions
Contamination
A device may not consist in whole or in part of any filthy, putrid, or decomposed substance1 or contain an unsafe color additive.2
Container Contamination
The container of a device may not be composed in whole or in part of any poisonous or deleterious substances that may render its contents injurious to health.3
False Claims of Compliance
The marketer of a device may not claim that it complies with a performance standard established by or recognized by the FDA, unless the device conforms in all respects to the standard.4
False or Misleading Labeling
The labeling of a device may not be false or misleading in any particular.5 It may not fail to make all required disclosures conspicuous and accessible to potential users under customary conditions of sale.6 Whether labeling is misleading is determined both by what is said and by what material information is omitted in light of what is said.7
Unsafe When Used in Accordance with Instructions
A device may not be dangerous to health when used as suggested in its labeling.8
Mandates
Registration of Manufacturers
A US-located manufacturer, processor, packager (or reprocessor and repackager9) of a medical device must register with the FDA the business’s
___________________
1Federal Food, Drug, and Cosmetic Act [hereinafter FFDCA] § 501(a)(1); 21 USC § 351(a)(1) (2006).
2FFDCA § 501(a)(4), 21 USC § 351(a)(4)(B) (2006).
3FFDCA § 501(a)(3), 21 USC § 351(a)(3) (2006).
4FFDCA § 501(e), 21 USC § 351(a)(1) (2006).
5FFDCA § 502(a), 21 USC § 352(a) (2006).
6FFDCA § 502(c), 21 USC § 352(c) (2006).
7FFDCA § 201(n), 21 USC § 321(n) (2006).
8FFDCA § 502(j), 21 USC § 352(j) (2006).
9Reprocessors and repackagers may sterilize, refurbish, or repackage previously used devices. There are special requirements for those who reprocess single-use medical devices. Section 502(v); 21 USC 352(v) (2006).
name and all places of business.10 Any additional place of business must be registered immediately.11 Registration is also required for establishments outside the United States where devices are made for importation into the United States.12 Registration is to be electronic.13
Listing of Products
Each registered person must file with the FDA a list identifying each device made or processed for commercial distribution in the United States.14 The label and labeling15 for each listed device and a representative sample of other labeling must be provided.16 The FDA may request a registrant to provide a statement as to why it believes that any product listed is not subject to a performance standard or a premarket approval (PMA) application requirement.17 If a device previously listed is no longer made, the registrant must provide a notice of discontinuance regarding the product.18 Listings are to be electronic.19
Label Information
The label is affixed to the device or its immediate container.20 The label of a device must prominently disclose the company name, trade name, or trade symbol of the original manufacturer.21 It must also disclose the name and place of business of the manufacturer, packager, or distributor and the quantity of contents in the package.22 The device must be identified by its established or common nonproprietary name (if any).23
Labeling with Adequate Instructions for Use
The labeling of a device must provide adequate directions for use and adequate warnings against unsafe use.24 The FDA has historically required
___________________
10FFDCA § 510(c), 21 USC § 360(c) (2006).
11FFDCA § 510(d), 21 USC § 360(d) (2006).
12FFDCA § 510(i), 21 USC § 360(i) (2006 & Supp. II 2008).
13FFDCA § 510(p), 21 USC § 360(p) (2006).
14FFDCA § 510(j)(1), 21 USC § 360(j)(1) (2006).
15A label is written matter affixed to a device or its immediate packaging. Labeling includes labels and other written materials accompanying the device (for example, an operator’s manual or detailed product information). The FDA also includes some promotional materials in the scope of labeling. For this chapter, promotional labeling is treated with advertising, below.
16FFDCA §§ 510(j)(1)(A)–(B), 21 USC § 360(j)(1)(A)–(B) (2006).
17FFDCA § 510(j)(1)(D), 21 USC §§ 360(j)(1)(D) (2006).
18FFDCA § 510(j)(2)(B), 21 USC § 360(j)(2)(B) (2006).
19FFDCA § 510(p), 21 USC § 360(p) (2006).
20FFDCA §§ 201(k)–(l), 21 USC §§ 321(k)–(l) (2006).
21FFDCA § 502(u), 21 USC § 352(u) (2006).
22FFDCA § 502(b), 21 USC § 352(b) (2006).
23FFDCA §§ 502(e)(2), (4), 21 USC §§ 352(e)(2), (4) (2006).
24FFDCA § 502(f), 21 USC § 352(f) (2006).
that directions be adequate for a layperson, both as to the conditions for which the device is to be used and as to the safe and effective way to use the device.25 The statute permits the FDA to exempt devices from these requirements, which the agency has done by declaring them (or allowing their manufacturers to declare them) to be prescription devices,26 for which the labeling need only be written for healthcare professionals, not laypersons.27 To take advantage of that exemption, however, the labeling must also provide information that permits a healthcare practitioner to use the product safely and for the purpose for which it is intended.28
If a device is intended for use in healthcare facilities or by healthcare professionals, labeling required for a prescription device may be made available solely by electronic means and need not be included physically with the device package.29
Design and Manufacture of Products
Every device must be designed, manufactured, packed, stored, and installed in conformity with current good manufacturing practice regulations established by the FDA.30 The regulations mandate use of design validation, investigation of complaints, a corrective and preventive action plan to identify root causes of product nonconformance with standards and specifications and to implement effective actions to prevent recurrence, and a quality system to oversee and ensure compliance with the FDA requirements and internal company procedures.31
Reports of Removals and Corrections
A manufacturer or importer must report to the FDA any device correction (for example, a software upgrade, change in operating instructions, or field modification) or removal (for example, recall, field recovery, or replacement) if the correction or removal was undertaken to reduce a risk to health posed by the device or to remedy a violation of the FFDCA caused by the device that may have presented a risk to health.32 The FDA has promulgated regulations to implement this provision.33
___________________
2521 CFR § 801.5 (2009).
26Prescription devices are not the same as restricted devices, although an individual device may be both. See below regarding restricted devices.
27FFDCA § 502(f), 21 USC § 352(f) (2010); 21 CFR § 801.109 (2009).
2821 CFR § 801.109 (2009).
29FFDCA § 502(f), 21 USC § 352(f) (2006).
30FFDCA §§ 501(h), 520(f)(1), 21 USC §§ 351(h), 360j(f)(1) (2006).
3121 CFR pt. 820 (2009).
32FFDCA § 519(g), 21 USC § 360i(g) (2006).
3321 CFR pt. 806 (2009).
Reporting of Adverse Medical Events by Manufacturers and Importers
In general, each manufacturer or importer of a medical device legally marketed in the United States must keep records and make reports to the FDA regarding deaths or serious injuries that the device may have caused or to which it may have contributed.34 Malfunctions that did not cause or contribute to deaths or serious injuries must also be reported if a death or serious injury would be likely to result should the malfunction recur.35 The agency has implemented this authority through regulations.36
Reporting of Adverse Medical Events by Device-User Facilities (Not Manufacturers)
Hospitals, ambulatory surgical facilities, nursing homes, and outpatient diagnostic and treatment facilities (other than physician offices) are required to report to the FDA information that reasonably suggests that a device has or may have caused or contributed to the death or serious illness of or serious injury to a patient of the facility.37 There is no comparable user-facility reporting requirement for drugs. This requirement has been implemented through regulations issued by the FDA.38 (Adverse-event reporting is discussed in detail in Chapter 5.)
Controls Applicable Only to Higher-Risk Devices
Restricted Devices
If the FDA determines that a device’s potential for harm or collateral measures necessary for its use are such that there cannot be reasonable assurance of its safety and effectiveness without the restriction, the agency is authorized to require that the device be restricted to sale, distribution, or use, only on the written or oral authorization of a healthcare practitioner or “upon such other conditions as [the FDA] may prescribe.”39 The statute goes on to suggest both the types of other conditions that the FDA might consider and limitations of these conditions if used:
No condition … may restrict the use of a device to persons with specific training or experience in its use or to persons for use in certain facilities unless [the FDA] determines that such a restriction is required for the safe and effective use of the device. No such condition may exclude a person
___________________
34FFDCA § 519(a), 21 USC § 360i(a) (2006). See 21 CFR pt. 803 (2009) (implementing regulations).
35FFDCA § 519(a)(8), 21 USC § 360i(a)(8) (2006).
3621 CFR pt. 803, subpts. D–E (2009).
37FFDCA § 519(b), 21 USC § 360i(b) (2006).
3821 CFR pt. 803, subpt. C (2006).
39FFDCA § 520(e), 21 USC § 360j(e) (2006).
from using a device solely because the person does not have the training or experience to make him eligible for certification by a certifying board recognized by the American Board of Medical Specialties or has not been certified by such a Board.40
That authority is exercised at the FDA’s discretion and may by imposed in any of three ways: by regulation issued through a notice-and-comment rule-making (usable for devices in any class),41 as part of a performance standard established by notice-and-comment rule-making (usable only for Class II devices),42 or as a condition for the approval of a PMA application (usable only for Class III devices).43
Although the language is quite similar to that used by the FDA to determine whether a device should be a prescription device,44 the designations as “restricted device” and “prescription device” are technically distinct. Numerous devices are recognized as “prescription devices” by their manufacturers and the FDA through labeling and fall under exemptions from the “adequate directions for use” requirements.45 Those devices, however, are not “restricted devices” unless separately designated by the FDA.
The FDA has generally used the restricted-device authority via the PMA application process and rarely by regulation (Hutt et al., 2007). Only a very few Class II devices are formally “restricted.”46
As early as 1983, however, the FDA was being criticized in Congress for failing to use its power to impose other conditions on use:
The legislative history [of the 1976 amendments] explains that Congress sought to supersede and add to the existing authority used by the FDA to limit sale or use of certain devices except by prescription. Authority beyond prescription was necessary because many … believed that major problems arose from misuse of devices by practitioners, and not just from manufacturing or design defects. Establishing conditions under which devices could be used, or limiting or describing the facilities where they could be used, or the training or qualification of those who use them in treatment, was envisioned as a way to address user-related problems. Controls such as these were not contemplated by the lone previously existing authority to limit devices to a practitioner’s prescription.47
___________________
40Id.
41Id.
42FFDCA § 514(a)(1), (b), 21 USC § 360d(a)(1), (b) (2006).
43FFDCA § 515(d)(1)(B)(ii), 21 USC § 360e(d)(1)(B)(ii) (2006).
4421 CFR § 801.109(a) (2009).
45See above regarding “Labeling with Adequate Instructions for Use.”
4621 CFR § 801.420, 21 CFR § 809.30, 21 CFR § 864.4020(d).
47Subcomm. on Oversight and Investigations of the H. Comm. on Energy and Commerce, 98th Cong., Medical Device Regulation: The FDA’s Neglected Child (Comm. Print 98-F).
Once a device is designated as a restricted device, two additional important legal consequences—apart from limiting access to the device to or through physicians—result:
• Advertising and Promotional Materials. The promotion of medical products is generally regulated by the Federal Trade Commission (FTC), with no official role for the FDA with two exceptions. The FDA has jurisdiction to regulate the advertising of prescription drugs. With regard to prescription devices, however, the agency is given jurisdiction only to regulate advertising for prescription devices that are also restricted devices. The marketer is prohibited from advertising a restricted device with materials that are “false or misleading in any particular.”48 In addition, all advertisements for a restricted device must provide the device’s established or common name (if any) and a brief statement of the product’s intended uses, side effects, and contraindications.49 Because the FDA has declined to restrict almost all Class II devices, responsibility for preventing false or misleading advertising for these devices has remained exclusively with FTC.
• Inspections. During inspections of manufacturing plants, the FDA is authorized by one provision of the statute to see the production and distribution records related to restricted devices but not to devices that are not restricted.50 A separate provision, however, empowers the FDA to inspect any records that are required by law to be kept.51 The agency has directed that specific production and distribution records on all devices be kept as part of good manufacturing practice requirements.52 Thus, the failure to designate devices as restricted has not adversely affected the FDA’s ability to examine manufacturing records.
Controls Related Only to Class II or Class III
Device Tracking
The FDA may require a manufacturer to adopt a method for tracking a Class II or Class III device whose failure might be reasonably likely to have serious adverse health consequences, that is intended to be implanted for more than 1 year, or that is a life-sustaining or a life-supporting device
___________________
48FFDCA § 502(q)(1), 21 USC § 352(q)(1) (2006).
49FFDCA § 502(r), 21 USC § 352(r) (2006).
50FFDCA § 704(a), 21 USC § 374(a) (2006).
51FFDCA § 301(e), 21 USC § 331(e) (2006).
52FFDCA §§ 501(h), 520(f)(1), 21 USC §§ 351(h), 360j(f)(1); 21 CFR pt. 320, subpt. M.
used outside a device-user facility.53 In 2002, the FDA set forth three new nonbinding criteria in addition to those in the statute to determine whether device tracking might be required: the likelihood of sudden, catastrophic failure; the likelihood of serious adverse clinical outcomes; and the need for prompt professional intervention.54
Tracking requires that the manufacturer keep records of the name and address of each patient who received the device, the physician who prescribed the device, and (if different) the physician who is following the patient. The agency has issued regulations creating the general requirements for complying with this provision.55 The authority to impose tracking requirements is discretionary with the FDA and is exercised by an “order” to individual manufacturers covering each affected device.56
Postmarket Surveillance
The FDA may require a manufacturer to conduct surveillance of a Class II or Class III device that meets any of the criteria for device tracking (above) or is expected to have substantial use in pediatric populations.57 “Postmarket surveillance” is a specific activity defined by the statute and is not to be confused with “postmarketing surveillance,” which encompasses a wide array of programs, including adverse-event reporting by manufacturers and user facilities, third-party safety monitoring, and FDA–academic collaborations. Surveillance generally cannot last more than 36 months and must be designed to provide useful data that could reveal unforeseen adverse events or other information necessary to protect public health.58 The FDA has issued general regulations governing requirements when surveillance is ordered.59 Imposing a requirement for surveillance is discretionary with the FDA60 and is done by an “order” to the manufacturer of the affected device or, in the case of pediatric devices, as a condition for clearance of a Class II device or approval of a Class III device.61
___________________
53FFDCA § 519(e), 21 USC § 360i(e) (2006).
54Medical Devices; Device Tracking, 67 Fed. Reg. 5,943, 5,944 (Feb. 6, 2002) (codified at 21 CFR pt. 821).
5521 CFR pt. 821 (2009).
5621 CFR § 821.20 (2009).
57FFDCA § 522(a), 21 USC § 360l(a) (2006).
58FFDCA § 522(b), 21 USC § 360l(b) (2006).
5921 CFR pt. 822 (2009).
60FFDCA § 522(a)(1), 21 USC § 360l(a)(1) (2006).
61FFDCA §§ 522(a)(1)(A)–(B), 21 USC §§ 360l(a)(1)(A)–(B) (2006).
Special Controls
Class II devices may be subject to one or more “special controls” to supplement the general controls to provide reasonable assurance of safety and effectiveness.62 The definition of a Class II device specifies that it is
a device which cannot be classified as a Class I device because the general controls by themselves are insufficient to provide reasonable assurance of the safety and effectiveness of the device, and for which there is sufficient information to establish special controls to provide such assurance, including the promulgation of performance standards, postmarket surveillance, patient registries, development and dissemination of guidelines …, recommendations, and other appropriate actions as [the FDA] deems necessary to provide such assurance63 [emphasis added].
In parallel, the definition of Class III covers devices that cannot be classified as Class I because general controls are insufficient to ensure safety and effectiveness and that cannot be classified as Class II because “insufficient information exists to determine that the special controls … would provide reasonable assurance of its safety and effectiveness.”64
The statute does not require, however, that every Class II device be subject to special controls. Although the definitional sentences just quoted state that “general controls … are insufficient,” the definition of Class II goes on to provide that
for a device that is purported or represented to be for a use in supporting or sustaining human life, [the FDA] shall examine and identify the special controls, if any, that are necessary to provide adequate assurance of safety and effectiveness and describe how such controls provide such assurance65 [emphasis added].
By implication, for a Class II device that is not so represented, the FDA is not even obliged to consider special controls. In addition, the various types of special controls identified in the statute (discussed below) are all written to be discretionary with the agency. As a practical matter, the FDA has always treated special controls as a discretionary matter. Today, roughly 15% of all device types classified in Class II are subject to special controls (Desjardins, 2010).
The statutorily authorized “special controls” can include one or more of the following:
___________________
62FFDCA § 513(a)(1)(B), 21 USC § 360c(a)(1)(B) (2006).
63Id.
64FFDCA § 513(a)(1)(C), 21 USC § 360c(a)(1)(C) (2006).
65FFDCA § 513(a)(1)(B), 21 USC § 360c(a)(1)(B) (2006).
• Device tracking (patient registries) and postmarket surveillance, as discussed above. Each authority is exercised in individual cases by order of the agency.
• “Performance standards” that are promulgated either by the FDA (through an elaborate notice-and-comment rule-making)66 or by a third party and recognized by the FDA (through a simple published notice).67 Performance standards may cover the construction, components, ingredients, and properties of a device and its compatibility with power systems and connections with these systems; testing requirements; provisions for measurement of performance; and requirements for submission of test results to demonstrate conformity. A performance standard may also specify that the device is a restricted device in the same manner in which the FDA might restrict the device under separate authority (see above).68
• FDA guidelines, including guidelines on the submission of clinical data with a 510(k) notification.69 The FDA has adopted a form of notice-and-comment procedure for the adoption of guidelines (which the FDA now calls guidances).70
• FDA recommendations. The statute refers only to “recommendations” without specifying what they might address or how a recommendation would become an enforceable control.71 The agency may, in issuing recommendations, use the same procedures as for guidances.72
• Any “other appropriate actions.” The FDA is authorized to adopt any other special control that it deems necessary to provide a reasonable assurance of safety and effectiveness.73
For a number of reasons, however, special controls have not transformed and cannot transform the 510(k) process into one based on safety and effectiveness rather than one based on substantial equivalence to a predicate device. First, as noted above, only about 15% of all Class II device types are subject to special controls. Second, some premarket special controls are focused on a particular aspect of the device, such as medical devices that include an antimicrobial agent, or on generic procedures for conducting research for a class of devices, such as animal studies for cardiovascular
___________________
66FFDCA § 514(b), 21 USC § 360d(b) (2006).
67FFDCA § 514(c), 21 USC § 360d(c) (2006).
68FFDCA § 514(a)(2), 21 USC § 360d(a)(2) (2010).
69FFDCA § 513(a)(1)(B), 21 USC § 360c(a)(1)(B) (2006).
7021 CFR § 10.115 (2009).
71FFDCA § 513(a)(1)(B), 21 USC § 360c(a)(1)(B) (2006).
7221 CFR § 10.90(c) (2009).
73FFDCA § 513(a)(1)(B), 21 USC § 360c(a)(1)(B) (2006).
devices (FDA, 2007b, 2010d). These guidances are not intended to supplant the standard for premarket clearance, which remains substantial equivalence to a predicate device, not safety and effectiveness. Third, many special controls are nonmandatory guidance documents, and they bind neither the FDA nor the regulated industry. For example, the guidance document for tissue adhesive with an adjunct wound-closure device intended for the topical approximation of skin explains that it “does not create or confer any rights for or on any person and does not operate to bind FDA or the public” and states that manufacturers are free to “use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations” (FDA, 2010c). Manufacturers using an alternate must, however, show that the binding legal requirements are fulfilled. Fourth, some special controls, such as postmarket surveillance and device tracking, are postmarket forms of regulation that are based on the recognition that the premarket data on safety and effectiveness on some Class II devices may not identify long-term risks or rare events. Finally, practical considerations probably preclude the issuance of special controls for each component of every Class II device. Developing a special control is highly resource-intensive, and in the case of a novel device, the necessary expertise to do so may be lacking (GAO, 1988). Thus, resource constraints presumably would prevent the FDA from taking on such a task. In addition, a proliferation of special controls could become a hurdle to innovation as a further barrier to bringing new devices to market.
The committee believes that special controls can provide a reasonable assurance of safety and effectiveness for some Class II devices. The FDA’s use of those controls, however, neither has transformed the 510(k) process into a form of comprehensive safety and effectiveness review nor represents a mechanism for doing so in the future.
Food and Drug Administration Monitoring Powers
Monitoring the Marketplace and Clinical Experience
Much of the FDA’s awareness of problems comes from information voluntarily submitted by consumers, physicians, insurers, and competing manufacturers; from reports in the mass media; and from routine surveillance of the marketplace. However, the agency has tools that require parties to submit information to permit it to oversee what products are in clinical use, whether they are being used safely, and whether manufacturers are complying with the requirements of the law.
Information Provided by Manufacturers and Importers
• Who is making what? The requirements for registration and product listing provide the FDA with an inventory of those supplying medical devices to the market and the devices being supplied.
• Product labeling. The FDA reviews only draft labeling, not final labeling, during the 510(k) review process, and marketers may make some types of changes in labeling without seeking additional FDA review. The FDA can obtain by inspection, or in some cases via the Internet, the current labeling of any device. It thus can assess the adequacy of directions for use, warnings, and other critical information for patients or healthcare professionals for that device.
• Product defects. Reports of removals and corrective actions allow the FDA early warning of potential safety issues and an opportunity to evaluate the adequacy of a manufacturer’s remedial and preventive actions.
• Product-related adverse events. Reporting both of individual cases and of trends permit the FDA to identify potential safety issues.
• Postmarketing surveillance. Reports from structured surveillance programs (including those mandated by the FDA order to individual manufacturers, those funded by the FDA, and those run through third-party collaborations) may supply a more comprehensive database (with both numerator and denominator data) in which to look for risks posed by medical devices.
Information Provided by Third Parties
• Device-related adverse events at device-user facilities. Mandatory and voluntary reporting of individual cases by end users in health facilities provides the FDA with an alternative source of potential safety information.
• Voluntary reports of adverse events from physicians, patients and third parties. Although not legally obliged to do so, many healthcare practitioners and patients submit MedWatch reports to the FDA regarding medical devices.
• Other voluntary reports and submissions of information. Competitors are a frequent source of potential violations of the FFDCA.
Inspections
The FDA is authorized to enter factories and warehouses in which devices are manufactured, packed, or held for shipment and to inspect a facility and equipment, finished and unfinished goods, and containers and labeling on the premises.74 The agency may obtain records related to move-
___________________
74FFDCA § 704(a), 21 USC § 374(a) (2006).
ment of devices and components in interstate commerce.75 And the FDA may inspect and copy records related to adverse medical events associated with devices, device tracking, and corrections and removals of devices.76 The statute says that the FDA may also inspect manufacturing records or other documents related to a medical device if the device is a restricted device.77 In that case, the FDA may examine all records (other than financial data, sales data, pricing data, personnel data, and research data) bearing on whether the device is in compliance with the FFDCA.78 With respect to devices that are not designated as restricted, the FDA nevertheless has been able to gain inspectional access to some manufacturing records. The agency has used its powers related to good manufacturing practice regulations to require that some manufacturing records be maintained for all devices;79 accordingly, the FDA may inspect these records.80
FOOD AND DRUG ADMINISTRATION ENFORCEMENT AND OTHER POWERS
When the agency discovers violations of the law or products that pose unacceptable risks to consumers, it has a wide variety of tools available to try to remedy the situation and to sanction the violators. The committee requested information from the FDA about its use of these authorities, and it was able to provide some information on the numbers and types of enforcement actions that it has taken. Given the different mechanisms needed to use these authorities, however, the agency does not have a centralized system for tracking the actions and was not able to provide the committee with some of the specifics about the actions, including whether they were used with products cleared under the 510(k) program or through other premarket-review pathways.
Judicial Enforcement Powers
The statute provides three remedies or sanctions that can be imposed, but only by order of a federal court, when the FDA finds a product or a manufacturer not in compliance with the law. To obtain such orders, the FDA must work through the DOJ Office of Consumer Litigation and the US attorney’s offices around the United States. Procedurally, the FDA must
___________________
75FFDCA § 703, 21 USC § 373 (2006).
76FFDCA § 704(e), 21 USC § 374(e) (2006) (referring to FFDCA § 519, 21 USC § 360i (2006)).
77FFDCA § 704(e), 21 USC § 374(e) (2006).
78FFDCA § 704(a), 21 USC § 374(e) (2006).
79FFDCA § 520(f), 21 USC § 360j(e) (2006); 21 CFR pt. 820, subpt. M.
80FFDCA § 301(e), 21 USC § 331(e) (2006).
first convince the DOJ attorneys that a case is meritorious and worth bringing from among the array of legal violations presented for action by other agencies. Then, the DOJ attorneys must present the matter before a federal judge (and perhaps a lay jury) and win a judgment or verdict.
To get a judicial action brought, CDRH must first prepare the case and then persuade the FDA associate commissioner for regulatory affairs (and the Office of Enforcement) that the case is meritorious. The associate commissioner is charged with balancing the enforcement initiatives of all components of the FDA, so in a sense CDRH may be competing for a place in line. The proposed action is also reviewed by the FDA Office of Chief Counsel for legal sufficiency. If the associate commissioner for regulatory affairs and chief counsel are favorably moved, the agency refers the matter to DOJ, which conducts an independent review for both legal merit and the likelihood of convincing a judge (or jury) of the merits of government’s case. Again, the FDA is competing with other agencies for the attention of DOJ. Thus, the process could appear very cumbersome and time-consuming for CDRH personnel.
The statute sets forth three remedies or sanctions:
• Seizure and forfeiture of violative product. When a medical device is found to be in violation of the prohibitions or mandates applicable to it, the law deems it to be “adulterated” or “misbranded.” The FDA, through DOJ, can obtain a judicial order authorizing a US marshall to seize the product and either secure it on site or remove it to another secure location. Thereafter, a notice is published announcing the action and permitting any interested party (for example, the manufacturer, distributor, retailer, or owner) to file a claim as to the product. If a claim is filed, the case proceeds as a civil action in which the government must prove, by a preponderance of the evidence, that the product is illegally adulterated or misbranded. If the FDA is successful or if no claim is filed, the device is forfeited to the government and can be destroyed. (In some cases, the claimant may seek to bring the product back into compliance under the FDA’s supervision.) If the claimant prevails, the device is returned as is.81 The FDA has advised the committee that in the period FY 2001–2008, it has successfully brought 13 seizure actions (Desjardins, 2011).
• Injunction. The FDA can seek a federal court order restraining persons from violations of the FFDCA. Again, there is an opportunity for the defendant to be tried by a judge, and the government must prove the violation by a preponderance of the evidence.82 If the
___________________
81FFDCA § 304, 21 USC § 334 (2006 & Supp. II 2008).
82FFDCA § 302, 21 USC § 332 (2006).
government prevails, the court can, in appropriate cases, also order restitution to the customers of the violator, disgorgement of unjust profits to the government, or both.83 Once an injunction is issued, any further violations can be enforced through a contempt-of-court proceeding. The FDA has informed the committee that in the period FY 2001–2008, it obtained 12 injunctions related to medical devices (Desjardins, 2011).
• Criminal prosecution. The FDA, through DOJ, can prosecute companies and individuals criminally for violations of the law.84 Guilt must be proved—usually to a jury—beyond a reasonable doubt. Fines have been substantially increased in recent decades, and prison time for individuals is a possibility although rarely imposed. The FDA reported that its Office of Criminal Investigations successfully obtained 212 criminal convictions for violations of the law in the period FY 2001–2010, which resulted in fines and restitution in excess of $577 million (Desjardins, 2011).
Administrative Enforcement Powers
Over the years, Congress has supplemented the judicial enforcement tools with others that the agency can use without involving DOJ or a federal court. Some tools “punish” the violator; others deal only with a violative or otherwise unsafe or ineffective product. The FDA is empowered to issue orders—usually after (or concurrent with) administrative proceedings, including formal or informal hearings—to accomplish specific tasks. Most of the authorities apply a criterion related to the risks posed by the device in question; the criteria are quite similar but not identical throughout the authorizing language.
Banning a Device
The FDA may ban a device from sale if it finds that the device “presents substantial deception or an unreasonable and substantial risk of illness or injury.”85 If the problem could be corrected or eliminated by labeling changes, the FDA is directed first to provide the manufacturer notice and a reasonable time to modify the labeling before starting a proceeding to ban the device.86 To ban a device, the FDA must engage in a notice-and-comment proposal-and-final-order rule-making proceeding to promulgate
___________________
83US v Lane Labs—USA Inc., 427 F.3d 219 (3d Cir. 2005); US v Rx Depot, Inc., 438 F.3d 1052 (10th Cir. 2006).
84FFDCA § 303(a), 21 USC § 333(a) (2006).
85FFDCA § 516(a), 21 USC § 360f(a) (2006).
86FFDCA § 516(a)(2), 21 USC § 360f(a)(2) (2006).
a regulation.87 The agency may make the rule effective on publication of the proposal if the deception or risk of illness or injury “presents an unreasonable, direct, and substantial danger to the health of individuals.”88 Once a device is banned, it becomes an adulterated device subject to the same enforcement powers as any other adulterated device.89 According to the FDA, this authority has been used only once since it was established in 1976 (Desjardins, 2011).
Recalling a Device
If the FDA finds that “there is a reasonable probability that a device … would cause serious, adverse health consequences or death,” it may order the manufacturer and other appropriate persons (for example, distributors and retailers) to cease distribution of the device immediately and to instruct healthcare professionals and device-user facilities (for example, hospitals and ambulatory surgical centers) to cease use of the device.90 After issuance of the order, the FDA must provide an opportunity for an informal hearing, which must consider both the merits of the order and whether the device should be physically recalled; the FDA is to vacate the order if the grounds for the action prove inadequate.91 After the hearing (if any), the FDA may amend the order to require the physical recall of the device, but no device may be recalled from individuals and a device should not be recalled from a device-user facility if the FDA determines that recalling the device presents a greater health risk than not recalling it.92 The FDA was given the power to order recalls in 1990. Nevertheless, most recalls remain voluntary (GAO, 2011). The agency advised that it has not formally tracked recall orders but believes that the authority has been used at least three times in the past 20 years (Desjardins, 2011).
Ordering Risk Notification about a Device
If the FDA finds that a device “presents an unreasonable risk of substantial harm to the public health”, that notification is “necessary to eliminate” this risk, and that “no more practicable means is available” to eliminate the risk, it may order that notification be given by “persons and means best suited under the circumstances involved, to all health professionals who prescribe or use the device and to any other person (including … device
___________________
87FFDCA § 516(a), 21 USC § 360f(a) (2006).
88FFDCA § 516(b)(1), 21 USC § 360f(b)(1) (2006).
89FFDCA § 501(g), 21 USC § 351(g) (2006).
90FFDCA § 518(e)(1), 21 USC § 360h(e)(1) (2006).
91Id.
92FFDCA § 518(e)(2), 21 USC § 360h(e)(2) (2006).
users) who should properly receive notification in order to eliminate such risk.”93 The statute allows the FDA to direct the order to any person (not just a manufacturer or distributor of the device), so healthcare practitioners who have treated patients with the device may be required to notify their patients. Before issuing any notification order, the FDA is to consult with the persons who are to give notice, but no formal or informal hearing is required. The FDA has not tracked the use of this authority (conferred in 1976) but advises that it has not been used many times. It was used once in 2010 (Desjardins, 2011).
Ordering Repair of, Replacement of, or Refund for a Device
If the FDA finds that a device “presents an unreasonable risk of substantial harm to the public health,” that there are reasonable grounds for believing that the device was not properly designed or manufactured with reference to the “state of the art” at the time it was designed or made, that there are grounds for believing that the unreasonable risk was not caused by failure of a person other than the manufacturer or supplier to exercise due care in the installation, maintenance, repair, or use of the device, and that simple risk notification would not by itself be sufficient to eliminate the risk but that repair, replacement, or refund is necessary to do so, it may order the manufacturer to repair the device, to replace it with an equivalent device that does not pose the risk, or to refund the purchase price of the device. The manufacturer is entitled to an opportunity for an informal hearing before the order takes final effect.94 The FDA has not used this provision since it was enacted in 1976, because the agency has found it difficult to establish that a device did not conform to the “state of the art” when first manufactured. The FDA did enter into a consent decree of permanent injunction in 2010 that required the defendant to replace or refund the price of a device, but this order was not issued under the statutory provision (Desjardins, 2011).
Imposing Civil Money Penalties
If the FDA finds that any company or individual (or both) has violated any requirement of the FFDCA related to a medical device, it may impose a civil money penalty in an amount not exceeding $15,000 for each violation and not exceeding $1 million for all violations adjudicated in a single proceeding.95 Exceptions are made for some technical violations, such as a violation of good manufacturing requirements that involves devices that
___________________
93FFDCA § 518(a), 21 USC § 360h(a) (2006).
94FFDCA § 518(b), 21 USC § 360h(b) (2006).
95FFDCA § 303(f)(1)(A), 21 USC § 333(f)(1)(A) (2006 & Supp. II 2008).
are not defective.96 The FDA must provide a formal evidentiary proceeding before the penalty can be enforced.97 According to the FDA, civil money penalties have been imposed seven times in the period FY 2001–2008 (Desjardins, 2011).
Detaining a Device in Anticipation of Seizure
If an FDA investigator finds during an inspection of a device that she or he has reason to believe the device violates the FFDCA, the investigator may order the device detained for a reasonable period, not to exceed 20 days. The detention period is to permit the FDA to decide whether to initiate a seizure action or undertake other actions. The manufacturer is entitled to a prompt informal hearing on any detention order.98 The FDA does not track the numbers of detention orders that precede seizure. The agency informed the committee, however, that use of this authority is difficult because of the statutory timeframe of 30 days, which is generally not sufficient to process a seizure action (Desjardins, 2011).
Detaining and Denying Entry of Devices Offered for Import into the United States
When an imported device is presented to US Customs and Border Protection (CBP) for release into US commerce, the FDA is to be notified and given an opportunity to inspect the product. The agency may request that CBP detain the device (that is, delay the release) while the FDA determines its compliance with the FFDCA. If the FDA finds that the device fails to comply with a requirement or has violated a prohibition or that the manufacturer did not comply with good manufacturing practice requirements, it can direct CBP to refuse release. The owner or consignee may seek to correct the violation, if that is possible, under FDA supervision. Any device not permitted entry may be destroyed if it cannot be brought into compliance or, in some instances, returned to the country of origin.99
In the period 2002–2010, more than 17,500 devices and almost 1,200 diagnostics were refused entry into the United States by the FDA (Desjardins, 2011).
___________________
96FFDCA § 303(f)(1)(B), 21 USC § 333(f)(1)(B) (2006 & Supp. II 2008).
9721 CFR pt. 17 (2009).
98FFDCA § 304(g), 21 USC § 334(g) (2006).
99FFDCA §801(a), 21 USC § 381(a) (2006).
Suspending Approval of a Premarket Approval Application
If the FDA finds that continued distribution of a Class III device under an approved PMA application “would cause serious, adverse health consequences or death,” it may temporarily suspend the approval while initiating proceedings to withdraw approval permanently in accordance with formal legal procedures.100 The manufacturer is entitled to an informal hearing on the suspension order, separate from the formal hearing on the withdrawal. There is no counterpart authority with respect to a 510(k) clearance.
Procedural Requirements
The foregoing has identified a variety of procedures that the FDA is required to use when exercising specific powers. Because CDRH has referred in its preliminary report to “important limitations” of its postmarket controls, it is appropriate to examine what each of the procedures entails.
Notice-and-Comment Rule-Making
The Administrative Procedure Act provides that regulations generally may be issued only after notice of the proposed rule is provided to interested persons and they are given an opportunity to comment before the rule is made final.101 The FDA has adopted detailed procedures for promulgating regulations.102 This can lead to improvements in the program or the development of an alternative. Given the authority of the FDA or any federal agency, it seems appropriate that stakeholders have the opportunity to comment before requirements are imposed. The process begins with publication of a proposal in the Federal Register that sets forth the factual grounds on which the FDA relies for its proposal, the legal authority for the proposed rule, and information on where and how people may submit comments. The FDA will almost always provide at least 60 days after the publication for the public to comment. A final order may be issued any time after the comment period closes. It, too, is published in the Federal Register and contains a summary of the comments received, the agency’s conclusions, and whatever changes have been made in light of the comments. At least 30 days must elapse before the order may become final, except in some public-health emergencies.
Those simple statutory procedures have been supplemented by two requirements. First, under a presidential executive order, each rule that could affect the economy, increase costs or prices, or substantially and adversely
___________________
100FFDCA § 515(e)(3), 21 USC § 360e(e)(3) (2006).
1015 USC § 553 (2006).
10221 CFR § 10.40.
affect competition, productivity, or innovation must be reviewed by the Office of Management and Budget before it is proposed and again before a final order is issued.103 Second, the Office of the Secretary of Health and Human Services (the Department of Health and Human Services is the parent organization of the FDA) must review and approve any FDA proposals or final rules that address substantial public issues, such as those affecting the availability, marketability, cost, or quality of regulated products.104 The process of issuing regulations through notice-and-comment rule-making has thus become more time-consuming and burdensome for the FDA.
The FDA has adopted similar procedures for obtaining public input on guidances or guidelines.105
Formal Evidentiary Hearing
A formal evidentiary hearing, the counterpart of a civil trial in a courtroom, occurs before an administrative law judge (ALJ) with sworn witnesses, cross-examination, written evidence, and briefs by counsel.106 Such hearings take considerable time to prepare and conduct and are often preceded by various motions and submissions to frame the issues, determine the admissible evidence, and resolve procedural disputes. After the hearing, the ALJ prepares findings of fact and conclusions and makes an initial decision. Either side may appeal the decision to the commissioner of the FDA, who reviews the record, the initial decision, and the arguments of the parties and issues a final decision. In many situations, a private party who loses a decision after a formal evidentiary hearing may seek review by a federal court.
Informal Hearing
The FDA has established procedures for a “regulatory hearing” to provide an expeditious opportunity for an adversely affected party to be heard on a proposed or actual order.107 In contrast with a formal hearing, the rules of evidence do not apply. Both sides present their views, a limited amount of cross-questioning may occur, and a written report is issued. But the timeframe for initiating, holding, and completing the process is tightly controlled.
___________________
103Exec. Order 12,291, 46 Fed. Reg. 13,193 (Feb. 17, 1981).
10446 Fed. Reg. 26,052 (May 11, 1981).
10521 CFR § 10.115.
10621 CFR pt. 12; see also 5 USC § 554 (2006).
10721 CFR pt. 16.
Order
The FDA may issue an order simply by issuing a written directive to an affected party, provided that the factual predicates for the order are satisfied. The statute may require the agency to provide an opportunity for an informal hearing before or soon after the issuance of an order.
Finding 3-1 The Food and Drug Administration has a wide array of tools to address safety risks that are discovered to be posed by marketed devices.
Finding 3-2 The Food and Drug Administration has not used the tools at its disposal extensively. The Center for Devices and Radiological Health has suggested that there are important limitations in their use. The committee identified some procedural burdens on the exercise of these tools, but these burdens do not in themselves explain the historical and continuing sparse use of the tools.
In contrast with the system designed for medical devices, the FDA’s drug regulatory framework after the Drug Amendments of 1962 required a more standardized, clinical-trial–based premarket review of both safety and effectiveness of all new drugs and placed less emphasis on postmarket surveillance and management of risks.108 Until passage of the Food and Drug Administration Amendments Act of 2007 (FDAAA),109 it could be fairly said that the FDA had better risk-management authorities for marketed devices than it had for approved drugs. The 2007 legislation made important changes in the FDA’s powers, which implemented some of the recommendations of the Institute of Medicine report The Future of Drug Safety (IOM, 2007). In essence, after 4 decades of extensive, if not often exclusive, reliance on authority to approve new drugs and to remove them later, Congress and the FDA recognized that this model had inherent limitations in ensuring the safety and effectiveness of drug products. The results of premarket clinical trials conducted by trained investigators on relatively small numbers of carefully selected research subjects could not be extrapolated to the use of a drug by thousands of physicians and millions of patients. Unexpected adverse reactions, interactions with other medicines, the
___________________
108Drug Amendments of 1962 (Harris-Kefauver Act), Pub. L. No. 87-781, 76 Stat. 780 (codified as amended in scattered sections of 21 USC).
109Pub. L. No. 110-85, 121 Stat. 823 (codified as amended in scattered sections of 21 USC).
need for dose adjustments for concomitant diseases, and new uses for other diseases or conditions emerged after approval. Removal of a drug from the market was not always a rational option for addressing the new issues. Thus, although it did not alter the FDA’s premarket approval process for drugs, the FDAAA added important new authorities for the FDA to evaluate and manage drug-related risks after approval (Evans, 2009).
Drugs and devices present distinct regulatory issues, and the authorities that the FDA has in connection with drugs may not always be appropriate for devices. Nevertheless, it is instructive to compare the regulatory frameworks as they exist today.
It appears that the FDA already has many authorities over marketed devices that closely resemble the new postmarket authorities that the FDAAA created for drugs. The FDAAA enables the agency to institute a Risk Evaluation and Mitigation Strategy (REMS) plan for an individual drug when necessary to ensure that the benefits of the drug outweigh the risks that it poses. If there is a substantial discrepancy, it lies in the procedural requirements to use the powers granted by Congress. For example, the FDA may be required to promulgate a regulation to exercise an authority for devices, whereas the equivalent authority for drugs can be exercised by means of an order. This procedural complexity may have contributed to CDRH’s stated concern about limitations on its statutory authorities, and it tends to increase the effect of FDA’s resource constraints.
Authority to Gather Evidence During the Postmarket Period
Table 3-1 compares the FDA’s authorities to obtain information regarding risks posed by drugs and medical devices after they enter the marketplace. Here, the term passive refers to evidence-gathering that is passive from the regulator’s perspective—that is, the agency passively awaits data collected and submitted by other parties. Active surveillance involves active development of systems to allow the FDA to acquire safety data on its own initiative, for example, through the creation of information systems that allow mining existing administrative or clinical databases.
The FDA is also authorized to engage in the active surveillance of clinical experience for medical products through the Sentinel Initiative (device-specific active surveillance programs are discussed in detail in Chapter 5). In 2007, the FDAAA directed the FDA to develop methods to obtain access to data from disparate sources (including federal health-related data from the Medicare and Department of Veterans Affairs programs and private health-insurance claims) and to construct and validate methods for linking and analyzing safety data from these sources.110
___________________
110FFDCA § 1005, Pub. L. No. 110-85, 121 Stat 823 (2007).
|
Passive Gathering of Clinical Experience |
Approved Drugs |
Marketed Devices |
|
Reporting by manufacturers and importer of adverse-event information received by them |
Mandatory for all drugs |
Mandatory for all devices |
|
Reporting by hospitals and other user facilities of observed adverse events |
No authority to require |
Mandatory for manufacturer or the FDA |
|
Tracking of users; user registry |
Discretionary authority to require as part of REMS |
Discretionary authority to require |
|
Reporting by manufacturers and importers of corrections and removals to address risks |
No authority to require |
Mandatory for all devices |
|
Assessing specific safety issues |
Discretionary authority to require as part of REMS or as condition of approvala |
Discretionary authority to require “postmarket surveillance” study or as condition of Class III approval |
|
Requiring prospective clinical trials |
Discretionary authority to require any time that new safety information emergesb |
Discretionary authority as condition of Class III approval |
aFFDCA § 505(o), 21 USC § 355(o) (Supp. 2007).
bFFDCA § 505(o), 21 USC § 355(o) (Supp. 2007).
The FDAAA also directed the agency to prepare an analysis of adverse-event reports for each newly approved drug within 18 months after approval or after use by 10,000 people, whichever is later.111
Authority to Take Action to Manage Emerging Safety Issues
The FDA has several authorities that allow it to manage safety issues as they occur. These authorities are summarized in Table 3-2.
___________________
111FFDCA § 505(r)(2)(D), 21 USC § 355(r)(2)(D) (Supp. 2007).
Labeling Changes
The FDA has various authorities to address serious problems of device labeling, but these are procedurally more cumbersome than the process now in effect for drugs. In practice, device labeling changes may require voluntary cooperation of the manufacturer. In contrast, the FDAAA substantially expanded the FDA’s authority to require mandatory safety-related labeling changes for already-approved drugs at any point in their life cycle.112 The process calls for the FDA to notify manufacturers of new safety information that the agency believes should be placed in the drug’s labeling. There are brief periods for response (30 days) and discussion (30 days), after which the FDA can order the change if agreement has not been reached.
Restrictions on Sale, Distribution, and Use
The FDA has always had authority, after following due-process procedures, to revoke a drug’s approval and force it from the market. However, the agency lacked tools for selective risk management that would let a drug remain on the market but require specific measures to manage its risks. The FDAAA provided such authorities. The FDA can condition the sale of a drug on a specific risk-management measure, a REMS.113 The FDA can require a REMS on initial approval or later if emerging evidence reveal that a REMS is needed to ensure that the drug’s benefits outweigh its risks. A REMS must provide for continuing evaluation of risk. Additional elements may be included, such as medication guides, patient package inserts, and warning letters to inform healthcare providers or patients of a risk. The FDA also has authority to impose specific restrictions on the use, distribution, or prescribing of a drug in cases in which a drug is effective but has known risks so serious that the drug otherwise would have to be taken off the market. Those specific restrictions are called elements to ensure safe use and can include such measures as requiring providers to have special training to prescribe the drug, requiring that the drug be used only in specific settings, requiring a registry to monitor patients’ outcomes, and requiring patients to be screened before use of the product or requiring them to be monitored for adverse events.
For devices, the FDA has authority to restrict a device of any class if it determines that the restriction is necessary to provide reasonable assurance of safety and effectiveness. The types of use restrictions that can be imposed are in many respects similar to those that can be imposed for drugs under REMS. An important difference is that REMS use restrictions potentially make it possible to limit the use of a drug to patients who have particular
___________________
112FFDCA § 505(o)(4), 21 USC § 355(o)(4) (Supp. 2007).
113FFDCA §§ 505(p), 505-1, 21 USC §§ 355(p), 355-1 (Supp. 2007).
TABLE 3-2 Comparison of FDA Authorities to Take Remedial Actions in Light of New Safety Findings
| FDA Authority | Approved Drugs | Marketed Devices |
|
Banning a product |
Discretion to revoke marketing approval; may suspend immediately as an imminent hazarda |
Discretion to declare product a banned device; discretion (for Class III premarket approval only) to revoke marketing approval |
|
Recalling a product |
No authority to compelb |
Discretion to compel by order |
|
Changing labeling |
Discretion to compel labeling change by orderc |
No direct authority to compel by order |
|
Notifying (healthcare practitioners or users) of risk |
Discretion to compel by REMS pland |
Discretion to compel by order |
|
Restricting distribution or use |
Discretion to restrict by REMS plane |
Discretion to restrict under restricted-device powers |
|
Compelling special labeling for patients |
Discretion to compel by REMS plan or MedGuide programf |
Discretion to compel through special controls (by notice-and-commentrule-making regarding Class II device) or premarket approval (as condition of approval for Class III) |
|
Ordering repair of, replacement of, or refund for a product |
No authority to compel |
Discretionary authority to compel by order |
characteristics; there is no explicit provision for similarly limiting the use of a device. Another major difference is procedural: the FDA can restrict devices of any class by notice-and-comment rule-making, as part of a performance standard established by notice-and-comment rule-making for Class II devices, or as a condition of approval of a PMA device. Thus, in theory, the FDA has some authorities that would allow dispensers of devices to be subject to restrictions, but the method of imposing restrictions is so procedurally cumbersome as to make it impractical to apply in most circumstances, and there is no explicit provision for adding nuance to the limitations to reflect patient characteristics.
| FDA Authority | Approved Drugs | Marketed Devices |
|
Imposing civil money penalties for violations |
No authority (other than for false direct-to-consumer advertising of prescription drugs)g |
Discretionary authority to impose after formal hearing |
|
Detaining product in anticipation of seizure |
No authority to order |
Authority to detain upon order |
|
Detaining and denying products offered for import |
Discretionary authority identical for drugs and devices |
Discretionary authority identical for drugs and devices |
|
Communicating risk–benefit information on marketed products in timely fashion |
Required for drug productsh |
No explicit communication program or process, but the FDA has general authority to issue public-health notificationsi |
aFFDCA § 505(e), 21 USC § 355(e) (2006).
bThe FDA is authorized to undertake multiple seizures of a product. FFDCA § 304(a), 21 USC § 334(a) (2006). Often, the threat of a seizure will induce a recalcitrant company to undertake a voluntary recall.
cFFDCA § 505(o)(4), 21 USC § 355(o)(4) (Supp. 2007).
dFFDCA § 505-1(e), 21 USC § 355-1(e) (Supp. 2007).
eFFDCA § 505-1(f)(3), 21 USC § 355-1(f)(3) (Supp. 2007).
fFFDCA § 505-1(e)(2), 21 USC § 355-1(e)(2) (Supp. 2007); see also 21 CFR pt. 208.
gFFDCA § 303(g), 21 USC § 333(g) (Supp. 2007).
hFFDCA § 505(r), 21 USC § 355(r) (Supp. 2007).
iFFDCA § 705(b), 21 USC § 375(b) (2006).
Timely Communication of Postmarket Risk–Benefit Information
The FDAAA did not alter the FDA’s traditional drug-labeling requirements. The statute, however, recognizes that labeling may not be an ideal medium for communicating timely, specific information about emerging drug-safety problems. The FDAAA established an Internet-based system for disseminating risk information to patients and providers (FDA, 2010e).114 The FDA met its statutory deadline to have a basic system in place 1 year after the enactment of the FDAAA.115 The system’s functionality continues to develop. The FDAAA envisions that this system will provide a searchable,116
___________________
114FDAAA § 915, 21 USC § 355(r) (Supp. 2007).
115Id., 21 USC § 355(r)(1).
116Id., 21 USC § 355(r)(2)(A).
centralized source of information on drug-related risks and how to manage them. The system already incorporates information that traditionally has been provided in paper form, such as labeling, package inserts, medication guides,117 and safety alerts.118 In addition, the system is intended to provide continuous, close-to-real-time feedback of emerging postmarket data, such as early signals of potential safety problems that are not clear enough to support labeling changes.119 The Internet system also will provide risk-management information, including REMS information.120
There appears to be no statutory impediment that would prevent the FDA from developing a similar communication system for device labeling and other safety information. Rather, it appears to be a question of resources and priorities.
DEVICE CLASSIFICATION AND PREMARKET REVIEW
As noted earlier, all devices must comply with FDA standards of safety and performance. The 1976 Medical Devices Amendments (MDA) adopted a three-tier system to determine the need for additional regulatory controls, in the form of premarket review, to protect and promote public health. The tiers correspond to the perceived risks posed by the devices. In that sense, the law represented a sharp break with the approach taken with respect to pharmaceutical agents, for which a uniform and rigorous system was established for all new agents and an only slightly less demanding and uniform system for generic copies of the agents. Because of the wide variety of devices, Congress recognized that uniformity was neither necessary nor ideal to attain the ultimate public-health goal of a “reasonable assurance of the safety and effectiveness” of each marketed device. A detailed description of device classification criteria is included in Chapter 2.
Using the standards outlined in Chapter 2, the FDA was tasked with reviewing the universe of devices on the market when the 1976 law was enacted—and therefore called preamendment devices—and placing them into the appropriate Class. In addition, the 1976 MDA directed that any device that was not on the market at the time the bill became law (so-called postamendment devices) would be automatically placed in Class III, at least until reclassified.121
It took the FDA until 1988 to complete the classification of preamendment devices (Hutt et al., 2007).
___________________
117Id., 21 USC §§ 355(r)(2)(B)(i)–(ii), 355(r)(3).
118Id., 21 USC § 355(r)(2)(B)(iv).
119Id.
120FDAAA § 915, 21 USC § 355(r)(2)(B)(v).
121FFDCA § 513(f)(1), 21 USC § 360c(f)(1) (2006).
Premarket Approval Application122
For Class III devices, Congress required affirmative FDA approval before marketing.123 The application for PMA must contain the following major elements: full reports of all information known to or reasonably known to the applicant regarding investigations to assess the safety and effectiveness of the device; a full statement of the components and properties of the device and of the principles of its operation; a full description of the methods used in and facilities and controls used for its manufacture, processing, and (when relevant) packaging and installation; specimens of the labeling proposed to be used for the device; and any other information relevant to the PMA application that the FDA (with the concurrence of an advisory panel) may require.124 The statute does not give the FDA authority to waive any of those requirements, but the FDA permits an applicant to omit information that is not applicable to the device if the omission can be justified.125
In 2002 and again in 2007, Congress enacted 5-year user-fee programs under which persons submitting PMA applications and 510(k) submissions must pay a fee to underwrite some of the cost of FDA review. Thus, a new PMA application must be accompanied by payment of a fee for FDA review; in FY 2011, the standard fee for a new PMA application was $236,298, although small businesses can have the fee reduced to $59,075 (FDA, 2011d).
The agency has 180 days to review a PMA application and either to grant or to deny its approval.126 Moreover, the FDA, if requested, must meet with the applicant not later than 100 days after the PMA application is filed and discuss the status of the review, identifying any deficiencies and describing the information needed to correct them.127 During the review process of a novel device, the FDA may on its own initiative (and must on request by an applicant) refer the PMA application to an outside advisory panel, which must report its conclusions and recommendations.128
Grounds for denial are the lack of demonstration of a reasonable assurance of either safety or effectiveness, noncompliance with good manufacturing practices, and false or misleading labeling.129 If the agency denies
___________________
122This discussion is highly abbreviated from Appendix A. Its purpose is to provide an overview of the principal premarket-review alternative to the 510(k) submission.
123FFDCA §§ 513(a)(1)(C), 515(a), 515(a), 21 USC §§ 360c(a)(1)(C), 360e(a) (2006).
124FFDCA § 515(c), 21 USC § 360e(c) (2006); see 21 CFR § 814.20 for FDA’s regulations detailing the required format and contents of a PMA.
12521 CFR § 814.20(d).
126FFDCA § 515(d)(1), 21 USC § 360e(d)(1) (2006).
127FFDCA § 515(d)(3), 21 USC § 360e(d)(3) (2006).
12821 CFR § 814.44(a), (b).
129FFDCA § 515(d)(2), 21 USC § 360e(d)(2) (2006).
a PMA application, the applicant may request a second review by and a hearing before a hearing officer or a standing outside FDA advisory panel.130
The FDA may condition approval on compliance with one or more of a variety of postapproval requirements, including the completion of studies to confirm the safety, effectiveness, and reliability of the device for its intended use.131
Once a PMA application is approved, the applicant must obtain additional FDA approvals before making changes in the labeling of the device, its indications for use, its packaging or sterilization procedures, its performance or design specifications, or its components, principles of operation, or physical layout.132 In addition, the applicant may not make any changes in the manufacturing procedures without submitting to the FDA a notice describing the change and summarizing the supporting data, which the agency has 30 days to review; if the FDA finds the notice inadequate, the applicant may not implement the modification.133 Finally, after approval of the PMA application, the applicant must make periodic reports to the FDA containing new unpublished reports, if any, of clinical or nonclinical investigations involving the device or related devices and reports from the scientific literature concerning the device.134 Revisions of devices approved through the PMA application process require a supplemental PMA application as opposed to a 510(k) submission. Thus, the obligations of the PMA application process extend well past the initial review and approval.
User fees for PMA supplements in FY 2011 range from $3,800 to $236,000, depending on the type of supplement and hence the FDA resources needed to review it. There are substantial reductions for small businesses (FDA, 2011d).
510(k) Clearance-Process Submission
The 1976 law contemplated that the sponsor of a new product that was classified in Class I would submit a notice to the FDA at least 90 days before marketing; the notice would set forth the simple fact that the device was so classified. That notice became known as a 510(k) notification after the section of the MDA that required a manufacturer to notify the FDA of a new product before marketing it.135 If the agency concurred (or failed to respond within 90 days), the product could enter the market; if not, the
___________________
130FFDCA § 515(g), 21 USC § 360e(g) (2006).
13121 CFR § 814.82.
132FFDCA § 515(d)(6), 21 USC § 360e(d)(6) (2006); limited exceptions are provided in 21 CFR § 820.39(d).
133FFDCA § 515(d)(6), 21 USC § 360e(d)(6) (2006).
13421 CFR § 814.44.
135FFDCA § 510(k), 21 USC § 360(k) (2006).
sponsor was notified that the product could not enter the market without further action, such as approval of a PMA application. In 1990, Congress modified the law to prohibit the launch of a new product until the FDA had issued a written response to the 510(k) submission.136 In 1997, Congress again amended the 1976 law and eliminated the requirement of a 510(k) submission for most Class I devices.137
For premarket review of a Class II device, the 1976 law seemed to contemplate that the sponsor of a new product would submit to the FDA a notice under Section 510(k) setting forth the fact that the device was classified in Class II and, if a performance standard had been promulgated, that appropriate certification or evidence that the product conformed to the standard.138 As with Class I, the original law did not require FDA action on the 510(k) submission; but in 1990, Congress amended the law to require a written FDA response to the submission before marketing. At the same time, the broader concept of “special controls” was substituted for (but still included) the narrower “performance standard” in the definition of Class II. In 1997, Congress exempted a small proportion of Class II device types from 510(k) submission requirements.
There is no standardized format for a 510(k) submission, although the FDA has issued guidance for manufacturers (see Chapter 4) (FDA, 2005). The 510(k) submission needs to set forth a device’s proposed intended use, the device to which substantial equivalence is claimed, and evidence demonstrating the equivalence. That information may be based on bench testing, animal studies, or clinical trials. Although a 510(k) submission is usually far leaner than a PMA application, the number of pages in the average 510(k) submission has grown over the years and in some cases might be as high as in a regular PMA (FDA, 2010a).
The same legislation that established user fees for PMA applications required fees for the review of 510(k) submissions. In FY 2011, the standard fee is $4,348, discounted to $2,174 for small businesses (FDA, 2011d).
The most important development after 1976 was the evolution of the 510(k) clearance process from a transitional tool for preclearance of postamendment devices to a permanent and dominant means of premarket review for most postamendment devices.
___________________
136FFDCA § 513(i)(1)(A), 21 USC § 360c(i)(1)(A) (2006) (added by SMDA § 12, 104 Stat. at 4523).
137FFDCA § 510(l), 21 USC § 360(l) (2006) (added by FDAMA, § 206(a), 111 Stat. 2338-39 (1997).
138FFDCA § 510(k), 21 USC § 360(k); § 514(a)(1)(B), 21 USC § 360d(a)(1)(B) 2006).
Comparison of 510(k) and Premarket Approval Application Requirements
From the perspective of a device manufacturer, the 510(k) clearance process currently offers (and historically has offered) a number of advantages over the PMA application pathway. Table 3-3 summarizes some of the key differences.
Because the 510(k) submission does not include much of the information required in a PMA application, the time needed for preparation of the
TABLE 3-3 Summary of Key Differences Between PMA Applications and 510(k) Submissions
|
Premarket Approval Application |
510(k) Submission |
|
|
Detailed description of device components |
Required |
Not required |
|
Detailed description of methods of manufacture |
Required |
Not required |
|
Specimens of draft labeling |
Required, reviewed, and approved |
Required and reviewed, but not approved |
|
Studies regarding safety and effectiveness |
Reports of all studies relevant to safety and effectiveness required; clinical trials commonly required |
Only studies demonstrating substantial equivalence to predicate needed; clinical trials infrequently needed |
|
Filing fees (FY 2011) (regular business; small business) |
$236,298; $59,075 |
$4,348; $2,174 |
|
Statutory time for FDA review |
180 days |
90 days |
|
Conditions on final FDA action |
Postapproval conditions authorized |
Postclearance conditions not authorized |
|
Need for FDA action on changes in manufacturing, labeling? |
Generally, FDA approval required; thus, changes are subject to user fees |
Generally, FDA clearance not required for manufacturing and labeling changes; thus, these changes are not subject to user fees; other changes—in design, materials, and intended uses—do require a new 510(k) submission |
|
Subject to FDA revocation or rescission? |
Yes |
Generally, no |
submission is usually less, and the scope of issues that can emerge during FDA review is narrower. The committee was advised by industry that it prefers the flexibility and economy of the 510(k) process (IOM, 2010). It is important to note, however, that the 510(k) process was never intended to be a substitute for the PMA process.
Finding 3-3 The 510(k) clearance pathway is generally more economical, faster, and less burdensome to industry and the Food and Drug Administration than the premarket approval application route and has substantially fewer postmarketing controls.
From a public-health standpoint, the differences between the two pathways are less clear. For example, some patient advocates advised the committee that they wanted premarket clinical trials of safety and effectiveness for more devices, but other experts questioned the feasibility of such trials in light of the time required to plan, execute, and analyze the studies; the small numbers of subjects that might be eligible for trials in any given period (limiting the power of studies to detect differences between the new product and alternate treatments); and the pace of technologic change (IOM, 2010, 2011). Determining the “value added” to device safety and effectiveness by requiring a PMA application, as opposed to a 510(k) submission, would be complex and difficult and was not part of the committee’s charge.
INFRASTRUCTURE OF THE CENTER FOR DEVICES AND RADIOLOGICAL HEALTH
As noted in Chapter 2, the FDA and CDRH have faced resource and capability limitations and competing priorities over the life of the medical-device regulatory programs. The limitations have shaped the organization’s approach to fulfilling its mission and created shortcomings in the programs that are difficult to address. Given the increasing number and complexity of devices and combination products that include devices, CDRH’s mission would be difficult even under ideal circumstances. This section explores the resource constraints and strategic issues that affect CDRH and its work.
Financial Resources
The total budget (including mandatory and discretionary spending) of the Department of Health and Human Services in FY 2008 was $722 billion (see Figure 3-1). Of the $1.2 billion used to support the FDA’s medical-products programs, $275 million supported the medical-devices program, compared with $681 million for the drug program and $234 million for the biologics program. All the FDA’s programs are considered discretion-
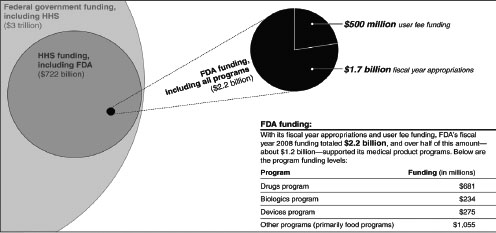
FIGURE 3-1 Federal government, Department of Health and Human Services, and FDA funding, FY 2008.
SOURCE: GAO, 2009a.
ary spending (GAO, 2009b). CDRH’s funding is augmented by user fees. However, as described in Chapter 2, there are limitations to how those funds can be used.
The funding for CDRH has grown from $159 million in 1999 to $275.3 million in 2008—a 73% increase (GAO, 2009a). Despite the increase in funding, agency officials reported that the limitations placed on the use of the user-fee funds seriously undermine their ability to meet their responsibilities in programs not supported by user fees (GAO, 2002, 2009a). About two-thirds of all the funding for medical-products program centers (both user-fee funding and discretionary funding) supports user-fee–related activities (for example, submission review). Funding for the program centers increased three times as fast as funding for the FDA’s field operations, which are responsible for inspecting manufacturing facilities (GAO, 2009b).
Staffing
The FDA is concerned about its ability to attract and retain key staff who have essential expertise, such as biologists, chemists, computer programmers, and epidemiologists (GAO, 2009b). For all the FDA centers—CDRH, the Center for Biologics Evaluation and Research (CBER), and the Center for Drug Evaluation and Research (CDER)—full-time equivalents (FTEs) funded by user fees increased by 113% from 1999 (856 FTEs) to 2008 (1,825 FTEs), whereas FTEs funded through appropriations declined
by 7% from 1999 (4,069 FTEs) to 2008 (3,802 FTEs).139 In an effort to reduce staffing levels in programs funded through appropriations, the agency offered buyouts to some employees in FY 2004–2006 (GAO, 2009b). CDRH and the Office of Regulatory Affairs (ORA) estimated that they increased their use of contractors to fulfill their responsibilities during that period but were unable to provide the Government Accountability Office (GAO) documentation of total number of contractor hours. The decrease in FTEs resulted in a backlog in work (GAO, 2009b).
CDRH staff are required to respond within 90 days of receipt of a 510(k) notification. In 2009, the FDA’s goal was to review 90% of 510(k) submissions within 90 days and 98% within 150 days (GAO, 2009b).140 Given the variability in the 510(k) submissions, FDA staff report that review times did not allow sufficient review of complex issues (FDA, 2011a). Over a 7-year period, the number of FTEs dedicated to processing 510(k) submissions has risen dramatically, from 166 in 2003 to 249 in 2009. The number of FTEs dedicated to processing PMA applications has grown from 133 to 152 in the same period (Desjardins, 2011). The FDA reported that it takes about 2 years to train new staff to review applications (GAO, 2009b). This long training period makes average annual turnover of 11–13% in the centers (CDER, CBER, and CDRH) an important issue for the FDA (GAO, 2009b).
In 1989, GAO recommended that the FDA collect basic management information, such as staffing (including contractors), to estimate its future needs better (GAO, 1989). The FDA disagreed with that recommendation, indicating that the resources needed to collect the information would detract from the agency’s oversight responsibilities. In a 2009 report, GAO reiterated its recommendation with the assertion that the collection of this information is essential for the agency to be able to fulfill its mission adequately. Without the information, it is also impossible for external groups to verify whether the FDA is fulfilling its oversight requirements and optimizing its performance (GAO, 2009b).
Third-Party Review
In August 1996, the FDA initiated a voluntary third-party review pilot program for selected devices. The pilot’s purpose was to provide manufacturers of eligible devices an alternative review process that might yield more rapid marketing clearances and enable the FDA to use its scientific review resources for higher-risk devices. All Class I and 30 select Class II device types were eligible for third-party review. The eligible Class II devices were
___________________
139These numbers do not include contractors.
140Compared with PMA reviews, for which the goals are 60% of original PMA submissions within 180 days and 90% within 295 days (GAO-09190).
subject to a device-specific guidance or a recognized consensus standard. Well-recognized road maps for establishing device performance were considered important in choosing products to be reviewed in the pilot because such documents would, it was believed, standardize the review process among third-party organizations. The FDA developed an accreditation and training process to ensure that third-party reviewers had the capacity to perform credible reviews and to avoid conflict of interest.
The 1997 FDAMA formally recognized and extended the program. The FDAMA directed the FDA to accredit third parties to review devices of low to moderate risk and to set limits on the number of Class II devices that would be ineligible for third-party review. In September 1998, the FDA published an expanded list of Class II device types eligible for third-party review for which there were no device-specific guidances or standards. For a third party to review a device not subject to a guidance or standard, it must (FDA, 2009)
• Have completed three successful 510(k) reviews in the program.
• Agree to contact the appropriate CDRH branch chief or designee to discuss issues and review criteria.
• Prepare a summary of the discussion and submit it to the Office of Device Evaluation with its 510(k) review.
The third party charges a fee to submitters for review. Once a review has been completed, it must be submitted with a cover letter, a letter from the submitter authorizing submission, a summary of the presubmission discussion described above, the complete 510(k) submission, and a completed review. In addition, there must be a certification that the reported information accurately reflects the data reviewed. The FDA supervisory official is expected to review the third-party submission and make a decision on the submission within 30 days of receipt.
GAO found that often the third-party review could be faster than FDA review for traditional 510(k) submissions but that, as FDA review has become more efficient, the number of third-party reviews is likely to decrease (GAO, 2009c).
As of February 2011, 670 Class I and Class II device types were eligible for third-party review. No Class II devices that are intended to be permanently implanted, that are intended to be life-sustaining or life-supporting, or whose 510(k) submission requires clinical data are eligible for third-party review (GAO, 2009c). Since 2004, about 250 510(k) submissions have participated in the third-party process per year (see Figure 3-2).
Quality assessments were completed on 75% of third-party reviews received during the last 9 months of FY 2005. Overall, major problems were observed by supervisors who reviewed submissions in 31% of reviews;
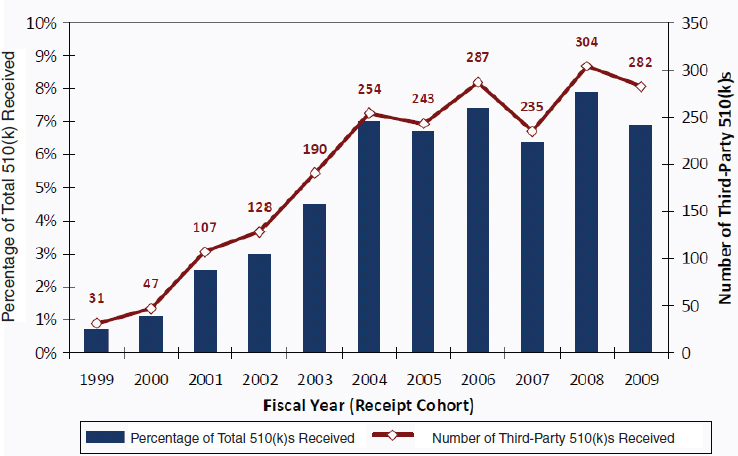
SOURCE: FDA, 2010a.
the most common problems were lack of rationale for conclusions and recommendations, failure to provide an adequate comparison with a legally marketed device, and failure to resolve deficiencies in the 510(k) submission or to address FDA requests (see Table 3-4) (von Eschenbach, 2007). A review of recall data from 2003–2009 found that 510(k) devices cleared via third-party review were more common among recalled devices than among nonrecalled devices (9.9% vs 7.3%) (IOM, 2011). Given the rate of review deficiencies and the higher recall rate, it is not certain that the program has met its goal of assisting the FDA in reducing its work burden.
Information-Sharing and Technology
Inadequacies in information-sharing and technology in the FDA are felt at all levels and centers in the agency. In 2007, the FDA produced a strategic action plan that focused on four strategic goals: strengthening the FDA, improving the safety of patients and consumers, increasing access to new medical and food products, and improving the safety and quality of manufactured products and the supply chain (FDA, 2007a). Improvement of the medical-product review process to increase the predictability and transparency of decisions was identified as a priority. The initiatives included in the plan were the integration of premarket-decision information into a
TABLE 3-4 Frequency of Problems with Third-Party Reviews of 510(k) Submissions
|
Review Element |
% Rated as Minor Issue |
% Rated as Major Issue |
|
Presubmission consultation with the FDA |
15 |
4 |
|
Rationale for conclusions and recommendations |
11 |
6 |
|
Comparison with legally marketed devices—identification and analysis of key similarities and differences |
11 |
5 |
|
Summary of device characteristics, intended use, performance, and reason for 510(k) |
10 |
4 |
|
Organization and format of review documentation |
13 |
1 |
|
Use of guidance and standards |
10 |
2 |
|
Scope of reviewer expertise |
8 |
3 |
|
Resolution of 510(k) deficiencies and FDA requests |
3 |
5 |
|
Determination of device eligibility for third-party review |
3 |
4 |
|
Determination of 510(k) administrative completeness |
6 |
1 |
NOTE: Data based on quality assessments completed by FDA supervisors when making final determinations on 510(k)s with third-party review. The FDA initiated the quality assessments in January 2005.
SOURCE: Adapted from von Eschenbach, 2007.
single comprehensive tracking warehouse accessible to all staff and the pilot testing and evaluation of a Web-based tracking system for premarket review of medical devices (GAO, 2009d).
In a 2009 report, GAO found that although the FDA has an information-technology (IT) modernization project underway, it has failed to develop a comprehensive IT strategic plan to inform the modernization process. A plan would include a well-defined set of goals, strategies, milestones, and performance measures and would allow the agency to consider the human, infrastructure, and funding resources needed. Without a clear plan, GAO asserts, the FDA lacks a roadmap needed to develop an effective system (GAO, 2009d). In addition, CDRH has acknowledged that it is difficult for staff to share knowledge throughout the center (FDA, 2011a). The inability to communicate with other staff, seek essential expertise, and share relevant information can be attributed to problems of individual workload, the lack
of a system to institutionalize cross-center communication, the rigidity of review-turnaround expectations, and the presence of multiple information systems (FDA, 2010b).
In an effort to address its information-systems issues, the FDA has implemented a number of pilot programs that have met with various degrees of success. A program called eConsults is intended to facilitate the exchange of scientific information and staff expertise throughout CDRH. The program, in place for 5 years, fosters communication within CDRH by managing expert-consultation requests and allowing staff to access information and analysis as needed (Desjardins, 2011). It prompts discussion among CDRH offices to access different types of expertise in premarket device submissions, postapproval studies, and compliance and enforcement actions (FDA, 2011b).
CDRH included several items related to the improvement of IT systems in its 2010 strategic priorities. For example, the iReview program, a pilot application begun in 2008, automated the premarket certification of medical devices. The iReview system is a workflow-management tool intended to automate the end-to-end 510(k) review process and to ensure that work is not duplicated in multiple systems, that technical risk is reduced, that future changes in the 510(k) process can be incorporated easily without affecting other applications, and that reviewers, supervisors, and consultants can use one system as a single repository for all 510(k) review status and work products (FDA, 2011c). However, during user-acceptance testing in May 2010, reviewers determined that iReview did not meet the center’s most pressing needs, namely, internal searches, reporting, and electronic document routing. As a result, iReview implementation was canceled, and CDRH will work to improve existing tracking systems to perform key functions (Desjardins, 2011).
Another pilot that proved unsuitable was the Appian business-management program introduced in the 2010 strategic priorities. In November 2010, the FDA announced that it would sign a 5-year contract to use this platform in all its centers. The program was intended to improve and align processes between groups and departments and to improve management of several core business processes. Because most of CDRH’s core functions involve knowledge creation, the business-management program did not survive the evaluation process and was determined to be the wrong tool for CDRH’s work (Desjardins, 2011).
One successful pilot program is the @work toolset, internally known as Traction. Traction is a collaborative tool that combines the attributes of a wiki and a blog with networking capability to forge connections between people throughout the organization. Traction was piloted by CDRH and is now used throughout the FDA (Desjardins, 2011).
Finding 3-4 The Center for Devices and Radiological Health faces persistent challenges because of a lack of or limitations on human, fiscal, and technologic resources and capabilities.
Quality Assurance
The preliminary report of the CDRH 510(k) Working Group finds that “CDRH does not currently have an adequate mechanism to regularly assess the quality, consistency, and effectiveness of the 510(k) program” (FDA, 2010a). That finding is amply supported by the results of the reviewer survey in Appendix D to the preliminary report. The survey asked CDRH personnel who review 510(k) submissions and their managers about their understanding of and opinions on a variety of issues related to making 510(k) substantial-equivalence decisions. In a number of cases, reviewers selected the correct answer only 50–60% of the time, and managers did not do much better (FDA, 2010a).
Problems surrounding quality assurance in the 510(k) process are not new. The first public guidance to aid reviewers and industry in the 510(k) decision-making process was not issued until the program had been operating for a decade (FDA, 1986).141 In 1988, GAO found a lack of a clear officewide policy and a lack of coordination among review divisions, and it recommended steps to improve consistency in 510(k) decision-making and documentation (GAO, 1988). The Department of Health and Human Services inspector general (IG) in 1990 determined that CDRH lacked a comprehensive quality-control program to evaluate and critique the adequacy of the 510(k) review process independently.142 Three years later, the inspector general issued a follow-up report that concluded that CDRH had focused its quality efforts primarily on the administrative aspects of the 510(k) process, not on the scientific validity of the review decisions.143
CDRH has long required device manufacturers to operate a quality system.144 In an oversimplified description, the regulations prescribe a continuous-improvement process in which specifications and operating procedures are established, persons who have appropriate backgrounds
___________________
141Memorandum Re: Medical Device Hearing, May 4, 1987, Medical Devices and Drug Issues: Hearing Before the Subcomm. on Health and the Env’t of the H. Comm. on Energy and Commerce, 100th Cong. 336-47 (1987) (referred to in the statement of Rep. Henry A. Waxman, Chairman, H. Subcomm. on Health and the Env’t).
142Memorandum Re: Internal Control Weaknesses in the Food and Drug Administration’s Medical Device 510(k) Review Process, from the HHS inspector general to the HHS assistant secretary for health (July 5, 1990), 2.
143Memorandum from the principal deputy inspector general to the acting assistant secretary for health, Re: Follow-Up Review on Internal Control Weaknesses in the Food and Drug Administration’s Medical Device 510(k) Review Process (Feb. 26. 1993), 19.
14421 CFR pt. 820.
are trained in the procedures, execution of the procedures is properly documented, and the resulting output is monitored for conformity to the specifications. When a deviation occurs, the manufacturer is to undertake a root-cause analysis and then implement a corrective-action and preventive-action plan to address the root problem and prevent its recurrence (for example, by revising operating procedures, retraining employees, or revising specifications). The continuous-improvement process is overseen by a quality manager or group and executive management of the company. Those basic principles are as applicable to the making of 510(k) clearance decisions—and any other regulatory decisions (such as PMA application approvals and the use of postmarketing tools to address emerging safety concerns)—as to the production of medical devices.
The absence of a quality system for the 510(k) process since its inception has important consequences for the future of the process. Prior 510(k) clearance decisions are by law binding on the FDA unless a predicate product is removed from the market by the FDA or declared adulterated or misbranded by a federal court.145 The agency is concerned that its authority to rescind prior decisions may be subject to challenge (Shuren, 2011).
About 120,000 510(k) submissions have been cleared over the past 35 years (Tillman, 2010). As described in Chapter 2, those actions have by and large built on a chain of devices that link a new postamendment device to earlier postamendment devices that ultimately could be traced back to a preamendment device from 1976. CDRH has never had an effective quality-assurance system in the 510(k) process. In addition, at least in the early years of implementation, the FDA may have biased the review process in favor of finding substantial equivalence to avoid the administrative consequences of placing too many devices in Class III (OTA, 1984). Today, CDRH cannot reconstruct the “piggy-backing” of devices without a manual review of perhaps thousands of files. Even if a computerized database allowed easy access to the history, the agency would have to review every decision manually to identify questionable ones. The cost of the exercise would be staggering; the benefit would be, it is hoped, small in terms of identifying devices that should not have gotten to the market by a 510(k) clearance. However, it cannot be assumed that the number is zero.
Finding 3-5 The committee agrees with the CDRH 510(k) Working Group that the Center for Devices and Radiological Health does not have “an adequate mechanism to regularly assess the quality, consistency, and effectiveness of the 510(k) program.”
___________________
145FFDCA § 513(i)(2), 21 USC § 360c(i)(2) (2006).
• Finding 3-1 The Food and Drug Administration has a wide array of tools to address safety risks that are discovered to be posed by marketed devices.
• Finding 3-2 The Food and Drug Administration has not used the tools at its disposal extensively. The Center for Devices and Radiological Health has suggested that there are important limitations in their use. The committee identified some procedural burdens on the exercise of these tools, but these burdens do not in themselves explain the historical and continuing sparse use of the tools.
• Finding 3-3 The 510(k) clearance pathway is generally more economical, faster, and less burdensome to industry and the Food and Drug Administration than the premarket approval application route and has substantially fewer postmarketing controls.
• Finding 3-4 The Center for Devices and Radiological Health faces persistent challenges because of a lack of or limitations on human, fiscal, and technologic resources and capabilities.
• Finding 3-5 The committee agrees with the CDRH 510(k) Working Group that the Center for Devices and Radiological Health does not have “an adequate mechanism to regularly assess the quality, consistency, and effectiveness of the 510(k) program.”
Desjardins, P. R. 2010. FDA response to information inquiry from the committee on the public health effectiveness of the 510(k) clearance process. Silver Spring, MD, 09/17/2010.
_______. 2011. FDA response to information inquiry from the committee on the public health effectiveness of the 510(k) clearance process. Silver Spring, MD, 01/07/2011.
Evans, B. 2009. Seven pillars of a new evidentiary paradigm: The Food, Drug, and Cosmetic Act enters the genomic era. Notre Dame Law Review 85(2):419-524.
FDA (Food and Drug Administration). 1986. Guidance on the CDRH Premarket Notification Review Program (K86-3) (June 30, 1986). http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm081383.htm (accessed 02/16/2011).
_______. 2005. Guidance for industry and FDA staff—format for traditional and abbreviated 510(k)s. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm084396.pdf (accessed 03/14/2011).
_______. 2007a. FDA strategic action plan. http://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/StrategicActionPlan/default.htm (accessed 12/3/2010).
_______. 2007b. Premarket notification [510(k)] submissions for medical devices that include antimicrobial agents. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071380.htm (accessed 05/31/2011).
_______. 2009. Third party review. http://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/thirdparyreview/default.htm (accessed 02/16/2011).
_______. 2010a. CDRH preliminary internal evaluations—Volume I: 510(k) working group preliminary report and recommendations. Silver Spring, MD: Food and Drug Administration.
_______. 2010b. CDRH preliminary internal evaluations—Volume II: Task force on the utilization of science in regulatory decision making: preliminary report and recommendations. Silver Spring, MD: Food and Drug Administration.
_______. 2010c. Class II special controls guidance document: Tissue adhesive with adjunct wound closure device intended for the topical approximation of skin. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm233027.htm (accessed 05/24/2011).
_______. 2010d. General considerations for animal studies for cardiovascular devices. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm220760.htm (accessed 05/24/2011).
_______. 2010e. Postmarket drug safety information for patients and providers. http://www.fda.gov/cder/drugSafety.htm (accessed 12/6/2009).
_______. 2011a. 510(k) and science report recommendations: Summary and overview of comments and next steps. Silver Spring, MD: Food and Drug Administration.
_______. 2011b. FDA-track CDRH office of surveillance and biometrics (OSB) dashboard. http://www.fda.gov/AboutFDA/Transparency/track/ucm203271.htm (accessed 02/03/2011).
_______. 2011c. FDA-track CDRH premarket dashboard. http://www.fda.gov/AboutFDA/Transparency/track/ucm201070.htm (accessed 02/15/2011).
_______. 2011d. Important information on the medical device user fee rates for FY2011. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/MedicalDeviceUserFeeandModernizationActMDUFMA/ucm109179.htm (accessed 01/12/2011).
GAO (Government Accountability Office). 1988. Medical devices: FDA’s 510(k) operations could be improved. Report to the chairman, Subcommittee on Health and the Environment, Committee on Energy and Commerce, House of Representatives (PEMD-88-14). Washington, DC: General Accounting Office.
_______. 1989. FDA resources: Comprehensive assessment of staffing, facilities, and equipment needed (HRD-89-142). Washington, DC: General Accounting Office.
_______. 2002. Food and drug administration: Effect of user fees on drug approval times, withdrawals, and other agency activities (GAO-02-958). Washington, DC: General Accounting Office.
_______. 2009a. FDA’s medical product resources (GAO-09-581). Washington, DC: Government Accountability Office.
_______. 2009b. FDA faces challenges meeting its growing medical product responsibilities and should develop complete estimates of its resource needs (GAO-09-581). Washington, DC: Government Accountability Office.
_______. 2009c. FDA should take steps to ensure that high-risk device types are approved through the most stringent premarket review process (GAO-09-190). Washington, DC: Government Accountability Office.
_______. 2009d. Information technology: FDA needs to establish key plans and processes for guiding systems modernization efforts (GAO-09-523). Washington, DC: Government Accountability Office.
_______. 2011. Testimony before the Special Committee on Aging, U.S. Senate. Medical devices: FDA’s premarket review and postmarket safety efforts (GAO-11-556T). 04/13/2011.
Hutt, P. B., R. A. Merrill, and L. A. Grossman. 2007. Food and drug law: Cases and materials. Third edition. New York: Foundation Press.
IOM (Institute of Medicine). 2007. The future of drug safety: Promoting and protecting the health of the public. Washington, DC: The National Academies Press.
_______. 2010. Public health effectiveness of the FDA 510(k) clearance process: Balancing patient safety and innovation. Washington, DC: The National Academies Press.
_______. 2011. Public health effectiveness of the FDA 510(k) clearance process: Measuring postmarket performance and other select topics. Washington. DC: The National Academies Press.
Kahan, J. S. 2009. Medical device development: Regulation and law, Needham, MA: Parexel International.
OTA (Office of Technology Assessment). 1984. Federal policies and the medical devices industry. Washington, DC: Congress of the United States, Office of Technology Assessment.
Shuren, J. 2011. CDRH summary of public comments; issues for committee to consider. Silver Spring, MD, 01/21/2011.
Tillman, D. B. 2010. Understanding the premarket notification (510(k)) process. Presentation given at Public Health Effectiveness of the FDA 510(k) Clearance Process Committee, Meeting 1, March 1, 2010, Washington, DC.
von Eschenbach, A. C. 2007. Third party review of medical device premarket notifications. Report to the Committee on Energy and Commerce, U.S. House of Representatives, and the Committee on Health, Education, Labor, and Pensions, U.S. Senate. Washington, DC.












































