Congress passed the National Childhood Vaccine Injury Act (P.L. 99-660) in 1986. The legislation was intended to bolster vaccine research and develop ment through federal coordination of vaccine efforts in government by providing relief to vaccine manufacturers who reported at the time that financial burdens from awards in the tort system threatened their financial viability. The legislation was also intended to address concerns about the safety of vaccines through a multipronged approach involving instituting a compensation program financed by an excise tax on covered vaccines, setting up a passive surveillance system for vaccine adverse events, and providing information to consumers.
Sections 312 and 313 of the legislation required the secretary of the U.S. Department of Health and Human Services to consult with the Institute of Medicine (IOM) to conduct a review of the scientific literature related to a set of serious adverse events1 following immunizations recommended for use in children. Two reports were issued (IOM, 1991, 1994). These reports contain a framework for causality assessment of adverse events following vaccination. The reports embraced all vaccines covered by the National Vaccine Injury Compensation Program (VICP) up to that point: diphtheria- and tetanus-toxoids and whole cell pertussis (DTwP) vaccine2 and other tetanus toxoid–containing vaccines; measles, mumps, and rubella
___________
1 Adverse events are distinguished from adverse effects in that an event is something that occurs but may not be causally associated, whereas an adverse effect implies causation. All adverse effects are adverse events, but not all adverse events are adverse effects.
2 Acellular pertussis vaccine (aP) has replaced whole cell pertussis vaccine in the United States.
(MMR) vaccines; Haemophilus influenzae type B vaccine; hepatitis B vaccine; and both inactivated and oral polio vaccines.3 The reports informed the secretary’s review of the Vaccine Injury Table. The reports have also been referenced extensively as a source of definitive scientific understanding of the evidence by Special Masters in decisions regarding injuries not listed on the Vaccine Injury Table.
The IOM was subsequently asked to review specific vaccine safety concerns in a series of reports requested by the Centers for Disease Control and Prevention (CDC). These reports (IOM, 2001a,b, 2002a,b, 2003a,b, 2004a,b) included causality assessments similar to the previous IOM reports, but included other conclusions and recommendations regarding research, communications, and policy review.
In 2009 the IOM entered into a contract with the Health Resources and Services Administration (HRSA)4 to convene a committee of experts to review the epidemiologic, clinical, and biological evidence regarding adverse health events associated with specific vaccines covered by the VICP. The committee was composed of individuals with expertise in pediatrics, internal medicine, neurology, immunology, immunotoxicology, neurobiol-ogy, rheumatology, epidemiology, biostatistics, and law.
The vaccines to be reviewed included varicella zoster vaccine; influenza vaccines;5 hepatitis B vaccine; human papillomavirus vaccine (HPV); tetanus toxoid–containing vaccines other than those containing the whole cell pertussis component; measles, mumps, and rubella vaccines; hepatitis A vaccine; and meningococcal vaccines. It is expected that the report will provide the scientific basis for review and adjudication of claims of vaccine injury by the VICP.
HRSA presented a list of specific adverse events for the committee to review (see Table S-1). The selection criteria was described at the first committee meeting (Johann-Liang, 2009) as including the vast majority of adverse events in the claims for compensation. The committee added adverse events to the list if it identified epidemiologic studies or case reports for an adverse event not originally assigned by HRSA. These additions were all-cause mortality and seizures following influenza vaccine; optic neuritis
___________
3 Vaccines are included in the VICP if they are recommended by the CDC for routine ad ministration in children and are subject to an excise tax. Adults who experience an adverse reaction to one of these “childhood” vaccines are also covered by the program.
4 The CDC and the National Vaccine Program Office also provided funds for the project via the contract with HRSA.
5 The 2009 H1N1 influenza vaccine is covered by the Countermeasures Injury Compensation Program, and evidence about its safety is not covered in this report.
TABLE S-1 Adverse Events and Causality Conclusions Included in the Vaccine Chapters
|
|
|||||||||
| Adverse event | MMR Vaccine Chapter 4 | Varicella Vaccine Chapter 5 | Influenza Vaccine Chapter 6 | Hepatitis A Vaccine Chapter 7 | Hepatitis B Vaccine Chapter 8 | HPV Vaccine Chapter 9 | DT—, TT—, and aP—Containing Vaccines Chapter 10 |
Meningococcal Vaccine Chapter 11 | Injection-Related Events Chapter 12 |
|
|
|||||||||
| Disseminated Oka VZV without Other Organ Involvement | CS | ||||||||
| Disseminated Oka VZV with Subsequent Infection Resulting in Pneumonia, Meningitis, or Hepatitis | CSa | ||||||||
| Vaccine Strain Viral Reactivation without Other Organ Involvement | CS | ||||||||
| Vaccine Strain Viral Reactivation with Subsequent Infection Resulting in Meningitis or Encephalitis | CS | ||||||||
| Measles Inclusion Body Encephalitis | CSa,b | ||||||||
| Encephalitis | I | I | I | I | I | ||||
| Encephalopathy | I | i | I | I | I | I | |||
| Infantile Spasms | I | ||||||||
|
|
|||||||||
| Adverse event | MMR Vaccine Chapter 4 | Varicella Vaccine Chapter 5 | Influenza Vaccine Chapter 6 | Hepatitis A Vaccine Chapter 7 | Hepatitis B Vaccine Chapter 8 | HPV Vaccine Chapter 9 | DT—, TT—, and aP—Containing Vaccines Chapter 10 |
Meningococcal Vaccine Chapter 11 | Injection-Related Events Chapter 12 |
|
|
|||||||||
| Febrile Seizures | CS | ||||||||
| Afebrile Seizures | I | ||||||||
| Seizures | I | Ic | I | I | |||||
| Meningitis | Ic | ||||||||
| Cerebellar Ataxia | I | ||||||||
| Ataxia | I | I | |||||||
| Autism | FR | I | |||||||
| Acute Disseminated Encephalomyelitis | I | I | I | I | I | I | I | I | |
| Transverse Myelitis | I | I | I | I | I | I | I | I | |
| Optic Neuritis | Ic | Ic | Ic | Ic | |||||
| Neuromyelitis Optica | Ic | I | I | I | |||||
| Multiple Sclerosis Onset in Adults | I | I | I | I | |||||
| Multiple Sclerosis Onset in Children | I | I | |||||||
| Multiple Sclerosis Relapse in Adults | I | I | I | ||||||
| Multiple Sclerosis Relapse in Children | I | I | |||||||
| Multiple Sclerosis | I | I | I | ||||||
| First Dcmychnating Event in Adults | I | ||||||||
| First Demy el mating Event in Children | I | ||||||||
| Guillain-Barré Syndrome | I | I | I | I | I | I | I | I | |
| Chronic Inflaminatory Disseminated Polyneuropathy | I | I | I | I | I | I | I | ||
| Opsoclonus Myoclonus Syndrome | I | I | |||||||
| Bell's Palsy | FR | I | I | ||||||
| Brachial Neuritis | I | I | I | I | |||||
| Amyotrophic Lateral Sclerosis | I | ||||||||
| Small Fiber Neuropathy | Ic | I | |||||||
| Anaphylaxis | CS | CS | CS | I | CSd | FA | CSe | CS | |
| Chronic Urticaria | I | ||||||||
| Scrum Sickness | I |
|
|
|||||||||
| Adverse event | MMR Vaccine Chapter 4 | Varicella Vaccine Chapter 5 | Influenza Vaccine Chapter 6 | Hepatitis A Vaccine Chapter 7 | Hepatitis B Vaccine Chapter 8 | HPV Vaccine Chapter 9 | DT—, TT—, and aP—Containing Vaccines Chapter 10 |
Meningococcal Vaccine Chapter 11 | Injection-Related Events Chapter 12 |
|
|
|||||||||
| Inactivated Influenza | FR | ||||||||
| Vaccine and Asthma | |||||||||
| Exacerbation or Reactive | |||||||||
| Airway Disease Episodes in Children and Adults | |||||||||
| Live Attenuated Influenza | I | ||||||||
| Vaccine and Asthma Exacerbation or Reactive | |||||||||
| Airway Disease Episodes in Children Younger Than 5 Years of Age | |||||||||
| Live Attenuated Influenza | I | ||||||||
| Vaccine and Asthma | |||||||||
| Exacerbation or Reactive | |||||||||
| Airway Disease Episodes in Persons 5 Years of Age | |||||||||
| or Older | |||||||||
| Erythema Nodosum | Ic | ||||||||
| Systemic Lupus Erythematosus | I | I | |||||||
| Vasculitis | I | I | |||||||
| Polyarteritis Nodosa | I | I | |||||||
| Psoriatic Arthritis | I | ||||
| Reactive Arthritis | I | ||||
| Rheumatoid Arthritis | I | ||||
| Juvenile Idiopathic Arthritis | I | ||||
| Transient Arthralgia in Women | FAf | ||||
| Transient Arthralgia in Children | FA | ||||
| Transient Arthralgia | I | ||||
| Chronic Arthralgia in Women | I | ||||
| Chronic Arthritis in Women | I | ||||
| Chronic Arthropathy in Children | I | ||||
| Arthropathy in Men | I | ||||
| Arthropathy | I | I | I | ||
| Type 1 Diabetes | FR | I | FR | ||
| Autoimmune Hepatitis | I | ||||
| Myocarditis | I | ||||
| Pancreatitis | I |
|
|
|||||||||
| Adverse event | MMR Vaccine Chapter 4 | Varicella Vaccine Chapter 5 | Influenza Vaccine Chapter 6 | Hepatitis A Vaccine Chapter 7 | Hepatitis B Vaccine Chapter 8 | HPV Vaccine Chapter 9 | DT—, TT—, and aP—Containing Vaccines Chapter 10 |
Meningococcal Vaccine Chapter 11 | Injection-Related Events Chapter 12 |
|
|
|||||||||
| Hepatitis | I | ||||||||
| Thromboembolic Events | I | ||||||||
| Stroke | Ic | I | |||||||
| Hypcrcoagulablc States | I | ||||||||
| Myocardial Infarction | I | ||||||||
| Chronic Fatigue Syndrome | I | ||||||||
| Chronic Headache | I | ||||||||
| Fibromyalgia | I | I | I | I | |||||
| Sudden Infant Death Syndrome | I | ||||||||
| Hearing Loss | I | ||||||||
| All Cause Mortality | Ic | ||||||||
| Oculorespiratory Syndrome | FAg | ||||||||
| Thrombocytopenia | I | ||||||||
| Immune Thrombocytopenic Purpura | I | ||||||||
| Complex Regional Pain Syndrome | I | ||||||||
| Deltoid Bursitis | CS | ||||||||
| Syncope | CS | ||||||||
|
|
|||||||||
NOTE: CS = convincingly supports a causal relationship; FA = favors acceptance of a causal relationship; FR = favors rejection of a causal relationship; I = inadequate to accept or reject a causal relationship.
a The committee attributes causation to individuals with demonstrated immunodeficiencies.
b The committee attributes causation to the measles component of the vaccine.
c Although not originally charged to the committee by the sponsor, the committee considered this adverse event in its review of the literature.
d The committee attributes causation to yeast-sensitive individuals.
e The committee attributes causation to the tetanus toxoid vaccine. The evidence is inadequate to accept or reject a causal relationship between anaphylaxis and diphtheria toxoid or acellular pertussis vaccine.
f The committee attributes causation to the rubella component of the vaccine.
g The committee attributes causation to two particular vaccines used in three particular years in Canada.
following MMR, influenza, hepatitis B, and DTaP vaccines; neuromyelitis optica and meningitis following MMR vaccine; erythema nodosum following hepatitis B vaccine; and stroke and small fiber neuropathy following varicella vaccine.
It is important to note that the committee was not tasked with assessing the benefits (effectiveness) of vaccines or any policy issues related to vaccination. The committee’s task is focused only on an assessment of the risk of vaccines.
ASSESSING THE WEIGHT OF EVIDENCE
Two streams of evidence support the committee’s causality conclusions: epidemiologic evidence derived from studies of populations (most often based on observational designs but randomized trials when available), and mechanistic evidence derived primarily from biological and clinical studies in animals and individual humans (see Figure S-1). Some studies provide evidence capable of addressing both epidemiologic and mechanistic questions. Drawing from both sources of evidence to support causal inference is well established in the literature.
The committee made three assessments for each relationship reviewed. The first assessment applies to the weight of evidence from the epidemiologic literature; the second applies to the weight of evidence from the mechanistic literature. Each individual article (or findings within an article if more than one outcome or vaccine was studied) was evaluated for its strengths and weaknesses. The committee then synthesized the body of evidence of each type (epidemiologic or mechanistic) and assigned a “weight-of-evidence” for each. These weights-of-evidence represent the committee’s assessment of the quality and quantity of evidence. The two weight-of-evidence assessments contributed to the third assessment, a conclusion about the causal relationship.
Weight of Epidemiologic Evidence
Each peer-reviewed epidemiologic study was evaluated for its methodologic limitations (e.g., flawed measurement of either vaccine administration or adverse event, or failure to adequately control confounding variables) and for the precision of the reported results (e.g., the width of the 95% confidence interval around an effect estimate, reflecting the statistical power to detect a significantly increased risk of an adverse event). A specific study involving multiple outcomes or vaccines could have fewer limitations for the analysis of some vaccines or some outcomes than for others. Small clinical studies can be well conducted but the low number of subjects may limit the ability to detect most adverse events. Although most efficacy studies
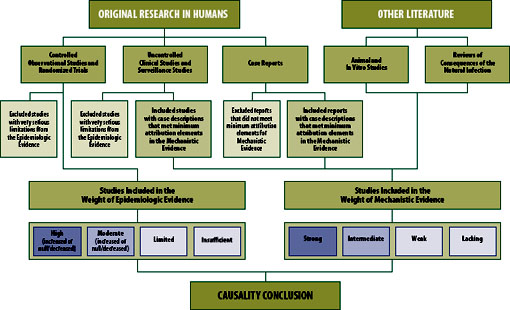
FIGURE S-1 Epidemiologic and mechanistic evidence reviewed by the committee.
include a safety component, the results are often nonspecific (e.g., “no serious adverse events were detected”). The committee was rigorous in assessing the strengths and weaknesses of each epidemiologic study. Some studies reviewed are likely the most reasonably methodologically sound given the nature of the exposure and the outcomes, even if the studies have some residual limitation due to the challenges that often attend such research. Summary paragraphs describe the epidemiologic evidence (as well as the mechanistic evidence and in some circumstances the causality conclusion) more fully than can be captured with the formal and consistent wording of the assessments used in this report.
The committee used a summary classification scheme that incorporates both the quality and quantity of the individual epidemiologic studies and the consistency of the group of studies in terms of direction of effect (i.e., whether the vaccine increases risk, decreases risk, or has no effect on risk). Integral to the assessment is the confidence the committee has that the true effect lies close to the average overall effect estimate for the body of evidence (i.e., collection of reports) reviewed (Schunemann et al., 2011).
The four weight-of-evidence assessments for the epidemiologic evidence are
- High: Two or more studies with negligible methodological limitations that are consistent in terms of the direction of the effect, and taken together provide high confidence.
- Moderate: One study with negligible methodological limitations, or a collection of studies generally consistent in terms of the direction of the effect, that provides moderate confidence.
- Limited: One study or a collection of studies lacking precision or consistency that provides limited, or low, confidence.
- Insufficient: No epidemiologic studies of sufficient quality.
Assessments of high and moderate include a direction of effect. These are to indicate increased risk of the adverse event, decreased risk of the adverse event, or no change in risk of the adverse event or “null.” Assessments of limited or insufficient include no direction of effect.
Weight of Mechanistic Evidence
The committee assessed the mechanisms by which the vaccine could cause a specific adverse event by identifying and evaluating clinical and biological evidence. First, the committee searched for evidence in the peer-reviewed literature that a vaccine was or may be a cause of an adverse event in one or more persons (from case reports or clinical studies) in a reasonable time period after the vaccination. Then the committee looked
for other information from the clinical and biological (human, animal, or in vitro studies) literature that would provide evidence of a pathophysiological process or mechanism that is reasonably likely to cause the adverse event or to occur in response to a specific immunization. Chapter 3 contains a discussion of the major mechanisms the committee invokes as possible explanations of how a given adverse event can occur after vaccination.
The committee identified many case reports in the literature describing adverse events following vaccination. For the purposes of this report, case report refers to a description of an individual patient; one publication could describe multiple case reports. The committee evaluated each case report using a well-established set of criteria called attribution elements for case evaluation (Miller et al., 2000). At a minimum, for a case to factor into the weight-of-evidence assessment, it had to include specific mention of the vaccine administered, evidence of a clinician-diagnosed health outcome, and a specified and reasonable time interval (i.e., temporality or latency) between vaccination and symptoms. Case descriptions that did not have the three basic elements described above were not considered in the mechanistic weight-of-evidence assessments. These three criteria were only necessary but not sufficient to affect the weight of mechanistic evidence. After identifying cases with the three basic elements, the committee looked for evidence in the case descriptions and in other clinical or biological literature of a possible operative mechanism(s) that would support a judgment that the vaccination was related to the adverse event. See Chapter 3 for a description of possible mechanisms identified by the committee.
An important attribute in the evaluation of the clinical evidence from case reports is rechallenge, an adverse event that occurred after more than one administration of a particular vaccine in the same individual. Each challenge in a patient, however, must meet the same attributes of reasonable latency, documentation of vaccination receipt, and clinician diagnosis of the health outcome. The value of any case report is much greater if the clinical workup eliminated well-accepted alternative explanations for the condition, thus increasing the possibility that the vaccine could be associated with the adverse event. A particularly strong piece of evidence in the case description is laboratory-confirmed isolation of a vaccine strain virus in the patient.
The committee follows the convention of previous IOM committees in considering the effects of the natural infection as one type, albeit minor, of clinical or biological evidence in support of mechanisms.6 Other evidence,
___________
6 The committee relied on standard textbooks of infectious disease or internal medicine for this evaluation; the committee did not review original research to come to this determination. This is consistent with previous IOM committees tasked with reviewing evidence of causality for vaccine safety. Evidence consisting only of parallels with the natural infections is never sufficient to merit a conclusion other than the evidence is inadequate to accept or reject a causal relationship.
described above, provided much stronger evidence in support of the mechanistic assessment. Evidence from animal studies is also informative if the model of the disease (adverse outcome) is well established as applicable to humans, or if the basic immunology of the vaccine reaction is well understood. In vitro studies can also be informative, but such data were eyed with skepticism regarding their relationship to the human experience.
The committee developed categories for the mechanistic weight-of-evidence assessment. Each assessment includes consideration of the clinical information from case reports and consideration of clinical and experimental evidence from other sources. The four weight-of-evidence assessments for the mechanistic evidence are
- Strong: One or more cases in the literature, for which the committee concludes the vaccine was a contributing cause of the adverse event, based on an overall assessment of attribution in the available cases and clinical, diagnostic, or experimental evidence consistent with relevant biological response to vaccine.
- Intermediate: At least two cases, taken together, for which the committee concludes the vaccine may be a contributing cause of the adverse event, based on an overall assessment of attribution in the available cases and clinical, diagnostic, or experimental evidence consistent with relevant biological response to vaccine. On occasion, the committee reviewed evidence consisting of at least two cases that, taken together, while suggestive, are nonetheless insufficient to conclude that the vaccine may be a contributing cause of the adverse event. This evidence has been categorized as “low-intermediate.”
- Weak: Insufficient evidence from cases in the literature for the committee to conclude the vaccine may be a contributing cause of the adverse event, based on an overall assessment of attribution in the available cases and clinical, diagnostic, or experimental evidence consistent with relevant biological response to vaccine.
- Lacking: No clinical, diagnostic, or experimental evidence consistent with relevant biological response to vaccine, regardless of the presence of individual cases in the literature.
The committee adopted the approach to causation developed by previous IOM committees. Implicit in these categories is that “the absence of evidence is not evidence of absence.” That is, the committee began its assessment from the position of neutrality; until all evidence was reviewed, it presumed neither causation nor lack of causation. The committee then
moved from that position only when the combination of epidemiologic evidence and mechanistic evidence suggested a more definitive assessment regarding causation, either that vaccines might or might not pose an increased risk of an adverse effect. The categories of causation used by the committee are the following:
- Evidence convincingly supports7 a causal relationship—This applies to relationships in which the causal link is convincing, as with the oral polio vaccine and vaccine-associated paralytic polio.
- Evidence favors acceptance of a causal relationship—Evidence is strong and generally suggestive, although not firm enough to be described as convincing or established.
- Evidence is inadequate to accept or reject a causal relationship— The evidence is not reasonably convincing either in support of or against causality; evidence that is sparse, conflicting, of weak quality, or merely suggestive—whether toward or away from causality—falls into this category. Where there is no evidence meeting the standards described above, the committee also uses this causal conclusion.
- Evidence favors rejection of a causal relationship—The evidence is strong and generally convincing, and suggests there is no causal relationship.
The category of “establishes or convincingly supports no causal relationship” is not used because it is virtually impossible to prove the absence of a relationship with the same certainty that is possible in establishing the presence of one. Even in the presence of a convincing protective effect of a vaccine based on epidemiology, studies may not rule out the possibility that the reaction is caused by vaccine in a subset of individuals. Thus, the framework for this and previous IOM reports on vaccine safety is asymmetrical. The committee began not by assuming the causal relationship does not exist, but by requiring evidence to shift away from the neutral position that the evidence is “inadequate to accept or reject” a causal relationship.
The committee established a general framework by which the two streams of evidence (epidemiologic and mechanistic) influence the final causality conclusion (see Figure S-2). This framework needed to accommodate the reality that—for any given adverse event relationship reviewed—one or both of the types of evidence could be lacking, the two types of evidence could conflict, or neither type of evidence might definitively influence the causality conclusion. The framework does not accommodate any information regarding the benefit of the vaccine to either population or individual
___________
7 Previous IOM committees used the term “establishes” instead of “convincingly supports.”
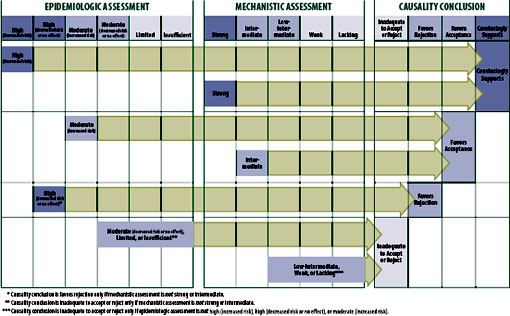
FIGURE S-2 Strength of evidence that determined the causality conclusions.
health. The focus of this particular committee is only on the question of what particular vaccines can cause particular adverse effects.
The framework also had to accommodate known strengths and limitations of both types of evidence. Mechanistic evidence can only support causation, but epidemiologic evidence can support a causal association or can support the absence of (“rejection of”) a causal association in the general population. Mechanistic evidence, particularly that emerging from case reports, occasionally provides compelling evidence of an association between exposure to a vaccine and an adverse event in the individual being studied, but it provides no meaningful information about the risk to the population. Epidemiologic analyses are usually unable to detect an increased or decreased risk that is small, unless the study population is very large or the between-group (e.g., vaccinated vs. unvaccinated) difference in risk is very high (e.g., smoking increases the risk of lung cancer by at least 10-fold). Epidemiologic analyses also cannot identify with certainty which individual in a population at risk will develop the condition.
The committee does not consider a single epidemiologic study—regardless of how well it is designed, the size of the estimated effect, or the narrowness of the confidence interval—sufficient to merit a weight of “high” or, in the absence of strong or intermediate mechanistic evidence, sufficient evidence to support a causality conclusion other than “inadequate to accept or reject a causal relationship.” This requirement might seem overly rigorous to some readers. However, the Agency for Healthcare Research and Quality advises the Evidence-based Practice Centers that it has funded to produce evidence reports on important issues in health care to view an evidence base of a single study with caution (Owens et al., 2010). It does so due to the inability to judge consistency of results, an important contributor to a strength of evidence, because one cannot “be certain that a single trial, no matter how large or well designed, presents the definitive picture of any particular clinical benefit or harm for a given treatment” (Owens et al., 2010). It is acknowledged by the committee and others (Owens et al., 2010) that policy makers must often make decisions based on only one study. However, the committee is not recommending policy, rather evaluating the evidence using a transparent and justifiable framework.
Convincingly Supports
The framework allows for a causality conclusion of “convincingly supports” based on an epidemiologic weight-of-evidence assessment of high in the direction of increased risk (which requires at least two well-conducted epidemiologic studies). Strong mechanistic evidence, which requires at least
one case report in which compelling evidence exists that the vaccine indeed did cause the adverse event, always carries sufficient weight for the committee to conclude the evidence convincingly supports a causal relationship. The committee considered the detection of laboratory-confirmed, vaccine-strain virus compelling evidence to attribute the disease to the vaccine-strain virus and not other etiologies. This conclusion can be reached even if the epidemiologic evidence is rated high in the direction of no increased risk or even decreased risk.
The simplest explanation in this circumstance is that the adverse effect is real but also very rare. Stating this another way, if the vaccine did cause the adverse effect in one person, then it can cause the adverse effect in someone else; however, the isolated report of one convincing case provides no information about the risk of the adverse effect in the total population of vaccinated individuals compared with unvaccinated individuals.
The committee concluded the evidence convincingly supports 14 specific vaccine–adverse event relationships. In all but one of these relationships, the conclusion was based on strong mechanistic evidence with the epidemiologic evidence rated as either limited confidence or insufficient. The convincing evidence regarding varicella vaccine and disseminated Oka varicella zoster virus (VZV) and Oka VZV viral reactivation depended on identification of vaccine-strain virus as documented by polymerase chain reaction, as was the evidence regarding MMR vaccine and measles inclusion body encephalitis. Epidemiologic evidence, as well as mechanistic evidence, convincingly supported the causal relationship between MMR vaccine and febrile seizures. Clinical evidence from case reports and a well-identified mechanism of hypersensitivity reactions convincingly supported the conclusions regarding anaphylaxis and six vaccines (MMR, varicella, influenza, hepatitis B, meningococcal, and tetanus toxoid vaccine). Mechanistic evidence provided the convincing support for the conclusion that injection of vaccine, independent of the antigen involved, can lead to two adverse events: syncope and deltoid bursitis (see Table S-2).
Favors Acceptance
A conclusion of “favors acceptance of a causal relationship” must be supported by either epidemiologic evidence of moderate certainty of an increased risk or by mechanistic evidence of intermediate weight. The committee concluded the evidence favors acceptance of four specific vaccine– adverse event relationships. These include HPV vaccine and anaphylaxis, MMR vaccine and transient arthralgia in female adults, MMR vaccine and transient arthralgia in children, and certain trivalent influenza vaccines used in Canada and a mild and temporary oculorespiratory syndrome. The conclusion regarding anaphylaxis was supported by only mechanistic evidence.
TABLE S-2 Summary of Causality Conclusionsa
|
|
||||||||
| Chapter | Vaccine | Adverse Event | Epidemiologic Assessment | Studies Contributing to the Epidemiologic Assessment |
Mechanistic Assessment | Cases Contributing to the Mechanistic Assessment |
Causality Conclusion | |
|
|
||||||||
| S | Varicella | Disseminated Oka VZV without Other Organ Involvement | Insufficient | None | Strong | _ b | Convincingly Supports | |
| 5 | Varicella | Disseminated Oka | Limited (subsequent | 1 | Strong | 9 | Convincingly | |
| VZV with Subsequent | infection resulting in | (in individuals | Supportsc | |||||
| Infection Resulting | pneumonia) | with demonstrated | ||||||
| in Pneumonia, Meningitis, or Hepatitis | Insufficient (subsequent infection resulting in meningitis or hepatitis) | immunodeficiencies) | ||||||
| S | Varicella | Vaccine Strain Viral Reactivation without Other Organ Involvement | Insufficient | None | Strong | _ b | Convincingly Supports | |
| 5 | Varicella | Vaccine Strain Viral Reactivation with Subsequent Infection Resulting in Meningitis or Encephalitis | Limited (subsequent infection resulting in encephalitis) Insufficient (subsequent infection resulting in meningitis) | 1 | Strong | 6 | Convincingly Supports | |
|
|
||||||||
| Chapter | Vaccine | Adverse Event | Epidemiologic Assessment | Studies Contributing to the Epidemiologic Assessment |
Mechanistic Assessment | Cases Contributing to the Mechanistic Assessment |
Causality Conclusion | |
|
|
||||||||
| 4 | MMR | Measles Inclusion Body Encephalitis | Insufficient | None | Strong (measles; in individuals with demonstrated immunodeficiencies) Lacking (mumps or rubella) | 1 None | Convincingly Supportsc,d | |
| 4 | MMR | Febrile Seizures | High (increase) | 7 | Intermediate | 12 | Convincingly Supports | |
| 4 | MMR | Anaphylaxis | Insufficient | None | Strong | 43e | Convincingly Supports | |
| 5 | Varicella | Anaphylaxis | Limited | 1 | Strong | 76f | Convincingly Supports | |
| 6 | Influenza | Anaphylaxis | Limited | 1 | Strong | 22 | Convincingly Supports | |
| 8 | Heparins B | Anaphylaxis | Insufficient | None | Strong (in yeast-sensitive individuals) | 10 | Convincingly Supportsg | |
| 10 | Tetanus Toxoid | Anaphylaxis | Insufficient | None | Strong | 6 | Convincingly Supports | |
| 11 | Meningococcal | Anaphylaxis | Insufficient | None | Strong | 1 | Convincingly Supports | |
| 12 | Injection-Related Event | Deltoid Bursitis | Limited | 1 | Strong | 16 | Convincingly Supports |
| 12 | Injection-Related Event | Syncope | Insufficient | None | Strong | 35b | Convincingly Supports |
| 9 | HPV | Anaphylaxis | Insufficient | None | Intermediate | 36 | Favors Acceptance |
| 4 | MMR | Transient Arthralgia in Women | Moderate (increase) (rubella) Insufficient (measles or mumps) | 4 None | Intermediate (rubella) Lacking (measles or mumps) | 13 None | Favors Acceptancei |
| 4 | MMR | Transient Arthralgia in Children | Moderate (increase) | 7 | Weak (rubella) Lacking (measles or mumps) | None None | Favors Acceptance |
| 6 | Influenza | Oculorespiratory Syndrome | Moderate (increase) | 3 | Intermediate | _ j | Favors Acceptancek |
| 4 | MMR | Autism | High (null) | 4 | Lacking | None | Favors Rejection |
| 6 | Influenza | Inactivated Influenza Vaccine and Bell's palsy | High (null) | 2 | Lacking | None | Favors Rejection |
|
|
||||||||
| Chapter | Vaccine | Adverse Event | Epidemiologic Assessment | Studies Contributing to the Epidemiologic Assessment |
Mechanistic Assessment | Cases Contributing to the Mechanistic Assessment |
Causality Conclusion | |
|
|
||||||||
| 6 | Influenza | Inactivated Influenza Vaccine and Asthma Exacerbation or Reactive Airway Disease Episodes in Children and Adults | High (null) | 9 | Weak | 6 | Favors Rejection | |
| 4 | MMR | Type 1 Diabetes | High (null) | 5 | Lacking | None | Favors Rejection | |
| 10 | DT, TT, or aP | Type 1 Diabetes | High (null) | 5 | Lacking | None | Favors | |
| containing | Rejection | |||||||
|
|
||||||||
a All other causality conclusions are the evidence is inadequate to accept or reject a causal relationship.
b Due to the use of the same surveillance systems in some publications it is likely that some of the cases were presented more than once; thus, it is difficult to determine the number of unique cases.
c The committee attributes causation to individuals with demonstrated immunodeficiencies.
d The committee attributes causation to the measles component of the vaccine.
e Some cases were from passive surveillance systems; however, it is not possible to know how many represent unique cases or were reported elsewhere.
fIn addition, at least 30 cases were reported to passive surveillance systems; however, it is not possible to know how many represent unique cases or were reported elsewhere.
gThe committee attributes causation to yeast-sensitive individuals.
hIn addition, hundreds of cases have been reported to passive surveillance systems; however, it is not possible to known how many represent unique cases or were reported elsewhere.
i The committee attributes causation to the rubella component of the vaccine.
jDue to the use of the same sample population in some studies it is likely that some of the cases were presented in more than one publication; thus, it is difficult to determine the number of unique cases.
kThe committee attributes causation to two particular vaccines used in three particular years in Canada.
The other conclusions were supported by both epidemiologic evidence and by mechanistic evidence (see Table S-2).
Favors Rejection
The framework allows the committee to “favor rejection” of a causal relationship only in the face of epidemiologic evidence rated as high or moderate in the direction of no effect (the null) or of decreased risk and in the absence of strong or intermediate mechanistic evidence in support of a causal relationship. The committee concluded the evidence favors rejection of five vaccine–adverse event relationships. These include MMR vaccine and type 1 diabetes, diphtheria, tetanus, and pertussis (DTaP) vaccine and type 1 diabetes, MMR vaccine and autism, inactivated influenza vaccine and asthma exacerbation or reactive airway disease episodes, and inactivated influenza vaccine and Bell’s palsy. The evidence base for these conclusions consisted of epidemiologic studies reporting no increased risk; this evidence was not countered by mechanistic evidence (see Table S-2).
Inadequate to Accept or Reject
The committee identified two main pathways by which it concludes that the evidence is “inadequate to accept or reject” a causal relationship. The most common pathway to this conclusion occurs when the epidemiologic evidence was of limited certainty or insufficient and the mechanistic evidence was weak or lacking. Another pathway occurs when the epidemiologic evidence is of moderate certainty of no effect but the mechanistic evidence is intermediate in support of an association. The committee analyzed these sets of apparently contradictory evidence and ultimately depended upon their expert judgment in deciding if a conclusion to favor acceptance based on the intermediate mechanistic data was warranted, or if the conclusion remained as “inadequate to accept or reject” a causal relationship.
The vast majority of causality conclusions in the report are that the evidence was inadequate to accept or reject a causal relationship. Some might interpret that to mean either of the following statements:
- Because the committee did not find convincing evidence that the vaccine does cause the adverse event, the vaccine is safe.
- Because the committee did not find convincing evidence that the vaccine does not cause the adverse event, the vaccine is unsafe.
Neither of these interpretations is correct. “Inadequate to accept or reject” means just that—inadequate. If there is evidence in either direction that is suggestive but not sufficiently strong about the causal relationship, it
will be reflected in the weight-of-evidence assessments of the epidemiologic or the mechanistic data. However suggestive those assessments might be, in the end the committee concluded that the evidence was inadequate to accept or reject a causal association.
A list of all conclusions, including the weights of evidence for both the epidemiologic evidence and the mechanistic evidence, can be found in Appendix D.
The literature supporting several of the causality conclusions discussed in the previous section indicates that individuals with certain characteristics are more likely to suffer adverse effects from particular immunizations. Individuals with an acquired or genetic immunodeficiency are clearly recognized as at increased risk for specific adverse reactions to live viral vaccines such as MMR and varicella vaccine. Age is also a risk factor; seizures after immunization, for example, are more likely to occur in young children. Thus, the committee was able at times to reach more limited conclusions that did not generalize to the entire population.
Committee members spent an enormous amount of time reading thousands of articles. The committee makes 158 causality conclusions in this report. It tried to apply consistent standards when reviewing individual articles and when assessing the bodies of evidence. Some of the conclusions were easy to reach; the evidence was clear and consistent or, in the extreme, completely absent. Some conclusions required substantial discussion and debate. Inevitably, there are elements of expert clinical and scientific judgment involved.
The committee used the best evidence available at the time. The committee hopes that the report is sufficiently transparent such that when new information emerges from either the clinic or the laboratory, others will be able to assess the importance of that new information within the approach and set of conclusions presented in this report.
IOM (Institute of Medicine). 1991. Adverse effects of pertussis and rubella vaccines: A report of the committee to review the adverse consequences of pertussis and rubella vaccines. Washington, DC: National Academy Press.
IOM. 1994. Adverse events associated with childhood vaccines: Evidence bearing on causality. Washington, DC: National Academy Press.
IOM. 2001a. Immunization safety review: Measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press.
IOM. 2001b. Immunization safety review: Thimerosal-containing vaccines and neuro-developmental disorders. Washington, DC: National Academy Press.
IOM. 2002a. Immunization safety review: Hepatitis B vaccine and demyelinating neurological disorders. Washington, DC: The National Academies Press.
IOM. 2002b. Immunization safety review: Multiple immunizations and immune dysfunction. Washington, DC: National Academy Press.
IOM. 2003a. Immunization safety review: SV40 contamination of polio vaccine and cancer. Washington, DC: The National Academies Press.
IOM. 2003b. Immunization safety review: Vaccinations and sudden unexpected death in infancy. Washington, DC: The National Academies Press.
IOM. 2004a. Immunization safety review: Influenza vaccines and neurological complications. Washington, DC: The National Academies Press.
IOM. 2004b. Immunization safety review: Vaccines and autism. Washington, DC: The National Academies Press.
Johann-Liang, R. 2009. Charge to the Institute of Medicine committee to review adverse effects of vaccines. Paper read at the Meeting of the Institute of Medicine Committee to Review Adverse Effects of Vaccines, Washington, DC.
Miller, F. W., E. V. Hess, D. J. Clauw, P. A. Hertzman, T. Pincus, R. M. Silver, M. D. Mayes, J. Varga, T. A. Medsger, Jr., and L. A. Love. 2000. Approaches for identifying and defining environmentally associated rheumatic disorders. Arthritis & Rheumatism 43(2):243-249.
Owens, D. K., K. N. Lohr, D. Atkins, J. R. Treadwell, J. T. Reston, E. B. Bass, S. Chang, and M. Helfand. 2010. AHRQ series paper 5: Grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health Care Program. Journal of Clinical Epidemiology 63(5):513-523.
Schunemann, H. J., S. Hill, G. H. Guyatt, E. A. Akl, and F. Ahmed. 2011. The GRADE approach and Bradford Hill’s criteria for causation. Journal of Epidemiology and Community Health 65(5):392-395.


























