3
Case Study of a Mitigation-Selection Decision
This chapter describes a detailed case study of the use of the risk-characterization framework to evaluate a hypothetical decision on whether to withdraw a vaccine from the market and provides information on the human-health consequences of two mitigation options that could be used as input in a comprehensive decision-making process. The case study described was selected because it involves a mitigation-selection decision, it is related to several decision scenarios provided to the committee by the Food and Drug Administration (FDA), and several committee members had knowledge of the case history. The data used were gleaned from publicly available Web sites or publications or provided by FDA. The committee did not conduct exhaustive literature searches or reviews, and all information is illustrative. It emphasizes that the case study simply provides an illustration of how the committee’s framework might be used for a mitigation-selection decision.
FRAMING THE ISSUE: VACCINE WITHDRAWAL
Before vaccine availability, the burden of rotavirus infection in the United States was estimated to be 3.5 million casesper year in children less than 5 years old (Murphy et al. 2001). Rotavirus caused gastroenteritis in nearly all infected children and serious complications in a small fraction of that number. Using several previously published studies, Glass et al. (1996) estimated that the annual number of hospitalizations for rotavirus diarrhea in the United States ranged from 23,000 to 110,000; the best projected estimate was 55,000 hospitalizations per year. They estimated the number of deaths from rotavirus diarrhea in the United States at 20-40 per year. It was also projected that physician visits were less frequent than cases of diarrhea.
In three randomized prelicensure trials, the first available tetravalent rhesus-human reassortant rotavirus vaccine, RRV-TV (RotaShield, Wyeth Lederle Vaccines, Philadelphia), was shown to be highly effective (80-100%) for the prevention of severe rotavirus gastroenteritis in infants (Rennels et al. 1996; Perez-Schael et al. 1997; Santosham et al. 1997; Joensuu et al. 1998).1 In the conduct of 27 prelicensure trials of RRV-TV, five cases of intussusception—a rare form of bowel obstruction in which a portion of the bowel prolapses into a more distal portion—were reported in 10,054 infants who received the vaccine compared with only one case in 4,633 recipients of placebo; the rates were not statistically significantly different (p > 0.45) (Rennels et al. 1998). After much deliberation about the potential risk of intussusception after RRV-TV, FDA approved RRV-TV on August 31, 1998, for administration at the ages of 2, 4, and 6 months. Intussusception was listed as a possible adverse reaction in the manufacturer's product insert and in the published recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics (AAP 1998; CDC 1999a).
for future vaccine development and evaluation in developed and developing countries, but that discussion is outside the scope of this exercise.
DECISION CONTEXT FOR THE CASE STUDY
The committee chose this real-world case to illustrate the use of the risk attributes defined in this report to organize, evaluate, and compare the public-health consequences associated with a mitigation-selection decision similar to others that FDA often faces. To complete the case study, the committee attempted to look at the situation and the decision options as they were understood in late summer to early fall 1999. Although Wyeth withdrew the vaccine from the market at that point, the committee assumed for purposes of the case study that FDA was in a position in which it had to make a decision about vaccine withdrawal.
The committee considered two options: leave the vaccine on the market or withdraw it from the market. Two types of health consequences are particularly relevant for this comparison. First, adverse effects after vaccination would affect all infants who were vaccinated and would be relevant only if it were decided to leave the vaccine on the market. Second, although the likelihood of contracting rotavirus disease is much lower with vaccination, adverse effects from rotavirus disease would be relevant for both options. Both types of effects would have to be considered for FDA to make a fully informed decision between the two options, so both are included as key parts of the risk characterization.
CHARACTERIZING THE PUBLIC-HEALTH CONSEQUENCES
The committee used data that were available in late 1999 (the hypothesized time of this decision) to develop estimates of the human-health consequences of the two options. Although data were available, as indicated in the detailed development of the estimates below, characterizing the public-health consequences of the two decision options required the committee to make informed judgments, some assumptions, and a variety of calculations. For example, estimates of the number or percentage of children who would receive the vaccine were required. Although substantial data on vaccination rates in the United States are available from CDC, the committee had to make judgments about the relevance of various vaccinations rates to the rate of RRV-TV vaccination. Furthermore, developing estimates of the number of children who would suffer adverse health effects of vaccination required combining estimates of the number of children vaccinated and the adverse-effect rate for the vaccine, both of which are uncertain.
Table 3-1 summarizes the results of the risk characterization. The bases of the estimates, including assumptions and intermediate calculations, are discussed below.
TABLE 3-1 Risk Attributes for Mitigation-Selection Decision for Biologics
|
Attribute |
Metric |
Decision option |
|
|
RRV-TV remains on market |
RRV-TV is removed from market |
||
|
Exposed population |
Number vaccinated annually |
3 million (1.5-3.8 million) |
0 |
|
|
Number who are susceptible to rotavirus disease annually |
6.5 million (2.9-13.2 million) |
20 million |
|
|
Populations of concern |
Infants are vaccinated at 2, 4, and 6 months old; susceptible population for rotavirus disease in the unvaccinated population is the same as if vaccine were removed from market |
All children under 5 years old are at risk for rotavirus disease; children under 2 years old are most at risk (~75% of cases) and experience more severe effects |
|
Mortality |
Number of deaths per year |
15 (6.5-24) total: 9.8 (2.9-21) from rotavirus, 5.5 (0-10) from vaccine-induced intussusception |
30 (20-40) from severe rotavirus disease |
|
Morbidity |
Number experiencing severe adverse health effects per year |
18,300 (5,800-44,200) total: 17,900 (5,500-43,900) from rotavirus, 420 (0-620) from vaccine-induced intussusception |
55,000 (23,000-110,000) |
|
|
Number experiencing less severe adverse health effects per year |
162,500 (52,800-348,000) from rotavirus |
500,000 (365,000-672,000) |
|
Attribute |
Metric |
Decision option |
|
|
RRV-TV remains on market |
RRV-TV is removed from market |
||
|
|
Number per year experiencing adverse health effects that affect only quality of life |
1 million (375,000 to 2.05 million) total: 960,000 (324,000 to 2 million) from rotavirus, 42,000 (0-60,000) from post-vaccine fever or diarrhea |
2.9 million (2.2-3.5 million) |
|
Personal controllability |
For vaccine-induced adverse effects |
Parents of 80-90% of vaccine-eligible children have the ability to avoid or reduce the risks to their child |
Not applicable |
|
|
For rotavirus disease |
Not controllable |
Not controllable |
|
Ability to detect adverse health effects |
Ability of informed institution to detect population-level effects associated with product being evaluated |
Adverse effects would have to occur at a rate at least 5 times higher than normally expected to be detected; very-long-latency adverse effects are unlikely to be detected |
Not applicable |
|
Ability to mitigate adverse health effects |
Probability that an informed institution will be able to reduce or mitigate adverse health effects associated with the product being evaluated if such a problem is known to exist |
For RRV-TV induced effects: >99% |
Not applicable |
Exposed Population
By the age of 5 years, almost everyone has been infected with rotavirus, and if people contract it after the age of 5 years, they generally do not suffer the complications of severe dehydration that are much more common in younger children. All children under 5 years old are considered to be at risk for symptomatic rotavirus infection and are identified as part of the exposed population for this case study. The susceptible population was estimated to be 20 million (assuming a birth cohort of 4 million each year) if RRV-TV were removed from the market. Longitudinal studies and placebo-controlled field trials of rotavirus vaccines that used active surveillance methods, including home visits twice each week, indicated that the incidence of rotavirus diarrhea reached 0.30 episodes per child per year during the first 2 years of life with a cumulative incidence approaching 0.80 episode per child by the age of 5 years (Glass et al. 1996). Those estimates suggest that about 75% of all rotavirus cases each year occur in children under 2 years old. Older children and adults can be reinfected with rotavirus, but the clinical symptoms are generally much less severe because previous infections confer some degree of immunity.
With the vaccine on the market, two populations were of interest: those exposed to the risks of adverse effects after RRV-TV vaccination (all infants who received the vaccine) and those exposed to the risks of rotavirus disease (all children under 5 years old who did not receive the RRV-TV and those who did receive the vaccine but who remained susceptible). Estimating the size of the two populations required estimates of vaccination rates and the vaccine effectiveness.
Because RRV-TV was not on the market for very long before it was withdrawn, the committee did not think it was appropriate to use the vaccination rates associated with it to calculate the vaccination rates that would have been accomplished by a fully mature program. Instead, data on overall vaccination rates in the United States for vaccines available at that time were reviewed. Using publicly available data from CDC, the committee examined the national average rates of vaccination for vaccines that were recommended in 1998 for children up to 24 months old. The rates ranged from 38% for varicella vaccination to 95% for three or more doses of DTP vaccine (CDC 2001). The committee concluded that the ultimate acceptance and adoption of RRV-TV would probably be less than those of DTP vaccines and decided to use the 95% vaccination rate as an estimate of the highest rate of vaccination with RRV-TV, representing about the 95th percentile of the distribution. Varicella vaccination rate was used as the estimate of the lowest rate of vaccination with RRV-TV, representing the 5th percentile of the distribution.
The committee notes that there are two possible reasons for the varicella vaccination rate to have been so much lower than the DTP rate, each of which is relevant for RRV-TV. First, the vaccine was approved and recommended for use in the United States in 1995; by 1998, it would have been offered to all children under 2 years old but would still be relatively novel in the market. Second,
varicella is a common disease that is viewed as manageable by parents, so they may be less inclined to vaccinate. As a median estimate of the rate of RRV-TV use, the committee used 75% on the basis of the rates of having received a full complement of recommended vaccines in 24-month-olds in 1998. As noted above, estimating the rates required informed judgments to be made about the relevant data and their applicability to the future vaccination rates with RRV-TV.
Vaccination rate and vaccine effectiveness, with the sizes of the birth cohort and the exposed population, are sufficient to develop estimates of the exposed populations of interest: those exposed to the risk of vaccine-associated side effects and those exposed to the risks of rotavirus disease. All children who receive RRV-TV will be exposed to the risk of adverse effects associated with the vaccine, including intussusception; the size of the exposed population is simply the birth cohort of 4 million multiplied by the vaccination rate. The population exposed to the risk of adverse effects after vaccination is therefore 3 million, with a range of 1.5 million to 3.8 million.
The children who would be susceptible to rotavirus disease if it were decided to leave the vaccine on the market include those who were not vaccinated and those who received the vaccine but for whom the vaccine is not effective. As stated above, RRV-TV was estimated to be 80-100% effective in eliminating severe rotavirus disease. For the purposes of this case study only, the committee applied the estimates of vaccine effectiveness (80-100% with a median of 90%) to all incidences of rotavirus disease rather than only to that of severe rotavirus disease. That simplification likely underestimates the size of the exposed population with the vaccine on the market and underestimates the number of less severe adverse effects (discussed in the next section). Thus, the number susceptible to rotavirus disease can be estimated from the following equation:
That equation can be used to calculate the number susceptible to rotavirus disease for any given estimates of vaccination rate and vaccine effectiveness. However, using the estimates to derive the 5th and 95th percentiles of the number of susceptible children requires some additional calculations; it is not as simple as using the 5th and 95th percentiles of the estimated vaccination rate and vaccine effectiveness. One approach is to use an event tree to evaluate all the possible combinations of vaccination rate and vaccine effectiveness and then to calculate the probabilities of the resulting estimates of susceptible children to identify which estimates are the 5th, 50th, and 95th percentiles. Figure 3-1 illustrates the event tree; the number of susceptible children under 5 years old is shown on the right side of the figure for every combination of vaccination rate and vaccine effectiveness considered. For example, given a vaccination rate of 38% and vaccine effectiveness of 80%, the number of susceptible children under 5 years old is 13,920,000. Using standard decision-analysis methods to assign a
probability to each branch of the event tree (Keefer and Bodily 1983; Clemen 1996), the committee calculated a probability associated with each outcome and selected the 5th, 50th, and 95th percentiles of that combined distribution (highlighted) to represent the size of the population susceptible to rotavirus disease in the scenario in which RRV-TV is left on the market.
Table 3-1 summarizes the estimates of the number of infants at risk for vaccine-induced adverse effects and those at risk for rotavirus disease for the mitigation-decision options being compared.
Mortality and Morbidity
To estimate mortality and morbidity for the two decision options, the committee developed rough estimates of the following factors on the basis of publicly available data:
-
The number of infants vaccinated each year (see discussion above).
-
The effectiveness of the vaccine in protecting infants from rotavirus infection (see discussion above).
-
The rotavirus infection rate in the unprotected population.
-
The direct effects of rotavirus disease, characterized in terms of the rates of death and severe, less severe, and adverse quality-of-life health effects given rotavirus infection.
-
The rate of adverse effects attributable to RRV-TV.
-
The adverse effects attributable to RRV-TV, characterized in terms of rates of death and severe, less severe, and adverse quality-of-life health effects.
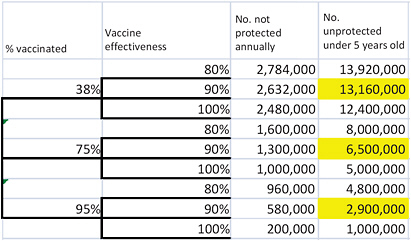
FIGURE 3-1 Estimating the size of the unprotected population given uncertainty in vaccination rates and vaccine effectiveness. Highlighted cells show the 5th, 50th, and 95th percentiles of the combined distribution.
Rotavirus affects most infants in the United States but causes severe gastroenteritis in only a small fraction of those infected. As noted earlier, before the introduction of a vaccine, there were an estimated 3.5 million cases per year in children less than 5 years old in the United States, which led to 500,000 office visits (“less adverse events”), 55,000 hospitalizations (“severe adverse events”), and about 30 deaths (Glass et al. 1996; Fischer et al. 2007)—none of those numbers is known with precision. Table 3-2 summarizes the estimates of the number of rotavirus cases and the morbidity and mortality from rotavirus developed by the committee. The lower half of the table shows incidence rates calculated directly from the median estimates in the upper half of the table; footnotes to the table document the basis of the estimates. The incidence rates were used to calculate the numbers of deaths and other adverse health effects in various vaccination scenarios.
The committee recognized that the risk of serious rotavirus disease was much higher in some countries other than the United States (Dennehy 2008). However, because the focus of this study is on U.S. users of FDA-regulated products, the committee relied on and presents only estimates for the U.S. population, although it understands that other public-health agencies may be interested in health consequences that are outside the scope of FDA’s authority to consider.
RRV-TV was administered to nearly 7,000 infants in placebo-controlled studies and to 4,740 infants in three non-placebo-controlled trials before licensure (CDC 1999a). In those studies, 2% of all vaccine recipients experienced fever greater than 102.2ºF compared with 1% of all placebo recipients. In the placebo-controlled trials, investigators found no overall difference in the rate of diarrhea; but in studies in Finland, vaccinated children had a significantly higher rate of diarrhea after the first dose of vaccine than did placebo recipients (2.8% vs 1.4%; p < 0.05) (CDC 1999a). Because the adverse effects associated with vaccine administration were well tolerated (Joensuu et al. 1997) and generally did not require additional physician or emergency-department visits, the committee characterized them as adverse health consequences that affect only quality of life, similar to those cases of symptomatic rotavirus disease that do not require medical intervention. A 1.4% rate of postvaccine adverse effects was used as the median estimate of the rate of adverse effects affecting only quality of life, with a range of 0-2%.
At the time of the decision concerning withdrawal of the vaccine from the market, there was substantial uncertainty about the quantitative relationship between RRV-TV and increased rates of intussusception. A large case-control study that included 19 states where 80% of the RRV-TV vaccine was administered confirmed the association and was published in the New England Journal of Medicine in February 2001. The report concluded that there was an increased risk of intussusception 3-14 days after both the first and second doses of RRV-TV but that the risk after the second dose was less than that after the first dose.
TABLE 3-2 Estimated Numbers and Rates of Deaths and Illnesses from Rotavirus Disease in the Absence of Vaccination
|
|
Low Estimate (5th percentile) |
Median Estimate |
High Estimate (95th percentile) |
|
Number of children |
|||
|
Susceptible populationa |
|
20,000,000 |
|
|
Total cases |
2,800,000c |
3,500,000b |
4,000,000d |
|
Deathsb |
20 |
30 |
40 |
|
Severe adverse health effectsb |
23,000 |
55,000 |
110,000 |
|
Less severe adverse health effects |
365,000e |
500,000b |
672,000e |
|
Adverse health effects affecting only quality of lifef |
|
2,944,970 |
|
|
Rates among susceptible children |
|||
|
Deaths |
0.000001 |
0.0000015 |
0.000002 |
|
Severe adverse health effects |
0.00115 |
0.00275 |
0.0055 |
|
Less severe adverse health effects |
0.018 |
0.025 |
0.034 |
|
Adverse health effects affecting only quality of life |
|
0.147 |
|
|
aUncertainty in the number of children under 5 years old is insignificant relative to other uncertainties, so only a best estimate is used. bBased on Glass et al. (1996). cBased on Tucker et al. (1998), with an estimate of a 70% cumulative incidence by the age of 5 years. dAssumes that all children under 5 years old get rotavirus disease; some cases are sufficiently mild that they might not be recognized as rotavirus disease. eBased on assumptions described in Tucker et al. (1998). Low estimate based on assumption that 10% of all physician, clinic, and emergency-room visits for diarrhea are due to rotavirus; high estimate based on assumption that 25% of physician and clinic visits and 30% of emergency-room visits for diarrhea are due to rotavirus. fCalculated value: all cases that do not lead to the more serious effects are assumed to have an adverse effect only on quality of life. 5th and 95th percentiles, not shown in this table, are calculated by using the event-tree approach described above and assuming independence between the rates for more serious effects. |
|||
With an assumption of full implementation of a national program of vaccination with RRV-TV, it was estimated that one case of intussusception attributable to the vaccine would occur in every 4,670-9,474 infants vaccinated (Murphy et al. 2001). The committee chose the midpoint of that range as the estimate of the median value, that is, about 1 in 7,000. It would have been reasonable to consider the possibility that there was no RRV-TV-attributable increase in the rate of intussusceptions because prelicensure studies had not established the association, and the 15 cases reported after RRV-TV vaccination were consistent with what would have been expected from base rates in the population (CDC 1999b). On that basis, the committee included a rate of RRV-TV-attributable intussusception of 0 as the estimate of the 5th percentile and a rate of 1 in 4,670 as the estimate of the 95th percentile.
The final estimates required are those of mortality and morbidity rates associated with intussusception. The mortality rate associated with intussusceptions has recently been estimated at about 1.3% (Cortese et al. 2009), which is consistent with data that would have been available in 1999, and was chosen as the median estimate for this case study. Parashar et al. (2000) used data from 1994-1997 to provide a range of estimates of the incidence of intussusception hospitalization of infants; the estimates provided were 18-56 cases per 100,000 children (1994-1996) and about 2.3 intussusception-caused deaths per million live births (1995-1997). Using those estimates, the committee projected the 5th and 95th percentiles of the distribution on the mortality rate with intussusception to be 0.4% and 1.9%, respectively. For purposes of this case study, the committee assumed that all occurrences of intussusceptions led to hospitalization (severe adverse health effects), and thus the rate of severe adverse effects of RRV-TV was assumed to be the same as the rate of vaccine-induced intussusception.
Estimates of Deaths and Morbidity for Option to Remove RRV-TV from the Market
The health consequences of a decision to withdraw the vaccine from the market can be estimated directly from the number of cases that occurred per year before the vaccine was available and the severity of the disease effects as described above and summarized in Table 3-2.
Estimates of Deaths and Morbidity for Option to Leave RRV-TV on the Market
Estimating the health consequences of a decision to keep the vaccine on the market requires two sets of estimates: one for the effects of rotavirus disease in susceptible people who did not receive vaccine or people who were vaccinated but in whom the vaccine was not effective and the other for the adverse effects associated with the vaccine itself. For purposes of this case study, the committee applied the mortality and morbidity rates for rotavirus disease esti-
mated from the rates in the general population before 1998 to the unprotected population under different vaccination assumptions. That approach is a substantial simplification of what might be expected to occur. For example, widespread use of the vaccine could reduce the overall incidence in the unprotected population simply because prevalence of the circulating virus is reduced. That concept of vaccine protection is called herd immunity and probably would be present in a fully mature rotavirus-vaccination program. Similarly, the vaccine itself could be more effective in reducing the most severe cases of the disease and less effective in reducing the overall incidence. Again, for purposes of this case study, the committee assumed a single estimate of vaccine effectiveness and used it to reduce the size of the susceptible population rather than adjusting the incidence for the degree of severity of health effects in the vaccinated.
To estimate the numbers of deaths and severe adverse health effects attributable to vaccination, the committee used the rates of intussusception after RRV-TV and the mortality rate from intussusception described above and applied them to the size of the vaccinated population. The number of deaths for this decision option was estimated as follows:
(Eq. 3-1a)
(Eq. 3-1b)
(Eq. 3-1c)
where
Nf = total number of deaths, Rf = deaths from rotavirus disease, and If = deaths from vaccine-induced intussusception,
P<5 and P<1 represent the total population under the ages of 5 years and 1 year, respectively,
VR = vaccination rate,
VE = vaccine effectiveness,
I|V = rate of RRV-TV-induced intussusception, and
MR and MI represent mortality rates from rotavirus disease and intussusception, respectively.
Four of the factors (VR, VE, I|V, and MR) are considered sufficiently uncertain that a range of estimates was developed and are described above. The relationships of the various factors are illustrated graphically in Figure 3-2.
Table 3-3 uses Equations 3-1(a-c) and median estimates for each of the factors documented above to illustrate a sample calculation of the number of deaths from RRV-TV-induced intussusception and from rotavirus disease with a decision to leave the vaccine on the market. Because the vaccination rate affects the number of deaths from rotavirus disease and the number of deaths from vaccine-induced intussusception in opposite directions (that is, the more children who are vaccinated, the fewer the deaths from rotavirus disease and the more deaths from vaccine-induced intussusception), estimating the range for the total
number of deaths (or other adverse health effects) is not as simple as adding the low (5th percentile) or high (95th percentile) estimates of the number from each cause; doing so would overestimate the range. The committee used the decision-analysis approach described previously to propagate uncertainties in each factor and derive the final calculated value of the total number of deaths. The 5th, 50th, and 95th percentiles of that distribution are summarized in Table 3-1.
Simply substituting the rates of severe adverse health effects and less severe adverse health effects for the mortality rates in Equations 3-1b and 3-1c yields estimates of the total number of children who would experience those effects if the vaccine were retained (see example calculation in Table 3-3). Those estimates are also summarized in Table 3-1.
Personal Controllability
The primary way in which people can eliminate or reduce the RRV-TV-induced risks to their children is by declining to vaccinate, which would eliminate the risk of vaccine-induced illnesses. Controlling or reducing risks after vaccination is more difficult. Vaccine information sheets are provided to all parents before children are vaccinated and should make them aware of potential risks, normal side effects, and what adverse side effects warrant medical attention. Although those factors suggest that the personal controllability of vaccine-induced risks is high in theory, it can be argued that vaccinations are not perceived as being voluntary. For some vaccines, refusal to vaccinate may carry important consequences that make it impractical not to do so, such as the inability to attend day care or public school or to enlist in the armed forces.
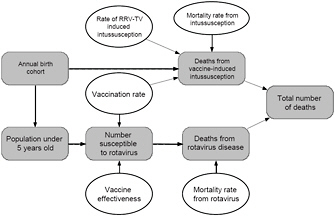
FIGURE 3-2 Relationship among various factors used to estimate the number of deaths from rotavirus disease and adverse effects of vaccination for the hypothetical decision to leave RRV-TV on the market. Quantities for which estimates of ranges of values were available are shown with ovals, and calculated values or quantities for which only a single estimate was available are shown as rounded rectangles.
TABLE 3-3 Example Calculation of Estimated Number of Deaths and Illnesses from Rotavirus Disease and RRV-TV-Induced Intussusceptions for Option to Leave the Vaccine on Market (Based on Median Estimates of all Factors)
|
Exposure estimates |
|
|
No. of children under age of 5 years |
20,000,000 |
|
Size of annual birth cohort |
4,000,000 |
|
Vaccine effectiveness |
90% |
|
Vaccination rate |
75% |
|
No. vaccinated per year |
3,000,000 |
|
No. of susceptible children |
6,500,000 |
|
Rotavirus infection and rate of adverse effects in the susceptible populationa |
|
|
Rotavirus infection rate |
17.5% |
|
Mortality rate |
0.00015% |
|
Severe-disease rate |
0.28% |
|
Less-severe-disease rate |
2.5% |
|
Rate of only quality-of-life effects |
14.7% |
|
No. of deaths |
9.8 |
|
No. of severe adverse effects |
17,875 |
|
No. of less severe adverse effects |
162,500 |
|
No. of only quality-of-life effects |
957,115 |
|
Adverse health effects from RRV-TV vaccination |
|
|
Vaccine-induced intussusception rate |
0.014% |
|
Mortality rate of intussusception |
1.3% |
|
Rate of severe adverse effects of intussusceptionb |
98.7% |
|
Rate of only quality-of-life effects of vaccination (postvaccination fever and diarrhea) |
1.4% |
|
No. of deaths from RRV-TV attributable to intussusception |
5.5 |
|
No. experiencing severe adverse health effects from RRV-TV-induced intussusception |
423 |
|
No. experiencing only quality-of-life effects (from vaccination side effects) |
42,000 |
|
Totals |
|
|
No. of deaths |
15 |
|
No. of severe adverse health effects (hospitalizations) |
18,298 |
|
No. of less severe adverse health effects (doctor visits) |
162,500 |
|
No. of only quality-of-life effects |
999,115 |
|
aRates are calculated by dividing the median estimate of the number of cases of each type of health effect by the size of the susceptible population of 20 million; rates shown in the table are rounded to two or three significant figures, but the actual rates are used in the calculations. Differences in the number of effects shown in this table from the results of hand calculations using the rates shown in this table are due to rounding of the rates in the table. bAll incidents of intussusception that do not lead to death result in a “severe adverse health effect.” |
|
The committee considered the varicella vaccine to be a reasonable analogue to the rotavirus vaccine, for reasons discussed above. When the varicella vaccine was first recommended in 1997, only about 26% of children received the vaccine. Immunization rates have increased substantially, but the relatively low early rates likely reflect a reluctance of physicians to recommend the vaccine soon after the universal recommendations and the choice of parents to decline the vaccine at that time. Of the 26% of parents who chose to have their children vaccinated, however, it is reasonable to assume that most considered the benefits of the vaccine to be worth the known risks posed by vaccination rather than feeling that they had no choice in the matter. Although the vast majority of parents choose to have their children vaccinated with recommended vaccines, the committee estimates that 80-90% of parents understand that they have the right to decline vaccinations and to control the RRV-TV-induced risks if they so desire.
In the absence of the vaccine, the ability of parents to prevent their child from contracting rotavirus disease is virtually zero. Rotavirus affects almost all children at least once during the first 5 years of life in developed and developing countries alike (Bernstein 2009).
Ability to Detect Adverse Health Effects
Some adverse effects after vaccination are expected; side effects that were identified during the testing and licensing process would be expected to occur at about the same rate in the vaccinated population. Of interest here are adverse effects that are not expected or that occur at a much higher rate than expected; such effects are an indication of a problem with the vaccine that could be of concern. In the case of vaccines, reporting systems are in place to track adverse effects after vaccination on a regular basis, such as VAERS and the CDC Vaccine Safety Datalink (VSD). In the near future, the Post-licensure Rapid Immunization Safety Monitoring (PRISM) system, which is part of FDA’s Sentinel Initiative, will also contribute information. Those systems are intended to identify or refine signals of adverse effects that occur with increased frequency after vaccination.
In the case of RRV-TV, VAERS reporting and the fact that vaccine-induced intussusceptions had been identified as a potential issue led to rapid identification of the signal, although reports of intussusceptions were about equal to the expected size of the effect. It is widely recognized, however, that passive surveillance systems, such as VAERS, have issues with under-reporting. For example, one study (Braun 2006) estimated that the proportion of adverse events reported to VAERS after vaccination was 4% for one vaccine and 68% for another; the findings demonstrated that under-reporting can vary greatly depending on the type of adverse event and other factors. So the number of intussusceptions reports after RRV-TV vaccination was interpreted as implying a higher rate in practice. Additional vaccine-safety systems, such as the CDC
VSD, provided data to establish more definitive assessments of the risk (Kramarz et al. 2001). More advanced vaccine-safety monitoring programs are in place now2 than in 1998, when RRV-TV was licensed; however, the ability to detect near-term adverse effects that were occurring at rates higher than expected was high even then. Using the metrics proposed in Chapter 2 and considering the many difficulties associated with interpreting data from passive surveillance (VAERS) (Ellenberg and Chen 1997), the committee estimated that in 1998-1999, the rate of an adverse effect of a vaccine would have to be at least 5 times the expected rate for it to be rapidly detected through the surveillance systems in operation at that time.
For any drug or vaccine, there is the possibility of unexpected adverse effects—effects too rare to be detected in prelicensure testing. If such effects arose with RRV-TV on the market and the effects were serious (ones that led to death or severe adverse health effects) and occurred at a rate substantially higher than would be expected for this population, the reporting systems in place at that time would have identified those effects quickly. The committee estimates that serious unexpected adverse effects from RRV-TV that occurred at a rate at least 5 times higher than normally occurs in the population would be detected. Detecting unexpected vaccine-induced adverse health outcomes that have a long latency, such as adverse effects that occur many years after vaccination, would be much more difficult. The committee estimates that the ability to detect such effects and determine that they are associated with a specific vaccine (RRV-TV) would be virtually zero.
The committee notes that at the time of this hypothetical evaluation, RRV-TV had been in use for less than a year. If it were removed from the market, the only possible adverse effect would be the loss of protection against rotavirus disease provided by the vaccine, and that is captured in the mortality and morbidity estimates for the vaccine-withdrawal option. Thus, there would be no other adverse effects of the absence of the vaccine to be detected, so this attribute does not apply to the decision option to withdraw the vaccine.
Ability to Mitigate Adverse Health Effects
As described in Chapter 2, this attribute refers to the ability of institutions to manage, reduce, or otherwise control adverse effects of the product being evaluated. Mitigation of adverse effects is relevant for effects that are expected and for effects that are not. For example, the routine use of an antipyretic before vaccination reduces the likelihood of fever after vaccination, a common and expected side effect. For adverse effects that occur at rates higher than expected or acceptable, other mitigation steps might be necessary. Because vaccines are
given in the controlled setting of clinics and health-care centers, immediate adverse effects—such as anaphylaxis and hypersensitivity reactions—can be quickly identified and managed. Similarly, vaccine-induced intussusception can be managed and permanent harm almost always avoided. If an unacceptable level of risk after vaccination is identified, administration of the vaccine can be halted almost immediately and the risk to future populations eliminated, providing for high institutional ability to mitigate: the committee estimated that over 99% of the time RRV-TV-induced adverse effects (once detected) could be mitigated.
USING THE RISK CHARACTERIZATION TO SUPPORT DECISION-MAKING
Table 3-1 highlights the differences in public-health consequences that could be expected if the vaccine is withdrawn or left on the market. If the vaccine remained on the market, the number of children who would suffer adverse health effects—ranging from diarrhea to death—from rotavirus and RRV-TV would be substantially less than the number who would suffer similar effects of rotavirus disease if the vaccine were removed from the market. If the vaccine remained on the market, most of the adverse effects would result from rotavirus disease in the unvaccinated population, not from the vaccine. If the vaccine were assumed to be more effective and used more widely, the number of deaths and illnesses from rotavirus would decrease, and those from vaccine-induced effects would increase. However, examining the ranges of effects in Table 3-1 indicates that such changes in assumptions would result in even fewer adverse health effects with the vaccine on the market. The table also indicates that the vaccine-related risks are controllable, and adverse effects from the vaccine are readily detectable and treated. On the basis of this risk characterization alone, it appears that a case could be made to retain the vaccine on the market.
However, as described in Chapter 2, the risk characterization conducted here is designed to capture only the relatively direct public-health consequences associated with different decisions. This information should be relevant and important to FDA decision-making, but many other factors outside the scope of the risk characterization offered here are also relevant and should be considered. For example, the public has a high standard for and expectation of vaccine safety, and that public confidence in the safety of vaccines is important for public health and has implications extending far beyond the case of a single vaccine. A much more detailed analysis could be conducted to evaluate those indirect health consequences. Such an evaluation would require an even greater number of assumptions and estimates, such as whether (and the degree to which) confidence in vaccines would be eroded by the retention of RRV-TV, how erosion of confidence would affect the willingness of parents to vaccinate their children against other diseases, and whether and when those other diseases would increase in prevalence as a result of decreased immunizations.
A similar analysis could be conducted for situations in which the decision options being considered are more complex than the simple retain-withdraw options considered here. For mitigation-selection decisions that FDA would face, the options are likely to be much more nuanced than those described here. For example, mitigation options for any vaccine or drug could include changes in the recommended use or dosage or recommendations for preadministration screening or postadministration monitoring. Comparing more than two mitigation options by using the framework simply requires FDA to define each mitigation option clearly, to characterize the consequences of each by using the attributes, and to follow a logic similar to that illustrated in this case study. An attribute table similar to Table 3-1 would summarize the outcomes of all decisions being considered on a common basis (in terms of the same set of attributes). The decision-making step remains complicated by additional factors, as discussed above, but the use of this framework will enable FDA to compare the public-health consequences of multiple mitigation approaches on a common basis and to describe the consequences with a common language.
In the actual events on which this case study was based, the decision of whether the vaccine remained on the market was made by the manufacturer, not FDA. The manufacturer had its own set of decision criteria that may have included some of the risk-characterization information above but almost certainly included other considerations, including liability and financial issues. Research on the rates of intussusception and the risk associated with RRV-TV continued after 1999. In the interest of completeness, the committee reviewed data that are available now but were not when a decision about withdrawal of RRV-TV had to be made. In particular, later assessments of the risk of RRV-TV-induced intussusception yielded reduced risk estimates of one case in every 10,000-32,000 vaccinations (Murphy et al. 2003a; Murphy et al. 2003b). The mean annual rate of intussusception in the absence of vaccine was recently published in a retrospective analysis involving infants in three children’s hospitals over a 5-year period. The mean annual intussusception rate was found to be 49.3 cases per 100,000 live births (inpatient cases, 27.1 cases per 100,000 live births; short-stay or emergency-department cases, 22.3 cases per 100,000 live births) with a case-fatality of about 1.3% (2 of 156 cases) (Cortese et al. 2009).
REFERENCES
AAP (American Academy of Pediatrics). 1998. Prevention of rotavirus disease: Guidelines for use of rotavirus vaccine. Pediatrics 102(6):1483-1491.
Alpert, J.J. 1999. PedComm: AAP Member Alert, July 15, 1999. American Academy of Pediatrics [online]. Available: http://www.westchestergov.com/Health/PCaaprecomm.htm [accessed Oct. 26, 2010].
Bernstein, D.I. 2009. Rotavirus overview. Pediatr. Infect. Dis. J. 28(Suppl. 3):S50-S53.
Braun, M.M. 2006. Vaccine Adverse Event Reporting System (VAERS): Usefulness and Limitations. Institute for Vaccine Safety, John Hopkins Bloomberg School of
Public Health [online]. Available: http://www.vaccinesafety.edu/VAERS.htm [accessed Mar. 18, 2011].
CDC (U.S. Centers for Disease Control and Prevention). 1999a. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 48 (RR-2):1-23 [online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00056669.htm [accessed Oct. 26, 2010].
CDC (U.S. Centers for Disease Control and Prevention). 1999b. Intussusception among recipients of rotavirus vaccine -United States, 1998-1999. MMWR 48(27):577-581 [online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4827a1.htm [accessed Oct. 27, 2010].
CDC (U.S. Centers for Disease Control and Prevention). 1999c. Withdrawal of rotavirus vaccine recommendation. MMWR 48(43):1007 [online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4843a5.htm [accessed Oct. 27, 2010].
CDC (U.S. Centers for Disease Control and Prevention). 2001. Immunization Coverage in the U.S.: January-December 1998 Table Data [online]. Available: http://www.test.cdc.gov/vaccines/stats-surv/nis/data/tables_1998.htm [accessed Oct. 27, 2010].
CDC (Centers for Disease Control and Prevention). 2009. Prevention of rotavirus gastro-enteritis among infants and children recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 58(RR02):1-25[online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5802a1.htm [accessed Mar. 15, 2011].
Clemen, R.T. 1996. Making Hard Decisions: An Introduction to Decision Analysis. Belmont, CA: Duxbury Press. 664 pp.
Cortese, M.M., M.A. Staat, G.A. Weinberg, K. Edwards, M.A. Rice, P.G. Szilagyi, C.B. Hall, D.C. Payne, and U.D. Parashar. 2009. Underestimates of intussusception rates among U.S. infants based on inpatient discharge data: Implications for monitoring the safety of rotavirus vaccines. J. Infect. Dis. 2000(Suppl. 1):S264-S270.
Dennehy, P.H. 2008. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 21(1):198-208.
Ellenberg, S.S., and R.T. Chen. 1997. The complicated task of monitoring vaccine safety. Public Health Rep. 112(1):10–21.
Fischer, T.K., C. Viboud, U. Parashar, M. Malek, C. Steiner, R. Glass, and L. Simonsen. 2007. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993-2003. J. Infect. Dis. 195(8):1117-1125.
Glass, R.I., P.E. Kilgore, R.C. Holman, S. Jin, J.C. Smith, P.A. Woods, M.J. Clarke, M.S. Ho, and J.R. Gentsch. 1996. The epidemiology of rotavirus diarrhea in the United States: Surveillance and estimates of disease burden. J. Infect. Dis. 174(Suppl. 1):S5-S11.
Joensuu, J., E. Koskenniemi, X.L. Pang, and T. Vesikari. 1997. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet 350(9086):1205-1209.
Joensuu, J., E. Koskenniemi, and T. Vesikari. 1998. Symptoms associated with rhesus-human reassortant rotavirus vaccine in infants. Pediatr. Infect. Dis. J. 17(4):334-340.
Keefer, D.L., and S.E. Bodily. 1983. Three-point approximations for continuous random variables. Manage. Sci. 29(5):595-609.
Kramarz, P., E.K. France, F. Destefano, S.B. Black, H. Shinefield, J.I. Ward, E.J. Chang, R.T. Chen, D. Shatin, J. Hill, T. Lieu, and J.M. Ogren. 2001. Population-based study of rotavirus vaccination and intussusception. Pediatr. Infect. Dis. J. 20(4):410-416.
Lieu, T.A., M. Kulldorff, R.L. Davis, E.M. Lewis, E. Weintraub, K. Yih, R. Yin, J.S. Brown, and R. Platt. 2007. Real-time vaccine safety surveillance for the early detection of adverse events. Med. Care 45(10 Suppl. 2):S89-S95.
Murphy, T.V., P.M. Gargiullo, M.S. Massoudi, D.B. Nelson, A.O. Jumaan, C.A. Okoro, L.R. Zanardi, S. Setia, E. Fair, C.W. LeBaron, B. Schwartz, M. Wharton, and J.R. Livengood. 2001. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344(8):564-572.
Murphy, B.R., D.M. Morens, L. Simonsen, R.M. Chanock, J.R. La Montagne, and A.Z. Kapikian. 2003a. Reappraisal of the association of intussusception with the licensed live rotavirus vaccine challenged initial conclusions. J. Infect. Dis. 187(8):1301-1308.
Murphy, T.V., P.J. Smith, P.M. Gargiullo, and B. Schwartz. 2003b. The first rotavirus vaccine and intussusception: Epidemiological studies and policy decisions. J. Infect. Dis. 187(8):1309-1313.
Parashar, U.D., R.C. Holman, K.C. Cummings, N.W. Staggs, A.T. Curns, C.M. Zimmerman, S.F. Kaufman, J.E. Lewis, D.J. Vugia, K.E. Powell, and R.I. Glass. 2000. Trends in intussusception-associated hospitalizations and deaths among U.S. infants. Pediatrics. 10696:1413-1421.
Perez-Schael, I., M.J. Guntinas, M. Perez, V. Pagone, A.M. Rojas, R. Gonzalez, W. Cunto, Y. Hoshino, and A.Z. Kapikian. 1997. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N. Engl. J. Med. 337(17):1181-1187.
Rennels, M.B., R.I. Glass, P.H. Dennehy, D.I. Bernstein, M.E. Pichichero, E.T. Zito, M.E. Mack, B.L. Davidson, and A.Z. Kapikian. 1996. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines—report of the National Multicenter Trial. Pediatrics 97(1):7-13.
Rennels, M.B., U.D. Parashar, R.C. Holman, C.T. Le, H.G. Chang, and R.I. Glass. 1998. Lack of an apparent association between intussusception and wild or vaccine rotavirus infection. Pediatr. Infect. Dis. J. 17(10):924-925.
Santosham, M., L.H. Moulton, R. Reid, J. Croll, R. Weatherholt, R. Ward, J. Forro, E. Zito, M. Mack, G. Brenneman, and B.L. Davidson. 1997. Efficacy and safety of high-dose rhesus-human reassortant rotavirus vaccine in Native American populations. J. Pediatr. 131(4):632-638.
Tucker, A.W., A.C. Haddix, J.S. Bresee, R.C. Holman, U.D. Parashar, and R.I. Glass. 1998. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 279(17):1371-1376.




















