4
Case Study of a Targeting Decision
This chapter describes a case study that uses the risk-characterization framework to evaluate several food categories and the potential human-health consequences of foodborne illnesses associated with them. The case study was selected because it is an example of the kind of evaluation and analysis that might be used to support a targeting decision, it is relevant to a scenario provided to the committee by the Food and Drug Administration (FDA), and several committee members had relevant expertise. As noted in Chapter 2, targeting decisions are the most closely related to risk-ranking questions that are of interest to some FDA centers. The data used were gleaned from publicly available Web sites or publications or provided by FDA. The committee did not conduct exhaustive literature searches or reviews, and all information is illustrative. The case study simply illustrates how the committee’s framework might be used for a targeting decision.
FRAMING THE ISSUE: FOOD SAFETY
FDA has the responsibility for ensuring the safety of about 80% of the U.S. food supply and regulates most foods and food ingredients, which range from raw commodities to highly processed foods (GAO 2008). The extent of FDA oversight is variable and ranges from relatively little public oversight of some products, such as fresh vegetables, to a highly regulated system for others, such as canned foods. The diversity and number of food products and varied regulations pose challenges to any attempt to characterize or rank food categories on the basis of health consequences. The task is complicated by the rapid adjustments of the global food system, which changes with market access and availability. Several recent reports have identified important gaps and deficiencies in the oversight of foods by the federal government (GAO 2010; IOM/NRC 2010), and a report recently released by the Institute of Medicine (IOM/NRC 2010) described and compared a number of risk-ranking models for foods (see Box 4-1).
|
BOX 4-1 Risk-Ranking Models for Foods Among FDA-regulated products, foods are probably the category that has been the focus of more risk-assessment and ranking studies than any other products. Several government agencies and research groups have developed risk-ranking approaches and models, and the National Research Council (NRC) and the Institute of Medicine (IOM) have published several reports addressing the need for ensuring the safety of foods (IOM/NRC 1998, 2003, 2010). In the latest report, different risk-ranking models developed by researchers and regulators in different countries were contrasted. The “degree of complexity, level of quantification, and approach to model construction” differed among the models (IOM/NRC 2010). However, some of the common criteria identified were “(1) burden of illness…(2) illness severity, (3) population susceptibility, (4) likelihood of contamination, (5) potential for agent amplification, and (6) breadth of exposure” (IOM/NRC 2010, p. 87). The risk-characterization framework proposed here considers all those factors although some are not called out explicitly: (3) and (6) are included in exposed population; (1) and (2) are covered and expanded under mortality and adverse health effects; (4) and (5) must be considered to develop estimates of the number of deaths and other adverse health effects and thus are implicitly included in this framework. However, like the risk-ranking models described in the recent IOM/NRC report, the risk-characterization framework proposed here was designed for a specific purpose, which was to characterize the public-health consequences of various decisions at FDA among all its programs, and therefore does not include program-specific attributes that would not be universally applicable, such as the probability of contamination and amplification in the food supply. |
DECISION CONTEXT FOR THE CASE STUDY
The decision context considered in this case study was one of allocating food-safety inspection resources; that is, if additional inspection resources were available, how should they be allocated among the various food categories to maximize public-health protection? That general decision problem is too large to be undertaken as a case study, so a much simplified decision context and evaluation were selected. Rather than considering all the different options for what types of food could be inspected, this case study considers only three specific food categories: leafy greens, shrimp, and canned foods. The categories were chosen to highlight products that are inherently different with respect to level of processing, origin, and potential risks. Furthermore, rather than identifying all the possible allocations of inspection resources that could potentially be compared, this case focuses only on characterizing the public-health consequences associated with each food category assuming the current regulatory and inspection regime. The results of this evaluation could be used directly for ranking or
comparing the food categories on the basis of risk or could serve as input into decisions for allocating resources among the three food categories to maximize protection of the public from foodborne illnesses. Possible extensions of the analysis that would support resource-allocation decisions more directly are described in the final section of this chapter.
CHARACTERIZING THE PUBLIC-HEALTH CONSEQUENCES
Data on foodborne illnesses are generally available from the Centers for Disease Control and Prevention (CDC), but estimating the number of illnesses caused by the food categories evaluated in this case study required additional data and various assumptions. The committee consulted three primary sources of data—CDC (2010), Scallan et al. (2011a,b), and Hoffmann et al. (2007)—to estimate the number and severity of foodborne illnesses associated with each food category. CDC (2010) provides data on reported outbreaks of foodborne illnesses caused by various food commodities by pathogen for 1998-2007. Data are also reported on foodborne illnesses of unknown etiology. The actual cases of foodborne illness, however, are thought to exceed the number of reported cases associated with outbreaks substantially (Mead et al. 1999; Scallan et al. 2011a), so the reported cases represent only a fraction of the actual cases. Scallan et al. (2011a) provided pathogen-specific estimates of under-reporting and under-diagnosis that range from a factor of 2 to factors over 700.1 Scallan et al. (2011a,b) also provided estimates of the total number of cases of foodborne illness, hospitalizations, and deaths in the United States annually attributed to specific pathogens and those from unspecified agents. However, the data provided by Scallan et al. do not attribute the cases to specific food groups or food types. Hoffmann et al. (2007) described an expert elicitation study in which 42 nationally recognized food-safety experts were asked to provide estimates of the percentage of cases of foodborne illness caused by a specific pathogen that are attributable to 11 food categories. The committee combined the number of cases by pathogen from Scallan et al. (2011a) with the estimated percentage of illnesses from that pathogen attributed to specific food groups from Hoffmann et al. (2007) to estimate the annual number of cases from consumption of each food category. In each of the cases, some additional assumptions were necessary to extrapolate from the food categories of Hoffmann et al. to the more specific, narrower categories evaluated in this case study. CDC outbreak data were examined to support the estimates developed by the committee.
The following discussion provides some background information on the three food categories selected for evaluation and describes the basis of the risk characterization. The attributes and their estimates are summarized in Table 4-1.
TABLE 4-1 Risk Attributes for Targeting Decision for Foods
|
Attribute |
Metric |
Food Category |
||
|
Domestic Leafy Greens |
Shrimp |
Canned food |
||
|
Exposed population |
Number exposed annually (number of U.S. population that consume product) |
294 million (232-304 million) |
263 million (155-279 million) |
308 million (307-310 million) |
|
|
Populations of concern |
Children under 5 years old, the elderly, and the immunosuppressed are more susceptible and suffer more severe effects from foodborne illnesses; pregnant women are of special concern for specific pathogens, such as Listeria. |
||
|
Mortality |
Number of deaths per year |
280 (5-590) total: 60 (5-150) from known pathogens 220 (0-440) from unspecified agents |
56 (0-110) total: 10 (0-19) from known pathogens 46 (0-91) from unspecified agents |
0.2 (0.1-2.8) |
|
Morbidity |
Number experiencing severe adverse health effects per year |
13,900 (280-28,200) total: 4,600 (280-7,900) from known pathogens 9,300 (0-20,300) from unspecified agents |
2,730 (15-6,700) total: 730 (15-1,200) from known pathogens 2,000 (0-5,500) from unspecified agents |
20 (20-400) |
|
|
Number experiencing less severe adverse health effects per year |
165,000 (1,500-266,000) total: 35,000 (1,500-56,000) from known pathogens 130,000 (0-210,000) from unspecified agents |
34,800 (120-54,500) total: 6,800 (120-10,500) from know pathogens 28,000 (0-44,000) from unspecified agents |
110 (110-200) |
|
Attribute |
Metric |
Food Category |
||
|
Domestic Leafy Greens |
Shrimp |
Canned food |
||
|
|
Number per year experiencing adverse health effects that affect only quality of life |
6.3 million (58,000 to 9.9 million) total: 1.3 million (58,000 to 2 million) from known pathogens 5 million (0 to 7.9 million) from unspecified agents |
1.6 million (7,000 to 2.4 million) total: 255,000 (7,000-390,000) from known pathogens 1.3 million (0 to 2 million) from unspecified agents |
4,100 (4,100-84,000) |
|
Personal controllability |
Degree to which a person can eliminate or reduce his or her own risks through voluntary actions |
40-50% of cases of foodborne illnesses from leafy greens could be eliminated or reduced by personal action by the consumer |
10-15% of cases of foodborne illnesses from shrimp could be eliminated or reduced by personal action by the consumer |
45-60% of cases of foodborne illnesses from commercially canned foods could be eliminated or reduced by personal action by the consumer |
|
Ability to detect adverse health effects |
Ability of informed institution to detect population-level effects associated with product being evaluated |
3% or fewer of all cases of foodborne illness caused by leafy greens could be detected and successfully attributed. |
3-5% of all cases of foodborne illness caused by shrimp could be detected and successfully attributed. |
About 50% of cases of botulism from commercial canned foods could be detected; 3% or fewer of all other types of foodborne illness caused by commercially canned foods could be detected and successfully attributed. |
|
Ability to mitigate adverse health effects |
Probability that an informed institution will be able to reduce or mitigate adverse health effects associated with the product being evaluated if such a problem is known to exist |
<10% |
10-50% |
50-75% |
Domestic Leafy Greens
Domestic leafy greens are widely consumed in the United States; although many of the leafy greens are available in frozen or canned form, the fresh product dominates the market. For example, fresh spinach made up 65.5% of total per capita spinach consumption in 2008, an increase from 58.5% in 1990 and from only 17.0% in 1970 (USDA 2010). Today, annual per capita consumption of fresh leafy greens is about 29 lb; romaine lettuce, leaf lettuce, head lettuce, and spinach make up most of the fresh product. However, other, less well-known greens—such as arugula, radicchio, and mizuna—have increased in consumption, often as components in fresh bagged mixed salads.
Most of the fresh greens are grown domestically. In 2006, only 3% of spinach, 2% of head lettuce, and 1% of leaf and romaine lettuce were imported for domestic consumption (Calvin et al. 2009). Monterey, Santa Clara, and San Benito Counties produce more than half the U.S. fresh market for spinach and supply up to 80% of other leafy greens sold in the United States (Calvin et al. 2009). Products are widely and quickly shipped around the country. Although the greens are often shipped directly from the field, they may be mixed and repackaged by processors before being shipped to retail and food-service outlets.
Fresh leafy greens are often consumed raw with little preparation, and the shift toward fresh products has increased the associated food-safety risk because products consumed raw have not been treated with heat or other kill steps. Outbreaks associated with leafy greens were 38.6% more frequent in 1996-2005 than in 1986-1995 (Mandrell 2009). However, there does not seem to be a well-identified cause of outbreaks associated with leafy greens: risk factors are numerous and include the potential for contamination before and after harvest (Mandrell 2009).
Although reported outbreaks associated with leafy greens are relatively rare, there have been some major ones. In 2006, for example, an outbreak of Escherichia coli O157:H7 in spinach resulted in illness in consumers in 26 states; of 204 cases, 31 cases of hemolytic-uremic syndrome (a serious complication of E. coli O157:H7 exposure) and three deaths were reported and attributed to the exposure (Calvin 2007). Mandrell (2009) noted a review by CDC that found that leafy greens were associated with 502 outbreaks, more than 18,000 cases of illness, and 15 deaths from 1973 to 2006.
Exposed Population
Although most people 2 years old and older consume lettuce or fresh leafy greens at some point in a year, the average daily consumption of greens is relatively low, less than 0.1 cup per day (NCI 2010). On the basis of nationally representative data, Tooze et al. (2006) estimated that 48% of men and 57% of women consume dark-green vegetables in a day. Therefore, although the average amount consumed per day may be small, the population exposed over a year
is potentially large. For purposes of this case study, the committee estimated that the number of people who consume leafy greens in a year could range from 75% to 98% of the U.S. population. The committee’s best estimate is that 95% of the U.S. population consumes some leafy greens during the year and thus would be exposed to the potential for foodborne pathogens from this food product. Multiplying those percentages by an assumed U.S. population of 310 million (U.S. Census Bureau 2010) yields the estimates shown in Table 4-1.
Outbreaks associated with leafy greens have typically been attributed to microbiologic contamination (Beuchat 1996). Although some investigations have shown that the incidence of pathogens on greens is relatively low (Mandrell 2009), the infectious dose of some pathogens is also low, so the effect of even low pathogen concentrations on leafy greens can be important. In general, infants and young children, older people, and immunosuppressed populations are more susceptible to foodborne illnesses and are likely to suffer more severe effects if they contract the illnesses. Accordingly, those groups are highlighted as populations of special concern.
Mortality and Morbidity
To estimate the mortality and morbidity attributable to leafy greens, the committee used a variety of data to make two types of estimates:
-
The annual number of cases of foodborne illness caused by leafy greens attributable to specific pathogens or of an unknown etiology. Those estimates were based on data from CDC (2010), Scallan et al. (2011a,b), and Hoffmann et al. (2007).
-
The rates of death and hospitalization for foodborne illness caused by each pathogen and of unknown etiology. Those rates were derived from data in Scallan et al. (2011a,b).
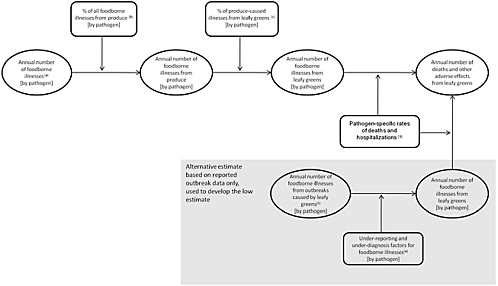
The numbers of cases and the rates of death and hospitalizations were used to calculate the estimated numbers of deaths and other adverse health effects summarized in Table 4-1. Figure 4-1 illustrates the general approach taken by the committee.
Annual Number of Cases of Foodborne Illnesses Associated with Leafy Greens
As noted above, the committee used three primary sources to estimate the annual number of cases of foodborne illness caused by leafy greens. An estimate of the average annual number of reported cases attributed to various pathogens (and of unknown etiology) and caused by consumption of leafy greens was developed directly from the CDC (2010) data. A second estimate of the number of
cases of illness from leafy greens was developed by using data from Scallan et al. (2011a,b) and Hoffmann et al. (2007) as follows. Combining the total number of cases of foodborne illnesses by pathogen from Scallan et al. (2011a,b) with the attribution to food categories from Hoffmann et al. (2007) yielded an estimate of the annual number of cases from consumption of produce by pathogen. The CDC (2010) data were used to estimate the percentage of cases of illness caused by consumption of produce that were attributable to leafy greens by pathogen.
Rates of Foodborne Illnesses from Leafy Greens
From the data in Scallan et al. (2011a,b), hospitalization and death rates were calculated for each pathogen and for foodborne illnesses from unspecified agents. The rates and the “multiplier” for each pathogen that indicates the degree to which illnesses associated with a given pathogen are under-diagnosed and under-reported were used to estimate the number of deaths and adverse health effects of different severity from leafy greens, as described below.
Estimates of the Number of Deaths from and Other Adverse Health Effects of Leafy Greens
There is substantial uncertainty in the estimated total number of foodborne illnesses that occur annually in the United States and in how many of those illnesses are caused by leafy greens and by what pathogens. That uncertainty is reflected in the committee’s approach described below to derive the low, high, and best estimates for the number of foodborne illnesses (by pathogen and from unspecified causes) from consumption of leafy greens.
-
Low Estimate. The CDC (2010) data were used to calculate the reported illnesses for leafy greens. Those estimates were then scaled up with the pathogen-specific multipliers in Scallan et al. (2011a) to yield the pathogen-specific numbers of cases of illness from leafy greens. For the low estimate, no foodborne illnesses from unspecified agents were attributed to leafy greens.
-
Best Estimate. The cases of illness attributable to produce were first calculated by using either the mean or modal estimates of the number of illnesses by pathogen as reported by Scallan et al. (2011a) and either the pathogen-specific or average expert-based attribution calculated from Hoffmann et al. (2007). Thirty percent of all the cases of foodborne illness from unspecified agents reported in Scallan et al. (2011b) were also included; this percentage is based on the overall percentage of total foodborne illnesses attributed to produce in Hoffmann et al. (2007). Next, the cases attributed to leafy greens were calculated by using either the pathogen-specific or the average attributions from all produce-caused illnesses that could be calculated from the CDC (2010) data.
-
High Estimate. Scallan et al. (2011a,b) provided not only the mean or modal estimates of the number of foodborne illnesses but a 90% credible interval for those numbers. The high estimate was calculated analogously to the best estimate except that the high end of the range provided by Scallan et al. was used rather than the mean or modal estimates.
For all three estimates (low, best, and high estimates), the pathogen-specific hospitalization and death rates derived from Scallan et al. (2011a,b) were used to calculate numbers of severe health effects (assumed to be hospitalizations) and deaths. Generic rates derived from Scallan et al. were used for illnesses for which no pathogen-specific rates were available. Illnesses that did not lead to death or to hospitalization were classified as producing less severe adverse effects or effects that were related only to quality of life. Those illnesses were apportioned to the two morbidity categories by using the under-diagnosis and under-reporting factors from Scallan et al. (2011a,b). For example, if Salmonella is assumed to have a combined factor of 29 for under-diagnosis and under-reporting, the committee assumed that there will be about 29 times as many illnesses that affect only quality of life compared with those which will give rise to “less severe adverse effects.”
The committee notes that for the best and high estimates the vast majority of the cases of foodborne illness are from unspecified agents. Of the pathogen-specific illnesses, a large proportion are attributed to Norovirus, as described in Mead et al. (1999) and updated in Scallan et al. (2011a); such illnesses may not typically be thought of as foodborne.
Personal Controllability
People consuming leafy greens at home have several options available to reduce their risks of foodborne illnesses from those greens: primarily by refrigerating foods at proper temperatures; checking for signs of spoilage; thoroughly washing hands, utensils, and the greens before use; and maintaining strict separation of the greens from utensils used to prepare other foods, especially raw meats (FSIS 2006). People consuming leafy greens in restaurants, in contrast, have little ability to reduce their risks of contracting a foodborne illness other than not ordering or consuming foods that contain raw leafy greens. About 27% of all meals consumed in the United States are prepared outside the home (Lin et al. 1998). Lacking any data to the contrary, the committee assumed that leafy greens were just as likely to be consumed at home as away from home, so at least 27% of leafy greens consumed are prepared outside the home, and at least 27% of the cases of foodborne illnesses from leafy greens are not personally controllable by the individual consumer.
The cases remaining arise from home use. Although those cases are theoretically controllable, people must have knowledge that they are potentially at risk and knowledge of the steps that they can take to reduce those risks for them
to have practical controllability over their risks. Reports on consumers’ behavior before, during, and after the 2006 spinach outbreak show confusion about details of the recall announcement (for example, which spinach products were involved) and of the associated symptoms of E. coli O157:H7-related illness (Hallman et al. 2009); this suggests that consumers have imperfect knowledge of how to prevent illnesses from leafy greens. Even during the recall with considerable media attention, only two-thirds of those surveyed reported that bloody diarrhea was a symptom of the infection. Furthermore, there may be some contamination that cannot be removed even if all proper precautions and procedures are followed. Given the uncertainty surrounding consumer knowledge and the ability to remove contamination, the committee estimated that 55-70% of those preparing leafy greens at home have practical control over the risks. Given the estimate that 73% of cases of foodborne illnesses from leafy greens result from home preparation, about 40-50% of the total cases of illnesses from leafy greens are personally controllable.
Ability to Detect Adverse Health Effects
It is difficult in general to detect foodborne illness and to attribute it correctly to a specific cause, especially foodborne illness that occurs sporadically as opposed to illness that is associated with a specific outbreak. Use of surveillance data and advanced genotyping techniques has improved identification of outbreaks and the ability to identify and trace back potentially contaminated products associated with them. However, sporadic cases of foodborne illness occur much more frequently and in far greater numbers than cases associated with outbreaks (Mead et al. 1999).
The types of foodborne illnesses associated with leafy greens—such as those caused by Norovirus, Campylobacter, and Salmonella—are also those estimated to be largely under-reported (Mead et al. 1999); incidents of illnesses are far more likely to occur as individual cases than as concentrated outbreaks, and this compounds the difficulty in detecting those cases. On the basis of the estimated under-reporting, the committee estimates that fewer than 3% of all cases of foodborne illnesses from leafy greens can be detected and successfully attributed to the causal agent.
Ability to Mitigate Adverse Health Effects
In food safety, risk mitigation often refers to prevention or intervention—that is, preventing contamination or treating to ensure that contamination is reduced. In 1998, FDA published voluntary guidelines (Good Agricultural Practices) to help growers reduce the risk of contamination on the farm. Since the 2006 outbreak linked to spinach, the California leafy-greens industry, through the Leafy Greens Marketing Agreement (LGMA), developed best-practice standards to guide production practices and control and monitoring of produce. The
preharvest and harvest handling have been identified as key points where hazards may be introduced through exposure to environmental and production hazards, such as contaminated water, manure, and poor field sanitation. However, postharvest handling, produce mixing in fresh packaging, and distribution are also important. Although FDA and others have developed guidance to reduce health risks, control of product movement and the ability to prevent contamination rest with growers, processors, retailers, food-service industries, and ultimately consumers (as discussed above in connection with personal controllability). As noted, testing of produce has increased throughout the production process (at time of field harvest, during initial processing, and by buyers and distributors) in the private sector, and the effectiveness of the activities is reflected in the overall rates of foodborne illnesses from leafy greens.
In this framework, the ability to mitigate refers to the ability to manage, reduce, or otherwise control any adverse health effects of the products being evaluated, assuming that such effects occur and are detected. Because of the complexity of the food supply and the diversity of risks associated with foods, mitigation of adverse health effects from foodborne illness is challenging. At an institutional level, mitigation efforts could include efforts to prevent additional cases from occurring through recalls of tainted products and efforts to reduce disease symptoms through outreach and treatment education. For leafy greens (and other food products), prevention of additional cases rarely occurs because of the inability to identify the food vehicle. For a highly perishable food product with a short shelf-life, such as leafy greens, even when a problem has been detected most of the product has already been consumed, and this limits further the ability of institutions to mitigate adverse health effects. On the basis of those factors, the committee estimated that the probability that institutions can mitigate adverse effects from foodborne illness caused by consumption of leafy greens through effective recalls or better treatment after a potential problem has been detected is less than 10%.
Shrimp
About 90% of the shrimp consumed in the United States is imported (NOAA 2010). According to the U.S. National Marine Fisheries Service, the United States imported more than 548,000 metric tons of shrimp in 2009 (NOAA 2010). The increase in shrimp importation mirrors a dramatic increase in overall seafood importation. The United States receives 318,000 tons of its imported shrimp from Thailand, Ecuador, and China (NOAA 2010). Other countries that supply shrimp to the United States include Indonesia, Bangladesh, Mexico, Vietnam, and India (FAO 2010). Most of the shrimp imported into the United States are fresh or frozen whole. Recently, there has been a dramatic increase in imported breaded shrimp; however, this product accounted for less than 10% (37,427 metric tons) of shrimp imports in 2009 (NOAA 2010).
Frozen shrimp imported into the United States are directly transported, further processed, or placed into cold storage. Directly transported shrimp products are delivered to restaurants or grocery chains. Further-processed shrimp products are delivered to other restaurants or local grocery chains. Shrimp products can remain in cold storage for long periods. It is said that 80% of imported shrimp is consumed in restaurants and the rest sold in grocery stores (R. Fischer, FDA, personal communication, March 2010).
In 2005-2009, nearly 100,000 lines2 of shrimp were imported into the United States (mostly raw and frozen); 2,030 lines were refused. Major reasons for refusal included evidence of filth (21%), Salmonella (16%), and drug residues (23%). Those refusal data are similar to published data on the 2001-2003 experience (Wan Norhana et al. 2009). Most of the reported outbreaks associated with shrimp for which an infectious agent has been identified have been attributed to Salmonella contamination, and salmonellosis has been linked to shrimp from aquaculture ponds (Koonse et al. 2005). Consequently, the United States has a zero-tolerance policy regarding Salmonella (that is, it requires the absence of Salmonella) for raw or cooked and ready-to-eat shrimp (Koonse et al. 2005; Wan Norhana et al. 2009).
Exposed Population
The per capita U.S. consumption of shrimp increased from 2.4 kg in 1997 to a peak of 4.6 kg in 2006 (The Fish Site 2008). It is estimated that 85% of the U.S. population consumes some shrimp each year (IOM 2007). For purposes of this case study, it is assumed that 50-90% of the U.S. population consumes shrimp; the best estimate is 85%. Combining those estimates with a U.S. population size of 310 million yields the estimates of the exposed population shown in Table 4-1.
As described above in the discussion of leafy greens, infants and young children, older people, and immunosuppressed populations are more susceptible to foodborne illnesses generally and are more likely to suffer more severe effects if they do contract the illnesses. Accordingly, those groups are highlighted as populations of special concern.
Mortality and Morbidity
According to outbreak reports to CDC, 62 outbreaks of illness were linked to shrimp in 2001-2005, and they were associated with 618 cases of illness (see
Table 4-2). Cases of illness in 2001-2005 ranged from 25 to 184 per year (Tsutumi 2007). Shrimp outbreaks can be confounded because shrimp are often mixed with various other food ingredients, and this complicates investigations that are trying to identify outbreak sources. Most outbreaks cannot be linked to an identified pathogen or agent, as shown in Table 4-2; the data in the table reflect only reported outbreaks and cases.
Mortality and morbidity attributable to shrimp were calculated by using the methods described above for leafy greens and data from Tsutumi (2007), CDC (2010), Scallan et al. (2011a,b), and Hoffmann et al. (2007). CDC (2010) reported data on illnesses associated with shrimp and seafood, and Hoffmann et al. (2007) provided estimates of the percentages of cases of foodborne illness from specific pathogens attributable to seafood (that is, seafood was one of the food categories evaluated in the expert-elicitation study). Specifically, the values shown in Table 4-1 were estimated as follows:
-
Low Estimate. The low estimate was calculated by using the outbreak data shown in Table 4-2. Those estimates were then scaled up by using the pathogen-specific multipliers in Scallan et al. (2011a) to yield the pathogen-specific numbers of cases of illness. Numbers of deaths, severe health effects, less severe health effects, and adverse quality-of-life health effects were then calculated according to the general method used for leafy greens.
TABLE 4-2 Agents Linked to Shrimp-Associated Outbreaks and Cases in the United States, 2001-2005
|
Infectious Agents |
Number of Outbreaks |
Number of Cases of Illness |
|
Clostridium perfringens |
0 |
0 |
|
Shigella sonnei |
1 |
2 |
|
Salmonella spp. |
5 |
58 |
|
Staphylococcus spp. |
0 |
0 |
|
Vibrio parahaemolyticus |
4 |
121 |
|
Multiple bacteria |
0 |
0 |
|
Norovirus/Norwalk viruses |
3 |
182 |
|
Unknown |
49 |
255 |
|
Total |
62 |
618 |
|
Source: Adapted from Tsutumi 2007. |
||
-
Best Estimate. Cases of illness attributable to seafood were first calculated by using the data on pathogen-specific illnesses reported by Scallan et al. (2011a) and the pathogen-specific or average expert-based attribution from Hoffmann et al. (2007). Twenty-five percent of all the cases of foodborne illnesses from unspecified agents as reported in Scallan et al. (2011b) were also included; this percentage is based on the overall percentage of cases attributed to seafood in Hoffmann et al. (2007). Next, the cases attributed to shrimp were calculated by using the pathogen-specific or average attributions that could be calculated from the CDC (2010) data. The numbers of death, severe health effects, less severe health effects, and adverse quality-of-life health effects were calculated according to the general method described for leafy greens.
-
High Estimate. The high estimate was calculated analogously to the best estimate except that the high end of the range of number of foodborne illnesses provided in Scallan et al. (2011a,b) was used.
Like the estimates of the number of adverse health effects attributable to leafy greens, the best and high estimates for illness associated with shrimp include a large number of cases due to Norovirus.
Personal Controllability
The probability of contracting foodborne illnesses from shrimp can be largely minimized through proper hygiene in food preparation and cooking shrimp to an appropriate temperature; both activities can be practiced during home preparation of shrimp. However, as mentioned above, it is estimated that about 80% of shrimp consumed in the United States is consumed in restaurants rather than prepared at home. For food prepared in restaurants, the only action available to a person to minimize (or control) risks of foodborne illnesses from shrimp is not to order or consume it. Although that option is available, there is no reason, in the absence of any specific information about heightened risk from shrimp, for an individual consumer to believe that another food choice would carry lower risks and thus no reason to choose a different food as a way to control personal risks.
At most 20% of the total number of cases of foodborne illnesses from shrimp are potentially controllable by individual action on the basis of the percentage of shrimp consumed at home. Following the same logic described for leafy greens, the committee assumes that 60-80% of those consuming shrimp at home have sufficient knowledge to be able to reduce the likelihood of foodborne illnesses, resulting in an estimate that about 10-15% of all cases of foodborne illnesses from shrimp are personally controllable, that is, could be avoided by the actions of individual consumers.
Ability to Detect Adverse Health Effects
Because a variety of pathogens and agents can be linked to contaminated shrimp, the disease manifestations are varied. Hence, an institution’s ability to detect a problem is based on common symptoms and requires a clinic or hospital visit where appropriate diagnostic tests are performed. On the basis of studies that examined a health department’s ability to detect a contamination problem, at least five or six patients would need to be identified to establish a possible link to a contaminated food item (Bender et al. 1997; Rounds et al. 2010). With appropriate diagnostics and reporting to a public-health official, baseline epidemiologic features can be determined and can generate hypotheses as to likely sources of illness. Mild cases or cases with long incubation periods are not likely to be reported or linked to a specific food item, nor are sporadic cases, which make up the vast majority of cases of foodborne illness.
As in the case of leafy greens, most foodborne illnesses associated with shrimp have high estimated rates of under-reporting. On the other hand, because most shrimp is consumed in restaurants, there may be a higher likelihood that illnesses will be reported and a higher likelihood that multiple people will be affected at one time and an “outbreak” identified than when foods are prepared at home. On the basis of the under-reporting and under-diagnosis estimates in Scallan et al. (2011a) and the specific pathogens associated with shrimp, the committee estimated that about 3-5% of foodborne illnesses caused by shrimp would be detected.
Ability to Mitigate Adverse Health Effects
In the United States, the Federal Food, Drug, and Cosmetic Act gives FDA the responsibility of ensuring that no adulterated or misbranded food, including seafood, enters interstate commerce [21 USC §331]. The Office of Food Safety in FDA’s Center for Food Safety and Applied Nutrition (CFSAN) oversees seafood safety. Its regulatory activities include control of foodborne pathogens and contaminants (such as methylmercury, Vibrio, and drug and chemical residues), inspection and compliance, and importation and exportation. Three other federal agencies work with FDA to protect the food supply system: the National Oceanic and Atmospheric Administration of the Department of Commerce conducts fee-for-service inspection for the industry and controls domestic fishing activities by prohibiting harvesting when the water is unsafe because of pollution or contamination, the Environmental Protection Agency sets tolerances for pesticide-residue limits and ensures cleanliness of air and water, and the U.S. Department of Agriculture is involved in seafood regulations and is responsible for promoting the aquaculture industry.
Under the current Hazard Analysis and Critical Control Points (HACCP) regulations, domestic and foreign seafood processors must have and implement a written HACCP plan and follow appropriate corrective action, verification, record-keeping, and training. The industry’s implementation of the seafood HACCP regulation is investigated as a part of FDA’s compliance programs. CFSAN provides the guidance for its field officers who inspect the industries. Domestic and foreign seafood processors are inspected for their compliance with HACCP regulations and other, non-HACCP attributes, such as filth and decomposition. The effectiveness of those programs in preventing contaminated products from reaching consumers is reflected in the estimates of the number of cases of foodborne illnesses that occur, as summarized in Table 4-1
The ability to mitigate a problem with shrimp if such a problem is detected and successfully attributed relies on the ability of appropriate institutions to remove the contaminated product from the marketplace through product recalls and consumer education. If the source of the problem is fresh shrimp, the situation is analogous to that described above for leafy greens: most of the product will have been consumed by the time the problem is identified. If the problem occurs in frozen and packaged shrimp, the ability to track the problem to specific lines and to recall the product successfully is somewhat greater because frozen shrimp may be stored for up to 2 years before being distributed to consumers. The next section on canned foods includes a discussion of the effectiveness of recalls. Canned foods are characterized by a longer shelf-life than frozen shrimp, but it is unclear whether the longer shelf-life makes it easier or harder to recall a product from the market. For example, canned foods that remain on the market may be purchased months after a recall and stored by consumers for many more months or even years before being consumed. Frozen shrimp may be stored for relatively long periods before distribution; thus, if they are recalled, a higher percentage of the product may be in centralized storage than in consumers’ homes at the time of the recall. Considering both fresh and frozen shrimp and the estimated ability to mitigate adverse health effects of consumption of leafy greens and canned foods described elsewhere in this case study, the committee estimated the ability of institutions to mitigate adverse health effects of shrimp consumption to be 10-50%.
Commercial Canned Foods
According to the Economic Research Service (ERS), the United States produces about 150 lb of domestic canned foods per capita per year (USDA 2009). They consist of a wide array of acidified and low-acid categories and include meats, vegetables, fruits, dairy foods, beverages, seafood, cereals, and multi-ingredient foods. Canned foods imported into this country are similar to domestic canned foods and also consist of a wide array of products. There is no available information on the geographic source distribution of nondomestic canned foods, but they are probably imported from at least 50 countries.
In the canning process, food products are subjected to a thermal treatment in sealed metal containers or cans to kill as many microorganisms present in the food in the form of vegetative cells or spores as possible. Because of the potential risk of botulism caused by improperly treated cans contaminated with spores of Clostridium botulinum, canned foods are among the most strictly regulated food categories. Modern canning technologies have been optimized thanks to years of research and strict regulations and typically are capable of delivering an equivalent bacterial killing of 12D or 12 log CFU of C. botulinum spores.
Recalls of canned foods are relatively unusual. Despite the large volume of product manufactured in the United States and overseas, there are rarely more than five recalls of canned foods in a year (FDA 2010b). From January to August 2010, there were three recalls; in 2009, only one recall was reported. The most common causes of recalls are under-processing, presence of allergens, and mislabeling, and they rarely involved a proven health risk.
According to FDA records, the shipments of imported canned foods have averaged about 97,240 lines in the last 5 years. About 2-3% of those lines were refused at the port of entry. The main reason for refusal was the lack of registration by the manufacturer; other frequent reasons were mislabeling and failure to file a process schedule with FDA.
Exposed Population
No data are available on the percentage of the U.S. population that regularly consumes canned foods. However, given the prevalence of these products in our food supply and the relatively high annual per capita production of 150 lb of canned foods (USDA 2009), the committee assumed that an overwhelming majority of Americans consume canned foods during the course of the year—that is, between 99-99.9% (best estimate, 99.5%), which accounts for people who prefer to consume nonprocessed food. Multiplying those percentages by an assumed U.S. population of 310 million yields the estimates shown in Table 4-1.
As described above in the discussion of leafy greens and shrimp, infants and young children, older people, and immunosuppressed populations are more susceptible to foodborne illnesses generally and are more likely to suffer severe effects if they do contract the illnesses. Accordingly, those groups are highlighted as populations of special concern.
Mortality and Morbidity
Commercially canned foods have rarely been linked to documented deaths in the United States. Botulism is the pathogen generally considered to be of most concern in regard to canned foods, but almost all recent cases of botulism caused by canned foods in this country have been linked to domestic home-canned goods. According to CDC, a total of six botulism deaths in 10 years were recorded as due to other food products (CDC 2010), none of them associated spe-
cifically with commercial canned foods. Scallan et al. (2011a) estimate 0-55 deaths per year from botulism poisoning.
According to outbreak surveillance data from CDC (2010), from 1998 to 2008 a total of 11 cases of foodborne botulism were attributed to canned foods; the data include illnesses from consumption of home-prepared products. In recent years, there has been only one recorded event in which commercially canned foods were linked to botulism. In 2007, four people got sick after consumption of a domestic brand of beans in the first recorded outbreak of botulism linked to commercially canned foods in 30 years (CDC 2007). As discussed above, foodborne illnesses are assumed to be under-reported, but Scallan et al. (2011a) estimated the under-reporting and under-diagnosis rate of botulism as relatively low (about a factor of 2).
A number of other natural and artificial toxicants may be present in canned foods, such as pesticide residues, mycotoxins, and chemicals that leach from packaging into the product. Available data are not sufficient to assess their effects on health. Reported cases of any of the agents in canned foods are also rare. In 1989, imported canned mushrooms from China were responsible for as many as four outbreaks of staphylococcal intoxication in different parts of the United States (CDC 1989). The outbreaks involved 102 people, 7 of whom required hospitalization, but no deaths were reported. In a 2003 outbreak involving 65 cases of salmonellosis, canned mushrooms were again implicated, but it was not clear whether the contamination occurred after cans were opened. In 2007, three people suffered scombroid intoxication from canned tuna (CDC 2010). The recent isolated outbreaks were documented as linked to domestic or imported canned products. There is no record of any death associated with commercially produced canned foods in the last 30 years.
To estimate mortality and morbidity attributable to canned foods, the committee used the data on deaths and illness described above, mortality and hospitalization rates from Scallan et al. (2011a) for each of the pathogens described above, and methods similar to those described above for leafy greens and shrimp. Specifically, the values presented in Table 4-1 were estimated at follows:
-
Low Estimate. The committee assumed that no botulism cases attributed to commercially canned food occurred for the low estimate. The outbreak data described above were annualized and then adjusted by using the multipliers described in Scallan et al. (2011a) for under-reporting to obtain estimates of numbers of cases of illness associated with canned goods (by pathogen). Numbers of deaths, severe health effects, less severe health effects, and adverse quality-of-life health effects were then calculated according to the general method used for leafy greens.
-
Best Estimate. The best estimate differs from the low estimate only in the attribution of botulism cases. For the best estimate, the committee assumed that 10% of botulism cases could be attributed to commercially canned foods.
-
High Estimate. The high estimates were calculated by assuming that half the botulism cases in a year could be attributed to commercially canned foods and that the total incidence of the other foodborne illnesses described above (that is, 186 reported illnesses from canned foods) occurs in a single year. Those data were then adjusted by using the multipliers described in Scallan et al. (2011a,b) for under-reporting and under-diagnosis. Numbers of deaths, severe health effects, less severe health effects, and adverse quality-of-life health effects were then calculated according to the general method used for leafy greens.
Personal Controllability
As with the other food categories discussed, people theoretically have the ability to eliminate their personal risks of foodborne illnesses from commercially canned foods by avoiding the use of canned foods entirely. Given the prevalence of canned foods, that solution is not practical. Avoiding the use of any canned foods that show signs of spoilage—such as bulging, leaking, or dented cans—can help to minimize the chances of illnesses. Assuming that commercially canned foods are used just as frequently in home preparation of foods as in restaurant preparation, that about 27% of all meals consumed in the United States are prepared outside the home (Lin et al. 1998), and that 60-80% of consumers are aware of the precautions that should be taken with damaged commercially canned foods, about 45-60% of all cases of foodborne illnesses from commercially canned foods could be avoided through actions by individual consumers
Ability to Detect Adverse Health Effects
As noted above in connection with leafy greens and shrimp, the ability of an institution to detect adverse effects is problematic because illness has to be identified and then correctly attributed to a specific food product. Canned foods have a much longer shelf-life than leafy greens and shrimp and a well-documented production process and distribution system, so the possibility of attributing illness to them may be greater. However, canned foods will most likely be consumed over a longer period, so any illnesses associated with them may be seen as sporadic cases and be more difficult to identify and attribute to a source. Furthermore, because commercially canned foods are rarely associated with foodborne illnesses, individual cases are more likely to be attributed to other foods than to canned foods. The committee, however, notes that the ability to detect botulism poisoning caused by consumption of canned foods is quite high given that public-health officials must report a single case to CDC so that it can be investigated. Overall, however, other foodborne illnesses associated with canned foods are much more likely to occur than botulism poisoning, and they are more difficult to detect and attribute successfully to canned foods. On the basis of the under-reporting and under-diagnosis multipliers used in Scallan et
al. (2011a), the committee estimated that about half the cases of botulism poisoning from commercially canned foods would be detected, and less than 3% of all other types of foodborne illnesses from canned foods would be detected.
Ability to Mitigate Adverse Health Effects
Industrial canning processes are typically designed to deliver products “commercially sterile” to minimize the presence of pathogenic organisms and to extend the shelf-life of foods. Commercial sterility is defined in 21 CFR 113.3 as the process in which heat is applied to render food free from “(a) microorganisms capable of reproducing in the food under normal non-refrigerated conditions of storage and distribution; and (b) viable microorganisms (including spores) of public health significance.” Canned foods are typically produced by packing at near-boiling temperatures in tightly sealed containers. The hot-fill process eliminates most of the oxygen and creates a strictly anaerobic condition that minimizes oxidative reactions and inhibits aerobic microorganisms. At the same time, the initial high-temperature step kills all microbial vegetative cells. The tight seal of canned foods protects the product from external contamination during further processing and during distribution. The key step in the manufacture of canned foods is the treatment of recently sealed cans at temperatures above 100C for several minutes (Murano 2003). That heat treatment is typically conducted inside retorts, pressurized containers that use steam to attain temperatures as high as 121C (Potter and Hotchkiss 1995). After thermal processing, cans are normally cooled with water and stored until they enter distribution.
In addition, canned foods are tightly regulated (see, for example, 21 CFR 113, 108.25, and 108.35), including requirements for the proper operation and design of facilities that produce canned foods (Section 113). Section 113 defines two types of canned foods—acidified and low-acid foods—on the basis of the ability of C. botulinum to grow at a pH above 4.6. The regulations described in Section 113 apply mostly to low-acid foods. The rules in Section 113 include the characteristics of the equipment, controls, facilities, and product preparation that producers need to comply with. The regulations also require reporting any deviation in process characteristics of and continuous recordkeeping on every processed batch. As a result of that heightened oversight, canned foods have rarely been linked to cases of foodborne disease in the last few decades. The effectiveness of the steps to prevent contamination of canned foods can be seen in the very low rates of foodborne illnesses from canned foods shown in Table 4-1.
If a problem with canned foods does occur and is detected the ability of institutions to mitigate adverse effects through successful recall of a contaminated product before it is consumed is substantially higher than for the other two kinds of products considered in this case study, primarily because of the longer shelf-life of canned foods. The supply chain for canned foods is complex; a single manufacturing facility sometimes produces products that carry many differ-
ent labels. The process, however, is well understood and relatively easily tracked; if a problem is detected and successfully traced to a particular facility, the potentially contaminated products can be readily identified in appropriate recall notices. For canned foods, the potentially contaminated product is likely to be widely distributed by the time a problem is detected, so the recall itself can be challenging; that is, all affected retail outlets and consumers must be made aware of and respond to the recall if it is to be completely effective.
After the 2007 Castleberry recall of tens of millions of cans because of potential C. botulinum contamination, a review by the North Carolina Department of Agriculture and Consumer Services found that the product remained on the shelves of 38% of the retail outlets that handled it (Seltzer et al. 2008). Most were smaller retail outlets, so this does not necessarily represent 38% of all recalled product, but it highlights a weakness in the effectiveness of recalls even for a well-understood supply chain and a product with a long shelf-life. Recalls are even less effective for product that has already been purchased by consumers. Patrick et al. (2007) report on the result of a random telephone survey conducted after a large-scale nationwide recall: only 45% of all adults were even aware of the recall. Again, depending on the severity of the foodborne illness caused by the canned food, a recall may come early enough for most of the product to still be in stores and not in individual consumers’ homes, so that does not necessarily imply that 55% of the recalled product will be consumed. On the basis of those studies, the committee estimated that the ability of institutions to mitigate the adverse effects of foodborne illness caused by consumption of canned foods after a potential problem has been detected is about 50-75%.
USING THE RISK CHARACTERIZATION TO SUPPORT DECISION-MAKING
A review of Table 4-1 reveals a clear “ranking” of the three food categories in terms of the number of foodborne illnesses associated with each: domestic leafy greens appear to cause substantially more illnesses than do shrimp, and both appear to cause far more illnesses than do commercially canned foods. Other factors, however, also differ between the food categories and could be relevant to decision-makers and policy-makers. For example, people are estimated to have a higher degree of control over their own risk of contracting a foodborne illness from leafy greens than from shrimp. That information could be interpreted in several ways. It might suggest, for example, that efforts to improve awareness of how to prepare foods safely at home would do more to reduce the number of foodborne illnesses from leafy greens than to reduce the number from shrimp, whereas restaurant-focused efforts might be more effective in reducing the number of shrimp-related illnesses. Similarly, the higher ability to detect and mitigate risks associated with foods with well-understood and controlled distribution channels and longer shelf-lives, such as commercially canned foods, could suggest that supply-chain management would be helpful for leafy
greens or that efforts to reduce the number of foodborne illnesses from leafy greens should focus on the source because mitigation after exposure is more difficult.
As discussed in Chapter 2, although a risk ranking is not necessarily directly useful for a decision-maker, it can be a useful first step in making targeting or resource-allocation decisions. For example, if FDA were considering a high-level decision about whether to focus newly available inspection resources on leafy greens, shrimp, or commercially canned foods, the risk characterization developed here would provide information on the current levels of adverse health effects associated with each category and on where the maximum potential for risk reduction exists. Deciding how to allocate the resources would require additional analysis and more detailed understanding of how the resources would be used. If resources were available to inspect a particular number of agricultural suppliers in the field or some number of import locations or some number of canned-food production facilities, additional risk characterizations (attribute tables) would need to be developed that describe the likely outcomes of the increased inspections. Developing those risk characterizations would require consideration of how much of the particular food type could be inspected and the effectiveness of the inspections in reducing contamination in addition to all the factors considered above about numbers, types, and severity of foodborne illnesses. The differences between the attribute tables developed above and the attribute tables describing the public-health consequences of each food category in the enhanced inspection would be a measure of the relative benefits of different resource allocations.
REFERENCES
Bender, J.B., C.W. Hedberg, J.M. Besser, D.J. Boxrud, K.L. MacDonald, and M.T. Osterholm. 1997. Surveillance by molecular subtype for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N. Engl. J. Med. 337(6):388-394.
Beuchat, L.R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Protect. 59(2):204-216.
Calvin, L. 2007. Outbreak Linked to Spinach Forces Reassessment of Food Safety Practices. Amber Waves (June):25-31 [online]. Available: http://www.ers.usda.gov/AmberWaves/June07/PDF/Spinach.pdf [accessed Oct. 26, 2010].
Calvin, L., H.H. Jensen, and J. Liang. 2009. The economics of food safety: The 2006 foodborne illness outbreak linked to spinach. Pp. 399-418 in Microbial Safety of Fresh Produce, X. Fan, B.A. Niemira, C.J. Doona, F.E. Feeherry, and R.B. Gravani, eds. Ames, IA: IFT Press.
CDC (U.S. Centers for Disease Control and Prevention). 1989. Epidemiologic notes and reports multiple outbreaks of staphylococcal food poisoning caused by canned mushrooms. MMWR 38(24):417-418 [online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00001410.htm [accessed Oct. 26, 2010].
CDC (U.S. Centers for Disease Control and Prevention). 2007. Botulism associated with commercially canned chili sauce--Texas and Indiana, July 2007. MMWR 56:1-3
[online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm56d730a1.htm [accessed Oct. 27, 2010].
CDC (U.S. Centers for Disease Control and Prevention). 2010. Outbreak Net, Foodborne Outbreak Online Database. Available: http://wwwn.cdc.gov/foodborneoutbreaks/Default.aspx [accessed Oct. 27, 2010].
FAO (Food and Agricultural Organization of United Nations). 2010. Shrimp Market Report. GLOBEFISH, Fisheries Department, Food and Agricultural Organization of United Nations. May 2010 [online]. Available: http://www.globefish.org/shrimp-market-reports.html [accessed Oct. 27, 2010].
FDA (U.S. Food and Drug Administration). 2010a. Glossary of import term: Line (Line item). Subchapter 6.7.27 in Investigations Operations Manual. Inspections, Compliance, Enforcement, and Criminal Investigations, U.S. Food and Drug Administration [online]. Available: http://www.fda.gov/ICECI/Inspections/IOM/ucm063172.htm [accessed Mar. 21, 2011].
FDA (U. S. Food and Drug Administration). 2010b. Archive for Recalls, Market Withdrawals & Safety Alerts. U. S. Food and Drug Administration [online]. Available: http://www.fda.gov/Safety/Recalls/ArchiveRecalls/default.htm [accessed Oct. 27, 2010].
The Fish Site. 2008. U.S. Shrimp Market Report - April 2008 [online]. Available: http://www.thefishsite.com/articles/431/us-shrimp-market-report-april-2008 [accessed July 2010].
FSIS (Food Safety and Inspection Service). 2006. Safe Food Handling: Cleanliness Helps Prevent Foodborne Illness. Fact Sheet. Food Safety and Inspection Service, U.S. Department of Agriculture [online]. Available: http://www.fsis.usda.gov/factsheets/Cleanliness_Helps_Prevent_Foodborne_Illness/index.asp [accessed Mar. 22, 2011].
GAO (U.S. Government Accountability Office). 2008. Federal Oversight of Food Safety: FDA’s Food Protection Plan Proposes Positive First Steps, but Capacity to Carry Them Out Is Critical. GAO-08-435T. Washington, DC: U.S. Government Accountability Office [online]. Available: http://www.gao.gov/new.items/d08435t.pdf [accessed Mar. 21, 2011].
GAO (U.S. General Accountability Office). 2010. FDA Could Strengthen Oversight of Imported Food by Improving Enforcement and Seeking Additional Authorities. GAO-10-699T. Washington, DC: GAO [online]. Available: http://www.gao.gov/new.items/d10699t.pdf [accessed Oct.27, 2010].
Hallman, W.K, C.L. Cuite, J.E. Dellava, M.L. Nucci, and S.C. Condry. 2009. Public response to the 2006 recall of contaminated spinach. Pp. 351-368 in Microbial Safety of Fresh Produce, X. Fan, B.A. Niemira, C.J. Doona, F.E. Feeherry, and R.B. Gravani, eds. Ames, IA: IFT Press.
Hoffmann, S., P. Fischbeck, A. Krupnick, and M. McWilliams. 2007. Using expert elicitation to link foodborne illnesses in the United States to foods. J. Food. Prot. 70(5):1220-1229.
IOM (Institute of Medicine). 2007. Seafood Choices: Balancing Benefits and Risks. Washington, DC: The National Academies Press.
IOM/NRC (Institute of Medicine and National Research Council). 1998. Ensuring Safe Food: From Production to Consumption. Washington, DC: National Academy Press.
IOM/NRC (Institute of Medicine and National Research Council). 2003. Scientific Criteria to Ensure Safe Food. Washington, DC: The National Academies Press.
IOM/NRC (Institute of Medicine and National Research Council). 2010. Enhancing Food Safety: The Role of the Food and Drug Administration. Washington, DC: The National Academies Press.
Koonse, B., W. Burkhardt, S. Chirtel, and G. Hoskin. 2005. Salmonella and sanitary quality of aquacultured shrimp. J. Food Protect. 68(12):2527-2532.
Lin, B.H., J. Guthrie, and E. Frazao. 1998. Popularity of dinning out present barrier to dietary improvements. Food Rev. 21(2):2-10 [online]. Available: http://www.ers. usda.gov/publications/foodreview/may1998/may98a.pdf [accessed Mar. 22, 2011].
Mandrell, R.E. 2009. Enteric human pathogens associated with fresh produce: Sources, transport and ecology. Pp. 5-42 in Microbial Safety of Fresh Produce, X. Fan, B.A. Niemira, C.J. Doona, F.E. Feeherry, and R.B. Gravani, eds. Ames, IA: IFT Press.
Mead, P.S., L. Slutsker, V. Dietz, L.F. McCaig, J.S. Bresee, C. Shapiro, P.M. Griffin, and R.V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5(5):607-625.
Murano, P.S. 2003. Understanding Food Science and Technology. Belmont, CA: Thomson/Wadsworth.
NCI (National Cancer Institute). 2010. Usual Dietary Intake: Food Intakes, U.S. Population 2001-04. Risk Factor Monitoring and Methods. National Cancer Institute, U.S. National Institute of Health [online]. Available: http://riskfactor.cancer.gov/diet/usualintakes/pop/ [accessed Oct. 26, 2010].
NOAA (National Oceanic Atmospheric Administration). 2010. Fisheries of the United States – 2009, E.S. Pritchard, ed. National Marine Fisheries Service, Office of Science and Technology, National Oceanic Atmospheric Administration, Silver Spring, MD [online]. Available: http://www.st.nmfs.noaa.gov/st1/fus/fus09/fus_2009.pdf [accessed June 18, 2010].
Patrick, M.E., P.M. Griffin, A.C. Voetsch, and P.S. Mead. 2007. Effectiveness of recall notification: Community response to a nationwide recall of hot dogs and deli meats. J. Food Prot. 70(10):2373-2376.
Potter, N.N., and J.H. Hotchkiss. 1995. Food Science, 5th Ed. New York: Chapman and Hall.
Rounds, J.M., C.W. Hedberg, S. Meyer, D.J. Boxrud, and K.E. Smith. 2010. Salmonella enterica pulsed-field gel electrophoresis clusters, Minnesota, USA, 2001–2007. Emerg. Infect. Dis. 16(11):1678-1685.
Scallan, E., R.M. Hoekstra, F.J. Angulo, R.V. Tauxe, M.A. Widdowson, S.L. Roy, J.L. Jones, and P.M. Griffin. 2011a. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 17(1):7-15.
Scallan, E., P.M. Griffin, F.J. Angulo, R.V. Tauxe , and R.M. Hoekstra. 2011b. Foodborne illness acquired in the United States--unspecified agents. Emerg. Infect. Dis. 17(1):16-22.
Seltzer, J., J. Rush, and J. Kinsey. 2008. Castleberry’s: 2007 Botulism Recall; A Case Study by the Food Industry Center, August 2008. Food Industry Center, University of Minnesota [online]. Available: http://ageconsearch.umn.edu/bitstream/46055/2/Castelberry%20Study-%20Final.pdf [accessed Oct. 28, 2010].
Tooze, J.A., D Midthune, K.W. Dodd, L.S. Freedman, S.M. Krebs-Smith, A.F. Subar, P.M. Guenther, R.J. Carrol, and V. Kipnis. 2006. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J. Am. Diet. Assoc. 106(10):1575-1587.
Tsutsumi, H. 2007. A Food Predictive Modeling and Risk Estimation for Imported Shrimp into Japan and the United States. MS Thesis, University of Minnesota, St. Paul, MN. March 2007.
USDA (U.S. Department of Agriculture). 2009. Canned Fruits, Canned Vegetables, Canned Meats. Food Availability (Per Capita) Data System. Economic Research Services, U.S. Department of Agriculture [online]. Available: http://www.ers.usda.gov/data/foodconsumption/FoodAvailspreadsheets.htm#vegcan [accessed June 18, 2010].
USDA (U.S. Department of Agriculture). 2010. U.S. per Capita Food Availability: Custom Report-Individual Vegetable-Spinach. Economic Research Services, U.S. Department of Agriculture [online]. Available: http://www.ers.usda.gov/Data/FoodConsump-tion/app/reports/displayCommodities.aspx?reportName=Individual+vegetable&id=24#startForm [accessed Oct.26, 2010].
U. S. Census Bureau. 2010. Population Estimates, Population Characteristics, National Sex and Age [online]. Available: http://www.census.gov/popest/national/asrh/NC-EST2009-sa.html [accessed Oct. 26, 2010].
Wan Norhana, M.N., S.E. Poole, H.C. Deeth, and G.A. Dykes. 2009. Prevalence, persistence and control of Salmonella and Listeria in shrimp and shrimp products: A review. Food Control 21(4):343-361.